Measurement of streaming potential coupling coefficient in sandstones saturated with natural and artificial brines at high salinity
|
|
|
- Curtis Clarke
- 5 years ago
- Views:
Transcription
1 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi: /2010jb007593, 2010 Measurement of streaming potential coupling coefficient in sandstones saturated with natural and artificial brines at high salinity J. Vinogradov, 1 M. Z. Jaafar, 1,2 and M. D. Jackson 1 Received 26 March 2010; revised 14 July 2010; accepted 3 August 2010; published 3 December [1] We report experimental measurements of the streaming potential coupling coefficient in sandstones saturated with NaCl dominated artificial and natural brines up to 5.5 M (321.4 g L 1 of NaCl; electrical conductivity of 23 S m 1 ). We find that the magnitude of the coupling coefficient decreases with increasing brine salinity, as observed in previous experimental studies and predicted by models of the electrical double layer. However, the magnitude of the coupling coefficient remains greater than zero up to the saturated brine salinity. The magnitude of the zeta potential we interpret from our measurements also decreases with increasing brine salinity in the low salinity domain (<0.4 M; 23.4 g L 1 of NaCl and 3.4 S m 1 ) but reaches a constant value at higher salinity (>0.4 M). We hypothesize that the constant value of zeta potential observed at high salinity reflects the maximum packing of counterions in the diffuse part of the electrical double layer. Our hypothesis predicts that the zeta potential becomes independent of brine salinity when the diffuse layer thickness is similar to the diameter of the hydrated counterion. This prediction is confirmed by our experimental data and also by published measurements on alumina in KCl brine. At high salinity (>0.4 M), values of the streaming potential coupling coefficient and the corresponding zeta potential are the same within experimental error regardless of sample mineralogy and texture and the composition of the brine. Citation: Vinogradov, J., M. Z. Jaafar, and M. D. Jackson (2010), Measurement of streaming potential coupling coefficient in sandstones saturated with natural and artificial brines at high salinity, J. Geophys. Res., 115,, doi: /2010jb Introduction [2] Streaming potentials in fluid saturated rocks arise from the electrical double layer, which forms at solid fluid interfaces [e.g., Hunter, 1981]. In the fluid immediately adjacent to the charged mineral surface, there is a layer of adsorbed countercharge termed the Stern layer. This excess of countercharge is immobile. However, there is an excess of countercharge in the fluid adjacent to the Stern layer, which is mobile; this is termed the diffuse or Gouy Chapman layer. Within the diffuse layer, the concentration of excess countercharge decreases away from the mineral surface until the fluid becomes electrically neutral; this is termed the free electrolyte. Overall, the electrical double layer remains neutral, as the excess countercharge in the fluid balances the surface charge on the mineral. However, if the fluid is induced to flow by an external potential (pressure) gradient, then some of the excess charge within the diffuse layers is transported with the flow, giving rise to a streaming current. Divergence 1 Department of Earth Science and Engineering, Imperial College London, London, UK. 2 Now at the Petroleum Engineering Department, Universiti Teknologi Malaysia, Johor Bahru, Malaysia. Copyright 2010 by the American Geophysical Union /10/2010JB of the streaming current density establishes an electrical potential, termed the streaming potential. The closest plane to the mineral surface at which flow occurs in the diffuse layer is termed the shear plane; the electrical potential at this plane is termed the zeta potential. [3] Measurements of streaming potential have been used to characterize the subsurface flow of relatively low salinity brine in numerous Earth science applications (see Corwin and Hoovert [1979] for geothermal examples and Ishido [2004] for examples of volcano monitoring amongst numerous publications). They have also been proposed to characterize flow in subsurface environments, such as hydrocarbon reservoirs and deep saline aquifers, which are fully or partially saturated with brine of considerably higher salinity [e.g., Saunders et al., 2006, 2008]. However, to interpret measurements of streaming potential in these more saline environments requires knowledge of the behavior of the streaming potential coupling coefficient at high salinity. The coupling coefficient (C) is a key petrophysical property that relates the fluid (rp) and streaming (rv) potential gradients when the total current density ( j) is zero[sill, 1983], rv ¼ CrPj j¼0 : The magnitude and sign of the coupling coefficient depends upon the formation factor (F; measured when surface elec- ð1þ 1of18
2 trical conductivity is negligible) and electrical conductivity of the brine saturated rock (s rw ), the dielectric permittivity (" w ) and dynamic viscosity (m w ) of the brine, and the zeta potential (z) [e.g., Jouniaux and Pozzi, 1995], C ¼ " w w rw F : When surface conductivity is negligible, equation (2) simplifies to the well known Helmholtz Smoluchowski equation [e.g., Hunter, 1981], C ¼ " w w w ; where s w is the electrical conductivity of the brine saturating the rock. [4] Most experimental measurements of the streaming potential coupling coefficient in Earth materials have been obtained using samples saturated with relatively low salinity NaCl or KCl brines (less than 1 M, which corresponds to a concentration of 58.4 g L 1 and electrical conductivity of 8.5 S m 1 for NaCl brine) [e.g., Morgan et al., 1989; Sprunt et al., 1994; Jouniaux and Pozzi, 1995, 1997; Li et al., 1995; Jiang et al., 1998; Pengra et al., 1999; Reppert et al., 2001; Reppert and Morgan, 2003; Revil et al., 2003; Alkafeef and Alajmi, 2006; Block and Harris, 2006]. Only Jaafar et al. [2009] have presented data obtained at higher salinity. They measured the streaming potential coupling coefficient in intact sandstone samples saturated with artificial NaCl brine and found that the magnitude of the coupling coefficient decreased with increasing brine salinity but was nonzero up to the saturated brine concentration at laboratory temperature (5.5 M or g L 1 of NaCl; electrical conductivity of 23 S m 1 ). The measured coupling coefficient was always negative, so the zeta potential interpreted from the measurements using equation (2) was also negative. At low salinity, their results were consistent with those obtained previously. [5] Jaafar et al. [2009] also found that, at low salinity, the magnitude of the zeta potential decreased with increasing brine salinity, as observed in previous studies [Gaudin and Fuerstenau, 1955; Li and de Bruyn, 1966; Kirby and Hasselbrink, 2004; Bolève et al., 2007; Kosmulski and Dahlsten, 2006; see also the compilation in Pride and Morgan, 1991] and predicted by models of the electrical double layer based on the Boltzmann equation [e.g., Hunter, 1981; Revil et al., 1999a]. However, at salinities higher than approximately 0.4 M (23.4 g L 1 of NaCl; electrical conductivity of 3.4 S m 1 ), they found that the zeta potential reached a constant value within experimental error. At high salinity, models based on the Boltzmann equation predict that the diffuse layer thickness collapses to zero, in which case the countercharge resides entirely within the Stern layer. Consequently, the zeta potential is also predicted to fall to zero [Hunter, 1981]. Jaafar et al. [2009] suggested that ion interactions cause the reduction in thickness of the diffuse layer at high salinity to be less than predicted by the Boltzmann equation, in which it is assumed that the ions are point charges. Moreover, the countercharge required to balance the mineral surface charge is not accommodated entirely within the Stern layer, so the diffuse layer does not collapse to zero. Some of the countercharge remains mobile within ð2þ ð3þ the diffuse layer, at a maximum charge density which is limited by the size of the hydrated counterions. They noted that the Debye length, calculated for the salinity at which the zeta potential reaches a constant value, is comparable with the diameter of a hydrated sodium ion, which suggests that the constant zeta potential they observed at high salinity reflects the maximum charge density in the diffuse layer. This behavior is not captured by current models of the electrical double layer. [6] Jaafar et al. [2009] measured the coupling coefficient in only two samples of the same sandstone, saturated with artificial NaCl brine. Consequently, it is not clear whether the same results would be obtained in samples with differing mineralogy or texture, saturated with brines containing a wider range of ionic species. Moreover, Jaafar et al. [2009] did not report in detail how they were able to obtain reliable measurements at high salinity, when the magnitude of the streaming potential is very small. The aims of this paper are therefore twofold. The first is to present measured values of streaming potential coupling coefficient in intact sandstone cores of varying mineralogy and texture, saturated with both natural and artificial brines, at salinities up to 5.5 M. The results have application to the interpretation of streaming potential measurements to characterize fluid flow in deep saline aquifers, hydrocarbon reservoirs, and other saline subsurface environments; they also have implications for our understanding of the charge distribution within the electrical double layer in natural porous media saturated with highsalinity brine. The second is to clearly explain the experimental methodology required to acquire streaming potentials of very small magnitude in natural porous media. This same methodology was followed by Jaafar et al. [2009]. We place particular emphasis on reporting our quality control procedures. 2. Experimental Methodology 2.1. Apparatus [7] The experimental setup is shown schematically in Figure 1a and is the same as that used by Jaafar et al. [2009]. The experiments are carried out on cylindrical (1.5 and 1 inch diameter) sandstone core samples, which are tightly confined within a core holder. A cross sectional view of the core holder containing a sample is shown in Figure 1b. To prevent brine flowing along the external surface of the sample, the latter is held within a rubber sleeve embedded in the core holder. Three different core holders of similar design are used in the experiments, two of stainless steel which can hold 1.5 and 1 inch diameter cores and one of acrylic which can hold 1.5 inch diameter cores. The samples are held in place by nonmetallic end caps in the stainless steel core holders which, in conjunction with the rubber sleeve, ensure that steel does not come into contact with the sample or brine. The stainless steel body of the core holders was connected to Earth and served as a Faraday enclosure, while the acrylic core holder is wrapped in an earthed, conductive mesh. The cores are held within the holders by a confining pressure applied via a hydraulic fluid (oil in the stainless steel 1.5 inch cell; nitrogen in the other two) pumped through the purge opening into the space between the outer hull and the inner sleeve. The confining pressure is kept several hundreds of kpa higher than the maximum pressure used in experiments. 2of18
3 Figure 1. Experimental apparatus for measuring the streaming potential coupling coefficient. (a) Pressure vessel, brine reservoirs, pump, flow lines (solid lines), and electrical connections (dashed lines). (b) Cross section through the external electrodes. (c) Cross section through the coreholder. For details, see Jaafar et al. [2009]. 3of18
