Metaphysics in Early Modern Philosophy. Monday, September 25, 17
|
|
|
- Sophia Wilcox
- 5 years ago
- Views:
Transcription
1 Metaphysics in Early Modern Philosophy
2 The Atomic Theory of Matter The atomic theory poses a challenge to theories of substances or objects Atomic theory: things are composed of atoms; properties of things depend on nature and motion of atoms Things are not as they appear
3 Appearance and Reality Aristotle: objects cause perceptions, and are represented in them Causes of perception = objects of perception Atomic Theory: No causes are the atoms which are real objects are appearances
4
5 Causes and Effects Causes of perception are the atoms We don t see atoms, but their effects What we see doesn t exist in reality How can we distinguish the aspects of the effects (appearances) that do match the causes?
6 Primary and Secondary Primary qualities: mathematical properties, assigned to atoms by physical theory e.g., size, shape, mass, motion Secondary qualities: effects of primary qualities on us e.g., color, texture, moral and aesthetic qualities
7 Primary Qualities Descartes: We perceive clearly and distinctly only the mathematical properties of objects: size, shape, motion Only they reflect the true natures of things
8 Primary Qualities Locke: Primary qualities are inseparable from objects; atoms have them Primary qualities are those objects possess according to the atomic theory of matter They produce simple ideas in us that resemble the primary qualities in the objects
9 Secondary Qualities Secondary qualities are effects of objects on our nervous systems They produce ideas in us that do NOT resemble them
10 Secondary Qualities Secondary qualities depend on primary qualities Secondary qualities are response-dependent: to have one is just to produce a certain effect in a (normal) perceiver
11 Primary and Secondary To be red is just to look red to a standard perceiver in standard conditions
12 Aristotle on Essence The essence of x = what it is to be x what x is by virtue of itself what x is by its very nature what is expressed by a definition of x (a formula for the nature of x)
13 Aquinas on Essence The essence of x = the properties necessary to x, without which x would not be what it is The quiddity of x = what corresponds to x s definition in the world The nature of x = what makes x what it is
14 Aquinas on Essence The essence of wax = the properties necessary to wax, without which wax would not be wax The quiddity of wax = what corresponds to wax s definition in the world The nature of wax = what makes wax what it is
15 Aquinas on Essence The essence of wax =?? a combination of: The quiddity of wax = compound that is malleable near ambient temperatures The nature of wax = organic compound that consists of long alkyl chains (CnH2n+1 or CnH2n-1).
16 Real and Nominal Essence Aristotle and Aquinas identify: The essence of x = the properties necessary to x The quiddity of x = the definition of x in re The nature of x = what makes x what it is
17 Real and Nominal Essence Locke: Nominal essence = quiddity: uses secondary qualities Real essence = nature: real internal constitution
18 Real and Nominal Essence Gold
19 Real and Nominal Essence Gold Nominal essence: heavy yellow metal
20 Real and Nominal Essence Gold Nominal essence: heavy yellow metal Real essence: element with atomic number 79
21 Real and Nominal Essence Water
22 Real and Nominal Essence Water Nominal essence: colorless, tasteless liquid (at common temperatures) necessary to life
23 Real and Nominal Essence Water Nominal essence: colorless, tasteless liquid (at common temperatures) necessary to life Real essence: H 2 O
24 Elms and Beeches Same nominal essence: North American deciduous tree?
25 Elms and Beeches Different real essences: Ulmus Americana and Fagus Grandifolia
26 Real Essences Aristotle and Aquinas: We know objects by grasping their essences Locke: Which essence? Nominal: concept; secondary qualities; conditioned; so, mind-dependent Real: real internal constitution; primary qualities; unconditioned; so, mind-independent
27 Categorematic Terms means, connotes Triangle Essence: Triangularity Daniel refers to, denotes A triangle
28 Categorematic Terms Which essence? means, connotes Triangle Essence: Triangularity Daniel refers to, denotes A triangle
29 Natural Kind Terms means, connotes Elm Essence: Being an elm Daniel refers to, denotes An elm
30 Natural Kind Terms Which essence? means, connotes Elm Essence: Being an elm Daniel refers to, denotes An elm
31 Natural Kind Terms means, connotes Elm Nominal Essence: NA deciduous tree Daniel refers to, denotes An elm
32 Natural Kind Terms means, connotes Elm Real Essence: Ulmus Americana Daniel refers to, denotes An elm
07. Descartes ( ) I. Cartesian Metaphysics
 07. Descartes (1596-1650) I. Cartesian Metaphysics Principia Philosophiae (The Principles of Philosophy) (1644). Three things exist: minds = thinking substance bodies = extended substance God = infinite
07. Descartes (1596-1650) I. Cartesian Metaphysics Principia Philosophiae (The Principles of Philosophy) (1644). Three things exist: minds = thinking substance bodies = extended substance God = infinite
Causality 03: The Modern "Epistemic Turn" and Causality
 Causality 03: The Modern "Epistemic Turn" and Causality Announcement Register to BEEF! https://beef.center.kobe-u.ac.jp/course/view.php?id=8559 Reading assignment J.L. Macke, Causes and Conditions, excerpt
Causality 03: The Modern "Epistemic Turn" and Causality Announcement Register to BEEF! https://beef.center.kobe-u.ac.jp/course/view.php?id=8559 Reading assignment J.L. Macke, Causes and Conditions, excerpt
An Ontology Diagram for Coordination of the Hylomorphically Treated Entities
 An Ontology Diagram for Coordination of the Hylomorphically Treated Entities Algirdas [0000-0001-6712-3521] Vilnius University, Vilnius, Universiteto g. 3, LT-01513, Lithuania algirdas.budrevicius@kf.vu.lt
An Ontology Diagram for Coordination of the Hylomorphically Treated Entities Algirdas [0000-0001-6712-3521] Vilnius University, Vilnius, Universiteto g. 3, LT-01513, Lithuania algirdas.budrevicius@kf.vu.lt
What constitutes space? : The development of Leibniz's theory of constituting space
 What constitutes space? : The development of Leibniz's theory of constituting space Hiroyuki INAOKA (Kobe, Japan) X.Internationalen Leibniz Kongress: Leibniz Universität Hannover, 18-23. July 2016 Outline
What constitutes space? : The development of Leibniz's theory of constituting space Hiroyuki INAOKA (Kobe, Japan) X.Internationalen Leibniz Kongress: Leibniz Universität Hannover, 18-23. July 2016 Outline
Families of Elements. Harley Defelice College Chemistry Period 6
 Families of Elements Harley Defelice College Chemistry Period 6 1 Valence electron Atomic number: 1 Symbol: H Hydrogen is the most abundant element it makes up 75% of the universe s mass. Hydrogen can
Families of Elements Harley Defelice College Chemistry Period 6 1 Valence electron Atomic number: 1 Symbol: H Hydrogen is the most abundant element it makes up 75% of the universe s mass. Hydrogen can
2.1 Properties of Matter > Chapter 2 Matter and Change. 2.1 Properties of Matter. 2.2 Mixtures 2.3 Elements and Compounds 2.4 Chemical Reactions
 21 Properties of Matter > 1 Copyright Pearson Education, Inc, or its affiliates All Rights Reserved Chapter 2 Matter and Change 21 Properties of Matter 22 Mixtures 23 Elements and Compounds 24 Chemical
21 Properties of Matter > 1 Copyright Pearson Education, Inc, or its affiliates All Rights Reserved Chapter 2 Matter and Change 21 Properties of Matter 22 Mixtures 23 Elements and Compounds 24 Chemical
CONTENTS INTRODUCTION... EXISTENCE, PERCEPTION, OBSERVATION, AND PHYSICAL PHENOMENA... MASS AND SPACE... IMPACT ON PHYSICS...
