Worksheet 14 - Hybridization
|
|
|
- Myles Holmes
- 6 years ago
- Views:
Transcription
1 Workheet 4 - ybridization When atom bond to form molecule, they ue molecular orbital. Thee are formed through the of the atomic orbital that we have already dicued,,, and d orbital. The hybridized molecular orbital have different hae and level than the atomic orbital. The number of molecular orbital created by deend on the number of atomic orbital that are mixed to form them. In forming hybridized orbital, four atomic orbital are mixed, one and three. The diagram for thi roce i hown below. The hybridized orbital are higher in than the orbital, but lower in than the orbital. atomic orbital hybridized orbital arbon ha 4 valence electron. Add thee electron to the atomic and molecular orbital. Thi give tetrahedral geometry. With thi, will form four equivalent σ bond. Draw a imilar diagram for hybridized oxygen. ow many σ bond will be formed? ow are the other orbital ued? Do the ame for hybridized nitrogen.
2 In ome Lewi tructure, there are only three equivalent bond formed. To create three equivalent hybridized orbital, mix three atomic orbital. Draw and name the orbital formed in thi, then add the electron for ulfur. Since the hybridized orbital are cloe in, every orbital i filled with one electron before electron are aired. The hybridized orbital will form σ bond(). The unhybridized orbital will form π bond(). There will be lone air(). Thi give trigonal lanar geometry. In linear molecule, like, the central atom ha only two equivalent bonding orbital. Draw the level and name the orbital formed in thi. Fill in the electron for carbon and determine the number and tyed of bond formed. In, determine the of the oxygen atom. omlete the diagram for the oxygen. Draw the tructure of.
3 In atom with n= or larger, the d orbital can alo be hybridized. In molecule with five molecular orbital, five atomic orbital are mixed: Thi will give trigonal biyramidal geometry and i called d. Finally, molecule with octahedral geometry, will have molecular orbital. Thi i called. Shown below i a ortion of the chart from Workheet. Fill in the for each of the comound. comound bond lone air geometry hae SF octahedral octahedral tetrahedral trigonal yramidal Il 4-4 octahedral quare lanar F tetrahedral tetrahedral S 0 trigonal lanar SF 4 4 trigonal biyramidal trigonal lanar eeaw 0 linear linear tetrahedral V-haed - trigonal lanar V-haed
4 Fill in the chart below and then comlete the Lewi tructure for the molecule hown below and fill in thoe chart. element Lewi ymbol # bond # lone air alogen acetic acid σ bond π bond bond angle acrylonitrile σ bond π bond bond angle σ bond π bond bond angle caffeine
5 The molecule hown to the left i riboflavin (vitamin B). Anwer the following quetion about it tructure. a) how many carbon are hybridized? hybridized? hybridized? b) ow many nitrogen are hybridized? hybridized? hybridized? c) ow many oxygen are hybridized? hybridized? hybridized? d) ow many σ bond are there in total? e) ow many π bond are there in total? f) Which of the three ring are lanar? The acetate ion, -, ha both oxygen bonded to the ame carbon. a) Draw the Lewi tructure and all reonance form. b) Label the around each carbon. c) Pick one reonance tructure and label the of each oxygen. d) ow many σ and π bond are reent? e) Which atom carrie the formal negative charge?
4/5/2010. Orbitals. Figure 12.18: Three representations of the hydrogen 1s. Figure 12.19b: Representation of the 2p orbitals.
 The Central Theme of VB Theory Baic Princile Covalent Bonding: rbital A covalent bond form when the of two atom overla and are occuied by a air of electron that have the highet robability of being located
The Central Theme of VB Theory Baic Princile Covalent Bonding: rbital A covalent bond form when the of two atom overla and are occuied by a air of electron that have the highet robability of being located
CHAPTER 10 CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS
 APTER 10 EMIAL BNDING II: MLEULAR GEMETRY AND YBRIDIZATIN ATMI RBITALS 10.7 (a) The Lewi tructure of P 3 i hown below. Since in the VSEPR method the number of bonding pair and lone pair of electron around
APTER 10 EMIAL BNDING II: MLEULAR GEMETRY AND YBRIDIZATIN ATMI RBITALS 10.7 (a) The Lewi tructure of P 3 i hown below. Since in the VSEPR method the number of bonding pair and lone pair of electron around
Hybridization of Orbitals
 Hybridization of Orbitals Structure & Properties of Matter 1 Atomic Orbitals and Bonding Previously: Electron configurations Lewis structures Bonding Shapes of molecules Now: How do atoms form covalent
Hybridization of Orbitals Structure & Properties of Matter 1 Atomic Orbitals and Bonding Previously: Electron configurations Lewis structures Bonding Shapes of molecules Now: How do atoms form covalent
Homework #7. Chapter 14. Covalent Bonding Orbitals
 omework #7 hapter 14 ovalent Bonding rbitals 7. Both M theory and LE model use quantum mechanics to describe bonding. In the LE model, wavefunctions on one atom are mixed to form hybridized orbitals. In
omework #7 hapter 14 ovalent Bonding rbitals 7. Both M theory and LE model use quantum mechanics to describe bonding. In the LE model, wavefunctions on one atom are mixed to form hybridized orbitals. In
Chapter 9. Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory
 Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
CHEMISTRY - ZUMDAHL 2E CH.4 - MOLECULAR STRUCTURE AND ORBITALS.
