Build Your Own Periodic. The Bottom Line Grade W/S 15 Lewis Dot Structures Homework 3 Build Your Own Periodic Table
|
|
|
- Mitchell Mosley
- 5 years ago
- Views:
Transcription
1 Build Your Own Periodic Table The Bottom Line Grade W/S 15 Lewis Dot Structures Homework 3 Build Your Own Periodic Table
2 The Bottom Line FACE IT, Nobody owes you a living, What you achieve or fail to achieve in your lifetime, is directly related to what you do or fail to do. No one chooses their parents or childhood, but you can choose your own direction. Everyone has problems and obstacles to overcome, but that, too, is relative to each individual.
3 NOTHING IS CARVED IN STONE, you can change anything in your life, if you want to badly enough, Excuses are for losers; Those who take responsibility for their actions are the real winners in life. Winners meet life s challenges head on, knowing there are no guarantees, and give it all they ve got. And never think it s too late or too early to begin. Time plays no favorites and will pass whether you act or not. TAKE CONTROL OF YOUR LIFE.
4 Dare to dream and take risks Compete. If you aren t willing to work for your goals, don t expect others to. ---Believe in Yourself---
5 Grade Worksheet Metals, Non-Metals and Valence Electrons Worksheet
6 Lewis Structures 1) Find your element on the periodic table. 2) Determine the number of valence electrons. 3) This is how many electrons you will draw.
7 Lewis Structures Find out which group (column) your element is in. This will tell you the number of valence electrons your element has. You will only draw the valence electrons.
8 Groups - Review Group 1 = 1 electron Group 2 = 2 electrons Group 8 = 8 electrons Except for He, it has 2 electrons Each column is called a group Each element in a group has the same number of electrons in their outer orbital, also known as shells. The electrons in the outer shell are called valence electrons
9 Lewis Structures 1) Write the element symbol. 2) Carbon is in the 4 th group, so it has 4 valence electrons. 3) Starting at the top, draw 4 electrons, or dots, clockwise around the element symbol.
10 Lewis Structures 1) Check your work. 2) Using your periodic table, check that Carbon is in the 4 th group. 3) You should have 4 total electrons, or dots, drawn in for Carbon.
11 Lewis Structures On your worksheet, try these elements on your own: a) H b) P c) Ca d) Ar e) Cl f) Al
12 Lewis Structures On your worksheet, try these elements on your own: a) H b) P c) Ca d) Ar e) Cl f) Al
13 Lewis Structures On your worksheet, try these elements on your own: a) H b) P c) Ca d) Ar e) Cl f) Al
14 Lewis Structures On your worksheet, try these elements on your own: a) H b) P c) Ca d) Ar e) Cl f) Al
15 Lewis Structures On your worksheet, try these elements on your own: a) H b) P c) Ca d) Ar e) Cl f) Al
16 Lewis Structures On your worksheet, try these elements on your own: a) H b) P c) Ca d) Ar e) Cl f) Al
17 You will be building your own periodic tables and organizing your elements based on your knowledge of atomic structure You will be giving information about the element by filling out the tiles of the periodic table You will organize the elements according to groups and periods You will identify the group names you learned in class To conclude, you will write three paragraphs about the periodic table using our academic vocabulary
18 Title You will be creating your periodic table on provided poster paper Give your Periodic Table a Title
19 Tiles You will be giving the following information for each tile given to you This information is based upon the periodic table On your poster, create a key to your tile identifying the following Atomic Number Chemical Name Atomic Mass
20 Organization You will organize the tiles of the elements in the same manner the periodic table is organized Number the Groups accordingly
21 Identify Group Names Of the tiles that you have been given, identify the Group (Family) Names by using a different color for each group Hint-There are four Create a key of the colors you are using with the family name
22 Concluding Paragraphs On the half sheet provided you, write a concluding paragraph (3-5 sentences long each) for each vocabulary word listed below Atomic Number Valence Electron Subatomic Particles Glue your Concluding Paragraphs to the back of your poster
23 Your poster is due next period Next Class Unit 2 Test review Binder Prep
24 Homework 3
25 On the provided poster paper, give your Periodic Table a Title You will be giving the following information for each tile given to you This information is based upon the periodic table On your poster, create a key to your tile identifying the following Atomic Number Chemical Name Atomic Mass Organization You will organize the tiles of the elements in the same manner the periodic table is organized Number the Groups accordingly Identify Group Names Of the tiles that you have been given, identify the Group (Family) Names by using a different color for each group Hint-There are four Create a key of the colors you are using with the family name Concluding Paragraphs On the half sheet provided you, write a concluding paragraph (3-5 sentences long each) for each vocabulary word listed below Atomic Number Valence Electron Subatomic Particles Glue your Concluding Paragraphs to the back of your poster
Metals, Nonmetals and
 Metals, Nonmetals and Valence Electrons The Bottom Line Grade W/S 13 & 14 Homework 2 Valence Electrons Groups and Families The Bottom Line FACE IT, Nobody owes you a living, What you achieve or fail to
Metals, Nonmetals and Valence Electrons The Bottom Line Grade W/S 13 & 14 Homework 2 Valence Electrons Groups and Families The Bottom Line FACE IT, Nobody owes you a living, What you achieve or fail to
Bohr Models are NOT Boring! How to Draw Bohr Diagrams
 Bohr Models are NOT Boring! How to Draw Bohr Diagrams 1) Find the element on the periodic table. 2)Determine the number of electrons--it is the same as the atomic number. 3)This is how many electrons you
Bohr Models are NOT Boring! How to Draw Bohr Diagrams 1) Find the element on the periodic table. 2)Determine the number of electrons--it is the same as the atomic number. 3)This is how many electrons you
The Particle Theory of Matter
 Identifying Elements TEKS: 8.5B - identify that protons determine an element s identity and valence electrons determine its chemical properties, including reactivity. 8.5C interpret the arrangement of
Identifying Elements TEKS: 8.5B - identify that protons determine an element s identity and valence electrons determine its chemical properties, including reactivity. 8.5C interpret the arrangement of
All images are from
 www.middleschoolscience.com 2008 All images are from www.chem4kids.com What is the Periodic Table of Elements? The Periodic Table of Elements is a way of organizing the elements in relation to each other
www.middleschoolscience.com 2008 All images are from www.chem4kids.com What is the Periodic Table of Elements? The Periodic Table of Elements is a way of organizing the elements in relation to each other
base 2 4 The EXPONENT tells you how many times to write the base as a factor. Evaluate the following expressions in standard notation.
