Continent-Ocean Interaction: Role of Weathering
|
|
|
- Chester Russell
- 5 years ago
- Views:
Transcription
1 Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 Phone: th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling: why? 2 Model development: general principles 3 Illustration: simple carbon cycle model 4 Conclusions and outlook
2 Carbon Cycle: Processes and Time Scales ATMOSPHERE deforestation assimilation respiration assimilation water exchange Dissolved inorganic carbon Dissolved organic carbon Particulate organic carbon Mantle degassing air sea exchange Marine biota weathering surface water respiration particulate flux OCEAN deep water Seafloor alteration BIOSPHERE riverborne material sedimentation (organic & inorganic) litterfall Litter Humus Peat Carbonate Coal, oil, gas Carbonate minerals Dispersed carbon fossil fuel burning LITHOSPHERE volcanism (Adapted from Holmén, 1992) Natural Processes with long time scales Natural Processes with short time scales Human Perturbations Modelling OBSERVATION (Data) HYPOTHESES (Theory) STATISTICAL LAWS MECHANISTIC LAWS CALIBRATION VALIDATION MODEL - statistical - mechanistic DIAGNOSTIC USAGE (comprehension) PROGNOSTIC USAGE (prediction)
3 Model Development: General Principles Four stages 1 Problem Identification 2 Model Formulation 3 Model Solution 4 Interpretation of the results Equal importance for each stage Not a uni-directional procedure (following Boudreau, 1997) Development of a Model Formulation processes to include / exclude mathematical representation of the processes approximations adopted hypotheses made Solution depends on the situation Interpretation secondary results: consequences model to be refined or to simplified (following Boudreau, 1997)
4 Illustration: Application to an Actual Question Question How much CO 2 is released by volcanic and hydrothermal activity (metamorphic fluxes included)? How does this compare to the amount of CO 2 released by human activity? Model Formulation: Hypotheses and Simplifications Time Scale: 1,000 10,000 years and more little variability of volcanic and hydrothermal fluxes biosphere at steady state : fluxes have no influence burial of organic matter counter-balanced by kerogen carbon weathering: fluxes cancel out sea-floor weathering poorly known and small: neglected Steady state
5 Carbon Cycle Model: Processes Considered volcanic output C vol 01 silicate weathering C sil a Exchange of carbon between ocean and atmosphere Exchange of carbon between ocean/atmosphere and crust 01 ATMOSPHERE carbonate deposition air sea exchange C o a C a o Ccar a OCEAN carbonate weathering C car r C sed C hyd CRUST hydrothermal processes Carbonate Chemistry in Seawater Carbonate system equilibria CO 2(aq) + 2 H 2 O HCO 3 + H 3O + HCO 3 + H 2O CO H 3O + Special roles played by particular species atmospheric p CO2 [CO 2(aq) ] surface CaCO 3 burial [CO 2 3 ] deep sea Speciation calculated from combinations Dissolved Inorganic Carbon C T = [CO 2(aq) ] + [HCO 3 ] + [CO2 3 ] Total Alkalinity A T [HCO 3 ] + 2[CO2 3 ] + [B(OH) 4 ] + [OH ] [H 3 O + ]
6 Carbon Cycle Model: Fluxes Considered volcanic output C vol 01 C sil a C sed silicate weathering Creation and destruction of alkalinity Exchange of carbon between ocean and atmosphere Exchange of carbon between ocean/atmosphere and crust A sil ATMOSPHERE carbonate deposition air sea exchange C o a C a o Ccar a A car OCEAN carbonate weathering C car r C hyd A sed CRUST hydrothermal processes Carbon Cycle Model: Conservation Equations C atm : total amount of C in the atmosphere C oce : total amount of C in the ocean C atm + C oce = C A : total amount of alkalinity in the ocean dc atm dc oce = C vol C sil a C car a + C o a C a o = C hyd + C sil a + C car a + C car r C o a + C a o C sed dc atm + dc oce = dc da = C hyd + C vol + C car r C sed = A sil + A car A sed