4 Table 1. Mineralogy and Properties of Rock Samples Used in This Study a Fontainebleau Stainton St. Bees 1 Sandpack Porosity 7.2% 17% 19% 43% Grain size 250 mm mm mm mm Permeability 25 md 38 md 70 md 3 D Mineralogy >99% quartz 90% quartz 5% clay and feldspar 90% quartz 5% white >99% quartz <5% white mica <5% calcite mica 5% clay Formation factor N/A Dimensions 47.8 cm length 2.54 cm diameter 6.92 cm length 3.73 cm diameter 7.76 cm length cm diameter 7.2 cm length 3.9 cm diameter a Sample St. Bees 1 was also used by Jaafar et al. [2009]. [8] A syringe pump (either a GDS Standard Pressure/ Volume Controller or a GDS Advanced Pressure/Volume Controller) is used to flow brine through the sample from reservoirs connected to each side of the pressure vessel, and the resulting pressure difference across the sample is measured using a pair of calibrated Druck PDCR 810 pressure transducers (accuracy 0.1% of measured value, resolution 70 Pa) (Figure 1a). Synthetic oil is used to translate the pressure from the pump to the brine in the inlet reservoir, which allows air bubbles in the brine to be captured at the top of the oil layer in each reservoir, prevents exposure of the brine to air which may cause a change in ph, eliminates the flow of electrical current through the brine along a path parallel to the core sample, ensures that the stainless steel pump cylinder does not come into contact with the brine, and reduces corrosion of the pump. Each syringe pump maintains constant rate to high accuracy, and flow can be directed in either direction through the samples by adjusting the flow valves (denoted V1 V6 in Figure 1a). [9] The streaming potential induced across the sample is measured using two pairs of nonpolarizing Ag/AgCl electrodes and either an HP3490A voltmeter (internal impedance 10 GW, accuracy 0.15%, resolution 10 nv) or an NI9219 voltmeter (internal impedance >1 GW, accuracy 0.18%, resolution 50 nv). The electrodes are connected to the voltmeter using copper coaxial cables, with the outer stainless steel cable earthed to shield the inner cable which carries the signal. One electrode in each pair is earthed. The noise level of the measurements is dictated by the stability of the electrodes rather than the performance of the voltmeters. One pair of electrodes is positioned out of the flow path to eliminate electrode flow effects [e.g., Korpi and debruyn, 1972]. These external electrodes are located in a brine reservoir which is in electrical contact with the flowing brine via a lowpermeability ceramic disc (Figure 1c) and provide voltage measurements which are stable to approximately 10 mv. The other pair of electrodes is located on each face of the core sample and comprises a permeable AgCl membrane of the same diameter as the core sample, connected via Ag rods through the nonmetallic end caps and through both the fine and the coarse meshes placed on the core faces (Figure 1b). These nylon meshes of the same diameter as the core sample are used as flow dispersers. The internal electrodes, which are in the path of the flow, are less stable than the external electrodes and, as discussed in section 3.1, record flow ratedependent voltages at high salinity which are independent of flow direction. However, they can be used to measure the conductivity of the saturated core. To confirm these measurements, the saturated samples are occasionally removed from the core holder, and the conductivity across them is measured using two Ag membranes of 1.5 inch diameter which are clamped to each end of the sample using an adjustable jig. The conductivity of the brine is measured using a Metrohm 712 conductometer, and the ph of the brine is measured using a Hanna H8519 ph meter. Both of these meters are regularly calibrated Samples and Sample Preparation [10] We used three different sandstones samples and one unconsolidated sandpack (Table 1). The St. Bees 1 sample is the same as that used by Jaafar et al. [2009]. The brines used in our experiments were simple NaCl solutions in deionized water, two natural brines (seawater sampled from the Dorset coast, UK, and Dead Sea water) and tap water from Rijswijk, Netherlands. The chemical composition of the natural brines is given in Table 2. [11] Prior to conducting any experiments, each sample is cleaned using a 50:50 toluene/methanol solution for 24 h and then flushed with a methanol solution for 24 h. This is a standard method for cleaning core samples [e.g., Byrne and Patey, 2004]. The cleaned sample is then dried at 80 C for 48 h. Finally, the sample is saturated with the brine to be used in the streaming potential measurements for 24 h in a vacuum chamber. The sample is then loaded into the pressure vessel, and the same brine flowed repeatedly through the sample from one reservoir to the other and back again, until the conductivity and ph of the brine in each reservoir remain constant and equal within a 10% tolerance. Measurements of streaming potential then begin Measurements of Streaming Potential [12] The experiments for each core sample and brine composition begin with a measurement of the initial voltage across the sample. We term this voltage, when there is no Table 2. Composition of the Natural Brines Used in This Study a Composition (M) Species UK Seawater Dead Sea Water Cl SO Na Mg Ca K HCO Br Sr Li Mn a The data for seawater are taken from Adams and Bachu [2002], and the data for the Dead Sea water are taken from Nissenbaum [1977]. 4of18
5 fluid flow, the static voltage. The syringe pump is then used to induce brine flow through the sample at constant rate, resulting in a change in pressure and voltage across the sample, which is recorded at a frequency of 1 Hz (e.g., Figure 2), along with the volume of fluid in the pump cylinder, from which we can calculate the flow rate and confirm the target setting on the pump controller. Flow is terminated once stable voltage and pressure are recorded across the sample, defined as a variation of <10 mv and <2 kpa over 1200 s (20 min). The system is then allowed to relax, and the conductivity of the saturated sample and the conductivity and ph of the brine in each reservoir are measured. [13] We then adjust the valves V1 V6 and set the syringe pump so that flow through the sample occurs in the opposite direction, but at the same flow rate, until stable voltage and pressure are again recorded across the sample (e.g., Figure 2). The conductivity of the saturated sample and the conductivity and ph of the brine in each reservoir are then measured. As discussed later, these paired experiments (which we term the paired stabilization method), in which flow is induced through the sample at the same rate but in opposing directions, ensure that electrode polarization effects are negligible [e.g., Ball and Fuerstenau, 1973] and eliminate the effect of temporal variations in the static voltage. The paired experiments are conducted using at least four different flow rates for each sample and brine composition and repeated 3 times for each flow rate to evaluate experimental uncertainty (e.g., Figure 2). [14] To validate the voltage measurements obtained using the paired stabilization method, we also use a second approach termed the pressure ramping method. The pressure difference across the sample is increased linearly with time, from zero to a maximum value of approximately 0.5 MPa over a period of 120 s, and the pressure difference and voltage across the sample are recorded at a frequency of 1 Hz (e.g., Figure 3). The advantage of the pressure ramping method is that it is much quicker to implement than the paired stabilization method; the disadvantage is that poor results can be obtained if the pressure is ramped too quickly, because an equilibrium state is not achieved at which the streaming and conduction currents balance and equation (1) is valid. [15] To ensure that electrochemical and thermoelectric potentials are eliminated during measurements of the streaming potential, uniform and constant brine conductivity and ph (6 8) in each reservoir, and uniform and constant temperature (23 C), are maintained within a 5% tolerance. Redox potentials are minimized by ensuring that the Ag/AgCl electrodes are the only metal in contact with the samples and brine Measurements of Saturated Rock Conductivity [16] The conductivity of the saturated sample is measured using a two electrode configuration (either the internal Ag/AgCl electrodes shown in Figure 1b or similar external Ag electrodes with the sample removed from the pressure vessel) over the frequency range 10 Hz to 2 MHz. The measured parameters are frequency ( f), impedance (Z), and resistance (R). The resulting reactance (X) is then calculated, p X ¼ ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi ðz 2 R 2 Þ: ð4þ The value of measured resistance that corresponds to the minimum reactance is taken to be the resistance of the sample to direct current (e.g., Figure 4). Values of frequency that correspond to the minimum reactance are normally 1 5 khz. The conductivity of the saturated sample (s rw ) is calculated using rw ¼ L Rr 2 ; where r is the radius of the cylindrical core sample and L is the length. There is typically a small difference (<5%) in conductivity values obtained using the internal and external electrodes; the mean of the two measurements is taken as the final value Interpretation of the Streaming Potential Coupling Coefficient [17] Interpretation of the results from the paired stabilization experiments follows from the observation that at steady state, the streaming current induced by the flow is balanced by a conduction current to maintain overall electrical neutrality. It is reasonable to assume that the currents follow approximately the same 1 D path along the samples, so equation (1) can be used to determine the coupling coefficient with rp and rv replaced by the stabilized pressure difference (DP m ) and voltage (V m ) measured across the sample. Typically, the streaming potential coupling coefficient is given by the slope of a linear regression through a plot of stabilized voltage against pressure difference obtained for a number of different flow rates [e.g., Jouniaux and Pozzi, 1995, 1997; Lorne et al., 1999] (see Figures 5a and 5b). This approach works well in samples saturated with brine of relatively low salinity (<0.1 M) when the streaming potential is large compared to the static potential, and the absence of electrode polarization effects is confirmed if the calculated coupling coefficient is independent of flow direction (e.g., Figures 5a and 5b). However, in samples saturated with brine of higher salinity, the streaming potential is much smaller in magnitude and it is necessary to account for temporal variations in the static potential (V static ; e.g., Figures 5c and 5d). We eliminate this by conducting paired experiments over short time intervals (ca. 1 h) during which the variation in static voltage is small (<5 mv) compared to the measured streaming potential (>30 mv). The stabilized voltage measured in each experiment within a pair (denoted 1 and 2) is given by V m1 ¼ V s þ V static1 ; V m2 ¼ V s þ V static2 ; ð5þ ð6aþ ð6bþ where V s is the streaming potential. Assuming V static1 V static2 and subtracting the stabilized voltage and pressure difference measured in experiment 1 of a pair from those measured in experiment 2 yields V m1 V m2 ¼ 2V s ; DP m1 DP m2 ¼ 2DP: ð7aþ ð7bþ The streaming potential coupling coefficient is given by the slope of a linear regression through a plot of (V m1 V m2 )/2 against (DP m1 DP m2 )/2 (e.g., Figures 5e and 5f). The 5of18
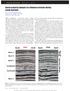
6 Figure 2. Typical results for measured pressure difference and voltage against time obtained using the paired stabilization method for different rock samples and brines. (a) Stainton, 0.57 M NaCl, 3 ml/min; (b) Stainton, 0.1 M NaCl, 3 ml/min; (c) Stainton, M NaCl, 4.3 ml/min; (d) Stainton, 3.98 M NaCl, 3 ml/min; (e) Fontainebleau, M NaCl, 3 ml/min; (f) Fontainebleau, 0.23 M NaCl, 4.3 ml/min. 6of18
7 Figure 3. Measured voltage against pressure difference obtained using the pressure ramping method. The slope of the regression yields the coupling coefficient. (a) Stainton, 3.98 M NaCl, C = mv MPa 1, R 2 = ; (b) Stainton, 0.5 M NaCl, C = mv MPa 1, R 2 = ; (c) Stainton, Dead Sea water, C = mv MPa 1, R 2 = ; (d) St. Bees 1, 0.9 M NaCl, C = 1.35 mv MPa 1, R 2 = advantage of this approach is that we do not need to monitor or identify a value for the static voltage in order to interpret the measured data; moreover, we can still interpret the data for a given sample and brine salinity even if considerable time (e.g., 48 h over a weekend) has elapsed between measurements at different flow rates, so long as paired experiments for a given rate are conducted over a short time interval. A good fit of the data to a linear regression confirms that electrode polarization effects and variations in the static voltage are both small. Data for three repeat experiments are shown in Figures 5e and 5f Interpretation of the Zeta Potential [18] We calculate zeta potential from the measured streaming potential coupling coefficient using equation (2). We calculate the formation factor F by plotting the measured values of saturated sample conductivity against brine conductivity (Figure 6). During the establishment of thermodynamic equilibrium between the brine and the samples, a change in brine conductivity is recorded, which corresponds to a change in salinity. We relate conductivity and salinity using an empirical correlation [Worthington et al., 1990] and published data [CRC Handbook of Chemistry and Physics, 1989; Sen and Goode, 1992] at 23 C (Figures 7a and 7b). A comparison between these sources shows good agreement at low brine conductivity but significant differences for conductivity >1 S m 1. We therefore combined the sources, using the expression of Worthington et al. [1990] for brine conductivity <1 S m 1, and at higher conductivity, a new polynomial function of the same order as that suggested by Worthington et al. [1990] fitted to published data in the CRC Handbook of Chemistry and Physics [1989] and Sen and Goode [1992], C f ¼ 5: : þ 7: : þ 4: þ 3: þ 3: ; ð8þ 7of18
