Available online at
|
|
|
- June Flynn
- 5 years ago
- Views:
Transcription
1 Available online at Earth and Planetary Science Letters 265 (2008) Towards a consistent mantle carbon flux estimate: Insights from volatile systematics (H 2 O/Ce, δd, CO 2 /Nb) in the North Atlantic mantle (14 N and 34 N) Pierre Cartigny, Françoise Pineau, Cyril Aubaud, Marc Javoy Laboratoire de Géochimie des Isotopes Stables de l'institut de Physique du Globe de Paris, 2 place Jussieu, T54-64 E1, Paris Cedex 05, France Received 6 October 2006; received in revised form 2 November 2007; accepted 2 November 2007 Available online 17 November 2007 Editor: R.W. Carlson Abstract In order to better characterise mantle CO 2 /Nb-variability, we obtained and compiled major and trace elements, content and isotope composition of both CO 2 and water on two series of mid-ocean ridge basalt (MORB) samples dredged at 14 N (n=6) and 34 N (n=11) on the mid-atlantic ridge. All samples are carbon-saturated. One, the so-called popping rock 2ΠD43 kept its vesicles, the initial (pre-degassing) C-contents of the 16 other samples being reconstructed from their assumed degassing history. For water, the samples show large variations, from 1300 to 6900 ppm and from 1900 to 7900 ppm with associated δd-values ranging from 55 to 79 and from 55 to 88 for samples at 14 N and 34 N respectively. For carbon, the inferred initial predegassing contents vary greatly, from 660 to 14,700 ppmco 2 and from 1400 to 57,600 ppmco 2 for samples at 14 N and 34 N respectively. Measured Nb-contents range from 4.5 to 29.6 ppm show both good agreement with previously published data and positive correlations with reconstructed initial CO 2 -contents. The mean CO 2 /Nb range from 570 to 730 at 14 N and 34 N respectively. CO 2 and Nb data for the two undegassed samples available so far (i.e. the popping rock of the present study and the basaltic glasses from the Siqueiros transform fault from the study of Saal et al., 2002) show significant variations in CO 2 /Nb over a factor of 2 and thus questions the constant CO 2 /Nb previously emphasised for these two samples, this view being supported by CO 2 /Nb-ratios of samples whose initial C-contents were reconstructed. For incompatible elements such as Ce, K and including water, a comparison of the geochemical characteristics of transform fault basaltic magmatism with other MORB systems shows magma transform fault magmatism to be unrepresentative of mantle compositions. Assuming a more appropriate average MORB CO 2 /Nb-ratio of 530 and a mean MORB Nb-content of 3:31 þ3:99 1:8, we computed a mantle carbon flux of 2:3 1:3 þ2: mol/yr, a value actually consistent with that derived from C/ 3 He systematics Elsevier B.V. All rights reserved. Keywords: MORB; volatiles; carbon; niobium; degassing Corresponding author. Tel.: address: cartigny@ipgp.jussieu.fr (P. Cartigny) X/$ - see front matter 2007 Elsevier B.V. All rights reserved. doi: /j.epsl
2 P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008) Introduction A reliable estimate of mantle carbon flux degassed to the atmosphere is essential due to its impact on Earth's climate (Berner and Kothavala, 2001; François and Goddéris, 1998; Hayes and Waldbauer, 2006; Javoy et al., 1982). It also bears on our evaluation of mantle carbon contents which has implications not only on the initiation and amount of mantle partial melting (Dasgupta and Hirschmann, 2006) but also on our understanding of the Earth's mantle structure and convection, i.e. as to whether one or two layers of convection cells are required (Ballentine et al., 2000). Compared with margin and intra-plate volcanisms (Crisp, 1984), the main CO 2 flux from the mantle is emitted at mid-ocean ridges with estimates varying greatly from 1.0 to mol/year (e.g. Javoy et al., 1982; Marty and Tolstikhin, 1998; Cartigny et al., 2001). Given the rather low carbon solubility in basaltic melts ( μmol/bar/g at Pb0.2 GPa Dixon et al., 1995; Jendrzejewski et al., 1997), almost every MORB sample underwent N 80% carbon degassing and the range in CO 2 flux estimates reflects the various methods used to reconstruct initial, i.e. prior to degassing, MORB C- contents. Every method suffers from a series of assumptions and simplifications (see Cartigny et al., 2001 for discussion). In this respect, key constraints can be brought by considering samples which have kept their volatiles. Two samples are presently available. The first, often referred to as popping-rock, as the sample spontaneously jumpswhenover-pressuredgas-richvesiclesexplode, was dredged at 14 N on the mid-atlantic ridge (MAR) (Sarda and Graham, 1990). Its high vesicularity ( 17%), high gas content of 4000 ppmc ( 14,700 ppmco 2 ), 4 He 10 4 ccstp/g, isotopes ratios such as 4 He/ 40 Ar 1.5 being similar within error to the mantle production ratio (Javoy and Pineau, 1991; Moreira et al., 1998; Sarda and Graham, 1990; Staudacher et al., 1988), all strongly support the idea that the sample did not lose its volatiles, i.e. the MORB was supersaturated with respect to carbon, had an associated exsolved vapor phase, but its vesicles stayed within the magma while degassing (i.e. closed system) and the reader is referred to Bottinga and Javoy (1990) for a discussion of the conditions that led to their preservation. This specific sample has been considered by numerous authors as a reference for MORB volatile studies. The possibility that the high gas contents might correspond to gas accumulation (see Javoy and Pineau, 1991) is unlikely and so far nobody has questioned that conclusion (Cartigny et al., 2001; Javoy and Pineau, 1991; Marty and Tolstikhin, 1998; Moreira et al., 1998; Saal et al., 2002; Sarda and Graham, 1990; Staudacher et al., 1988). Given the importance of this sample in the following discussion, it is worth noting that this conclusion is actually supported by most recent rare gas data on magmatic CO 2 from well gases in New Mexico (Holland and Ballentine, 2006). Given the very different solubilities of rare gases in geologic systems, volatile accumulation in 2πD43 would led to rare gas patterns different from those of New Mexico gas wells (Holland and Ballentine, 2006). This is not the case: gas wells and MORB popping rock showing very similar rare gas patterns. The second sample was recovered from the Siqueiros fault zone in the South Pacific (Saal et al., 2002). In this specific case it corresponds to a series of samples unusually depleted in volatiles (i.e. very low dissolved H 2 O and CO 2 contents of ppm and ppm respectively) erupted deep enough (N 3500 m) to prevent carbon supersaturation, i.e. the sample did not degas. For these two samples, initial carbon contents are preserved and can thus be studied (as it is commonly done for water see Dixon et al., 2002; Michael, 1995; Simons et al., 2002) in relation with another non-volatile elements with similar incompatibility, among them Nb (Saal et al., 2002). Such a method is extremely promising as, in contrast with previous approaches (e.g. Javoy and Pineau, 1991; Marty and Jambon, 1987), it normalises carbon abundance to another, non-volatile, incompatible element. Furthermore, the suggestion that both Siqueiros and popping rock samples would have similar CO 2 /Nb-ratios ( 239±49 Saal et al., 2002) supports its use in determining mante carbon contents and the carbon flux degassed at mid-ocean ridges. To better address this issue, we analysed and compiled C-contents and isotope compositions, and Nb contents of MORB glass samples dredged at 14 N and at 34 N along the MAR. The origin of CO 2 /Nb variability is discussed in light of both other volatile systematics, in particular H 2 O/Ce and H-isotopes, and source enrichment indexes such as K/Ti and La/Sm. 2. Samples studied and analytical techniques The sample set is composed of two series of MORB glass samples dredged around 14 N (between 12.4 to 16.4 N) (n=6) and at 34 N (n=11) respectively along the MAR (Bougault et al., 1988; Hékinian et al., 2000; Niu et al., 2001). When not available, predegassing CO 2 -contents, H 2 O-contents, major and trace element data were obtained. Water and carbon contents and their isotope compositions (δd, δ 13 C) were measured using
3 674 P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008) both vacuum-crushing and step-heating techniques (see Pineau and Javoy, 1983; Pineau and Javoy, 1994). Errors are 5% on volatile contents, ±3 and ±0.1 (2σ) for δd and δ 13 C respectively (Pineau and Javoy, 1983, 1994). Major and trace elements (including Nb- and Cecontents) were reported previously for most of the investigated samples. However, in order to provide the most consistent dataset, when enough material remains (i.e. for 14 of them, Supplemental Table 1), samples on which volatile contents and isotope compositions have been determined, were systematically (re)analysed for major and trace elements. MORB glasses were mounted in araldite, polished and analysed for major elements using a Cameca SX 50 electron micro-probe at the Service Camparis of University of Paris 6. Analytical conditions were 15 kv accelerating voltage, 10 na beam current, 20 μm defocused beam size, 10 s peak acquisition time and 5 s for background. Relative uncertainties for each oxide are as follows: SiO 2 1%, TiO 2 2%, Al 2 O 3 1%, FeO 2%, MnO 20%, MgO 2%, CaO 2%, Na 2 O 2.5% and K 2 O 5%. Trace elements concentrations were measured at the Mineralogishes Institut of Frankfurt by laser-icp-ms using a 0.25 mj laser beam ( μm) delivered by NewwaveUP213 operating at 10 Hz and attached to a Finnigan Element2 ICPMS, 44 Ca and BHVO2G glass standard (Gao et al., 2002) being used as internal and external standards respectively. Accuracies are better than 10% (2σ) for all elements and data obtained for BHVO2G standard are given in Table 2 in the Appendix. 3. Results 3.1. Major and trace element data Measured and previously measured Nb, Ce-contents, K 2 O, TiO 2 and (La/Sm) N -ratios (Dosso et al., 1993; Hémond et al., 2006; Javoy and Pineau, 1991; Niu et al., 2001; Pineau and Javoy, 1994; Pineau et al., 2004) are reported in Table 1. As shown previously, samples from 14 N comprise N- to T-MORB (K 2 O/TiO 2 from 0.02 to 0.32) (Bougault et al., 1988) whereas samples from 34 N cover the whole range of composition from N-MORB to alkali-basalt (K 2 O/TiO 2 from 0.07 to 0.53) (Hékinian et al., 2000; Niu et al., 2001). Major element compositions (n=14, Table 1 in the Appendix) show overall good agreement with previously published data. All samples (n = 17) are characterised by rather primitive compositions (6.78 b MgO b 8.48 wt.%, average 7.63 wt.%, n =14, Table 1 in the Appendix plus 3 glass analyses in Bougault et al., 1988; Hékinian et al., 2000). As our samples display little variable MgO-contents, the samples set shows the expected relationships between (La/Sm) N with both Nbcontents and K 2 O/TiO 2 (not shown). Nb-data range from 1.1 to 41.3 ppm and there is an overall good agreement between new and previously published data. Furthermore a close comparison between our data and previously measured Nb-data (Bézos, 2003; Bougault et al., 1988; Dosso et al., 1993; Hémond et al., 2006; Niu et al., 2001) show the former to be lower by 5% on average. Ce-contents range between 3.8 and 41.6 ppm (n = 16; Table 1) and compared with previously data published on the same samples (n=9 Bougault et al., 1988; Niu et al., 2001), show the former to be lower by 9% on average, differences within the range reported among different laboratories (e.g. Kelley et al., 2003). As trace element contents have not all been determined with/within a single equipement/ laboratory, in order to provide the most consistent dataset, new Nb- and Ce-data reported in Table 1 have been multiplied by a factor 1.05 and 1.09 respectively. This choice is arbitrary but it is worth noting that choosing to normalise previous data to the present dataset would strengthen our main conclusion that mantle CO 2 /Nb ratio is higher than initially suggested. Trace elements data reported in Table 3 in the Appendix correspond to measured (i.e. uncorrected) values Measured and reconstructed (i.e. pre-degassing) volatile data Samples show large water content variations from 1300 to 6900 ppm and from 2300 to 7700 ppm for samples at 14 N and 34 N respectively (Table 1). Associated δd-values range from 55 to 79 and from 55 to 88 respectively. Because of a water solubility much higher than that of carbon (Hamilton et al., 1964), most samples remained water-undersaturated or were only slightly affected by degassing (i.e. limited amounts of water in vesicles) (Javoy and Pineau, 1991; Pineau and Javoy, 1994; Pineau et al., 2004). Water contents and H-isotope compositions reported in Table 1, thus mostly correspond to measured values for dissolved water. Table 1 summarises the reconstructed initial (i.e. predegassing) carbon-contents (see below), water contents and H-isotope compositions. Volatile data for 34 N samples having been published earlier, the reader is referred to the original publication for details (Pineau et al., 2004). The data, including dissolved and exsolved carbon contents and isotope compositions, used to estimate reconstructed carbon contents of 14 N samples of Table 1 are new data and the following paragraphs will
