MIAMI UNIVERSITY The Graduate School. Certificate for approving the Dissertation. We hereby approve the dissertation SARITA HEBBAR
|
|
|
- Amy Leonard
- 5 years ago
- Views:
Transcription
1 MIAMI UNIVERSITY The Graduate School Certificate for approving the Dissertation We hereby approve the dissertation Of SARITA HEBBAR Candidate for the Degree: Doctor of Philosophy Dr. Joyce J. Fernandes (Advisor) Dr. Lori G. Isaacson (Reader) Dr. Kathleen A. Killian Dr. Katia Del Rio-Tsonis Dr. Paul F. James Dr. Stephen D. Berry (Graduate School Representative)
2 ABSTRACT Patterning the DLM innervation in Drosophila: cellular interactions and molecular mechanisms by Sarita Hebbar Connections made during early nervous system development are subject to modifications such as the strengthening of connections, elimination of neurons, projections or synapses. These remodeling events serve to refine existing connections and thus ensure proper circuit formation. A remarkable example of remodeling occurs during the lifecycle of holometablous insects. For instance, in Drosophila, elements of the larval nervous system are extensively restructured to generate adult specific circuitry. As a result adult specific behaviors are executed. This thesis has utilized patterning of innervation on a set of prominent adult thoracic muscle fibers in Drosophila, to examine cellular processes, interactions and molecular mechanisms during remodeling. The Dorsal Longitudinal (flight) muscle (DLM) is an important component of the flight musculature in the thorax. These muscles display a stereotypic pattern of innervation, wherein a motor axon makes multiple contact points along each muscle fiber (Chapter 2). This has been referred to as multi-terminal innervation in the insect literature. The stereotypic pattern of innervation is generated during metamorphosis in 3 steps: Outgrowth and elaboration, during which adult specific motor axons branch and elaborate over the developing fibers; pruning (Chapter 2) and stabilization (Chapter 3) during which a majority of branches are eliminated and only a third of secondary branches and their higher order arbors are retained; and finally arbor expansion, during which boutons make an appearance and the stabilized arbors increase their expanse in association with the increase in muscle mass. In addition to interactions between the pre- and post-synaptic cells, neuron-glial interactions also play significant roles in influencing branch patterning (Chapter 4). The cell-adhesion molecule, FasII is an important mediator of these cellular interactions. This thesis also presents the ventral abdominal NMJ as a second adult model for developmental plasticity because of its unique advantages over the DLMs (Chapter 5).
3 PATTERNING THE DLM INNERVATION IN DROSOPHILA: CELLULAR INTERACTIONS AND MOLECULAR MECHANISMS A DISSERTATION Submitted to the Faculty of Miami University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Zoology by Sarita Hebbar Miami University Oxford, Ohio 2005 Dissertation Advisor: Dr. Joyce J. Fernandes
4 TABLE OF CONTENTS 1 Introduction 1 2 Pruning of motor neuron branches establishes the DLM innervation pattern in Drosophila. Journal of Neurobiology (2004). 60 (4), Abstract Introduction Materials and Methods Results Discussion References Tables Figures Appendix A role for Fas II in the stabilization of motor neuron branches during pruning in Drosophila. Manuscript in press, Developmental Biology Abstract Introduction Materials and Methods Results Discussion References Tables Figures Appendix Glial-Neuronal Interactions via Fas II influence stabilization of motor neuronal branches in Drosophila. Abstract Introduction Materials and Methods Results Discussion References Tables ii
5 Figures The adult abdominal neuromuscular junction of Drosophila: a model for synaptic plasticity. Manuscript in Review, Journal of Neurobiology Abstract Introduction Materials and Methods Results Discussion References Tables Figures Conclusions 129 iii
6 Acknowledgements I am grateful to my wonderful mentor, Joyce Fernandes. She has given me the freedom to think and develop my projects and, when it was necessary, she has stepped in to channel my efforts in a more fruitful direction. I am especially thankful for her inputs that have improved my writing skills. Members of the Drosophila community have been very generous in providing fly stocks and antibodies. For this, I am grateful to Drs. Haig Keshishian, Barry Ganetzky, Vivian Budnik, Graeme Davis, Richard Baines, Sujata Rao, Ben White, Troy Littleton, Bradley Jones and Aaron DiAntonio. The technical assistance of Richard Edelmann and Matt Duley in the use of imaging software and the confocal microscope in the Electron Microscope Facility has been invaluable to my work. I thank members of my graduate committee, Drs. Lori Isaacson, Kathleen Killian, Katia Del Rio- Tsonis, Paul James and Stephen Berry, for their input and time commitment towards my PhD training. I am grateful to Dr. Isaacson for being a reader for the final version of my thesis. I thank Drs. Haig Keshishian, Richard Levine, Karla Kent, John Jellies, Kathleen Killian, and Louise Nicholson for their inputs and useful comments on versions of my manuscripts. I also acknowledge the reviewing and editing efforts of Mayur Madhavan and Jason Spence. I have enjoyed the company of my colleagues in the Fernandes laboratory and in the zoology department. Life in Pearson Hall would not have been the same without some of my interactions especially those with Kathy or Mayur. Likewise, time in the lab was definitely made more interesting thorough my conversations with Tom Dockendorff. I have also enjoyed my interactions with undergraduates, Allison, Sarah, Rachel, Jay, Melanie, Andrea, Maureen and Omar, with whom I have worked closely on related or independent projects. I thank Rachel Hall and Sarah Demski for help with quantification of boutons in Chapter 5. Finally I thank my parents, siblings, family and friends for their support and encouragement. iv
7 Chapter 1 Introduction 1.1 Remodeling of the nervous system Behavior exhibited by an organism is a result of an appropriate neural circuitry. One major area of research is the manner in which neural circuits generate behaviors. This research encompasses three levels of study: identifying the components of a circuit, understanding the resulting physiology and, finally, the manner in which neural circuits are precisely assembled during development. It is well known that, during embryonic development, there is an initial phase of exuberant outgrowth during which excessive connections are made. A later phase of fine-tuning occurs during which excessive connections are removed and/or new ones are added (Kantor and Kolodkin, 2003). This fine-tuning has been referred to as remodeling or restructuring, and serves to appropriately match the pre and postsynaptic partners. As a consequence of remodeling, the efficiency of the circuit is potentially enhanced. Remodeling could involve the death of neurons or the removal of axonal, dendritic branches and the removal/strengthening of synapses. Of these possibilities, the removal or elimination of established inputs (axons, dendrites and or synapses), is an interesting event that is the least studied remodeling event (Kantor and Kolodkin, 2003). An estimated 50% of synaptic contacts in rats and a comparable number in humans are lost during development (Sanes et al., 2000; Goodman and Shatz, 1993). Remodeling is not limited to the developmental phase but is also seen in the mature adult nervous system. In the adult nervous system it is most often a consequence of learning and the associated behavioral changes. Here, remodeling is reflected in a change in the number and/or strength of terminals or synapses. Another instance of remodeling in the adult nervous system occurs at the onset of puberty under the influence of steroid hormones. In females, during the estrous cycle, there is a transient disconnection of certain inputs to the arcuate nucleus in the hypothalamus. This synaptic remodeling results in organizational changes in connectivity in the arcuate nucleus (Garcia-Segura et al., 1994). 1.2 Developmental remodeling of the vertebrate nervous system 1
8 The biology of nervous system remodeling via elimination of projections or established connections has been studied in a variety of model systems. The classic work of Weisel and Hubel in 1960s first demonstrated the elimination of afferents to the mammalian visual cortex as the right and left eye projection fields begin to segregate (Weisel and Hubel, 1963; Hubel and Weisel, 1970; LeVay et al 1978). In the visual system, there are two instances of refinement that occur during distinct stages of development. The first phase of elimination occurs in-utero and involves the removal of transient projections made by the retinal ganglion cells onto the Lateral Geniculate Nuclei, LGN (Sur et al., 1984; Sretavan and Shatz, 1986). The second phase of elimination occurs post-natally and involves the removal of a fraction of thalamic inputs that constitute the ocular dominance columns in Layer IV of the visual cortex (LeVay et al, 1978). Interestingly, both kinds of elimination are dependent on electrical activity (Sur et al., 1984; Katz and Shatz, 1996). Input elimination has subsequently been described in the amphibian midbrain (Reh and Constantine-Paton, 1984), the mammalian cerebellum (Mason and Gregory, 1984), the mammalian hippocampus (Bagri et al., 2003) and the mammalian neuromuscular junction (Kuffler et al., 1977). Although these events of input elimination have been described, we know very little about the biology of this process. What factors influence the stabilization of some inputs and the elimination of others? Does the process of elimination commence specifically at one synaptic partner and progress to the other? What factors mediate the removal of projections/synapses? Only recently, semaphorins, a class of molecules that are known to play a role in axon guidance, have been implicated in remodeling in the developing hippocampus. It has been demonstrated that mice deficient in the semaphorin-3 signaling pathway exhibit defects in elimination of arbors specific to the hippocampus (Bagri et al., 2003). The sheer complexity of the mammalian brain has made it difficult to address remodeling at the level of individual inputs under in-vivo conditions. Some of these questions have been addressed at the vertebrate NMJ which has the advantages of being more accessible. At birth, mammalian skeletal muscles are innervated by multiple motorneurons ( Kuffler et al., 1977; Balice-Gordon and Lichtman, 1990). Within the first two postnatal weeks, synapses and axonal inputs are eliminated such that eventually one muscle fiber is innervated by only one motor neuron (Kuffler et al., 1977; Balice-Gordon and Lichtman, 1990; Colman et al., 1997). This phase is activity dependent (Thompson, 1983) and competition between innervating motorneurons is thought to drive the process of elimination (Ribchester et al, 1987; Callaway et al, 1987). Synapse elimination commences at the postsynaptic side with the dismantling of the acetylcholine receptor cluster (Balice-Gordon and Lichtman, 1990) and ends with the disappearance of the axonal membrane. Evidence of input loss comes from the 2
9 morphological observations of disconnected axons that sometimes end in enlarged tips (Balice-Gordon et al., 1993; Balice-Gordon and Lichtman, 1993). Recent time-lapse imaging using transgenic mice have revealed interesting details about the manner in which inputs are lost during development. It has been confirmed that disconnected axon like structures are the distal tips of axons that have been eliminated (Keller-Peck et al., 2001; Walsh and Lichtman, 2003). Using transgenic mice to visualize a subset of axons undergoing synapse elimination in combination with electron microscopy, it has been demonstrated that as axons withdraw, they shed pieces of their membrane (Bishop et al., 2004). These axonal membrane pieces or axosomes are engulfed by the neighboring Schwann cells. At this point, it remains unclear if Schwann cells passively engulf the axosomes or if they actively mediate the process of input elimination (Bishop et al., 2004; Koirala and Ko, 2004) 1.3 Post-embryonic remodeling in invertebrate models. As with many problems in biology, a reductionist approach has been successfully applied to study remodeling in relatively simple nervous systems. A surprising, yet effective, model is the nervous system of holometabolous insects, examples of which include the moth, Manduca and the fruit fly, Drosophila (Fernandes and Keshishian, 1999; Consoulas et al., 2000). A hallmark of the holometabolous lifecycle is the phase of metamorphosis when the larva transitions into the adult stage and during which almost all larval tissues including the nervous system, the musculature, the gut and the body wall undergo transformations. The most dramatic changes are seen in the case of the nervous system, which has to be extensively remodeled in order to accommodate the vastly different repertoire of behaviors, demanded by a change in body form. For example, olfactory preferences, visual perception and locomotion in the adult are completely different from the larval stage. An interesting aspect of this remodeling is that the larval nervous system is not simply eliminated to make way for adult structures. Instead, generation of adult specific circuits involves the generation of new neurons as well as the respecification of persistent larval/embryonic neurons. For example, most of the adult sensory neurons and some interneurons are born during metamorphosis (Tissot and Stocker, 2000) whereas almost all motorneurons (Fernandes and VijayRaghavan, 1993; Consoulas et al., 2002) and some interneurons (Tissot and Stocker, 2000) are born in the embryonic phase but are respecified into adult counterparts as a part of the remodeling. Since this process involves dramatic changes in structure and physiology of neurons in order to execute very different behaviors, holometabolous insects serve as excellent model systems to study plasticity of the nervous system. 3
10 a. Postembryonic remodeling in Manduca. The strength of Manduca as a model system lies in its relatively large size. The ability to perform in-vivo surgical and in-vitro manipulations have given many insights into the remodeling of motorneurons, most important being the role of steroid hormones (Weeks, 2003). Both in the CNS and the periphery, as a part of the respecification of motorneurons, larval processes regress and make way for new adult specific processes (Truman and Reiss, 1990; Kent and Levine, 1993). By surgically dissecting the source of the ecdysteriod or injection of ecdysteriod analogs, it has been demonstrated that ecdysteriods play an important role in the regression and regrowth of motorneuronal processes (Prugh et al., 1992; Kent and Levine, 1993; Truman and Reiss, 1995; Knittel and Kent, 2005). Theses studies have been accompanied by electrophysiological manipulations indicating that phases of regression and regrowth are characterized by distinct patterns of activity in the motorneurons (Duch and Mentel, 2003). However, one of the drawbacks of Manduca as a model system is the lack of genetic or molecular tools to dissect these processes. Recently studies in Manduca have begun using RNA interference or subtractive hybridization to elucidate genes that may be regulating steroid dependent processes during metamorphosis (Weeks, 2003). b. Post-embryonic remodeling in Drosophila. Besides having a simple nervous system, where preand post-synaptic partners can be uniquely identified, the strength of Drosophila as a model system lies in its amenability for cellular, molecular and physiological studies (Keshishian et al., 1996). It has been used as a model genetic organism for over a century. The recent completion of the Drosophila genomesequencing project has revealed remarkable similarities to genes involved in human physiological processes and disease. About 60% of human genes are orthologous to Drosophila genes, and further analysis of these genes will be crucial in bettering our understanding of the nature of signaling pathways linked to disease. Drosophila is now being used as a genetic model to address questions linked to neurodegeneration (Muqit and Feany, 2002) including Parkinson s disease (Feany and Bender, 2000), Huntington s syndrome (Jackson et al., 1998) and Fragile X linked mental retardation (Wan et al., 2000; Dockendorff et al., 2002) among others. Postembryonic remodeling in Drosophila has been studied in three systems; the mushroom body (a collection of interneurons in the brain), the larval neuromuscular junction (NMJ), and the adult NMJ. Each is described in detail below: 4
11 1. The mushroom bodies are important centers for processing learning and memory in the adult. Two classes of identifiable neurons, the γ and the α /β are present within this structure. The γ neurons are present within the larval mushroom body whereas the α /β neurons are born later in the larval stages. At the onset of metamorphosis, the dendritic and axonal arbors of the γ neurons prune and thus make way for new adult specific projections. In contrast the α /β neurons do not restructure their projections during metamorphosis (Lee et al., 2000). Using genetic tools available in Drosophila, it has been demonstrated that the selective removal of γ projections occurs in a cell-autonomous fashion and is mediated by the ecdysteriod receptor, Ultraspiracle (Lee et al., 2000) and the TGF-β signaling system (Zheng et al., 2003). Interestingly, pruning occurs through a degeneration (in contrast to retraction) process that is mediated by the ubiquitin-protease system (Watts et al., 2003). Interestingly, just as in the vertebrate NMJ (Bishop et al., 2004), glia have been implicated in engulfing the degenerating axonal membrane (Watts et al., 2004; Awasaki and Ito, 2004). 2. The embryonic NMJ is generated after 17 hours of egg laying and functions in larval hatching, crawling and feeding. The embryonic NMJ undergoes a continuous remodeling throughout the larval stages (see schematic in Figure 1). Accompanying the increase in larval size is a growth in the muscles of the body wall and an associated presynaptic elaboration (Keshishian et al., 1996). Presynaptic elaboration occurs through the addition of synaptic varicosities (also referred to as boutons) and active zones (Schuster et al., 1996). Interestingly, the phase of synaptic growth is also characterized by elimination via local synaptic disassembly. Thus, the resulting NMJ is an outcome of the balance between synapse addition and disassembly (Eaton et al., 2002). The dyenin/dynactin complex (Eaton et al., 2002), coupled with the retrograde transport of P-Mad (McCabe et al., 2003), a component of the TGF-β signaling pathway, is believed to be important for synapse stabilization during the growth phase. 3. The adult NMJs develop during metamorphosis through a restructuring of the embryonic NMJ (Figure 1). At the end of the pupal stage an adult fly emerges with a remodeled set of NMJs that executes adult specific behaviors such as flight, walking and copulation. This second phase of neuromuscular remodeling is significant because it results in morphological and behavioral changes. Interestingly, the adult motorneurons are believed to be embryonic in origin just as in Manduca (Truman, 1990; Consoulas et al., 2002). The remodeling involves changes in central as well as peripheral projections such that a functional adult circuitry is established. My project focuses on this 5
12 phase of remodeling using a specific set of adult muscles, the dorsal longitudinal muscles or the DLMs as a model system. 1.4 Scope of this thesis The indirect Flight muscles (IFMs) of the adult thorax are some of the largest muscles in Drosophila. The IFMs are made up of two groups of muscles with opposing functions; the dorsal longitudinal muscles (DLMs) are the wing depressors, while the dorso-ventral muscles (DVMs) are the wing elevators. The IFMs are an integral part of a very important neural circuit, the Giant Fiber pathway, which controls the escape response of the fruit fly. The muscles and their motor neurons are singly identifiable and therefore highly accessible (Fernandes and Keshishian, 1999). The primary neuromuscular pattern of the IFMs develops during day 1 of the four-day period of metamorphosis (Figure 1;Table 1, Fernandes and VijayRaghavan, 1993). As the third instar larva enters the pupal phase, 0h APF (hours after puparium formation), most of the larval muscles degenerate. In the mesothorax, three larval muscles persist to serve as scaffolds for the development of DLMs. Adult myoblasts gather around these fibers and form the DLM fibers (Figure 1, Fernandes et al, 1991). Innervation to the DLMs and to most adult muscles develops from the restructuring of larval innervation (Fernandes and VijayRaghavan, 1993; Consoulas et al., 2002). Initially, as larval muscles degenerate, neuromuscular endings are withdrawn, and the nerve maintains minimal contact with persistent muscles. Subsequently, adult specific branches are formed, which elaborate over the developing adult musculature (Fernandes and VijayRaghavan, 1993). The primary branching pattern seen on the DLMs at the end of day 1 (24h APF) of pupal development is similar to what has been reported in the adult. The adult emerges at the end of the 4th pupal day (96h APF). Thus, formation of the adult fibers and their primary nerve branching pattern is established at 25% of adult development. Relatively nothing is known about the developmental fate of their neuronal contacts after 24 hapf. Some interesting features of the remodeling of DLM innervation are as follows: a. Myogenesis and innervation occur in parallel, allowing neuromuscular interactions to shape the emerging innervation pattern (Fernandes and Keshishian, 1998). This feature is absent during embryogenesis where the motor neuron innervates an established muscle pattern (Broadie and Bate, 1993). Thus, the DLMs are a useful model to study neuromuscular interactions during development. 6
13 b. Formation of DLM innervation is also distinct from the embryonic process because it is not complicated by events such as axon guidance and connectivity that are important influences during embryogenesis (Fernandes and Keshishian, 1995). c. Since the adult NMJs develop in the second wave of development, it would be of interest to examine if embryonic genes are reutilized or new genes are deployed for adult development. Although certain events in day 1 of metamorphosis have been outlined (see above section); the following questions remain unanswered: a. What is the morphology of the adult innervation? What is the morphology and molecular architecture of adult synapses? b. Do the adult specific branches at 24h APF undergo changes during the remainder of metamorphosis? How does the innervation pattern at 24hAPF relate to the mature pattern of innervation in adults (96-100hAPF)? c. What factors influence patterning of DLM innervation through metamorphosis? 1.5 Remodeling at the DLMs: Overview of accomplishments Chapter 2: The adult innervation pattern at the DLMs. Rationale: EM studies and intracellular dye fills have been used to identify the DLM motor neurons, their location in the CNS (Ikeda and Koenig, 1988) and the pattern of connectivity (Ikeda et al., 1980; Cogshall, 1990). In order to examine how the adult innervation is established during the remainder of metamorphosis, a first step to define the morphological attributes of the adult DLM innervation was necessary. Approach: Whole mount thoracic preparations were processed for Anti-HRP (a nervous system specific marker; Jan and Jan, 1982) immunocytochemistry to examine the entire DLM innervation. Outcome: The pattern of adult DLM innervation is multi-terminal (Hebbar and Fernandes, 2004). This terminology (Hoyle, 1990) has been used to describe a pattern wherein the innervating motorneuron 7
14 makes multiple contacts along the length of the DLM fiber. This is in contrast to the single terminal innervation of the larval muscles wherein the motorneurons have one entry point on the muscles before they branch into boutons (Johansen et al 1989). At the adult DLM, each entry point made by the motor axon and its terminal arbor is referred to as a contact point (CP). Interestingly, the adult DLM innervation is stereotypic at the level of the number of CPs or axonal entry points and their terminal arbors. For instance DLMa typically exhibits 5 CPs in a majority of animals investigated. It is the higher order arbors of each terminal that bear the boutons or presynaptic swellings. Synaptic markers such as synaptotagmin, vesicular glutamate transporter, Discs large and Microtubule marker, Futsch/22C10 were localized to these presynaptic swellings or boutons (Chapter 2; Hebbar and Fernandes, 2004; Appendix 1). Chapter 2: A time-line of events in the generation of the multi-terminal innervation pattern. Rationale: The characteristic multi-terminal innervation pattern of the DLMs is at the level of secondorder branching of the motor axon. It had been previously established that adult primary branching pattern is established by the end of Day 1 (24h APF) of metamorphosis (Fernandes et al., 1991). Second order branching seen at this time point did not resemble the adult pattern. Therefore, it was necessary to identify the event(s) subsequent to this stage that lead to the multi-terminal innervation pattern. Approach: Morphology of second order branching was qualitatively and quantitatively examined from early metamorphosis (14h APF) to the emergence of a multi-terminal innervation pattern (38h APF). In addition, the onset of differentiation of branches into morphologically identifiable synapses was also examined. Finally, second order branch development was also examined when levels of electrical activity were altered using hyperexcitable K + channel double mutants and triple mutant combinations with Na + channel mutants. Outcome: In a manner similar to the formation of vertebrate nervous systems, there is a period of excessive outgrowth (14-24h APF) followed by a phase of pruning (24-38h APF) (Figure 1; Hebbar and Fernandes, 2004). Pruning occurs at the level of second order branches and establishes the stereotypic multi-terminal innervation pattern seen in the adult. Interestingly, more than 75% of secondary branches are pruned while the remainder is stabilized to generate the adult innervation pattern. Morphological swellings or boutons appear after the pruning is completed at 38h APF. By 48h APF, the presynaptic marker synaptotagmin, which was previously along branches, becomes localized to the boutons. This is also the time another presynaptic marker, DVGLUT gets localized to boutons (Appendix 1). Lastly, 8
15 electrical activity influences both the outgrowth of excessive branches and the pruning process. As a result, innervation in the hyperexcitable K + mutant, eag 1 Sh 120b is altered with a reduced number of CPs. This phenotype can be genetically rescued by introducing a hypoexcitable Na + channel mutant, nap ts1 in the eag 1 Sh 120b background. Chapter 3: Role of cell adhesion molecule, FasII, in patterning the adult innervation pattern. Rationale: During the pruning phase, a large fraction of second order branches are eliminated. This raises the question of how the remaining branches and their arbors are stabilized. Cell adhesion molecule, Fasciclin II (FasII), has been implicated in synapse stabilization (Schuster et al., 1996), making it an ideal candidate. Expression of FasII revealed that it is present on a subset of secondary branches and their arbors, making it a candidate for influencing branch stabilization. Approach: FasII expression pattern was followed during metamorphosis using a FasII antibody. Adult innervation patterns were examined when FasII levels were decreased in FasII hypomorphs, and when FasII levels were increased using the Gal4/UAS system of targeted overexpression (Brand and Perrimon, 1993). Since FasII alterations resulted in an altered adult innervation pattern, pupal stages were also examined to investigate the developmental origins of the phenotype. Finally, the effect of decreased FasII levels on the number of synapses was also examined by using DVGLUT as a synapse specific marker (Appendix 2) Outcome: FasII is expressed during second order branch development in a subset of secondary branches and their arbors. In FasII hypomorphs, the number of CPs is increased and this phenotype is rescued by targeted expression of FasII in either synaptic partner. Thus, FasII is required for generating the stereotypic adult innervation pattern at the DLMs. Although FasII is expressed in a subset of branches; not all FasII positive branches are retained. Therefore, FasII is likely to prime branches for stabilization. By examining pupal development, we find that FasII restricts secondary branch length and arbor expanse. Finally, FasII is absent at the adult NMJ implicating other molecules to be involved in the maintenance of the junction (Hebbar and Fernandes, 2005). FasII hypomorphs exhibit no differences in the morphology of DLM synapses (Appendix 2). Chapter 4: Glial-neuronal interactions via FasII in patterning DLM innervation. 9
16 Rationale: FasII was implicated in regulating arbor size in the first 24 hours of metamorphosis (Chapter 3). However, it continues to be expressed in the secondary branches and in their surrounding glial component. Interestingly, the glial component around secondary branches is evident only after pruning. Does FasII play any role beyond 24h, in conjunction with the glia, to pattern the DLM innervation? Approach: The progression of glial ensheathment of DLM axonal branches during metamorphosis was followed using glial specific marker, repo and the Gal 4/UAS system with reporter gene, UAS-2xEGFP. Next, patterning of DLM innervation in the absence of glial processes was examined. For this, glial membrane function was suppressed by overexpressing a dominant negative shibire (Kitamoto, 2002) specifically in glia. The role of FasII in glia was investigated by examining innervation when FasII levels were altered using UAS-FasII and repo-gal 4. Finally, FasII hypomorphic phenotype was rescued by increasing FasII levels in glia using the Gal 4/UAS system. Outcome: Glial processes maintain close association with the innervation during metamorphosis. Prior to the establishment of the adult innervation pattern, glial processes migrate towards the secondary branches. Subsequent to pruning, every stabilized CP is ensheathed by the glial process. Suppressing glial membrane function and therefore its migration towards the secondary branches, results in an enhanced pruning at 31h APF. This is reflected in a reduced number of CPs in the adult. Overexpression of FasII in the glia has an effect only on the pruning at 31h APF and not on earlier events. Additionally, FasII hypomorphic phenotypes can be rescued with glial FasII overexpression. Thus, glial neuronal interactions via FasII are important in patterning DLM innervation. Chapter 5: A characterization of the adult ventral abdominal synapse. Rationale: There are three sets of neuromuscular systems in the adult: the head, thoracic, and abdominal systems. Studies on peripheral remodeling have focused on NMJs of one group of flight muscles, the Dorsal Longitudinal Muscles (DLMs) (Fernandes et al., 1991; Fernandes and VijayRaghavan, 1993; Fernandes and Keshishian, 1998; Consoulas et al., 2002). In contrast, very little is known about the adult abdominal NMJ. The adult abdominal system, although not as large as the flight muscles in the thorax, present two major advantages as a model system. First, there are seven segments, thus allowing many more observations from a single animal (Currie and Bate, 1991). Secondly, the abdominal muscles are very accessible. The abdominal muscles are organized into three groups, dorsal, lateral, and ventral. The ventral muscles are the largest group of muscles and have an innervation pattern that resembles the single terminal larval innervation (Currie and Bate, 1991). The 10
17 goal of this study is to develop the abdominal NMJ as model system to examine plasticity during metamorphosis. The larval abdominal NMJ is a well characterized system (Keshishian et al., 1996) that has been extensively studied in isolation from its adult counterpart. An investigation of the adult abdominal system and its development will serve as the basis to increase our understanding of the similarities and differences in the development of the two distinct NMJs. Approach: The adult abdominal NMJ was characterized using anti HRP, a nervous system marker (Jan and Jan, 1982) in dissected preparations. Synapse numbers and size were quantified. The localization of synaptic markers was examined. Next, as a step towards understanding synapse development, boutons were visualized as they appeared during development and the localization of presynaptic markers was examined at these stages. In the final set of experiments, morphological attributes of the adult abdominal NMJ including bouton numbers, size and expanse, were quantified in hyperexcitable K + channel mutants and in FasII hypomorphs in order to examine the effects of electrical activity and cell adhesion molecule FasII on adult synapse development. Outcome: The strength of the ventral abdominal muscle system lies in the large, identifiable synapses that can be easily accessed. We have quantified synapses as larges as 8.0µm. These synapses are identifiable as early as 55h APF as presynaptic swellings. In addition, presynaptic markers synaptotagmin and DVGLUT localize to these boutons at 55h APF. Finally, both activity and FasII affect synapse development at the adult abdominal NMJ. However, their influence on the adult NMJ is different from that seen at the larval NMJ. 1.6 Significance of these studies Synaptic remodeling at the DLMs involves a significant amount of axon pruning. This phenomenon is known to occur at the vertebrate NMJ and is not seen during the formation of the embryonic NMJ in Drosophila. a. Significance for Drosophila NMJ formation: There are two sets of NMJs made in Drosophila: an embryonic set and an adult set that develop during metamorphosis. While synaptic plasticity in the mature larval stage has been well established (Keshishian et al., 1996), there is relatively little known about the mechanisms of plasticity seen during the formation of the adult NMJ. These studies have described pruning as a significant process in the generation of the adult innervation pattern. Additionally, 11
18 the genetic analyses carried out can serve as a basis for future screens to identify molecules involved in pruning. Often in biology, pathways used in one process of development are recapitulated in a completely different process. Recently, it has been shown that molecules implicated in axon guidance (early nervous system development) such as semaphorins are necessary for later events of remodeling (Bagri et al., 2003). In the case of Drosophila, cellular and molecular mechanisms of embryonic/larval NMJ development have been extensively studied (Keshishian et al., 1996) however, in isolation from adult NMJ development. These studies will add to our understanding of the molecular basis of the differences and similarities between these two NMJs. b. Significance for vertebrate development: Adult NMJ formation in Drosophila has many similarities with vertebrate NMJ formation unlike its embryonic counterpart (Fernandes and Keshishian, 1995; Fernandes and Keshishian, 1999). Two prominent similarities are the involvement of neuromuscular interactions and a significant amount of activity dependent pruning that sculpts the adult pattern. Thus, a thorough study of adult NMJ formation becomes necessary to compare the similarities and differences in the development of NMJs, specifically, in the pruning process between vertebrate models and Drosophila. c. Significance for disease: In the developing mammalian nervous system, a large amount of remodeling occurs in the post-natal phase. It, therefore, follows that defects in this phase affect the efficient functioning of the nervous system and can underlie some neurodevelopmental disorders. Retts Syndrome is a diseased condition that affects 1 in 10,000 female children. These patients are normal at birth and as a consequence of postnatal developmental defects they begin to show signs of mental retardation by the age of sixteen months. For most cases, a mutation in a gene, MeCP2, has been linked to the disorder. This gene is a transcriptional repressor of other genes and its actions are responsible for the maintenance of connections during the remodeling of the postnatal brain (Segawa and Nomura, 2005). Therefore, understanding how neuronal projections and synapses are stabilized during development will be important in the treatment of developmental disorders. Understanding how synapses and axonal processes are eliminated during the course of remodeling will also give insights into the biology of neurodegeneration. Since pruning at the NMJ occurs largely as a result of axonal breakdown, future studies identifying the molecules involved will be important in understanding neurodegeneration. 12
