1) Organize the matter into the four types of matter and explain your answer. Matter Type Explanation
|
|
|
- Olivia Freeman
- 5 years ago
- Views:
Transcription
1 Dougherty Valley HS Chemistry Fall Test #1 - Practice Packet S-6* This practice packet is a general guideline to help you study. It is NOT a definitive list. There are potentially things on here that will not show up on the test, and there are potentially things not on this list that will show up on the test. Material that appeared in Warm Ups, Notes, Homework, Classwork, Labs, Study Materials, etc are all have the potential to appear on the test. 1) Organize the matter into the four types of matter and explain your answer. Matter Type Explanation Gasoline Uranium Orange Juice Methane (CH 4) 2) Organize the changes below as physical or chemical and explain your answer. Action Change Explanation Melting of gold Cooking meat Digesting food Charcoal drawing 3) Explain the difference between the mass number and the average atomic mass number. 4) How many protons neutrons and electrons are in the elements below? Element Proton # Electron # Neutron # Mass # Nitrogen Pu Most abundant Iron atom Cl 5) Chlorine has two isotopes 35 Cl with a mass of g and 37 Cl with a mass of The percentage of these isotopes are 75.77% and 24.23% respectively. What is the average atomic mass unit of chlorine? 6) A sample of element X contains 100 atoms with a mass of and 10 atoms with a mass of Calculate the average atomic mass (in amu) of element X. 7) What is an alpha particle and what caused it to change course in the gold foil experiment? 8) Describe in detail everything the gold foil experiment taught us about the structure of the atom 9) Carbon-14 measurements on the linen wrappings from the Book of Isaiah on the Dead Sea Scrolls indicated that the scrolls contained about 79.5% of the carbon-14 found in living tissue. Approximately how old are these scrolls? The half-life of carbon-14 is 5730 years. 10) Phosphorus-32 is a radioactive isotope used as a tracer in the liver. How much phosphorus-32 was originally used if there is only 3.50 mg left in a sample after 288 h? (The half-life is 14.3 days.) 11) Show plutonium 239 going through two alpha decays. 12) The isotope Uranium 238 undergoes a alpha decay and then two beta decays what is your final elemental isotope product?
2 Dougherty Valley HS Chemistry Fall Test #1 Practice Test 1. Express in scientific notation. A) B) C) D) E) Express in scientific notation. A) B) C) D) E) The number expressed in scientific notation is A) B) C) D) E) Express the number in scientific notation. A) B) C) D) kilogram(s) contains this many grams:.5 x 10 2 B) 1.5 x 10 3 C) 15 D) 0.15 E) 1.5 x The volume of a helium balloon is 2.4 L. What is this volume in cm 3? (1 L = 1 dm 3 ) A) 24. cm 3 B) cm 3 C) cm 3 D) 0.24 cm 3 E) cm 3 7. The element curium (Z = 242, A = 96) can be produced by positive-ion bombardment when an alpha particle collides with which of the following nuclei? Recall that a neutron is also a product of this bombardment. A) B) C) D) E) The iodine-131 nuclide has a half-life of 8.0 days. If you originally have a 623-g sample, after 2.0 months you will have (Ignore sig figs for this problem.) A) 46 g B) 54 g C) 120 g D) 3.4 g E) less than 1 g 9. A radioactive element has a half-life of 2.00 weeks. What % of the original sample is left after 19.5 days? A) 38.1% B) 60.8% C) 61.9% D) 1.39% 10. A sample of a radioactive element decays to 27.5% of its original amount of radioactive nuclides in 15 years. What is the half-life of this radioactive element? A) 32. years B) 2.5 years C) 8.1 years D) 91.9 years E) 8.6 years 11. A radioactive element has a half-life of 1.20 years. What % of the original sample is left after days? A) 23.4% B) 76.6% C) 38.3% D) 25.5% E) 16.4% 12. The measurement 3.3 x10 3 g also could be written as A) 3.3 g B) 3.3 mg C) 3.3 pg D) 3.3 kg E) 3.3 dg 13. Which metric prefix is used to designate 1000? A) m B) M C) k D) c E) d 14. Which of the following is an SI unit for expressing the mass of a block of Au? A) m B) g C) L D) pound 24. Calculate the mass of a rectangular solid that has a density of 3.87 g/cm 3 and measures 2.50 cm by 1.80 cm by 3.00 cm. A) 3.49 g B) 52.2 g C) 9.68 g D) 28.3 g E) 55.2 g 25. Find the volume of an object that has a density of 3.14 g/ml and a mass of 55.0 g. 7.5 ml B) 5.71 x 10 2 ml C) 173 ml D) 1.75 x 10 2 ml E) 1.73 x 10 5 ml 26. An object has a mass of 40.1 g and occupies a volume of 6.09 ml. The density of this object is A) 244 g/ml B) g/ml C) 6.58 g/ml D) too low to measure E) 40.1 g/ml 27. The density of an object that has a mass of 4.48 g and occupies a volume of 1.20 ml equals A) 4.48 g/ml B) 1.20 g/ml C) 3.73 g/ml D) 0.27 g/ml E) 5.38 g/ml
3 31. The half-life of a radioactive nuclide is A) that period of time in which 25% of the original number of atoms undergoes radioactive decay. B) the time at which the isotope becomes nonradioactive. C) that period of time in which 50% of the original number of atoms undergoes radioactive decay. D) the period of time it takes to reduce the radioactivity by 100%. E) none of the above 33. The state of matter for an object that has neither definite shape nor definite volume is A) solid B) liquid C) gaseous D) elemental E) mixed 34. Which of the following involves a chemical change? A) boiling water B) melting ice C) chopping wood D) cooking an egg 35. Which of the following is a physical change? A) burning gasoline B) cooking an egg C) decomposing meat D) evaporating water E) rusting iron 36. Which of these is a chemical property? A) Ice melts at 0 C. B) Oxygen is a gas. C) Helium is very nonreactive. D) Sodium is a soft, shiny metal. E) Water has a high specific heat. 37. Which of the following involves no chemical change? A) burning paper B) boiling water C) baking a cake D) lighting a match E) driving a car 38. Which of the following is only a physical change? A) Sugar dissolves in coffee. B) Cookies burn in the oven. C) A banana ripens. D) Leaves turn colors in the fall. E) At least two of the above (a-d) exhibit only a physical change. 39. Which of the following is a chemical change? A) Water condenses on a mirror. B) A damp towel dries. C) Peanuts are crushed. D) A tin can rusts. E) At least two of the above (a-d) exhibit a chemical change. 40. An example of a chemical change is A) boiling alcohol B) grinding coffee beans. C) digesting a pizza D) coffee spilled on a shirt E) an ice cube melting in a drink 41. In a chemical change, A) a phase change must occur B) the original material can never be regenerated C) a phase change never occurs D) the products are different substances from the starting materials 42. Which of the following describes a chemical property of gold? A) Gold is a yellow metal. B) Gold is an inert (nonreactive) metal. C) Gold is a soft metal. D) Gold is a very dense metal. E) Gold is a good conductor of heat and electricity. 43. Which of the following is a chemical change? A) water boiling B) gasoline evaporating C) butter melting D) sugar dissolving in water E) paper burning 44. How many of the following are pure compounds? sodium, sugar, oxygen, air, iron B) 2 C) 3 D) 4 E) Which of the following is an element? A) air B) water C) salt D) helium E) sugar 46. An example of a mixture is A) hydrogen fluoride B) purified water C) gold D) the air in this room E) all of these 47. An example of a pure substance is A) elements B) compounds C) pure water D) carbon dioxide E) all of these 48. A homogeneous mixture is also called. A) a heterogeneous mixture. B) a pure substance. C) a compound. D) a solution. E) an element. 49. Which of the following processes require(s) chemical methods? A) Separating a homogeneous mixture into pure substances. B) Separating a heterogeneous mixture into pure substances. C) Distilling a saltwater mixture. D) Breaking a compound into its constituent elements. E) At least two of the above (a-d) require chemical methods. 50. Which of the following processes is a chemical change? A) Dry ice sublimes when left on the demo table in lecture. B) The light on a candle burns until a bell jar is placed over it for a period of time. C) When a few drops of red food coloring are added to a beaker of hot water, the water immediately turns red. D) Liquid nitrogen dumped onto the floor vaporizes at room temperature. E) None of the above processes are chemical changes.
