Lecture Notes Set 3a: Probabilities, Microstates and Entropy
|
|
|
- Gary Austin
- 5 years ago
- Views:
Transcription
1 Lecture Notes Set 3a: Probabilities, Microstates and Entropy
2 Thus far.. In Sections 1 and 2 of the module we ve covered a broad variety of topics at the heart of the thermal and statistical behaviour of matter. These topics include: distributions and mean values, the ideal gas law, Boltzmann factors, the Maxwell-Boltzmann distribution, equipartition of energy, specific heats, blackbody radiation, and diffusion and thermal conductivity in a gas. However, we still don t yet fully understand why heat flows from a hot to a cold object (and not vice versa) nor why molecules diffuse from regions of high to low concentration so that at equilibrium they are evenly distributed across the available space. Furthermore, although we (correctly) think of temperature as being related to the mean energy of a system, we ve yet to quantifiably define the relationship between temperature and the direction of heat flow. In this section of the module we ll explore distributions in even more depth. A key focus will be a consideration of the very many arrangements of quanta of energy that are possible even for very small numbers of atoms and quanta. This will lead to a discussion of entropy and the related - second law of thermodynamics. These are conceptually challenging topics which have a history of being misrepresented in popular science writing. The final part of this section of the module is therefore concerned with attempting to dispel some of the myths associated with the 2 nd law. 3.1 Energy transfer In Section 2 we found that there was a very close connection between temperature and the average kinetic energy of the molecules in a system, i.e., ½ m<v 2 > = 3kT/2 a fundamental result of equipartition of energy theory. Intuitively, we know that a hot block of material will lose energy to a colder block of material and we can explain this by reasoning that the average energy of the atoms in the hot block is greater than the average energy of the atoms in the cold block. Therefore, it is more likely that an atom in the hotter block will lose energy to an atom in the colder block. f(v ) Speed Velocity (m/sec) (m/s) Fig. 3.1 Maxwell-Boltzmann distribution of speeds of molecules in two (separate) gases the taller peak is the distribution associated with the cooler gas. Note that for both gases a substantial fraction of the molecules have speeds well below and well above the mean speed. The question then arises as to why energy doesn t transfer from the cold to the hot gas if the two gases are mixed (rather than from hot to cold, as is observed). However, how much more likely is a transfer of energy from the hot to the cold block The atoms have a distribution of energies. If we consider the Maxwell-Boltzmann distribution for gas molecules shown in Fig. 3.1 it is clear that many molecules in the hot gas have energies well below the mean energy. Similarly, many molecules in the cold gas have energies well in excess of the mean energy of the gas.
3 Similarly, why don t the atoms in a cold block of material tend to transfer their energy to the hot block, rather than the other way around What is it that defines the direction of energy transfer (A closely related question is what defines the arrow of time a topic we ll also discuss). T + T Q T Fig. 3.2 The direction of heat transfer shown above doesn t violate the 1 st law of thermodynamics but is prohibited by the 2 nd law of thermodynamics. A primary aim of Section 3 of the module is to explain the physical principles underlying the 2 nd law. The direction of heat flow shown in Fig. 3.2 wouldn t violate the conservation of energy the 1 st law of thermodynamics - because the total energy remains the same. However, throughout Section 3 of the module we ll be concerned with determining just what physical principle would be violated if heat were to flow from a cold to a hot body so that, at equilibrium, the temperature of the hot body was increased. Later on, when we explore heat engines in Section 4 of the module, we ll see that this principle - which is, in fact, the 2 nd law of thermodynamics - also makes it impossible to convert heat into work at a single temperature.
4 3.2 An introduction to statistical mechanics To answer the questions raised in Section 3.1, the statistical behaviour of large assemblies or ensembles of atoms will need to be explored in some detail. This area of physics is called statistical mechanics. We will touch on only the basic concepts underlying statistical mechanics you will cover this topic in much more depth and with much more rigour in your 2 nd year course. However, even the basic statistical mechanics concepts we ll cover in the Thermal & Kinetic module lead to powerful and extremely important insights into the behaviour of matter. First, consider a model of a solid which comprises a collection of simple harmonic oscillators (see Fig. 3.3). (You might ask why, after we spent so much time discussing gases in Section 2 of the module, we ve now switched to a discussion of solids. We ll return to gases soon enough! Solids are easier to consider at the moment because the atoms are fixed in place.) We can simplify the model shown in Fig. 3.3 even further (as first devised by Einstein in 1907). Instead of considering coupled harmonic oscillators, we envisage each atom in the solid as moving independently of its neighbours. (This is obviously a big assumption but bear in mind that we re interested only in the distribution of energy amongst the atoms (the oscillators) not the detailed dynamics of the atomic motion). A second assumption we make (as did Einstein) is that each 3D oscillator (i.e. each atom) may be replaced with three - also independent 1D simple harmonic oscillators (i.e. one for each of the x, y, and z directions). Fig. 3.3 A model of a solid comprising a collection of interacting simple harmonic oscillators (movie frame taken from Chabay and Sherwood s website at mi_vol1_files.html) U(x) The energies of the simple harmonic oscillators comprising the solid are quantized (see Section 2 and Fig. 3.4): 1 E n = ( n + ) hω E 0 E 3 E 2 E 1 Fig. 3.4 Quantised energy levels of simple harmonic oscillator. x As discussed for the Planck model of blackbody radiation (Set 2b of the lecture notes), the energy difference between consecutive energy levels is h ω0 and we ll ignore the zero point energy that is, we ll set E 0 to zero. Having set up this model (known, unsurprisingly, as the Einstein model), we can now address the key question:
5 how is energy distributed amongst the available energy states Energy distributions Consider bringing two identical blocks of material which are at different temperatures (say, T 1 and T 2 ) into contact. What is the final temperature of the blocks (i.e. the temperature when they reach thermal equilibrium) The most probable distribution of energy is intuitively that where the total thermal energy is shared equally between the two blocks. However, what is the probability that at equilibrium the first block has more energy than the second, or, indeed, that the first block ends up with all the thermal energy To answer these questions, the possible arrangements of energy quanta must be considered. We have 3N independent 1D oscillators (where N is the total number of atoms in the solid) we now need to determine how many ways there are of distributing a certain number of quanta of energy amongst these oscillators. Mathematical principles related to counting combinations and permutations of objects will prove to be very useful. Let s start with a straight-forward example: 3 h ω 0 0 h ω 0 Oscillator 1 Oscillator 2 Fig. 3.5 One way of distributing 3 quanta of energy between two oscillators. The arrangement shown in Fig. 3.5 is one possible distribution of 3 quanta of energy amongst two oscillators. Sketch the remaining possibilities. How many possibilities in total are there
6 Counting arrangements: permutations and combinations Clearly we are not going to count by hand every arrangement of energy possible for 3N oscillators in, for example, a mole of material (N ~ 6 x ). We need to determine the appropriate formula to use and this involves spending a little time thinking about permutations and combinations. A permutation is an arrangement of a collection of objects where the ordering of the arrangement is important. The number of permutations of r objects selected from a set of n distinct objects is denoted by n P r, where n P r = n!/(n-r)! Consider the following question: A CD reviewer is asked to choose her top 3 CDs from a list of 10 CDs and rank them in order of preference. How many different lists can be formed
7 Now consider the following case: A CD club member is asked to pick 3 CDs from a list of 10 CDs. How many different choices are possible The number of combinations of r objects selected from a set of n distinct objects is denoted by n C r, where n n! C r = r!( n r)! One final example: Take a collection of 10 pool (billiard) balls, 6 of which are yellow and 4 of which are red. How many different arrangements of the coloured balls are possible (eg RRYYYYYYRR)
