Refractive Index of Salt (KCl) From Aqueous Solution
|
|
|
- Edward Montgomery
- 5 years ago
- Views:
Transcription
1 Refractive Index of Salt (KCl) From Aqueous Solution U.V. Biradar 1, S.M. Dongarge 2 and N.V.Wani 3 1 Department of Physics, Mahatma Basweshwar Mahavidyalaya, Latur, Maharashtra (India) 2 Department of Physics, Mahatma Basweshwar Mahavidyalaya, Latur, Maharashtra (India) 3 Department of Physics, Mahatma Basweshwar Mahavidyalaya, Latur, Maharashtra(India) ABSTRACT There are different methods to determine the refractive index of salts, solids (crystalline) and liquids. These methods are quite tedious. A simple and reliable method is developed by developing mathematical equation for aqueous solutions. The technique was employed to study refractive index of salt and water by varying concentrations. A hollow prism is used along with optical spectrometer at room temperature to determine refractive index of aqueous solution of KCl. The results were compared with the results obtained from commercial refractometers and it was found that this technique is quite reliable and can be safely used in the study of the optical properties of any crystal clear liquids and solids. 1.INTRODUCTION:- Refractive index is one of the most important optical properties of a medium. It plays an important role in many areas of material science with special reference to thin film technology and fiber optics. Similarly, measurement of refractive index is widely used in analytical chemistry to determine the concentration of solutions. Recent studies [Schwartz 1999, Olesberg 2000, Shlichta 1986] provide more detailed discussion on the concentration mapping by the measurement of refractive index of liquids. Temperature coefficient of refractive index can also be used to calculate thermal expansion coefficient [Miller 1975]. Several techniques are reported in literature for the measurement of concentration dependence of refractive index of liquids [McPherson 1999, Garcia 1999, Otalora 1999, Miyashita 1994]. The present project reports a relatively simple and effective technique, which can be used to measure the refractive index of the liquid at different concentrations. M.T. Teli developed the equation for volume shrinkage of salt when added to water which is taken into account for this experiment[1997 Radiation physics and chemistry].. This effect is known as dispersion. A prism divides white light into its constituent spectral colours. For a given colour, the refractive index of a medium depends on the density of the medium. In 1928, C.V. Raman discovered another (much weaker) type of light scattering in which the frequency changes when the light is scattered. The frequency shift Δν occurs when some of the energy of the scattered photon is taken up by a molecule, which is excited into vibration motion. Most of the molecules are initially in the ground state but because of thermal agitation some molecules will be in an excited state. The absolute refractive index of a medium is the ratio of the speed of electromagnetic radiation in free space to the speed of the radiation in that medium. The relative refractive index is the ratio of the speed of light in one medium to that in the adjacent medium. Refraction occurs with all types of waves but is most familiar with light waves. The refractive index of a medium differs with frequencythe scattering process can be thought of as the incoming photon raising the molecule to a virtual (i.e. non-existent) excited state. 2.Equipment used for the study:- It is a compact apparatus for obtaining a pure spectrum. It is used for the study of spectra and for finding the refractive index of a material in the form of a prism. In its simplest form it has following main parts. 29
2 ( Fig 1. Optical Spectrometer Hollow Prism ) 2.1Collimator :- Its purpose is to produce a parallel beam of light. It consists of a tube mounted horizontally on the arm of the spectrometer. The tube has a converging achromatic lens at one end and a sliding tube having and adjustable vertical slit at the other end. The focal length of the lens is almost equal to the length of the collimator tube. The distance between the slit and the lens can be alter by a rack and pinion arrangement or by sliding the inner tube to obtain parallel rays. The tube rests on two screws by which it can be slightly tilted up or down if necessary as shown in figure Slit :- It consists of two sharp edges. One of the edges is fixed while the other can be moved parallel to it by working the screw Sprovided at its side as shown in figure Telescope :- It is an astronomical telescope having an achromatic objective and a Ramsden s eye-piece. It is mounted on another arm fixed rigidly to the circular scale in half degrees. The telescope along with scale can be turned round a vertical axis passing through the centre of the spectrometer. It can be fixed in any position by screw and then can be given a slow rotation or fine adjustment by a tangent screw. The position of the telescope can be read by the two vernier V 1 and V 2,180 0 apart fixed to the prism table. The telescope rests on the screws by which it can be slightly tilted up or down, for adjustment of its axis if necessary. A rack and pinion arrangement is provided on the side of the telescope for focusing. 2.4 Prism table: - It consists of an upper plate and a lower plate separated by three spring through which passes the leveling screws. A set of parallel equidistant lines are engraved on the upper plate. These lines are parallel to the line joining any two of the screws. The prism is always placed with one of its reflecting faces perpendicular to these lines. A series of circles concentric with the axis of rotation of the prism are also ruled on this plate. This help in placing the prism correctly. The height of prism table can be adjusted by camping screw, which is used to fix the prism table to the vernier V 1 and V 2. The table can rotate about vertical axes and may be fixed in any desired position by means of the screw. When fixed by the screw, it can be turned very slowly by a tangent screw placed at a base of the spectrometer. The position of table can be accurately read by vernier moving on the circular scale. Fig 2.: Prism table 30
3 3.THEORY DEVELOPED FOR THE DETERMINATION OF R.I.OF SALT :- Spectroscopy is the study of interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength by a prism. Latter the concept was expanded greatly to comprise any interaction with radiant energy as a function of its wavelength or frequency. Electromagnetic radiation was the first source of energy used for spectroscopic studies, There are different type of spectroscopy such as microwave spectroscopy, UV spectroscopy, IR spectroscopy, Gamma ray spectroscopy, acoustic spectroscopy.etc. Spectrometers are used as spectral measurement devices. A convenient formula for refractive index is obtained in the minimum deviation case when a ray of light suffers deviation while passing through a prism. The deviation produced by the prism depends on the angle of incidence. For a certain value of the angle of incidence, the angle of deviation is minimum. If m denotes the angle of minimum deviation for a given prism of refractive angle A, then the refractive index of the material of the prism is given by = sin(a+ m/2)/sin(a/2) (i) Equation (i) is used to calculate the refractive index of the liquids (water) where A is angle of hollow prism and m is angle of minimum deviation. If we consider V w as the volume of water taken in the hollow prism and V s is the volume of salt (KCl) added to the water.then total volume (V s +V w ) in the hollow prism is V = V s + V w When the salt (KCl) is added to the water