19 1.7 References Awasaki, T., and Ito, K. (2004). Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol 14, Bagri, A., Cheng, H. J., Yaron, A., Pleasure, S. J., and Tessier-Lavigne, M. (2003). Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell 113, Balice-Gordon, R. J., Chua, C. K., Nelson, C. C., and Lichtman, J. W. (1993). Gradual loss of synaptic cartels precedes axon withdrawal at developing neuromuscular junctions. Neuron 11, Balice-Gordon, R. J., and Lichtman, J. W. (1990). In vivo visualization of the growth of pre- and postsynaptic elements of neuromuscular junctions in the mouse. J Neurosci 10, Balice-Gordon, R. J., and Lichtman, J. W. (1993). In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. J Neurosci 13, Bishop, D. L., Misgeld, T., Walsh, M. K., Gan, W. B., and Lichtman, J. W. (2004). Axon branch removal at developing synapses by axosome shedding. Neuron 44, Brand, A. H., and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, Broadie, K., and Bate, M. (1993). Muscle development is independent of innervation during Drosophila embryogenesis. Development 119, Colman, H., Nabekura, J., and Lichtman, J. W. (1997). Alterations in synaptic strength preceding axon withdrawal. Science 275, Consoulas, C., Duch, C., Bayline, R. J., and Levine, R. B. (2000). Behavioral transformations during metamorphosis: remodeling of neural and motor systems. Brain Res Bull 53, Consoulas, C., Restifo, L. L., and Levine, R. B. (2002). Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. J Neurosci 22, Currie, D. A., and Bate, M. (1991). The development of adult abdominal muscles in Drosophila: myoblasts express twist and are associated with nerves. Development 113, Dockendorff, T. C., Su, H. S., McBride, S. M., Yang, Z., Choi, C. H., Siwicki, K. K., Sehgal, A., and Jongens, T. A. (2002). Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron 34, Duch, C., and Mentel, T. (2003). Stage-specific activity patterns affect motoneuron axonal retraction and outgrowth during the metamorphosis of Manduca sexta. Eur J Neurosci 17, Eaton, B. A., Fetter, R. D., and Davis, G. W. (2002). Dynactin is necessary for synapse stabilization. Neuron 34, Feany, M. B., and Bender, W. W. (2000). A Drosophila model of Parkinson's disease. Nature 404, Fernandes, J., Bate, M., and Vijayraghavan, K. (1991). Development of the indirect flight muscles of Drosophila. Development 113, Fernandes, J., and Keshishian, H. (1995). Neuromuscular development in Drosophila: insights from embryos and pupae. Curr Opin Neurobiol 5, Fernandes, J., and VijayRaghavan, K. (1993). The development of indirect flight muscle innervationin Drosophila melanogaster. Development 118, Fernandes, J. J., and Keshishian, H. (1998). Nerve-muscle interactions during flight muscle development in Drosophila. Development 125,
20 Fernandes, J. J., and Keshishian, H. (1999). Development of the adult neuromuscular system. Int Rev Neurobiol 43, Garcia-Segura, L. M., Chowen, J. A., Duenas, M., Torres-Aleman, I., and Naftolin, F. (1994). Gonadal steroids as promoters of neuro-glial plasticity. Psychoneuroendocrinology 19, Goodman, C. S., and Shatz, C. J. (1993). Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell 72 Suppl, Hebbar, S., and Fernandes, J. J. (2004). Pruning of motor neuron branches establishes the DLM innervation pattern in Drosophila. J Neurobiol 60, Hebbar, S and Fernandes, J.J. (2005). A role for FasII in the stabilization of motor neuronal branches during pruning. Dev Biol, m.s in press Hubel D and Weisel TN (1970). Steroscopic vision in maccaque monkey. Cells sensitive to binoccular depth in area 18. Nature 3, Ikeda, K., and Koenig, J. H. (1988). Morphological identification of the motor neurons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Comp Neurol 273, Ikeda, K., Koenig, J. H., and Tsuruhara, T. (1980). Organization of identified axons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Neurocytol 9, Jackson, G. R., Salecker, I., Dong, X., Yao, X., Arnheim, N., Faber, P. W., MacDonald, M. E., and Zipursky, S. L. (1998). Polyglutamine-expanded human huntingtin transgenes induce degeneration of Drosophila photoreceptor neurons. Neuron 21, Jan, L.Y. and Jan, Y.N. (1982). Antibodies to HRP as specific neuronal markers in Drosophila and grasshopper embryos. Proc Natl Acad Sci U S A 79, Kantor, D. B., and Kolodkin, A. L. (2003). Curbing the excesses of youth: molecular insights into axonal pruning. Neuron 38, Katz, L. C., and Shatz, C. J. (1996). Synaptic activity and the construction of cortical circuits. Science 274, Keller-Peck, C. R., Walsh, M. K., Gan, W. B., Feng, G., Sanes, J. R., and Lichtman, J. W. (2001). Asynchronous synapse elimination in neonatal motor units: studies using GFP transgenic mice. Neuron 31, Kent, K. S., and Levine, R. B. (1993). Dendritic reorganization of an identified neuron during metamorphosis of the moth Manduca sexta: the influence of interactions with the periphery. J Neurobiol 24, Keshishian, H., Broadie, K., Chiba, A., and Bate, M. (1996). The Drosophila neuromuscular junction: a model system for studying synaptic development and function. Annu Rev Neurosci 19, Kitamoto, T. (2002). Conditional disruption of synaptic transmission induces male-male courtship behavior in Drosophila. Proc Natl Acad Sci U S A 99, Knittel, L. M., and Kent, K. S. (2005). Remodeling of an identified motoneuron during metamorphosis: hormonal influences on the growth of dendrites and axon terminals. J Neurobiol 63, Koirala, S., and Ko, C. P. (2004). Pruning an axon piece by piece: a new mode of synapse elimination. Neuron 44, Kuffler, D., Thompson, W., and Jansen, J. K. (1977). The elimination of synapses in multiply-innervated skeletal muscle fibres of the rat: dependence on distance between end-plates. Brain Res 138, Lee, T., Marticke, S., Sung, C., Robinow, S., and Luo, L. (2000). Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron 28, Mason, C. A., and Gregory, E. (1984). Postnatal maturation of cerebellar mossy and climbing fibers: transient expression of dual features on single axons. J Neurosci 4,
21 McCabe, B. D., Marques, G., Haghighi, A. P., Fetter, R. D., Crotty, M. L., Haerry, T. E., Goodman, C. S., and O'Connor, M. B. (2003). The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39, Muqit, M. M., and Feany, M. B. (2002). Modelling neurodegenerative diseases in Drosophila: a fruitful approach? Nat Rev Neurosci 3, Prugh, J., Della Croce, K., and Levine, R. B. (1992). Effects of the steroid hormone, 20-hydroxyecdysone, on the growth of neurites by identified insect motoneurons in vitro. Dev Biol 154, Reh, T. A., and Constantine-Paton, M. (1984). Retinal ganglion cell terminals change their projection sites during larval development of Rana pipiens. J Neurosci 4, Schuster, C. M., Davis, G. W., Fetter, R. D., and Goodman, C. S. (1996). Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17, Segawa, M., and Nomura, Y. (2005). Rett syndrome. Curr Opin Neurol 18, Sretavan, D. W., and Shatz, C. J. (1986). Prenatal development of cat retinogeniculate axon arbors in the absence of binocular interactions. J Neurosci 6, Sur, M., Weller, R. E., and Sherman, S. M. (1984). Development of X- and Y-cell retinogeniculate terminations in kittens. Nature 310, Tissot, M., and Stocker, R. F. (2000). Metamorphosis in drosophila and other insects: the fate of neurons throughout the stages. Prog Neurobiol 62, Truman, J. W. (1990). Metamorphosis of the central nervous system of Drosophila. J Neurobiol 21, Truman, J. W., and Reiss, S. E. (1995). Neuromuscular metamorphosis in the moth Manduca sexta: hormonal regulation of synapses loss and remodeling. J Neurosci 15, Walsh, M. K., and Lichtman, J. W. (2003). In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron 37, Wan, L., Dockendorff, T. C., Jongens, T. A., and Dreyfuss, G. (2000). Characterization of dfmr1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol 20, Watts, R. J., Hoopfer, E. D., and Luo, L. (2003). Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron 38, Watts, R. J., Schuldiner, O., Perrino, J., Larsen, C., and Luo, L. (2004). Glia engulf degenerating axons during developmental axon pruning. Curr Biol 14, Weeks, J. C. (2003). Thinking globally, acting locally: steroid hormone regulation of the dendritic architecture, synaptic connectivity and death of an individual neuron. Prog Neurobiol 70, Weisel, TN and Hubek DH (1963).Effects of visual deprivation on morphology and physiology of cells in cat LGN body. J. Neurophysiology Zheng, X., Wang, J., Haerry, T. E., Wu, A. Y., Martin, J., O'Connor, M. B., Lee, C. H., and Lee, T. (2003). TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell 112,
22 Table 1: Stages and corresponding events of NMJ formation during the life cycle of Drosophila DAY STAGE EVENT 1 Embryonic Formation of functional NMJ 2 1 st Larval instar Assembly/disassembly of synapses Muscles increase in size. 3 2 nd Larval instar rd Larval instar Assembly/disassembly of synapses Muscles increase in size. Assembly/disassembly of synapses Muscles increase in size (0h APF-96h APF*) *h APF : hours after puparium formation Metamorphosis Muscles histolyze; adult myogenesis Larval NMJs retract, outgrowth and elaboration of adult branches 10 Adult Flight and walking 16
23 Oh APF pre pupal stage Embryo Functional NMJ Larva NMJ expands as the muscle size increases PUPA (8h APF) Larval NMJs retract Persistent larval muscle surrounded by myoblasts 14-18h APF Motor neuronal outgrowth Electrical activity promotes outgrowth Arbor elaboration Muscle surface dictates motor neuronal elaboration FasII restricts elaboration 24h APF Primary branch pattern established FasII in a subset of branches Stabilization process underway; Futsch/22C10 in a subset of branches Pruning of second order branches Neural activity enhances pruning Glial processes are required for pruning/stabilization 38h APF Differentiation of neuromuscular contacts into terminals Terminal arbor expansion Maturation of DLM synapse Terminal Arbor expansion Adult Mature, Functional NMJ Figure 1: Overview of Drosophila neuromuscular development 17
24 Chapter 2 Pruning of motor neuron branches establishes the DLM innervation pattern in Drosophila 2.1 Abstract During the Drosophila life-cycle two sets of neuromuscular junctions are generated: the embryonic/larval NMJs develop during the first half, followed by the period of metamorphosis during which the adult counterpart is generated. Development of the adult innervation pattern is preceded by a withdrawal of larval NMJs, which occurs at the onset of metamorphosis, and is followed by adult-specific motor neuron outgrowth to innervate the newly developing adult fibers. Establishment of the adult innervation pattern occurs in the context of a broader restructuring of the nervous system, which results in the development of neural circuits that are necessary to carry out behaviors specific to the adult. Here, we follow development of the Dorsal Longitudinal Muscle innervation pattern through metamorphosis. We find that the initial period of motor neuron elaboration is followed by a phase of extensive pruning resulting in a three-fold reduction of neuromuscular contacts. This event establishes the adult pattern of second order branching. Subsequent higher order branching from the second order contact points generates the characteristic multi-terminal innervation pattern of the DLMs. Boutons begin to appear after the pruning phase, and are much smaller than their larval counterparts. Additionally, we demonstrate that the DLM innervation is altered in the hyperexcitable double mutant, ether a go-go, Shaker, and that the phenotype is suppressed by the hypoexcitable mutant, nap ts1. Our results demonstrate that electrical activity regulates the patterning of DLM innervation during metamorphosis. 2.2 Introduction During development of a nervous system, neurons must grow out and establish precise connections with post-synaptic targets in order to establish neural circuits necessary to control a wide variety of behaviors in the organism. It is a well-known fact that during embryonic development of 18
25 vertebrate nervous systems, several inappropriate connections are generated during the initial period of neuron outgrowth. These fall into two general categories- ectopically placed afferents or an excess number of afferents (Sanes et al., 2000). An estimated 50% of synaptic contacts in rats and a comparable number in humans are lost during development. During a critical post-natal period, addition or retraction of synaptic contacts occurs to generate the precise connectivity that is found in the mature organism. This remodeling serves to fine-tune the system such that the resulting network is then capable of precise co-ordination of sensory input and motor output. The best-known example in the vertebrate CNS is one that occurs during visual pathway formation, where remodeling results in the establishment of ocular dominance columns in the visual cortex (Goodman and Shatz, 1993). Here, the terminals of the lateral geniculate nucleus (LGN) neurons (that receive retinal ganglionic inputs) become localized in eye-specific zones. The retraction of existing synapses is also seen at the neuromuscular junction (NMJ), a peripheral synapse (Sanes and Lichtman, 1999). In this case, the polyneuronal innervation of neonatal muscle fibers is converted into the mature mononeuronal innervation. The terms refinement, pruning, and synapse elimination, have been used interchangeably to refer to this process of fine-tuning. Post-embryonic remodeling is the hallmark of metamorphosis in holometabolous insects such as Drosophila and the hawkmoth, Manduca sexta (Levine et al., 1995). In such animals, two distinct body forms are generated during the course of the life-cycle, each with its specific repertoire of behaviors. It is during metamorphosis that the larval nervous system is reorganized to generate an adult nervous system that will execute adult-specific behaviors such as walking, flight, and reproduction. Generation of the adult CNS in these insects is brought about by three main events: neurogenesis that mainly generates interneurons for the new neural circuits, selective death of some larval neurons and respecification of persistent larval motor neurons to innervate adult-specific muscle targets (Truman, 1990). A key feature of respecification is the retraction of larval neuronal processes, axonal and dendritic, followed by the outgrowth and elaboration of adult specific branches. Remodeling of branches is not restricted to motor neurons but is also seen in the mushroom bodies (Technau and Heisenberg, 1982) which are regions of higher-order processing in the brain and thought to be the seat of learning and memory. Studies on motor neuron reorganization during metamorphosis, as described in Manduca and Drosophila, have mainly focused on a morphological description of retraction of larval branches followed by the elaboration of adultspecific processes, mostly dendritic in Manduca, and NMJs in Drosophila (Consoulas et al., 2000). The studies that have attempted to detail subsequent maturation of the peripheral processes into synapses 19
26 have been conducted in Manduca (Consoulas et al., 1996; Knittel et al., 2001). Such studies have yet to be carried out for the neuromuscular system in Drosophila. The Dorsal Longitudinal Muscles (DLMs) of Drosophila are some of the largest muscles of the thorax. The muscles and their motor neurons are easily identifiable (Cogshall, 1978; Ikeda and Koenig, 1988) making it possible to monitor and manipulate the synaptic partners (Fernandes and Keshishian, 1999). Some aspects of neuromuscular development of the DLMs have been previously described (Fernandes et al., 1991; Farrell et al., 1996; Fernandes and Keshishian, 1996) and a brief summary is as follows. At the onset of metamorphosis, most larval bodywall muscles histolyze, to give way to the formation of adult muscle fibers. Some larval muscles persist, and among them are three larval fibers in the mesothorax (9, 10 and 19 ) that serve as fusion targets for DLM myoblasts. Fusion begins around 14h APF (hours after puparium formation), and fiber formation is well underway by 18h APF. Unlike in the embryo (Johansen et al., 1989b), where motor neurons contact muscle fibers after myogenesis is completed, during adult myogenesis, motor neurons make contact with developing fibers (Currie and Bate, 1991; Fernandes and VijayRaghavan, 1993). This allows for nerve-muscle interactions to shape the emerging neuromuscular pattern (Fernandes and Keshishian, 1998). Several aspects of the neuromuscular pattern are evident within the first 24 hours of metamorphosis including the number of adult fibers and the primary nerve branching pattern. In the current study, we have followed higher order (second and third order) branch development through metamorphosis and show that an initial exuberant outgrowth of neuromuscular contacts is followed by a phase of pruning to establish the mature adult pattern. This pruning process occurs during the first half of metamorphosis and is followed by bouton formation and expansion of the motor neuron arbor. Using mutants with altered membrane excitability we have shown that the patterning of DLM innervation is activity dependent. 2.3 Materials and Methods Fly strains: Oregon R raised on standard Drosophila food at 25 C was used as the wild type strain. A hyperexcitable double mutant, eag 1 Sh 120b was used for comparative studies. Both genes encode K + channel subunits. eag 1 preferentially removes I K current (Wu et al., 1983)while Sh 120b reduces I A current (Ganetzky and Wu, 1983). The double mutant combination synergistically increases nerve excitability and neurotransmitter release (Ganetzky and Wu, 1983). An additional double mutant combination eag 4PM Sh KS133 was also tested for adult branching. To test suppression of branching phenotypes seen in 20
27 eag 1 Sh 120b, a triple mutant combination was generated using the hypoactive mutant, nap ts1, which blocks action potentials (Wu et al., 1978). The suppression of hyperexcitable phenotypes is known to occur at permissive (25 C ) temperatures (Ganetzky and Wu, 1982a; Ganetzky and Wu, 1982b; Budnik et al., 1990; Engel and Wu, 1992). Staging and dissection: White prepupae (0h After Puparium Formation, APF) were collected and placed on moist filter paper on a petri dish. They were raised at 25 C to the following stages: 14h, 24h, 38 and 48h APF, dissected in insect saline, fixed in 4% paraformaldehyde (in PBS, Phosphate Buffered Saline) for 40 minutes and processed for immunocytochemistry. The 30-36h APF period was not amenable to analysis of innervation patterns due to shortening of muscles associated with the development of attachment sites (Fernandes et al., 1991; Reedy and Beall, 1993). Two day-old adults were bisected following anaesthetization on ice. The hemithoraces were fixed in 4% paraformaldehyde (in PBS) at 4 C for hours. Immunocytochemistry: Following fixation, all preparations were rinsed in phosphate buffered saline, washed with 0.3% Triton-X (TBS), blocked in 1 % solution of Bovine Serum Albumin (BSA, Sigma) made in TBS or in 5% Normal Goat Serum (Jackson Labs), and incubated overnight at 4 C in primary antibody. The following primary antibodies were used: goat anti-hrp (1:200, Jackson Labs), rabbit anti-synaptotagmin (1:500, dsyt2, gift from Troy Littleton) and MAb 22C10 (1:25, Hybridoma Bank, Iowa). After incubation in anti-hrp, the dissected tissue was washed in TBS and incubated with peroxidase-coupled secondary antibody (Cappel) for 2 hours at room temperature or overnight at 4 C. For labeling with dsyt2 and MAb 22C10, washed tissue was incubated in biotinylated secondary antibodies (Vector Labs) for 2 hours at room temperature, washed and incubated in ABC reagent (Vector Labs) according to manufacturer s recommendation % Diaminobenzidene (Sigma) was used to develop the color reaction in the presence of 0.003% H The tissue was then rinsed, dehydrated using an alcohol series, cleared in xylene and mounted in Permount. Adult hemithoraces were cleared in methyl salicylate and mounted in Canada balsam. All immunostained tissues were visualized with DIC optics on a Nikon E600 microscope. Data analysis: Camera Lucida drawings of anti-hrp stained preparations were used to illustrate the entire motor neuron branching pattern and to obtain qualitative information on branching patterns. Primary outgrowth at 14-24h APF was defined as the branches growing longitudinally along the length of the fiber. 21
28 Lengths of primary branches were determined from camera lucida drawings. Secondary branches were defined as the short processes off the primary branch, usually growing perpendicular to the primary branch (See inset in camera lucida drawing of 14h APF, Fig.1). Secondary processes at 14, 18, 24 and 38hAPF were quantified at a magnification of 600x. All statistical analyses were performed using the Minitab program (Minitab Inc., State College, PA; A two sample Student s t test was used to determine differences. Numbers represent mean ± S.E.M. Digital images were captured using Magnafire 2.0. For imaging motor neuron arbors and boutons, several focal planes were merged in Adobe Photoshop to arrive at the final image. 2.4 Results Outgrowth and elaboration of higher order branches is followed by pruning: 14-38h APF The six dorsal longitudinal muscle fibers (DLMs-a-f) develop from three persistent larval muscles in the mesothorax, 9, 10 and 19 that act as scaffolds (Fernandes et al., 1991). The dorsal- most pair of fibers, DLMs a and b, are innervated by a single motor neuron, MN5, while the remaining 4 fibers, DLMs c-f, are singly innervated by MNs 1-4 (Cogshall, 1978; Ikeda and Koenig, 1988). After the initial withdrawal of larval neuromuscular junctions (NMJs) at the onset of metamorphosis, adult-specific branches grow over the larval scaffolds (Fernandes and VijayRaghavan, 1993). At 14h APF, two primary branches (anteriorly and posteriorly directed) extend off the main nerve trunk and begin growing along the long axis of the muscle. Numerous secondary branches off the primary branches are also seen (Fig. 1, top and middle panels). By 18h APF, the primary branches have extended along the length of the muscles (Fig 1, top and middle panels) and additional secondary branches are present. Primary branches on most of the developing fibers (DLMs c-f) at this time usually consist of two fasciculated axons (Fernandes and VijayRaghavan, 1993), which begin to defasciculate by 24hAPF. By 38h APF (Fig 1, top panel), the defasciculation process has progressed proximally (toward CNS) such that a single nerve trunk is no longer evident in the region of the DLMs. This is particularly evident for the branches that innervate DLMs a and b, the most dorsal pair of fibers. As a result, the primary branches no longer lie along the length of the muscle fiber, and we refer to the secondary branches as contact points. To assess higher order branch development, we first counted the number of secondary branches from 14h-38h APF, and compared it to the adult pattern (Table 1; Figure 2). We focused on DLMs a and b, which are innervated by MN5. Secondary branch number significantly increases from 22.6 ± 1.0 at 14h APF to 27.2 ± 1.0 at 18h APF (p<0.004), accounting for a 20% increase. By 24h APF, the number of 22
29 secondary branches, 37.4 ± 1.4, increases significantly relative to 18h APF (p<0.0001), accounting for a 37% increase. However, by 38h APF, there is a significant reduction (a little over 3-fold) in the numbers of second order branches (10.87 ± 0.2; p<0.0001). This reduction in the number of motor neuron processes after an initial period of exuberant growth is a phenomenon characteristic to vertebrates and has not been previously reported for Drosophila or any invertebrate NMJ. We refer to this reduction of secondary branches as pruning. The number of contact points seen at 38h APF are not statistically different from those found on the adult fibers (Fig 2; Table 1). Taken together these data indicate that pruning occurs between 24h and 38h APF, and that this process sets up the adult pattern of second order branching. Another aspect of motor neuron development that we have examined is the appearance of tertiary branches (Fig. 2). These branches are first seen as early as 14h APF; they continue to increase steadily during the elaboration phase and we have used their presence as an indicator of the extent of elaboration. At 14h APF, 16% of secondary branches show tertiary processes and this number increases by 18h APF. By 24h APF, when the number of secondary branches is maximal, 60% of secondary contacts exhibit tertiary processes. By 38h APF, when the secondary branch pattern resembles the adult, all second order branches (contact points) have tertiary processes. The adult branching pattern and the neuromuscular junction In order to better understand how motor neuron elaboration during metamorphosis results in the appearance of morphologically identifiable terminals, we first examined anti-hrp labeled junctions in bisected thoraces. Most of our observations are made on the dorsal pair of DLMs a&b. Each contact point (secondary branch) has a flattened base where it makes contact with the proximal edge of a muscle fiber (Fig. 3A). This morphological feature is evident as early as 38h APF. Tertiary branches radiate from these points and project across the width of the fiber (Fig. 3A-C). These branches along with their higher order branches comprise a single terminal (or unit) of the adult neuromuscular junction. The DLM (and DVM) fibers are cylindrical, and motor neuron branches run circumferentially around the muscle. At 38h APF, when muscles are one-third of their final size, higher order branches are present only at muscle surface closest to the nerve entry point (Fig. 1, upper panel; also see Fig. 5). Higher order branches may traverse the circumference of the muscle fiber or they may elongate along the length of the muscle. In both cases, they extend below the muscle surface and make embedded contacts between myofibers. These contacts can be visualized as punctate HRP-immunoreactivity along the muscle length (Fig. 3), and have been previously described for the DLMs using silver-staining techniques on tissue sections (Ikeda et al., 1980). A 23
30 recent study has demonstrated expression of the sodium channel, DSC1, in a similar pattern, suggesting that the embedded contacts are neuronal in origin (Castella et al., 2001). Both DLMs and DVMs, the second group of indirect flight muscle, exhibit such contacts that are present at a maximum depth of 20-25µm below the muscle surface (the muscle diameter is about 70 µm). Terminal varicosities or boutons are seen on tertiary and higher order branches. DVM and DLM boutons are similar in morphology (Fig. 4A,B), and are of varying sizes. They range from µm in diameter. Most of the boutons are small in size, with a few large ones interspersed. The smallest boutons are smaller than Type II larval boutons, which have an average size of 1.4µm (Johansen et al., 1989a), while the largest boutons are not more than 1.7µm in diameter. When observed using anti-synaptotagmin (SYT), the labeling is concentrated in a smaller area than the anti-hrp label (compare Fig 4C and C ), and this is because SYT is associated with vesicles (Littleton et al., 1993) while HRP is localized to membranes (Jan and Jan, 1982). Boutons on the leg and abdominal muscles (Fig. 4C, D) are similar in size to the larger, Type I larval boutons, which have an average size of 3.1µm. During development, large bouton-like structures are present in a node like fashion along a single branch at each terminal and may represent branch points. They can be seen at the abdominal muscles (Fig. 4E) as well as at the DLM (Fig. 4F). Differentiation of motor neuron branches into terminal varicosities Our studies have shown that neuromuscular elaboration is maximal at 24h APF and is followed by a phase of pruning, which generates the adult complement of second order nerve branching. Interestingly, boutons are rarely observed during branch outgrowth and elaboration, but begin to appear by 38h APF, following the pruning process. At this stage, the boutons have a barely discernible punctate morphology (Fig. 5, WT) and range in size from µm. As development progresses, there is an associated increase in the punctate appearance of the tertiary branches, (Fig. 5), suggesting an increase in bouton number and size. The estimated bouton sizes at 48h APF are in the range of µm. These include the larger boutons (Fig. 5; inset). They are seen in the adult, but less frequently (Fig 4). Expression of synaptotagmin during motor neuron outgrowth Pruning or synapse elimination is known to occur at the vertebrate NMJ and in the brain, where it involves the removal of already formed synapses. This raises the question of whether the motor neuron elaborations seen on the DLM fibers prior to pruning and prior to the appearance of any terminal structures (boutons/varicosities) are some form of nascent or immature synapses. We, therefore, followed the 24
31 expression of synaptotagmin, a well-known synaptic marker, through the stages of branch elaboration and pruning (Fig 1, lower panel). Our results indicate that this synaptic marker is present at neuromuscular contacts as early as 18h APF. At 14h APF, when branch elaboration has just begun, the immunoreactivity is punctate and is present in primary branches where it is confined to a region closest to the nerve trunk. By 18h APF, as primary branches extend along the length of the developing fiber, synaptotagmin is seen all along the primary branches and begins to appear in secondary branches. By 24h APF, synaptotagmin continues to be localized to secondary branches and can be better visualized. By 38h APF, when pruning is complete, synaptotagmin is no longer present in primary nerve branches, and punctate expression can be visualized near the proximal edge of the DLM fibers. This expression is likely to be present in the first forming boutons which are barely discernible at this stage. By 48h APF, when the higher order branches are expanding over the muscle surface, synaptotagmin is present in the boutons (Fig. 5), thus defining the higher order expanse seen with anti-hrp as being a nascent NMJ. At the adult NMJ, synaptotagmin immunoreactivity (Fig 4B ) reveals a delicate array of boutons, unlike the leg muscles, which have larger boutons (Fig. 4C ) and are arrayed in a manner that resembles the larval organization (4C). Motor neuron branching and pruning are altered in hyperexcitable mutant, eag 1 Sh 120 In order to investigate a possible role for electrical activity in shaping arbor development, a hyperexcitable double mutant, eag 1 Sh 120b was examined at stages that were identified to be key events in the patterning of DLM motor neuron outgrowth. Both genes encode K + channel subunits. While eag 1 preferentially removes I K current and Sh 120b reduces I A current, the double mutant combination synergistically increases nerve excitability and neurotransmitter release (Ganetzky and Wu, 1983). Motor neuron outgrowth and elaboration (14-24h APF): At 14h APF, as primary branches begin to grow along the muscle, there is a 30% increase in second order branches as compared to the wild-type (Table 1). During the subsequent phase of continued outgrowth and elaboration (14-24h APF) the double mutant displayed significant increases in the number of secondary branches as compared to wild type (Fig 2, 6; Table 1). At 18 and 24h APF, there is an increased density of second order branching when compared with the wild-type (p<0.05). The extent of motor neuron elaboration as determined by the number of secondary branches that exhibited tertiary processes, also increases during 14-24h APF (Fig. 2). Interestingly, the rate of development of secondary branches is similar in wild-type and mutant during 14-24h APF (Fig. 2; Table 1). Between 14h APF and 18h APF, secondary branches increase by 20% and 23% in the wild-type and in 25
32 the mutant respectively. Between 18h APF and 24h APF, the increase is 37% in the wild-type and 28% in the mutant. Muscle morphologies can be revealed using 22C10 (Fernandes and VijayRaghavan, 1993). We examined the 18h APF and 24h APF stages to rule out the possibility that pupal development is simply advanced in the mutant. At 18h APF, myoblast fusion initiates splitting of the larval scaffolds (Fernandes and Keshishian, 1998; Roy and VijayRaghavan, 1998) and by 24h APF, the six DLM fibers are evident. We observed no difference in the timing of muscle development between the mutant and wild-type (Fig. 7). Further, comparison of muscle areas for DLMs a and b showed no significant differences between mutant and wild-type (data not shown). Motor neuron pruning (24h-38h): Another striking feature of the development of innervation in eag 1 Sh 120 is the significant reduction in number of contact points compared to wild-type. Also, pruning is more dramatic when considering the reduction in second order branches on DLMs a and b between 24h and 38h APF (Fig. 2, Table 1). In the wild-type, pruning results in a three-fold decrease (from 24h to 38h), whereas in mutants we observe a six- fold reduction (Table 1). Bouton formation: Another difference between the mutant and wild-type is in the timing of bouton formation (Fig. 5). While boutons are not clearly visible at 38h APF in the wild-type, they are well defined at this time in the mutant (0.4µm-1.1µm). In addition, there are many more of the larger-sized boutons. At 48h APF, bouton sizes range from 0.7µm- 1µm. Interestingly, adult boutons in the mutant appear to be similar in size to the wild-type (0.4µm-1.6µm). Adult: In the 2-day old adult, the number of contact points is significantly reduced as compared to the wildtype (Figure 2, Table 1; p<0.0001). We also examined a different allelic combination, eag 4PM Sh KS133, and found that the number of contact points on DLMa (4.1 ± 0.16; n=20) were not significantly different from eag 1 Sh 120b (4.3 ± 0.16; n=26). Does the altered number of terminals have a bearing on the morphology of each arbor/terminal? It is possible that higher order (tertiary and fourth order) branch lengths and/numbers are increased, reduced, or unchanged as compared to wild-type. In order to address this, it was necessary to examine the expanse of the unit NMJ in the adult. Since branching of the terminal arbor is not stereotypic, it was difficult to assess the extent to which this attribute is altered in mutants. Also, since some higher order branches extend below the muscle surface and come to lie in between myofibers, and 26
33 others wrap around the muscle fiber, it is not possible to get accurate counts of branch tips as a measure of expanse. A qualitative examination of the unit NMJ (branching at single contact point; Fig. 3) did not reveal an obvious difference in higher order branching. Bouton sizes were not altered, although during the pupal phase, the mutant displayed larger boutons that wild-type. Since the adult boutons are very small in size (0.4µm-1.1µm) it is not possible to reliably count bouton numbers as a measure of NMJ expanse. Interestingly, muscle length in the mutants is significantly reduced at the adult stage (WT: 559µm ± 12, eag 1 Sh 120b : 493µm ± 11, p<0.001). We next examined why fewer contact points were present in the mutant and focused on the branching patterns on DLMa. (Table 2). In the wild-type, DLM a receives more contact points than the mutant (WT: 5.2± 0.14; eag 1 Sh 120b : 4.3± 0.16; p<0.0001). DLMs a and b are innervated by the central dorsal branch of the posterior dorsal mesothoracic nerve (PDMN) which has three additional branches (Ikeda et al., 1980). We refer to these branches as the anterior branch A, the posterior branch B, and the medial branch C (Table 2). The medial branch usually comes off branch A (73%), and in the remainder of cases (27%), it comes off branch B. In the eag 1 Sh 120b mutants, 50% of thoraces examined (n=16) had no medial branch, which partially explains the reduction in number of contact points seen on DLMa. In animals with a medial branch, the average number of contact points made on DLMa by branches A and B is significantly reduced (Table 2). Thus, the reduction in number of contact points is only partly due to absence of the medial branch that normally innervates DLMs a. nap ts1 suppresses the adult DLM innervation patterns of eag 1 Sh 120b An alteration in the number of contact points (or terminals) in eag 1 Sh 120b suggests a role for electrical activity in establishing the DLM innervation pattern. Previous studies (Budnik et al., 1990) on the larval NMJ have demonstrated that altered innervation patterns seen in hyperexcitable mutants are rescued by generating a triple combination (eag 1 Sh 120b ;nap ts1 ). The mutation in nap ts1 reduces I Na by decreasing the expression of para, the structural gene for a sodium channel (Loughney et al., 1989). nap ts1 is known to suppress several phenotypes of hyperexcitable mutants such as leg shaking (Ganetzky and Wu, 1982b) abnormal wing positioning (Stern et al., 1990), motor neuronal activity (Ganetzky and Wu, 1982a; Engel and Wu, 1992) and morphology of larval NMJs (Budnik et al., 1990). We generated the triple mutant combination to determine if DLM innervation patterns were similarly suppressed. Prior to testing innervation phenotypes, we confirmed that the leg shaking and wing posture phenotypes were suppressed in the triple mutant (Table 3). Leg shaking behavior showed a complete suppression, while the wing phenotype was 27
