UNSATURATED HYDROCARBONS
|
|
|
- Louisa Jones
- 5 years ago
- Views:
Transcription
1 C20 09/16/ :47:28 Page 295 CHAPTER 20 UNSATURATED HYDROCARBONS SOLUTIONS TO REVIEW QUESTIONS 1. The sigma bond in the double bond of ethene is formed by the overlap of two sp 2 electron orbitals and is symmetrical about a line drawn between the nuclei of the two carbon atoms. The pi bond is formed by the sidewise overlap of two p orbitals which are perpendicular to the carbon-carbon sigma bond. The pi bond consists of two electron clouds, one above and one below the plane of the carbon-carbon sigma bond. 2. The restricted rotation around the carbon-carbon double bond in 1,2-dichloroethene means two different structural isomers exist, one with two chlorines on the same side of the double bond (cis) and when the chlorines are on opposite sides of the double bond (trans). For 1,2-dichloroethyne, the triple bond also has restricted rotation. However, as all atoms lie in a line, there is only one way to arrange the atoms for 1,2-dichloroethyne pentene is a liquid at room temperature (25 C) because its boiling point is above room temperature (30 C). 2-methylpropene is a gas at room temperature because its boiling point ( 7 C) is much lower than 25 C butene has a much lower melting point ( 185 C) than 2-methylpropene ( 14 C) although their boiling points are almost the same (1-butene, 6 C; 2-methylpropene, 7 C). 1-butene has a much different molecular shape than 2-methylpropene. This shape difference has a great effect on melting points because the molecules fit closely together in a solid. In a liquid the molecules are rapidly moving and molecular shape differences are much less important. Thus, the boiling points for these two molecules are almost the same. 5. Trans fats contain double bonds in the trans isomer form. In contrast, most naturally occurring unsaturated fats have double bonds in the cis form. Our bodies can t metabolize the trans fats because they are the wrong shape. The trans fats accumulate over time and increase the risk of cardiovascular disease. 6. During the 10-year period from 1935 to 1945, the major source of aromatic hydrocarbons shifted from coal tar to petroleum due to the rapid growth of several industries which used aromatic hydrocarbons as raw material. These industries include drugs, dyes, detergents, explosives, insecticides, plastics, and synthetic rubber. Since the raw material needs far exceeded the aromatics available from coal tar, another source had to be found, and processes were developed to make aromatic compounds from alkanes in petroleum. World War II, which occurred during this period, put high demands on many of these industries, particularly explosives. 7. Benzene does not undergo the typical reactions of an alkene. Benzene does not decolorize bromine rapidly and it does not destroy the purple color of permanganate ions. The reactions of benzene are more like those of an alkane. Reaction of benzene with chlorine requires a catalyst. Benzene does not readily add 2, but rather a hydrogen atom is replaced by a chlorine atom. C 6 H 6 þ 2! Fe C 6 H 5 þ H
2 C20 09/16/ :47:28 Page The 11-cis isomer of retinal combines with a protein (opsin) to form the visual pigment rhodopsin. When light is absorbed, the 11-cis double bond is converted to a trans-double bond. This process initiates the mechanism of visual excitation which our brains perceive as light or light forms. 9. Polycyclic aromatic hydrocarbons are potent carcinogens. 10. Cyclohexane carbons form bond angles of about 109 in a tetrahedron; this causes the ring to be either a boat or a chair shape. Benzene carbons form bond angles of 120 in a plane. Therefore, the benzene molecule must be planar. 11. According to the mechanism, the addition of an unsymmetrical module such as HX adds to a carboncarbon double bond. In the first step, the H adds to the carbon of the carbon-carbon double bond that has the most hydrogen atoms on it, according to Markovnikoff s rule. The second step completes the addition by adding the more negative element, X of the HX. 12. Cracking means breaking into pieces, which, according to the pyrolysis products, is what occurs in the reaction. 