 CONTENTS I. II. III. IV. V. VI. VII. INTRODUCTION........................... EXISTENCE, PERCEPTION, OBSERVATION, AND PHYSICAL PHENOMENA.................. MASS AND SPACE......................... IMPACT
CONTENTS I. II. III. IV. V. VI. VII. INTRODUCTION........................... EXISTENCE, PERCEPTION, OBSERVATION, AND PHYSICAL PHENOMENA.................. MASS AND SPACE......................... IMPACT
Textbook: Section B, Chapter 1
 Atoms and the Periodic Table Review Sheet Textbook: Section B, Chapter 1 1. What is the Atomic number of nitrogen? 2. How many protons does nitrogen have? 3. How many electrons does nitrogen have? 4. How
Atoms and the Periodic Table Review Sheet Textbook: Section B, Chapter 1 1. What is the Atomic number of nitrogen? 2. How many protons does nitrogen have? 3. How many electrons does nitrogen have? 4. How
Chemistry. Chapter 14
 Chemistry Chapter 14 Central Science Study of matter Transformations it can make Basic research how things work Applied research making useful things Submicroscopic world Made of atoms Link to make molecules
Chemistry Chapter 14 Central Science Study of matter Transformations it can make Basic research how things work Applied research making useful things Submicroscopic world Made of atoms Link to make molecules
Matter Notes (Part 1)
 Matter Notes (Part 1) Matter is anything that has mass and takes up space. Matter is identified by its properties; shape, size, color, mass, etc. There are two classifications of properties - physical
Matter Notes (Part 1) Matter is anything that has mass and takes up space. Matter is identified by its properties; shape, size, color, mass, etc. There are two classifications of properties - physical
Chapter 3 Matter and Energy
 Introductory Chemistry, 3 rd Edition Nivaldo Tro Matter and Energy The chapter opening (page 52) showing a room and highlighting the structure of water and the carbon atoms in a graphite tennis racket
Introductory Chemistry, 3 rd Edition Nivaldo Tro Matter and Energy The chapter opening (page 52) showing a room and highlighting the structure of water and the carbon atoms in a graphite tennis racket
What s the Matter with Matter?
 What s the Matter with Matter? Thinking about Matter Sort the following into two categories, matter and not matter. Peanut butter, water, fish, garbage, time, motion, the human brain, carbon dioxide gas,
What s the Matter with Matter? Thinking about Matter Sort the following into two categories, matter and not matter. Peanut butter, water, fish, garbage, time, motion, the human brain, carbon dioxide gas,
Elements of a Bahá í-inspired Natural Theology
 Elements of a Bahá í-inspired Natural Theology Willliam S. Hatcher Source: The Library. Can be used under terms of the Library s 1 Besides the moral and spiritual teachings they contain, the Bahá í Writings
Elements of a Bahá í-inspired Natural Theology Willliam S. Hatcher Source: The Library. Can be used under terms of the Library s 1 Besides the moral and spiritual teachings they contain, the Bahá í Writings
Lecture 30 Descartes on Matter and Motion
 Lecture 30 Descartes on Matter and Motion Patrick Maher Scientific Thought I Fall 2009 Introduction This lecture concerns Part II of Descartes s Principles of Philosophy. It deals with matter and motion.
Lecture 30 Descartes on Matter and Motion Patrick Maher Scientific Thought I Fall 2009 Introduction This lecture concerns Part II of Descartes s Principles of Philosophy. It deals with matter and motion.
Matter and Its Properties
 Section 2 4A, 4B, 4C, 4D Main Ideas Atoms are the building blocks of matter. All substances have characteristic properties. Matter can be a pure substance or a mixture. 4A differentiate between physical
Section 2 4A, 4B, 4C, 4D Main Ideas Atoms are the building blocks of matter. All substances have characteristic properties. Matter can be a pure substance or a mixture. 4A differentiate between physical
Structuralism and the Limits of Skepticism. David Chalmers Thalheimer Lecture 3
 Structuralism and the Limits of Skepticism David Chalmers Thalheimer Lecture 3 Skepticism and Realism I Skepticism: We don t know whether external things exist Realism: External things exist Anti-Realism:
Structuralism and the Limits of Skepticism David Chalmers Thalheimer Lecture 3 Skepticism and Realism I Skepticism: We don t know whether external things exist Realism: External things exist Anti-Realism:
TEST NAME: Chemistry TEST ID: GRADE:08 SUBJECT:Life and Physical Sciences TEST CATEGORY: My Classroom
 TEST NAME: Chemistry TEST ID: 199257 GRADE:08 SUBJECT:Life and Physical Sciences TEST CATEGORY: My Classroom Chemistry Page 1 of 9 Student: Class: Date: 1. How can mixtures best be described? A. made of
TEST NAME: Chemistry TEST ID: 199257 GRADE:08 SUBJECT:Life and Physical Sciences TEST CATEGORY: My Classroom Chemistry Page 1 of 9 Student: Class: Date: 1. How can mixtures best be described? A. made of
Aristotle Metaphysics. Aristotle Metaphysics
 Aristotle Metaphysics I. What is Metaphysics? tôn meta ta phusika = the things after the Physics. Not to be confused with the study of anything non-physical. Not to be confused with later conceptions of
Aristotle Metaphysics I. What is Metaphysics? tôn meta ta phusika = the things after the Physics. Not to be confused with the study of anything non-physical. Not to be confused with later conceptions of
Determiniation of % Iron. The goal of this experiment is to determine the percent Iron in an unknown sample.
