Global Carbon Cycle - I Systematics: Reservoirs and Fluxes
|
|
|
- Mae Ophelia Johns
- 5 years ago
- Views:
Transcription
1 OCN Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes
2 The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes: atmospheric exchange, weathering and the rock cycle, the physical chemistry of the oceans Linked to the global cycles of oxygen, nitrogen and phosphorus
3 Photosynthesis links the C & O Cycles Photosynthetic uptake of C to synthesize tissues releases O 2 : CO 2 + H 2 O CH 2 O + O 2 Weathering of rocks consumes O 2 : CH 2 O + O 2 CO 2 + H 2 O - Burial of organic matter (reduced C) equates to an increase in the atmospheric O 2 reservoir - Photosynthesis and weathering impact form and locus and transformations of bioelements N, P and S - Anthropogenic activity has impacted the global C cycle, and global climate change through the greenhouse effect
4 Earth s Carbon Reservoirs Total Sedimentary Rocks Surficial Active Pools *Largest near-surface pool of C; ocean contains 56 X more C than the atmosphere; therefore has an enormous capacity to buffer changes in atmospheric CO 2 via Henry s Law, which describes partitioning of gases between the gaseous and dissolved phase (eqn. 2.7): S = kp 1 x g 8 x g 4 x g TDC in Ocean 3.8 x g (Fig. 11.1)* Extractable Fossil Fuels 4 x g Soil Organic Carbon 1.5 x g (Table 5.3) S is solubility of gas in a liquid k = solubility constant P = overlying pressure in atmosphere
5 The Contemporary Global Carbon Cycle [(surficial active pools (10 15 g C) and fluxes between pools (10 15 g C yr -1 )] *Largest fluxes link atm CO 2 to land vegetation and the surface ocean. * * * * *
6 Fluxes and Residence Times (all units x g) Global NPP = GPP - R p = 60 g/yr T R of Atm-CO 2 wrt terrestrial vegetation: 750 g / 60 g yr -1 = 12.5 yr Thus, each molecule of CO 2 in the atm has the potential to be taken up in terrestrial NPP every 12.5 years. T R of Atm-CO 2 wrt the ocean: 750 g / 90 g yr -1 = 8 yr Mean T R of Atm-CO 2 wrt the ocean: 750 g / ( ) g yr -1 = 5 yr. Mean T R only slightly longer than the mixing time of the atmosphere, so only minor seasonal variations are evident about the mean global average concentration of 360 ppm (in 1996).
7 Seasonal Variations in Atmospheric CO 2 Seasonal uptake of CO 2 results in oscillations in atm CO 2 content
8 Seasonal Variations in Atmospheric CO 2 (cont d.) Carbon dioxide is only a small fraction of the Earth s surficial Carbon reservoir, but its role in photosynthesis, climate regulation and rock weathering make it a critical component of the system Globally, 2/3 of terrestrial vegetation occurs in regions with seasonal biomass growth Atm CO 2 fluctuations are greatest in the N. Hemisphere, where most of the continental landmass resides S. Hemisphere fluctuations believed due to exchange with surface ocean
9 Carbon Reservoirs
10 Listed in order of size (oceans): g C Carbonate sediments 6.53 x Organic matter in seds 1.25x DIC in Ocean 3.74 x DOC in oceans 1.00 x Ocean biota 3.00 x Carbonate sediments are the largest reservoir larger than organic matter reservoir by ~ 5:1 Ocean water is next largest reservoir Inorganic (DIC) is ~40 x organic (DOC) ocean reservoir Listed in order of size (land + atmosphere)): CaCO 3 in soils 7.2 x Land biota 7.0 x Soil organic matter 2.5 x Atmosphere CO 2 6 x Soils are next largest reservoir Living biotic reservoir is ~same as inorganic reservoir Dead organic matter is 1/3 of the inorganic reservoir Phytomass ~100 x bacteria and animal reservoirs Atmosphere is the smallest reservoir, similar to size of all living biomass
11 Transfers between organic reservoirs (on land and in the oceans) can occur on short time scales Buried reservoirs of organic carbon are large relative to atmosphere
12 Reactions linking carbon and oxygen C + O 2 = CO 2 Under conditions at Earth s surface this inorganic reaction proceeds to the right, i.e. CO 2 favored over C and O 2 thermodynamically --lots of Gibbs free energy Burning of fossil fuels or forest fires --> oxidation of carbon But kinetics are slow, activation energy is needed to promote the reaction
13 Reactions linking C and O: photosynthesis and respiration CO 2 + 2H 2 O = CH 2 O + H 2 O + O 2 Photosynthesis rxn proceeding to the right, releases oxygen to the atmosphere Respiration, rxn proceeding to the left, consumes oxygen from the atmosphere Both of these reactions proceed rapidly on annual cycles
14 Organic C burial links CO 2 to atmospheric O 2 cycle
15 Carbonate and Silicate Rock Cycle Weathering on land CaCO 3 + CO 2 + H 2 O = Ca HCO 3 - CaSiO 3 +2CO 2 +3H 2 O = Ca HCO H 4 SIO 4 --> Uptake of atmospheric CO 2 during weathering on land, delivery of dissolved form to oceans Deposition in the oceans Ca HCO 3 - = CaCO 3 + CO 2 + H 2 O H 4 SiO 4 = SiO 2 + 2H 2 O --> Release of CO 2 during carbonate precipitation Metamorphic reactions CaCO 3 + SiO 2 = CaSiO 3 + CO 2 --> Release of CO 2 and return to atm via volcanic/hydrothermal activity
16 Carbonate and Silicate Rock Cycle CaCO 3 weathering on land and re-precipitation in ocean has no net effect on atmospheric CO 2 Weathering of silicates on land and re-precipitation in ocean results in net uptake of atmospheric CO 2 Balance of weathering types affects atmospheric CO 2 Subduction of sediments and volcanic activity returns CO 2 to atmosphere In the absence of recycling, weathering would remove all CO 2 from atmosphere in ~ 1 million years Residence time of CO 2 in atmosphere relative to weathering and volcanic input is ~ 6,000 years, i.e. the rock cycle exerts long term control on atm CO 2 Rock cycle does not control decadal to century scale changes seen in modern C cycle
17 History of the C cycle Long term changes in atmospheric CO 2 driven by rock cycle and biological cycle Initial high levels of atmospheric CO 2 Weak sun Silicate weathering and carbonate precipitation in ocean reduced atmospheric CO 2 levels Evolution of life ~3.9 Ga sequestered organic C Leads to production of oxygen
18 Initial production of O 2 used up by oxidation of Fe in seawater Terrestrial weathering also consumed early O 2 production Multi-cellular organisms appear as O 2 levels in atmosphere increase