34 suppressed in a majority (87%) of animals. With respect to eag 1 Sh 120b, the mean number of contact points or terminals on DLMa showed a statistically significant increase (Table 3, ; p<0.05). These results confirm that the DLM innervation phenotypes seen in hyperexcitable mutants are a result of increased membrane excitability. To provide another means of demonstrating the suppression of innervation phenotypes by nap ts1 we also analyzed the distribution of absolute numbers of contact points or terminals in each animal (Table 3). In wild-type animals, 89% of animals show 5-6 contact points, whereas only 30% of eag 1 Sh 120b animals display 5-6 contact points. In the triple mutant, this number increases to 62%, which tends toward the wildtype phenotype (89%). Interestingly, 100% of nap ts1 animals display 5-6 contact points, which is slightly higher than the wildtype, a trend that mimics observations at the larval NMJ (Budnik et al., 1990). 2.5 Discussion Some distinctive features of DLM innervation Drosophila makes two sets of muscles, an embryonic set which continues to grow in size through the larval instars and an adult set which is generated during metamorphosis, soon after the larval set is histolyzed. Innervation of larval muscles and most adult abdominal muscles is similar- upon arriving at its muscle target, the motor neuron elaborates stereotypically arranged branches that bear terminal varicosities (Johansen et al., 1989a; Currie and Bate, 1991; Currie and Bate, 1995). Unlike the single terminal that makes up the larval/adult abdominal NMJ, the DLMs exhibit a multi-terminal innervation pattern (this study). Motor axons run along the length of each muscle fiber, and make transverse contacts or secondary contact points. From each secondary contact point a branched terminal emerges, whose expanse includes branches that run along the long axis of the myofibers and also those that run circumferentially, around the muscle fiber. On average, each DLM fiber has five branched terminals. DLM innervation is established during metamorphosis (summarized in Fig. 8) and occurs within the context of nervous system restructuring, which includes selective death of larval neurons, generation of adult-specific neurons and remodeling of persistent larval motor neurons (Truman, 1990; Levine et al., 1995; Consoulas et al., 2000). Nervous system restructuring is a prominent feature of insects that undergo a complete metamorphosis. Here, two distinct body forms have specific motor behaviors, and most adult motor neurons have their origins in larval motor neurons that are respecified during metamorphosis. The respecification includes withdrawal of larval branches and/synapses in the CNS and in the periphery, followed by an outgrowth of adult specific branches. In addition, the motor neurons connect with newly 28
35 generated interneurons and become incorporated into adult-specific circuits. The six DLM fibers are innervated by five motor neurons (Cogshall, 1978; Ikeda and Koenig, 1988). DLMs c-d are singly innervated by MNs1-4. The dorsal most pair of DLMs, a and b, which this study focuses on, are innervated by MN5. Recently, it has been shown that although MN5 has an embryonic origin like most motorneurons, it does not have a larval muscle target, and its axon arrives in the vicinity of the DLMs around 4-5h APF, much prior to the onset of longitudinal growth along the developing muscle fibers (Consoulas et al., 2002). Pruning of second order branches at the DLMs The period of motor neuron outgrowth and elaboration (14h-24h APF) on the DLMs is followed by a 3-fold reduction of second order branches. We refer to this reduction of adult-specific branch outgrowth as pruning, which must be distinguished from the earlier withdrawal of larval NMJs at the onset of metamorphosis (Fernandes and VijayRaghavan, 1993). Our observations of pruning at the level of new adult specific branches reveal an additional level of synaptic plasticity that is the hallmark of nervous system restructuring during metamorphosis. Pruning or the withdrawal of exuberant processes is a common mechanism to fine-tune a developing nervous system. In the CNS it is seen at the level of axons and dendrites in both vertebrates and invertebrates. For example, cortical visual neurons (O'Leary and Stanfield, 1986) and specific hippocampal mossy fibers and pyramidal axons (Bagri et al., 2003) undergo pruning of those projections that are transient or temporary. During insect metamorphosis, mushroom body neurons undergo axonal as well as dendritic pruning of larval processes prior to the outgrowth of adult-specific processes (Technau and Heisenberg, 1982; Lee et al., 2000). Pruning also occurs at neuromuscular junctions and the term synapse elimination is used to refer to the resizing of terminal arbors as seen in vertebrates (Bernstein and Lichtman, 1999). Pruning in this case is followed by an increase in the expanse of the terminal arbor. Interestingly, synapse elimination eventually results in the removal of axonal branches. Why does pruning occur during the establishment of DLM innervation? We consider two possibilities- first that pruning removes motor neuron contacts that arise from non-native motor neurons and second, that pruning maximizes expanse of the native motor neuron arbor. Pruning as a possible mechanism to match pre- and post-synaptic cells: At the vertebrate NMJ, each muscle fiber is multiply innervated at birth, and pruning results in a mononeuronal innervation within two weeks, thereby achieving a one-to-one matching of pre- and post-synaptic cells (Sanes and Lichtman, 29
36 1999). Similarly, it is possible that the initial exuberant outgrowth at the DLM includes native as well as nonnative motor neuron branches, and that pruning serves to match correct pre- and postsynaptic partners. According to this model, each muscle would later become singly innervated as a result of pruning. Experimental manipulations have shown that DLM muscle fibers can attract inputs from non-native motor neurons (Fernandes and Keshishian, 1998). The six DLMs develop from three persistent larval muscles that act as scaffolds for myogenesis (Fernandes et al., 1991). When the larval muscle scaffold for DLMs a and b is ablated at the onset of the pupal phase, muscle development is delayed or abolished (Fernandes and Keshishian, 1998). Under these circumstances, MN5 can establish contacts with the neighboring DLMc. However, when a muscle does eventually develop in the region of DLMs a and b, MN5 directs most of its arbor to the de novo developing fiber, thus exhibiting target specificity. Given the capability of MN5 to explore and to exhibit target specificity, it is not unreasonable to hypothesize that during the initial stages of outgrowth and elaboration, motor neurons may be promiscuous, and that the later event of pruning serves to match pre- and post-synaptic cells. Pruning as a means of maximizing expanse on the target: The pattern of innervation on the DLM is referred to as multiterminal (reviewed in Hoyle, 1983). Here, a single motor neuron makes several contacts with the muscle, each contact branching further into progressively finer processes. The multi-terminal innervation of the DLM makes it possible for local depolarizations to occur almost simultaneously across the entire length of the large muscle. The large fibers are known to be isopotential in nature (Salkoff and Wyman, 1983). It is conceivable that the purpose of pruning is to reduce the initial exuberance of neuronal contacts from a single motor neuron in order to ensure maximal expanse of each terminal on a DLM fiber. Interestingly, the exuberance of outgrowth coincides with a nerve dependent phase of myoblast proliferation, which is necessary to generate an appropriately sized myoblast pool for DLM myogenesis (Fernandes and Keshishian, 1998; Fernandes and Keshishian, 2005). Pruning is evident at a time when the muscle fiber is only one-third of its final size, having just completed the period of myogenesis. It is during the remaining 2.5 days of metamorphosis, that the muscle reaches its final size, accompanied by expansion of the third and higher order branches and their differentiation into terminal varicosities or boutons. Thus, an earlier reduction in the number of secondary contact points can allow for continued higher order branching to generate the combined expanse of the adult terminals. 30
37 From neuromuscular contact to bouton It is interesting to note that terminal varicosities are not seen prior to pruning and only those branches that are stabilized as a result of pruning, eventually harbor morphologically identifiable boutons. Because pruning in our case does not involve resizing of the terminal arbor and the associated removal of terminal varicosities as it does in the case of vertebrate skeletal muscles (Sanes and Lichtman, 1999), it raises questions regarding the differentiated state of motor neuron arbors present on the developing adult muscle surface prior to pruning. Are these second order branches (or neuromuscular contacts) some form of nascent synapses that are later stabilized or eliminated? It is not necessary that a newly arising synapse display mature morphological characteristics. In the early Drosophila embryo, the first motor neuron contacts do not exhibit characteristic mature bouton morphology- they appear as swellings or prevaricosities that contain synaptotagmin and which later give rise to the first boutons (Broadie and Bate, 1993; Yoshihara et al., 1997). The presence of synaptotagmin (SYT) immunoreactivity in the DLM motor neuron branches prior to pruning, may suggest that nascent synapses are present. When boutons make an appearance after pruning, synaptotagmin reactivity which was previously found along the primary axon and higher order branches becomes localized to the boutons. Our preliminary studies on the developmental expression of another synaptic marker, discs-large (DLG) indicate that this protein is not present in motor neuron branches prior to pruning. The developmental localization of SYT follows a pattern similar to that described for NMJs of leg muscles in Manduca (Consoulas et al., 1996). Studies in Manduca have also demonstrated that pre-synaptic release sites coincide with synaptotagmin immunoreactivity and that transient sites of release present during an earlier phase of neuronal outgrowth are later replaced by new sites, to parallel muscle growth (Consoulas and Levine, 1998). Bouton arrangements at the adult DLMs as well as the DVMs are not as distinct as the terminal varicosities seen at larval NMJs. The arrangement of boutons at the larval NMJ is characteristic to each muscle fiber (Johansen et al., 1989a). By contrast, the arrangement of boutons at the DLMs and DVMs is not ordered. The stereotypy exists at the level of the number of terminals on each adult DLM fiber. The layout of the DLM boutons bears more of a resemblance to en passant synapses seen in the vertebrate CNS. The adult boutons differ from their larval counterparts in other respects. The smaller boutons range from µm, with an average size of 0.8µm. This is smaller than the Type II boutons found in larvae (average size: 1.4µm). The larger boutons, which are less abundant, do not exceed 1.7µm, which is smaller than the average size of the Type I larval boutons (3.1µm). The Type I and Type II boutons at the 31
38 larval NMJ arise form separate motor neurons (Hoang and Chiba, 2001). Since each DLM fiber is innervated by a single motor neuron, we can conclude that both the large and small sized boutons arise from the same motor neuron. Consistently, the large and small boutons are intermingled, unlike in the larvae, where Type I and Type II boutons are on separate processes or branches. Leg and abdominal muscles on the other hand, have boutons that resemble their counterparts in the larva. Thus it is likely that developmental events such as pruning that set up the DLM pattern of innervation are specific to the larger muscles. We do not know if pruning also sets up innervation patterns of the DVM and the TDT, two other groups of large muscle fibers in the thorax. A role for electrical activity in the development of DLM innervation In examining the development of DLM innervation in the hyperexcitable mutant eag 1 Sh 120b, we find that motor neuron outgrowth, pruning and bouton formation are altered. The genes encode potassium channel subunits, which are expressed in both muscle and neurons. This raises two important questions about the involvement of electrical activity in shaping the DLM innervation pattern. (1) Are both muscle and neuron electrically active during the establishment of DLM innervation? (2) Is electrical activity required in the muscle or in the neuron, or in both? Our present knowledge of neuromuscular development allows us to consider some interesting possibilities. Motor neuron outgrowth and elaboration: The hyperexcitable mutant, eag 1 Sh 120b exhibits an increase in secondary and tertiary branches, a phenotype that parallels the excessive branching seen at the larval stages (Budnik et al., 1990). The excessive branching in pupae is seen as early as 14h APF, at which time the larval muscle scaffolds have dedifferentiated and myoblast fusions are yet to begin (Fernandes et al., 1991). We have evidence that development is not simply hastened in the mutant. Taken together, a role for muscle activity in the outgrowth of secondary branches can be ruled out. It is therefore likely that branching in the mutant is initiated earlier than in the wild-type due to the hyperactive nature of the motor neurons. In support of this hypothesis, a recent study in Manduca has demonstrated that motor neuron outgrowth on the developing DLM is accompanied by characteristic ecdysis motor patterns. During the preceeding period of NMJ withdrawal, the motor neurons are silent. Extracellular stimulation during this silent period with ecdysis-like patterns caused a significant advancement of new outgrowth (Duch and Mentel, 2003). At the developing DLMs, there are increased numbers of secondary branches between 14-24hAPF. However since the number of second order branches increases at the same rate in both the wild type and the mutant 32
39 during this period, we are cautious in declaring addition of secondary branches during this phase to be a direct consequence of hyperactivity. In studies that manipulated hormonal levels in Manduca, outgrowth of primary branches along the muscle length was affected and not elaboration (secondary branches in our case), which is thought to be regulated by properties of the muscle surface (Truman and Reiss, 1995). In experimental manipulations that delayed the development of DLM fibers in Drosophila, it was seen that longitudinal outgrowth could occur in the absence of muscle, but higher order branches did not elaborate unless a muscle fiber was present (Fernandes and Keshishian, 1998). Since myogenesis is well underway during the 14-24h period, it is unlikely that electrical membrane properties are in place. Thus, any contribution of the muscle in regulating neuronal branching is not due to membrane excitability of the muscle, but perhaps brought about by molecules such as cell adhesion molecules. It is well established that altering expression of neural recognition molecules such as Fasciclin II in the Drosophila embryo can alter branching (reviewed in Chiba and Keshishian, 1996) Motor neuron pruning: Development of the frog neuromuscular junction is the best example of activity dependent synapse elimination. Blocking electrical activity is known to prolong synapse elimination/refinement, whereas increased stimulation accelerates the process (Thompson et al., 1979; Thompson, 1983). Is the pruning of DLM innervation similarly dependent on electrical activity? Our observations of pruning in the hyperexcitable mutant eag 1 Sh 120b indicate that this is likely to be the case. Pruning occurs between 24 and 38h APF and involves a 3-fold reduction in branch number. In the mutant, we find that the reduction is a little over 6-fold (Table 1). This is similar to the scenario at the frog NMJ. A role for neuronal activity in shaping DLM innervation: In our case, we must again consider if the muscle and/or neuronal activity plays a role during pruning. Several significant developmental events occur during the period of pruning (24-38h APF). First, myogenesis is completed (by 30h APF) and myofibers begin to emerge (Fernandes et al., 1991). Second, voltage gated channels on the muscles develop only later at 55hAPF (Salkoff and Wyman, 1981a; Salkoff and Wyman, 1981b; Salkoff and Wyman, 1983). Together, these data suggest that muscle activity is an unlikely influence on the pruning of secondary branches between 24-38hAPF. We are left with the possibility that neuronal activity is an important player in the pruning of secondary branches. Our experiments with the triple mutant provide evidence for this hypothesis. eag 1 Sh 120b exhibits reduced number of terminals on the DLM (Table 3), and this phenotype is suppressed by nap ts1. The suppression cannot be due to muscle activity as the DLM has no I Na component 33
40 and uses Ic a instead (Salkoff and Wyman, 1983), which comes on just prior to eclosion. Thus, the suppression of eag 1 Sh 120b phenotypes by nap ts1 must occur presynaptically. A role for neuronal activity in shaping DLM innervation during the pruning phase must be considered in the context of developmental events in the CNS. It is during this period that the giant fiber (GF) escape circuit is being established (Hummon and Costello, 1988; Allen et al., 1999; Allen et al., 2000). A significant connection that develops during this time period involves the PS interneuron that connects the giant fiber interneurons in the central brain with the DLM neurons in the thoracic ganglion. Another major morphological change that occurs with respect to MN5 is that size of the motor neuron soma and the dendritic arbor undergo a dramatic increase between 25-40hAPF (Consoulas et al., 2002), implicating an increased surface area for receiving neuronal inputs. Given that establishment of the GF circuit and occurrence of pruning occur in close temporal proximity, it is tempting to speculate that pre-synaptic activity may influence pruning. Thus, it may well be that at the earlier stage of secondary branch outgrowth, spontaneous neuronal activity of the DLM motor neurons regulates motor neuron branching and that at a later stage, branch pruning is regulated by a recently developed GF circuit, as well as other synaptic inputs. After 55h APF, when muscle activity has previously been recorded (Salkoff and Wyman, 1983), correlated activity between the neuron and muscle may shape further expansion of the terminal arbor on the DLMs. Our future studies will investigate these possible scenarios. Consequences of hyperactivity on attributes of the terminal arbor In eagsh mutants, there is a direct correlation between hyper-excitability and NMJ expanse (Budnik et al., 1990). Although a similar correlation occurs during the outgrowth and elaboration phase in the pupa, there isn t an increase in branching or synapse size at the adult stage. There are some instances where a correlation between morphology and hyperexcitability does not occur (Featherstone and Broadie, 2000). For example, Frequenin is a Drosophila calcium binding protein whose over expression causes functional abnormalities similar to eag 1 Sh 120b. However, these mutants have reduced NMJ size (Angaut- Petit et al., 1998). During the development of DLM innervation in the hyperexcitable mutant, increased pruning results in fewer terminals at the adult fibers in comparison to the wild-type. However, unlike in the larva, the adult terminals do not increase in expanse. Interestingly, the length of muscle fibers in mutants is significantly reduced as compared to wild-type. Since terminals are spaced out along the DLM fibers to ensure rapid local depolarization, it is possible that subsequent to pruning, the pre- and post-synaptic cell communicate 34
41 to ensure appropriate expansion of individual terminals, so as to achieve isopotential characteristics of the adult fibers. The occurrence of such homeostatic mechanisms that involve pre- and post-synaptic communication has been well documented for the larval neuromuscular junction in Drosophila (Davis and Goodman, 1998). Most studies on synapse formation and maturation in Drosophila have focused on the embryonic and the larval system (Budnik and Gramates, 1999) while development of the adult synapse remains to be explored in the same detail. Our studies establish the use of the DLM as an adult motor system to address questions of synaptic plasticity during post-embryonic development. We demonstrated that the occurrence of developmental pruning in the periphery is an important aspect of patterning, and show that it is an additional example of the synaptic plasticity, which is a hallmark of the restructuring nervous system. By using hyper- and hypoexcitable mutants, we have also shown that DLM innervation is patterned by electrical activity. Further studies will be aimed at understanding the electrophysiological basis for the process, to dissect the role of electrical excitability in individual synaptic partners and to investigate if, as in vertebrates, pruning occurs to cause a polyneuronally innervated muscle fiber to become mononeuronally innervated. 35
42 2.6 References Allen MJ, Shan X, Caruccio P, Froggett SJ, Moffat KG, Murphey RK Targeted expression of truncated glued disrupts giant fiber synapse formation in Drosophila. J Neurosci 19: Allen MJ, Shan X, Murphey RK A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. Mol Cell Neurosci 16: Angaut-Petit D, Toth P, Rogero O, Faille L, Tejedor FJ, Ferrus A Enhanced neurotransmitter release is associated with reduction of neuronal branching in a Drosophila mutant overexpressing frequenin. Eur J Neurosci 10: Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell 113: Bernstein M, Lichtman JW Axonal atrophy: the retraction reaction. Curr Opin Neurobiol 9: Broadie KS, Bate M Development of the embryonic neuromuscular synapse of Drosophila melanogaster. J Neurosci 13: Budnik V, Gramates S Neuromuscular Development of Drosophila. International review of Neurobiology 44. Budnik V, Zhong Y, Wu CF Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci 10: Castella C, Amichot M, Berge JB, Pauron D DSC1 channels are expressed in both the central and the peripheral nervous system of adult Drosophila melanogaster. Invert Neurosci 4: Chiba A, Keshishian H Neuronal pathfinding and recognition: roles of cell adhesion molecules. Dev Biol 180: Cogshall J Neurons associated with the Dorsal Longitudinal flight muscles of Drosophila melanogaster. J. Comp. Neurol. 177: Consoulas C, Duch C, Bayline RJ, Levine RB Behavioral transformations during metamorphosis: remodeling of neural and motor systems. Brain Res Bull 53: Consoulas C, Kent KS, Levine RB Remodeling of the peripheral processes and presynaptic terminals of leg motoneurons during metamorphosis of the hawkmoth, Manduca sexta. J Comp Neurol 372: Consoulas C, Levine RB Presynaptic function during muscle remodeling in insect metamorphosis. J Neurosci 18: Consoulas C, Restifo LL, Levine RB Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. J Neurosci 22: Currie DA, Bate M The development of adult abdominal muscles in Drosophila: myoblasts express twist and are associated with nerves. Development 113: Currie DA, Bate M Innervation is essential for the development and differentiation of a sex-specific adult muscle in Drosophila melanogaster. Development 121: Davis GW, Goodman CS Genetic analysis of synaptic development and plasticity: homeostatic regulation of synaptic efficacy. Curr Opin Neurobiol 8: Duch C, Mentel T Stage-specific activity patterns affect motoneuron axonal retraction and outgrowth during the metamorphosis of Manduca sexta. Eur J Neurosci 17: Engel JE, Wu CF Interactions of membrane excitability mutations affecting potassium and sodium currents in the flight and giant fiber escape systems of Drosophila. J Comp Physiol [A] 171: Farrell ER, Fernandes J, Keshishian H Muscle organizers in Drosophila: the role of persistent larval fibers in adult flight muscle development. Dev Biol 176:
43 Featherstone DE, Broadie K Surprises from Drosophila: genetic mechanisms of synaptic development and plasticity. Brain Res Bull 53: Fernandes J, Bate M, Vijayraghavan K Development of the indirect flight muscles of Drosophila. Development 113: Fernandes J, VijayRaghavan K The development of indirect flight muscle innervationin Drosophila melanogaster. Development 118: Fernandes JJ, Keshishian H Patterning the dorsal longitudinal flight muscles (DLM) of Drosophila: insights from the ablation of larval scaffolds. Development 122: Fernandes JJ, Keshishian H Nerve-muscle interactions during flight muscle development in Drosophila. Development 125: Fernandes JJ, Keshishian H Development of the adult neuromuscular system. Int Rev Neurobiol 43: Ganetzky B, Wu C. 1982b. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics 100: Ganetzky B, Wu CF. 1982a. Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J Neurophysiol 47: Ganetzky B, Wu CF Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet 1: Goodman CS, Shatz CJ Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell 72 Suppl: Hoang B, Chiba A Single-cell analysis of Drosophila neuromuscular synapses. Developmental Biology 229: Hoyle G Muscles and their neural control. New York: John Wiley. Hummon MR, Costello WJ Induced neuroma formation and target muscle perturbation in the giant fiber pathway of the Drosophila temperature-sensitive mutant, shibire. Roux's Archives of Developmental Biology 197: Ikeda K, Koenig JH Morphological identification of the motor neurons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Comp Neurol 273: Ikeda K, Koenig JH, Tsuruhara T Organization of identified axons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Neurocytol 9: Jan LY, Jan YN Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc Natl Acad Sci U S A 79: Johansen J, Halpern ME, Johansen KM, Keshishian H. 1989a. Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J Neurosci 9: Johansen J, Halpern ME, Keshishian H. 1989b. Axonal guidance and the development of muscle fiberspecific innervation in Drosophila embryos. J Neurosci 9: Knittel LM, Copenhaver PF, Kent KS Remodeling of motor terminals during metamorphosis of the moth Manduca sexta: expression patterns of two distinct isoforms of Manduca fasciclin II. J Comp Neurol 434: Lee T, Marticke S, Sung C, Robinow S, Luo L Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron 28: Levine RB, Morton DB, Restifo LL Remodeling of the insect nervous system. Curr Opin Neurobiol 5: Littleton JT, Bellen HJ, Perin MS Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development 118: Loughney K, Kreber R, Ganetzky B Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell 58:
44 O'Leary DD, Stanfield BB A transient pyramidal tract projection from the visual cortex in the hamster and its removal by selective collateral elimination. Brain Res 392: Reedy MC, Beall C Ultrastructure of developing flight muscle in Drosophila. I. Assembly of myofibrils. Dev Biol 160: Roy S, VijayRaghavan K Patterning muscles using organizers: larval muscle templates and adult myoblasts actively interact to pattern the dorsal longitudinal flight muscles of Drosophila. J Cell Biol 141: Salkoff L, Wyman R. 1981a. Genetic modification of potassium channels in Drosophila Shaker mutants. Nature 293: Salkoff L, Wyman R. 1981b. Outward currents in developing Drosophila flight muscle. Science 212: Salkoff L, Wyman RJ Ion currents in Drosophila flight muscles. Journal of Physiology 337: Sanes D, Reh T, Harris W Development of the nervous system. : Academic Press. Sanes JR, Lichtman JW Development of the vertebrate neuromuscular junction. Annu Rev Neurosci 22: Stern M, Kreber R, Ganetzky B Dosage effects of a Drosophila sodium channel gene on behavior and axonal excitability. Genetics 124: Technau G, Heisenberg M Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster. Nature 295: Thompson W Synapse elimination in neonatal rat muscle is sensitive to pattern of muscle use. Nature 302: Thompson W, Kuffler DP, Jansen JK The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience 4: Truman JW Metamorphosis of the central nervous system of Drosophila. J Neurobiol 21: Truman JW, Reiss SE Neuromuscular metamorphosis in the moth Manduca sexta: hormonal regulation of synapses loss and remodeling. J Neurosci 15: Wu C, Gantetzky B, Haughland F, Liu A Potassium currents in Drosophila: Different componentsaffected by mutations of two genes. Science 220: Wu CF, Ganetzky B, Jan LY, Jan YN A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc Natl Acad Sci U S A 75: Yoshihara M, Rheuben MB, Kidokoro Y Transition from growth cone to functional motor nerve terminal in Drosophila embryos. J Neurosci 17:
45 Table 1: Features of DLM motor neuron branching in wild-type and eag 1 Sh 120b 14h APF 18h APF 24h APF 38h APF adult Number of 2 branches on DLM a and b # wt eag 1 Sh 120b 22.6 ± 1.0 (13) 29.6± 2.1 (5)* 27.2 ± 1.0 (15) 36.7± 1.7 (9)** 37.4 ± 1.4 (20) 51.2 ± 3.3 (10)* 10.8 ± 0.2 (8) 7.7 ± 0.3 (8)** 10.5 ± 0.29 (10) 8.3 ± 0.2 (6)** Rate of change during development wt eag 1 Sh 120b n.a. n.a. 20% 23% 37% 28% 71% 84% n.a. n.a. Number of 2 wt n.a. 1.2 ± 0.1(6) 1.3 ± 0.1(6) n.a. n.a. branches/ 10µm of primary # eag 1 Sh 120b n.a. 1.8 ± 0.1 (9)* 2.0 ± 0.2(6)* n.a. n.a. Number of 2 branches with tertiary processes on DLM a and b # wt 3.6 ± 0.6 (13) 12.8± 0.6 (15) 22.7 ± 1.4 (20) 10.8 ± 0.2 (8) n.a. eag 1 Sh 120b 18.0 ± 1.7 (5)** 26.3 ± 1.5 (9) ** 36.6±2.7 (10)** 7.7 ± 0.3 (8)** n.a. # Mean ± S.E.M. Mutants showed significant differences when compared with the wild-type. *p<0.05; **p<0.0001; n.a- not applicable. Numbers in parenthesis indicate sample size. 39
46 C A B Table 2: Branching patterns in the adult thorax Presence of medial branch C Contact points from branch A # Contact points from branch B # Wild-type 95% 2.8 ±0.23 (16) 2.4 ± 0.20 (16) eag 1 Sh 120b 50% 1.4 ± 0.16* (10) 1.3 ± 0.15* (10) # Mean ± S.E.M. Mutants showed significant differences when compared with the wild-type. *p< Numbers in parenthesis indicate sample size. Table 3: nap ts1 suppresses adult phenotypes of eag 1 Sh 120b Genotype Leg shaking % animals with wings down phenotype Number of contact points on adult DLMa # % animals with 5-6 contact points on DLM a Wild-type * (28) 89 (28) (Oregon R) eag 1 Sh 120b (26) 30 (26) nap ts * (6) 100 (6) eag 1 Sh 120b ;nap ts * (13) 62 (13) Mean + S.E.M. Numbers in parenthess indicate sample size. All genotypes were compared with eag 1 Sh 120b. *p<
47 HRP f e d c b a f e d c ba 14h 18h 24h 38h N N h 18h 24h 38h adult SYT Fig. 1: Outgrowth, elaboration and subsequent pruning of secondary branches between 14-24hAPF in wild-type. Top panel: Anti HRP localization showing the developmental progression of DLM innervation. 14h APF: Adult specific primary branches grow out along the length of three persistent larval muscles (outlined with dotted lines) that act as scaffolds for the six adult DLM fibers. 18h APF: Primary branches increase in length, and higher order branching is evident. 24h APF: Adult DLM fibers (a-f) have formed and further higher order branching occurs. Some primary branches begin to defasciculate (black arrows). 38h APF: Pruning results in the reduction of secondary branches, which are now evident as contact points. The terminal arborization from each contact point on DLMa is indicated by arrowheads. Middle panel: Camera lucida drawings correspond to stages in the upper panel and show the entire DLM arbor. Inset is a schematic that depicts the relationship between the main nerve trunk (N), and the primary, secondary and tertiary branches. Lower Panel: anti-synaptotagmin (SYT) immunoreactivity. 14h APF: Staining is restricted to primary branches h APF: SYT localizes to primary branches and begins to appear in some secondary branches. 38h APF: SYT immunoreactivity corresponds to newly arising boutons (arrowheads). Adult: SYT is present at boutons (arrowheads). Anterior is to the top and dorsal midline to the right in all images. Bar=50µm. 41
48 A. WT Number of secondary branches Number of secondary branches with tertiary outgrowths * adult Hours after puparium formation * * B. eag 1 Sh 120b * * * adult Hours after puparium formation Fig. 2: Secondary and tertiary branch development in wild-type (A) and the hyperactive (eag 1 Sh 120b ) mutant (B). (A) Secondary branch development (solid bars) in the wild-type increases steadily from 14-24h APF and is maximal at 24h APF. By 38h APF, the completion of pruning causes a significant reduction to generate the adult complement. Between 14-24h APF, only a fraction of second order branches elaborate tertiary processes (open bars). All values represent the mean numbers of branches ± SEM. (B) Hyperactive mutants, eag 1 Sh 120b exhibit increased numbers of secondary branches compared with the wild-type. Additionally, there is about a sixfold decrease in secondary branches as a result of pruning, which is significantly higher than in the wild-type. The extent of development as determined by the number of tertiary branches is also enhanced. All values represent the mean numbers of branches ± SEM. Significant differences (p<0.05) in branch numbers between two consecutive stages (for example between 14 and 18hAPF) are represented by asterisks. 42
49 A * * * * * D * * * * B WT eag 1 Sh 120b E C * * * * * * F * * * a b Fig. 3: Innervation patterns at the adult DLM in wild-type and eag 1 Sh 120b.(A-C) Wild-type (D-F): eag 1 Sh 120b. DLM innervation patterns visualized using anti-hrp in a bisected thoracic preparation. Asterisks mark the contact points on DLMa. (B) and (E) are camera lucida representations of branching patterns on DLMa in the wild-type and mutant. Boxed areas in (A) and (D) represent one terminal arbor and are shown in C and F respectively. Arrowheads (in C) point to embedded contacts of motor neuron branches along the myofibers. Anterior is to the left and dorsal midline is at the top in all images Bar=100 µm (A and D). Bar=25 µm (C and F). 43
50 DLM DVM leg abdominal 48h A B C D E A B C abdominal F SYT leg SYT DLMa Fig. 4: Bouton morphology at adult NMJs. All boutons have been visualized with anti-hrp except in B and C where anti SYT has been used. (A) Boutons at a DLM fiber. Arrowheads indicate the larger sized boutons. An array of smaller boutons is also visible (arrow). (A ) A string of boutons emerging from a motor neuron branch on a DLM fiber. (B and B ) Boutons on a DVM fiber. (C and C ) Boutons on leg muscles in the metathorax. (D) Boutons on transverse abdominal muscles. (E) Developing innervation in the transverse abdominal muscles. The filled bouton-like structures (arrowheads) are also seen at the developing arbor of the DLMs (F). They appear to be nodal points from which bouton-bearing branches (arrow) arise. Bar= 25µm. 44
51 Wild-type Adult-HRP eag 1 Sh 120b Adult-HRP 38h- HRP 38h-HRP * * 48h-HRP 48h-SYT * 48h-SYT Figure 5: Bouton formation at the DLMs of wild-type and eag 1 Sh 120b. Left panel (Wild-type). Adult: Part of a higher-order branch is shown here. Anti-HRP reveals punctuate appearance of boutons, similar to type-ii boutons of third-instar larvae. 38h APF: Two adjacent contact points (asterisks) and their arbor on DLMa are seen. The arrow points to boutons on a tertiary branch. 48h APF: A single contact point (asterisk) with its terminal arbor on DLMa is seen. Larger boutons are indicated with an arrowhead. Inset shows a magnified view. Synaptotagmin is present in boutons along higher order branches. Right panel: Bouton formation in the hyperexcitable mutant, eag 1 Sh 120. Adult: Morphology of individual boutons is similar to the wild-type. 38h APF: Boutons appear larger than their wild-type counterparts. Larger boutons are also present (arrowhead). 48h APF: A well-defined NMJ is present. Anterior is to the left and dorsal midline is at the top in all images. Bar=25µm 45