 Determiniation of % Iron The goal of this experiment is to determine the percent Iron in an unknown sample. What do we need so solve our objective? Percent mass of Iron. Need mass of Iron sample. Need
Determiniation of % Iron The goal of this experiment is to determine the percent Iron in an unknown sample. What do we need so solve our objective? Percent mass of Iron. Need mass of Iron sample. Need
Tree morphology and identification. TreeKeepers March 18, 2017
 Tree morphology and identification TreeKeepers March 18, 2017 Tree identification keys What if you don t know what kind of tree it is? Dichotomous keys work you through it. Ask a series of yes or no questions
Tree morphology and identification TreeKeepers March 18, 2017 Tree identification keys What if you don t know what kind of tree it is? Dichotomous keys work you through it. Ask a series of yes or no questions
For example! Matter and Its Phases. Matter and Its Changes Chemistry 11. Observations vs. Interpretations 2/1/09
 Matter and Its Phases Matter: Anything that has mass and occupies space. Matter and Its Changes Chemistry 11 Matter is anything that has mass means that matter is anything that has inertia and requires
Matter and Its Phases Matter: Anything that has mass and occupies space. Matter and Its Changes Chemistry 11 Matter is anything that has mass means that matter is anything that has inertia and requires
SCIENCE WRAP Game 1a (26 cards)
 SCIENCE WRAP Game 1a (26 cards) Directions: Print this file and cut out the 26 cards. (Pages will line up to make this easier). Mix up the cards and distribute all to the class or group. Each student may
SCIENCE WRAP Game 1a (26 cards) Directions: Print this file and cut out the 26 cards. (Pages will line up to make this easier). Mix up the cards and distribute all to the class or group. Each student may
Word of the Day for August 27, Definition - any property of a substance that must be observed during a chemical change.
 Word of the Day for August 27, 2008 Chemical Property Definition - any property of a substance that must be observed during a chemical change. One chemical property of magnesium is that it will combine
Word of the Day for August 27, 2008 Chemical Property Definition - any property of a substance that must be observed during a chemical change. One chemical property of magnesium is that it will combine
Chemistry Review- Packet 11, Page 1
 Chemistry Review- Packet 11, Page 1 Atom- smallest particle of an element with the same properties as that element In size the entire atom has been thought to be approximately four-billionths of an inch,
Chemistry Review- Packet 11, Page 1 Atom- smallest particle of an element with the same properties as that element In size the entire atom has been thought to be approximately four-billionths of an inch,
Electronegativity Scale F > O > Cl, N > Br > C, H
 Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
10 February 2009 Aim: How can I tell the difference between substances, compounds, and mixtures? Engagement: write three to four sentences describing
 10 February 2009 Aim: How can I tell the difference between substances, compounds, and mixtures? Engagement: write three to four sentences describing what you know about atoms. HW: Make note cards for
10 February 2009 Aim: How can I tell the difference between substances, compounds, and mixtures? Engagement: write three to four sentences describing what you know about atoms. HW: Make note cards for
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 CHM 210 Chemistry Homework #2 Atoms and Elements (Ch. 3) Due: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Helium is a(n) A) heterogeneous mixture.
CHM 210 Chemistry Homework #2 Atoms and Elements (Ch. 3) Due: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Helium is a(n) A) heterogeneous mixture.
2.3 Elements and Compounds > Chapter 2 Matter and Change. 2.3 Elements and Compounds. 2.1 Properties of Matter 2.2 Mixtures. 2.4 Chemical Reactions
 Chapter 2 Matter and Change 2.1 Properties of Matter 2.2 Mixtures 2.3 Elements and Compounds 2.4 Chemical Reactions 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chapter 2 Matter and Change 2.1 Properties of Matter 2.2 Mixtures 2.3 Elements and Compounds 2.4 Chemical Reactions 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Potter Name: Date: Hour: Earth Science Unit 1: Earth Science Overview, Energy and Matter
 Unit 1: Overview, Energy and Matter #1 LT 1.1: Matter and Energy: I can explain the difference between matter and energy and understand their role in earth systems. Yes I can: 1. Can you define matter
Unit 1: Overview, Energy and Matter #1 LT 1.1: Matter and Energy: I can explain the difference between matter and energy and understand their role in earth systems. Yes I can: 1. Can you define matter
Are Objects Ontologically Dependent on Processes?
 Are Objects Ontologically Dependent on Processes? Antony Galton Department of Computer Science University of Exeter, UK CAOS Symposium University of Bath 20 April 2017 Things vs Processes Traditional substance-based
Are Objects Ontologically Dependent on Processes? Antony Galton Department of Computer Science University of Exeter, UK CAOS Symposium University of Bath 20 April 2017 Things vs Processes Traditional substance-based
Qualitative Chemistry Unit 2. Matter A Central Idea in Chemistry
 Qualitative Chemistry Unit 2 Matter A Central Idea in Chemistry Unit Warm-Up 1. What do chemists study? 2. How do atoms differ from molecules? 3. Describe a chemical change (chemical reaction) you have
Qualitative Chemistry Unit 2 Matter A Central Idea in Chemistry Unit Warm-Up 1. What do chemists study? 2. How do atoms differ from molecules? 3. Describe a chemical change (chemical reaction) you have
Name Date Period Molecular Nature of Water
 Name Date Period Molecular Nature of Water Purpose: To determine how water molecules react using molecular models and Lab demos. Materials: I cup of 12 water molecules (red & white), 1 Na (blue), 1 Cl
Name Date Period Molecular Nature of Water Purpose: To determine how water molecules react using molecular models and Lab demos. Materials: I cup of 12 water molecules (red & white), 1 Na (blue), 1 Cl
Welcome to chemistry
 Welcome to chemistry Science a search for facts about the world around us understanding the past, predicting the future Aristotle (384-322 B.C.) one can work out all the laws that govern the universe by
Welcome to chemistry Science a search for facts about the world around us understanding the past, predicting the future Aristotle (384-322 B.C.) one can work out all the laws that govern the universe by
Name Date Period Unit 3 Review: Electrons and the periodic table
 Name Date Period Unit 3 Review: Electrons and the periodic table G Chem; Coleman SHOW YOUR WORK ON ANY AND ALL CALCULATIONS. SIG FIGS MATTER. UNITS MATTER. General Questions: 1. Use the following terms
Name Date Period Unit 3 Review: Electrons and the periodic table G Chem; Coleman SHOW YOUR WORK ON ANY AND ALL CALCULATIONS. SIG FIGS MATTER. UNITS MATTER. General Questions: 1. Use the following terms
What Does Quantum Mechanics Suggest About Our Perceptions of Reality?