8 Arranging quanta Returning to distributing quanta of energy amongst a collection of oscillators, we can now establish a formula for the number of possible arrangements. (What follows is based heavily on Chabay and Sherwood, p.348). Take the two oscillator, three quanta problem considered in Fig 3.5 above. Another possible arrangement (one of the three you should have sketched earlier) is shown on the left hand side of Fig = Fig. 3.6 We can consider the distribution of 3 quanta of energy between 2 oscillators as a problem involving the arrangement of 4 objects, namely 3 dots representing the energy quanta and a bar representing a separator between the oscillators. If there are N oscillators there are N-1 bars. On the right hand side of Fig. 3.6 the dots represent the three energy quanta and the vertical bar represents a separator between the two oscillators. With N oscillators, there will be N-1 separators. Counting up the number of arrangements of dots and bars (i.e. the arrangements of quanta amongst the oscillators) thus reduces to the pool ball problem except instead of red and yellow pool balls we need to arrange and objects. The total number of arrangements of three 4! quanta amongst two oscillators is thus:. More generally: 31!! The number of ways to arrange q quanta of energy amongst N 1D oscillators is: ( q + N 1)! q!( N 1)! How many ways can 4 quanta of energy be arranged amongst four 1D oscillators
9 3.3 Microstates and Macrostates Taking another example, five quanta of energy can be arranged in 21 different ways among 3 oscillators. Each of the 21 different distributions of energy is a microstate (i.e. an individual microscopic configuration of the system); The 21 different microstates all correspond to the same macrostate - the total energy of the system is 5 h ω0. This is just one example we could also have considered the distribution of 27 quanta of energy amongst 300 oscillators, or the distribution of 10 7 quanta of energy amongst 1 mole of oscillators. Regardless of the number of microstates we re considering, the following fundamental hypothesis is at the very core of statistical mechanics (and thus underlies thermodynamics): FUNDAMENTAL ASSUMPTION OF STATISTICAL MECHANICS Each microstate corresponding to a given macrostate is equally probable. We can draw an analogy between a game of poker and the concept of microstates and macrostates. (NB Do not take this analogy too literally. We ll see later how it is dangerous to associate a concept such as entropy with everyday objects such as decks of cards, arrangements of socks in a drawer, or a messy ( disordered ) bedroom.) Ask yourself what are the chances of being dealt the following hand of cards from a well shuffled, 52 card deck: Now, take a new well shuffled deck of 52 cards and deal out five cards. What are the chances of being dealt the following hand
10 The probability of being dealt either of the hands of cards shown on the previous page is exactly the same. Why then are we so much happier to be dealt the royal flush rather than what might best be described as a junk hand This is because, as eloquently stated by a student during Lecture 10, the rules of the game state that some combinations of cards are worth more than others. A total of over 2½ million different possible hands of 5 cards may be drawn from a 52 card deck (make sure you can work out precisely how many combinations there are). Each one of these hands is analogous to a microstate a single combination of 5 cards. However, we can define a variety of macrostates for the hands of cards. For example, we could have the following: Royal Flush, Straight, 4 of a kind, Pair, Two Pair, Junk where Junk is a worthless hand of cards. Although every microstate is equally likely, the macrostate Royal Flush is associated with very many less microstates than is the macrostate Pair or the macrostate Junk. (You ll explore this in more detail in Coursework Set 6). Macrostates associated with low numbers of microstates are much less likely to be observed.
11 Two interacting atoms: six 1D oscillators Remember, we re ultimately trying to establish why the total thermal energy is shared uniformly between two blocks, originally of different temperatures, when they re brought into contact. Let s consider the smallest possible blocks two interacting atoms. As each atom comprises three 1D oscillators this means we have six 1D oscillators in total. Try answering the following questions: How many ways are there of distributing 4 quanta of energy amongst six 1D oscillators If all four quanta of energy are given to one atom or the other, how many ways are there of distributing the energy If three quanta are given to one atom, and one quantum to the other, how many ways are there of distributing the energy If the four quanta are shared equally between the atoms, how many ways are there of distributing the energy
12 The results from the questions on the previous page are shown as a histogram in Fig It is clear that it is most probable that the thermal energy is shared equally between the two atoms. Note that there are two ways to consider Fig. 3.7: (i) if frequent observations of the two atom system are made, in 29% of the observations (36 out of 126), the energy will be split equally; (ii) for 100 identical two atom systems, at any given instant 29% of the systems will have the energy split equally between the two atoms. Number of distributions Number of quanta in atom 1 Fig. 3.7 A histogram showing the number of microstates associated with having 0, 1, 2, 3, or 4 quanta of energy on atom 1 of a two atom system. Increasing the number of atoms What happens if we increase the number of atoms in the system If we consider only a few more atoms (say, 10) and a few more quanta of energy (again, let s choose 10) it turns out that there are 635 million ways of arranging the quanta amongst the oscillators. This is for only a few atoms! So you can see that the number of arrangements increases very quickly for small changes in the number of oscillators. For example, for 300 oscillators (i.e. 100 atoms), there are 1.7 x ways of distributing 100 quanta of energy. However, there is only 1 way of placing all the quanta on one particular oscillator (i.e. the quanta are given to one special oscillator we ve chosen). The probability of finding all the energy concentrated on one atom in a 100 atom ensemble is thus unimaginably small. For a mole of solid (~ 6 x atoms), although it is possible that rather than finding that the energy is uniformly distributed throughout the system we observe that all the energy is concentrated on one atom (or a small group of atoms), we would have to wait on average for a period many times the age of the Universe to see this exceptionally unlikely event occur. (The phrase exceptionally unlikely does not do justice to the staggeringly small odds associated with this possibility!). Fig. 3.8 Instead of single atom blocks, we now consider two blocks comprising 6 atoms and 3 atoms respectively. The total energy to be distributed amongst the oscillators in the blocks is 100 quanta. Again, let s put this argument on a more quantitative footing and examine how the distribution of energy changes as the number of atoms is increased. Instead of using single atom blocks, let s first choose two slightly larger blocks one comprising 6 atoms, the other comprising three atoms. 100 quanta of energy are placed in the blocks (which are, as before, in contact, see Fig. 3.8). Note also that the blocks are adibatically isolated i.e. they re not thermally coupled to their environment.
13 2.5x10 25 Number of ways of distributing 100 quanta Total number of microstates 2.0x x x x10 24 of vibrational energy between two blocks having 3 6 and 6 3 atoms respectively Number of quanta in block 1 Fig. 3.9 A histogram showing the total number of microstates (arrangements of quanta of energy) vs. the number of quanta of energy in block 1. Note that the histogram has a well defined width and mean value. A total of 100 quanta of energy have been distributed between the blocks (which comprise six and three atoms (18 and 9 oscillators) respectively). It is clear that a plot of the total number of microstates (i.e. W, where W is the product of the number of microstates associated with block 1 (Ω 1 ) and the number of microstates associated with block 2 (Ω 2 )) vs the number of quanta of energy in block 1 is peaked at a certain value of energy. In Fig. 3.9, to the nearest integer, where on the x-axis is the maximum of the distribution curve located Why
14 Fig illustrates what happens when the number of atoms in each block shown in Fig. 3.8 is increased by an order of magnitude. Note that the distribution has narrowed appreciably. Fig. 3.9 represents a system containing less than 100 atoms. If we were to carry out this calculation for a typical macroscopic system comprising of the order of atoms, the distribution would be extremely narrow (..another understatement!). For a macroscopic system, the probability of finding the system in a state other than the most probable (which involves an equal sharing of the energy amongst all the oscillators) is an unimaginably small number. The width of the distribution is, in fact, proportional to 1/ N where N is the total number of oscillators. Total number of microstates 1.4x10 91 Number of ways of distributing 100 quanta of vibrational energy between two blocks 1.2x10 91 having 60 and 30 atoms respectively. 1.0x x x x x Number of quanta in block 1 Fig When the number of atoms is increased by an order of magnitude, the mean of the distribution doesn t change but its width decreases considerably. This distribution is for a system containing less than 100 atoms. For a typical macroscopic system comprising ~ atoms, the distribution will be extremely narrow. The probability of finding the system in a state other than that where the energy is shared equally between the two blocks is unimaginably small.