8 Figure 4. Measurements of saturated sample conductivity. (a) Impedance and resistance against frequency for the Fontainebleau sample saturated with 0.01 M NaCl brine. (b) Reactance against resistance for the same sample and brine as Figure 4a. The sample conductivity of S m 1 is obtained at the minimum reactance which corresponds to a frequency of 5.5 khz. (c) Impedance and resistance against frequency for the Stainton sample saturated with 1.92 M NaCl brine. (d) Reactance against resistance for the same sample and brine as Figure 4c. The sample conductivity of S m 1 is obtained at the minimum reactance which corresponds to a frequency of 1.4 khz. where the brine salinity (C f ) is expressed in M and conductivity (s f )isinsm 1. To relate the brine viscosity to salinity, we linearly interpolated the measured data at 25 C reported by Zhang and Han [1996] and that at 20 C reported in the CRC Handbook of Chemistry and Physics [1989] to yield values at 23 C (Figure 7c). The electrical permittivity as a function of brine salinity is calculated using the correlation suggested by Malmberg and Maryott [1956], which is similar to that used by Revil et al. [1999b]. 3. Results 3.1. Measurements of Streaming Potential [19] Figure 2 shows typical measurements of voltage and pressure difference across each sample against time for the paired stabilization experiments at different flow rates and brine salinity. Each plot shows a single paired experiment at a given flow rate for a given sample and brine composition. Our data set comprises approximately 200 of these paired experiments, so only a small subset is shown here. Brine is flowed through the sample in first one direction, until stable voltage and pressure differences are observed, at which time the pump is stopped and the voltage across the sample returns to approximately its initial (static) value. The brine is then flowed through the sample in the opposite direction but at the same rate, which causes the pressure and voltage to respond in the opposite sense but with the same magnitude. This gives us confidence that electrode polarization effects are small. [20] Figures 5a 5d shows typical plots of stabilized voltage against pressure difference. Each datapoint is obtained from a single flow experiment of the type described above. In samples saturated with low salinity brine (<0.1 M), the 8of18
9 stabilized voltage varies linearly with pressure difference and the streaming potential coupling coefficient is given by the slope of a linear regression through the data (Figures 5a and 5b). However, in samples saturated with higher salinity brine, the measured voltages are much smaller and a linear relationship is no longer observed because of temporal variations in the static potential across the sample (Figures 5c and 5d). However, as described in section 2.5, we can eliminate Figure 5 9of18
10 Figure 6. Saturated sample conductivity versus brine conductivity. The slope of the linear regression at brine conductivities greater than 1 S m 1 yields the formation factor (Table 1). the effect of the static potential by plotting the difference in stabilized pressure and voltage obtained between a pair of flow experiments (equation (7)). Figures 5e and 5f show the same data as Figures 5c and 5d, but each datapoint represents a pair of flow experiments, as described above. The voltage varies linearly with pressure difference, and the coupling coefficient is given by the slope of a linear regression through the data. [21] Figure 3 shows measured voltage against pressure difference from pressure ramping experiments on a range of different samples saturated with brines of different salinity and composition. The voltage varies linearly with pressure difference and the streaming potential coupling coefficient is given by the slope of a linear regression through the data. Figure 3a shows pressure ramping data from the same sample, saturated with brine of the same salinity (Stainton, 3.98 M NaCl brine), as the paired stabilization data shown in Figures 5c and 5e. The pressure ramping method yields a value of mv MPa 1 for the coupling coefficient (Figure 3a), while the paired stabilization method yields a value of mv MPa 1 (Figure 5e). In general, the results of the paired stabilized and pressure ramping experiments differ by less than 10%. [22] Figure 8 compares the stabilized voltage versus the pressure difference obtained using the internal and external electrodes. At low salinities, the electrodes yield similar values of the streaming potential coupling coefficient ( and mv MPa 1 for the internal and external electrodes, respectively; Figure 8a). However, at higher salinities (>0.1 M), the internal electrodes record flow rate dependent voltages, which are independent of flow direction (Figure 8b) Measurements of Saturated Rock Conductivity [23] Figure 4 shows typical measurements of saturated sample resistance and impedance against frequency and reactance against resistance. At low frequency, electrode polarization effects become significant, while at high frequency, capacitance effects become significant. The conductivity of the saturated sample is obtained using the approach outlined in section 2.4. Figure 6 shows the relationship between saturated sample conductivity and the conductivity of the saturating brine for three of the four samples investigated; the saturated sample conductivity was not measured over the range of brine salinities for the sandpack. The data follow a linear trend at brine conductivities >1 S m 1 when bulk conductivity dominates, but the behavior at lower salinity reflects the increasing contribution of surface conductivity [e.g., Lorne et al., 1999]. Including only data at brine conductivities >1 S m 1, we calculate the formation factor F as the reciprocal of the slope of a linear regression through the data. Values are reported in Table Relationship Between Flow Rate and Pressure Drop [24] At high salinities, the change in voltage which results from an applied pressure difference is small, so a large pressure difference is required to record a measurable voltage. However, a large pressure difference may result in nonlaminar flow, in which case Darcy s law is no longer obeyed and equation (2) is not valid [e.g., Lorne et al., 1999]. To verify that the flow is laminar, we plot flow rate Q as a function of applied pressure difference DP (e.g., Figure 9) and confirm that a linear relationship is observed with slope k/m w, where k is the sample permeability (reported in Table 1) and m w is the brine viscosity (Figure 7c). In all experiments, we find that a linear regression fits the measured data with R 2 >0.99, and the value of permeability calculated from the slope varies by <5%. Note also that the Reynolds number for these experiments, calculated using equations (22) and (23) of Bolève et al. [2007], is always less than 10 5, suggesting that inertial forces are negligible Salinity Dependence of the Streaming Potential Coupling Coefficient [25] Figure 10 shows the streaming potential coupling coefficient as a function of brine salinity along with data from previous studies on consolidated sandstones and unconsolidated sand. Also shown are values of the coupling coefficient Figure 5. Stabilized voltage (V m ) against stabilized pressure difference (DP m ) for different rock samples and brines. (a) V m against DP m for the Stainton sample saturated with M NaCl brine. The temporal variation of static voltage is negligible compared to the measured voltage. A linear regression through data yields C = mv MPa 1 with R 2 = (b) V m against DP m for the Fontainebleau sample saturated with M NaCl brine. A linear regression through data yields C = mv MPa 1 with R 2 = (c) V m against DP m for the Stainton sample saturated with 3.98 M NaCl brine. The temporal variation of static voltage is comparable to the measured voltage, so the data are scattered. (d) V m against DP m for the Fountainbleau sample saturated with 3.08 M NaCl brine. The temporal variation of static voltage is comparable to the measured voltage, so the data are scattered. (e) (V m1 V m2 )/2 against (DP 1 DP 2 )/2 for the same data as shown in Figure 5c. A linear regression through these data yields C = mv MPa 1 with R 2 = (f) (V m1 V m2 )/2 against (DP 1 DP 2 )/2 for the same data as shown in Figure 5d. A linear regression through these data yields C = mv MPa 1 with R 2 = of 18
11 Figure 7. Brine salinity versus brine conductivity plotted (a) log log scale and (b) linear scale, showing our expression to relate the two (equation (8)) compared against that of Worthington et al. [1990] and published data in the CRC Handbook of Chemistry and Physics [1989] and Sen and Goode [1992]. (c) Brine viscosity versus brine salinity showing our expression to relate the two. predicted in two ways. The first uses equation (3) and a relationship between zeta potential and brine salinity, which we discuss in section 3.5. The second uses an empirical relation between the coupling coefficient and brine salinity obtained directly from a regression through the available data, C ¼ 1:36C 0:9123 f ; ð9þ where the coupling coefficient is expressed in mv MPa 1 and the brine salinity is expressed in M. This regression matches the available data with R 2 = [26] The magnitude of the measured coupling coefficient decreases with increasing salinity. At low salinity, the values we record are consistent with those obtained in previous studies. At high salinity, the coupling coefficient does not fall to zero, but remains nonzero until the NaCl saturation limit is approached, in agreement with the data obtained by Jaafar et al. [2009]. The value of the measured coupling coefficient for a given brine salinity, particular at high salinity (>0.5 M), is similar regardless of the composition of the brine or the mineralogy and texture of the sample. The coupling coefficient is always negative, so the zeta potential is also negative Salinity Dependence of the Zeta Potential [27] Figure 11a shows the zeta potential as a function of brine salinity along with data from previous studies on quartz, silica, and glass. At low salinity, the magnitude of the zeta potential decreases with increasing salinity, as observed in previous studies and predicted by models of the electrical double layer based on the Boltzmann transport equation [e.g., Hunter, 1981; Revil et al., 1999a]. However, at salinities above approximately 0.4 M, the zeta potential reaches a constant value within experimental error, which is consistent with the findings of Jaafar et al. [2009]. [28] Also shown in Figure 11a are values of the zeta potential predicted using a relationship between zeta potential and brine salinity given by ¼ a þ b log C f ; C f < 0:4M ¼ c; C f 0:4M; ð10aþ ð10bþ 11 of 18
12 Figure 8. Comparison between stabilized voltages measured using the external and internal electrodes. (a) Stabilized voltage versus pressure difference for the Stainton sample saturated with 0.04 M NaCl brine yields C = mv MPa 1 and R 2 = using the external electrodes and C = mv MPa 1 and R 2 = using the internal electrodes. (b) Stabilized voltage versus pressure difference for the Stainton sample saturated with 0.31 M NaCl brine yields C = 3.77 mv MPa 1 and R 2 = using the external electrodes and C = 4.06 mv MPa 1 and R 2 = using the internal electrodes. where z is expressed in mv and C f is expressed in M. A relationship of the form of equation (10a) between zeta potential and salinity was originally proposed based on measured zeta potential data in the low salinity range (<0.1 M) [Pride and Morgan, 1991] and can also be derived from a model of the electrical double layer which captures the partitioning of countercharge between the Stern and diffuse layers and invokes the Boltzmann equation to describe the charge density in the diffuse layer [Revil et al., 1999a]. Jaafar et al. [2009] suggested that the zeta potential is constant at high salinity (>0.4 M; equation (10b)). We find the best fit to the data shown in Figure 11 is obtained with a = 9.67 mv, b = mv, and c = 17 mv. The quality of the fit is given by R 2 = Pride and Morgan [1991] reported a = 8 mv and b = 26 mv, Boléve et al. [2007] suggested a = 14.6 mv and b = 29.1, while Revil et al. [1999a] estimate a 10 mv and b 20 mv. All of these studies implicitly assumed c =0.Jaafar et al. [2009] suggest a = 6.43 mv, b = mv and c = 20 mv, which are similar to the values proposed here Time Dependence of the Streaming Potential Coupling Coefficient [29] Reppert and Morgan [2003] found that measured values of the streaming potential coupling coefficient decreased significantly with time. For example, in a Fontainebleau sample saturated with approximately M NaCl brine (i.e., much lower salinity than the brines considered in this Figure 9. Flow rate against applied pressure difference. (a) Stainton saturated with 0.1 M NaCl brine, linear regression through the origin fits with R 2 = (b) Fountainbleau saturated with 0.56 M NaCl brine, linear regression through the origin fits with R 2 = of 18