 What Does Quantum Mechanics Suggest About Our Perceptions of Reality? Quantum mechanics suggests that we perceive at most a tiny sliver of reality. Of course we already knew that! We knew that the visible
What Does Quantum Mechanics Suggest About Our Perceptions of Reality? Quantum mechanics suggests that we perceive at most a tiny sliver of reality. Of course we already knew that! We knew that the visible
Physical Properties Explained (hopefully)
 Physical Properties Explained (hopefully) Melting and Boiling Points: in order to melt or boil a compound, the solid form of the compound must be broken up into small particles what these particles are,
Physical Properties Explained (hopefully) Melting and Boiling Points: in order to melt or boil a compound, the solid form of the compound must be broken up into small particles what these particles are,
The grand theory of astrology
 Astrology for Aquarius Sharing our Knowledge Hermetic astrology The grand theory of astrology The Brotherhood of Light The grand theory of astrology The Brotherhood of Light 1 Introduction Astrology was
Astrology for Aquarius Sharing our Knowledge Hermetic astrology The grand theory of astrology The Brotherhood of Light The grand theory of astrology The Brotherhood of Light 1 Introduction Astrology was
Chapter 4 Atoms Practice Problems
 Chapter 4 Atoms Practice Problems 1) The primary substances of which all other things are composed are A) molecules. B) compounds. C) elements. D) electrons. E) protons. 2) Which of the following is a
Chapter 4 Atoms Practice Problems 1) The primary substances of which all other things are composed are A) molecules. B) compounds. C) elements. D) electrons. E) protons. 2) Which of the following is a
Review. 8th grade science STAAR. Name Class. Underline your strong TEKS and circle your weak TEKS: 8.5A Atoms. 7.5C Energy Flow Through Ecosystems
 8th grade science STAAR Review Name Class Underline your strong TEKS and circle your weak TEKS: 8.5A Atoms 7.5C Energy Flow Through Ecosystems 8.5B Reactivity 8.5C Periodic Table 8.5D Chemical Formulas
8th grade science STAAR Review Name Class Underline your strong TEKS and circle your weak TEKS: 8.5A Atoms 7.5C Energy Flow Through Ecosystems 8.5B Reactivity 8.5C Periodic Table 8.5D Chemical Formulas
PYP 001 First Major Code: Term: 132 Tuesday, March 11, 2014 Page: 1
 Term: 132 Tuesday, March 11, 2014 Page: 1 *The correct answer is the choice (A) for all questions. 1 is NOT a pure substance. A) An orange. B) Iron rust. C) Carbon dioxide. D) Neon (Ne). E) Dry ice. 2
Term: 132 Tuesday, March 11, 2014 Page: 1 *The correct answer is the choice (A) for all questions. 1 is NOT a pure substance. A) An orange. B) Iron rust. C) Carbon dioxide. D) Neon (Ne). E) Dry ice. 2
Periodic Table of Elements
 Periodic Table of Elements chlorine nitrogen helium gold oxygen silver mercury hydrogen neodymium sodium niobium carbon Elements Science has come along way since Aristotle s theory of Air, Water, Fire,
Periodic Table of Elements chlorine nitrogen helium gold oxygen silver mercury hydrogen neodymium sodium niobium carbon Elements Science has come along way since Aristotle s theory of Air, Water, Fire,
Knowledge, Truth, and Mathematics
 Knowledge, Truth, and Mathematics Philosophy 405 Russell Marcus Hamilton College, Fall 2010 September 27 Class 9: Kant II Marcus, Knowledge, Truth, and Mathematics, Fall 2010 Slide 1 Necessity/Contingency
Knowledge, Truth, and Mathematics Philosophy 405 Russell Marcus Hamilton College, Fall 2010 September 27 Class 9: Kant II Marcus, Knowledge, Truth, and Mathematics, Fall 2010 Slide 1 Necessity/Contingency
Atoms & Their Interactions
 Lecture 2 Atoms & Their Interactions Si: the heart of electronic materials Intel, 300mm Si wafer, 200 μm thick and 48-core CPU ( cloud computing on a chip ) Twin Creeks Technologies, San Jose, Si wafer,
Lecture 2 Atoms & Their Interactions Si: the heart of electronic materials Intel, 300mm Si wafer, 200 μm thick and 48-core CPU ( cloud computing on a chip ) Twin Creeks Technologies, San Jose, Si wafer,
States of Matter. We can explain the properties that we observe in the various states of matter with these postulates.
 States of Matter Kinetic Molecular Theory When discussing the properties of matter, it is not enough just to classify them. We must also create a model that helps to explain the properties that we see.
States of Matter Kinetic Molecular Theory When discussing the properties of matter, it is not enough just to classify them. We must also create a model that helps to explain the properties that we see.
Chapter 14. Liquids and Solids
 Chapter 14 Liquids and Solids Review Solid - Has a definite (fixed) shape and volume (cannot flow). Liquid - Definite volume but takes the shape of its container (flows). Gas Has neither fixed shape nor
Chapter 14 Liquids and Solids Review Solid - Has a definite (fixed) shape and volume (cannot flow). Liquid - Definite volume but takes the shape of its container (flows). Gas Has neither fixed shape nor
CHAPTER 2. Solid Liquid Gas (vapor) Matter and Change IDENTIFYING SUBSTANCES THE STATES OF MATTER INTENSIVE PROPERTY:
 CHAPTER 2 Matter and Change 2.1 PROPERTIES OF MATTER EXTENSIVE PROPERTY: Depends on the amount of matter in a sample Comparing the same substances. Diamonds to Diamonds INTENSIVE PROPERTY: Depends on the
CHAPTER 2 Matter and Change 2.1 PROPERTIES OF MATTER EXTENSIVE PROPERTY: Depends on the amount of matter in a sample Comparing the same substances. Diamonds to Diamonds INTENSIVE PROPERTY: Depends on the
Properties of Matter
 Chemistry 1 of 26 Properties of Matter Bamboo has properties that make it a good choice for use in chopsticks. It has no noticeable odor or taste. It is hard, yet easy to split, and it is heat resistant.