15 3.4 Thermal equilibrium and entropy For two systems that are not at thermal equilibrium, there will be a flow of energy until a balance is reached and the flow of energy ceases. Having considered the distribution of microstates for the two block system, we are now in a position to understand just why energy flows so that a balance is reached, i.e. so that both blocks reach the same equilibrium temperature (rather than one block ending up with all the energy). In Fig. 3.11, the logarithms of Ω 1 (the number of microstates associated with Block 1), Ω 2 (the number of microstates associated with Block 2), and W, the total number of microstates (= Ω 1 Ω 2 ) are plotted against the number of quanta of energy in Block 1. (Although one reason for the introduction of logarithms is that they make the consideration of very large numbers rather more straight-forward, there is a more fundamental reason which will be outlined below.) Note that the maximum of ln(ω 1 Ω 2 ) is located at the same position on the x-axis as was the peak in Fig This is not so surprising if Ω 1 Ω 2 has a maximum at x max then so too will ln(ω 1 Ω 2 ). ln (number of microstates) vs no. of quanta in block 1 ln (number of microstates) ln(ω 2 ) ln(ω 1 Ω 2 ) ln(ω 1 ) Number of quanta of energy in block 1 Fig Plot of natural log of microstates vs quanta of energy in Block1. If Block 2 were three times the size of Block 1, what value on the x-axis would correspond to the maximum of ln(ω 1 Ω 2 )
16 A definition of entropy Entropy is a measure of the number of possible microstates available to a system. Boltzmann s expression for entropy is a very simple (yet far-reaching) equation: S = k ln (W) 3.2 where W is the total number of accessible microstates (note the word accessible we ll have more to say on this later!) and k is Boltzmann s constant. As for the expression given for the Boltzmann factor in Section 2.3 of the notes, I strongly recommend that you commit Eqn. 3.2 to memory! In fact, as we ll soon see, the Boltzmann factor expression (i.e. the probability of occupying a state equals α exp (- E/kT), where α is a constant and E is the energy of the state above the ground state) and Eqn 3.2 are just two different ways of expressing precisely the same physical law. Due to the properties of logarithms, we can get the total entropy of a system by simply adding up the entropies of the individual parts of the system: S S = k ln( Ω Ω = k ln( Ω ) ) + k ln( Ω 2 ) To summarise.. If we now collate the various ideas outlined in this component of the module, we can write down the following important statement. At thermal equilibrium, the most probable energy distribution is that associated with the greatest number of possible microstates. This distribution maximizes the total entropy of the system. In the next set of lecture notes we ll focus on defining just what we mean by the term system, consider the importance of reversiblility when considering entropy, introduce the concept of increasing entropy in a closed system, and discuss in some detail the 2 nd law of thermodynamics.
Lecture Notes Set 3b: Entropy and the 2 nd law
 Lecture Notes Set 3b: Entropy and the 2 nd law 3.5 Entropy and the 2 nd law of thermodynamics The st law of thermodynamics is simply a restatement of the conservation of energy principle and may be concisely
Lecture Notes Set 3b: Entropy and the 2 nd law 3.5 Entropy and the 2 nd law of thermodynamics The st law of thermodynamics is simply a restatement of the conservation of energy principle and may be concisely
Physics 172H Modern Mechanics
 Physics 172H Modern Mechanics Instructor: Dr. Mark Haugan Office: PHYS 282 haugan@purdue.edu TAs: Alex Kryzwda John Lorenz akryzwda@purdue.edu jdlorenz@purdue.edu Lecture 22: Matter & Interactions, Ch.
Physics 172H Modern Mechanics Instructor: Dr. Mark Haugan Office: PHYS 282 haugan@purdue.edu TAs: Alex Kryzwda John Lorenz akryzwda@purdue.edu jdlorenz@purdue.edu Lecture 22: Matter & Interactions, Ch.
Lecture 8. The Second Law of Thermodynamics; Energy Exchange
 Lecture 8 The Second Law of Thermodynamics; Energy Exchange The second law of thermodynamics Statistics of energy exchange General definition of temperature Why heat flows from hot to cold Reading for
Lecture 8 The Second Law of Thermodynamics; Energy Exchange The second law of thermodynamics Statistics of energy exchange General definition of temperature Why heat flows from hot to cold Reading for
Lecture 8. The Second Law of Thermodynamics; Energy Exchange
 Lecture 8 The Second Law of Thermodynamics; Energy Exchange The second law of thermodynamics Statistics of energy exchange General definition of temperature Why heat flows from hot to cold Reading for
Lecture 8 The Second Law of Thermodynamics; Energy Exchange The second law of thermodynamics Statistics of energy exchange General definition of temperature Why heat flows from hot to cold Reading for
Let s start by reviewing what we learned last time. Here is the basic line of reasoning for Einstein Solids
 Chapter 5 In this chapter we want to review the concept of irreversibility in more detail and see how it comes from the multiplicity of states. In addition, we want to introduce the following new topics:
Chapter 5 In this chapter we want to review the concept of irreversibility in more detail and see how it comes from the multiplicity of states. In addition, we want to introduce the following new topics:
Entropy, free energy and equilibrium. Spontaneity Entropy Free energy and equilibrium
 Entropy, free energy and equilibrium Spontaneity Entropy Free energy and equilibrium Learning objectives Discuss what is meant by spontaneity Discuss energy dispersal and its relevance to spontaneity Describe
Entropy, free energy and equilibrium Spontaneity Entropy Free energy and equilibrium Learning objectives Discuss what is meant by spontaneity Discuss energy dispersal and its relevance to spontaneity Describe
Lecture 9 Examples and Problems
 Lecture 9 Examples and Problems Counting microstates of combined systems Volume exchange between systems Definition of Entropy and its role in equilibrium The second law of thermodynamics Statistics of
Lecture 9 Examples and Problems Counting microstates of combined systems Volume exchange between systems Definition of Entropy and its role in equilibrium The second law of thermodynamics Statistics of
213 Midterm coming up
 213 Midterm coming up Monday April 8 @ 7 pm (conflict exam @ 5:15pm) Covers: Lectures 1-12 (not including thermal radiation) HW 1-4 Discussion 1-4 Labs 1-2 Review Session Sunday April 7, 3-5 PM, 141 Loomis
213 Midterm coming up Monday April 8 @ 7 pm (conflict exam @ 5:15pm) Covers: Lectures 1-12 (not including thermal radiation) HW 1-4 Discussion 1-4 Labs 1-2 Review Session Sunday April 7, 3-5 PM, 141 Loomis
Thermodynamics: More Entropy
 Thermodynamics: More Entropy From Warmup Yay for only having to read one section! I thought the entropy statement of the second law made a lot more sense than the other two. Just probability. I haven't
Thermodynamics: More Entropy From Warmup Yay for only having to read one section! I thought the entropy statement of the second law made a lot more sense than the other two. Just probability. I haven't
Lecture Notes Set 4c: Heat engines and the Carnot cycle
 ecture Notes Set 4c: eat engines and the Carnot cycle Introduction to heat engines In the following sections the fundamental operating principles of the ideal heat engine, the Carnot engine, will be discussed.
ecture Notes Set 4c: eat engines and the Carnot cycle Introduction to heat engines In the following sections the fundamental operating principles of the ideal heat engine, the Carnot engine, will be discussed.