 !! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
!! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
Orbitals, Shapes and Polarity Quiz
 Orbital, Shae and Polarity Quiz Name: /17 Knowledge. Anwer the following quetion on foolca. /2 1. Exlain why the ub-level can aear to be herical like the ub-level? /2 2.a) What i the maximum number of
Orbital, Shae and Polarity Quiz Name: /17 Knowledge. Anwer the following quetion on foolca. /2 1. Exlain why the ub-level can aear to be herical like the ub-level? /2 2.a) What i the maximum number of
CHAPTER 10 CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS
 APTER 10 EMIAL BNDING II: MLEULAR GEMETRY AND YBRIDIZATIN ATMI RBITALS 10.7 (a) The Lewi tructure of P 3 i hown below. Since in the VSEPR method the number of bonding pair and lone pair of electron around
APTER 10 EMIAL BNDING II: MLEULAR GEMETRY AND YBRIDIZATIN ATMI RBITALS 10.7 (a) The Lewi tructure of P 3 i hown below. Since in the VSEPR method the number of bonding pair and lone pair of electron around
CH 222 Sample Exam Exam I Name: Lab Section:
 222 Sample Exam Exam I Name: Lab Section: Part I: Multiple hoice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. Which of the following statements
222 Sample Exam Exam I Name: Lab Section: Part I: Multiple hoice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. Which of the following statements
Covalent Compounds: Bonding Theories and Molecular Structure
 CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
Homework - Chapter 1 Chem 2310
 omework - hapter 1 hem 2310 ame I. Introduction to rganic hemistry 1. Explain in your own words what organic chemistry is, and what it is useful for. 2. Why do you think the field of study that you are
omework - hapter 1 hem 2310 ame I. Introduction to rganic hemistry 1. Explain in your own words what organic chemistry is, and what it is useful for. 2. Why do you think the field of study that you are
CHAPTER 1 HW SOLUTIONS: STRUCTURE
 APTER 1 W SLUTIS: STRUTURE FRMAL ARGE 1. Indicate the formal charge on any atom that has a non-zero formal charge. a. b. c. d. e. 6-7 = -1 4-3 = +1 4-5 = -1 5-4 = +1 f. g. h. i. j. 6-5 = +1 P 5-5 = 0 6-6
APTER 1 W SLUTIS: STRUTURE FRMAL ARGE 1. Indicate the formal charge on any atom that has a non-zero formal charge. a. b. c. d. e. 6-7 = -1 4-3 = +1 4-5 = -1 5-4 = +1 f. g. h. i. j. 6-5 = +1 P 5-5 = 0 6-6
Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity
 Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
At the end of this lesson, students should be able to :
 At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
; (c) [Li] [: O :] [Li]. 5a. The electrostatic potential map that corresponds to IF is the one with the most red in it. ... C C H
![; (c) [Li] [: O :] [Li]. 5a. The electrostatic potential map that corresponds to IF is the one with the most red in it. ... C C H ; (c) [Li] [: O :] [Li]. 5a. The electrostatic potential map that corresponds to IF is the one with the most red in it. ... C C H](/thumbs/87/96516624.jpg) hapter 10 Answers ractice Examples 1a Mg 1b n, Ge, [: Br :], K, : e: + 2 : : +, [Tl ] +, 2 : : [] 2a (a) [a] [ ] [a] ; (b) [Mg] [: :] [Mg] [: :] [Mg] 2+ 3 2+ 3 2+ 2+ 2b (a) [: I :] [a] [: I :] 2+ 2 ; (b)
hapter 10 Answers ractice Examples 1a Mg 1b n, Ge, [: Br :], K, : e: + 2 : : +, [Tl ] +, 2 : : [] 2a (a) [a] [ ] [a] ; (b) [Mg] [: :] [Mg] [: :] [Mg] 2+ 3 2+ 3 2+ 2+ 2b (a) [: I :] [a] [: I :] 2+ 2 ; (b)
Chapter 6 Molecular Structure
 hapter 6 Molecular Structure 1. Draw the Lewis structure of each of the following ions, showing all nonzero formal charges. Indicate whether each ion is linear or bent. If the ion is bent, what is the
hapter 6 Molecular Structure 1. Draw the Lewis structure of each of the following ions, showing all nonzero formal charges. Indicate whether each ion is linear or bent. If the ion is bent, what is the
Part A: There is a table attached to the end of this document. For each molecule or ion in the table, provide entries for columns I through IX.
 Chemistry 400 Discussion: VSEPR Theory and Molecular Shapes Instructions: Work through the exercises below. If you have access to molecular model kits, use them to build three dimensional models of the
Chemistry 400 Discussion: VSEPR Theory and Molecular Shapes Instructions: Work through the exercises below. If you have access to molecular model kits, use them to build three dimensional models of the
Chemical Bonding I: Basic Concepts
 Chemical Bonding I: Basic Concepts Chapter 9 Chang & Goldsby Modified by Dr. Hahn Copyright McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent
Chemical Bonding I: Basic Concepts Chapter 9 Chang & Goldsby Modified by Dr. Hahn Copyright McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
CHEMISTRY 110 EXAM 2 Feb 25, 2013 FORM A
 EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
Lewis Structure. Lewis Structures & VSEPR. Octet & Duet Rules. Steps for drawing Lewis Structures
 Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
Formal Charge. Formal Charge. Formal Charge. Formal Charge. Most Lewis structures do not require any formal charges.
