A Guide to. Limestone Products. Selecting. Agricultural. Goodwin. Jonathan H. /V-6S: IMN 73. ILLINOIS MINERAL NOTE 73 November 1979
|
|
|
- Lorraine Booker
- 5 years ago
- Views:
Transcription
1 /V-6S: ex IMN 73 CLJS*^] ILLINOIS MINERAL NOTE 73 November 1979 A Guide to Agricultural Selecting Limestone Products Jonathan H. Goodwin ILLINOIS STATE GEOLOGICAL SURVEY Jack A. Simon, Chief Urbana, II
2 Goodwin, Jonathan H A guide to selecting agricultural limestone products / by Jonathan H. Goodwin. -- Urbana : Illinois State Geological Survey, November p. : ill. ; 28cm. -- (Illinois Mineral Note Geological Survey ; 73) References: p Illinois State 1. Limestone. I. Title. II. Series. ILLINOIS STATE GEOLOGICAL SURVEY
3 A Guide to Agricultural Selecting Limestone Products Jonathan H. Goodwin ABSTRACT Application of agricultural limestone or dolomite is an important means of maintaining soil acidity at levels that are best for maximum economical crop production. Illinois farmers apply more agricultural limestone per acre than any other farmers in the United States. To select the best agricultural limestone product, farmers must consider such geologic, agronomic, and economic factors as the chemical composition of the stone, the particle size of the crushed limestone product, and the cost per ton of purchasing and transporting the agricultural limestone. The calculated effective neutralizing value (I'NV) of a limestone involves the effects of both particle size and chemical composition on the acid-neutralizing capacity of the stone. KNV calculations provide an easy means of comparing one agricultural limestone product with another. To objectively select the best agricultural limestone to use in a particular case, careful cost comparisons that consider all factors must be made. INTRODUCTION Crushed limestone and dolomite for agricultural applications are major industrial mineral commodities in Illinois. Illinois farmers apply more agricultural limestone per acre than any other farmers in the United States. In 1976, of the 61.8 million tons of crushed stone produced in Illinois, more than 10 percent, 6.4 million tons, was agricultural limestone or dolomite. In 1977, the state of Illinois ranked second in the United States in total production of crushed stone and produced 7 percent of the total United States output (U.S. Bureau of Mines, 1978). Limestone and dolomite are quarried in about 60 of the 1 02 counties in Illinois. For many of the smaller operators, agricultural limestone or dolomite constitutes a major proportion of total annual production. The purpose of this note is to provide farmers with some basic facts about limestone and dolomite and agricultural limestone products in order to make it easier for them to select the best product to suit their particular needs. The question of which quarry product is the best in a particular area often has no easy answer. As will be shown, geologic, agronomic, and economic factors all affect any judgement of the best product for use on a particular farm. It is not the intent of this publication to supply the detailed information necessary to prepare a management program for agricultural liming. The local Agricultural Extension Service advisor can provide much more complete information on the effects of liming on yields, the influence of soil type on limestone requirements, and the overall economics of regular lime applications. The University of Illinois Cooperative Extension Service at Urbana- Champaign has several publications available on the use and correct application of agricultural limestone products. The Agronomy Handbook (University of Illinois Cooperative Extension Service, , pages 29-57) has an extensive treatment of soil testing and fertility, and Agronomy Facts, SF-79 (Tucker and Peck, 1972), discusses the importance and effects of application of agricultural limestone and dolomite. Portions of these publications have been quoted in this note. CARBONATE ROCKS Agricultural limestone and dolomite are quarried and crushed from rocks called carbonate rocks because of their chemical composition. In Illinois, carbonate rocks are sedimentary rocks originally deposited on the bottoms of shallow seas that repeatedly covered the area. The major constituents of these rocks are the whole and fragmented shells of animals and plants that lived in these oceans several hundreds of millions of years ago. Like the shells found along the ocean beaches of today, the shells of these ancient organisms were made mostly of the mineral calcite, having the chemical composition calcium carbonate (CaC0 3 ). Sedimentary rocks made mostly of the mineral calcite are called limestone. Most carbonate rocks found in the central and southern thirds of Illinois are limestone. Under some circumstances, chemical reactions in limestone can alter its chemical and mineral composition. From a rock that is originally wholly calcite (CaC0 3 ), the chemical reaction can make a rock that consists entirely of the mineral dolomite, having the chemical composition calcium magnesium carbonate (CaMg(C0 3 ) 2 ). a Most carbonate rocks in the northern third of Illinois are made of dolomite. Such rocks made mostly of the mineral dolomite GUIDE TO SELECTING AGRICULTURAL LIMESTONE PRODUCTS
4 are called dolomite or dolostone. Throughout this note, the term dolomite will be used to refer to the rock; if t he mineral dolomite is the substance being discussed, the distinction will be made clear in the sentence. Mineral grains with chemical compositions intermediate between those of pure calcite and pure dolomite are almost unknown in ancient carbonate rocks. But carbonate rocks do occur that are mixtures of grains of calcite and dolomite. On a larger scale, carbonate rocks mined in a quarry may consist of interlayered beds of limestone and dolomite rocks, or patches of dolomite surrounded by limestone, or vice versa. Quarry operators rarely bother to separate such layers or patches of different kinds of rock; instead, they market a mixed product. For convenience, geologists use the following terms for such mixed carbonate rocks: limestone: dolomitic limestone: calcareous dolomite: dolomite: to IMPURITIES 10% dolomite 10 to 50% dolomite 50 to 90% dolomite 90 to 100% dolomite The carbonate minerals in a limestone or dolomite do the work of neutralizing soil acidity. Impurities decrease the acid-neutralizing capacity of a rock by diluting the amount of carbonate mineral per unit volume of rock. Because limestone and dolomite form as accumulations of sediment on the bottom of the ocean, sediment grains other than carbonate minerals are the most common impurities. As is the case with the oceans of today, the principal contaminants found in ancient carbonate rocks are quartz sand and silt and clay minerals eroded from the land and transported to the sea by rivers. Because carbonate rocks are made largely of the whole and fragmented shells of marine animals and plants, it not unexpected that some limestones and dolomites contain small amounts of organic matter. Although rarely present in amounts greater than about 5 percent, organicmatter can change the color of a limestone or dolomite. Color and impurities The color of a carbonate rock can give only a vague indication of its purity. A perfectly pure limestone or dolomite should be a brilliant, paper-white color, but even small amounts of some impurities can drastically alter the color. The degree of color change depends on both the amount and the nature of the impurities in the rock. Among the impurities that can affect the color of a carbonate rock to varying degrees are organic matter, silt and clay minerals, finely divided iron sulfide minerals, and iron oxide or hydroxide minerals. Even small amounts of organic matter can drastically darken the color of a carbonate rock. Contamination with clay minerals may also result in some darkening of the color is of the rock, but much more clay contamination is required to achieve the same amount of darkening as a very small amount of organic matter. Thus, two carbonate rocks of almost the same color may have widely differing carbonate contents because the color of the one is caused by organic matter and of the other by clay mineral contamination. Iron sulfide minerals such as pyrite can occur in some limestones and dolomites. Pyrite is commonly associated with organic matter. When the grains of pyrite in a limestone or dolomite are extremely small and scattered throughout the rock, they can impart a pronounced dark gray or black coloi to the rock. Small amounts of both pyrite and organic matter can turn a limestone or dolomite almost black. Carbonate rocks are often somewhat porous and permeable, allowing ground water to pass through them. Ground water can transport small amounts of iron oxide into the rock or can cause oxidation of iron-bearing minerals already in the rock. Such iron oxide or hydroxide minerals can result in a uniform or blotchy, orange or yellow stain. It should be noted that, of all the possible coloring contaminants in carbonate rocks, only clay minerals and silt result in substantial contamination of the carbonate content of the rock. The fact that one quarry produces a whiter-looking agricultural limestone product than another is no indication that the whiter product is more effective in neutralizing soil acidity. As will be shown, there are many factors that affect the acid-neutralizing capacity and efficiency of agricultural limestone products. The only way to be certain of choosing the most effective product is by examining and working with the chemical analysis and other characteristics of the products using the methods outlined in this note. EVALUATING LIMESTONES Calcium carbonate equivalent Because limestone and dolomite are applied to agricultural acreage to neutralize excess soil acidity, it is important to be able to compare the theoretical acid-neutralizing capacities of various products. A useful figure for making this comparison is the calcium carbonate equivalent or CCE. This number expresses the acid-neutralizing capacity of a carbonate rock relative to the capacity of pure calcium carbonate (calcite). An absolutely pure limestone (all calcite) has a CCE of 100 percent. When measured relative to calcite, dolomite has a greater acid-neutralizing capacity per unit of weight because of the difference between the atomic weights of calcium (40) and magnesium (24). Pure dolomite has a CCE of percent, but the greater value is essentially the result of the method of calculation. Dolomite should not automatically be considered more effective than limestone for agricultural purposes solely because of its higher CCE value. As will be shown, there are other factors to consider. ILLINOIS STATE GEOLOGICAL SURVEY/ILLINOIS MINERALS NOTE 73