then there will be shrinkage of volume( ) in the salt (KCl) which is to be considered by Teli s equation not by the above equation =V V i.e.v = V =V (1 / V )=V (1- ) Where = / V =V V/V =Degree of shrinkage If we consider this equation for the solution for determining refractive index combining together (salt and water) for different concentrations we get an equation of straight line in the following form. = w + ( s w ) V s /V (ii) Where is the refractive index of solution, w is the refractive index of the water and s is the refractive index of the salt. The equation (ii) can be used for any concentration of solution. These values are given in table 1. Experimental arrangement used in our study is shown in Fig1.Specially constructed hollow prism is used to measure the refractive index of liquid with the help of an optical spectrometer. A mercury lamp is used as a source of light and a collimated beam is allowed to fall on one reflecting face of the liquid prism and the angle of minimum deviation is determined for sodium light(at nm). Mean of two values are taken for each angle of minimum deviation( m). To study the variation of refractive index of salt solution as a function of concentration, an electronic balance is used to measure the mass of salt and solutions of required concentration are prepared by dissolving the salt(kcl) in 100 ml of water. Thus, prepared solutions are filtered before pouring into the hollow prism. The hollow prism was rinsed carefully after every measurement. Solutions of lower concentrations(like 0.05 M,0.10 M,0.15 M,0.20 M,0.25 M, 0.30 M,0.35 M,0.40 M,0.45 M, 0.50 M )are made by concentrating the water with proper weight of salt. Accordingly the concentration is converted to volume as required in formula. Refractive index of KCl salt solution as a function of concentration is shown in Fig.3. For 0.50 M solution, refractive index is as high as 1.350, which reduces to when the solution is diluted to a concentration of 0.05 M.With the decrease in concentration, the density of the solution also decreases and also refractive index decreases. The results showed that the refractive index of the solution of concentration less than 0.05 M measures nearly the same as that of the pure water. The resultindicates that the effect of concentration on refractive index is dominant upto the concentration of 0.05 M. By using above mathematical equations we can calculate refractive index of water ( w ) and KCl by using graph as shown in fig.4. A graph between R.I. and concentrationof KCl solution gives the slope of R.I.ofsolution (KCl salt and water)and intercept gives the R.I.of water. By subtracting intercept from slope we get R.I.ofKCl. For this purpose, we made the calculations mentioned in table 1. It requires to be converted in V s and V w form. For 31
4 Refractive Index International Journal of Computer & Mathematical Sciences this we have considered least square method. The refractive index of KCl salt is and water is Both these values are within the acceptable limit,which satisfies our experimental and theoretical 4.CONCLUSION :- We have been able to use a hollow prism suitable for the measurement of refractive index of crystal clear aqueous solutions. Experimental results calculated by using formula considered by DongargeS.M. isemployed and the technique is easier than the other methods mentioned here. Our results are in good agreement with other methods employed. Here the study of dependence ofconcentration on refractive index of solutions is not only carried out but also the refractive indexof salts whichare dissolved in water are also calculated. This is an alternate method to determine the R.I. of crystalline material without taking them into prism form. 5. ACKNOWLEDGEMENT :- We are thankful to Prof. Dr. J S Dargad, Principal,Dayanand Science College, Latur for giving the permission to work in their research lab.we are also thankful to Dr. Dindore U B, Adarsh College, Omerga. Table 1 : Observation table for R.I. of aqueous solution of KCl solution Sr. No. Concentratio n In mol Ver-I Ver-II Refractive Index (Experimental) Refractive Index (Theroetical) o o o o o o o o o o o o o o o o o o o o o o Molar Concentration v/s Refractive Index n Pract. n Theo Molar Concentration Fig.3 :Refractive index of potassium chloride solution as a function of its concentration expressed in Molar. 32
5 R.I. of Solution International Journal of Computer & Mathematical Sciences Refractive Index of KCl in water with concentration Series1 Series2 Concentration Vs/Vs+Vw Fig.4:Refractive index of potassium chloride solution as a function of its concentration expressed in Volume. REFERENCES:- [1]U.V. Biradar and S.M. Dongarge, Refractive Index of Salt (NaCl) from Aquous Solution, International Journal of Computer & Mathematical Sciences Volume 4, Issue 12 December [2] Schwartz, A.M., Berglund, K.A., The use of Raman spectroscopy for in situ monitoring of lysozyme concentration during crystallization in a hanging drop. J. Crystal Growth, 203, 599. [3]Olesberg, J.T, Arnold, M.A, Hu, S-Y.B., Wiencek, J.M., Temperature insensitive near infrared method for determination of protein concentration during protein crystal growth.analytical Chemistry, 72, [4]Shlichta, P. J., Feasibility of mapping solution properties during the growth of protein crystals. J. Crystal Growth, 76, 656. [5] Miller, A., Hussmann, E. K., McLaughlin, W. L., Interferometer for measuring fast changes of refractive index and temperature in transparent liquids Review of Scientific instruments, 46, [6] McPherson, A., Malkin, A. J., Kuznetsov, Y. G., Koszelak, S., Wells, M., Jenkins, G., Howard, J., Lawson, G., Effects of microgravity on protein crystallization: evidence for concentration gradients around growing crystals, J. Crystal Growth, 196, 572. [7] Garcia-Ruiz, J. M., Novella, M. L., Otalora, F., Supersaturation patterns in counter-diffusion crystallisation methods followed by Mach-Zehnder interferometry, Crystal Growth, 196, [8]Otalora F, Novella ML, Rondon D, Garcia-Ruiz JM, Growth of lysozyme crystals under micro gravity conditions in the LMS (STS-78) mission Journal of Crystal Growth, 196, 649. [9] Miyashita, S., Komatsu, H., Suzuki, Y., Nakada, T., Observation of the concentration distribution around a growing lysozyme crystal, J. Crystal Growth, 141, 419. [10]Lukin, O.V., Magunov, A. N., Temperature measurement of glass and quartz plates by laser interferometry, Opt. Spectroscopy., 74, 630. [11] R. S. Longhurst, Geometrical and Physical Optics (Wiley, NewYork, 1967). [12] B. W. Grange, W. H. Stevenson, and R. Viskanta, "Refractive Index of Liquid Solutions at Low Temperatures: An Accurate Measurement," Appl. Opt. 15, 858 (1976). [13] D. D. Jenkin, "Refractive Index of Solution," Phys. Educ. 17, 82 (1982). [14] J. M. Cariou, J. Dugas, L. Martin, and P. Michel, "Refractive- Index Variations with Temperature of PMMA and Polycarbonate," Appl. Opt. 25, ). [15] J. D. Bass and D. J. Weidner, "Method for Measuring the Refractive Index of Transparent Solids," Rev. Sci. Instrum. 55, 1569 (1984). [16] M. T. Teli on attenuation coefficient of X-ray and ɤ-ray on aqueous solutions of salts. Radiation physics and chemistry. 33
Study of Refractive Index of Potassium Dichromate Solution as a Function of its Concentration
 Study of Refractive Index of Potassium Dichromate Solution as a Function of its Concentration N.V.Wani 1,S.M. Dongarge 2 andu.v. Biradar 3 1,2,3 Department of Physics, Mahatma Basweshwar Mahavidyalaya,
Study of Refractive Index of Potassium Dichromate Solution as a Function of its Concentration N.V.Wani 1,S.M. Dongarge 2 andu.v. Biradar 3 1,2,3 Department of Physics, Mahatma Basweshwar Mahavidyalaya,