13 Figure 10. Streaming potential coupling coefficient as a function of brine salinity, plotted with published data. Also shown are curves calculated using equation (10) in conjunction with equation (3) and equation (9). All values of the coupling coefficient are negative; only the magnitude of the coupling coefficient is presented. (a) Data from (1) sandstone and NaCl brine [Jiang et al., 1998; Spruntetal., 1994; Jouniaux and Pozzi, 1995; Jouniaux and Pozzi, 1997; Li et al., 1995; Pengra et al., 1999], (2) sandstone and KCl brine [Alkafeef and Alajmi, 2006], (3) sand and NaCl brine [Ahmad, 1964; Block and Harris, 2006; Guichet et al., 2003; Ogilvy et al., 1969; Schriever and Bleil, 1957], (4) silica nanochannels and KCl brine, (5) glass beads and NaCl brine [Block and Harris, 2006; Li et al., 1995; Pengra et al., 1999], (6) St. Bees 1 and NaCl brine [Jaafar et al., 2009], (7) St. Bees 2 and NaCl brine [Jaafar et al., 2009], (8) Stainton and NaCl brine, (9) Fountainbleau and NaCl brine, (10) Fountainbleau and seawater, (11) Stainton and Dead Sea water (plotted at the NaCl salinity which yields the same electrical conductivity), (12) sandpack and tap water, (13) St. Bees 2 and seawater. (b) The inset focusing on the high salinity domain. The axis labels and data legend are the same as in Figure 10a. paper), they found that the coupling coefficient decreased in magnitude from approximately 360 mv MPa 1 to approximately 250 mv MPa 1 over a period of approximately 46 days (their Figure 6). In general, the values of coupling coefficient and zeta potential they report for their sandstone samples are smaller than those reported previously, and they ascribed this to the increased equilibration time in their study. To confirm that our reported values of the streaming potential coupling coefficient and zeta potential reflect equilibrium conditions, we ran an extended experiment for one sample in which the coupling coefficient, and brine conductivity and ph in each column were measured twice a week over a period of 77 days (Figure 12). We recorded no change in the measured streaming potential, brine conductivity, or ph within experimental error and conclude that our preparation of the samples prior to conducting streaming potential measurements (see section 2.2) ensures equilibrium between samples and saturating brines Uncertainties in the Reported Values of Streaming Potential Coupling Coefficient and Zeta Potential [30] The error bars shown in Figures 10 and 11a are representative of all samples and brines investigated. Uncertainty in the measured value of the coupling coefficient is assessed from the reproducibility of experiments. The uncertainty in measured voltage increases with increasing salinity, since the magnitude of the voltage decreases. Consequently, the reproducibility of results deteriorates with increasing brine salinity, resulting in an increased scatter of data and reducing the quality of fit of a linear regression through the voltage and pressure data from R 2 = at 1 M to R 2 = at 5 M (e.g., Figure 5). The resulting uncertainties in 13 of 18
14 Figure 11. (a) Zeta potential as a function of brine salinity, compared with published data. The curve represents equation (10) fitted to the data for silica, quartz, and glass in NaCl brine (including the data from this study) with values a = 9.67 mv, b = mv, and c = 17 mv; R 2 = The transition from equation (10a) to equation (10b) occurs at a salinity of 0.4 M. Data from (1) silica [Gaudin and Fuerstenau, 1955; Li and de Bruyn, 1966; Kirby and Hasselbrink, 2004], (2) quartz [Pride and Morgan, 1991; Kosmulski et al., 2003], (3) glass beads [Bolève et al., 2007],(4)St.Bees1[Jaafar et al., 2009], (5) St. Bees 2 [Jaafar et al., 2009], (6) Stainton, (7) Fountainbleau, (8) Fountainbleau and UK seawater, (9) Stainton and Dead Sea water (plotted at the NaCl salinity which yields the same electrical conductivity), and (10) St. Bees 2 and UK seawater. (b) Zeta potential as a function of brine salinity measured on alumina particles in KCl [Dukhin et al., 2005]. Note that the zeta potential is positive, reflecting the positively charged alumina surfaces. The curve represents equation (10) fitted to their data with a = 9 mv, b = 27 mv and c = 9 mv; R 2 = The transition from equation (10a) to equation (10b) occurs at a salinity of 1 M. the coupling coefficient are carried through into the interpreted values of zeta potentials using equation (2). 4. Discussion 4.1. Dependence of the Zeta Potential on Brine Salinity [31] The values of zeta potential we interpret from our streaming potential measurements on different sandstone samples are consistent with those reported by Jaafar et al. [2009]. The magnitude of the zeta potential decreases with increasing brine salinity, up to a threshold value above which the zeta potential remains constant. The threshold salinity and the constant value of zeta potential above this threshold are both uncertain given the scatter in the data. [32] At high brine salinity, models of the double layer based on the Boltzmann equation predict that the diffuse layer thickness collapses to zero, in which case the countercharge must reside entirely within the Stern layer and the zeta potential falls to zero, as predicted by equation (10a) when extrapolated into the high salinity domain. Jaafar et al. [2009] suggested that ion interactions cause the reduction in thickness of the diffuse layer at high salinity to be less than predicted by the Boltzmann equation, in which it is assumed that the ions are point charges. Moreover, the countercharge required to balance the mineral surface charge is not accommodated entirely within the Stern layer, so the diffuse layer does not collapse to zero. Rather, some of the countercharge remains mobile within the diffuse layer, at a maximum charge density which is limited by the size of the hydrated counterions. Jaafar et al. [2009] noted that the zeta potential reaches a constant value at a salinity of approximately 0.4 M, while the Debye length, which is a measure of the thickness of the diffuse layer derived from the Boltzmann model [e.g., Hunter, 1981], is approximately 0.47 nm at this salinity, which is comparable with the diameter of a hydrated sodium ion [e.g., Yang et al., 2004; Figure 13]. This suggests that the constant zeta potential observed at salinities higher than 0.4 M Figure 12. Coupling coefficient and brine conductivity measured over 77 days for the Stainton sample saturated with 1 M NaCl brine. 14 of 18
15 Figure 13. Debye length versus salinity of 1 1 brine. Also shown are the estimated diameters of hydrated Na + and K + ions. reflects the maximum charge density in the diffuse layer, which is reached when the diffuse layer thickness approaches the diameter of the counterions. At high brine concentration, the double layer therefore resembles the Helmholtz model, in which the excess charge distribution approximates that of a parallel capacitor, with a single layer of hydrated, immobile ions accommodated within the Stern layer, and a single layer of hydrated, mobile ions accommodated within the diffuse layer, and the so called shear plane located at the boundary between the Stern and diffuse layers (a boundary sometimes termed the Outer Helmholtz Plane) [Bard and Faulkner, 2001]. [33] This hypothesis is supported by our new data and also by data obtained by Dukhin et al. [2005] in the high salinity domain. They measured the zeta potential of alumina particles in KCl solutions up to 2 M using the electroacoustic method, in which the application of ultrasound waves causes relative motion between the suspended particles and the solution, resulting in the generation of an alternating current and an associated electrical potential. They also found that the zeta potential decreased with increasing salinity but reached a constant value of approximately 10 mv at salinities higher than approximately 1 M (Figure 11b). Our hypothesis predicts that the Debye length at this salinity should be similar to the radius of a hydrated potassium ion, which is indeed thecase(figure13).themeasurementsofdukhin et al. [2005] are supported by those of Johnson et al. [1999a, 1999b] on the same materials, which yielded zeta potentials of approximately mv at 1 M Dependence of the Streaming Potential Coupling Coefficient on Brine Salinity [34] We have presented a new regression to the measured values of streaming potential coupling coefficient as a function of salinity (equation (9)). However, we can also use our new regression for zeta potential (equation (10)) in conjunction with equation (3) to predict the value of the coupling coefficient as a function of salinity (Figure 10a). Equation (3) neglects the effect of surface conductivity, which depends upon the texture of the porous medium and becomes more significant at lower salinity (<0.1 M). Consequently, predicted values of the coupling coefficient obtained using a regression to zeta potential data are larger in magnitude than those obtained experimentally at low salinity, so our two approaches to predicting the salinity dependence of the coupling coefficient yield different values (Figure 10). However, they agree to within 5% at high salinity, when surface conductivity is less important Effect of Rock Texture and Mineralogy [35] The samples investigated in this paper and by Jaafar et al. [2009] vary in texture and mineralogy. However, the values of coupling coefficient obtained at salinities above approximately 0.1 M, where the surface electrical conductivity is negligible (Figure 6; a salinity of 0.1 M corresponds to a brine conductivity of approximately 1 S m 1 ) are the same within experimental error, regardless of rock texture and mineralogy (Figure 14). Zeta potentials for salinities above 0.4 M are also found to be independent of rock texture and mineralogy, which agrees with equation (10). Figure 14 shows data from one consolidated sample with low porosity and high formation factor which is entirely composed of quartz and two consolidated samples which have higher porosity and lower formation factor, but which also contain mica and clay minerals (Table 1). The surface charge of clay minerals is known to differ from that of quartz, because the crystalline structure of clay minerals promotes the exchange of cations with the brine [e.g., van Olphen, 1963]. However, we do not need to invoke the high surface charge on clay minerals to explain the zeta potentials we record at high salinity. We record the same zeta potential, within experimental error, in samples which do not contain clay Effect of Brine Composition [36] The presence of multivalent ions in high enough concentration can lead to charge inversion, which occurs when the accumulation of multivalent ions within the Stern layer causes the apparent surface electrical charge to change sign, and is characterized by a change in sign of the measured streaming potential coupling coefficient (and hence zeta potential) [Hunter, 1981]. We have investigated simple NaCl brines which contain no multivalent ions and natural brines (seawater and Dead Sea water) which contain multivalent ions in concentrations up to 1.62 M (Table 2). However, we record the same coupling coefficient and zeta potential for a given sample and brine salinity regardless of brine composition, and observe no evidence of charge inversion (Figure 15). The zeta potentials we obtain from samples saturated with seawater are consistent in both sign and magnitude with those obtained by Kim [2000] for quartz sand powders in seawater, but are rather larger in magnitude than those reported by Kosmulski et al. [2003] for amorphous quartz (Figure 11). Multivalent ions in natural brines do not appear to be present in high enough concentration to cause charge inversion in quartz rich sandstones. This is consistent with the observations of van der Heyden et al. [2005], who found that charge inversion in quartz nanochannels was suppressed at high ionic strength (ca M, which spans the value of 0.6 M typical of seawater) in KCl dominated 15 of 18
Measurement of streaming potential coupling coefficient in sandstones saturated with high salinity NaCl brine
 GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L21306, doi:10.1029/2009gl040549, 2009 Measurement of streaming potential coupling coefficient in sandstones saturated with high salinity NaCl brine M. Z. Jaafar,
GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L21306, doi:10.1029/2009gl040549, 2009 Measurement of streaming potential coupling coefficient in sandstones saturated with high salinity NaCl brine M. Z. Jaafar,
Point of Zero Charge for Sandstone and Carbonate Rocks by Streaming Potential