 : a charge assigned to each atom in a structure by assuming that the shared electrons are divided equally between the bonded atoms. Most Lewis structures do not require any formal charges. tructures that
: a charge assigned to each atom in a structure by assuming that the shared electrons are divided equally between the bonded atoms. Most Lewis structures do not require any formal charges. tructures that
Outline for Today. Monday, Nov. 12. Wednesday Friday. Chapter 8: Chemical Bonding. Bond Enthalpies. Chapter 9: Theories of Bonding
 Outline for Today Monday, Nov. 12 Chapter 8: Chemical Bonding Bond Enthalpies Chapter 9: Theories of Bonding VSEPR (Valence Shell Electron Pair Repulsion) Theory Valence Bond Orbital ybridization Molecular
Outline for Today Monday, Nov. 12 Chapter 8: Chemical Bonding Bond Enthalpies Chapter 9: Theories of Bonding VSEPR (Valence Shell Electron Pair Repulsion) Theory Valence Bond Orbital ybridization Molecular
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. Dr. V.M. Williamson Texas A & M University Student Version
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. VSEPR: Electronic Geometries VSEPR
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Valence Bond Theory - Description
 Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
Lecture 16 C1403 October 31, Molecular orbital theory: molecular orbitals and diatomic molecules
 Lecture 16 C1403 October 31, 2005 18.1 Molecular orbital theory: molecular orbitals and diatomic molecules 18.2 Valence bond theory: hybridized orbitals and polyatomic molecules. From steric number to
Lecture 16 C1403 October 31, 2005 18.1 Molecular orbital theory: molecular orbitals and diatomic molecules 18.2 Valence bond theory: hybridized orbitals and polyatomic molecules. From steric number to
Chapter 9 Molecular Geometries. and Bonding Theories
 Chapter 9 Molecular Geometries and Bonding Theories Coverage of Chapter 9 9.1 All 9.2 All 9.3 All 9.4 All 9.5 Omit Hybridization Involving d Orbitals 9.6 All 9.7 and 9.8 Omit ALL MOLECULAR SHAPES The shape
Chapter 9 Molecular Geometries and Bonding Theories Coverage of Chapter 9 9.1 All 9.2 All 9.3 All 9.4 All 9.5 Omit Hybridization Involving d Orbitals 9.6 All 9.7 and 9.8 Omit ALL MOLECULAR SHAPES The shape
AP Chemistry- Practice Bonding Questions for Exam
 AP Chemistry- Practice Bonding Questions for Exam Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is a correct Lewis structure for
AP Chemistry- Practice Bonding Questions for Exam Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is a correct Lewis structure for
Localized Electron Model
 Localized Electron Model Models for Chemical Bonding Localized electron model (Valence bond model) Molecular orbital model Localized Electron Model Useful for explaining the structure of molecules especially
Localized Electron Model Models for Chemical Bonding Localized electron model (Valence bond model) Molecular orbital model Localized Electron Model Useful for explaining the structure of molecules especially
BONDING THEORIES Chapter , Carey
 BONDING THEORIES Chapter 10.6-10.7, Carey The Covalent Chemical Bond (9.2) FIG I Potential Energy Change to Form H2 What is a chemical bond? Why do chemical bonds occur? Descriptions of bonding: Valence
BONDING THEORIES Chapter 10.6-10.7, Carey The Covalent Chemical Bond (9.2) FIG I Potential Energy Change to Form H2 What is a chemical bond? Why do chemical bonds occur? Descriptions of bonding: Valence
Chapter 9 Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories molecular shapes the VSEPR model molecular shape and molecular polarity covalent bonding and orbital overlap hybrid orbitals multiple bonds 9.1 Molecular
Chapter 9 Molecular Geometry and Bonding Theories molecular shapes the VSEPR model molecular shape and molecular polarity covalent bonding and orbital overlap hybrid orbitals multiple bonds 9.1 Molecular
Fill in the chart below to determine the valence electrons of elements 3-10
 Chemistry 11 Atomic Theory IV Name: Date: Block: 1. Lewis Diagrams 2. VSEPR Lewis Diagrams Lewis diagrams show the bonding between atoms of a molecule. Only the outermost electrons of an atom (called electrons)
Chemistry 11 Atomic Theory IV Name: Date: Block: 1. Lewis Diagrams 2. VSEPR Lewis Diagrams Lewis diagrams show the bonding between atoms of a molecule. Only the outermost electrons of an atom (called electrons)
Carbon-based molecules are held together by covalent bonds between atoms
 hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
Chapter 9 - Covalent Bonding: Orbitals
 Chapter 9 - Covalent Bonding: Orbitals 9.1 Hybridization and the Localized Electron Model A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new