4 Table 1 Measured and compiled volatile, major and trace elements contents used in the present study Sample H 2 O (wt%) TiO2 (wt %) K 2 O (wt %) Ce (ppm) (La/Sm) N MgO (wt %) δd ( ) [CO2] initial Nb (this study) Nb (literature) Nb CO 2 /Nb TiO 2 /H 2 O K 2 O/TiO 2 H 2 O/Ce ppm ppm +/ ppm average +/ (ppm/ppm) +/ 2πD πD πD πD πD πD OT OT OT OT OT OT OT OT OT OT OT Siqueiros n.d Trace element data for 14 N samples are from Bougault et al. (1988), Siqueiros data are from Saal et al. (2002), trace element data for 34 N are from Niu et al. (2001) and volatile data for 34 N samples are from Pineau et al. (2004). Average Nb contents corresponds to averaged values obtained in this and previous studies and errors on CO 2 /Nb reflects only Nb variability. P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008)
5 676 P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008) detail the method to evaluate initial (pre-degassing) C- contents. Measured contents and C-isotope compositions of both dissolved and exsolved carbon are given in Table 4 in the Appendix. Measured carbon contents (vesicles+dissolved) range greatly from 100 to 4000 ppmc (i.e. 370 to 14,700 ppmco 2 ) (Table 4 in the Appendix). Initial carbon contents have been reconstructed assuming a degassing scenario for all samples (but for undegassed 2πD43 sample) from dissolved C-content and isotope composition data (Table 4 in the Appendix). The degassing model was that developed earlier in Pineau and Javoy, (1983, 1994) and assumes that degassing mostly occurred as batch equilibrium degassing as required by limited δ 13 C range of both CO 2 -vesicles and dissolved carbon ( 4 to 8 and 8 to 12 respectively) and/or large carbon contents of most MORB [see Pineau and Javoy (1983) for justification], a model supported by recent rare-gas content data (Moreira and Sarda, 2000). As illustrated by Fig. 1, isotopic fractionation during magma degassing takes place in two main steps during the MORB magma ascent: during most of the ascent the velocity is low, the bubbles move very little relative to the magma, there is plenty of time for gas-magma isotopic reequilibration and that step corresponds to isotopic equilibrium in a closed system between the volatiles dissolved in the magma and the gas. Isotopic fractionation results from a change of carbon speciation when carbonate ions dissolved in the melt exsolve as CO 2 in the vesicles leading in a decrease in 13 Cof dissolved carbon. As the magma further rises, and until vesicles escape, volatiles further concentrate in the gas phase and still remain in equilibrium with the magma. The equations driving degassing at depths are thus those under closed-system conditions (batch equilibrium degassing) and can be written as follows (larger grey curve in Fig. 1): d 13 C o C o ¼ d 13 C dis f þ ð1 f Þ d 13 C dis þ D C where δ 13 C o is the initial δ 13 C (i.e. 4.5 ), δ 13 C dis and f=c dis /C o is the remaining fraction of carbon. Δ C = (δ 13 C ves δ 13 C dis =+3.5 ) is the carbon isotope fractionation between dissoved and exsolved carbon. The second step for isotopic fractionation begins when the whole of a gas bubble cannot any more reequlibrate continuously with the magma: this is because the bubbles become larger and less dense because of the decreasing pressure, and because the rate of magma ascent decreases. For all these reasons bubbles ascend now much faster than the magma, leaving no time for isotopic reequilibration (Pineau and Javoy, 1983; Pineau and Javoy, 1994). The conditions for that step to take place depend on individual tectonic conditions and the depth where that change occurs can vary greatly, from very near the ocean bottom (degassing at equilibrium all along the ascent), the basis of oceanic crust, or, in extreme cases deep in the oceanic lithosphere. These different cases are translated in the isotopic composition of the residual dissolved carbon. The onset of that second step (hereafter referred to as stage A) corresponds to some kind of transition between a tranquil, slow style of ascent and the beginning of a rapid, more eruptive-like way. The bubbles now continuously Fig. 1. Illustration of the variations in carbon contents and carbon isotope compositions for samples from 14 N on the MAR trough closed (batch equilibrium, large grey curve) and open (Rayleigh, fine grey curve) system degassing. Crosses and diamonds correspond to samples after open and closed system degassing respectively.
6 P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008) escape their original magma site and have no time to reequilibrate with the magma they are going through, corresponding to an open system degassing, or Rayleigh distillation (fine grey curve on Fig. 1). The equations governing the isotope of both carbon phase can be written as follows: d 13 C diss d 13 C A ¼ D C ln f A where δ 13 C A denotes for the δ 13 C at stage A and f A = C diss /C A is the remaining fraction of carbon at stage A. As illustrated by Fig. 1, all samples would follow the same batch equilibrium degassing path but will differ in both the scale of open and closed degassing. In practice reconstructing initial carbon contents consists first in estimating the scale of open degassing allowing estimates of C-contents and isotope compositions at stage A (given in Table 4 in the Appendix), further allowing the value for C o to be inferred. The two main underlying assumptions of this reconstruction are (1) all samples had similar initial δ 13 C- values 4.5, a hypothesis supported by the little variable δ 13 C-values of mantle-derived samples (e.g. Cartigny et al., 1998, 2003) and (2) the fractionation factor value Δ C =+3.5, a value supported by most recent experiments (Appora, 1998) and observed in natural samples containing homogeneous vesicles in terms of δ 13 C(Pineau and Javoy, 1994). Errors are further depending on the assumption that some kinetic fractionation did not occur during the emplacement on the sea floor (see Aubaud et al., 2004; Pineau and Javoy, 1994). Significant kinetic fractionation is associated with high (N 3) carbon supersaturation (Aubaud et al., 2004) and is thus unlikely on the basis of the rather limited supersaturation degree of 14 N samples (b2, Table 4 in the Appendix). On the basis of the variability among replicates volatile content and isotope measurements errors associated with the present volatile reconstructions are estimated to be 50% (deviation from average value range from 1.5% for sample 2πD43 n=3 to 37% for sample 2πD45 n=3). A further source of error is attached to the assumed δ 13 C o - value, higher and lower δ 13 C o corresponding to higher and lower C o 's respectively. Yet, for two samples from 14 N (2πD40 and 2πD43, Table 4 in the Appendix, Fig. 1) it would be tempting to argue for a higher δ 13 C o - value: in that respect our assumed δ 13 C o does not tend to systematically over-evaluate reconstructed initial (i.e. pre-degassing) MORB C-contents. Reconstructed initial carbon contents given in Table 1 are mean values of data displayed in Table 4 in the Appendix. Inferred initial carbon contents vary greatly, from 660 to 14,300 ppm CO 2 and from 1400 to 57,600 ppm CO 2 for samples at 14 N and 34 N respectively. 4. Discussion 4.1. On the CO 2 /Nb-ratio of the MORB popping rock The Siqueiros samples are the only carbon-undersaturated samples available so far in the literature. Whether their volatile to non-volatile incompatible element ratios such as CO 2 /Nb-ratio can be extended to other carbon-saturated MORB samples is a key issue. In this respect, the fact that these [i.e. the MORB popping rock] undegassed samples have the same CO 2 / Nb-ratios than the Siqueiros melt inclusions (Saal et al., 2002, p.454) was taken in support for the idea that the CO 2 /Nb-ratios measured in carbon-undersaturated Siqueiros samples could be applied to all MORB samples. This statement is erroneous, because published data shows that the MORB popping rock has a much higher CO 2 /Nb-ratio, up to 500 (Bougault et al., 1988; Javoy and Pineau, 1991; Sarda and Graham, 1990, Fig. 2B, Table 1). Available estimates for MORB popping rock CO 2 - contents may appear confusing and, given the importance of this sample in the present discussion, the following paragraph provides a more detailed discussion of these contents and of the resulting CO 2 /Nb variability. Given its high vesicularity ( 17%) and the size of MORB chunks (b10 mm, Javoy and Pineau, 1991) selected for analyses, a significant (N 25% according to Javoy and Pineau, 1991) fraction of the gas, in particular that within the largest vesicles, was lost prior to analysis. Volatile content was estimated from vesicularity measurements (Gerlach, 1991; Graham and Sarda, 1991; Javoy and Pineau, 1991) using the following formula: CðAg=gÞ ¼ V P T T V m ð1þ V m where V is the volume fraction of vesicles in 1 g of lava, P is the CO 2 partial pressure in bars, T=273 K, T is the temperature of the vitreous transition (K), m is the molecular mass of carbon and V m the volume of 1 μmol of gas at standard conditions. It led to carbon concentrations varying by a factor 2, ranging from 3897±100 ppm C ( 14,667 ppm CO 2 ) (Javoy and Pineau, 1991), 2730 ppm C ( 10,000 ppm CO 2 ) (Gerlach, 1991; Javoy and Pineau, 1991) to 2320 ppm C ( 8500 ppm CO 2 )(Gerlach, 1991; Graham and Sarda, 1991). Part of this variability originates in an