13. H þ adds to C-1 forming the more stable cation, C þ HCH 2 A then adds to C-2 to form the product, CHCH chloropropene meets the requirement of two different groups on each carbon atom of the carboncarbon-double bond to have cis-trans isomers. 2-chloropropene does not meet this requirement. H H C C C C C C H H H H trans-1-chloropropene cis-1-chloropropene 2-chloropropene 15. They are the same compound. Both structures have the same name, 2,3-dimethylcyclohexene
3 C20 09/16/ :47:28 Page 297 SOLUTIONS TO EXERCISES 1. (a) 2. (a) 3. CH sp3 3 CH sp2 CH sp2 CH sp HC sp C sp sp 3 sp 3 sp 3 CH 2 CH 2 5. Isomeric iodobutenes, C 4 H 7 I
4 C20 09/16/ :47:29 Page (a) 7. (a) CH CCH CH 3-penten-1-yne (d) (e) (f) 8. (a) (d)
5 C20 09/16/ :47:29 Page 299 (e) (f) 9. (a) 23 sigma bonds, 1 pi bond; 17 sigma bonds, 1 pi bond; 10 sigma bonds, 3 pi bonds; (d) 17 sigma bonds, 1 pi bond; (e) 15 sigma bonds, 2 pi bonds; (f) 33 sigma bonds, 3 pi bonds. 10. (a) 23 sigma bonds, 1 pi bond; 27 sigma bonds, 7 pi bonds; 20 sigma bonds, 5 pi bonds; (d) 13 sigma bonds, 1 pi bond; (e) 19 sigma bonds, 1 pi bond; (f) 21 sigma bonds, 1 pi bond. 11. (a) trans-6-chloro-3-heptene; 4,4-dimethyl-2-pentyne; 2-ethyl-l-pentene. 12. (a) 3-phenyl-l-butyne; 2-methyl-2-hexene; cis-3,4-dimethyl-3-hexene. 13. All the hexynes, C 6 H 10 CH 2 CH 2 CH 2 C CH CH 2 CH 2 C C CH 2 C CCH 2 1-hexyne 2-hexyne 3-hexyne 14. All the pentynes, C 5 H 8 CH 2 CH 2 C CH 1-pentyne CH 2 C C 2-pentyne 15. (a) 1-butyne (The smallest numbered carbon that is involved in the triple bond is used to number the triple bond.); 2-hexyne (The parent chain is the longest continuous carbon chain that contains the triple bond.); propyne (The triple bond has only one possible location in propyne and no number is needed.) 16. (a) 4-methyl-2-pentyne (The parent chain is numbered from the end closest to the triple bond.); 4-methyl-2-hexyne (The parent chain is the longest continuous carbon chain that contains the triple bond.); 3-bromo-l-butyne (The parent chain is numbered from the end closest to the triple bond.). 17. Cis-trans isomers exist for only. Alkynes do not have cis-trans isomerism. Alkenes have cis-trans isomerism only when each double-bonded carbon is attached to two different groups
6 C20 09/16/ :47:30 Page Cis-trans isomers exist for only. Alkynes do not have cis-trans isomerism. Alkenes have cis-trans isomerism only when each double-bonded carbon is attached to two different groups. 19. (a) cis neither trans 20. (a) trans trans neither 21. (a) CH 2 CH 2 CH CH 2 þ Br 2! CH 2 CH 2 CHBrCH 2 Br (d) (e) 22. (a) CH 2 CH CH þ HBr! CH 2 CHBrCH 2 þ CH 2 CH 2 CHBr (CH 2 CH þ Br 2! CH 2 BrCHBr (d) (e) 23. (a) C C þ Br 2 ð1 moleþ! CBr CBr Two-step reaction: H CH CH þ H! CH 2 CH! CH 2 CH 2 CH 2 C CH þ H 2 ð1 moleþ! 3CH 25 2 CH 2 CH CH 2 C
7 C20 09/16/ :47:30 Page 301 Pt; (a) C CH þ H 2 ð1 molþ! C 1 atm CH CH 2 C C þ Br 2 ð2 molþ! CBr 2 CBr 2 Two-step reaction: C CH þ H! C ¼ CH 2! H C When cyclohexene, reacts with: (a) Br 2, the product is Br 1,2-dibromocyclohexane Br HI, the product is iodocyclohexane H 2 O, H þ, the product is cyclohexanol (d) KMnO 4 (aq), the product is cyclohexene glycol or 1,2-dihydroxycyclohexane 26. When cyclopentene, reacts with: (a) 2, the product is 1,2-dichlorocyclopentane HBr, the product is bromocyclopentane H 2, Pt, the product is cyclopentane 27. (d) H 2 O, H þ, the product is cyclopentanol (a)
8 C20 09/16/ :47:30 Page (a) 29. (a) meta meta ortho 30. (a) para meta para O 31. (a) CH (d) CH CH3 OH NO 2 (e) Br NH 2 Br CH 2 (f)
9 C20 09/16/ :47:31 Page 303 O 32. (a) COH (d) CH CH 2 Br (e) NO 2 NO 2 (f) 33. (a) bromodichlorobenzenes