Chapter 9 - Covalent Bonding: Orbitals 9.1 Hybridization and the Localized Electron Model A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new
Organic Chemistry. Review Information for Unit 1. VSEPR Hybrid Orbitals Polar Molecules
 rganic hemistry Review Information for Unit 1 VSEPR ybrid rbitals Polar Molecules VSEPR The valence shell electron pair repulsion model (VSEPR) can be used to predict the geometry around a particular atom
rganic hemistry Review Information for Unit 1 VSEPR ybrid rbitals Polar Molecules VSEPR The valence shell electron pair repulsion model (VSEPR) can be used to predict the geometry around a particular atom
Chemical Bonding II. Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds Hybridization MO theory
 Chemical Bonding II Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds ybridization MO theory 1 Molecular Geometry 3-D arrangement of atoms 2 VSEPR Valence-shell
Chemical Bonding II Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds ybridization MO theory 1 Molecular Geometry 3-D arrangement of atoms 2 VSEPR Valence-shell
Review Chapter 10: Theories of Bonding & Structure. Chemistry: The Molecular Nature of Matter, 6 th edition By Jesperson, Brady, & Hyslop
 Review Chapter 10: Theories of Bonding & Structure Chemistry: The Molecular Nature of Matter, 6 th edition By Jesperson, Brady, & Hyslop Chapter 10 Concepts q VESPR theory q Predict molecular geometry
Review Chapter 10: Theories of Bonding & Structure Chemistry: The Molecular Nature of Matter, 6 th edition By Jesperson, Brady, & Hyslop Chapter 10 Concepts q VESPR theory q Predict molecular geometry
: O: (1) (2) (3) (4) Page 1 of 6 : : : : : : (8) H H
 Experiment #12 MOLECULAR MODELS An aspect of chemistry, which traditionally proves to be difficult to many students, is the visualization of compounds, ions, and molecules in three dimensional space. In
Experiment #12 MOLECULAR MODELS An aspect of chemistry, which traditionally proves to be difficult to many students, is the visualization of compounds, ions, and molecules in three dimensional space. In
Lecture Presentation. Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
Models of Molecular Structure
 19 Models of Molecular tructure Molecular model kits are designed to give a reasonably accurate picture of how atoms are arranged in molecules. They follow common bonding patterns and produce the geometric
19 Models of Molecular tructure Molecular model kits are designed to give a reasonably accurate picture of how atoms are arranged in molecules. They follow common bonding patterns and produce the geometric
Molecular Geometry and Bonding Theories. Chapter 9
 Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Valence Bond Model and Hybridization
 Valence Bond Model and ybridization APPENDIX 4 1 Concepts The key ideas required to understand this section are: Concept Book page reference VSEPR theory 65 More advanced ideas about electronic structure
Valence Bond Model and ybridization APPENDIX 4 1 Concepts The key ideas required to understand this section are: Concept Book page reference VSEPR theory 65 More advanced ideas about electronic structure
Part 1. Reading: Gray: (4-1), (4-2), and (4-4) OGN: (16.2)
 Part 1 Reading: Gray: (4-1), (4-2), and (4-4) OGN: (16.2) The story so far: MO-LCAO works great for diatomic molecules! But... What about other numbers of atoms? Will MO-LCAO work for polyatomic molecules?
Part 1 Reading: Gray: (4-1), (4-2), and (4-4) OGN: (16.2) The story so far: MO-LCAO works great for diatomic molecules! But... What about other numbers of atoms? Will MO-LCAO work for polyatomic molecules?
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9 Covalent Bonding: Orbitals Localized electron model A bond is made when a half-filled orbital of one atom overlaps with a half-filled orbital of another.! Bond: orbitals overlap straight on p
Chapter 9 Covalent Bonding: Orbitals Localized electron model A bond is made when a half-filled orbital of one atom overlaps with a half-filled orbital of another.! Bond: orbitals overlap straight on p
Molecular Geometry and Bonding Theories. Molecular Shapes. Molecular Shapes. Chapter 9 Part 2 November 16 th, 2004
 Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Activity Formal Charge and VSEPR Theory for Expanded Octets
 Activity 201 7 Formal Charge and VSEPR Theory for Expanded Octets Directions: This Guided Learning Activity (GLA) goes over formal charge and the structures of molecules with expanded octets. Part A introduces
Activity 201 7 Formal Charge and VSEPR Theory for Expanded Octets Directions: This Guided Learning Activity (GLA) goes over formal charge and the structures of molecules with expanded octets. Part A introduces
How does the number of bonds and nonbonded pairs of electrons affect the shape of a molecule?