 EXPONENTIALS Exponential is a number written with an exponent. The rules for exponents make computing with very large or very small numbers easier. Students will come across exponentials in geometric sequences
EXPONENTIALS Exponential is a number written with an exponent. The rules for exponents make computing with very large or very small numbers easier. Students will come across exponentials in geometric sequences
Glue in Periodic Table and Modeling Atoms Complete Bell work.
 Bell Work Glue in Periodic Table and Modeling Atoms 1 10. Complete Bell work. Divide a page in your composition book into 2 columns. In one column, complete Bohr's models for elements 1, 3, and 11. In
Bell Work Glue in Periodic Table and Modeling Atoms 1 10. Complete Bell work. Divide a page in your composition book into 2 columns. In one column, complete Bohr's models for elements 1, 3, and 11. In
Lesson Plan. This lesson plan depicts a hands-on, minds-on activity and worksheet
 Lesson Plan This lesson plan depicts a hands-on, minds-on activity and worksheet involving knowledge students have gained about atoms, valence electrons, and energy levels. Students use this knowledge
Lesson Plan This lesson plan depicts a hands-on, minds-on activity and worksheet involving knowledge students have gained about atoms, valence electrons, and energy levels. Students use this knowledge
Periodic Table Basics
 Periodic Table asics Step 1: Complete the square for each element by filling in the atomic number, name, & atomic mass. Step 2: Determine the number of protons, neutrons, and electrons in an atom of each
Periodic Table asics Step 1: Complete the square for each element by filling in the atomic number, name, & atomic mass. Step 2: Determine the number of protons, neutrons, and electrons in an atom of each
SWBAT draw Lewis Dot Structure for covalent bonding
 Unit 5 NAME Class Work 1/4/14 5.5 Covalent Bonding SPARK (Take out your 5.4 WS) Name the following compounds: 1) Si 3 N 4 2) BaSO 4 3) Ca(ClO 3 ) 2 Be ready to quickly go over the homework! ObjecSve SWBAT
Unit 5 NAME Class Work 1/4/14 5.5 Covalent Bonding SPARK (Take out your 5.4 WS) Name the following compounds: 1) Si 3 N 4 2) BaSO 4 3) Ca(ClO 3 ) 2 Be ready to quickly go over the homework! ObjecSve SWBAT
Take the Anxiety Out of Word Problems
 Take the Anxiety Out of Word Problems I find that students fear any problem that has words in it. This does not have to be the case. In this chapter, we will practice a strategy for approaching word problems
Take the Anxiety Out of Word Problems I find that students fear any problem that has words in it. This does not have to be the case. In this chapter, we will practice a strategy for approaching word problems
You are looking at a textile fabric pattern. Please answer the following questions.
 Introductory Activity: You are looking at a textile fabric pattern. Please answer the following questions. 1.) What different patterns do you see inside the fabric? 2.) How are the shapes in the pattern
Introductory Activity: You are looking at a textile fabric pattern. Please answer the following questions. 1.) What different patterns do you see inside the fabric? 2.) How are the shapes in the pattern
Lewis Dot Structures. Team Chemistry Lanier H.S.
 Lewis Dot Structures Team Chemistry Lanier H.S. Part 1: Review of Lewis Dot Symbols To Draw a Lewis Dot Symbol: 1. Write the symbol for the atom 2. Find the number of valence electrons (use Periodic Table)
Lewis Dot Structures Team Chemistry Lanier H.S. Part 1: Review of Lewis Dot Symbols To Draw a Lewis Dot Symbol: 1. Write the symbol for the atom 2. Find the number of valence electrons (use Periodic Table)
An atom refresher Matter
 Elements & Atoms An atom refresher Matter is anything that takes up space and has mass. All matter is made of atoms Atoms are the building blocks of matter, sort of how bricks are the building blocks of
Elements & Atoms An atom refresher Matter is anything that takes up space and has mass. All matter is made of atoms Atoms are the building blocks of matter, sort of how bricks are the building blocks of
Line Integrals and Path Independence
 Line Integrals and Path Independence We get to talk about integrals that are the areas under a line in three (or more) dimensional space. These are called, strangely enough, line integrals. Figure 11.1
Line Integrals and Path Independence We get to talk about integrals that are the areas under a line in three (or more) dimensional space. These are called, strangely enough, line integrals. Figure 11.1
4.1 Atomic Theory and Bonding
 4.1 Atomic Theory and Bonding An atom is the that still has the 50 million atoms, = An atom = (s) + (s) + (s) Atoms join together. A compound is a that is composed of combined in a. and are atoms/elements;
4.1 Atomic Theory and Bonding An atom is the that still has the 50 million atoms, = An atom = (s) + (s) + (s) Atoms join together. A compound is a that is composed of combined in a. and are atoms/elements;
WORKSHEET 1 REVIEW OF GRADE 9 CHEMISTRY
 WORKSHEET 1 REVIEW OF GRADE 9 CHEMISTRY PART A: DEFINITIONS/THEORY Matter: is anything that has mass and takes up space. -Matter can be classified according to its composition, ex. a mixture or a pure
WORKSHEET 1 REVIEW OF GRADE 9 CHEMISTRY PART A: DEFINITIONS/THEORY Matter: is anything that has mass and takes up space. -Matter can be classified according to its composition, ex. a mixture or a pure
! Chemical!Bond!! Lewis!Diagram!(HI!#13)! o Ionic!and!covalent!bond!(M!+!NM!or!NM!+!NM)!(Complete!transfer!of!e S!or!sharing!of!e S )!
 !! Unit*2.*Atomic*Theory*! Molar!mass!calculation!using!the!abundance!of!isotopes!of!an!element!!!! Electron!configuration!(both!full!notation!and!core!notation)!(HI!#12)! o Neutral!atom,!anion,!cation!(ensure!you!know!the!rules!associated!with!ions)!
!! Unit*2.*Atomic*Theory*! Molar!mass!calculation!using!the!abundance!of!isotopes!of!an!element!!!! Electron!configuration!(both!full!notation!and!core!notation)!(HI!#12)! o Neutral!atom,!anion,!cation!(ensure!you!know!the!rules!associated!with!ions)!