10 C20 09/16/ :47:31 Page 304 The toluene derivatives of formula C 9 H 12 : 1,2,3-trimethylbenzene 1,2,4-trimethylbenzene H 3 C 1,3,5-trimethylbenzene CH2 o-ethyltoluene 34. (a) trichlorobenzenes CH 2 m-ethyltoluene CH 2 p-ethyltoluene 1,2,3-trichlorobenzene 1,2,4-trichlorobenzene The benzene derivatives of formula C 8 H 10 : 1,3,5-trichlorobenzene o-xylene or 1,2-dimethylbenzene m-xylene or 1,3-dimethylbenzene CH 2 H 3 C p-xylene or 1,4-dimethylbenzene ethylbenzene 35. (a) p-chloroethylbenzene (d) p-bromophenol propylbenzene (e) triphenylmethane m-nitroaniline 36. (a) styrene (d) isopropylbenzene m-nitrotoluene (e) 2,4,6-tribromophenol 2,4-dibromobenzoic acid
11 C20 09/16/ :47:31 Page (a) 2-chloro-1,4-diiodobenzene o-dibromobenzene m-chloroaniline 38. (a) 2,3-dibromophenol 1,3,5-trichlorobenzene m-iodotoluene 39. (a) 40. (a) 41. When reacts with HBr, two products are possible: The first will strongly predominate. This is the product according to Markovnikov s rule and forms because the tertiary carbocation intermediate formed is more stable than a primary carbocation. 42. Two tests can be used. (1) Baeyer test hexene will decolorize KMnO 4 solution; cyclohexane will not. (2) In the absence of sunlight, hexene will react with and decolorize bromine; cyclohexane will not
12 C20 09/16/ :47:32 Page
13 C20 09/16/ :47:32 Page (Cont.) 44. (a) C þ H 2 primary carbocation (a positively charged carbon bonded to one other carbon) C þ H secondary carbocation (a positively charged carbon bonded to two other carbons) tertiary carbocation (a positively charged carbon bonded to three other carbons) 45. (a)
14 C20 09/16/ :47:33 Page 308 (d) 46. The reaction mechanism by which benzene is brominated in the presence of FeBr 3 : (a) FeBr 3 þ Br 2! FeBr 4 þ Brþ Formation of a bromonium ion (Br þ ), an electrophile. The bromonium ion adds to benzene forming a carbocation intermediate. A hydrogen ion is lost from the carbocation forming the product bromobenzene. H 47. (a) CHCH 2! CH 2 CHCH 2 þ CH CH H CH 2 CH 2 CH 2 CH 2! CH 2 CHCH 2 CH Yes, there will be a color change (loss of Br 2 color). The fact that there is no HBr formed indicates that the reaction is not substitution but addition. Therefore, C 4 H 8 must contain a carbon-carbon double bond. Three structures are possible. 49. Baeyer test: Add KMnO 4 solution to each sample. The KMnO 4 will lose its purple color with 1-heptene. There will be no reaction (no color change) with heptane
15 C20 09/16/ :47:33 Page I would not expect graphene to react easily with H in an addition reaction. Graphene is composed of sheets of aromatic rings that do not easily undergo addition reactions The double bonds in fatty acids are oxidized. This reaction adds oxygen and the double bond converts to a single bond. This reaction is thought to start the process that eventually results in hardening of the arteries. This is an oxidation reaction. 53. (a) HC sp C sp sp (a) Four isomers: CH 2 CHCH 2 C 4 H 8 1-butene CH 2 ¼ Cð Þ 2 2-methyl-1-propene One isomer C 5 H 8 CH ¼ CH 2-butene (cis and trans)
16 C20 09/16/ :47:34 Page is the correct structure, (a) is an incorrect structure because the carbons where the two rings are fused have five bonds, not four bonds to each carbon. 56. Carbon-2 has two methyl groups on it. The configuration of two of the same groups on a carbon of a carbon-carbon double bond does not show cis-trans isomerism. 57. (a) alkyne or cycloalkene alkene alkane (d) alkyne or cycloalkene 58. Chemically distinguishing between benzene, 1-hexene, and 1-hexyne Step 1. Add KMnO 4 solution to a sample of each liquid. Benzene is the only one in which the KMnO 4 does not lose its purple color. Step 2. To 0.5 ml samples of 1-hexene and 1-hexyne add bromine solution dropwise until there is no more color change of the bromine (from reddish-brown to colorless). 1-hexyne (with a triple bond) will decolor about twice as many drops of bromine as 1-hexene. Thus the three liquids are identified. 59. (a) C CH! H C CH 2! HBr CBr C CH! Br 2 CBr CHBr! H CBrCH 2 Br C CH! 2 2 C 2 CH
3. What number would be used to indicate the double bond position in the IUPAC name for CH 3 CH 2 CH=CH CH 3 a. 1 b. 2 c. 3 d.