 Reading: Chapter 9, sections 9.1-9.6 As you read these sections ask yourself: ow does the number of bonds and nonbonded pairs of electrons affect the shape of a molecule? Why is the repulsion between two
Reading: Chapter 9, sections 9.1-9.6 As you read these sections ask yourself: ow does the number of bonds and nonbonded pairs of electrons affect the shape of a molecule? Why is the repulsion between two
Activity Hybrid Atomic Orbitals
 Activity 201 8 Hybrid Atomic Orbitals Directions: This Guided Learning Activity (GLA) discusses Hybrid Atomic Orbitals, which are the basis for Valence Bond Theory. Part A introduces σ- and π-bonds. Part
Activity 201 8 Hybrid Atomic Orbitals Directions: This Guided Learning Activity (GLA) discusses Hybrid Atomic Orbitals, which are the basis for Valence Bond Theory. Part A introduces σ- and π-bonds. Part
Lecture 17 - Covalent Bonding. Lecture 17 - VSEPR and Molecular Shape. Lecture 17 - Introduction. Lecture 17 - VSEPR and Molecular Shape
 Chem 103, Section F0F Unit VI - Compounds Part II: Covalent Compounds Lecture 17 Using the Valence-Shell Electron-Pair Repulsion (VSEPR) Theory to predict molecular shapes Molecular shape and polarity
Chem 103, Section F0F Unit VI - Compounds Part II: Covalent Compounds Lecture 17 Using the Valence-Shell Electron-Pair Repulsion (VSEPR) Theory to predict molecular shapes Molecular shape and polarity
2011, Robert Ayton. All rights reserved.
 Chemical Bonding Outline 1. Lewis Dot Structures 2. Bonds 3. Formal Charges 4. VSEPR (Molecular Geometry and Hybridzation) 5. Common Resonance Structures and Dimerization Review 1. Lewis Dot Structures
Chemical Bonding Outline 1. Lewis Dot Structures 2. Bonds 3. Formal Charges 4. VSEPR (Molecular Geometry and Hybridzation) 5. Common Resonance Structures and Dimerization Review 1. Lewis Dot Structures
Chapter 9 Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
Covalent Bonding - Orbitals
 Covalent Bonding - Orbitals ybridization - The Blending of Orbitals + = Poodle + Cocker Spaniel = Cockapoo + = s orbital + p orbital = sp orbital What Proof Exists for ybridization? We have studied electron
Covalent Bonding - Orbitals ybridization - The Blending of Orbitals + = Poodle + Cocker Spaniel = Cockapoo + = s orbital + p orbital = sp orbital What Proof Exists for ybridization? We have studied electron
HourEx4 Practice Chapt 8 & 9 Kotz
 HourEx4 Practice Chapt 8 & 9 Kotz Multiple Choice 5 pts apiece Identify the letter of the choice that best completes the statement or answers the question. PUT YOUR ANSWER ON THE BUBBLE SHEET PROVIDED.You
HourEx4 Practice Chapt 8 & 9 Kotz Multiple Choice 5 pts apiece Identify the letter of the choice that best completes the statement or answers the question. PUT YOUR ANSWER ON THE BUBBLE SHEET PROVIDED.You
Chemical Bonds, Orbital Shapes, and Orbital Hybridization
 Chemical Bonds, Orbital Shapes, and Orbital Hybridization PRELAB ASSIGNMENT Read the entire laboratory write up. Write an objective and answer the following questions in your laboratory notebook before
Chemical Bonds, Orbital Shapes, and Orbital Hybridization PRELAB ASSIGNMENT Read the entire laboratory write up. Write an objective and answer the following questions in your laboratory notebook before
CHAPTER TEN MOLECULAR GEOMETRY MOLECULAR GEOMETRY V S E P R CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS
 CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
Electron Geometry Hybrid Orbitals
 Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2014 Introduction: In chemistry, the three dimensional shape of a molecule is as important
Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2014 Introduction: In chemistry, the three dimensional shape of a molecule is as important
CHAPTER 8. Molecular Structure & Covalent Bonding Theories
 CAPTER 8 Molecular Structure & Covalent Bonding Theories 1 Chapter Goals 1. A Preview of the Chapter 2. Valence Shell Electron Pair Repulsion (VSEPR) Theory 3. Polar Molecules:The Influence of Molecular
CAPTER 8 Molecular Structure & Covalent Bonding Theories 1 Chapter Goals 1. A Preview of the Chapter 2. Valence Shell Electron Pair Repulsion (VSEPR) Theory 3. Polar Molecules:The Influence of Molecular
Find the difference in electronegativity between the hydrogen and chlorine atoms
 Answers Questions 16.2 Molecular polarity 1. Write a dot diagram for the HCl molecule. Find the difference in electronegativity between the hydrogen and chlorine atoms Difference in electronegativity =
Answers Questions 16.2 Molecular polarity 1. Write a dot diagram for the HCl molecule. Find the difference in electronegativity between the hydrogen and chlorine atoms Difference in electronegativity =
Valence Shell Electron Pair Repulsion Model
 Activity 22 Valence hell Electron Pair Repulsion Model Why? Molecules adopt a shape that minimizes their energy. In many cases it is possible to predict the geometry of a molecule simply by considering
Activity 22 Valence hell Electron Pair Repulsion Model Why? Molecules adopt a shape that minimizes their energy. In many cases it is possible to predict the geometry of a molecule simply by considering
Molecular Geometry and Chemical Bonding Theory
 Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
Covalent Bonds: overlap of orbitals σ-bond π-bond Molecular Orbitals
 Covalent Bonding What is covalent bonding? Covalent Bonds: overlap of orbitals σ-bond π-bond Molecular Orbitals Hybrid Orbital Formation Shapes of Hybrid Orbitals Hybrid orbitals and Multiple Bonds resonance
Covalent Bonding What is covalent bonding? Covalent Bonds: overlap of orbitals σ-bond π-bond Molecular Orbitals Hybrid Orbital Formation Shapes of Hybrid Orbitals Hybrid orbitals and Multiple Bonds resonance
Homework #2. Chapter 14. Covalent Bonding Orbitals
 Homework # Chapter 14 Covalent Bonding Orbitals 1. Single bonds have their electron density concentrated between the two atoms (on the internuclear axis). Therefore an atom can rotate freely on the internuclear
Homework # Chapter 14 Covalent Bonding Orbitals 1. Single bonds have their electron density concentrated between the two atoms (on the internuclear axis). Therefore an atom can rotate freely on the internuclear
Localized Electron Model
 Localized Electron Model Models for Chemical Bonding Localized electron model (Valence bond model) Molecular orbital model Localized Electron Model Useful for explaining the structure of molecules especially
Localized Electron Model Models for Chemical Bonding Localized electron model (Valence bond model) Molecular orbital model Localized Electron Model Useful for explaining the structure of molecules especially
Lewis structures show the number and type of bonds between atoms in a molecule or polyatomic ion.