Refractive Index of Salt (NaCl) from Aquous Solution
 Refractive Index of Salt (NaCl) from Aquous Solution Department of Physics, Mahatma Basweshwar Mahavidyalaya Latur 413512 (M.S.)INDIA. ABSTRACT There are different methods to determine the refractive index
Refractive Index of Salt (NaCl) from Aquous Solution Department of Physics, Mahatma Basweshwar Mahavidyalaya Latur 413512 (M.S.)INDIA. ABSTRACT There are different methods to determine the refractive index
RESEARCH ON STUDY OF CONCENTRATION DEPENDENCE OF REFRACTIVE INDEX OF OIL USING A NOVEL TECHNIQUE
 RESEARCH ON STUDY OF CONCENTRATION DEPENDENCE OF REFRACTIVE INDEX OF OIL USING A NOVEL TECHNIQUE Department of Physics, Mid Western University, Campus of Science and Technology email:dhakaldharma605@gmail.com
RESEARCH ON STUDY OF CONCENTRATION DEPENDENCE OF REFRACTIVE INDEX OF OIL USING A NOVEL TECHNIQUE Department of Physics, Mid Western University, Campus of Science and Technology email:dhakaldharma605@gmail.com
Optical Characterization of Sucrose (C 12 H 21 O 11 ) at Different Temperatures
 Optical Characterization of Sucrose (C 12 H 21 O 11 ) at Different Temperatures N. K. Wagholikar 1, S. B. Jagtap 2 * Asst. Professor, Dept. of Physics, Sir Parashurambhau College, Pune, Maharashtra State,
Optical Characterization of Sucrose (C 12 H 21 O 11 ) at Different Temperatures N. K. Wagholikar 1, S. B. Jagtap 2 * Asst. Professor, Dept. of Physics, Sir Parashurambhau College, Pune, Maharashtra State,
Experiment #4: Optical Spectrometer and the Prism Deviation
 Experiment #4: Optical Spectrometer and the Prism Deviation Carl Adams October 2, 2011 1 Purpose In the first part of this lab you will set up and become familiar with an optical spectrometer. In the second
Experiment #4: Optical Spectrometer and the Prism Deviation Carl Adams October 2, 2011 1 Purpose In the first part of this lab you will set up and become familiar with an optical spectrometer. In the second
DISPERSION OF A GLASS PRISM
 PH2 page 1 DISPERSION OF A GLASS PRISM OBJECTIVE The objective of this experiment is to analyze the emission spectrum of helium and to analyze the dispersion of a glass prism by measuring the index of
PH2 page 1 DISPERSION OF A GLASS PRISM OBJECTIVE The objective of this experiment is to analyze the emission spectrum of helium and to analyze the dispersion of a glass prism by measuring the index of
Determination of Cauchy s Contants
 8. Determination of Cauchy s Contants 8.1 Objective: To determine Cauchy s Constants using a prism and spectrometer. Apparatus: Glass prism, spectrometer and mercury vapour lamp. 8. Theory: The wavelength
8. Determination of Cauchy s Contants 8.1 Objective: To determine Cauchy s Constants using a prism and spectrometer. Apparatus: Glass prism, spectrometer and mercury vapour lamp. 8. Theory: The wavelength
DISPERSION AND SPECTRA CHAPTER 20
 CHAPTER 20 DISPERSION AND SPECTRA 20.1 DISPERSION As mentioned earlier, the refractive index of a material depends slightly on the wavelength of light. The relation between the two may be approximately
CHAPTER 20 DISPERSION AND SPECTRA 20.1 DISPERSION As mentioned earlier, the refractive index of a material depends slightly on the wavelength of light. The relation between the two may be approximately
Any first year text, sections on atomic structure, spectral lines and spectrometers
 Physics 33 Experiment 5 Atomic Spectra References Any first year text, sections on atomic structure, spectral lines and spectrometers Any modern physics text, eg F.K. Richtmeyer, E.H. Kennard and J.N.
Physics 33 Experiment 5 Atomic Spectra References Any first year text, sections on atomic structure, spectral lines and spectrometers Any modern physics text, eg F.K. Richtmeyer, E.H. Kennard and J.N.
Measurement of Temperature and Concentration Dependences of. Refractive Index of Hen-egg-white Lysozyme Solution
 Measurement of Temperature and Concentration Dependences of Refractive Index of Hen-egg-white Lysozyme Solution Corresponding author: Y. Inatomi Postal Address: Space Utilization Research Center, The Institute
Measurement of Temperature and Concentration Dependences of Refractive Index of Hen-egg-white Lysozyme Solution Corresponding author: Y. Inatomi Postal Address: Space Utilization Research Center, The Institute
THE DIFFRACTION GRATING SPECTROMETER
 Purpose Theory THE DIFFRACTION GRATING SPECTROMETER a. To study diffraction of light using a diffraction grating spectrometer b. To measure the wavelengths of certain lines in the spectrum of the mercury
Purpose Theory THE DIFFRACTION GRATING SPECTROMETER a. To study diffraction of light using a diffraction grating spectrometer b. To measure the wavelengths of certain lines in the spectrum of the mercury
Study of concentration dependence of Refractive index of Sugar and Salt Solution
 KATHMANDU UNIVERSITY SCHOOL OF SCIENCE DEPARTMENT OF NATURAL SCIENCE (PHYSICS) Study of concentration dependence of Refractive index of Sugar and Salt Solution A 2 nd Year 1 st Semester Project Report
KATHMANDU UNIVERSITY SCHOOL OF SCIENCE DEPARTMENT OF NATURAL SCIENCE (PHYSICS) Study of concentration dependence of Refractive index of Sugar and Salt Solution A 2 nd Year 1 st Semester Project Report
Optics. Measuring the line spectra of inert gases and metal vapors using a prism spectrometer. LD Physics Leaflets P
 Optics Spectrometer Prism spectrometer LD Physics Leaflets P5.7.1.1 Measuring the line spectra of inert gases and metal vapors using a prism spectrometer Objects of the experiment Adjusting the prism spectrometer.
Optics Spectrometer Prism spectrometer LD Physics Leaflets P5.7.1.1 Measuring the line spectra of inert gases and metal vapors using a prism spectrometer Objects of the experiment Adjusting the prism spectrometer.
EXPERIMENT 5:Determination of the refractive index (µ) of the material of a prism using sprectometer
 EXPERIMENT 5:Determination of the refractive index (µ) of the material of a prism using sprectometer Debangshu Mukherjee B.Sc Physics,1st year Chennai Mathematical Institute 17.10.008 1 Aim of Experiment
EXPERIMENT 5:Determination of the refractive index (µ) of the material of a prism using sprectometer Debangshu Mukherjee B.Sc Physics,1st year Chennai Mathematical Institute 17.10.008 1 Aim of Experiment
Lab 5: Spectroscopy & the Hydrogen Atom Phy248 Spring 2009
 Lab 5: Spectroscopy & the Hydrogen Atom Phy248 Spring 2009 Name Section Return this spreadsheet to your TA that will use it to score your lab. To receive full credit you must use complete sentences and
Lab 5: Spectroscopy & the Hydrogen Atom Phy248 Spring 2009 Name Section Return this spreadsheet to your TA that will use it to score your lab. To receive full credit you must use complete sentences and
Laboratory #29: Spectrometer
 INDIANA UNIVERSITY, DEPARTMENT OF PHYSICS, P309 LABORATORY Laboratory #29: Spectrometer Goal: Learn to adjust an optical spectrometer, use a transmission grating to measure known spectral lines of mercury,
INDIANA UNIVERSITY, DEPARTMENT OF PHYSICS, P309 LABORATORY Laboratory #29: Spectrometer Goal: Learn to adjust an optical spectrometer, use a transmission grating to measure known spectral lines of mercury,
ARC SPECTRUM OF IRON /COPPER / BRASS
 ARC PECTRUM OF IRON /COPPER / BRA Aim : To determine the wavelength of prominent lines in the emission spectrum of iron/ copper/ brass. Apparatus : Constant deviation spectrometer, dc voltage source, metal
ARC PECTRUM OF IRON /COPPER / BRA Aim : To determine the wavelength of prominent lines in the emission spectrum of iron/ copper/ brass. Apparatus : Constant deviation spectrometer, dc voltage source, metal
CHEM*3440. Photon Energy Units. Spectrum of Electromagnetic Radiation. Chemical Instrumentation. Spectroscopic Experimental Concept.