 Chapter 2 Unsaturated Hydrocarbons MULTIPLE CHOICE 1. Name a difference between a saturated and an unsaturated hydrocarbon. a. Saturated hydrocarbons are composed of only carbon and hydrogen, and unsaturated
Chapter 2 Unsaturated Hydrocarbons MULTIPLE CHOICE 1. Name a difference between a saturated and an unsaturated hydrocarbon. a. Saturated hydrocarbons are composed of only carbon and hydrogen, and unsaturated
Unsaturated hydrocarbons. Chapter 13
 Unsaturated hydrocarbons Chapter 13 Unsaturated hydrocarbons Hydrocarbons which contain at least one C-C multiple (double or triple) bond. The multiple bond is a site for chemical reactions in these molecules.
Unsaturated hydrocarbons Chapter 13 Unsaturated hydrocarbons Hydrocarbons which contain at least one C-C multiple (double or triple) bond. The multiple bond is a site for chemical reactions in these molecules.
Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 INTRODUCTION TO ORGANIC AND BIOCHEMISTRY QUIZ 5 Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name
INTRODUCTION TO ORGANIC AND BIOCHEMISTRY QUIZ 5 Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name
Chapter 13 Alkenes and Alkynes & Aromatic Compounds
 Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Chapter 12: Unsaturated Hydrocarbons
 Chapter 12: Unsaturated Hydrocarbons UNSATURATED HYDROCARBONS contain carbon-carbon multiple bonds. Alkenes C=C double bonds Alkynes triple bonds Aromatics benzene rings 1 2 NAMING ALKENES Step 1: Name
Chapter 12: Unsaturated Hydrocarbons UNSATURATED HYDROCARBONS contain carbon-carbon multiple bonds. Alkenes C=C double bonds Alkynes triple bonds Aromatics benzene rings 1 2 NAMING ALKENES Step 1: Name
Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Vision. Cis-trans isomerism is key to vision. How rods work H 3 C CH 3. Protein opsin. 11-cis-retinal. Opsin. Rhodopsin.
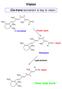 Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes.
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
A. They all have a benzene ring structure in the molecule. B. They all have the same molecular formula. C. They all have carbon and hydrogen only
 Ch 21 G12 CoreI- Choose the best answer, then transfer your answers to page (1) [32 marks; 2 each] 1. What characteristic do all aromatic hydrocarbons share? A. They all have a benzene ring structure in
Ch 21 G12 CoreI- Choose the best answer, then transfer your answers to page (1) [32 marks; 2 each] 1. What characteristic do all aromatic hydrocarbons share? A. They all have a benzene ring structure in
Chapter 3 Alkenes and Alkynes. Excluded sections 3.15&3.16
 Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
CHAPTER 15. Practice exercises 15.1
 CAPTER 15 Practice exercises 15.1 15.3 (a) C 9 18 : isobutylcyclopentane C 11 22 : sec-butylcycloheptane C 6 2 : 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene 2,3-dimethyl-2-butene 3,3-dimethyl-1-butyne
CAPTER 15 Practice exercises 15.1 15.3 (a) C 9 18 : isobutylcyclopentane C 11 22 : sec-butylcycloheptane C 6 2 : 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene 2,3-dimethyl-2-butene 3,3-dimethyl-1-butyne
Chapter 13 Unsaturated Hydrocarbons
 hapter 1 1 hapter 1 Unsaturated ydrocarbons Solutions to In-hapter Problems 1.1 To draw a complete structure for each condensed structure, first draw in the multiple bonds. Then draw in all the other s