 International Journal of Petroleum & Geoscience Engineering (IJPGE) 1 (2): 82-90, ISSN 2289-4713 Academic Research Online Publisher Research Article Point of Zero Charge for Sandstone and Carbonate Rocks
International Journal of Petroleum & Geoscience Engineering (IJPGE) 1 (2): 82-90, ISSN 2289-4713 Academic Research Online Publisher Research Article Point of Zero Charge for Sandstone and Carbonate Rocks
A study on the Variation of Streaming Potential Coefficient with Physical Parameters of Rocks
 VNU Journal o Science: Mathematics Physics, Vol. 33, No. 1 (2017) 60-68 A study on the Variation o Streaming Potential Coeicient with Physical Parameters o Rocks Luong Duy Thanh 1,*, Rudol Sprik 2 1 Thuy
VNU Journal o Science: Mathematics Physics, Vol. 33, No. 1 (2017) 60-68 A study on the Variation o Streaming Potential Coeicient with Physical Parameters o Rocks Luong Duy Thanh 1,*, Rudol Sprik 2 1 Thuy
UvA-DARE (Digital Academic Repository) Electrokinetics in porous media Luong, D.T. Link to publication
 UvA-DARE (Digital Academic Repository) Electrokinetics in porous media Luong, D.T. Link to publication Citation for published version (APA): Luong, D. T. (2014). Electrokinetics in porous media General
UvA-DARE (Digital Academic Repository) Electrokinetics in porous media Luong, D.T. Link to publication Citation for published version (APA): Luong, D. T. (2014). Electrokinetics in porous media General
The Zeta Potential Measurements in Sandpacks Saturated with Monovalent Electrolytes
 VNU Journal o cience: Mathematics Physics, Vol. 34, No. 2 (218) 8-19 The Zeta Potential Measurements in andpacks aturated with Monovalent Electrolytes Luong Duy Thanh * Thuy Loi University, 175 Tay on,
VNU Journal o cience: Mathematics Physics, Vol. 34, No. 2 (218) 8-19 The Zeta Potential Measurements in andpacks aturated with Monovalent Electrolytes Luong Duy Thanh * Thuy Loi University, 175 Tay on,
water L v i Chapter 4 Saturation
 4. Resistivity The presence of hydrocarbons is identified by the electrical resistance of the formation. These electrical properties of rocks depend on the pore geometry and fluid distribution. That is,
4. Resistivity The presence of hydrocarbons is identified by the electrical resistance of the formation. These electrical properties of rocks depend on the pore geometry and fluid distribution. That is,
ELECTROKINETIC GROUNDWATER EXPLORATION: A NEW GEOPHYSICAL TECHNIQUE
 181 ELECTROKINETIC GROUNDWATER EXPLORATION: A NEW GEOPHYSICAL TECHNIQUE Sukhyoun Kim, Graham Heinson & John Joseph CRC LEME, School of Earth and Environment Sciences, University of Adelaide, SA, 5005 ABSTRACT
181 ELECTROKINETIC GROUNDWATER EXPLORATION: A NEW GEOPHYSICAL TECHNIQUE Sukhyoun Kim, Graham Heinson & John Joseph CRC LEME, School of Earth and Environment Sciences, University of Adelaide, SA, 5005 ABSTRACT
Simultaneous Measurement of Capillary Pressure and Dielectric Constant in Porous Media
 PIERS ONLINE, VOL. 3, NO. 4, 2007 549 Simultaneous Measurement of Capillary Pressure and Dielectric Constant in Porous Media W. J. Plug, L. M. Moreno, J. Bruining, and E. C. Slob Delft University of Technology,
PIERS ONLINE, VOL. 3, NO. 4, 2007 549 Simultaneous Measurement of Capillary Pressure and Dielectric Constant in Porous Media W. J. Plug, L. M. Moreno, J. Bruining, and E. C. Slob Delft University of Technology,
Modelling ph-dependent and Microstructure-Dependent Streaming Potential Coefficient and Zeta Potential of Porous Sandstones
 Transp Porous Med (2018) 124:31 56 https://doi.org/10.1007/s11242-018-1036-z Modelling ph-dependent and Microstructure-Dependent Streaming Potential Coefficient and Zeta Potential of Porous Sandstones
Transp Porous Med (2018) 124:31 56 https://doi.org/10.1007/s11242-018-1036-z Modelling ph-dependent and Microstructure-Dependent Streaming Potential Coefficient and Zeta Potential of Porous Sandstones
Deviation of linear relation between streaming potential and pore fluid pressure difference in granular material at relatively high Reynolds numbers
 Earth Planets Space, 58, 1045 1051, 2006 Deviation of linear relation between streaming potential and pore fluid pressure difference in granular material at relatively high Reynolds numbers Tohru Watanabe
Earth Planets Space, 58, 1045 1051, 2006 Deviation of linear relation between streaming potential and pore fluid pressure difference in granular material at relatively high Reynolds numbers Tohru Watanabe
INVESTIGATION ON THE EFFECT OF STRESS ON CEMENTATION FACTOR OF IRANIAN CARBONATE OIL RESERVOIR ROCKS
 SCA4-41 1/7 INVESTIGATION ON THE EFFECT OF STRESS ON CEMENTATION FACTOR OF IRANIAN CARBONATE OIL RESERVOIR ROCKS R. Behin, RIPI, NIOC This paper was prepared for presentation at the International Symposium
SCA4-41 1/7 INVESTIGATION ON THE EFFECT OF STRESS ON CEMENTATION FACTOR OF IRANIAN CARBONATE OIL RESERVOIR ROCKS R. Behin, RIPI, NIOC This paper was prepared for presentation at the International Symposium
Part II: Self Potential Method and Induced Polarization (IP)
 Part II: Self Potential Method and Induced Polarization (IP) Self-potential method (passive) Self-potential mechanism Measurement of self potentials and interpretation Induced polarization method (active)
Part II: Self Potential Method and Induced Polarization (IP) Self-potential method (passive) Self-potential mechanism Measurement of self potentials and interpretation Induced polarization method (active)
Estimating Permeability from Acoustic Velocity and Formation Resistivity Factor
 5th Conference & Exposition on Petroleum Geophysics, Hyderabad-2004, India PP 582-587 and Formation Resistivity Factor Majid Nabi-Bidhendi Institute of Geophysics, University of Tehran, P.O. Box 14155-6466,
5th Conference & Exposition on Petroleum Geophysics, Hyderabad-2004, India PP 582-587 and Formation Resistivity Factor Majid Nabi-Bidhendi Institute of Geophysics, University of Tehran, P.O. Box 14155-6466,
Zeta Potential of Intact Natural Limestone: Impact of
 1 Zeta Potential of Intact Natural Limestone: Impact of Potential-Determining Ions Ca, Mg and SO 4 A. Alroudhan 1, J. Vinogradov 1, and M.D. Jackson 1 1 Department of Earth Science and Engineering, Imperial
1 Zeta Potential of Intact Natural Limestone: Impact of Potential-Determining Ions Ca, Mg and SO 4 A. Alroudhan 1, J. Vinogradov 1, and M.D. Jackson 1 1 Department of Earth Science and Engineering, Imperial
Zeta potential changes at mineralbrine and oil-brine interfaces control improved oil recovery during smart waterflooding
 Zeta potential changes at mineralbrine and oil-brine interfaces control improved oil recovery during smart waterflooding Matthew Jackson Dawoud Al-Mahrouqi, Shuai Li Department of Earth Science and Engineering,
Zeta potential changes at mineralbrine and oil-brine interfaces control improved oil recovery during smart waterflooding Matthew Jackson Dawoud Al-Mahrouqi, Shuai Li Department of Earth Science and Engineering,
Modeling the electrochemical properties and the complex conductivity of calcite
 Modeling the electrochemical properties and the complex conductivity of calcite Shuai Li Philippe Leroy Damien Jougnot André Revil Nicolas Devau Frank Heberling Mohamed Azaroual Complex conductivity in
Modeling the electrochemical properties and the complex conductivity of calcite Shuai Li Philippe Leroy Damien Jougnot André Revil Nicolas Devau Frank Heberling Mohamed Azaroual Complex conductivity in
Self-potential anomalies induced by water injection into hydrocarbon reservoirs
 GEOPHYSICS, VOL. 76, NO. 4 (JULY-AUGUST 2011); P. F283 F292, 8 FIGS. 10.1190/1.3596010 Self-potential anomalies induced by water injection into hydrocarbon reservoirs Murtaza Y. Gulamali 1, Eli Leinov
GEOPHYSICS, VOL. 76, NO. 4 (JULY-AUGUST 2011); P. F283 F292, 8 FIGS. 10.1190/1.3596010 Self-potential anomalies induced by water injection into hydrocarbon reservoirs Murtaza Y. Gulamali 1, Eli Leinov
Examination of a Theoretical Model of Streaming Potential Coupling Coefficient Luong, D.T.; Sprik, R.
 UvA-DARE (Digital Academic Repository) Examination of a Theoretical Model of Streaming Potential Coupling Coefficient Luong, D.T.; Sprik, R. Published in: ISRN Geophysics DOI: 1.1155/214/471819 Link to
UvA-DARE (Digital Academic Repository) Examination of a Theoretical Model of Streaming Potential Coupling Coefficient Luong, D.T.; Sprik, R. Published in: ISRN Geophysics DOI: 1.1155/214/471819 Link to
Lecture 3 Charged interfaces
 Lecture 3 Charged interfaces rigin of Surface Charge Immersion of some materials in an electrolyte solution. Two mechanisms can operate. (1) Dissociation of surface sites. H H H H H M M M +H () Adsorption
Lecture 3 Charged interfaces rigin of Surface Charge Immersion of some materials in an electrolyte solution. Two mechanisms can operate. (1) Dissociation of surface sites. H H H H H M M M +H () Adsorption
Chem 321 Lecture 17 - Potentiometry 10/24/13
 Student Learning Objectives Chem 321 Lecture 17 - Potentiometry 10/24/13 Electrodes The cell described in the potentiometric chloride titration (see 10/22/13 posting) consists of a Ag/AgCl reference electrode
Student Learning Objectives Chem 321 Lecture 17 - Potentiometry 10/24/13 Electrodes The cell described in the potentiometric chloride titration (see 10/22/13 posting) consists of a Ag/AgCl reference electrode
Numerical Modeling of the Bistability of Electrolyte Transport in Conical Nanopores
 Numerical Modeling of the Bistability of Electrolyte Transport in Conical Nanopores Long Luo, Robert P. Johnson, Henry S. White * Department of Chemistry, University of Utah, Salt Lake City, UT 84112,
Numerical Modeling of the Bistability of Electrolyte Transport in Conical Nanopores Long Luo, Robert P. Johnson, Henry S. White * Department of Chemistry, University of Utah, Salt Lake City, UT 84112,
ROCK PHYSICS DIAGNOSTICS OF NORTH SEA SANDS: LINK BETWEEN MICROSTRUCTURE AND SEISMIC PROPERTIES ABSTRACT
 ROCK PHYSICS DIAGNOSTICS OF NORTH SEA SANDS: LINK BETWEEN MICROSTRUCTURE AND SEISMIC PROPERTIES PER AVSETH, JACK DVORKIN, AND GARY MAVKO Department of Geophysics, Stanford University, CA 94305-2215, USA
ROCK PHYSICS DIAGNOSTICS OF NORTH SEA SANDS: LINK BETWEEN MICROSTRUCTURE AND SEISMIC PROPERTIES PER AVSETH, JACK DVORKIN, AND GARY MAVKO Department of Geophysics, Stanford University, CA 94305-2215, USA
957 Lecture #13 of 18
 Lecture #13 of 18 957 958 Q: What was in this set of lectures? A: B&F Chapter 2 main concepts: Section 2.1 : Section 2.3: Salt; Activity; Underpotential deposition Transference numbers; Liquid junction
Lecture #13 of 18 957 958 Q: What was in this set of lectures? A: B&F Chapter 2 main concepts: Section 2.1 : Section 2.3: Salt; Activity; Underpotential deposition Transference numbers; Liquid junction
PETROPHYSICAL EVALUATION CORE COPYRIGHT. Saturation Models in Shaly Sands. By the end of this lesson, you will be able to:
 LEARNING OBJECTIVES PETROPHYSICAL EVALUATION CORE Saturation Models in Shaly Sands By the end of this lesson, you will be able to: Explain what a shale is and how to distinguish between a shaly sand and
LEARNING OBJECTIVES PETROPHYSICAL EVALUATION CORE Saturation Models in Shaly Sands By the end of this lesson, you will be able to: Explain what a shale is and how to distinguish between a shaly sand and
Derivation of soil-specific streaming potential electrical parameters from hydrodynamic characteristics of partially saturated soils
 Derivation of soil-specific streaming potential electrical parameters from hydrodynamic characteristics of partially saturated soils D. Jougnot 1, N. Linde 1, A. Revil 2,3, and C. Doussan 4 1 Institute
Derivation of soil-specific streaming potential electrical parameters from hydrodynamic characteristics of partially saturated soils D. Jougnot 1, N. Linde 1, A. Revil 2,3, and C. Doussan 4 1 Institute
Electrochemical Properties of Materials for Electrical Energy Storage Applications
 Electrochemical Properties of Materials for Electrical Energy Storage Applications Lecture Note 3 October 11, 2013 Kwang Kim Yonsei Univ., KOREA kbkim@yonsei.ac.kr 39 Y 88.91 8 O 16.00 7 N 14.01 34 Se
Electrochemical Properties of Materials for Electrical Energy Storage Applications Lecture Note 3 October 11, 2013 Kwang Kim Yonsei Univ., KOREA kbkim@yonsei.ac.kr 39 Y 88.91 8 O 16.00 7 N 14.01 34 Se
Electrophoretic Deposition. - process in which particles, suspended in a liquid medium, migrate in an electric field and deposit on an electrode
 Electrophoretic Deposition - process in which particles, suspended in a liquid medium, migrate in an electric field and deposit on an electrode no redox differs from electrolytic in several ways deposit
Electrophoretic Deposition - process in which particles, suspended in a liquid medium, migrate in an electric field and deposit on an electrode no redox differs from electrolytic in several ways deposit
Rock Physics Perturbational Modeling: Carbonate case study, an intracratonic basin Northwest/Saharan Africa
 Rock Physics Perturbational Modeling: Carbonate case study, an intracratonic basin Northwest/Saharan Africa Franklin Ruiz, Carlos Cobos, Marcelo Benabentos, Beatriz Chacon, and Roberto Varade, Luis Gairifo,