Guided Inquiry Worksheet 2: Interacting Einstein Solids & Entropy
 Guided Inquiry Worksheet 2: Interacting Einstein Solids & Entropy According to the fundamental assumption of statistical mechanics, AT EQUILIBRIUM, ALL ALLOWED MICROSTATES OF AN ISOLATED SYSTEM ARE EQUALLY
Guided Inquiry Worksheet 2: Interacting Einstein Solids & Entropy According to the fundamental assumption of statistical mechanics, AT EQUILIBRIUM, ALL ALLOWED MICROSTATES OF AN ISOLATED SYSTEM ARE EQUALLY
Random arrang ement (disorder) Ordered arrangement (order)
 2 CHAPTER 3 In any spontaneous energy conversion, the entropy of the system increases. Regardless of the nature of energy conversion, the entropy of the universe tends toward a maximum. (more probable)
2 CHAPTER 3 In any spontaneous energy conversion, the entropy of the system increases. Regardless of the nature of energy conversion, the entropy of the universe tends toward a maximum. (more probable)
Statistical Mechanics
 42 My God, He Plays Dice! Statistical Mechanics Statistical Mechanics 43 Statistical Mechanics Statistical mechanics and thermodynamics are nineteenthcentury classical physics, but they contain the seeds
42 My God, He Plays Dice! Statistical Mechanics Statistical Mechanics 43 Statistical Mechanics Statistical mechanics and thermodynamics are nineteenthcentury classical physics, but they contain the seeds
Chapter 20 The Second Law of Thermodynamics
 Chapter 20 The Second Law of Thermodynamics When we previously studied the first law of thermodynamics, we observed how conservation of energy provided us with a relationship between U, Q, and W, namely
Chapter 20 The Second Law of Thermodynamics When we previously studied the first law of thermodynamics, we observed how conservation of energy provided us with a relationship between U, Q, and W, namely
Thermodynamics: Entropy
 Thermodynamics: Entropy From Warmup I've heard people say that the Entropy Statement of the Second Law of Thermodynamics disproves God. Why is this? What are your thoughts? The second law of thermodynamics
Thermodynamics: Entropy From Warmup I've heard people say that the Entropy Statement of the Second Law of Thermodynamics disproves God. Why is this? What are your thoughts? The second law of thermodynamics
Thermodynamics: Entropy
 Thermodynamics: Entropy From Warmup I still do not understand the benefit of using a reversible process to calculate data when it is not possible to achieve. What makes a reversible process so useful?
Thermodynamics: Entropy From Warmup I still do not understand the benefit of using a reversible process to calculate data when it is not possible to achieve. What makes a reversible process so useful?
Spontaneity: Second law of thermodynamics CH102 General Chemistry, Spring 2012, Boston University
 Spontaneity: Second law of thermodynamics CH102 General Chemistry, Spring 2012, Boston University three or four forces and, as capstone, a minimalist cosmic constitution to legislate their use: Article
Spontaneity: Second law of thermodynamics CH102 General Chemistry, Spring 2012, Boston University three or four forces and, as capstone, a minimalist cosmic constitution to legislate their use: Article
Thermodynamics: More Entropy
 Thermodynamics: More Entropy From Warmup On a kind of spiritual note, this could possibly explain how God works some miracles. Supposing He could precisely determine which microstate occurs, He could heat,
Thermodynamics: More Entropy From Warmup On a kind of spiritual note, this could possibly explain how God works some miracles. Supposing He could precisely determine which microstate occurs, He could heat,
Thermodynamics & Statistical Mechanics
 hysics GRE: hermodynamics & Statistical Mechanics G. J. Loges University of Rochester Dept. of hysics & Astronomy xkcd.com/66/ c Gregory Loges, 206 Contents Ensembles 2 Laws of hermodynamics 3 hermodynamic
hysics GRE: hermodynamics & Statistical Mechanics G. J. Loges University of Rochester Dept. of hysics & Astronomy xkcd.com/66/ c Gregory Loges, 206 Contents Ensembles 2 Laws of hermodynamics 3 hermodynamic
UNDERSTANDING BOLTZMANN S ANALYSIS VIA. Contents SOLVABLE MODELS
 UNDERSTANDING BOLTZMANN S ANALYSIS VIA Contents SOLVABLE MODELS 1 Kac ring model 2 1.1 Microstates............................ 3 1.2 Macrostates............................ 6 1.3 Boltzmann s entropy.......................
UNDERSTANDING BOLTZMANN S ANALYSIS VIA Contents SOLVABLE MODELS 1 Kac ring model 2 1.1 Microstates............................ 3 1.2 Macrostates............................ 6 1.3 Boltzmann s entropy.......................
Lecture 6. Statistical Processes. Irreversibility. Counting and Probability. Microstates and Macrostates. The Meaning of Equilibrium Ω(m) 9 spins
 Lecture 6 Statistical Processes Irreversibility Counting and Probability Microstates and Macrostates The Meaning of Equilibrium Ω(m) 9 spins -9-7 -5-3 -1 1 3 5 7 m 9 Lecture 6, p. 1 Irreversibility Have
Lecture 6 Statistical Processes Irreversibility Counting and Probability Microstates and Macrostates The Meaning of Equilibrium Ω(m) 9 spins -9-7 -5-3 -1 1 3 5 7 m 9 Lecture 6, p. 1 Irreversibility Have
Chemistry 2000 Lecture 9: Entropy and the second law of thermodynamics
 Chemistry 2000 Lecture 9: Entropy and the second law of thermodynamics Marc R. Roussel January 23, 2018 Marc R. Roussel Entropy and the second law January 23, 2018 1 / 29 States in thermodynamics The thermodynamic
Chemistry 2000 Lecture 9: Entropy and the second law of thermodynamics Marc R. Roussel January 23, 2018 Marc R. Roussel Entropy and the second law January 23, 2018 1 / 29 States in thermodynamics The thermodynamic
Thermodynamics Second Law Entropy
 Thermodynamics Second Law Entropy Lana Sheridan De Anza College May 9, 2018 Last time entropy (macroscopic perspective) Overview entropy (microscopic perspective) Reminder of Example from Last Lecture
Thermodynamics Second Law Entropy Lana Sheridan De Anza College May 9, 2018 Last time entropy (macroscopic perspective) Overview entropy (microscopic perspective) Reminder of Example from Last Lecture
PHYS 172: Modern Mechanics Fall 2009
 PHYS 172: Modern Mechanics Fall 2009 Lecture 14 Energy Quantization Read 7.1 7.9 Reading Question: Ch. 7, Secs 1-5 A simple model for the hydrogen atom treats the electron as a particle in circular orbit
PHYS 172: Modern Mechanics Fall 2009 Lecture 14 Energy Quantization Read 7.1 7.9 Reading Question: Ch. 7, Secs 1-5 A simple model for the hydrogen atom treats the electron as a particle in circular orbit
Before the Quiz. Make sure you have SIX pennies
 Before the Quiz Make sure you have SIX pennies If you have more than 6, please share with your neighbors There are some additional pennies in the baskets up front please be sure to return them after class!!!
Before the Quiz Make sure you have SIX pennies If you have more than 6, please share with your neighbors There are some additional pennies in the baskets up front please be sure to return them after class!!!
