31. GEOCHEMISTRY OF INTERSTITIAL WATERS INTERSTITIAL WATER STUDIES ON SMALL CORE SAMPLES FROM THE MEDITERRANEAN SEA 1
|
|
|
- Percival Arnold
- 5 years ago
- Views:
Transcription
1 31. GEOCHEMISTRY OF INTERSTITIAL WATERS INTERSTITIAL WATER STUDIES ON SMALL CORE SAMPLES FROM THE MEDITERRANEAN SEA 1 F. L. Sayles and L. S. Waterman, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts and F. T. Manheim, U. S. Geological Survey, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts ABSTRACT Of ten Leg 13 sites studied by us, eight give definite evidence of the existence of halite-containing sediments beneath the seabed. This conclusion is based on the existence of continuous sodium and chloride enrichments in interstitial waters with depth. This is the only direct evidence of the existence of salt at these sites, for only evaporitic dolomite, gypsum, and/or anhydrite were recovered in cores. Among other ionic relationships, influence of evaporites is seen in calcium, magnesium and sulfate concentrations. Record high barium (21 ppm) and strontium (34 ppm) concentrations were observed in sulfate depleted pore fluids of Site 12 in the Hellenic Trench. INTRODUCTION The sediments cored on Leg 13 are, for the most part, characterized by high sedimentation rates and usually contain large proportions of terrigenous detritus. Upper Miocene evaporites are widespread throughout the deep basins, and underlie most of the Leg 13 sites. It is to be expected that the influence of both normal diagenesis and underlying evaporites will be seen in the composition of interstitial solutions. Previous experience has demonstrated that diffusion of salts from evaporite sequences into overlying sediments may strongly influence the composition of interstitial solutions in the superjacent sediments. Increases in interstitial Na and Cl concentration with depth have been found to be characteristic of sediments overlying known salt deposits in the Gulf of Mexico (Manheim and Bischoff, 199; Manheim and Sayles, 199; Manheim et al., 192). Data from Leg of the Deep Sea Drilling Project in the Gulf of Mexico and Manheim and Bischoff (199) demonstrate that large Mg concentration increases may occur with the more characteristic Na and Cl enrichments. Increases in Ca and SO 4 concentration may occur at least locally through the dissolution of gypsum and anhydrite. Concentration changes resulting from diagenetic reactions not related to evaporites are readily distinguishable from those associated with dissolution and diagenesis of evaporites. Normal marine diagenesis produces little or no significant change in Na and Cl concentrations in most cases. In the rare cases where changes are noted, they are depletions. Mg and SO 4 have not been found to be enriched 'Site 12 was located below the evaporites in a deep cleft in the Mediterranean Ridge which cuts through the inferred evaporite layer. in normal marine sedimentary sequences in previous DSDP studies, but depletions of both are common. Characteristically, the greatest diagenetic changes in pore fluid composition are observed in rapidly deposited sediments (> cm/ 3 years) containing appreciable amounts of terrigenous material. The analytical methods utilized are those previously employed in this laboratory and described in earlier reports. We gratefully acknowledge the assistance of James O'Neill and Joanne Goudreau in the analysis of these samples. RESULTS The analytical data are presented in Tables 1 and 2 (major and minor constituents, respectively). The data are subject to uncertainties arising chiefly from two sources: Ca loss due to CaCθ3 precipitation, and temperature-ofsqueezing effects. In sediments where SO 4 is strongly reduced and alkalinities are increased above meq/kg, Ca and alkalinity loss due to CaCO 3 precipitation after squeezing has been noted (Gieskes, 192). Extensive reduction of SO 4 is common in the Leg 13 samples, but alkalinities have not exceeded meq/kg. Serious Ca and alkalinity losses have not been found in studies of such samples (Gieskes, 192; Sayles et al, 192). Small losses, however, cannot be ruled out completely. While we believe the depletions of Ca found are real, the in situ depletions may be somewhat smaller than our data indicate, and the alkalinities reported may be low. A second source of uncertainty arises from the effects of warming sediments from in situ to laboratory temperatures prior to squeezing. Such treatment leads to depletions of Ca and Mg, and enrichments of Na and K (Mangelsdorf, et al., 199). Silica is also enriched upon warming (Fanning and Pilson, 191). These changes do not obscure the existing major ion trends, and with the exception of K, concentra- 01
2 00 s TABLE 1 Major Constituents of Pore Fluids. Values in g/kg (% ) Except as Noted Sample Designation Depth (m) Age Description Na d Na [ Total Cations Ca Mg (meq/kg) Hole 121 (3 09.'N, 'W, water depth 113m, Alboran Basin) Alk. Cl SO4 (meq/kg) Total Anions Refrac- H2O (meq/kg) Sum d tometer (%o) ph Surface ocean water Dark greenish gray marl, plastic Dark greenish gray marl, plastic Dark greenish gray marl, plastic Hole 122 (40 2.'N, 'E, water depth 214m, Valencia Trough) Upper Varicolored brown and gray alternations of fine sands and clays Hole 1 (40 3.3'N,02 c 0.2'E, water depth 20m, Valencia Trough) Olive gray clays and sand, turbidites Lower Varicolored gray and brown nanno to marl, fine layers of silt or fine sand Hole 124 (3 2.3'N, 'E, water depth 22m, Balearic Basin) 124-1, CC 12 Pleistocene 3,CC 30 Lower 4, CC 9 Lower Varicolored gray terrigenous nanno, plastic, mottling Light gray nanno Dark grayish brown marl Holes 12 & 12A ( 'N, 20 2.'E, water depth 22m, Mediterranean Ridge in the Ionian Basin) Surface ocean water Upper 12A-3-CC 3 Upper Upper Brownish and greenish gray nanno with foram and sapropel beds Brown to yellowish brown nanno, deformed Light gray nanno with foram s Light yellowish brown nanno, disturbed by drilling
3 TABLE 1 - Continued Holes 12 and 12A (Continued) 12A--CC 0 Lower 9-CC 2 Upper Miocene Olive green nanno Dark gray dolomite, plastic Hole 12 (3 09.2'N, 21, 2.3'E, water depth 330m, Mediterranean Ridge Cleft in the Ionian Basin) Olive gray marl homogenous, plastic Gray and brown nanno and marl Holes 12 & 12A ( 'N, 22.1'E, water depth 44m, Northeast Margin, Hellenic Trench) Surface ocean water A , CC 3, CC 24 11, CC 30 12, CC 1, CC Gray and olive gray nanno to marl with sand, disturbed by drilling Olive gray marl disturbed by drilling Dark gray to olive gray marl, homogeneous Gray and dark gray marl, plastic, gassy Dark greenish gray marl, graded sand and silt Dark greenish gray marl, plastic Olive gray nanno with olive black sand layer below Varicolored gray silt to clay, nannos abundant Olive gray nanno Light gray nanno Olive gray nanno, plastic to stiff Olive gray nanno, plastic to stiff Hole 12 (3 42.'N, 22 2.'E, water depth 440m, SW margin Hellenic Trench) oo o Olive gray marl, gassy Olive gray nanno with graded layers of sand, gassy - 22 Olive gray nanno, plastic to stiff, gassy,cc 313 Olive gray and dark greenish gray marl
4 00 TABLE 1 - Continued Sample Desig- 1Depth nation (m) Age Description Na a Na b K Ca Mg Hole 130 ( 3.31'N, 'E, water depth 9, Mediterranean Ridge in the Levantine Basin) Total Cations (meq/kg) Cl SO 4 Alk. (meq/kg) HCO3 C Total Anions (meq/kg) Sum α Refractometer H 2 O e ( ) ph ,CC Light gray to olive gray nanno, odor of H2S Dark gray clay and greenish gray nanno, sharp contact between Nile clay and pelagic deposits > H w á > z H S > z X Hole 132 (40 1.0' N, 'E, water depth 23m, Tyrrhenian Basin) Upper Upper Lower Lower Lower Light olive gray nanno Yellow brown nanno Light olive gray nanno, moderately deformed Olive gray nanno, deformed Brown foram-nanno Light olive gray foramnanno Pale yellow foram-nanno Light olive gray nanno Light olive gray nanno _ (1.24) _ Sodium determined by difference between anions and cations excluding Na. Sodium determined directly. c HCθ3 i s calculated from total alkalinity, assuming this is entirely due to bicarbonate ion. The sum incorporates the calculated Na values. Minor constituents are not included, but with the exception of strontium in some samples contribute less than 0.1 to the sum. ph and water content are taken from shipboard summaries. Value determined by difference.
