ARTICLE Probing Synaptic Transmission and Behavior in Drosophila with Optogenetics: A Laboratory Exercise
|
|
|
- Annabella Oliver
- 5 years ago
- Views:
Transcription
1 ARTICLE Probing Synaptic Transmission and Behavior in Drosophila with Optogenetics: A Laboratory Exercise Ilya Vilinsky 1, Karen L. Hibbard 2, Bruce R. Johnson 3 & David L. Deitcher 3 1 Department of Biological Sciences, University of Cincinnati, Cincinnati, OH 45221; 2 Janelia Research Campus, Howard Hughes Medical Institute, Ashburn, Virginia 2017; 3 Department of Neurobiology and Behavior, Cornell University, Ithaca, NY Optogenetics is possibly the most revolutionary advance in neuroscience research techniques within the last decade. Here, we describe lab modules, presented at a workshop for undergraduate neuroscience educators, using optogenetic control of neurons in the fruit fly Drosophila melanogaster. Drosophila is a genetically accessible model system that combines behavioral and neurophysiological complexity, ease of use, and high research relevance. One lab module utilized two transgenic Drosophila strains, each activating specific circuits underlying startle behavior and backwards locomotion, respectively. The red-shifted channelrhodopsin, CsChrimson, was expressed in neurons sharing a common transcriptional profile, with the expression pattern further refined by the use of a Split GAL4 intersectional activation system. Another set of strains was used to investigate synaptic transmission at the larval neuromuscular junction. These expressed Channelrhodopsin 2 (ChR2) in glutamatergic neurons, including the motor neurons. The first strain expressed ChR2 in a wild type background, while the second contained the SNAP-25 ts mutant allele, which confers heightened evoked potential amplitude and greatly increased spontaneous vesicle release frequency at the larval neuromuscular junction. These modules introduced educators and students to the use of optogenetic stimulation to control behavior and evoked release at a model synapse, and establish a basis for students to explore neurophysiology using this technique, through recapitulating classic experiments and conducting independent research. Key words: optogenetics; Drosophila; neuromuscular junction; behavior; SNAP-25; electrophysiology The recent optogenetics revolution has changed the landscape of neuroscience research (Boyden et al., 2005; Fenno et al., 2011; Sjulson et al., 2016). Remote control of neuronal activity using light has given researchers an unprecedented level of access to specific, transcriptionallydefined neural circuits, even within intact and freely behaving animals (Arenkiel et al., 2007; Pulver et al., 2009; Nieh et al., 2013; Smith and Graybiel, 2013). From the first widely applicable system, reported in 2005 (Boyden et al., 2005), the optogenetic toolbox has greatly expanded to include excitatory, inhibitory, and biochemically active molecules. In addition to their usefulness in research, optogenetics-based clinical applications are being actively explored. These include potential treatments for macular degeneration and retinal disease (Scholl et al., 2016), deafness (Moser, 2015), peripheral pain (Liu et al., 2016), and as a substitute for deep brain stimulation for diseases such as Parkinson s and Tourette s (Kalanithi and Henderson, 2012). Most optogenetic methods consist of co-opting a photosensitive ion channel or pump from a bacterium or algae and expressing them in genetically accessible organism under the control of a particular promoter or enhancer. With the appropriate enhancer, selected neurons express the photosensitive ion channel which can be activated by a light stimulus such as a laser, or a high-power LED, the latter of which is well within the capabilities of a budget- conscious research laboratory or an undergraduate teaching lab (Pulver et al., 2011; Titlow et al., 2015; Rose, 2018, this issue; Pokala and Glater, 2018). Here, we use Drosophila melanogaster as a model organism to demonstrate optogenetic control of neural circuits and use this technique as a foundation for investigation of neurophysiology and behavior. In Drosophila, transgenes can be expressed with exquisite control by utilizing the binary system, GAL4/UAS, which utilizes the tissue-specific expression of the yeast transcriptional activator (GAL4) to drive expression of genes that are under control of the GAL4 upstream activation sequence (UAS). GAL4 and UAS lines can be mixed and matched to express virtually any gene in a multitude of patterns (Pulver and Berni, 2012). In this case, channelrhodopsins are expressed in adult neuronal circuits to elicit behavioral responses and to trigger action potentials in motor neurons of the larva. Further refinement of the GAL4 system is also possible by expressing the two halves of the protein with different promoters, and only the intersecting cells would contain the functional GAL4, further restricting the neurons being activated (Luan et al., 2006; Pfeiffer et al., 2010; Dionne et al., 2018). At the 2017 Faculty for Undergraduate Neuroscience Workshop at Dominican University, participants used optogenetic activation of neural circuits in transgenic and mutant Drosophila lines to observe behavioral and neurophysiological effects of activating specific neurons, and to conduct simple experiments demonstrating basic principles of neurophysiology and behavior. Two transgenic lines expressed CsChrimson, an algal opsin with an activation peak in the orange-red range at about 600 nm (Klapoetke et al., 2014), in restricted groups of neurons JUNE is a publication of Faculty for Undergraduate Neuroscience (FUN)
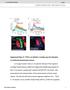
2 Vilinsky et al. Drosophila Optogenetics A290 within the CNS (Jenett et al., 2012). Of the two lines exhibiting light-triggered behavior, the first, Moonwalker, drives expression in a specific cluster of descending neurons that integrate input from the visual system and drive a backward walking behavior (Sen et al., 2017). The second has an expression pattern that includes bilateral neurons, which elicit startle and escape response when activated. These were used for behavioral observation. A third line expressed Channelrhodopsin 2 (ChR2), the first widely implemented optogenetic construct with an activation peak in the blue range at 460 nm, in glutamatergic neurons (Hornstein et al., 2009). This expression pattern includes motor neurons innervating the body wall muscles of the Drosophila third instar larva, a classic neuromuscular junction research prep often used as a genetically accessible synapse (Zhang and Stewart, 2010). The line expressing ChR2 in glutamatergic neurons causes tetanic muscle contraction when activated and can be used to record evoked potentials and miniature endplate potentials in the body wall muscles innervated by motor neurons. The workshop had two broad teaching aims. The first was familiarization of the participants with Drosophila as a model organism for investigating and teaching neuroscience. Participants began by directly handling Drosophila, in both larval and adult forms. Those who had no experience with this model system were guided in handling food vials containing fly stocks, transferring the animals into clear vials, anesthetizing them on ice, and observing them under the dissection scope. Hands-on familiarization with the system is often key to overcoming the barrier between conceptually understanding the value of Drosophila modules, and actually trying the experiments for the first time in their own teaching labs. Participants then observed free roaming behavior of adult Drosophila, followed by their response to specific circuit activation. Then, they observed third instar larval locomotion, and the response of larvae to motor neuron activation. Finally, they practiced dissecting larvae to expose the body wall muscles and associated nerves and observed evoked junctional potentials (EJPs) and