Chapter 15 Organic Matter Diagenesis Jim Murray (5/09/01) Univ. Washington NO 3
|
|
|
- Erin Gordon
- 5 years ago
- Views:
Transcription
1 Chapter 15 Organic Matter Diagenesis Jim Murray (5/09/01) Univ. Washington 15-1 Oxidation-reduction reactions Many elements in the periodic table can exist in more than one oxidation state. Oxidation states are indicated by Roman numerials in parantheses (e.g. (+I), (-II), etc). The oxidation state represents the "electron content" of an element which can be expressed as the excess or deficiency of electrons relative to the elemental state. If you have a compound and you want the oxidation state of an element, assign oxygen (O) to be (-II) and hydrogen (H) to be (+I) and calculate the oxidation state of the element of interest, taking into account the charge on the compound. For example: Element Oxidation State Species Nitrogen N (+V) - NO 3 N (+III) - NO 2 N (O) N 2 N (-III) + NH 3, NH 4 Sulfur S (+VI) 2- SO 4 S (+II) 2- S 2 O 3 S (O) S S(-II) H 2 S, HS -, S 2- Iron Fe (+III) Fe 3+ Fe (+II) Fe 2+ Manganese Mn (+VI) 2- MnO 4 Mn (+IV) MnO 2 (s) Mn (+III) MnOOH (s) Mn (+II) Mn Redox half-reactions Redox reactions are written as half-reactions which are in the form of reductions (which means an element is transformed from a higher oxidation state (e.g. +II) to a lower oxidation state (e.g. +I)): Ox + ne - = Red; G ; K where the more oxidized form of an element is on the left and the reduced form is on the right. n is the number of electrons transferred. We can write an equilibrium constant for this reaction as we can any other reaction. Formally the concentrations should be expressed as activities. Thus: K = (Red) / (Ox)(e - ) n 1
2 We can also rearrange the equation to determine the activity of the electron for any redox couple: (e - ) = [ (Red) / K (Ox) ] 1/n Electron activities are usually expressed on either the pe or Eh scales as shown below. or pe = - log (e - ) = 1/n [logk - log (Red)/(Ox) ] Eh = 2.3 RT pe / F The pe provides a nondimensional scale (like ph) that expresses the activity of electrons in factors of 10. Eh (called the redox potential) is measured in volts. F is the Faraday which is the electric charge of one mole of electrons (96,500 coulombs). The ratio 2.3 RT.F has a value of V at 25 C. With these equations you can express the energy available from a reaction in terms of either G, Eh or pe. We can express the equilibrium constants for half reactions in different forms as well (pe, Eh, K, G ). Remember that G = - RT ln K. Then: pe = F Eh / 2.3 RT = 1/n log K = - 1/n G /2.3RT It is most conventient to write the various half reactions in terms of one electron although this introduces odd fractions for the species coefficients. The species that loses an electron is the e- donor (thus it is oxidized but is a reductant for other elements) and the species that accepts an electron is the e- acceptor (so it is reduced but is an oxidant). A summary of many redox half-reactions that may occur in natural waters (with values for pe = log K) are given in Table 15-1 (from Morel and Hering, 1993). Note that all halfreactions are written in terms of one electron. The pe equation can be expressed as: pe = pe - log (Red) / (Ox) where pe can be calculated from log K. 2
3 If you know the equilibrium distribution of (Red)/(Ox), this equation can be used to solve for the pe of the environment. Conversely, if you know the pe of the environment you can calculate the equilibrium activity ratio of the reduced and oxidized forms. Because the half reactions have different ph dependencies a common approach is to compare the various half reactions by calculating the equilibrium constant at ph = 7. This constant is called pe (w). For example, consider the SO 4 /HS half reaction: 1/8 SO /8 H + + e- = 1/8 HS - + 1/2 H 2 O pe = 4.25 we can write: pe = pe - log (HS - ) 1/8 / (SO 4 2- ) 1/8 (H + ) 9/8 = /8 log (HS - )/(SO 4 ) + 9/8 log (H + ) at ph = 7 we calculate pe(w) pe (w) = pe + 9/8 log (H+) pe (w) = /8 (7) = Values of pe(w) for the main half-reactions are summarized in Table
4 Table 15-1 Redox Half Reactions (from Morel and Hering, 1993) 4
5 Table 15-1, cont. 5
6 Table 15-1, cont. 6
7 Table
8 15-3 Redox Calculations There are two main types of redox calculations. The first is the calculation of what controls the pe of the environment. This is analogous to calculating the ph of the environment, for example when it is controlled by the H 2 CO 3 system in equilibrium with atmospheric P CO2. The second type of calculation is to determine how trace species respond or distribute themselves with respect to that pe. Again by analogy when we know the ph we can calculate the ph dependent speciation of trace species, like Fe for example. I.What is the pe of water at ph 8 in equilibrium with oxygen (P O2 = 0.20) in the atmosphere. This is the typical pe for oxic conditions. We assume that the pe is controlled by the oxygen-water half reaction from Table 15-1, Note that the pe is relatively insensitive to P O2 but is very dependent on ph. 1/4O 2 + H + + e - = 1/2 H 2 O pe = K = 1 / (P O2 ) 1/4 (H + ) (e - ) = pe = pe + 1/n log (oxid/red) pe = log (P O2 ) 1/4 (H + ) (because n = 1) pe = /4 log ph pe = pe = II. What is the pe of water at ph = 8 in equilibrium with the SO 4 /H 2 S couple? Assume that HS - = and SO 4 = This is the typical of the anoxic end-member condition. 1/8SO /8H + + e - = 1/8HS - + 1/2H 2 O pe = pe = pe + log (SO 2-4 ) 1/8 (H + ) 9/8 / (HS - ) 1/8 pe = pe + 1/8 log (SO 2-4 ) + 9/8 log (H + ) - 1/8 log (HS - ) pe = /8pH pe = pe =
9 III. When the pe is externally controlled calculate the distribution of trace components. For example, assume a natural seawater at ph = 8 in equilibrium with P O2 = Then pe = as calculated above. What would be the Mn 2+ in equilibrium with γmno 2? Assume: 1/2 γmno 2 (s) + 2 H+ + 1e- = 1/2 Mn2+ + H 2 O log K = pe = 20.8 pe = log (H + ) 2 / (Mn 2+ ) 1/ = log (H + ) - 1/2 log (Mn 2+ ) log (Mn 2+ ) = 2 ( ph) log (Mn 2+ ) = 2 ( ) log (Mn 2+ ) = or (Mn 2+ ) = In oxygenated surface seawater dissolved Mn 2+ = Equilibrium does not exist. This is a typical case with redox reactions. 9
10 15-4 Balanced Redox Reactions A balanced reaction has an electron passed from an electron donor to an electron acceptor. Thus: Ox 1 + Red 2 = Red 1 + Ox 2 In this case Red 2 is the electron donor, passing electrons to Ox 1 which is the electron acceptor. Thus Red 2 is oxidized to Ox 2 and Ox 1 is reduced to Red 1. The equilibrium constant for an oxidation-reduction reaction can be determined by combining the constants from Table 1 as follows. Say we want to study the reaction of oxygen with glucose. The two half reactions (written as reductions in terms of one electron) with their appropriate values of log K, are: (Rxn 1) 1/4 O 2 (g) + H + + e - = H 2 O pe = log K = (Rxn 2) 1/4 CO 2 (g) + H + + e - = 1/24 C 6 H 12 O 6 + 1/4 H 2 O pe = We reverse reaction 2 (now it's log K = +0.20) and add it to reaction 1 to get: 1/4 O 2 (g) + 1/24 C 6 H 12 O 6 = 1/4 CO 2 (g) + 1/4 H 2 O log K = = or 6 O 2 (g) + C 6 H 12 O 6 = 6 CO 2 (g) + 6 H 2 O log K = x 24 =