19 Phanerozoic C cycle Driven by evolution of land plants - C storage Ma 30 x moles C buried in sediments yr -1 releases oxygen to atmosphere Atmos. reservoir O 2 38 x moles, T r = 1 x 10 6 yrs wrt sedimentary C Uplift of rocks and weathering of kerogen balances process Atmospheric O 2 is balance of organic C burial and its weathering
20 Evolution of angiosperms ~ 150 Ma Deeper roots increase Si weathering rates, leads to drop in atm CO 2
21 from wikipedia.org
22 O 2 levels in atmosphere track maximum burial of organic C ~ 350 Ma Microbial processes then pull down O 2 levels as organic C is oxidized
23
24 Fluxes of C
Global Carbon Cycle - I
 Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I
 Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
XI. the natural carbon cycle. with materials from J. Kasting (Penn State)
 XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
Figure 65: Reservoir in a steady state condition where the input flux is equal to the output flux and the reservoir size remains constant.
 7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks. Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7.
 The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7. Overhead Transparencies Faint Faint Young Sun Paradox Young
The Biogeochemical Carbon Cycle: CO 2,the greenhouse effect, & climate feedbacks Assigned Reading: Kump et al. (1999) The Earth System, Chap. 7. Overhead Transparencies Faint Faint Young Sun Paradox Young
This Week: Biogeochemical Cycles. Hydrologic Cycle Carbon Cycle
 This Week: Biogeochemical Cycles Hydrologic Cycle Carbon Cycle Announcements Reading: Chapters 4 (p. 74 81) and 8 Another Problem Set (Due next Tuesday) Exam 2: Friday Feb 29 My office hours today and
This Week: Biogeochemical Cycles Hydrologic Cycle Carbon Cycle Announcements Reading: Chapters 4 (p. 74 81) and 8 Another Problem Set (Due next Tuesday) Exam 2: Friday Feb 29 My office hours today and
/ Past and Present Climate
 MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
key to long-term sustainability is recycling..
 .. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
.. to support life over ~ 4 billion years, Earth must be sustainable system.. key to long-term sustainability is recycling.. Earth System how are key elements needed for life (C, N, P) recycled on the
Long-term Climate Change. We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold.
 Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
Long-term Climate Change We are in a period of relative warmth right now but on the time scale of the Earth s history, the planet is cold. Long-term Climate Change The Archean is thought to have been warmer,
CO2 in atmosphere is influenced by pco2 of surface water (partial pressure of water is the CO2 (gas) that would be in equilibrium with water).
 EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
SCOPE 35 Scales and Global Change (1988)
 1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
Part II: Past climates
 Part II: Past climates This week Solid Earth - excerpts of Ch 7 Carbon cycle - Ch 8 Early unexplainable things about the Earth Continental Drift (Alfred Wegener, 1920s) Ocean basins: trenches and midocean
Part II: Past climates This week Solid Earth - excerpts of Ch 7 Carbon cycle - Ch 8 Early unexplainable things about the Earth Continental Drift (Alfred Wegener, 1920s) Ocean basins: trenches and midocean
Carbon Dioxide, Alkalinity and ph
 Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
Cycles in the Phanerozoic
 Cycles in the Phanerozoic Evolutionary trends: extinctions, adaptive radiations, diversity over time Glaciations Sea level change Ocean chemistry Atmospheric CO 2 biosphere Mass extinctions in the..you
Cycles in the Phanerozoic Evolutionary trends: extinctions, adaptive radiations, diversity over time Glaciations Sea level change Ocean chemistry Atmospheric CO 2 biosphere Mass extinctions in the..you
Chapter 7: Environmental Systems and Ecosystem Ecology
 Chapter 7: Environmental Systems and Ecosystem Ecology Vocabulary words to know: Hypoxia Negative feedback Dynamic equilibrium Emergent properties Lithosphere Biosphere Gross primary production Nutrients
Chapter 7: Environmental Systems and Ecosystem Ecology Vocabulary words to know: Hypoxia Negative feedback Dynamic equilibrium Emergent properties Lithosphere Biosphere Gross primary production Nutrients
Lecture 20. Origin of the atmosphere (Chap. 10) The carbon cycle and long-term climate (Chap. 8 of the textbook: p )
 Lecture 20 Origin of the atmosphere (Chap. 10) The carbon cycle and long-term climate (Chap. 8 of the textbook: p.158-170) end of last ice-age; begin civilization beginning of modern era of ice-ages asteroid
Lecture 20 Origin of the atmosphere (Chap. 10) The carbon cycle and long-term climate (Chap. 8 of the textbook: p.158-170) end of last ice-age; begin civilization beginning of modern era of ice-ages asteroid
The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet
 The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet Celebrating 5 years of world-leading collaborative and multidisciplinary research
The Global Carbon Cycle Recording the Evolution of Earth, from the origin of life to the industrialization of the planet Celebrating 5 years of world-leading collaborative and multidisciplinary research
Continent-Ocean Interaction: Role of Weathering
 Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Atmospheric Evolution: Earth s s Oxidation
 Earth s s Atmosphere Thematic Questions about the Atmosphere Observations of the Modern Atmosphere What is its structure and composition? What controls atmospheric dynamics? Information from the Rock Record
Earth s s Atmosphere Thematic Questions about the Atmosphere Observations of the Modern Atmosphere What is its structure and composition? What controls atmospheric dynamics? Information from the Rock Record
PHOTOSYNTHESIS. Joseph Priestly 1772 experiment. SFSU Geography 316 Fall 2006 Dr. Barbara A. Holzman
 Nutrient Cycling I. A.Photosynthesis B. Respiration C. Production Primary productivity Gross Production Net Production II. Types of photosynthesis A. C3, B. C4, C. CAM D. Comparisons III. General Carbon
Nutrient Cycling I. A.Photosynthesis B. Respiration C. Production Primary productivity Gross Production Net Production II. Types of photosynthesis A. C3, B. C4, C. CAM D. Comparisons III. General Carbon
Sun. Photosynthesis (performed by plants, algae, and some bacteria) Respiration (performed by all organisms) 6 O 2 6 CO 2.