 VSEPR & Geometry Lewis structures show the number and type of bonds between atoms in a molecule or polyatomic ion. Lewis structures are not intended to show the 3-dimensional structure (i.e. shape or geometry)
VSEPR & Geometry Lewis structures show the number and type of bonds between atoms in a molecule or polyatomic ion. Lewis structures are not intended to show the 3-dimensional structure (i.e. shape or geometry)
Chem 121 Exam 4 Practice Exam
 Chem 121 Exam 4 Practice Exam 1. What is the correct electron configuration for bromine? b. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9 4s 2 4p 6 c. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5 d. 1s 2 2s 2 2p 6 3s 2 3p
Chem 121 Exam 4 Practice Exam 1. What is the correct electron configuration for bromine? b. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9 4s 2 4p 6 c. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5 d. 1s 2 2s 2 2p 6 3s 2 3p
4.Chemical bonding and Molecular Structure
 4.Chemical bonding and Molecular Structure Some Imortant Points and Terms of the Chater 1. Lewis dot structures are shorthand to reresent the valence electrons of an atom. The structures are written as
4.Chemical bonding and Molecular Structure Some Imortant Points and Terms of the Chater 1. Lewis dot structures are shorthand to reresent the valence electrons of an atom. The structures are written as
Orbital Shapes Carbon: Electron configuration Carbon: Full. Short form. Orbital energy diagram. Orbital energy levels diagram
 rganic hemistry involves mostly NPS and the halogens. rganic compounds use valence shell electrons to bond. Usually only in the s and p orbitals. rbital Shapes arbon: z y z y z y z y z y x x x x x 1s n=1
rganic hemistry involves mostly NPS and the halogens. rganic compounds use valence shell electrons to bond. Usually only in the s and p orbitals. rbital Shapes arbon: z y z y z y z y z y x x x x x 1s n=1
CHEM 110 Exam 2 - Practice Test 1 - Solutions
 CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
Name: Period: Date: What Is VSEPR? Now explore the Compare Two Structures link. Try changing the display to explore different combinations.
 Name: Period: Date: What Is VSEPR? Exploring The Valence Shell Electron Pair Repulsion (VSEPR) model. Go to the Purdue University website to explore VSEPR theory. http://www.chem.purdue.edu/gchelp/vsepr/structur2.html
Name: Period: Date: What Is VSEPR? Exploring The Valence Shell Electron Pair Repulsion (VSEPR) model. Go to the Purdue University website to explore VSEPR theory. http://www.chem.purdue.edu/gchelp/vsepr/structur2.html
16. NO 3, 5 + 3(6) + 1 = 24 e. 22. HCN, = 10 valence electrons
 Solution to Chapts 9 & 10 Problems: 16. N 3, 5 + 3(6) + 1 = 24 e 22. HCN, 1 + 4 + 5 = 10 valence electrons Assuming N is hybridized, both C and N atoms are sp hybridized. The C H bond is formed from overlap
Solution to Chapts 9 & 10 Problems: 16. N 3, 5 + 3(6) + 1 = 24 e 22. HCN, 1 + 4 + 5 = 10 valence electrons Assuming N is hybridized, both C and N atoms are sp hybridized. The C H bond is formed from overlap
2. (10%) Correct answers are given below. Every correct answer gives a student 1⅔=1.666 point. There are no negative points for incorrect answers
 1. (9%) There are 9 correct answers: (0,0), (1,-1), (1,0), (1,1), (2,-2), (2,-1), (2,0), (2,1), and (2,2). Each correct answer gives a student +1 point. All other answers are incorrect. Every incorrect
1. (9%) There are 9 correct answers: (0,0), (1,-1), (1,0), (1,1), (2,-2), (2,-1), (2,0), (2,1), and (2,2). Each correct answer gives a student +1 point. All other answers are incorrect. Every incorrect
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Chang & Goldsby Modified by Dr. Juliet Hahn Copyright McGraw-Hill Education. All rights reserved. No reproduction
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Chang & Goldsby Modified by Dr. Juliet Hahn Copyright McGraw-Hill Education. All rights reserved. No reproduction
Which of the following is the most polar bond?