miniature endplate potentials (minis) in muscle cells through intracellular recording, while stimulating presynaptic motor neurons with brief pulses of blue light. Participants could in this way merge theoretical understanding of genetic manipulation with actual data that they personally obtained in the workshop. The second aim was to familiarize participants with the principles of optogenetics, and the application of these principles in a lab setting. By experimenting with the techniques required to prepare animals or neuromuscular junction preps, to set up intracellular recording, and to deliver light stimuli so as to elicit tightly controlled responses, participants could put their theoretical understating into an immediate, applied context. They experienced the physical setup, the range of light amplitudes, pulse durations, and stimulation frequencies that would result in the desired physiological effects. Participants could explore the dynamics of specific elicited behaviors, and experiment with short-term synaptic plasticity in a genetically accessible model synapse. As with handling flies, this served to overcome another barrier to implementation of the demonstrated exercises. MATERIALS AND METHODS Fly Genetics All optogenetic behavior lines were raised on standard cornmeal-molasses fly food with the addition 0.5mM alltrans retinal at 25 degrees Celsius in foil-covered vials. The adult behavior lines used were the Moonwalker -20XUAS- IVS-CSChrimson.mVenus attp18;; VT GAL4 attp2 and the Jumping - 20XUAS-IVS-CSChrimson.mVenus attp18;; R42E06-GAL4 attp2. The optogenetic line used in the electrophysiology recording from the larval NMJ is w; OK371-GAL4, UAS-ChR2-H134-Cherry, MHC-GFP;+, in a wild type background and with the mutant allele SNAP -25 ts on chromosome III. Adult Behavior Adult flies, 3-5 days old were anesthetized on ice and loaded into glass test tubes with cotton caps and covered with aluminum foil. Behavioral activity was observed by eye or under a dissecting microscope during optogenetic stimulation. Larval Electrophysiology Wandering third instar larvae (large white, mobile larva found on the walls of the vial) were selected with forceps, placed in a sylgard petri dish, and rinsed once in HL3 saline lacking Ca 2+ consisting of (in mm): 70 NaCl, 20 MgCl2, 5 KCl, 10 NaHCO3, 5 trehalose, 115 sucrose (Stewart et. al., 1994). Larvae were prepared as described in Hornstein et al., 2009 and Pulver et al., Briefly, larvae were oriented trachea side up, pinned at the tail and mouth hooks with Minuten pins (Fine Science Tools). HL3 saline lacking Ca 2+ was added, a superficial cut was made with microscissors from tail to mouth hooks, and the internal organs were carefully removed without damaging the body wall muscles or the CNS and its associated axons. Four more pins were used to splay out the larva at each corner so it resembled a fillet (Hornstein et. al., 2009). After rinsing the fillet with HL3 saline lacking Ca 2+ (to prevent muscle contractions), the prep bathing solution was replaced with HL3 saline containing 3 mm CaCl2. Sharp glass electrodes (5-30 M ) filled with 3M KCl were used for intracellular recordings. A ground electrode was placed in the dish and the intracellular electrode was advanced slowly into one of the body wall muscles. Altering the fiber lights so they illuminate from the side often helps to visualize the musculature. Commonly, muscle 6 or 7 were used as shown in Fig. 2A. A resting potential below -40 mv is usually sufficient to see minis in wild type and SNAP-25ts larvae. Signals were amplified with a model AM-Systems The resulting signals were digitized at 20 KHz with a Powerlab 8/30 data acquisition system (ADInstruments, Colorado Springs, CO, USA) and recorded and analyzed in LabChart 8 (ADInstruments). Once a satisfactory resting potential was achieved, the dissection scope illumination was extinguished, and minis could be recorded (Fig. 3). To elicit evoked release, a high intensity blue LED was positioned closed to the preparation with the light aimed directly at the
3 A291 larva. A single stimulus of 2-5 ms, at maximum intensity, was usually sufficient to observe evoked release. The length of the pulse could be increased to 50 ms if no initial response was observed. Optogenetic Stimulation Construction of a simple, voltage controllable LED current driver (Fig. 1) was based on the BuckBLock DC LED driver by LEDdynamics (LEDsupply.com, part # 0A009-D-V- 1000). The blue and red LEDs used were Luxeon Rebel 3up LED packages on star bases (LEDsupply, parts # PB000-D and PD000-F, respectively). LEDs were mounted with thermal adhesive on a star heat sink (Digikey.com, part # ND). Light intensity was controlled by the 0-10 Volt analog output of a PowerLab 26T (ADInstruments, Sydney, Australia). Free crawling larvae were stimulated with 500 ms pulses of 460 nm blue light using a computer controlled LED (Fig. 1). Dissected larval fillets were stimulated with this wavelength, but with brief pulses lasting 2-50 ms. Optogenetic stimulation for behavioral responses was induced with brief exposure to light activation with a red ( nm) CREE XP-E LED Flashlight available from Amazon or from a computer controlled red LED light source (Fig. 1). Imaging The adult CNS was imaged with the methods detailed in Jennet et al., OK371-GAL4, UAS-ChR2-H134-Cherry, MHC-GFP;+, larvae was dissected as described for electrophysiology in HL3 saline lacking Ca2+. The native mcherry and GFP were imaged with a Zeiss LSM 880 with a 10X water immersion objective. The mcherry signal in the inset of the image was intensified using ImageJ. Workshop Participant Evaluations Feedback on our fly workshop sessions was gathered by Figure 1. LED and power supply assembly. (A) A voltage controlled current source is mounted in an enclosure that holds input, output, power, and switch components. (B) Blue (460 nm) or Red (625 nm) LED is mounted on a heatsink. (C) Port for voltage control. (D) Power output ports for LED. (E) Switching power supply and input. LED light source apparatus modified and upgraded from Pulver et al., asking participants to answer the following questions that the workshop organizers provided, on a Likert Scale of 1-5, with 1- strongly agree to 5- strongly disagree: (1) this workshop met my expectations, 2) this workshop increased my understanding of the topic material, 3) this workshop was well organized with clear objectives, 4) the exercise presented at this workshop is a good vehicle for teaching undergraduates principles of neuroscience, 5) I am likely to incorporate this material as a classroom activity, and 6) I am likely to use the material in this workshop as a resource for my class lectures and my own neuroscience background. RESULTS Behavioral responses to optogenetic stimulation The first exercise examined the effects of red LED light on the adult flies as they freely moved in an empty glass test tubes and the effects of blue light on crawling larvae. Illumination was accomplished through a high intensity blue or red LED powered by a simple current source and controlled via software, using the device depicted in Figure 1. Adult flies could also be stimulated by an inexpensive red LED flashlight. The larvae were of the same strain used later for intracellular recording at the NMJ, which expressed ChR2 under control of the OK371 promoter, driving expression in glutamatergic neurons (Fig. 2A). Illumination of optogenetically activatable larvae resulted in a characteristic seizure behavior. Larvae stopped crawling and contracted into a tight oval for the duration of the light stimulus. Sometimes, if the larvae were crawling in a particular direction, the brief seizure acted as an aversive shock, causing them to quickly change direction. For adult behavior, two different genotypes were tested: the Moonwalker-GAL4 and the Jumping-GAL4, each expressing CsChrimson, the red-sensitive channelrhodopsin, in different sets of