5 Calculating CCE values Particle size If the analysis of a carbonate rock does not list a CCE value for the stone, the value can be calculated from the following: CCE = (wt% CaC0 3 ) + (I. 187 x wt% MgCOJ. If the analysis does not list values for CaC0 3 and MgC0 3, but instead lists values for CaO, MgO, and C0 2, the CCE can be calculated from the following: CCE = ( x wt% CaO) + (2.483 x wt% MgO). Some care should be exercised in evaluating and comparing CCE values of various products. Although a pure dolomite has a CCE value higher than a pure calcite, dolomite is more slowly soluble in acid than calcite. Thus, an impure dolomite may take considerably longer to neutralize the same amount of acid as an impure, nondolomitic limestone having the same CCE value. For comparison, the relative amounts of dolomite and limestone required for similar results in a 2- to 4-year period are shown in figure 1. The figure shows that dolomite, unless finely ground, may be much less efficient than limestone in rapidly neutralizing soil acidity (Tucker and Peck, 1972, p. 9). Although there are no specific legal standards for minimum CCE values of agricultural limestone and dolomite, impurities that decrease the CCE value below about 80 percent are undesirable for either construction or agricultural purposes. Such rocks are quarried and sold only where no other satisfactory sources exist in the area. The particle size of an agricultural limestone or dolomite product strongly affects the rate at which the product dissolves to neutralize soil acidity. The coarsest particles, those retained on an 8-mesh U.S. Standard sieve, have little immediate effect on soil acidity because they may take several years to dissolve. Particles in the range of 10 mesh to 28 mesh are only 14 percent as effective as particles finer than 100 mesh in neutralizing soil acidity in a given period of time (Tucker and Peck, 1972, p. 9). Table 1 solution for various size compiles the results of some studies of rates of fractions of an agricultural limestone. Even after 8 years, only 25 percent of the particles more than 8 mesh in size have dissolved. Because the coarsest particles contribute little to the acid-neutralizing capacity of the product, additional limestone must be applied to compensate. TABLE 1. Weight percent of limestone dissolved in several size Size fraction Passing 60 mesh 30 to 60 mesh 8 to 30 mesh Retained on 8 mesh fractions in 1,4, and 8 years after application to soil (from Aldrich, 1961). Years a fter application Table 2 shows the average particle-size distributions DOLOMITE of agricultural limestone products at five east-central Illinois quarries. Only one of the quarries crushes a product in which more than 80 percent of the particles pass a number 8 sieve. As will be shown, the effects of particle size on rates of dissolution can be compensated for when determining the amount of a particular product to use for a particular application. TABLE 2. Particle-size analyses of agricultural limestone products from selected quarries in east-central Illinois. HI Weight percent through 60 mesh 100 ISGS 1979 figure 1. Tons per aere of limestone and dolomite of equivalent partiele size required to give equivalent neutralizing of soil acidity over a 2-to 4-s ear period. Quarry number 8 mesh Weight percent passing 30 mesh 60 mesh Values arc averages of 8- to 10-sieve analyses of samples collected monthly from stockpiles. GUIDE TO SELECTING AGRICULTURAL LIMESTONE PRODUCTS
6 . Fineness efficiency The amounts of each size fraction dissolved in 1 year, as shown in table 1, can be used as efficiency factors to determine the relative efficiencies of various agricultural limestone products (University of Illinois Cooperative Extension Service, , p. 31). Table 3 shows the particle-size analyses of the five east-central Illinois quarries from table 2 recalculated for the same particle-size intervals as those given in table 1. The weight percent of the stone in each particle-size interval, when multiplied by the corresponding 1-year efficiency factor selected from table 1, gives a number called a fineness efficiency factor. The sum of the fineness efficiency factors calculated for a particular agricultural limestone product gives the total fineness efficiency of the product. As an illustration of this calculation, the total fineness efficiency of the product from Quarry 3 has been determined in table 4. TABLE 3. Size fraction Particle-size analyses of agricultural limestone products from five selected quarries in east-central in a Illinois. Quarry number Passing 60 mesh to 60 mesh to 30 mesh Retained on 8 mesh Caculated from average values in table 2. duct must be applied to give the same acid neutralizing effect as another (University of Illinois Cooperative Extension Service, , p ). The effective neutralizing value (ENV) of an agricultural limestone product is determined by multiplying the CCE value of the stone by the total fineness efficiency. Samples from Quarry 3, for example, have an average CCE of 92 percent. So, the ENV of the stone from Quarry 3 is x 0.92 = percent. Comparing application rates Figure 2 shows the charts used by the University of Illinois Cooperative Extension Service to determine the application rates for agricultural limestone. The charts take into account the general soil type, the soil acidity or ph, and the cropping system. A 9-inch plowing depth is assumed. Applications rates from these charts apply to a stone having a total fineness efficiency of 51.5 and a CCE of 90 percent. Thus, the ENV of the typical limestone assumed for the charts is percent. To calculate the application rate for a stone having a different ENV, due predominantly to a difference of fineness efficiency, divide the ENV of that stone into the ENV of the typical stone of the chart, and multiply the result by the tonnage rate originally determined from the chart. For example, for grain farming in soil type C and an initial soil ph of 5.5, Chart 1 of figure 2 indicates an application rate of 3 tons per acre of typical limestone to bring the soil ph to about 6. If stone from Quarry 3 of table 4 is to be used, then the calculated tonnage multiplier would be: TABLE 4. Calculation of fineness efficiency of limestone from Quarry 3 of table 3. a ENV of Chart 1 ENV of Quarry % 30.30% = 1.53 Weight % Efficiency Fineness Size fraction 100 factor efficiency Passing 60 mesh to 60 mesh to 30 mesh Retained on 8 mesh Total fineness efficiency Efficiency factor is from 1-year values of table 1 Effective neutralizing value The effective neutralizing value is perhaps the ultimate tool for making comparisons among various agricultural limestone products. This calculated percentage combines the fineness efficiency of the stone and the calcium carbonate equivalent. The number can be used to calculate how much more or less of one agricultural limestone pro- Multiplying this tonnage multiplier by the tonnage rate of 3 tons per acre originally determined from Chart 1 gives an application rate of 4.59 tons per acre for stone from Quarry 3 to produce the same acid-neutralizing effect as the typical stone of the charts. Because limestone or dolomite ground to a fineness comparable to that of the typical stone of the charts in figure 2 is expensive to produce, a premium price will therefore be asked for such a product. As will be shown, however, the cost of higher application rates for a coarser stone can be less than the cost of importing a smaller amount of higher-priced, finer-ground stone from some distance away. Careful cost comparisons should govern the choice of stone to use for agricultural liming. ECONOMIC CONSIDERATIONS When stone for liming is purchased from a nearby quarry (no more than about 35 miles distant) the cost of the stone is the dominant expense. Commercial ILLINOIS STATE GEOLOGICAL SURVEY/ILLINOIS MINERALS NOTE 73