 !"#$%&'()*++,*#-.$*/* ct 14, 2013 FRM A 1. A neutral atom has the following electron configuration. What is the maximum number of covalent bonds this atom could form with hydrogen atoms? 3. Which of the
!"#$%&'()*++,*#-.$*/* ct 14, 2013 FRM A 1. A neutral atom has the following electron configuration. What is the maximum number of covalent bonds this atom could form with hydrogen atoms? 3. Which of the
Chapter 13: Phenomena
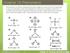 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Lewis Dot Structures. a. Duet Rule: 2 electrons needed to satisfy valence shell. i. What follows this rule? Hydrogen and Helium
 1. Important points about Lewis Dot: Lewis Dot Structures a. Duet Rule: 2 electrons needed to satisfy valence shell. i. What follows this rule? Hydrogen and Helium b. Octet Rule: 8 electrons needed to
1. Important points about Lewis Dot: Lewis Dot Structures a. Duet Rule: 2 electrons needed to satisfy valence shell. i. What follows this rule? Hydrogen and Helium b. Octet Rule: 8 electrons needed to
Valence Shell Electron Pair repulsion
 Molecular Geometry Valence Shell Electron Pair repulsion The valence shell electron pair repulsion model (VSEPR model) assumes that electron pairs repel one another. (VSEPR) model gives helps determine
Molecular Geometry Valence Shell Electron Pair repulsion The valence shell electron pair repulsion model (VSEPR model) assumes that electron pairs repel one another. (VSEPR) model gives helps determine
1.14 the orbital view of bonding:
 1.14 the orbital view of bonding: The sigma bond Dr. Abdullah Saleh/236-3 1 A limitation of Lewis Theory of Bonding It does not explain the three dimensional geometries of molecules! Dr. Abdullah Saleh/236-3
1.14 the orbital view of bonding: The sigma bond Dr. Abdullah Saleh/236-3 1 A limitation of Lewis Theory of Bonding It does not explain the three dimensional geometries of molecules! Dr. Abdullah Saleh/236-3
CHEM1101 Worksheet 6: Lewis Structures
 CHEM1101 Worksheet 6: Lewis Structures Model 1: Simple Compounds of C, N, O and F The octet rule tells us that C, N, O and F will form covalent bonds so that they are surrounded by eight electrons. For
CHEM1101 Worksheet 6: Lewis Structures Model 1: Simple Compounds of C, N, O and F The octet rule tells us that C, N, O and F will form covalent bonds so that they are surrounded by eight electrons. For
1. Which is the most electronegative atom in the compound below?
 1. Which is the most electronegative atom in the compound below? A) Carbon C) Oxygen B) Nitrogen D) Bromine 2. Which of the following correctly describes the electrons of a carbon atom in its ground state?
1. Which is the most electronegative atom in the compound below? A) Carbon C) Oxygen B) Nitrogen D) Bromine 2. Which of the following correctly describes the electrons of a carbon atom in its ground state?
Shapes of Molecules. Lewis structures are useful but don t allow prediction of the shape of a molecule.
 Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals 1 Chemical Bonding II Molecular Geometry (10.1) Dipole Moments (10.2) Valence Bond Theory (10.3) Hybridization of Atomic Orbitals
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals 1 Chemical Bonding II Molecular Geometry (10.1) Dipole Moments (10.2) Valence Bond Theory (10.3) Hybridization of Atomic Orbitals
Chapter One MULTIPLE CHOICE QUESTIONS. Topic: General Section: 1.1 Difficulty Level: Easy
 Chapter ne MULTIPLE CICE QUESTIS Topic: General Section: 1.1 1. Credit for the first synthesis of an organic compound from an inorganic precursor is usually given to: A) Berzelius B) Arrhenius C) Kekule
Chapter ne MULTIPLE CICE QUESTIS Topic: General Section: 1.1 1. Credit for the first synthesis of an organic compound from an inorganic precursor is usually given to: A) Berzelius B) Arrhenius C) Kekule
Chemical Bonding 4.8. Valence Bond Theory Hybrid Orbital Theory Multiple Bonds High School Chem Solutions. All rights reserved.