Rock Physics Perturbational Modeling: Carbonate case study, an intracratonic basin Northwest/Saharan Africa Franklin Ruiz, Carlos Cobos, Marcelo Benabentos, Beatriz Chacon, and Roberto Varade, Luis Gairifo,
Measurement of the organic saturation and organic porosity in. shale
 Measurement of the organic saturation and organic porosity in shale Qian Sang a,b, Shaojie Zhang a, Yajun Li a, Mingzhe Dong a,b Steven Bryant b a College of Petroleum Engineering, China University of
Measurement of the organic saturation and organic porosity in shale Qian Sang a,b, Shaojie Zhang a, Yajun Li a, Mingzhe Dong a,b Steven Bryant b a College of Petroleum Engineering, China University of
SENSITIVITY ANALYSIS OF THE PETROPHYSICAL PROPERTIES VARIATIONS ON THE SEISMIC RESPONSE OF A CO2 STORAGE SITE. Juan E. Santos
 SENSITIVITY ANALYSIS OF THE PETROPHYSICAL PROPERTIES VARIATIONS ON THE SEISMIC RESPONSE OF A CO2 STORAGE SITE Juan E. Santos Instituto del Gas y del Petróleo, Facultad de Ingeniería UBA and Department
SENSITIVITY ANALYSIS OF THE PETROPHYSICAL PROPERTIES VARIATIONS ON THE SEISMIC RESPONSE OF A CO2 STORAGE SITE Juan E. Santos Instituto del Gas y del Petróleo, Facultad de Ingeniería UBA and Department
The Electrical Properties of Saturated Crustal Rocks: Measurement and Implications. Paul Glover University Montpellier II
 The Electrical Properties of Saturated Crustal Rocks: Measurement and Implications Paul Glover University Montpellier II 1 Structure Basics Ignoring the difficult mathematics Past Experiments Benchtop
The Electrical Properties of Saturated Crustal Rocks: Measurement and Implications Paul Glover University Montpellier II 1 Structure Basics Ignoring the difficult mathematics Past Experiments Benchtop
NEW SATURATION FUNCTION FOR TIGHT CARBONATES USING ROCK ELECTRICAL PROPERTIES AT RESERVOIR CONDITIONS
 SCA2016-055 1/6 NEW SATURATION FUNCTION FOR TIGHT CARBONATES USING ROCK ELECTRICAL PROPERTIES AT RESERVOIR CONDITIONS Oriyomi Raheem and Hadi Belhaj The Petroleum Institute, Abu Dhabi, UAE This paper was
SCA2016-055 1/6 NEW SATURATION FUNCTION FOR TIGHT CARBONATES USING ROCK ELECTRICAL PROPERTIES AT RESERVOIR CONDITIONS Oriyomi Raheem and Hadi Belhaj The Petroleum Institute, Abu Dhabi, UAE This paper was
ROCK PHYSICS MODELING FOR LITHOLOGY PREDICTION USING HERTZ- MINDLIN THEORY
 ROCK PHYSICS MODELING FOR LITHOLOGY PREDICTION USING HERTZ- MINDLIN THEORY Ida Ayu PURNAMASARI*, Hilfan KHAIRY, Abdelazis Lotfy ABDELDAYEM Geoscience and Petroleum Engineering Department Universiti Teknologi
ROCK PHYSICS MODELING FOR LITHOLOGY PREDICTION USING HERTZ- MINDLIN THEORY Ida Ayu PURNAMASARI*, Hilfan KHAIRY, Abdelazis Lotfy ABDELDAYEM Geoscience and Petroleum Engineering Department Universiti Teknologi
A Review of Log-Based Techniques for Measuring Clay Volume
 A Review of Log-Based Techniques for Measuring Clay Volume Sherif Farag Schlumberger Richard Holland Lundin Rt/Ro (log scale) The Effect of Archie s m and n S n w R R o t R t a m R S w n w Rt/Ro (log scale)
A Review of Log-Based Techniques for Measuring Clay Volume Sherif Farag Schlumberger Richard Holland Lundin Rt/Ro (log scale) The Effect of Archie s m and n S n w R R o t R t a m R S w n w Rt/Ro (log scale)
ISO Colloidal systems Methods for zetapotential. Part 1: Electroacoustic and electrokinetic phenomena
 INTERNATIONAL STANDARD ISO 13099-1 First edition 2012-06-15 Colloidal systems Methods for zetapotential determination Part 1: Electroacoustic and electrokinetic phenomena Systèmes colloïdaux Méthodes de
INTERNATIONAL STANDARD ISO 13099-1 First edition 2012-06-15 Colloidal systems Methods for zetapotential determination Part 1: Electroacoustic and electrokinetic phenomena Systèmes colloïdaux Méthodes de
Pore radius distribution and fractal dimension derived from spectral induced polarization
 Pore radius distribution and fractal dimension derived from spectral induced polarization Zeyu Zhang 1 & Andreas Weller 2 1) Southwest Petroleum University, China & Bundesanstalt für Materialforschung
Pore radius distribution and fractal dimension derived from spectral induced polarization Zeyu Zhang 1 & Andreas Weller 2 1) Southwest Petroleum University, China & Bundesanstalt für Materialforschung
Electroosmotic Flow Mixing in a Microchannel
 *c: Unmarked Revised manuscript Click here to view linked References 1 1 1 0 1 0 1 0 1 0 1 0 1 Effects of Ionic Concentration Gradient on Electroosmotic Flow Mixing in a Microchannel Ran Peng and Dongqing
*c: Unmarked Revised manuscript Click here to view linked References 1 1 1 0 1 0 1 0 1 0 1 0 1 Effects of Ionic Concentration Gradient on Electroosmotic Flow Mixing in a Microchannel Ran Peng and Dongqing
Shear Wave Velocity Estimation Utilizing Wireline Logs for a Carbonate Reservoir, South-West Iran
 Iranian Int. J. Sci. 4(2), 2003, p. 209-221 Shear Wave Velocity Estimation Utilizing Wireline Logs for a Carbonate Reservoir, South-West Iran Eskandari, H. 1, Rezaee, M.R., 2 Javaherian, A., 3 and Mohammadnia,
Iranian Int. J. Sci. 4(2), 2003, p. 209-221 Shear Wave Velocity Estimation Utilizing Wireline Logs for a Carbonate Reservoir, South-West Iran Eskandari, H. 1, Rezaee, M.R., 2 Javaherian, A., 3 and Mohammadnia,
Chemistry Instrumental Analysis Lecture 31. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 31 High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) Solvent Delivery
Chemistry 4631 Instrumental Analysis Lecture 31 High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) Solvent Delivery
WM 00 Conference, February 27 March 2, 2000, Tucson, AZ DIFFUSION COEFFICIENTS OF CRITICAL RADIONUCLIDES FROM RADIOACTIVE WASTE IN GEOLOGICAL MEDIUM
 DIFFUSION COEFFICIENTS OF CRITICAL RADIONUCLIDES FROM RADIOACTIVE WASTE IN GEOLOGICAL MEDIUM ABSTRACT: C. Bucur, A.Popa, C. Arsene and M.Olteanu Institute for Nuclear Research, P.O. Box 78, 0300 Pitesti
DIFFUSION COEFFICIENTS OF CRITICAL RADIONUCLIDES FROM RADIOACTIVE WASTE IN GEOLOGICAL MEDIUM ABSTRACT: C. Bucur, A.Popa, C. Arsene and M.Olteanu Institute for Nuclear Research, P.O. Box 78, 0300 Pitesti
Available online at ScienceDirect. Energy Procedia 63 (2014 ) GHGT-12
 Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 4273 4280 GHGT-12 Laboratory measurements of ultrasonic velocities in CO saturated brines Maxim Lebedev a,b *, Olga Bilenko
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 63 (2014 ) 4273 4280 GHGT-12 Laboratory measurements of ultrasonic velocities in CO saturated brines Maxim Lebedev a,b *, Olga Bilenko
LARGE-SCALE PHYSICAL MODELING OF WATER INJECTION INTO GEOTHERMAL RESERVOIRS AND CORRELATION TO SELF POTENTIAL MEASUREMENTS
 PROCEEDINGS, Twenty-Eighth Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 27-29, 23 SGP-TR-173 LARGE-SCALE PHYSICAL MODELING OF WATER INJECTION INTO GEOTHERMAL
PROCEEDINGS, Twenty-Eighth Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 27-29, 23 SGP-TR-173 LARGE-SCALE PHYSICAL MODELING OF WATER INJECTION INTO GEOTHERMAL
Membrane Electrodes. Several types
 Membrane Electrodes Electrical connection Several types - Glass membrane electrode - Liquid membrane electrode - Solid State membrane electrode - Permeable membrane electrode seal 0.1 M HCl Filling solution
Membrane Electrodes Electrical connection Several types - Glass membrane electrode - Liquid membrane electrode - Solid State membrane electrode - Permeable membrane electrode seal 0.1 M HCl Filling solution
Zeta Potential of Artificial and Natural Calcite in
 1 2 Zeta Potential of Artificial and Natural Calcite in Aqueous Solution 3 4 5 Dawoud Al Mahrouqi 1, 2*, Jan Vinogradov 1, 3 and Matthew D. Jackson 1 1 Department of Earth Science and Engineering, Imperial
1 2 Zeta Potential of Artificial and Natural Calcite in Aqueous Solution 3 4 5 Dawoud Al Mahrouqi 1, 2*, Jan Vinogradov 1, 3 and Matthew D. Jackson 1 1 Department of Earth Science and Engineering, Imperial
Module for: Resistivity Theory (adapted/modified from lectures in PETE 321 (Jensen/Ayers))
 (PETE 663 Formation Evaluation and the Analysis of Reservoir Performance (Fall 2003)) Module for: Resistivity Theory (adapted/modified from lectures in PETE 321 (Jensen/Ayers)) J. L. Jensen W.B. Ayers
(PETE 663 Formation Evaluation and the Analysis of Reservoir Performance (Fall 2003)) Module for: Resistivity Theory (adapted/modified from lectures in PETE 321 (Jensen/Ayers)) J. L. Jensen W.B. Ayers
Darcy's Law. Laboratory 2 HWR 531/431
 Darcy's Law Laboratory HWR 531/431-1 Introduction In 1856, Henry Darcy, a French hydraulic engineer, published a report in which he described a series of experiments he had performed in an attempt to quantify
Darcy's Law Laboratory HWR 531/431-1 Introduction In 1856, Henry Darcy, a French hydraulic engineer, published a report in which he described a series of experiments he had performed in an attempt to quantify
CO 2 Rock Physics: A Laboratory Study
 CO 2 Rock Physics: A Laboratory Study Helen Yam* University of Alberta, Edmonton, Alberta, Canada hyam@ualberta.ca and Douglas R. Schmitt University of Alberta, Edmonton, Alberta, Canada Summary It is
CO 2 Rock Physics: A Laboratory Study Helen Yam* University of Alberta, Edmonton, Alberta, Canada hyam@ualberta.ca and Douglas R. Schmitt University of Alberta, Edmonton, Alberta, Canada Summary It is
Electrophoretic Light Scattering Overview
 Electrophoretic Light Scattering Overview When an electric field is applied across an electrolytic solution, charged particles suspended in the electrolyte are attracted towards the electrode of opposite
Electrophoretic Light Scattering Overview When an electric field is applied across an electrolytic solution, charged particles suspended in the electrolyte are attracted towards the electrode of opposite
The Hangingstone steam-assisted gravity drainage
 SPECIAL Heavy SECTION: oil H e a v y o i l Elastic property changes in a bitumen reservoir during steam injection AYATO KATO, University of Houston, USA SHIGENOBU ONOZUKA, JOGMEC, Chiba, Japan TORU NAKAYAMA,
SPECIAL Heavy SECTION: oil H e a v y o i l Elastic property changes in a bitumen reservoir during steam injection AYATO KATO, University of Houston, USA SHIGENOBU ONOZUKA, JOGMEC, Chiba, Japan TORU NAKAYAMA,
LINK BETWEEN ATTENUATION AND VELOCITY DISPERSION
 LINK BETWEEN ATTENUATION AND VELOCITY DISPERSION Jack Dvorkin Stanford University and Rock Solid Images April 25, 2005 SUMMARY In a viscoelastic sample, the causality principle links the attenuation of
LINK BETWEEN ATTENUATION AND VELOCITY DISPERSION Jack Dvorkin Stanford University and Rock Solid Images April 25, 2005 SUMMARY In a viscoelastic sample, the causality principle links the attenuation of
DIELECTRIC PROPERTIES OF MIXTURES OF CLAY-WATER-ORGANIC COMPOUNDS
 DIELECTRIC PROPERTIES OF MIXTURES OF CLAY-WATER-ORGANIC COMPOUNDS By Birsen Canan ABSTRACT The propagation of an electromagnetic wave through a material is dependent on the electrical and magnetic properties
DIELECTRIC PROPERTIES OF MIXTURES OF CLAY-WATER-ORGANIC COMPOUNDS By Birsen Canan ABSTRACT The propagation of an electromagnetic wave through a material is dependent on the electrical and magnetic properties
STRESS-DEPENDENT POROSITY AND PERMEABILITY OF A SUITE OF SAMPLES FROM SAUDI ARABIAN SANDSTONE AND LIMESTONE RESERVOIRS
 STRESS-DEPENDENT POROSITY AND PERMEABILITY OF A SUITE OF SAMPLES FROM SAUDI ARABIAN SANDSTONE AND LIMESTONE RESERVOIRS M. A. Mohiuddin 1, G. Korvin 2, A. Abdulraheem 1, M. R. Awal 1, K. Khan 1, M. S. Khan
STRESS-DEPENDENT POROSITY AND PERMEABILITY OF A SUITE OF SAMPLES FROM SAUDI ARABIAN SANDSTONE AND LIMESTONE RESERVOIRS M. A. Mohiuddin 1, G. Korvin 2, A. Abdulraheem 1, M. R. Awal 1, K. Khan 1, M. S. Khan
Laboratory Characterization of Chemico-osmotic, Hydraulic and Diffusion Properties of Rocks: Apparatus Development
 Laboratory Characterization of Chemico-osmotic, Hydraulic and Diffusion Properties of Rocks: Apparatus Development - 9134 M. Takeda, T. Hiratsuka, K. Ito Institute for Geo-Resources and Environment, National
Laboratory Characterization of Chemico-osmotic, Hydraulic and Diffusion Properties of Rocks: Apparatus Development - 9134 M. Takeda, T. Hiratsuka, K. Ito Institute for Geo-Resources and Environment, National
A NOVEL FLOW CELL AND INTEGRATED SENSOR TECHNIQUE FOR SIMULTANEOUS MAGNETIC MONITORING OF CORE SAMPLES DURING FLUID FLOW EXPERIMENTS
 SCA2010-14 1/12 A NOVEL FLOW CELL AND INTEGRATED SENSOR TECHNIQUE FOR SIMULTANEOUS MAGNETIC MONITORING OF CORE SAMPLES DURING FLUID FLOW EXPERIMENTS Shahjahan Khan, *David K. Potter and Ergun Kuru Department
SCA2010-14 1/12 A NOVEL FLOW CELL AND INTEGRATED SENSOR TECHNIQUE FOR SIMULTANEOUS MAGNETIC MONITORING OF CORE SAMPLES DURING FLUID FLOW EXPERIMENTS Shahjahan Khan, *David K. Potter and Ergun Kuru Department
Geoelectricity. ieso 2010
 Geoelectricity ieso 2010 1 RESISTIVITY SURVEY AT VENETO VILLA GRITTI AT THE TOWN OF TREVISO (VENETO REGION) The survey was carried out to verify the underground presence of the fondations of a rustic building.