Do now: Brainstorm how you would draw the Lewis diagram for: H 2 O CO 2
 Do now: Brainstorm how you would draw the Lewis diagram for: 2 O CO 2 Shapes of molecules C 4 N 3 2 O C 2 O CO 2 Shapes of molecules Shapes of molecules are determined by the number of bonding and non-bonding
Do now: Brainstorm how you would draw the Lewis diagram for: 2 O CO 2 Shapes of molecules C 4 N 3 2 O C 2 O CO 2 Shapes of molecules Shapes of molecules are determined by the number of bonding and non-bonding
When I lecture we will add more info, so leave spaces in your notes
 Title and Highlight Topic: EQ: Date Reflect Question: Reflect on the material by asking a question (its not suppose to be answered from notes) NOTES: Write out the notes from my website. Use different
Title and Highlight Topic: EQ: Date Reflect Question: Reflect on the material by asking a question (its not suppose to be answered from notes) NOTES: Write out the notes from my website. Use different
DRAWING YOUR CONTINENT
 CONTINENT PROJECT PART I On the attached sheet, you will draw your own continent. Use your creativity to create a continent that is uniquely your own. Give the continent a name. When you are finished with
CONTINENT PROJECT PART I On the attached sheet, you will draw your own continent. Use your creativity to create a continent that is uniquely your own. Give the continent a name. When you are finished with
K. Diagram or construct models of simple hydrocarbons (four or fewer carbons) with single, double or triple bonds. Name: Period:
 Name: Period: Final Due Date: Friday, March 2, 2007 Atomic Structure Objectives: Students will be able to: A. Distinguish among symbols for atoms, ions, molecules, and equations for chemical reactions.
Name: Period: Final Due Date: Friday, March 2, 2007 Atomic Structure Objectives: Students will be able to: A. Distinguish among symbols for atoms, ions, molecules, and equations for chemical reactions.
Name: Unit 3 Guide-Electrons In Atoms
 Name: Unit 3 Guide-Electrons In Atoms Importance of Electrons Draw a complete Bohr model of the atom. Write an element s electron configuration. Know how the symbols used in ECs relate to electron properties
Name: Unit 3 Guide-Electrons In Atoms Importance of Electrons Draw a complete Bohr model of the atom. Write an element s electron configuration. Know how the symbols used in ECs relate to electron properties
Ch 10 Chemical Bonding, Lewis Structures for Ionic & Covalent Compounds, and Predicting Shapes of Molecules
 Fructose Water Ch 10 Chemical Bonding, Lewis Structures for Ionic & Covalent Compounds, and Predicting Shapes of Molecules Carbon Dioxide Ammonia Title and Highlight TN Ch 10.1 Topic: EQ: Right Side NOTES
Fructose Water Ch 10 Chemical Bonding, Lewis Structures for Ionic & Covalent Compounds, and Predicting Shapes of Molecules Carbon Dioxide Ammonia Title and Highlight TN Ch 10.1 Topic: EQ: Right Side NOTES
7.2 The Bohr Theory of the Atom
 7.2 The Bohr Theory of the Atom John Dalton Michael Faraday showed that atoms could gain electric charges J.J. Thompson The atomic theory was once again revised, to include his ideas: Ernest Rutherford
7.2 The Bohr Theory of the Atom John Dalton Michael Faraday showed that atoms could gain electric charges J.J. Thompson The atomic theory was once again revised, to include his ideas: Ernest Rutherford
Electron Counting. It s easier to show than to explain, I think.
 Electron Counting This, for some reason I ve never worked out, isn t as easy as it should be. It is, however, important. It allows us to say things about the stability of organometallic complexes and make
Electron Counting This, for some reason I ve never worked out, isn t as easy as it should be. It is, however, important. It allows us to say things about the stability of organometallic complexes and make
The Atom & Periodic Table. Unit 2 Topics 4-6
 The Atom & Periodic Table Unit 2 Topics 4-6 Electrons in Atoms Topic 4 Describe Bohr s model of the atom. Sketch it! Bohr - A review electrons exist in orbits around the nucleus. Bohr - IB Information
The Atom & Periodic Table Unit 2 Topics 4-6 Electrons in Atoms Topic 4 Describe Bohr s model of the atom. Sketch it! Bohr - A review electrons exist in orbits around the nucleus. Bohr - IB Information
Q25: Record the wavelength of each colored line according to the scale given.
 C. Measurement Errors and Uncertainties The term "error" signifies a deviation of the result from some "true" value. Often in science, we cannot know what the true value is, and we can only determine estimates
C. Measurement Errors and Uncertainties The term "error" signifies a deviation of the result from some "true" value. Often in science, we cannot know what the true value is, and we can only determine estimates
Slide 1. Slide 2 Total Number of Valence Electrons. Slide 3. More Lewis Structures. Lewis Dot Structures
 Slide 1 More Lewis Structures Lewis Dot Structures Slide 2 Total Number of Valence Electrons The total number of available valence electrons is just the sum of the number of valence electrons that each
Slide 1 More Lewis Structures Lewis Dot Structures Slide 2 Total Number of Valence Electrons The total number of available valence electrons is just the sum of the number of valence electrons that each
A bit of review. Atoms are made of 3 different SUB-ATOMIC PARTICLES: 1. ELECTRONS 2. PROTONS 3. NEUTRONS
 Chemistry in Action A bit of review Chemistry is the study of MATTER and ENERGY. Matter is anything that has MASS. All matter is made of super small particles called ATOMS. Atoms are made of 3 different
Chemistry in Action A bit of review Chemistry is the study of MATTER and ENERGY. Matter is anything that has MASS. All matter is made of super small particles called ATOMS. Atoms are made of 3 different
What does acid base theory tell us is the difference between acids and bases? Name at least 2 parts.
 Monday, March 16th Learning Target : I can determine the relative acidity of solutions using indicators to measure ph. Homework: Worksheet due Thursday What does acid base theory tell us is the difference
Monday, March 16th Learning Target : I can determine the relative acidity of solutions using indicators to measure ph. Homework: Worksheet due Thursday What does acid base theory tell us is the difference
MS Algebra A-CED-1 Ch. 1.4 Write Equations & Inequalities
 MS Algebra A-CED-1 Ch. 1.4 Write Equations & Inequalities ALGEBRA SUPPORT Write verbal phrases as algebraic equations or inequalities using appropriate terms, factors, and coefficients Title: 1.4 Write
MS Algebra A-CED-1 Ch. 1.4 Write Equations & Inequalities ALGEBRA SUPPORT Write verbal phrases as algebraic equations or inequalities using appropriate terms, factors, and coefficients Title: 1.4 Write
Good morning, please do the following:
 Good morning, please do the following: Get out your What is Cellular Respiration? reading for a stamp Get out your Assignment Sheet Get out your concept maps for a check-in If you were absent on Monday,
Good morning, please do the following: Get out your What is Cellular Respiration? reading for a stamp Get out your Assignment Sheet Get out your concept maps for a check-in If you were absent on Monday,
COM INC COM* 1. What is each row called in the periodic table? The elements in each row of the periodic table have the.