11 15-5 Ideal redox sequence There is an ideal sequence of redox reactions that is based on the energy available. In this sequence organic matter is combusted in order by O 2, NO 3, MnO 2, Fe 2 O 3 then SO Most of these reactions have slow kinetics if left to occur on their own abiologically. Bacteria mediate most of these reactions and get the energy for their life processes. Because the energy of the sun is trapped in the C-C bonds, bacteria are indirectly using sunlight when they combust natural organic matter to CO 2. Bacteria use the electron acceptors in the order of decreasing energy availability. Using the half-reactions in Tables 15-1 and 15-2 we can calculate the log K (e.g. peº) and log Kw values for generic organic matter (CH 2 O) oxidation by various oxidants (or electron acceptors) (Table 15-3). Table 15-3 Oxidation-Reduction reaction log K log Kw Aerobic Respiration 1/4CH 2 O + 1/4O 2 = 1/4H 2 O + 1/4CO 2 (g) Denitrification 1/4CH 2 O + 1/5NO 3 + 1/5H + = 1/4CO 2 (g) + 1/10N 2 (g) +7/20H 2 O Mnaganese Reduction 1/4CH 2 O + 1/2MnO 2 (s) + H + = 1/4CO 2 (g) + 1/2Mn /4H 2 O Iron Reduction 1/4CH 2 O + Fe(OH) 3 (s) + 2H + = 1/4CO 2 (g) + Fe /4H 2 O Sulfate Reduction 1/4CH 2 O + 1/8SO /8H + = 1/4CO 2 (g) + 1/8HS - + 1/4H 2 O Methane Fermentation 1/4CH 2 O = 1/8CO 2 (g) + 1/8CH We can also write a sequence of Redfield-like reactions to examine the stoichiometric relationships in more detail. These reactions are shown in Table 15-4 where OM represents the Redfield organic matter. Note that during aerobic respiration and the organic NH 4 ends up as NO 3. During denitrification both NH 4 and NO 3 end up as N 2. For the remaining reactions NH 4 simply accumulates. The "tracer" species commonly used to identify where you are in the redox sequences are underlined. Another approach is to calculate the accumulated alkalinity as diagenesis proceeds. Alkalinity will decrease slightly during aerobic respiration then increase slowly during MnO 2 and Fe(OH) 3 reduction. Alkalinity increases rapidly during SO 4 reduction, because SO 4 in seawater is about 25 mm and alkalinity is produced in a 2:1 ratio to SO 4. 11
12 Table 15-4 Redfield-like equations for organic matter respiration. Indicator species are underlined. 12
13 The ideal sequence of indicator species can be seen in the following example modified from Froelich et al (1979) by including O 2, SO 4 and CH 4 profiles. The insert shows how dissolved and particulate Mn are related. Fig
14 15-6: Case Study-Aerobic Diagenesis Equatorial Pacific at about 180 W. See Murray and Grundmanis (1980) and Grundmanis and Murray (1982). Figure 15-2 Table 15-5 Flux O 2 = -D O2 φ (D O2 /D z ) z=0 where D O2 is the molecular diffusion coefficient for O 2 and φ is the porosity. The average O 2 flux was mol O 2 m -2 y
15 15-7 Case Study-Denitrification When O 2 2 µm bacteria use NO 3 to oxidize organic carbon. The NO 3 is reduced to NO 2 then to N 2. The NH 3 on the organic matter is either oxidized to N 2 or it accumulates as NH 4 +. The following Figure 15-5 is from Cline and Richards (1972) and shows data from the oxygen minimum zone of the eastern tropical North Pacific. δn was calculated as the NO 3 predicted from AOU minus the measued NO 3. The other panel shows NO 2 -, which is an intermediate in the NO 3 reduction sequence. Both suggest that denitrification begins at an O 2 concentration of about 2 µm. Fig
16 15-8 The Global Nitrogen Cycle Denitrification in the ocean keeps N 2 in the atmosphere. Figure 15-6 shows the regions of the world's oceans where O 2 < 20 µm (Broecker and Peng, 1982). Figure 15-7 shows a cross section through the oxygen minimum off Mexico (Cline and Richards, 1972). Figure 15-8 shows a cartoon diagram of the nitrogen cycle. 16
17 15-9 Case Study-Suboxic Diagenesis in the Guatemala Basin (Emerson et al, 1980) Fig 15-9: Site M = metaliferous site on the East Pacific Rise (3100m). Evidence for Mn, Fe and SO 4 reduction. Fig 15-10: Site H = Hemipelagic site in the Guatemala Basin (3500m). Mn reduction only. 17
18 15-10 Case Study-Sulfate Reduction Saanich Inlet is a Fjord on Vancouver Island where the water column is anoxic through most of the year. Sulfate reduction and methane production occur in the sediments (Murray et al,1978; Devol et al 1984). Comparison of SO 4 and CH 4 distributions and the SO 4 reduction and CH 4 oxidation rates. The distributions suggest an anaerobic methane oxidation pathway such as: CH 4 + SO 4 2- HS - + HCO H 2 O. In Saanich sediments about 40% of the downward SO 4 flux is consumed by methane oxidation. Fig
19 Anoxic environments Interspecies hydrogen transfer Interspecies hydrogen transfer is a central process in anaerobic environments linking fermentation of organic compounds to sulfate reduction and methanogenesis through transfer of reducing power from organic molecules to inorganic electron acceptors via H 2 (Valentine, 2001). H 2 is a key intermediate in virtually all anoxic environments. In the absence of O 2, fermentation is the primary path by which biochemical monomers (sugars, nucleosides, amino acids, fatty acids) are degraded. The bacteria which carry out this fermentation cannot utilize inorganic electron acceptors, so instead they release organic acids, H 2 or both as electron sinks. Other bacteria and archaea, which are otherwise unable to degrade the monomers, can oxidize the volatile fatty acids and H 2 using inorganic electron receptors, such as sulfate and CO 2. Tha partnership between the two groups is true syntrophy and in particulare H 2 concentration is kept very low (see Wolin, 1982). The steady-state concentration of H 2 in anaerobic environments is extremely low (nm to pm) (Novelli et al., 1987). The full extent of this interspecies hydrogen transfer in natural environments remains unknown. The use of H 2 as a carrier of reducing power is facilitated by hydrogenase enzymes, which catalyze the reversible reaction 2e - + 2H + H 2. Fermenting organisms utilize the forward reaction to shunt electrons to H 2, while H 2 consumers catalyze the reverse reaction. These reactions can result in H/D fractionation depending on whether they take place inside or outside the cells. H 2 produced by fermentation is strongly depleted in D (by up to ~600%o) relative to water. The standard picture of H 2 oxidation is that the hydrogenase enzymes are located in the periplasm. The oxidation of H 2 to H 2 O results in transport of 2 electrons across the cell membrane (e.g., to reduce sulfate). The hydrogens are left behind. This separation of electrons from protons generates a membrane potential that is used for ATP synthesis. It is possible that H 2 is a source for organic-bound H in H 2 -consuming prokaryotes. Because H 2 is generally strongly depleted in D relative to other hydrogen sources this may lead to strongly negative δd val;ues in organisim that consume H 2. Wolin M.J. (1982) Hydrogen transfer in microbial communities. In: (J.H. Slater, ed.) Microbial interactions and communities. Academic Press, London, Novelli et al (1987) Hydrogen distributions in marine sediments. Limnology and Oceanography, 32, Valentine D.L. (2001) Thermodynamic ecology of hydrogen based syntrophy. In: (J. Seckbach., ed.) Symbiosis: Mechanisms and Model Systems. Kluwer, Dordrecht,