 Photosynthesis (performed by plants, algae, and some bacteria) Sun 6 O 6 CO 6 H O C 6 H O 6 (glucose) Solar energy + 6 H O + 6 CO C 6 H O 6 + 6 O Energy Respiration (performed by all organisms) 6 O 6 CO
Photosynthesis (performed by plants, algae, and some bacteria) Sun 6 O 6 CO 6 H O C 6 H O 6 (glucose) Solar energy + 6 H O + 6 CO C 6 H O 6 + 6 O Energy Respiration (performed by all organisms) 6 O 6 CO
8. Carbon Cycle. Carbon ( 炭素 ) Family. Earth Watch: Antarctic lake hides bizarre ecosystem 無機탄소, 有機탄소
 arbon ( 炭素 ) Family 8. arbon ycle 지구시스템의이해읽기 : 탄소이야기 3 Earth Watch: Antarctic lake hides bizarre ecosystem Extremely alkaline waters with high dissolved 4 bacterial stromatolites 無機탄소, 有機탄소 Inorganic carbon:
arbon ( 炭素 ) Family 8. arbon ycle 지구시스템의이해읽기 : 탄소이야기 3 Earth Watch: Antarctic lake hides bizarre ecosystem Extremely alkaline waters with high dissolved 4 bacterial stromatolites 無機탄소, 有機탄소 Inorganic carbon:
Carbon Cycling Internal
 Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
NUTRIENT CYCLES. Water Carbon Nitrogen
 NUTRIENT CYCLES Water Carbon Nitrogen NUTRIENT CYCLES Energy transfer through an ecosystem is ONE WAY Most energy is lost as heat Nutrients such as nitrogen, water and carbon are able to cycle through
NUTRIENT CYCLES Water Carbon Nitrogen NUTRIENT CYCLES Energy transfer through an ecosystem is ONE WAY Most energy is lost as heat Nutrients such as nitrogen, water and carbon are able to cycle through
S= 95.02% S= 4.21% 35. S=radioactive 36 S=0.02% S= 0.75% 34 VI V IV III II I 0 -I -II SO 4 S 2 O 6 H 2 SO 3 HS 2 O 4- S 2 O 3
 SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
Wednesday week 12. These ions move through the soil to streams and eventually to the ocean. In the ocean; CaCO 3 + H 2 O + CO 2 H 2 O + H 2 O
 Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Weathering and Soils
 OCN 401-11 Oct. 13, 2011 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
OCN 401-11 Oct. 13, 2011 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.)
 Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
Lecture 16 - Stable isotopes
 Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
BIOGEOCHEMICAL CYCLES
 BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
BIOGEOCHEMICAL CYCLES BASICS Biogeochemical Cycle: The complete path a chemical takes through the four major components, or reservoirs, of Earth s system (atmosphere, lithosphere, hydrosphere and biosphere)
Weathering and Soils
 OCN 401-17 Aug. 29, 2016 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
OCN 401-17 Aug. 29, 2016 KCR Weathering and Soils Biogeochemistry Chapter 4: The Lithosphere Introduction: the context Rock Weathering Soil Chemical Reactions Soil Development (see text) Weathering Rates
Global phosphorus cycle
 Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
Global phosphorus cycle OCN 623 Chemical Oceanography 11 April 2013 2013 Arisa Okazaki and Kathleen Ruttenberg Outline 1. Introduction on global phosphorus (P) cycle 2. Terrestrial environment 3. Atmospheric
Evolution of Earth Environments Bio-Geo-Chemical Cycling
 Evolution of Earth Environments Bio-Geo-Chemical Cycling Evolution of the Earliest Atmospheres of Mars and Earth Volcanic Outgassing Evolving to Equilibrium Atmosphere To Atmosphere Lost to space (Abundant)
Evolution of Earth Environments Bio-Geo-Chemical Cycling Evolution of the Earliest Atmospheres of Mars and Earth Volcanic Outgassing Evolving to Equilibrium Atmosphere To Atmosphere Lost to space (Abundant)
The Cycling of Matter. Day 1
 The Cycling of Matter Day 1 Objective I will learn the rock cycle is the series of processes in which rock changes from one form to another. I will learn in the water cycle, water condenses, precipitates
The Cycling of Matter Day 1 Objective I will learn the rock cycle is the series of processes in which rock changes from one form to another. I will learn in the water cycle, water condenses, precipitates
Geol. 656 Isotope Geochemistry
 THE ARBON YLE, ISOTOPES, AND LIMATE II THE LONG-TERM ARBON YLE On geologic times scales, the carbon cycle model must be augmented by 3 reservoirs, sedimentary carbonate, sedimentary organic carbon, and
THE ARBON YLE, ISOTOPES, AND LIMATE II THE LONG-TERM ARBON YLE On geologic times scales, the carbon cycle model must be augmented by 3 reservoirs, sedimentary carbonate, sedimentary organic carbon, and
Electrons, life and the evolution of Earth s chemical cycles*
 Electrons, life and the evolution of Earth s chemical cycles* 4H2O > 4e - + 4H + + O2 CO2 + 4e - + 4H + > (CH2O) + H2O OCN 623 Chemical Oceanography 25 April 2017 *largely based on Falkowski and Godfrey
Electrons, life and the evolution of Earth s chemical cycles* 4H2O > 4e - + 4H + + O2 CO2 + 4e - + 4H + > (CH2O) + H2O OCN 623 Chemical Oceanography 25 April 2017 *largely based on Falkowski and Godfrey
Part II: Past climates
 Part II: Past climates This week Early unexplainable things about the Earth Solid Earth - excerpts of Ch 7 Continental Drift (Alfred Wegener, 1920s) Carbon cycle - Ch 8 Ocean basins: trenches and midocean
Part II: Past climates This week Early unexplainable things about the Earth Solid Earth - excerpts of Ch 7 Continental Drift (Alfred Wegener, 1920s) Carbon cycle - Ch 8 Ocean basins: trenches and midocean
OCN 401. Photosynthesis
 OCN 401 Photosynthesis Photosynthesis Process by which carbon is reduced from CO 2 to organic carbon Provides all energy for the biosphere (except for chemosynthesis at hydrothermal vents) Affects composition
OCN 401 Photosynthesis Photosynthesis Process by which carbon is reduced from CO 2 to organic carbon Provides all energy for the biosphere (except for chemosynthesis at hydrothermal vents) Affects composition
(4) Give an example of important reactions that are responsible for the composition of river water.
 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Chapter 14: The Changing Climate
 Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
 Carbon - I This figure from IPCC, 2001 illustrates the large variations in atmospheric CO 2 (a) Direct measurements of atmospheric CO 2 concentration, and O 2 from 1990 onwards. O 2 concentration is the
Carbon - I This figure from IPCC, 2001 illustrates the large variations in atmospheric CO 2 (a) Direct measurements of atmospheric CO 2 concentration, and O 2 from 1990 onwards. O 2 concentration is the
Reference pg and in Textbook
 Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
Energy and Matter. Principles of Biology. Organisms interact with their environment, exchanging energy and matter. Topics Covered in this Module
 Principles of Biology contents 2 Energy and Matter Organisms interact with their environment, exchanging energy and matter. The Sun. Most ecosystems receive their energy from the Sun's radiation. NASA/European
Principles of Biology contents 2 Energy and Matter Organisms interact with their environment, exchanging energy and matter. The Sun. Most ecosystems receive their energy from the Sun's radiation. NASA/European
: 1.9 ppm y -1
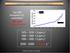 Atmospheric CO 2 Concentration Year 2006 Atmospheric CO 2 concentration: 381 ppm 35% above pre-industrial Atmoapheric [CO2] (ppmv) 4001850 1870 1890 1910 1930 1950 1970 1990 2010 380 360 340 320 300 280
Atmospheric CO 2 Concentration Year 2006 Atmospheric CO 2 concentration: 381 ppm 35% above pre-industrial Atmoapheric [CO2] (ppmv) 4001850 1870 1890 1910 1930 1950 1970 1990 2010 380 360 340 320 300 280
Effect of Life on the Atmosphere: The Rise of Oxygen and Ozone
 Some preliminary chemistry Chapter 11 Effect of Life on the Atmosphere: The Rise of Oxygen and Ozone Chemical reactions involve the giving and taking of electrons between atoms. the nucleus is not affected
Some preliminary chemistry Chapter 11 Effect of Life on the Atmosphere: The Rise of Oxygen and Ozone Chemical reactions involve the giving and taking of electrons between atoms. the nucleus is not affected
Ecosystems. 1. Population Interactions 2. Energy Flow 3. Material Cycle
 Ecosystems 1. Population Interactions 2. Energy Flow 3. Material Cycle The deep sea was once thought to have few forms of life because of the darkness (no photosynthesis) and tremendous pressures. But
Ecosystems 1. Population Interactions 2. Energy Flow 3. Material Cycle The deep sea was once thought to have few forms of life because of the darkness (no photosynthesis) and tremendous pressures. But
Ocean Acidification the other CO2 problem..
 Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
Geochemical Reservoirs and Transfer Processes
 Geochemical Reservoirs and Transfer Processes Ocn 623 Dr. Michael J. Mottl Dept. Of Oceanography Three Basic Questions 1. Why does Earth have oceans? 2. Why does Earth have dry land? 3. Why are the seas
Geochemical Reservoirs and Transfer Processes Ocn 623 Dr. Michael J. Mottl Dept. Of Oceanography Three Basic Questions 1. Why does Earth have oceans? 2. Why does Earth have dry land? 3. Why are the seas
Lecture 3. - Global Sulfur, Nitrogen, Carbon Cycles - Short-term vs. Long-term carbon cycle - CO 2 & Temperature: Last 100,000+ years
 Lecture 3 - Global Sulfur, Nitrogen, Carbon Cycles - Short-term vs. Long-term carbon cycle - CO 2 & Temperature: Last 100,000+ years METR 113/ENVS 113 Spring Semester 2011 March 1, 2011 Suggested Reading
Lecture 3 - Global Sulfur, Nitrogen, Carbon Cycles - Short-term vs. Long-term carbon cycle - CO 2 & Temperature: Last 100,000+ years METR 113/ENVS 113 Spring Semester 2011 March 1, 2011 Suggested Reading
1 General Introduction
 GENERAL INTRODUCTION 1 General Introduction 1.1 The Carbon Cycle Carbon dioxide (CO 2 ) present in our atmosphere absorbs the infrared (IR) radiation emitted by the Earth but is transparent to incoming
GENERAL INTRODUCTION 1 General Introduction 1.1 The Carbon Cycle Carbon dioxide (CO 2 ) present in our atmosphere absorbs the infrared (IR) radiation emitted by the Earth but is transparent to incoming
Earth systems the big idea guiding questions Chapter 1 & 2 Earth and Earth Systems review notes are in purple
 Earth systems the big idea guiding questions Chapter 1 & 2 Earth and Earth Systems review notes are in purple How can you describe Earth? What are the composition and the structure of the atmosphere? How
Earth systems the big idea guiding questions Chapter 1 & 2 Earth and Earth Systems review notes are in purple How can you describe Earth? What are the composition and the structure of the atmosphere? How
GEOL/ENVS 3520 Spring 2009 Hour Exam #2
 GEOL/ENVS 3520 Spring 2009 Hour Exam #2 Enter your name, the date, your ID number, and a made-up 4-digit code (for later recall and identification of your test results) on the separate test sheet. Carefully
GEOL/ENVS 3520 Spring 2009 Hour Exam #2 Enter your name, the date, your ID number, and a made-up 4-digit code (for later recall and identification of your test results) on the separate test sheet. Carefully
Communities Structure and Dynamics
 Communities Structure and Dynamics (Outline) 1. Community & niche. 2. Inter-specific interactions with examples. 3. The trophic structure of a community 4. Food chain: primary, secondary, tertiary, and
Communities Structure and Dynamics (Outline) 1. Community & niche. 2. Inter-specific interactions with examples. 3. The trophic structure of a community 4. Food chain: primary, secondary, tertiary, and
Lungs of the Planet with Dr. Michael Heithaus
 Lungs of the Planet with Dr. Michael Heithaus Problem Why do people call rain forests the lungs of the planet? Usually it is because people think that the rain forests produce most of the oxygen we breathe.
Lungs of the Planet with Dr. Michael Heithaus Problem Why do people call rain forests the lungs of the planet? Usually it is because people think that the rain forests produce most of the oxygen we breathe.
Biogeographic Processes
 Biogeographic Processes Energy and Matter Flow in Ecosystems Ecological Biogeography Ecological Succession Historical Biogeography Biogeographic Processes Biogeography examines the distribution of plants
Biogeographic Processes Energy and Matter Flow in Ecosystems Ecological Biogeography Ecological Succession Historical Biogeography Biogeographic Processes Biogeography examines the distribution of plants
Lungs of the Planet. 1. Based on the equations above, describe how the processes of photosynthesis and cellular respiration relate to each other.
 Lungs of the Planet Name: Date: Why do people call rain forests the lungs of the planet? Usually it is because people think that the rain forests produce most of the oxygen we breathe. But do they? To
Lungs of the Planet Name: Date: Why do people call rain forests the lungs of the planet? Usually it is because people think that the rain forests produce most of the oxygen we breathe. But do they? To
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions
 Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
S Illustrate and explain how carbon, nitrogen, and oxygen are cycled through an ecosystem.
