,-- ,,,., -,L , ' ~.. ' ''. -i-~-...1.,,.". '., ~-. L ' $!.." ~ "' ~ )$' I o,j,(' Jc>o-'..o" ~ i!.'.., / \ -, ~ r ~ ""'
|
|
|
- Erik Erick Leonard
- 5 years ago
- Views:
Transcription
1 ; _ ; L ) L_ = _) _L L: y : ;Ly ; Q eeoc OF R:L _ 4 $ L ee;; ; : ] :2 o 5 aa u :> * ; L L : _ :_ L : L_ LL L L ) + LL : :::;;= L B L+ _ L L )$ o( : 9 :2 :) :4: a _ c c + L f E:LE:VTON O O :: o a e: ec> CMNS NC f : ON <; o ecc;;;& c>oo 386 ; :; ( f 4 L f f L L > 4 2: ;:: T L (; L: f _; L 4 f 4 7d _ : o:: o: ::o::::: o_: :: o:: :_a::_: o: :: : o : :a_:== H f [?=:::::o:::_a::::c < ===;:==d::::::a::::o LocTON PLN e _ 4 7 ) TO GE:T o CL;: MQ>T o PFZ:fTf :::e:ctffc TPCXo o 5U ST CQVffC ac ff>qk L ; L L L L L L ) < f : 5CCTON ee f o ) : SE:CTON :T <_ : 4 L L L {o f&:; TON: C TKCH ;o T NQLE TO OF SR<:;E:) S:CTON cc : f (GUTT::; FQNT P_oT L_L;:::::::::=E::::::::::=: ELE:VTON E L =o THE WESTf RNPCF C RRCO ENFORCE cocete BRQE T MYSVLLE CL TO RE: PLCE: 6 R OGE: 7B 7;}5f Ce:: ;;
2 CUT WSH C 6Lf:EV ) To V w P c To Vc::;w PCR B e: f _ <f ; S PC:q a BOCS f:)(pn:on FOR SLBS UNER 5oCWLK CLSLE:CVC [ ::;q f :: ::: :: :;:;;;;:; : : :::::::::::: :::: =::::::: :=:: ::: c:: ::::::;: :;::::;:: ;;;;: :_;;::=;;:;;:::::= =:=:=:::::::::;; :::::: :=::::::::: =: ::;;;: ::::: :::::::= ==:::::: :::: ±: ±::::::;z;:;: :::;::;;: 9 SCC:TON HCTo EF c : + + F<FM<CCHT OUZOfTL > c> L P: S h ; f p f f : f d : ff ff f : f : L n u P P H ; n ( : 7: : PCR:S C ; >4LF E:LCVTONS 34 a:f PCR 6 + e CLCVTO PC S S NOCTeC 5:CTON P::R PLTf: E:LCVTOM P C : _ L ::: ; ;;::: ;};L : : _: : : _ :; _: _ ;_ : ; 9 PCP TWO TC:::55=: OF TfPQ f:t MN SLB ::XPNSON f PCR f3 TH WESTERNPCfC RRCO R:HFORCE: CONCE:TE BCE T MYSVLLE CL TO RE: PLCE: BR COE: 787::: OYE:FfS:fST e5 C4f:: S OTE:C CE;F E:NGNEf:R6 OFFCE 5N frmc:sco CL MRCH 5 >d 5 :T N 2 Of W:P N 3842
3 ese o f L f _ cd ;o: : ) + o {: :: 6 & ( b ) _ f { 4q ( :o _6o _L: L g O }: ; : ; : {; > : : ::_ : ;;_: } : : : ; ;> : ; ; E:LE:VTON C C 5 :CTON e }LF ELCVTON SE:CTON 6 6 M &:: 3 fltc o 5CWK SLe (V:fTCU f: O ;4:> Pc>:>:5 PVCT O TO PTC PLUSM m Nb C:T:O ) ) PLTE FOf MN Le 5 o NoT;:; f: CH o e e;: CORHZ:R FOM CfO(::: 4:T TO_ WTM: CXNO:O MCTL &e:ht ;T ( MO P;C: 5OWH ( VS ::::=::S:> :T7:; F_ <C: a WP +T ON) _:+=======::;;::=======:::===== TWO HC:MN:&e: o TffC EL TO fcv:nt SONO 8 TW:M 5Lcf$ N_ &UTM E:NT cxhoh HT of eutm fts ; ; o y ;: ;7 HEWESTERNPCFC RRCO fehforce COCFETE BRC T MRV:S VLLE CL 3TRC:C:T L f: S Cf::: s HoT::O CEF ::NCME:E:fa OCE: SN frnc ecq C MRC :23 >a :_ PLH ; ;_:;;;;+: 5e:e:T N 3 o o; 3843 # f
4 d ;: ; 5 o ; f : : ) 6 : $ < o : 9 : : ( _ M H 4 Mf PN [ o a ; SSE OF C _ SOC C&EVTOH :P 7 : ff ; e 5LB : _ s L ]o ; _;+S :> S6 ;::s 5ECTONCC 6 _:::;:::) ;: : ( :: f} } SECTON E: C b <: fl: u;c ; :: ; _ :::: < : <: :;:;: : {:::=;f ;;:;:::::=: ::::::;2:::=:;::z: : =;; C B Raco HCMo Bo Ts <;UT WT4 SCC:T ON mr =ft +L;( THE WESTERN PCFC RRCO EHFOCCE CONC:FETE BGE T MYSVLLE CL :5 <:> L;:: ;L f: c c < +++ ; 5 CC: ;: 8: o CH:F E:Kc; <::E:Fs OFTGE:: 5N FK<;:soq CL RWH< N 6&: :S;u:&T N 4 OF ;;; 3844
5 _::; L B FCQ L E:TLS o 8sc 4 Fe:q ;: fct &: L:T :TLS Of COF>N o h F <;> QHT & 2 :FT ote: otr oh$ bot g:tm<<(bpcxwork) E:RUSE::O Ou 4: 5:f> :STFE;TCMf:R : COUM:SE:SMT SONO#S f:vs:ry 3V 8RCK u ) s : ===:e===::::=e5::=::::===:=e==o=========:::==ee:==:=e:==e:: :u? : f q o f WEH $L$ f: Be:T M5 $C:: WT CE T <;fou : : ; SccT ONE E : ; : < :: + SECTON + SE:CTON c <; G ; o FLH OF SSE: a sux:wlk SLeus 6 & +:LL : ESON OHT G <: ; : z a_: SECT ON H H :TLS OF PE:EST5 LT : : : f G +a o ::Q PLN Of CO N : [ : : : f ) : f : ; f ; c c+=: f : f ;f + Q { : f : ( ) + P _ : > cxffa offt ; 9: : oee:: TEQ : N TH:S 54T TO EVZHT BONO ) f($u:: FS:C >( MSo T : OF : R Ne; SOT O ffc;o a u cows: N:5 TT 6; :: Re:q _a Uf; h C HH Ls OF f PoST Me:; CNCfL C>ST FOf OT sy M5$ ) f =:+L=+= ===fu CWN >(OCTe:;:> OY _H:$ k:>ht wo& LL WT M$TC TH WESTERN PCFC RRCO FfENFORCE CoNCfETE BR4E T MRYSVLLE CL S<::E;: a ;; Fo CHE:F E:NGMCCR:e Oce 5f FRNC5COCL _ G :6 5; N: s Of :; e 384 5
6 LST OF BS _ 38 TEM _ o c ( $TT: WC<HT fo:: + LS o ee:ft _fs OLY 7o LBS SLB C SKCTCH G SKCTC> N SKCTC> KCTCH SKCTCH K SLB SKCTCH M f CR C :S ) SKCTCH OTE:: use TWS: CCTL YO L BR:S WCRE: 4K S NCTf:C ON5)(S::Tc;X $ ;::> TH WEST RN PCFC RRCO REMfORCE COHCETE: BRGE T MRYSVLLE CL NoTc: soc ::SKCTCHCS SHOW OHC :MT ER o e:<=:> <O e fc FOF<: CNCH( ose:s OHY o COMLE:TE: f:f;e:me:nt OF f3f$ TO E PLCE: SR <:4E: 757> OYER52s :Sce: :5 c C:: ENc;::::s occ :5N FRHcscoCL PRL 2 9e Sf S: :T N C> o G> 3846