Oxidation States. 1. Redox potential Oxic vs. anoxic Simple electrochemical cell Redox potential in nature
 1. Redox potential Oxic vs. anoxic Simple electrochemical cell Redox potential in nature 2. Redox reactions Redox potential of a reaction Eh ph diagrams Redox reactions in nature 3. Biogeochemical reactions
1. Redox potential Oxic vs. anoxic Simple electrochemical cell Redox potential in nature 2. Redox reactions Redox potential of a reaction Eh ph diagrams Redox reactions in nature 3. Biogeochemical reactions
Lecture Summary. Physical properties of water exert profound control on nutrient cycling and NPP in lakes
 Lecture Summary Physical properties of water exert profound control on nutrient cycling and NPP in lakes Lakes respond dynamically to seasonal climate change The biogeochemical character of lakes is directly
Lecture Summary Physical properties of water exert profound control on nutrient cycling and NPP in lakes Lakes respond dynamically to seasonal climate change The biogeochemical character of lakes is directly
Lecture 5. More Aqueous Geochemistry of Natural Waters OXIDATION/REDUCTION (aka Redox)
 Lecture 5 More Aqueous Geochemistry of Natural Waters OXIDATION/REDUCTION (aka Redox) Redox state and ph are two fundamental controls on chemical make up of natural and non-natural waters. Pease read chapter
Lecture 5 More Aqueous Geochemistry of Natural Waters OXIDATION/REDUCTION (aka Redox) Redox state and ph are two fundamental controls on chemical make up of natural and non-natural waters. Pease read chapter
Metabolic diversity is based on the Electron donors, acceptors, and carbon sources available - thermodynamics
 To date you have covered microbial community sampling using molecular techniques to identify who is present in the environment. You have also looked at various genetic mechanisms to understand how organisms
To date you have covered microbial community sampling using molecular techniques to identify who is present in the environment. You have also looked at various genetic mechanisms to understand how organisms
OCN 623 pe and ph. OCN 623 Chemical Oceanography
 OCN 623 pe and ph OCN 623 Chemical Oceanography Thermodynamics applied to redox speciation Redox speciation has profound effects on chemical and biological processes Photosynthetic organisms, altered Earth
OCN 623 pe and ph OCN 623 Chemical Oceanography Thermodynamics applied to redox speciation Redox speciation has profound effects on chemical and biological processes Photosynthetic organisms, altered Earth
Structure & properties of water
 OCN 623 Chemical Oceanography Reading: Libes, Chapter 7 Structure & properties of water Water accounts for 96.5 weight percent of seawater Innate characteristics affect nearly all properties of seawater
OCN 623 Chemical Oceanography Reading: Libes, Chapter 7 Structure & properties of water Water accounts for 96.5 weight percent of seawater Innate characteristics affect nearly all properties of seawater
Microbial Biogeochemistry
 Microbial Biogeochemistry Chemical reactions occurring in the environment mediated by microbial communities Outline Metabolic Classifications. Winogradsky columns, Microenvironments. Redox Reactions. Microbes
Microbial Biogeochemistry Chemical reactions occurring in the environment mediated by microbial communities Outline Metabolic Classifications. Winogradsky columns, Microenvironments. Redox Reactions. Microbes
The Tree of Life. Metabolic Pathways. Calculation Of Energy Yields
 The Tree of Life Metabolic Pathways Calculation Of Energy Yields OCN 401 - Biogeochemical Systems 8/27/09 Earth s History (continental crust) 170 Oldest oceanic crust Ga = billions of years ago The Traditional
The Tree of Life Metabolic Pathways Calculation Of Energy Yields OCN 401 - Biogeochemical Systems 8/27/09 Earth s History (continental crust) 170 Oldest oceanic crust Ga = billions of years ago The Traditional
Physiological diversity
 Physiological diversity Principles Energetic considerations Biochemical pathways Organisms Ecological relevance Physiological diversity Sulfate- and nitrate reducers (5. Nov.) Methanogens and homoacetogens
Physiological diversity Principles Energetic considerations Biochemical pathways Organisms Ecological relevance Physiological diversity Sulfate- and nitrate reducers (5. Nov.) Methanogens and homoacetogens
Nutrients; Aerobic Carbon Production and Consumption
 Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Why is organic matter such a good electron donor? Every (other) breath you take is a
Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Why is organic matter such a good electron donor? Every (other) breath you take is a
OCN 623 pe and ph. OCN 623 Chemical Oceanography. Thermodynamics applied to redox speciation
 OCN 623 pe and ph OCN 623 Chemical Oceanography Thermodynamics applied to redox speciation Redox speciation has profound effects on chemical and biological processes Photosynthetic organisms, altered Earth
OCN 623 pe and ph OCN 623 Chemical Oceanography Thermodynamics applied to redox speciation Redox speciation has profound effects on chemical and biological processes Photosynthetic organisms, altered Earth
Nutrients; Aerobic Carbon Production and Consumption
 Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Term paper topics, due February 9
 Term paper topics, due February 9 ODV mini-projects, due March 14 (10% final grade) Individuals or teams of two Using any available datasets, put together a ~7-10 minute talk to present in class on March
Term paper topics, due February 9 ODV mini-projects, due March 14 (10% final grade) Individuals or teams of two Using any available datasets, put together a ~7-10 minute talk to present in class on March
Term paper topics, due February 8
 Term paper topics, due February 8 ODV mini-projects, due March 13 (10% final grade) Individuals or teams of two Using any available datasets, put together a ~7-10 minute talk to present in class on March
Term paper topics, due February 8 ODV mini-projects, due March 13 (10% final grade) Individuals or teams of two Using any available datasets, put together a ~7-10 minute talk to present in class on March
Nutrients; Aerobic Carbon Production and Consumption
 Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Oxidation-Reduction (Redox) Reactions
 Oxidation-Reduction (Redox) Reactions Def n: Reactions in which one or more electrons is shifted from one element to another (In acid/base, gas transfer, and precipitation reactions discussed previously,
Oxidation-Reduction (Redox) Reactions Def n: Reactions in which one or more electrons is shifted from one element to another (In acid/base, gas transfer, and precipitation reactions discussed previously,
Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.)
 Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
Physiological diversity. Recommended text books. Physiological diversity. Sulfate and nitrate reducers. ! Principles. ! Energetic considerations
 Physiological diversity Recommended text books! Principles! Energetic considerations! Biochemical pathways! Organisms! Ecological relevance Physiological diversity! Sulfate- and nitrate reducers (11. Nov.)!
Physiological diversity Recommended text books! Principles! Energetic considerations! Biochemical pathways! Organisms! Ecological relevance Physiological diversity! Sulfate- and nitrate reducers (11. Nov.)!
The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet
 The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet Celebrating 5 years of world-leading collaborative and multidisciplinary research
The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet Celebrating 5 years of world-leading collaborative and multidisciplinary research
Metabolism. Fermentation vs. Respiration. End products of fermentations are waste products and not fully.
 Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Outline: Metabolism Part I: Fermentations Part II: Respiration Part III: Metabolic Diversity Learning objectives are: Learn about respiratory metabolism, ATP generation by respiration linked (oxidative)
Redox reactions.
 Redox reactions http://eps.mcgill.ca/~courses/c220/ Redox reactions In a similar way that acids and bases have been defined as proton donors and proton acceptors, reductants and oxidants are defined as
Redox reactions http://eps.mcgill.ca/~courses/c220/ Redox reactions In a similar way that acids and bases have been defined as proton donors and proton acceptors, reductants and oxidants are defined as
Anaerobic processes. Annual production of cells a -1 Mean generation time in sediments
 Anaerobic processes Motivation Where are they? Number of prokaryotes on earth 4-6 * 10 30 Cells in open ocean 1.2 * 10 29 in marine sediments 3.5 * 10 30 in soil 2.6 * 10 29 sub-terrestrial 0.5 2.5 * 10
Anaerobic processes Motivation Where are they? Number of prokaryotes on earth 4-6 * 10 30 Cells in open ocean 1.2 * 10 29 in marine sediments 3.5 * 10 30 in soil 2.6 * 10 29 sub-terrestrial 0.5 2.5 * 10
Chemistry in Sediments: Aerobic to Anaerobic Diagenesis
 Chemistry in Sediments: Aerobic to Anaerobic Diagenesis OCN 623 Chemical Oceanography Reading: Libes, Chapter 12 Why Study Sediments? Very large surface area of sediments with respect to the volume of
Chemistry in Sediments: Aerobic to Anaerobic Diagenesis OCN 623 Chemical Oceanography Reading: Libes, Chapter 12 Why Study Sediments? Very large surface area of sediments with respect to the volume of
BBS2710 Microbial Physiology. Module 5 - Energy and Metabolism
 BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
REDOX CHEMISTRY and Eh-pH Diagrams
 REDOX CHEMISTRY and Eh-pH Diagrams As we have seen at various previous portions of this course thus far, the redox state of the system is important in understanding speciation, solubility, transport, degradation
REDOX CHEMISTRY and Eh-pH Diagrams As we have seen at various previous portions of this course thus far, the redox state of the system is important in understanding speciation, solubility, transport, degradation
The products have more enthalpy and are more ordered than the reactants.
 hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
hapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Biology Reading Assignment: Chapter 9 in textbook
 Biology 205 5.10.06 Reading Assignment: Chapter 9 in textbook HTTP://WUNMR.WUSTL.EDU/EDUDEV/LABTUTORIALS/CYTOCHROMES/CYTOCHROMES.HTML What does a cell need to do? propagate itself (and its genetic program)
Biology 205 5.10.06 Reading Assignment: Chapter 9 in textbook HTTP://WUNMR.WUSTL.EDU/EDUDEV/LABTUTORIALS/CYTOCHROMES/CYTOCHROMES.HTML What does a cell need to do? propagate itself (and its genetic program)
A staggering number of organism-organism and organism- environment interactions underlie global biogeochemistry These can be studied at vastly
 Geobiology Week 3 How do microbes garner energy and carbon? Review of redox couples, reaction potential and free energy yields Hydrogen as an energy currency for subsurface microbes. Acknowledgements:
Geobiology Week 3 How do microbes garner energy and carbon? Review of redox couples, reaction potential and free energy yields Hydrogen as an energy currency for subsurface microbes. Acknowledgements:
Microbial Biogeochemistry & Global Change SWES 410/510 Dr. Jon Chorover 1/31/14
 Thermodynamics of Biogeochemical Reactions Microbial Biogeochemistry & Global Change SWES 410/510 Dr. Jon Chorover 1/31/14 How define biogeochemical reactions? Reactions that involve biological and geochemical
Thermodynamics of Biogeochemical Reactions Microbial Biogeochemistry & Global Change SWES 410/510 Dr. Jon Chorover 1/31/14 How define biogeochemical reactions? Reactions that involve biological and geochemical
CHAPTER 2. Stoichiometry a nd and Bacterial Energetics
 CHAPTER 2. Stoichiometry and Bacterial Energetics 2. Stoichiometry and Bacterial Energetics Mass balance: the important concept in the engineering design of system for biological treatment Determine the
CHAPTER 2. Stoichiometry and Bacterial Energetics 2. Stoichiometry and Bacterial Energetics Mass balance: the important concept in the engineering design of system for biological treatment Determine the
Global Biogeochemical Cycles and. II. Biological Metabolism
 Global Biogeochemical Cycles and Biological Metabolism I. Biogeochemistry & Biogeochemical Cycles A. Global cycles: nitrogen, water, carbon B. Carbon cycle through time II. Biological Metabolism A. Redox
Global Biogeochemical Cycles and Biological Metabolism I. Biogeochemistry & Biogeochemical Cycles A. Global cycles: nitrogen, water, carbon B. Carbon cycle through time II. Biological Metabolism A. Redox
Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington
 Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington Last lecture was concerned with gas exchange and one example we looked at was the solubility of CO 2. Next we have
Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington Last lecture was concerned with gas exchange and one example we looked at was the solubility of CO 2. Next we have
Photosynthetic autotrophs use the energy of sunlight to convert low-g CO 2 and H 2 O into energy-rich complex sugar molecules.
 Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Chapters 7 & 10 Bioenergetics To live, organisms must obtain energy from their environment and use it to do the work of building and organizing cell components such as proteins, enzymes, nucleic acids,
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Geology 560, Prof. Thomas Johnson, Fall 2007 Class Notes: 3-6: Redox #2 Eh-pH diagrams, and practical applications
 Geology 56, Prof. Thomas Johnson, Fall 27 Class Notes: 36: Redox #2 Eh diagrams, and practical applications Reading: White, Section 3...3; Walther Ch. 4 Goals: Recognize that, because many redox reactions
Geology 56, Prof. Thomas Johnson, Fall 27 Class Notes: 36: Redox #2 Eh diagrams, and practical applications Reading: White, Section 3...3; Walther Ch. 4 Goals: Recognize that, because many redox reactions
Thermodynamic Laws, Gibbs Free Energy & pe/ph
 Thermodynamic Laws, Gibbs Free Energy & pe/ph or how to predict chemical reactions without doing experiments OCN 623 Chemical Oceanography Definitions Extensive properties Depend on the amount of material
Thermodynamic Laws, Gibbs Free Energy & pe/ph or how to predict chemical reactions without doing experiments OCN 623 Chemical Oceanography Definitions Extensive properties Depend on the amount of material
Chapter 4: Oxidation Reduction Reactions
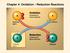 Chapter 4: Oxidation Reduction Reactions Oxidation Reduction Reactions involve the gain and loss of electrons. The species involved in these reactions may be atoms, molecules,or ions. Cation: a positively
Chapter 4: Oxidation Reduction Reactions Oxidation Reduction Reactions involve the gain and loss of electrons. The species involved in these reactions may be atoms, molecules,or ions. Cation: a positively
Ch/APh2 Bioenergetics Section Lecture of May 14, The thermodynamics of biological energy production.
 Ch/APh2 Bioenergetics Section Lecture of May 14, 2009 Introduction to bioenergetics. The thermodynamics of biological energy production. Kinetic aspects of bioenergetic processes. The molecular and cellular
Ch/APh2 Bioenergetics Section Lecture of May 14, 2009 Introduction to bioenergetics. The thermodynamics of biological energy production. Kinetic aspects of bioenergetic processes. The molecular and cellular
SCOPE 35 Scales and Global Change (1988)
 1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
METABOLISM CHAPTER 04 BIO 211: ANATOMY & PHYSIOLOGY I. Dr. Lawrence G. Altman Some illustrations are courtesy of McGraw-Hill.
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
CELL METABOLISM OVERVIEW Keep the big picture in mind as we discuss the particulars!
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
- Cation exchange capacity
 What is Necessary for Plant Growth? - ph (~5 to 8); - water - structural support - minerals and nutrients Macronutrients (C HOPKNS CaFe Mg) (Nutrients must be in a chemical form that plants can use.) -
What is Necessary for Plant Growth? - ph (~5 to 8); - water - structural support - minerals and nutrients Macronutrients (C HOPKNS CaFe Mg) (Nutrients must be in a chemical form that plants can use.) -
3. Organic Geochemisty Organic Chemistry is the chemistry... of Carbon -Morrison and Boyd
 3. Organic Geochemisty Organic Chemistry is the chemistry... of Carbon -Morrison and Boyd Definitions, Nomenclature Organic Compound Solubility Octanol-Water Partition Coefficient Organic Compound Sorption
3. Organic Geochemisty Organic Chemistry is the chemistry... of Carbon -Morrison and Boyd Definitions, Nomenclature Organic Compound Solubility Octanol-Water Partition Coefficient Organic Compound Sorption
Lecture 11: Petrology of Mineralizing Aqueous Solutions GY303 Igneous & Metamorphic Petrology
 Lecture 11: Petrology of Mineralizing Aqueous Solutions GY303 Igneous & Metamorphic Petrology H2O Dominated Fluids Excluding Magmatic and Metamorphic systems leaves hydrothermal H2O fluids as the most
Lecture 11: Petrology of Mineralizing Aqueous Solutions GY303 Igneous & Metamorphic Petrology H2O Dominated Fluids Excluding Magmatic and Metamorphic systems leaves hydrothermal H2O fluids as the most
Early diagenesis in marine sediments
 Early diagenesis in marine sediments Why study this part of the ocean? Particle flux to the sea floor ocean surface sediments early diagenesis layer Biogeochemical reactions Why study this part of the
Early diagenesis in marine sediments Why study this part of the ocean? Particle flux to the sea floor ocean surface sediments early diagenesis layer Biogeochemical reactions Why study this part of the
Biochemical Pathways
 Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
Biochemical Pathways Living organisms can be divided into two large groups according to the chemical form in which they obtain carbon from the environment. Autotrophs can use carbon dioxide from the atmosphere
Energy Transformation. Metabolism = total chemical reactions in cells.
 Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Energy Transformation Metabolism = total chemical reactions in cells. metabole = change Metabolism is concerned with managing the material and energy resources of the cell -Catabolism -Anabolism -Catabolism
Section A: The Principles of Energy Harvest
 CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
Unit 5 Cellular Energy
 Unit 5 Cellular Energy I. Enzymes (159) 1.Are CATALYSTS: Speed up chemical reactions that would otherwise happen too slowly to support life. Catalysts DO NOT make reactions happen that couldn t happen
Unit 5 Cellular Energy I. Enzymes (159) 1.Are CATALYSTS: Speed up chemical reactions that would otherwise happen too slowly to support life. Catalysts DO NOT make reactions happen that couldn t happen
Bio-electrochemistry course
 Bio-electrochemistry course LabMET short course on microbial energy management and microbial fuel cells Peter Aelterman and Korneel Rabaey Course overview Bacterial metabolism and the redox potential Basic
Bio-electrochemistry course LabMET short course on microbial energy management and microbial fuel cells Peter Aelterman and Korneel Rabaey Course overview Bacterial metabolism and the redox potential Basic
METABOLISM. What is metabolism? Categories of metabolic reactions. Total of all chemical reactions occurring within the body
 METABOLISM What is metabolism? METABOLISM Total of all chemical reactions occurring within the body Categories of metabolic reactions Catabolic reactions Degradation pathways Anabolic reactions Synthesis
METABOLISM What is metabolism? METABOLISM Total of all chemical reactions occurring within the body Categories of metabolic reactions Catabolic reactions Degradation pathways Anabolic reactions Synthesis
Principles of Bioenergetics. Lehninger 3 rd ed. Chapter 14
 1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
1 Principles of Bioenergetics Lehninger 3 rd ed. Chapter 14 2 Metabolism A highly coordinated cellular activity aimed at achieving the following goals: Obtain chemical energy. Convert nutrient molecules
AP Biology Energy Exam Study Guide. Enzymes, Cellular Respiration, Metabolic Patterns, and Photosynthesis
 AP Biology Energy Exam Study Guide Enzymes, Cellular Respiration, Metabolic Patterns, and Photosynthesis 1. In which orientation must these two amino acids be brought together to form a dipeptide bond?
AP Biology Energy Exam Study Guide Enzymes, Cellular Respiration, Metabolic Patterns, and Photosynthesis 1. In which orientation must these two amino acids be brought together to form a dipeptide bond?
Unit 3: Cell Energy Guided Notes
 Enzymes Unit 3: Cell Energy Guided Notes 1 We get energy from the food we eat by breaking apart the chemical bonds where food is stored. energy is in the bonds, energy is the energy we use to do things.
Enzymes Unit 3: Cell Energy Guided Notes 1 We get energy from the food we eat by breaking apart the chemical bonds where food is stored. energy is in the bonds, energy is the energy we use to do things.
Global phosphorus cycle
 Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
Outline 10: Origin of Life. Better Living Through Chemistry
 Outline 10: Origin of Life Better Living Through Chemistry What is Life? Internal chemical activity providing growth, repair, and generation of energy. The ability to reproduce. The capacity to respond
Outline 10: Origin of Life Better Living Through Chemistry What is Life? Internal chemical activity providing growth, repair, and generation of energy. The ability to reproduce. The capacity to respond
Redox and Electrochemistry (BLB chapter 20, p.723)
 Redox and Electrochemistry (BLB chapter 20, p.723) Redox is short for reduction/oxidation Redox chemistry deals with changes in the oxidation states of atoms Oxidation States All atoms have an oxidation
Redox and Electrochemistry (BLB chapter 20, p.723) Redox is short for reduction/oxidation Redox chemistry deals with changes in the oxidation states of atoms Oxidation States All atoms have an oxidation
Chapter 5: Photosynthesis: The Energy of Life pg : Pathways of Photosynthesis pg
 UNIT 2: Metabolic Processes Chapter 5: Photosynthesis: The Energy of Life pg. 210-240 5.2: Pathways of Photosynthesis pg. 220-228 Light Dependent Reactions Photosystem II and I are the two light capturing
UNIT 2: Metabolic Processes Chapter 5: Photosynthesis: The Energy of Life pg. 210-240 5.2: Pathways of Photosynthesis pg. 220-228 Light Dependent Reactions Photosystem II and I are the two light capturing
Chapter 8 Photosynthesis
 Chapter 8 Photosynthesis 8-1 NRG and Living Things n Where does the NRG we use come from. n Directly or indirectly from the sun n Plants get their NRG directly from the sun n How? n Plants use photosynthesis
Chapter 8 Photosynthesis 8-1 NRG and Living Things n Where does the NRG we use come from. n Directly or indirectly from the sun n Plants get their NRG directly from the sun n How? n Plants use photosynthesis
Oxidation-reduction (redox) reactions
 Oxidation-reduction (redox) reactions Reactions in which there are changes in oxidation state (oxidation number) between reactants and products 2 MnO 4- + 10 Br - + 16 H + 2 Mn 2+ + 5 Br 2 + 8 H 2 O One
Oxidation-reduction (redox) reactions Reactions in which there are changes in oxidation state (oxidation number) between reactants and products 2 MnO 4- + 10 Br - + 16 H + 2 Mn 2+ + 5 Br 2 + 8 H 2 O One
Cellular Respiration: Harvesting Chemical Energy. 9.1 Catabolic pathways yield energy by oxidizing organic fuels
 Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Energy and Cells. Appendix 1. The two primary energy transformations in plants are photosynthesis and respiration.
 Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
Energy and Cells Appendix 1 Energy transformations play a key role in all physical and chemical processes that occur in plants. Energy by itself is insufficient to drive plant growth and development. Enzymes
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES. Bio 107 Week 6
 RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 107 Week 6 Procedure 7.2 Label test tubes well, including group name 1) Add solutions listed to small test tubes 2)
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 107 Week 6 Procedure 7.2 Label test tubes well, including group name 1) Add solutions listed to small test tubes 2)
Chapter 5. The Chloroplast. 5.1 Matter and Energy Pathways in Living Systems. Photosynthesis & Cellular Respiration
 Chapter 5 Photosynthesis & Cellular Respiration 5.1 Matter and Energy Pathways in Living Systems Both cellular respiration and photosynthesis are examples of biological processes that involve matter &
Chapter 5 Photosynthesis & Cellular Respiration 5.1 Matter and Energy Pathways in Living Systems Both cellular respiration and photosynthesis are examples of biological processes that involve matter &
BIOCHEMISTRY. František Vácha. JKU, Linz.
 BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
BIOCHEMISTRY František Vácha http://www.prf.jcu.cz/~vacha/ JKU, Linz Recommended reading: D.L. Nelson, M.M. Cox Lehninger Principles of Biochemistry D.J. Voet, J.G. Voet, C.W. Pratt Principles of Biochemistry
10/4/2016. Matter, Energy, and Life
 DISCLAIMER: Principles and concepts on atomic structure, the Periodic Table, atoms, ions, ionic and covalent compounds, metals, and nonmetals will not be covered in this course. You are expected to know
DISCLAIMER: Principles and concepts on atomic structure, the Periodic Table, atoms, ions, ionic and covalent compounds, metals, and nonmetals will not be covered in this course. You are expected to know
What are the mechanisms of C preservation?