 Biogeochemical Cycles S2-1-01 Illustrate and explain how carbon, nitrogen, and oxygen are cycled through an ecosystem. Biogeochemical Cycles Let s take a closer look at the interactions between LIVING
Biogeochemical Cycles S2-1-01 Illustrate and explain how carbon, nitrogen, and oxygen are cycled through an ecosystem. Biogeochemical Cycles Let s take a closer look at the interactions between LIVING
Global Biogeochemical Cycles and. II. Biological Metabolism
 Global Biogeochemical Cycles and Biological Metabolism I. Biogeochemistry & Biogeochemical Cycles A. Global cycles: nitrogen, water, carbon B. Carbon cycle through time II. Biological Metabolism A. Redox
Global Biogeochemical Cycles and Biological Metabolism I. Biogeochemistry & Biogeochemical Cycles A. Global cycles: nitrogen, water, carbon B. Carbon cycle through time II. Biological Metabolism A. Redox
Aquatic Chemistry (10 hrs)
 Aquatic Chemistry (10 hrs) Water -The quality and quantity of water available to human have been vital factors in determining their well-being. -More then 70% of the earth is covered by water. Living cells
Aquatic Chemistry (10 hrs) Water -The quality and quantity of water available to human have been vital factors in determining their well-being. -More then 70% of the earth is covered by water. Living cells
Natural Climate Variability: Longer Term
 Natural Climate Variability: Longer Term Natural Climate Change Today: Natural Climate Change-2: Ice Ages, and Deep Time Geologic Time Scale background: Need a system for talking about unimaginable lengths
Natural Climate Variability: Longer Term Natural Climate Change Today: Natural Climate Change-2: Ice Ages, and Deep Time Geologic Time Scale background: Need a system for talking about unimaginable lengths
The Chemistry of Global Warming
 The Chemistry of Global Warming Venus Atmospheric pressure is 90x that of Earth 96% CO 2 and sulfuric acid clouds Average temperature = 450 C Expected temperature based on solar radiation and distance
The Chemistry of Global Warming Venus Atmospheric pressure is 90x that of Earth 96% CO 2 and sulfuric acid clouds Average temperature = 450 C Expected temperature based on solar radiation and distance
Communities Structure and Dynamics
 Communities Structure and Dynamics (Outline) 1. Community & niche. 2. Inter-specific interactions with examples. 3. The trophic structure of a community 4. Food chain: primary, secondary, tertiary, and
Communities Structure and Dynamics (Outline) 1. Community & niche. 2. Inter-specific interactions with examples. 3. The trophic structure of a community 4. Food chain: primary, secondary, tertiary, and
Chapter 5. The Biogeochemical Cycles. Botkin & Keller Environmental Science 5e
 Chapter 5 The Biogeochemical Cycles How Chemicals Cycle Biogeochemical Cycle The complete path a chemical takes through the four major components or reservoirs of Earth s systems 1. Atmosphere 2. Hydrosphere
Chapter 5 The Biogeochemical Cycles How Chemicals Cycle Biogeochemical Cycle The complete path a chemical takes through the four major components or reservoirs of Earth s systems 1. Atmosphere 2. Hydrosphere
CHAPTER 5 WARM UPS. Mrs. Hilliard
 CHAPTER 5 WARM UPS Mrs. Hilliard CHAPTER 5 VOCABULARY 1. Photosynthesis 2. Cellular respiration 3. Producer 4. Consumer 5. Decomposer 6. Food chain 7. Food web 8. Trophic level 9. Carbon cycle 10. Nitrogen-fixing
CHAPTER 5 WARM UPS Mrs. Hilliard CHAPTER 5 VOCABULARY 1. Photosynthesis 2. Cellular respiration 3. Producer 4. Consumer 5. Decomposer 6. Food chain 7. Food web 8. Trophic level 9. Carbon cycle 10. Nitrogen-fixing
Lecture 5. Introduction to Stable Isotopes
 Lecture 5 Introduction to Stable Isotopes Stable Isotope Geochemistry Primarily concerned with the isotope ratios of H, C, N, O, and S Si and B often included and new instrumentation has opened up others
Lecture 5 Introduction to Stable Isotopes Stable Isotope Geochemistry Primarily concerned with the isotope ratios of H, C, N, O, and S Si and B often included and new instrumentation has opened up others
THE CHANGING SURFACE OF THE EARTH
 THE CHANGING SURFACE OF THE EARTH Key words Drain geological agent weathering erosion Sediment deposition transport The landscape is a consequence of the action of two types of geological processes; internal
THE CHANGING SURFACE OF THE EARTH Key words Drain geological agent weathering erosion Sediment deposition transport The landscape is a consequence of the action of two types of geological processes; internal
Mycorrhizal Fungi. Symbiotic relationship with plants -- form sheath around fine roots and extend hyphae into soil and sometimes into root cells
 Mycorrhizal Fungi Symbiotic relationship with plants -- form sheath around fine roots and extend hyphae into soil and sometimes into root cells Mycorrhizae transfer nutrients to roots (important in infertile
Mycorrhizal Fungi Symbiotic relationship with plants -- form sheath around fine roots and extend hyphae into soil and sometimes into root cells Mycorrhizae transfer nutrients to roots (important in infertile
Nutrient Cycling in Land Vegetation and Soils
 Nutrient Cycling in Land Vegetation and Soils OCN 401 - Biogeochemical Systems 13 September 2012 Reading: Schlesinger, Chapter 6 Outline 1. The annual Intrasystem Nutrient Cycle 2. Mass balance of the
Nutrient Cycling in Land Vegetation and Soils OCN 401 - Biogeochemical Systems 13 September 2012 Reading: Schlesinger, Chapter 6 Outline 1. The annual Intrasystem Nutrient Cycle 2. Mass balance of the
Terrestrial Climate Change Variables
 Terrestrial Climate Change Variables Content Terrestrial Climate Change Variables Surface Air Temperature Land Surface Temperature Sea Level Ice Level Aerosol Particles (acid rain) Terrestrial Climate
Terrestrial Climate Change Variables Content Terrestrial Climate Change Variables Surface Air Temperature Land Surface Temperature Sea Level Ice Level Aerosol Particles (acid rain) Terrestrial Climate
Water Carbon Nitrogen. Nutrient Cycles
 Water Carbon Nitrogen Nutrient Cycles Nutrient Cycles Energy transfer through an ecosystem is ONE WAY Most energy is lost as heat Matter such as nitrogen, water and carbon are able to cycle through an
Water Carbon Nitrogen Nutrient Cycles Nutrient Cycles Energy transfer through an ecosystem is ONE WAY Most energy is lost as heat Matter such as nitrogen, water and carbon are able to cycle through an
Chapter 12 - Long term climate regulation. Chapter 10-11* -Brief History of the Atmosphere. What is p really about? New and improved!
 What is p16164 really about? New and improved! 1) When CO 2 dissolves in water, some reacts with water to produce acid and ions, making gas exchange NOT just CO 2 (g in atm) CO 2 (aq in ocn) 2) If
What is p16164 really about? New and improved! 1) When CO 2 dissolves in water, some reacts with water to produce acid and ions, making gas exchange NOT just CO 2 (g in atm) CO 2 (aq in ocn) 2) If
2 Complete the following sentences to describe the properties of the different layers of the Earth s structure. Use the words given below.