neurons. Red-shifted channelrhodopsin was used in the adult experiment since red light is easily transmitted through the adult cuticle than blue. After removing the foil and observing fly behavior under low light conditions, a red light beam from an inexpensive LED flashlight or a high intensity LED was used to briefly illuminate the vial (see Supplementary Video for a depiction of Jumping-GAL4 stimulation). Workshop participants observed, either with the naked eye or under a dissecting scope, changes in behavior when the light was on. Vials were tested several times to determine the reproducibility and the nuances of the behavior. The red light triggers the moonwalker flies to walk backwards while the jumping flies jump excitedly (as if exhibiting a startle response) in the tube as long as the light shone. The expression pattern of Moonwalker-GAL4 is shown in Figure 2B. Here, the Moonwalker Gal4 driven GFP is expressed in a set of central neurons in sensory and motor neuropils. These neurons are sufficient to elicit the backward walking escape response. Electrophysiological responses to optogenetic stimulation The second exercise examined neurotransmitter release at the third instar larval neuromuscular junction (NMJ). Instead
4 Vilinsky et al. Drosophila Optogenetics A292 of electrically stimulating specific motor neuron axons to trigger neurotransmitter release, blue light is used to generate action potentials in all the motor neurons and an intracellular electrode inserted in the muscle records the synaptic events. This is a much easier way to stimulate Drosophila motor neurons than using conventional direct electrical stimulation (Pulver et al., 2011). Either wild type larva or SNAP-25 ts mutant larva expressing the channelrhodopsin2 can be used. The SNAP-25 ts mutant has the advantage of having a very high frequency of spontaneous vesicle release (minis) allowing students to observe these small synaptic events easily. Wandering third instar larva from vials expressing mcherry tagged channelrhodopsin2 in motor neurons (Fig. 2A) were investigated. A sample fillet can be viewed under a fluorescent dissecting scope to acquaint students with the anatomy of the ventral ganglion and body wall muscles with the fluorescent proteins mcherry and GFP, respectively (Fig. 2A). A typical evoked response from wild type animals is observed in Fig. 3A. The amplitude of the evoked potential varies with resting potential: the best recordings were obtained with resting potentials of -60 mv or more negative, but reasonable data could be obtained even with suboptimal resting potentials of as low as -30. Minis were generally not visible at resting potentials less negative than -40 mv. Fig. 3B shows a typical response in SNAP-25 ts larvae, which displays a characteristically elevated mini frequency, and occasionally demonstrates increased evoked potential amplitude. Students may vary the frequency of the stimulus to observe either synaptic potentiation or depression. Figure 2. Drosophila adult and larval CNS. (A) Larval OK371- GAL4 driving expression of mcherry-tagged channelrhodopsin2 in motor neurons of the ventral ganglion (VG) and GFP expression in muscle. Note expression of channelrhodopsin2 in motor neuron axons. Location of muscles 6 and 7 are indicated. The inset has increased brightness of mcherry to show the innervation of the neurons at the muscles. (B) Confocal image of Moonwalker-GAL4 driving expression of GFP in adult brain (green) and the presynaptic marker BRP (magenta). Workshop Session Feedback Feedback on our fly workshop sessions by 42 of the 75 participants who attended and signed our mailing list indicated an overall very positive response to our activities. Participants rated our workshop sessions with the following questions on a scale of 1 (strongly agree) to 5 (strongly disagree); mean responses +- SD after each question: 1) this workshop met my expectations ( ); 2) This workshop increased my understanding of the topic material ( ); 3) This workshop was well organized with clear objectives ( ; 4) The exercise presented at this workshop is a good vehicle for teaching undergraduates principles of neuroscience ( ); 5) I am likely to incorporate this material as a classroom activity ( ), and 6) I am likely to use the material in this workshop as a resource for my class lectures and my own neuroscience background ( ). Mean participant responses expressed satisfaction with the workshop material as useful and interesting neuroscience teaching resources. Less positive scores for using the material in class or as a teaching resource may be due to inexperience with fly biology and the lack of electrophysiological equipment in some neuroscience programs. DISCUSSION Neuroscience research is characterized by constant change, adaptation, and adoption of new techniques. The
5 A293 Figure 3. Sample student recordings from the Drosophila third install larva neuromuscular junction. Evoked potentials are elicited by brief pulses of blue light from a high power LED in transgenic strains expressing Channelrhodopsin 2 in glutamatergic neurons. (A) Wild type evoked potential and miniature endplate potentials. (B) SNAP-25 ts, showing characteristically elevated frequency of miniature endplate potentials indicated by arrowheads. described laboratories at the 2017 Faculty for Undergraduate Neuroscience Workshop highlighted the use of optogenetics, arguably the most influential and transformative technology to have been developed in recent years. This method uses molecular genetic techniques to make a wide variety of neural circuits controllable remotely by pulses of light. Though powerful, the technique is easily implemented in the teaching environment as well as the research lab. The prep used was Drosophila, the classical genetic model organism that combines ease of use, cost effectiveness, and unparalleled versatility in demonstrating behavior and neuronal physiology. Optogenetic control of specific central circuits in the Drosophila brain can drive a variety of behaviors. Our lab exercises focused on locomotor behaviors, because these feature the most robust responses and are most easily observed by students and researchers new to the model system. The startle response is elicited by a small group of neurons within the brain, and is readily apparent, even without the use of a dissection scope. The collection of transgenic lines that drive expression in specific circuits and elicit specific behaviors is increasing, and these can often be readily incorporated into teaching labs. Wellcharacterized examples include a strain that expresses ChR2 in gustatory neurons and drives a proboscis extension reflex upon activation (Inagaki et al., 2014), and a strain that expresses CsChrimson in a central pattern that includes the giant fiber neurons, driving an escape response similar to the one presented here (Klapoetke et al., 2014, Titlow et al., 2015). Many lines driving expression in sparse groups of neurons remain to be characterized (Jenett et al., 2012). Undergraduates in a lab setting can observe and record videos of the response using their now-ubiquitous cell phones. Behavioral responses can be categorized by the use of ethogram analysis, where discrete, stereotyped actions are defined, quantified, and linked into series of more complex behavioral response repertoires (Chen et al., 2002). Students can use their observations, whether live or reviewed on video, and develop their own ethogram definitions. These can then be compared to literature reports (Branson et al., 2009), and be used as a basis for selfdirected research projects comparing different transgenic and mutant lines in an optogenetically activatable background (McKellar and Wyttenbach, 2017). Synaptic plasticity is a primary component of development, learning, and memory (Harris and Littleton, 2015). A key goal of neuroscience education is to give students the training and the platform to discover and