T h e C S E T I P r o j e c t
 T h e P r o j e c t T H E P R O J E C T T A B L E O F C O N T E N T S A r t i c l e P a g e C o m p r e h e n s i v e A s s es s m e n t o f t h e U F O / E T I P h e n o m e n o n M a y 1 9 9 1 1 E T
T h e P r o j e c t T H E P R O J E C T T A B L E O F C O N T E N T S A r t i c l e P a g e C o m p r e h e n s i v e A s s es s m e n t o f t h e U F O / E T I P h e n o m e n o n M a y 1 9 9 1 1 E T
176 5 t h Fl oo r. 337 P o ly me r Ma te ri al s
 A g la di ou s F. L. 462 E l ec tr on ic D ev el op me nt A i ng er A.W.S. 371 C. A. M. A l ex an de r 236 A d mi ni st ra ti on R. H. (M rs ) A n dr ew s P. V. 326 O p ti ca l Tr an sm is si on A p ps
A g la di ou s F. L. 462 E l ec tr on ic D ev el op me nt A i ng er A.W.S. 371 C. A. M. A l ex an de r 236 A d mi ni st ra ti on R. H. (M rs ) A n dr ew s P. V. 326 O p ti ca l Tr an sm is si on A p ps
A L A BA M A L A W R E V IE W
 A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
A L A BA M A L A W R E V IE W Volume 52 Fall 2000 Number 1 B E F O R E D I S A B I L I T Y C I V I L R I G HT S : C I V I L W A R P E N S I O N S A N D TH E P O L I T I C S O F D I S A B I L I T Y I N
i;\-'i frz q > R>? >tr E*+ [S I z> N g> F 'x sa :r> >,9 T F >= = = I Y E H H>tr iir- g-i I * s I!,i --' - = a trx - H tnz rqx o >.F g< s Ire tr () -s
 5 C /? >9 T > ; '. ; J ' ' J. \ ;\' \.> ). L; c\ u ( (J ) \ 1 ) : C ) (... >\ > 9 e!) T C). '1!\ /_ \ '\ ' > 9 C > 9.' \( T Z > 9 > 5 P + 9 9 ) :> : + (. \ z : ) z cf C : u 9 ( :!z! Z c (! $ f 1 :.1 f.
5 C /? >9 T > ; '. ; J ' ' J. \ ;\' \.> ). L; c\ u ( (J ) \ 1 ) : C ) (... >\ > 9 e!) T C). '1!\ /_ \ '\ ' > 9 C > 9.' \( T Z > 9 > 5 P + 9 9 ) :> : + (. \ z : ) z cf C : u 9 ( :!z! Z c (! $ f 1 :.1 f.
OH BOY! Story. N a r r a t iv e a n d o bj e c t s th ea t e r Fo r a l l a g e s, fr o m th e a ge of 9
 OH BOY! O h Boy!, was or igin a lly cr eat ed in F r en ch an d was a m a jor s u cc ess on t h e Fr en ch st a ge f or young au di enc es. It h a s b een s een by ap pr ox i ma t ely 175,000 sp ect at
OH BOY! O h Boy!, was or igin a lly cr eat ed in F r en ch an d was a m a jor s u cc ess on t h e Fr en ch st a ge f or young au di enc es. It h a s b een s een by ap pr ox i ma t ely 175,000 sp ect at
I N A C O M P L E X W O R L D
 IS L A M I C E C O N O M I C S I N A C O M P L E X W O R L D E x p l o r a t i o n s i n A g-b eanste d S i m u l a t i o n S a m i A l-s u w a i l e m 1 4 2 9 H 2 0 0 8 I s l a m i c D e v e l o p m e
IS L A M I C E C O N O M I C S I N A C O M P L E X W O R L D E x p l o r a t i o n s i n A g-b eanste d S i m u l a t i o n S a m i A l-s u w a i l e m 1 4 2 9 H 2 0 0 8 I s l a m i c D e v e l o p m e
P a g e 5 1 of R e p o r t P B 4 / 0 9
 P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
P a g e 5 1 of R e p o r t P B 4 / 0 9 J A R T a l s o c o n c l u d e d t h a t a l t h o u g h t h e i n t e n t o f N e l s o n s r e h a b i l i t a t i o n p l a n i s t o e n h a n c e c o n n e
P a g e 3 6 of R e p o r t P B 4 / 0 9
 P a g e 3 6 of R e p o r t P B 4 / 0 9 p r o t e c t h um a n h e a l t h a n d p r o p e r t y fr om t h e d a n g e rs i n h e r e n t i n m i n i n g o p e r a t i o n s s u c h a s a q u a r r y. J
P a g e 3 6 of R e p o r t P B 4 / 0 9 p r o t e c t h um a n h e a l t h a n d p r o p e r t y fr om t h e d a n g e rs i n h e r e n t i n m i n i n g o p e r a t i o n s s u c h a s a q u a r r y. J
Executive Committee and Officers ( )
 Gifted and Talented International V o l u m e 2 4, N u m b e r 2, D e c e m b e r, 2 0 0 9. G i f t e d a n d T a l e n t e d I n t e r n a t i o n a2 l 4 ( 2), D e c e m b e r, 2 0 0 9. 1 T h e W o r
Gifted and Talented International V o l u m e 2 4, N u m b e r 2, D e c e m b e r, 2 0 0 9. G i f t e d a n d T a l e n t e d I n t e r n a t i o n a2 l 4 ( 2), D e c e m b e r, 2 0 0 9. 1 T h e W o r
THIS PAGE DECLASSIFIED IAW EO IRIS u blic Record. Key I fo mation. Ma n: AIR MATERIEL COMM ND. Adm ni trative Mar ings.