7 Typical Weathering Reactions for Silicate Minerals Dissolution of albite with precipitation of kaolinite 2NaAlSi 3 O 8 + 2CO H 2 O Al 2 Si 2 O 5 (OH) 4 + 2Na + + 2HCO 3 + 4H 4 SiO 4 Dissolution of anorthite with precipitation of kaolinite CaAl 2 Si 2 O 8 + 2CO 2 + 3H 2 O Al 2 Si 2 O 5 (OH) 4 + Ca HCO 3 Dissolution of microcline with precipitation of pyrophillite 2KAlSi 3 O 8 + 2CO 2 + 6H 2 O Al 2 Si 4 O 10 (OH) 2 + 2K + + 2HCO 3 + 2H 4 SiO 4 Typical Weathering Reactions for Silicate Minerals Dissolution of chlorite with precipitation of kaolinite Mg 5 Al 2 Si 3 O CO 2 + 5H 2 O Al 2 Si 2 O 5 (OH) 4 + 5Mg HCO 3 + H 4SiO 4 Dissolution of microcline with precipitation of gibbsite KAlSi 3 O 8 + CO 2 + 4H 2 O Al(OH) 3 + K + + HCO 3 + H 4SiO 4
8 Sources and Sinks of DIC and TA in the Ocean Sources : continental weathering carbonate rocks: congruent dissolution CaCO 3 + CO 2 + H 2 O Ca HCO 3 silicate rocks: incongruent dissolution silicate mineral + b CO 2 + water secondary minerals + cations + b HCO 3 + s H 4SiO 4 Sinks : burial of biogenic carbonates Ca HCO 3 CaCO 3 + CO 2 + H 2 O Cycles to the Carbon Cycle: Ca, K, Mg, Na, Si Residence times of coupled cycles elements in the oceans Element τ oc Note (10 6 yr) Ca 1 Mg 13 K 12 Na 83 Si 0.02 DIC 0.07 org. and inorg. sinks 0.10 inorg. sinks only Alk 0.05
9 Carbon Cycle Silicate Cycle Coupling Geochemical carbonate and silicate cycles Simplification: neglect K and Na contributions No significant K- or Na-carbonate depositions Only 5% of the total riverine HCO 3 flux provided by Na- and K-silicate dissolution (Berner, 2004, based upon Berner and Berner, 1996) According to Gaillardet et al. (1999), this fraction is 19%, to be compared with 21% from Ca- and Mg-silicates This oceanic HCO 3 source is counterbalanced by the HCO 3 sink represented by authigenic Na- and K-mineral precipitation in marine sediments (reverse weathering, very slow process): 2K + + 3Al 2 Si 2 O 5 (OH) 4 + 2HCO 3 2KAl 3 Si 3 O 10 (OH) 2 + 5H 2 O + CO 2 Carbon Cycle Silicate Cycle Coupling Geochemical carbonate and silicate cycles Weathering of Ca- (or Mg-) carbonate CaCO 3 + CO 2 + H 2 O Ca HCO 3 Precipitation and sediment burial of carbonates Ca HCO 3 CaCO 3 + CO 2 + H 2 O Net balance:
10 Carbon Cycle Silicate Cycle Coupling Geochemical carbonate and silicate cycles Weathering of Ca- (or Mg-) silicate CaSiO 3 + 2CO 2 + 3H 2 O Ca 2+ + H 4 SiO 4 + 2HCO 3 Precipitation and sediment burial of carbonate and opal Ca HCO 3 CaCO 3 + CO 2 + H 2 O H 4 SiO 4 SiO 2 + 2H 2 O Net balance CaSiO 3 + CO 2 CaCO 3 + SiO 2 Combined with reverse reaction (metamorphism) CaSiO 3 + CO 2 CaCO 3 + SiO 2 Urey s Reaction Global Balance of the Ocean-Atmosphere System Relationships between carbon and alkalinity fluxes C car r = C car a A sil = C sil a A car = C car a + C car r = 2C car r A sed = 2C sed Upon introduction into the C et A balance equations: dc da = C hyd + C vol + C car r C sed = A sil + A car A sed
11 Carbon Cycle Model: Resolution dc da = C hyd + C vol + C car r C sed = C sil a + 2C car r 2C sed Carbon Cycle Model: Resolution Steady state conditions: t > 10 6 yr dc = 0 et da = 0 Accordingly, the balance equations for C et A become C hyd + C vol + C car r C sed = 0 (1) C sil a + 2C car r 2C sed = 0 (2) Finally, equation (1) 1 2 equation (2) yields C hyd + C vol = 1 2 C sil a
12 Carbon Cycle Model: Resolution Initial problem reduced to: C sil a =? 66% {}}{ C riv = C sil a +C car a }{{}}{{} 32% 34% 34% {}}{ + C car r }{{} 34% Riverine HCO 3 data analysis total amount: 31,6 37, molhco 3 66% stem from the atmosphere per year Hence: and thus C sil a = 0.32 C riv C hyd + C vol = 0.16 C riv. Solution and Interpretation Result Since we find that C riv = ( ) mol C/yr, C hyd + C vol = ( ) mol C/yr
13 Solution and Interpretation Interpretation Comparison with anthropogenic CO 2 emissions Secondary result: sedimentary flux C sed C sed = C hyd + C vol + C car r (equation (1)) = 1 2 C sil a + C car r = 1 2 C sil a C car a C car r = 1 2 C riv Hence: C sed = ( ) mol C/an Solution and Interpretation CO 2 Emissions (10 12 gc/yr) Fossil Fuel Combustion and Cement Production Oil Coal Gas Cement Production Flaring Total Emissions Year CO 2 Emissions (10 12 molc/an) Coal Oil Gas Cement Flaring Total Units: Tmol C/yr (original data in Tg C/yr). Data sources: Boden et al. (2011), for years before 2008; Boden and Blasing (2012) for (preliminary).