 Spectrum of Electromagnetic Radiation Electromagnetic radiation is light. Different energy light interacts with different motions in molecules. CHEM*344 Chemical Instrumentation Topic 7 Spectrometry Radiofrequency
Spectrum of Electromagnetic Radiation Electromagnetic radiation is light. Different energy light interacts with different motions in molecules. CHEM*344 Chemical Instrumentation Topic 7 Spectrometry Radiofrequency
Dispersion and resolving power of the prism and grating spectroscope (Item No.: P )
 Dispersion and resolving power of the prism and grating spectroscope (Item No.: P2210300) Curricular Relevance Area of Expertise: Physics Education Level: University Topic: Light and Optics Subtopic: Diffraction
Dispersion and resolving power of the prism and grating spectroscope (Item No.: P2210300) Curricular Relevance Area of Expertise: Physics Education Level: University Topic: Light and Optics Subtopic: Diffraction
To determine the wavelengths of light emitted by a mercury vapour lamp by using a diffraction grating.
 12. Diffraction grating OBJECT To determine the wavelengths of light emitted by a mercury vapour lamp by using a diffraction grating. INTRODUCTION: Consider a light beam transmitted through an aperture
12. Diffraction grating OBJECT To determine the wavelengths of light emitted by a mercury vapour lamp by using a diffraction grating. INTRODUCTION: Consider a light beam transmitted through an aperture
Ph 3455/MSE 3255 Experiment 2: Atomic Spectra
 Ph 3455/MSE 3255 Experiment 2: Atomic Spectra Background Reading: Tipler, Llewellyn pp. 163-165 Apparatus: Spectrometer, sodium lamp, hydrogen lamp, mercury lamp, diffraction grating, watchmaker eyeglass,
Ph 3455/MSE 3255 Experiment 2: Atomic Spectra Background Reading: Tipler, Llewellyn pp. 163-165 Apparatus: Spectrometer, sodium lamp, hydrogen lamp, mercury lamp, diffraction grating, watchmaker eyeglass,
Instruments and Related Topics
 1. Traveling Microscope The traveling microscope [Fig.1] is an instrument well-suited for the purpose of measuring small vertical or horizontal distances with high accuracy. It consists of a compound microscope
1. Traveling Microscope The traveling microscope [Fig.1] is an instrument well-suited for the purpose of measuring small vertical or horizontal distances with high accuracy. It consists of a compound microscope
UNIT-5 EM WAVES UNIT-6 RAY OPTICS
 UNIT-5 EM WAVES 2 Marks Question 1. To which regions of electromagnetic spectrum do the following wavelengths belong: (a) 250 nm (b) 1500 nm 2. State any one property which is common to all electromagnetic
UNIT-5 EM WAVES 2 Marks Question 1. To which regions of electromagnetic spectrum do the following wavelengths belong: (a) 250 nm (b) 1500 nm 2. State any one property which is common to all electromagnetic
E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 3081, 4051 FRAUNHOFER DIFFRACTION
 E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 3081, 4051 FRAUNHOFER DIFFRACTION References for Fraunhofer Diffraction 1. Jenkins and White Fundamentals of Optics. Chapters on Fraunhofer diffraction and
E. K. A. ADVANCED PHYSICS LABORATORY PHYSICS 3081, 4051 FRAUNHOFER DIFFRACTION References for Fraunhofer Diffraction 1. Jenkins and White Fundamentals of Optics. Chapters on Fraunhofer diffraction and
Pre-lab Quiz/PHYS 224. Your name Lab section
 Pre-lab Quiz/PHYS 224 THE DIFFRACTION GRATING AND THE OPTICAL SPECTRUM Your name Lab section 1. What are the goals of this experiment? 2. If the period of a diffraction grating is d = 1,000 nm, where the
Pre-lab Quiz/PHYS 224 THE DIFFRACTION GRATING AND THE OPTICAL SPECTRUM Your name Lab section 1. What are the goals of this experiment? 2. If the period of a diffraction grating is d = 1,000 nm, where the
Lab 10: Spectroscopy & the Hydrogen Atom Phy208 Fall 2008
 Lab 10: Spectroscopy & the Hydrogen Atom Phy208 Fall 2008 Name Section This sheet is the lab document your TA will use to score your lab. It is to be turned in at the end of lab. To receive full credit
Lab 10: Spectroscopy & the Hydrogen Atom Phy208 Fall 2008 Name Section This sheet is the lab document your TA will use to score your lab. It is to be turned in at the end of lab. To receive full credit
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications
 Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
1 WHAT IS SPECTROSCOPY?
 1 WHAT IS SPECTROSCOPY? 1.1 The Nature Of Electromagnetic Radiation Anyone who has been sunburnt will know that light packs a punch: in scientific terms, it contains considerable amounts of energy. All
1 WHAT IS SPECTROSCOPY? 1.1 The Nature Of Electromagnetic Radiation Anyone who has been sunburnt will know that light packs a punch: in scientific terms, it contains considerable amounts of energy. All
NORTHERN ILLINOIS UNIVERSITY PHYSICS DEPARTMENT. Physics 211 E&M and Quantum Physics Spring Lab #9: Diffraction Spectroscopy
 NORTHERN ILLINOIS UNIVERSITY PHYSICS DEPARTMENT Physics 211 E&M and Quantum Physics Spring 2018 Lab #9: Diffraction Spectroscopy Lab Writeup Due: Mon/Wed/Thu/Fri, April 30/ May 2/3/4, 2018 Background All
NORTHERN ILLINOIS UNIVERSITY PHYSICS DEPARTMENT Physics 211 E&M and Quantum Physics Spring 2018 Lab #9: Diffraction Spectroscopy Lab Writeup Due: Mon/Wed/Thu/Fri, April 30/ May 2/3/4, 2018 Background All
AS 101: Day Lab #2 Summer Spectroscopy
 Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
The Quantum Model of the Hydrogen Atom
 Physics 109 Science 1 Experiment 1 1 The Quantum Model of the Hydrogen Atom In this experiment you will use a spectrometer to determine the wavelengths of the visible lines of atomic hydrogen. The goal
Physics 109 Science 1 Experiment 1 1 The Quantum Model of the Hydrogen Atom In this experiment you will use a spectrometer to determine the wavelengths of the visible lines of atomic hydrogen. The goal
Engineering Physics 1 Prof. G.D. Vermaa Department of Physics Indian Institute of Technology-Roorkee
 Engineering Physics 1 Prof. G.D. Vermaa Department of Physics Indian Institute of Technology-Roorkee Module-04 Lecture-02 Diffraction Part - 02 In the previous lecture I discussed single slit and double
Engineering Physics 1 Prof. G.D. Vermaa Department of Physics Indian Institute of Technology-Roorkee Module-04 Lecture-02 Diffraction Part - 02 In the previous lecture I discussed single slit and double
Atomic and nuclear physics
 Atomic and nuclear physics Atomic shell Normal Zeeman effect LEYBOLD Physics Leaflets Observing the normal Zeeman effect in transverse and longitudinal Objects of the experiment Observing the line triplet
Atomic and nuclear physics Atomic shell Normal Zeeman effect LEYBOLD Physics Leaflets Observing the normal Zeeman effect in transverse and longitudinal Objects of the experiment Observing the line triplet
LAB 10: OPTICAL MATERIALS AND DISPERSION I
 OPTI 202L - Geometrical and Instrumental Optics Lab LAB 10: OPTICAL MATERIALS AND DISPERSION I 10-1 Measuring the refractive index of a material is one of the most fundamental optical measurements, and
OPTI 202L - Geometrical and Instrumental Optics Lab LAB 10: OPTICAL MATERIALS AND DISPERSION I 10-1 Measuring the refractive index of a material is one of the most fundamental optical measurements, and
DAY LABORATORY EXERCISE: SPECTROSCOPY
 AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
MOLEBIO LAB #4: Using a Spectrophotometer
 Introduction: Spectrophotometry MOLEBIO LAB #4: Using a Spectrophotometer Many kinds of molecules interact with or absorb specific types of radiant energy in a predictable fashion. For example, when while
Introduction: Spectrophotometry MOLEBIO LAB #4: Using a Spectrophotometer Many kinds of molecules interact with or absorb specific types of radiant energy in a predictable fashion. For example, when while
Solution 3: A glass prism deviates the violet light most and the red light least.