Chapter 12- The Law of Increasing Disorder
 Second Law of Thermodynamics Changes occurring in natural systems always proceed in such a way that the total amount of entropy in the universe is either unchanged or increased. If total disorder is increased,
Second Law of Thermodynamics Changes occurring in natural systems always proceed in such a way that the total amount of entropy in the universe is either unchanged or increased. If total disorder is increased,
PAPER No. 6: PHYSICAL CHEMISTRY-II (Statistical
 Subject Paper No and Title Module No and Title Module Tag 6: PHYSICAL CHEMISTRY-II (Statistical 1: Introduction to Statistical CHE_P6_M1 TABLE OF CONTENTS 1. Learning Outcomes 2. Statistical Mechanics
Subject Paper No and Title Module No and Title Module Tag 6: PHYSICAL CHEMISTRY-II (Statistical 1: Introduction to Statistical CHE_P6_M1 TABLE OF CONTENTS 1. Learning Outcomes 2. Statistical Mechanics
Phys 172 Modern Mechanics Summer 2010
 Phys 172 Modern Mechanics Summer 2010 r r Δ p = F Δt sys net Δ E = W + Q sys sys net surr r r Δ L = τ Δt Lecture 14 Energy Quantization Read:Ch 8 Reading Quiz 1 An electron volt (ev) is a measure of: A)
Phys 172 Modern Mechanics Summer 2010 r r Δ p = F Δt sys net Δ E = W + Q sys sys net surr r r Δ L = τ Δt Lecture 14 Energy Quantization Read:Ch 8 Reading Quiz 1 An electron volt (ev) is a measure of: A)
Entropy. Physics 1425 Lecture 36. Michael Fowler, UVa
 Entropy Physics 1425 Lecture 36 Michael Fowler, UVa First and Second Laws of A quick review. Thermodynamics First Law: total energy conserved in any process: joules in = joules out Second Law: heat only
Entropy Physics 1425 Lecture 36 Michael Fowler, UVa First and Second Laws of A quick review. Thermodynamics First Law: total energy conserved in any process: joules in = joules out Second Law: heat only
Chapter 19 Chemical Thermodynamics
 Chapter 19 Chemical Thermodynamics Spontaneous Processes Entropy and the Second Law of Thermodynamics The Molecular Interpretation of Entropy Entropy Changes in Chemical Reactions Gibbs Free Energy Free
Chapter 19 Chemical Thermodynamics Spontaneous Processes Entropy and the Second Law of Thermodynamics The Molecular Interpretation of Entropy Entropy Changes in Chemical Reactions Gibbs Free Energy Free
Lecture 2: Intro. Statistical Mechanics
 Lecture 2: Intro. Statistical Mechanics Statistical mechanics: concepts Aims: A microscopic view of entropy: Joule expansion reviewed. Boltzmann s postulate. S k ln g. Methods: Calculating arrangements;
Lecture 2: Intro. Statistical Mechanics Statistical mechanics: concepts Aims: A microscopic view of entropy: Joule expansion reviewed. Boltzmann s postulate. S k ln g. Methods: Calculating arrangements;
CHAPTER FIVE FUNDAMENTAL CONCEPTS OF STATISTICAL PHYSICS "
 CHAPTE FIVE FUNDAMENTAL CONCEPTS OF STATISTICAL PHYSICS " INTODUCTION In the previous chapters we have discussed classical thermodynamic principles which can be used to predict relationships among the
CHAPTE FIVE FUNDAMENTAL CONCEPTS OF STATISTICAL PHYSICS " INTODUCTION In the previous chapters we have discussed classical thermodynamic principles which can be used to predict relationships among the
The goal of equilibrium statistical mechanics is to calculate the diagonal elements of ˆρ eq so we can evaluate average observables < A >= Tr{Â ˆρ eq
 Chapter. The microcanonical ensemble The goal of equilibrium statistical mechanics is to calculate the diagonal elements of ˆρ eq so we can evaluate average observables < A >= Tr{Â ˆρ eq } = A that give
Chapter. The microcanonical ensemble The goal of equilibrium statistical mechanics is to calculate the diagonal elements of ˆρ eq so we can evaluate average observables < A >= Tr{Â ˆρ eq } = A that give
Chapter 20. Heat Engines, Entropy and the Second Law of Thermodynamics. Dr. Armen Kocharian
 Chapter 20 Heat Engines, Entropy and the Second Law of Thermodynamics Dr. Armen Kocharian First Law of Thermodynamics Review Review: The first law states that a change in internal energy in a system can
Chapter 20 Heat Engines, Entropy and the Second Law of Thermodynamics Dr. Armen Kocharian First Law of Thermodynamics Review Review: The first law states that a change in internal energy in a system can
Introduction. Chapter The Purpose of Statistical Mechanics
 Chapter 1 Introduction 1.1 The Purpose of Statistical Mechanics Statistical Mechanics is the mechanics developed to treat a collection of a large number of atoms or particles. Such a collection is, for
Chapter 1 Introduction 1.1 The Purpose of Statistical Mechanics Statistical Mechanics is the mechanics developed to treat a collection of a large number of atoms or particles. Such a collection is, for
Quiz 3 for Physics 176: Answers. Professor Greenside
 Quiz 3 for Physics 176: Answers Professor Greenside True or False Questions ( points each) For each of the following statements, please circle T or F to indicate respectively whether a given statement
Quiz 3 for Physics 176: Answers Professor Greenside True or False Questions ( points each) For each of the following statements, please circle T or F to indicate respectively whether a given statement
Irreversibility. Have you ever seen this happen? (when you weren t asleep or on medication) Which stage never happens?
 Lecture 5: Statistical Processes Random Walk and Particle Diffusion Counting and Probability Microstates and Macrostates The meaning of equilibrium 0.10 0.08 Reading: Elements Ch. 5 Probability (N 1, N
Lecture 5: Statistical Processes Random Walk and Particle Diffusion Counting and Probability Microstates and Macrostates The meaning of equilibrium 0.10 0.08 Reading: Elements Ch. 5 Probability (N 1, N
Lecture 4: Classical Illustrations of Macroscopic Thermal Effects
 Lecture 4: Classical Illustrations of Macroscopic Thermal Effects Heat capacity of solids & liquids Thermal conductivity Irreversibility References for this Lecture: Elements Ch 3,4A-C Reference for Lecture
Lecture 4: Classical Illustrations of Macroscopic Thermal Effects Heat capacity of solids & liquids Thermal conductivity Irreversibility References for this Lecture: Elements Ch 3,4A-C Reference for Lecture
CHEM-UA 652: Thermodynamics and Kinetics
 1 CHEM-UA 652: Thermodynamics and Kinetics Notes for Lecture 2 I. THE IDEAL GAS LAW In the last lecture, we discussed the Maxwell-Boltzmann velocity and speed distribution functions for an ideal gas. Remember
1 CHEM-UA 652: Thermodynamics and Kinetics Notes for Lecture 2 I. THE IDEAL GAS LAW In the last lecture, we discussed the Maxwell-Boltzmann velocity and speed distribution functions for an ideal gas. Remember
Lecture 7: Statistics and the Central Limit Theorem. Philip Moriarty,
 Lecture 7: Statistics and the Central Limit Theorem Philip Moriarty, philip.moriarty@nottingham.ac.uk NB Notes based heavily on lecture slides prepared by DE Rourke for the F32SMS module, 2006 7.1 Recap
Lecture 7: Statistics and the Central Limit Theorem Philip Moriarty, philip.moriarty@nottingham.ac.uk NB Notes based heavily on lecture slides prepared by DE Rourke for the F32SMS module, 2006 7.1 Recap
Physics 213 Spring 2009 Midterm exam. Review Lecture
 Physics 213 Spring 2009 Midterm exam Review Lecture The next two questions pertain to the following situation. A container of air (primarily nitrogen and oxygen molecules) is initially at 300 K and atmospheric
Physics 213 Spring 2009 Midterm exam Review Lecture The next two questions pertain to the following situation. A container of air (primarily nitrogen and oxygen molecules) is initially at 300 K and atmospheric
Heat Capacities, Absolute Zero, and the Third Law
 Heat Capacities, Absolute Zero, and the hird Law We have already noted that heat capacity and entropy have the same units. We will explore further the relationship between heat capacity and entropy. We
Heat Capacities, Absolute Zero, and the hird Law We have already noted that heat capacity and entropy have the same units. We will explore further the relationship between heat capacity and entropy. We
With certain caveats (described later) an object absorbs as effectively as it emits
 Figure 1: A blackbody defined by a cavity where emission and absorption are in equilibrium so as to maintain a constant temperature Blackbody radiation The basic principles of thermal emission are as follows:
Figure 1: A blackbody defined by a cavity where emission and absorption are in equilibrium so as to maintain a constant temperature Blackbody radiation The basic principles of thermal emission are as follows:
International Physics Course Entrance Examination Questions
 International Physics Course Entrance Examination Questions (May 2010) Please answer the four questions from Problem 1 to Problem 4. You can use as many answer sheets you need. Your name, question numbers
International Physics Course Entrance Examination Questions (May 2010) Please answer the four questions from Problem 1 to Problem 4. You can use as many answer sheets you need. Your name, question numbers
Chapter 4 - Second Law of Thermodynamics
 Chapter 4 - The motive power of heat is independent of the agents employed to realize it. -Nicolas Léonard Sadi Carnot David J. Starling Penn State Hazleton Fall 2013 An irreversible process is a process
Chapter 4 - The motive power of heat is independent of the agents employed to realize it. -Nicolas Léonard Sadi Carnot David J. Starling Penn State Hazleton Fall 2013 An irreversible process is a process
The Methodology of Statistical Mechanics
 Chapter 4 The Methodology of Statistical Mechanics c 2006 by Harvey Gould and Jan Tobochnik 16 November 2006 We develop the basic methodology of statistical mechanics and provide a microscopic foundation