7 CHART I CHART II GRAIN FARMING SYSTEMS A By 8 7 CROPPING SYSTEMS WITH ALFALFA, CLOVER OR LESPEDEZA C Bj C D 6 5 * 4 o None needed if naturally ph 6.2 ' or above N D j E Application is optional Application 1 is optional ph ph Slightly Moderately Strongly Slighty Moderately Strongly acid acid acid acid acid acid STEPS TO FOLLOW 1. Use Chart I for grain systems, Chart II for alfalfa, clover, or lespedeza. 2. Decide which soil class fits your soil: A. Silty clays and silty clay loams (dark). B. Silty clays and silty clay loams (light and medium). Silt and clay loams (dark). C. Silt and clay loams (light and medium). Sandy loams (dark). Loams (dark and medium). D. Loams (light). Sandy loams (light and medium). All sands. E. Muck and peat. 3. Find your soil's ph along the bottom of the chart. 4. Follow up the vertical line until it intersects the diagonal line A, B, C, D, or E that fits your soil. 5. Read the suggested rate of limestone along the right side. ISGS 1979 Figure 2. Charts for determining tonnage of limestone or dolomite necessary to maintain soil acidity at recommended levels for maximum production for the two main cropping systems of Illinois. trucking rates for hauling less than 5,000 tons of stone are about equal to, or less than, the cost of the stone for hauls of up to 35 miles. For longer hauls, the trucking rates for each ton of stone are higher than the cost per ton of the stone at the quarry (FOB price). Hauls across state lines may be even more costly than those within a state because of the differing rate structures in interstate commerce. To demonstrate the influence of transportation costs and to illustrate the use of effective neutralizing values (ENV) in comparing the costs of agricultural limestone products, four cases will be outlined below. Let us say that a farmer has learned from soil testing that he should apply limestone having an ENV of percent at an average rate of 5 tons per acre to his 500 acres of corn and soybean ground. A local quarry about 35 miles from the farm sells a stone that has an ENV of 30 percent, primarily because of its coarseness. A more distant quarry, about 80 miles from the farm, sells a stone having a CCE almost equal to the local quarry, but the ENV of this stone is 40 percent because of its higher total fineness efficiency value. Because of the increased cost of fine grinding, the distant quarry charges a premium price GUIDE TO SELECTING AGRICULTURAL LIMESTONE PRODUCTS
8 . for its agricultural limestone. The farmer evaluated the two cases in the following way: : ENV correction is = Case 1 Local Quarry, 30% ENV So, x 5 tons /acre gives an application rate of tons/acre. Price of limestone is S3. 75 per ton for sales of 1,000 tons or more tons/acre x 500 acres = tons <s> S3. 75 per ton = $14, Commerical trucking rates are S3. 02 per ton for a 35-mile one-way loaded haul to a single dump point. $3.02 per ton x 3,865 tons - $1 1, Total delivered cost of stone = $26, Case 2 Distant Quarry. 40% ENV ENV correction is So, 1.16 x 5 tons/acre gives an application rate of 5.8 tons /acre. Price of limestone is $4.25 per ton. 5.8 tons /acre x 500 acres = $4.25 per ton = $12, Commercial trucking rates are $5.78 per ton for an 80 mile one-way loaded haul to a single dump point. $5. 78 per ton x 2,900 tons = $16, Total delivered cost of stone = $29, Although the price of the stone at the distant quarry is higher than that at the local quarry, the lower tonnage requirements of the higher ENV stone result in a lower cost for the stone at the distant quarry. However, the much greater cost of transporting the stone from the distant quarry increases the total delivered cost of the stone by almost $3,000 over that from the local quarry. Stone from the distant quarry would have to have an ENV of more than percent to bring the total delivered cost down to that of the local quarry. The farmer also calculated the cost of transporting the stone in his own 20-ton spreader truck. He estimates that it costs him about 3 cents per ton-mile to operate the truck. To transport the 3,865 tons of stone from the local quarry would require loads, each requiring a round trip of 70 miles at a cost of $0.60 per mile (20 tons x 3 cents per ton-mile). The total haulage cost would thus be $8, ( loads x 70 miles x $0.60 per mile). This, added to the price of the stone at the quarry, gives a total delivered cost of $22, for the stone. For stone from the distant quarry, the 2,900 tons of stone would require 145 round trips of 160 miles at $0.60 per mile, or a cost of $13, Total delivered cost of the stone from the distant quarry would then be $26, This exceeds the cost of the stone from the local quarry by almost $4,000. Only if the stone from the distant quarry had an ENV of percent, that of a "'typical limestone" from the charts in figure 2, would its total delivered cost equal that of the local quarry stone. Although these cases provide only a partial cost analysis, they do illustrate the strong influence of transportation charges on the total delivered cost of agricultural limestone. Costs of transportation can outweigh the total cost of the stone at the quarry. Given two quarries an equal distance from a farm, the limestone having the higher ENV will certainly be less expensive to transport because of the lower application rate and, therefore, lower total tonnage of stone required. However, a higher price charged for stone of higher ENV may make it more expensive, overall, than stone of lower ENV and lower price. Careful cost analysis, including transportation costs, is the best way to ensure choosing the most economical product for use in liming agricultural fields. BENEFITS OF LIMING Application of agricultural limestone or dolomite is an important means of maintaining soil acidity at levels that are best for maximum economical crop production. The University of Illinois Cooperative Extension Service lists the following benefits from maintaining soil acidity at recommended levels by regular liming of fields: 1 Manganese and aluminum, which may be available in amounts great enough to be toxic in acid soils, are much less soluble and less available in soils with near-neutral acidity. 2. The micro-organisms that speed the decay of plant materials, thereby releasing nitrogen and phosphorus into the soil, grow more rapidly and operate more effectively in neutral to slightly acid soils. 3. Growth of nodule-forming, nitrogen-fixing bacteria on alfalfa, clover, and soybeans is favored in neutral to only slightly acid soils. 4. The minor elements essential for plant growth have the best balance of availability in neutral to slightly acid soils. 5. Phosphorus is more available in neutral to slightly acid soils than in strongly acid soils. Under prolonged production, Illinois soils tend to become strongly acid unless limestone or dolomite is applied at regular intervals. Annual application of large amounts of nitrogen (more than 150 pounds per acre) can cause severe increases of soil acidity. Acidification of soil by nitrogen application is caused by both direct and indirect effects. Plants take up nitrogen from the soil in the ILLINOIS STATE GEOLOGICAL SURVEY/ILLINOIS MINERALS NOTE 73
9 form of soluble nitrate ions. When nitrogen is applied to the soil in the form of anhydrous ammonia (NH 3 ), ammonium nitrate (NH 4 N0 3 ), ammonium sulfate ((NH 4 ) 2 S0 4 ), or urea (H 2 NCONH 2 ), the released ammonia is converted to nitrate ions (N0 3 ) in the soil. This releases hydrogen ions (H ) that increase the soil acidity. Indirectly, the increased plant growth caused by the nitrogen application takes up greater amounts of the base ions calcium (Ca ), magnesium (Mg ) and potassium (K ), thus relatively enriching the soil in hydrogen ions. Greater plant growth also increases the production of carbonic acid (H 2 C0 3 ) from plant respiration. Figure 3 shows the effects of the addition of various amounts of nitrogen in various forms to the Pullman clay loam soil. Figure 3. Five years of annual nitrogen application caused the ph change shown for a Pullman clay loam soil. AS = ammonium sulfate AIM = ammonium nitrate U = urea AA = anhydrous ammonium Effect o(' nitrogen application on soil ph. ISGS 1979 pound of nitrogen supplied to the soil by anhydrous ammonia, ammonium nitrate, and urea fertilizers. For ammonium sulfate applications, the amounts of limestone required are even higher. CONCLUSIONS 1. Outward appearance, especially color, of agricultural limestone or dolomite is a poor indicator of the quality and effectiveness of a product for liming agricultural fields. 2. The particle-size distribution of an agricultural limestone or dolomite product has a strong influence on the effectiveness of the product for neutralizing excess soil acidity. Coarser particles may take years to dissolve and therefore contribute little to the neutralizing process. 3. When the particle-size distribution of an agricultural limestone product is known, efficiency factors can be used to calculate a total fineness efficiency value that is useful for examining the influence of particle-size distributions of various products. 4. The calcium carbonate equivalent (CCE) can be easily calculated from chemical analyses of carbonate rocks and provides a satisfactory means of comparing agricultural limestone products when grain-size distributions are unknown. 5. The effective neutralizing value (ENV) of an agricultural limestone combines total fineness efficiency value and the calcium carbonate equivalent to provide a highly useful tool for comparing various agricultural limestone products. 6. Careful economic analysis, taking into account the differing ENV of the limestone and the varying costs of transportation, should be the major method used select the best product for agricultural liming. ACKNOWLEDGMENTS The manuscript of this note was reviewed by Dr. Theodore R. Peck, Professor of Soil Chemistry and Extension, University of Illinois, and others at the Cooperative Extension Service. The author would like to thank them for their useful comments and information. The publication was greatly improved by their efforts. to On the ph scale, a value of 7 is neutral. Values greater than 7 are considered basic and values less than 7 are acidic. In figure 3, although almost all the ph values are greater than 7, the application of nitrogen causes the ph of soil to decrease from more basic to less basic values, indicating increasing acidity of the soil. The most drastic effects are found when applying ammonium sulfate fertilizers. Regular applications of agricultural limestone or dolomite can prevent this increase of acidity. A common rule of thumb used by the Cooperative Extension Service is that 3 to 4 pounds of agricultural limestone should be aplied for each REFERENCES Aldrich, S. R., 1961, Limestone tor more profitable tanning: University of Illinois Cooperative Extension Service, Circular 721 (revised), 8 p. Tucker, B. B., and T. R. Peek, 1972, Agronomy tacts, Si -79: University of Illinois Cooperative I xtension Service, 6 p. U.S. Bureau of Mines, 1978, Minerals in the economy of Illinois: State Mineral Profiles, SMP-42, Pittsburgh. PA, 10 p. University of Illinois Cooperative Extension Service, , Agronomy handbook: Circular , p GUIDE TO SELECTING AGRICULTURAL LIMESTONE PRODUCTS (2000 1/80) Printed by authority of State of Illinois