 T H S PA G E D E CLA SSFED AW E O 2958 RS u blc Recod Key fo maon Ma n AR MATEREL COMM ND D cumen Type Call N u b e 03 V 7 Rcvd Rel 98 / 0 ndexe D 38 Eneed Dae RS l umbe 0 0 4 2 3 5 6 C D QC d Dac A cesson
T H S PA G E D E CLA SSFED AW E O 2958 RS u blc Recod Key fo maon Ma n AR MATEREL COMM ND D cumen Type Call N u b e 03 V 7 Rcvd Rel 98 / 0 ndexe D 38 Eneed Dae RS l umbe 0 0 4 2 3 5 6 C D QC d Dac A cesson
Last 4 Digits of USC ID:
 Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
THIS PAGE DECLASSIFIED IAW E
 THS PAGE DECLASSFED AW E0 2958 BL K THS PAGE DECLASSFED AW E0 2958 THS PAGE DECLASSFED AW E0 2958 B L K THS PAGE DECLASSFED AW E0 2958 THS PAGE DECLASSFED AW EO 2958 THS PAGE DECLASSFED AW EO 2958 THS
THS PAGE DECLASSFED AW E0 2958 BL K THS PAGE DECLASSFED AW E0 2958 THS PAGE DECLASSFED AW E0 2958 B L K THS PAGE DECLASSFED AW E0 2958 THS PAGE DECLASSFED AW EO 2958 THS PAGE DECLASSFED AW EO 2958 THS
35H MPa Hydraulic Cylinder 3.5 MPa Hydraulic Cylinder 35H-3
 - - - - ff ff - - - - - - B B BB f f f f f f f 6 96 f f f f f f f 6 f LF LZ f 6 MM f 9 P D RR DD M6 M6 M6 M. M. M. M. M. SL. E 6 6 9 ZB Z EE RC/ RC/ RC/ RC/ RC/ ZM 6 F FP 6 K KK M. M. M. M. M M M M f f
- - - - ff ff - - - - - - B B BB f f f f f f f 6 96 f f f f f f f 6 f LF LZ f 6 MM f 9 P D RR DD M6 M6 M6 M. M. M. M. M. SL. E 6 6 9 ZB Z EE RC/ RC/ RC/ RC/ RC/ ZM 6 F FP 6 K KK M. M. M. M. M M M M f f
Element Cube Project (x2)
 Element Cube Project (x2) Background: As a class, we will construct a three dimensional periodic table by each student selecting two elements in which you will need to create an element cube. Helpful Links
Element Cube Project (x2) Background: As a class, we will construct a three dimensional periodic table by each student selecting two elements in which you will need to create an element cube. Helpful Links
F l a s h-b a s e d S S D s i n E n t e r p r i s e F l a s h-b a s e d S S D s ( S o-s ltiad t e D r i v e s ) a r e b e c o m i n g a n a t t r a c
 L i f e t i m e M a n a g e m e n t o f F l a-b s ah s e d S S D s U s i n g R e c o v e r-a y w a r e D y n a m i c T h r o t t l i n g S u n g j i n L e, e T a e j i n K i m, K y u n g h o, Kainmd J
L i f e t i m e M a n a g e m e n t o f F l a-b s ah s e d S S D s U s i n g R e c o v e r-a y w a r e D y n a m i c T h r o t t l i n g S u n g j i n L e, e T a e j i n K i m, K y u n g h o, Kainmd J
[ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34.
![[ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34. [ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34.](/thumbs/74/69911105.jpg) .. - 2-2009 [661.87.+661.88]:543.4(075.8) 35.20:34.2373-60..,..,..,..,.. -60 : /... ;. 2-. : -, 2008. 134. ISBN 5-98298-299-7 -., -,,. - «,, -, -», - 550800,, 240600 «-», -. [661.87.+661.88]:543.4(075.8)
.. - 2-2009 [661.87.+661.88]:543.4(075.8) 35.20:34.2373-60..,..,..,..,.. -60 : /... ;. 2-. : -, 2008. 134. ISBN 5-98298-299-7 -., -,,. - «,, -, -», - 550800,, 240600 «-», -. [661.87.+661.88]:543.4(075.8)
Geometric Predicates P r og r a m s need t o t es t r ela t ive p os it ions of p oint s b a s ed on t heir coor d ina t es. S im p le exa m p les ( i
 Automatic Generation of SS tag ed Geometric PP red icates Aleksandar Nanevski, G u y B lello c h and R o b ert H arp er PSCICO project h ttp: / / w w w. cs. cm u. ed u / ~ ps ci co Geometric Predicates
Automatic Generation of SS tag ed Geometric PP red icates Aleksandar Nanevski, G u y B lello c h and R o b ert H arp er PSCICO project h ttp: / / w w w. cs. cm u. ed u / ~ ps ci co Geometric Predicates
5 questions, 3 points each, 15 points total possible. 26 Fe Cu Ni Co Pd Ag Ru 101.
 Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
Solutions and Ions. Pure Substances
 Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr
 Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Speed of light c = m/s. x n e a x d x = 1. 2 n+1 a n π a. He Li Ne Na Ar K Ni 58.
 Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
30 Zn(s) 45 Rh. Pd(s) Ag(s) Cd(s) In(s) Sn(s) white. 77 Ir. Pt(s) Au. Hg(l) Tl. 109 Mt. 111 Uuu. 112 Uub. 110 Uun. 65 Tb. 62 Sm. 64 Gd. 63 Eu.
 Enthalpy changes: experimentally it is much easier to measure heat flow at const pressure - this is enthalpy q p = )H : also nearly all chemical reactions are done at constant pressure. Enthalpy (heat)
Enthalpy changes: experimentally it is much easier to measure heat flow at const pressure - this is enthalpy q p = )H : also nearly all chemical reactions are done at constant pressure. Enthalpy (heat)
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
". :'=: "t',.4 :; :::-':7'- --,r. "c:"" --; : I :. \ 1 :;,'I ~,:-._._'.:.:1... ~~ \..,i ... ~.. ~--~ ( L ;...3L-. ' f.':... I. -.1;':'.
 = 47 \ \ L 3L f \ / \ L \ \ j \ \ 6! \ j \ / w j / \ \ 4 / N L5 Dm94 O6zq 9 qmn j!!! j 3DLLE N f 3LLE Of ADL!N RALROAD ORAL OR AL AOAON N 5 5 D D 9 94 4 E ROL 2LL RLLAY RL AY 3 ER OLLL 832 876 8 76 L A
= 47 \ \ L 3L f \ / \ L \ \ j \ \ 6! \ j \ / w j / \ \ 4 / N L5 Dm94 O6zq 9 qmn j!!! j 3DLLE N f 3LLE Of ADL!N RALROAD ORAL OR AL AOAON N 5 5 D D 9 94 4 E ROL 2LL RLLAY RL AY 3 ER OLLL 832 876 8 76 L A
CHEM 167 FINAL EXAM MONDAY, MAY 2 9:45 11:45 A.M GILMAN HALL
 PROF. JOHN VERKADE SPRING 2005 THIS EXAM CONSISTS OF 12 QUESTIONS ON 9 PAGES CHEM 167 HOUR EXAM IV APRIL 20, 2005 SEAT NO. NAME RECIT. INSTR. RECIT. SECT. GRADING PAGE Page 2 Page 3 Page 4 Page 5 Page
PROF. JOHN VERKADE SPRING 2005 THIS EXAM CONSISTS OF 12 QUESTIONS ON 9 PAGES CHEM 167 HOUR EXAM IV APRIL 20, 2005 SEAT NO. NAME RECIT. INSTR. RECIT. SECT. GRADING PAGE Page 2 Page 3 Page 4 Page 5 Page
The Periodic Table. Periodic Properties. Can you explain this graph? Valence Electrons. Valence Electrons. Paramagnetism
 Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
ATTACHMENT 1. MOUNTAIN PARK LAND Page 2 of 5
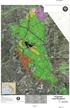 ATTACHMENT 53 YOBA LINDA Gyp Yb gl 9 Gt Fthly gl t 247 Mt Utct cl Mt Gyp 248 248X A0 A0 Hghy G:\jct\K00074232_p_Iv_Cp_L_Dt_204\MXD\p_Iv_Cp_L_Dt_(K00074232)_0-29-204.x C ( l t b t f y p b lc ly ) Ch Hll
ATTACHMENT 53 YOBA LINDA Gyp Yb gl 9 Gt Fthly gl t 247 Mt Utct cl Mt Gyp 248 248X A0 A0 Hghy G:\jct\K00074232_p_Iv_Cp_L_Dt_204\MXD\p_Iv_Cp_L_Dt_(K00074232)_0-29-204.x C ( l t b t f y p b lc ly ) Ch Hll
The Periodic Table of Elements
 The Periodic Table of Elements 8 Uuo Uus Uuh (9) Uup (88) Uuq (89) Uut (8) Uub (8) Rg () 0 Ds (9) 09 Mt (8) 08 Hs (9) 0 h () 0 Sg () 0 Db () 0 Rf () 0 Lr () 88 Ra () 8 Fr () 8 Rn () 8 At (0) 8 Po (09)
The Periodic Table of Elements 8 Uuo Uus Uuh (9) Uup (88) Uuq (89) Uut (8) Uub (8) Rg () 0 Ds (9) 09 Mt (8) 08 Hs (9) 0 h () 0 Sg () 0 Db () 0 Rf () 0 Lr () 88 Ra () 8 Fr () 8 Rn () 8 At (0) 8 Po (09)
8. Relax and do well.
 CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
INSTRUCTIONS: Exam III. November 10, 1999 Lab Section
 CHEM 1215 Exam III John III. Gelder November 10, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 1215 Exam III John III. Gelder November 10, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
Guide to the Extended Step-Pyramid Periodic Table
 Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
CHEM 10113, Quiz 5 October 26, 2011
 CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
Faculty of Natural and Agricultural Sciences Chemistry Department. Semester Test 1. Analytical Chemistry CMY 283. Time: 120 min Marks: 100 Pages: 6
 Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 120 min
Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 120 min
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Agenda Rationale for ETG S eek ing I d eas ETG fram ew ork and res u lts 2
 Internal Innovation @ C is c o 2 0 0 6 C i s c o S y s t e m s, I n c. A l l r i g h t s r e s e r v e d. C i s c o C o n f i d e n t i a l 1 Agenda Rationale for ETG S eek ing I d eas ETG fram ew ork
Internal Innovation @ C is c o 2 0 0 6 C i s c o S y s t e m s, I n c. A l l r i g h t s r e s e r v e d. C i s c o C o n f i d e n t i a l 1 Agenda Rationale for ETG S eek ing I d eas ETG fram ew ork
SPECIFICATION SHEET : WHSG4-UNV-T8-HB
 SPECIFICATION SHEET : WHSG4UNVT8HB ELECTRICAL DATA (120V APPLICATION) INPUT VO LT : 120V ± 10%, 50/60H z LAM P W ATTS/T YPE F17T8 F25T8 F30T8 F 32T8 F32T 8( 25W ) F32T8(28W ) F32T8(30W ) FB31T 8 FB32T8
SPECIFICATION SHEET : WHSG4UNVT8HB ELECTRICAL DATA (120V APPLICATION) INPUT VO LT : 120V ± 10%, 50/60H z LAM P W ATTS/T YPE F17T8 F25T8 F30T8 F 32T8 F32T 8( 25W ) F32T8(28W ) F32T8(30W ) FB31T 8 FB32T8
Secondary Support Pack. be introduced to some of the different elements within the periodic table;
 Secondary Support Pack INTRODUCTION The periodic table of the elements is central to chemistry as we know it today and the study of it is a key part of every student s chemical education. By playing the
Secondary Support Pack INTRODUCTION The periodic table of the elements is central to chemistry as we know it today and the study of it is a key part of every student s chemical education. By playing the
Chemistry 185 Exam #2 - A November 5, Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
If anything confuses you or is not clear, raise your hand and ask!
 CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
CHEM 4641 Fall questions worth a total of 32 points. Show your work, except on multiple-choice questions. 1 V α=
 Physical Chemistry I Final Exam Name: KEY CHEM 4641 Fall 017 15 questions worth a total of 3 points. Show your work, except on multiple-choice questions. 1 V 1 V α= κt = V T P V P T Gas constant R = 8.314
Physical Chemistry I Final Exam Name: KEY CHEM 4641 Fall 017 15 questions worth a total of 3 points. Show your work, except on multiple-choice questions. 1 V 1 V α= κt = V T P V P T Gas constant R = 8.314
HANDOUT SET GENERAL CHEMISTRY II
 HANDOUT SET GENERAL CHEMISTRY II Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
HANDOUT SET GENERAL CHEMISTRY II Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
(C) Pavel Sedach and Prep101 1
 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach
(C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach
fur \ \,,^N/ D7,,)d.s) 7. The champion and Runner up of the previous year shall be allowed to play directly in final Zone.
 OUL O GR SODRY DUTO, ODS,RT,SMTUR,USWR.l ntuctin f cnuct f Kbi ( y/gil)tunent f 2L-Lg t. 2.. 4.. 6. Mtche hll be lye e K ule f ene f tie t tie Dutin f ech tch hll be - +0 (Rece)+ = M The ticint f ech Te
OUL O GR SODRY DUTO, ODS,RT,SMTUR,USWR.l ntuctin f cnuct f Kbi ( y/gil)tunent f 2L-Lg t. 2.. 4.. 6. Mtche hll be lye e K ule f ene f tie t tie Dutin f ech tch hll be - +0 (Rece)+ = M The ticint f ech Te
Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Kr7. wd /J-t- To pick up your graded exam from a box outside 5100A Pacific Hall sign here:
 wd /J-t- To pick up your graded exam from a box outside 5100A Pacific Hall sign here: Kr7 chemtsrry 140A FTNAL EXAM 1v & s 6" Eo1 DEC 8,2011 NAME (please print) SIGNATURE FIBST LAST ID NUMBER LAST NAME
wd /J-t- To pick up your graded exam from a box outside 5100A Pacific Hall sign here: Kr7 chemtsrry 140A FTNAL EXAM 1v & s 6" Eo1 DEC 8,2011 NAME (please print) SIGNATURE FIBST LAST ID NUMBER LAST NAME
A0A TEXAS UNIV AT AUSTIN DEPT OF CHEMISTRY F/G 7/5 PHOTOASSISTED WATER-GAS SHIFT REACTION ON PLATINIZED T7TANIAI T--ETCIUI
 AA113 878 TEXAS UNIV AT AUSTIN DEPT OF CHEMISTRY F/G 7/5 PHOTOASSISTED WATER-GAS SHIFT REACTION ON PLATINIZED T7TANIAI T--ETCIUI APR 82 S FANG. 8 CHEN..J M WHITE N14-75-C-922 UNCLASSIFIED NL OFFICE OF
AA113 878 TEXAS UNIV AT AUSTIN DEPT OF CHEMISTRY F/G 7/5 PHOTOASSISTED WATER-GAS SHIFT REACTION ON PLATINIZED T7TANIAI T--ETCIUI APR 82 S FANG. 8 CHEN..J M WHITE N14-75-C-922 UNCLASSIFIED NL OFFICE OF
8. Relax and do well.
 CHEM 15 Exam II John II. Gelder March 4, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last two pages includes a periodic table, a solubility
CHEM 15 Exam II John II. Gelder March 4, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last two pages includes a periodic table, a solubility
8. Relax and do well.
 CHEM 1314.03 Exam I John I. Gelder September 25, 1997 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
CHEM 1314.03 Exam I John I. Gelder September 25, 1997 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Made the FIRST periodic table
 Made the FIRST periodic table 1869 Mendeleev organized the periodic table based on the similar properties and relativities of certain elements Later, Henri Moseley organized the elements by increasing
Made the FIRST periodic table 1869 Mendeleev organized the periodic table based on the similar properties and relativities of certain elements Later, Henri Moseley organized the elements by increasing
8. Relax and do well.
 CHEM 1215 Exam III John III. Gelder November 11, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 1215 Exam III John III. Gelder November 11, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
Atoms and the Periodic Table
 Atoms and the Periodic Table Parts of the Atom Proton Found in the nucleus Number of protons defines the element Charge +1, mass 1 Parts of the Atom Neutron Found in the nucleus Stabilizes the nucleus
Atoms and the Periodic Table Parts of the Atom Proton Found in the nucleus Number of protons defines the element Charge +1, mass 1 Parts of the Atom Neutron Found in the nucleus Stabilizes the nucleus
Radiometric Dating (tap anywhere)
 Radiometric Dating (tap anywhere) Protons Neutrons Electrons Elements on the periodic table are STABLE Elements can have radioactive versions of itself called ISOTOPES!! Page 1 in your ESRT has your list!
Radiometric Dating (tap anywhere) Protons Neutrons Electrons Elements on the periodic table are STABLE Elements can have radioactive versions of itself called ISOTOPES!! Page 1 in your ESRT has your list!
CHM 101 PRACTICE TEST 1 Page 1 of 4
 CHM 101 PRACTICE TEST 1 Page 1 of 4 Please show calculations (stuffed equations) on all mathematical problems!! On the actual test, "naked answers, with no work shown, will receive no credit even if correct.
CHM 101 PRACTICE TEST 1 Page 1 of 4 Please show calculations (stuffed equations) on all mathematical problems!! On the actual test, "naked answers, with no work shown, will receive no credit even if correct.