 Chemical Bonding 4.8 Valence Bond Theory Hybrid Orbital Theory Multiple Bonds Valence Bond Theory Combines Lewis theory of filling octets by sharing pairs of electrons with the electron configuration of
Chemical Bonding 4.8 Valence Bond Theory Hybrid Orbital Theory Multiple Bonds Valence Bond Theory Combines Lewis theory of filling octets by sharing pairs of electrons with the electron configuration of
Electron Geometry Hybrid Orbitals
 Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University Introduction: In chemistry, the three dimensional shape of a molecule is as important as the
Molecular Shape and Hybridized Orbitals CH2000: Introduction to General Chemistry, Plymouth State University Introduction: In chemistry, the three dimensional shape of a molecule is as important as the
5 Polyatomic molecules
 s manual for Burrows et.al. Chemistry 3 Third edition 5 Polyatomic molecules Answers to worked examples WE 5.1 Formal charges in N 2 (on p. 221 in Chemistry 3 ) Use formal charges to decide whether oxygen
s manual for Burrows et.al. Chemistry 3 Third edition 5 Polyatomic molecules Answers to worked examples WE 5.1 Formal charges in N 2 (on p. 221 in Chemistry 3 ) Use formal charges to decide whether oxygen
Chapter 9. Molecular Geometry and Bonding Theories
 9.1 Molecular Shapes Read Sec. 9.1 and 9.2, then complete the Sample and Practice Exercises in these sections. Sample Exercise 9.1 (p. 347) Use the VSEPR model to predict the molecular geometries of a)
9.1 Molecular Shapes Read Sec. 9.1 and 9.2, then complete the Sample and Practice Exercises in these sections. Sample Exercise 9.1 (p. 347) Use the VSEPR model to predict the molecular geometries of a)
CHEMISTRY 121 AUTUMN 2009 CHAPTER 8 & 9 PROBLEMS
 Dr. Fus AU 2009 EM 121 EMISTRY 121 AUTUMN 2009 APTER 8 & 9 PROBLEMS All questions listed below are problems taken from old hemistry 121 exams given here at The Ohio State University. Read hapters 8 and
Dr. Fus AU 2009 EM 121 EMISTRY 121 AUTUMN 2009 APTER 8 & 9 PROBLEMS All questions listed below are problems taken from old hemistry 121 exams given here at The Ohio State University. Read hapters 8 and
CHEMISTRY. Chapter 10 Theories of Bonding and Structure. The Molecular Nature of Matter. Jespersen Brady Hyslop SIXTH EDITION
 CHEMISTRY The Molecular Nature of Matter SIXTH EDITION Jespersen Brady Hyslop Chapter 10 Theories of Bonding and Structure Copyright 2012 by John Wiley & Sons, Inc. Molecular Structures Molecules containing
CHEMISTRY The Molecular Nature of Matter SIXTH EDITION Jespersen Brady Hyslop Chapter 10 Theories of Bonding and Structure Copyright 2012 by John Wiley & Sons, Inc. Molecular Structures Molecules containing
Chapter 9. Molecular Geometries and Bonding Theories. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
CHEMISTRY - MCMURRY 7E CH.7 - COVALENT BONDING AND ELECTRON DOT STRUCTURES
 !! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
!! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
Chapters 8 and 9. Octet Rule Breakers Shapes
 Chapters 8 and 9 Octet Rule Breakers Shapes Bond Energies Bond Energy (review): The energy needed to break one mole of covalent bonds in the gas phase Breaking bonds consumes energy; forming bonds releases
Chapters 8 and 9 Octet Rule Breakers Shapes Bond Energies Bond Energy (review): The energy needed to break one mole of covalent bonds in the gas phase Breaking bonds consumes energy; forming bonds releases
Valence Shell Electron Pair Repulsion Model
 Valence Shell Electron Pair Repulsion Model Why? Molecules adopt a shape that minimizes their energy. In most cases simply considering the repulsive energy of electron pairs is sufficient to predict molecular
Valence Shell Electron Pair Repulsion Model Why? Molecules adopt a shape that minimizes their energy. In most cases simply considering the repulsive energy of electron pairs is sufficient to predict molecular
CHEM 109A Organic Chemistry. CHEM 109A Organic Chemistry
 CEM 109A Organic Chemistry Wait Lists and Add code requests: Got you emails, working on it! Questions? (non chemistry) Chika Anyiwo, undergraduate advisor anyiwo@chem.ucsb.edu CEM 109A Organic Chemistry
CEM 109A Organic Chemistry Wait Lists and Add code requests: Got you emails, working on it! Questions? (non chemistry) Chika Anyiwo, undergraduate advisor anyiwo@chem.ucsb.edu CEM 109A Organic Chemistry
Molecular Orbitals. Chapter 9. Sigma bonding orbitals. Sigma bonding orbitals. Pi bonding orbitals. Sigma and pi bonds
 Molecular Orbitals Chapter 9 Orbitals and Covalent Bond The overlap of atomic orbitals from separate atoms makes molecular orbitals Each molecular orbital has room for two electrons Two types of MO Sigma
Molecular Orbitals Chapter 9 Orbitals and Covalent Bond The overlap of atomic orbitals from separate atoms makes molecular orbitals Each molecular orbital has room for two electrons Two types of MO Sigma
Hybridization and Molecular Orbital (MO) Theory
 ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
UNIVERSITY OF VICTORIA. CHEMISTRY 101 Mid-Term Test 2, November
 NAME Student No. SECTIN (circle one): A01 (Codding) A02 (Sirk) A03 (Briggs) Version A UNIVERSITY F VICTRIA CEMISTRY 101 Mid-Term Test 2, November 19 2010 Version A This test has two parts and 8 pages,
NAME Student No. SECTIN (circle one): A01 (Codding) A02 (Sirk) A03 (Briggs) Version A UNIVERSITY F VICTRIA CEMISTRY 101 Mid-Term Test 2, November 19 2010 Version A This test has two parts and 8 pages,