10 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign
11 MINERAL ECONOMICS BRIEFS SERIES (in print) 5. Summary of Illinois Mineral Production in Shipments of Illinois Crushed Stone, Mineral Resources and Mineral Industries of the East St. Louis Region, Illinois Mineral Resources and Mineral Industries of the Extreme Southern Illinois Region Mineral Resources and Mineral Industries of the Springfield Region, Illinois Mineral Resources and Mineral Industries of the Western Illinois Region Mineral Resources and Mineral Industries of the Northwestern Illinois Region Mineral Resources and Mineral Industries of the Northeastern Illinois Region Directory of Illinois Mineral Producers INDUSTRIAL MINERALS NOTES SERIES (in print) 13. Summary of Illinois Mineral Industry, Pelletizing Illinois Fluorspar Binding Materials Used in Making Pellets and Briquets Chemical Composition of Some Deep Limestones and Dolomites in Livingston County, Illinois Illinois Natural Resources-An Industrial Development Asset Limestone Resources of Jefferson and Marion Counties, Illinois Thermal Expansion of Certain Illinois Limestones High-Purity Limestones in Illinois Gay and Shale Resources of Clark, Crawford, Cumberland, Edgar, Effingham, Jasper, and Vermilion Counties Lightweight Bricks Made with Clay and Expanded Plastic Clays as Binding Materials Silica Sand Briquets and Pellets As a Replacement for Quartzite Computer-Calculated Lambert Conformal Conic Projection Tables for Illinois (7.5-Minute Intersections) Kankakee Dune Sands As a Commercial Source of Feldspar Alumina Content of Carbonate Rocks As an Index to Sodium Sulfate Soundness Colloidal-Size Silica Produced from Southern Illinois Tripoli Two-Dimensional Shape of Sand Made by Crushing Illinois Limestones of Different Textures An Investigation of Sands on the Uplands Adjacent to the Sangamon River Floodplain: Possibilities As a "Blend Sand" Resource Lower Mississippi River Terrace Sands As a Commercial Source of Feldspar Clay and Shale Resources of Madison, Monroe, and St. Clair Counties, Illinois ILLINOIS MINERALS NOTES SERIES (in print) (The Illinois Minerals Notes Series continues the Industrial Minerals Notes Series and incorporates the Mineral Economics Briefs Series) 49. Clay and Shale Resources of Peoria and Tazewell Counties, Illinois By-Product Gypsum in Illinois A New Resource? Coal Resources of Illinois Properties of Carbonate Rocks Affecting Soundness of Aggregate- A Progress Report The Energy Crisis and Its Potential Impact on the Illinois Clay Products Industry Commercial Feldspar Resources in Southeastern Kankakee County, Illinois Electric Utility Plant Flue-Gas Desulfurization: A Potential New Market for Lime, Limestone, and Other Carbonate Materials The Distribution and Physical Properties of Chert Gravel in Pike County, Illinois Factors Responsible for Variation in Productivity of Illinois Coal Mines Behavior of Coal Ash in Gasification Beds of Ignifiuid Boilers Place of Coal in the Total Energy Needs of the United States Directory of Illinois Mineral Producers, Illinois Coal: Development Potential Illinois Mineral Industry in Market Potential for Coals of the Illinois Basin Illinois Mineral Industry in 1975 and Review of Preliminary Mineral Production Data for Industrial Minerals Publications of the Illinois State Geological Survey, through December Illinois Mineral Industry in 1976 and Review of Preliminary Mineral Production Data for Abundance and Recovery of Sphalerite and line Coal from Mine Waste in Illinois Roof Strata of the Herrin (No. 6) Coal Member in Mines of Illinois: Their Geology and Stability
12
ph and Liming Practices Kent Martin Stafford County 1/5/2010
 ph and Liming Practices Kent Martin Stafford County 1/5/2010 Outline What is ph Normal ph ranges Acid Soil Importance of soil ph Factors affecting soil ph Acid types and measurement Neutralizing value
ph and Liming Practices Kent Martin Stafford County 1/5/2010 Outline What is ph Normal ph ranges Acid Soil Importance of soil ph Factors affecting soil ph Acid types and measurement Neutralizing value
UmllluIfiW, SfOLOGICAL SURVEY
 UmllluIfiW, SfOLOGICAL SURVEY 3 3051 00005 8770 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/heavymineralsini01shro s
UmllluIfiW, SfOLOGICAL SURVEY 3 3051 00005 8770 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/heavymineralsini01shro s
Sedimentology & Stratigraphy. Thanks to Rob Viens for slides
 Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Sediment and sedimentary rocks Sediment
 Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
Be sure to show all calculations so that you can receive partial credit for your work!
 Agronomy 365T Exam 1 Spring 2004 Exam Score: Name TA Lab Hour Be sure to show all calculations so that you can receive partial credit for your work! 1) List 14 of the plant essential nutrient for plant
Agronomy 365T Exam 1 Spring 2004 Exam Score: Name TA Lab Hour Be sure to show all calculations so that you can receive partial credit for your work! 1) List 14 of the plant essential nutrient for plant
UNIT 4 SEDIMENTARY ROCKS
 UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
Geology 229 Engineering Geology. Lecture 6. Basic Rock Classification and Engineering Considerations (West, Chs. 2, 3, 4, 5)
 Geology 229 Engineering Geology Lecture 6 Basic Rock Classification and Engineering Considerations (West, Chs. 2, 3, 4, 5) Outline of this Lecture 1. Rock types and rock cycle 2. Geological and engineering
Geology 229 Engineering Geology Lecture 6 Basic Rock Classification and Engineering Considerations (West, Chs. 2, 3, 4, 5) Outline of this Lecture 1. Rock types and rock cycle 2. Geological and engineering
Sediment. Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface
 Sediment Some basic terminology Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface Erosion: removal of weathered rock and minerals from one place to
Sediment Some basic terminology Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface Erosion: removal of weathered rock and minerals from one place to
What is a sedimentary rock?
 Sedimentary Rocks What is a sedimentary rock? Sedimentary rocks are products of mechanical and chemical weathering They account for only 5% of the top 10 miles of the outer crust, yet most of the earth
Sedimentary Rocks What is a sedimentary rock? Sedimentary rocks are products of mechanical and chemical weathering They account for only 5% of the top 10 miles of the outer crust, yet most of the earth
2 Aggregates in Indiana
 2 Aggregates in Indiana Origin of Aggregates Gravel and Natural Sands Crushed Stone Slag Distribution of Aggregates Glacial Deposits Bedrock Deposits Aggregate Types Natural Aggregates Artificial Aggregates
2 Aggregates in Indiana Origin of Aggregates Gravel and Natural Sands Crushed Stone Slag Distribution of Aggregates Glacial Deposits Bedrock Deposits Aggregate Types Natural Aggregates Artificial Aggregates
EPS 50 Lab 4: Sedimentary Rocks
 Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Sedimentary Environments Chapter 8
 Sedimentary Environments Chapter 8 Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. What is a sedimentary rock? Sedimentary rocks are products of
Sedimentary Environments Chapter 8 Does not contain complete lecture notes. To be used to help organize lecture notes and home/test studies. What is a sedimentary rock? Sedimentary rocks are products of
ROCK CLASSIFICATION AND IDENTIFICATION
 Name: Miramar College Grade: GEOL 101 - Physical Geology Laboratory SEDIMENTARY ROCK CLASSIFICATION AND IDENTIFICATION PRELAB SECTION To be completed before labs starts: I. Introduction & Purpose: The
Name: Miramar College Grade: GEOL 101 - Physical Geology Laboratory SEDIMENTARY ROCK CLASSIFICATION AND IDENTIFICATION PRELAB SECTION To be completed before labs starts: I. Introduction & Purpose: The
Sedimentary Rocks. Weathering. Mechanical & Chemical Weathering. Sediments. Lithification. Deposition. Transport. Erosion.
 Lithification Sedimentary Rocks Sediments Deposition Transport Erosion Weathering Weathering The sediments that make up sedimentary rocks are produced by: Mechanical & Chemical Weathering Mechanical Weathering
Lithification Sedimentary Rocks Sediments Deposition Transport Erosion Weathering Weathering The sediments that make up sedimentary rocks are produced by: Mechanical & Chemical Weathering Mechanical Weathering
PREDICTION OF ACID MINE DRAINAGE POTENTIAL FROM COAL MINES
 PREDICTION OF ACID MINE DRAINAGE POTENTIAL FROM COAL MINES Arthur W. Rose, Professor of Geochemistry Eugene G. Williams, Professor of Geology Richard R. Parizek, Professor of Hydrogeology Acid mine drainage
PREDICTION OF ACID MINE DRAINAGE POTENTIAL FROM COAL MINES Arthur W. Rose, Professor of Geochemistry Eugene G. Williams, Professor of Geology Richard R. Parizek, Professor of Hydrogeology Acid mine drainage
Lecture Outline Wednesday - Friday February 14-16, 2018
 Lecture Outline Wednesday - Friday February 14-16, 2018 Quiz 2 scheduled for Friday Feb 23 (Interlude B, Chapters 6,7) Questions? Chapter 6 Pages of the Past: Sedimentary Rocks Key Points for today Be
Lecture Outline Wednesday - Friday February 14-16, 2018 Quiz 2 scheduled for Friday Feb 23 (Interlude B, Chapters 6,7) Questions? Chapter 6 Pages of the Past: Sedimentary Rocks Key Points for today Be
Sedimentary Rocks, our most Valuable Rocks. Or, what you will probably find when you are outdoors exploring.