 The Earth Task 1: Structure of the Earth 1 Label the diagram below, which shows the structure of the Earth. 2 Complete the following sentences to describe the properties of the different layers of the
The Earth Task 1: Structure of the Earth 1 Label the diagram below, which shows the structure of the Earth. 2 Complete the following sentences to describe the properties of the different layers of the
PTYS 214 Spring Announcements Midterm #4: two weeks from today!
 PTYS 214 Spring 2018 Announcements Midterm #4: two weeks from today! 1 Previously Radiometric Dating Compare parent / daughter to determine # of half lives 14C, 40K, 238U, 232Th, 87Ru Evidence for Early
PTYS 214 Spring 2018 Announcements Midterm #4: two weeks from today! 1 Previously Radiometric Dating Compare parent / daughter to determine # of half lives 14C, 40K, 238U, 232Th, 87Ru Evidence for Early
Summary. The Ice Ages and Global Climate
 The Ice Ages and Global Climate Summary Earth s climate system involves the atmosphere, hydrosphere, lithosphere, and biosphere. Changes affecting it operate on time scales ranging from decades to millions
The Ice Ages and Global Climate Summary Earth s climate system involves the atmosphere, hydrosphere, lithosphere, and biosphere. Changes affecting it operate on time scales ranging from decades to millions
Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments
 Peter Köhler 1 October 2015 Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments enhanced weathering, ocean alkalinization, ocean fertilization Peter Köhler, Judith Hauck
Peter Köhler 1 October 2015 Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments enhanced weathering, ocean alkalinization, ocean fertilization Peter Köhler, Judith Hauck
Biogeography. Fig. 12-6a, p. 276
 Biogeography Fig. 12-6a, p. 276 Biogeographic Processes Energy and Matter Flow in Ecosystems Ecological Biogeography Ecological Succession Historical Biogeography Biogeographic Processes Biogeography examines
Biogeography Fig. 12-6a, p. 276 Biogeographic Processes Energy and Matter Flow in Ecosystems Ecological Biogeography Ecological Succession Historical Biogeography Biogeographic Processes Biogeography examines
The Lithosphere. Definition
 10/12/2015 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2015 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/12/2015 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2015 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
3. Carbon Dioxide (CO 2 )
 3. Carbon Dioxide (CO 2 ) Basic information on CO 2 with regard to environmental issues Carbon dioxide (CO 2 ) is a significant greenhouse gas that has strong absorption bands in the infrared region and
3. Carbon Dioxide (CO 2 ) Basic information on CO 2 with regard to environmental issues Carbon dioxide (CO 2 ) is a significant greenhouse gas that has strong absorption bands in the infrared region and
Soil ph: Review of Concepts
 Soils and Water, Spring 008 Soil ph: Review of Concepts Acid: substance that can donate a proton Base: substance that can accept a proton HA H A HA and A - are called conjugate acid-base pairs. The strength
Soils and Water, Spring 008 Soil ph: Review of Concepts Acid: substance that can donate a proton Base: substance that can accept a proton HA H A HA and A - are called conjugate acid-base pairs. The strength
Section 7.1. Weathering. SES3a. Objectives
 SES3a. Objectives Distinguish between mechanical and chemical weathering. Describe the different factors that affect mechanical and chemical weathering. Identify variables that affect the rate of weathering.
SES3a. Objectives Distinguish between mechanical and chemical weathering. Describe the different factors that affect mechanical and chemical weathering. Identify variables that affect the rate of weathering.
Nutrient Cycling in Land Plants
 Nutrient Cycling in Land Plants OCN 401 - Biogeochemical Systems 10 September 2015 Reading: Chapter 6 2015 Frank Sansone Outline 1. Plant nutrient requirements and sources 2. Nutrient uptake by plants
Nutrient Cycling in Land Plants OCN 401 - Biogeochemical Systems 10 September 2015 Reading: Chapter 6 2015 Frank Sansone Outline 1. Plant nutrient requirements and sources 2. Nutrient uptake by plants
Atmosphere - Part 2. High and Low Pressure Systems
 Atmosphere - Part 2 High and Low Pressure Systems High Pressure vs. Low Pressure H regions : cool air sinks, increasing the air density, thus resulting in an area of high pressure L regions: warm air rises,
Atmosphere - Part 2 High and Low Pressure Systems High Pressure vs. Low Pressure H regions : cool air sinks, increasing the air density, thus resulting in an area of high pressure L regions: warm air rises,
What s up with Earth s Climate? David Archer Department of the Geophysical Sciences University of Chicago
 What s up with Earth s Climate? David Archer Department of the Geophysical Sciences University of Chicago This Sesson Began Sept. 29, 2014 Will begin again March 2015 Coursera.org Understanding the forecast
What s up with Earth s Climate? David Archer Department of the Geophysical Sciences University of Chicago This Sesson Began Sept. 29, 2014 Will begin again March 2015 Coursera.org Understanding the forecast
Nutrients; Aerobic Carbon Production and Consumption
 Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Chapter Introduction. Chapter Wrap-Up. Earth Systems
 Chapter Introduction Lesson 1 Lesson 2 Chapter Wrap-Up Earth Systems Interactions of Earth Systems How can you describe Earth? What do you think? Before you begin, decide if you agree or disagree with