7 678 P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008) CO 2 /Nb-variability among MORB samples Fig. 2. (A) CO 2 (initial) vs. Nb content variations and (B) CO 2 (initial)/ Nb-ratio (ppm/ppm) vs. Nb content among MORB from 14 N and 34 N on the MAR and Siqueiros Transform fault magmas. incorrect quotation of the glass transition temperature (T') by Sarda and Graham (1990) of 1273 K instead of 1000 K (i.e. glass transition temperature determinations for basaltic glass vary little between 998 to 1003 K (Ryan and Sammis, 1981)). Recalculated carbon contents for MORB popping rock gives a consistent range between 3897 (Javoy and Pineau, 1991) and 3475 to 2953 ppmc (Gerlach, 1991; Graham and Sarda, 1991; Sarda and Graham, 1990), thus a mean value of 3500±500 ppmc (i.e ppm CO 2 ). The variability among the different estimates reflect variations in both vesicularities measurements (from 15.9 to 17.8% Sarda and Graham, 1990)and chemical compositions (with CO 2 ranging from 73 to 97 vol% Javoy and Pineau, 1991). Together with Nb-contents of about 25.7 ± 0.8 ppm (n=2; Bézos, 2003; Bougault et al., 1988; Dosso et al., 1993), it corresponds to CO 2 /Nb-ratios ranging from 573 to 408 respectively (Table 1). This is in all cases about 2 times higher than the CO 2 /Nb-ratio measured among the Siqueiros melt inclusions of 239±46 (Fig. 2). As shown by Fig. 2A, MORB samples at 14 N define a linear relationship over a large range of Nb-contents, from 1 to 25 ppm defining a slope of 515 close to the average CO 2 /Nb 537±112 (n=6) and includes the undegassed MORB popping-rock 2ΠD43. In Fig. 2A, MORB glasses dredged at 34 N define a rough positive correlation with a slope of 1130 and an average CO 2 / Nb-ratio 724±514 (n=10). Although we introduced a series of assumptions (see above) when reconstructing initial carbon contents, the small variability of CO 2 /Nb-ratios ( ) over such a large range of initial CO 2 -contents (up to 14,300 ppm), and Nb-contents (up to 28 ppm), plead for the accuracy of our degassing corrections. We acknowledge some circularity in our argumentation, but believe that the coherent CO 2 /Nb-systematics, in particular for 14 N samples, cannot result from a coincidence. This allows us to validate the accuracy of our degassing corrections and use the corresponding CO 2 /Nb-ratios in further discussing mantle CO 2 /Nb-ratio(s). Furthermore, we emphasise that, for 3 out of 6 new data from 14 N, the CO 2 /Nb-ratios corresponding simply to measured CO 2 -contents (Table 4 in the Appendix, CO 2 content in vesicles+dissolved CO 2, i.e. no degassing correction introduced) are already higher than 280, and thus higher than the Siqueiros value. For samples from 14 N specifically, the near-to-zero intercept is in agreement with the idea that carbon behaves similarly to niobium [i.e. incompatible, Saal et al., 2002] supporting previous experimental evidences (e.g. Canil and Scarfe, 1989). The situation for samples from 34 N is more complex. The rough positive trend between CO 2 and Nb-contents is illustrated in Fig. 2A and CO 2 /Nb-ratios are distinct among samples dredged at 14 N and 34 N defining average CO 2 /Nb-ratios of 537 and 724 respectively. Within the 34 N dataset, the difference in averaged CO 2 /Nb-ratios is actually imposed by the three enriched (K/TiN0.45, (La/Sm) N N2.9) samples with CO 2 / NbN850. The rough positive trend among CO 2 /Nb and Nb (Fig. 2B) may be taken in support for either, some inaccuracy in our degassing correction that applies to most volatile-rich or from slight source heterogeneity. It cannot correspond to a fractionation process occurring during melting processes, because it would require unrealistically low or high bulk partition coefficient for CO 2 and Nb respectively. Although not a definitive conclusion, in support for source heterogeneity, we note that samples from 14 and 34 N having similar e.g. K/ Ti or La/Sm show similar ranges in CO 2 /Nb-ratios (Fig. 3A; Table 1). Yet, sample from 34 N (OT 03-02
8 P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008) CO 2 /Nb 190) showing a CO 2 /Nb-ratio lower than the Siqueiros value ( 239±46 Saal et al., 2002) is actually characterised by a high K/Ti-ratio. If the conclusions that can be drawn from 34 N samples are less straightforward than at 14 N, we emphasise that CO 2 /Nb-ratios are higher than that measured among the Siqueiros melt inclusions, on average 2 to 3 times (and up to 8) and thus supports the conclusions drawn from 14 N samples Representativity of volatiles within Siqueiros and mid-atlantic 34 and 14 N samples Because most MORB samples are carbon-saturated and are therefore degassed, volatile variability is usually rather addressed considering water (i.e. which in most cases remained vapor-undersaturated) in relation with elements behaving similarly, usually Ce, La or K (Michael, 1995; Jambon and Zimmermann, 1990; Danyushevsky et al., 2000; Dixon et al., 2002; Simons et al., 2002) and/or its isotopic composition (Poreda et al., 1986; Kingsley et al., 2002). The advantage in using H 2 O/Ce-ratios is that the two elements have very similar degree of incompatibility (e.g. Michael, 1995) and are thus little affected by magmatic processes such as partial melting and crystal fractionation. Fig. 3B illustrates that MORB samples with (La/Sm) N 1 from 14 N (except 2πD40, see below) have lower H 2 O/Ce that those from 34 N, a result in agreement, with the earlier suggestion that N-MORB north of 22 N on the MAR have generally higher H 2 O/Ce 260 (Michael, 1995) (see Fig. 3B). Thus, the heterogeneity in H 2 O/Ce is not associated with significant heterogeneity in CO 2 /Nb. Volatile heterogeneity can be further addressed through water and H-isotope variations using mixing diagrams, such as δd versus Ce/H 2 O(Fig. 4C) (Kingsley et al., 2002; Poreda et al., 1986) andtio 2 /H 2 O-ratios (Fig. 3A) (Aubaud, 2002). With the exception of 2πD40, samples from 14 N and 34 N both define a trend (r 2 0.4) between δd and Ce/H 2 O(Fig. 3C) which is compatible with a two components mixing between a source inferred to have δd 80, TiO 2 /H 2 O 6 and H 2 O/Ce 170 and a second source inferred to have δd 54, TiO 2 /H 2 O 2 and H 2 O/Ce 80. Binary mixing is supported by previous studies usually inferred from either δd or Ce/H 2 O-systematics (see also Fig. 6A in Kingsley et al., 2002). The second endmember corresponds to enriched MORB whose characteristics correspond to values inferred previously, such as high δd-values (Kingsley et al., 2002; Poreda et al., 1986) or low H 2 O/Ce (e.g. Dixon et al., 2002). The depleted endmember likely corresponds to N-MORB, the inferred characteristics such as δd 80 (Lecuyer et al., 1998; Pineau et al., 2004)andH 2 O/Ce 170 (Dixon et al., 2002; Michael, 1995) corresponding to values generally quoted in the literature. The relationship between TiO 2 /H 2 O-ratios, δd (Fig. 4A) in relation to K 2 O/TiO 2 (Fig. 4B) allows to consider volatile heterogeneity in relation with other index(es) of enrichment. TiO 2 /H 2 O versus δd (Fig. 3A) depicts a mixing trend similar to that inferred from Fig. 3C with end-member values given above. In the detail however, the characteristics of the enriched endmembers differ slightly from one location to the other, e.g. Easter and samples being characterised by δd 40 and b 50 for TiO 2 /H 2 O-ratios Fig. 3. Variations of (A) CO 2 (initial)/nb-ratios and H 2 O/Ce (all ppm/ppm) with (La/Sm) N illustrating the possible dependence of CO 2 /Nb with source enrichment.
9 680 P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008) close to 2 (not shown). Supporting previous evidence, Fig. 4 clearly illustrates that our sample set is dominated by enriched samples. One sample, 2πD40, deserves closer examination. The sample is water-depleted with water contents b 2500 ppm, high TiO 2 /H 2 ON10, low H 2 O/Ce 70 and low K 2 O/ TiO 2 b0.05 (Table 1 in the Appendix, Fig. 4). It therefore falls out of the broad correlation illustrated in Fig. 4A and C and at the very end of the K 2 O TiO 2 H 2 Ovariations. In our view, the depleted sample 2πD40isunlikelytobe the most appropriate sample to infer any canonical mantle value; this being supported by the evidence that this sample falls out the mixing relationship described in Fig. 4C. Yet, this sample shows striking similarities with the Siqueiros samples investigated by Saal et al. (2002) (Figs. 4C and 5). The similarity in major-element composition of the Siqueiros samples to primitive normal MORB was emphasised by Saal and coworkers, p. 453). Yet, Siqueiros (Perfit et al., 1996; Saal et al., 2002, see also Michael, 2002 for discussion), as do some other active transform fault-related active magmatism such as Garrett in the Pacific (Wendt et al., 1999) or some low-k basalts (Danyushevsky et al., 2000) are characterised by low concentrations of incompatible trace elements and have lower ratios of more incompatible to less incompatible trace elements such as K 2 O/TiO 2 compared to other MORB, including lavas from adjacent segments. The variations are best explained by remelting of a MORB source that underwent prior melt extraction (Danyushevsky et al., 2000; Michael, 2002; Wendt et al., 1999). Given that K is more incompatible than La, H 2 O, Ce and Sm (e.g. Danyushevsky et al., 2000; Hofmann, 1988), remelting of a previously depleted source would lead to basalts with lower abundance of trace elements. These would also show lower incompatible to less incompatible element ratios such as low K 2 O/TiO 2, but only slightly modified (yet still higher) H 2 O/Ce, and (lower) La/Sm and La/H 2 O-ratio (see Table 1 and data in Danyushevsky et al., 2000; Michael, 1995; Saal et al., 2002; Wendt et al., 1999). According to a model where depletion of the most incompatible elements result from Fig. 4. Variations of (A) δd vs. TiO 2 /H 2 O (B) K 2 O/TiO 2 vs. TiO 2 /H 2 O and (C) δd vs. H 2 O/Ce among MORB from 14 N and 34 N on the MAR and Siqueiros Transform fault magmas.
10 P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008) Fig. 5. Histogram of Nb-contents in worldwide MORB with MgO=8±1 wt.%. Average and standard deviation are calculated from the log-normal distribution adjusted to the data (see text for details). a prior melt-extraction event, the residual peridotite source would be characterised by fractionated values of e.g. CO 2 /Nb except if the two elements have exactly the same bulk partition coefficient. The fact that 2πD40 sample has a CO 2 /Nb-ratio within the range of other 14 N samples (i.e. from 405 to 733; Table 1) supports the idea that transform fault magmas may provide constraints on (i.e. unmodified) mantle CO 2 /Nb ratios. In this case, low CO 2 /Nb-ratios among Siqueiros samples would trace mantle heterogeneity in CO 2 /Nb. Yet, 2πD40 samples have rather high e.g. Nb/La (Table 3 in the Appendix; Fig. 6) which might reflect refertilisation of a residual mantle by partial melts of a more fertile source. In this case, low CO 2 /Nb-ratios among Siqueiros samples would reflect a bulk partition coefficient of CO 2 during partial melting slightly lower than Nb and be unrepresentative of (original) mantle CO 2 /Nb-ratios. An alternative hypothesis would consist in describing Siqueiros samples as the most representative samples of an ultra-depleted mantle source; according to this latter possibility these samples cannot be considered any further to infer mantle carbon fluxes and contents. In both cases, we want to stress out the dubious significance of transform fault basalts to infer any canonical true mantle values, for CO 2 /Nb-ratios or any volatile to incompatible elements ratios (e.g. Saal et al., 2002; Salters and Stracke, 2004; Workman et al., 2006) On the use of CO 2 /Nb-ratio in estimating mantle carbon content When establishing mantle volatile contents and fluxes, it is very often assumed that the mantle is homogeneous in terms of mantle volatile ratios (such as Fig. 6. Normalised trace elements pattern of MORB from 14 N and 34 N on the MAR and Siqueiros Transform fault magmas.