experiment with these fundamental processes. The Drosophila third instar neuromuscular junction demonstrates the principles of synaptic transmission in an easily accessible and controllable prep. By varying pulse number and frequency, the participants could demonstrate short-term synaptic dynamics. At typical physiological levels of extracellular calcium (about 3 mm), the neuromuscular junction at the large central muscles in Drosophila larvae tend to show mild synaptic depression (Kidokoro et al., 2004). Reducing extracellular calcium or recording from some smaller muscles within the larval segment, yields smaller amplitude EJPs, but allows observation of shortterm synaptic facilitation, as each evoked potential amplitude is larger than the previous one. Participants began to conduct their own explorations, testing the precise dynamics of the synapse they were recording. Experiments included testing the frequency dependence of synaptic depression or facilitation, the maximal firing rate of the synapse, the recovery time required to return to baseline synaptic strength, and longer- term adaptation or potentiation dynamics. In addition, these synapses often show detectable minis. Thus, participants could estimate the mini frequency and potentially perform simple quantal analysis to determine the average quantal content of an EJP. Recent studies have begun to address questions of synaptic transmission and synaptic plasticity using optogenetics (Watanabe et al., 2013). However, these processes remain relatively little studied in the Drosophila larval NMJ model synapse. This offers an opportunity for students to design protocols to explore various aspects of synaptic transmission with this novel way of remotely controlling neuronal activity (Pulver et al., 2011). The workshop participants had the opportunity to try optogenetic
6 Vilinsky et al. Drosophila Optogenetics A294 activation of motor neurons in an electrophysiologically interesting mutant, SNAP-25 ts, a temperature sensitive paralytic mutant allele of the SNAP-25 gene (Rao et al., 2001). In the larval stage, this mutation results in a greatly elevated mini frequency, and often a slightly elevated EJP amplitude. Other mutants that affect synaptic transmission and synaptic dynamics are open to exploration by students using the application of optogenetic techniques. For example, ion channel mutants such as Shaker (Salkoff et al., 1992), or vesicle recycling mutants such as Shibire (van der Bliek and Meyerowitz, 1991), are prime candidates for application in the teaching lab. These experiments are all the more impactful because all of the molecular components listed have direct homologues in humans, with fundamentally conserved function. Taken together, these exercises gave the participants a way to demonstrate and explore the molecular and physiological principles of synaptic transmission though optogenetic stimulation. Tying together genetics, molecular biology, neurophysiolology and behavior was a key aim of our workshop presentation. Organismal behavior can be directly observed, and neuronal activity can be directly recorded, but the genes and proteins underlying these processes are, for most undergraduates, mostly hypothetical and inaccessible. Optogenetics applied to a highly genetically manipulable animal allows students to make direct connections between molecules and behavior. These connections become tangible as students perform these experiments themselves, using the same techniques that are at the cutting edge of current research. This link between theory and practice not only promotes learning and understanding, but also serves as a platform for discovery in the teaching lab that can be used to conduct original investigations. Finally, the lab modules presented serve to familiarize participants with the tools of optogenetic stimulation, and the Drosophila prep, in both the adult and larval form, in order to overcome the novelty barrier that can delay adoption of new approaches to teaching and research. REFERENCES Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G (2007) In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54: Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: Branson K, Robie AA, Bender J, Perona P, Dickinson MH (2009) High-throughput ethomics in large groups of Drosophila. Nat Methods 6: Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA (2002) Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci U S A 99: Dionne H, Hibbard KL, Cavallaro A, Kao J-C, Rubin GM (2018) Genetic reagents for making split-gal4 lines in Drosophila. Genetics 209: Fenno L, Yizhar O, Deisseroth K (2011) The development and application of optogenetics. Annu Rev Neurosci 34: Harris KP, Littleton JT (2015) Transmission, development, and plasticity of synapses. Genetics 201: Hornstein NJ, Pulver SR, Griffith LC (2009) Channelrhodopsin2 mediated stimulation of synaptic potentials at Drosophila neuromuscular junctions. J Vis Exp 16(25) pii:1133 Inagaki HK, Jung Y, Hoopfer ED, Wong AM, Mishra N, Lin JY, Tsien RY, Anderson DJ (2014) Optogenetic control of Drosophila using a red-shifted channelrhodopsin channelrhodopsin reveals experience-dependent influences on courtship. Nat Methods 11: Jenett A, Rubin GM, Ngo T-TB, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. (2012) A GAL4- driver line resource for Drosophila neurobiology. Cell Rep 2: Kalanithi PS, Henderson JM (2012) Optogenetic neuromodulation. Int Rev Neurobiol 107: Kidokoro Y, Kuromi H, Delgado R, Maureira C, Oliva C, Labarca P (2004) Synaptic vesicle pools and plasticity of synaptic transmission at the Drosophila synapse. Brain Res Brain Res Rev 47: Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, et al. (2014) Independent optical excitation of distinct neural populations. Nat Methods 11: Liu S, Li C, Xing Y, Wang Y, Tao F (2016) Role of neuromodulation and optogenetic manipulation in pain Treatment. Curr Neuropharmacol 14: Luan H, Peabody NC, Vinson CR, White BH (2006) Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron 52: McKellar CE, Wyttenbach RA (2017) A protocol demonstrating 60 different Drosophila behaviors in one assay. J Undergrad Neurosci Educ 15:A110 A116. Moser T (2015) Optogenetic stimulation of the auditory pathway for research and future prosthetics. Curr Opin Neurobiol 34: Nieh EH, Kim S-Y, Namburi P, Tye KM (2013) Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain Res 1511: Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM (2010) Refinement of tools for targeted gene expression in Drosophila. Genetics 186: Pokala K, Glater EE (2018) Using optogenetics to understand neuronal mechanisms underlying behavior in C. elegans. J Undergrad Neurosci Educ 16:A152-A158. Pulver SR, Berni J (2012) The fundamentals of flying: simple and inexpensive strategies for employing Drosophila genetics in neuroscience teaching laboratories. J Undergrad Neurosci Educ 11:A139-A148. Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC (2009) Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol 101: Pulver SR, Hornstein NJ, Land BL, Johnson BR (2011) Optogenetics in the teaching laboratory: Using channelrhodopsin-2 to study the neural basis of behavior and synaptic physiology in Drosophila. Adv Physiol Educ 35: Rao SS, Stewart BA, Rivlin PK, Vilinsky I, Watson BO, Lang C, Boulianne G, Salpeter MM, Deitcher DL (2001) Two distinct effects on neurotransmission in a temperature-senitive SNAP-25 mutant. EMBO J 20: Rose JK (2018) Demonstrating connections between neuron signaling and behavior using C. elegans learning assays and optogenetics in a laboratory class. J Undergrad Neurosci Educ 16:A223-A231. Salkoff L, Baker K, Butler A, Covarrubias M, Pak MD, Wei A (1992) An essential set of K+ channels conserved in flies, mice and humans. Trends Neurosci 15: Scholl HPN, Strauss RW, Singh MS, Dalkara D, Roska B, Picaud S, Sahel JA (2016) Emerging therapies for inherited retinal degeneration. Sci Transl Med 8:368rv6.