14 Carbon Cycle: Present-day and Pre-industrial ATMOSPHERE ~ CO 2 (preindustrial ) organic C = CO + HCO + CO OCEAN LITHOSPHERE CaCO 3 Riverine HCO 3 Weathering CO 2 surface deep Deep sea CaCO deposition 3 Volcanic CO Shallow water CaCO deposition Carbonate Rock Weathering Reservoir sizes ~ VEGETATION & SOIL in mol C Fluxes in 1012 mol C/yr Carbon Cycle: Present-day and Pre-industrial ATMOSPHERE CO 2 (preindustrial ) = CO + HCO +CO Riverine HCO 3 Weathering CO 2 surface Volcanic CO ~ deep ~ Carbonate Rock Weathering VEGETATION & SOIL Shallow water organic C CaCO 3 CaCO deposition OCEAN LITHOSPHERE Deep sea CaCO 3 deposition 7 11 Deep sea CaCO dissolution 3 Reservoir sizes in mol C Fluxes in 1012 mol C/yr
15 Connecting the Carbon and Alkalinity Budgets dc da = C hyd + C vol + C car r C sed = C sil a + 2C car r 2C sed da 2 dc = C sil a 2 (C hyd + C vol ) Basic Constraints of the System: Time Scales > 1 Myr τ carbon 100 kyr τ alkalinity 50 kyr Long time-scales (typically > 1 Myr): Global budgets of C and of A balanced da = 0 = C sil a = 2 (C vol + C hyd ) dc = 0
16 Basic Constraints of the System: Time Scales < 1 Myr On time scales of kyr constraint fulfilled on average only fluctuations possible classically, it has been assumed that hydrothermal and volcanic activity exhibit only small variability on these time scales C hyd + C vol = Chyd + C vol = 1 2 C sil a Hence da 2 dc = (C sil a C sil a ) da 2 dc = C sil a Sensitivity Analysis: Variable Silicate Weathering C hyd+vol 0 Constant C car r mol/yr Riverine HCO 3 supply t mol/yr CaCO 3 deposition t Full Carbon Compensation Full Alkalinity Compensation Intermediate Response Constant CO3 Response eq Alkalinity (Ocean) mol Carbon (Ocean+Atmosphere) t t ppm Atmospheric CO 2 t
17 Sensitivity Analysis: Variable Carbonate Weathering C hyd+vol 0 Constant C sil a mol/yr Riverine HCO 3 supply t mol/yr CaCO 3 deposition No compensation t Compensation for both Carbon and Alkalinity eq Alkalinity (Ocean) mol Carbon (Ocean+Atmosphere) t t ppm Atmospheric CO 2 t Sensitivity Analysis: How Does it Work in a Model? MBM Multi-Box Model of ocean-atmosphere carbon cycle ten oceanic and one atmospheric reservoirs realistic hypsometry fully coupled to copies of MEDUSA Model of Early Diagenesis in the Upper Sediment (A) bioturbated mixed-layer with 21 grid-points on top of a stack of thin layers (sediment core) solves time-dependent transport-reaction equations solids: calcite, aragonite, POM, clay solutes: CO 2, HCO 3, CO2 3, O 2 fully bi-directional exchange between the two zones
18 Bicarbonate Production Rate Scenarios 30 Carbonate Weathering HCO 3 Production 30 Silicate Weathering HCO 3 Production HCO 3 (Tmol/yr) HCO 3 (Tmol/yr) Age (kyr BP) Age (kyr BP) CO 2 Consumption Rate Scenarios 30 Carbonate Weathering CO 2 Consumption 30 Silicate Weathering CO 2 Consumption CO 2 (Tmol/yr) CO 2 (Tmol/yr) Age (kyr BP) Age (kyr BP)
19 pco 2 and Calcite Saturation Horizon Variations 360 Atmospheric CO Indo-Pacific Calcite Saturation Horizon CO 2 (ppm) Depth BSL (m) Age (kyr BP) Age (kyr BP) Summary Geochemical Carbon Cycle: complex system quantitative study requires models Four stages for development of a model 1 Identification of the problem 2 Formulation of the model 3 Resolution of the model 4 Interpretation of the results Illustration on an actual example
20 References cited T. A. Boden, G. Marland, and R. J. Andres. Global, regional, and national fossil-fuel CO 2 emissions. Data base, Carbon Dioxide Information Analysis Center (CDIAC), Oak Ridge, TN, URL T. Boden and T. J. Blasing. Record high 2010 global carbon dioxide emissions from fossil-fuel combustion and cement manufacture posted on CDIAC site. Data base, Carbon Dioxide Information Analysis Center (CDIAC), Oak Ridge, TN, URL B. P. Boudreau. Diagenetic Models and Their Implementation. Springer-Verlag, Berlin, 1997.
/ Past and Present Climate
 MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
Global Carbon Cycle - I
 Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I
 Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks. Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7.
 The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7. Overhead Transparencies Faint Faint Young Sun Paradox Young
The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7. Overhead Transparencies Faint Faint Young Sun Paradox Young
(4) Give an example of important reactions that are responsible for the composition of river water.
 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Global Carbon Cycle - I Systematics: Reservoirs and Fluxes
 OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions
 Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
XI. the natural carbon cycle. with materials from J. Kasting (Penn State)
 XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
Lecture 4 What Controls the Composition of Seawater
 Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Carbon Dioxide, Alkalinity and ph
 Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
THE OCEAN CARBON CYCLE
 THE OCEAN CARBON CYCLE 21st February 2018 1 Box-model of the global ocean phosphorus, alkalinity, carbon 2 Pre-industrial model 3 Evolution during the industrial period 4 13 C isotopic evolution BOX-MODEL
THE OCEAN CARBON CYCLE 21st February 2018 1 Box-model of the global ocean phosphorus, alkalinity, carbon 2 Pre-industrial model 3 Evolution during the industrial period 4 13 C isotopic evolution BOX-MODEL
Figure 65: Reservoir in a steady state condition where the input flux is equal to the output flux and the reservoir size remains constant.
 7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
organisms CaCO 3 + H 2 O + CO 2 shallow water
 Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Processes affecting continental shelves
 Marine Sediments Continental Shelves Processes affecting continental shelves 1. Glaciation 2. Sea-level change (±130 m during continental glaciation) 3. Waves and currents 4. Sedimentation 5. Carbonate
Marine Sediments Continental Shelves Processes affecting continental shelves 1. Glaciation 2. Sea-level change (±130 m during continental glaciation) 3. Waves and currents 4. Sedimentation 5. Carbonate
Mid-Term #1 (125 points total)
 Ocean 520 Name: Chemical Oceanography 20 October 2009 Fall 2009 Points are in parentheses (show all your work) (use back if necessary) MidTerm #1 (125 points total) 1. Doney et al (2008) Ocean Acidification
Ocean 520 Name: Chemical Oceanography 20 October 2009 Fall 2009 Points are in parentheses (show all your work) (use back if necessary) MidTerm #1 (125 points total) 1. Doney et al (2008) Ocean Acidification
This Week: Biogeochemical Cycles. Hydrologic Cycle Carbon Cycle
 This Week: Biogeochemical Cycles Hydrologic Cycle Carbon Cycle Announcements Reading: Chapters 4 (p. 74 81) and 8 Another Problem Set (Due next Tuesday) Exam 2: Friday Feb 29 My office hours today and
This Week: Biogeochemical Cycles Hydrologic Cycle Carbon Cycle Announcements Reading: Chapters 4 (p. 74 81) and 8 Another Problem Set (Due next Tuesday) Exam 2: Friday Feb 29 My office hours today and
Long-term Climate Change. We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold.
 Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza
 Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza General Concepts for Natural Controls on Fresh Water Composition 1. Generalizations about freshwater compositions 2. Conservative/nonconservative
Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza General Concepts for Natural Controls on Fresh Water Composition 1. Generalizations about freshwater compositions 2. Conservative/nonconservative
CO2 in atmosphere is influenced by pco2 of surface water (partial pressure of water is the CO2 (gas) that would be in equilibrium with water).
 EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
Hydrological Cycle Rain and rivers OUTLINE
 Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
Ocean Acidifica+on: past analogies, present concerns, future predic+ons. Sco8 Wieman
 Ocean Acidifica+on: past analogies, present concerns, future predic+ons Sco8 Wieman Effects Planktonic calcifica+on Carbon and nutrient assimila+on Primary produc+on Acid-base balance Larval stages of
Ocean Acidifica+on: past analogies, present concerns, future predic+ons Sco8 Wieman Effects Planktonic calcifica+on Carbon and nutrient assimila+on Primary produc+on Acid-base balance Larval stages of
Ocean Sediments. Key Concepts
 Ocean Sediments Key Concepts 1. What are the processes that control what types of sediments are deposited in which places? 2. Conversely, how can we use the sedimentary record to figure out tectonic and
Ocean Sediments Key Concepts 1. What are the processes that control what types of sediments are deposited in which places? 2. Conversely, how can we use the sedimentary record to figure out tectonic and
1/31/2013. Weathering Includes Physical, Chemical, Biological processes. Weathering Mechanisms. Wind abrasion forming Ventifacts
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
key to long-term sustainability is recycling..
 .. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
.. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
Ocean Sediments OCN Nov 2016
 Ocean Sediments OCN 401 10 Nov 2016 Outline Significance & terms Origin & distribution of major types of marine sediments Delivery - dissolution destruction mid-ocean ridges Significance of ocean sediments
Ocean Sediments OCN 401 10 Nov 2016 Outline Significance & terms Origin & distribution of major types of marine sediments Delivery - dissolution destruction mid-ocean ridges Significance of ocean sediments
Hydrothermal Chemistry/ Reverse Weathering. Marine Chemistry Seminar
 Hydrothermal Chemistry/ Reverse Weathering Marine Chemistry Seminar 1974 Drever, The Sea Chapter 10:The Magnesium Problem 1979 Edmonds et al., Ridge Crest Hydrothermal Activity and the Balances of Major
Hydrothermal Chemistry/ Reverse Weathering Marine Chemistry Seminar 1974 Drever, The Sea Chapter 10:The Magnesium Problem 1979 Edmonds et al., Ridge Crest Hydrothermal Activity and the Balances of Major
Lecture 6 - Determinants of Seawater Composition. Sets up electric dipole because O is more electronegative A o. Figure 3.
 12.742 - Marine Chemistry Fall 2004 Lecture 6 - Determinants of Seawater Composition Prof. Scott Doney What is seawater? Water Dissolved inorganic salts (major ions) Trace species, organics, colloids,
12.742 - Marine Chemistry Fall 2004 Lecture 6 - Determinants of Seawater Composition Prof. Scott Doney What is seawater? Water Dissolved inorganic salts (major ions) Trace species, organics, colloids,
Part II: Past climates
 Part II: Past climates This week Solid Earth - excerpts of Ch 7 Carbon cycle - Ch 8 Early unexplainable things about the Earth Continental Drift (Alfred Wegener, 1920s) Ocean basins: trenches and midocean
Part II: Past climates This week Solid Earth - excerpts of Ch 7 Carbon cycle - Ch 8 Early unexplainable things about the Earth Continental Drift (Alfred Wegener, 1920s) Ocean basins: trenches and midocean
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and
 1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
MAR 510 Chemical Oceanography
 Carbonate Equilibrium -Key Concepts- Major buffer system influencing ph (master variable) Linked to geological, biological and climatological cycles Complex chemistry involving gaseous, dissolved, and
Carbonate Equilibrium -Key Concepts- Major buffer system influencing ph (master variable) Linked to geological, biological and climatological cycles Complex chemistry involving gaseous, dissolved, and
Global phosphorus cycle
 Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments
 Peter Köhler 1 October 2015 Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments enhanced weathering, ocean alkalinization, ocean fertilization Peter Köhler, Judith Hauck
Peter Köhler 1 October 2015 Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments enhanced weathering, ocean alkalinization, ocean fertilization Peter Köhler, Judith Hauck
SoilGen2 model: data requirements and model output
 SoilGen2 model: data requirements and model output 1. Essential plot data... 1 2. Essential soil data... 2 3. Precipitation data (for a typical year)... 2 4. Evapotranspiration and Air temperature data
SoilGen2 model: data requirements and model output 1. Essential plot data... 1 2. Essential soil data... 2 3. Precipitation data (for a typical year)... 2 4. Evapotranspiration and Air temperature data
Physics of Aquatic Systems II
 Physics of Aquatic Systems II 10. C-Dating Werner Aeschbach-Hertig Institute of Environmental Physics University of Heidelberg 1 Contents of Session 10 General principles of C dating Conventional C age,
Physics of Aquatic Systems II 10. C-Dating Werner Aeschbach-Hertig Institute of Environmental Physics University of Heidelberg 1 Contents of Session 10 General principles of C dating Conventional C age,
Wednesday week 12. These ions move through the soil to streams and eventually to the ocean. In the ocean; CaCO 3 + H 2 O + CO 2 H 2 O + H 2 O
 Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
8. Carbon Cycle. Carbon ( 炭素 ) Family. Earth Watch: Antarctic lake hides bizarre ecosystem 無機탄소, 有機탄소
 arbon ( 炭素 ) Family 8. arbon ycle 지구시스템의이해읽기 : 탄소이야기 3 Earth Watch: Antarctic lake hides bizarre ecosystem Extremely alkaline waters with high dissolved 4 bacterial stromatolites 無機탄소, 有機탄소 Inorganic carbon:
arbon ( 炭素 ) Family 8. arbon ycle 지구시스템의이해읽기 : 탄소이야기 3 Earth Watch: Antarctic lake hides bizarre ecosystem Extremely alkaline waters with high dissolved 4 bacterial stromatolites 無機탄소, 有機탄소 Inorganic carbon:
SCOPE 35 Scales and Global Change (1988)
 1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
BIOGEOCHEMICAL CYCLES
 BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
Geochemical Reservoirs and Transfer Processes
 Geochemical Reservoirs and Transfer Processes Ocn 623 Dr. Michael J. Mottl Dept. Of Oceanography Three Basic Questions 1. Why does Earth have oceans? 2. Why does Earth have dry land? 3. Why are the seas
Geochemical Reservoirs and Transfer Processes Ocn 623 Dr. Michael J. Mottl Dept. Of Oceanography Three Basic Questions 1. Why does Earth have oceans? 2. Why does Earth have dry land? 3. Why are the seas
 Carbon - I This figure from IPCC, 2001 illustrates the large variations in atmospheric CO 2 (a) Direct measurements of atmospheric CO 2 concentration, and O 2 from 1990 onwards. O 2 concentration is the
Carbon - I This figure from IPCC, 2001 illustrates the large variations in atmospheric CO 2 (a) Direct measurements of atmospheric CO 2 concentration, and O 2 from 1990 onwards. O 2 concentration is the
Part 1. Ocean Composition & Circulation
 OCN 401 Biogeochemical Systems (10.19.17) (Schlesinger: Chapter 9) Part 1. Ocean Composition & Circulation 1. Introduction Lecture Outline 2. Ocean Circulation a) Global Patterns in T, S, ρ b) Thermohaline
OCN 401 Biogeochemical Systems (10.19.17) (Schlesinger: Chapter 9) Part 1. Ocean Composition & Circulation 1. Introduction Lecture Outline 2. Ocean Circulation a) Global Patterns in T, S, ρ b) Thermohaline
Part II: Past climates
 Part II: Past climates This week Early unexplainable things about the Earth Solid Earth - excerpts of Ch 7 Continental Drift (Alfred Wegener, 1920s) Carbon cycle - Ch 8 Ocean basins: trenches and midocean
Part II: Past climates This week Early unexplainable things about the Earth Solid Earth - excerpts of Ch 7 Continental Drift (Alfred Wegener, 1920s) Carbon cycle - Ch 8 Ocean basins: trenches and midocean
ph and Acid-Base reactions
 ph and Acid-Base reactions http://eps.mcgill.ca/~courses/c220/ Interaction of water with H + (H 3 O + ) Although it is a minor component of most aqueous solutions, the proton, hydrogen ion or H + concentration
ph and Acid-Base reactions http://eps.mcgill.ca/~courses/c220/ Interaction of water with H + (H 3 O + ) Although it is a minor component of most aqueous solutions, the proton, hydrogen ion or H + concentration
CO 2 -water-rock reactivity at hydrothermal temperatures: The BigRig2 experiment
 CO 2 -water-rock reactivity at hydrothermal temperatures: The BigRig2 experiment C.A. ROCHELLE 1 *, K. BATEMAN 1, A. LACINSKA 1, D. WAGNER 1, J. LIONS 2 AND I. GAUS 2 1 British Geological Survey, Keyworth,
CO 2 -water-rock reactivity at hydrothermal temperatures: The BigRig2 experiment C.A. ROCHELLE 1 *, K. BATEMAN 1, A. LACINSKA 1, D. WAGNER 1, J. LIONS 2 AND I. GAUS 2 1 British Geological Survey, Keyworth,
: 1.9 ppm y -1
 Atmospheric CO 2 Concentration Year 2006 Atmospheric CO 2 concentration: 381 ppm 35% above pre-industrial Atmoapheric [CO2] (ppmv) 4001850 1870 1890 1910 1930 1950 1970 1990 2010 380 360 340 320 300 280
Atmospheric CO 2 Concentration Year 2006 Atmospheric CO 2 concentration: 381 ppm 35% above pre-industrial Atmoapheric [CO2] (ppmv) 4001850 1870 1890 1910 1930 1950 1970 1990 2010 380 360 340 320 300 280
S= 95.02% S= 4.21% 35. S=radioactive 36 S=0.02% S= 0.75% 34 VI V IV III II I 0 -I -II SO 4 S 2 O 6 H 2 SO 3 HS 2 O 4- S 2 O 3
 SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
Sedimentary Rocks and Processes
 Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Weathering and Soils
 OCN 401-17 Aug. 29, 2016 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
OCN 401-17 Aug. 29, 2016 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
Early diagenesis in marine sediments
 Early diagenesis in marine sediments Why study this part of the ocean? Particle flux to the sea floor ocean surface sediments early diagenesis layer Biogeochemical reactions Why study this part of the
Early diagenesis in marine sediments Why study this part of the ocean? Particle flux to the sea floor ocean surface sediments early diagenesis layer Biogeochemical reactions Why study this part of the
Ocean Chemical Dynamics. EAS 2200 Lecture 21
 Ocean Chemical Dynamics EAS 2200 Lecture 21 Summary of Water s H-bonding accounts for high heat capacity, latent heats of fusion and evaporation. important for heat transport, transfer to atmosphere, and
Ocean Chemical Dynamics EAS 2200 Lecture 21 Summary of Water s H-bonding accounts for high heat capacity, latent heats of fusion and evaporation. important for heat transport, transfer to atmosphere, and
The Lithosphere. Definition
 10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
Marine Sediments EPSS15 Spring 2017 Lab 4
 Marine Sediments EPSS15 Spring 2017 Lab 4 Why Sediments? Record of Earth s history - Tectonic plate movement - Past changes in climate - Ancient ocean circulation currents - Cataclysmic events 1 Classification
Marine Sediments EPSS15 Spring 2017 Lab 4 Why Sediments? Record of Earth s history - Tectonic plate movement - Past changes in climate - Ancient ocean circulation currents - Cataclysmic events 1 Classification
Lecture 6. Physical Properties. Solid Phase. Particle Composition
 Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero.