11 682 P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008) Fig. 7. Covariations of CO 2 (initial)/h 2 O vs. (A) (La/Sm)N, (B) CO 2 (initial)/nb and (C) TiO 2 /H 2 O among MORB from 14 N and 34 N on the MAR and Siqueiros Transform fault magmas. Dashed lines and grey field indicate average and 1σ standard deviation respectively of the log-normal distribution adjusted to the data (see text for details). C/ 3 He, C/N 2,N 2 /Ar) and volatile contents ( 3 He, C). This assumption is often supported for volatiles having not too different solubilities, (i.e. being the less fractionated by degassing) such as N 2 /Ar (Marty, 1995; Marty and Humbert, 1997; Marty and Zimmermann, 1999; Nishio et al., 1999; Cartigny et al., 2001)or C/ 3 He (Javoy and Pineau, 1991; Marty and Jambon, 1987; Marty and Tolstikhin, 1998), but usually remain difficult for volatile ratios highly affected by degassing (e.g. C/Ar, C/N 2 ) and for absolute volatile contents (C, He). From the present dataset, we infer a large range in initial carbon contents over an order of magnitude (from 1400 to 57,600 ppmco 2 ). Given the restricted range in Na 2 O or MgO, the estimated initial MORB CO 2 - contents little depend upon partial melting or fractional crystallisation rates respectively. The large range in initial carbon contents closely related to Nb-contents (Fig. 1) reflect mantle variability in C-contents and therefore questions the assumption, that our group followed for many years, of a homogeneous mantle carbon source content (e.g. Javoy and Pineau, 1991). The consideration of a hypotetical depleted endmember (see Salters and Stracke, 2004) would lead to consider the most volatile depleted endmember and accordingly the lowest volatile flux. In constraining mantle carbon contents/flux, this is an average mantle that has to be considered. Our sample set is not appropriate either, being biaised towards enriched samples. We computed mantle carbon flux using a MORB CO 2 / Nb-ratio of 530. This value is compatible (Fig. 7) with both the average MORB CO 2 /H 2 O( 1) and TiO 2 /H 2 O 5:74 þ3:21 2:08 (mean of the log-normal distribution adjusted to the data; n=326 MORB with MgO 8±1wt.%(PetDBdatabase)). With a mean MORB Nb-content of 3:31 1:81 þ3:99 (Fig. 5, mean of the log-normal distribution adjusted to the data, n=767 MORB with MgO 8±1 wt.% (PetDB), a value in agreement with that of Hofmann (1988), a mean oceanic crust production of 21 km 3 /yr (Cogné and Humler, 2006; Crisp, 1984), this corresponds to a mantle carbon flux of 2:3 1:3 þ2: mol/yr (i.e gc/ yr gco 2 /yr) and about 2.5 higher than the estimate of Saal et al. (2002). It is within the lowest range of estimates published so far (Marty and Jambon, 1987; Marty and Tolstikhin, 1998) and reflects the evidence that the MORB is likeley heteregenous in carbon contents, concentrations up to 4000 ppm C representing more an exception that the rule, an average MORB CO 2 -content of 1800 ppmco 2 (i.e. 490 ppm C) being more representative. Furthermore, the present C-flux is compatible with C/ 3 He-systematics of (MORB popping rock data, Javoy and Pineau, 1991 see also Marty and Jambon, 1987; Marty and Tolstikhin, 1998) anda 3 He-flux of 10 3 mol/yr (Craig et al., 1975; Farley et al., 1993; Welhan and Craig, 1983). This is also consistent with the range estimated by Dasgupta and Hirschmann (2006) based on efficient removal of incipient carbonatite melt from mantle peridotite at a depth of 300 km and taking into account the present day crust production rate. Thus the mantle carbon flux estimates now point towards broadly consistent values. It is however unclear if the present estimates of mantle carbon flux can be extended on a worldwide scale, i.e. as to whether a geographical dependence of CO 2 /Nb-ratios may exist or not. Available data do not support such a
12 P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008) statement (Fig. 2A) since so far CO 2 does correlate with Nb. Yet the existence of distinct mantle domains is significant if one considers CO 2 /Nb-ratios not only from the point of view of melting but also from recycling. Constant mantle CO 2 /Nb might be envisaged only if CO 2 and Nb behave similarly during both partial melting and subduction. More precisely, recent experimental data show that both during subduction and in the mantle (Dasgupta and Hirschmann, 2006; Dasgupta et al., 2004; Hammouda, 2003), carbonates may be liberated from the subducting slab at corresponding depths N 300 km. Compelling evidence shows carbonatitic metasomatism to be HFSE-depleted. In other words, carbon and niobium might be fractionated during subduction and metasomatism and distinct mantle domains may exist. More data will precise such a possibility. 5. Conclusions Most of our samples are enriched (e.g. high Nb-contents, high K/Ti) and show exceptionally high CO 2 -contents (Nten times as much CO 2 than average MORB) that contributed to their explosive behavior. Because gas loss occurred in all but one sample, our conclusions, in part, strongly relies on the recalculated (pre-degassing) gas content estimates. Yet, supporting a previous observation (Saal et al., 2002), CO 2 -contents correlates with Nb in 14 N and 34 N MORB from the mid-atlantic ridge and include the sample widely considered as undegassed in the literature, and for which no degassing correction was applied. CO 2 /Nb in the mantle however appears to be about 530, or about 473 if samples with less than 5 ppm Nb are considered. This value is higher than suggested earlier (Saal et al., 2002) from the study of highly depleted samples; a conclusion further supported by three of our samples having higher measured CO 2 /Nb-ratios. This change cannot be accounted for by melting processes but reflects higher mantle CO 2 /Nb-ratio(s). The CO 2 /Nb-systematics are in favor of a carbon flux from the mantle of mol/yr, although this value is likely to be adjusted with increasing amount of data (available data are almost exclusively from the mid-atlantic ridge). CO 2 /Nb-systematics are thus in favor of rather low carbon flux. The moderate variability of CO 2 /Nb-ratios over restricted areas makes the CO 2 /Nb-ratio a powerful test to evaluate the accuracy of degassing corrections. Acknowledgments Yann Lahaye (U. of Frankfurt) and Frédéric Couffignal (U. of Paris 6) are thanked for their help with trace and major element analyses respectively. Eric Humler is thanked for discussions. The PetDB-initiative is greatly thanked for allowing the use of a worldwide database in minimum time. We would like to thank the two reviewers for their comments and suggestions that greatly helped improving the manuscript. IPGP contribution Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi: /j. epsl References Appora, I., Étude expérimentale du fractionnement isotopique du carbone et de l'oxygène dans les systèmes CO 2 -Carbonates liquides: application aux contextes carbonatitiques. PhD thesis, University Paris 7, France, pp Aubaud, C., Processus de dégazage et sources mantelliques dans les magmas de type MORB et OIB. PhD-thesis, University Paris 7, pp Aubaud, C., Pineau, F., Jambon, A., Javoy, M., Kinetic disequilibrium of C, He, Ar and carbon isotopes during degassing of mid-ocean ridge basalts. Earth Planet. Sci. Lett. 222, Ballentine, C., Van Keken, P.E., Porcelli, D., Hauri, E.H., Numerical models, geochemistry and the zero-paradox noble-gas mantle. Philos. Trans. R. Soc. Lond. A 360, Berner, R.A., Kothavala, Z., GEOCARB III:. A revised model of atmospheric CO 2 over Phanerozoic time. Am. J. Sci. 301, Bézos, A., Study of lithophile and siderophile elements (Ir, Ru, Pt and Pd) in mid-oceanic ridge basalts. PhD-thesis, University Paris 7, pp Bottinga, Y., Javoy, M., MORB degassing: bubble growth and ascent. Chem. Geol. 81, Bougault, H., Dimitriev, L., Schilling, J.G., Sobolev, A., Joron, J.-L., Needham, H.D., Mantle heterogeneity from trace elements: MAR triple junction near 14 N. Earth Planet. Sci. Lett. 88, Canil, D., Scarfe, C.M., Phase relations in peridotite +CO 2 systems to 12 GPa: implications for the origin of kimberlite and carbonate stability in the Earth's upper mantle. J. Geophys. Res. 95, Cartigny, P., Harris, J.W., Phillips, D., Boyd, S.R., Javoy, M., Subduction-related diamonds: the evidence for a mantle-derived origin based on δ 13 C δ 15 N measurements. Chem. Geol. 147, Cartigny, P., Jendrzejewski, N., Pineau, F., Petit, E., Javoy, M., Volatile (C, N, Ar) variability in MORB and the respective roles of mantle source heterogeneity and degassing: the case of the Southwest Indian Ridge. Earth Planet. Sci. Lett. 194, Cartigny, P., Harris, J.W., Taylor, A., Davis, R., Javoy, M., On the possibility of a kinetic fractionation of nitrogen stable isotopes during natural diamond growth. Geochim. Cosmochim. Acta 67, Cogné, J.-P., Humler, E., Trends and rhythms in global seafloor generation rate. Geochem. Geophys. Geosyst. 7. doi: / 2005GC Craig, H., Clarke, W.B., Beg, M.A., Excess 3He in deep water on the East Pacific Rise. Earth Planet. Sci. Lett. 26,
13 684 P. Cartigny et al. / Earth and Planetary Science Letters 265 (2008) Crisp, J.A., Rates of magma emplacement and volcanic output. J. Volcanol. Geotherm. Res. 20, Danyushevsky, L.V., Eggins, S.M., Falloon, T.J., Christie, D.M., H 2 O abundance in depleted to moderately enriched mid-ocean ridge magmas; Part I: incompatible behaviour, implications for mantle storage, and origin of regional variations. J. Petrol. 41, Dasgupta, R., Hirschmann, M.M., Melting in the Earth's deep upper mantle caused by carbon dioxide. Nature 440, Dasgupta, R., Hirschmann, M.M., Withers, A.C., Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite under upper mantle conditions. Earth Planet. Sci. Lett. 227, Dixon, J.E., Leist, L., Langmuir, C., Schilling, J.-G., Recycled dehydrated lithosphere observed in plume-influenced mid-oceanridge basalt. Nature 420, Dixon, J.E., Stolper, E., Holloway, J.R., An experimental study of H 2 O and carbon dioxide solubilities in mid-ocean ridge basaltic liquids. Part I: calibration and solubility results. J. Petrol. 36, Dosso, L., Bougault, H., Joron, J.-L., Geochemical morphology of the North Mid-Atlantic Ridge, N: trace element-isotope complementarity. Earth Planet. Sci. Lett. 120, Farley, K., Maier-Reimer, E., Schlosser, P., Broecker, W.S., Constraints on mantle He fluxes and deep-sea circulation from an oceanic general circulation model. Geophys. Res. Lett. 100, François, L.M., Goddéris, Y., Isotopic constraints on the Cenozoic evolution of the carbon cycle. Chem. Geol. 145, Gao, S., Liu, X.M., Yuan, H.L., Hattendorf, B., Gunther, D., Chen, L., Hu, S.H., Determination of forty two major and trace elements in USGS and NIST SRM glasses by laser ablation-inductively coupled plasma-mass spectrometry. Geostand. Newsl. J. Geostand. Geoanal. 26, Gerlach, T.M., Comment on Mid-ocean ridge popping rocks: implications for degassing at ridge crests ; by P. Sarda and D. Graham. Earth Planet. Sci. Lett. 105, Graham, D., Sarda, P., Reply to comment by T.M. Gerlach on Mid-ocean ridge popping rocks: implications for degassing at ridge crests. Earth Planet. Sci. Lett. 105, Hamilton, D.L., Burnham, C.W., Osborn, E.F., The solubility of water and effects of oxygen fugacity and water content on crystallisation in mafic magmas. J. Petrol. 5, Hammouda, T., High-pressure melting of carbonated eclogite and experimental constraints on carbon recycling and storage in the mantle. Earth Planet. Sci. Lett. 214, Hayes, J.M., Waldbauer, J.R., The carbon cycle and associated redox processes through time. Philos. Trans. R. Soc. Lond. A 361, Hékinian, R., Pineau, F., Shilobreeva, S., Bideau, R.D., Gracia, E., Javoy, M., Deep sea explosive activity on the mid-atlantic ridge near N: Magma composition, vesicularity and volatile content. J. Volcanol. Geotherm. Res. 98, Hémond, C., Hofmann, A.W., Vlastélic, I., Nauret, F., Origin of MORB enrichment and relative trace element compatibilities along the Mid-Atlantic Ridge between 10 and 24 N. Geochem. Geophys. Geosyst. 12. doi: /2006gc Hofmann, A.W., Chemical differentiation of the Earth: the relationship between mantle, continental crust, and oceanic crust. Earth Planet. Sci. Lett. 90, Holland, G., Ballentine, C.J., Seawater subduction controls the heavy noble gas composition of the mantle. Nature 441, Jambon, A., Zimmermann, J.L., Water in oceanic basalts: evidence for dehydration of recycled crust. Earth Planet. Sci. Lett. 101, Javoy, M., Pineau, F., The volatiles record of a popping rock from the Mid-Atlantic ridge at 14 N: chemical and isotopic composition of gas trapped in the vesicles. Earth Planet. Sci. Lett. 107, Javoy, M., Pineau, F., Allègre, C.J., Carbon geodynamic cycle. Nature 300, Jendrzejewski, N., Trull, T.W., Pineau, F., Javoy, M., Carbon solubility in Mid-Ocean Ridge basaltic melt at low pressures ( bar). Chem. Geol. 138, Kelley, K.A., Plank, T., Ludden, J., Staudigel, H., Composition of altered oceanic crust at ODP Sites 801 and Geochem. Geophys. Geosyst. 4, doi: /2002gc Kingsley, R.H., Schilling, J.-G., Dixon, J.E., Swart, P., Poreda, R., Simons, K., D/H ratios in basalt glasses from the Salas y Gomez mantle plume interacting with the East Pacific Rise: water from old D-rich recycled crust or primordial water from the lower mantle? Geochem. Geophys. Geosyst. 3. doi: /2001gc Lecuyer, C., Gillet, P., Robert, F., The hydrogen isotope composition of seawater and the global water cycle. Chem. Geol. 145, Marty, B., Nitrogen content of the mantle inferred from N 2 Ar correlation in oceanic basalts. Nature 377, Marty, B., Jambon, A., C/3He in volatiles fluxes from the solid Earth: implications for carbon geodynamics. Earth Planet. Sci. Lett. 83, Marty, B., Humbert, F., Nitrogen and argon isotopes in oceanic basalts. Earth Planet. Sci. Lett. 152, Marty, B., Tolstikhin, I.N., CO 2 fluxes from mid-ocean ridges, arc and plumes. Chem. Geol. 145, Marty, B., Zimmermann, L., Volatiles (He, C, N, Ar) in midocean ridge basalts: assesment of shallow-level fractionation and characterisation of source composition. Geochim. Cosmochim. Acta 63, Michael, P., Regionally distinctive sources of depleted MORB: evidence from trace elements and H 2 O. Earth Planet. Sci. Lett. 131, Michael, P., Lava without the fizz. Nature 419, Moreira, M., Sarda, P., Noble gas constraints on degassing processes. Earth Planet. Sci. Lett. 176, Moreira, M., Kunz, J., Allègre, C.J., Rare gas systematics in Popping Rock: isotopic and elemental compositions in the upper mantle. Science. Nishio, Y., Ishii, T., Gamo, T., Sano, Y., Volatile element isotopic systematics of the Rodriguez Triple Junction Indian Ocean MORB: implications for mantle heterogeneity. Earth Planet. Sci. Lett. 170, Niu, Y., Bideau, D., Hekinian, R., Batiza, R., Mantle compositional control on the extent of mantle melting, crust production, gravity anomaly, ridge morphology, and ridge segmentation: a case study at the mid-atlantic Ridge N. Earth Planet. Sci. Lett. 186, Perfit, M.R., Fornari, D.J., Ridley, W.I., Kirk, P.D., Casey, J., Kastens, K.A., Reynolds, J.R., Edwards, M., Desonie, D., Shuster, R., Paradis, S., Recent volcanism in the Siqueiros transform fault: picritic basalts and implications for MORB magma genesis. Earth Planet. Sci. Lett. 141, PetDB, Petrological Database of the Ocean Floor, petdb.org/. Pineau, F., Javoy, M., Carbon isotopes and concentrations in mid-oceanic ridge basalts. Earth Planet. Sci. Lett. 62, Pineau, F., Javoy, M., Strong degassing at ridge crests: the behavior of dissolved carbon and water in basalt glasses at 14 N, Mid-Atlantic Ridge. Earth Planet. Sci. Lett. 123,