Chemistry 1 of 26 Properties of Matter Bamboo has properties that make it a good choice for use in chopsticks. It has no noticeable odor or taste. It is hard, yet easy to split, and it is heat resistant.
What s da matter? Matter anything that takes up space and has mass
 MATTER Chapter 2 What s da matter? Matter anything that takes up space and has mass Classification of Matter (p. 22 table 21-A) Physical Properties physical relationships between particles in the matter
MATTER Chapter 2 What s da matter? Matter anything that takes up space and has mass Classification of Matter (p. 22 table 21-A) Physical Properties physical relationships between particles in the matter
Molecules have to move past one another to flow, and stronger attractions between molecules make that more difficult!
 40 VISCOSITY - viscosity can also be explained (at least partially) by looking at INTERMOLECULAR FORCES! - For a liquid to FLOW, its molecules must move past one another. This means that some of the molecules
40 VISCOSITY - viscosity can also be explained (at least partially) by looking at INTERMOLECULAR FORCES! - For a liquid to FLOW, its molecules must move past one another. This means that some of the molecules
Chapters 1 & 2 Atoms and Elements and Chemical Reactions (Period 5) CHEMISTRY REVIEW
 Chapters 1 & 2 Atoms and Elements and Chemical Reactions (Period 5) CHEMISTRY REVIEW T = CHAPTER 1 ATOMS AND ELEMENTS Elements and Atoms Matter - anything that takes up and has mass Elements A substance
Chapters 1 & 2 Atoms and Elements and Chemical Reactions (Period 5) CHEMISTRY REVIEW T = CHAPTER 1 ATOMS AND ELEMENTS Elements and Atoms Matter - anything that takes up and has mass Elements A substance
Chemistry and Measurement
 Chapter 1 Chemistry and Measurement Concept Check 1.1 Matter can be represented as being composed of individual units. For example, the smallest individual unit of matter can be represented as a single
Chapter 1 Chemistry and Measurement Concept Check 1.1 Matter can be represented as being composed of individual units. For example, the smallest individual unit of matter can be represented as a single
Matter and Energy. What is matter? Properties of Matter 9/15/15. EQ: How do I describe and classify matter? EQ: How do I describe and classify matter?
 Matter and Energy Pt. 1 Properties of Matter What is matter? ± Material that makes up the universe is called matter 2 Properties of Matter 1. Has mass 2. Occupies space (has volume) 1 4 Phases of Matter
Matter and Energy Pt. 1 Properties of Matter What is matter? ± Material that makes up the universe is called matter 2 Properties of Matter 1. Has mass 2. Occupies space (has volume) 1 4 Phases of Matter
The electronic structure of three Alkali Metals The alkali metals appearance
 The electronic structure of three Alkali Metals Notice that in each of these the outermost shell only has 1 electron. This is the valance electron which is easily removed during chemical reactions. Cs
The electronic structure of three Alkali Metals Notice that in each of these the outermost shell only has 1 electron. This is the valance electron which is easily removed during chemical reactions. Cs
Lesson 6: Periodic Table and Atomic Theory
 NOTES Name: _ Date: Class: Lesson 6: Periodic Table and Atomic Theory Element: fundamental substance that ; all matter consists of ~100 elements Atom: _ that can exist; smallest unit of an element that
NOTES Name: _ Date: Class: Lesson 6: Periodic Table and Atomic Theory Element: fundamental substance that ; all matter consists of ~100 elements Atom: _ that can exist; smallest unit of an element that
PYP 001 First Major Code: Term: 133 Saturday, June 28, 2014 Page: 1
 Term: 133 Saturday, June 28, 2014 Page: 1 *Read the following (20) questions and choose the right answer: 1 is Not a compound. A) Smoke. B) Iron rust. C) Carbon dioxide. D) Water. E) Dry ice. 2 In the
Term: 133 Saturday, June 28, 2014 Page: 1 *Read the following (20) questions and choose the right answer: 1 is Not a compound. A) Smoke. B) Iron rust. C) Carbon dioxide. D) Water. E) Dry ice. 2 In the
Unit 2: Atoms and Elements
 Review: Do you remember... Unit 2: Atoms and Elements matter mass volume Review: Do you remember... Unit 2: Atoms and Elements Matter: anything that has mass and takes up space. Mass: measue of the amount
Review: Do you remember... Unit 2: Atoms and Elements matter mass volume Review: Do you remember... Unit 2: Atoms and Elements Matter: anything that has mass and takes up space. Mass: measue of the amount
Experiment 2 - Using Physical Properties to Identify an Unknown Liquid
 Experiment 2 - Using Physical Properties to Identify an Unknown Liquid We usually think of chemists as scientists who do things with chemicals. We can picture a chemist's laboratory with rows of bottles
Experiment 2 - Using Physical Properties to Identify an Unknown Liquid We usually think of chemists as scientists who do things with chemicals. We can picture a chemist's laboratory with rows of bottles
Chapter 1 The Atomic Nature of Matter 1-1 Chemistry: Science of Change 1-2 The Composition of Matter 1-3 The Atomic Theory of Matter 1-4 Chemical
 Chapter 1 The Atomic Nature of Matter 1-1 Chemistry: Science of Change 1-2 The Composition of Matter 1-3 The Atomic Theory of Matter 1-4 Chemical Formulas and Relative Atomic Masses 1-5 The Building Blocks
Chapter 1 The Atomic Nature of Matter 1-1 Chemistry: Science of Change 1-2 The Composition of Matter 1-3 The Atomic Theory of Matter 1-4 Chemical Formulas and Relative Atomic Masses 1-5 The Building Blocks
Biogeochemical Review
 Biogeochemical Review Name KEY LT 1 1. Name and define 5 processes in the water cycle. Precipitation moisture falls back to the earth as rain, snow, sleet, or hail. Evaporation liquid water changes into
Biogeochemical Review Name KEY LT 1 1. Name and define 5 processes in the water cycle. Precipitation moisture falls back to the earth as rain, snow, sleet, or hail. Evaporation liquid water changes into
Introduction to Philosophy Philosophy 110W Fall 2014 Russell Marcus
 Introduction to Philosophy Philosophy 110W Fall 2014 Russell Marcus Class #8: Newton and Leibniz on Space and Time Marcus, Introduction to Philosophy, Fall 2014 Slide 1 Business P Return Exegeses P Thursday
Introduction to Philosophy Philosophy 110W Fall 2014 Russell Marcus Class #8: Newton and Leibniz on Space and Time Marcus, Introduction to Philosophy, Fall 2014 Slide 1 Business P Return Exegeses P Thursday
Temporal Extension. 1. Time as Quantity
 1 Boris Hennig IFOMIS Saarbrücken Draft version as of July 17, 2005 Temporal Extension Abstract. Space and time are continuous and extensive quantities. But according to Aristotle, time differs from space
1 Boris Hennig IFOMIS Saarbrücken Draft version as of July 17, 2005 Temporal Extension Abstract. Space and time are continuous and extensive quantities. But according to Aristotle, time differs from space
1. Volume=amount of an object takes up Ways we can measure volume:
 Chemistry Ms. Ye Name Date Block A physical property is something that can be measured or observed without changing the chemical composition of the substance. Mass, volume, and density or examples of physical
Chemistry Ms. Ye Name Date Block A physical property is something that can be measured or observed without changing the chemical composition of the substance. Mass, volume, and density or examples of physical
WHAT IS CHEMISTRY? [pg. vii]
![WHAT IS CHEMISTRY? [pg. vii] WHAT IS CHEMISTRY? [pg. vii]](/thumbs/89/100753863.jpg) CH 11 T1 INTRODUCING MATTER & ATOMIC THEORY 1 You have mastered this topic when you can: 1) define the terms CHEMISTRY, ELEMENT, ATOM, COMPOUND, MOLECULE, ION, MATTER, MASS, WEIGHT and INERTIA 2) define
CH 11 T1 INTRODUCING MATTER & ATOMIC THEORY 1 You have mastered this topic when you can: 1) define the terms CHEMISTRY, ELEMENT, ATOM, COMPOUND, MOLECULE, ION, MATTER, MASS, WEIGHT and INERTIA 2) define
Test 3: Lab Safety, Measurements, Matter and Periodic Table
 Name: Grade/Group: Subject: Chemistry-7 Teacher: Mrs. Raj Date: Test 3: Lab Safety, Measurements, Matter and Periodic Table Directions: Determine the best answer for each question. Circle your answer on
Name: Grade/Group: Subject: Chemistry-7 Teacher: Mrs. Raj Date: Test 3: Lab Safety, Measurements, Matter and Periodic Table Directions: Determine the best answer for each question. Circle your answer on
Matter Properties and Changes
 Matter Properties and Changes What is matter? anything that takes up space (volume) and has mass everything around you is made up of matter matter has 3 main states: solid, liquid, and gas Physical Property
Matter Properties and Changes What is matter? anything that takes up space (volume) and has mass everything around you is made up of matter matter has 3 main states: solid, liquid, and gas Physical Property
Vocabulary atom atomos Dalton's atomic theory law of constant composition law of definite proportions law of multiple proportions matter.
 1.3 Early Atomic Theory Lesson Objectives The student will: define matter and explain how it is composed of building blocks known as atoms. give a short history of how the concept of the atom developed.
1.3 Early Atomic Theory Lesson Objectives The student will: define matter and explain how it is composed of building blocks known as atoms. give a short history of how the concept of the atom developed.
Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na
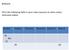 Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
Elements and the Periodic Table
 Chapter 7 Elements and the Periodic Table What are metals like? Think of things that are made with metals like aluminum, copper, iron, and gold. What do they have in common? They are usually shiny, and
Chapter 7 Elements and the Periodic Table What are metals like? Think of things that are made with metals like aluminum, copper, iron, and gold. What do they have in common? They are usually shiny, and
Copyright 2008 NSTA. All rights reserved. For more information, go to Pennies
 Pennies A shiny new penny is made up of atoms. Put an X next to all the things on the list that describe the atoms that make up the shiny new penny. hard soft I N G O D L I B E R T Y W E T R U S T 2 0
Pennies A shiny new penny is made up of atoms. Put an X next to all the things on the list that describe the atoms that make up the shiny new penny. hard soft I N G O D L I B E R T Y W E T R U S T 2 0
Is Consciousness a Nonspatial Phenomenon?
 KRITIKE VOLUME FIVE NUMBER ONE (JUNE 2011) 91-98 Article Is Consciousness a Nonspatial Phenomenon? Ståle Gundersen Abstract: Colin McGinn has argued that consciousness is a nonspatial phenomenon. McGinn
KRITIKE VOLUME FIVE NUMBER ONE (JUNE 2011) 91-98 Article Is Consciousness a Nonspatial Phenomenon? Ståle Gundersen Abstract: Colin McGinn has argued that consciousness is a nonspatial phenomenon. McGinn
Chemical and physical properties of elements
 Chemical and physical properties of elements What makes you, you? List a few characteristics or properties about yourself: Just like you are unique, every element/compound is unique in some way from all
Chemical and physical properties of elements What makes you, you? List a few characteristics or properties about yourself: Just like you are unique, every element/compound is unique in some way from all
02. Plato's Cosmology
 02. Plato's Cosmology Outline: World of Forms eternal, changeless, perfect ORDER & REASON blueprints Demiurge craftsman CHAOS & NECESSITY material The Cosmos (World of sensible objects) product Creation
02. Plato's Cosmology Outline: World of Forms eternal, changeless, perfect ORDER & REASON blueprints Demiurge craftsman CHAOS & NECESSITY material The Cosmos (World of sensible objects) product Creation
2.3 CHEMICAL PROPERTIES
 2.3 CHEMICAL PROPERTIES n Vocabulary: n chemical property n flammability n reactivity n chemical change n precipitate n Objectives: n Describe chemical properties of matter. n Describe clues that indicate
2.3 CHEMICAL PROPERTIES n Vocabulary: n chemical property n flammability n reactivity n chemical change n precipitate n Objectives: n Describe chemical properties of matter. n Describe clues that indicate
The Fundamental Laws of Chemistry
 The Fundamental Laws of Chemistry As we have discussed, matter can be classified into different categories based on directly observable properties, be they physical or chemical. Heterogeneous systems contain
The Fundamental Laws of Chemistry As we have discussed, matter can be classified into different categories based on directly observable properties, be they physical or chemical. Heterogeneous systems contain
States of Matter. Chemistry The Four States of Matter
 States of Matter Chemistry The Four States of Matter 1 What is matter? Any substance that has mass and takes up space. Brian Pop Video http://glencoe.mcgrawhill.com/sites/dl/free/0078600472/164155/0004468
States of Matter Chemistry The Four States of Matter 1 What is matter? Any substance that has mass and takes up space. Brian Pop Video http://glencoe.mcgrawhill.com/sites/dl/free/0078600472/164155/0004468
Cartesian Thinking System Thinking Quantum Thinking
 Cartesian Thinking System Thinking Quantum Thinking CVEN4700L15 For good or ill, ideas guide our economic, social and cultural development. Ideas and History We must torture nature to reveal her secrets
Cartesian Thinking System Thinking Quantum Thinking CVEN4700L15 For good or ill, ideas guide our economic, social and cultural development. Ideas and History We must torture nature to reveal her secrets
Matter, Singularity, Universe and Time
 Matter, Singularity, Universe and Time Robert Yusupov dialectical materialist, free researcher Virtual university, laboratory of dialectical materialism, physics and cosmology 690018 Vladivostok, Russian
Matter, Singularity, Universe and Time Robert Yusupov dialectical materialist, free researcher Virtual university, laboratory of dialectical materialism, physics and cosmology 690018 Vladivostok, Russian
Mendeleev s Periodic Table Mendeleev arranged the elements in his periodic table in order of increasing atomic mass.