 Last name: First name: Date: Period: Cornell Notes Title: Periodic Table Book Section: Ch 12 I CAN explain how the Periodic Table is an organization of elements based on their physical and chemical properties.
Last name: First name: Date: Period: Cornell Notes Title: Periodic Table Book Section: Ch 12 I CAN explain how the Periodic Table is an organization of elements based on their physical and chemical properties.
= proton (positive charge) = electron (negative charge) = neutron (no charge) A Z. ,, and are notations that represent isotopes of carbon.
 ChemQuest 8 Name: Date: Hour: Information: Structure of the Atom Note the following symbols: (they are not to scale) = proton (positive charge) = electron (negative charge) = neutron (no charge) The following
ChemQuest 8 Name: Date: Hour: Information: Structure of the Atom Note the following symbols: (they are not to scale) = proton (positive charge) = electron (negative charge) = neutron (no charge) The following
Molecular Structure and Bonding. Assis.Prof.Dr.Mohammed Hassan Lecture 2
 Molecular Structure and Bonding Assis.Prof.Dr.Mohammed Hassan Lecture 2 Lewis structures: Lewis Theory The octet rule All elements except hydrogen ( hydrogen have a duet of electrons) have octet of electrons
Molecular Structure and Bonding Assis.Prof.Dr.Mohammed Hassan Lecture 2 Lewis structures: Lewis Theory The octet rule All elements except hydrogen ( hydrogen have a duet of electrons) have octet of electrons
CH 59 SQUARE ROOTS. Every positive number has two square roots. Ch 59 Square Roots. Introduction
 59 CH 59 SQUARE ROOTS Introduction W e saw square roots when we studied the Pythagorean Theorem. They may have been hidden, but when the end of a right-triangle problem resulted in an equation like c =
59 CH 59 SQUARE ROOTS Introduction W e saw square roots when we studied the Pythagorean Theorem. They may have been hidden, but when the end of a right-triangle problem resulted in an equation like c =
Electron Configurations: Assigning each electron in an atom to the energy level and sublevel it occupies in the atom. Number of Electrons
 First some terms and more information about the structure of the atom: 1) Energy level is no longer an orbit but more like a boundary or maximum distance from the nucleus that electrons occupy. 1, 2, 3
First some terms and more information about the structure of the atom: 1) Energy level is no longer an orbit but more like a boundary or maximum distance from the nucleus that electrons occupy. 1, 2, 3
Name... Class... Date... In this activity you will have an opportunity to explore the nuclear model of the atom by building your own.
 Model of an atom Specification references: C1.1.4 Relative electrical charges of subatomic particles C1.1.5 Size and mass of atoms WS 1.2 Aims In this activity you will have an opportunity to explore the
Model of an atom Specification references: C1.1.4 Relative electrical charges of subatomic particles C1.1.5 Size and mass of atoms WS 1.2 Aims In this activity you will have an opportunity to explore the
Name: Period: Date: What Is VSEPR? Now explore the Compare Two Structures link. Try changing the display to explore different combinations.
 Name: Period: Date: What Is VSEPR? Exploring The Valence Shell Electron Pair Repulsion (VSEPR) model. Go to the Purdue University website to explore VSEPR theory. http://www.chem.purdue.edu/gchelp/vsepr/structur2.html
Name: Period: Date: What Is VSEPR? Exploring The Valence Shell Electron Pair Repulsion (VSEPR) model. Go to the Purdue University website to explore VSEPR theory. http://www.chem.purdue.edu/gchelp/vsepr/structur2.html
Vocabulary: covalent bond, diatomic molecule, Lewis diagram, molecule, noble gases, nonmetal, octet rule, shell, valence, valence electron
 Covalent Bonds Answer Key Vocabulary: covalent bond, diatomic molecule, Lewis diagram, molecule, noble gases, nonmetal, octet rule, shell, valence, valence electron Prior Knowledge Questions (Do these
Covalent Bonds Answer Key Vocabulary: covalent bond, diatomic molecule, Lewis diagram, molecule, noble gases, nonmetal, octet rule, shell, valence, valence electron Prior Knowledge Questions (Do these
Chemical Bonding Basics
 Chemical Bonding Basics Reading: Ch 9, sections 5-9 Homework: Chapter 9: 49, 51, 53, 59, 63*, 69, 71*,73 * = important homework question Recap and overview: We have already investigated the structure of
Chemical Bonding Basics Reading: Ch 9, sections 5-9 Homework: Chapter 9: 49, 51, 53, 59, 63*, 69, 71*,73 * = important homework question Recap and overview: We have already investigated the structure of
Atomic Structure Chapter 4 Mr. Hines
 Atomic Structure Chapter 4 Mr. Hines Part A Standard model of the atom Learning Targets and I can statements 1 List, label, and describe the parts of an atom. 2 Identify the atomic number and the atomic
Atomic Structure Chapter 4 Mr. Hines Part A Standard model of the atom Learning Targets and I can statements 1 List, label, and describe the parts of an atom. 2 Identify the atomic number and the atomic
Valence electrons octet rule. Lewis structure Lewis structures
 Lewis Dot Diagrams Valence electrons are the electrons in the outermost energy level of an atom. An element with a full octet of valence electrons has a stable configuration. The tendency of bonded atoms
Lewis Dot Diagrams Valence electrons are the electrons in the outermost energy level of an atom. An element with a full octet of valence electrons has a stable configuration. The tendency of bonded atoms
Atomic Structure Chapter 4 Mr. Hines
 Atomic Structure Chapter 4 Mr. Hines Part A Standard model of the atom Learning Targets and I can statements 1 List, label, and describe the parts of an atom. 2 Identify the atomic number and the atomic
Atomic Structure Chapter 4 Mr. Hines Part A Standard model of the atom Learning Targets and I can statements 1 List, label, and describe the parts of an atom. 2 Identify the atomic number and the atomic
St Robert of Newminster Catholic School and Sixth Form College
 St Robert of Newminster Catholic School and Sixth Form College Year 12 Pre-Course Tasks: CHEMISTRY Exercise Mark Grade Atomic structure Chemical bonding Chemical equations Maths for chemists Moles Name:
St Robert of Newminster Catholic School and Sixth Form College Year 12 Pre-Course Tasks: CHEMISTRY Exercise Mark Grade Atomic structure Chemical bonding Chemical equations Maths for chemists Moles Name:
Elements and Chemical Bonds
 Elements and Chemical Bonds Electrons and Energy Levels What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree
Elements and Chemical Bonds Electrons and Energy Levels What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree
Unit 2 ~ Learning Guide Name:
 Unit 2 ~ Learning Guide Name: Instructions: Using a pencil, complete the following notes as you work through the related lessons. Show ALL work as is explained in the lessons. You are required to have
Unit 2 ~ Learning Guide Name: Instructions: Using a pencil, complete the following notes as you work through the related lessons. Show ALL work as is explained in the lessons. You are required to have
Midterm Exam I. CHEM 181: Introduction to Chemical Principles September 24, 2015 Key
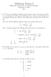 Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Lewis Dot diagrams. Developing and using models to predict formulas for stable, binary ionic compounds based on balance of charges
 Lewis Dot diagrams 1. Developing and using models to predict formulas for stable, binary ionic compounds based on balance of charges 1 Lewis Dot Diagrams Refresher Element symbol is the centerpiece, surrounded
Lewis Dot diagrams 1. Developing and using models to predict formulas for stable, binary ionic compounds based on balance of charges 1 Lewis Dot Diagrams Refresher Element symbol is the centerpiece, surrounded
Name: Per: Date: Teacher: Official Class: Chemistry. Unit 1: The Atom
 Unit 1: The Atom The following pages are practice questions for this unit, and will be submitted for homework! You must complete: Unit Vocabulary ALL QUESTIONS What is an Atom? ALL QUESTIONS Calculating
Unit 1: The Atom The following pages are practice questions for this unit, and will be submitted for homework! You must complete: Unit Vocabulary ALL QUESTIONS What is an Atom? ALL QUESTIONS Calculating
Explain how you got your answer.