What can noble gases really say about mantle. 2) Extent of mantle degassing
 What can noble gases really say about mantle convection and the deep Earth volatile cycles? 1) Constraints on mass flow 1) Constraints on mass flow 2) Extent of mantle degassing Outline: -Noble gas geochemistry
What can noble gases really say about mantle convection and the deep Earth volatile cycles? 1) Constraints on mass flow 1) Constraints on mass flow 2) Extent of mantle degassing Outline: -Noble gas geochemistry
What can isotopes tell us about mantle dynamics? Sujoy Mukhopadhyay. Harvard University
 What can isotopes tell us about mantle dynamics? Sujoy Mukhopadhyay Harvard University The mantle zoo Hofmann, 1997 187 Os/ 188 Os 0.168 0.156 0.144 0.132 EM1 Hawaii Pitcairn DMM peridotites Shield Basalts
What can isotopes tell us about mantle dynamics? Sujoy Mukhopadhyay Harvard University The mantle zoo Hofmann, 1997 187 Os/ 188 Os 0.168 0.156 0.144 0.132 EM1 Hawaii Pitcairn DMM peridotites Shield Basalts
Effect of tectonic setting on chemistry of mantle-derived melts
 Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
Effect of tectonic setting on chemistry of mantle-derived melts Lherzolite Basalt Factors controlling magma composition Composition of the source Partial melting process Fractional crystallization Crustal
Lecture 38. Igneous geochemistry. Read White Chapter 7 if you haven t already
 Lecture 38 Igneous geochemistry Read White Chapter 7 if you haven t already Today. Magma mixing/afc 2. Spot light on using the Rare Earth Elements (REE) to constrain mantle sources and conditions of petrogenesis
Lecture 38 Igneous geochemistry Read White Chapter 7 if you haven t already Today. Magma mixing/afc 2. Spot light on using the Rare Earth Elements (REE) to constrain mantle sources and conditions of petrogenesis
Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m
 Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m (b). Dashed lines represent the two successive ridge
Supplementary Figure 1 Map of the study area Sample locations and main physiographic features of the study area. Contour interval is 200m (a) and 40m (b). Dashed lines represent the two successive ridge
Radiogenic Isotope Systematics and Noble Gases. Sujoy Mukhopadhyay CIDER 2006
 Radiogenic Isotope Systematics and Noble Gases Sujoy Mukhopadhyay CIDER 2006 What I will not cover.. U-Th-Pb sytematics 206 Pb 204 Pb 207 Pb 204 Pb 208 Pb 204 Pb = t = t = t 206 Pb 204 Pb 207 Pb 204 Pb
Radiogenic Isotope Systematics and Noble Gases Sujoy Mukhopadhyay CIDER 2006 What I will not cover.. U-Th-Pb sytematics 206 Pb 204 Pb 207 Pb 204 Pb 208 Pb 204 Pb = t = t = t 206 Pb 204 Pb 207 Pb 204 Pb
Composition of the Earth and its reservoirs: Geochemical observables
 Composition of the Earth and its reservoirs: Geochemical observables Cin-Ty A. Lee Rice University MYRES-I 2004 The Earth is dynamic and heterogeneous Atmosphere Midocean Ridge Plume Ocean Crust Oceanic
Composition of the Earth and its reservoirs: Geochemical observables Cin-Ty A. Lee Rice University MYRES-I 2004 The Earth is dynamic and heterogeneous Atmosphere Midocean Ridge Plume Ocean Crust Oceanic
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10326 Supplementary Discussion All known modern terrestrial mantle reservoirs evolved from a primitive precursor with superchondritic 143 Nd/ 144 Nd. What is this reservoir? The terms
doi:10.1038/nature10326 Supplementary Discussion All known modern terrestrial mantle reservoirs evolved from a primitive precursor with superchondritic 143 Nd/ 144 Nd. What is this reservoir? The terms
GSA DATA REPOSITORY
 GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
GSA DATA REPOSITORY 2013019 Supplemental information for The Solidus of Alkaline Carbonatite in the Deep Mantle Konstantin D. Litasov, Anton Shatskiy, Eiji Ohtani, and Gregory M. Yaxley EXPERIMENTAL METHODS
Lecture 12 COMPLEX MELTING MODELS. (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007))
 Lecture 12 COMPLEX MELTING MODELS (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007)) Thus far we have considered two end-member melting models, batch melting
Lecture 12 COMPLEX MELTING MODELS (see books by Shaw, Trace Elements in Magmas (2006) and Zou, Quantitative Geochemistry (2007)) Thus far we have considered two end-member melting models, batch melting
Evidences for geochemically distinct mantle components
 Evidences for geochemically distinct mantle components 1 Mantle Array Oceanic basalts, including seamounts, oceanic islands and middle ocean ridge basalts, were used. 2 Binary All analyses fall between
Evidences for geochemically distinct mantle components 1 Mantle Array Oceanic basalts, including seamounts, oceanic islands and middle ocean ridge basalts, were used. 2 Binary All analyses fall between
Constitution of Magmas. Magmas. Gas Law. Composition. Atomic Structure of Magma. Structural Model. PV = nrt H 2 O + O -2 = 2(OH) -
 Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
A deep mantle source for high 3 He/ 4 He ocean island basalts (OIB) inferred from Pacific near-ridge seamount lavas
 GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L20316, doi:10.1029/2009gl040560, 2009 A deep mantle source for high 4 He ocean island basalts (OIB) inferred from Pacific near-ridge seamount lavas D. Hahm, 1,2
GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L20316, doi:10.1029/2009gl040560, 2009 A deep mantle source for high 4 He ocean island basalts (OIB) inferred from Pacific near-ridge seamount lavas D. Hahm, 1,2
GSA Data Repository
 GSA Data Repository 218145 Parolari et al., 218, A balancing act of crust creation and destruction along the western Mexican convergent margin: Geology, https://doi.org/1.113/g39972.1. 218145_Tables DR1-DR4.xls
GSA Data Repository 218145 Parolari et al., 218, A balancing act of crust creation and destruction along the western Mexican convergent margin: Geology, https://doi.org/1.113/g39972.1. 218145_Tables DR1-DR4.xls
GSA Data Repository
 GSA Data Repository 2015244 1. Method of Statistical Analysis Appendix DR1 One has to be careful and use only samples with complete Sm-Eu-Gd concentration data to study Eu/Eu* in the crust. This is because
GSA Data Repository 2015244 1. Method of Statistical Analysis Appendix DR1 One has to be careful and use only samples with complete Sm-Eu-Gd concentration data to study Eu/Eu* in the crust. This is because
Volcanology and Petrology of the Taney Seamounts, Northeast Pacific Ocean
 MSc Research Proposal Volcanology and Petrology of the Taney Seamounts, Northeast Pacific Ocean Jason Coumans Introduction: Short chains of seamounts are observed near mid-ocean ridges and have been previously
MSc Research Proposal Volcanology and Petrology of the Taney Seamounts, Northeast Pacific Ocean Jason Coumans Introduction: Short chains of seamounts are observed near mid-ocean ridges and have been previously
Archimer
 Please note that this is an author-produced PDF of an article accepted for publication following peer review. The definitive publisher-authenticated version is available on the publisher Web site Earth
Please note that this is an author-produced PDF of an article accepted for publication following peer review. The definitive publisher-authenticated version is available on the publisher Web site Earth
Composition of bulk silicate Earth and global geodynamics
 Composition of bulk silicate Earth and global geodynamics Jun Korenaga Department of Geology and Geophysics Yale University March 23, 2007 @ Hawaii Geoneutrino Workshop Overview Motivation: Thermal evolution
Composition of bulk silicate Earth and global geodynamics Jun Korenaga Department of Geology and Geophysics Yale University March 23, 2007 @ Hawaii Geoneutrino Workshop Overview Motivation: Thermal evolution
Earth and Planetary Science Letters
 Earth and Planetary Science Letters 355 356 (2012) 244 254 Contents lists available at SciVerse ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl The heavy
Earth and Planetary Science Letters 355 356 (2012) 244 254 Contents lists available at SciVerse ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl The heavy
Mantle geochemistry: How geochemists see the deep Earth
 Geochemistry: Overview: the geochemist's Earth (reservoirs, budgets and processes) Mantle geochemistry: How geochemists see the deep Earth Don DePaolo/Stan Hart CIDER - KITP Summer School Lecture #1, July
Geochemistry: Overview: the geochemist's Earth (reservoirs, budgets and processes) Mantle geochemistry: How geochemists see the deep Earth Don DePaolo/Stan Hart CIDER - KITP Summer School Lecture #1, July
Nitrogen speciation in upper mantle fluids and the origin of Earth s nitrogen-rich atmosphere
 Supporting Online Material for Nitrogen speciation in upper mantle fluids and the origin of Earth s nitrogen-rich atmosphere Sami Mikhail & Dimitri Sverjensky S1. Supplementary discussion S1.1 The selection
Supporting Online Material for Nitrogen speciation in upper mantle fluids and the origin of Earth s nitrogen-rich atmosphere Sami Mikhail & Dimitri Sverjensky S1. Supplementary discussion S1.1 The selection
Alternative Mechanisms for Volcanic Activity in Hotspot-Ridge Systems: The Northern Galapagos Province
 ABSTRACT for the Plume IV Penrose Conference Alternative Mechanisms for Volcanic Activity in Hotspot-Ridge Systems: The Northern Galapagos Province Karen S. Harpp, Colgate University, Department of Geology,
ABSTRACT for the Plume IV Penrose Conference Alternative Mechanisms for Volcanic Activity in Hotspot-Ridge Systems: The Northern Galapagos Province Karen S. Harpp, Colgate University, Department of Geology,
Fluids, melts, and supercriticality in the MSH system and element transport in subduction zones
 cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
cosmic rays Fluids, s, and supercriticality in the MSH system and element transport in subduction zones 10 Be volcanic front N, O 10 Be ocean water + CO 2 tracing petrologic and geotectonic processes (trace)
Earth and Planetary Science Letters
 Earth and Planetary Science Letters 274 (2008) 142 150 Contents lists available at ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl He, Ne and Ar systematics
Earth and Planetary Science Letters 274 (2008) 142 150 Contents lists available at ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl He, Ne and Ar systematics
ARTICLE IN PRESS. Available online at Earth and Planetary Science Letters 6836 (2003) 1^8. Discussion
 R Available online at www.sciencedirect.com Earth and Planetary Science Letters 6836 (2003) 1^8 Discussion A comment on The nitrogen record of crust^mantle interaction and mantle convection from Archean
R Available online at www.sciencedirect.com Earth and Planetary Science Letters 6836 (2003) 1^8 Discussion A comment on The nitrogen record of crust^mantle interaction and mantle convection from Archean
Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics
 Chemical Geology 183 (2002) 143 168 www.elsevier.com/locate/chemgeo Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics Marc D. Norman a, *, Michael
Chemical Geology 183 (2002) 143 168 www.elsevier.com/locate/chemgeo Olivine-hosted melt inclusions in Hawaiian picrites: equilibration, melting, and plume source characteristics Marc D. Norman a, *, Michael
Subduction zones 3 arc magmatism
 5. 3 Subduction zones 3 arc magmatism Where can we observe magmatic/volcanic activities along subduction zones? Characteristics of arc magmatism (vs. mid-ocean ridge/intraplate magmatism) Structure of
5. 3 Subduction zones 3 arc magmatism Where can we observe magmatic/volcanic activities along subduction zones? Characteristics of arc magmatism (vs. mid-ocean ridge/intraplate magmatism) Structure of
Worked Example of Batch Melting: Rb and Sr
 Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Michele Lustrino a,b,* Rome Italy. Studi di Roma La Sapienza, P.le A. Moro, 5, Rome Italy. * address:
 Pyroxenites everywhere. Comment on High-pressure melting experiments on garnet clinopyroxenite and the alkalic to tholeiitic transition in ocean-island basalts by Keshav et al. [Earth Planet. Sci. Lett.