5 TABLE 2 Minor Constituents of Pore Fluids. Concentrations in mg/kg (ppm) INTERSTITIAL WATER STUDIES ON SMALL CORE SAMPLES Sample Designation Depth (m) Age Description B Sr Ba Si(col.) a Si(sρec.) b Hole 121 Surface ocean water Dark greenish gray marl, plastic Dark greenish gray marl, plastic Dark greenish gray marl, plastic to stiff _ Hole Upper Varicolored brown and gray alternations of fine sands and clays Hole Lower Olive gray clays and sand, turbidites Varicolored gray and brown nanno to marl, fine layers of silt or fine sand 4 9. Hole , CC 12 Pleistocene 3,CC 4,CC 30 9 Lower Lower Varicolored gray terrigenous nanno, plastic, 4 < mottling Light gray nanno < 2 Dark grayish brown marl 1 < 0.3 Holes 12 and 12A Surface ocean water Brownish and greenish gray nanno with 1 foram and sapropel beds Upper Brown to yellowish brown nanno, deformed 19 12A-3,CC 3 Upper Light gray nanno with forams Upper Light yellowish brown nanno, disturbed by 9 19 drilling 12A-,CC 0 Early Olive green nanno 30 9,CC 2 Upper Miocene Dark gray dolomite, plastic 9 3 < <2 1 Hole Olive gray marl homogeneous, plastic Gray and brown nanno and marl Holes 12 and 12A Surface ocean water 9 <2 < Gray and olive gray nanno to marl with.0 sand, disturbed by drilling 3-42 Olive gray marl disturbed by drilling A Dark gray to olive gray marl, homogeneous Gray and dark gray marl, plastic, gassy Dark greenish gray marl, graded sand and silt - Dark greenish gray marl, plastic Olive gray nanno with olive black sand layer below 9,CC 3 Varicolored gray silt to clay, nannos abundant , CC 24 Olive gray nanno , CC 30 Light gray nanno , CC Olive gray nanno, plastic to stiff , CC 42 Olive gray nanno, plastic to stiff 13 <2 9 Hole Olive gray marl, gassy Olive gray nanno, with graded layers of <2 sand, gassy - 22 Olive gray nanno, plastic to stiff, gassy <2 4,CC 313 Olive gray and dark greenish gray marl
6 F. L. SAYLES, L S. WATERMAN, F. T. MANHEIM TABLE 2 - Continued Sample Designation Depth (m) Age Description B Sr Ba Si(coir Si (spec.) Hole , CC Light gray to olive gray nanno, odor of H2S Dark gray clay and greenish gray nanno, sharp contact between Nile clay and pelagic deposits Hole Upper Upper Lower Lower Lower Light olive gray nanno Yellow brown nanno Light olive gray nanno, moderately deformed Olive gray nanno, deformed Brown foram-nanno Light olive gray foram-nanno Pale yellow foram-nanno Light olive gray nanno Light olive gray nanno <2. 9 a(col.) (spec.) = Colorimetric determination Emission spectographic determination. tions are altered per cent or less (Sayles et al., 192). K concentrations may be significantly affected. Gains of up to.0 g/kg have been observed (Bischoff et al., 190; Sayles et al, 192). Enrichments of Na and Cl are found at all of the sites sampled for interstitial water studies. At Sites 122, Valencia Trough, and 12, Mediterranean Ridge, the Cl increases are small but above analytical uncertainty. Evaporation prior to squeezing or inadequate sealing of storage vessels could have produced these small increases, but the increase in Cl with depth at Site 12 suggests the conclusion that real Cl gradients exist at least at this site. The increases in Na and Cl found at all of the other sites sampled on Leg 13 are large enough to avoid uncertainty as to the existence of in situ enrichments. Where sufficient samples exist, these increases can be seen to be roughly linear as a function of depth. Cl concentrations of 2 to 30 g/kg are common with maximum values of 9 and 9 g/kg occurring at Sites 12 and 12, respectively. At Sites 122 and 124 in the western Mediterranean, 12 and 12 in the eastern Mediterranean, and 132 in the Tyrrhenian Sea, the influence of underlying evaporites appears to exert a dominant influence on SO 4 concentrations and in some instances on Mg (124, 12, 132). Ca concentrations appear to reflect the interaction of both diagenetic and evaporitic influences. Gypsum or anhydrite was recovered in cores from each of these sites except 12 where penetration was terminated before reaching the level of the evaporite formation. Not surprisingly, the pore waters commonly are characterized by Ca and SO 4 enrichments. Relative to standard seawater (Ca = 0.41 g/kg, SO 4 = 2.1 g/kg), nine-fold Ca increases (to 3.2 g/kg) and two-fold SO 4 enrichments (to.9 g/kg) are found. Mg concentrations in excess of seawater (1. g/kg) are observed at Sites 124 (slight), 12, 12 and 132, reaching concentrations of 2.4 g/kg at Site 12 with values exceeding 1. g/kg common. At Sites 121 (Alboran Basin), 1 (Valencia Trough), and 12 and 130 on the Mediterranean Ridge, Ca, Mg and SO 4 concentrations chiefly reflect the influence of diagenetic reactions rather than that of evaporites at depth. In the absence of evaporites, Mg concentrations have been found to invariably decrease with depth. Depletions of Mg may be related to dolomitization (Sayles et al., 192; Broecker, 192) or to silicate reactions such as those proposed by Drever (191). Mg depletions are found in sediments at Sites 121, 12, 130 and the upper portion of 12. The depletions of Mg (to g/kg) are compatible with earlier DSDP pore water data. Depletions of SO4 are also a common product of diagenesis and result from bacterial reduction of SO 4. Depletion of more than 0 per cent of the original SO 4 present is found at Sites 121, 12 and 130. Smaller losses are found at Site 1. Ca depletion commonly accompanies strong SO 4 reduction at least in the upper portions of such sites (Gieskes, 192; Sayles, et al., 192). Losses of Ca are found at Sites 121, 12 and 130, those sites marked by SO 4 depletion. With the possible exception of Sample , we believe the Ca losses are too large to be explained as an artifact resulting from the precipitation of CaCO 3 after squeezing. Once SO 4 reduction is virtually complete, further Ca depletion does not occur. At depth in these sites, depletion actually gives way to gradual Ca enrichment. This effect can be seen in the lowermost samples of Sites 121, 12 and 130. At sites where no appreciable SO 4 reduction occurs, Ca enrichment commonly is seen over the entire hole (cf. data Legs and 1). Thus, all of the Ca, Mg and SO 4 changes occurring at Sites 121, 1, 12 and 130 can be adequately explained within the framework of diagenetic alterations noted in previous interstitial water studies. Site 12 in the Hellenic Trench exhibits behavior characteristic of both evaporite influence and diagenesis. Concentrations of SO 4 are below our detection limit (.0 g/kg) in all samples from this site, reflecting complete 0
7 31.1. INTERSTITIAL WATER STUDIES ON SMALL CORE SAMPLES utilization in diagenetic reduction. The observed Ca enrichments (below m) may reflect either diagenetic or evaporitic influence. Mg is strongly enriched reaching concentrations of 3.2 g/kg, the highest value recorded on Leg 13. As noted above, such enrichment has only been found where evidence of underlying evaporites exists. Site 12, located a few kilometers from Site 12, also exhibits a somewhat split personality. In the upper portions of this site (< 1 m) diagenetic reactions hold sway, but below this the influence of evaporites on Mg and SO 4 can be seen. The concentrations of minor elements in the samples from Leg 13 exhibit some of the largest deviations from seawater found during the Deep Sea Drilling Project. Enrichments of Sr reached ppm and 34 ppm in samples from Sites 12 and 12, respectively. Modest enrichments of 20 to 0 ppm are found in all of the other sites for which samples are available. Such modest enrichments are quite common in interstitial solutions of calcareous sediments from the Pacific Ocean and have been discussed in earlier leg reports (e.g. Legs and ). Ba concentrations markedly higher than any reported previously by us are found at Site 12 (up to 21 ppm), and unusually high values are found at Sites 121 and 12. As has been noted previously, Ba enrichments are characteristic of sites exhibiting extensive SO 4 reduction, indicating that barite solubility is the primary control of concentration. The extremely high values at Site 12 may well represent other unidentified influences, however. The reduction of SO 4 to our detection limit is common, but concentrations even approaching those at 12 have not been seen previously. The unusually high Mg and Sr also suggest abnormal conditions at this site. While the B data exhibit considerable scatter, real enrichment relative to seawater is usually found at the Leg 13 sites where evaporites appear to influence Mg or SO 4. Concentrations commonly fall in the range to 9 ppm at these sites. At 12, B is strongly enriched to 20 to ppm in the lower portion of the hole. DISCUSSION The existence of Na and Cl gradients at all but two sites for which interstitial water sampling was carried out indicate that halite or brine-containing evaporites exist at depth at all of these sites. The two remaining sites (122 and 12) exhibit small chloride enrichments, but sampling was too sparse and shallow to definitely prove the existence of Na and Cl enrichments and gradients characteristic of sediments overlying salt deposits. Although halite was not recovered at any of the above sites, we believe that Na and Cl data provide sufficient evidence for concluding that such sediments do exist at depth at all of the sites except perhaps 122 and This conclusion is in agreement with