4 51. A change involves a change in one or more physical properties, but no change in the fundamental components that make up the substance. A) chemical B) physical C) mixed D) Potential E) Kinetic 52. A change involves a change in the fundamental components of the substance; a given substance changes into a different substance or substances. A) chemical B) physical C) mixed D) potential E) kinetic 58. How many of the following did Dalton not discuss in his atomic theory? Isotopes, ions, protons, electrons, neutrons B) 2 C) 3 D) 4 E) Which of the following statements are true? I. Models are always wrong unless they are proved by a theory. II. Elements, such as lead, are made of tiny particles that mostly consist of open space. III. The air you breathe is an example of a heterogeneous mixture. IV. Because NH 3 always contains the same relative numbers of atoms, it will always contain 4.6 g of nitrogen for every 1.0 g of hydrogen. A) II only B) II, IV C) I, II, IV D) I, III E) All of the above statements are true. 63. Which particle has the smallest mass? A) neutron B) proton C) electron D) helium nucleus 64. How many protons, electrons, and neutrons, respectively, does 127 I have? A) 53, 127, 74 B) 53, 74, 53 C) 53, 53, 127 D) 74, 53, 127 E) 53, 53, How many protons, electrons, and neutrons, respectively, does 16 O have? A) 8, 18, 8 B) 8, 8, 8 C) 8, 10, 8 D) 8, 14, 8 E) 8, 18, An atom with 15 protons and 16 neutrons is an atom of A) P B) Ga C) S D) Pd E) Rh 67. A certain isotope X + contains 54 electrons and 78 neutrons. What is the mass number for this element? 33 B) 132 C) 131 D) 55 E) 53 Answer Key 1. C 2. B 3. D 4. A 5. B 6. B 7. E 8. D 9. A 10. C 11. B 12. D 13. C 14. B 24. B 25. A 26. C 27. C 31. C 33. C 34. D 35. D 36. C 37. B 38. A 39. D 40. C 41. D 42. B 43. E 44. A 45. D 46. D 47. E 48. D 49. D 50. B 51. B 52. A 58. E 59. B 63. C 64. E 65. B 66. A 67. A 4. A line spectrum is produced when an electron moves from one energy level a. to a higher energy level. b. to a lower energy level. c. into the nucleus. d. to another position in the same sublevel. 5. Because excited hydrogen atoms always produce the same lineemission spectrum, scientists concluded that hydrogen a. had no electrons. b. did not release photons. c. released photons of only certain energies. d. could only exist in the ground state. 6. For an electron in an atom to change from the ground state to an excited state, a. energy must be released. b. energy must be absorbed. c. radiation must be emitted. d. the electron must make a transition from a higher to a lower energy level. 8. According to the quantum theory of an atom, in an orbital a. an electron's position cannot be known precisely. b. an electron has no energy. c. electrons cannot be found. d. electrons travel around the nucleus on paths of specific radii. 13.The set of orbitals that are dumbbell shaped and directed along the x, y, and z axes are called a. d orbitals. b. p orbitals. c. f orbitals. d. s orbitals. 14.A spherical electron cloud surrounding an atomic nucleus would best represent a. an s orbital. b. a px orbital. c. a combination of px and py orbitals. d. a combination of an s and a px orbital.
5 15.The major difference between a 1s orbital and a 2s orbital is that a. the 2s orbital can hold more electrons. b. the 2s orbital has a slightly different shape. c. the 2s orbital is at a higher energy level. d. the 1s orbital can have only one electron. 16.An orbital that can never exist according to the quantum description of the atom is a. 3d. b. 7s. c. 6d. d. 3f. 18.The number of orientations for the d orbitals is a. 1. b. 3. c. 5. d How many orientations can an s orbital have about the nucleus? a. 1 b. 2 c. 3 d One main energy level can hold 18 electrons. What is n? a. b. 3 c. 6 d Electron occupies the lowest available energy orbital is a. Hund's rule. b. the Aufbau principle. c. Bohr's law. d. the Pauli exclusion principle. 22."Orbitals of equal energy are each occupied by one electron before any is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin" is a. the Pauli exclusion principle. b. the Aufbau principle. c. the quantum effect. d. Hund's rule. 23.Which of the following lists atomic orbitals in the correct order they are filled according to the Aufbau principle? a. 1s 2s 2p 3s 4s 3p 3d 4p 5s b. 1s 2s 2p 3s 3p 4s 3d 4p 5s c. 1s 2s 2p 3s 3p 4s 4p 3d 4d d. 1s 2s 2p 3s 3p 3d 4s 4p 5s 24.In the ground state, the 3d and 4s orbitals of the chromium atom (atomic number 24) are represented as a. 3d 6 4s 1. b. 3d 4 4s 2. c. 3d 5 4s 1. d. 4s 2 3d The element with electron configuration 1s 2 2s 2 2p 6 3s 2 3p 2 is a. Mg (Z = 12). b. C (Z = 6). c. S (Z = 16). d. Si (Z = 14). 26.The electron notation for aluminum (atomic number 13) is a. 1s 2 2s 2 2p 3 3s 2 3p 3 3d 1. b. 1s 2 2s 2 2p 6 3s 2 2d 1. c. 1s 2 2s 2 2p 6 3s 2 3p 1. d. 1s 2 2s 2 2p The number of electrons in the highest energy level of the argon atom (atomic number 18) is a. 10. b. 2. c. 6. d. 8. Problem Use periodic table below to answer the following Qs 28.Which element has the following electron configuration: [Ar] 4s 2 3d 10 4p 5? 29.Write the noble-gas electron configuration for silicon. 30.Draw the orbital diagram for phosphorus. 31.Draw the orbital diagram for argon. 32.Write the noble-gas electron configuration represented in the orbital diagram below. 33.Argon, krypton, and xenon are a. alkaline earth metals. b. noble gases. c. actinides. d. lanthanides. 4. B 5. C 6. B Answers 8. A 13. B 14. A 15. C 16. D 17. C 18. C 19. A 20. B 21. B 22. D 23. B 24. C 25. D 26. C 27. D 28. Br 29. [Ne] 3s 2 3p X 31. X 32. [Ne] 3s 2 3p B
6 Dougherty Valley HS Chemistry Fall Test #1 Practice Test 1. Which color of visible light has the least amount of energy per photon? A) violet B) blue C) green D) yellow E) red 2. The energy levels of the hydrogen atom (and all atoms) are, meaning that only certain discrete energy levels are allowed. A) varied B) quantized C) ramp-like D) continuous E) two of these 3. True or false? A packet of energy of electromagnetic radiation is called a electrons. A) True B) False 5. The probability map for an electron is called A) an orbit B) a photon C) an orbital D) an electron configuration 6. The maximum number of electrons allowed in each of the p orbitals is A) 2 B) 4 C) 8 D) A given set of d orbitals consists of orbital(s). B) 3 C) 5 D) 6 8. The maximum electron capacity of an f sublevel is 8 B) 14 C) 10 D) 6 E) 2 9. A d sublevel can hold a maximum of A) 5 electrons B) 10 electrons C) 14 electrons D) 32 electrons 10. The number of d orbitals in the second principal energy level is A) 2 B) 6 C) 10 D) The electron configuration for the sulfur atom is s 2 2s 2 2p 6 3s 2 3p 2 B) 1s 2 2s 2 2p 6 3s 2 3p 4 C) 1s 2 2s 2 2p 6 3s 5 D) 1s 2 2s 2 2p 6 3s 2 3p How many electrons are in the third principal energy level (n = 3) of one atom of Fe? A) 2 B) 8 C) 14 D) The noble gases contain how many valence electrons? B) 7 C) 0 D) The maximum number of electrons in the second principal energy level of an atom is A) 2 B) 6 C) 8 D) 18 E) Which element has the fewest electrons in its valence shell? A) Cs B) Mg C) P D) O E) Br 17. Which one of the following atoms has a partly filled d sublevel? A) Ca B) Ni C) Zn D) As E) Ar 19. What is the expected ground-state electron configuration for Te 2-? A) [Kr]5s 2 5d 10 5p 4 B) [Kr]5s 2 4d 10 5p 4 C) [Kr]5s 2 4d 10 4f 14 5p 6 D) [Kr]5s 2 4d 10 5p 6 E) [Ar]5s 2 4d 10 5p The correct electron configuration for Mn is s 2 2s 2 2p 6 3s 2 3p 6 3d 7 B) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 C) 1s 2 2s 2 2p 6 2d 10 3s 2 3p 3 D) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d The electron configuration for manganese is A) [Ar] 3d 7 B) 1s 2 2s 2 2p 6 3s 1 3d 6 C) [Ar] 4s 2 3d 5 D) 1s 2 2s 2 2p 6 3s 2 3d 4 E) [Ar] 4s 2 4p Which of the following has the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5? A) Cr B) Ca C) Mn D) Br