hapter 1 1 hapter 1 Unsaturated ydrocarbons Solutions to In-hapter Problems 1.1 To draw a complete structure for each condensed structure, first draw in the multiple bonds. Then draw in all the other s
EXPERIMENT 8 Reactions of Hydrocarbons
 EXPERIMENT 8 Reactions of Hydrocarbons Properties and Identification of Hydrocarbons Purpose: a) To identify saturated and unsaturated hydrocarbons using properties and reactions. b) Study substitution
EXPERIMENT 8 Reactions of Hydrocarbons Properties and Identification of Hydrocarbons Purpose: a) To identify saturated and unsaturated hydrocarbons using properties and reactions. b) Study substitution
ST. JOSEPH S COLLEGE OF ARTS & SCIENCE (AUTONOMOUS) ST. JOSEPH S COLLEGE ROAD, CUDDALORE CH101T ORGANIC CHEMISTRY I (SEMESTER-I)
 UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
Organic Chemistry. February 18, 2014
 Organic Chemistry February 18, 2014 What does organic mean? Organic Describes products Grown through natural biological process Without synthetic materials In the 18 th century Produced by a living system
Organic Chemistry February 18, 2014 What does organic mean? Organic Describes products Grown through natural biological process Without synthetic materials In the 18 th century Produced by a living system
Unsaturated Hydrocarbons
 Unsaturated ydrocarbons hemical Formulas and Unsaturation n n 2n n 2n+2 n 2n+2 hemical Formulas and Unsaturation n n n n 2n n 2n hemical Formulas and Unsaturation ydrocarbons Saturated ydrocarbons Unsaturated
Unsaturated ydrocarbons hemical Formulas and Unsaturation n n 2n n 2n+2 n 2n+2 hemical Formulas and Unsaturation n n n n 2n n 2n hemical Formulas and Unsaturation ydrocarbons Saturated ydrocarbons Unsaturated
CHAPTER 3 ALKENES, ALKYNES & CONJUGATE DIENES
 CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
Class XI Chapter 13 Hydrocarbons Chemistry
 Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Chem 145 Unsaturated hydrocarbons Alkynes
 Dr. Seham ALTERARY Chem 145 Unsaturated hydrocarbons Alkynes Chapter 4 1434-1435 2013-2014 2 st semester By the end of this chapter you should be familiar with: Definition for Alkynes. Nomenclature of
Dr. Seham ALTERARY Chem 145 Unsaturated hydrocarbons Alkynes Chapter 4 1434-1435 2013-2014 2 st semester By the end of this chapter you should be familiar with: Definition for Alkynes. Nomenclature of
Ch09. Alkenes & Alkynes. Unsaturated hydrocarbons. Double and triple bonds in our carbon backbone. version 1.0
 Ch09 Alkenes & Alkynes Unsaturated hydrocarbons. Double and triple bonds in our carbon backbone. version 1.0 Nick DeMello, PhD. 2007-2015 Ch09 Alkenes & Alkynes Alkenes & Alkynes Definition and bond angles.
Ch09 Alkenes & Alkynes Unsaturated hydrocarbons. Double and triple bonds in our carbon backbone. version 1.0 Nick DeMello, PhD. 2007-2015 Ch09 Alkenes & Alkynes Alkenes & Alkynes Definition and bond angles.
Chapter 12 Alkenes and Alkynes
 BR M 102 lass Notes hapter 12 Page 1 of 8 hapter 12 Alkenes and Alkynes * alkenes = double bonds * alkynes triple bonds * aromatics or arenes alternating double and single bonds such as in benzene * saturated
BR M 102 lass Notes hapter 12 Page 1 of 8 hapter 12 Alkenes and Alkynes * alkenes = double bonds * alkynes triple bonds * aromatics or arenes alternating double and single bonds such as in benzene * saturated
16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond.