Chapter 4 The Methodology of Statistical Mechanics c 2006 by Harvey Gould and Jan Tobochnik 16 November 2006 We develop the basic methodology of statistical mechanics and provide a microscopic foundation
PHYS Statistical Mechanics I Course Outline
 PHYS 449 - Statistical Mechanics I Course Outline There is no official textbook for this course. Suggested References: An Introduction to Thermal Physics, by Daniel V. Schroeder: this was the textbook
PHYS 449 - Statistical Mechanics I Course Outline There is no official textbook for this course. Suggested References: An Introduction to Thermal Physics, by Daniel V. Schroeder: this was the textbook
Physics 132- Fundamentals of Physics for Biologists II. Statistical Physics and Thermodynamics
 Physics 132- Fundamentals of Physics for Biologists II Statistical Physics and Thermodynamics QUIZ 2 25 Quiz 2 20 Number of Students 15 10 5 AVG: STDEV: 5.15 2.17 0 0 2 4 6 8 10 Score 1. (4 pts) A 200
Physics 132- Fundamentals of Physics for Biologists II Statistical Physics and Thermodynamics QUIZ 2 25 Quiz 2 20 Number of Students 15 10 5 AVG: STDEV: 5.15 2.17 0 0 2 4 6 8 10 Score 1. (4 pts) A 200
Physics Oct A Quantum Harmonic Oscillator
 Physics 301 5-Oct-2005 9-1 A Quantum Harmonic Oscillator The quantum harmonic oscillator (the only kind there is, really) has energy levels given by E n = (n + 1/2) hω, where n 0 is an integer and the
Physics 301 5-Oct-2005 9-1 A Quantum Harmonic Oscillator The quantum harmonic oscillator (the only kind there is, really) has energy levels given by E n = (n + 1/2) hω, where n 0 is an integer and the
Lecture 9 Overview (Ch. 1-3)
 Lecture 9 Overview (Ch. -) Format of the first midterm: four problems with multiple questions. he Ideal Gas Law, calculation of δw, δq and ds for various ideal gas processes. Einstein solid and two-state
Lecture 9 Overview (Ch. -) Format of the first midterm: four problems with multiple questions. he Ideal Gas Law, calculation of δw, δq and ds for various ideal gas processes. Einstein solid and two-state
DAY 28. Summary of Primary Topics Covered. The 2 nd Law of Thermodynamics
 DAY 28 Summary of Primary Topics Covered The 2 nd Law of Thermodynamics The 2 nd Law of Thermodynamics says this - - Heat energy naturally flows from hotter objects to colder objects. We know this happens,
DAY 28 Summary of Primary Topics Covered The 2 nd Law of Thermodynamics The 2 nd Law of Thermodynamics says this - - Heat energy naturally flows from hotter objects to colder objects. We know this happens,
84 My God, He Plays Dice! Chapter 12. Irreversibility. This chapter on the web informationphilosopher.com/problems/reversibility
 84 My God, He Plays Dice! This chapter on the web informationphilosopher.com/problems/reversibility Microscopic In the 1870 s, Ludwig Boltzmann developed his transport equation and his dynamical H-theorem
84 My God, He Plays Dice! This chapter on the web informationphilosopher.com/problems/reversibility Microscopic In the 1870 s, Ludwig Boltzmann developed his transport equation and his dynamical H-theorem
Revision Guide for Chapter 13
 Matter: very simple Revision Guide for Chapter Contents Student s Checklist Revision Notes Ideal gas... Ideal gas laws... Assumptions of kinetic theory of gases... 5 Internal energy... 6 Specific thermal
Matter: very simple Revision Guide for Chapter Contents Student s Checklist Revision Notes Ideal gas... Ideal gas laws... Assumptions of kinetic theory of gases... 5 Internal energy... 6 Specific thermal
Conduction. Heat Transfer Methods. Conduction. Conduction
 Heat Transfer Methods Conduction: Thermal kinetic energy passed from particle-to-particle along a length of material. Convection: Thermal energy carried by moving fluid. Radiation: Thermal energy carried
Heat Transfer Methods Conduction: Thermal kinetic energy passed from particle-to-particle along a length of material. Convection: Thermal energy carried by moving fluid. Radiation: Thermal energy carried
Lecture 6 Examples and Problems
 Lecture 6 Examples and Problems Heat capacity of solids & liquids Thermal diffusion Thermal conductivity Irreversibility Hot Cold Random Walk and Particle Diffusion Counting and Probability Microstates
Lecture 6 Examples and Problems Heat capacity of solids & liquids Thermal diffusion Thermal conductivity Irreversibility Hot Cold Random Walk and Particle Diffusion Counting and Probability Microstates
Basic Concepts and Tools in Statistical Physics
 Chapter 1 Basic Concepts and Tools in Statistical Physics 1.1 Introduction Statistical mechanics provides general methods to study properties of systems composed of a large number of particles. It establishes
Chapter 1 Basic Concepts and Tools in Statistical Physics 1.1 Introduction Statistical mechanics provides general methods to study properties of systems composed of a large number of particles. It establishes
Lecture 8: Differential Equations. Philip Moriarty,
 Lecture 8: Differential Equations Philip Moriarty, philip.moriarty@nottingham.ac.uk NB Notes based heavily on lecture slides prepared by DE Rourke for the F32SMS module, 2006 8.1 Overview In this final
Lecture 8: Differential Equations Philip Moriarty, philip.moriarty@nottingham.ac.uk NB Notes based heavily on lecture slides prepared by DE Rourke for the F32SMS module, 2006 8.1 Overview In this final
...Thermodynamics. Lecture 15 November 9, / 26
 ...Thermodynamics Conjugate variables Positive specific heats and compressibility Clausius Clapeyron Relation for Phase boundary Phase defined by discontinuities in state variables Lecture 15 November
...Thermodynamics Conjugate variables Positive specific heats and compressibility Clausius Clapeyron Relation for Phase boundary Phase defined by discontinuities in state variables Lecture 15 November
Statistical Mechanics Victor Naden Robinson vlnr500 3 rd Year MPhys 17/2/12 Lectured by Rex Godby
 Statistical Mechanics Victor Naden Robinson vlnr500 3 rd Year MPhys 17/2/12 Lectured by Rex Godby Lecture 1: Probabilities Lecture 2: Microstates for system of N harmonic oscillators Lecture 3: More Thermodynamics,
Statistical Mechanics Victor Naden Robinson vlnr500 3 rd Year MPhys 17/2/12 Lectured by Rex Godby Lecture 1: Probabilities Lecture 2: Microstates for system of N harmonic oscillators Lecture 3: More Thermodynamics,
Introduction Statistical Thermodynamics. Monday, January 6, 14
 Introduction Statistical Thermodynamics 1 Molecular Simulations Molecular dynamics: solve equations of motion Monte Carlo: importance sampling r 1 r 2 r n MD MC r 1 r 2 2 r n 2 3 3 4 4 Questions How can
Introduction Statistical Thermodynamics 1 Molecular Simulations Molecular dynamics: solve equations of motion Monte Carlo: importance sampling r 1 r 2 r n MD MC r 1 r 2 2 r n 2 3 3 4 4 Questions How can
13! (52 13)! 52! =
 Thermo and Intro to Stat Mech 018 Homework assignment 1, Problem 1: What is the probability that all 13 cards on a hand (in bridge for example) are of the same kind, for example all spades? There are 5
Thermo and Intro to Stat Mech 018 Homework assignment 1, Problem 1: What is the probability that all 13 cards on a hand (in bridge for example) are of the same kind, for example all spades? There are 5
MP203 Statistical and Thermal Physics. Problem set 7 - Solutions
 MP203 Statistical and Thermal Physics Problem set 7 - Solutions 1. For each of the following processes, decide whether or not they are reversible. If they are irreversible, explain how you can tell that
MP203 Statistical and Thermal Physics Problem set 7 - Solutions 1. For each of the following processes, decide whether or not they are reversible. If they are irreversible, explain how you can tell that
a. 4.2x10-4 m 3 b. 5.5x10-4 m 3 c. 1.2x10-4 m 3 d. 1.4x10-5 m 3 e. 8.8x10-5 m 3
 The following two problems refer to this situation: #1 A cylindrical chamber containing an ideal diatomic gas is sealed by a movable piston with cross-sectional area A = 0.0015 m 2. The volume of the chamber
The following two problems refer to this situation: #1 A cylindrical chamber containing an ideal diatomic gas is sealed by a movable piston with cross-sectional area A = 0.0015 m 2. The volume of the chamber
PHYSICS DEPARTMENT, PRINCETON UNIVERSITY PHYSICS 301 FINAL EXAMINATION. January 13, 2005, 7:30 10:30pm, Jadwin A10 SOLUTIONS
 PHYSICS DEPARTMENT, PRINCETON UNIVERSITY PHYSICS 301 FINAL EXAMINATION January 13, 2005, 7:30 10:30pm, Jadwin A10 SOLUTIONS This exam contains five problems. Work any three of the five problems. All problems
PHYSICS DEPARTMENT, PRINCETON UNIVERSITY PHYSICS 301 FINAL EXAMINATION January 13, 2005, 7:30 10:30pm, Jadwin A10 SOLUTIONS This exam contains five problems. Work any three of the five problems. All problems
Statistical Mechanics
 Statistical Mechanics Newton's laws in principle tell us how anything works But in a system with many particles, the actual computations can become complicated. We will therefore be happy to get some 'average'
Statistical Mechanics Newton's laws in principle tell us how anything works But in a system with many particles, the actual computations can become complicated. We will therefore be happy to get some 'average'
Statistical Mechanics in a Nutshell
 Chapter 2 Statistical Mechanics in a Nutshell Adapted from: Understanding Molecular Simulation Daan Frenkel and Berend Smit Academic Press (2001) pp. 9-22 11 2.1 Introduction In this course, we will treat
Chapter 2 Statistical Mechanics in a Nutshell Adapted from: Understanding Molecular Simulation Daan Frenkel and Berend Smit Academic Press (2001) pp. 9-22 11 2.1 Introduction In this course, we will treat
Physics 404: Final Exam Name (print): "I pledge on my honor that I have not given or received any unauthorized assistance on this examination.