7 A295 Sen R, Wu M, Branson K, Robie A, Rubin GM, Dickson BJ (2017) Moonwalker descending neurons mediate visually evoked retreat in Drosophila. Curr Biol 27: Sjulson L, Cassataro D, DasGupta S, Miesenböck G (2016) Cellspecific targeting of genetically encoded tools for neuroscience. Annu Rev Genet 50: Smith KS, Graybiel AM (2013) Using optogenetics to study habits. Brain Res 1511: Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF (1994) Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A 175: Titlow JS, Johnson BR, Pulver SR (2015) Light activated escape circuits: a behavior and neurophysiology lab module using Drosophila optogenetics. J Undergrad Neurosci Educ 13:A166- A173. van der Bliek AM, Meyerowitz EM (1991) Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351: Watanabe S, Rost BR, Camacho-Pérez M, Davis MW, Söhl- Kielczynski B, Rosemund C, Jorgensen EM (2013) Ultrafast endocytosis at mouse hippocampal synapses. Nature 504(7479): Zhang B, Stewart B (2010) Synaptic electrophysiology of the Drosophila neuromuscular junction. In: Drosophila neurobiology: a laboratory manual (Zhang B, Freeman MR, Waddell S, eds) pp Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. Received August 07, 2018; revised August 21, 2018; accepted August 22, This work was supported by the College of Arts and Sciences, University of Cincinnati and the Departmental of Neurobiology and Behavior, Cornell University. Support for Karen Hibbard comes from HHMI, Janelia Research Farms Campus. We thank Rajyashree Sen for the Moonwalker image, Gudrun Ihrke for assistance with imagining the larval CNS, and Annette Stowasser for technical advice on constructing the LED current source circuit. The lab exercises presented here are part of the CrawFly Faculty Workshops supported by ADInstruments, Inc. as part of the Classroom of Excellence program at Cornell, Emory, the University of Cincinnati and other CrawFly hosting institutions. Address correspondence to: Dr. Ilya Vilinsky, Department of Biological Sciences, University of Cincinnati, Cincinnati, OH ilya.vilinsky@uc.edu Copyright 2018 Faculty for Undergraduate Neuroscience
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae. BioNB4910 Cornell University.
 Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Lecture 11 : Simple Neuron Models. Dr Eileen Nugent
 Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Microsystems for Neuroscience and Medicine. Lecture 9
 1 Microsystems for Neuroscience and Medicine Lecture 9 2 Neural Microsystems Neurons - Structure and behaviour Measuring neural activity Interfacing with neurons Medical applications - DBS, Retinal Implants
1 Microsystems for Neuroscience and Medicine Lecture 9 2 Neural Microsystems Neurons - Structure and behaviour Measuring neural activity Interfacing with neurons Medical applications - DBS, Retinal Implants
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Maximum)individuals:!2! Shape)requirements:!rodent0like 4! Identity)method:!crosses!solved 5!
 Supplementary Material Automated image-based tracking and its application in ecology Anthony I. Dell*, John A. Bender, Kristin Branson, Iain D. Couzin, Gonzalo G. de Polavieja, Lucas P.J.J. Noldus, Alfonso
Supplementary Material Automated image-based tracking and its application in ecology Anthony I. Dell*, John A. Bender, Kristin Branson, Iain D. Couzin, Gonzalo G. de Polavieja, Lucas P.J.J. Noldus, Alfonso
Illustrating concepts of quantal analysis with an intuitive classroom model
 Adv Physiol Educ 37: 11 11, 13; doi:.115/advan..1. Illustrating concepts of quantal analysis with an intuitive classroom model Matthew A. Xu-Friedman Department of Biological Sciences, University at Buffalo,
Adv Physiol Educ 37: 11 11, 13; doi:.115/advan..1. Illustrating concepts of quantal analysis with an intuitive classroom model Matthew A. Xu-Friedman Department of Biological Sciences, University at Buffalo,
Math in systems neuroscience. Quan Wen
 Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Mr.checker Wikipedia Commons. Louise Docker from sydney, Australia, Wikimedia Commons. Angelique Paulk
 Mr.checker Wikipedia Commons Louise Docker from sydney, Australia, Wikimedia Commons Angelique Paulk Objectives How does learning and memory operate at the behavioral level? How can you train an insect?
Mr.checker Wikipedia Commons Louise Docker from sydney, Australia, Wikimedia Commons Angelique Paulk Objectives How does learning and memory operate at the behavioral level? How can you train an insect?
"Small Brains, Big Ideas: The value of model organisms to science. Dr. Yuly Fuentes-Medel Executive Director Descience
 "Small Brains, Big Ideas: The value of model organisms to science Dr. Yuly Fuentes-Medel Executive Director Descience Outline 1. What is a model organism? 2. Big questions solved by small-brained organisms
"Small Brains, Big Ideas: The value of model organisms to science Dr. Yuly Fuentes-Medel Executive Director Descience Outline 1. What is a model organism? 2. Big questions solved by small-brained organisms
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Neurons and Nervous Systems
 34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
Biosciences in the 21st century
 Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
Nervous Tissue. Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation
 Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling
 Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
How to read a burst duration code
 Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila
 Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
SUPPLEMENTARY INFORMATION
 Supplemental Figure S1. WISL is not elicited by courtship song and is dependent on chordotonal mechanosensory neurons. (a) Average locomotor velocity vs. time plots for wild-type CS flies exposed to recordings
Supplemental Figure S1. WISL is not elicited by courtship song and is dependent on chordotonal mechanosensory neurons. (a) Average locomotor velocity vs. time plots for wild-type CS flies exposed to recordings
Ch 33. The nervous system
 Ch 33 The nervous system AP bio schedule Tuesday Wed Thursday Friday Plant test Animal behavior lab Nervous system 25 Review Day (bring computer) 27 Review Day (bring computer) 28 Practice AP bio test
Ch 33 The nervous system AP bio schedule Tuesday Wed Thursday Friday Plant test Animal behavior lab Nervous system 25 Review Day (bring computer) 27 Review Day (bring computer) 28 Practice AP bio test
me239 mechanics of the cell - syllabus me239 mechanics of the cell me239 mechanics of the cell - grading me239 mechanics of the cell - overview
 6 mechanotransduction wong, goktepe, kuhl [2010] me239 mechanics of the cell add l information http://biomechanics.stanford.edu and coursework 1 me239 mechanics of the cell - syllabus favorite topics in
6 mechanotransduction wong, goktepe, kuhl [2010] me239 mechanics of the cell add l information http://biomechanics.stanford.edu and coursework 1 me239 mechanics of the cell - syllabus favorite topics in
Control and Integration. Nervous System Organization: Bilateral Symmetric Animals. Nervous System Organization: Radial Symmetric Animals
 Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
MEMBRANE POTENTIALS AND ACTION POTENTIALS:
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
Nature Methods: doi: /nmeth Supplementary Figure 1. In vitro screening of recombinant R-CaMP2 variants.
 Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
Mutations in palmitoyl-protein thioesterase 1 alter exocytosis and endocytosis at synapses in Drosophila larvae
 Paper Type Fly7:4, 267 279; October/November/December 2013; 2013 Landes Bioscience RESEARCH PAPER Mutations in palmitoyl-protein thioesterase 1 alter exocytosis and endocytosis at synapses in Drosophila
Paper Type Fly7:4, 267 279; October/November/December 2013; 2013 Landes Bioscience RESEARCH PAPER Mutations in palmitoyl-protein thioesterase 1 alter exocytosis and endocytosis at synapses in Drosophila
Hopfield Neural Network and Associative Memory. Typical Myelinated Vertebrate Motoneuron (Wikipedia) Topic 3 Polymers and Neurons Lecture 5
 Hopfield Neural Network and Associative Memory Typical Myelinated Vertebrate Motoneuron (Wikipedia) PHY 411-506 Computational Physics 2 1 Wednesday, March 5 1906 Nobel Prize in Physiology or Medicine.