 Monday, March 16th Learning Target : I can determine the relative acidity of solutions using indicators to measure ph. Homework: Worksheet due Thursday What does acid base theory tell us is the difference
Monday, March 16th Learning Target : I can determine the relative acidity of solutions using indicators to measure ph. Homework: Worksheet due Thursday What does acid base theory tell us is the difference
Elements combine to form compounds chemical bonds. Chemical Bonding
 Elements combine to form compounds chemical bonds Chemical Bonding Review Valence electrons Using periodic table to determine them. General bonding rules: If an atom has 1 to 3 valence electrons, it will
Elements combine to form compounds chemical bonds Chemical Bonding Review Valence electrons Using periodic table to determine them. General bonding rules: If an atom has 1 to 3 valence electrons, it will
Homework from last time. Your Homework Question: What is the difference between an Atom and an Element? Out of 3 marks
 Homework from last time Your Homework Question: What is the difference between an Atom and an Element? Out of 3 marks How is this possible? Bohr Models S1-2-05 Objective: Assemble or draw Bohr atomic models
Homework from last time Your Homework Question: What is the difference between an Atom and an Element? Out of 3 marks How is this possible? Bohr Models S1-2-05 Objective: Assemble or draw Bohr atomic models
DO NOW: Complete 4.2 Exit Ticket! Take out page 3 and 5 of your 4.2 packet and staple these papers together. Write your name on top and Lab #9.
 Unit 4 NAME Class Work 11/25/13 4.3 Valence Electrons and Lewis Dot Diagrams DO NOW: Complete 4.2 Exit Ticket! Take out page 3 and 5 of your 4.2 packet and staple these papers together. Write your name
Unit 4 NAME Class Work 11/25/13 4.3 Valence Electrons and Lewis Dot Diagrams DO NOW: Complete 4.2 Exit Ticket! Take out page 3 and 5 of your 4.2 packet and staple these papers together. Write your name
Chapter 9 Bonding - 1. Dr. Sapna Gupta
 Chapter 9 Bonding - 1 Dr. Sapna Gupta Lewis Dot Symbol Lewis dot symbols is a notation where valence electrons are shown as dots. Draw the electrons symmetrically around the sides (top, bottom, left and
Chapter 9 Bonding - 1 Dr. Sapna Gupta Lewis Dot Symbol Lewis dot symbols is a notation where valence electrons are shown as dots. Draw the electrons symmetrically around the sides (top, bottom, left and
Investigating Similar Triangles and Understanding Proportionality: Lesson Plan
 Investigating Similar Triangles and Understanding Proportionality: Lesson Plan Purpose of the lesson: This lesson is designed to help students to discover the properties of similar triangles. They will
Investigating Similar Triangles and Understanding Proportionality: Lesson Plan Purpose of the lesson: This lesson is designed to help students to discover the properties of similar triangles. They will
Stratford School Academy Schemes of Work GEOG: RESTLESS EARTH
 Case studies Drawing maps, graphs and diagrams. Stratford School Academy Year 10 Restless Earth Geography: YEAR 10 Restless Earth Number of weeks Content of the unit Assumed prior learning (tested at the
Case studies Drawing maps, graphs and diagrams. Stratford School Academy Year 10 Restless Earth Geography: YEAR 10 Restless Earth Number of weeks Content of the unit Assumed prior learning (tested at the
Chapter 4 Lecture Outline. Copyright McGraw-Hill Education. Permission required for reproduction or display.