![2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero. 2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero.](/thumbs/91/107062387.jpg) OCN400 Winter 2015 PS2 - Key 1. You might think it is well known, but there is some debate about what controls the magnesium concentration in seawater. The main input is rivers (see Power Point Lecture
OCN400 Winter 2015 PS2 - Key 1. You might think it is well known, but there is some debate about what controls the magnesium concentration in seawater. The main input is rivers (see Power Point Lecture
/ Past and Present Climate
 MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Climate on Geologic
MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Climate on Geologic
Activity and Concentration
 Activity and Concentration Activity effective concentration Ion-ion and ion-h 2 O interactions (hydration shell) cause number of ions available to react chemically ("free" ions) to be less than the number
Activity and Concentration Activity effective concentration Ion-ion and ion-h 2 O interactions (hydration shell) cause number of ions available to react chemically ("free" ions) to be less than the number
Overview of CO 2 -induced Changes in Seawater Chemistry
 Overview of CO 2 -induced Changes in Seawater Chemistry Joan Kleypas & Chris Langdon I. Background II. Facts vs Hypotheses III. Future directions 10-Oct-00 9th Int Coral Reef Symp. 1 Effects of CO 2 on
Overview of CO 2 -induced Changes in Seawater Chemistry Joan Kleypas & Chris Langdon I. Background II. Facts vs Hypotheses III. Future directions 10-Oct-00 9th Int Coral Reef Symp. 1 Effects of CO 2 on
What s up with Earth s Climate? David Archer Department of the Geophysical Sciences University of Chicago
 What s up with Earth s Climate? David Archer Department of the Geophysical Sciences University of Chicago This Sesson Began Sept. 29, 2014 Will begin again March 2015 Coursera.org Understanding the forecast
What s up with Earth s Climate? David Archer Department of the Geophysical Sciences University of Chicago This Sesson Began Sept. 29, 2014 Will begin again March 2015 Coursera.org Understanding the forecast
Where is all the water?
 Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
Where is all the water? The distribution of water at the Earth's surface % of total Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes 0.01 Soils 0.005 Atmosphere (as vapour) 0.001 Rivers 0.0001
Lecture 13 More Surface Reactions on Mineral Surfaces. & Intro to Soil Formation and Chemistry
 Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
Problem Set #4 ANSWER KEY Fall 2009 Due: 9:30, Monday, Nov 30
 OCN 520 Problem Set #4 ANSWER KEY Fall 2009 Due: 9:30, Monday, Nov 30 1. Two-Box Ocean Model The B Flux Using a 2 box model like the one you have worked on in problem set #4 (question 1) assume the following
OCN 520 Problem Set #4 ANSWER KEY Fall 2009 Due: 9:30, Monday, Nov 30 1. Two-Box Ocean Model The B Flux Using a 2 box model like the one you have worked on in problem set #4 (question 1) assume the following
Carbon Cycle Activity
 David Faure, InThinking www.biology-inthinking.co.uk Carbon Cycle Activity IB Biology Different types of Carbon Carbon Dioxide Gas Dissolved Carbon dioxide Carbohydrates (e.g. glucose) Hydrogen carbonate
David Faure, InThinking www.biology-inthinking.co.uk Carbon Cycle Activity IB Biology Different types of Carbon Carbon Dioxide Gas Dissolved Carbon dioxide Carbohydrates (e.g. glucose) Hydrogen carbonate
Redox, ph, pe OUTLINE 9/12/17. Equilibrium? Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox
 Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
Destabilization of Carbon Dioxide Hydrates in the Ocean. Resherle Verna Department of Earth Science, University of Southern California
 Verna 1 Destabilization of Carbon Dioxide Hydrates in the Ocean Resherle Verna Department of Earth Science, University of Southern California I. ABSTRACT This paper features the investigation of the change
Verna 1 Destabilization of Carbon Dioxide Hydrates in the Ocean Resherle Verna Department of Earth Science, University of Southern California I. ABSTRACT This paper features the investigation of the change
Weathering Cycle Teacher Notes
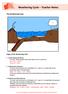 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.)
 Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
Lecture 29: Soil Formation
 Lecture 29: Soil Formation Factors Controlling Soil Formation 1. Parent material: Soil precursor 2. Climate: Temperature and precipitation 3. Biota: Native vegetation, microbes, soil animals, humans 4.
Lecture 29: Soil Formation Factors Controlling Soil Formation 1. Parent material: Soil precursor 2. Climate: Temperature and precipitation 3. Biota: Native vegetation, microbes, soil animals, humans 4.