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
M10/4/CHEMI/SPM/ENG/TZ2/XX+ CHEMISTRY. Wednesday 12 May 2010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
single-layer transition metal dichalcogenides MC2
 single-layer transition metal dichalcogenides MC2 Period 1 1 H 18 He 2 Group 1 2 Li Be Group 13 14 15 16 17 18 B C N O F Ne 3 4 Na K Mg Ca Group 3 4 5 6 7 8 9 10 11 12 Sc Ti V Cr Mn Fe Co Ni Cu Zn Al Ga
single-layer transition metal dichalcogenides MC2 Period 1 1 H 18 He 2 Group 1 2 Li Be Group 13 14 15 16 17 18 B C N O F Ne 3 4 Na K Mg Ca Group 3 4 5 6 7 8 9 10 11 12 Sc Ti V Cr Mn Fe Co Ni Cu Zn Al Ga
Faculty of Natural and Agricultural Sciences Chemistry Department. Semester Test 1 MEMO. Analytical Chemistry CMY 283
 Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 MEMO Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 90
Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 MEMO Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 90
Spin Cut-off Parameter of Nuclear Level Density and Effective Moment of Inertia
 Commun. Theor. Phys. (Beijing, China) 43 (005) pp. 709 718 c International Academic Publishers Vol. 43, No. 4, April 15, 005 Spin Cut-off Parameter of Nuclear Level Density and Effective Moment of Inertia
Commun. Theor. Phys. (Beijing, China) 43 (005) pp. 709 718 c International Academic Publishers Vol. 43, No. 4, April 15, 005 Spin Cut-off Parameter of Nuclear Level Density and Effective Moment of Inertia
(please print) (1) (18) H IIA IIIA IVA VA VIA VIIA He (2) (13) (14) (15) (16) (17)
 CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
MANY ELECTRON ATOMS Chapter 15
 MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
Marks for each question are as indicated in [] brackets.
![Marks for each question are as indicated in [] brackets. Marks for each question are as indicated in [] brackets.](/thumbs/77/75878580.jpg) Name Student Number CHEMISTRY 140 FINAL EXAM December 10, 2002 Numerical answers must be given with appropriate units and significant figures. Please place all answers in the space provided for the question.
Name Student Number CHEMISTRY 140 FINAL EXAM December 10, 2002 Numerical answers must be given with appropriate units and significant figures. Please place all answers in the space provided for the question.
PART 1 Introduction to Theory of Solids
 Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:1 Trim:165 240MM TS: Integra, India PART 1 Introduction to Theory of Solids Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:2
Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:1 Trim:165 240MM TS: Integra, India PART 1 Introduction to Theory of Solids Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:2
PERIODIC TABLE OF THE ELEMENTS
 Useful Constants and equations: K = o C + 273 Avogadro's number = 6.022 x 10 23 d = density = mass/volume R H = 2.178 x 10-18 J c = E = h = hc/ h = 6.626 x 10-34 J s c = 2.998 x 10 8 m/s E n = -R H Z 2
Useful Constants and equations: K = o C + 273 Avogadro's number = 6.022 x 10 23 d = density = mass/volume R H = 2.178 x 10-18 J c = E = h = hc/ h = 6.626 x 10-34 J s c = 2.998 x 10 8 m/s E n = -R H Z 2
COMPILATION OF AUTOMATA FROM MORPHOLOGICAL TWO-LEVEL RULES
 Kimmo Koskenniemi Re se ar ch Unit for Co mp ut at io na l Li ng ui st ic s University of Helsinki, Hallituskatu 11 SF-00100 Helsinki, Finland COMPILATION OF AUTOMATA FROM MORPHOLOGICAL TWO-LEVEL RULES
Kimmo Koskenniemi Re se ar ch Unit for Co mp ut at io na l Li ng ui st ic s University of Helsinki, Hallituskatu 11 SF-00100 Helsinki, Finland COMPILATION OF AUTOMATA FROM MORPHOLOGICAL TWO-LEVEL RULES
M11/4/CHEMI/SPM/ENG/TZ2/XX CHEMISTRY STANDARD LEVEL PAPER 1. Monday 9 May 2011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M11/4/CHEMI/SPM/ENG/TZ/XX 116116 CHEMISTRY STANDARD LEVEL PAPER 1 Monday 9 May 011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M11/4/CHEMI/SPM/ENG/TZ/XX 116116 CHEMISTRY STANDARD LEVEL PAPER 1 Monday 9 May 011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
Physical Chemistry I CHEM 4641 Final Exam 13 questions, 30 points
 Physical Chemistry I CHEM 4641 Final Exam 13 questions, 30 points Name: KEY Gas constant: R = 8.314 J mol -1 K -1 = 0.008314 kj mol -1 K -1. Boltzmann constant k = 1.381 10-23 J/K = 0.6950 cm -1 /K h =
Physical Chemistry I CHEM 4641 Final Exam 13 questions, 30 points Name: KEY Gas constant: R = 8.314 J mol -1 K -1 = 0.008314 kj mol -1 K -1. Boltzmann constant k = 1.381 10-23 J/K = 0.6950 cm -1 /K h =
8. Relax and do well.
 CHEM 1225 Exam I John I. Gelder February 4, 1999 Name KEY TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do
CHEM 1225 Exam I John I. Gelder February 4, 1999 Name KEY TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do
INSTRUCTIONS: CHEM Exam I. September 13, 1994 Lab Section
 CHEM 1314.05 Exam I John I. Gelder September 13, 1994 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
CHEM 1314.05 Exam I John I. Gelder September 13, 1994 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
Nucleus. Electron Cloud
 Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
M09/4/CHEMI/HPM/ENG/TZ2/XX+ CHEMISTRY. Monday 18 May 2009 (afternoon) 1 hour INSTRUCTIONS TO CANDIDATES
 M09/4/CEMI/PM/ENG/TZ/XX+ 096113 CEMISTRY igher level Paper 1 Monday 18 May 009 (afternoon) 1 hour INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer all the
M09/4/CEMI/PM/ENG/TZ/XX+ 096113 CEMISTRY igher level Paper 1 Monday 18 May 009 (afternoon) 1 hour INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer all the
CMSC 313 Lecture 17 Postulates & Theorems of Boolean Algebra Semiconductors CMOS Logic Gates
 CMSC 313 Lecture 17 Postulates & Theorems of Boolean Algebra Semiconductors CMOS Logic Gates UMBC, CMSC313, Richard Chang Last Time Overview of second half of this course Logic gates &
CMSC 313 Lecture 17 Postulates & Theorems of Boolean Algebra Semiconductors CMOS Logic Gates UMBC, CMSC313, Richard Chang Last Time Overview of second half of this course Logic gates &
SOUTHWESTERN ELECTRIC POWER COMPANY SCHEDULE H-6.1b NUCLEAR UNIT OUTAGE DATA. For the Test Year Ended March 31, 2009
 Schedule H-6.lb SOUTHWSTRN LCTRIC POWR COMPANY SCHDUL H-6.1b NUCLAR UNIT OUTAG DATA For the Test Year nded March 31, 29 This schedule is not applicable to SVvPCO. 5 Schedule H-6.1 c SOUTHWSTRN LCTRIC POWR
Schedule H-6.lb SOUTHWSTRN LCTRIC POWR COMPANY SCHDUL H-6.1b NUCLAR UNIT OUTAG DATA For the Test Year nded March 31, 29 This schedule is not applicable to SVvPCO. 5 Schedule H-6.1 c SOUTHWSTRN LCTRIC POWR
Chemistry 2 Exam Roane State Academic Festival. Name (print neatly) School
 Name (print neatly) School There are fifteen question on this exam. Each question is weighted equally. n the answer sheet, write your name in the space provided and your answers in the blanks provided.