seismic data which also suggest that a halite layer exists below the deepest penetration at these sites. Site 121 was terminated in a schist which, in the preliminary report, is presumed to be basement. The interstitial waters of Site 121 are characterized by Na and Cl gradients typical of sediments overlying salt. This site was drilled over a basement high onto which and Miocene sediments lap unconformably (W. B. F. Ryan, personal communication). Thus, some lateral movement of brines from the Miocene or sediments must occur to account for the observed gradients. There is little question that halite or brine-containing sediments do exist near this site. Enrichments of SO 4 have not been found previously in interstitial waters sampled by the DSDP. There is little doubt that the enhanced SO 4 concentrations, common in these pore fluids, result from the dissolution of gypsum or anhydrite. With one exception, the SO 4 enrichments are found at sites where evaporitic gypsum or anhydrite was recovered (Sites 122, 124, 12, 132). Site 132 appears to provide the most straightforward example of the influence of anhydrite dissolution upon overlying sediments. As seen in Figure 1, ΔCa and ΔSO 4 (Δ = change in pore water concentration relative to seawater) vary linearly suggesting that dissolution and diffusion are responsible for the observed concentration increases. The slope of the ΔCa-ΔSO 4 correspondence is not 1 as required by simple dissolution, but rather 1.. Consequently, some concurrent reaction must either supply Ca or remove a fraction of the SO 4 released by dissolution. Both reactions have been frequently observed in previous DSDP studies. At the other sites where evaporitic sediments were cored, no simple and consistent relationship between Ca and SO 4 can be determined. Insufficient data are available at Sites 122 and 124, and the data of 12 exhibit no consistent trend. In several earlier legs of the DSDP (e.g.,,, and 1) a nearly linear correspondence between Ca and Sr enrichment was found to exist. A similar correspondence appears to hold for all of the Leg 13 sites where sufficient data are available for interpretation. At least at Site 132 the linear variations of ΔCa, ΔSO 4 and ΔSr indicate that the Sr is 1 So µ (πim/kg) Figure 1. The variation in Ca enrichment with SO 4 enrichment (A = pore water concentration - standard seawater concentration.) Data are for Site 132 only. 0
8 F. L. SAYLES, L S. WATERMAN, F. T. MANHEIM released during dissolution and possibly recrystallization of gypsum and anhydrite. The slopes of the data plots in Figure 2 are in the range 0 to 0. This requires that between 1 and 2 per cent of the Ca ion sites in a presumed CaSO 4 phase be occupied by Sr if solution of CaSO 4 is responsible for the observed enrichments. Since most marine gypsum and anhydrite rocks contain < 0.2 per cent Sr (Stewart, 193, F. T. Manheim, unpublished data), we presume that much of the Sr is released during recrystallization. The abnormally high concentrations of Mg observed at Sites 12, 12 and 132 are difficult to explain. Normal diagenetic changes lead to the depletion of Mg, whereas the increases noted above are believed due to the influence of evaporitic sediments. Late stage evaporites, in particular, contain readily soluble Mg-bearing minerals and could supply Mg. The data argue against any simple explanation, however, for at least Sites 12 and 12, Mg concentrations pass through a maximum. This is also true of Mg at 132, but the drop in the lowermost sample is small and a maximum is not well established. Maxima in Mg vs. depth 0 ;T) Site 12 Ca vs Sr JDA Site 132 Ca vs Sr ( )+ Site 132 Soi, vs Sr Φ Sites 124 & 12 Ca vs Sr 0 Sr (m M/kg x 1Q 3 00 Figure 2. The variation of Sr enrichment with Ca and SO j enrichment for Site 132 and Ca enrichment only for Sites 124, 12 and 12. (A = pore water concentration - standard seawater concentration). profiles have also been found associated with known or inferred evaporites in the Gulf of Mexico by Manheim and Bischoff (199) in core 2 and at DSDP Site 92 (Leg ). Such maxima cannot be explained by diffusive fluxes of Mg from late stage evaporites at depth, and diagenetic reactions must be responsible. This type of Mg vs. depth distribution is associated with evaporitic sediments, but we do not know the nature of the reactions occurring. REFERENCES Bischoff, J. L., Greer, R. E. and Luistros, A. O., 190. Composition of interstitial waters of marine sediments: temperature of squeezing effect. Science. 1, 124. Broecker, W. S., 192. Initial Reports of the Deep Sea Drilling Project, Volume 1 (in press). Drever, J. I., 191. Magnesium-iron replacement in clay minerals in anoxic marine sediments. Science. 12, 14. Fanning, D. K. and Pilson, M. E. Q., 191. Interstitial silica and ph in marine sediments: some effects of sampling procedure. Science. 13, 122. Gieskes, J. M. Interstitial water studies, Leg 1. In Edgar, N. T. and Saunders, J. B., et al., 192. Initial Reports of the Deep Sea Drilling Project, Volume 1. Washington (U.S. Government Printing Office) (in preparation). Mangelsdorf, P. C, Jr., Wilson, T. R. S. and Daniell, E., 199. Potassium enrichments in interstitial waters of marine sediments. Science. 1, 11. Manheim, F. T. and Bischoff, J. L., 199. Geochemistry of pore waters from Shell Oil Company drill holes on the continental slope of the northern Gulf of Mexico. Chern. Geol. 4,3. Manheim, F. T. and Sayles, F. L., 199. Interstitial Water studies on small core samples, Deep Sea Drilling Project, Leg 1. In Ewing, M. and Worzel, J. L., et al., 199. Initial Reports of the Deep Sea Drilling Project, Volume 1. Washington (U.S. Government Printing Office), 403. Manheim, F. T., Sayles, F. L. and Waterman, L. S. Interstitial water studies on small core samples, Leg. In Worzel, J. L. and Bryant, W. R., et al, Initial Reports of the Deep Sea Drilling Project, Volume. Washington, D. C. (U.S. Government Printing Office) (in preparation). Sayles, F. L., Manheim, F. T. and Waterman, L. S. Interstitial water studies on small core samples, Leg 1. In Edgar, N. T. and Saunders, J. B., et al, Initial Reports of the Deep Sea Drilling Project, Volume 1. Washington, D.C. (U.S. Government Printing Office) (in preparation). Stewart, F. H., 193. Marine Evaporites. U.S. Geol. Surv. Prof. Paper, 440-y. 0
15. INTERSTITIAL WATER STUDIES ON SMALL CORE SAMPLES, DEEP SEA DRILLING PROJECT, LEG 2 1
 15. INTERSTITIAL WATER STUDIES ON SMALL CORE SAMPLES, DEEP SEA DRILLING PROJECT, LEG 2 1 K. M. Chan, Woods Hole Oceanographic Institution, Woods Hole, Mass. and F. T. Manheim2, U.S. Geological Survey,
15. INTERSTITIAL WATER STUDIES ON SMALL CORE SAMPLES, DEEP SEA DRILLING PROJECT, LEG 2 1 K. M. Chan, Woods Hole Oceanographic Institution, Woods Hole, Mass. and F. T. Manheim2, U.S. Geological Survey,
12. INTERSTITIAL WATER STUDIES ON SMALL CORE SAMPLES DEEP SEA DRILLING PROJECT, LEG 7 1
 12. INTERSTITIAL WATER STUDIES ON SMALL CORE SAMPLES DEEP SEA DRILLING PROJECT, LEG 7 1 F. L. Sayles, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts and F. T. Manheim, 2 U.S. Geological
12. INTERSTITIAL WATER STUDIES ON SMALL CORE SAMPLES DEEP SEA DRILLING PROJECT, LEG 7 1 F. L. Sayles, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts and F. T. Manheim, 2 U.S. Geological
13. INTERSTITIAL WATER CHEMISTRY: DEEP SEA DRILLING PROJECT, LEG 7 1,2
 13. INTERSTITIAL WATER CHEMISTRY: DEEP SEA DRILLING PROJECT, LEG 7 1,2 B. J. Presley 3 and I. R. Kaplan, Department of Geology and Institute of Geophysics and Planetary Physics, University of California,
13. INTERSTITIAL WATER CHEMISTRY: DEEP SEA DRILLING PROJECT, LEG 7 1,2 B. J. Presley 3 and I. R. Kaplan, Department of Geology and Institute of Geophysics and Planetary Physics, University of California,
Sedimentary Rocks and Processes
 Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
UNIT 4 SEDIMENTARY ROCKS
 UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
48. A SUMMARY OF INTERSTITIAL-WATER GEOCHEMISTRY OF LEG 133 1
 McKenzie, J.A., Davies, P.J., Palmer-Julson, A., et al., 1993 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 133 48. A SUMMARY OF INTERSTITIAL-WATER GEOCHEMISTRY OF LEG 133 1 Peter
McKenzie, J.A., Davies, P.J., Palmer-Julson, A., et al., 1993 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 133 48. A SUMMARY OF INTERSTITIAL-WATER GEOCHEMISTRY OF LEG 133 1 Peter
17. CARBONATE SEDIMENTARY ROCKS FROM THE WESTERN PACIFIC: LEG 7, DEEP SEA DRILLING PROJECT
 17. CARBONATE SEDIMENTARY ROCKS FROM THE WESTERN PACIFIC: LEG 7, DEEP SEA DRILLING PROJECT Ralph Moberly, Jr., Hawaii Institute of Geophysics, University of Hawaii, Honolulu, Hawaii and G. Ross Heath,
17. CARBONATE SEDIMENTARY ROCKS FROM THE WESTERN PACIFIC: LEG 7, DEEP SEA DRILLING PROJECT Ralph Moberly, Jr., Hawaii Institute of Geophysics, University of Hawaii, Honolulu, Hawaii and G. Ross Heath,
Hydrothermal Chemistry/ Reverse Weathering. Marine Chemistry Seminar
 Hydrothermal Chemistry/ Reverse Weathering Marine Chemistry Seminar 1974 Drever, The Sea Chapter 10:The Magnesium Problem 1979 Edmonds et al., Ridge Crest Hydrothermal Activity and the Balances of Major
Hydrothermal Chemistry/ Reverse Weathering Marine Chemistry Seminar 1974 Drever, The Sea Chapter 10:The Magnesium Problem 1979 Edmonds et al., Ridge Crest Hydrothermal Activity and the Balances of Major
Where is all the water?
 Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
(4) Give an example of important reactions that are responsible for the composition of river water.
 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
EPS 50 Lab 4: Sedimentary Rocks
 Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Lecture 3 questions Temperature, Salinity, Density and Circulation
 Lecture 3 questions Temperature, Salinity, Density and Circulation (1) These are profiles of mean ocean temperature with depth at various locations in the ocean which in the following (a, b, c) figures
Lecture 3 questions Temperature, Salinity, Density and Circulation (1) These are profiles of mean ocean temperature with depth at various locations in the ocean which in the following (a, b, c) figures
Module 9 Sedimentary Rocks
 Module 9 Sedimentary Rocks SEDIMENTARY ROCKS Rocks formed from material derived from preexisting rocks by surfacial processes followed by diagenesis There are two main classes of sedimentary rocks Clastic
Module 9 Sedimentary Rocks SEDIMENTARY ROCKS Rocks formed from material derived from preexisting rocks by surfacial processes followed by diagenesis There are two main classes of sedimentary rocks Clastic
32. GEOCHEMISTRY OF CARBON: DEEP SEA DRILLING PROJECT LEGS 42A AND 42B
 32. GEOCHEMISTRY OF CARBON: DEEP SEA DRILLING PROJECT LEGS 42A AND 42B J.G. Erdman and K.S. Schorno, Phillips Petroleum Company, Bartlesville, Oklahoma INTRODUCTION A total of 14 core sections from Legs
32. GEOCHEMISTRY OF CARBON: DEEP SEA DRILLING PROJECT LEGS 42A AND 42B J.G. Erdman and K.S. Schorno, Phillips Petroleum Company, Bartlesville, Oklahoma INTRODUCTION A total of 14 core sections from Legs
SW Density = kg/l at 20 o C (Pilson 1998)
 Composition of SW To Date We Have Covered: Descriptive Oceanography (Millero chapter 1) Special Properties of H 2 O (Millero chapter 4) Ion-Water & Ion-Ion Interactions (Millero chap 4) Continuing Coverage
Composition of SW To Date We Have Covered: Descriptive Oceanography (Millero chapter 1) Special Properties of H 2 O (Millero chapter 4) Ion-Water & Ion-Ion Interactions (Millero chap 4) Continuing Coverage
Foundations of Earth Science, 6e Lutgens, Tarbuck, & Tasa
 Foundations of Earth Science, 6e Lutgens, Tarbuck, & Tasa Oceans: The Last Frontier Foundations, 6e - Chapter 9 Stan Hatfield Southwestern Illinois College The vast world ocean Earth is often referred
Foundations of Earth Science, 6e Lutgens, Tarbuck, & Tasa Oceans: The Last Frontier Foundations, 6e - Chapter 9 Stan Hatfield Southwestern Illinois College The vast world ocean Earth is often referred
Chapter 6 Pages of Earth s Past: Sedimentary Rocks
 Chapter 6 Pages of Earth s Past: Sedimentary Rocks Introduction! Drilling into the bottom of the North Sea, we encounter: " Soft mud and loose sand, silt, pebbles, and shells. Then: " Similar materials
Chapter 6 Pages of Earth s Past: Sedimentary Rocks Introduction! Drilling into the bottom of the North Sea, we encounter: " Soft mud and loose sand, silt, pebbles, and shells. Then: " Similar materials
Bahamian Dolomites. Occurrences in the Bahamas 2/25/2009. Platform Dolomites. Cretaceous Dolomite. San Salvador Little Bahama Bank.
 Bahamian Dolomites A Short Course VU March, 2009 Peter Swart University of Miami Occurrences in the Bahamas Platform Dolomites San Salvador Little Bahama Bank Bahamas Drilling Project Unda Clino Cretaceous
Bahamian Dolomites A Short Course VU March, 2009 Peter Swart University of Miami Occurrences in the Bahamas Platform Dolomites San Salvador Little Bahama Bank Bahamas Drilling Project Unda Clino Cretaceous
CEE 437 Lecture 10 Rock Classification. Thomas Doe
 CEE 437 Lecture 10 Rock Classification Thomas Doe Igneous Origins Intrusive Batholithic or plutonic: phaneritic Dikes or sills that chill rapidly: aphanitic Extrusive deposition as melt (lava) pyroclastic
CEE 437 Lecture 10 Rock Classification Thomas Doe Igneous Origins Intrusive Batholithic or plutonic: phaneritic Dikes or sills that chill rapidly: aphanitic Extrusive deposition as melt (lava) pyroclastic
Geochemical mobility of chemical elements in saline lake systems in Khakassia (Russia)
 Available online at www.sciencedirect.com Procedia Earth and Planetary Science 7 ( 2013 ) 325 329 Water Rock Interaction [WRI 14] Geochemical mobility of chemical elements in saline lake systems in Khakassia
Available online at www.sciencedirect.com Procedia Earth and Planetary Science 7 ( 2013 ) 325 329 Water Rock Interaction [WRI 14] Geochemical mobility of chemical elements in saline lake systems in Khakassia
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions
 Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Sediment. Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface
 Sediment Some basic terminology Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface Erosion: removal of weathered rock and minerals from one place to
Sediment Some basic terminology Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface Erosion: removal of weathered rock and minerals from one place to
Subduction Zone Conditions
 Summary Students investigate core samples obtained across a subduction zone off the east coast of Japan and compare the rock found in each drill hole. Learning Objectives Students will be able to: Use
Summary Students investigate core samples obtained across a subduction zone off the east coast of Japan and compare the rock found in each drill hole. Learning Objectives Students will be able to: Use
Lecture 6 - Determinants of Seawater Composition. Sets up electric dipole because O is more electronegative A o. Figure 3.