 Chapter 4 Lecture Outline 1 Copyright McGraw-ill Education. Permission required for reproduction or display. 4.1 Introduction to Covalent Bonding Covalent bonds result from the sharing of electrons between
Chapter 4 Lecture Outline 1 Copyright McGraw-ill Education. Permission required for reproduction or display. 4.1 Introduction to Covalent Bonding Covalent bonds result from the sharing of electrons between
Drexel-SDP GK-12 ACTIVITY
 Drexel-SDP GK-12 ACTIVITY Subject Area(s): Chemistry Associated Unit: None Associated Lesson: None Activity Title : Adopt an Element Grade Level: 7 and 8 (7-9) Activity Dependency: None Time Required:
Drexel-SDP GK-12 ACTIVITY Subject Area(s): Chemistry Associated Unit: None Associated Lesson: None Activity Title : Adopt an Element Grade Level: 7 and 8 (7-9) Activity Dependency: None Time Required:
CHM Simple Lewis Structures (r14) Charles Taylor 1/5
 CHM 110 - Simple Lewis Structures (r14) - 2014 Charles Taylor 1/5 Introduction In the previous note pack, you learned some about Lewis dot structures, which represent chemical compounds by showing how
CHM 110 - Simple Lewis Structures (r14) - 2014 Charles Taylor 1/5 Introduction In the previous note pack, you learned some about Lewis dot structures, which represent chemical compounds by showing how
Chemistry 11 Lewis Structures Study Guide. After discussing about ionic and covalent bonds, there are times when it is valuable to draw
 Chemistry 11 Lewis Structures Study Guide After discussing about ionic and covalent bonds, there are times when it is valuable to draw Lewis structure ( electron dot diagrams ) to visualize how valence
Chemistry 11 Lewis Structures Study Guide After discussing about ionic and covalent bonds, there are times when it is valuable to draw Lewis structure ( electron dot diagrams ) to visualize how valence
Organic Chemistry. Review Information for Unit 1. Atomic Structure MO Theory Chemical Bonds
 Organic Chemistry Review Information for Unit 1 Atomic Structure MO Theory Chemical Bonds Atomic Structure Atoms are the smallest representative particle of an element. Three subatomic particles: protons
Organic Chemistry Review Information for Unit 1 Atomic Structure MO Theory Chemical Bonds Atomic Structure Atoms are the smallest representative particle of an element. Three subatomic particles: protons
CHEMICAL BONDING SUTHERLAND HIGH SCHOOL GRADE 10 PHYSICAL SCIENCE TB. 103 K. FALING EDITED: R. BASSON
 CHEMICAL BONDING SUTHERLAND HIGH SCHOOL K. FALING EDITED: R. BASSON GRADE 10 PHYSICAL SCIENCE TB. 103 HOW DOES BONDING WORK? The chemical reaction between elements leads to compounds, which have new physical
CHEMICAL BONDING SUTHERLAND HIGH SCHOOL K. FALING EDITED: R. BASSON GRADE 10 PHYSICAL SCIENCE TB. 103 HOW DOES BONDING WORK? The chemical reaction between elements leads to compounds, which have new physical
8.2 Another Look at Bonding Lewis Diagrams
 8.2 Another Look at Bonding Lewis Diagrams To learn more about the work of G.N. Lewis, go to www.science.nelson.com G You have learned that bonding types (ionic and covalent) can be used to classify all
8.2 Another Look at Bonding Lewis Diagrams To learn more about the work of G.N. Lewis, go to www.science.nelson.com G You have learned that bonding types (ionic and covalent) can be used to classify all
Algebra 1B notes and problems March 12, 2009 Factoring page 1
 March 12, 2009 Factoring page 1 Factoring Last class, you worked on a set of problems where you had to do multiplication table calculations in reverse. For example, given the answer x 2 + 4x + 2x + 8,
March 12, 2009 Factoring page 1 Factoring Last class, you worked on a set of problems where you had to do multiplication table calculations in reverse. For example, given the answer x 2 + 4x + 2x + 8,
Reasons for the Seasons WebQuest Worksheet
 Name per Reasons for the Seasons WebQuest Worksheet Misconceptions About the Reasons for the Seasons What are misconceptions? A misconception is an incorrect idea about something. Your task is to find
Name per Reasons for the Seasons WebQuest Worksheet Misconceptions About the Reasons for the Seasons What are misconceptions? A misconception is an incorrect idea about something. Your task is to find
Democritus s ideas don t explain chemical behavior & lacked experimental support.
 A1: Atomic Structure Worksheet Key (Goals 1 3, Chapter 4) 1. Democritus, who lived in Greece during the 4 th century B.C., suggested that matter is made up of tiny particles that cannot be divided. He
A1: Atomic Structure Worksheet Key (Goals 1 3, Chapter 4) 1. Democritus, who lived in Greece during the 4 th century B.C., suggested that matter is made up of tiny particles that cannot be divided. He
Distributive property and its connection to areas
 February 27, 2009 Distributive property and its connection to areas page 1 Distributive property and its connection to areas Recap: distributive property The distributive property says that when you multiply
February 27, 2009 Distributive property and its connection to areas page 1 Distributive property and its connection to areas Recap: distributive property The distributive property says that when you multiply
What disease has been tamed to some degree through the help of chemical bonding theory?
 Chapter 9 Reading Guide Bonding I Dr. Baxley Tro 4 th edition 1 See end of this document for suggested (strongly urged) problems. Section 9.1, Why is chemical bonding important? What disease has been tamed
Chapter 9 Reading Guide Bonding I Dr. Baxley Tro 4 th edition 1 See end of this document for suggested (strongly urged) problems. Section 9.1, Why is chemical bonding important? What disease has been tamed
Science 1.5 AS Demonstrate understanding of aspects of acids and bases WORKBOOK. Working to Excellence
 Science 1.5 AS 90944 Demonstrate understanding of aspects of acids and bases WORKBOOK Working to Excellence CONTENTS 1. Writing Excellence answers to Ion Formation questions 2. Writing Excellence answers
Science 1.5 AS 90944 Demonstrate understanding of aspects of acids and bases WORKBOOK Working to Excellence CONTENTS 1. Writing Excellence answers to Ion Formation questions 2. Writing Excellence answers
UNIT 2 - ATOMIC THEORY
 UNIT 2 - ATOMIC THEORY VOCABULARY: Allotrope Electron Configuration Nuclear Charge Anion Element Nucleons Atom Excited state Nucleus Atomic Mass Ground state Orbital Atomic Mass unit (a.m.u.) Ion Proton
UNIT 2 - ATOMIC THEORY VOCABULARY: Allotrope Electron Configuration Nuclear Charge Anion Element Nucleons Atom Excited state Nucleus Atomic Mass Ground state Orbital Atomic Mass unit (a.m.u.) Ion Proton
Unit 7: The Periodic Table
 Unit 7: The Periodic Table Name Class Website: http://pilarz.weebly.com PS:7 TLW categorize elements of the periodic table according to common properties and explain how elements differ in structural parts
Unit 7: The Periodic Table Name Class Website: http://pilarz.weebly.com PS:7 TLW categorize elements of the periodic table according to common properties and explain how elements differ in structural parts
Activity 06.3a Periodic Trends Inquiry
 Background In this investigation you will examine several periodic trends, including atomic radius, ionization energy and ionic radius. You will be asked to interact with select atoms as you investigate
Background In this investigation you will examine several periodic trends, including atomic radius, ionization energy and ionic radius. You will be asked to interact with select atoms as you investigate
IONIC BONDING NOTES (Chapter 7 Section 1)
 IONIC BONDING NOTES (Chapter 7 Section 1) I. Introduction Because all atoms want to have a total of, atoms will,, or electrons to form bonds. One of these bonds is an. A. Ionic Bond - is when a charged
IONIC BONDING NOTES (Chapter 7 Section 1) I. Introduction Because all atoms want to have a total of, atoms will,, or electrons to form bonds. One of these bonds is an. A. Ionic Bond - is when a charged
Remember Bohr s Explanation: Energy Levels of Hydrogen: The Electronic Structure of the Atom 11/28/2011
 The Electronic Structure of the Atom Bohr based his theory on his experiments with hydrogen he found that when energy is added to a sample of hydrogen, energy is absorbed and reemitted as light When passed
The Electronic Structure of the Atom Bohr based his theory on his experiments with hydrogen he found that when energy is added to a sample of hydrogen, energy is absorbed and reemitted as light When passed
Unit 3 Ray Tedder s Chemistry I Test Prep Guide page 1
 Unit 3 Ray Tedder s Chemistry I Test Prep Guide page 1 Bonding Unit 3: Chemistry I In this unit all students must be able to Understand that the structure of molecules is the result of nonmetals sharing
Unit 3 Ray Tedder s Chemistry I Test Prep Guide page 1 Bonding Unit 3: Chemistry I In this unit all students must be able to Understand that the structure of molecules is the result of nonmetals sharing
1920 s Charge Cloud Model (developed by Louis debroglie, Erwin Schrodinger, Werner Heisenberg and others)
 1920 s Charge Cloud Model (developed by Louis debroglie, Erwin Schrodinger, Werner Heisenberg and others) A new model was developed through complex mathematical equations which predicted the The Schrodinger
1920 s Charge Cloud Model (developed by Louis debroglie, Erwin Schrodinger, Werner Heisenberg and others) A new model was developed through complex mathematical equations which predicted the The Schrodinger
protons electrons neutrons nucleus Center of the atom; contains protons and neutrons. The Atom Molecules are made up of two or more atoms.