 Sedimentary Rocks, our most Valuable Rocks Or, what you will probably find when you are outdoors exploring. Sedimentary rocks give us evidence to earth s earlier history. We look at processes happening
Sedimentary Rocks, our most Valuable Rocks Or, what you will probably find when you are outdoors exploring. Sedimentary rocks give us evidence to earth s earlier history. We look at processes happening
Sedimentary Rocks Chapter 6
 Sedimentary Rocks Chapter 6 I. What is a sedimentary rock? A. Sedimentary rock 1) Rock made of detrital sediments (such as sand) or inorganic/organic chemical precipitates (such as calcite) 2) Detrital
Sedimentary Rocks Chapter 6 I. What is a sedimentary rock? A. Sedimentary rock 1) Rock made of detrital sediments (such as sand) or inorganic/organic chemical precipitates (such as calcite) 2) Detrital
The physical breakdown and chemical alteration of rocks and minerals at or near Earth s surface.
 The physical breakdown and chemical alteration of rocks and minerals at or near Earth s surface. The material that is chemically and mechanically weathered to yield sediment and soil. Regolith consisting
The physical breakdown and chemical alteration of rocks and minerals at or near Earth s surface. The material that is chemically and mechanically weathered to yield sediment and soil. Regolith consisting
Fossils and Geology of Litzsinger Road Ecology Center
 Fossils and Geology of Litzsinger Road Ecology Center Table of Content Key Terms 1 Key Terms 2 What you need to know Geologic Map of Missouri Geologic Time Chart More of what you need to know Digital map
Fossils and Geology of Litzsinger Road Ecology Center Table of Content Key Terms 1 Key Terms 2 What you need to know Geologic Map of Missouri Geologic Time Chart More of what you need to know Digital map
A rock is a naturally occurring solid mixture of one or more minerals, or organic matter
 A rock is a naturally occurring solid mixture of one or more minerals, or organic matter Rocks are classified by how they are formed, their composition, and texture Rocks change over time through the rock
A rock is a naturally occurring solid mixture of one or more minerals, or organic matter Rocks are classified by how they are formed, their composition, and texture Rocks change over time through the rock
Sediment and Sedimentary rock
 Sediment and Sedimentary rock Sediment: An accumulation of loose mineral grains, such as boulders, pebbles, sand, silt or mud, which are not cemented together. Mechanical and chemical weathering produces
Sediment and Sedimentary rock Sediment: An accumulation of loose mineral grains, such as boulders, pebbles, sand, silt or mud, which are not cemented together. Mechanical and chemical weathering produces
Aggregates for Concrete
 Fine Aggregate Sand and/or crushed stone < 5 mm (0.2 in.) F.A. content usually 35% to 45% by mass or volume of total aggregate Coarse Aggregate Gravel and crushed stone 5 mm (0.2 in.) typically between
Fine Aggregate Sand and/or crushed stone < 5 mm (0.2 in.) F.A. content usually 35% to 45% by mass or volume of total aggregate Coarse Aggregate Gravel and crushed stone 5 mm (0.2 in.) typically between
Sediments and Sedimentary Rocks
 Sediments and Sedimentary Rocks (Shaping Earth s Surface, Part 2) Science 330 Summer 2005 What is a sedimentary rock? Products of mechanical and chemical weathering Account for about 5 percent of Earth
Sediments and Sedimentary Rocks (Shaping Earth s Surface, Part 2) Science 330 Summer 2005 What is a sedimentary rock? Products of mechanical and chemical weathering Account for about 5 percent of Earth
Minerals and Rocks Chapter 20
 Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Liz LaRosa Images from Geology.com unless otherwise noted
 Liz LaRosa http://www.middleschoolscience.com 2010 Images from Geology.com unless otherwise noted A rock is a naturally occurring solid mixture of one or more minerals, or organic matter Rocks are classified
Liz LaRosa http://www.middleschoolscience.com 2010 Images from Geology.com unless otherwise noted A rock is a naturally occurring solid mixture of one or more minerals, or organic matter Rocks are classified
Emily and Megan. Earth System Science. Elements of Earth by weight. Crust Elements, by weight. Minerals. Made of atoms Earth is mostly iron, by weight
 Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Gain a better understanding of soil ph and how it is measured. Understand how lime requirement is determined.
 LABORATORY 7 SOIL REACTION (ph) AND LIME REQUIREMENT I Objectives Gain a better understanding of soil ph and how it is measured. Understand how lime requirement is determined. II Introduction A Soil Reaction
LABORATORY 7 SOIL REACTION (ph) AND LIME REQUIREMENT I Objectives Gain a better understanding of soil ph and how it is measured. Understand how lime requirement is determined. II Introduction A Soil Reaction
CLASS EXERCISE 5.1 List processes occurring in soils that cause changes in the levels of ions.
 5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
GEOL Lab 9 (Carbonate Sedimentary Rocks in Hand Sample and Thin Section)
 GEOL 333 - Lab 9 (Carbonate Sedimentary Rocks in Hand Sample and Thin Section) Sedimentary Rock Classification - As we learned last week, sedimentary rock, which forms by accumulation and lithification
GEOL 333 - Lab 9 (Carbonate Sedimentary Rocks in Hand Sample and Thin Section) Sedimentary Rock Classification - As we learned last week, sedimentary rock, which forms by accumulation and lithification
How to Identify and Properly Classify Drill Cuttings
 How to Identify and Properly Classify Drill Cuttings (Creating Useful Borehole Logs) Dave Larson Hydrogeology and Geophysics Section Accurate information about the borehole location and a careful description
How to Identify and Properly Classify Drill Cuttings (Creating Useful Borehole Logs) Dave Larson Hydrogeology and Geophysics Section Accurate information about the borehole location and a careful description
Your teacher will show you a sample or diagram of each, and show you a settling column. Draw these, and label your diagrams (8 pts) Ungraded:
 From Sand to Stone: How do we recognize and interpret sedimentary rocks in the rock record? (Based closely on the University of Washington ESS 101 Lab 5: Sedimentary Rocks) Introduction: This lab consists
From Sand to Stone: How do we recognize and interpret sedimentary rocks in the rock record? (Based closely on the University of Washington ESS 101 Lab 5: Sedimentary Rocks) Introduction: This lab consists
EROSION, DEPOSITION AND SEDIMENTARY ROCKS. Reading: Earth Science Tarbuck and Lutgens Chapter 5: pages Chapter 3: pages 52-54, 61-69
 EROSION, DEPOSITION AND SEDIMENTARY ROCKS Reading: Earth Science Tarbuck and Lutgens Chapter 5: pages 124-133 Chapter 3: pages 52-54, 61-69 Base Level Resistant bed Resistant bed creates a local base level
EROSION, DEPOSITION AND SEDIMENTARY ROCKS Reading: Earth Science Tarbuck and Lutgens Chapter 5: pages 124-133 Chapter 3: pages 52-54, 61-69 Base Level Resistant bed Resistant bed creates a local base level
Clastic Textures. I. What is the sorting of sample numbers 60, 61, and 62? Answers on last page.
 Sed Rock s Sel f-instruction N ame Geology 100 Harbor Secti on Sedimentary rocks are usually identified in the field by their stratification or layering, which originates by the successive deposition of
Sed Rock s Sel f-instruction N ame Geology 100 Harbor Secti on Sedimentary rocks are usually identified in the field by their stratification or layering, which originates by the successive deposition of
List of Equipment, Tools, Supplies, and Facilities:
 Unit D: ph of Soil Lesson 2: Identifying ph Connection With Plant Growth Student Learning Objectives: Instruction in this lesson should result in the students achieving the following objectives: 1. Explain
Unit D: ph of Soil Lesson 2: Identifying ph Connection With Plant Growth Student Learning Objectives: Instruction in this lesson should result in the students achieving the following objectives: 1. Explain
Name Class Date. In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements.