7 Dougherty Valley HS Chemistry Fall Test #1 Practice Test 23. Which of the following atoms has the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1? A) Sc B) Ca C) Sr D) Ar 24. How many unpaired electrons does the element cobalt (Co) have in its lowest energy state? A) 0 B) 1 C) 2 D) 3 E) Which electron configuration indicates a transitional element? s 2 2s 2 2p 6 3s 1 3p 6 B) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 3 C) 1s 2 2s 2 2p 5 D) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p The element with the electron configuration [Kr] 5s 2 4d 10 5p 3 is A) As B) Sb C) Nb D) Pr 27. How many of the following electron configurations for the species in their ground state are correct? I. Ca: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 II. Mg: 1s 2 2s 2 2p 6 3s 1 III. V: [Ar] 3s 2 3d 3 IV. As: [Ar] 4s 2 3d 10 4p 3 V. P: 1s 2 2s 2 2p 6 3p 5 B) 2 C) 3 D) 4 E) Consider the following representation of the one orbital below. The points represent various electron locations. 31. What element has the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5? A) Cl B) Se C) I D) Kr E) Br 32. What element has the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 3? A) N B) P C) S D) Al E) Cl 33. What element has the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 6? A) Ar B) Cl C) Kr D) S 34. What atom below will have the same number of electrons as Fe 2+ A) Cr B) Ni C) Kr D) Ar E) K 42. How many f orbitals have the value n = 3? A) 0 B) 3 C) 5 D) 7 E) Which Ion below has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p6 A) Ar B) Cl 2- C) Ne D) S 2- E) O 2- Where could an electron be located in the representation above? A) Point A B) Point B C) Point C D) Point D E) An electron could be located at any of these points.
8
Q1 and Q2 Review large CHEMISTRY
 Q1 and Q2 Review large CHEMISTRY Multiple Choice Identify the choice that best completes the statement or answers the question. 1. E = hv relates the following a. Energy to Planck s constant & wavelength
Q1 and Q2 Review large CHEMISTRY Multiple Choice Identify the choice that best completes the statement or answers the question. 1. E = hv relates the following a. Energy to Planck s constant & wavelength
CHEMISTRY I - HONORS MIDTERM REVIEW* *Test may cover other topics not included on this review, yet have been covered throughout the semester.
 Name Period CHEMISTRY I - HONORS MIDTERM REVIEW* *Test may cover other topics not included on this review, yet have been covered throughout the semester. Chapter 2 Measurement & Calculations Describe the
Name Period CHEMISTRY I - HONORS MIDTERM REVIEW* *Test may cover other topics not included on this review, yet have been covered throughout the semester. Chapter 2 Measurement & Calculations Describe the
Unit Two Test Review. Click to get a new slide. Choose your answer, then click to see if you were correct.
 Unit Two Test Review Click to get a new slide. Choose your answer, then click to see if you were correct. According to the law of definite proportions, any two samples of water, H2O, A. will be made up
Unit Two Test Review Click to get a new slide. Choose your answer, then click to see if you were correct. According to the law of definite proportions, any two samples of water, H2O, A. will be made up
Unit 1: Analyzing Data 1. Measure the following using the appropriate number of significant digits. Name Hour Date. b. o C
 Name Hour Date Chemistry Semester 1 Review!!! If you have completed ALL of the review on the assigned days you are allowed to use a cheat sheet that is created on the back of the periodic table found on
Name Hour Date Chemistry Semester 1 Review!!! If you have completed ALL of the review on the assigned days you are allowed to use a cheat sheet that is created on the back of the periodic table found on
Honors Chemistry - 1st Semester Final Practice Exam
 Honors Chemistry - 1st Semester Final Practice Exam Mr. Matthew Totaro MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which statement about the
Honors Chemistry - 1st Semester Final Practice Exam Mr. Matthew Totaro MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which statement about the
ATOMIC THEORY, PERIODICITY, and NUCLEAR CHEMISTRY
 ATOMIC THEORY, PERIODICITY, and NUCLEAR CHEMISTRY Note: For all questions referring to solutions, assume that the solvent is water unless otherwise stated. 1. The nuclide is radioactive and decays by the
ATOMIC THEORY, PERIODICITY, and NUCLEAR CHEMISTRY Note: For all questions referring to solutions, assume that the solvent is water unless otherwise stated. 1. The nuclide is radioactive and decays by the
new experimental data, and can be modified
 Mass in grams 10 20 30 40 50 Name: Date: Period: CP Chemistry Semester 1 Final Test Review CHAPTERS 1 & 2: Scientific Method, Density, Metric Conversions, Accuracy/Precision, Significant Figures 1. Know
Mass in grams 10 20 30 40 50 Name: Date: Period: CP Chemistry Semester 1 Final Test Review CHAPTERS 1 & 2: Scientific Method, Density, Metric Conversions, Accuracy/Precision, Significant Figures 1. Know
273 TC. Mass Length Volume 1 lb = g * 1 in = 2.54 cm 1 L = qt* 1 kg = lb * 1 m = yd * 1 ft 3 = L * 1 mi = 1.
 Chem 106 Midterm Study Questions Name: Chapters 1-5,11-12 Review Mon 10/10/2016 Due 10/13/2016 (Midterm exam) This is a homework assignment. Please show your work for full credit. If you do work on separate
Chem 106 Midterm Study Questions Name: Chapters 1-5,11-12 Review Mon 10/10/2016 Due 10/13/2016 (Midterm exam) This is a homework assignment. Please show your work for full credit. If you do work on separate
Chem 106 Midterm Study Questions Chapters 1-5,11-12
 Chem 106 Midterm Study Questions Chapters 1-5,11-12 Name: Review Mon 10/12/15 Due 10/13/15 (Midterm exam) This is a homework assignment. Please show your work for full credit. If you do work on separate
Chem 106 Midterm Study Questions Chapters 1-5,11-12 Name: Review Mon 10/12/15 Due 10/13/15 (Midterm exam) This is a homework assignment. Please show your work for full credit. If you do work on separate
Regents review Atomic & periodic
 2011-2012 1. The diagram below represents the nucleus of an atom. What are the atomic number and mass number of this atom? A) The atomic number is 9 and the mass number is 19. B) The atomic number is 9
2011-2012 1. The diagram below represents the nucleus of an atom. What are the atomic number and mass number of this atom? A) The atomic number is 9 and the mass number is 19. B) The atomic number is 9
Teacher Workbooks. Science and Nature Series. Atomic Structure, Electron Configuration, Classifying Matter and Nuclear Chemistry, Vol.
 Teacher Workbooks Science and Nature Series Atomic Structure, Electron Configuration, Classifying Matter and Nuclear Chemistry, Vol. 1 Copyright 23 Teachnology Publishing Company A Division of Teachnology,
Teacher Workbooks Science and Nature Series Atomic Structure, Electron Configuration, Classifying Matter and Nuclear Chemistry, Vol. 1 Copyright 23 Teachnology Publishing Company A Division of Teachnology,
Chapter 2 Atoms and the Periodic Table
 Chapter 2 1 Chapter 2 Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element.