 6.1 Searching For an Organizing Principle Chemists used the properties of elements to sort them into groups. Mendeleev s Periodic Table Mendeleev arranged the elements in his periodic table in order of
6.1 Searching For an Organizing Principle Chemists used the properties of elements to sort them into groups. Mendeleev s Periodic Table Mendeleev arranged the elements in his periodic table in order of
Chapter 6 The Periodic Table
 Chapter 6 The Periodic Table Section 6.1 Organizing the Elements OBJECTIVES: Explain how elements are organized in a periodic table. Section 6.1 Organizing the Elements OBJECTIVES: Compare early and modern
Chapter 6 The Periodic Table Section 6.1 Organizing the Elements OBJECTIVES: Explain how elements are organized in a periodic table. Section 6.1 Organizing the Elements OBJECTIVES: Compare early and modern
Philosophy 5340 Epistemology. Topic 3: Analysis, Analytically Basic Concepts, Direct Acquaintance, and Theoretical Terms. Part 2: Theoretical Terms
 Philosophy 5340 Epistemology Topic 3: Analysis, Analytically Basic Concepts, Direct Acquaintance, and Theoretical Terms Part 2: Theoretical Terms 1. What Apparatus Is Available for Carrying out Analyses?
Philosophy 5340 Epistemology Topic 3: Analysis, Analytically Basic Concepts, Direct Acquaintance, and Theoretical Terms Part 2: Theoretical Terms 1. What Apparatus Is Available for Carrying out Analyses?
Experiment 6 Alcohols and Phenols
 Experiment 6 Alcohols and Phenols Alcohols are organic molecules that contain a hydroxyl (-) group. Phenols are molecules that contain an group that is directly attached to a benzene ring. Alcohols can
Experiment 6 Alcohols and Phenols Alcohols are organic molecules that contain a hydroxyl (-) group. Phenols are molecules that contain an group that is directly attached to a benzene ring. Alcohols can
World Scientific News
 Available online at www.worldscientificnews.com World Scientific News 6 (2014) 43-49 EISSN 2392-2192 Time from the Aristotle's perspective Fatemeh Rassi Department of Philosophy, Science and Research branch,
Available online at www.worldscientificnews.com World Scientific News 6 (2014) 43-49 EISSN 2392-2192 Time from the Aristotle's perspective Fatemeh Rassi Department of Philosophy, Science and Research branch,
Physical Properties: Mass, Volume, Density, Conductivity, Magnetism, State of Matter, Solubility Mixtures, Heterogeneous mixtures, suspension,
 Physical Properties: Mass, Volume, Density, Conductivity, Magnetism, State of Matter, Solubility Mixtures, Heterogeneous mixtures, suspension, Homogeneous mixtures, colloid, solution Unit: Physical Properties
Physical Properties: Mass, Volume, Density, Conductivity, Magnetism, State of Matter, Solubility Mixtures, Heterogeneous mixtures, suspension, Homogeneous mixtures, colloid, solution Unit: Physical Properties
UNIT 2 Atomic Structure
 UNIT 2 Atomic Structure Section 1: History & Development of Atomic Theory (Chapter 3) History of the Atom Video The Greeks Democritus World made of empty space and tiny particles ( atoms ) Thought there
UNIT 2 Atomic Structure Section 1: History & Development of Atomic Theory (Chapter 3) History of the Atom Video The Greeks Democritus World made of empty space and tiny particles ( atoms ) Thought there
What is Matter? How can matter be classified? Every sample of matter is either an element, a compound, or a mixture.
 Matter Section 1 What is Matter? How can matter be classified? Every sample of matter is either an element, a compound, or a mixture. matter: anything that has mass and takes up space Matter Section 1
Matter Section 1 What is Matter? How can matter be classified? Every sample of matter is either an element, a compound, or a mixture. matter: anything that has mass and takes up space Matter Section 1
How Einstein came to employ the action-reaction principle in justifying general relativity
 How Einstein came to employ the action-reaction principle in justifying general relativity Harvey Brown Faculty of Philosophy H.R.B. and Dennis Lehmkuhl, Einstein, the reality of space, and the action-reaction
How Einstein came to employ the action-reaction principle in justifying general relativity Harvey Brown Faculty of Philosophy H.R.B. and Dennis Lehmkuhl, Einstein, the reality of space, and the action-reaction
Chemistry. Objectives 1-9
 Chemistry Objectives 1-9 Matter anything that has mass and takes up space (volume). It can be in the form of a solid, liquid or gas (States of Matter) Determine Particle Arrangement of solids, liquids,
Chemistry Objectives 1-9 Matter anything that has mass and takes up space (volume). It can be in the form of a solid, liquid or gas (States of Matter) Determine Particle Arrangement of solids, liquids,
Introduction to the Periodic Table. Chapter 4.5
 Introduction to the Periodic Table Chapter 4.5 History of the Periodic Table Dmitri Mendeleev, Russian Chemist Organized the first periodic table (1860) Organized elements according to properties 1.Ordered
Introduction to the Periodic Table Chapter 4.5 History of the Periodic Table Dmitri Mendeleev, Russian Chemist Organized the first periodic table (1860) Organized elements according to properties 1.Ordered
UNIT 4 NOTES: ATOMIC THEORY & STRUCTURE
 S T U D E N T N O T E S P r e - A P C h e m i s t r y U N I T 4 Page 1 NAME PERIOD UNIT 4 NOTES: ATOMIC THEORY & STRUCTURE STUDENT OBJECTIVES: Your fascinating teachers would like you amazing learners
S T U D E N T N O T E S P r e - A P C h e m i s t r y U N I T 4 Page 1 NAME PERIOD UNIT 4 NOTES: ATOMIC THEORY & STRUCTURE STUDENT OBJECTIVES: Your fascinating teachers would like you amazing learners
Title: Chem Review 3 PART 1 TOPIC: HISTORY OF PERIODIC TABLE. EQ: How was the first PT organized and how has it changed over the years?