 CHAPTER 5 Igneous Rocks SECTION 5.1 What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. basaltic
CHAPTER 5 Igneous Rocks SECTION 5.1 What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. basaltic
Bowen s Chemical Stability Series
 Lab 5 - Identification of Sedimentary Rocks Page - Introduction Sedimentary rocks are the second great rock group. Although they make up only a small percentage of the rocks in the earth s crust (~5%)
Lab 5 - Identification of Sedimentary Rocks Page - Introduction Sedimentary rocks are the second great rock group. Although they make up only a small percentage of the rocks in the earth s crust (~5%)
Chapter 6. Weathering, Erosion, and Soil
 Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Report on samples from the Great Basin Science Sample and Records Library
 Jonathan G. Price, Ph.D. State Geologist and Director Nevada Bureau of Mines and Geology Office telephone: 775-784-6691 extension 5 1664 North Virginia Street Home telephone: 775-329-8011 University of
Jonathan G. Price, Ph.D. State Geologist and Director Nevada Bureau of Mines and Geology Office telephone: 775-784-6691 extension 5 1664 North Virginia Street Home telephone: 775-329-8011 University of
Weathering, Soil, and Mass Movements
 Tarbuck Lutgens Weathering, Soil, and Mass Movements 5.1 Weathering Mechanical Weathering Mechanical weathering occurs when physical forces break rock into smaller and smaller pieces without changing the
Tarbuck Lutgens Weathering, Soil, and Mass Movements 5.1 Weathering Mechanical Weathering Mechanical weathering occurs when physical forces break rock into smaller and smaller pieces without changing the
Chapter 10. Chapter Rocks and the Rock Cycle. Rocks. Section 1 Rocks and the Rock Cycle
 Chapter 10 Rocks 1 Chapter 10 Section 1 Rocks and the Rock Cycle 2 10.1 Rocks and the Rock Cycle Magma is the parent material for all rocks. Once the magma cools and hardens, many changes can occur. Geology:
Chapter 10 Rocks 1 Chapter 10 Section 1 Rocks and the Rock Cycle 2 10.1 Rocks and the Rock Cycle Magma is the parent material for all rocks. Once the magma cools and hardens, many changes can occur. Geology:
LAB 2 IDENTIFYING MATERIALS FOR MAKING SOILS: ROCK AND PARENT MATERIALS
 LAB 2 IDENTIFYING MATERIALS FOR MAKING SOILS: ROCK AND PARENT MATERIALS Learning outcomes The student is able to: 1. understand and identify rocks 2. understand and identify parent materials 3. recognize
LAB 2 IDENTIFYING MATERIALS FOR MAKING SOILS: ROCK AND PARENT MATERIALS Learning outcomes The student is able to: 1. understand and identify rocks 2. understand and identify parent materials 3. recognize
For Sale - 56 Acre Sandstone Quarry State Route 511, Oberlin/Kipton, OH 44075
 14647 State Route 511, Oberlin/Kipton, OH 44075 56 ACRES Vermilion Rd Gifford Rd Haigh Rd 44 Summary The Kipton sandstone quarry is included in 56 acre tract owned by Terry A. Johnson, of Huron, Ohio.
14647 State Route 511, Oberlin/Kipton, OH 44075 56 ACRES Vermilion Rd Gifford Rd Haigh Rd 44 Summary The Kipton sandstone quarry is included in 56 acre tract owned by Terry A. Johnson, of Huron, Ohio.
A. IGNEOUS Rocks formed by cooling and hardening of hot molten rock called magma (within crust or at its surface).
 EARTH SCIENCE 11 CHAPTER 5 NOTES KEY How Earth's Rocks Were Formed Early geologists believed that the physical features of the Earth were formed by sudden spectacular events called CATASTROPHES. Modern
EARTH SCIENCE 11 CHAPTER 5 NOTES KEY How Earth's Rocks Were Formed Early geologists believed that the physical features of the Earth were formed by sudden spectacular events called CATASTROPHES. Modern
Engineering Geology ECIV 2204
 Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (6) : Sedimentary Rocks Chapter 6: Sedimentary Rocks Chapter 6: Sedimentary Rocks Origin and nature of sedimentary rocks: Sedimentary
Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (6) : Sedimentary Rocks Chapter 6: Sedimentary Rocks Chapter 6: Sedimentary Rocks Origin and nature of sedimentary rocks: Sedimentary
Igneous Rocks. Sedimentary Rocks. Metamorphic Rocks
 Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
Rocks Rock- A group of minerals, glass, mineroid bound together in some way.
 Rocks Rock- A group of minerals, glass, mineroid bound together in some way. All rocks fit into one of three categories: Igneous- formed by the cooling and hardening of hot molten rock Sedimentary- formed
Rocks Rock- A group of minerals, glass, mineroid bound together in some way. All rocks fit into one of three categories: Igneous- formed by the cooling and hardening of hot molten rock Sedimentary- formed
This document downloaded from vulcanhammer.net vulcanhammer.info Chet Aero Marine
 This document downloaded from vulcanhammer.net vulcanhammer.info Chet Aero Marine Don t forget to visit our companion site http://www.vulcanhammer.org Use subject to the terms and conditions of the respective
This document downloaded from vulcanhammer.net vulcanhammer.info Chet Aero Marine Don t forget to visit our companion site http://www.vulcanhammer.org Use subject to the terms and conditions of the respective
Soil Mechanics/Geotechnical Engineering I Prof. Dilip Kumar Baidya Department of Civil Engineering Indian Institute of Technology, Kharagpur
 Soil Mechanics/Geotechnical Engineering I Prof. Dilip Kumar Baidya Department of Civil Engineering Indian Institute of Technology, Kharagpur Lecture - 01 Rock Cycle Good morning. I welcome you to this
Soil Mechanics/Geotechnical Engineering I Prof. Dilip Kumar Baidya Department of Civil Engineering Indian Institute of Technology, Kharagpur Lecture - 01 Rock Cycle Good morning. I welcome you to this
Sedimentary Rocks. Origin, Properties and Identification. Geology Laboratory GEOL 101 Lab Ray Rector - Instructor
 Sedimentary Rocks Origin, Properties and Identification Geology Laboratory GEOL 101 Lab Ray Rector - Instructor Sedimentary Rock Origin and Identification Lab Pre-Lab Internet Link Resources 1) http://www.rockhounds.com/rockshop/rockkey/index.html
Sedimentary Rocks Origin, Properties and Identification Geology Laboratory GEOL 101 Lab Ray Rector - Instructor Sedimentary Rock Origin and Identification Lab Pre-Lab Internet Link Resources 1) http://www.rockhounds.com/rockshop/rockkey/index.html
Chapter 6 Pages of Earth s Past: Sedimentary Rocks
 Chapter 6 Pages of Earth s Past: Sedimentary Rocks Introduction! Drilling into the bottom of the North Sea, we encounter: " Soft mud and loose sand, silt, pebbles, and shells. Then: " Similar materials
Chapter 6 Pages of Earth s Past: Sedimentary Rocks Introduction! Drilling into the bottom of the North Sea, we encounter: " Soft mud and loose sand, silt, pebbles, and shells. Then: " Similar materials
13. Sedimentary Rocks I (p )
 13. Sedimentary Rocks I (p. 194-208) Sediment Deposition Weathering results in rock being broken down into smaller fragments, called regolith. This regolith is then broken down to form soil. The regolith
13. Sedimentary Rocks I (p. 194-208) Sediment Deposition Weathering results in rock being broken down into smaller fragments, called regolith. This regolith is then broken down to form soil. The regolith
2 Igneous Rock. How do igneous rocks form? What factors affect the texture of igneous rock? BEFORE YOU READ. Rocks: Mineral Mixtures
 CHAPTER 4 2 Igneous Rock SECTION Rocks: Mineral Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: How do igneous rocks form? What factors affect the texture
CHAPTER 4 2 Igneous Rock SECTION Rocks: Mineral Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: How do igneous rocks form? What factors affect the texture
Weathering Cycle Teacher Notes
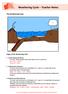 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
GEOL.3250 Geology for Engineers Sedimentary & Metamorphic Rocks
 GEOL.3250 Geology for Engineers Sedimentary & Metamorphic Rocks Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give
GEOL.3250 Geology for Engineers Sedimentary & Metamorphic Rocks Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give
Lab 7: Sedimentary Structures
 Name: Lab 7: Sedimentary Structures Sedimentary rocks account for a negligibly small fraction of Earth s mass, yet they are commonly encountered because the processes that form them are ubiquitous in the
Name: Lab 7: Sedimentary Structures Sedimentary rocks account for a negligibly small fraction of Earth s mass, yet they are commonly encountered because the processes that form them are ubiquitous in the
GEOL Lab #11 Information (Guidelines for Student Soil Presentations on April 8)
 GEOL 333 - Lab #11 Information (Guidelines for Student Soil Presentations on April 8) Assignment During Lab on April 8, you will give an oral presentation about one of the 12 soil orders (categories).
GEOL 333 - Lab #11 Information (Guidelines for Student Soil Presentations on April 8) Assignment During Lab on April 8, you will give an oral presentation about one of the 12 soil orders (categories).