 12.742 - Marine Chemistry Fall 2004 Lecture 6 - Determinants of Seawater Composition Prof. Scott Doney What is seawater? Water Dissolved inorganic salts (major ions) Trace species, organics, colloids,
12.742 - Marine Chemistry Fall 2004 Lecture 6 - Determinants of Seawater Composition Prof. Scott Doney What is seawater? Water Dissolved inorganic salts (major ions) Trace species, organics, colloids,
SCOPE 35 Scales and Global Change (1988)
 1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
I. CORE-DISCING AND OTHER DRILLING EFFECTS IN DSDP LEG 42A MEDITERRANEAN SEDIMENT CORES
 I. CORE-DISCING AND OTHER DRILLING EFFECTS IN DSDP LEG 42A MEDITERRANEAN SEDIMENT CORES Robert B. Kidd, Institute of Oceanographic Sciences, Wormley, Surrey, United Kingdom INTRODUCTION Since deep-sea
I. CORE-DISCING AND OTHER DRILLING EFFECTS IN DSDP LEG 42A MEDITERRANEAN SEDIMENT CORES Robert B. Kidd, Institute of Oceanographic Sciences, Wormley, Surrey, United Kingdom INTRODUCTION Since deep-sea
Lecture Outlines PowerPoint. Chapter 13 Earth Science 11e Tarbuck/Lutgens
 Lecture Outlines PowerPoint Chapter 13 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
Lecture Outlines PowerPoint Chapter 13 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
Sediment and sedimentary rocks Sediment
 Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
Lecture 4 What Controls the Composition of Seawater
 Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Sedimentología Ayudantía Lectura 1 Carbonate minerals
 Carbonate minerals The most common minerals in this group are the calcium carbonates, calcite and aragonite, while dolomite (a magnesium calcium carbonate) and siderite (iron carbonate) are also frequently
Carbonate minerals The most common minerals in this group are the calcium carbonates, calcite and aragonite, while dolomite (a magnesium calcium carbonate) and siderite (iron carbonate) are also frequently
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and
 1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
32. GEOCHEMISTRY OF CARBON: DSDPLEG31
 . GEOCHEMISTRY OF CARBON: DSDPLEG J. G. Erdman, K. S. Schorno, and R. S. Scalan, Phillips Petroleum Company, Bartlesville, Oklahoma INTRODUCTION Eleven core sections from Sites 99 and 0, on DSDP Leg in
. GEOCHEMISTRY OF CARBON: DSDPLEG J. G. Erdman, K. S. Schorno, and R. S. Scalan, Phillips Petroleum Company, Bartlesville, Oklahoma INTRODUCTION Eleven core sections from Sites 99 and 0, on DSDP Leg in
predictive mineral discovery*cooperative Research Centre A legacy for mineral exploration science Mineral Systems Q3 Fluid reservoirs
 Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
CEE 437 Lecture 11 Rock Classification. Thomas Doe
 CEE 437 Lecture 11 Rock Classification Thomas Doe Translation of Mineral Properties to Rock Properties Comparison of mineral properties to rock properties Rocks have lower strength, especially tensile
CEE 437 Lecture 11 Rock Classification Thomas Doe Translation of Mineral Properties to Rock Properties Comparison of mineral properties to rock properties Rocks have lower strength, especially tensile
GEOL Lab 9 (Carbonate Sedimentary Rocks in Hand Sample and Thin Section)
 GEOL 333 - Lab 9 (Carbonate Sedimentary Rocks in Hand Sample and Thin Section) Sedimentary Rock Classification - As we learned last week, sedimentary rock, which forms by accumulation and lithification
GEOL 333 - Lab 9 (Carbonate Sedimentary Rocks in Hand Sample and Thin Section) Sedimentary Rock Classification - As we learned last week, sedimentary rock, which forms by accumulation and lithification
14.2 Ocean Floor Features Mapping the Ocean Floor
 14.2 Ocean Floor Features Mapping the Ocean Floor The ocean floor regions are the continental margins, the ocean basin floor, and the mid-ocean ridge. 14.2 Ocean Floor Features Continental Margins A continental
14.2 Ocean Floor Features Mapping the Ocean Floor The ocean floor regions are the continental margins, the ocean basin floor, and the mid-ocean ridge. 14.2 Ocean Floor Features Continental Margins A continental
2/25/2009. Dolomitization
 Dolomitization A Short Course VU March, 29 www.aoqz76.dsl.pipex.com/ Landscapes.htm Peter Swart University of Miami Dieudonné Sylvain Guy Tancrede de Gratet de Dolomieu usually known as Déodat de Dolomieu
Dolomitization A Short Course VU March, 29 www.aoqz76.dsl.pipex.com/ Landscapes.htm Peter Swart University of Miami Dieudonné Sylvain Guy Tancrede de Gratet de Dolomieu usually known as Déodat de Dolomieu
Question. What caused the recent explosive eruptions of hot ash and gas at Kilauea s Halema uma u crater:
 OCN 201 Deep Sea Sediments Question What caused the recent explosive eruptions of hot ash and gas at Kilauea s Halema uma u crater: A. The interaction of lava with seawater B. Drainage of the lava lake
OCN 201 Deep Sea Sediments Question What caused the recent explosive eruptions of hot ash and gas at Kilauea s Halema uma u crater: A. The interaction of lava with seawater B. Drainage of the lava lake
14. THE CHEMISTRY OF INTERSTITIAL WATERS FROM THE UPPER OCEAN CRUST, SITE 395, DEEP SEA DRILLING PROJECT LEG 78B 1
 14. THE CHEMISTRY OF INTERSTITIAL WATERS FROM THE UPPER OCEAN CRUST, SITE 395, DEEP SEA DRILLING PROJECT LEG 78B 1 Russell E. McDuff, School of Oceanography, University of Washington 2 ABSTRACT Chemical
14. THE CHEMISTRY OF INTERSTITIAL WATERS FROM THE UPPER OCEAN CRUST, SITE 395, DEEP SEA DRILLING PROJECT LEG 78B 1 Russell E. McDuff, School of Oceanography, University of Washington 2 ABSTRACT Chemical
Sedimentology & Stratigraphy. Thanks to Rob Viens for slides
 Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
 26. MIXED-LAYER ILLITE/MONTMORILLONITE CLAYS FROM SITES 146 AND 149 Herman E. Roberson, State University of New York, Binghamton, New York INTRODUCTION The purpose of this report is to describe the clay
26. MIXED-LAYER ILLITE/MONTMORILLONITE CLAYS FROM SITES 146 AND 149 Herman E. Roberson, State University of New York, Binghamton, New York INTRODUCTION The purpose of this report is to describe the clay
1. Base your answer to the following question on The diagram below represents a part of the crystal structure of the mineral kaolinite.