Pyroxenites everywhere. Comment on High-pressure melting experiments on garnet clinopyroxenite and the alkalic to tholeiitic transition in ocean-island basalts by Keshav et al. [Earth Planet. Sci. Lett.
Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2.
 Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2. Screening for olivine interference in Fe-µ-XANES spectra When collecting Fe-µ-XANES
Electronic Appendix A: Supplementary material to accompany the manuscript, Fe 3+ / Fe in Mariana Arc basalts and primary fo 2. Screening for olivine interference in Fe-µ-XANES spectra When collecting Fe-µ-XANES
Magmatic Processes at Subduction Zones
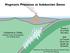 Magmatic Processes at Subduction Zones Katherine A. Kelley Graduate School of Oceanography Univ. of Rhode Island Thanks to Terry Plank Erik Hauri GVP: Liz Cottrell Simon Carn Jennifer Jay Ed Venzke Subduction
Magmatic Processes at Subduction Zones Katherine A. Kelley Graduate School of Oceanography Univ. of Rhode Island Thanks to Terry Plank Erik Hauri GVP: Liz Cottrell Simon Carn Jennifer Jay Ed Venzke Subduction
Mantle Dynamics and Convective Mixing
 Mantle Dynamics and Convective Mixing ( Chemical Geodynamics ) Henri Samuel Mantle Dynamics and Convective Mixing ( Dynamical Geochemistry ) Henri Samuel General Motivation [Ballentine et al., 2002] [Ballentine
Mantle Dynamics and Convective Mixing ( Chemical Geodynamics ) Henri Samuel Mantle Dynamics and Convective Mixing ( Dynamical Geochemistry ) Henri Samuel General Motivation [Ballentine et al., 2002] [Ballentine
Supplementary material to accompany the manuscript, Redox variations in HSDP2
 Supplementary material to accompany the manuscript, Redox variations in HSDP2 Mauna Kea lavas, the oxygen fugacity of the Hawaiian plume, and the role of volcanic gases in Earth s oxygenation by M. Brounce,
Supplementary material to accompany the manuscript, Redox variations in HSDP2 Mauna Kea lavas, the oxygen fugacity of the Hawaiian plume, and the role of volcanic gases in Earth s oxygenation by M. Brounce,
Heterogeneities from the first 100 million years recorded in deep mantle noble gases from the Northern Lau Back-arc Basin
 University of Rhode Island DigitalCommons@URI Graduate School of Oceanography Faculty Publications Graduate School of Oceanography 2013 Heterogeneities from the first 100 million years recorded in deep
University of Rhode Island DigitalCommons@URI Graduate School of Oceanography Faculty Publications Graduate School of Oceanography 2013 Heterogeneities from the first 100 million years recorded in deep
Rare Earth Elements in some representative arc lavas
 Rare Earth Elements in some representative arc lavas Low-K (tholeiitic), Medium-K (calc-alkaline), and High-K basaltic andesites and andesites. A typical N-MORB pattern is included for reference Notes:
Rare Earth Elements in some representative arc lavas Low-K (tholeiitic), Medium-K (calc-alkaline), and High-K basaltic andesites and andesites. A typical N-MORB pattern is included for reference Notes:
Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere. 07/11/2017 GEO-DEEP 9300 Claire Aupart
 Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere 07/11/2017 GEO-DEEP 9300 Claire Aupart Introduction Introduction Getting samples Cores: Maximum depth reach in
Geochemical and mineralogical technics to investigate the lithosphere and the asthenosphere 07/11/2017 GEO-DEEP 9300 Claire Aupart Introduction Introduction Getting samples Cores: Maximum depth reach in
LETTERS. Preserving noble gases in a convecting mantle. Helge M. Gonnermann 1 & Sujoy Mukhopadhyay 2
 Vol 459 28 May 29 doi:.38/nature88 Preserving noble gases in a convecting Helge M. Gonnermann & Sujoy Mukhopadhyay 2 High 3 He/ 4 He ratios sampled at many ocean islands are usually attributed to an essentially
Vol 459 28 May 29 doi:.38/nature88 Preserving noble gases in a convecting Helge M. Gonnermann & Sujoy Mukhopadhyay 2 High 3 He/ 4 He ratios sampled at many ocean islands are usually attributed to an essentially
Week 7 Submarine Pyroclastic Activity (there was no lecture for week 6)
 Week 7 Submarine Pyroclastic Activity (there was no lecture for week 6) Note: some of these slides were provided by Dave Clague, MBARI Two topics: fluidal clasts and bubble wall fragments internal water
Week 7 Submarine Pyroclastic Activity (there was no lecture for week 6) Note: some of these slides were provided by Dave Clague, MBARI Two topics: fluidal clasts and bubble wall fragments internal water
12. Data from Ito et al. (1987) Chemical Geology, 62, ; Figure ; and LeRoex et al. (1983) J. Petrol., 24,
 Announcements Reading for Wed: p.363-399!!! p.362-366; p.373-378; p.383-386; p.392-394; p.395-399 Last lecture on Wednesday Bring food for pizza party Bring class notes, labs, book N-MORBs: 87 Sr/ 86 Sr
Announcements Reading for Wed: p.363-399!!! p.362-366; p.373-378; p.383-386; p.392-394; p.395-399 Last lecture on Wednesday Bring food for pizza party Bring class notes, labs, book N-MORBs: 87 Sr/ 86 Sr
BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO
 GSA DATA REPOSITORY 2015057 BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO DATA REPOSITORY for Thermal and chemical evolution of the subarc mantle revealed by spinel-hosted melt
GSA DATA REPOSITORY 2015057 BONINITIC MELT INCLUSIONS IN CHROME SPINEL FROM THE OGASAWARA ARCHIPELAGO DATA REPOSITORY for Thermal and chemical evolution of the subarc mantle revealed by spinel-hosted melt
Continental Alkaline Magmatism. The East African Rift
 Announcements No lecture on Friday Lab final begins at 1 PM Today s agenda: Lecture/Demo Go through field trip pics Call for pizza Lab review Lecture Review Make poster Continental Alkaline Magmatism.
Announcements No lecture on Friday Lab final begins at 1 PM Today s agenda: Lecture/Demo Go through field trip pics Call for pizza Lab review Lecture Review Make poster Continental Alkaline Magmatism.
N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0
 N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0 is initial number of parents, D* is number of radiogenic daughter atoms, and λ is the decay
N = N 0 e -λt D* = N 0 -N D* = N 0 (1-e -λt ) or N(e λt -1) where N is number of parent atoms at time t, N 0 is initial number of parents, D* is number of radiogenic daughter atoms, and λ is the decay
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION DOI: 1.13/NGEO11 Deep-sea mud in the Pacific Ocean as a potential resource for rare-earth elements Yasuhiro Kato 1, Koichiro Fujinaga 1, Kentaro Nakamura, Yutaro Takaya 1, Kenichi
SUPPLEMENTARY INFORMATION DOI: 1.13/NGEO11 Deep-sea mud in the Pacific Ocean as a potential resource for rare-earth elements Yasuhiro Kato 1, Koichiro Fujinaga 1, Kentaro Nakamura, Yutaro Takaya 1, Kenichi
Systems? Climate Systems. Earth Systems. Earth Interior Systems. Atmospheric/Biospheric Systems: Human Impact Hydrologic Cycle.
 Chapter 15 Climate Systems Systems? What is a system? Geologic phenomena are complex. All processes are related to, and interact with, other processes. So it is useful to think of geologic processes as
Chapter 15 Climate Systems Systems? What is a system? Geologic phenomena are complex. All processes are related to, and interact with, other processes. So it is useful to think of geologic processes as
Remote Sensing of the Earth s Interior
 Remote Sensing of the Earth s Interior Earth s interior is largely inaccessible Origin and Layering of the Earth: Geochemical Perspectives Composition of Earth cannot be understood in isolation Sun and
Remote Sensing of the Earth s Interior Earth s interior is largely inaccessible Origin and Layering of the Earth: Geochemical Perspectives Composition of Earth cannot be understood in isolation Sun and
TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS
 TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS W.L. Griffin 1, 3, Sonal Rege 1, Rondi M. Davies 1, 2, Simon Jackson 1, Suzanne Y. O Reilly 1 1.ARC National Key
TRACE ELEMENT ANALYSIS OF DIAMOND BY LAM ICPMS: STANDARDISATION, RESULTS AND DIRECTIONS W.L. Griffin 1, 3, Sonal Rege 1, Rondi M. Davies 1, 2, Simon Jackson 1, Suzanne Y. O Reilly 1 1.ARC National Key
Summary and Conclusions
 Chapter 9 Summary and Conclusions 9.1 Summary The contents of this thesis revolve around the question of what type of geodynamics was active in the Early Earth and other terrestrial planets. The geology
Chapter 9 Summary and Conclusions 9.1 Summary The contents of this thesis revolve around the question of what type of geodynamics was active in the Early Earth and other terrestrial planets. The geology
A trapdoor mechanism for slab tearing and melt generation in the northern Andes
 GSA Data Repository 201418 Geology DOI:10.1130/G45429.1 (Rosenbaum et al.) A trapdoor mechanism for slab tearing and melt generation in the northern Andes Gideon Rosenbaum, Mike Sandiford, John Caulfield
GSA Data Repository 201418 Geology DOI:10.1130/G45429.1 (Rosenbaum et al.) A trapdoor mechanism for slab tearing and melt generation in the northern Andes Gideon Rosenbaum, Mike Sandiford, John Caulfield
G 3. AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society
 Geosystems G 3 AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society Article Volume 3, Number 4 13 April 2002 10.1029/2001GC000199 ISSN: 1525-2027 D/H ratios in basalt
Geosystems G 3 AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society Article Volume 3, Number 4 13 April 2002 10.1029/2001GC000199 ISSN: 1525-2027 D/H ratios in basalt
GEOL 2312 Igneous and Metamorphic Petrology Spring 2009 Sc ore / 40
 GEOL 2312 Igneous and Metamorphic Petrology Name Spring 2009 Sc ore / 40 QUIZ 3 1) Name two geologic features that provide physical evidence for the mineralogy of the earth s mantle (2 pts) Ophiolites,
GEOL 2312 Igneous and Metamorphic Petrology Name Spring 2009 Sc ore / 40 QUIZ 3 1) Name two geologic features that provide physical evidence for the mineralogy of the earth s mantle (2 pts) Ophiolites,
Trace Elements - Definitions
 Trace Elements - Definitions Elements that are not stoichiometric constituents in phases in the system of interest For example, IG/MET systems would have different trace elements than aqueous systems Do
Trace Elements - Definitions Elements that are not stoichiometric constituents in phases in the system of interest For example, IG/MET systems would have different trace elements than aqueous systems Do
The influence of short wavelength variations in viscosity on subduction dynamics
 1 Introduction Deformation within the earth, driven by mantle convection due primarily to cooling and subduction of oceanic lithosphere, is expressed at every length scale in various geophysical observations.