Sedimentary Rocks. Origin, Properties and Identification. Physical Geology GEOL 100. Ray Rector - Instructor
 Sedimentary Rocks Origin, Properties and Identification Physical Geology GEOL 100 Ray Rector - Instructor Sedimentary Rock Origin and Identification Lab Pre-Lab Internet Link Resources 1) http://www.rockhounds.com/rockshop/rockkey/index.html
Sedimentary Rocks Origin, Properties and Identification Physical Geology GEOL 100 Ray Rector - Instructor Sedimentary Rock Origin and Identification Lab Pre-Lab Internet Link Resources 1) http://www.rockhounds.com/rockshop/rockkey/index.html
STUDENT SOIL PRESENTATIONS
 STUDENT SOIL PRESENTATIONS Soil Order (and informal name) Student Name(s) Alfisol = deciduous forest soil Andisol = formed on volcanic ash Aridisol = desert soil Entisol = alluvium soil Gelisol = tundra
STUDENT SOIL PRESENTATIONS Soil Order (and informal name) Student Name(s) Alfisol = deciduous forest soil Andisol = formed on volcanic ash Aridisol = desert soil Entisol = alluvium soil Gelisol = tundra
Marine Sediments. Introductory Oceanography. Ray Rector: Instructor
 Marine Sediments Introductory Oceanography Ray Rector: Instructor Ocean Basins are Vast Sinks for Huge Amounts of Sediment from Numerous Different Sources Four Major Types of Seafloor Sediments 1. Lithogenous
Marine Sediments Introductory Oceanography Ray Rector: Instructor Ocean Basins are Vast Sinks for Huge Amounts of Sediment from Numerous Different Sources Four Major Types of Seafloor Sediments 1. Lithogenous
Geotechnical Engineering I CE 341
 Geotechnical Engineering I CE 341 What do we learn in this course? Introduction to Geotechnical Engineering (1) Formation, Soil Composition, Type and Identification of Soils (2) Soil Structure and Fabric
Geotechnical Engineering I CE 341 What do we learn in this course? Introduction to Geotechnical Engineering (1) Formation, Soil Composition, Type and Identification of Soils (2) Soil Structure and Fabric
Crust Elements. Elements of Earth. Minerals. Crystals. Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air
 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Name: Grade: GEOL Physical Geology Laboratory Sedimentaryand Metamorphic Rocks Lab #6
 Name: GEOL 101 - Physical Geology Laboratory Sedimentaryand Metamorphic Rocks Lab #6 Grade: PRELAB SECTION To be completed before labs starts: I. Introduction & Purpose: The purpose of this laboratory
Name: GEOL 101 - Physical Geology Laboratory Sedimentaryand Metamorphic Rocks Lab #6 Grade: PRELAB SECTION To be completed before labs starts: I. Introduction & Purpose: The purpose of this laboratory
1. Gravel-size 2. Sand-size 3. Silt-size 4. Clay-size 5. Microcrystalline 6. Macrocrystalline
 Name: GEOL 101 - Physical Geology Lab Grade: SEDIMENTARY & METAMORPHIC ROCK CLASSIFICATION and IDENTIFICATION SEDIMENTARY PRE-ID SECTION To be completed before observing hand samples: I. Introduction &
Name: GEOL 101 - Physical Geology Lab Grade: SEDIMENTARY & METAMORPHIC ROCK CLASSIFICATION and IDENTIFICATION SEDIMENTARY PRE-ID SECTION To be completed before observing hand samples: I. Introduction &
GLG Chapter 7 Sedimentary Environments & Rocks
 GLG 101 - Chapter 7 Sedimentary Environments & Rocks Name Note, Oct 11: I ll be writing this study sheet over the next few days. Each day I will add questions until the entire chapter is done, hopefully
GLG 101 - Chapter 7 Sedimentary Environments & Rocks Name Note, Oct 11: I ll be writing this study sheet over the next few days. Each day I will add questions until the entire chapter is done, hopefully
Tell me what the word aggregate means and at least three things aggregate is used to make.
 Lesson Plan Scout s Geology Objective: After today s lesson, you will be able to Tell me what the word aggregate means and at least three things aggregate is used to make. List the steps in the mining
Lesson Plan Scout s Geology Objective: After today s lesson, you will be able to Tell me what the word aggregate means and at least three things aggregate is used to make. List the steps in the mining
Minerals and Rocks. Environmental Learning Community CORC 1332 Sept 21, 2010
 Minerals and Rocks Environmental Learning Community CORC 1332 Sept 21, 2010 Outline Quiz More on minerals Twinkies Rocks How can you identify one mineral from another? Distinguishing One Mineral from Another
Minerals and Rocks Environmental Learning Community CORC 1332 Sept 21, 2010 Outline Quiz More on minerals Twinkies Rocks How can you identify one mineral from another? Distinguishing One Mineral from Another
Lecture # 02 DEPARTMENT OF CIVIL ENGINEERING SWEDISH COLLEGE OF ENGINEERING & TECHNOLOGY, WAH CANTT. 14th December,
 Lecture # 02 DEPARTMENT OF CIVIL ENGINEERING SWEDISH COLLEGE OF ENGINEERING & TECHNOLOGY, WAH CANTT Instructor: Date: Engr. Imran Mehmood 14th December, 2011 1 SEDIMENTARY ROCKS SEDIMENTARY ROCKS The rocks
Lecture # 02 DEPARTMENT OF CIVIL ENGINEERING SWEDISH COLLEGE OF ENGINEERING & TECHNOLOGY, WAH CANTT Instructor: Date: Engr. Imran Mehmood 14th December, 2011 1 SEDIMENTARY ROCKS SEDIMENTARY ROCKS The rocks
Sedimentary rocks. Sedimentary rocks form from pre existing rock. igneous, metamorphic or. sedimentary.
 Sedimentary Rocks 1 Sedimentary rocks Sedimentary rocks form from pre existing rock particles: igneous, metamorphic or sedimentary. 2 3 The parent rock undergoes: Sedimentary rocks WEATHERING TRANSPORTATION
Sedimentary Rocks 1 Sedimentary rocks Sedimentary rocks form from pre existing rock particles: igneous, metamorphic or sedimentary. 2 3 The parent rock undergoes: Sedimentary rocks WEATHERING TRANSPORTATION
WEATHERING. Turning Rock to Sediment and Solutions 10/22/2012
 WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
I. Uniformitarianism- James Hutton s 2-part theory states: A. The geologic processes now at work were also active in the past B. The present physical
 How Earth s Rocks Were Formed I. Uniformitarianism- James Hutton s 2-part theory states: A. The geologic processes now at work were also active in the past B. The present physical features of Earth were
How Earth s Rocks Were Formed I. Uniformitarianism- James Hutton s 2-part theory states: A. The geologic processes now at work were also active in the past B. The present physical features of Earth were
COMPOSITIONAL TERMS: FELSIC : light colored INTERMEDIATE : medium shades MAFIC : dark colored ULTRAMAFIC : rare (composition of the mantle)
 GEOLOGY 306 Laboratory NAME: Instructor: TERRY J. BOROUGHS SECTION: Common Rocks (Chapter 2) For this assignment, you will require: a streak plate, glass plate, magnet, dilute hydrochloric (HCl) acid,
GEOLOGY 306 Laboratory NAME: Instructor: TERRY J. BOROUGHS SECTION: Common Rocks (Chapter 2) For this assignment, you will require: a streak plate, glass plate, magnet, dilute hydrochloric (HCl) acid,
Rocks and Weathering
 Rocks and Weathering The Effects of Weathering The process of mountain building thrusts rock up to Earth s surface. Weathering is the process that breaks down rock and other substances at Earth s surface.
Rocks and Weathering The Effects of Weathering The process of mountain building thrusts rock up to Earth s surface. Weathering is the process that breaks down rock and other substances at Earth s surface.
Cement in Cambrian Sandstone : Assessing the Potential for the Generation of Respirable Silica
 Cement in Cambrian Sandstone : Assessing the Potential for the Generation of Respirable Silica J. Brian Mahoney Kent M. Syverson Department of Geology University of Wisconsin-Eau Claire Geologic Setting
Cement in Cambrian Sandstone : Assessing the Potential for the Generation of Respirable Silica J. Brian Mahoney Kent M. Syverson Department of Geology University of Wisconsin-Eau Claire Geologic Setting
Engineering Geology ECIV 3302
 Engineering Geology ECIV 3302 Instructor : Dr. Jehad Hamad 2019-2018 Chapter (5) Weathering & Soil Chapter 5: Weathering, Soil, and Mass Wasting External processes include : (1) Weathering (2) Mass wasting
Engineering Geology ECIV 3302 Instructor : Dr. Jehad Hamad 2019-2018 Chapter (5) Weathering & Soil Chapter 5: Weathering, Soil, and Mass Wasting External processes include : (1) Weathering (2) Mass wasting
The Nature of Sedimentary Rocks
 The Nature of Sedimentary Rocks Sedimentary rocks are composed of: Fragments of other rocks Chemical precipitates Organic matter or biochemically produced materials The Nature of Sedimentary Rocks Sedimentary
The Nature of Sedimentary Rocks Sedimentary rocks are composed of: Fragments of other rocks Chemical precipitates Organic matter or biochemically produced materials The Nature of Sedimentary Rocks Sedimentary
Name Class Date. 1. In your own words, write a definition for the term rock cycle.
 Skills Worksheet Chapter Review USING KEY TERMS 1. In your own words, write a definition for the term rock cycle. Complete each of the following sentences by choosing the correct term from the word bank.
Skills Worksheet Chapter Review USING KEY TERMS 1. In your own words, write a definition for the term rock cycle. Complete each of the following sentences by choosing the correct term from the word bank.
Connecticut's Aquifers
 Page 1 of 5 DEP Search: Connecticut's Aquifers The technical definition of the word "aquifer" is: any geologic formation capable of yielding significant quantities of water to wells. By that definition,
Page 1 of 5 DEP Search: Connecticut's Aquifers The technical definition of the word "aquifer" is: any geologic formation capable of yielding significant quantities of water to wells. By that definition,
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Rocks & Minerals. Lesson 1 Properties of Minerals. What is a mineral? What is a mineral?