 1. Base your answer to the following question on The diagram below represents a part of the crystal structure of the mineral kaolinite. An arrangement of atoms such as the one shown in the diagram determines
1. Base your answer to the following question on The diagram below represents a part of the crystal structure of the mineral kaolinite. An arrangement of atoms such as the one shown in the diagram determines
21. GEOCHEMICAL ANALYSIS OF SAMPLES FROM SITES 397 AND 398
 21. GEOCHEMICAL ANALYSIS OF SAMPLES FROM SITES 397 AND 398 Daniel B. Pearson, The Superior Company, Houston, Texas and Wallace G. Dow, Getty Company, Houston, Texas 1 INTRODUCTION Economic interest in
21. GEOCHEMICAL ANALYSIS OF SAMPLES FROM SITES 397 AND 398 Daniel B. Pearson, The Superior Company, Houston, Texas and Wallace G. Dow, Getty Company, Houston, Texas 1 INTRODUCTION Economic interest in
Lab 7: Sedimentary Structures
 Name: Lab 7: Sedimentary Structures Sedimentary rocks account for a negligibly small fraction of Earth s mass, yet they are commonly encountered because the processes that form them are ubiquitous in the
Name: Lab 7: Sedimentary Structures Sedimentary rocks account for a negligibly small fraction of Earth s mass, yet they are commonly encountered because the processes that form them are ubiquitous in the
29. IMPLICATIONS OF DEEP SEA DRILLING, SITES 186 AND 187 ON ISLAND ARC STRUCTURE
 29. IMPLICATIONS OF DEEP SEA DRILLING, SITES 186 AND 187 ON ISLAND ARC STRUCTURE John A. Grow 1, Marine Physical Laboratory, Scripps Institution of Oceanography, La Jolla, California INTRODUCTION Pacific
29. IMPLICATIONS OF DEEP SEA DRILLING, SITES 186 AND 187 ON ISLAND ARC STRUCTURE John A. Grow 1, Marine Physical Laboratory, Scripps Institution of Oceanography, La Jolla, California INTRODUCTION Pacific
Weathering Cycle Teacher Notes
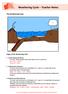 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
Marine Sediments. Introductory Oceanography. Ray Rector: Instructor
 Marine Sediments Introductory Oceanography Ray Rector: Instructor Ocean Basins are Vast Sinks for Huge Amounts of Sediment from Numerous Different Sources Four Major Types of Seafloor Sediments 1. Lithogenous
Marine Sediments Introductory Oceanography Ray Rector: Instructor Ocean Basins are Vast Sinks for Huge Amounts of Sediment from Numerous Different Sources Four Major Types of Seafloor Sediments 1. Lithogenous
I. CALCIUM-CARBONATE AND SAND-FRACTION ANALYSIS OF CENOZOIC AND MESOZOIC SEDIMENTS FROM THE MOROCCAN BASIN
 I. CALCIUM-CARBONATE AND SAND-FRACTION ANALYSIS OF CENOZOIC AND MESOZOIC SEDIMENTS FROM THE MOROCCAN BASIN Marthe Melguen, Centre Océanologique de Bretagne, BP, 9 Brest Cedex, France INODUCTION As the
I. CALCIUM-CARBONATE AND SAND-FRACTION ANALYSIS OF CENOZOIC AND MESOZOIC SEDIMENTS FROM THE MOROCCAN BASIN Marthe Melguen, Centre Océanologique de Bretagne, BP, 9 Brest Cedex, France INODUCTION As the
33. INTERSTITIAL WATER CHEMISTRY IN THE WESTERN MEDITERRANEAN: RESULTS FROM LEG 161 1
 Zahn, R., Comas, M.C., and Klaus, A. (Eds.), 1999 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 161 33. INTERSTITIAL WATER CHEMISTRY IN THE WESTERN MEDITERRANEAN: RESULTS FROM LEG
Zahn, R., Comas, M.C., and Klaus, A. (Eds.), 1999 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 161 33. INTERSTITIAL WATER CHEMISTRY IN THE WESTERN MEDITERRANEAN: RESULTS FROM LEG
PREDICTION OF ACID MINE DRAINAGE POTENTIAL FROM COAL MINES
 PREDICTION OF ACID MINE DRAINAGE POTENTIAL FROM COAL MINES Arthur W. Rose, Professor of Geochemistry Eugene G. Williams, Professor of Geology Richard R. Parizek, Professor of Hydrogeology Acid mine drainage
PREDICTION OF ACID MINE DRAINAGE POTENTIAL FROM COAL MINES Arthur W. Rose, Professor of Geochemistry Eugene G. Williams, Professor of Geology Richard R. Parizek, Professor of Hydrogeology Acid mine drainage
Lecture 11: Non-Carbonate Biogenic and Chemical Sedimentary Rocks
 Lecture 11: Non-Carbonate Biogenic and Chemical Sedimentary Rocks Siliceous Sediments & Chert Phosphorites Evaporites Banded Iron Siliceous Sedimentary Rocks Fine-grained, dense, hard rocks composed predominantly
Lecture 11: Non-Carbonate Biogenic and Chemical Sedimentary Rocks Siliceous Sediments & Chert Phosphorites Evaporites Banded Iron Siliceous Sedimentary Rocks Fine-grained, dense, hard rocks composed predominantly
Ocean Floor. Continental Margins. Divided into 3 major regions. Continental Margins. Ocean Basins. Mid-Ocean Ridges. Include:
 Ocean Floor Divided into 3 major regions Continental Margins Ocean Basins Mid-Ocean Ridges Continental Margins Include: Continental Shelves Continental Slopes Continental Rise 1 Continental Shelves Part
Ocean Floor Divided into 3 major regions Continental Margins Ocean Basins Mid-Ocean Ridges Continental Margins Include: Continental Shelves Continental Slopes Continental Rise 1 Continental Shelves Part
9. DATA REPORT: PORE WATER CHEMICAL AND ISOTOPIC COMPOSITIONS DRILLING PROGRAM SITE FROM THE WEST PHILIPPINE BASIN, OCEAN ABSTRACT
 Shinohara, M., Salisbury, M.H., and Richter, C. (Eds.) Proceedings of the Ocean Drilling Program, Scientific Results Volume 195 9. DATA REPORT: PORE WATER CHEMICAL AND ISOTOPIC COMPOSITIONS FROM THE WEST
Shinohara, M., Salisbury, M.H., and Richter, C. (Eds.) Proceedings of the Ocean Drilling Program, Scientific Results Volume 195 9. DATA REPORT: PORE WATER CHEMICAL AND ISOTOPIC COMPOSITIONS FROM THE WEST
APPENDIX III. COMPOSITION AND SOURCE OF DETRITAL SAND LAYERS FROM THE GUAYMAS BASIN 1
 APPENDIX III. COMPOSITION AND SOURCE OF DETRITAL SAND LAYERS FROM THE GUAYMAS BASIN J. Eduardo Aguayo> instituto Mexicano del Petróleo, Mexico, D.F., Mexico QAL 0 After: Geological chart, Instituto de
APPENDIX III. COMPOSITION AND SOURCE OF DETRITAL SAND LAYERS FROM THE GUAYMAS BASIN J. Eduardo Aguayo> instituto Mexicano del Petróleo, Mexico, D.F., Mexico QAL 0 After: Geological chart, Instituto de
Geology 252, Historical Geology, California State University, Los Angeles - professor: Dr. Alessandro Grippo
 LAB # 1 - CLASTIC ROCKS Background: - Mechanical and Chemical Weathering - Production of Clastic Sediment - Classification of Sediment according to size: Gravel, Sand, Silt, Clay - Erosion, Transportation
LAB # 1 - CLASTIC ROCKS Background: - Mechanical and Chemical Weathering - Production of Clastic Sediment - Classification of Sediment according to size: Gravel, Sand, Silt, Clay - Erosion, Transportation
Marine Sediments EPSS15 Spring 2017 Lab 4
 Marine Sediments EPSS15 Spring 2017 Lab 4 Why Sediments? Record of Earth s history - Tectonic plate movement - Past changes in climate - Ancient ocean circulation currents - Cataclysmic events 1 Classification
Marine Sediments EPSS15 Spring 2017 Lab 4 Why Sediments? Record of Earth s history - Tectonic plate movement - Past changes in climate - Ancient ocean circulation currents - Cataclysmic events 1 Classification
Chapter 9 Lecture Outline. Oceans: The Last Frontier
 Chapter 9 Lecture Outline Oceans: The Last Frontier The Vast World Ocean Earth is referred to as the blue planet 71% of Earth s surface is oceans and marginal seas Continents and islands comprise the remaining
Chapter 9 Lecture Outline Oceans: The Last Frontier The Vast World Ocean Earth is referred to as the blue planet 71% of Earth s surface is oceans and marginal seas Continents and islands comprise the remaining
15. GEOCHEMISTRY OF SEDIMENTS FROM THE EAST PACIFIC RISE AT 23 N!
 . GEOCHEMISTRY OF SEDIMENTS FROM THE EAST PACIFIC RISE AT 2 N! S. A. Moorby, A.G.R.G., Geology Department, Imperial College, London, United Kingdom S. P. Varnavas, Department of Geology, University of
. GEOCHEMISTRY OF SEDIMENTS FROM THE EAST PACIFIC RISE AT 2 N! S. A. Moorby, A.G.R.G., Geology Department, Imperial College, London, United Kingdom S. P. Varnavas, Department of Geology, University of
ROCK CLASSIFICATION AND IDENTIFICATION
 Name: Miramar College Grade: GEOL 101 - Physical Geology Laboratory SEDIMENTARY ROCK CLASSIFICATION AND IDENTIFICATION PRELAB SECTION To be completed before labs starts: I. Introduction & Purpose: The
Name: Miramar College Grade: GEOL 101 - Physical Geology Laboratory SEDIMENTARY ROCK CLASSIFICATION AND IDENTIFICATION PRELAB SECTION To be completed before labs starts: I. Introduction & Purpose: The
Ocean Chemical Dynamics. EAS 2200 Lecture 21
 Ocean Chemical Dynamics EAS 2200 Lecture 21 Summary of Water s H-bonding accounts for high heat capacity, latent heats of fusion and evaporation. important for heat transport, transfer to atmosphere, and
Ocean Chemical Dynamics EAS 2200 Lecture 21 Summary of Water s H-bonding accounts for high heat capacity, latent heats of fusion and evaporation. important for heat transport, transfer to atmosphere, and
The Ocean Floor Chapter 14. Essentials of Geology, 8e. Stan Hatfield and Ken Pinzke Southwestern Illinois College
 The Ocean Floor Chapter 14 Essentials of Geology, 8e Stan Hatfield and Ken Pinzke Southwestern Illinois College The vast world ocean Earth is often referred to as the water planet 71% of Earth s surface
The Ocean Floor Chapter 14 Essentials of Geology, 8e Stan Hatfield and Ken Pinzke Southwestern Illinois College The vast world ocean Earth is often referred to as the water planet 71% of Earth s surface
 UNIT DESCRIPTIONS: Artificial Fill, Undocumented (Afu): Locally derived sandy silt and silty sand, locally with clay and varying amounts of gravel and man-made debris. Abundant concrete rubble, in places
UNIT DESCRIPTIONS: Artificial Fill, Undocumented (Afu): Locally derived sandy silt and silty sand, locally with clay and varying amounts of gravel and man-made debris. Abundant concrete rubble, in places
Quartz or opaline silica solubility
 Quartz or opaline silica solubility The simplest process that might regulate the concentration of an element in solution is equilibrium with respect to a solid phase containing the element as a major component.