 _ Period: The Atom Ch. 18:1 Everything is made of atoms. Atoms are the smallest part of matter. Atoms are made up of 3 subatomic particles (particles smaller than the atom): electrons, protons, and neutrons.
_ Period: The Atom Ch. 18:1 Everything is made of atoms. Atoms are the smallest part of matter. Atoms are made up of 3 subatomic particles (particles smaller than the atom): electrons, protons, and neutrons.
Drawing Atoms. How many protons! How many neutrons? How many electrons? What element does thisatomrepresent?
 Drawing Atoms When we draw atoms, we are making a model of an atom. It is not EXACTLY like a real atom, but it shows important structures and helps us to understand similarities and differences among different
Drawing Atoms When we draw atoms, we are making a model of an atom. It is not EXACTLY like a real atom, but it shows important structures and helps us to understand similarities and differences among different
Inquiry 2.1 (Investigating Lunar Phases) Purpose: What causes you to see the moon going through eight different moon phases?
 Inquiry 2.1 (Investigating Lunar Phases) Purpose: What causes you to see the moon going through eight different moon phases? Background Information: What is an orbital plane? Does the moon make or reflect
Inquiry 2.1 (Investigating Lunar Phases) Purpose: What causes you to see the moon going through eight different moon phases? Background Information: What is an orbital plane? Does the moon make or reflect
Outline for Today. Monday, Nov. 12. Wednesday Friday. Chapter 8: Chemical Bonding. Bond Enthalpies. Chapter 9: Theories of Bonding
 Outline for Today Monday, Nov. 12 Chapter 8: Chemical Bonding Bond Enthalpies Chapter 9: Theories of Bonding VSEPR (Valence Shell Electron Pair Repulsion) Theory Valence Bond Orbital ybridization Molecular
Outline for Today Monday, Nov. 12 Chapter 8: Chemical Bonding Bond Enthalpies Chapter 9: Theories of Bonding VSEPR (Valence Shell Electron Pair Repulsion) Theory Valence Bond Orbital ybridization Molecular
Lesson 15 Part II: Ionic Bonding & Intro to Lewis Structures
 Lesson 15 Part II: Ionic Bonding & Intro to Lewis Structures Do Now: Follow all instructions to earn 5 points for participation grade. Take your CJs out write info down from board. Keep CJs out and open
Lesson 15 Part II: Ionic Bonding & Intro to Lewis Structures Do Now: Follow all instructions to earn 5 points for participation grade. Take your CJs out write info down from board. Keep CJs out and open
Atomic combinations: Covalent bonding and Lewis notation *
 OpenStax-CNX module: m38895 1 Atomic combinations: Covalent bonding and Lewis notation * Free High School Science Texts Project This work is produced by OpenStax-CNX and licensed under the Creative Commons
OpenStax-CNX module: m38895 1 Atomic combinations: Covalent bonding and Lewis notation * Free High School Science Texts Project This work is produced by OpenStax-CNX and licensed under the Creative Commons
CHEMISTRY OF LIFE. Composition of Matter. Composition of Matter 10/3/14
 CHEMISTRY OF LIFE Matter- occupies space and has mass Mass- the quantity of matter an object has Weight- the quantity of matter multiplied by the gravity of the planet you are on. Earth s gravity is 9.8
CHEMISTRY OF LIFE Matter- occupies space and has mass Mass- the quantity of matter an object has Weight- the quantity of matter multiplied by the gravity of the planet you are on. Earth s gravity is 9.8
PARCC Research Simulation Task Grade 3 Reading Lesson 5: Using Context Clues for the Vocabulary EBSR
 PARCC Research Simulation Task Grade 3 Reading Lesson 5: Using Context Clues for the Vocabulary EBSR Rationale Goal The vocabulary evidence-based selected response will test students not only on their
PARCC Research Simulation Task Grade 3 Reading Lesson 5: Using Context Clues for the Vocabulary EBSR Rationale Goal The vocabulary evidence-based selected response will test students not only on their
Name Honors Chemistry / /
 Name Honors Chemistry / / Lewis Structures & Resonance Structures Last chapter we studied ionic compounds. In ionic compounds electrons are gained or lost. In this chapter we are going to study covalent
Name Honors Chemistry / / Lewis Structures & Resonance Structures Last chapter we studied ionic compounds. In ionic compounds electrons are gained or lost. In this chapter we are going to study covalent
PHY2048 Physics with Calculus I
 PHY2048 Physics with Calculus I Section 584761 Prof. Douglas H. Laurence Exam 1 (Chapters 2 6) February 14, 2018 Name: Solutions 1 Instructions: This exam is composed of 10 multiple choice questions and
PHY2048 Physics with Calculus I Section 584761 Prof. Douglas H. Laurence Exam 1 (Chapters 2 6) February 14, 2018 Name: Solutions 1 Instructions: This exam is composed of 10 multiple choice questions and
T U T O R I A L : A M O D E L F O R C I R C U I T S
 South Pasadena Physics Name 10 Circuits Period Date T U T O R I A L : A M O D E L F O R C I R C U I T S Tutorial Instructions This Tutorial contains Activities and Exercises. Activities: These are intended
South Pasadena Physics Name 10 Circuits Period Date T U T O R I A L : A M O D E L F O R C I R C U I T S Tutorial Instructions This Tutorial contains Activities and Exercises. Activities: These are intended
The periodic table is divided up into blocks. You should be familiar with the s-block, p-block and d-block, as shown in Figure 1.