Chapter 2 1 Chapter 2 Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element.
Lab Chemistry - Final Exam Review 1. Express in scientific notation. A) D) B) E) C)
 Lab Chemistry - Final Exam Review 1. Express 30554000 in scientific notation. A) 3 10 7 D) 30554 10 3 B) 3.0554 10 7 E) 305540 10 7 C) 306 10 7 2. What is the result of the following multiplication expressed
Lab Chemistry - Final Exam Review 1. Express 30554000 in scientific notation. A) 3 10 7 D) 30554 10 3 B) 3.0554 10 7 E) 305540 10 7 C) 306 10 7 2. What is the result of the following multiplication expressed
Multiple Choice Identify the choice that best completes the statement or answers the question.
 Chemi1100pretest1 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Accordingly to the periodic law the properties of elements repeat at regular intervals
Chemi1100pretest1 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Accordingly to the periodic law the properties of elements repeat at regular intervals
Regular Chemistry - 1st Semester Final Practice Exam
 Regular Chemistry - 1st Semester Final Practice Exam Mr. Matthew Totaro MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which statement about the
Regular Chemistry - 1st Semester Final Practice Exam Mr. Matthew Totaro MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which statement about the
Atomic Theory. H. Cannon, C. Clapper and T. Guillot Klein High School
 Atomic Theory Unit 3 Development of the Atomic Theory 1. Where is the mass of the atom concentrated? 2. What is located in the nucleus? 3. What is the negative particle that orbits the nucleus? 4. What
Atomic Theory Unit 3 Development of the Atomic Theory 1. Where is the mass of the atom concentrated? 2. What is located in the nucleus? 3. What is the negative particle that orbits the nucleus? 4. What
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
Note that the protons and neutrons are each almost 2,000 times more massive than an electron; What is the approximate diameter of an atom?
 Atomic Structure and the Periodic Table Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Atomic Structure and the Periodic Table Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Full file at
 16 Chapter 2: Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element. a.
16 Chapter 2: Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element. a.
Review. 8th grade science STAAR. Name Class. Underline your strong TEKS and circle your weak TEKS: 8.5A Atoms. 7.5C Energy Flow Through Ecosystems
 8th grade science STAAR Review Name Class Underline your strong TEKS and circle your weak TEKS: 8.5A Atoms 7.5C Energy Flow Through Ecosystems 8.5B Reactivity 8.5C Periodic Table 8.5D Chemical Formulas
8th grade science STAAR Review Name Class Underline your strong TEKS and circle your weak TEKS: 8.5A Atoms 7.5C Energy Flow Through Ecosystems 8.5B Reactivity 8.5C Periodic Table 8.5D Chemical Formulas
Name: American River College Chemistry 310 Exam #1 Spring 2017
 Name: American River College Chemistry 310 Exam #1 Spring 2017 1). Which of the following is not a step in the scientific method? a. Make an observation. b. Formulate a hypothesis. c. Perform an experiment.
Name: American River College Chemistry 310 Exam #1 Spring 2017 1). Which of the following is not a step in the scientific method? a. Make an observation. b. Formulate a hypothesis. c. Perform an experiment.
8. Which of the following could be an isotope of chlorine? (A) 37 Cl 17 (B) 17 Cl 17 (C) 37 Cl 17 (D) 17 Cl 37.5 (E) 17 Cl 37
 Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
Electronic Structure Worksheet 1 Given the following list of atomic and ionic species, find the appropriate match for questions 1-4. (A) Fe 2+ (B) Cl (C) K + (D) Cs (E) Hg + 1. Has the electron configuration:
What are the isotopes of hydrogen? H, 2 H, and 3 H
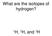 What are the isotopes of hydrogen? 1 H, 2 H, and 3 H The average mass of a boron atom is 10.81. Assuming you were able to isolate only one boron atom, the chance that you would randomly get one with a
What are the isotopes of hydrogen? 1 H, 2 H, and 3 H The average mass of a boron atom is 10.81. Assuming you were able to isolate only one boron atom, the chance that you would randomly get one with a
Review. 8th grade science STAAR. Name Class. Underline your strong TEKS and circle your weak TEKS: 8.5A Atoms. 7.5C Energy Flow Through Ecosystems
 8th grade science STAAR Review Name Class Underline your strong TEKS and circle your weak TEKS: 8.5A Atoms 7.5C Energy Flow Through Ecosystems 8.5B Reactivity 8.5C Periodic Table 8.5D Chemical Formulas
8th grade science STAAR Review Name Class Underline your strong TEKS and circle your weak TEKS: 8.5A Atoms 7.5C Energy Flow Through Ecosystems 8.5B Reactivity 8.5C Periodic Table 8.5D Chemical Formulas
EM SPECTRUM, WAVELENGTH, FREQUENCY, AND ENERGY WORKSHEET
 EM SPECTRUM, WAVELENGTH, FREQUENCY, AND ENERGY WORKSHEET 1.) Look at the EM spectrum below to answer this question. As you move across the visible light spectrum from red to violet (A) Does the wavelength
EM SPECTRUM, WAVELENGTH, FREQUENCY, AND ENERGY WORKSHEET 1.) Look at the EM spectrum below to answer this question. As you move across the visible light spectrum from red to violet (A) Does the wavelength
Which of the following chemical elements corresponds to the symbol Cu?
 Which of the following chemical elements corresponds to the symbol Cu? A) copper B) gold C) lead D) silver E) none of the above Which of the following chemical elements corresponds to the symbol Cu? A)
Which of the following chemical elements corresponds to the symbol Cu? A) copper B) gold C) lead D) silver E) none of the above Which of the following chemical elements corresponds to the symbol Cu? A)
Chapter 2: The Structure of the Atom and the Periodic Table
 Chapter 2: The Structure of the Atom and the Periodic Table 1. What are the three primary particles found in an atom? A) neutron, positron, and electron B) electron, neutron, and proton C) electron, proton,
Chapter 2: The Structure of the Atom and the Periodic Table 1. What are the three primary particles found in an atom? A) neutron, positron, and electron B) electron, neutron, and proton C) electron, proton,
Chemistry CRT Study Guide First Quarter
 Number AL COS # 1. #1.0 Classify sodium chloride as an element, mixture, compound, or colloid. Compound 2. #1.0 Classify air as an element, mixture, compound, or colloid. Mixture 3. #1.0 Classify a blueberry
Number AL COS # 1. #1.0 Classify sodium chloride as an element, mixture, compound, or colloid. Compound 2. #1.0 Classify air as an element, mixture, compound, or colloid. Mixture 3. #1.0 Classify a blueberry
6. How many nonmetal atoms are there in the formula: NaH 2 PO 4? a. 1 b. 2 c. 4 d. 6 e. 7
 C101 Chapters 1 and 2 90 representative questions from old exams The questions are grouped by topic. Do these problems as homework to prepare for Exam 1. 1. Carbon is a metal/nonmetal that has the symbol.
C101 Chapters 1 and 2 90 representative questions from old exams The questions are grouped by topic. Do these problems as homework to prepare for Exam 1. 1. Carbon is a metal/nonmetal that has the symbol.
Modern Atomic Theory
 Modern Atomic Theory Review of the Discovery of the Atom 1803 John Dalton discovered that elements are made of atoms. He thought that atoms were solid, like a marble. 1875 Crooks discovered the electron.
Modern Atomic Theory Review of the Discovery of the Atom 1803 John Dalton discovered that elements are made of atoms. He thought that atoms were solid, like a marble. 1875 Crooks discovered the electron.
Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT.