 EXERCISE- 6 (A) Question 1: Name three factors on which the deviation produces by a prism depends and state how does it depend on the factors stated by you. Solution 1: The deviation produced by the prism
EXERCISE- 6 (A) Question 1: Name three factors on which the deviation produces by a prism depends and state how does it depend on the factors stated by you. Solution 1: The deviation produced by the prism
n(λ) = c/v(λ). Figure 1: Dispersion curves for some common optical glass types.
 Physics 2310 Lab 2: The Dispersion of Optical Glass Dr. Michael Pierce (Univ. of Wyoming) Based on a lab by Dr. M. Kruger (Univ. of Missouri, Kansas City) Purpose: The purpose of this lab is to introduce
Physics 2310 Lab 2: The Dispersion of Optical Glass Dr. Michael Pierce (Univ. of Wyoming) Based on a lab by Dr. M. Kruger (Univ. of Missouri, Kansas City) Purpose: The purpose of this lab is to introduce
PHYSICS 116 SPECTROSCOPY: DETERMINATION OF THE WAVELENGTH OF LIGHT
 Name Date Lab Time Lab TA PHYSICS 116 SPECTROSCOPY: DETERMINATION OF THE WAVELENGTH OF LIGHT I. PURPOSE To use a diffraction grating to investigate the spectra produced by several unknown gas discharge
Name Date Lab Time Lab TA PHYSICS 116 SPECTROSCOPY: DETERMINATION OF THE WAVELENGTH OF LIGHT I. PURPOSE To use a diffraction grating to investigate the spectra produced by several unknown gas discharge
School. Team Number. Optics
 School Team Number Optics Physical Optics (30%) Proceed to the laser shoot (40%) when your team number is called. 1. What are the four colors used in the CMYK color model? (2 points) 2. Muscae Volitantes
School Team Number Optics Physical Optics (30%) Proceed to the laser shoot (40%) when your team number is called. 1. What are the four colors used in the CMYK color model? (2 points) 2. Muscae Volitantes
Telescopes (Chapter 6)
 Telescopes (Chapter 6) Based on Chapter 6 This material will be useful for understanding Chapters 7 and 10 on Our planetary system and Jovian planet systems Chapter 5 on Light will be useful for understanding
Telescopes (Chapter 6) Based on Chapter 6 This material will be useful for understanding Chapters 7 and 10 on Our planetary system and Jovian planet systems Chapter 5 on Light will be useful for understanding
Experiment #5: Cauchy s Formula
 Experiment #5: Cauchy s Formula Carl Adams October 14, 2011 1 Purpose This experiment is a continuation of Experiment #4. It is assumed you have an aligned spectrometer. 2 Safety/Protocol 1. The gas discharge
Experiment #5: Cauchy s Formula Carl Adams October 14, 2011 1 Purpose This experiment is a continuation of Experiment #4. It is assumed you have an aligned spectrometer. 2 Safety/Protocol 1. The gas discharge
Skoog Chapter 6 Introduction to Spectrometric Methods
 Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Application of IR Raman Spectroscopy
 Application of IR Raman Spectroscopy 3 IR regions Structure and Functional Group Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10-1 Mid-IR Mid-IR absorption Samples Placed in cell (salt)
Application of IR Raman Spectroscopy 3 IR regions Structure and Functional Group Absorption IR Reflection IR Photoacoustic IR IR Emission Micro 10-1 Mid-IR Mid-IR absorption Samples Placed in cell (salt)
Preview from Notesale.co.uk Page 1 of 38
 F UNDAMENTALS OF PHOTONICS Module 1.1 Nature and Properties of Light Linda J. Vandergriff Director of Photonics System Engineering Science Applications International Corporation McLean, Virginia Light
F UNDAMENTALS OF PHOTONICS Module 1.1 Nature and Properties of Light Linda J. Vandergriff Director of Photonics System Engineering Science Applications International Corporation McLean, Virginia Light
Reflection = EM strikes a boundary between two media differing in η and bounces back
 Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
Chapter 4. Dispersion of Glass. 4.1 Introduction. 4.2 Apparatus
 Chapter 4 Dispersion of Glass 4.1 Introduction This experiment will develop skills in choosing a suitable fit for data and plotting the resulting curve. Curve fitting will count for a big chunk of the
Chapter 4 Dispersion of Glass 4.1 Introduction This experiment will develop skills in choosing a suitable fit for data and plotting the resulting curve. Curve fitting will count for a big chunk of the
Spectroscopy. Page 1 of 8 L.Pillay (2012)
 Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
SPECTRUM. Dispersion. This phenomenon can be observed in a lab environment using a
 SPECTRUM Dispersion The phenomenon due to which a polychromatic light, like sunlight, splits into its component colours, when passed through a transparent medium like a glass prism, is called dispersion
SPECTRUM Dispersion The phenomenon due to which a polychromatic light, like sunlight, splits into its component colours, when passed through a transparent medium like a glass prism, is called dispersion
PC1144 Physics IV. Atomic Spectra
 PC1144 Physics IV Atomic Spectra 1 Objectives Investigate how well the visible light wavelengths of hydrogen predicted by the Bohr theory agree with experimental values. Determine an experimental value
PC1144 Physics IV Atomic Spectra 1 Objectives Investigate how well the visible light wavelengths of hydrogen predicted by the Bohr theory agree with experimental values. Determine an experimental value
EXPERIMENT 12 THE GRATING SPECTROMETER AND ATOMIC SPECTRA
 OBJECTIVES Learn the theory of the grating spectrometer Observe the spectrum of mercury and hydrogen Measure the grating constant of a diffraction grating Measure the Rydberg Constant EXPERIMENT THE GRATING
OBJECTIVES Learn the theory of the grating spectrometer Observe the spectrum of mercury and hydrogen Measure the grating constant of a diffraction grating Measure the Rydberg Constant EXPERIMENT THE GRATING
10. Wavelength measurement using prism spectroscopy
 Spk 0. Wavelength measurement using prism spectroscopy 0. Introduction The study of emitted spectra of electromagnetic waves by excited atoms makes for one of the most important methods to investigate
Spk 0. Wavelength measurement using prism spectroscopy 0. Introduction The study of emitted spectra of electromagnetic waves by excited atoms makes for one of the most important methods to investigate
LABORATORY WRITE-UP MICHELSON INTERFEROMETER LAB AUTHOR S NAME GOES HERE STUDENT NUMBER:
 LABORATORY WRITE-UP MICHELSON INTERFEROMETER LAB AUTHOR S NAME GOES HERE STUDENT NUMBER: 111-22-3333 MICHELSON INTERFEROMETER 1. PURPOSE The purpose of this experiment is to give some practice in using
LABORATORY WRITE-UP MICHELSON INTERFEROMETER LAB AUTHOR S NAME GOES HERE STUDENT NUMBER: 111-22-3333 MICHELSON INTERFEROMETER 1. PURPOSE The purpose of this experiment is to give some practice in using