Name (print neatly) School There are fifteen question on this exam. Each question is weighted equally. n the answer sheet, write your name in the space provided and your answers in the blanks provided.
CHEM 10123/10125, Exam 2
 CHEM 10123/10125, Exam 2 March 7, 2012 (50 minutes) Name (please print) Please box your answers, and remember that significant figures, phases (for chemical equations), and units do count! 1. (13 points)
CHEM 10123/10125, Exam 2 March 7, 2012 (50 minutes) Name (please print) Please box your answers, and remember that significant figures, phases (for chemical equations), and units do count! 1. (13 points)
Chemistry 431 Practice Final Exam Fall Hours
 Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
SAMPLE LITANY OF THE SAINTS E/G. Dadd9/F. Aadd9. cy. Christ, have. Lord, have mer cy. Christ, have A/E. Dadd9. Aadd9/C Bm E. 1. Ma ry and. mer cy.
 LTNY OF TH SNTS Cntrs Gnt flwng ( = c. 100) /G Ddd9/F ll Kybrd / hv Ddd9 hv hv Txt 1973, CL. ll rghts rsrvd. Usd wth prmssn. Musc: D. Bckr, b. 1953, 1987, D. Bckr. Publshd by OCP. ll rghts rsrvd. SMPL
LTNY OF TH SNTS Cntrs Gnt flwng ( = c. 100) /G Ddd9/F ll Kybrd / hv Ddd9 hv hv Txt 1973, CL. ll rghts rsrvd. Usd wth prmssn. Musc: D. Bckr, b. 1953, 1987, D. Bckr. Publshd by OCP. ll rghts rsrvd. SMPL
Circle the letters only. NO ANSWERS in the Columns! (3 points each)
 Chemistry 1304.001 Name (please print) Exam 4 (100 points) April 12, 2017 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Chemistry 1304.001 Name (please print) Exam 4 (100 points) April 12, 2017 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Using the Periodic Table
 MATH SKILLS TRANSPARENCY WORKSHEET Using the Periodic Table 6 Use with Chapter 6, Section 6.2 1. Identify the number of valence electrons in each of the following elements. a. Ne e. O b. K f. Cl c. B g.
MATH SKILLS TRANSPARENCY WORKSHEET Using the Periodic Table 6 Use with Chapter 6, Section 6.2 1. Identify the number of valence electrons in each of the following elements. a. Ne e. O b. K f. Cl c. B g.
Circle the letters only. NO ANSWERS in the Columns!
 Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
APPENDIX F WATER USE SUMMARY
 APPENDX F WATER USE SUMMARY From Past Projects Town of Norman Wells Water Storage Facltes Exstng Storage Requrements and Tank Volume Requred Fre Flow 492.5 m 3 See Feb 27/9 letter MACA to Norman Wells
APPENDX F WATER USE SUMMARY From Past Projects Town of Norman Wells Water Storage Facltes Exstng Storage Requrements and Tank Volume Requred Fre Flow 492.5 m 3 See Feb 27/9 letter MACA to Norman Wells
7. Relax and do well.
 CHEM 1215 Exam II John II. Gelder October 7, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 5 different pages. The last page includes a periodic table and a solubility
CHEM 1215 Exam II John II. Gelder October 7, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 5 different pages. The last page includes a periodic table and a solubility
Chemistry Standard level Paper 1
 M15/4/CHEMI/SPM/ENG/TZ1/XX Chemistry Standard level Paper 1 Thursday 14 May 2015 (afternoon) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all
M15/4/CHEMI/SPM/ENG/TZ1/XX Chemistry Standard level Paper 1 Thursday 14 May 2015 (afternoon) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all
9/20/2017. Elements are Pure Substances that cannot be broken down into simpler substances by chemical change (contain Only One Type of Atom)
 CAPTER 6: TE PERIODIC TABLE Elements are Pure Substances that cannot be broken down into simpler substances by chemical change (contain Only One Type of Atom) The Periodic Table (Mendeleev) In 1872, Dmitri
CAPTER 6: TE PERIODIC TABLE Elements are Pure Substances that cannot be broken down into simpler substances by chemical change (contain Only One Type of Atom) The Periodic Table (Mendeleev) In 1872, Dmitri
< < or a. * or c w u. "* \, w * r? ««m * * Z * < -4 * if # * « * W * <r? # *» */>* - 2r 2 * j j. # w O <» x <» V X * M <2 * * * *
 - W # a a 2T. mj 5 a a s " V l UJ a > M tf U > n &. at M- ~ a f ^ 3 T N - H f Ml fn -> M - M. a w ma a Z a ~ - «2-5 - J «a -J -J Uk. D tm -5. U U # f # -J «vfl \ \ Q f\ \ y; - z «w W ^ z ~ ~ / 5 - - ^
- W # a a 2T. mj 5 a a s " V l UJ a > M tf U > n &. at M- ~ a f ^ 3 T N - H f Ml fn -> M - M. a w ma a Z a ~ - «2-5 - J «a -J -J Uk. D tm -5. U U # f # -J «vfl \ \ Q f\ \ y; - z «w W ^ z ~ ~ / 5 - - ^
Practice Final Exam CH 201
 Practice Final Exam CH 201 Name: (please print) Student I D : Instructions - Read Carefully 1. Please show your work, and put your final answers in the spaces provided. 2. Point values for each question
Practice Final Exam CH 201 Name: (please print) Student I D : Instructions - Read Carefully 1. Please show your work, and put your final answers in the spaces provided. 2. Point values for each question