 Physics 404: Final Exam Name (print): "I pledge on my honor that I have not given or received any unauthorized assistance on this examination." May 20, 2008 Sign Honor Pledge: Don't get bogged down on
Physics 404: Final Exam Name (print): "I pledge on my honor that I have not given or received any unauthorized assistance on this examination." May 20, 2008 Sign Honor Pledge: Don't get bogged down on
What Is Air Temperature?
 2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
1 What is the area model for multiplication?
 for multiplication represents a lovely way to view the distribution property the real number exhibit. This property is the link between addition and multiplication. 1 1 What is the area model for multiplication?
for multiplication represents a lovely way to view the distribution property the real number exhibit. This property is the link between addition and multiplication. 1 1 What is the area model for multiplication?
First Law Limitations
 First Law Limitations First Law: During any process, the energy of the universe is constant. du du du ZERO!!! universe sys surroundings Any energy transfer between system and surroundings is accomplished
First Law Limitations First Law: During any process, the energy of the universe is constant. du du du ZERO!!! universe sys surroundings Any energy transfer between system and surroundings is accomplished
Concepts in Materials Science I. StatMech Basics. VBS/MRC Stat Mech Basics 0
 StatMech Basics VBS/MRC Stat Mech Basics 0 Goal of Stat Mech Concepts in Materials Science I Quantum (Classical) Mechanics gives energy as a function of configuration Macroscopic observables (state) (P,
StatMech Basics VBS/MRC Stat Mech Basics 0 Goal of Stat Mech Concepts in Materials Science I Quantum (Classical) Mechanics gives energy as a function of configuration Macroscopic observables (state) (P,
Combinatorics. But there are some standard techniques. That s what we ll be studying.
 Combinatorics Problem: How to count without counting. How do you figure out how many things there are with a certain property without actually enumerating all of them. Sometimes this requires a lot of
Combinatorics Problem: How to count without counting. How do you figure out how many things there are with a certain property without actually enumerating all of them. Sometimes this requires a lot of
The SI unit for Energy is the joule, usually abbreviated J. One joule is equal to one kilogram meter squared per second squared:
 Chapter 2 Energy Energy is an extremely loaded term. It is used in everyday parlance to mean a number of different things, many of which bear at most a passing resemblance to the term as used in physical
Chapter 2 Energy Energy is an extremely loaded term. It is used in everyday parlance to mean a number of different things, many of which bear at most a passing resemblance to the term as used in physical
Algebra Exam. Solutions and Grading Guide
 Algebra Exam Solutions and Grading Guide You should use this grading guide to carefully grade your own exam, trying to be as objective as possible about what score the TAs would give your responses. Full
Algebra Exam Solutions and Grading Guide You should use this grading guide to carefully grade your own exam, trying to be as objective as possible about what score the TAs would give your responses. Full
Big-oh stuff. You should know this definition by heart and be able to give it,
 Big-oh stuff Definition. if asked. You should know this definition by heart and be able to give it, Let f and g both be functions from R + to R +. Then f is O(g) (pronounced big-oh ) if and only if there
Big-oh stuff Definition. if asked. You should know this definition by heart and be able to give it, Let f and g both be functions from R + to R +. Then f is O(g) (pronounced big-oh ) if and only if there
Boltzmann Distribution Law (adapted from Nash)
 Introduction Statistical mechanics provides a bridge between the macroscopic realm of classical thermodynamics and the microscopic realm of atoms and molecules. We are able to use computational methods
Introduction Statistical mechanics provides a bridge between the macroscopic realm of classical thermodynamics and the microscopic realm of atoms and molecules. We are able to use computational methods
Module 5 : MODERN PHYSICS Lecture 23 : Particle and Waves
 Module 5 : MODERN PHYSICS Lecture 23 : Particle and Waves Objectives In this lecture you will learn the following Radiation (light) exhibits both wave and particle nature. Laws governing black body radiation,
Module 5 : MODERN PHYSICS Lecture 23 : Particle and Waves Objectives In this lecture you will learn the following Radiation (light) exhibits both wave and particle nature. Laws governing black body radiation,
Entropy online activity: accompanying handout I. Introduction
 Entropy online activity: accompanying handout I. Introduction The goal of this activity is to provide insight into the ways modern science views the effects of temperature on chemical reactions, including
Entropy online activity: accompanying handout I. Introduction The goal of this activity is to provide insight into the ways modern science views the effects of temperature on chemical reactions, including
SCED 204 Sample Activity SCED 204: Matter and Energy in Chemical Systems
 SCED 204 Sample Activity SCED 204: Matter and Energy in Chemical Systems Activity #2: DO THE SMALL PARTICLES OF MATTER MOVE? IF SO, HOW? Purpose: We have been developing a model of matter that involves
SCED 204 Sample Activity SCED 204: Matter and Energy in Chemical Systems Activity #2: DO THE SMALL PARTICLES OF MATTER MOVE? IF SO, HOW? Purpose: We have been developing a model of matter that involves
Dr.Salwa Alsaleh fac.ksu.edu.sa/salwams
 Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams Lecture 5 Basic Ideas of Statistical Mechanics General idea Macrostates and microstates Fundamental assumptions A simple illustration: tossing
Dr.Salwa Alsaleh Salwams@ksu.edu.sa fac.ksu.edu.sa/salwams Lecture 5 Basic Ideas of Statistical Mechanics General idea Macrostates and microstates Fundamental assumptions A simple illustration: tossing
MATH2206 Prob Stat/20.Jan Weekly Review 1-2
 MATH2206 Prob Stat/20.Jan.2017 Weekly Review 1-2 This week I explained the idea behind the formula of the well-known statistic standard deviation so that it is clear now why it is a measure of dispersion
MATH2206 Prob Stat/20.Jan.2017 Weekly Review 1-2 This week I explained the idea behind the formula of the well-known statistic standard deviation so that it is clear now why it is a measure of dispersion
Figure 1: Doing work on a block by pushing it across the floor.