Fig. 8.1 illustrates the three measurements. air medium A. ray 1. air medium A. ray 2. air medium A. ray 3. Fig For Examiner s Use
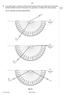 9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
A system of two lenses is achromatic when the separation between them is
 L e c t u r e 1 5 1 Eyepieces Single eye lens in a telescope / microscope produces spherical and chromatic aberrations. The field of view is also narrow. The eye lens is replaced by a system of lenses
L e c t u r e 1 5 1 Eyepieces Single eye lens in a telescope / microscope produces spherical and chromatic aberrations. The field of view is also narrow. The eye lens is replaced by a system of lenses
Lab report 30 EXPERIMENT 4. REFRACTION OF LIGHT
 30 EXPERIMENT 4. REFRACTION OF LIGHT Lab report Go to your course homepage on Sakai (Resources, Lab templates) to access the online lab report worksheet for this experiment. The worksheet has to be completed
30 EXPERIMENT 4. REFRACTION OF LIGHT Lab report Go to your course homepage on Sakai (Resources, Lab templates) to access the online lab report worksheet for this experiment. The worksheet has to be completed
Operating Instructions Spectro-Goniometer Student. 1 Functional Elements. 2 Safety Precautions. Figure 1: Spectro-Goniometer Student
 Operating Instructions Spectro-Goniometer Student 1 Functional Elements Figure 1: Spectro-Goniometer Student 1. Adjustable entrance slit, holding screw for slit cover 2. Lock ring fixing entrance slit
Operating Instructions Spectro-Goniometer Student 1 Functional Elements Figure 1: Spectro-Goniometer Student 1. Adjustable entrance slit, holding screw for slit cover 2. Lock ring fixing entrance slit
Protokoll. Grundpraktikum II - Optical Spectroscopy
 Protokoll Grundpraktikum II - Optical Spectroscopy 1 Elaboration 1.1 Optical Spectroscopy Student: Hauke Rasch, Martin Borchert Tutor: Madsen Date: 22.October2014 This experiment is about the fundamental
Protokoll Grundpraktikum II - Optical Spectroscopy 1 Elaboration 1.1 Optical Spectroscopy Student: Hauke Rasch, Martin Borchert Tutor: Madsen Date: 22.October2014 This experiment is about the fundamental
HYDROGEN SPECTRUM. Figure 1 shows the energy level scheme for the hydrogen atom as calculated from equation. Figure 1 Figure 2
 15 Jul 04 Hydrogen.1 HYDROGEN SPECTRUM In this experiment the wavelengths of the visible emission lines of hydrogen (Balmer series) will be measured and compared to the values predicted by Bohr s quantum
15 Jul 04 Hydrogen.1 HYDROGEN SPECTRUM In this experiment the wavelengths of the visible emission lines of hydrogen (Balmer series) will be measured and compared to the values predicted by Bohr s quantum
Build and Use a Simple Spectroscope
 Build and Use a Simple Spectroscope Subject Area: Physical Sciences Grade Level: 9 12 Overview In this activity students will build a spectroscope to analyze the composition of light. Our scope is inexpensive,
Build and Use a Simple Spectroscope Subject Area: Physical Sciences Grade Level: 9 12 Overview In this activity students will build a spectroscope to analyze the composition of light. Our scope is inexpensive,
Physics 30: Chapter 5 Exam Wave Nature of Light
 Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
Dispersion of light by a prism
 Dispersion of light by a prism Aim: (i) To calculate refractive index µ of a prism for various wavelengths (λ) of Hg and to find dispersive power of the material of the prism (ii) To plot µ-/λ curve and
Dispersion of light by a prism Aim: (i) To calculate refractive index µ of a prism for various wavelengths (λ) of Hg and to find dispersive power of the material of the prism (ii) To plot µ-/λ curve and
Vibrational Spectroscopies. C-874 University of Delaware
 Vibrational Spectroscopies C-874 University of Delaware Vibrational Spectroscopies..everything that living things do can be understood in terms of the jigglings and wigglings of atoms.. R. P. Feymann Vibrational
Vibrational Spectroscopies C-874 University of Delaware Vibrational Spectroscopies..everything that living things do can be understood in terms of the jigglings and wigglings of atoms.. R. P. Feymann Vibrational
1901 Application of Spectrophotometry
 1901 Application of Spectrophotometry Chemical Analysis Problem: 1 Application of Spectroscopy Organic Compounds Organic compounds with single bonds absorb in the UV region because electrons from single
1901 Application of Spectrophotometry Chemical Analysis Problem: 1 Application of Spectroscopy Organic Compounds Organic compounds with single bonds absorb in the UV region because electrons from single
The Nature of Light. We have a dual model
 Light and Atoms Properties of Light We can come to understand the composition of distant bodies by analyzing the light they emit This analysis can tell us about the composition as well as the temperature
Light and Atoms Properties of Light We can come to understand the composition of distant bodies by analyzing the light they emit This analysis can tell us about the composition as well as the temperature
Atomic emission spectra experiment
 Atomic emission spectra experiment Contents 1 Overview 1 2 Equipment 1 3 Measuring the grating spacing using the sodium D-lines 4 4 Measurement of hydrogen lines and the Rydberg Constant 5 5 Measurement
Atomic emission spectra experiment Contents 1 Overview 1 2 Equipment 1 3 Measuring the grating spacing using the sodium D-lines 4 4 Measurement of hydrogen lines and the Rydberg Constant 5 5 Measurement
Astronomy. Optics and Telescopes
 Astronomy A. Dayle Hancock adhancock@wm.edu Small 239 Office hours: MTWR 10-11am Optics and Telescopes - Refraction, lenses and refracting telescopes - Mirrors and reflecting telescopes - Diffraction limit,
Astronomy A. Dayle Hancock adhancock@wm.edu Small 239 Office hours: MTWR 10-11am Optics and Telescopes - Refraction, lenses and refracting telescopes - Mirrors and reflecting telescopes - Diffraction limit,
University of Massachusetts, Amherst
 PHYSICS 286: Modern Physics Laboratory SPRING 2010 (A. Dinsmore and K. Kumar) Feb 2009 Experiment 4: THE FRANCK HERTZ EXPERIMENT Electronic Excitations of a Gas, and Evidence for the Quantization of Atomic
PHYSICS 286: Modern Physics Laboratory SPRING 2010 (A. Dinsmore and K. Kumar) Feb 2009 Experiment 4: THE FRANCK HERTZ EXPERIMENT Electronic Excitations of a Gas, and Evidence for the Quantization of Atomic
Atomic Spectra. d sin θ = mλ (1)
 Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
CBSE QUESTION PAPER. PHYSICS (Theory)