 ELECTRONS IN ATOMS Chapter Quiz Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. The orbitals of a principal energy level are lower in energy than the orbitals
ELECTRONS IN ATOMS Chapter Quiz Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. The orbitals of a principal energy level are lower in energy than the orbitals
Which order of statements represents the historical development of the atomic model? A) C D A B B) C D B A C) D B A C D) D B C A
 1. The mass of a proton is approximately equal to the mass of A) an electron B) a neutron C) an alpha particle D) a beta particle 2. What is the number of electrons in an atom that has 20 protons and 17
1. The mass of a proton is approximately equal to the mass of A) an electron B) a neutron C) an alpha particle D) a beta particle 2. What is the number of electrons in an atom that has 20 protons and 17
ATOMIC MATH HOMEWORK
 Name: Block: ATOMIC MATH HOMEWORK True/False: Indicate if each of the following is true or false. If it is false, CHANGE the underlined portion to make it true. 1. In a neutral atom of an element, the
Name: Block: ATOMIC MATH HOMEWORK True/False: Indicate if each of the following is true or false. If it is false, CHANGE the underlined portion to make it true. 1. In a neutral atom of an element, the
Honors Chemistry: Chapter 4- Problem Set (with some 6)
 Honors Chemistry: Chapter 4- Problem Set (with some 6) All answers and work on a separate sheet of paper! Classify the following as always true (AT), sometimes true (ST), or never true (NT) 1. Atoms of
Honors Chemistry: Chapter 4- Problem Set (with some 6) All answers and work on a separate sheet of paper! Classify the following as always true (AT), sometimes true (ST), or never true (NT) 1. Atoms of
E J The electron s energy difference between the second and third levels is J. = J
 The wavelength of light emitted is 654 nm. = c f c 3.00 10 8 m/s f c 3.00 108 m 1s 6.54 10 7 m f 4.59 4.59 10 14 z 1 s 10 14 The frequency of the light emitted is 4.59 10 14 z. E hf h 6.63 10 34 J/z E
The wavelength of light emitted is 654 nm. = c f c 3.00 10 8 m/s f c 3.00 108 m 1s 6.54 10 7 m f 4.59 4.59 10 14 z 1 s 10 14 The frequency of the light emitted is 4.59 10 14 z. E hf h 6.63 10 34 J/z E
PYP 001 First Major Code: Term: 132 Tuesday, March 11, 2014 Page: 1
 Term: 132 Tuesday, March 11, 2014 Page: 1 *The correct answer is the choice (A) for all questions. 1 is NOT a pure substance. A) An orange. B) Iron rust. C) Carbon dioxide. D) Neon (Ne). E) Dry ice. 2
Term: 132 Tuesday, March 11, 2014 Page: 1 *The correct answer is the choice (A) for all questions. 1 is NOT a pure substance. A) An orange. B) Iron rust. C) Carbon dioxide. D) Neon (Ne). E) Dry ice. 2
Observation information obtained through the senses; observation in science often involves measurement
 Review Sheet Unit 1: The Atom Chemistry the study of the composition of matter and the changes matter undergoes Scientific Method Scientific method a logical, systematic approach to the solution of a scientific
Review Sheet Unit 1: The Atom Chemistry the study of the composition of matter and the changes matter undergoes Scientific Method Scientific method a logical, systematic approach to the solution of a scientific
Chapter 2: Atoms and the Periodic Table
 1. Which element is a nonmetal? A) K B) Co C) Br D) Al Ans: C Difficulty: Easy 2. Which element is a metal? A) Li B) Si C) Cl D) Ar E) More than one of the elements above is a metal. Ans: A Difficulty:
1. Which element is a nonmetal? A) K B) Co C) Br D) Al Ans: C Difficulty: Easy 2. Which element is a metal? A) Li B) Si C) Cl D) Ar E) More than one of the elements above is a metal. Ans: A Difficulty:
Reporting Category 1: Matter and Energy
 Name: Science Teacher: Reporting Category 1: Matter and Energy Atoms 8.5A Fill in the missing information to summarize what you know about atomic structure. Name of Subatomic Particle Location within the
Name: Science Teacher: Reporting Category 1: Matter and Energy Atoms 8.5A Fill in the missing information to summarize what you know about atomic structure. Name of Subatomic Particle Location within the
Electron Configurations
 Section 3 Electron Configurations Key Terms electron configuration Pauli exclusion principle noble gas Aufbau principle Hund s rule noble-gas configuration Main Ideas Electrons fill in the lowest-energy
Section 3 Electron Configurations Key Terms electron configuration Pauli exclusion principle noble gas Aufbau principle Hund s rule noble-gas configuration Main Ideas Electrons fill in the lowest-energy
Name Hour Date. B. Provide an example for the labs that we did to support your explanation.
 Name Hour Date Chemistry Semester 1 Review!!! If you have completed ALL of the review on the assigned days you are allowed to use a cheat sheet that is created on the back and of the periodic table found
Name Hour Date Chemistry Semester 1 Review!!! If you have completed ALL of the review on the assigned days you are allowed to use a cheat sheet that is created on the back and of the periodic table found
Ch 9 Electrons in Atoms & the Periodic Table Study Sheet Acc. Chemistry SCANTRON. Name /99. 3) Light is a type of matter. 3)
 Ch 9 Electrons in Atoms & the Periodic Table Study Sheet Acc. Chemistry SCANTRON Name /99 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. 1) When the elements are arranged
Ch 9 Electrons in Atoms & the Periodic Table Study Sheet Acc. Chemistry SCANTRON Name /99 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. 1) When the elements are arranged
Name: Section: Matter: Atoms and Properties Practice Test
 Name: Section: Matter: Atoms and Properties Practice Test Directions: For each of the questions or incomplete statements below, choose the best of the answer choices given and write your answer on the
Name: Section: Matter: Atoms and Properties Practice Test Directions: For each of the questions or incomplete statements below, choose the best of the answer choices given and write your answer on the
Ch. 3 Answer Key. O can be broken down to form two atoms of H and 1 atom of O. Hydrogen and oxygen are elements.
 Ch. 3 Answer Key 1. The Greeks believed that all matter is made of elements. We currently believe the same thing. However, the Greeks believed that there were 4 elements: earth, water, air and fire. Instead,
Ch. 3 Answer Key 1. The Greeks believed that all matter is made of elements. We currently believe the same thing. However, the Greeks believed that there were 4 elements: earth, water, air and fire. Instead,
Full file at Chapter 2 The Chemical View of Matter
 Chapter 2 The Chemical View of Matter MULTIPLE CHOICE 1. Which of the following is not one of the common states of matter? a. solid b. plasma c. liquid d. gas 2. A pure substance which can be decomposed
Chapter 2 The Chemical View of Matter MULTIPLE CHOICE 1. Which of the following is not one of the common states of matter? a. solid b. plasma c. liquid d. gas 2. A pure substance which can be decomposed
Test Topics: Periodic Table, Atomic Theory, Physical/Chemical Properties, Atom, Isotopes, Average Atomic Mass
 Elemental Properties Review Worksheet Test Topics: Periodic Table, Atomic Theory, Physical/Chemical Properties, Atom, Isotopes, Average Atomic Mass Periodic Table 1. List the element symbols for the following
Elemental Properties Review Worksheet Test Topics: Periodic Table, Atomic Theory, Physical/Chemical Properties, Atom, Isotopes, Average Atomic Mass Periodic Table 1. List the element symbols for the following
5. The outermost principal energy level electron configuration of the element bromine is: a. 4s 2 c. 4s 2 4p 5 b. 4p 5 d.