Weathering and Soils
 OCN 401-11 Oct. 13, 2011 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
OCN 401-11 Oct. 13, 2011 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
Acid Soil. Soil Acidity and ph
 Acid Soil Soil Acidity and ph ph ph = - log (H + ) H 2 O H + + OH - (H + ) x (OH - )= K w = 10-14 measures H + activity with an electrode (in the lab), solutions (in the field) reflects the acid intensity,
Acid Soil Soil Acidity and ph ph ph = - log (H + ) H 2 O H + + OH - (H + ) x (OH - )= K w = 10-14 measures H + activity with an electrode (in the lab), solutions (in the field) reflects the acid intensity,
Chapter 3: Acid Base Equilibria. HCl + KOH KCl + H 2 O acid + base salt + water
 Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
Search and Discovery Article #50999 (2014)** Posted August 18, Abstract
 Oil Degradation in the Gullfaks Field (Norway): How Hydrogeochemical Modeling can Help to Decipher Organic- Inorganic Interactions Controlling CO 2 Fate and Behavior* Wolfgang van Berk 1, Yunjiao Fu 2,
Oil Degradation in the Gullfaks Field (Norway): How Hydrogeochemical Modeling can Help to Decipher Organic- Inorganic Interactions Controlling CO 2 Fate and Behavior* Wolfgang van Berk 1, Yunjiao Fu 2,
CO 2 and the carbonate system. 1/45
 CO 2 and the carbonate system http://eps.mcgill.ca/~courses/c542/ 1/45 The Atmospheric CO 2 -Climate Connection From: http://www.google.ca/imgres?imgurl=http:/ /www.tallbergfoundation.org/ From: Ruddiman,
CO 2 and the carbonate system http://eps.mcgill.ca/~courses/c542/ 1/45 The Atmospheric CO 2 -Climate Connection From: http://www.google.ca/imgres?imgurl=http:/ /www.tallbergfoundation.org/ From: Ruddiman,
UNIT 4 SEDIMENTARY ROCKS
 UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
Lecture 16 - Stable isotopes
 Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
Topics: The Layers of the Earth and its Formation Sources of Heat Volcanos and Earthquakes Rock Cycle Rock Types Carbon Tax
 Topics: The Layers of the Earth and its Formation Sources of Heat Volcanos and Earthquakes Rock Cycle Rock Types Carbon Tax Essay Question on Carbon Tax 1. Drilling 2. Volcanic Activity 3. Laboratory experiments
Topics: The Layers of the Earth and its Formation Sources of Heat Volcanos and Earthquakes Rock Cycle Rock Types Carbon Tax Essay Question on Carbon Tax 1. Drilling 2. Volcanic Activity 3. Laboratory experiments
Ocean Acidification the other CO2 problem..
 Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
Cycles in the Phanerozoic
 Cycles in the Phanerozoic Evolutionary trends: extinctions, adaptive radiations, diversity over time Glaciations Sea level change Ocean chemistry Atmospheric CO 2 biosphere Mass extinctions in the..you
Cycles in the Phanerozoic Evolutionary trends: extinctions, adaptive radiations, diversity over time Glaciations Sea level change Ocean chemistry Atmospheric CO 2 biosphere Mass extinctions in the..you
predictive mineral discovery*cooperative Research Centre A legacy for mineral exploration science Mineral Systems Q3 Fluid reservoirs
 Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
Chapter 7: Environmental Systems and Ecosystem Ecology
 Chapter 7: Environmental Systems and Ecosystem Ecology Vocabulary words to know: Hypoxia Negative feedback Dynamic equilibrium Emergent properties Lithosphere Biosphere Gross primary production Nutrients
Chapter 7: Environmental Systems and Ecosystem Ecology Vocabulary words to know: Hypoxia Negative feedback Dynamic equilibrium Emergent properties Lithosphere Biosphere Gross primary production Nutrients
1 General Introduction
 GENERAL INTRODUCTION 1 General Introduction 1.1 The Carbon Cycle Carbon dioxide (CO 2 ) present in our atmosphere absorbs the infrared (IR) radiation emitted by the Earth but is transparent to incoming
GENERAL INTRODUCTION 1 General Introduction 1.1 The Carbon Cycle Carbon dioxide (CO 2 ) present in our atmosphere absorbs the infrared (IR) radiation emitted by the Earth but is transparent to incoming
Geol. 656 Isotope Geochemistry
 THE ARBON YLE, ISOTOPES, AND LIMATE II THE LONG-TERM ARBON YLE On geologic times scales, the carbon cycle model must be augmented by 3 reservoirs, sedimentary carbonate, sedimentary organic carbon, and
THE ARBON YLE, ISOTOPES, AND LIMATE II THE LONG-TERM ARBON YLE On geologic times scales, the carbon cycle model must be augmented by 3 reservoirs, sedimentary carbonate, sedimentary organic carbon, and
Water percolating through hot lava dissolves soluble minerals containing chlorine, bromine and sulphur compounds
 Figure 5 The sources of dissolved ions in sea water. Water falls as rain Compounds containing mainly calcium, magnesium, carbonate and silicate ions are leached from the soil Rivers carry ions in solution
Figure 5 The sources of dissolved ions in sea water. Water falls as rain Compounds containing mainly calcium, magnesium, carbonate and silicate ions are leached from the soil Rivers carry ions in solution
Chapter 5. The Biogeochemical Cycles. Botkin & Keller Environmental Science 5e
 Chapter 5 The Biogeochemical Cycles How Chemicals Cycle Biogeochemical Cycle The complete path a chemical takes through the four major components or reservoirs of Earth s systems 1. Atmosphere 2. Hydrosphere
Chapter 5 The Biogeochemical Cycles How Chemicals Cycle Biogeochemical Cycle The complete path a chemical takes through the four major components or reservoirs of Earth s systems 1. Atmosphere 2. Hydrosphere
Atmospheric Evolution: Earth s s Oxidation
 Earth s s Atmosphere Thematic Questions about the Atmosphere Observations of the Modern Atmosphere What is its structure and composition? What controls atmospheric dynamics? Information from the Rock Record