Hopfield Neural Network and Associative Memory Typical Myelinated Vertebrate Motoneuron (Wikipedia) PHY 411-506 Computational Physics 2 1 Wednesday, March 5 1906 Nobel Prize in Physiology or Medicine.
Neurons, Synapses, and Signaling
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
Course Descriptions Biology
 Course Descriptions Biology BIOL 1010 (F/S) Human Anatomy and Physiology I. An introductory study of the structure and function of the human organ systems including the nervous, sensory, muscular, skeletal,
Course Descriptions Biology BIOL 1010 (F/S) Human Anatomy and Physiology I. An introductory study of the structure and function of the human organ systems including the nervous, sensory, muscular, skeletal,
NOTES: CH 48 Neurons, Synapses, and Signaling
 NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
Independent optical excitation of distinct neural populations
 CORRECTION NOTICE Nat. Methods 11, 338 346 (214) Independent optical excitation of distinct neural populations Nathan C Klapoetke, Yasunobu Murata, Sung Soo Kim, Stefan R Pulver, Amanda Birdsey-Benson,
CORRECTION NOTICE Nat. Methods 11, 338 346 (214) Independent optical excitation of distinct neural populations Nathan C Klapoetke, Yasunobu Murata, Sung Soo Kim, Stefan R Pulver, Amanda Birdsey-Benson,
Early consolidation of development and physiology of an identified presynaptic nerve terminal
 Laviolette and Stewart BMC Neuroscience 2013, 14:124 RESEARCH ARTICLE Open Access Early consolidation of development and physiology of an identified presynaptic nerve terminal Matthew Laviolette and Bryan
Laviolette and Stewart BMC Neuroscience 2013, 14:124 RESEARCH ARTICLE Open Access Early consolidation of development and physiology of an identified presynaptic nerve terminal Matthew Laviolette and Bryan
COMPUTATIONAL APPROACH OF OPTOELECTRONIC TRANSDUCTION IN THE COMPOUND EYE
 Journal of Optoelectronics and Advanced Materials Vol. 7, No. 6, December 005, p. 90-905 COMPUTATIONAL APPROACH OF OPTOELECTRONIC TRANSDUCTION IN THE COMPOUND EYE C. Stan, C. P. Cristescu, D. Creanga a*
Journal of Optoelectronics and Advanced Materials Vol. 7, No. 6, December 005, p. 90-905 COMPUTATIONAL APPROACH OF OPTOELECTRONIC TRANSDUCTION IN THE COMPOUND EYE C. Stan, C. P. Cristescu, D. Creanga a*
Supplementary material: Orbitofrontal and striatal circuits dynamically encode the shift. between goal-directed and habitual actions
 Supplementary material: Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions Christina M. Gremel 1 & Rui M. Costa 1,2 1 Laboratory for Integrative
Supplementary material: Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions Christina M. Gremel 1 & Rui M. Costa 1,2 1 Laboratory for Integrative
Synapses. Electrophysiology and Vesicle release
 Synapses Electrophysiology and Vesicle release Major point Cell theory (cells being separated) implies that cells must communicate with each other through extracellular connections most communication is
Synapses Electrophysiology and Vesicle release Major point Cell theory (cells being separated) implies that cells must communicate with each other through extracellular connections most communication is
Developmental genetics: finding the genes that regulate development
 Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
Hair Cells: The Sensory Transducers of the Inner Ear
 Chapter 1 Hair Cells: The Sensory Transducers of the Inner Ear Hair cells are specialized cells that transform a mechanical motion into changes in membrane potential. Such changes, whereby one form of
Chapter 1 Hair Cells: The Sensory Transducers of the Inner Ear Hair cells are specialized cells that transform a mechanical motion into changes in membrane potential. Such changes, whereby one form of
Nervous System AP Biology
 Nervous System 2007-2008 Why do animals need a nervous system? What characteristics do animals need in a nervous system? fast accurate reset quickly Remember Poor think bunny! about the bunny signal direction
Nervous System 2007-2008 Why do animals need a nervous system? What characteristics do animals need in a nervous system? fast accurate reset quickly Remember Poor think bunny! about the bunny signal direction
BIOLOGY 11/10/2016. Neurons, Synapses, and Signaling. Concept 48.1: Neuron organization and structure reflect function in information transfer
 48 Neurons, Synapses, and Signaling CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Concept 48.1: Neuron organization
48 Neurons, Synapses, and Signaling CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Concept 48.1: Neuron organization
Experiment 3: Nerve Conduction
 Experiment 3: Nerve Conduction Overview The interior of a cell is negatively charged with respect to the outside, and the magnitude of the potential difference is usually between 50 and 80 mv. Some cells,
Experiment 3: Nerve Conduction Overview The interior of a cell is negatively charged with respect to the outside, and the magnitude of the potential difference is usually between 50 and 80 mv. Some cells,
An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding
 NOTE Communicated by Michael Hines An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding A. Destexhe Z. F. Mainen T. J. Sejnowski The Howard Hughes Medical
NOTE Communicated by Michael Hines An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding A. Destexhe Z. F. Mainen T. J. Sejnowski The Howard Hughes Medical
Introduction to Cellular Communication *
 OpenStax-CNX module: m53235 1 Introduction to Cellular Communication * Steven Telleen This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 1 Why Cells Communicate
OpenStax-CNX module: m53235 1 Introduction to Cellular Communication * Steven Telleen This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 1 Why Cells Communicate
Membrane Potentials, Action Potentials, and Synaptic Transmission. Membrane Potential
 Cl Cl - - + K + K+ K + K Cl - 2/2/15 Membrane Potentials, Action Potentials, and Synaptic Transmission Core Curriculum II Spring 2015 Membrane Potential Example 1: K +, Cl - equally permeant no charge
Cl Cl - - + K + K+ K + K Cl - 2/2/15 Membrane Potentials, Action Potentials, and Synaptic Transmission Core Curriculum II Spring 2015 Membrane Potential Example 1: K +, Cl - equally permeant no charge
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Overexpression of YFP::GPR-1 in the germline.
 Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR
 REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR Grade Requirement: All courses required for the Biochemistry major (CH, MATH, PHYS, BI courses) must be graded and passed with a grade of C- or better. Core Chemistry
REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR Grade Requirement: All courses required for the Biochemistry major (CH, MATH, PHYS, BI courses) must be graded and passed with a grade of C- or better. Core Chemistry
Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions:
 Nervous System Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions: Sensory Input: Obtaining stimulation from the environment (light, heat, pressure, vibration, chemical,
Nervous System Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions: Sensory Input: Obtaining stimulation from the environment (light, heat, pressure, vibration, chemical,
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell
 Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Chapter 48 Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
purpose of this Chapter is to highlight some problems that will likely provide new
 119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
Nervous System Organization
 The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals?
 LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
Open Syntaxin Docks Synaptic Vesicles
 Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Neurons. The Molecular Basis of their Electrical Excitability
 Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR
 REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR Grade Requirement: All courses required for the Biochemistry major (CH, MATH, PHYS, BI courses) must be graded and passed with a grade of C- or better. Core Chemistry
REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR Grade Requirement: All courses required for the Biochemistry major (CH, MATH, PHYS, BI courses) must be graded and passed with a grade of C- or better. Core Chemistry
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Neurophysiology. + = Na + - = Cl - Proteins HOW? HOW?