Geoelectricity ieso 2010 1 RESISTIVITY SURVEY AT VENETO VILLA GRITTI AT THE TOWN OF TREVISO (VENETO REGION) The survey was carried out to verify the underground presence of the fondations of a rustic building.
VIBRATION-INDUCED CONDUCTIVITY FLUCTUATION (VICOF) TESTING OF SOILS *
 VIBRATION-INDUCED CONDUCTIVITY FLUCTUATION (VICOF) TESTING OF SOILS * L. B. KISH, Department of Electrical and Computer Engineering, Texas A&M University, College Station, TX 77843-3128, USA C. L. S. MORGAN,
VIBRATION-INDUCED CONDUCTIVITY FLUCTUATION (VICOF) TESTING OF SOILS * L. B. KISH, Department of Electrical and Computer Engineering, Texas A&M University, College Station, TX 77843-3128, USA C. L. S. MORGAN,
Determination of zeta potential by means of a rotating disk: planar, particulate, and porous samples!
 Determination of zeta potential by means of a rotating disk: planar, particulate, and porous samples! Paul J Sides! Zetametrix Inc! 5821 Kentucky Avenue! Pittsburgh, PA 15232! 1! ZetaSpin: universal tool
Determination of zeta potential by means of a rotating disk: planar, particulate, and porous samples! Paul J Sides! Zetametrix Inc! 5821 Kentucky Avenue! Pittsburgh, PA 15232! 1! ZetaSpin: universal tool
CHARACTERIZATION OF FRACTURES IN GEOTHERMAL RESERVOIRS USING RESISTIVITY
 PROCEEDINGS, Thirty-Seventh Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 30 - February 1, 01 SGP-TR-194 CHARACTERIZATION OF FRACTURES IN GEOTHERMAL RESERVOIRS
PROCEEDINGS, Thirty-Seventh Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 30 - February 1, 01 SGP-TR-194 CHARACTERIZATION OF FRACTURES IN GEOTHERMAL RESERVOIRS
AN EXPERIMENTAL INVESTIGATION OF BOILING HEAT CONVECTION WITH RADIAL FLOW IN A FRACTURE
 PROCEEDINGS, Twenty-Fourth Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 25-27, 1999 SGP-TR-162 AN EXPERIMENTAL INVESTIGATION OF BOILING HEAT CONVECTION
PROCEEDINGS, Twenty-Fourth Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 25-27, 1999 SGP-TR-162 AN EXPERIMENTAL INVESTIGATION OF BOILING HEAT CONVECTION
Chapter Four. Experimental
 Chapter Four 4.1 Materials N,N-Diethyl monoethanolamine (purity 98%) used in all experiments was purchased from Spectrochem Pvt. Ltd., Mumbai. N-Ethyl monoethanolamine, N-(- aminoethyl)ethanolamine, diethanolamine,
Chapter Four 4.1 Materials N,N-Diethyl monoethanolamine (purity 98%) used in all experiments was purchased from Spectrochem Pvt. Ltd., Mumbai. N-Ethyl monoethanolamine, N-(- aminoethyl)ethanolamine, diethanolamine,
Role of pore scale heterogeneities on the localization of dissolution and precipitation reactions
 Role of pore scale heterogeneities on the localization of dissolution and precipitation reactions Linda Luquot María García-Ríos, Gabriela Davila, Laura Martinez, Tobias Roetting, Jordi Cama, Josep Soler,
Role of pore scale heterogeneities on the localization of dissolution and precipitation reactions Linda Luquot María García-Ríos, Gabriela Davila, Laura Martinez, Tobias Roetting, Jordi Cama, Josep Soler,
Effect of Gas Hydrate Saturation on Hydraulic Conductivity of Marine Sediments
 Effect of Gas Hydrate Saturation on Hydraulic Conductivity of Marine Sediments *Chul-Whan Kang 1), Ah-Ram Kim 2), Hak-Sung Kim 3), Gye-Chun Cho 4) and Joo-Yong Lee 5) 1), 2), 3), 4) Department of Civil
Effect of Gas Hydrate Saturation on Hydraulic Conductivity of Marine Sediments *Chul-Whan Kang 1), Ah-Ram Kim 2), Hak-Sung Kim 3), Gye-Chun Cho 4) and Joo-Yong Lee 5) 1), 2), 3), 4) Department of Civil
INTRODUCTION TO LOGGING TOOLS
 BY: MUHAMMAD ZAHID INTRODUCTION TO LOGGING TOOLS 1- SPONTANEOUS POTENTIAL (SP) The Spontaneous potential survey, (sp) was one of the first measurements, which was carried out, in a well bore. The SP log
BY: MUHAMMAD ZAHID INTRODUCTION TO LOGGING TOOLS 1- SPONTANEOUS POTENTIAL (SP) The Spontaneous potential survey, (sp) was one of the first measurements, which was carried out, in a well bore. The SP log
Treatment of Colloids in the Safety Case
 Treatment of Colloids in the Safety Case Clay Colloids in Aqueous Systems 3 4 February 2016, Berlin Dr Amy Shelton, Radioactive Waste Management (RWM) KBS-3 Concept Based on the multi- barrier principles
Treatment of Colloids in the Safety Case Clay Colloids in Aqueous Systems 3 4 February 2016, Berlin Dr Amy Shelton, Radioactive Waste Management (RWM) KBS-3 Concept Based on the multi- barrier principles
Copyright SOIL STRUCTURE and CLAY MINERALS
 SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
FORMATION EVALUATION PETE 663
 FORMATION EVALUATION PETE 663 SHALY SAND EVALUATION - B Summer 2010 SHALY FORMATION ISSUES Water Saturation Obstacles with Shaly Sands Fresh formation water can cause conventional analysis to overestimate
FORMATION EVALUATION PETE 663 SHALY SAND EVALUATION - B Summer 2010 SHALY FORMATION ISSUES Water Saturation Obstacles with Shaly Sands Fresh formation water can cause conventional analysis to overestimate
The effect of CO 2 -fluid-rock interactions on the porosity and permeability of calcite-bearing sandstone
 The effect of CO 2 -fluid-rock interactions on the porosity and permeability of calcite-bearing sandstone Benoit Lamy-Chappuis, Bruce Yardley, Carlos Grattoni School of Earth and Environment, University
The effect of CO 2 -fluid-rock interactions on the porosity and permeability of calcite-bearing sandstone Benoit Lamy-Chappuis, Bruce Yardley, Carlos Grattoni School of Earth and Environment, University
Clay Control and its Application in Fracture Design. Branden Ruyle Basin Engineering Staff Completions Engineer Consultant
 Clay Control and its Application in Fracture Design Branden Ruyle Basin Engineering Staff Completions Engineer Consultant Outline Agenda Characteristics Types of Clays and Mechanism Acidizing Control Additives
Clay Control and its Application in Fracture Design Branden Ruyle Basin Engineering Staff Completions Engineer Consultant Outline Agenda Characteristics Types of Clays and Mechanism Acidizing Control Additives
AE 3051, Lab #16. Investigation of the Ideal Gas State Equation. By: George P. Burdell. Group E3
 AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
READING A. INTRODUCTION CHE425L POTENTIOMETRY WITH K + ION-SELECTIVE ELECTRODE. Skoog, Holler and Crouch: Chapter 23 and Appendix 3.
 CHE425L POTENTIOMETRY WITH K + ION-SELECTIVE ELECTRODE READING Skoog, Holler and Crouch: Chapter 23 and Appendix 3. A. INTRODUCTION Potentiometry is a static electroanalytical method in which the potential
CHE425L POTENTIOMETRY WITH K + ION-SELECTIVE ELECTRODE READING Skoog, Holler and Crouch: Chapter 23 and Appendix 3. A. INTRODUCTION Potentiometry is a static electroanalytical method in which the potential
V. Electrostatics. MIT Student
 V. Electrostatics Lecture 26: Compact Part of the Double Layer MIT Student 1 Double-layer Capacitance 1.1 Stern Layer As was discussed in the previous lecture, the Gouy-Chapman model predicts unphysically
V. Electrostatics Lecture 26: Compact Part of the Double Layer MIT Student 1 Double-layer Capacitance 1.1 Stern Layer As was discussed in the previous lecture, the Gouy-Chapman model predicts unphysically
SECOND PUBLIC EXAMINATION. Honour School of Physics Part C: 4 Year Course. Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS
 2757 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS TRINITY TERM 2013 Monday, 17 June, 2.30 pm 5.45 pm 15
2757 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS TRINITY TERM 2013 Monday, 17 June, 2.30 pm 5.45 pm 15
The ph dependence of spectral induced polarization of silica sands: Experiment and modeling
 GEOPHYSICAL RESEARCH LETTERS, VOL. 38,, doi:10.1029/2011gl047748, 2011 The ph dependence of spectral induced polarization of silica sands: Experiment and modeling M. Skold, 1 A. Revil, 1,2 and P. Vaudelet
GEOPHYSICAL RESEARCH LETTERS, VOL. 38,, doi:10.1029/2011gl047748, 2011 The ph dependence of spectral induced polarization of silica sands: Experiment and modeling M. Skold, 1 A. Revil, 1,2 and P. Vaudelet
Fracture-Matrix Flow Partitioning and Cross Flow: Numerical Modeling of Laboratory Fractured Core Flood
 Fracture-Matrix Flow Partitioning and Cross Flow: Numerical Modeling of Laboratory Fractured Core Flood R. Sanaee *, G. F. Oluyemi, M. Hossain, and M. B. Oyeneyin Robert Gordon University *Corresponding
Fracture-Matrix Flow Partitioning and Cross Flow: Numerical Modeling of Laboratory Fractured Core Flood R. Sanaee *, G. F. Oluyemi, M. Hossain, and M. B. Oyeneyin Robert Gordon University *Corresponding
RP 2.6. SEG/Houston 2005 Annual Meeting 1521
 Ludmila Adam 1, Michael Batzle 1, and Ivar Brevik 2 1 Colorado School of Mines, 2 Statoil R&D Summary A set of carbonate plugs of different porosity, permeability, mineralogy and texture are measured at
Ludmila Adam 1, Michael Batzle 1, and Ivar Brevik 2 1 Colorado School of Mines, 2 Statoil R&D Summary A set of carbonate plugs of different porosity, permeability, mineralogy and texture are measured at
*blood and bones contain colloids. *milk is a good example of a colloidal dispersion.