1 Introduction Deformation within the earth, driven by mantle convection due primarily to cooling and subduction of oceanic lithosphere, is expressed at every length scale in various geophysical observations.
What processes control the chemical compositions of arc front stratovolcanoes?
 What processes control the chemical compositions of arc front stratovolcanoes? The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
What processes control the chemical compositions of arc front stratovolcanoes? The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
ESS 312 Geochemistry Simulating Earth degassing using radionuclides
 ESS 312 Geochemistry Simulating Earth degassing using radionuclides CHEMICAL AND ISOTOPIC EVOLUTION OF A TWO-RESERVOIR SYSTEM In lectures we discussed radioactive decay and build-up of a daughter isotope
ESS 312 Geochemistry Simulating Earth degassing using radionuclides CHEMICAL AND ISOTOPIC EVOLUTION OF A TWO-RESERVOIR SYSTEM In lectures we discussed radioactive decay and build-up of a daughter isotope
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION DOI: 10.1038/NGEO1250 Intensive hydration of the mantle transition zone beneath China caused by ancient slab stagnation Takeshi Kuritani 1,2 *, Eiji Ohtani 1, and Jun-Ichi Kimura
SUPPLEMENTARY INFORMATION DOI: 10.1038/NGEO1250 Intensive hydration of the mantle transition zone beneath China caused by ancient slab stagnation Takeshi Kuritani 1,2 *, Eiji Ohtani 1, and Jun-Ichi Kimura
Plate Tectonics. Continental Drift Sea Floor Spreading Plate Boundaries
 Plate Tectonics Continental Drift Sea Floor Spreading Plate Boundaries Continental Drift 1915, Alfred Wegener - Pangea hypothesis: suggested Earth s continents were part of a large super-continent 200
Plate Tectonics Continental Drift Sea Floor Spreading Plate Boundaries Continental Drift 1915, Alfred Wegener - Pangea hypothesis: suggested Earth s continents were part of a large super-continent 200
Tectonic-Igneous Associations
 Tectonic-Igneous Associations Associations on a larger scale than the petrogenetic provinces An attempt to address global patterns of igneous activity by grouping provinces based upon similarities in occurrence
Tectonic-Igneous Associations Associations on a larger scale than the petrogenetic provinces An attempt to address global patterns of igneous activity by grouping provinces based upon similarities in occurrence
Trace Elements. Today s lecture
 Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
Trace Elements 300 Ni 200 ppm 100 0 300 Zr 200 100 0 40 50 60 70 80 SiO 2 wt. % Updates: M&M due date: Tuesday Today s lecture Topics: Trace element compositions Trace element behavior Partitioning Spider(
A model to explain the various paradoxes associated with mantle noble gas geochemistry
 Proc. Natl. Acad. Sci. USA Vol. 95, pp. 9087 9092, August 1998 Geophysics A model to explain the various paradoxes associated with mantle noble gas geochemistry DON L. ANDERSON Seismological Laboratory
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 9087 9092, August 1998 Geophysics A model to explain the various paradoxes associated with mantle noble gas geochemistry DON L. ANDERSON Seismological Laboratory
Volatiles in the terrestrial planets. Sujoy Mukhopadhyay University of California, Davis CIDER, 2014
 Volatiles in the terrestrial planets Sujoy Mukhopadhyay University of California, Davis CIDER, 2014 Atmophiles: Elements I will talk about rock-loving iron-loving sulfur-loving Temperatures in Protoplanetary
Volatiles in the terrestrial planets Sujoy Mukhopadhyay University of California, Davis CIDER, 2014 Atmophiles: Elements I will talk about rock-loving iron-loving sulfur-loving Temperatures in Protoplanetary
Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints
 REFERENCES RELEVANT TO PERIDOTITE PARTIAL MELTING IN THE MANTLE WEDGE Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints at converging plate boundaries - I: Dehydration in
REFERENCES RELEVANT TO PERIDOTITE PARTIAL MELTING IN THE MANTLE WEDGE Anderson RN, Uyeda S, Miyashiro A. (1976) Geophysical and geochemical constraints at converging plate boundaries - I: Dehydration in
The application of olivine geothermometry to infer crystallization temperatures of parental liquids: Implications for the temperature of MORB magmas
 Chemical Geology 241 (2007) 207 233 www.elsevier.com/locate/chemgeo The application of olivine geothermometry to infer crystallization temperatures of parental liquids: Implications for the temperature
Chemical Geology 241 (2007) 207 233 www.elsevier.com/locate/chemgeo The application of olivine geothermometry to infer crystallization temperatures of parental liquids: Implications for the temperature
Major and trace element composition of the high
 Article Volume 14, Number 8 22 August 2013 doi: ISSN: 1525-2027 Major and trace element composition of the high 3 He/ 4 He mantle: Implications for the composition of a nonchonditic Earth Matthew G. Jackson
Article Volume 14, Number 8 22 August 2013 doi: ISSN: 1525-2027 Major and trace element composition of the high 3 He/ 4 He mantle: Implications for the composition of a nonchonditic Earth Matthew G. Jackson
SEA-FLOOR SPREADING. In the 1950 s and early 1960 s detailed study of the oceans revealed the following surprising information:-
 SEA-FLOOR SPREADING In the 1950 s and early 1960 s detailed study of the oceans revealed the following surprising information:- Detailed bathymetric (depth) studies showed that there was an extensive submarine
SEA-FLOOR SPREADING In the 1950 s and early 1960 s detailed study of the oceans revealed the following surprising information:- Detailed bathymetric (depth) studies showed that there was an extensive submarine
Plate Tectonics. entirely rock both and rock
 Plate Tectonics I. Tectonics A. Tectonic Forces are forces generated from within Earth causing rock to become. B. 1. The study of the origin and arrangement of Earth surface including mountain belts, continents,
Plate Tectonics I. Tectonics A. Tectonic Forces are forces generated from within Earth causing rock to become. B. 1. The study of the origin and arrangement of Earth surface including mountain belts, continents,
G 3. AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society
 Geosystems G 3 AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society Article Volume 5, Number 1 28 January 2004 Q01E16, doi:10.1029/2003gc000568 ISSN: 1525-2027 A hydrous
Geosystems G 3 AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society Article Volume 5, Number 1 28 January 2004 Q01E16, doi:10.1029/2003gc000568 ISSN: 1525-2027 A hydrous
MAR110 Lecture #4 Fundamentals of Plate Tectonics
 1 MAR110 Lecture #4 Fundamentals of Plate Tectonics The Ocean Sea Floor is formed Along the Mid-Ocean Ridge Spreading Centers The Ocean Sea Floor is destroyed in the Subduction Zones Figure 4.2 Convection
1 MAR110 Lecture #4 Fundamentals of Plate Tectonics The Ocean Sea Floor is formed Along the Mid-Ocean Ridge Spreading Centers The Ocean Sea Floor is destroyed in the Subduction Zones Figure 4.2 Convection
Chapter Introduction Lesson 1 Earthquakes Lesson 2 Volcanoes Chapter Wrap-Up
 Chapter Introduction Lesson 1 Earthquakes Lesson 2 Volcanoes Chapter Wrap-Up What causes earthquakes and volcanic eruptions? What do you think? Before you begin, decide if you agree or disagree with each
Chapter Introduction Lesson 1 Earthquakes Lesson 2 Volcanoes Chapter Wrap-Up What causes earthquakes and volcanic eruptions? What do you think? Before you begin, decide if you agree or disagree with each
Earth and Planetary Science Letters
 Earth and Planetary Science Letters 365 (2013) 209 220 Contents lists available at SciVerse ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl Constraints
Earth and Planetary Science Letters 365 (2013) 209 220 Contents lists available at SciVerse ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl Constraints
Earthquakes. Earthquakes are caused by a sudden release of energy
 Earthquakes Earthquakes are caused by a sudden release of energy The amount of energy released determines the magnitude of the earthquake Seismic waves carry the energy away from its origin Fig. 18.1 Origin
Earthquakes Earthquakes are caused by a sudden release of energy The amount of energy released determines the magnitude of the earthquake Seismic waves carry the energy away from its origin Fig. 18.1 Origin
BIBLIOGRAPHIC REFERENCE
 BIBLIOGRAPHIC REFERENCE Chambefort, I; Bignall, G. 2013. Preliminary stable isotope study on the Lahendong geothermal system, Indonesia, GNS Science Report 2013/14. 9p. I. Chambefort, GNS Science, Wairakei
BIBLIOGRAPHIC REFERENCE Chambefort, I; Bignall, G. 2013. Preliminary stable isotope study on the Lahendong geothermal system, Indonesia, GNS Science Report 2013/14. 9p. I. Chambefort, GNS Science, Wairakei
Isotopic record of the atmosphere and hydrosphere
 Isotopic record of the atmosphere and hydrosphere W. F. McDonough 1 1 Department of Earth Sciences and Research Center for Neutrino Science, Tohoku University, Sendai 980-8578, Japan (Dated: March 7, 2018)
Isotopic record of the atmosphere and hydrosphere W. F. McDonough 1 1 Department of Earth Sciences and Research Center for Neutrino Science, Tohoku University, Sendai 980-8578, Japan (Dated: March 7, 2018)
Part A GEOLOGY 12 CHAPTER 4 WORKSHEET VOLCANOES. Name
 GEOLOGY 12 CHAPTER 4 WORKSHEET VOLCANOES Name Part A 1. The rough, jumbled blocky or jagged surface of a lava flow is called a. pahoehoe b. lahar c. aa d. phreatic 2. The Cascade volcanoes like Mt. St.
GEOLOGY 12 CHAPTER 4 WORKSHEET VOLCANOES Name Part A 1. The rough, jumbled blocky or jagged surface of a lava flow is called a. pahoehoe b. lahar c. aa d. phreatic 2. The Cascade volcanoes like Mt. St.
Answer ALL questions in Section A, and TWO questions from Section B.
 UNIVERSITY OF EAST ANGLIA School of Environmental Sciences Main Series Undergraduate Examination 2012-2013 Candidate s no.: GEODYNAMICS: EARTH S ENGINE ENV-2A43 Time allowed: 2 hours. Answer ALL questions
UNIVERSITY OF EAST ANGLIA School of Environmental Sciences Main Series Undergraduate Examination 2012-2013 Candidate s no.: GEODYNAMICS: EARTH S ENGINE ENV-2A43 Time allowed: 2 hours. Answer ALL questions
Ore deposits related to mafic igneous rocks Diamonds - GLY 361 Lecture 4
 Ore deposits related to mafic igneous rocks Diamonds - GLY 361 Lecture 4 More on kimberlites Classification of kimberlites Historically, kimberlites have been subdivided into two distinct varieties based
Ore deposits related to mafic igneous rocks Diamonds - GLY 361 Lecture 4 More on kimberlites Classification of kimberlites Historically, kimberlites have been subdivided into two distinct varieties based
New evidence for lunar basalt metasomatism by underlying regolith.