 All animal cells have electric potential differences (voltages) across plasma s only electrically excitable cells can respond with APs Luigi Galvani (1791) Animal electricity Electrical fluid passed through
All animal cells have electric potential differences (voltages) across plasma s only electrically excitable cells can respond with APs Luigi Galvani (1791) Animal electricity Electrical fluid passed through
NERVOUS SYSTEM. Some of the most exciting things about animals HOW TO BUILD A INTERACTIONS SONIA SEN. What is the nervous system?
 HOW TO BUILD A NERVOUS SYSTEM INTERACTIONS SONIA SEN Animals constantly interact with their environment for the most vital of functions - finding food, avoiding predators or courting a prospective mate.
HOW TO BUILD A NERVOUS SYSTEM INTERACTIONS SONIA SEN Animals constantly interact with their environment for the most vital of functions - finding food, avoiding predators or courting a prospective mate.
Coordination of Cellular Pattern-Generating Circuits that Control Limb Movements: The Sources of Stable Differences in Intersegmental Phases
 The Journal of Neuroscience, April 15, 2003 23(8):3457 3468 3457 Coordination of Cellular Pattern-Generating Circuits that Control Limb Movements: The Sources of Stable Differences in Intersegmental Phases
The Journal of Neuroscience, April 15, 2003 23(8):3457 3468 3457 Coordination of Cellular Pattern-Generating Circuits that Control Limb Movements: The Sources of Stable Differences in Intersegmental Phases
ECEN 4606, UNDERGRADUATE OPTICS LAB
 ECEN 4606, UNDERGRADUATE OPTICS LAB Lab 6: Polarization Original: Professor McLeod SUMMARY: In this lab you will become familiar with the basics of polarization and learn to use common optical elements
ECEN 4606, UNDERGRADUATE OPTICS LAB Lab 6: Polarization Original: Professor McLeod SUMMARY: In this lab you will become familiar with the basics of polarization and learn to use common optical elements
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Parametric down-conversion
 Parametric down-conversion 1 Introduction You have seen that laser light, for all its intensity and coherence, gave us the same PP(mm) counts distribution as a thermal light source with a high fluctuation
Parametric down-conversion 1 Introduction You have seen that laser light, for all its intensity and coherence, gave us the same PP(mm) counts distribution as a thermal light source with a high fluctuation
R7.3 Receptor Kinetics
 Chapter 7 9/30/04 R7.3 Receptor Kinetics Professional Reference Shelf Just as enzymes are fundamental to life, so is the living cell s ability to receive and process signals from beyond the cell membrane.
Chapter 7 9/30/04 R7.3 Receptor Kinetics Professional Reference Shelf Just as enzymes are fundamental to life, so is the living cell s ability to receive and process signals from beyond the cell membrane.
Characterization of development, behavior and neuromuscular physiology in the phorid fly, Megaselia scalaris
 Comparative Biochemistry and Physiology Part A 136 (2003) 427 439 Characterization of development, behavior and neuromuscular physiology in the phorid fly, Megaselia scalaris Douglas A. Harrison, Robin
Comparative Biochemistry and Physiology Part A 136 (2003) 427 439 Characterization of development, behavior and neuromuscular physiology in the phorid fly, Megaselia scalaris Douglas A. Harrison, Robin
Eye size in Drosophila melanogaster and how it affects peripheral motion vision. Abstract
 Varkey 1 Justin Varkey Anthony McGoron 4 December 2015 Eye size in Drosophila melanogaster and how it affects peripheral motion vision Abstract Drosophila melanogaster, the fruit fly, is a holometabolous
Varkey 1 Justin Varkey Anthony McGoron 4 December 2015 Eye size in Drosophila melanogaster and how it affects peripheral motion vision Abstract Drosophila melanogaster, the fruit fly, is a holometabolous
CELL BIOLOGY - CLUTCH CH. 9 - TRANSPORT ACROSS MEMBRANES.
 !! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
!! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34
 NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests
 Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Overview Organization: Central Nervous System (CNS) Peripheral Nervous System (PNS) innervate Divisions: a. Afferent
 Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
(Received 15 June 1973)
 J. Phyeiol. (1974), 236, pp. 363-371 363 With 5 text-fighurem Printed in Great Britain STOCHASTIC PROPERTIES OF SPONTANEOUS TRANSMITTER RELEASE AT THE CRAYFISH NEUROMUSCULAR JUNCTION BY IRA COHEN, HIROSHI
J. Phyeiol. (1974), 236, pp. 363-371 363 With 5 text-fighurem Printed in Great Britain STOCHASTIC PROPERTIES OF SPONTANEOUS TRANSMITTER RELEASE AT THE CRAYFISH NEUROMUSCULAR JUNCTION BY IRA COHEN, HIROSHI
All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model
 All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model S. A. Sadegh Zadeh, C. Kambhampati International Science Index, Mathematical and Computational Sciences waset.org/publication/10008281
All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model S. A. Sadegh Zadeh, C. Kambhampati International Science Index, Mathematical and Computational Sciences waset.org/publication/10008281
Chapter 9. Nerve Signals and Homeostasis
 Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Synaptotagmin 2 Mutations Cause Autosomal-Dominant Form of Lambert-Eaton Myasthenic Syndrome and Nonprogressive Motor Neuropathy
 The American Journal of Human Genetics, Volume 95 Supplemental Data Synaptotagmin 2 Mutations Cause AutosomalDominant Form of LambertEaton Myasthenic Syndrome and Nonprogressive Motor Neuropathy David
The American Journal of Human Genetics, Volume 95 Supplemental Data Synaptotagmin 2 Mutations Cause AutosomalDominant Form of LambertEaton Myasthenic Syndrome and Nonprogressive Motor Neuropathy David
2401 : Anatomy/Physiology
 Dr. Chris Doumen Week 6 2401 : Anatomy/Physiology Action Potentials NeuroPhysiology TextBook Readings Pages 400 through 408 Make use of the figures in your textbook ; a picture is worth a thousand words!
Dr. Chris Doumen Week 6 2401 : Anatomy/Physiology Action Potentials NeuroPhysiology TextBook Readings Pages 400 through 408 Make use of the figures in your textbook ; a picture is worth a thousand words!