 CAPTER 16 Practice Exercises 16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond. 16.3 (a) The more stable conformation
CAPTER 16 Practice Exercises 16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond. 16.3 (a) The more stable conformation
1. How do you account for the formation of ethane during chlorination of methane?
 1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
Chapter 22. Organic and Biological Molecules
 Chapter 22 Organic and Biological Molecules The Bonding of Carbon Organic chemistry is the chemistry of compounds containing carbon. Because carbon can form single, double, and triple bonds, the following
Chapter 22 Organic and Biological Molecules The Bonding of Carbon Organic chemistry is the chemistry of compounds containing carbon. Because carbon can form single, double, and triple bonds, the following
8. What is the slow, rate-determining step, in the acidcatalyzed dehydration of 2-methyl-2-propanol?
 CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
General Chemistry Unit 7A ( )
 Organic Chemistry Allotropes Isomers Hydrocarbons o Alkanes o Alkenes o Alkynes o Aromatics Alkyl Halides General Chemistry Unit 7A (2017-2018) 1 2 3 4 Parent Chain: Methane Ethane CH4 C2H6 Propane C3H8
Organic Chemistry Allotropes Isomers Hydrocarbons o Alkanes o Alkenes o Alkynes o Aromatics Alkyl Halides General Chemistry Unit 7A (2017-2018) 1 2 3 4 Parent Chain: Methane Ethane CH4 C2H6 Propane C3H8
CHAPTER HYDROCARBONS. Chapterwise Previous year Qs. (a) Na (b) HCl in H2O (c) KOH in C2H5OH (d) Zn in alcohol. Ans: (c)
 122 CHAPTER HYDROCARBONS 1. Acetylenic hydrogens are acidic because [1989] Sigma electron density of C Hbond in acetylene is nearer to carbon, which has 50% s- character Acetylene has only open hydrogen
122 CHAPTER HYDROCARBONS 1. Acetylenic hydrogens are acidic because [1989] Sigma electron density of C Hbond in acetylene is nearer to carbon, which has 50% s- character Acetylene has only open hydrogen
Chemistry 11. Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons
 Chemistry 11 Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons 2 1. Unsaturated hydrocarbons So far, we have studied the hydrocarbons in which atoms are connected exclusively by
Chemistry 11 Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons 2 1. Unsaturated hydrocarbons So far, we have studied the hydrocarbons in which atoms are connected exclusively by
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Unit 2 Review: Organic Chemistry. 1. Terms for which you should be able to write or apply the definitions:
 Unit 2 Review: Organic Chemistry 1. Terms for which you should be able to write or apply the definitions: organic compound aliphatic hydrocarbons saturated miscible functional group aromatic hydrocarbons
Unit 2 Review: Organic Chemistry 1. Terms for which you should be able to write or apply the definitions: organic compound aliphatic hydrocarbons saturated miscible functional group aromatic hydrocarbons
Chapter 20 (part 2) Organic Chemistry
 Chapter 20 (part 2) Organic Chemistry Section 20.7 Alkenes and Alkynes Alkenes: hydrocarbons that contain a carbon carbon double bond. [C n H 2n ] CH 3 CH=CH 2 propene Alkynes: hydrocarbons containing
Chapter 20 (part 2) Organic Chemistry Section 20.7 Alkenes and Alkynes Alkenes: hydrocarbons that contain a carbon carbon double bond. [C n H 2n ] CH 3 CH=CH 2 propene Alkynes: hydrocarbons containing
Alkenes. Alkenes are unsaturated aliphatic hydrocarbons.
 Alkenes Alkenes Each member contains one double covalent bond between two C atoms. Draw condensed structural formulas of first three members of alkenes family. Alkenes are unsaturated aliphatic hydrocarbons.
Alkenes Alkenes Each member contains one double covalent bond between two C atoms. Draw condensed structural formulas of first three members of alkenes family. Alkenes are unsaturated aliphatic hydrocarbons.
Name Date Class HYDROCARBONS
 22.1 HYDROCARBONS Section Review Objectives Describe the relationship between number of valence electrons and bonding in carbon Define and describe alkanes Relate the polarity of hydrocarbons to their
22.1 HYDROCARBONS Section Review Objectives Describe the relationship between number of valence electrons and bonding in carbon Define and describe alkanes Relate the polarity of hydrocarbons to their
Introduction to Organic Chemistry. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Introduction to Organic Chemistry Copyright The McGraw-ill Companies, Inc. Permission required for reproduction or display. 1 Common Elements in Organic Compounds 2 Classification of ydrocarbons ydrocarbons
Introduction to Organic Chemistry Copyright The McGraw-ill Companies, Inc. Permission required for reproduction or display. 1 Common Elements in Organic Compounds 2 Classification of ydrocarbons ydrocarbons
Organic Chemistry is the chemistry of compounds containing.
 Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Hydrocarbons. Table Alkanes
 Hydrocarbons Hydrocarbons may be divided into two groups, aliphatic and aromatic. The aliphatics can be subdivided into i) alkanes, ii) alkenes, and iii) alkynes. The alkanes contain single covalent bonds
Hydrocarbons Hydrocarbons may be divided into two groups, aliphatic and aromatic. The aliphatics can be subdivided into i) alkanes, ii) alkenes, and iii) alkynes. The alkanes contain single covalent bonds
4.4 Nomenclature of Cycloalkanes
 4.4 Nomenclature of Cycloalkanes 4.4A Monocyclic Compounds Cycloalkanes with only one ring are named by attaching the prefix cyclo- to the names of the alkanes possessing the same number of carbon atoms.
4.4 Nomenclature of Cycloalkanes 4.4A Monocyclic Compounds Cycloalkanes with only one ring are named by attaching the prefix cyclo- to the names of the alkanes possessing the same number of carbon atoms.
Organic Chemistry. REACTIONS Grade 12 Physical Science Mrs KL Faling
 Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
ORGANIC CHEMISTRY. Classification of organic compounds
 ORGANIC CHEMISTRY Organic chemistry is very important branch of chemistry and it study the compounds which contain carbon (C) and hydrogen (H), in general, and may contains other atoms such as oxygen (O),
ORGANIC CHEMISTRY Organic chemistry is very important branch of chemistry and it study the compounds which contain carbon (C) and hydrogen (H), in general, and may contains other atoms such as oxygen (O),
nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes
 240 Chem nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes 1 Chapter 3 Alkenes or Olefines Crabon-Carbon double bond C n H 2n Hybridization in Alkenes: 1.34 A 2 Nomenclature of Alkenes and Cycloalkenes
240 Chem nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes 1 Chapter 3 Alkenes or Olefines Crabon-Carbon double bond C n H 2n Hybridization in Alkenes: 1.34 A 2 Nomenclature of Alkenes and Cycloalkenes
Reactions of Alkenes and Alkynes
 5 2 2 2 2 2 2 2 Reactions of Alkenes and Alkynes APTER SUMMARY Addition is the characteristic reaction of alkenes and alkynes. Since the carbons of a double or triple bond do not have the maximum number
5 2 2 2 2 2 2 2 Reactions of Alkenes and Alkynes APTER SUMMARY Addition is the characteristic reaction of alkenes and alkynes. Since the carbons of a double or triple bond do not have the maximum number
Organic Chemistry. Pre-lab Assignment. Purpose. Background. Experiment 14. Before coming to lab: Hydrocarbons. Read the lab thoroughly.
 Experiment 14 Pre-lab Assignment Before coming to lab: Read the lab thoroughly. rganic hemistry Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered
Experiment 14 Pre-lab Assignment Before coming to lab: Read the lab thoroughly. rganic hemistry Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered
Organic Chemistry Worksheets
 Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
Arrange the following alkene in increasing order of their enthalpy of hydrogenation ( )
 Q.1. Which of the statements is correct? (I) Melting point of alkane increases with increase of C atoms and with increase in branching. (II) Boiling point of alkane increases with increase of C atoms but
Q.1. Which of the statements is correct? (I) Melting point of alkane increases with increase of C atoms and with increase in branching. (II) Boiling point of alkane increases with increase of C atoms but
Reading Skill Practice
 system. This process is explained on page 698. create a flowchart that describes the steps for naming branched-chain alkanes using the IUPAC A flowchart can help you to remember the order in which events
system. This process is explained on page 698. create a flowchart that describes the steps for naming branched-chain alkanes using the IUPAC A flowchart can help you to remember the order in which events
BENZENE & AROMATIC COMPOUNDS
 BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
Some Reactions of Hydrocarbons Experiment #2
 Some Reactions of Hydrocarbons Experiment #2 Objective: To distinguish alkanes, alkenes and aromatic hydrocarbons by their chemical reactions and reactivity. Introduction Hydrocarbons are organic compounds
Some Reactions of Hydrocarbons Experiment #2 Objective: To distinguish alkanes, alkenes and aromatic hydrocarbons by their chemical reactions and reactivity. Introduction Hydrocarbons are organic compounds
Ashwani Gupta. Mb: Class IX-X: X: Math & Science Class XI-XII: XII: Accts., Eco. & B. Stds. Carbon and its compounds.