52 f e d c b a 14h 18h 24h 38h Fig. 6: Branch elaboration and pruning are altered in the hyperactive mutant, eag 1 Sh 120b Top Panel: The progression of DLM innervation from 14h APF through 38h APF as revealed by anti HRP. The motor neuron arbor elaborated at each contact point at 38hAPF is indicated by arrowheads. Bottom panel: Camera lucida representations of the DLM arbor at the corresponding stages in the upper panel. Anterior is to the top and dorsal midline to the right in all images. Bar=50µm 46
53 WT f e d c b a 18h eag 1 Sh 120b 24h f e d c b a 18h 24h Fig. 7: A comparison of muscle profiles between wild type (WT) and mutant (eag 1 Sh 120b ) as revealed by 22C10. There is no apparent difference in the size of muscles between mutant and the wild type at 18h APF and 24h APF. Anterior is to the top and dorsal midline to the right in all images. Bar=100µm. 47
54 * * Age 14h APF 24h APF 38h APF 48h APF Adult Innervation Myogenesis Outgrowth of primary neuronal branches Dedifferentiation of larval scaffolds, Myoblast proliferation Elongated primary neuronal branches containing SYT, maximal elaboration of secondary branches Myoblast fusion and proliferation Defasiculation of primary neuronal branches, 3-fold reduction of secondary branches (pruning), SYT present in the first boutons. Muscle growth Tertiary branches grow out on the muscle surface, some branches wrap around the outer muscle surface, SYT present at boutons. Muscle growth Well defined boutons, nested contacts along myofibers, HRP and SYT positive. Mature muscle Fig. 8: Schematic representation of major events during the establishment of DLM innervation during metamorphosis. The major events are outgrowth of primary branches and initiation of second order branching (14h APF), elaboration of second and third order branches (24h APF), pruning of secondary branches followed by the initiation of bouton formation (38h APF), and the subsequent expansion of the NMJ (48h- Adult). Red dots represent unfused myoblasts. Light purple dots represent nuclei of fused myoblasts. At 48hAPF and in the adult, a single contact point (asterisk) and its terminal arbor are shown. Solid lines represent higher order branches on the inner muscle surface and dashed lines indicate branches that are on the outer muscle surface. Black dots represent embedded contacts of motor neuron branches along the myofibers. Anterior is to the top and dorsal midline to the right. 48
55 Appendix 1: Localization of synaptic markers during development Figure Legend: In most cases preparations have been double labeled with anti HRP (green) and a synaptic marker (see below; except for SYT, where the second label is 22C10) Top Panel (24-28h APF): Localization of synaptic markers, 22C10/Futsch (blue, left), and Synaptotagmin (red, middle) at 24h APF and 22C10/Futsch (blue, right) at 28h APF, on DLMs (a-f shown here). Futsch/22C10 localizes to muscle fibers. At 24h APF, it is seen along the primary branch and in select secondary branches (yellow arrowhead). Many secondary branches do not show localization of Futsch/22C10. Synaptotagmin (Red, middle) is present in discrete puncta along the primary branches and at secondary branches (yellow arrowhead) Middle Panel (48h APF): Localization of synaptic markers, 22C10/Futsch (blue, left), and Synaptotagmin (red, middle) and DVGLUT (Drosophila vesicular glutamate transporter, red; right) on DLMs. Expression of Futsch/22C10 from the muscle decreases. All the stabilized secondary branches show presence of Futsch/22C10. CPs and their terminal arbors are indicated by red asterisks. Higher magnification of a CP and its terminal arbor (red asterisk) is shown for the SYT and DVGLUT. Both SYT and DVGLUT are seen localizing to morphological swellings (white arrowhead). That some higher order branches have not elaborated into boutons is also evident. Bottom Panel (Adult): Localization of synaptic markers, 22C10/Futsch (blue; left: lower magnification and right: higher magnification), and Synaptotagmin (red, top inset) and DLG (red, bottom inset) on DLMs. At a low magnification, expression of Futsch/22C10 resembles that seen at 48h APF; it is present in all stabilized secondary branches. At a higher magnification, it appears that only some boutons (arrowhead) of the terminal arbor (red asterisk) show localization of Futsch/22C10. There are boutons at the distal end (arrow) of the arbor that do not bear Futsch/22C10. Synaptotagmin is seen localized to the boutons (arrowhead, top inset). Postsynaptic marker DLG also localizes to the outer edge of a bouton (arrowhead). It is obvious that DLG displays an extra synaptic localization as well. Low magnification scale bar = 50 microns and high magnification scale bar = 10 microns 49
56 Table 1: Summary of onset of synaptic markers in development 14-18h APF 24h APF 48h APF Adult 22C10/Futsch (Microtubule associated protein) Along primary branches Localizes to stabilized secondary branches Synaptotagmin (Vesicle associated protein) DVGLUT (vesicular glutamate transporter) DLG (post synaptic, PDZ protein) Along primary branches and in a subset of secondary branches Localizes to stabilized secondary branches and in some boutons of the terminal arbor Along primary branches Along primary branches and in a subset of secondary branches In boutons In boutons?? In boutons In boutons? Cannot be visualized Cannot be visualized Localization around boutons (indicated by pre synaptic marker) and also in extra synaptic areas. 50
57
58 Chapter 3 A role for FasII in the stabilization of motor neuron branches during pruning in Drosophila 3.1 Abstract During insect metamorphosis, the nervous system is extensively remodeled resulting in the development of new circuits that will execute adult-specific behaviors. The peripheral remodeling seen during development of innervation to the Dorsal Longitudinal (flight) Muscle (DLM) in Drosophila, involves an initial retraction of larval neuromuscular junctions followed by adult-specific branch outgrowth. Subsequently, a phase of pruning occurs during which motor neuron branches are pruned back to reveal the stereotypic pattern of multiple contact points (or arbors) along the length of each DLM fiber. Here, we show that the cell adhesion molecule, Fasciclin II (FasII), is important for generating the stereotypic pattern. In FasII hypomorphs, the number of contact points is increased, and the phenotype is rescued by targeted expression of FasII in either synaptic partner. Arbor development has three distinct phases: outgrowth and elaboration, pruning and stabilization, and expansion of stabilized arbors. FasII is expressed during the first two phases. A subset of branches is labeled during the elaboration phase, which is likely to initiate a stabilization pathway allowing branches to survive the pruning phase. However, since not all FasII positive branches are retained, we propose that it primes branches for stabilization. Our data suggest that FasII functions to restrict branch length and arbor expanse. 3.2 Introduction A common theme in the formation of nervous systems is the initial exuberance of neuronal outgrowth that is seen both in the central nervous system as well as in the periphery. Outgrowth is followed by a process of refinement, which results in the mature pattern of connectivity and/or expanse of arbors. Refinement in the periphery is exemplified by the vertebrate NMJ where muscle fibers initially innervated by multiple neurons become singly innervated as a result of synapse elimination (Sanes and Lichtman, 1999). 52
59 In the CNS, pruning of axonal and dendritic branches is well known. Examples of developmental axon pruning (reviewed in Kantor and Kolodkin, 2003) include the retino-tectal system, where it is necessary for establishment of topographic maps; in the visual cortex, where it is important for segregating projections of cortical neurons to appropriate target regions and in the hippocampus, where it serves to prune back mossy fiber projections to a shorter adult length. During insect metamorphosis, the nervous system undergoes extensive remodeling. This reorganization provides useful models to study developmental pruning. One such model in Drosophila is exemplified by the mushroom bodies, centers for learning and memory. Prior to the onset of adult-specific outgrowth, axons of larval γ neurons are pruned back. This is a selective process, as axons of the α /β neurons are not altered. The pruning is a result of degeneration that is initiated by steroid hormones and mediated by cell intrinsic mechanisms involving a ubiquitin-proteosome system acting in concert with ecdysteriod receptor B/USP (Lee et al., 2000). Another instance of pruning occurs during the development of innervation to the dorsal longitudinal muscle (Hebbar and Fernandes, 2004) and is distinct from what occurs in the mushroom bodies. In this case, following retraction of larval synapses, there is an excessive outgrowth of adult specific motor neuron branches, followed by a phase of pruning. More than 75% of the branches are eliminated through pruning and, consequently, the adult pattern of innervation emerges. Each motor neuron makes multiple contacts along the length of the muscle, and has been referred to as multi-terminal (Hoyle, 1983). A terminal refers to the higher order arbor and its collection of boutons. This innervation pattern is distinct from the single terminal innervation typical of larval muscles (Johansen et al., 1989). When excessive branches are pruned, two aspects must be considered - the selective removal of some branches and the stabilization of those that persist. For the well-studied event of synapse elimination at the vertebrate NMJ (Sanes and Lichtman, 1999), it is proposed that a protective signal stabilizes some synapses, whereas an elimination signal initiates withdrawal. Very little is known about the mechanisms that bring about stabilization (reviewed in Zito, 2003). In the case of hippocampal pruning, there is evidence that molecules that initially promote outgrowth, can also promote axon pruning at a later stage (Kantor and Kolodkin, 2003). For example, ephrins are thought to regulate both early hippocampal outgrowth and pruning of axons (Gao et al., 1999), while semaphorins and their receptors (plexins) have been implicated in axon guidance and stereotypic pruning of hippocampo-septal projections (Bagri et al., 2003). It is conceivable that in Drosophila, molecules initially involved in establishing synapses during the embryonic/larval stages, may have distinct later roles in the context of pruning and stabilization. 53
60 The cell adhesion molecule, FasII, is a good candidate to be involved in stabilizing branches during the formation of DLM innervation. In its classical role during insect (Drosophila and grasshopper) embryogenesis, it mediates selective axon fasciculation (Lin et al., 1994), guidance of growth cones (Harrelson and Goodman, 1988; Grenningloh et al., 1991), and target selection (Davis et al., 1997). In Drosophila it also functions to regulate post embryonic stabilization of larval NMJs (Schuster et al., 1996a) and of cholinergic inputs on the dendrites of motorneurons (Baines et al., 2002). We were interested in examining how FasII may be re-used during the formation of adult NMJs. We focused our investigations on the formation of innervation to the Dorsal Longitudinal (flight) Muscle (DLM). Early during metamorphosis, FasII is expressed in a subset of branches that elaborate over the muscle surface, suggesting a possible role in stabilization. Analysis of hypomorphic mutants revealed that the adult muscles have many more terminal arbors, and that this phenotype can be rescued through targeted expression of FasII in motor neurons and muscle. Our studies demonstrate that FasII is important for establishing the stereotypic pattern of terminal arbors on each DLM fiber, and that FasII enables stabilization of subsets of branches during metamorphosis by influencing the length of second order branches and their expanse of higher order branches. 3.3 Materials and Methods Fly strains: Oregon R raised on standard Drosophila food at 25ºC was used as the wild-type strain. The following FasII alleles (described in Grenningloh et al., 1991) were used: FasII e93 (precise excision of a P- element insertion-source: R.Baines, University of Warwick), hypomorphs FasII e86 (50% of wild-type levelssource: G.Davis, UCSF) and FasII e76 (10% of wild-type levels-source: V. Budnik, U.Mass Med School). Since the null allele, FasII EB112 is lethal by the larval stage; we generated a transhetereozygote, FasII e76 /FasII EB112. FasII overexpression was achieved by using UAS-FasII (transmembrane FasII) under the control of neuronal drivers elav-gal 4 (Robinow and White, 1988; source: B. White, NIH) and D42-Gal 4 (Sweeney and Davis, 2002; source: S. Rao, Cornell University). Reporter gene expression for elav-gal 4 has been observed in neuronal components up until 24hAPF. However, elav-gal 4 also drives expression in the muscles from 16hAPF onwards (Fernandes and Keshishian, unpublished observations). D42-Gal 4 drives reporter gene expression in motor neuronal branches as early as 14hAPF and continues throughout the rest of metamorphosis. However, reporter expression for the early pupal stages is not as intense as with elav-gal 4 (Hebbar and Fernandes, unpublished observations). For muscle specific overexpression of 54
61 FasII, we used MHC (Myosin Heavy Chain)-Gal 4 (source: A.Chiba, Univ of Illinois, Urbana-Champaign). The larval promoter for the MHC gene is active until about 10h APF and subsequently the adult specific promoter switches on by 26h APF (Fernandes et al., 1991). For rescue experiments we crossed (female) homozygous FasII e76 ; UAS-FasII animals to appropriate Gal 4 drivers and the male progeny heterozygous for the driver and transgene were examined. A hyperexcitable, K + channel double mutant, eag 1 Sh 120b (source: H. Keshishian, Yale University) was used as an activity mutant. In these animals, eag 1 preferentially removes the I K current (Wu et al., 1983) while Sh 120 reduces I A (Ganetzky and Wu, 1983). The double mutant synergistically increases nerve excitability and neurotransmitter release (Ganetzky and Wu, 1983). Staging: White prepupae (0h APF: hours after puparium formation) were collected and placed on moist filter paper on a Petri dish. They were raised at 25 ºC to the following stages; 18h, 24h, 28h and 38h APF. The stages were confirmed using muscle morphology (Fernandes et al., 1991). Two day old adults were used for analyses of adult muscle and innervation as described previously (Hebbar and Fernandes, 2004). Immunochemistry: The general protocol followed was as described previously (Hebbar and Fernandes, 2004). Pupae and adults were fixed with 4% paraformaldehyde (Ted Pella, Inc, CA). 10% donkey serum in 0.1% BSA and 0.3% Triton-X buffered saline was used as a blocking solution prior to primary antibody application. The following primary antibodies were used: anti-hrp (1:200, raised in goat, source: Jackson ImmunoResearch Laboratories, Inc, PA), MAb1D4 (1:2 mouse anti-transmembrane FasII, source: Hybridoma Bank, Iowa), MAb 22C10 (1:25 mouse anti-futsch, source: Hybridoma Bank, Iowa), anti β-3 Tubulin (1:5000, raised in rabbit and used to label muscle outlines at 24h APF, source: R. Pohl, Germany). Synapses were marked with anti DVGLUT (1:100, raised in rabbit and recognizes Drosophila vesicular glutamate transporter, source: A. DiAntonio, Washington University School of Medicine). The following secondary antibodies were used: Alexa Fluor 488 Donkey anti-goat, Alexa Fluor 555 Donkey anti-rabbit and Alexa Fluor 594 or 555 Donkey anti-mouse (all at 1:200; Molecular Probes, OR). Adult muscles were visualized with Alexa Fluor 594 phalloidin (Molecular Probes, OR). Image Acquisition: All immunostained tissues were visualized using an Olympus FV500 confocal microscope. Fluorescent dyes were excited using Ar 488 and HeNe 543nm lasers. Optical sections of 1µ thickness (for pupal preps) and 1-3 µ (for adult preps) were taken and stacked using Fluoview Software to 55
62 obtain a 2D projection. These stacked images were used for further analysis. Image panels were prepared using Adobe Photoshop 6.0 (Adobe Systems Incorporated, CA). Data Analysis: All morphometric measurements were carried out on 2D projections. For adult muscle lengths, hemithoracic preparations of 2-day-old female flies were used. Phalloidin staining was used to outline the muscle and measurements were made in Image-Pro Plus 4.5 (Media Cybernetics, MD). In the pupal stages, our analysis focused on primary branches that innervate dorsal muscles, DLMs a and b. Both of these muscles are innervated by one motor neuron, MN5 (Ikeda and Koenig, 1988). The axon of MN5 divides into 2-3 longitudinal branches (each defined as a primary branch). These include the anterior (a), medial (b) and posterior (c) branch (Ikeda et al., 1980). Secondary branches are transverse outgrowths off a primary branch. Secondary branch length was defined as length of the branch up to the first tertiary branch outgrowth. Lengths were measured in Image-Pro Plus 4.5 (see schematic in Figure 5 for representative traces). Density of second order branching is expressed as number of secondary branches per 10 µ of a primary branch. In addition, at 24h APF, secondary branches with tertiary and higher order branches on DLMa were quantified. The area occupied by the secondary branch arbor on DLMa was outlined and measured in Image-Pro Plus 4.5 (see schematic in Figure 5 for representative traces). Intensity analyses were carried out as reported for larval NMJs (Mathew et al., 2003; Albin and Davis, 2004). Briefly, 5 regions along an axon within the main nerve trunk (at 24h APF) were highlighted and these areas were used to measure anti-hrp and anti-fasii intensity on double labeled (anti-hrp and anti-fasii) samples using Fluoview software. All values represent mean ± s.e.m. Basic statistical functions such as mean, standard error of mean and two sample Students t test were performed using Microsoft Excel. Chi Square test was performed and interpreted using Minitab program (Minitab Inc, State College, PA). 3.4 Results Each DLM fiber is innervated by a single motor neuron, which makes multiple contacts along the length of the muscle. This innervation pattern is characteristic of insect DLMs and has been referred to as multi-terminal (Hoyle, 1983). We refer to each terminal as a contact point (CP), which includes the axon entry point as well as the arbor of higher order branches that emanates from it (Figure 1A and Hebbar and Fernandes, 2004). It is the higher order branches that bear presynaptic swellings or boutons, and can be visualized by the presence of Drosophila vesicular glutamate transporter, DVGLUT (Daniels et al., 2004; see Figure 1B). The characteristic DLM innervation pattern is established by developmental events that 56
63 occur during the first two days of metamorphosis (Fernandes and VijayRaghavan, 1993; Hebbar and Fernandes, 2004). These include outgrowth of adult specific branches (14-24h APF), and the subsequent pruning of exuberant outgrowths (24-38h APF). To examine a role for FasII during this period, we have followed its expression pattern and analyzed innervation patterns under conditions of increased and decreased FasII levels. FasII is expressed in developing neuronal branches on the DLMs At 14h APF, FasII is detected in the posterior dorsal mesothoracic nerve (PDMN), where it labels axons of the DLM motor neurons. FasII expression is also detectable in the primary branches (Figure 2) that project along the length of the dedifferentiated larval muscle scaffolds. At this time, higher order branches do not display detectable levels of FasII. At 18h APF, when the larval scaffolds are in the process of splitting into DLM fibers (Fernandes et al., 1991), FasII is detected along the primary branches that have now increased in length. FasII is additionally detected in the secondary and higher order branches that elaborate over the muscle surface (Figure 2). By 24h APF, muscle splitting is complete and the primary branching pattern is established (Fernandes et al., 1991; Fernandes and VijayRaghavan, 1993). At this time, FasII continues to be present along the entire length of the primary branch, in secondary branches and in higher order branches (Figure 2). It is the second order branch that prefigures the contact point seen in the adult, and as early as 38h APF (Hebbar and Fernandes, 2004). A closer examination of FasII expression between 18-24h APF revealed some interesting features. We focused our analysis on DLMs a and b, since this dorsal pair of muscles is innervated by a single motor neuron (Ikeda and Koenig, 1988). First, FasII is expressed in a subset of second order branches. At 18h APF, 22.5% (n = 4) of the total pool of second order branches is FasII positive, while at 24h APF FasII is expressed in 38.0% of branches (n = 6). Secondly, the number of FasII positive branches increases approximately three fold during this time period. At 18h APF, there is an average of 8.5 ± 2.3 (n = 4) FasII positive branches, whereas by 24h, this number increases to 30.0 ± 3.8 (n = 6), indicating that additional branches become FasII positive. The pool of FasII positive branches is largely made up of those that bear a higher order arbor (86%). We have previously reported that 50% of branches with higher order arbors undergo pruning (Hebbar and Fernandes, 2004; see also Table 5). Consistent with this observation, we find that not all FasII positive second order branches that bear an arbor are stabilized to give rise to the adult pattern. At 24h APF, there are 8.3 ± 1.25 FasII positive branches on DLMa, whereas at 38h APF, 5 branches are seen. These five branches correspond to the stabilized contact points that emerge after 57
64 pruning, and are, therefore, prefigured by second order branches during the 14-24h period (Hebbar and Fernandes, 2004). This pattern of 5 CPs comprises the stereotypy of the adult innervation pattern. At 38h APF, FasII is detected in the axon of each CP (Figure 3A). It can also be detected in tertiary and higher-order branches of the arbor. FasII is subsequently down regulated from the arbors after 48h APF (data not shown). Consistent with these observations, at the adult stage, FasII is not detected in motor neuron branches or at the terminals (Figure 3B). This is in contrast to the larval NMJ where FasII is detected in boutons (Schuster et al., 1996b). When a pan-neuronal driver, elav-gal 4 (Robinow and White, 1988) is used to overexpress FasII, the protein is seen in individual CPs (Figure 3C), but is still undetectable at the boutons. An interesting aspect of FasII expression at 38h APF is that in addition to being present in the DLM motor axons, it is also present in a compartment that extends around them (Fig 3A ). This is likely to be a glial compartment due to the lack of anti HRP staining. Thus, FasII expression is detected in secondary and higher order branches from 18h APF onward and is coincident with the period of branch elaboration. It becomes downregulated after the pruning phase and is absent from the adult NMJs. FasII alleles exhibit altered adult innervation patterns Immunostaining studies indicated that FasII is expressed in a subset of higher order branches during the period of branch elaboration (Figure 2). To test if this restricted pattern of expression has a bearing on branch elaboration and/pruning, innervation patterns in hypomorphic alleles of FasII (Grenningloh et al., 1991) were examined. The following alleles were used: FasII e86 (50% of wild-type levels) and FasII e76 (10% of wild-type levels). Since the null FasII EB112 is lethal at the larval stages (Grenningloh et al., 1991), a transhetereozygote, FasII e76 /FasII EB112, was generated which has the least amount of FasII in a viable allele (less than 5%). FasII e93 (precise excision of a P-insert which was used to derive the above lines) and Oregon-R were used as controls. Adult innervation patterns were observed in these mutants by immunostaining for anti-hrp (Figure 4). We focused on examining the number of CPs on the dorsal-most muscle, DLMa. In the wild-type, DLMa displays an average of CPs. Each CP originates from a stabilized second order branch (Hebbar and Fernandes, 2004). The number of CPs in FasII e93 and FasII e86 was not significantly different from the wild-type (Figure 4). However, both FasII e76 and FasII e76 /FasII EB112 exhibited an increase in the number and range of CPs (Table 1, Figure 4). The mean number of CPs in FasII e76 and FasII e76 /FasII EB112 were and respectively, an increase that was statistically significant. The increased number of 58
65 CPs is also evident when the range of CPs is examined (Table 1, Figure 4). In the wild-type, the number of CPs ranged from 4-6 with 69% of animals exhibiting 5 CPs (Table 1). By contrast, FasII e76 animals display 5-7 CPs with 36% exhibiting 5 CPs. In FasII e76 /FasII EB112 animals, the range of CPs remains the same as in FasII e76 ; however, fewer animals (25%) exhibit 5 CPs. Thus decreasing FasII levels causes an increase in the number and range of CPs (Table 1 and Figure4). Associated with the increase in number of CPs, an increase in muscle length is observed (Figure 4, top panel and Table 2). This trend is statistically significant only in the case of the most severe hypomorph, FasII e76 /FasII EB112. In these animals there is an 11% increase in muscle length. More importantly, the increase in length occurs after the pruning event as there is no significant alteration at 24h APF (data not shown). It is of interest to note that CPs are uniformly spaced along the length of the muscle (Figures 1 and 4). This has a bearing on the occurrence of rapid local depolarizations, ensuring that the muscle membrane is isopotential through the entire length (Hoyle, 1983). This direct relationship between muscle length and number of contact points indicates that a homeostatic event that involves neuron-muscle communication must occur along with or subsequent to the pruning process to ensure co-ordinate expansion of terminal arbors and muscle length (Hebbar and Fernandes, 2004). The FasII phenotype is rescued using neuronal and muscle drivers. To confirm that the observed FasII phenotypes were due to decreased levels of FasII, the Gal4/UAS system of targeted expression (Brand and Perrimon, 1993) was used to deliver FasII in a tissue specific manner. In these rescue experiments, a neuronal driver, elav-gal 4 was used to drive UAS-FasII in the FasII e76 background. In the mutant, we have shown that the number of CPs is increased (Table 1). This is also evident in the number of animals displaying more than the mean number of wild-type CPs (more than 5). In FasII e76, 64% of animals displayed the phenotype, whereas in the wild-type, only 23% of animals display more than 5 branches, a difference that is statistically significant (p=0.04). The mutant phenotypes were rescued by targeted FasII expression (Table 1). Two genetic controls were also examined: FasII e76 ; UAS-FasII (homozygotes) which showed a phenotype similar to the mutant alone, and FasII e76 /+, which exhibited a wild-type phenotype (data not shown). A second neuronal driver, D42-Gal 4, was unable to rescue the FasII e76 phenotype (Table 1). Interestingly, the elav-gal 4 driver is expressed in DLM fibers during metamorphosis (Fernandes and Keshishian, unpublished observations). We therefore considered the possibility that muscle expression of elav-gal 4 may contribute to the rescue. When a muscle driver MHC-Gal 4 was used to overexpress 59
66 UAS-FasII in the background of FasII e76, the mutant phenotype was rescued, as determined by the decrease in number of animals that displayed more than 5 CPs (Table 1). However, the mean number of CPs was significantly reduced in comparison to wild-type (Table 1). Thus, it seems likely that a balance between muscle and neuronal FasII may exist to promote appropriate branch development. We have not been able to detect muscle FasII to the extent that we can detect it in the neuronal component, and this may simply indicate that there are low levels of muscle FasII. Associated with the decrease in the number of CPs, the muscle length is also significantly reduced (Table 2). How are the additional CPs generated in FasII hypomorphs? The adult DLM innervation pattern is established within the first two days of metamorphosis (0-38h APF) as a result of adult-specific neuronal outgrowth followed by the subsequent pruning of more than 75% of second order branches (Hebbar and Fernandes, 2004). The additional contact point seen in FasII hypomorphic adults could be a result of excessive initial outgrowth or due to the stabilization of a greater number of secondary branches. In order to determine the developmental origins of the extra CP in the hypomorph, we analyzed representative pupal stages in FasII e76 /FasII EB112. In order to better characterize outgrowth and expanse of secondary branches, morphometric measurements such as number and density of second order branches (for outgrowth), length of secondary branches and areas occupied by arbors (for expanse) were carried out (Table 3, 4). We quantified the number of secondary branches that bear higher order arbors as another measure of developmental progression. Our measurements focused on primary branches that innervate DLMa. At 18h APF, second order branches are well defined and it is at this time that FasII expression is evident in second order branches (Figure 2). At this stage, the innervation pattern seen in FasII e76 /FasII EB112 shows no significant differences in secondary branch density or in the number or lengths of secondary branches (Table 3), suggesting that decreased FasII levels do not impact early events. Next, the innervation pattern was examined at 24h APF (Figure 5), when second order branching is maximal (Fernandes and VijayRaghavan, 1993). There is no statistical difference in the numbers or density of second-order branches between the wild-type and the mutant. Likewise, there is no change in the number of secondary branches that bear higher order branches (Table 4). However, in FasII e76 /FasII EB112, the average length of secondary branches on DLMa is significantly increased. In addition, the area occupied by higher order arbors of a secondary branch on the muscle is enhanced in the hypomorph (Table 4). Taken together, these data suggest that a reduction in FasII does not affect outgrowth of second order branches, but that 60
67 during the period of branch elaboration (18-24h), the reduced FasII levels promote an increase in the secondary branch length and expanse of the developing arbor, which in turn has a bearing on branch stabilization. Not all secondary branches with an arbor are stabilized (Hebbar and Fernandes, 2004), and this is reflected in the case of FasII positive branches as well. At 24h APF, DLMa has as many 10 secondary branches that bear arbors (Figure 5A). A subset of these (8.3) is positive for FasII (Table 5), and only 5 are incorporated into the adult pattern. This suggests a role for FasII in priming branches for stabilization during metamorphosis. The earliest evidence that specific branches will be stabilized prior to the pruning phase, comes from studies that examined the labeling of 22C10, an antibody that binds to a microtubule associated protein, Futsch (Hummel et al., 2000). In the wild type at 24h APF (Figure 6A), 3.8 secondary branches with arbors are positive for Futsch/22C10 (n=9). By 28h APF (Figure 6B) when pruning is in progress, more secondary branches, 4.4 (n=7) are labeled by futsch/22c10. By the completion of pruning at 38h, all stabilized secondary branches express Futsch/22C10 (n=4, Table 5). Thus, the onset of Futsch/22C10 between 24-38h AP in secondary branches is a signature of their stabilization. When Futsch/22C10 localization was examined at 24h in FasII e76 /FasII EB112 many more secondary branches (4.7; n=9), are seen to be positive for Futsch/22C10 (Figure 6C). Overexpression of FasII using Gal 4 drivers Our analysis of FasII hypomorphs has shown that lowering FasII levels results in more CPs. Does the converse hold true- i.e., when FasII levels are increased, will it result in fewer CPs? We were able to create a hypermorphic condition by using the UAS/Gal4 system (Brand and Perrimon, 1993) to overexpress FasII in the wild-type background. A neuronal driver, elav-gal 4 and a muscle driver MHC-Gal 4 were used. In UAS-FasII; elav-gal 4 adults, the mean number of CPs is lower than the wild type (4.4 ± 0.15), a difference that is statistically significant when compared to the wild-type (Table 1). The range of CPs in these animals has shifted (3-6; Table 1), also an indicator of the decrease in CPs. A majority of animals, 58%, exhibit less than the typical 5 CPs at the adult DLMs (p=0.005; Table 1). Thus, as expected, increased FasII expression with elav-gal 4 results in fewer CPs. What brings about the generation of fewer CPs? From our results with the hypomorphic mutants, it can be predicted that increasing FasII levels would restrict axonal outgrowth and arbor elaboration. When branching patterns were examined at 24h APF, we found that the number of secondary branches along a primary is decreased. In the wild type there are 61
68 typically ± 1.7 branches along a primary branch. In UAS-FasII; elav-gal 4 pupae, there are fewer second order branches along a primary (19.30 ± 1.70; Table 4). This reduction is statistically significant (p=0.013). As a result, the pool of secondary branches that can elaborate into higher order branches is also reduced (Table 4, Figure 6) and consequently by 38h APF fewer branches are stabilized. This is reflected in the decreased number of CPs in the adults. We next examined the effects of overexpressing FasII in the muscle. In UAS-FasII; MHC-Gal 4 animals, the number of CPs is significantly reduced (4.3 ± 0.2; Table 1). The reduction in the number of CPs is reflected in the range of CPs in these animals (3-5; Table 1). Thus, increasing FasII levels in muscles alone has an effect on the eventual number of CPs in the adult. Does muscle overexpression of FasII also result in fewer branches at 24h APF? This is indeed the case. At 24h, UAS-FasII; MHC-Gal 4 animals display reduced numbers of secondary branches (Table 4). This overall reduction is further reflected in the reduced number of secondary branches that bear tertiary branches (Table 4 and Figure 5). Thus, increasing FasII levels either in muscle alone or in both neuron and muscle, results in fewer secondary branches. Our morphometric analyses also revealed that FasII over expression in muscle results in an increase in the expanse of the secondary branch in terms of its length and area occupied on muscle surface (Table 4). This is different from observations with the elav-gal 4 driver and reflects the onset of MHC promoter activity and/or the need for a balance between pre- and post-synaptic FasII (see discussion). Investigating the relationship between FasII and electrical activity for the patterning of adult innervation Studies on the development of larval NMJs have demonstrated that hyperactive mutants have excessive branches and boutons (Budnik et al., 1990) and that this is due to a lowering of FasII levels (Schuster et al., 1996b). Our own studies have shown that the hyperactive mutant, eag 1 Sh 120 has fewer CPs at the DLM (Hebbar and Fernandes, 2004), a phenotype distinct from the larva. This raises two relevant questions. First, is branch length and expanse reduced in hyperactive mutants during development? Second, are FasII levels altered in the hyperactive mutant? eag 1 Sh 120b displays an increase in the number and density of second order branching during pupal development. 75% of animals exhibit less than the typical 5 CPs in the adult; at 24h APF, in eag 1 Sh 120b animals, there is an increase in the number of secondary branches (Hebbar and Fernandes, 2004) and in the density of branching (Figure 5; Table 4). The secondary branches are as long as their wild-type 62