 CBSE QUESTION PAPER PHYSICS (Theory) Time allowed : 3 hours Maximum Marks : 70 General Instructions: (i) (ii) (iii) All questions are compulsory. There are 30 questions in total. Questions 1 to 8 carry
CBSE QUESTION PAPER PHYSICS (Theory) Time allowed : 3 hours Maximum Marks : 70 General Instructions: (i) (ii) (iii) All questions are compulsory. There are 30 questions in total. Questions 1 to 8 carry
Experiment 24: Spectroscopy
 Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
DISPERSION VERY SHORT ANSWER QUESTIONS. Two identical prisms made of the same material placed with their based on opposite sides (of the
 DISPERSION VERY SHORT ANSWER QUESTIONS Q-1. What will be the spectrum of sun during a total solar eclipse? Q-2. Why the secondary rainbow is always fainter than the primary rainbow? Q-3. Two identical
DISPERSION VERY SHORT ANSWER QUESTIONS Q-1. What will be the spectrum of sun during a total solar eclipse? Q-2. Why the secondary rainbow is always fainter than the primary rainbow? Q-3. Two identical
4. Circular Dichroism - Spectroscopy
 4. Circular Dichroism - Spectroscopy The optical rotatory dispersion (ORD) and the circular dichroism (CD) are special variations of absorption spectroscopy in the UV and VIS region of the spectrum. The
4. Circular Dichroism - Spectroscopy The optical rotatory dispersion (ORD) and the circular dichroism (CD) are special variations of absorption spectroscopy in the UV and VIS region of the spectrum. The
Grade 8 Science Unit 2: Optics Chapters 4, 5 and 6
 Grade 8 Science Unit 2: Optics Chapters 4, 5 and 6 At the end of this unit, students will be expected to 1. Provide examples of ideas and theories of light used in the past to explain observed properties.
Grade 8 Science Unit 2: Optics Chapters 4, 5 and 6 At the end of this unit, students will be expected to 1. Provide examples of ideas and theories of light used in the past to explain observed properties.
The Grating Spectrometer and Atomic Spectra
 PHY 192 Grating Spectrometer Spring 2012 1 The Grating Spectrometer and Atomic Spectra Introduction In the previous experiment diffraction and interference were discussed and at the end a diffraction grating
PHY 192 Grating Spectrometer Spring 2012 1 The Grating Spectrometer and Atomic Spectra Introduction In the previous experiment diffraction and interference were discussed and at the end a diffraction grating
EXPERIMENT 09 OBSERVATION OF SPECTRA
 EXPERIMENT 09 OBSERVATION OF SPECTRA INTRODUCTION: In physics, as in very other area of study, one of the most valuable questions a student can learn to ask is, How do they know that? Thus, when you read
EXPERIMENT 09 OBSERVATION OF SPECTRA INTRODUCTION: In physics, as in very other area of study, one of the most valuable questions a student can learn to ask is, How do they know that? Thus, when you read
Course Details. Analytical Techniques Based on Optical Spectroscopy. Course Details. Textbook. SCCH 211: Analytical Chemistry I
 SCCH 211: Analytical Chemistry I Analytical Techniques Based on Optical Spectroscopy Course Details September 22 October 10 September 22 November 7 November 17 December 1 Topic Period Introduction to Spectrometric
SCCH 211: Analytical Chemistry I Analytical Techniques Based on Optical Spectroscopy Course Details September 22 October 10 September 22 November 7 November 17 December 1 Topic Period Introduction to Spectrometric
INTERFEROMETERS. There are 4 principal types of measurements that can be made with this type of interferometer.
 INTERFEROMETERS NOTE: Most mirrors in the apparatus are front surface aluminized. Do not touch the surfaces nor wipe them. they can be easily permanently damaged. Introduction This experiment provides
INTERFEROMETERS NOTE: Most mirrors in the apparatus are front surface aluminized. Do not touch the surfaces nor wipe them. they can be easily permanently damaged. Introduction This experiment provides
Department of Physics, Colorado State University PH 425 Advanced Physics Laboratory The Zeeman Effect. 1 Introduction. 2 Origin of the Zeeman Effect
 Department of Physics, Colorado State University PH 425 Advanced Physics Laboratory The Zeeman Effect (a) CAUTION: Do not look directly at the mercury light source. It is contained in a quartz tube. The
Department of Physics, Colorado State University PH 425 Advanced Physics Laboratory The Zeeman Effect (a) CAUTION: Do not look directly at the mercury light source. It is contained in a quartz tube. The
Experiment #4 Nature of Light: Telescope and Microscope and Spectroscope
 Experiment #4 Nature of Light: Telescope and Microscope and Spectroscope In this experiment, we are going to learn the basic principles of the telescope and the microscope that make it possible for us
Experiment #4 Nature of Light: Telescope and Microscope and Spectroscope In this experiment, we are going to learn the basic principles of the telescope and the microscope that make it possible for us
Probing Atomic Crystals: Bragg Diffraction
 1 Probing Atomic Crystals: Bragg Diffraction OBJECTIVE: To learn how scientists probe the structure of solids, using a scaled-up version of X-ray Diffraction. APPARATUS: Steel ball "crystal", microwave
1 Probing Atomic Crystals: Bragg Diffraction OBJECTIVE: To learn how scientists probe the structure of solids, using a scaled-up version of X-ray Diffraction. APPARATUS: Steel ball "crystal", microwave
For more sample papers visit :
 PHYSICS (THEORY) (Three hours) For more sample papers visit : www.4ono.com Answer all questions in Part I and six questions from Part II, choosing two questions from each of the Sections A, B and C. All
PHYSICS (THEORY) (Three hours) For more sample papers visit : www.4ono.com Answer all questions in Part I and six questions from Part II, choosing two questions from each of the Sections A, B and C. All
Measurement of linear and mass attenuation coefficient of alcohol soluble compound for gamma rays at energy MeV
 Available online at www.scholarsresearchlibrary.com Archives of Applied Science Research, 2012, 4 (4):1748-1752 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-508X CODEN (USA) AASRC9 Measurement
Available online at www.scholarsresearchlibrary.com Archives of Applied Science Research, 2012, 4 (4):1748-1752 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-508X CODEN (USA) AASRC9 Measurement
Rb, which had been compressed to a density of 1013
 Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Rydberg constant from atomic spectra of gases
 Page 1 of 8 Rydberg constant from atomic spectra of gases Objective - Calibrating a prism spectrometer to convert the scale readings in wavelengths of spectral lines. - Observing the Balmer series of atomic