 What are the mechanisms of C preservation? Primary production: The higher PP, the higher the flux of OM, the more rapidly the C/N/P transits the reactive zone of active C degradation. Oxygen: Anaerobic
What are the mechanisms of C preservation? Primary production: The higher PP, the higher the flux of OM, the more rapidly the C/N/P transits the reactive zone of active C degradation. Oxygen: Anaerobic
Worksheet 25 - Oxidation/Reduction Reactions
 Worksheet 25 Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II In addition to the elemental oxidation state of 0, Group I has an oxidation state
Worksheet 25 Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II In addition to the elemental oxidation state of 0, Group I has an oxidation state
ET Life #17. Today: Reminders: Energy of Life. Paper Proposal Due Friday First Mid-term Next Monday
 ET Life #17 Today: Energy of Life Reminders: Paper Proposal Due Friday First Mid-term Next Monday Origin of Life: Summary 1. Early Organic Molecules 2. Complex organics developed (mineral templates?).
ET Life #17 Today: Energy of Life Reminders: Paper Proposal Due Friday First Mid-term Next Monday Origin of Life: Summary 1. Early Organic Molecules 2. Complex organics developed (mineral templates?).
This is an example of cellular respiration, which can be used to make beer and wine using different metabolic pathways For these reasons we call this
 Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
Chapter 6 Carvings from ancient Egypt show barley being crushed and mixed with water (left) and then put into closed vessels (centre) where airless conditions are suitable for the production of alcohol
7.014 Quiz I Handout
 7.014 Quiz I andout Quiz I announcements: Quiz I: Friday, February 27 12:05 12:55 Walker Gym, rd floor (room 5040) **This will be a closed book exam** Quiz Review Session: Wednesday, February 25 7:00 9:00
7.014 Quiz I andout Quiz I announcements: Quiz I: Friday, February 27 12:05 12:55 Walker Gym, rd floor (room 5040) **This will be a closed book exam** Quiz Review Session: Wednesday, February 25 7:00 9:00
Sunday, August 25, 2013 PHOTOSYNTHESIS
 PHOTOSYNTHESIS PREFACE The sun is the ultimate source of energy. The sun powers nearly all life forms. Photosynthesis converts solar energy into chemical energy. Photoautotrophs use solar energy to synthesize
PHOTOSYNTHESIS PREFACE The sun is the ultimate source of energy. The sun powers nearly all life forms. Photosynthesis converts solar energy into chemical energy. Photoautotrophs use solar energy to synthesize
1/25/2018. Bio 1101 Lec. 5, Part A Chapter 6: Cellular Respiration
 1 2 3 4 5 Bio 1101 Lec. 5, Part A Chapter 6: Cellular Respiration Energy is needed by cells to do work Chemical energy, a form of potential energy, is stored in bonds of food molecules (such as glucose)
1 2 3 4 5 Bio 1101 Lec. 5, Part A Chapter 6: Cellular Respiration Energy is needed by cells to do work Chemical energy, a form of potential energy, is stored in bonds of food molecules (such as glucose)
CEE 370 Environmental Engineering Principles. Equilibrium Chemistry
 Updated: 9 September 015 Print version CEE 370 Environmental Engineering Principles Lecture #6 Environmental Chemistry IV: Thermodynamics, Equilibria, Acids-bases I Reading: Mihelcic & Zimmerman, Chapter
Updated: 9 September 015 Print version CEE 370 Environmental Engineering Principles Lecture #6 Environmental Chemistry IV: Thermodynamics, Equilibria, Acids-bases I Reading: Mihelcic & Zimmerman, Chapter
Biology Reading Assignments:
 Biology 205 5.13.08 Reading Assignments: Chapter 3 Energy, Catalysis and Biosynthesis pgs. 83-94; 106-116 (Note the various roles of nucleotide based carrier molecules); work questions 3-2 and 3-3 Chapter
Biology 205 5.13.08 Reading Assignments: Chapter 3 Energy, Catalysis and Biosynthesis pgs. 83-94; 106-116 (Note the various roles of nucleotide based carrier molecules); work questions 3-2 and 3-3 Chapter
Oil. Oil. Early common mistakes in the oil business.
 Oil www.priweb.org/ed/pgws/history/pennsylvania/pennsylvania.html Early common mistakes in the oil business Oil www.priweb.org/ed/pgws/history/pennsylvania/pennsylvania.html 1 Climate More recent common
Oil www.priweb.org/ed/pgws/history/pennsylvania/pennsylvania.html Early common mistakes in the oil business Oil www.priweb.org/ed/pgws/history/pennsylvania/pennsylvania.html 1 Climate More recent common
2 4 Chemical Reactions and Enzymes Chemical Reactions
 Chemical Reactions A chemical reaction occurs when chemical bonds are broken and reformed. Rust forms very slowly, while rocket fuel combustion is explosive! The significance of this comparison is that
Chemical Reactions A chemical reaction occurs when chemical bonds are broken and reformed. Rust forms very slowly, while rocket fuel combustion is explosive! The significance of this comparison is that
Judith Herzfeld 1997,1999. These exercises are provided here for classroom and study use only. All other uses are copyright protected.
 Judith Herzfeld 1997,1999 These exercises are provided here for classroom and study use only. All other uses are copyright protected. 5.5-010 Which of the following statements is not valid concerning a
Judith Herzfeld 1997,1999 These exercises are provided here for classroom and study use only. All other uses are copyright protected. 5.5-010 Which of the following statements is not valid concerning a
Biogeochemical Cycles
 s. 16 2553 Hydrologic cycle cycle Carbon cycle Contents 2 Did you know? 3 (bio) (chemical) (geo;, ) biogeochemical cycles Biogeochemistry = the study of the exchange or flux of materials between living
s. 16 2553 Hydrologic cycle cycle Carbon cycle Contents 2 Did you know? 3 (bio) (chemical) (geo;, ) biogeochemical cycles Biogeochemistry = the study of the exchange or flux of materials between living
Pathways that Harvest and Store Chemical Energy
 6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
6 Pathways that Harvest and Store Chemical Energy Energy is stored in chemical bonds and can be released and transformed by metabolic pathways. Chemical energy available to do work is termed free energy
Elementary Reactions
 Updated: 3 September 2013 Print version Lecture #5 Kinetics and Thermodynamics: Fundamentals of Kinetics and Analysis of Kinetic Data (Benjamin, 1.6) (Stumm & Morgan, Chapt.2 ) (pp.16-20; 69-81) David
Updated: 3 September 2013 Print version Lecture #5 Kinetics and Thermodynamics: Fundamentals of Kinetics and Analysis of Kinetic Data (Benjamin, 1.6) (Stumm & Morgan, Chapt.2 ) (pp.16-20; 69-81) David
Biology Chapter 8 Test: Cellular Energy
 Class: Date: Biology Chapter 8 Test: Cellular Energy True/False Indicate whether the statement is true or false. 1. During the light-independent reactions of photosynthesis, light energy is used to split
Class: Date: Biology Chapter 8 Test: Cellular Energy True/False Indicate whether the statement is true or false. 1. During the light-independent reactions of photosynthesis, light energy is used to split
Lecture 2 Carbon and Energy Transformations
 1.018/7.30J Fall 2003 Fundamentals of Ecology Lecture 2 Carbon and Energy Transformations READINGS FOR NEXT LECTURE: Krebs Chapter 25: Ecosystem Metabolism I: Primary Productivity Luria. 1975. Overview
1.018/7.30J Fall 2003 Fundamentals of Ecology Lecture 2 Carbon and Energy Transformations READINGS FOR NEXT LECTURE: Krebs Chapter 25: Ecosystem Metabolism I: Primary Productivity Luria. 1975. Overview
In Cellular Respiration, are removed from sugar and transferred to
 1 2 3 4 5 Bio 1101 Lec. 5, Part A (Guided Notes) Chapter 6: Cellular Respiration Energy is needed by cells to do work Chemical energy, a form of potential energy, is stored in bonds of food molecules (such
1 2 3 4 5 Bio 1101 Lec. 5, Part A (Guided Notes) Chapter 6: Cellular Respiration Energy is needed by cells to do work Chemical energy, a form of potential energy, is stored in bonds of food molecules (such
Oxidation number. The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred.
 Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na,
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na,
Cellular Energy: Respiration. Goals: Anaerobic respiration
 Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Tracers for Redox Environments: GEOTRACES in the Black Sea. James W. Murray School of Oceanography University of Washington
 Tracers for Redox Environments: GEOTRACES in the Black Sea James W. Murray School of Oceanography University of Washington 3 Oct 2010 GEOTRACES Themes 1.Fluxes and processes at ocean interfaces oxic/suboxic/anoxic
Tracers for Redox Environments: GEOTRACES in the Black Sea James W. Murray School of Oceanography University of Washington 3 Oct 2010 GEOTRACES Themes 1.Fluxes and processes at ocean interfaces oxic/suboxic/anoxic
Global Carbon Cycle - I Systematics: Reservoirs and Fluxes
 OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
6CO 2 + 6H 2 O C 6 H 12 O 6 + 6O 2. sun. Occurs in chloroplasts ATP. enzymes CO 2 O 2 H 2 O. sugars
 4.2 8.2 Overview Photosynthesis: of Photosynthesis An Overview Photosynthesis process by which plants make food using energy from the sun Plants are autotrophs that make their own source of chemical energy.
4.2 8.2 Overview Photosynthesis: of Photosynthesis An Overview Photosynthesis process by which plants make food using energy from the sun Plants are autotrophs that make their own source of chemical energy.
%Pluvial Input to the Ocean* Ocean Conc Range (nm) Major dissolved inorganic species in seawater yrs. Al
 Table 6.1 Estimated relative input of metals and metalloids to the ocean from the atmosphere, compared to other sources. Also listed are the range and average concentrations for open ocean waters, the
Table 6.1 Estimated relative input of metals and metalloids to the ocean from the atmosphere, compared to other sources. Also listed are the range and average concentrations for open ocean waters, the
AP Biology Review Chapters 6-8 Review Questions Chapter 6: Metabolism: Energy and Enzymes Chapter 7: Photosynthesis Chapter 8: Cellular Respiration
 AP Biology Review Chapters 6-8 Review Questions Chapter 6: Metabolism: Energy and Enzymes 1. Understand and know the first and second laws of thermodynamics. What is entropy? What happens when entropy
AP Biology Review Chapters 6-8 Review Questions Chapter 6: Metabolism: Energy and Enzymes 1. Understand and know the first and second laws of thermodynamics. What is entropy? What happens when entropy
Chemical Oceanography 14 March 2012 Points are in parentheses (show all your work) Final Exam
 Ocean 400 Name: Chemical Oceanography 14 March 2012 Winter 2012 Points are in parentheses (show all your work) (give as much detail as you can) (use back if necessary) Final Exam 1. Sarmiento and Gruber
Ocean 400 Name: Chemical Oceanography 14 March 2012 Winter 2012 Points are in parentheses (show all your work) (give as much detail as you can) (use back if necessary) Final Exam 1. Sarmiento and Gruber
Cellular Respiration. Mitochondria Rule! Mr. Kurt Kristensen
 Cellular Respiration Mitochondria Rule! Mr. Kurt Kristensen Harvard Biovisions Mitochondria Summer Session Week 1: Cellular Respiration Students should. 1) Understand the locations, and functions of the
Cellular Respiration Mitochondria Rule! Mr. Kurt Kristensen Harvard Biovisions Mitochondria Summer Session Week 1: Cellular Respiration Students should. 1) Understand the locations, and functions of the
Chapter 02 Chemical Basis of Life. Multiple Choice Questions
 Seeleys Essentials of Anatomy and Physiology 8th Edition VanPutte Test Bank Full Download: http://testbanklive.com/download/seeleys-essentials-of-anatomy-and-physiology-8th-edition-vanputte-test-bank/
Seeleys Essentials of Anatomy and Physiology 8th Edition VanPutte Test Bank Full Download: http://testbanklive.com/download/seeleys-essentials-of-anatomy-and-physiology-8th-edition-vanputte-test-bank/
Electron Transfer Reactions
 ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
Activity: Identifying forms of energy
 Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
Activity: Identifying forms of energy INTRODUCTION TO METABOLISM Metabolism Metabolism is the sum of all chemical reactions in an organism Metabolic pathway begins with a specific molecule and ends with
ELECTROCHEMISTRY OXIDATION-REDUCTION
 ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
8.2 Photosynthesis Draw and label a diagram showing the structure of a chloroplast as seen in electron micrographs
 8.2 Photosynthesis 8.2.1 - Draw and label a diagram showing the structure of a chloroplast as seen in electron micrographs double membrane starch grain grana thylakoid internal membrane - location of the
8.2 Photosynthesis 8.2.1 - Draw and label a diagram showing the structure of a chloroplast as seen in electron micrographs double membrane starch grain grana thylakoid internal membrane - location of the
Academic Biology: Midterm Review
 Academic Biology: Midterm Review Quarter #1 Chapter 1: The Science of Biology Biology the study of the living world Scientific Method Step 1: Observation Step 2: Forming a Question Step 3: Form a Hypothesis
Academic Biology: Midterm Review Quarter #1 Chapter 1: The Science of Biology Biology the study of the living world Scientific Method Step 1: Observation Step 2: Forming a Question Step 3: Form a Hypothesis
Chemistry 102 Chapter 19 OXIDATION-REDUCTION REACTIONS
 OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