69 counterparts (Table 4). Interestingly, the expanse of the secondary branch arbor measured as area occupied by the arbor is significantly greater than the wild-type (280 ± 63 µm 2 vs. 131 ± µm 2 in the wild type, p=0.03). Since secondary branch length is increased in FasII hypomorphs (with respect to wild-type) and remains unchanged in eag 1 Sh 120b animals, the prediction would be that FasII levels in eag 1 Sh 120b are similar to the wild-type. In studies of the larval NMJ, intensity of immunostaining has been used as a measure of FasII levels (Schuster et al., 1996b; Mathew et al., 2003). Intensity analyses were carried out along axons at 24h APF. FasII levels in eag 1 Sh 120b were within 10% of the wild type levels. The hypomorph, FasII e76 /FasII EB112 exhibited a 44% decrease in intensity, indicating reduced FasII levels. In elavgal4; UAS-FasII animals a 79% increase in staining intensity was detected (Figure 7). The staining intensity of anti-hrp was measured as an independent control. Anti HRP staining intensity remained constant in the genotypes that were tested for levels of FasII intensity (Figure 7). Thus, unlike in the larva, FasII levels along the axons of DLM motor neurons remain unchanged in eag 1 Sh 120b pupae, indicating that activity and FasII may act in different pathways leading to establishment of the DLM innervation pattern. 3.5 Discussion The stereotypy of the DLM innervation pattern is established within the first 48 hours of metamorphosis (Hebbar and Fernandes, 2004). Eight hours after the larva turns into an immobile pupa, the larval NMJs are completely withdrawn and the nerve maintains contact with the larval scaffolds that will give rise to the DLMs (Fernandes and VijayRaghavan, 1993). A phase of adult specific outgrowth then begins. Primary branches grow along the dedifferentiating larval scaffolds (12h APF) and even as they are extending, second order branches begin appearing. This coincides with the onset of myoblast fusions that initiate fiber formation (Fernandes et al., 1991). By 18h APF, third order branches are visible, and by the end of the first day (24h APF), higher order branching is maximal (Hebbar and Fernandes, 2004). Only a small subset of the pool of second order branches contributes to the adult innervation pattern; the remainder is pruned back between 24-38h APF, revealing the adult pattern of multiple contact points (CPs). The stereotypy of the adult pattern of innervation lies in the number of stabilized CPs along the length of the muscle fiber. Interestingly, boutons first become visible after the pruning event. For the remainder of metamorphosis, arbors corresponding to each contact point grow in size and match the growth of the muscle fiber. Thus, there are three major phases of arbor development (summarized in Figure 8): outgrowth and elaboration when adult specific outgrowth of branches occurs, pruning, when excessive 63
70 branches and their arbors are removed to reveal the adult pattern of CPs, and the expansion phase, during which the stabilized arbors expands in tandem with the muscle, which is known to grow three times in size (Finlayson, 1975). In this study we have investigated the role of FasII in stabilizing second order branches to thereby generate the adult pattern of multiple CPs. We present a model (Figure 8) wherein FasII regulates the length of second order branches and the expansion of arbors during the outgrowth and elaboration phase. These are important first steps in the pathway that leads to branch stabilization, a process that is initiated prior to the pruning phase. FasII levels begin to decline subsequent to the pruning phase, and we propose that this may have a bearing on the co-ordinate expansion of the stabilized arbor and the muscle fiber during the final phase of arbor development. The dynamic nature of FasII expression Although second order branches are first seen as early as 12h APF (Fernandes and VijayRaghavan, 1993), they do not express FasII until about 18h APF. It is likely that during this span of six hours a neuron-muscle communication or an interaction among neuronal branches may result in the onset of FasII expression. Each second order branch prefigures a potential contact point for the adult pattern of innervation. Thus, the expression of FasII in subsets of second order branches may be significant for stabilization. Interestingly, a large fraction (86%) of FasII branches is comprised of those that have elaborated an arbor. Of these, 60% are incorporated into the adult innervation pattern. The fact that not all FasII arbors survive the pruning phase indicates that the expression of FasII initiates a stabilization pathway that must involve other molecules that act together to regulate branch properties. It is likely that the muscle may be a participant in the stabilization process, in a manner similar to vertebrate NMJs, where it has been proposed to be the source of protective/survival factors that may be limiting (Nguyen and Lichtman, 1996; Chang and Balice-Gordon, 1997). As the pool of arbors marked by FasII goes through the pruning phase, 22C10, which detects a microtubule associated protein, begins to appear in branches that will be stabilized into the adult CPs. The number of 22C10 positive branches gradually increases from 24h APF to 28h APF, and by 38h APF (completion of pruning) all the retained branches express FasII as well as 22C10. Once branches are stabilized, and the adult pattern of multiple CPs emerges, FasII expression in the stabilized CPs begins to decline, a feature that is evident by 48h APF (data not shown). The downregulation of FasII may be significant for continued expansion of the arbors, which occurs through the remainder of metamorphosis, and presumably matches muscle growth (Hebbar and Fernandes, 2004). 64
71 FasII is absent from the adult NMJ, a feature that distinguishes the synapses of the adult DLM from its larval counterpart. FasII is an important component at the mature larval synapse (Schuster et al., 1996a). Thus, FasII plays a role during development of the adult innervation pattern of the DLM, and it is likely that other cell adhesion molecules or a different form of FasII may be present at the mature NMJ. In Manduca, it has been reported that during development of leg muscle innervation, a transmembrane FasII is present, whereas a GPI linked FasII later becomes localizes to the adult NMJs (Knittel et al., 2001). Another potentially interesting aspect of FasII expression is that at 38h APF, it is present in a compartment that encompasses the axons of the PDMN. This is likely to be glia, as it is not HRP positive. Since the glial expression is evident after the pruning phase is completed, it may have a bearing on the subsequent phase of arbor expansion and synapse formation. Recent studies in vertebrates have shown that thrombospondin released from glia bring about synapse formation (Christopherson et al., 2005). This may be relevant to synapse formation at the DLM, as bouton formation is initiated only after the pruning phase is completed (Hebbar and Fernandes, 2004). Regulating arbor development The onset of FasII expression is coincident with the onset of a phase of motor neuron elaboration (18-24h APF), during which higher order branching occurs, resulting in the formation of arbors. In addition to the expansion of arbors, new secondary branches are also being added, and these presumably comprise the 20% of FasII positive branches that have not elaborated into higher order branches. It is important to bear in mind that branch elaboration and branch addition occurs during the time that myogenesis is ongoing. The continued fusion of myoblasts serves to increase muscle surface area, which may be conducive for branch addition. During this period, both electrical activity (Hebbar and Fernandes, 2004) and the muscle surface (Fernandes and Keshishian, 1998) are known to promote branch elaboration, and therefore a role for FasII must be considered in this context. In FasII hypomorphs, the numbers of CPs (or stabilized secondary branches) that make up the adult innervation pattern are significantly increased. This is brought about not by increasing the number of second order branches, but by generating longer and more elaborated secondary branches during the pupal phase. A possible explanation is that FasII normally restricts branch outgrowth and that the loss of adhesion allows second order branches to explore and elaborate over the muscle surface. In restricting branch elaboration, it is likely that FasII acts on secondary branches through local interactions, with FasII on other secondary branches and/or on the muscle restricts expansion of secondary branch arbors. Similar 65
72 spatio-local interactions through Ca 2+ mediated signaling are believed to regulate the size of dendritic arbors in developing optic tectal neurons of Xenopus (Cline, 2001). The increased expanse is likely to be favorable for stabilization perhaps by access to protective signals, and this is reflected in the increased number of stabilized secondary branches/cps in the adults. Since FasII is a homophilic cell adhesion molecule (Grenningloh et al., 1991), it is possible that two molecules on the axonal branch interact to restrict secondary branch elaboration. Alternatively, FasII on the axon may interact with muscle FasII to bring about the restriction. Increasing the levels of FasII by using elav-gal 4 and MHC-Gal4 to overexpress FasII, results in fewer CPs. In this instance, the pool of second order branches is reduced, and changes in adhesion can be used to explain the phenotype. It is possible as a result of increased FasII levels; there is increased adhesion between axons (or between axon and muscle) that in turn inhibits the formation of secondary branches. Neuronal FasII or muscle FasII? FasII expression in the presynaptic compartment is obvious during the 18-38h period, and it is not surprising that a neuronal driver (elav-gal 4) is able to rescue the hypomorphic phenotypes. However, the ability of a muscle driver to suppress FasII hypomorphic phenotypes was surprising. This suggested a role for muscle-derived FasII, which is undetectable in our immunostaining experiments presumably due to low levels. Interestingly, elav-gal 4, a reported neuronal driver is also expressed in muscle fibers starting at about 16h APF (Fernandes and Keshishian, unpublished observations), and must be considered in explaining how the innervation phenotype is rescued. When the elav-gal 4 driver is used to express FasII in the hypomorphic background, there is a complete rescue of the number of CPs seen in the adult. However, when the MHC-Gal4 driver is used, the number of CPs is actually reduced. MHC driven expression is likely to increase FasII disproportionately in one compartment, while elav-gal 4 would increase it in both pre- and post-synaptic compartments. Thus, the difference in number of CPs observed in the two conditions of overexpression suggests that a balance between pre-and post-synaptic FasII regulates aspects of arbor development. A novel role for FasII in adult NMJ formation. An important question implicit in our studies is the manner in which molecules are re-utilized in the second round of NMJ formation during metamorphosis. In embryonic/larval NMJ development, FasII is important for growth cone guidance (Grenningloh et al., 1991), target selection (Davis et al., 1997) and 66
73 subsequently in synapse growth and maintenance (Schuster et al., 1996a). Our results show that FasII plays a role in stabilizing branches, thus influencing patterning of the adult NMJ. Two major attributes of FasII expression suggest that the mechanism(s) by which it operates in the adult context is distinct from the embryo/larva. This is not completely unexpected since the developmental events in the formation of the embryonic/larval and adult NMJs are distinct (Fernandes and Keshishian, 1995; Fernandes and Keshishian, 1999). First, FasII is only present during metamorphosis, when innervation is being patterned, and is absent from the adult NMJ. This transient expression of FasII is unlike the situation in the larval NMJ. FasII is downregulated at the early stage of synapse formation in the late embryo but is subsequently seen at the mature larval NMJ and regulates synapse maintenance (Schuster et al., 1996a). At the adult NMJ, since FasII is absent, it appears that other cell adhesion molecules may be involved. The absence of FasII from the adult synapse also suggests that the molecular architecture of the adult synapse differs from its larval counterpart. Second, at the larval NMJ, reductions in FasII levels result in a reduced NMJ (Schuster et al., 1996a), and it can be argued that the NMJ at the DLM is expanded. The extremely small size of boutons on the DLM fibers is a challenge for the purposes of obtaining bouton counts (Hebbar and Fernandes, 2004). However, since the higher order branches of each CP bear boutons (Figure 1), and given the increase in number of CPs in FasII hypomorphs, it is conceivable that the NMJ is expanded. Average expanse of arbors measured by muscle area occupied, as well as number of branch tips is not significantly different between controls and FasII hypomorphs (data not shown). Although not directly related to size of the NMJ, it has been documented that axonal projections of developing adult wing sensory neurons in the CNS also display increased branching when FasII levels are severely reduced (Whitlock, 1993). Relationship between FasII and electrical activity In several allelic combinations of the hyperactive mutant, eag 1 Sh 120b, larval NMJs are expanded through increases in bouton number as well as through increased higher order branching (Budnik et al., 1990). It has been demonstrated that an increase in electrical activity is associated with reduced FasII levels, which in turn cause expansion of the larval NMJ, suggesting that electrical activity and FasII act in the same pathway (Schuster et al., 1996b). Our studies with the development of DLM innervation suggest that the relationship between electrical activity and FasII is different. Although the establishment of stereotypic branch points of DLM motor axons (multiterminal innervation) on the muscle and the terminal arbor expanse at each branch point may not have a direct parallel with the larval NMJ (single-terminal 67
74 innervation), it is nevertheless useful to dissect apart the relationship between electrical activity and FasII in the context of patterning an adult NMJ (Table 6). The number of CPs or terminal arbors is increased in FasII hypomorphs and decreased in electrical activity mutants. Although FasII is absent at the adult NMJ, it is present during the patterning of innervation earlier in the metamorphic phase. At 24h APF, FasII is present at wild-type levels in eag 1 Sh 120b, as measured along second order branches of the motor axons. This is different from the larval NMJ, where FasII levels are reduced. At 24h APF, the length of secondary branches is normal, but branch addition (measured by density of second order branching) is altered. Thus, branch length is not regulated by electrical activity, and possibly influenced by FasII. Each second order branch develops an arbor, and the expanse is increased in hyperactive mutants. How can this be reconciled with increased arbor expanse that is also seen in hypomorphs? We propose that elaboration of higher order branches that takes place prior to the pruning phase is regulated by two opposing forceselectrical activity expands the arbor, and FasII serves to restrict the expansion. In FasII mutants, therefore, loss of FasII allows unrestricted expansion; whereas in eag 1 Sh 120b, hyperactivity drives the expansion, overriding the effects of normal FasII levels. Despite the presence of arbors with increased expanses, why then are fewer arbors stabilized in hyperactive mutants? As we have suggested in a previous study (Hebbar and Fernandes, 2004) this effect is likely due to enhanced pruning and resembles events at the developing vertebrate NMJ, where chronic stimulation of muscle accelerates synapse elimination (Thompson, 1983). Although pruning occurs prior to the appearance of morphologically identifiable boutons, it is likely that the terminal arbors are nascent synapses, and consistently, they do express the pre-synaptic marker synaptotagmin (Hebbar and Fernandes, 2004). In conclusion, we have identified a role for FasII in patterning the DLM innervation in the context of branch stabilization. We show that manipulating FasII levels does not grossly alter the innervation pattern, but that it affects attributes such as number of contact points, branch length and expanse of arbors. Our data indicate that FasII primes branches for stabilization and future studies will aim to identify molecules that act in concert with FasII to regulate branch development of adult motor neurons during metamorphosis. 68
75 3.6 References Albin, S. D., and Davis, G. W. (2004). Coordinating structural and functional synapse development: postsynaptic p21-activated kinase independently specifies glutamate receptor abundance and postsynaptic morphology. J Neurosci 24, Bagri, A., Cheng, H. J., Yaron, A., Pleasure, S. J., and Tessier-Lavigne, M. (2003). Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell 113, Baines, R. A., Seugnet, L., Thompson, A., Salvaterra, P. M., and Bate, M. (2002). Regulation of synaptic connectivity: levels of Fasciclin II influence synaptic growth in the Drosophila CNS. J Neurosci 22, Brand, A. H., and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, Budnik, V., Zhong, Y., and Wu, C. F. (1990). Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci 10, Chang, Q., and Balice-Gordon, R. J. (1997). Nip and tuck at the neuromuscular junction: a role for proteases in developmental synapse elimination. Bioessays 19, Christopherson, K. S., Ullian, E. M., Stokes, C. C., Mullowney, C. E., Hell, J. W., Agah, A., Lawler, J., Mosher, D. F., Bornstein, P., and Barres, B. A. (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, Cline, H. T. (2001). Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol 11, Daniels, R. W., Collins, C. A., Gelfand, M. V., Dant, J., Brooks, E. S., Krantz, D. E., and DiAntonio, A. (2004). Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci 24, Davis, G. W., Schuster, C. M., and Goodman, C. S. (1997). Genetic analysis of the mechanisms controlling target selection: target-derived Fasciclin II regulates the pattern of synapse formation. Neuron 19, Fernandes, J., Bate, M., and Vijayraghavan, K. (1991). Development of the indirect flight muscles of Drosophila. Development 113, Fernandes, J., and Keshishian, H. (1995). Neuromuscular development in Drosophila: insights from embryos and pupae. Curr Opin Neurobiol 5, Fernandes, J., and VijayRaghavan, K. (1993). The development of indirect flight muscle innervationin Drosophila melanogaster. Development 118, Fernandes, J. J., and Keshishian, H. (1998). Nerve-muscle interactions during flight muscle development in Drosophila. Development 125, Fernandes, J. J., and Keshishian, H. (1999). Development of the adult neuromuscular system. Int Rev Neurobiol 43, Finlayson, L. H. (1975). Development and degeneration. In "Insect Muscle" (P. N. Usherwood, Ed.), pp Academic Press, London. Ganetzky, B., and Wu, C. F. (1983). Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet 1, Gao, P. P., Yue, Y., Cerretti, D. P., Dreyfus, C., and Zhou, R. (1999). Ephrin-dependent growth and pruning of hippocampal axons. Proc Natl Acad Sci U S A 96, Grenningloh, G., Rehm, E. J., and Goodman, C. S. (1991). Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell 67,
76 Harrelson, A. L., and Goodman, C. S. (1988). Growth cone guidance in insects: fasciclin II is a member of the immunoglobulin superfamily. Science 242, Hebbar, S., and Fernandes, J. J. (2004). Pruning of motor neuron branches establishes the DLM innervation pattern in Drosophila. J Neurobiol 60, Hoyle, G. (1983). "Muscles and their neural control." John Wiley, New York. Hummel, T., Krukkert, K., Roos, J., Davis, G., and Klambt, C. (2000). Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron 26, Ikeda, K., and Koenig, J. H. (1988). Morphological identification of the motor neurons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Comp Neurol 273, Ikeda, K., Koenig, J. H., and Tsuruhara, T. (1980). Organization of identified axons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Neurocytol 9, Johansen, J., Halpern, M. E., Johansen, K. M., and Keshishian, H. (1989). Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J Neurosci 9, Kantor, D. B., and Kolodkin, A. L. (2003). Curbing the excesses of youth: molecular insights into axonal pruning. Neuron 38, Knittel, L. M., Copenhaver, P. F., and Kent, K. S. (2001). Remodeling of motor terminals during metamorphosis of the moth Manduca sexta: expression patterns of two distinct isoforms of Manduca fasciclin II. J Comp Neurol 434, Lee, T., Marticke, S., Sung, C., Robinow, S., and Luo, L. (2000). Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron 28, Mathew, D., Popescu, A., and Budnik, V. (2003). Drosophila amphiphysin functions during synaptic Fasciclin II membrane cycling. J Neurosci 23, Nguyen, Q. T., and Lichtman, J. W. (1996). Mechanism of synapse disassembly at the developing neuromuscular junction. Curr Opin Neurobiol 6, Robinow, S., and White, K. (1988). The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol 126, Sanes, J. R., and Lichtman, J. W. (1999). Development of the vertebrate neuromuscular junction. Annu Rev Neurosci 22, Schuster, C. M., Davis, G. W., Fetter, R. D., and Goodman, C. S. (1996a). Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17, Schuster, C. M., Davis, G. W., Fetter, R. D., and Goodman, C. S. (1996b). Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron 17, Sweeney, S. T., and Davis, G. W. (2002). Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron 36, Thompson, W. (1983). Synapse elimination in neonatal rat muscle is sensitive to pattern of muscle use. Nature 302, Whitlock, K. E. (1993). Development of Drosophila wing sensory neurons in mutants with missing or modified cell surface molecules. Development 117, Wu, C. F., Ganetzky, B., Haugland, F. N., and Liu, A. X. (1983). Potassium currents in Drosophila: different components affected by mutations of two genes. Science 220, Zito, K. (2003). The flip side of synapse elimination. Neuron 37,
77 Table 1: Range of CPs as displayed by various genotypes Genotype No. of CPs (mean ± s.e.m) Range of CPs <5 CPs % 5 CPs % >5CPs % Oregon R 5.2 ± 0.12 (20) FasII e ± 0.16 (14)*** * FasII e76 /FasII EB ± 0.17 (17)*** ** FasII e76 ; UAS-FasII; elav-gal ± 0.26 (16) FasII e76 ; UAS-FasII; MHC-Gal4 4.6 ± 0.18 (13)** * 46 8 FasII e76 ; UAS-FasII; D42-Gal4 5.7 ± 0.14 (11)** UAS-FasII; elav-gal ± 0.15 (19)*** *** 37 5 UAS-FasII; MHC-Gal ± 0.20 (11)*** * 46 0 *p<0.05, **p<0.025 and ***p<0.005 Number in parenthesis represents sample size Table 2: Adult muscle length is increased in FasII e76 /FasII EB112 animals Genotype DLMa muscle length (µ) Oregon R (females) ± (7) FasII e93 (females) ± (6) FasII e86 (females) ± (5) FasII e76 (females) ± 8.6 (5) FasII e76 /FasII EB112 (females) ± 10.93* (6) FasII e76 ; UAS -FasII (males) ± (5) FasII e76 ; UAS-FasII; MHC-Gal4 (males) ± a (6) Values are mean + s.e.m (n). * p< 0.05 between Oregon R and FasII e76 /FasII EB112 a p< 0.05 between FasII e76 ; UAS -FasII and FasII e76 ; UAS-FasII; MHC-Gal4 71
78 Table 3: Morphometric characteristics at 18h APF Morphology Oregon R FasII e76 /FasII EB112 (11) (8) Density of second-order branching 3.85 ± ± 0.33 Values are mean ± s.e.m Number in parenthesis represents sample size No. Of secondary branches ± ± 2.01 Length of secondary branch 4.90 ± ± 0.43 Table 4: Morphometric characteristics at 24h APF Morphology Oregon R (20) FasII e76 /FasII EB112 (16) UAS-FasII; elav-gal4 (13) UAS-FasII; MHC-Gal4 (11) eag 1 Sh 120b (10) Density of secondorder branching No. Of secondary branches Length of secondary branch No. Of secondary branches with arbors Average area occupied by arbors of a secondary branch (µm 2 ) 2.26 ± ± ± ± ± 0.38*** ± ± ± 1.70 * ± 1.77 * 40.5 ± 5.0* 6.49 ± ± *** 5.58 ± ± ± ± ± ± 0.62 * 2.90 ± 0.5 * 4.0 ± ± ± *** ± ± ** ± 63.2* Values are mean ± s.e.m Number in parenthesis represents sample size *p<0.05, **p<0.025 and ***p<
79 Table 5: Secondary branches with arbors on DLMa before and after pruning. Age No. of secondary branches with arbors FasII positive branches 22C10 positive branches 24h APF 10.6 ± 0.88 (6) 8.33 ± 1.25 (6) 3.88 ± 0.30 (9) 38h APF 4.86 ± 0.14 (7) 5.0 ± 0.0 (3) 4.75 ± 0.25 (4) Values are mean ± s.e.m Number in parenthesis represents sample size Table 6: Relationship between FasII and electrical activity during the patterning of DLM innervation FasII e76 /FasII eb112 eag 1 Sh 120b # CPs (adult) FasII levels (24h) WT 2º branch length WT Density of 2º WT Arbor expanse 73
80
81
82
83
84
85
86
87 Retraction of larval NMJs Outgrowth & Elaboration Pruning & Stabilization Arbor expansion & Bouton formation 0h 12h 24h 36h 48h 96h/Adult 18h: A subset of 2º branches (and their arbors) is FasII positive 38h: Stabilized 2º branch expresses FasII and 22C10. Expression in the arbor declines. FasII absent from adult NMJs FasII expression FasII +ve arbors MT markers STABILIZATION FasII -ve arbors PRUNING Figure 8. A schematic representation of the role of FasII in patterning DLM innervation. There are 3 phases of arbor development that result in the adult pattern of DLM innervation: Outgrowth and elaboration of adult-specific motor neuron branches, pruning of excessive branches, and the expansion of stabilized arbors. FasII is expressed during the first two phases. Between 14-18h APF, secondary branches grow out and begin elaborate higher order branches on the muscle surface. By 18h APF, a subset of branches with higher order arbors is positive for FasII. At 24h APF, some branches that bear arbors also express Microtubule (MT) associated protein, Futsch/22C10, and are the ones that will be stabilized to generate the stereotypical multi-terminal innervation at the adult DLMs. The remainder of branches are either FasII (+)/Futsch/22C10 (-) or FasII(-) /Futsch/22C10 (-). Since not all FasII (+) branches are retained, we propose that FasII primes branches for stabilization, and that it initiates a series of events that result in the expression of molecules such as 22C10, which complete the stabilization process. These stabilized branches survive the pruning phase. The down regulation of FasII during the last phase (decline is indicated by the lighter bar, and absence by the open bar) is likely to have a bearing on the expansion of stabilized arbors during the remainder of metamorphosis. FasII is absent at the adult stage, and another cell adhesion molecule is likely to be involved in maintenance of the terminal arbor. 81
88 Appendix 2: Characterization of DLM synapses in FasII e76 /FasII EB112 Characteristics of the adult DLM terminal arbor Morphology Oregon R Fas II e76 /FasII EB112 (7) (5) Degree of higher-order branching 12.4 ± ± 3.0 (Number of branch tips) Number of synapses/100 (µ 2 ) 1.18 ± ± 0.09 Area occupied by a terminal arbor (µ 2 ) 8633 ± ± 1509 Values are mean ± s.e.m Number in parenthesis represents sample size 82
89
90 Chapter 4 Glial-Neuronal Interactions Influence Stabilization of Motor Neuronal Branches in Drosophila 4.1 Abstract Motorneurons that innervate the dorsal longitudinal (flight) muscles, DLMs, make multiple points of contact along the length of fibers. The stereotypy of the innervation lies in the number of contact points (CPs) made by each motorneuron and is established as a consequence of pruning that occurs during metamorphosis. Coincident with the onset of pruning is the arrival of glial processes that eventually ensheath persistent branches. To test a possible role for glia during pruning, we disrupted glial ensheathment using a targeted expression of dominant negative shibire. Absence of ensheathment results in fewer CPs. To determine if it is a consequence of increased pruning, we examined innervation at a time when pruning is underway. We quantified the number of branches displaying discontinuities in their membrane; an indicator of the level of pruning. An absence of glial ensheathment causes a two-fold increase in the discontinuities, indicating that pruning is enhanced. Thus glial-neuronal interactions, specifically during pruning are important for the patterning of adult innervation. We also present data that indicates a role for FasII in mediating this communication. At the end of the pruning phase, FasII is seen to localize to glia and envelop each of the stabilized contact points. When the levels of glial FasII are increased using the Gal 4/UAS system, pruning of secondary branches is enhanced. Our studies suggest that glia regulate pruning of secondary branches by influencing the balance between stabilization and pruning. This was confirmed by an observed rescue of the innervation phenotype in FasII hypomorphs by overexpressing FasII in glia. 84
91 4.2 Introduction Glia ensheath synapses in the central and peripheral nervous systems (CNS and PNS) and play roles in the proper functioning and maintenance of the adult nervous system (Araque et al., 1999). Synaptic glia ensure optimal functioning of synapses by clearing out ions and neurotransmitters from the cleft (Bergles and Jahr, 1998). Glia can also detect and respond to synaptic activity by regulating neurotransmitter release (Reist and Smith, 1992; Rochon et al., 2001). In addition to their role in the mature nervous system, glia are also important during development. In Drosophila glia are known to guide sensory axons into the CNS (Sepp et al., 2001). In vertebrates, astrocyte derived soluble factors have been shown to be important in the formation of functional synapses (Pfrieger and Barres, 1997, Ullian et al., 2001; Nagler et al., 2001). Recently it has been demonstrated that immature astrocytes express Thrombospondins that work along with other astrocyte derived signals to facilitate synapse assembly in the CNS (Christopherson et al., 2005). In the PNS, Schwann cells also promote synapse formation in spinal motor neurons (Ullian et al., 2004) and the development of the neuromuscular junction (Reddy et al., 2003). Glial influence is also important for the refinement or sculpting of existing connections. For instance, experimentally induced retraction of Bergman glia in the cerebellum decreases activity dependent elimination of climbing fiber synapses (Iino et al., 2001). At the vertebrate NMJ, glia are known to play a role in axon branch removal during synapse elimination (Bishop et a, 2004). The remodeling of insect nervous systems during metamorphosis also has instances of glial dependent axon branch removal. One such example has been demonstrated during pruning of axonal branches in the mushroom bodies of Drosophila (Awasaki and Ito, 2004; Watts et al., 2004). In this case, glia actively eliminates axonal branches by engulfment. Although glial-neuronal interactions have been implicated in remodeling, little is known about the molecular nature of the communication (Broadie, 2004). Here we present our investigations that have identified a role for FasII mediated neuron-glial interactions that influence pruning of axonal branches during the formation of flight muscle innervation in Drosophila. The Dorsal Longitudinal flight Muscles (DLMs) are innervated by motor axons that make multiple contacts along the length of the muscle fibers (Hebbar and Fernandes, 2004). The number of contact points (CPs) is stereotypic, and the stereotypy arises during metamorphosis as a consequence of pruning. More than 70% of adult-specific motor neuron branches are eliminated. We have shown that an earlier expression of FasII in a subset of branches initiates their stabilization (Hebbar and Fernandes, 2005). We also observed that at the end of the pruning phase, in addition to being expressed in the 85
92 stabilized CP, FasII is also expressed in a glial compartment. This led us to investigate a role for glia during the patterning of DLM innervation. We followed glial morphogenesis and show that like the innervation, they too undergo remodeling. However, the outgrowth of adult-specific glial processes is delayed with respect to innervation; they grow out just before the onset of pruning. By using the UAS/Gal 4 system of targeted overexpression, we expressed temperature sensitive dominant negative shibire (Kitamoto, 2002) to suppress functions including endocytosis, selectively at the glial membrane. This results in a nearly complete absence of glial processes, and adult DLMs display fewer CPs. During the pruning phase, there is an increase in the number of axonal branches displaying membrane discontinuities, which is a sign of degeneration. Thus, in the absence of glia, axonal branches undergo pruning, suggesting that glia have a protective role. Overexpression of FasII in glia has no effect on the earlier elaboration phase but instead observe a net enhancement of the pruning phase. This is also reflected in the reduced number of terminal arbors in the adult. In addition we have also rescued the phenotype of hypomorphic mutants by overexpression of FasII in glia. 4.3 Materials and Methods Fly strains: Oregon R raised on standard Drosophila food at 25 ºC was used as the wild-type strain. repo- Gal 4 (2 chromosome insert, source: B.Jones, NYU) was recombined with UAS-2xEGFP (source: H.Keshishian, Yale University, NH) to generate a combination repo Gal-4;UAS-2xEGFP. This line was used to examine glial remodeling during metamorphosis. Temperature shift regimes: To disrupt glial membrane endocytosis, UAS-shi ts (III) flies were crossed to the previously generated repo Gal-4; UAS-2xEGFP flies. The crosses were maintained at 18 ºC. Three heat pulse regimes were applied. In the first regime, third instar progeny were subjected to 31ºC till adulthood. These adults were then processed for immunocytochemistry. Controls included repo Gal-4;UAS-2xEGFP that were subjected to the same heat pulse. In the second regime, their instar larvae were heat pulsed for 12 hours at 31ºC and the resulting pupae exhibited head eversion. Control animals were aged based on their innervation and muscle profiles (Fernandes and VijayRaghavan, 1993) at 14h APF. The third regime was targeted for mid pupal period, third instar larvae were heat pulsed for 26 hours at 31ºC. At the end of the heat pulse, age of the animals was approximately 31h APF. To examined the role of FasII, FasII 86
93 overexpression in glia was carried out by crossing UAS-FasII (source: G.Davis, USCF) to +;repo Gal- 4;UAS-2xEGFP. Rescue experiments were carried out in male progeny of the cross involving FasII e76 ; UAS FasII and +;repo Gal-4;UAS-2xEGFP. Immunochemistry: The general protocol followed was as described previously (Hebbar and Fernandes, 2004) Tissues were fixed with 4% paraformaldehyde (Ted Pella, Inc, CA). 10% donkey serum in 0.1% BSA and 0.3% Triton-X buffered saline was used as a blocking solution prior to primary antibody application. The following primary antibodies were used: anti-hrp (1:200, raised in goat, source: Jackson ImmunoResearch Laboratories, Inc, PA), MAb1D4 (1:2 mouse anti transmembrane FasII, source: (Hybridoma Bank, Iowa), MAb 8C2 (1:25 mouse anti repo, source: Hybridoma Bank, Iowa), anti GFP (1:200, raised in rabbit, source: Molecular Probes, OR). A cocktail of the following secondary antibodies were used: Alexa Fluor 488 Donkey anti-rabbit, Alexa Fluor 555 Donkey anti-mouse and Alexa Fluor 660 Donkey anti-goat (all at 1:200; Molecular Probes, OR). Image Acquisition: All immunostained tissues were visualized using an Olympus FV500 Confocal Microscope. Fluorescent dyes were excited using Ar 488 and HeNe 543nm lasers. Optical Sections of 1µ thickness (for pupal preps) and 1-3 µ (for adult preps) were taken and stacked using Fluoview Software to obtain a 2D projection. The GFP channel has been inverted to black in all the panels presented. Image panels were prepared using Adobe Photoshop 6.0 (Adobe Systems Incorporated, CA). Data Analysis: All morphometric measurements were carried out on 2D projections using Image-Pro Plus 4.5 (Media Cybernetics, MD). In the pupal stages, our analysis focused on primary branches that innervate dorsal muscle, DLM a. DLMs a and b are innervated by one motor neuron, MN5 (Ikeda and Koenig, 1988). The axon of MN5 divides into 2-3 longitudinal branches (each defined as a primary branch). These include the anterior (a), medial (b) and posterior (c) branch (Ikeda et al., 1980). Secondary branches are transverse outgrowths off a primary branch. All values represent mean ± s.e.m. Basic statistical functions such as mean, standard error of mean and two sample Students t test were performed using Microsoft Excel. Chi Square test was performed and interpreted using Minitab program (Minitab Inc, State College, PA). 87