Page 1 of 8 Rydberg constant from atomic spectra of gases Objective - Calibrating a prism spectrometer to convert the scale readings in wavelengths of spectral lines. - Observing the Balmer series of atomic
Lab #13: Polarization
 Lab #13: Polarization Introduction In this experiment we will investigate various properties associated with polarized light. We will study both its generation and application. Real world applications
Lab #13: Polarization Introduction In this experiment we will investigate various properties associated with polarized light. We will study both its generation and application. Real world applications
The Emission Spectra of Light
 The Emission Spectra of Light Objectives: Theory: 1.... measured the wavelength limits of the color bands in the visible spectrum, 2.... measured the wavelengths of the emission lines of the hydrogen Balmer
The Emission Spectra of Light Objectives: Theory: 1.... measured the wavelength limits of the color bands in the visible spectrum, 2.... measured the wavelengths of the emission lines of the hydrogen Balmer
Raman and stimulated Raman spectroscopy of chlorinated hydrocarbons
 Department of Chemistry Physical Chemistry Göteborg University KEN140 Spektroskopi Raman and stimulated Raman spectroscopy of chlorinated hydrocarbons WARNING! The laser gives a pulsed very energetic and
Department of Chemistry Physical Chemistry Göteborg University KEN140 Spektroskopi Raman and stimulated Raman spectroscopy of chlorinated hydrocarbons WARNING! The laser gives a pulsed very energetic and
Advanced Spectroscopy Laboratory
 Advanced Spectroscopy Laboratory - Raman Spectroscopy - Emission Spectroscopy - Absorption Spectroscopy - Raman Microscopy - Hyperspectral Imaging Spectroscopy FERGIELAB TM Raman Spectroscopy Absorption
Advanced Spectroscopy Laboratory - Raman Spectroscopy - Emission Spectroscopy - Absorption Spectroscopy - Raman Microscopy - Hyperspectral Imaging Spectroscopy FERGIELAB TM Raman Spectroscopy Absorption
AST 101 Intro to Astronomy: Stars & Galaxies
 AST 101 Intro to Astronomy: Stars & Galaxies Telescopes Mauna Kea Observatories, Big Island, HI Imaging with our Eyes pupil allows light to enter the eye lens focuses light to create an image retina detects
AST 101 Intro to Astronomy: Stars & Galaxies Telescopes Mauna Kea Observatories, Big Island, HI Imaging with our Eyes pupil allows light to enter the eye lens focuses light to create an image retina detects
Instruction Manual and Experiment Guide for the PASCO scientific Model SP E 2/96 STUDENT SPECTROMETER. Copyright January 1991 $7.
 Instruction Manual and Experiment Guide for the PASCO scientific Model SP-9268 012-02135E 2/96 STUDENT SPECTROMETER Copyright January 1991 $7.50 012-02135E Spectrometer Table of Contents Section Page
Instruction Manual and Experiment Guide for the PASCO scientific Model SP-9268 012-02135E 2/96 STUDENT SPECTROMETER Copyright January 1991 $7.50 012-02135E Spectrometer Table of Contents Section Page
Optical/IR Observational Astronomy Telescopes I: Optical Principles. David Buckley, SAAO. 24 Feb 2012 NASSP OT1: Telescopes I-1
 David Buckley, SAAO 24 Feb 2012 NASSP OT1: Telescopes I-1 1 What Do Telescopes Do? They collect light They form images of distant objects The images are analyzed by instruments The human eye Photographic
David Buckley, SAAO 24 Feb 2012 NASSP OT1: Telescopes I-1 1 What Do Telescopes Do? They collect light They form images of distant objects The images are analyzed by instruments The human eye Photographic
Grade 8 Science: Unit 3-Optics Chapter 4: Properties of Light
 Grade 8 Science: Unit 3-Optics Chapter 4: Properties of Light Key Terms: Microscope, telescope, amplitude, crest, energy, force, frequency, hertz, medium, transverse wave, trough, wave, wavelength, reflection,
Grade 8 Science: Unit 3-Optics Chapter 4: Properties of Light Key Terms: Microscope, telescope, amplitude, crest, energy, force, frequency, hertz, medium, transverse wave, trough, wave, wavelength, reflection,
The Grating Spectrometer and Atomic Spectra
 PHY 192 Grating Spectrometer 1 The Grating Spectrometer and Atomic Spectra Introduction In the previous experiment diffraction and interference were discussed and at the end a diffraction grating was introduced.
PHY 192 Grating Spectrometer 1 The Grating Spectrometer and Atomic Spectra Introduction In the previous experiment diffraction and interference were discussed and at the end a diffraction grating was introduced.
IO.5 Elliptically Polarized Light
 1. Purpose: IO.5 Elliptically Polarized Light Analyze elliptically polarized light; determine the orientation of the vibration ellipse and the ratio of its semi-axes. 2. Apparatus: Gaertner Scientific
1. Purpose: IO.5 Elliptically Polarized Light Analyze elliptically polarized light; determine the orientation of the vibration ellipse and the ratio of its semi-axes. 2. Apparatus: Gaertner Scientific
DOWNLOAD OR READ : INFRARED AND RAMAN SPECTROSCOPY CONCEPTS AND APPLICATIONS PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : INFRARED AND RAMAN SPECTROSCOPY CONCEPTS AND APPLICATIONS PDF EBOOK EPUB MOBI Page 1 Page 2 infrared and raman spectroscopy concepts and applications infrared and raman spectroscopy
DOWNLOAD OR READ : INFRARED AND RAMAN SPECTROSCOPY CONCEPTS AND APPLICATIONS PDF EBOOK EPUB MOBI Page 1 Page 2 infrared and raman spectroscopy concepts and applications infrared and raman spectroscopy
THE ZEEMAN EFFECT PHYSICS 359E
 THE ZEEMAN EFFECT PHYSICS 359E INTRODUCTION The Zeeman effect is a demonstration of spatial quantization of angular momentum in atomic physics. Since an electron circling a nucleus is analogous to a current
THE ZEEMAN EFFECT PHYSICS 359E INTRODUCTION The Zeeman effect is a demonstration of spatial quantization of angular momentum in atomic physics. Since an electron circling a nucleus is analogous to a current
2001 Spectrometers. Instrument Machinery. Movies from this presentation can be access at
 2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
3 - Atomic Absorption Spectroscopy
 3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
3 - Atomic Absorption Spectroscopy Introduction Atomic-absorption (AA) spectroscopy uses the absorption of light to measure the concentration of gas-phase atoms. Since samples are usually liquids or solids,
Electromagnetic spectra
 Properties of Light Waves, particles and EM spectrum Interaction with matter Absorption Reflection, refraction and scattering Polarization and diffraction Reading foci: pp 175-185, 191-199 not responsible
Properties of Light Waves, particles and EM spectrum Interaction with matter Absorption Reflection, refraction and scattering Polarization and diffraction Reading foci: pp 175-185, 191-199 not responsible