 1 2 3 4 5 New evidence for lunar basalt metasomatism by underlying regolith. John F. Pernet-Fisher* School of Earth, Atmospheric, and Environmental Sciences, University of Manchester, Manchester, M13 2PL,
1 2 3 4 5 New evidence for lunar basalt metasomatism by underlying regolith. John F. Pernet-Fisher* School of Earth, Atmospheric, and Environmental Sciences, University of Manchester, Manchester, M13 2PL,
Volatile element isotopic systematics of the Rodrigues Triple Junction Indian Ocean MORB: implications for mantle heterogeneity
 ELSEVIER Earth and Planetary Science Letters 170 (1999) 241 253 www.elsevier.com/locate/epsl Volatile element isotopic systematics of the Rodrigues Triple Junction Indian Ocean MORB: implications for mantle
ELSEVIER Earth and Planetary Science Letters 170 (1999) 241 253 www.elsevier.com/locate/epsl Volatile element isotopic systematics of the Rodrigues Triple Junction Indian Ocean MORB: implications for mantle
Chemical Geology (2012) Contents lists available at SciVerse ScienceDirect. Chemical Geology
 Chemical Geology 330 331 (2012) 176 187 Contents lists available at SciVerse ScienceDirect Chemical Geology journal homepage: www.elsevier.com/locate/chemgeo Geochemical constraints on a mixed pyroxenite
Chemical Geology 330 331 (2012) 176 187 Contents lists available at SciVerse ScienceDirect Chemical Geology journal homepage: www.elsevier.com/locate/chemgeo Geochemical constraints on a mixed pyroxenite
Regional and local variations in geochemistry and tectonics along and across Central America
 Regional and local variations in geochemistry and tectonics along and across Central America Michael J. Carr, Department of Geological Sciences, Wright Lab Rutgers University, 610 Taylor Rd., Piscataway
Regional and local variations in geochemistry and tectonics along and across Central America Michael J. Carr, Department of Geological Sciences, Wright Lab Rutgers University, 610 Taylor Rd., Piscataway
Reconciling geochemical and geophysical observations of magma supply and melt distribution at the 9 N overlapping spreading center, East Pacific Rise
 Article Volume 13, Number 11 6 November 2012 Q11005, doi:10.1029/2012gc004168 ISSN: 1525-2027 Reconciling geochemical and geophysical observations of magma supply and melt distribution at the 9 N overlapping
Article Volume 13, Number 11 6 November 2012 Q11005, doi:10.1029/2012gc004168 ISSN: 1525-2027 Reconciling geochemical and geophysical observations of magma supply and melt distribution at the 9 N overlapping
Engineering Geology ECIV 2204
 Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Lecture 36. Igneous geochemistry
 Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Lecture 36 Igneous geochemistry Reading - White Chapter 7 Today 1. Overview 2. solid-melt distribution coefficients Igneous geochemistry The chemistry of igneous systems provides clues to a number of important
Mantle melting as a function of water content beneath back-arc basins
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 111,, doi:10.1029/2005jb003732, 2006 Mantle melting as a function of water content beneath back-arc basins Katherine A. Kelley, 1,2,3 Terry
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 111,, doi:10.1029/2005jb003732, 2006 Mantle melting as a function of water content beneath back-arc basins Katherine A. Kelley, 1,2,3 Terry
Earth and Planetary Science Letters
 Earth and Planetary Science Letters 361 (2013) 298 309 Contents lists available at SciVerse ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl Chemical heterogeneity
Earth and Planetary Science Letters 361 (2013) 298 309 Contents lists available at SciVerse ScienceDirect Earth and Planetary Science Letters journal homepage: www.elsevier.com/locate/epsl Chemical heterogeneity
Chapter 4 Up from the Inferno: Magma and Igneous Rocks
 Chapter 4 Up from the Inferno: Magma and Igneous Rocks Up from the Inferno: Magma and Igneous Rocks Updated by: Rick Oches, Professor of Geology & Environmental Sciences Bentley University Waltham, Massachusetts
Chapter 4 Up from the Inferno: Magma and Igneous Rocks Up from the Inferno: Magma and Igneous Rocks Updated by: Rick Oches, Professor of Geology & Environmental Sciences Bentley University Waltham, Massachusetts
The Lead 206/207 Dating Method
 The Lead 206/207 Dating Method 1 U Pb Zircon Ages, Chemical Geology, Volume 211 (2004) Pages 87 109 2 Lead Isotope Planetary Profiling, Chemical Geology, Volume 233 (2006) Pages 1 45 3 U Pb Step-Leaching
The Lead 206/207 Dating Method 1 U Pb Zircon Ages, Chemical Geology, Volume 211 (2004) Pages 87 109 2 Lead Isotope Planetary Profiling, Chemical Geology, Volume 233 (2006) Pages 1 45 3 U Pb Step-Leaching
G 3. AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society
 Geosystems G 3 AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society Article Volume 5, Number 5 13 May 2004 Q05B07, doi:10.1029/2003gc000597 ISSN: 1525-2027 Composition
Geosystems G 3 AN ELECTRONIC JOURNAL OF THE EARTH SCIENCES Published by AGU and the Geochemical Society Article Volume 5, Number 5 13 May 2004 Q05B07, doi:10.1029/2003gc000597 ISSN: 1525-2027 Composition
LATE ARCHAEAN FELSIC ALKALINE MAGMATISM: GEOLOGY, GEOCHEMISTRY, AND TECTONIC SETTING
 LATE ARCHAEAN FELSIC ALKALINE MAGMATISM: GEOLOGY, GEOCHEMISTRY, AND TECTONIC SETTING ZOZULYA DMITRY 1, EBY NELSON 2 1 - Geological Institute Kola Science Centre RAS, Apatity, Russia 2 - Department of Environmental,
LATE ARCHAEAN FELSIC ALKALINE MAGMATISM: GEOLOGY, GEOCHEMISTRY, AND TECTONIC SETTING ZOZULYA DMITRY 1, EBY NELSON 2 1 - Geological Institute Kola Science Centre RAS, Apatity, Russia 2 - Department of Environmental,
Origin of Basaltic Magma. Geology 346- Petrology
 Origin of Basaltic Magma Geology 346- Petrology 2 principal types of basalt in the ocean basins Tholeiitic Basalt and Alkaline Basalt Table 10-1 Common petrographic differences between tholeiitic and alkaline
Origin of Basaltic Magma Geology 346- Petrology 2 principal types of basalt in the ocean basins Tholeiitic Basalt and Alkaline Basalt Table 10-1 Common petrographic differences between tholeiitic and alkaline
Geol. 656 Isotope Geochemistry
 STABLE ISOTOPE APPLICATIONS I: HIGH TEMPERATURES INTRODUCTION Stable isotopes have a number of uses in high temperature geochemistry (i.e., igneous and metamorphic geochemistry), which we will treat in
STABLE ISOTOPE APPLICATIONS I: HIGH TEMPERATURES INTRODUCTION Stable isotopes have a number of uses in high temperature geochemistry (i.e., igneous and metamorphic geochemistry), which we will treat in
A statistical test of the two reservoir model for helium isotopes
 Earth and Planetary Science Letters 193 (2001) 77^82 www.elsevier.com/locate/epsl A statistical test of the two reservoir model for helium isotopes Don L. Anderson * California Institute of Technology,
Earth and Planetary Science Letters 193 (2001) 77^82 www.elsevier.com/locate/epsl A statistical test of the two reservoir model for helium isotopes Don L. Anderson * California Institute of Technology,
At the beginning. Matter + antimatter. Matter has the advantage. baryons quarks, leptons, electrons, photons (no protons or neutrons)
 At the beginning Matter + antimatter Matter has the advantage baryons quarks, leptons, electrons, photons (no protons or neutrons) Hadrons protons, neutrons Hydrogen, helium (:0 H:He) Origin of the Universe
At the beginning Matter + antimatter Matter has the advantage baryons quarks, leptons, electrons, photons (no protons or neutrons) Hadrons protons, neutrons Hydrogen, helium (:0 H:He) Origin of the Universe
Layer Composition Thickness State of Matter
 Unit 4.2 Test Review Earth and Its Layers 1. Label the layers of the earth. oceanic crust continental crust lithosphere asthenosphere mantle outer core inner core 2. Complete the Following Table about
Unit 4.2 Test Review Earth and Its Layers 1. Label the layers of the earth. oceanic crust continental crust lithosphere asthenosphere mantle outer core inner core 2. Complete the Following Table about
Depths of Partial Crystallization of H 2 O-bearing MORB: Phase Equilibria Simulations of Basalts at the MAR near Ascension Island (7^118S)
 JOURNAL OF PETROLOGY VOLUME 49 NUMBER1 PAGES 25^45 2008 doi:10.1093/petrology/egm068 Depths of Partial Crystallization of H 2 O-bearing MORB: Phase Equilibria Simulations of Basalts at the MAR near Ascension
JOURNAL OF PETROLOGY VOLUME 49 NUMBER1 PAGES 25^45 2008 doi:10.1093/petrology/egm068 Depths of Partial Crystallization of H 2 O-bearing MORB: Phase Equilibria Simulations of Basalts at the MAR near Ascension
NOBLE GASES AND HALOGENS IN ICELANDIC BASALTS
 NOBLE GASES AND HALOGENS IN ICELANDIC BASALTS A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Engineering and Physical Sciences by Bridget M.
NOBLE GASES AND HALOGENS IN ICELANDIC BASALTS A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Engineering and Physical Sciences by Bridget M.
MAR110 Lecture #3 Ocean Bathymetry / Plate Tectonics
 1 MAR110 Lecture #3 Ocean Bathymetry / Plate Tectonics Ocean Basin Geographic Zones The geographic zones of the North Atlantic are identified in the bird s eye view of the sea floor above. Below is shown
1 MAR110 Lecture #3 Ocean Bathymetry / Plate Tectonics Ocean Basin Geographic Zones The geographic zones of the North Atlantic are identified in the bird s eye view of the sea floor above. Below is shown
OCN 201: Earth Structure
 OCN 201: Earth Structure Eric Heinen Eric H. De Carlo, Carlo: OCN 201, OCN Sp2010 201, Fall 2004 Early History of the Earth Rapid accretion of Earth and attendant dissipation of kinetic energy caused tremendous
OCN 201: Earth Structure Eric Heinen Eric H. De Carlo, Carlo: OCN 201, OCN Sp2010 201, Fall 2004 Early History of the Earth Rapid accretion of Earth and attendant dissipation of kinetic energy caused tremendous
Lecture 25 Subduction Related Magmatism
 Lecture 25 Subduction Related Magmatism Monday, May 2 nd 2005 Subduction Related Magmatism Activity along arcuate volcanic chains along subduction zones Distinctly different from the mainly basaltic provinces
Lecture 25 Subduction Related Magmatism Monday, May 2 nd 2005 Subduction Related Magmatism Activity along arcuate volcanic chains along subduction zones Distinctly different from the mainly basaltic provinces
Plate Tectonics Lab II: Background Information
 Plate Tectonics Lab II: Background Information This lab is based on a UW ESS101 Lab. Note: Hand in only the Answer Sheet at the back of this guide to your Instructor Introduction One of the more fundamental
Plate Tectonics Lab II: Background Information This lab is based on a UW ESS101 Lab. Note: Hand in only the Answer Sheet at the back of this guide to your Instructor Introduction One of the more fundamental
EARTH S ENERGY SOURCES
 EARTH S ENERGY SOURCES The geological processes that shape the Earth s surface are powered by two major sources of energy; geothermal heat from the Earth s interior and external energy from the sun. The
EARTH S ENERGY SOURCES The geological processes that shape the Earth s surface are powered by two major sources of energy; geothermal heat from the Earth s interior and external energy from the sun. The