 1 c E = h 1. Sodium and potassium have similar properties because they have the same a. atomic radii. c. number of valence electrons. b. ionization energy. d. electronegativity. 2. Electrons must be added
1 c E = h 1. Sodium and potassium have similar properties because they have the same a. atomic radii. c. number of valence electrons. b. ionization energy. d. electronegativity. 2. Electrons must be added
 Electronic configurations, Auf-bau principle, Pauli principle, Hunds rule 1. Which of the following statements in relation to the hydrogen atom is correct? 1) 3s and 3p orbitals are of lower energy than
Electronic configurations, Auf-bau principle, Pauli principle, Hunds rule 1. Which of the following statements in relation to the hydrogen atom is correct? 1) 3s and 3p orbitals are of lower energy than
Duncan. Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1. Figure 2. Figure 3
 Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1 Figure 2 Figure 3 Light Calculation Notes Here s how the type/form of EM radiation can be determined The amount
Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1 Figure 2 Figure 3 Light Calculation Notes Here s how the type/form of EM radiation can be determined The amount
4.1 Atomic structure and the periodic table. GCSE Chemistry
 4.1 Atomic structure and the periodic table GCSE Chemistry All substances are made of atoms this is cannot be chemically broken down it is the smallest part of an element. Elements are made of only one
4.1 Atomic structure and the periodic table GCSE Chemistry All substances are made of atoms this is cannot be chemically broken down it is the smallest part of an element. Elements are made of only one
Unit 7 Study Guide: Name: KEY Atomic Concepts & Periodic Table
 Unit 7 Study Guide: Name: KEY Atomic Concepts & Periodic Table Focus Questions for the unit... How has the modern view of the atom changed over time? How does a chemist use symbols and notation to communicate
Unit 7 Study Guide: Name: KEY Atomic Concepts & Periodic Table Focus Questions for the unit... How has the modern view of the atom changed over time? How does a chemist use symbols and notation to communicate
Chemistry: A Molecular Approach, 2e (Tro) Chapter 2 Atoms and Elements. Multiple Choice Questions
 Chemistry: A Molecular Approach, 2e (Tro) Chapter 2 Atoms and Elements Multiple Choice Questions 1) In a chemical reaction, matter is neither created or destroyed. Which law does this refer to? A) Law
Chemistry: A Molecular Approach, 2e (Tro) Chapter 2 Atoms and Elements Multiple Choice Questions 1) In a chemical reaction, matter is neither created or destroyed. Which law does this refer to? A) Law
Unit 3. The Atom & Modern Atomic Theory
 Unit 3 The Atom & Modern Atomic Theory Theories of the Atom Early Models & Thoughts: Democritus Matter is made up of tiny particles called atoms. Smallest unit that retains the identity of the element
Unit 3 The Atom & Modern Atomic Theory Theories of the Atom Early Models & Thoughts: Democritus Matter is made up of tiny particles called atoms. Smallest unit that retains the identity of the element
1. What is the difference between a qualitative and quantitative observation? Give at least one example of each.
 1 st 9wks Exam Review Name Per. Chemistry 1H Scientific Skills Unit - Lab Safety & Equipment: KEY EQUIPMENT TO KNOW: +beaker +Erlenmeyer flask +beaker tongs +balance +graduated cylinder +test tube +test
1 st 9wks Exam Review Name Per. Chemistry 1H Scientific Skills Unit - Lab Safety & Equipment: KEY EQUIPMENT TO KNOW: +beaker +Erlenmeyer flask +beaker tongs +balance +graduated cylinder +test tube +test
4. The mass of a proton is approximately equal to the mass of A an alpha particle C a positron. B a beta particle D a neutron
 1. Which particles have approximately the same mass? A an electron and an alpha particle B an electron and a proton C a neutron and an alpha particle D a neutron and a proton 2. Which phrase describes
1. Which particles have approximately the same mass? A an electron and an alpha particle B an electron and a proton C a neutron and an alpha particle D a neutron and a proton 2. Which phrase describes
Unit 02 Review: Atomic Theory and Periodic Table Review
 Practice Multiple Choice Questions Unit 02 Review: Atomic Theory and Periodic Table Review 1. The number of neutrons in an atom of radioactive C 14 is: a) 6 c) 8 b) 12 d) 14 2. When a radioactive nucleus
Practice Multiple Choice Questions Unit 02 Review: Atomic Theory and Periodic Table Review 1. The number of neutrons in an atom of radioactive C 14 is: a) 6 c) 8 b) 12 d) 14 2. When a radioactive nucleus
Democritus s ideas don t explain chemical behavior & lacked experimental support.
 A1: Atomic Structure Worksheet Key (Goals 1 3, Chapter 4) 1. Democritus, who lived in Greece during the 4 th century B.C., suggested that matter is made up of tiny particles that cannot be divided. He
A1: Atomic Structure Worksheet Key (Goals 1 3, Chapter 4) 1. Democritus, who lived in Greece during the 4 th century B.C., suggested that matter is made up of tiny particles that cannot be divided. He
Chemistry Released Questions
 Name: Date: 1. What was Niels Bohr s prediction about the location of the electrons in an atom? 3. An atom with which atomic diagram has chemical properties most similar to calcium? A. Electrons pair with
Name: Date: 1. What was Niels Bohr s prediction about the location of the electrons in an atom? 3. An atom with which atomic diagram has chemical properties most similar to calcium? A. Electrons pair with
Chapter 2: Atoms and the Periodic Table
 1. Which element is a nonmetal? A) K B) Co C) Br D) Al Ans: C Difficulty: Easy 2. Which element is a metal? A) Li B) Si C) Cl D) Ar E) More than one of the elements above are metals. 3. Which element is
1. Which element is a nonmetal? A) K B) Co C) Br D) Al Ans: C Difficulty: Easy 2. Which element is a metal? A) Li B) Si C) Cl D) Ar E) More than one of the elements above are metals. 3. Which element is
Unit 3. Atoms and molecules
 Unit 3. Atoms and molecules Index. s and compounds...2.. Dalton's Atomic theory...2 2.-The atom...2 3.-Atomic number and mass number...2 4.-Isotopes, atomic mass unit and atomic mass...3 5.- configuration...3
Unit 3. Atoms and molecules Index. s and compounds...2.. Dalton's Atomic theory...2 2.-The atom...2 3.-Atomic number and mass number...2 4.-Isotopes, atomic mass unit and atomic mass...3 5.- configuration...3
TEST REVIEW GCAA Chemistry Atoms. A. Excited B. Energy C. Orbital D. Plum Pudding Model
 TEST REVIEW GCAA Chemistry Atoms From the Hangman Game-----Match the answers correctly! A. Excited B. Energy C. Orbital D. Plum Pudding Model E. Bohr F. Electron G. Frequency H. Neutron I. Thomson J. alpha
TEST REVIEW GCAA Chemistry Atoms From the Hangman Game-----Match the answers correctly! A. Excited B. Energy C. Orbital D. Plum Pudding Model E. Bohr F. Electron G. Frequency H. Neutron I. Thomson J. alpha
3.1 Classification of Matter. Copyright 2009 by Pearson Education, Inc.
 Chapter 3 Atoms and Elements 3.1 Classification of Matter Copyright 2009 by Pearson Education, Inc. 1 Matter Matter is the stuff that makes up all things. Copyright 2009 by Pearson Education, Inc. 2 Pure
Chapter 3 Atoms and Elements 3.1 Classification of Matter Copyright 2009 by Pearson Education, Inc. 1 Matter Matter is the stuff that makes up all things. Copyright 2009 by Pearson Education, Inc. 2 Pure
#9 Modern Atomic Theory Quantitative Chemistry
 Name #9 Modern Atomic Theory Quantitative Chemistry Student Learning Map Unit EQ: What is the current model of the atom? Key Learning: The current model of the atom is based on the quantum mechanical model.
Name #9 Modern Atomic Theory Quantitative Chemistry Student Learning Map Unit EQ: What is the current model of the atom? Key Learning: The current model of the atom is based on the quantum mechanical model.
Matter and Energy. Chapter 3
 Matter and Energy Chapter 3 Matter Anything that has mass and takes up space Two categories Pure substances Mixtures Pure Substances Matter with a fixed composition Either an element or compound Element
Matter and Energy Chapter 3 Matter Anything that has mass and takes up space Two categories Pure substances Mixtures Pure Substances Matter with a fixed composition Either an element or compound Element
MODERN ATOMIC THEORY AND THE PERIODIC TABLE
 C10 04/19/2013 13:34:14 Page 114 CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is
C10 04/19/2013 13:34:14 Page 114 CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is
Review # 3 Matter. Copyright Cengage Learning. All rights reserved. 3 1
 Review # 3 Matter Copyright Cengage Learning. All rights reserved. 3 1 What are the three states of matter? 1. solid, liquid, and gas 2. solid, liquid, and plasma 3. solid, glass, and compounds 4. compounds,
Review # 3 Matter Copyright Cengage Learning. All rights reserved. 3 1 What are the three states of matter? 1. solid, liquid, and gas 2. solid, liquid, and plasma 3. solid, glass, and compounds 4. compounds,
Anything occupying space and having mass. Matter exists in three states.