94 4.4 Results Adult neuromuscular junctions arise from the remodeling of larval NMJs during metamorphosis (reviewed in Fernandes and Keshishian, 1999). This has been well studied for the dorsal longitudinal flight muscles (DLMs) (Fernandes and VijayRaghavan, 1993; Hebbar and Fernandes, 2004). At the onset of the metamorphic period, larval NMJs retract and soon after, adult specific longitudinal or primary branches grow along the persistent larval muscles that serve as templates for the DLM fibers. This is followed by the development of transverse second order branches. As myoblasts fuse to initiate fiber formation, higher order branches elaborate on the muscle surface. By the end of the first day of metamorphosis, 24h APF, six DLM fibers have formed (Fernandes et al., 1991) and are innervated by an exuberance of second order branches and their higher order arbors (Hebbar and Fernandes, 2004). Between 24-38h APF, more than 75% of second order branches are pruned and results in generation of a stereotypic number of secondary branches, characteristic of the adult multi-terminal innervation (Hebbar and Fernandes, 2004). The cell adhesion molecule, FasII is expressed in a subset of branches during the elaboration phase (18-24h APF), and is important for branch stabilization (Hebbar and Fernandes, 2005). FasII continues to be expressed in the stabilized secondary branch and additionally in the glial ensheathment seen at 38h APF. We were therefore interested in examining a role for glial FasII in patterning the DLM innervation Glial processes closely associate with the nervous system during remodeling. We first sough to examine glial ensheathment of the Posterior Dorsal Mesothoracic Nerve (PDMN), which innervates the adult DLMs. Its larval counterpart is called the Inter segmemental nerve (ISN), which innervates dorsal muscle fibers (Johansen et al, 1988). To visualize glial processes, we used a GFP as a reporter gene in conjunction with a glial specific Gal4 driver, repo-gal 4. This driver is able to drive reporter gene expression in all post mitotic PNS and CNS glia during embryogenesis and has no expression in neurons (Sepp et al., 2001). We have additionally used anti-repo to label glial nuclei. as a marker for glial cells. In third instar larvae, glial processes ensheath segmental motor nerves along the entire length of the nerve until the nerve-entry point (NEP) where axons defasciculate and form a neuromuscular junction (Figure 1). A few repo-positive nuclei are visible along the length of the motor nerve. By 10h APF, larval boutons/varicosities have retracted, but the nerve trunk remains closely associated with the DLM larval templates (MFs 9, 10 and 19). By 12h APF when the outgrowth of longitudinal primary branches is visible, 88
95 the glial sheath is visible around the nerve trunk. A closer examination reveals that in some areas the nerve trunk is devoid of glia (Figure 1, arrowhead). These are usually more dorsal in location. By 18h APF, primary branches have extended along the developing muscle and the transverse second order branches are elaborating higher order branches over the developing muscle surface. At this stage, glial ensheathment appears to be gradually regressing in a dorsal to ventral direction. The dorsal part of the nerve trunk especially in the region of muscle fibers 9 and 10 is bare; by contrast the nerve trunk over the most ventral muscle, 19, remains ensheathed by the glia. Interestingly, repo positive nuclei at the point of PDMN entry into the DLM target area have visibly increased. By 24h APF, the six DLMs are formed (Fernandes et al., 1991) the primary branches have increased in length and there is an exuberance of secondary branches that are arborized over the muscle (Hebbar and Fernandes, 2004). At this time (Figure 1), glia have completely ensheathed the main nerve trunk. Additionally, glial processes are seen extending longitudinally along the dorsal-most primary branches. By 48h APF, pruning of exuberant branches is completed and the stabilized branches have been incorporated into the innervation pattern as the stereotypic number of contact points (CPs) and their arbors. The glial component has completely ensheathed the PDMN including all the contact points generated after pruning (Figure 1). The glial processes stop short of the terminal arbors that by this time have differentiated into boutons/presynaptic swellings. This pattern of glial ensheathment resembles what is seen in the adult. Disruption of glial endocytosis results in a reduced number of terminal arbors. Our examination of glial ensheathment revealed that glia regress and then begin ensheathing the primary branches around 24h APF, just prior to the onset of pruning. By 48h APF, each stabilized CP is ensheathed. To test if glia are important for patterning the adult innervation, we inhibited their migration by disrupting endocytosis. This approach has been previously described for the mushroom bodies (Awasaki and Ito, 2004). It involves the overexpression of a dominant negative, temperature sensitive shibire (Kitamoto, 2002) specifically in glia using repo-gal 4 (Sepp et al., 2001). The temperature sensitive allele of shibire, shi ts allows temporal control of the disruption of glial endocytosis, specifically during metamorphosis (Temperature shift regimes are described in Materials and methods). One group of crawling third instar larvae grown at 25C were shifted to the higher restrictive temperature of 31C for the entire duration of metamorphosis (4 days). The resulting adults displayed altered innervation patterns. was used as the control group. Controls (repo-gal 4) exhibited no differences in the innervation pattern as compared to the 89
96 wild-type Oregon R (see Table 1 and Figure 1C). In heat pulsed repo-gal 4/UAS-shi ts ; UAS 2xEGFP (experimental) animals, the number of stereotypic CPs is reduced (Figure 2A1 and 2B1). Whereas in the control animals there are CPs, in the experimental animals there are only CPs (Table 2); this reduction is statistically significant (p<0.0025). The reduction is also revealed in the range of CPs displayed by the two genotypes; the control animals exhibit 4-6 CPs (Table 2, Figure 1C) with the majority, 62.5 % displaying the stereotypic 5 CPs. By contrast, the experimental animals displayed 3-5 CPs (Table 1, Figure 1C) with only 11% displaying 5CPs and the majority 88.9 displaying less than 5CPs. This difference is also significant (p<0.05, Figure 1C). Thus, inhibition of glial specific endocytosis disrupts glial ensheathment and this has a bearing on the adult innervation. Interestingly, glial ensheathment is not as extensive in pulsed repo-gal 4/+; UAS 2xEGFP animals (Figure 2A2 and 2B2) as in controls as determined by the less robust reporter gene expression. Alterations in glial ensheathment during metamorphosis enhances pruning of motorneuronal branches. How does the lack or less extensive glial ensheathment result in the observed reduction in the number of CPs? Pupal stages were therefore examined to determine the onset of any developmental defect. Two heat pulse regimes were carried out. One group of animals was heat pulsed animals until the 12h stage, when adult specific outgrowths are growing along the muscle surface (regime 2, Table 1). Our qualitative observations indicate that there are no gross defects in outgrowth of longitudinal branches or in secondary branch outgrowth (data not shown). A second group of animals was heat pulsed animals (regime 3, Table 1) such that the glial specific migration is disrupted during elaboration of branches and their stabilization; our heat pulse spanned 24 hours. The end-point corresponds to the 30-31h APF equivalent at 25C. This is the time when the pruning process is known to be underway (Hebbar and Fernandes, 2004). To independently confirm that this event was indeed underway, we quantified second order branches that bear arbors. At 31h APF, there are secondary branches on DLMa. This number is reduced in comparison to what has been reported for controls at 24h APF ( ; Hebbar and Fernandes, 2004). We have also used another morphological indication that pruning is in progress. In the mushroom bodies (Lee et al., 2000;Awasaki and Ito, 2004) the presence of axon branches with membrane discontinuities have been used as an indicator of pruning via axon breakdown. Likewise, we have also observed the presence of secondary branches that appear to be discontinuous with the rest of the innervation (Figure 3A1). By 31h APF, in the wild type, glia have migrated to completely ensheath the 90
97 primary branches and are beginning to ensheath some secondary branches (Figure 3A1 and 3A2). On an average, almost 30% of the secondary branches undergoing pruning at this time (Table 3). In a few instances, pruning appears to be completed by this phase since we do not observe any discontinuities (Figure 3A2). As expected, heat pulses resulted in defects in glial ensheathment that range from an almost complete absence of ensheathment around the primary branches (Figure 3C2) to thinner ensheathment (Figure 3C1) and in one out of nineteen cases, surprisingly extra glial branches (Figure 3C3). In one case, we observed animals in which the main nerve trunk was severely disrupted such that the motor axons appeared separated from one another (Figure 3C1). We have examined innervation on those animals that exhibited absence of glial processes and no severe nerve trunk defects (for instance Figure 3C1). An obvious defect was a reduction in the number of secondary branches with higher order arbors (Figure 3C2). The experimental animals displayed branches versus in the wildtype (Table 3); a reduction that is statistically significant (p<0.005). This could arise either due to fewer secondary branches or an enhanced pruning. A qualitative observation of the secondary branches reveals that many of them exhibit membrane discontinuities (see arrowheads in Figure 3C2). Our quantification revealed that almost 60% of the secondary branches exhibit signs of pruning. This is in contrast to the control where only one third exhibits signs of breakdown (p<0.005, Table 2). Thus, absence or alterations in glial ensheathment result in an enhanced pruning, which eventually results in a reduction in the number of terminal arbors in the adult. Glial FasII levels are important for the adult innervation. Our results described above suggest that glial-neuronal interaction(s) shape the DLM innervation pattern. What could be the nature of this glial-neuronal interaction? One possible candidate is cell adhesion molecule, FasII. We have previously demonstrated that it appears to be localized to the neuronal and glial component at 38h APF (Hebbar and Fernandes, 2005). Does this localization of FasII within the glial processes have any bearing on their role in pruning/stabilization? To address this question, we examined the effects of altering FasII levels in the glia. Using the repo-gal driver, we overexpressed FasII in glia and examined the effect on the adult innervation. In the adult thorax, FasII is usually absent from the innervating neurons, muscle or glia (Hebbar and Fernandes, 2005). Targeted overexpression with repo-gal 4 results in the localization of FasII in adult glial processes (data not shown), confirming presence of the protein. In repo-gal 4/+; UAS FasII/+ adults the average number of CPs is (Table 2), a reduction which is statistically significant in 91
98 comparison to controls (p<0.05, Table 2). The number of contact points ranges from 4-6 CPs with only 32.6% of animals displaying 5 as compared to 69.3% in the wildtype (Table 2, Figure 1C). Looked at in a different way, 52.6% of adults, in comparison to 7.7% in the wildtype, display less than the stereotypic 5 CPs, a difference that is statistically significant (p<0.05, Figure 1C and Table 2). Thus, glial FasII levels are important in the generation of the stereotypic adult innervation. We had previously described that FasII hypomorphs exhibit an increased number of CPs (Hebbar and Fernandes, 2004), which is due to an enhanced stabilization. To determine if glial FasII may have a role in this process we asked if FasII overexpression in glia could rescue the FasII e76 hypomorphic phenotype. Our examination of the adult innervation in FasII e76 ; repo-gal 4/UAS FasII animals revealed that increasing FasII in glia can rescue the hypomorphic phenotype. Whereas FasII e76 ; UAS FasII animals displayed an increased number of CPs, (p<0.05 as compared to wildtype, Table 1), in FasII e76 ; repo-gal 4/UAS FasII there are CPs, that is no different than the wildtype (Table2). This rescue is also reflected in the range of CPs displayed. In FasII e76 ; UAS FasII animals, CPs range from 4-6 with only 7.7% displaying 5 CPs while a majority, 77% display more than the stereotypic 5 CPs (Table 2 and Figure 1C). As a result of the rescue, CPs range from 3-6 with 53.3 % displaying 5 CPs and only 20% display more than 6 CPs. Thus FasII overexpression in glia rescues the hypomorphic phenotype of increased CPs and is comparable to the wildtype (Table 2 and Figure 1C). Increased levels of FasII in glia enhance the pruning of secondary branches To examine how glial expression of FasII can rescue the hypomorphic phenotype of FasII e76, we examined innervation during pupal development. FasII functions as a cell adhesion molecule (Grenningloh et al., 1991) and it may be possible that experimentally increased FasII expression in glia can alter the course of its progression along the main nerve trunk. Therefore one possibility for the defect may be the absence of glial processes. When we qualitatively examined glia at 24 and 31h (Figure 3B) APF, no differences in the profile of glial processes was observed. This is not surprising, given that glia do not express FasII until 38h APF. We have previously reported that FasII is expressed during the earlier phase of branch elaboration, which initiates events that eventually bring about stabilization. This made it necessary to examine arbor size at 24h APF even though glia do not express FasII at this time. As expected, we found no difference either in the number of secondary branches with arbors and in the size of an individual arbor (data not shown); thus confirming that glial FasII does not have any contribution prior to 24h APF. 92
99 We then examined innervation in repo-gal 4; UAS FasII at 31h APF, a time when pruning is underway. As with the wildtype, we observed some intact secondary branches and a few that were in the process of breakdown (arrowhead in Figure 3B). As described in the earlier section, we have used two criteria to determine if the balance between pruning/stabilization is altered. We have quantified the total number of secondary branches with arbors and the number of branches that exhibited membrane discontinuities, a sign of axonal pruning. In repo-gal 4; UAS FasII animals there are only branches, significantly lower than in the wildtype ( ; p<0.0025, Table 3). Therefore the number of intact branches is decreased when FasII levels are increased in the glia. In understanding if overexpression of FasII in glia affects pruning by 31h APF, we must take into consideration that prior to pruning, at 24h APF, number of secondary branches with arbors is not different from the wildtype. However by 31h APF, there are fewer branches with arbors (Table 3), suggesting the Fas overexpression in glia results enhanced breakdown of axonal arbors between 24 and 31h APF. Since the numbers of intact and degenerating axons are the same as in the wildtype (Figure 4), it is possible that at 31h APF, in these animals, breakdown of arbors occurs at the same rate as in the wildtype. Therefore overexpression of FasII in the glia results in an enhanced pruning during metamorphosis. This in turn is reflected in a decreased number of terminal arbors at the adult DLMs. 4.5 Discussion The DLM innervation pattern is established during metamorphosis, which is a four-day period characterized by extensive remodeling of larval tissues to generate the adult form. Some aspects of the remodeling of larval innervation have been previously established (Fernandes and VijayRaghavan, 1993; Fernandes and Keshishian, 1998; Hebbar and Fernandes, 2004). Before adult-specific outgrowth can be initiated, larval NMJs retract, and in case of the DLM, the larval nerve maintains contact with persistent larval scaffolds that prefigure the adult fibers. The patterning of DLM innervation can be divided into three distinct phases: outgrowth and elaboration (12-24h APF), during which arbors of secondary branches expand on the muscle surface; pruning and stabilization (24h- 38h APF), during which a majority of secondary branches are pruned, while a few are stabilized; arbor expansion (38h APF- Adult), during which stabilized secondary branches and their arbors are incorporated into the adult innervation pattern, and begin to differentiate into synapses. Additionally, there is a co-ordinate expansion of muscle fibers and their arbors. 93
100 Coincident with the occurrence of pruning is the arrival of glial processes that eventually ensheath persistent branches. Are glial processes important for patterning DLM innervation? To address this question, we first examined glial ensheathment of the developing innervation and observed that it involves a process of remodeling, which is not synchronous with the remodeling of innervation. Thus, as adult specific neuronal outgrowths begin to elaborate over the muscle surface, glial processes are in the process of receding to a more ventral location. To examine the importance of glia, their migration was disrupted through the targeted expression of dominant negative shibire. As a result, the adult innervation pattern is altered, displaying fewer contact points (CPs). During metamorphosis, a two-fold increase in axonal membrane discontinuities is seen indicating that lack of glial processes results in an enhanced pruning. Thus, glial-neuronal interactions are important for pruning and we provide evidence that they affect the balance between stabilization and pruning. In addition, our studies have also revealed that the cell adhesion molecule, FasII mediates the glial-neuronal interactions. Peripheral glia are remodeled during metamorphosis Each larval motor nerve is ensheathed by glial processes up until the point where individual axons make contact with muscle targets and elaborate into boutons. During metamorphosis, when the innervation is restructured to establish adult-specific patterns, the glial ensheathment also undergoes dramatic changes. By 10h APF, when larval synapses have retracted and new adult specific outgrowth has initiated, the glial ensheathment appears to be morphologically intact in the mesothorax. This is in contrast to ultrastructural studies from Manduca where it has been observed that glial degeneration precedes the retraction of larval synapses (Rheuben, 1992). It is likely that at the light microscopic level any ultrastructural changes that occur cannot be visualized. By 18h APF, there is a loss of glial processes; especially at the dorsal most muscles glial ensheathment appears intact over the ventral muscles. Since the glial sheath is continuous in the ventral region, it is likely that loss over the dorsal muscles is due to regression rather than degeneration. By 18h APF, an increase in glial nuclei is observed, and these are mainly clustered at the point where the PDMN enters the target/muscle field. Whether peripheral influences, either from the innervation or the muscle, promote the proliferation of glia remains to be determined. Neuronal influences on glial proliferation are varied and depend on the system under consideration. One such system is the developing olfactory system of Drosophila and Manduca during metamorphosis. Here, the olfactory receptor axons from the antenna migrate towards the neuropil area in 94
101 the antennal lobe (Tolbert et al., 2004). As the axons migrate, they first encounter a few glial cells at the base of the antennal lobe, before the neuropil, also called the sorting zone. Axons have been shown to promote proliferation of these glial cells and their absence results in a failure of glial cells to proliferate (Rossler et al., 1999). However axons do not influence the proliferation of another class of glia, that are present around the neuropil that is situated within the antennal lobe itself (Oland and Tolbert, 1989). It is known that DLM motorneurons promote myoblast proliferation (Fernandes and Keshishian, 1998; Fernandes and Keshishian, 2005) and this occurs at the same time (18-24h APF) that glial cells are increasing in number. These studies have proposed that neurons may release a mitogen like signal (Fernandes and Keshishian, 2005), and therefore it is reasonable to envision a role for DLM motorneurons in regulating glial proliferation as well. Along with the increase in glial cell numbers, we observe the migration of glial nuclei and their processes towards the dorsal axonal branches. Interestingly, as they move in the dorsal direction, they also begin to send out processes in a longitudinal fashion to ensheath the longitudinal primary branches. It is likely that the DLM axons attract or guide the glial processes towards themselves. In the developing visual system in Drosophila, glial migration is controlled and directed by an axonal scaffold (Huang and Kunes, 1998; Dearborn and Kunes, 2004;Suh et al., 2002). Likewise, olfactory axons in the antennal lobe generate nitric oxide which in turn regulates glial migration (Gibson et al., 2001). Finally, the glial processes are seen enveloping persistent secondary branches after the pruning is completed suggesting that glial ensheathment is an important component of the stabilization phase (discussed further in the next section on the role of glia during metamorphosis). We have previously reported that the adult pattern of innervation becomes evident at 38h APF. These studies indicate that subsequent to pruning, the adult pattern of glial ensheathment is also established. Mechanisms underlying glial regulation of the DLM innervation pattern Patterning of DLM innervation can be classified into distinct stages; outgrowth and elaboration, pruning and arbor expansion of stabilized branches (Hebbar and Fernandes, 2004). Glia do not have a role during the intial stages of outgrowth, as at this time, the larval glial processes are in the process of regression. In the mushroom bodies during axon pruning, glia invade and actively breakdown axon branches (Awasaki and Ito, 2004; Watts et al., 2004). Similarly at the vertebrate NMJ, axons undergo degeneration by shedding their membrane into the surrounding glia (Bishop et al, 2004). In our case, glia are likely to have a protective role during pruning, thus influencing the stabilization of branches that survive the process. Three observations support this notion. First, glia migrate towards secondary branches and 95
102 their arbors when pruning occurs; 24-38h APF. Second, subsequent to pruning, the persistent secondary branches are enveloped by a glial ensheathment. Finally, upon suppression of glial migration, an increase in pruning is observed by 31h APF and many more secondary branches with discontinuous membranes are seen. In addition, there are also fewer intact secondary branches and this is reflected in the fewer CPs in the adult. This protective role for the glia in remodeling is novel and differs from its role in the mushroom bodies. Pruning is intrinsic to the motorneurons, as demonstrated by our previous studies in which increased levels of activity in motorneuron of hyperexcitable mutants, enhances pruning (Hebbar and Fernandes, 2004). Likewise at the mushroom bodies, pruning is intrinsic to the axons. Therefore suppression of glial processes does not eliminate pruning but instead severely delays it (Awasaki and Ito, 2004). Role for glia in remodeling events in insects In early development, glia are important for the development of connectivity specifically in axonal guidance, fasciculation and sorting. For instance, evidence from early grasshopper development implicated a role for glial guidepost cells in establishing segment boundaries (Bastiani and Goodman, 1986). In addition, at the sorting zone of the olfactory system of Manduca (Oland and Tolbert, 1989; Rossler et al., 1999) and in the optic lobes in Drosophila (Poeck et al., 2001), glial cells provide a key signal to the arriving axons. As a result, axons sort themselves and leave the sorting zone in distinct fascicles to reach appropriate targets. In our experiments, when dominant negative shibire is expressed, we are able to suppress glial migration. As a result of the suppression, there are instances where axons are completely separated from each other, suggesting that glial processes influence the fasciculation of axons. However this influence must be minimal since suppression of glial migration does not always result in defasciculation. Defasciculation almost always results in inappropriate targeting of the axons suggesting that axon targeting to appropriate muscles is probably related to their fasciculation. Role of glial-neuronal interactions via FasII in stabilization/pruning. We have previously shown that FasII is present along glial processes in addition to its presence in the stabilized secondary branch. This study suggests that FasII in the glia is likely to influence stabilization. It is likely that FasII mediates an adhesive reaction between the glia and the neuron and thus promotes stabilization during the pruning phase. When FasII is overexpressed using a glial driver, there is an accelerated rate of pruning in these animals and is also reflected by a decreased number of stabilized CPs 96
103 in the adult. That wild-type levels of glial FasII are important for the stabilization is supported by our rescue experiments. The phenotype of enhanced stabilization in hypomorphs is rescued by an overexpression of FasII in the glia. In conclusion, we have described peripheral glial remodeling events at the DLMs during metamorphosis. In addition, we have demonstrated the requirement of glial processes in influencing the balance between pruning and stabilization. We also show that this glial-neuronal interaction is mediated by FasII. Our studies have revealed that the patterning of DLM innervation has to be considered in the context of a tripartite NMJ with the neuron, muscle and glia playing significant and interdependent roles. 97
104 4.6 References Araque, A., Parpura, V., Sanzgiri, R. P., and Haydon, P. G. (1999). Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22, Awasaki, T., and Ito, K. (2004). Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol 14, Bastiani, M. J., and Goodman, C. S. (1986). Guidance of neuronal growth cones in the grasshopper embryo. III. Recognition of specific glial pathways. J Neurosci 6, Bergles, D. E., and Jahr, C. E. (1998). Glial contribution to glutamate uptake at Schaffer collateralcommissural synapses in the hippocampus. J Neurosci 18, Bishop, D. L., Misgeld, T., Walsh, M. K., Gan, W. B., and Lichtman, J. W. (2004). Axon branch removal at developing synapses by axosome shedding. Neuron 44, Broadie, K. (2004). Axon pruning: an active role for glial cells. Curr Biol 14, R Christopherson, K. S., Ullian, E. M., Stokes, C. C., Mullowney, C. E., Hell, J. W., Agah, A., Lawler, J., Mosher, D. F., Bornstein, P., and Barres, B. A. (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, Dearborn, R., Jr., and Kunes, S. (2004). An axon scaffold induced by retinal axons directs glia to destinations in the Drosophila optic lobe. Development 131, Fernandes, J., Bate, M., and Vijayraghavan, K. (1991). Development of the indirect flight muscles of Drosophila. Development 113, Fernandes, J., and Keshishian, H. (1995). Neuromuscular development in Drosophila: insights from embryos and pupae. Curr Opin Neurobiol 5, Fernandes, J., and VijayRaghavan, K. (1993). The development of indirect flight muscle innervationin Drosophila melanogaster. Development 118, Fernandes, J. J., and Keshishian, H. (1998). Nerve-muscle interactions during flight muscle development in Drosophila. Development 125, Fernandes, J. J., and Keshishian, H. (1999). Development of the adult neuromuscular system. Int Rev Neurobiol 43, Fernandes, J. J., and Keshishian, H. (2005). Motoneurons regulate myoblast proliferation and patterning in Drosophila. Dev Biol 277, Gibson, N. J., Rossler, W., Nighorn, A. J., Oland, L. A., Hildebrand, J. G., and Tolbert, L. P. (2001). Neuron-glia communication via nitric oxide is essential in establishing antennal-lobe structure in Manduca sexta. Dev Biol 240, Grenningloh, G., Rehm, E. J., and Goodman, C. S. (1991). Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell 67, Hebbar, S., and Fernandes, J. J. (2004). Pruning of motor neuron branches establishes the DLM innervation pattern in Drosophila. J Neurobiol 60, Hebbar, S., and. Fernandes, J. J. (2005). A role for Fas II in the stabilization of motor neuron branches during pruning in Drosophila. Dev Biol. m.s. in press. Huang, Z., and Kunes, S. (1998). Signals transmitted along retinal axons in Drosophila: Hedgehog signal reception and the cell circuitry of lamina cartridge assembly. Development 125, Iino, M., Goto, K., Kakegawa, W., Okado, H., Sudo, M., Ishiuchi, S., Miwa, A., Takayasu, Y., Saito, I., Tsuzuki, K., and Ozawa, S. (2001). Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 292, Kitamoto, T. (2002). Conditional disruption of synaptic transmission induces male-male courtship behavior in Drosophila. Proc Natl Acad Sci U S A 99,
105 Lee, T., Marticke, S., Sung, C., Robinow, S., and Luo, L. (2000). Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron 28, Nagler, K., Mauch, D. H., and Pfrieger, F. W. (2001). Glia-derived signals induce synapse formation in neurones of the rat central nervous system. J Physiol 533, Oland, L. A., and Tolbert, L. P. (1989). Patterns of glial proliferation during formation of olfactory glomeruli in an insect. Glia 2, Pfrieger, F. W., and Barres, B. A. (1997). Synaptic efficacy enhanced by glial cells in vitro. Science 277, Poeck, B., Fischer, S., Gunning, D., Zipursky, S. L., and Salecker, I. (2001). Glial cells mediate target layer selection of retinal axons in the developing visual system of Drosophila. Neuron 29, Reddy, L. V., Koirala, S., Sugiura, Y., Herrera, A. A., and Ko, C. P. (2003). Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron 40, Reist, N. E., and Smith, S. J. (1992). Neurally evoked calcium transients in terminal Schwann cells at the neuromuscular junction. Proc Natl Acad Sci U S A 89, Rheuben, M. B. (1992). Degenerative changes in the structure of neuromuscular junctions of Manduca sexta during metamorphosis. J Exp Biol 167, Rochon, D., Rousse, I., and Robitaille, R. (2001). Synapse-glia interactions at the mammalian neuromuscular junction. J Neurosci 21, Rossler, W., Oland, L. A., Higgins, M. R., Hildebrand, J. G., and Tolbert, L. P. (1999). Development of a glia-rich axon-sorting zone in the olfactory pathway of the moth Manduca sexta. J Neurosci 19, Sepp, K. J., Schulte, J., and Auld, V. J. (2001). Peripheral glia direct axon guidance across the CNS/PNS transition zone. Dev Biol 238, Suh, G. S., Poeck, B., Chouard, T., Oron, E., Segal, D., Chamovitz, D. A., and Zipursky, S. L. (2002). Drosophila JAB1/CSN5 acts in photoreceptor cells to induce glial cells. Neuron 33, Tolbert, L. P., Oland, L. A., Tucker, E. S., Gibson, N. J., Higgins, M. R., and Lipscomb, B. W. (2004). Bidirectional influences between neurons and glial cells in the developing olfactory system. Prog Neurobiol 73, Ullian, E. M., Harris, B. T., Wu, A., Chan, J. R., and Barres, B. A. (2004). Schwann cells and astrocytes induce synapse formation by spinal motor neurons in culture. Mol Cell Neurosci 25, Ullian, E. M., Sapperstein, S. K., Christopherson, K. S., and Barres, B. A. (2001). Control of synapse number by glia. Science 291, Watts, R. J., Schuldiner, O., Perrino, J., Larsen, C., and Luo, L. (2004). Glia engulf degenerating axons during developmental axon pruning. Curr Biol 14,
106 Table 1: Heat Pulse Regimes applied to +; repo-gal 4/+; UAS-2xEGFP/UAS-shi ts Kept in 18º C Raised to 31º C Age (equivalent to 25º C ) (Permissive temperature) (Restrictive temperature) 1. Embryo---3 rd instar larva Adulthood Adults 2. Embryo---3 rd instar larva 12 hours 14h APF 3. Embryo---3 rd instar larva 26 hours 31h APF Table 2: Range of CPs as displayed by various genotypes Genotype No. of CPs Range of CPs <5 CPs 5 CPs >5CPs (mean ± s.e.m) % % % Oregon R 5.2 ± 0.12 (13) ; repo-gal 4; UAS- 2xEGFP (Heat pulsed) 4.9 ± 0.22 (8) ; repo-gal 4; UAS- 2xEGFP/UAS-shi ts (Heat pulsed) 3.7 ± 0.23 (9)** /y; repo-gal 4/UAS- FasII; UAS- 2xEGFP/+ 4.6 ± 0.17 (19) FasII e76 /y; repo-gal 4/UAS- FasII; UAS- 2xEGFP/+ 4.9 ± 0.30 (15) FasII e76 ; UAS-FasII; ± 0.21(13) p<0.05 comparison with Oregon R, **p< comparison with +; repo-gal 4; UAS- 2xEGFP (Heat pulsed). Number in parenthesis represents sample size 100
107 Table 3: Morphometric characteristics at 31h APF Morphology repo-gal 4; UAS- 2xEGFP repo-gal 4; UAS- 2xEGFP/UAS-shi ts repo-gal 4/UAS- FasII; UAS- 2xEGFP (Heat pulsed) Number of secondary branches with arbors Percentage of degenerating branches (7) (16) (13) 7.85 ± ± 0.54*** 6.14 ± 0.45*** 30.0 ± ± 6*** 22.0 ± 5.0 Number in parenthesis represents sample size Values represent mean ± s.e.m 101
108
109
110
111
112 Chapter 5 The adult abdominal neuromuscular junction of Drosophila: a model for synaptic plasticity 5.1 Abstract During its life-cycle, Drosophila makes 2 sets of neuromuscular junctions (NMJs), embryonic/larval and adult, which serve distinct stage-specific functions. During metamorphosis, the larval NMJs are restructured to give rise to their adult counterparts, a process that is integrated into the overall remodeling of the nervous system. The NMJs of the prothoracic muscles and the mesothoracic dorsal longitudinal (flight) muscles have been previously described. Given the diversity and complexity of adult muscle groups, we set out to examine the less complex abdominal muscles. Specifically, we have characterized morphological attributes of the ventral abdominal NMJ and its associated synapses in dissected preparations. We quantified bouton numbers and size and examined the localization of synaptic markers. The large bouton sizes of these NMJs are particularly advantageous for easy visualization. We have also examined the formation of boutons during development and examined the localization of presynaptic markers at these stages. To test the usefulness of the ventral abdominal NMJs as a model system, we used the identified morphological attributes including bouton numbers, size and expanse, to examine the effects of electrical activity and cell adhesion molecule FasII on their development. Our results indicate that both activity and FasII affect development at the adult abdominal NMJ in ways that are distinct from their larval and adult thoracic counterparts. 106
113 5.2 Introduction In holometabolous insects such as Drosophila and Manduca, there are distinct differences in the organization and function of the nervous system at larval and adult stages. An extensive remodeling of the nervous system occurs during metamorphosis, which is the transition stage. Three major mechanisms contribute to the remodeling: neurogenesis, programmed cell death, and respecification (Truman, 1990). The existing larval nervous system is not completely eliminated but rather, some neurons are removed, others are restructured and new neurons are integrated as the remodeling occurs. The outcome of such radical changes is the generation of adult specific neural circuits that are responsible for the repertoire of behaviors such as flight and copulation which are characteristic of the adult stage. Remodeling during metamorphosis has, therefore, been effectively used as a model for the study of behavioral plasticity (reviewed in Consoulas et al., 2000). The neuromuscular junctions (NMJs) in Drosophila present an excellent model to study remodeling of peripheral synapses during metamorphosis. Two sets of muscles and correspondingly two sets of synapses are formed during the life cycle of the Drosophila. Each set of NMJs develop using distinct mechanisms (Fernandes and Keshishian, 1999). For example, during development of the embryonic/larval NMJ, motor neurons arrive at an already established muscle pattern, and thus neurons do not influence formation of their target muscles. However, during development of the adult NMJ, myogenesis and innervation occur simultaneously (Fernandes and VijayRaghavan,1993; Currie and Bate, 1995), allowing each synaptic partner to influence development of the other (Fernandes and Keshishian, 1998). There are three sets of neuromuscular systems in the adult; the head, thoracic, and abdominal systems. Studies on peripheral remodeling have focused on NMJs one group of flight muscles, the Dorsal Longitudinal Muscles (DLMs) (Fernandes et al., 1991; Fernandes and VijayRaghavan, 1993; Fernandes and Keshishian, 1998; Consoulas et al., 2002). Another set of NMJs on the prothoracic muscles has been recently characterized (Rivlin et al., 2004). By contrast, very little is known about the adult abdominal NMJ. The adult abdominal system, although not as large as the flight muscles in the thorax, present two major advantages as a model system. First, there are seven segments, thus allowing many more observations from a single animal, as opposed to thoracic muscles. Secondly, the abdominal muscles have a simple organization as evidenced by the three muscle groups: dorsal, lateral, and ventral. The goal of this study is to demonstrate the usefulness of the abdominal NMJ as a model to study synaptic plasticity at the abdominal NMJ. Toward this goal, we first characterized the adult abdominal NMJ using anti-hrp, a nervous system marker (Jan and Jan, 1982), to visualize the NMJs in dissected 107