 Rocks & Minerals What is a mineral? A mineral must have 5 specific characteristics to be considered a mineral a. b. c. d. e. Naturally occurring - formed by natural processes. Solid - must have a definite
Rocks & Minerals What is a mineral? A mineral must have 5 specific characteristics to be considered a mineral a. b. c. d. e. Naturally occurring - formed by natural processes. Solid - must have a definite
NC Earth Science Essential Standards
 NC Earth Science Essential Standards EEn. 2.1 Explain how processes and forces affect the Lithosphere. EEn. 2.1.1 Explain how the rock cycle, plate tectonics, volcanoes, and earthquakes impact the Lithosphere.
NC Earth Science Essential Standards EEn. 2.1 Explain how processes and forces affect the Lithosphere. EEn. 2.1.1 Explain how the rock cycle, plate tectonics, volcanoes, and earthquakes impact the Lithosphere.
Soil Fertility. Fundamentals of Nutrient Management June 1, Patricia Steinhilber
 Soil Fertility Fundamentals of Nutrient Management June 1, 2010 Patricia Steinhilber Ag Nutrient Management Program University of Maryland College Park Main Topics plant nutrition functional soil model
Soil Fertility Fundamentals of Nutrient Management June 1, 2010 Patricia Steinhilber Ag Nutrient Management Program University of Maryland College Park Main Topics plant nutrition functional soil model
INSIDE OUR EARTH. The Earth is primarily composed of rocks. They can be in solid, semiplastic GEOGRAPHY. Chapter
 Chapter 2 INSIDE OUR EARTH Unit-1 : OUR ENVIRONMENT GEOGRAPHY 12 Continental Crust and Oceanic Crust The Earth is primarily composed of rocks. They can be in solid, semiplastic (semi molten) or liquid
Chapter 2 INSIDE OUR EARTH Unit-1 : OUR ENVIRONMENT GEOGRAPHY 12 Continental Crust and Oceanic Crust The Earth is primarily composed of rocks. They can be in solid, semiplastic (semi molten) or liquid
AN APPLICATION OF ACID BASE ACCOUNTING FOR HIGHWAY CONSTRUCTION IN EAST TENNESSEE 1
 AN APPLICATION OF ACID BASE ACCOUNTING FOR HIGHWAY CONSTRUCTION IN EAST TENNESSEE 1 by J.T. Ammons C.B. Coburn, Jr. P.A. Shelton 2 Abstract. Concern over the environmental impact of highway construction
AN APPLICATION OF ACID BASE ACCOUNTING FOR HIGHWAY CONSTRUCTION IN EAST TENNESSEE 1 by J.T. Ammons C.B. Coburn, Jr. P.A. Shelton 2 Abstract. Concern over the environmental impact of highway construction
Paleo Lab #4 - Sedimentary Environments
 Paleo Lab #4 - Sedimentary Environments page - 1. CHARACTERISTICS OF SEDIMENT Grain size and grain shape: The sizes and shapes of sedimentary particles (grains) are modified considerably during their transportation
Paleo Lab #4 - Sedimentary Environments page - 1. CHARACTERISTICS OF SEDIMENT Grain size and grain shape: The sizes and shapes of sedimentary particles (grains) are modified considerably during their transportation
Mud Sand Gravel. Clastic Textures
 Sed Rocks Self-Instruction Lab Name Geology 100 Harbor Section Please see the questions online before you begin. Sedimentary rocks are usually identified in the field by their stratification or layering,
Sed Rocks Self-Instruction Lab Name Geology 100 Harbor Section Please see the questions online before you begin. Sedimentary rocks are usually identified in the field by their stratification or layering,
Mud Sand Gravel. Clastic Textures
 Sed Rocks Self-Instruction Lab Name Geology 100 Harbor Section Read the sedimentary rocks chapter before you start. Sedimentary rocks are usually identified in the field by their stratification or layering,
Sed Rocks Self-Instruction Lab Name Geology 100 Harbor Section Read the sedimentary rocks chapter before you start. Sedimentary rocks are usually identified in the field by their stratification or layering,
The use of local building materials in the Baltic States Vilnius
 The use of local building materials in the Baltic States 2012-04-12 Vilnius Mining industry is one of the most important branches of national economy, though Lithuania is not rich in natural resources.
The use of local building materials in the Baltic States 2012-04-12 Vilnius Mining industry is one of the most important branches of national economy, though Lithuania is not rich in natural resources.
Weathering is the process that breaks down rock and other substances at Earth s surface
 Chapter 8 Notes Weathering is the process that breaks down rock and other substances at Earth s surface Factors that contribute to weathering Heat Cold Water Ice O 2 & CO 2 in the atmosphere Examples of
Chapter 8 Notes Weathering is the process that breaks down rock and other substances at Earth s surface Factors that contribute to weathering Heat Cold Water Ice O 2 & CO 2 in the atmosphere Examples of
Cretaceous, Dakota Formation, Terra Cotta Member South Side of I-70, Salina County, Kansas
 Cretaceous, Dakota Formation, Terra Cotta Member South Side of I-70, Salina County, Kansas Written By: Steven D.J. Baumann G-102010-1A Outcrop looking southeast Photo taken by: Steven Baumann on 10-20-2010
Cretaceous, Dakota Formation, Terra Cotta Member South Side of I-70, Salina County, Kansas Written By: Steven D.J. Baumann G-102010-1A Outcrop looking southeast Photo taken by: Steven Baumann on 10-20-2010
A Brief Review of the Geology of Monhegan Island, Maine
 Maine Geologic Facts and Localities April, 2010 A Brief Review of the Geology of Monhegan Island, Maine 43 45 58.95 N, 69 18 47.45 W Text by R. G. Marvinney, Department of Agriculture, Conservation & Forestry
Maine Geologic Facts and Localities April, 2010 A Brief Review of the Geology of Monhegan Island, Maine 43 45 58.95 N, 69 18 47.45 W Text by R. G. Marvinney, Department of Agriculture, Conservation & Forestry
The more common classification systems are enlisted below:
 A number of systems of classification have been evolved for categorizing various types of soil. Some of these have been developed specifically in connection with ascertaining the suitability of soil for
A number of systems of classification have been evolved for categorizing various types of soil. Some of these have been developed specifically in connection with ascertaining the suitability of soil for
Rocks. Sedimentary Rocks. Before You Read. Read to Learn
 chapter 3 Rocks section 4 Sedimentary Rocks What You ll Learn how sedimentary rocks form how sedimentary rocks are classified Before You Read Imagine you are stacking slices of bread, one on top of the
chapter 3 Rocks section 4 Sedimentary Rocks What You ll Learn how sedimentary rocks form how sedimentary rocks are classified Before You Read Imagine you are stacking slices of bread, one on top of the
Introduction to Soil Mechanics Geotechnical Engineering-II
 Introduction to Soil Mechanics Geotechnical Engineering-II ground SIVA Dr. Attaullah Shah 1 Soil Formation Soil derives from Latin word Solum having same meanings as our modern world. From Geologist point
Introduction to Soil Mechanics Geotechnical Engineering-II ground SIVA Dr. Attaullah Shah 1 Soil Formation Soil derives from Latin word Solum having same meanings as our modern world. From Geologist point
Chapter 6 9/25/2012. Weathering, Erosion and Soils. Introduction. How Are Earth Materials Altered? Introduction. How Are Earth Materials Altered?
 Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Geotechnical Aspects of the Ohio River Bridges Project
 Geotechnical Aspects of the Ohio River Bridges Project Mark A. Litkenhus, PE Sr. Geotechnical Engineer Stephen H. Bickel, PE Sr. Geotechnical Engineer STGEC Ohio River Bridges at Louisville Geotechnical
Geotechnical Aspects of the Ohio River Bridges Project Mark A. Litkenhus, PE Sr. Geotechnical Engineer Stephen H. Bickel, PE Sr. Geotechnical Engineer STGEC Ohio River Bridges at Louisville Geotechnical
Sedimentary Rocks, Stratigraphy, and Geologic Time
 Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
Soils and Soil Minerals. Remember, most things can be too little or too much.
 Soils and Soil Minerals Remember, most things can be too little or too much. 1 2 3 Source of essential elements CO 2, O 2 from atmosphere H 2 0, O 2, minerals from soil NH 4, SO 4 can volatilize and be
Soils and Soil Minerals Remember, most things can be too little or too much. 1 2 3 Source of essential elements CO 2, O 2 from atmosphere H 2 0, O 2, minerals from soil NH 4, SO 4 can volatilize and be
Florida s Karst Geology
 Florida s Karst Geology Orange Creek Basin Interagency Working Group Public Workshop, November 5 th, 2015 Harley Means, P.G. Assistant State Geologist Florida Geological Survey Karst Karst a type of topography
Florida s Karst Geology Orange Creek Basin Interagency Working Group Public Workshop, November 5 th, 2015 Harley Means, P.G. Assistant State Geologist Florida Geological Survey Karst Karst a type of topography