 Chapter 3 Matter Section 3.1 Matter Matter Anything occupying space and having mass. Matter exists in three states. Solid Liquid Gas Section 3.1 Matter The Three States of Water Section 3.1 Matter Solid
Chapter 3 Matter Section 3.1 Matter Matter Anything occupying space and having mass. Matter exists in three states. Solid Liquid Gas Section 3.1 Matter The Three States of Water Section 3.1 Matter Solid
IPC Science Semester 1 Study Guide
 IPC Science Semester 1 Study Guide Completion Complete each statement. 1. A measurement must include both a number and a(an). 2. A material used for electrical wiring would need to have good. 3. In an
IPC Science Semester 1 Study Guide Completion Complete each statement. 1. A measurement must include both a number and a(an). 2. A material used for electrical wiring would need to have good. 3. In an
CHAPTER 2 & 3 WARM-UP
 Name Period Date 1. What is the definition of energy? 2. What is work? CHAPTER 2 & 3 WARM-UP 3. What are the three forms of energy? Give an example of each. 4. Explain chemical potential energy and give
Name Period Date 1. What is the definition of energy? 2. What is work? CHAPTER 2 & 3 WARM-UP 3. What are the three forms of energy? Give an example of each. 4. Explain chemical potential energy and give
Teacher: Mr. gerraputa. Name: Base your answer to the question on the information below. Given the electron dot diagram:
 Teacher: Mr. gerraputa Print Close Name: 1. Given the electron dot diagram: The valence electrons represented by the electron dot diagram could be those of atoms in Group 1. 13 3. 3 2. 15 4. 16 2. Which
Teacher: Mr. gerraputa Print Close Name: 1. Given the electron dot diagram: The valence electrons represented by the electron dot diagram could be those of atoms in Group 1. 13 3. 3 2. 15 4. 16 2. Which
2016 Phys PRACTICE Sci Quiz 1
 2016 Phys PRTIE Sci Quiz 1 Name: ate: 1. mixture of crystals of salt and sugar is added to water and stirred until all solids have dissolved. Which statement best describes the resulting mixture?. The
2016 Phys PRTIE Sci Quiz 1 Name: ate: 1. mixture of crystals of salt and sugar is added to water and stirred until all solids have dissolved. Which statement best describes the resulting mixture?. The
Unit 2 Atomic Theory and Periodicity Review
 Unit 2 Atomic Theory and Periodicity Review Section I: History In each box, write the name of the scientist(s) associated with the statement. Choose from among the following: Democritus Thomson Bohr Schroedinger
Unit 2 Atomic Theory and Periodicity Review Section I: History In each box, write the name of the scientist(s) associated with the statement. Choose from among the following: Democritus Thomson Bohr Schroedinger
Section 11: Electron Configuration and Periodic Trends
 Section 11: Electron Configuration and Periodic Trends The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 11.01 The Bohr Model of the Atom
Section 11: Electron Configuration and Periodic Trends The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 11.01 The Bohr Model of the Atom
Unit 1 Part 1 Atomic Structure and The Periodic Table Introduction to Atomic Structure UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE
 UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE PART 1 INTRODUCTION TO ATOMIC STRUCTURE Contents 1. Protons, Neutrons and Electrons 2. Early Models of the Atom 3. Isotopes and Atomic Mass 4. Atoms and Ions
UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE PART 1 INTRODUCTION TO ATOMIC STRUCTURE Contents 1. Protons, Neutrons and Electrons 2. Early Models of the Atom 3. Isotopes and Atomic Mass 4. Atoms and Ions
Multiple Choice Do Now!
 Name: Period: Date: KIPP NYC College Prep General Chemistry Quarter 1: Let s Review Multiple Choice Do Now! 1. Which substance can be decomposed by chemical means? (1) Aluminum (2) Octane (3) Silicon (4)
Name: Period: Date: KIPP NYC College Prep General Chemistry Quarter 1: Let s Review Multiple Choice Do Now! 1. Which substance can be decomposed by chemical means? (1) Aluminum (2) Octane (3) Silicon (4)
Period: Chemistry Semester 1 Final Exam Review Packet. 1. What is the difference between a hypothesis and a theory?
 Chemistry Name: Period: Chemistry Semester 1 Final Exam Review Packet 1. What is the difference between a hypothesis and a theory? 2. Distinguish between quantitative and qualitative observations. States
Chemistry Name: Period: Chemistry Semester 1 Final Exam Review Packet 1. What is the difference between a hypothesis and a theory? 2. Distinguish between quantitative and qualitative observations. States
Mid-Term Review Multiple Choice: Ch. 3 Identify the letter of the choice that best completes the statement or answers the question.
 HONORS CHEMISTRY MID-TERM PRACTICE January 2013 Mrs. Allen from HS North put together a collection of multiple choice questions for you to use as a study tool. Not all of the midterm topics are covered
HONORS CHEMISTRY MID-TERM PRACTICE January 2013 Mrs. Allen from HS North put together a collection of multiple choice questions for you to use as a study tool. Not all of the midterm topics are covered
Chemistry Mid-Term Exam Review Spring 2017
 Unit 1 Measurement & Math Accuracy & Precision (recognizing given lab data) Density calculations Number of SFs in a measurement, Round answers to correct number of SFs Percent Error Unit conversions in
Unit 1 Measurement & Math Accuracy & Precision (recognizing given lab data) Density calculations Number of SFs in a measurement, Round answers to correct number of SFs Percent Error Unit conversions in
Key Equations. Determining the smallest change in an atom's energy.
 ATOMIC STRUCTURE AND PERIODICITY Matter and Energy Key Equations λν = c ΔE = hν Relating speed of a wave to its wavelength and frequency. Determining the smallest change in an atom's energy. H( λ =R n
ATOMIC STRUCTURE AND PERIODICITY Matter and Energy Key Equations λν = c ΔE = hν Relating speed of a wave to its wavelength and frequency. Determining the smallest change in an atom's energy. H( λ =R n
Chapter 2: Atoms. 2.1 (a) NaClO 3 (b) AlF (a) The mass number is = 31. (b) The mass number is = 222.
 2.1 (a) NaClO 3 (b) AlF 3 2.2 (a) The mass number is 15 + 16 = 31. (b) The mass number is 86 + 136 = 222. 2.3 (a) The element has 15 protons, making it phosphorus (P); its symbol is 31 P 15. (b) The element
2.1 (a) NaClO 3 (b) AlF 3 2.2 (a) The mass number is 15 + 16 = 31. (b) The mass number is 86 + 136 = 222. 2.3 (a) The element has 15 protons, making it phosphorus (P); its symbol is 31 P 15. (b) The element
b. Na. d. So. 1 A basketball has more mass than a golf ball because:
 Chem I Semester Review All of the following are general characteristics of a substance in the liquid state except a. definite volume. c. not easily compressed. b. able to flow. d. definite shape. In the
Chem I Semester Review All of the following are general characteristics of a substance in the liquid state except a. definite volume. c. not easily compressed. b. able to flow. d. definite shape. In the
CHAPTER 2. Atoms,Elements, Periodic Table
 CHAPTER Atoms,Elements, Periodic Table 1 Vocabulary Chemistry Science that describes matter its properties, the changes it undergoes, and the energy changes that accompany those processes Matter Anything
CHAPTER Atoms,Elements, Periodic Table 1 Vocabulary Chemistry Science that describes matter its properties, the changes it undergoes, and the energy changes that accompany those processes Matter Anything
Chemistry Mid-Term Practice Exam
 Chemistry Mid-Term Practice Exam Multiple Choice. Identify the choice that best completes the statement or answers the question. 1. A measure of the 3-D space matter occupies is a. density. c. volume.
Chemistry Mid-Term Practice Exam Multiple Choice. Identify the choice that best completes the statement or answers the question. 1. A measure of the 3-D space matter occupies is a. density. c. volume.