Earth s s Atmosphere Thematic Questions about the Atmosphere Observations of the Modern Atmosphere What is its structure and composition? What controls atmospheric dynamics? Information from the Rock Record
Jochen Hoefs Stable Isotope Geochemistry
 Jochen Hoefs Stable Isotope Geochemistry Springer-Verlag Berlin Heidelberg GmbH Jochen Hoefs Stable Isotope Geochemistry 4th, Completely Revised, Updated, and Enlarged Edition With 73 Figures and 22 Tables
Jochen Hoefs Stable Isotope Geochemistry Springer-Verlag Berlin Heidelberg GmbH Jochen Hoefs Stable Isotope Geochemistry 4th, Completely Revised, Updated, and Enlarged Edition With 73 Figures and 22 Tables
Ocean chemistry and atmospheric CO 2 sensitivity to carbon perturbations throughout the Cenozoic
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 37,, doi:10.1029/2009gl041436, 2010 Ocean chemistry and atmospheric CO 2 sensitivity to carbon perturbations throughout the Cenozoic Malte
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 37,, doi:10.1029/2009gl041436, 2010 Ocean chemistry and atmospheric CO 2 sensitivity to carbon perturbations throughout the Cenozoic Malte
Sedimentology & Stratigraphy. Thanks to Rob Viens for slides
 Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Evolution of Earth Environments Bio-Geo-Chemical Cycling
 Evolution of Earth Environments Bio-Geo-Chemical Cycling Evolution of the Earliest Atmospheres of Mars and Earth Volcanic Outgassing Evolving to Equilibrium Atmosphere To Atmosphere Lost to space (Abundant)
Evolution of Earth Environments Bio-Geo-Chemical Cycling Evolution of the Earliest Atmospheres of Mars and Earth Volcanic Outgassing Evolving to Equilibrium Atmosphere To Atmosphere Lost to space (Abundant)
Geologic history of seawater: a MAGic approach to carbon chemistry
 Geologic history of seawater: a MAGic approach to carbon chemistry Rolf S. Arvidson 1, Michael W. Guidry 2, and Fred T. Mackenzie 3 1 MARUM/Geowissenschaften FB5, University of Bremen, Germany 2 Department
Geologic history of seawater: a MAGic approach to carbon chemistry Rolf S. Arvidson 1, Michael W. Guidry 2, and Fred T. Mackenzie 3 1 MARUM/Geowissenschaften FB5, University of Bremen, Germany 2 Department
Understanding Earth Fifth Edition
 Understanding Earth Fifth Edition Grotzinger Jordan Press Siever Chapter 5: SEDIMENTATION: Rocks Formed by Surface Processes Lecturer: H Mohammadzadeh Assistant professors, Department of Geology, FUM Copyright
Understanding Earth Fifth Edition Grotzinger Jordan Press Siever Chapter 5: SEDIMENTATION: Rocks Formed by Surface Processes Lecturer: H Mohammadzadeh Assistant professors, Department of Geology, FUM Copyright
Part 2. Oceanic Carbon and Nutrient Cycling. Lecture Outline. 1. Net Primary Production (NPP) a) Global Patterns b) Fate of NPP
 OCN 401 Biogeochemical Systems (10.25.16) (Schlesinger: Chapter 9) Part 2. Oceanic Carbon and Nutrient Cycling Lecture Outline 1. Net Primary Production (NPP) a) Global Patterns b) Fate of NPP 2. Sediment
OCN 401 Biogeochemical Systems (10.25.16) (Schlesinger: Chapter 9) Part 2. Oceanic Carbon and Nutrient Cycling Lecture Outline 1. Net Primary Production (NPP) a) Global Patterns b) Fate of NPP 2. Sediment
Regulation of atmospheric CO 2 by deep-sea sediments in an Earth system model
 Click Here for Full Article GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 21,, doi:10.1029/2006gb002764, 2007 Regulation of atmospheric CO 2 by deep-sea sediments in an Earth system model Andy Ridgwell 1,2 and J.
Click Here for Full Article GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 21,, doi:10.1029/2006gb002764, 2007 Regulation of atmospheric CO 2 by deep-sea sediments in an Earth system model Andy Ridgwell 1,2 and J.
Nutrients; Aerobic Carbon Production and Consumption
 Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
EPS 50 Lab 4: Sedimentary Rocks
 Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) There are 7 common ions in freshwater: (e.g.
 Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) A. Major ions 1. Common Ions There are 7 common ions in freshwater: (e.g. Table 13-3, p207) cations: Ca +2, Mg +2, Na +1, K +1 anions:
Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) A. Major ions 1. Common Ions There are 7 common ions in freshwater: (e.g. Table 13-3, p207) cations: Ca +2, Mg +2, Na +1, K +1 anions:
Sedimentary Rocks, Stratigraphy, and Geologic Time
 Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet
 The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet Celebrating 5 years of world-leading collaborative and multidisciplinary research
The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet Celebrating 5 years of world-leading collaborative and multidisciplinary research
Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington
 Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington Last lecture was concerned with gas exchange and one example we looked at was the solubility of CO 2. Next we have
Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington Last lecture was concerned with gas exchange and one example we looked at was the solubility of CO 2. Next we have
Finishing Ruddiman s Chapter 5. Tectonics and Long-Term Climate Change
 Finishing Ruddiman s Chapter 5. Tectonics and Long-Term Climate Change A 2cnd hypothesis that tries to explain how plate tectonics controls Earth s climate: Uplift-Weathering Hypothesis Maureen Raymo built
Finishing Ruddiman s Chapter 5. Tectonics and Long-Term Climate Change A 2cnd hypothesis that tries to explain how plate tectonics controls Earth s climate: Uplift-Weathering Hypothesis Maureen Raymo built