 Work Let s imagine I have a block which I m pushing across the floor, shown in Figure 1. If I m moving the block at constant velocity, then I know that I have to apply a force to compensate the effects
Work Let s imagine I have a block which I m pushing across the floor, shown in Figure 1. If I m moving the block at constant velocity, then I know that I have to apply a force to compensate the effects
Module 1. Probability
 Module 1 Probability 1. Introduction In our daily life we come across many processes whose nature cannot be predicted in advance. Such processes are referred to as random processes. The only way to derive
Module 1 Probability 1. Introduction In our daily life we come across many processes whose nature cannot be predicted in advance. Such processes are referred to as random processes. The only way to derive
Lecture 25 Goals: Chapter 18 Understand the molecular basis for pressure and the idealgas
 Lecture 5 Goals: Chapter 18 Understand the molecular basis for pressure and the idealgas law. redict the molar specific heats of gases and solids. Understand how heat is transferred via molecular collisions
Lecture 5 Goals: Chapter 18 Understand the molecular basis for pressure and the idealgas law. redict the molar specific heats of gases and solids. Understand how heat is transferred via molecular collisions
Lecture 4: Classical Illustrations of Macroscopic Thermal Effects. Heat capacity of solids & liquids. Thermal conductivity
 Lecture 4: Classical Illustrations of Macroscopic Thermal Effects Heat capacity of solids & liquids Thermal conductivity References for this Lecture: Elements Ch 3,4A-C Reference for Lecture 5: Elements
Lecture 4: Classical Illustrations of Macroscopic Thermal Effects Heat capacity of solids & liquids Thermal conductivity References for this Lecture: Elements Ch 3,4A-C Reference for Lecture 5: Elements
Introduction to thermodynamics
 Chapter 6 Introduction to thermodynamics Topics First law of thermodynamics Definitions of internal energy and work done, leading to du = dq + dw Heat capacities, C p = C V + R Reversible and irreversible
Chapter 6 Introduction to thermodynamics Topics First law of thermodynamics Definitions of internal energy and work done, leading to du = dq + dw Heat capacities, C p = C V + R Reversible and irreversible
THE SECOND LAW OF THERMODYNAMICS. Professor Benjamin G. Levine CEM 182H Lecture 5
 THE SECOND LAW OF THERMODYNAMICS Professor Benjamin G. Levine CEM 182H Lecture 5 Chemical Equilibrium N 2 + 3 H 2 2 NH 3 Chemical reactions go in both directions Systems started from any initial state
THE SECOND LAW OF THERMODYNAMICS Professor Benjamin G. Levine CEM 182H Lecture 5 Chemical Equilibrium N 2 + 3 H 2 2 NH 3 Chemical reactions go in both directions Systems started from any initial state
Outline. Today we will learn what is thermal radiation
 Thermal Radiation & Outline Today we will learn what is thermal radiation Laws Laws of of themodynamics themodynamics Radiative Radiative Diffusion Diffusion Equation Equation Thermal Thermal Equilibrium
Thermal Radiation & Outline Today we will learn what is thermal radiation Laws Laws of of themodynamics themodynamics Radiative Radiative Diffusion Diffusion Equation Equation Thermal Thermal Equilibrium
Brownian Motion and The Atomic Theory
 Brownian Motion and The Atomic Theory Albert Einstein Annus Mirabilis Centenary Lecture Simeon Hellerman Institute for Advanced Study, 5/20/2005 Founders Day 1 1. What phenomenon did Einstein explain?
Brownian Motion and The Atomic Theory Albert Einstein Annus Mirabilis Centenary Lecture Simeon Hellerman Institute for Advanced Study, 5/20/2005 Founders Day 1 1. What phenomenon did Einstein explain?
t = no of steps of length s
 s t = no of steps of length s Figure : Schematic of the path of a diffusing molecule, for example, one in a gas or a liquid. The particle is moving in steps of length s. For a molecule in a liquid the
s t = no of steps of length s Figure : Schematic of the path of a diffusing molecule, for example, one in a gas or a liquid. The particle is moving in steps of length s. For a molecule in a liquid the
Probability (Devore Chapter Two)
 Probability (Devore Chapter Two) 1016-345-01: Probability and Statistics for Engineers Fall 2012 Contents 0 Administrata 2 0.1 Outline....................................... 3 1 Axiomatic Probability 3
Probability (Devore Chapter Two) 1016-345-01: Probability and Statistics for Engineers Fall 2012 Contents 0 Administrata 2 0.1 Outline....................................... 3 1 Axiomatic Probability 3
Probability and the Second Law of Thermodynamics
 Probability and the Second Law of Thermodynamics Stephen R. Addison January 24, 200 Introduction Over the next several class periods we will be reviewing the basic results of probability and relating probability
Probability and the Second Law of Thermodynamics Stephen R. Addison January 24, 200 Introduction Over the next several class periods we will be reviewing the basic results of probability and relating probability
Physics 008 Tutorial 7: Energy Bands
 1 Physics 8 Tutorial 7: Bands In this tutorial we are going to investigate how electronic bands form when atoms are brought together. We saw in the last tutorial and in the lectures that atoms possess
1 Physics 8 Tutorial 7: Bands In this tutorial we are going to investigate how electronic bands form when atoms are brought together. We saw in the last tutorial and in the lectures that atoms possess
Heat Machines (Chapters 18.6, 19)
 eat Machines (hapters 8.6, 9) eat machines eat engines eat pumps The Second Law of thermodynamics Entropy Ideal heat engines arnot cycle Other cycles: Brayton, Otto, Diesel eat Machines Description The
eat Machines (hapters 8.6, 9) eat machines eat engines eat pumps The Second Law of thermodynamics Entropy Ideal heat engines arnot cycle Other cycles: Brayton, Otto, Diesel eat Machines Description The
Lecture 6 Examples and Problems
 Lecture 6 Examples and Problems Heat capacity of solids & liquids Thermal diffusion Thermal conductivity Irreversibility Hot Cold Random Walk and Particle Diffusion Counting and Probability Microstates
Lecture 6 Examples and Problems Heat capacity of solids & liquids Thermal diffusion Thermal conductivity Irreversibility Hot Cold Random Walk and Particle Diffusion Counting and Probability Microstates
Stuff 1st Law of Thermodynamics First Law Differential Form Total Differential Total Differential
 Stuff ---onight: Lecture 4 July ---Assignment has been posted. ---Presentation Assignment posted. --Some more thermodynamics and then problem solving in class for Assignment #. --Next week: Free Energy
Stuff ---onight: Lecture 4 July ---Assignment has been posted. ---Presentation Assignment posted. --Some more thermodynamics and then problem solving in class for Assignment #. --Next week: Free Energy
Thermodynamics: Free Energy and Entropy. Suggested Reading: Chapter 19
 Thermodynamics: Free Energy and Entropy Suggested Reading: Chapter 19 System and Surroundings System: An object or collection of objects being studied. Surroundings: Everything outside of the system. the
Thermodynamics: Free Energy and Entropy Suggested Reading: Chapter 19 System and Surroundings System: An object or collection of objects being studied. Surroundings: Everything outside of the system. the
Active Learners Reflective Learners Use both ways equally frequently More Inclined to be Active More inclined to be Reflective
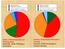 Active Learners Reflective Learners Use both ways equally frequently More Inclined to be Active More inclined to be Reflective Sensory Leaners Intuitive Learners Use both ways equally frequently More inclined
Active Learners Reflective Learners Use both ways equally frequently More Inclined to be Active More inclined to be Reflective Sensory Leaners Intuitive Learners Use both ways equally frequently More inclined
GRADE 9: Physical processes 4. UNIT 9P.4 6 hours. The electromagnetic spectrum. Resources. About this unit. Previous learning.
 GRADE 9: Physical processes 4 The electromagnetic spectrum UNIT 9P.4 6 hours About this unit This unit is the fourth of seven units on physical processes for Grade 9. The unit is designed to guide your
GRADE 9: Physical processes 4 The electromagnetic spectrum UNIT 9P.4 6 hours About this unit This unit is the fourth of seven units on physical processes for Grade 9. The unit is designed to guide your
1. Introductory Examples
 1. Introductory Examples We introduce the concept of the deterministic and stochastic simulation methods. Two problems are provided to explain the methods: the percolation problem, providing an example
1. Introductory Examples We introduce the concept of the deterministic and stochastic simulation methods. Two problems are provided to explain the methods: the percolation problem, providing an example