Quartz or opaline silica solubility The simplest process that might regulate the concentration of an element in solution is equilibrium with respect to a solid phase containing the element as a major component.
CORED SITE 941 HOLE A. Graphic Lith. Section Age. Disturb. Sample. Meter. Color. Description. Structure. 10YR 5/3 To 2.
 SITE 94 HOLE A eter mm : _ 3_ 4_ = α tv.v.v. a πj IΦ ----- ] Section Age a a a\ :.... 3 Holocene CORE {3 (3 {3 Disturb H Sample I Color 0YR 5/3. 6/ 5/ 4/ 5GY CORED 0.0-5.3 CALCAREOUS CLAY and CLAY mbsf
SITE 94 HOLE A eter mm : _ 3_ 4_ = α tv.v.v. a πj IΦ ----- ] Section Age a a a\ :.... 3 Holocene CORE {3 (3 {3 Disturb H Sample I Color 0YR 5/3. 6/ 5/ 4/ 5GY CORED 0.0-5.3 CALCAREOUS CLAY and CLAY mbsf
STUDENT SOIL PRESENTATIONS
 STUDENT SOIL PRESENTATIONS Soil Order (and informal name) Student Name(s) Alfisol = deciduous forest soil Andisol = formed on volcanic ash Aridisol = desert soil Entisol = alluvium soil Gelisol = tundra
STUDENT SOIL PRESENTATIONS Soil Order (and informal name) Student Name(s) Alfisol = deciduous forest soil Andisol = formed on volcanic ash Aridisol = desert soil Entisol = alluvium soil Gelisol = tundra
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Activity and Concentration
 Activity and Concentration Activity effective concentration Ion-ion and ion-h 2 O interactions (hydration shell) cause number of ions available to react chemically ("free" ions) to be less than the number
Activity and Concentration Activity effective concentration Ion-ion and ion-h 2 O interactions (hydration shell) cause number of ions available to react chemically ("free" ions) to be less than the number
Diagenetic processes in the Cenozoic sedimentary formations associated with the Chicxulub Impact Crater, northwestern Yucatan Peninsula, Mexico
 The Second International Conference on Saltwater Intrusion and Coastal Aquifers Monitoring, Modeling, and Management. Mérida, Yucatán, México, March 30 - April 2, 2003 Diagenetic processes in the Cenozoic
The Second International Conference on Saltwater Intrusion and Coastal Aquifers Monitoring, Modeling, and Management. Mérida, Yucatán, México, March 30 - April 2, 2003 Diagenetic processes in the Cenozoic
Marine Sediments Chapter Four Chapter Overview Marine Sediments Approaching the bottom (Alvin) Marine Sediments Classification of Marine Sediments
 1 2 3 4 5 6 7 8 9 10 11 Marine Sediments Chapter Four Chapter Overview Marine sediments contain a record of Earth history. Marine sediments provide many important resources. Marine sediments have origins
1 2 3 4 5 6 7 8 9 10 11 Marine Sediments Chapter Four Chapter Overview Marine sediments contain a record of Earth history. Marine sediments provide many important resources. Marine sediments have origins
The Nature of Sedimentary Rocks
 The Nature of Sedimentary Rocks Sedimentary rocks are composed of: Fragments of other rocks Chemical precipitates Organic matter or biochemically produced materials The Nature of Sedimentary Rocks Sedimentary
The Nature of Sedimentary Rocks Sedimentary rocks are composed of: Fragments of other rocks Chemical precipitates Organic matter or biochemically produced materials The Nature of Sedimentary Rocks Sedimentary
Rockall Plateau. OCN 201: Shelf Sediments
 Rockall Plateau OCN 201: Shelf Sediments Classification by Size Classification by Mode of Formation Detrital sediments Transported and deposited as particles Derived from weathering of pre-existing rocks
Rockall Plateau OCN 201: Shelf Sediments Classification by Size Classification by Mode of Formation Detrital sediments Transported and deposited as particles Derived from weathering of pre-existing rocks
Lab: Metamorphism: minerals, rocks and plate tectonics!
 Introduction The Earth s crust is in a constant state of change. For example, plutonic igneous rocks are exposed at the surface through uplift and erosion. Many minerals within igneous rocks are unstable
Introduction The Earth s crust is in a constant state of change. For example, plutonic igneous rocks are exposed at the surface through uplift and erosion. Many minerals within igneous rocks are unstable
Lecture 26: Marine Geology Read: Chapter 21 Homework due December 3
 Learning Objectives (LO) Lecture 26: Marine Geology Read: Chapter 21 Homework due December 3 What we ll learn today:! 1. Describe the world s five oceans! 2. Understand patterns of ocean circulation! 3.
Learning Objectives (LO) Lecture 26: Marine Geology Read: Chapter 21 Homework due December 3 What we ll learn today:! 1. Describe the world s five oceans! 2. Understand patterns of ocean circulation! 3.
Minerals and Rocks Chapter 20
 Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
1. are most likely to study the images sent back from Mars. A. Astronomers B. Geologists C. Doctors D. Engineers
 1. are most likely to study the images sent back from Mars. A. Astronomers B. Geologists C. Doctors D. Engineers 2. When did the Earth form? A. About 540 million years ago B. About 2.5 billion years ago
1. are most likely to study the images sent back from Mars. A. Astronomers B. Geologists C. Doctors D. Engineers 2. When did the Earth form? A. About 540 million years ago B. About 2.5 billion years ago
APPENDIX VI. GEOCHEMISTRY OF CARBON: DSDP LEGS 22, 24, 26, 27, AND 28
 APPENDIX VI. GEOCHEMISTRY OF CARBON: DSDP LEGS, 4, 6, 7, AND 8 J. G. Erdman, K. S. Schorno, and R. S. Scanlan, Phillips Petroleum Company, Bartlesville, Oklahoma INTRODUCTION A total of 8 samples from
APPENDIX VI. GEOCHEMISTRY OF CARBON: DSDP LEGS, 4, 6, 7, AND 8 J. G. Erdman, K. S. Schorno, and R. S. Scanlan, Phillips Petroleum Company, Bartlesville, Oklahoma INTRODUCTION A total of 8 samples from
Sedimentary Environments Chapter 8
 Sedimentary Environments Chapter 8 Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. What is a sedimentary rock? Sedimentary rocks are products of
Sedimentary Environments Chapter 8 Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. What is a sedimentary rock? Sedimentary rocks are products of
Your teacher will show you a sample or diagram of each, and show you a settling column. Draw these, and label your diagrams (8 pts) Ungraded:
 From Sand to Stone: How do we recognize and interpret sedimentary rocks in the rock record? (Based closely on the University of Washington ESS 101 Lab 5: Sedimentary Rocks) Introduction: This lab consists
From Sand to Stone: How do we recognize and interpret sedimentary rocks in the rock record? (Based closely on the University of Washington ESS 101 Lab 5: Sedimentary Rocks) Introduction: This lab consists
2/16/2014. Chapter Overview. Marine Sediments. Approaching the bottom (Alvin) Classification of Marine Sediments. Marine Sediments
 Chapter Overview Marine sediments contain a record of Earth history. Marine sediments provide many important resources. Marine sediments have origins from a variety of sources. Marine Sediments Chapter
Chapter Overview Marine sediments contain a record of Earth history. Marine sediments provide many important resources. Marine sediments have origins from a variety of sources. Marine Sediments Chapter
Lecture Outline Wednesday - Friday February 14-16, 2018
 Lecture Outline Wednesday - Friday February 14-16, 2018 Quiz 2 scheduled for Friday Feb 23 (Interlude B, Chapters 6,7) Questions? Chapter 6 Pages of the Past: Sedimentary Rocks Key Points for today Be
Lecture Outline Wednesday - Friday February 14-16, 2018 Quiz 2 scheduled for Friday Feb 23 (Interlude B, Chapters 6,7) Questions? Chapter 6 Pages of the Past: Sedimentary Rocks Key Points for today Be
Emily and Megan. Earth System Science. Elements of Earth by weight. Crust Elements, by weight. Minerals. Made of atoms Earth is mostly iron, by weight
 Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Hydrological Cycle Rain and rivers OUTLINE
 Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
17. OVERVIEW OF INTERSTITIAL FLUID AND SEDIMENT GEOCHEMISTRY, SITES (BAHAMAS TRANSECT) 1
 Swart, P.K., Eberli, G.P., Malone, M.J., and Sarg, J.F. (Eds.), 2 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 166 17. OVERVIEW OF INTERSTITIAL FLUID AND SEDIMENT GEOCHEMISTRY, SITES
Swart, P.K., Eberli, G.P., Malone, M.J., and Sarg, J.F. (Eds.), 2 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 166 17. OVERVIEW OF INTERSTITIAL FLUID AND SEDIMENT GEOCHEMISTRY, SITES
Log of Monitoring Well D58B
 Project: Motiva - Monitoring Well and Soil Boring Data Project Location: Delaware City Refinery Project Number: 20240412.W1000 Log of Monitoring Well D58B Sheet 1 of 7 Date(s) Drilled Drilling Method Drill
Project: Motiva - Monitoring Well and Soil Boring Data Project Location: Delaware City Refinery Project Number: 20240412.W1000 Log of Monitoring Well D58B Sheet 1 of 7 Date(s) Drilled Drilling Method Drill
Review - Unit 2 - Rocks and Minerals
 Review - Unit 2 - Rocks and Minerals Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral.