Chapter Introduction Lesson 1 Lesson 2 Chapter Wrap-Up Earth Systems Interactions of Earth Systems How can you describe Earth? What do you think? Before you begin, decide if you agree or disagree with
Lecture 13 More Surface Reactions on Mineral Surfaces. & Intro to Soil Formation and Chemistry
 Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
Lecture 13 More Surface Reactions on Mineral Surfaces & Intro to Soil Formation and Chemistry 3. charge transfer (e.g., ligand/donor sorption): Sorption involves a number of related processes that all
5 Stable and radioactive isotopes
 5 Stable and radioactive isotopes Outline 1 Stable isotopes Measuring stable isotopic abundances Equilibrium isotope effects Kinetic isotope effects Rayleigh distillation Isotopes: a mainstay of chemical
5 Stable and radioactive isotopes Outline 1 Stable isotopes Measuring stable isotopic abundances Equilibrium isotope effects Kinetic isotope effects Rayleigh distillation Isotopes: a mainstay of chemical
Our Planet Earth. I nteractions of Earth Systems
 CHAPTER 3 LESSON 2 Our Planet Earth I nteractions of Earth Systems Key Concepts How does the water cycle show interactions of Earth systems? How does weather show interactions of Earth systems? How does
CHAPTER 3 LESSON 2 Our Planet Earth I nteractions of Earth Systems Key Concepts How does the water cycle show interactions of Earth systems? How does weather show interactions of Earth systems? How does
Physics of Aquatic Systems II
 Physics of Aquatic Systems II 10. C-Dating Werner Aeschbach-Hertig Institute of Environmental Physics University of Heidelberg 1 Contents of Session 10 General principles of C dating Conventional C age,
Physics of Aquatic Systems II 10. C-Dating Werner Aeschbach-Hertig Institute of Environmental Physics University of Heidelberg 1 Contents of Session 10 General principles of C dating Conventional C age,
APES Fall Final REVIEW
 Class: Date: APES Fall Final REVIEW Short Answer 1. The difference between chemical and physical weathering of rock is that 2. The difference between weathering and erosion is that 3. Select the correct
Class: Date: APES Fall Final REVIEW Short Answer 1. The difference between chemical and physical weathering of rock is that 2. The difference between weathering and erosion is that 3. Select the correct
Carbon Cycle Activity
 David Faure, InThinking www.biology-inthinking.co.uk Carbon Cycle Activity IB Biology Different types of Carbon Carbon Dioxide Gas Dissolved Carbon dioxide Carbohydrates (e.g. glucose) Hydrogen carbonate
David Faure, InThinking www.biology-inthinking.co.uk Carbon Cycle Activity IB Biology Different types of Carbon Carbon Dioxide Gas Dissolved Carbon dioxide Carbohydrates (e.g. glucose) Hydrogen carbonate
Nutrients; Aerobic Carbon Production and Consumption
 Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
The Carbon Cycle and Energy Security
 The Carbon Cycle and Energy Security QU: Why is there no life on Mars? AIM: To explain the characteristics and understand the importance of different stores and fluxes within the carbon cycle. Which of
The Carbon Cycle and Energy Security QU: Why is there no life on Mars? AIM: To explain the characteristics and understand the importance of different stores and fluxes within the carbon cycle. Which of
CEE 370 Environmental Engineering Principles. Equilibrium Chemistry
 Updated: 9 September 015 Print version CEE 370 Environmental Engineering Principles Lecture #6 Environmental Chemistry IV: Thermodynamics, Equilibria, Acids-bases I Reading: Mihelcic & Zimmerman, Chapter
Updated: 9 September 015 Print version CEE 370 Environmental Engineering Principles Lecture #6 Environmental Chemistry IV: Thermodynamics, Equilibria, Acids-bases I Reading: Mihelcic & Zimmerman, Chapter
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and
 1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
Part 2. Oceanic Carbon and Nutrient Cycling. Lecture Outline. 1. Net Primary Production (NPP) a) Global Patterns b) Fate of NPP
 OCN 401 Biogeochemical Systems (10.25.16) (Schlesinger: Chapter 9) Part 2. Oceanic Carbon and Nutrient Cycling Lecture Outline 1. Net Primary Production (NPP) a) Global Patterns b) Fate of NPP 2. Sediment
OCN 401 Biogeochemical Systems (10.25.16) (Schlesinger: Chapter 9) Part 2. Oceanic Carbon and Nutrient Cycling Lecture Outline 1. Net Primary Production (NPP) a) Global Patterns b) Fate of NPP 2. Sediment
Chapter 03 Lecture Outline
 Chapter 03 Lecture Outline William P. Cunningham University of Minnesota Mary Ann Cunningham Vassar College Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1
Chapter 03 Lecture Outline William P. Cunningham University of Minnesota Mary Ann Cunningham Vassar College Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1
Biogeochemical Review
 Biogeochemical Review Name KEY LT 1 1. Name and define 5 processes in the water cycle. Precipitation moisture falls back to the earth as rain, snow, sleet, or hail. Evaporation liquid water changes into
Biogeochemical Review Name KEY LT 1 1. Name and define 5 processes in the water cycle. Precipitation moisture falls back to the earth as rain, snow, sleet, or hail. Evaporation liquid water changes into
Fig. 3.2 on Page 101. Warming. Evidence for CO 2. History of Global Warming-2. Fig. 3.2 Page 101. Drilled cores from ocean floors
 Chemistry in Context: Chapter 3:The Chemistry of Global Warming Practice Problems: All Ch. 3 problems with the blue codes or answers on Page 521. Venus Atmospheric pressure is 90x that of Earth 96% CO
Chemistry in Context: Chapter 3:The Chemistry of Global Warming Practice Problems: All Ch. 3 problems with the blue codes or answers on Page 521. Venus Atmospheric pressure is 90x that of Earth 96% CO