 Title: Chem Review 3 PART 1 TOPIC: HISTORY OF PERIODIC TABLE EQ: How was the first PT organized and how has it changed over the years? Title and Highlight Topic: EQ: Date Reflect Question: Reflect on the
Title: Chem Review 3 PART 1 TOPIC: HISTORY OF PERIODIC TABLE EQ: How was the first PT organized and how has it changed over the years? Title and Highlight Topic: EQ: Date Reflect Question: Reflect on the
Placeholder zeros, even though they aren't SIGNIFICANT, still need to be included, so we know how big the number is!
 28 A few more math with significant figures examples: 15047 11 0.9876 Placeholder zeros, even though they aren't SIGNIFICANT, still need to be included, so we know how big the number is! Addition: 147.3
28 A few more math with significant figures examples: 15047 11 0.9876 Placeholder zeros, even though they aren't SIGNIFICANT, still need to be included, so we know how big the number is! Addition: 147.3
MATTER Classification of Matter. Composition of Matter
 MATTER Classification of Matter Composition of Matter Pure Substances Matter is classified as substances or a mixture of substances. A pure substance, or simply a substance, is a type of matter with a
MATTER Classification of Matter Composition of Matter Pure Substances Matter is classified as substances or a mixture of substances. A pure substance, or simply a substance, is a type of matter with a
Chemistry of Life Essential Questions
 Chemistry of Life Essential Questions VMHS Standards 8.6b; 8.6c; 1h; 4e; 4f; 5a;1b; 1. What is an atom? What are elements? An atom is the basic unit of o Consist of,, and An element is a type of atom o
Chemistry of Life Essential Questions VMHS Standards 8.6b; 8.6c; 1h; 4e; 4f; 5a;1b; 1. What is an atom? What are elements? An atom is the basic unit of o Consist of,, and An element is a type of atom o
[Handout 23] John Hawthorne, Three-Dimensionalism vs. Four-Dimensionalism
![[Handout 23] John Hawthorne, Three-Dimensionalism vs. Four-Dimensionalism [Handout 23] John Hawthorne, Three-Dimensionalism vs. Four-Dimensionalism](/thumbs/79/79677569.jpg) Phil 420: Metaphysics Spring 2008 [Handout 23] John Hawthorne, Three-Dimensionalism vs. Four-Dimensionalism Professor JeeLoo Liu The debate: On the nature of persisting material objects [Three-Dimensionalism]:
Phil 420: Metaphysics Spring 2008 [Handout 23] John Hawthorne, Three-Dimensionalism vs. Four-Dimensionalism Professor JeeLoo Liu The debate: On the nature of persisting material objects [Three-Dimensionalism]:
Name: Class: Date: Multiple Choice Identify the choice that best completes the statement or answers the question.
 Name: Class: Date: Chapter 1 and 2 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. One difference between a mixture and a compound is that.
Name: Class: Date: Chapter 1 and 2 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. One difference between a mixture and a compound is that.
Periodic Table Workbook
 Key Ideas: The placement or location of elements on the Periodic Table gives an indication of physical and chemical properties of that element. The elements on the Periodic Table are arranged in order
Key Ideas: The placement or location of elements on the Periodic Table gives an indication of physical and chemical properties of that element. The elements on the Periodic Table are arranged in order
Understanding the Atom 1.3
 Understanding the Atom 1.3 Introduction to Atoms 1.What are some ways that scientists have described the atom? 2.What are the parts of the atom, and how are they arranged? 3.How are atoms of all elements
Understanding the Atom 1.3 Introduction to Atoms 1.What are some ways that scientists have described the atom? 2.What are the parts of the atom, and how are they arranged? 3.How are atoms of all elements
Chemistry Final Study Guide KEY. 3. Define physical changes. A change in any physical property of a substance, not in the substance itself.
 Chemistry Final Study Guide KEY Unit 2: Matter & Its Properties, Lesson 1: Physical and Chemical Properties & Changes 1. Define physical properties. The characteristics of a substance that can be observed
Chemistry Final Study Guide KEY Unit 2: Matter & Its Properties, Lesson 1: Physical and Chemical Properties & Changes 1. Define physical properties. The characteristics of a substance that can be observed
Physical Properties of Matter. Examples of Physical Properties. QUESTION: How could you find the volume of air in an "empty" room?
 QUESTION: How could you find the volume of air in an "empty" room? The volume of regularly shaped solids can be calculated from their dimensions. For example, the volume of a rectangular solid is the product
QUESTION: How could you find the volume of air in an "empty" room? The volume of regularly shaped solids can be calculated from their dimensions. For example, the volume of a rectangular solid is the product
GRADE 9 SCIENCE UNIT 1: ATOMS, ELEMENTS, AND COMPOUNDS C H A P T E R 1
 GRADE 9 SCIENCE UNIT 1: ATOMS, ELEMENTS, AND COMPOUNDS C H A P T E R 1 UNIT TERMS Use term handout to define all terms for this unit. 1.1 SAFETY IN THE SCIENCE CLASSROOM Read pages 8-9 Activity 1-1A Page
GRADE 9 SCIENCE UNIT 1: ATOMS, ELEMENTS, AND COMPOUNDS C H A P T E R 1 UNIT TERMS Use term handout to define all terms for this unit. 1.1 SAFETY IN THE SCIENCE CLASSROOM Read pages 8-9 Activity 1-1A Page