8. Relax and do well.
 CHEM 1014 Exam I John I. Gelder September 16, 1999 Name TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do not
CHEM 1014 Exam I John I. Gelder September 16, 1999 Name TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do not
8. Relax and do well.
 CHEM 1225 Exam III John III. Gelder April 8, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last two pages includes a periodic table and
CHEM 1225 Exam III John III. Gelder April 8, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last two pages includes a periodic table and
UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 READ ALL THE INSTRUCTIONS CAREFULLY
 WEDNESDAY MARCH 9th, 2016 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
WEDNESDAY MARCH 9th, 2016 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
Chapter 3: Stoichiometry
 Chapter 3: Stoichiometry Chem 6A Michael J. Sailor, UC San Diego 1 Announcements: Thursday (Sep 29) quiz: Bring student ID or we cannot accept your quiz! No notes, no calculators Covers chapters 1 and
Chapter 3: Stoichiometry Chem 6A Michael J. Sailor, UC San Diego 1 Announcements: Thursday (Sep 29) quiz: Bring student ID or we cannot accept your quiz! No notes, no calculators Covers chapters 1 and
CHEM 130 Exp. 8: Molecular Models
 CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
Ranking accounting, banking and finance journals: A note
 MPRA Munich Personal RePEc Archive Ranking accounting, banking and finance ournals: A note George Halkos and Nickolaos Tzeremes University of Thessaly, Department of Economics January 2012 Online at https://mpra.ub.uni-muenchen.de/36166/
MPRA Munich Personal RePEc Archive Ranking accounting, banking and finance ournals: A note George Halkos and Nickolaos Tzeremes University of Thessaly, Department of Economics January 2012 Online at https://mpra.ub.uni-muenchen.de/36166/
ADORO TE DEVOTE (Godhead Here in Hiding) te, stus bat mas, la te. in so non mor Je nunc. la in. tis. ne, su a. tum. tas: tur: tas: or: ni, ne, o:
 R TE EVTE (dhd H Hdg) L / Mld Kbrd gú s v l m sl c m qu gs v nns V n P P rs l mul m d lud 7 súb Fí cón ví f f dó, cru gs,, j l f c r s m l qum t pr qud ct, us: ns,,,, cs, cut r l sns m / m fí hó sn sí
R TE EVTE (dhd H Hdg) L / Mld Kbrd gú s v l m sl c m qu gs v nns V n P P rs l mul m d lud 7 súb Fí cón ví f f dó, cru gs,, j l f c r s m l qum t pr qud ct, us: ns,,,, cs, cut r l sns m / m fí hó sn sí
Example: Two Stochastic Process u~u[0,1]
![Example: Two Stochastic Process u~u[0,1] Example: Two Stochastic Process u~u[0,1]](/thumbs/86/93797001.jpg) Co o Slo o Coco S Sh EE I Gholo h@h. ll Sochc Slo Dc Slo l h PLL c Mo o coco w h o c o Ic o Co B P o Go E A o o Po o Th h h o q o ol o oc o lco q ccc lco l Bc El: Uo Dbo Ucol Sl Ab bo col l G col G col
Co o Slo o Coco S Sh EE I Gholo h@h. ll Sochc Slo Dc Slo l h PLL c Mo o coco w h o c o Ic o Co B P o Go E A o o Po o Th h h o q o ol o oc o lco q ccc lco l Bc El: Uo Dbo Ucol Sl Ab bo col l G col G col
Chem Exam 1. September 26, Dr. Susan E. Bates. Name 9:00 OR 10:00
 Chem 1711 Exam 1 September 26, 2013 Dr. Susan E. Bates Name 9:00 OR 10:00 N A = 6.022 x 10 23 mol 1 I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gases 1 H 1.008 3 Li
Chem 1711 Exam 1 September 26, 2013 Dr. Susan E. Bates Name 9:00 OR 10:00 N A = 6.022 x 10 23 mol 1 I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gases 1 H 1.008 3 Li
I n t e r n a t i o n a l E l e c t r o n i c J o u r n a l o f E l e m e n t a r y E.7 d u, c ai ts is ou n e, 1 V3 1o-2 l6, I n t h i s a r t
 I n t e r n a t i o n a l E l e c t r o n i c J o ue rlne am l e not fa r y E d u c a t i o n, 2 0 1 4, 1 37-2 ( 16 ). H o w R e a d i n g V o l u m e A f f e c t s b o t h R e a d i n g F l u e n c y
I n t e r n a t i o n a l E l e c t r o n i c J o ue rlne am l e not fa r y E d u c a t i o n, 2 0 1 4, 1 37-2 ( 16 ). H o w R e a d i n g V o l u m e A f f e c t s b o t h R e a d i n g F l u e n c y
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
 H NT Z N RT L 0 4 n f lt r h v d lt n r n, h p l," "Fl d nd fl d " ( n l d n l tr l t nt r t t n t nt t nt n fr n nl, th t l n r tr t nt. r d n f d rd n t th nd r nt r d t n th t th n r lth h v b n f
H NT Z N RT L 0 4 n f lt r h v d lt n r n, h p l," "Fl d nd fl d " ( n l d n l tr l t nt r t t n t nt t nt n fr n nl, th t l n r tr t nt. r d n f d rd n t th nd r nt r d t n th t th n r lth h v b n f
CHEM 251 (Fall-2003) Final Exam (100 pts)
 CEM 251 (Fall-2003) Final Exam (100 pts) Name: -------------------------------------------------------------------------------, SSN -------------------------------- LAST NAME, First (Circle the alphabet
CEM 251 (Fall-2003) Final Exam (100 pts) Name: -------------------------------------------------------------------------------, SSN -------------------------------- LAST NAME, First (Circle the alphabet
'NOTAS"CRITICAS PARA UNA TEDRIA DE M BUROCRACIA ESTATAL * Oscar Oszlak
 OVí "^Ox^ OqAÍ"^ Dcument SD-11 \ 'NOTAS"CRTCAS PARA UNA TEDRA DE M BUROCRACA ESTATAL * Oscr Oszlk * El presente dcument que se reprduce pr us exclusv de ls prtcpntes de curss de Prrms de Cpctcón, se h
OVí "^Ox^ OqAÍ"^ Dcument SD-11 \ 'NOTAS"CRTCAS PARA UNA TEDRA DE M BUROCRACA ESTATAL * Oscr Oszlk * El presente dcument que se reprduce pr us exclusv de ls prtcpntes de curss de Prrms de Cpctcón, se h