Atomic Emission Spectra. and. Flame Tests. Burlingame High School Chemistry
 Atomic Structure Atomic Emission Spectra and Flame Tests Flame Tests Sodium potassium lithium When electrons are excited they bump up to a higher energy level. As they bounce back down they release energy
Atomic Structure Atomic Emission Spectra and Flame Tests Flame Tests Sodium potassium lithium When electrons are excited they bump up to a higher energy level. As they bounce back down they release energy
CDO AP Chemistry Unit 5
 1. a. Calculate the wavelength of electromagnetic radiation that has a frequency of 5.56 MHz. b. Calculate the frequency of electromagnetic radiation that has a wavelength equal to 667 nm. 2. Electromagnetic
1. a. Calculate the wavelength of electromagnetic radiation that has a frequency of 5.56 MHz. b. Calculate the frequency of electromagnetic radiation that has a wavelength equal to 667 nm. 2. Electromagnetic
Solid Gas Liquid Plasma
 Unit 1: MATTER 1. Define CHEMISTRY: 2. Define MATTER: Use one of the states of matter to complete each statement. (Words will be used more than once.) Solid Gas Liquid Plasma 3. A has definite volume and
Unit 1: MATTER 1. Define CHEMISTRY: 2. Define MATTER: Use one of the states of matter to complete each statement. (Words will be used more than once.) Solid Gas Liquid Plasma 3. A has definite volume and
1. In the modern Periodic Table, the elements are arranged in order of increasing
 1. In the modern Periodic Table, the elements are arranged in order of increasing A atomic number B mass number C oxidation number D valence number 2. Berylium is classified as A an alkaline earth metal
1. In the modern Periodic Table, the elements are arranged in order of increasing A atomic number B mass number C oxidation number D valence number 2. Berylium is classified as A an alkaline earth metal
Questions 1 to 58 must be answered on the Scantron sheets.
 Questions 1 to 58 must be answered on the Scantron sheets. Base your answers to questions 1 to 5 on the heating curve for a pure substance that is shown below. 1. The freezing point of the substance is
Questions 1 to 58 must be answered on the Scantron sheets. Base your answers to questions 1 to 5 on the heating curve for a pure substance that is shown below. 1. The freezing point of the substance is
Chemistry. Chemistry is the study of the interactions between atoms and molecules. Atoms and Molecules
 Chemistry Chemistry is the study of the interactions between atoms and molecules. Atoms and Molecules An atom is a particle of matter that cannot be further divided without changing the chemical identity
Chemistry Chemistry is the study of the interactions between atoms and molecules. Atoms and Molecules An atom is a particle of matter that cannot be further divided without changing the chemical identity
CHEM Chapter 6. Basic Quantum Chemistry (Homework). WL36
 CHEM 1411. Chapter 6. Basic Quantum Chemistry (Homework). WL36 1. The Bohr model of the hydrogen atom found its greatest support in experimental work on the photoelectric effect. A) True B) False 2. A
CHEM 1411. Chapter 6. Basic Quantum Chemistry (Homework). WL36 1. The Bohr model of the hydrogen atom found its greatest support in experimental work on the photoelectric effect. A) True B) False 2. A
CP Physical Science Chemistry: Bell Work, Notes, Study Guides
 CP Physical Science Chemistry: Bell Work, Notes, Study Guides Mr. Banker Fall 2014 ian_banker@charleston.k12.sc.us http://wandohigh.ccsdschools.com/directory/science/banker_ian/physical_science/ Remind101.com
CP Physical Science Chemistry: Bell Work, Notes, Study Guides Mr. Banker Fall 2014 ian_banker@charleston.k12.sc.us http://wandohigh.ccsdschools.com/directory/science/banker_ian/physical_science/ Remind101.com
CP Chemistry Semester 1 Final Test Review
 Mass in grams 10 20 30 40 50 CP Chemistry Semester 1 Final Test Review 1. Know the symbol and the power of 10 for the following metric prefixes: A. Mega M 10 6 D. deka da 10 1 G. milli m 10 6 B. kilo k
Mass in grams 10 20 30 40 50 CP Chemistry Semester 1 Final Test Review 1. Know the symbol and the power of 10 for the following metric prefixes: A. Mega M 10 6 D. deka da 10 1 G. milli m 10 6 B. kilo k
AP Chapter 6 Study Questions
 Class: Date: AP Chapter 6 Study Questions True/False Indicate whether the statement is true or false. 1. The wavelength of radio waves can be longer than a football field. 2. Black body radiation is the
Class: Date: AP Chapter 6 Study Questions True/False Indicate whether the statement is true or false. 1. The wavelength of radio waves can be longer than a football field. 2. Black body radiation is the
Reporting Category 1: Matter and Energy
 Name: Science Teacher: Reporting Category 1: Matter and Energy Atoms Fill in the missing information to summarize what you know about atomic structure. Name of Subatomic Particle Location within the Atom
Name: Science Teacher: Reporting Category 1: Matter and Energy Atoms Fill in the missing information to summarize what you know about atomic structure. Name of Subatomic Particle Location within the Atom
Chapter 2: Atoms. 2.1 (a) NaClO3 (b) AlF (a) The mass number is = 31. (b) The mass number is = 222.
 2.1 (a) NaClO3 (b) AlF3 2.2 (a) The mass number is 15 + 16 = 31. (b) The mass number is 86 + 136 = 222. 2.3 (a) The element has 15 protons, making it phosphorus (P); its symbol is 31 P 15. (b) The element
2.1 (a) NaClO3 (b) AlF3 2.2 (a) The mass number is 15 + 16 = 31. (b) The mass number is 86 + 136 = 222. 2.3 (a) The element has 15 protons, making it phosphorus (P); its symbol is 31 P 15. (b) The element
Chapter 2 The Chemical View of Matter
 Chapter 2 The Chemical View of Matter MULTIPLE CHOICE 1. Which of the following is not one of the common states of matter? a. solid b. plasma c. liquid d. gas 2. Which of the following is one of the classes
Chapter 2 The Chemical View of Matter MULTIPLE CHOICE 1. Which of the following is not one of the common states of matter? a. solid b. plasma c. liquid d. gas 2. Which of the following is one of the classes
SCH4U1 Atomic & Molecular Structure Test Review
 SCH4U1 Atomic & Molecular Structure Test Review 1. Which object(s) would you use to describe the shape of the 2p orbital? a. a dumb-bell b. a circle c. a sphere d. two perpendicular dumb-bells e. a doughnut
SCH4U1 Atomic & Molecular Structure Test Review 1. Which object(s) would you use to describe the shape of the 2p orbital? a. a dumb-bell b. a circle c. a sphere d. two perpendicular dumb-bells e. a doughnut
1) What is the name given to the element with the symbol "K"? A) Potassium B) Krypton C) Phosphorus D) Kallium
 Practice Exam 1 (Chapters 1-4) Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the name given to the element with the symbol "K"?
Practice Exam 1 (Chapters 1-4) Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the name given to the element with the symbol "K"?
Worksheet #1: Atomic Spectra Answer the following questions using your Unit 3 notes.
 Worksheet #1: Atomic Spectra 1. How did Bohr expand on Rutherford s model of the atom? 2. Compare the energy of an electron in the ground state and an electron in the excited state. 3. When an electron
Worksheet #1: Atomic Spectra 1. How did Bohr expand on Rutherford s model of the atom? 2. Compare the energy of an electron in the ground state and an electron in the excited state. 3. When an electron
How many grams of sodium metal is required to completely react with 2545 grams of chlorine gas?
 146 EXAMPLE PROBLEM: How many grams of sodium metal is required to completely react with 2545 grams of chlorine gas? 1 - Convert 2545 grams of chlorine gas to moles. Use formula weight. 2 - Convert moles
146 EXAMPLE PROBLEM: How many grams of sodium metal is required to completely react with 2545 grams of chlorine gas? 1 - Convert 2545 grams of chlorine gas to moles. Use formula weight. 2 - Convert moles