114 preparations. We quantified bouton numbers and size and examined the localization of synaptic markers. The strength of the ventral abdominal muscle system lies in the large and therefore easily identifiable boutons. We also examined the formation of boutons during development and examined the localization of presynaptic markers at these stages. The easy accessibility of the NMJs during metamorphosis makes this an excellent system to study adult synaptogenesis. In the final set of experiments, we have used morphological attributes of the adult abdominal NMJ including bouton numbers, size and expanse, to examine the effects of electrical activity and the cell adhesion molecule FasII on their development. Our results indicate that both activity and FasII affect synapse development at the adult abdominal NMJ in ways that are distinct from their larval and adult DLM counterparts. 5.3 Materials and Methods Fly Strains: Oregon R raised on standard Drosophila media at 25 C was used as the wild-type strain. FasII hypomorphs, FasII e76 (expresses less than 10% of FasII, (Grenningloh et al., 1991), source: V.Budnik, U. Mass Med School, MA) and FasII EB112 (null, Grenningloh et al., 1991), source: G. Davis, UCSF, CA), were used to generate the transhetereozygote FasII e76 /FasII EB112. A hyperexcitable double mutant, eag 1 Sh 120b (Source: H. Keshishian, Yale Univ), was also used. Both genes encode K channel subunits. eag 1 preferentially removes K current (Wu et al., 1983), while Sh 120b reduces I A current (Ganetzky and Wu, 1983). The double mutant properties increase nerve excitability and neurotransmitter release. Staging and Dissection: Two day-old flies were dissected along the dorsal midline in insect saline to expose the ventral abdominal muscles. Wild-type pupae were aged as described previously (Fernandes et al., 1991). Pupae were dissected at 55h and 90h APF. Dissected preparations were fixed in 4% paraformaldehyde (Ted, Pella, CA) for 45 min at room temperature and processed for immunocytochemistry. Immunocytochemistry: Following fixation, all preparations were washed with 0.3% Triton-X (TBS), blocked in a 10% solution of donkey serum and incubated overnight at 4 C in primary antibody (goat anti-hrp at 1:250; Jackson Labs, rabbit anti-synaptotagmin at 1:100 (source: T. Littleton, MIT); rabbit anti-dvglut at 1:1000 (source: A. DiAntonio, Wash. Univ., Missouri); rabbit anti-dlg at 1:10,000 (source: V.Budnik, U. Mass Med School, MA); and mouse anti-fasii at 1:3 (source: Hybridoma Bank, U. Iowa, IA). Following 108
115 incubation, the preparations were washed in TBS and incubated overnight at 4 C in the secondary antibody. Peroxidase conjugated Donkey anti-goat (1:250; Cappel) was used in DAB visualization protocols. For fluorescent double labeling, the following Alexa Fluor (Molecular Probes, OR) secondary antibodies were used: 488 Donkey anti-goat (1:200); 555 Donkey anti-mouse or rabbit (1:200). After incubation, the preparations were washed in TBS. For muscle labeling, Alexa Fluor 594 phalloidin was used after the TBS washes. All preparations were mounted on a slide with either 70% glycerol (DAB preparations) or Vectasheild (Vector Labs, CA). Image acquisition: All fluorescently stained tissues were visualized using an Olympus FV500 confocal microscope. Fluorescent dyes were excited using Ar 488 and HeNe 543nm lasers. Optical sections of 1µ thickness (for pupal preps) and 1-3 µ (for adult preps) were taken and stacked using Fluoview Software to obtain a 2D projection. These stacked images were used for further analysis. Image panels were prepared using Adobe Photoshop 6.0 (Adobe Systems Incorporated, CA). Innervation on pupae was visualized with DIC optics on a Nikon E600 microscope. Digital images were captured using Magnafire 2.0. Images were captured at different focal planes and the different planes were stacked using Image ProPlus 4.0. Data Analysis: Branch and boutons numbers were quantified from images obtained with the confocal microscope. Bouton size (the long diameter) and area measurements were made in Image-Pro Plus 4.5 (Media Cybernetics, MD). All statistical analyses (including the mean, SD, SEM, and a t-test) were performed using Microsoft Excel. 5.4 Results In the abdomen, three muscle sets have been distinguished based on their position and orientation. These are the ventral longitudinal (closest to the ventral midline), the lateral pleural and the dorsal longitudinal muscles (Broadie and Bate, 1991; Currie and Bate, 1991 and Figure 1; top panel). We have used anti-hrp, a nervous system marker (Jan and Jan, 1982) to visualize the neuronal component on these adult muscles. Innervation on these different muscle groups is also distinct (Figure 1; bottom panel). In the case of the ventral and dorsal muscle groups there is a single and distinct nerve entry point at which axons branch onto the individual fibers. In the case of the lateral muscle group, the nerve entry point is not as distinct but instead appears to be at several points along the length of individual fibers. In all three 109
116 muscle sets, presynaptic swellings or boutons are evident. In the lateral muscle group they appear more like en-passant synapses (Figure 1C) while in the dorsal and ventral groups, the axons branch and bear distinct collection of boutons (Figure 1B and 1D). In this paper, we present our characterization of the NMJ at the ventral muscles on segments A3-A5. Single terminal innervation at the ventral abdominal muscles. In each hemisegment, the ventral muscle group is made of 8-10 fibers (Bate and Broadie, 1991). Each hemisegment receives a single nerve entry point (Figure 2) at the anterior end of the muscle group. Very close to the nerve entry point, axonal branches project onto individual fibers. We have quantified branch tips as an estimate of axon branch numbers. On average, each hemisegment receives axonal branches (Table 1). Typically these branches constitute primary and secondary branch points. Each axonal branch bears a cluster of tightly arranged morphological swellings or boutons. The entire cluster of boutons over each hemisegment covers 60-75% of the muscle area (Figure 2). On average, there are 35 boutons per hemisegment that emerge from the single nerve entry point (Table 1). Three bouton classes at the adult ventral abdominal muscles A closer examination reveals that boutons on the ventral muscles are diverse with respect to shape and size. Most boutons are compact but are either rounded or oval in appearance. We have used the long diameter as an estimate of bouton size. Boutons vary from 1.03 to 8.0 µ and the average size across segments is 3.0 µ (Table 1). We also classified boutons according to size (Figure 6), small (0-2µ), medium (2-4µ) and large (greater than 4µ). The medium sized boutons are most prevalent accounting for 71% of total bouton numbers. The larger boutons account for 18% while the smaller boutons are least prevalent accounting for only 11%. This trend is maintained across the segments examined (A3-A5, Figure 6). The larger boutons tend to be mostly oval; the medium sized boutons are rounded in shape while the small boutons are mostly oval in shape. In addition, the small sized boutons are usually present as a string of boutons at the distal (to the nerve entry point) end of the arbor. We have examined the expression of synaptic markers on the ventral abdominal synapses. The vesicle associated protein, synaptotagmin (Littleton et al., 1993) and vesicular glutamate transporter, DVGLUT (Daniels et al., 2004) were used as presynaptic markers. SYT (Figure 3A) localizes to the morphological swellings/boutons of all sizes. This is similar to their presynaptic localization in all larval boutons, Type I, II and III (Littleton et al., 1993). DVGLUT also localizes to all the 3 categories kinds of 110
117 presynaptic swellings (Figure 3B). At the larval NMJ, DVGLUT co-localizes with CSP at the Type I and II glutamatergic synapses, but is absent from the Type III (Daniels, R et al, 2004) which are known to express an insulin like peptide (Gorczyca et al., 1993). We next examined the localization of cell adhesion molecule, Fasciclin II (FasII). FasII is present in all three classes of boutons and, in addition, it also localizes to the axonal component (Figure 3C). This is also similar to FasII localization at larval boutons (Schuster et al., 1996a), but is distinct from the DLM NMJ where FasII is absent (Hebbar and Fernandes, 2005). The PDZ protein, discs large (DLG; Lahey et al., 1994) was used as a post-synaptic marker. DLG anchors ion channel Shaker, glutamate receptors and adhesion molecules such as FasII (Tejedor et al., 199;Zito et al., 1997). DLG is arranged in a rosette like pattern (Figure 3D) around the circumference of the presynaptic region (demarcated by anti HRP). Interestingly, we observe DLG in all three categories of boutons including the small sized boutons. This is distinct from the larval NMJ where the smaller boutons, Type II, lack DLG (Lahey et al., 1994). The abdominal synapse during metamorphosis Having characterized the adult abdominal NMJ, we examined the developing NMJ at two distinct stages in metamorphosis. From earlier studies (Currie and Bate, 1991), it has been established that the larval nerve is maintained through the early remodeling events and the first signs of outgrowth are observed as early as 26h APF. By 48hAPF, the muscle pattern is established and growth cones have reached their muscle targets (Currie and Bate, 1991). We followed the nervous system at 55h APF and at 90h APF (adults emerge between h). At 55h APF, the characteristic single terminal innervation pattern is evident (Figure 4); however, there are fewer branches than in the adult. By 90hAPF (Figure 4), the branching pattern resembles the adult innervation pattern. As early as 55h APF, there are tiny morphological swellings along the axon branches; these are likely to be nascent boutons (inset in Figure 4). These presynaptic swellings grow in size by 90h APF. At this point, the three classes of boutons are easily distinguishable. We have also used the localization of presynaptic markers to complement the morphological observations of boutons at 55h APF. We find that presynaptic markers SYT and DVGLUT localize to newly formed boutons by 55h APF (Figure 5). This suggests that presynaptic differentiation is well under way at this time. That synaptogenesis is still incomplete is evident from the presence of some branches that do not show any visible signs of boutons or presynaptic markers (Figure 5). Alterations in levels of electrical activity and FasII impact the morphology of adult abdominal NMJ. 111
118 Having characterized the adult abdominal NMJ, we attempted to test its usefulness as a model system. The role of electrical activity and cell adhesion molecule FasII during development of the NMJs was examined. These are known to be important for formation of NMJs on larval body wall muscles (Budnik et al., 1990; Jarecki and Keshishian, 1995; Schuster et al., 1996a) and on the adult thoracic muscles (Hebbar and Fernandes, 2004). For altering levels of activity, we used two alleles of hyperexcitable K + channel mutants, eag 1 Sh 120b and eag 4PM Sh 120. We examined innervation at abdominal muscles on segments, A3-A5. The overall innervation pattern and the number of branches remain unaffected with increased activity levels (Figure 7). However, we observed an increased number of boutons on each hemisegment (Table 1). In the wild type, there are usually 34.2 ± 2.8 boutons/hemisegment for A3; this increases to 97.1 ± 9.8 boutons in eag 1 Sh 120b and to 95.2 ±10.0 boutons in eag 4PM Sh 120 (p<0.0005). This increase in boutons was also observed for the other segments as well. An increase in bouton numbers associated with hyperexcitability is also seen at the larval NMJ (Budnik et al., 1990). At the abdominal NMJ this increase in boutons was accompanied by a 50% increase in the area occupied by the entire bouton cluster. In order to correct for the increased expanse of the bouton cluster, we calculated the number of boutons within a 10 µ 2 unit area of the muscle. In the wild type, there are typically 1.5 ± 0.9 boutons per 10 µ 2 of the muscle (Table 1). In the activity mutants, the increase in bouton number is reflected in the increase in number of bouonts/10 µ 2 of muscle ( (p<0.05) for eag 1 Sh 120b and (p<0.005) for eag 4PM Sh 120 ;Table 1). We also examined if this increase is restricted to any one kind of bouton. The number of smaller boutons is preferentially increased and occurs at the expense of the medium and large boutons (Figure 6). At the larval NMJ, increased levels of electrical activity is associated with reductions in FasII levels. We, therefore, examined the effect of reducing FasII levels at the abdominal NMJ. We made use of hypomorphic alleles of FasII, FasII e76 and FasII e76 /FasII EB112, that exhibit less than 10% levels of FasII (Grenningloh et al., 1991). In both allelic combinations, we did not observe a change in the overall innervation pattern or in the number of branches. However, the number of boutons is increased for FasII e76 and this trend is maintained across segments (Table 1). However, in case of both FasII e76 and FasII e76 /FasII EB112 there is a 50-60% increase in expanse of the entire bouton cluster on the muscle that is observed across segments (Table 1 and Figure 7). We therefore corrected for this difference by calculating the number of boutons per 10 µ of muscle area. In FasII e76, there are typically 1.1 ± 0.07 boutons/10 µ 2 of muscle, while in FasII e76 /FasII EB112 there are 0.85 ± 0.08 boutons/10 µ 2 of muscle (p<0.005; Table 1). Thus, severe reductions in FasII result in fewer boutons per unit area of the muscle and a net reduction in 112
119 the NMJ. This phenotype is similar to the reduced NMJ seen in case of null mutants and severe hypomorph, FasII e76 (Schuster et al., 1996a). Lastly, we observed that a reduced NMJ is not accompanied by an alteration in size of the boutons (Figure 6). 5.5 Discussion In Drosophila, the adult NMJs arise from the remodeling of larval counterparts. Remodeling of muscles and their innervation has been studied in one set of the thoracic muscles, the indirect flight muscles (Fernandes and Keshishian, 1999; Hebbar and Fernandes, 2005). Since there is much diversity among muscle groups not only within the thorax, but also in comparison to the abdomen, it is likely that remodeling events may also be distinct from what is known for the IFMs. To complement our ongoing studies on neuromuscular remodeling at the DLMs, we have investigated the adult abdominal NMJ as a system to study remodeling. Additionally, we were interested in examining if there are any parallels or differences with the well-established larval system. As a first step toward our goal, we have described morphological attributes of the NMJ on adult ventral abdominal muscles. One of the strengths of this system is the presence of morphologically identifiable synapses that can be as big as 8.0 µm in diameter. We have used several morphometric criteria to define the ventral abdominal NMJ; these include number of axon branches, number of boutons/synapses, size of boutons/synapses, their distribution and expanse per unit area of the muscle. Our results indicate that the ventral abdominal synapses are very different from their prothoracic (Rivlin et al., 2004) and mesothoracic counterparts (Hebbar and Fernandes, 2004). We also examined the development of NMJs on the ventral abdominal muscles during metamorphosis by following the differentiation of motorneuronal branches into morphological swellings/boutons, and determining the appearance of presynaptic markers such as Synaptotagmin (SYT) and DVGLUT. Finally, in an effort to use the abdominal NMJ as a model system to study synaptic plasticity, we have used the above-described morphological criteria to examine the effect of altered levels of activity and FasII in the adult NMJ. We have made use of FasII hypomorphs and hyperexcitable K + channel mutants to alter levels of FasII and electrical activity respectively. From our experiments we find that alterations in FasII and electrical activity are important in the resulting morphology of the synapses and their expanse on the surface. Interestingly, the effects on synapse number are similar to reported effects on the larval NMJ but effects on the size and expanse of the NMJ are different. 113
120 The ventral abdominal synapse is distinct from other adult synapses The adult abdominal muscles arise de-novo, from a population of twist positive myoblasts that have their origins in the embryo (Bate et al., 1991; Broadie and Bate, 1991; Currie and Bate, 1991). Myoblasts are found associated with segmental nerves and as adult specific outgrowths are seen, myoblasts continue to migrate along the new branches and form distinct muscle primordia that correspond to the dorsal, lateral and ventral muscles. Early events, such as the retraction of larval synapses and the outgrowth of neurites on these developing muscles, have been previously described (Currie and Bate, 1991). However, relatively little is known about the innervation and the synapses on these adult muscles. Our characterization of the ventral abdominal NMJ includes a record of the morphological and molecular organization of its synapses. We find that it is distinct from two other adult NMJs, the prothoracic muscles (Rivlin et al., 2004) and the mesothoracic DLMs (Hebbar and Fernandes, 2004). The innervation is single terminal, as in larvae (Johansen et al., 1989), with one nerve entry point and typically 11 axonal branches. Presynaptic swellings or boutons of varying sizes are present along each branch; the boutons are separated by intervening axonal membrane. Boutons are large and mostly elongate, oval or rounded in appearance. They vary in size from and can be upto 8.0 µm in diameter. Based on their diameters, we have classified them into three classes, small (0-2 µm), medium (2-4 µm) and large (greater than 4.0 µm); medium sized boutons being the most frequent. The overall organization of this NMJ is very different from the previously characterized NMJs on the DLMs (mesothoracic flight muscles; Hebbar and Fernandes, 2004) and on the prothoracic muscles (Rivlin et al., 2004). The DLMs exhibit a unique multi-terminal organization with each arbor/cluster of higher order axonal branches bearing small boutons, of the order of 1.0 µm in diameter (Hebbar and Fernandes, 2004). Prothoracic muscles exhibit a muscle-specific organization; for instance, synapses of muscle 25 resembled the organization of larval type I processes, synapses of muscle 27 are present on an elongated process and are not separated by much of an intervening axonal membrane and finally, in muscle 31, a single axon branch makes en-passant synapses (Rivlin et al., 2004). As with its overall morphological organization, boutons on the ventral abdominal muscles are distinct with respect to their molecular organization. All of the boutons, like their larval counterparts (Johansen et al., 1989), use glutamate as the neurotransmitter. We have used DVGLUT as an indicator for the presence of glutamate (Daniels et al., 2004) and find that it localizes to large, medium and small boutons. This is also the case for the DLMs, where DVGLUT localizes to all bouton sizes (Hebbar and Fernandes, 2005). However, the smaller, Type II-like boutons of the prothoracic muscles do not exhibit 114
121 glutamate localization (Rivlin et al, 2004). The presynaptic marker, SYT localize to all three kinds of boutons. This again is different from the prothoracic muscles where these presynaptic markers do not localize to the smaller Type II boutons (Rivlin et al., 2004). Boutons on the ventral abdominal muscles bear a novel localization pattern of the postsynaptic marker, DLG. In the medium and large boutons, it is present in a rosette fashion around the circumference of the boutons. This pattern resembles the rosetted pattern at the adult leg NMJs in the moth, Manduca (Knittel et al., 2001). Interestingly, DLG is also present in the smaller boutons. This marker is absent in the smaller, Type II boutons of the larvae (Lahey et al., 1994) and Type II-like boutons of the prothoracic muscles (Rivlin et al., 2004). A third synaptic marker, the cell adhesion molecule, FasII, is present in all three bouton classes of the ventral abdominal muscles, and correlates as expected with the presence of its anchor protein, DLG (Tejedor et al., 1997). Morphological swellings/boutons are evident during metamorphosis. The adult NMJs form during metamorphosis, a phase that is characterized by extensive remodeling of the nervous system As a part of the remodeling of larval innervation, larval synapses retract and new adult outgrowth is evident by 48h APF (Currie and Bate, 1991). Confocal analysis indicates that some of these outgrowths have differentiated into presynaptic swellings/boutons by 50h APF. This morphological attribute of development is supported by the presence of presynaptic markers, SYT and DVGLUT in the swellings/boutons. Boutons at this mid-pupal stage probably represent a nascent form that is smaller than the adult counterpart. Our observations of these boutons at 90h APF, suggest that they attain maturity in terms of their size just before emergence. Interestingly, the mid-pupal phase appears to be an important developmental time period for the appearance of presynaptic swellings/boutons. This is the case for the indirect flight muscles and other abdominal muscles such as the dorsal and lateral muscles. Another common feature is the presence of a few large, nodal swellings that give rise to smaller swellings along the branch. Future studies with other synaptic markers will help in describing this phase of adult synaptogenesis. The morphology of the adult abdominal NMJ is dependent on levels of electrical activity and cell adhesion molecule, FasII. We described the ventral, adult abdominal NMJ using morphological attributes such as number of axonal branches, the number and size of synapses and the expanse of these synapses on the muscle surface. Next, we applied these criteria and compared NMJs when cell adhesion molecule FasII levels or 115
122 electrical activity levels were altered. FasII and electrical activity are important in the post-embryonic formation of the mature 3 rd instar larval NMJ (Davis et al., 1996; Schuster et al., 1996a; b). Alterations in levels of FasII or activity alter the number of synapses. Specifically, chronic reductions in FasII result in a severely reduced NMJ, whereas mild reductions in FasII result in an overgrowth of the NMJ (Schuster et al., 1996b). Likewise, hyperactivity results in an overgrowth of the NMJ (Budnik et al., 1990), whereas lowered levels of activity result in ectopic synapse formation (Jarecki and Keshishian, 1995). Our studies at the ventral abdominal NMJ reveal some interesting trends. First, as with the larval NMJ, severe reductions in FasII result in a net reduced NMJ, and hyperexcitability results in an overgrowth at the NMJ (Table 1). In the case of the FasII mutants, it appears that the phenotype is more prominent for the more severe hypomorph, FasII e76 /FasII EB112. Nonetheless, these observations indicate that both electrical activity and FasII are important factors determining the morphology of the adult abdominal NMJ. Second, the net expansion or reduction seen with the mutants is not a consequence of a simple alteration in synapse numbers. In the larval stages of similar activity mutants or FasII hypomorphs, there are alterations in only the numbers of synapses (Budnik et al., 1990; Schuster et al., 1996a), and at times this is accompanied by changes in axonal branch numbers (Budnik et al., 1990). Surprisingly, the reduced NMJ in the case of the severe FasII hypomorphs arises from wild-type complement of boutons (or in a few instances reduced synapses numbers) that have expanded over a larger area (see Table1 for FasII e76 /FasII EB112 ). In both alleles of the activity mutants, the net increased NMJ arises from an increased synapse number. Interestingly, for one of the alleles, eag 1 Sh 120b, there is a small but correlated increase in the expanse of the corresponding muscle surface. However, the net effect is still an increase in the number of synapses per unit area. Coordinated development between synaptic partners can be attributed to homeostasis. At the larval NMJ, one instance of homeostasis is the physiological compensation for presynaptic overgrowth or reduction (Davis and Goodman, 1998). A second instance of homeostasis is the physiological compensation for altered levels of excitability in the postsynaptic partner, the muscle (Paradis et al., 2001). The ventral abdominal NMJ displays a third kind of compensation, at the morphological level. Here, in case of the FasII hypomorphs, an increase in the number of boutons is accompanied by the increase in their expanse such that the resulting NMJ is either reduced (FasII e76 /FasII EB112 ) or is not different from the wildtype (FasII e76 ). Likewise in the activity mutants, an increase in number of boutons is accompanied by a small, inconsequential increase in expanse over the muscle surface resulting in a larger NMJ. The developing DLMs and their innervation also display a similar coordinated development (Hebbar and 116
123 Fernandes, 2005). That developing adult synaptic partners display such remarkable structural homeostasis is not completely unexpected. The pre and postsynaptic partners develop in close spatial and temporal association of one another. Aspects of development of both abdominal (Currie and Bate, 1995) and thoracic musculature (Fernandes and Keshishian, 1998; Fernandes and Keshishian, 2005) are dependent on innervation and vice-versa. Our results suggest that expanse of adult abdominal NMJ is coordinated with the resulting number of synapses and that each synaptic partner possibly influences the development of the other. Finally, alterations in electrical activity affect synapse numbers in a specific manner; the number of small sized synapses increases dramatically. A possible explanation to this may be that the synapses arise from different motorneurons. Altered levels of activity may affect different motorneurons in a differential manner and thus affect synapse formation. Interestingly, the increase in the small synapses is at the expense of the large synapse numbers. From our studies during metamorphosis, we observed that synapse size is attained over a gradual period, thus allowing for potential regulations. Thus, an alternate explanation to this synapse-specific phenotype could be that there is a compensation for the overall increase in synapse numbers during development through an enhancement in the formation of smaller boutons. Identity of motor neurons that innervate the ventral abdominal muscles: Motor neurons that innervate adult muscles are believed to be largely larval motor neurons that undergo remodeling in the periphery as well as in their dendritic arbors (Truman, 1990; Tissot and Stocker, 2000). This remodeling has been demonstrated in Manduca (Levine and Truman, 1985) as well as for some motor neurons in the Drosophila adult head (Tissot et al., 1998). The DLMs are the only adult muscles for which the larval identity of adult motor neurons has been determined (Consoulas et al., 2002). Four of the motor neurons have larval counterparts that are dorsally located. The fifth motor neuron, MN5, is born in the embryo but doesn t have larval targets. It generates processes only during metamorphosis and innervates the most dorsal pair of DLM fibers. The larval identity of abdominal motor neurons has not been examined, and the results presented in this study provide a basis for these future studies. In conclusion, we have characterized the ventral abdominal NMJ. We have also examined representative stages during metamorphosis. In addition, we have used the adult abdominal system to examine the effect of altered FasII or electrical activity levels on the adult abdominal NMJ. Future studies 117
124 on this highly accessible and relatively easy model system will serve to provide an understanding of adult synaptogenesis and help complement studies on remodeling of adult NMJs during metamorphosis. 118
125 5.6 References Bate, M., Rushton, E., and Currie, D. A. (1991). Cells with persistent twist expression are the embryonic precursors of adult muscles in Drosophila. Development 113, Broadie, K. S., and Bate, M. (1991). The development of adult muscles in Drosophila: ablation of identified muscle precursor cells. Development 113, Budnik, V., Zhong, Y., and Wu, C. F. (1990). Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci 10, Consoulas, C., Duch, C., Bayline, R. J., and Levine, R. B. (2000). Behavioral transformations during metamorphosis: remodeling of neural and motor systems. Brain Res Bull 53, Consoulas, C., Restifo, L. L., and Levine, R. B. (2002). Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. J Neurosci 22, Currie, D. A., and Bate, M. (1991). The development of adult abdominal muscles in Drosophila: myoblasts express twist and are associated with nerves. Development 113, Currie, D. A., and Bate, M. (1995). Innervation is essential for the development and differentiation of a sexspecific adult muscle in Drosophila melanogaster. Development 121, Daniels, R. W., Collins, C. A., Gelfand, M. V., Dant, J., Brooks, E. S., Krantz, D. E., and DiAntonio, A. (2004). Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci 24, Davis, G. W., and Goodman, C. S. (1998). Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature 392, Davis, G. W., Schuster, C. M., and Goodman, C. S. (1996). Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity. Neuron 17, Fernandes, J., Bate, M., and Vijayraghavan, K. (1991). Development of the indirect flight muscles of Drosophila. Development 113, Fernandes, J., and VijayRaghavan, K. (1993). The development of indirect flight muscle innervation in Drosophila melanogaster. Development 118, Fernandes, J. J., and Keshishian, H. (1998). Nerve-muscle interactions during flight muscle development in Drosophila. Development 125, Fernandes, J. J., and Keshishian, H. (1999). Development of the adult neuromuscular system. Int Rev Neurobiol 43, Fernandes, J. J., and Keshishian, H. (2005). Motoneurons regulate myoblast proliferation and patterning in Drosophila. Dev Biol 277, Ganetzky, B., and Wu, C. F. (1983). Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet 1, Gorczyca, M., Augart, C., and Budnik, V. (1993). Insulin-like receptor and insulin-like peptide are localized at neuromuscular junctions in Drosophila. J Neurosci 13, Grenningloh, G., Rehm, E. J., and Goodman, C. S. (1991). Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell 67, Hebbar, S., and Fernandes, J. J. (2004). Pruning of motor neuron branches establishes the DLM innervation pattern in Drosophila. J Neurobiol 60, Hebbar, S. and. Fernandes, J. J. (2005). A role for Fas II in the stabilization of motor neuron branches during pruning in Drosophila. Dev Biol. m.s. in press. 119
126 Jarecki, J., and Keshishian, H. (1995). Role of neural activity during synaptogenesis in Drosophila. J Neurosci 15, Johansen, J., Halpern, M. E., Johansen, K. M., and Keshishian, H. (1989). Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J Neurosci 9, Knittel, L. M., Copenhaver, P. F., and Kent, K. S. (2001). Remodeling of motor terminals during metamorphosis of the moth Manduca sexta: expression patterns of two distinct isoforms of Manduca fasciclin II. J Comp Neurol 434, Lahey, T., Gorczyca, M., Jia, X. X., and Budnik, V. (1994). The Drosophila tumor suppressor gene DLG is required for normal synaptic bouton structure. Neuron 13, Levine, R. B., and Truman, J. W. (1985). Dendritic reorganization of abdominal motoneurons during metamorphosis of the moth, Manduca sexta. J Neurosci 5, Littleton, J. T., Bellen, H. J., and Perin, M. S. (1993). Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development 118, Paradis, S., Sweeney, S. T., and Davis, G. W. (2001). Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron 30, Rivlin, P. K., St Clair, R. M., Vilinsky, I., and Deitcher, D. L. (2004). Morphology and molecular organization of the adult neuromuscular junction of Drosophila. J Comp Neurol 468, Schuster, C. M., Davis, G. W., Fetter, R. D., and Goodman, C. S. (1996a). Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17, Schuster, C. M., Davis, G. W., Fetter, R. D., and Goodman, C. S. (1996b). Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron 17, Tejedor, F. J., Bokhari, A., Rogero, O., Gorczyca, M., Zhang, J., Kim, E., Sheng, M., and Budnik, V. (1997). Essential role for dlg in synaptic clustering of Shaker K+ channels in vivo. J Neurosci 17, Tissot, M., Gendre, N., and Stocker, R. F. (1998). Drosophila P[Gal4] lines reveal that motor neurons involved in feeding persist through metamorphosis. J Neurobiol 37, Tissot, M., and Stocker, R. F. (2000). Metamorphosis in drosophila and other insects: the fate of neurons throughout the stages. Prog Neurobiol 62, Truman, J. W. (1990). Metamorphosis of the central nervous system of Drosophila. J Neurobiol 21, Wu, C. F., Ganetzky, B., Haugland, F. N., and Liu, A. X. (1983). Potassium currents in Drosophila: different components affected by mutations of two genes. Science 220, Zito, K., Fetter, R. D., Goodman, C. S., and Isacoff, E. Y. (1997). Synaptic clustering of Fascilin II and Shaker: essential targeting sequences and role of Dlg. Neuron 19,
127 Table 1: Morphological attributes of the adult abdominal NMJ Wild-type eag 1 Sh 120b eag 4pm Sh 120 FasII e76 /FasII EB112 FasII e76 No. of Boutons A3 34.2± ±9.8 *** 95.2±10.0*** 35.1± ±4.4*** A4 A5 38± ± ±8.9 *** 81.9±7.7 *** 59.0± ±11.2*** 22.8±2.5** 33.9± ±9.2* 70.9±7.5*** Presynaptic Arbor A ± ±327.8 *** ± ±188.0* ±165.6*** Area (µ 2 ) A4 A ± ± ±423.1 *** ±379.4 * ± ± ±314.0*** ±280.8* ±438.8*** ±458.0*** No. of boutons/100 A3 1.5± ±0.29* 3.34±0.53** 0.85±0.85* 1.13±0.07 µ 2 A4 A5 1.98± ± ± ±0.44*** 2.53± ±0.20*** 0.71±0.13*** 1.08±0.13* 1.39± ±0.07 No. of Branches A3 10.5± ± ± ± ±1.0 A4 A5 10.9± ± ± ± ± ±0.8* 8.4±0.7* 10.0± ± ±0.9 Average A3 3.13± ± ±0.1* 3.5± ±0.2* Size (µ) A4 A5 2.99± ± ± ± ± ±0.0*** 3.8±0.2** 3.4± ± ±0.1 All values are mean ± s.e.m *p<0.05, **p<0.005, ***p< Sample sizes are Wild type: n=10 (A3), n=12 (A4) and n=12 (A5) eag 1 Sh 120b : n=14 (A3), n=11(a4) and n=17 (A5) eag 4pm Sh 120 : n=5 (A3), n=2 (A4) and n=6 (A5) FasII e76 /FasII EB112 : n=14 (A3), n=14 (A4) and n=20 (A5) FasII e76 : n=7 (A3), n=6 (A4) and n=9 (A5) 121
128
129
130
131
132
133 A3 > 4 microns 2-4 microns 0-2 microns A A Wild-type FasII e76 FasII e76 /FasII EB112 eag 1 Sh 120b eag 4PM Sh 120 Figure 6. Small, medium and large bouton distribution. Based on their diameters, boutons can be classified as small (0-2µ), medium (2-4µ) and large (greater than 4µ). Segments A3 (top), A4 (middle) and A5 (bottom) bear all three categories of boutons with the medium sized boutons being the most prevalent. In case of the FasII hypomorphs, FasII e76 and FasII e76 /FasII EB112, this trend is maintained. However in case of activity mutants, eag 1 Sh 120b and eag 4PM Sh 120, there is an increase in the number of small with a corresponding decrease in the medium and large boutons. Sample sizes are as indicated in Table
134
melanogaster The development of indirect flight muscle innervation in Drosophila Joyce Fernandes 1 and K. VijayRaghavan 1,2, * SUMMARY
 Development 118, 215-227 (1993) Printed in Great Britain The Company of Biologists Limited 1993 215 The development of indirect flight muscle innervation in Drosophila melanogaster Joyce Fernandes 1 and
Development 118, 215-227 (1993) Printed in Great Britain The Company of Biologists Limited 1993 215 The development of indirect flight muscle innervation in Drosophila melanogaster Joyce Fernandes 1 and
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila
 Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae. BioNB4910 Cornell University.
 Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo
 Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning
 MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening
 APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
Conclusions. The experimental studies presented in this thesis provide the first molecular insights
 C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
Cellular Neuroanatomy II The Prototypical Neuron: Neurites. Reading: BCP Chapter 2
 Cellular Neuroanatomy II The Prototypical Neuron: Neurites Reading: BCP Chapter 2 Major Internal Features of a Neuron The neuron is the functional unit of the nervous system. A typical neuron has a soma
Cellular Neuroanatomy II The Prototypical Neuron: Neurites Reading: BCP Chapter 2 Major Internal Features of a Neuron The neuron is the functional unit of the nervous system. A typical neuron has a soma
Neural development its all connected
 Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
C. elegans L1 cell adhesion molecule functions in axon guidance
 C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
purpose of this Chapter is to highlight some problems that will likely provide new
 119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
Dendritic Remodeling and Growth of Motoneurons during Metamorphosis of Drosophila melanogaster
 The Journal of Neuroscience, June 15, 2002, 22(12):4906 4917 Dendritic Remodeling and Growth of Motoneurons during Metamorphosis of Drosophila melanogaster Christos Consoulas, Linda L. Restifo, and Richard
The Journal of Neuroscience, June 15, 2002, 22(12):4906 4917 Dendritic Remodeling and Growth of Motoneurons during Metamorphosis of Drosophila melanogaster Christos Consoulas, Linda L. Restifo, and Richard
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
Single-Cell Analysis of Drosophila Larval Neuromuscular Synapses
 Developmental Biology 229, 55 70 (2001) doi:10.1006/dbio.2000.9983, available online at http://www.idealibrary.com on Single-Cell Analysis of Drosophila Larval Neuromuscular Synapses Bao Hoang 1 and Akira
Developmental Biology 229, 55 70 (2001) doi:10.1006/dbio.2000.9983, available online at http://www.idealibrary.com on Single-Cell Analysis of Drosophila Larval Neuromuscular Synapses Bao Hoang 1 and Akira
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis
 Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
Nervous Tissue. Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation
 Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis
 18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis An organism arises from a fertilized egg cell as the result of three interrelated processes: cell division, cell
18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis An organism arises from a fertilized egg cell as the result of three interrelated processes: cell division, cell
Physiology 2 nd year. Neuroscience Optional Lecture
 Academic year 2018/2019 Physiology 2 nd year Semester 1 Curricula Nervous system physiology Blood physiology Acid-base equilibrium Bibliography: Boron & Boulpaep Medical Physiology, 3 rd edition Physiology
Academic year 2018/2019 Physiology 2 nd year Semester 1 Curricula Nervous system physiology Blood physiology Acid-base equilibrium Bibliography: Boron & Boulpaep Medical Physiology, 3 rd edition Physiology
THE PROBLEMS OF DEVELOPMENT. Cell differentiation. Cell determination
 We emphasize these points from Kandel in Bi/CNS 150 Bi/CNS/NB 150: Neuroscience Read Lecture Lecture Friday, October 2, 2015 Development 1: pp 5-10 Introduction Brains evolved All higher animals have brains
We emphasize these points from Kandel in Bi/CNS 150 Bi/CNS/NB 150: Neuroscience Read Lecture Lecture Friday, October 2, 2015 Development 1: pp 5-10 Introduction Brains evolved All higher animals have brains
Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast
 Development 126, 4065-4076 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8625 4065 Development of the Drosophila mushroom bodies: sequential generation of three distinct types
Development 126, 4065-4076 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8625 4065 Development of the Drosophila mushroom bodies: sequential generation of three distinct types
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03
 Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8
 Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
Geert Geeven. April 14, 2010
 iction of Gene Regulatory Interactions NDNS+ Workshop April 14, 2010 Today s talk - Outline Outline Biological Background Construction of Predictors The main aim of my project is to better understand the
iction of Gene Regulatory Interactions NDNS+ Workshop April 14, 2010 Today s talk - Outline Outline Biological Background Construction of Predictors The main aim of my project is to better understand the
Neurons, Synapses, and Signaling
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Chapter 11. Development: Differentiation and Determination
 KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
Neurochemistry 1. Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906
 Neurochemistry 1 Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906 How Many Neurons Do We Have? The human brain contains ~86 billion neurons and
Neurochemistry 1 Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906 How Many Neurons Do We Have? The human brain contains ~86 billion neurons and
Neurite initiation. Neurite formation begins with a bud that sprouts from the cell body. One or several neurites can sprout at a time.
 Neurite initiation. Neuronal maturation initiation f-actin polarization and maturation tubulin stage 1: "spherical" neuron stage 2: neurons extend several neurites stage 3: one neurite accelerates its
Neurite initiation. Neuronal maturation initiation f-actin polarization and maturation tubulin stage 1: "spherical" neuron stage 2: neurons extend several neurites stage 3: one neurite accelerates its
Neuronal Differentiation: Synapse Formation NMJ
 Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
Genetics, brain development, and behavior
 Genetics, brain development, and behavior Jan. 13, 2004 Questions: Does it make sense to talk about genes for behavior? How do genes turn into brains? Can environment affect development before birth? What
Genetics, brain development, and behavior Jan. 13, 2004 Questions: Does it make sense to talk about genes for behavior? How do genes turn into brains? Can environment affect development before birth? What
Segment boundary formation in Drosophila embryos
 Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
9/4/2015 INDUCTION CHAPTER 1. Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology. Fig 1.
 INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
The majority of cells in the nervous system arise during the embryonic and early post
 Introduction Introduction The majority of cells in the nervous system arise during the embryonic and early post natal period. These cells are derived from population of neural stem cells first shown by
Introduction Introduction The majority of cells in the nervous system arise during the embryonic and early post natal period. These cells are derived from population of neural stem cells first shown by
Cells. Steven McLoon Department of Neuroscience University of Minnesota
 Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
"Small Brains, Big Ideas: The value of model organisms to science. Dr. Yuly Fuentes-Medel Executive Director Descience
 "Small Brains, Big Ideas: The value of model organisms to science Dr. Yuly Fuentes-Medel Executive Director Descience Outline 1. What is a model organism? 2. Big questions solved by small-brained organisms
"Small Brains, Big Ideas: The value of model organisms to science Dr. Yuly Fuentes-Medel Executive Director Descience Outline 1. What is a model organism? 2. Big questions solved by small-brained organisms
Glia Engulf Degenerating Axons during Developmental Axon Pruning
 Current Biology, Vol. 14, 678 684, April 20, 2004, 2004 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2004.03.035 Glia Engulf Degenerating Axons during Developmental Axon Pruning Ryan J. Watts,
Current Biology, Vol. 14, 678 684, April 20, 2004, 2004 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2004.03.035 Glia Engulf Degenerating Axons during Developmental Axon Pruning Ryan J. Watts,
Nature Neuroscience: doi: /nn.2717
 Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
BIOLOGY. 1. Overview of Neurons 11/3/2014. Neurons, Synapses, and Signaling. Communication in Neurons
 CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
Neurons and Nervous Systems
 34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
10/2/2015. Chapter 4. Determination and Differentiation. Neuroanatomical Diversity
 Chapter 4 Determination and Differentiation Neuroanatomical Diversity 1 Neurochemical diversity: another important aspect of neuronal fate Neurotransmitters and their receptors Excitatory Glutamate Acetylcholine
Chapter 4 Determination and Differentiation Neuroanatomical Diversity 1 Neurochemical diversity: another important aspect of neuronal fate Neurotransmitters and their receptors Excitatory Glutamate Acetylcholine
Early consolidation of development and physiology of an identified presynaptic nerve terminal
 Laviolette and Stewart BMC Neuroscience 2013, 14:124 RESEARCH ARTICLE Open Access Early consolidation of development and physiology of an identified presynaptic nerve terminal Matthew Laviolette and Bryan
Laviolette and Stewart BMC Neuroscience 2013, 14:124 RESEARCH ARTICLE Open Access Early consolidation of development and physiology of an identified presynaptic nerve terminal Matthew Laviolette and Bryan
The role of ephrins and structured retinal activity in the development of visual map topography
 The role of ephrins and structured retinal activity in the development of visual map topography David Feldheim, UC Santa Cruz KITP Brain08 March 21, 2008 Topographic map development in the mouse visual
The role of ephrins and structured retinal activity in the development of visual map topography David Feldheim, UC Santa Cruz KITP Brain08 March 21, 2008 Topographic map development in the mouse visual
Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling
 Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control
 Article The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control Carleton P. Goold 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics,
Article The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control Carleton P. Goold 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics,
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Nervous System Organization
 The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Reading. Lecture VI. Making Connections 9/17/12. Bio 3411 Lecture VI. Making Connections. Bio 3411 Monday September 17, 2012
 Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Lecture VI. Making Connections Bio 3411 Monday September 17, 2012!! 1! Reading NEUROSCIENCE: 5 th ed, pp!507?536! 4 th ed, pp 577-609 Bentley, D., & Caudy, M. (1983). Nature, 304(5921), 62-65. Dickson,
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Domain 6: Communication
 Domain 6: Communication 6.1: Cell communication processes share common features that reflect a shared evolutionary history. (EK3.D.1) 1. Introduction to Communication Communication requires the generation,
Domain 6: Communication 6.1: Cell communication processes share common features that reflect a shared evolutionary history. (EK3.D.1) 1. Introduction to Communication Communication requires the generation,
BIOLOGY 11/10/2016. Neurons, Synapses, and Signaling. Concept 48.1: Neuron organization and structure reflect function in information transfer
 48 Neurons, Synapses, and Signaling CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Concept 48.1: Neuron organization
48 Neurons, Synapses, and Signaling CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Concept 48.1: Neuron organization
Axon guidance I. Paul Garrity March 15, /9.013
 Axon guidance I Paul Garrity March 15, 2004 7.68/9.013 Neuronal Wiring: Functional Framework of the Nervous System Stretch reflex circuit Early theories of axonogenesis Schwann: many neurons link to form
Axon guidance I Paul Garrity March 15, 2004 7.68/9.013 Neuronal Wiring: Functional Framework of the Nervous System Stretch reflex circuit Early theories of axonogenesis Schwann: many neurons link to form
Cell Death & Trophic Factors II. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Clicker Question. Discussion Question
 Connectomics Networks and Graph Theory A network is a set of nodes connected by edges The field of graph theory has developed a number of measures to analyze networks Mean shortest path length: the average
Connectomics Networks and Graph Theory A network is a set of nodes connected by edges The field of graph theory has developed a number of measures to analyze networks Mean shortest path length: the average
Midterm 1. Average score: 74.4 Median score: 77
 Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Math in systems neuroscience. Quan Wen
 Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Neuronal cell death in grasshopper embryos: variable patterns in different species, clutches, and clones
 J. Embryol. exp. Morph. 78, 169-182 (1983) Printed in Great Britain The Company of Biologists Limited 1983 Neuronal cell death in grasshopper embryos: variable patterns in different species, clutches,
J. Embryol. exp. Morph. 78, 169-182 (1983) Printed in Great Britain The Company of Biologists Limited 1983 Neuronal cell death in grasshopper embryos: variable patterns in different species, clutches,
Control and Integration. Nervous System Organization: Bilateral Symmetric Animals. Nervous System Organization: Radial Symmetric Animals
 Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Chapter 3 BIOLOGY AND BEHAVIOR
 Chapter 3 BIOLOGY AND BEHAVIOR Section 1: The Nervous System Section 2: The Brain: Our Control Center Section 3: The Endocrine System Section 4: Heredity: Our Genetic Background 1 Section 1: The Nervous
Chapter 3 BIOLOGY AND BEHAVIOR Section 1: The Nervous System Section 2: The Brain: Our Control Center Section 3: The Endocrine System Section 4: Heredity: Our Genetic Background 1 Section 1: The Nervous
DEPARTMENT OF SCIENCE
 DEPARTMENT OF SCIENCE COURSE OUTLINE ZOOLOGY 2420 ANIMAL PHYSIOLOGY II: INTERCELLULAR COMMUNICATIONS INSTRUCTOR: Dr. Georgia Goth PHONE: 780-513-1041 OFFICE: J222 E-MAIL: ggoth@gprc.ab.ca OFFICE HOURS:
DEPARTMENT OF SCIENCE COURSE OUTLINE ZOOLOGY 2420 ANIMAL PHYSIOLOGY II: INTERCELLULAR COMMUNICATIONS INSTRUCTOR: Dr. Georgia Goth PHONE: 780-513-1041 OFFICE: J222 E-MAIL: ggoth@gprc.ab.ca OFFICE HOURS:
Neurite formation & neuronal polarization
 Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
The Role of G-Protein Coupled Estrogen Receptor (GPER) in Early Neurite Development. Kyle Pemberton
 The Role of G-Protein Coupled Estrogen Receptor (GPER) in Early Neurite Development Kyle Pemberton Acknowledgement Dr. Xu Lab Members Brittany Mersman Nicki Patel Pallavi Mhaskar Jason Cocjin Committee
The Role of G-Protein Coupled Estrogen Receptor (GPER) in Early Neurite Development Kyle Pemberton Acknowledgement Dr. Xu Lab Members Brittany Mersman Nicki Patel Pallavi Mhaskar Jason Cocjin Committee
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila July 9, 2008 Drosophila Development Overview Fertilization Cleavage Gastrulation Drosophila body plan Oocyte formation Genetic control
Developmental Biology Biology 4361 Axis Specification in Drosophila July 9, 2008 Drosophila Development Overview Fertilization Cleavage Gastrulation Drosophila body plan Oocyte formation Genetic control
Astrocytes Play a Key Role in Drosophila Mushroom Body Axon Pruning
 Astrocytes Play a Key Role in Drosophila Mushroom Body Axon Pruning Yaniv Hakim, Shiri P. Yaniv, Oren Schuldiner* Department of Molecular Cell Biology, Weizmann Institute of Sciences, Rehovot, Israel Abstract
Astrocytes Play a Key Role in Drosophila Mushroom Body Axon Pruning Yaniv Hakim, Shiri P. Yaniv, Oren Schuldiner* Department of Molecular Cell Biology, Weizmann Institute of Sciences, Rehovot, Israel Abstract
37 Neurons, Synapses, and Signaling
 CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: Lines of Communication
CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: Lines of Communication
Dimorphism and Homeostasis of Neuromuscular Synaptic Function
 Dimorphism and Homeostasis of Neuromuscular Synaptic Function - Epp m ε Release + + φ - q Sensitivity The nature of the problem Size, Strength, Resistance.. The Power of Drosophila The nature of the problem
Dimorphism and Homeostasis of Neuromuscular Synaptic Function - Epp m ε Release + + φ - q Sensitivity The nature of the problem Size, Strength, Resistance.. The Power of Drosophila The nature of the problem
1
 http://photos1.blogger.com/img/13/2627/640/screenhunter_047.jpg 1 The Nose Knows http://graphics.jsonline.com/graphics/owlive/img/mar05/sideways.one0308_big.jpg 2 http://www.stlmoviereviewweekly.com/sitebuilder/images/sideways-253x364.jpg
http://photos1.blogger.com/img/13/2627/640/screenhunter_047.jpg 1 The Nose Knows http://graphics.jsonline.com/graphics/owlive/img/mar05/sideways.one0308_big.jpg 2 http://www.stlmoviereviewweekly.com/sitebuilder/images/sideways-253x364.jpg
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34
 NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
How to read a burst duration code
 Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Neurons, Synapses, and Signaling
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
NOTES: CH 48 Neurons, Synapses, and Signaling
 NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
Sunday, September 30 th
 Sunday, September 30 th 3:00 pm Check-in 6:00 pm Reception (Lobby) 7:00 pm Dinner 8:00 pm Poster Introduction Blitz! 9:30 pm Refreshments available at Bob s Pub NOTE: Meals are in the Dining Room Talks
Sunday, September 30 th 3:00 pm Check-in 6:00 pm Reception (Lobby) 7:00 pm Dinner 8:00 pm Poster Introduction Blitz! 9:30 pm Refreshments available at Bob s Pub NOTE: Meals are in the Dining Room Talks
Lecture 7. Development of the Fruit Fly Drosophila
 BIOLOGY 205/SECTION 7 DEVELOPMENT- LILJEGREN Lecture 7 Development of the Fruit Fly Drosophila 1. The fruit fly- a highly successful, specialized organism a. Quick life cycle includes three larval stages
BIOLOGY 205/SECTION 7 DEVELOPMENT- LILJEGREN Lecture 7 Development of the Fruit Fly Drosophila 1. The fruit fly- a highly successful, specialized organism a. Quick life cycle includes three larval stages
Trophic Factors. Trophic Factors. History 2. History Growth Factors. Giles Plant
 217 - Growth Factors Giles Plant Role in: Growth and Trophic Factors Soluble/diffusible factors - polypeptides Proliferation Differentiation (ie Cancer) Survival (degenerative diseases) Innervation Maintenance
217 - Growth Factors Giles Plant Role in: Growth and Trophic Factors Soluble/diffusible factors - polypeptides Proliferation Differentiation (ie Cancer) Survival (degenerative diseases) Innervation Maintenance
Chapter 48 Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Highlights from Pesticides Lecture
 Highlights from Pesticides Lecture Prior to World War II pesticides were, while post-ww II they were. What is meant by the biomagnification of pesticides and what are its consequences? Differentiate between
Highlights from Pesticides Lecture Prior to World War II pesticides were, while post-ww II they were. What is meant by the biomagnification of pesticides and what are its consequences? Differentiate between
Biosciences in the 21st century
 Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
The neuron as a secretory cell
 The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
Mitochondria associate with the cytoskeleton. Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008)
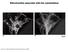 Mitochondria associate with the cytoskeleton Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008) Special arrangements of mitochondria Figure 14-6 Molecular Biology of the Cell ( Garland Science
Mitochondria associate with the cytoskeleton Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008) Special arrangements of mitochondria Figure 14-6 Molecular Biology of the Cell ( Garland Science
The Emergence of Modularity in Biological Systems
 The Emergence of Modularity in Biological Systems Zhenyu Wang Dec. 2007 Abstract: Modularity is a ubiquitous phenomenon in various biological systems, both in genotype and in phenotype. Biological modules,
The Emergence of Modularity in Biological Systems Zhenyu Wang Dec. 2007 Abstract: Modularity is a ubiquitous phenomenon in various biological systems, both in genotype and in phenotype. Biological modules,
Neurite formation & neuronal polarization. The cytoskeletal components of neurons have characteristic distributions and associations
 Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Cell biology: Death drags down the neighbourhood
 Cell biology: Death drags down the neighbourhood The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Vasquez,
Cell biology: Death drags down the neighbourhood The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Vasquez,
PROPERTY OF ELSEVIER SAMPLE CONTENT - NOT FINAL. The Nervous System and Muscle
 The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
Connectin Mediates Adhesion in Drosophila
 Neuron, Vol. 18, 873 880, June, 1997, Copyright 1997 by Cell Press Connectin Mediates Adhesion in Drosophila Srikala Raghavan and Robert A. H. White in little observable perturbation of the normal axonal
Neuron, Vol. 18, 873 880, June, 1997, Copyright 1997 by Cell Press Connectin Mediates Adhesion in Drosophila Srikala Raghavan and Robert A. H. White in little observable perturbation of the normal axonal
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Lecture 2: mrna localisation and decay in cells and in tissues
 Lecture 2: mrna localisation and decay in cells and in tissues Prof. Ilan Davis, Department of Biochemistry. Senior Research Fellow, Jesus College ilan.davis@bioch.ox.ac.uk http://www.ilandavis.com References
Lecture 2: mrna localisation and decay in cells and in tissues Prof. Ilan Davis, Department of Biochemistry. Senior Research Fellow, Jesus College ilan.davis@bioch.ox.ac.uk http://www.ilandavis.com References
Follow this and additional works at: Part of the Medical Sciences Commons
 Bucknell University Bucknell Digital Commons Master s Theses Student Theses 2010 The overexpression of homeotic complex gene Ultrabithorax in the post-embryonic neuronal lineages of the ventral nervous
Bucknell University Bucknell Digital Commons Master s Theses Student Theses 2010 The overexpression of homeotic complex gene Ultrabithorax in the post-embryonic neuronal lineages of the ventral nervous
Modeling retinal high and low contrast sensitivity lters. T. Lourens. Abstract
 Modeling retinal high and low contrast sensitivity lters T. Lourens Department of Computer Science University of Groningen P.O. Box 800, 9700 AV Groningen, The Netherlands E-mail: tino@cs.rug.nl Abstract
Modeling retinal high and low contrast sensitivity lters T. Lourens Department of Computer Science University of Groningen P.O. Box 800, 9700 AV Groningen, The Netherlands E-mail: tino@cs.rug.nl Abstract
Pioneering and pathfinding by an identified neuron in the embryonic leech
 J. Embryol. exp. Morph. 86, 155-167, (1985) 155 Printed in Great Britain The Company of Biologists Limited 1985 Pioneering and pathfinding by an identified neuron in the embryonic leech JOHN Y. KUWADA
J. Embryol. exp. Morph. 86, 155-167, (1985) 155 Printed in Great Britain The Company of Biologists Limited 1985 Pioneering and pathfinding by an identified neuron in the embryonic leech JOHN Y. KUWADA
Supplemental table S7.
 Supplemental table S7. GO terms significantly enriched in significantly up-regulated genes of the microarray. K: number of genes from the input cluster in the given category. F: number of total genes in
Supplemental table S7. GO terms significantly enriched in significantly up-regulated genes of the microarray. K: number of genes from the input cluster in the given category. F: number of total genes in
Animal structure and function
 Animal structure and function The nervous system Parts of the nervous system 43C, 44B, 45D Brain structure and function Eyes Retina Neurons: How neurons communicate: Resting potential: The resting
Animal structure and function The nervous system Parts of the nervous system 43C, 44B, 45D Brain structure and function Eyes Retina Neurons: How neurons communicate: Resting potential: The resting
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
HUMAN ANATOMY & PHYSIOLOGY STRUCTURE 30
 HUMAN ANATOMY & PHYSIOLOGY STRUCTURE 30 Description This second-year course in biology emphasizes the workings of the human body. The course is offered in the fall semester and meets six (6) periods per
HUMAN ANATOMY & PHYSIOLOGY STRUCTURE 30 Description This second-year course in biology emphasizes the workings of the human body. The course is offered in the fall semester and meets six (6) periods per
Ch 8: Neurons: Cellular and Network Properties, Part 1
 Developed by John Gallagher, MS, DVM Ch 8: Neurons: Cellular and Network Properties, Part 1 Objectives: Describe the Cells of the NS Explain the creation and propagation of an electrical signal in a nerve
Developed by John Gallagher, MS, DVM Ch 8: Neurons: Cellular and Network Properties, Part 1 Objectives: Describe the Cells of the NS Explain the creation and propagation of an electrical signal in a nerve
Developmental Biology
 Developmental Biology 366 (2012) 204 217 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology Developmental changes in
Developmental Biology 366 (2012) 204 217 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology Developmental changes in
1. What are the three general areas of the developing vertebrate limb? 2. What embryonic regions contribute to the developing limb bud?
 Study Questions - Lecture 17 & 18 1. What are the three general areas of the developing vertebrate limb? The three general areas of the developing vertebrate limb are the proximal stylopod, zeugopod, and
Study Questions - Lecture 17 & 18 1. What are the three general areas of the developing vertebrate limb? The three general areas of the developing vertebrate limb are the proximal stylopod, zeugopod, and