 Electron configuration and the periodic table Specification reference Elements of life (e) (f(i)) (f(iii)) Introduction Working out electronic configurations of atoms can be quite a challenge, requiring
Electron configuration and the periodic table Specification reference Elements of life (e) (f(i)) (f(iii)) Introduction Working out electronic configurations of atoms can be quite a challenge, requiring
Vocabulary Review. Atom Cathode Ray Electrons Protons Neutrons Nucleus
 2/19/16 Do Now On your half sheet of paper, identify the scientist that either discovered a part of the atom or developed a theory related to atomic structure. Vocabulary Review Atom Cathode Ray Electrons
2/19/16 Do Now On your half sheet of paper, identify the scientist that either discovered a part of the atom or developed a theory related to atomic structure. Vocabulary Review Atom Cathode Ray Electrons
For this you need to know covalent bonds, Lewis dots, electronegativity, geometric shapes, duet & octet, single/double/triple bonds, etc...
 Lewis Structure Lab For this you need to know covalent bonds, Lewis dots, electronegativity, geometric shapes, duet & octet, single/double/triple bonds, etc... I can t assume you have had all these, so
Lewis Structure Lab For this you need to know covalent bonds, Lewis dots, electronegativity, geometric shapes, duet & octet, single/double/triple bonds, etc... I can t assume you have had all these, so
 Name: 6 th Grade Plate Tectonics Journal Learning standards: E.SE.06.51 - Explain plate tectonic movement and how the lithospheric plates move centimeters each year. E.SE.06.52 - Demonstrate how major
Name: 6 th Grade Plate Tectonics Journal Learning standards: E.SE.06.51 - Explain plate tectonic movement and how the lithospheric plates move centimeters each year. E.SE.06.52 - Demonstrate how major
UNIT 2 CHEMISTRY IN ACTION. Mr.Yeung
 UNIT 2 CHEMISTRY IN ACTION Mr.Yeung ATOMS REVIEW Check out the Scale of the Universe http://htwins.net/scale2/ Keep the following questions in mind: 1. Are cells bigger or smaller than atoms? 2. Generally
UNIT 2 CHEMISTRY IN ACTION Mr.Yeung ATOMS REVIEW Check out the Scale of the Universe http://htwins.net/scale2/ Keep the following questions in mind: 1. Are cells bigger or smaller than atoms? 2. Generally
2. Atoms with nearly empty valence shells give up electrons. They are called
 Name: Date: Chemistry ~ Ms. Hart Class: Anions or Cations 4.8 Ions and Ionic Radius Directions: As we watch the video, answer these questions. 1. What is it called when an atom gains or loses an electron?
Name: Date: Chemistry ~ Ms. Hart Class: Anions or Cations 4.8 Ions and Ionic Radius Directions: As we watch the video, answer these questions. 1. What is it called when an atom gains or loses an electron?
Work hard. Be nice. Name: Period: Date: UNIT 3: Electrons Lesson 4: The Octet Rule. Nitrogen Neon Carbon He
 Name: Period: Date: UNIT 3: Electrons Lesson 4: The Octet Rule Do Now: By the end of today, you will have an answer to: What role do valence electrons play in chemical changes? Draw the following lewis
Name: Period: Date: UNIT 3: Electrons Lesson 4: The Octet Rule Do Now: By the end of today, you will have an answer to: What role do valence electrons play in chemical changes? Draw the following lewis
Clocks and Codes Patterns in Modular Arithmetic A Group Project for Math 104
 Clocks and Codes Patterns in Modular Arithmetic A Group Project for Math Clocks and Codes Patterns in Modular Arithmetic. The project is due as noted in the syllabus and will be presented in class. Late
Clocks and Codes Patterns in Modular Arithmetic A Group Project for Math Clocks and Codes Patterns in Modular Arithmetic. The project is due as noted in the syllabus and will be presented in class. Late
{ }. The dots mean they continue in that pattern to both
 INTEGERS Integers are positive and negative whole numbers, that is they are;... 3, 2, 1,0,1,2,3... { }. The dots mean they continue in that pattern to both positive and negative infinity. Before starting
INTEGERS Integers are positive and negative whole numbers, that is they are;... 3, 2, 1,0,1,2,3... { }. The dots mean they continue in that pattern to both positive and negative infinity. Before starting
Polynomial one or more monomials added or subtracted. (i.e. : 5x or 6xy-3 or 6xy - 5x + 3 or
 Polynomials Necessary Terms for Success Welcome back! We will now tackle the world of polynomials. Before we get started with performing operations or using polynomials for applications, we will need some
Polynomials Necessary Terms for Success Welcome back! We will now tackle the world of polynomials. Before we get started with performing operations or using polynomials for applications, we will need some
irst we need to know that there are many ways to indicate multiplication; for example the product of 5 and 7 can be written in a variety of ways:
 CH 2 VARIABLES INTRODUCTION F irst we need to know that there are many ways to indicate multiplication; for example the product of 5 and 7 can be written in a variety of ways: 5 7 5 7 5(7) (5)7 (5)(7)
CH 2 VARIABLES INTRODUCTION F irst we need to know that there are many ways to indicate multiplication; for example the product of 5 and 7 can be written in a variety of ways: 5 7 5 7 5(7) (5)7 (5)(7)
The structure of the Atom. Chemistry chapter 4
 The structure of the Atom Chemistry chapter 4 Rutherford-Bohr Model Niels Bohr (1922) Proposed improvements to Rutherford Atomic Model. For this reason the planetary model of the atoms is sometimes called
The structure of the Atom Chemistry chapter 4 Rutherford-Bohr Model Niels Bohr (1922) Proposed improvements to Rutherford Atomic Model. For this reason the planetary model of the atoms is sometimes called