 Chap. 3. Colloids 3.1. Introduction - Simple definition of a colloid: a macroscopically heterogeneous system where one component has dimensions in between molecules and macroscopic particles like sand
Chap. 3. Colloids 3.1. Introduction - Simple definition of a colloid: a macroscopically heterogeneous system where one component has dimensions in between molecules and macroscopic particles like sand
KOZENY-CARMAN EQUATION REVISITED. Jack Dvorkin Abstract
 KOZENY-CARMAN EQUATION REVISITED Jack Dvorkin -- 009 Abstract The Kozeny-Carman equation is often presented as permeability versus porosity, grain size, and tortuosity. When it is used to estimate permeability
KOZENY-CARMAN EQUATION REVISITED Jack Dvorkin -- 009 Abstract The Kozeny-Carman equation is often presented as permeability versus porosity, grain size, and tortuosity. When it is used to estimate permeability
EXPERIMENTAL AND NUMERICAL STUDIES OF A SPIRAL PLATE HEAT EXCHANGER
 THERMAL SCIENCE: Year 2014, Vol. 18, No. 4, pp. 1355-1360 1355 EXPERIMENTAL AND NUMERICAL STUDIES OF A SPIRAL PLATE HEAT EXCHANGER by Rangasamy RAJAVEL Department of Mechanical Engineering, AMET University,
THERMAL SCIENCE: Year 2014, Vol. 18, No. 4, pp. 1355-1360 1355 EXPERIMENTAL AND NUMERICAL STUDIES OF A SPIRAL PLATE HEAT EXCHANGER by Rangasamy RAJAVEL Department of Mechanical Engineering, AMET University,
16 Rainfall on a Slope
 Rainfall on a Slope 16-1 16 Rainfall on a Slope 16.1 Problem Statement In this example, the stability of a generic slope is analyzed for two successive rainfall events of increasing intensity and decreasing
Rainfall on a Slope 16-1 16 Rainfall on a Slope 16.1 Problem Statement In this example, the stability of a generic slope is analyzed for two successive rainfall events of increasing intensity and decreasing
ELECTRICAL AND THERMAL DESIGN OF UMBILICAL CABLE
 ELECTRICAL AND THERMAL DESIGN OF UMBILICAL CABLE Derek SHACKLETON, Oceaneering Multiflex UK, (Scotland), DShackleton@oceaneering.com Luciana ABIB, Marine Production Systems do Brasil, (Brazil), LAbib@oceaneering.com
ELECTRICAL AND THERMAL DESIGN OF UMBILICAL CABLE Derek SHACKLETON, Oceaneering Multiflex UK, (Scotland), DShackleton@oceaneering.com Luciana ABIB, Marine Production Systems do Brasil, (Brazil), LAbib@oceaneering.com
RADIONUCLIDE DIFFUSION IN GEOLOGICAL MEDIA
 GEOPHYSICS RADIONUCLIDE DIFFUSION IN GEOLOGICAL MEDIA C. BUCUR 1, M. OLTEANU 1, M. PAVELESCU 2 1 Institute for Nuclear Research, Pitesti, Romania, crina.bucur@scn.ro 2 Academy of Scientists Bucharest,
GEOPHYSICS RADIONUCLIDE DIFFUSION IN GEOLOGICAL MEDIA C. BUCUR 1, M. OLTEANU 1, M. PAVELESCU 2 1 Institute for Nuclear Research, Pitesti, Romania, crina.bucur@scn.ro 2 Academy of Scientists Bucharest,
Next layer is called diffuse layer----ions not held tightly----thickness is > 1000 angstroms-- exact distance depends on ionic strength of soln
 What does double layer of IPE look like?-see Fig.1.2.3 Also called Electrified Inteface At no external E appl -- inner Stern layer or Inner Helmholz plane (IHP) contains mostly solvent solvent molecules
What does double layer of IPE look like?-see Fig.1.2.3 Also called Electrified Inteface At no external E appl -- inner Stern layer or Inner Helmholz plane (IHP) contains mostly solvent solvent molecules
Permeability and fluid transport
 Permeability and fluid transport Thermal transport: Fluid transport: q = " k # $p with specific discharge (filter velocity) q [m s 1 ] pressure gradient p [N m 3 ] dynamic viscosity η [N s m 2 ] (intrinsic)
Permeability and fluid transport Thermal transport: Fluid transport: q = " k # $p with specific discharge (filter velocity) q [m s 1 ] pressure gradient p [N m 3 ] dynamic viscosity η [N s m 2 ] (intrinsic)
3/24/11. Introduction! Electrogenic cell
 March 2011 Introduction Electrogenic cell Electrode/electrolyte interface Electrical double layer Half-cell potential Polarization Electrode equivalent circuits Biopotential electrodes Body surface electrodes
March 2011 Introduction Electrogenic cell Electrode/electrolyte interface Electrical double layer Half-cell potential Polarization Electrode equivalent circuits Biopotential electrodes Body surface electrodes
MEASUREMENT OF CAPILLARY PRESSURE BY DIRECT VISUALIZATION OF A CENTRIFUGE EXPERIMENT
 MEASUREMENT OF CAPILLARY PRESSURE BY DIRECT VISUALIZATION OF A CENTRIFUGE EXPERIMENT Osamah A. Al-Omair and Richard L. Christiansen Petroleum Engineering Department, Colorado School of Mines ABSTRACT A
MEASUREMENT OF CAPILLARY PRESSURE BY DIRECT VISUALIZATION OF A CENTRIFUGE EXPERIMENT Osamah A. Al-Omair and Richard L. Christiansen Petroleum Engineering Department, Colorado School of Mines ABSTRACT A
Techniques for Fast Measurements of Low Dewpoint in Portable Hygrometers
 Techniques for Fast Measurements of Low Dewpoint in Portable Hygrometers Speaker / Author: Nick Malby, Michell Instruments Ltd, 48 Lancaster Way Business Park, Ely, Cambridgeshire. CB6 3NW. UK. +44 1353
Techniques for Fast Measurements of Low Dewpoint in Portable Hygrometers Speaker / Author: Nick Malby, Michell Instruments Ltd, 48 Lancaster Way Business Park, Ely, Cambridgeshire. CB6 3NW. UK. +44 1353
Impedance Spectroscopy on Carbonates
 SCA2014-039 1 / 6 Impedance Spectroscopy on Carbonates Matthias Halisch (1), Andreas Weller (2), Mohamed A. Kassab (3) (1) Leibniz Institute for Applied Geophysics (LIAG), Dept. 5 Petrophysics & Borehole
SCA2014-039 1 / 6 Impedance Spectroscopy on Carbonates Matthias Halisch (1), Andreas Weller (2), Mohamed A. Kassab (3) (1) Leibniz Institute for Applied Geophysics (LIAG), Dept. 5 Petrophysics & Borehole
CHEMICAL EFFECTS OF GOETHITE COLLOID ON THE TRANSPORT OF URANIUM (VI) THROUGH A SATURATED QUARTZ-PACKED COLUMN
 IAEA-CN-78/8 CHEMICAL EFFECTS OF GOETHITE COLLOID ON THE TRANSPORT OF URANIUM (VI) THROUGH A SATURATED QUARTZ-PACKED COLUMN A.A. ZAKI, M.Y. KHALIL, M.E. NAGY, H.F. ALY Atomic Energy Authority, Hot Laboratory
IAEA-CN-78/8 CHEMICAL EFFECTS OF GOETHITE COLLOID ON THE TRANSPORT OF URANIUM (VI) THROUGH A SATURATED QUARTZ-PACKED COLUMN A.A. ZAKI, M.Y. KHALIL, M.E. NAGY, H.F. ALY Atomic Energy Authority, Hot Laboratory
Experiment (4): Flow measurement
 Experiment (4): Flow measurement Introduction: The flow measuring apparatus is used to familiarize the students with typical methods of flow measurement of an incompressible fluid and, at the same time
Experiment (4): Flow measurement Introduction: The flow measuring apparatus is used to familiarize the students with typical methods of flow measurement of an incompressible fluid and, at the same time
Chapter 4 Influences of Compositional, Structural and Environmental Factors on. Soil EM Properties
 Chapter 4 Influences of Compositional, Structural and Environmental Factors on Soil EM Properties 4. 1 Introduction The measured soil electromagnetic properties can be affected by a large number of factors
Chapter 4 Influences of Compositional, Structural and Environmental Factors on Soil EM Properties 4. 1 Introduction The measured soil electromagnetic properties can be affected by a large number of factors
Chapter 6 ELECTRICAL CONDUCTIVITY ANALYSIS
 Chapter 6 ELECTRICAL CONDUCTIVITY ANALYSIS CHAPTER-6 6.1 Introduction The suitability and potentiality of a material for device applications can be determined from the frequency and temperature response
Chapter 6 ELECTRICAL CONDUCTIVITY ANALYSIS CHAPTER-6 6.1 Introduction The suitability and potentiality of a material for device applications can be determined from the frequency and temperature response
K.A. Terzi 1,2, I. Bountas 1,2 C.A. Aggelopoulos 1, C.D. Tsakiroglou 1
 K.A. Terzi 1,2, I. Bountas 1,2 C.A. Aggelopoulos 1, C.D. Tsakiroglou 1 1 Foundation for Research and Technology Hellas Institute of Chemical Engineering Sciences 2 Department of Chemical Engineering, Univ.
K.A. Terzi 1,2, I. Bountas 1,2 C.A. Aggelopoulos 1, C.D. Tsakiroglou 1 1 Foundation for Research and Technology Hellas Institute of Chemical Engineering Sciences 2 Department of Chemical Engineering, Univ.
2D Electrical Resistivity Tomography survey optimisation of solute transport in porous media
 ArcheoSciences Revue d'archéométrie 33 (suppl.) 2009 Mémoire du sol, espace des hommes 2D Electrical Resistivity Tomography survey optimisation of solute transport in porous media Gregory Lekmine, Marc
ArcheoSciences Revue d'archéométrie 33 (suppl.) 2009 Mémoire du sol, espace des hommes 2D Electrical Resistivity Tomography survey optimisation of solute transport in porous media Gregory Lekmine, Marc
1. Resistivity of rocks
 RESISTIVITY 1) Resistivity of rocks 2) General principles of resistivity surveying 3) Field procedures, interpretation and examples 4) Summary and conclusions INDUCED POLARIZATION 1) General principles
RESISTIVITY 1) Resistivity of rocks 2) General principles of resistivity surveying 3) Field procedures, interpretation and examples 4) Summary and conclusions INDUCED POLARIZATION 1) General principles
Differential Acoustic Resonance Spectroscopy Analysis of Fluids in Porous Media
 http://ijopaar.com; 2016 Vol. 2(1); pp. 22-30 Differential Acoustic Resonance Spectroscopy Analysis of Fluids in Porous Media Dr.Mohammad Miyan Associate Professor, Department of Mathematics, Shia P.G.College,
http://ijopaar.com; 2016 Vol. 2(1); pp. 22-30 Differential Acoustic Resonance Spectroscopy Analysis of Fluids in Porous Media Dr.Mohammad Miyan Associate Professor, Department of Mathematics, Shia P.G.College,
GENERAL INSTRUCTIONS GENERAL PREPARATION
 GENERAL INSTRUCTIONS Introduction The Van London-pHoenix Company Ammonium Ion Selective Electrode is used to quickly, simply, accurately, and economically measure potassium in aqueous solutions. Required
GENERAL INSTRUCTIONS Introduction The Van London-pHoenix Company Ammonium Ion Selective Electrode is used to quickly, simply, accurately, and economically measure potassium in aqueous solutions. Required
Analysis of cations and anions by Ion- Selective Electrodes (ISEs)
 Analysis of cations and anions by Ion- Selective Electrodes (ISEs) Purpose: The purpose of this assignment is to introduce potentiometric measurements of ionic species by ion selective electrodes (ISEs)
Analysis of cations and anions by Ion- Selective Electrodes (ISEs) Purpose: The purpose of this assignment is to introduce potentiometric measurements of ionic species by ion selective electrodes (ISEs)
Rock/water interaction in dielectric properties: Experiments with hydrophobic sandstones
 GEOPHYSICS, VOL. 60, NO. 2 (MARCH-APRIL 1995); P. 431-436, 9 FIGS., 4 TABLES. Rock/water interaction in dielectric properties: Experiments with hydrophobic sandstones Rosemary Knight* and Ana Abad ABSTRACT
GEOPHYSICS, VOL. 60, NO. 2 (MARCH-APRIL 1995); P. 431-436, 9 FIGS., 4 TABLES. Rock/water interaction in dielectric properties: Experiments with hydrophobic sandstones Rosemary Knight* and Ana Abad ABSTRACT
Supporting Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information Graphene transfer method 1 : Monolayer graphene was pre-deposited on both
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information Graphene transfer method 1 : Monolayer graphene was pre-deposited on both
Downloaded 02/05/15 to Redistribution subject to SEG license or copyright; see Terms of Use at
 Relationship among porosity, permeability, electrical and elastic properties Zair Hossain Alan J Cohen RSI, 2600 South Gessner Road, Houston, TX 77063, USA Summary Electrical resisivity is usually easier
Relationship among porosity, permeability, electrical and elastic properties Zair Hossain Alan J Cohen RSI, 2600 South Gessner Road, Houston, TX 77063, USA Summary Electrical resisivity is usually easier