MEMBRANE STRUCTURE. Lecture 9. Biology Department Concordia University. Dr. S. Azam BIOL 266/
 MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
Neuronal Differentiation: Synapse Formation NMJ
 Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
Propagation& Integration: Passive electrical properties
 Fundamentals of Neuroscience (NSCS 730, Spring 2010) Instructor: Art Riegel; email: Riegel@musc.edu; Room EL 113; time: 9 11 am Office: 416C BSB (792.5444) Propagation& Integration: Passive electrical
Fundamentals of Neuroscience (NSCS 730, Spring 2010) Instructor: Art Riegel; email: Riegel@musc.edu; Room EL 113; time: 9 11 am Office: 416C BSB (792.5444) Propagation& Integration: Passive electrical
Detecting local processing unit in drosophila brain by using network theory
 Detecting local processing unit in drosophila brain by using network theory Dongmei Shi 1,2, Chitin Shih 3,4, Chungchuan Lo 5, Yenjen Lin 1, and Annshyn Chiang 1,6,7 1. Brain Research Center, National
Detecting local processing unit in drosophila brain by using network theory Dongmei Shi 1,2, Chitin Shih 3,4, Chungchuan Lo 5, Yenjen Lin 1, and Annshyn Chiang 1,6,7 1. Brain Research Center, National
BIOLOGY. 1. Overview of Neurons 11/3/2014. Neurons, Synapses, and Signaling. Communication in Neurons
 CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
CSE/NB 528 Final Lecture: All Good Things Must. CSE/NB 528: Final Lecture
 CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
Domain 6: Communication
 Domain 6: Communication 6.1: Cell communication processes share common features that reflect a shared evolutionary history. (EK3.D.1) 1. Introduction to Communication Communication requires the generation,
Domain 6: Communication 6.1: Cell communication processes share common features that reflect a shared evolutionary history. (EK3.D.1) 1. Introduction to Communication Communication requires the generation,
Course plan Academic Year Qualification MSc on Bioinformatics for Health Sciences. Subject name: Computational Systems Biology Code: 30180
 Course plan 201-201 Academic Year Qualification MSc on Bioinformatics for Health Sciences 1. Description of the subject Subject name: Code: 30180 Total credits: 5 Workload: 125 hours Year: 1st Term: 3
Course plan 201-201 Academic Year Qualification MSc on Bioinformatics for Health Sciences 1. Description of the subject Subject name: Code: 30180 Total credits: 5 Workload: 125 hours Year: 1st Term: 3
37 Neurons, Synapses, and Signaling
 CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: Lines of Communication
CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: Lines of Communication
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1. Different crystal forms obtained for Sky
 Supplementary Figure 1 Different crystal forms obtained for Sky 1 353. (a) Crystal form 1 obtained in the presence of 20% PEG 3350 and 0.2 M ammonium citrate tribasic ph 7.0. (b) Crystal form 1 of the
Supplementary Figure 1 Different crystal forms obtained for Sky 1 353. (a) Crystal form 1 obtained in the presence of 20% PEG 3350 and 0.2 M ammonium citrate tribasic ph 7.0. (b) Crystal form 1 of the
Dendrites - receives information from other neuron cells - input receivers.
 The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
Ch 7. The Nervous System 7.1 & 7.2
 Ch 7 The Nervous System 7.1 & 7.2 SLOs Describe the different types of neurons and supporting cells, and identify their functions. Identify the myelin sheath and describe how it is formed in the CNS and
Ch 7 The Nervous System 7.1 & 7.2 SLOs Describe the different types of neurons and supporting cells, and identify their functions. Identify the myelin sheath and describe how it is formed in the CNS and
Neurons, Synapses, and Signaling
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
Lab I: Three-Point Mapping in Drosophila melanogaster
 Lab I: Three-Point Mapping in Drosophila melanogaster Makuo Aneke Partner: Christina Hwang BIO 365-004: Genetics with Laboratory TA: Dr. Hongmei Ma February 18, 2016 Abstract The purpose of this experiment
Lab I: Three-Point Mapping in Drosophila melanogaster Makuo Aneke Partner: Christina Hwang BIO 365-004: Genetics with Laboratory TA: Dr. Hongmei Ma February 18, 2016 Abstract The purpose of this experiment
Page AC : INSTRUMENTATION FOR SHOCK AND IMPACT ANALYSIS
 AC 2010-2123: INSTRUMENTATION FOR SHOCK AND IMPACT ANALYSIS Randy Buchanan, University of Southern Mississippi Steven Bunkley, University of Southern Mississippi American Society for Engineering Education,
AC 2010-2123: INSTRUMENTATION FOR SHOCK AND IMPACT ANALYSIS Randy Buchanan, University of Southern Mississippi Steven Bunkley, University of Southern Mississippi American Society for Engineering Education,
6.3.4 Action potential
 I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten. Lecture 2a. The Neuron - overview of structure. From Anderson (1995)
 Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Enduring understanding 1.A: Change in the genetic makeup of a population over time is evolution.
 The AP Biology course is designed to enable you to develop advanced inquiry and reasoning skills, such as designing a plan for collecting data, analyzing data, applying mathematical routines, and connecting
The AP Biology course is designed to enable you to develop advanced inquiry and reasoning skills, such as designing a plan for collecting data, analyzing data, applying mathematical routines, and connecting
This script will produce a series of pulses of amplitude 40 na, duration 1ms, recurring every 50 ms.
 9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
Biological membranes and bioelectric phenomena
 Lectures on Medical Biophysics Dept. Biophysics, Medical faculty, Masaryk University in Brno Biological membranes and bioelectric phenomena A part of this lecture was prepared on the basis of a presentation
Lectures on Medical Biophysics Dept. Biophysics, Medical faculty, Masaryk University in Brno Biological membranes and bioelectric phenomena A part of this lecture was prepared on the basis of a presentation
Live-cell imaging of alkyne-tagged small biomolecules by stimulated Raman scattering
 Live-cell imaging of alkyne-tagged small biomolecules by stimulated Raman scattering Lu Wei, Fanghao Hu, Yihui Shen, Zhixing Chen, Yong Yu, Chih-Chun Lin, Meng C. Wang, Wei Min * * To whom correspondence
Live-cell imaging of alkyne-tagged small biomolecules by stimulated Raman scattering Lu Wei, Fanghao Hu, Yihui Shen, Zhixing Chen, Yong Yu, Chih-Chun Lin, Meng C. Wang, Wei Min * * To whom correspondence
Imagent for fnirs and EROS measurements
 TECHNICAL NOTE Imagent for fnirs and EROS measurements 1. Brain imaging using Infrared Photons Brain imaging techniques can be broadly classified in two groups. One group includes the techniques that have
TECHNICAL NOTE Imagent for fnirs and EROS measurements 1. Brain imaging using Infrared Photons Brain imaging techniques can be broadly classified in two groups. One group includes the techniques that have
A Three-dimensional Physiologically Realistic Model of the Retina
 A Three-dimensional Physiologically Realistic Model of the Retina Michael Tadross, Cameron Whitehouse, Melissa Hornstein, Vicky Eng and Evangelia Micheli-Tzanakou Department of Biomedical Engineering 617
A Three-dimensional Physiologically Realistic Model of the Retina Michael Tadross, Cameron Whitehouse, Melissa Hornstein, Vicky Eng and Evangelia Micheli-Tzanakou Department of Biomedical Engineering 617
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03
 Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Sensory Encoding of Smell in the Olfactory System of Drosophila
 Sensory Encoding of Smell in the Olfactory System of Drosophila (reviewing Olfactory Information Processing in Drosophila by Masse et al, 2009) Ben Cipollini COGS 160 May 11, 2010 Smell Drives Our Behavior...
Sensory Encoding of Smell in the Olfactory System of Drosophila (reviewing Olfactory Information Processing in Drosophila by Masse et al, 2009) Ben Cipollini COGS 160 May 11, 2010 Smell Drives Our Behavior...