Review - Unit 2 - Rocks and Minerals Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral.
Chapter 1: Acoustic and elastic properties of calcareous sediments siliceous diagenetic front on the eastern U.S.
 1 Chapter 1: Acoustic and elastic properties of calcareous sediments siliceous diagenetic front on the eastern U.S. continental slope across a 1.1 Abstract The Ocean Drilling Program drilled Hole 904A
1 Chapter 1: Acoustic and elastic properties of calcareous sediments siliceous diagenetic front on the eastern U.S. continental slope across a 1.1 Abstract The Ocean Drilling Program drilled Hole 904A
I. Earth spheres A. Three major spheres 1. atmosphere, thin envelope 2. hydrosphere covers more than 71% of surface 3. geosphere from hydrosphere to
 I. Earth spheres A. Three major spheres 1. atmosphere, thin envelope 2. hydrosphere covers more than 71% of surface 3. geosphere from hydrosphere to center 4. Biosphere penetrates all three, a. only thin
I. Earth spheres A. Three major spheres 1. atmosphere, thin envelope 2. hydrosphere covers more than 71% of surface 3. geosphere from hydrosphere to center 4. Biosphere penetrates all three, a. only thin
organisms CaCO 3 + H 2 O + CO 2 shallow water
 Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Crust Elements. Elements of Earth. Minerals. Crystals. Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air
 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
IODP EXPEDITION 306: NORTH ATLANTIC CLIMATE II SITE U1314 SUMMARY
 IODP EXPEDITION 306: NORTH ATLANTIC CLIMATE II SITE U1314 SUMMARY Hole U1314A Latitude: 56 21.883'N, Longitude: 27 53.309'W Hole U1314B Latitude: 56 21.896'N, Longitude: 27 53.311'W Hole U1314C Latitude:
IODP EXPEDITION 306: NORTH ATLANTIC CLIMATE II SITE U1314 SUMMARY Hole U1314A Latitude: 56 21.883'N, Longitude: 27 53.309'W Hole U1314B Latitude: 56 21.896'N, Longitude: 27 53.311'W Hole U1314C Latitude:
6th Grade Science Sample Assessment Items S6E3c.
 Composition 6th Grade Science Sample Assessment Items Ocean water differs from freshwater in that it has. A. a lower temperature B. a higher temperature C. a higher concentration of silicon dioxide D.
Composition 6th Grade Science Sample Assessment Items Ocean water differs from freshwater in that it has. A. a lower temperature B. a higher temperature C. a higher concentration of silicon dioxide D.
Lecture 13. Hydrothermal Circulation
 Lecture 13. Hydrothermal Circulation The discovery of hot springs on the ocean floor during the 1970s was one of the most exciting events in the history of oceanography. Although hydrothermal activity
Lecture 13. Hydrothermal Circulation The discovery of hot springs on the ocean floor during the 1970s was one of the most exciting events in the history of oceanography. Although hydrothermal activity
Downloaded 11/20/12 to Redistribution subject to SEG license or copyright; see Terms of Use at
 AVO crossplot analysis in unconsolidated sediments containing gas hydrate and free gas: Green Canyon 955, Gulf of Mexico Zijian Zhang* 1, Daniel R. McConnell 1, De-hua Han 2 1 Fugro GeoConsulting, Inc.,
AVO crossplot analysis in unconsolidated sediments containing gas hydrate and free gas: Green Canyon 955, Gulf of Mexico Zijian Zhang* 1, Daniel R. McConnell 1, De-hua Han 2 1 Fugro GeoConsulting, Inc.,
Seismic stratigraphy, some examples from Indian Ocean, interpretation of reflection data in interactive mode
 Seismic stratigraphy, some examples from Indian Ocean, interpretation of reflection data in interactive mode K. S. Krishna National Institute of Oceanography, Dona Paula, Goa-403 004. krishna@nio.org Seismic
Seismic stratigraphy, some examples from Indian Ocean, interpretation of reflection data in interactive mode K. S. Krishna National Institute of Oceanography, Dona Paula, Goa-403 004. krishna@nio.org Seismic
Global phosphorus cycle
 Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
Samples Studied and Methods
 . MINERALOGIC STUDIES OF SEDIMENTS FROM THE NORWEGIANGREENLAND SEA (SITES, 33, 3, 3, AND 3) Edward A. Perry, Jr., Stephen J. Grady, and William M. Kelly, Department of Geology. University of Massachusetts,
. MINERALOGIC STUDIES OF SEDIMENTS FROM THE NORWEGIANGREENLAND SEA (SITES, 33, 3, 3, AND 3) Edward A. Perry, Jr., Stephen J. Grady, and William M. Kelly, Department of Geology. University of Massachusetts,
Petroleum Potential of the Application Area L12-4
 Petroleum Potential of the Application Area L12-4 The Application Area (L12-4) is underlain by the western Officer Basin, beneath the Gunbarrel Basin. The general basin architecture is outlined in Figure
Petroleum Potential of the Application Area L12-4 The Application Area (L12-4) is underlain by the western Officer Basin, beneath the Gunbarrel Basin. The general basin architecture is outlined in Figure
17. TRACE ELEMENT DISTRIBUTION IN DSDP SITES 372, 374, 375, AND 376 IN THE MEDITERRANEAN SEA
 17. TRACE ELEMENT DISTRIBUTION IN DSDP SITES 372, 374, 375, AND 376 IN THE MEDITERRANEAN SEA Francis Coumes and Cyrille Boltenhagen, Société Nationale des Pétroles d'aquitaine, Pau, France INTRODUCTION
17. TRACE ELEMENT DISTRIBUTION IN DSDP SITES 372, 374, 375, AND 376 IN THE MEDITERRANEAN SEA Francis Coumes and Cyrille Boltenhagen, Société Nationale des Pétroles d'aquitaine, Pau, France INTRODUCTION
Small area of the ocean that is partially surrounded by land. The Ocean Basins. Three Major Oceans. Three Major Oceans. What is a SEA?
 The Ocean Basins How Deep is the Ocean? 1 2 Three Major Oceans Three Major Oceans Pacific Atlantic the shallowest ocean (3.3km average depth) Indian second shallowest ocean (3.8km average depth) Pacific
The Ocean Basins How Deep is the Ocean? 1 2 Three Major Oceans Three Major Oceans Pacific Atlantic the shallowest ocean (3.3km average depth) Indian second shallowest ocean (3.8km average depth) Pacific
Sci.tanta.edu.eg PALEOECOLOGY, GE 2218
 Sci.tanta.edu.eg PALEOECOLOGY, GE 2218 Lec. 4 1 Biosphere Lithosphere Community Hydrosphere Atmosphere 2 1 Temperature Temperature range in the ocean is approximately 2 to 40 º C. Coldest waters are found
Sci.tanta.edu.eg PALEOECOLOGY, GE 2218 Lec. 4 1 Biosphere Lithosphere Community Hydrosphere Atmosphere 2 1 Temperature Temperature range in the ocean is approximately 2 to 40 º C. Coldest waters are found