 Carbon and its compounds MCQ s How many unshared pairs of electrons are present on a nitrogen atom in a molecule of ammonia? 1. 1 2. 2 3. 0 4. 3 What is the estimated number of carbon compounds whose formulae
Carbon and its compounds MCQ s How many unshared pairs of electrons are present on a nitrogen atom in a molecule of ammonia? 1. 1 2. 2 3. 0 4. 3 What is the estimated number of carbon compounds whose formulae
Unsaturated Hydrocarbons
 Interchapter G Unsaturated ydrocarbons The flame from an acetylene torch. Acetylene, which is used extensively in welding, is an example of an unsaturated hydrocarbon. University Science Books, 2011. All
Interchapter G Unsaturated ydrocarbons The flame from an acetylene torch. Acetylene, which is used extensively in welding, is an example of an unsaturated hydrocarbon. University Science Books, 2011. All
Organic Chemistry. Saturated Hydrocarbons: The Alkanes. ethane H C C H CH 3 CH 3
 rganic hemistry The classification of chemical compounds in to the general areas of organic and inorganic derives from the use of the "mineral, vegetable and animal" designation by the early workers in
rganic hemistry The classification of chemical compounds in to the general areas of organic and inorganic derives from the use of the "mineral, vegetable and animal" designation by the early workers in
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
Unit 2, Review for Quiz #1: Hydrocarbons
 Unit 2, Review for Quiz #1: Hydrocarbons 1. What is the simplest organic molecule? a) CH 4 c) HCN b) CO 2 d) HC CH 2. Which of the following molecules would be classified as organic? I) CaCO 3 II) C 2
Unit 2, Review for Quiz #1: Hydrocarbons 1. What is the simplest organic molecule? a) CH 4 c) HCN b) CO 2 d) HC CH 2. Which of the following molecules would be classified as organic? I) CaCO 3 II) C 2
3 free radical is most stable. Q.5. A + Cl 2 hv monochloro product To maximize the yield of monochloro product in the above reaction? Cl 2 must be add
 Q.1. I II III IV IV III II I I III II IV IV II III I I II III IV The decreasing order of the anti knocking value of octane number of the following is: (I) CH 4 (II) C 2 H 6 (III) C 3 H 8 (IV) C 4 H 10
Q.1. I II III IV IV III II I I III II IV IV II III I I II III IV The decreasing order of the anti knocking value of octane number of the following is: (I) CH 4 (II) C 2 H 6 (III) C 3 H 8 (IV) C 4 H 10
Chapter 13: Unsaturated Hydrocarbons
 Chemistry 121(01) Winter 2009 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@chem.latech.edu Office: 311 Carson Taylor all ; Phone:
Chemistry 121(01) Winter 2009 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@chem.latech.edu Office: 311 Carson Taylor all ; Phone:
Practice Packet Unit 11: Organic Chemistry
 Regents Chemistry: Mr. Palermo Practice Packet Unit 11: Organic Chemistry www.mrpalermo.com 1 LESSON 1: Introduction to Organic Chemistry 1. How many times does carbon bond and why? 2. A student investigated
Regents Chemistry: Mr. Palermo Practice Packet Unit 11: Organic Chemistry www.mrpalermo.com 1 LESSON 1: Introduction to Organic Chemistry 1. How many times does carbon bond and why? 2. A student investigated
Reading: Chapter 4 Practice Problems: in text problems and 19 22, 24, 26, 27, 29, 30, 33 35, 39, 40.
 Reading: Chapter Practice Problems: in text problems and 19 22, 24, 26, 27, 29, 30, 33 35, 39, 40. Alkenes: Structure, Nomenclature, Stability, and an Introduction to Reactivity Alkenes are unsaturated
Reading: Chapter Practice Problems: in text problems and 19 22, 24, 26, 27, 29, 30, 33 35, 39, 40. Alkenes: Structure, Nomenclature, Stability, and an Introduction to Reactivity Alkenes are unsaturated
Organic Chemistry. A. Introduction
 Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
(a) Give the general formula that applies to both alkenes and cycloalkanes. (1)
 1 Alkenes and cycloalkanes have the same general formula, but react very differently with halogens. (a) Give the general formula that applies to both alkenes and cycloalkanes. (b) Using structural formulae,
1 Alkenes and cycloalkanes have the same general formula, but react very differently with halogens. (a) Give the general formula that applies to both alkenes and cycloalkanes. (b) Using structural formulae,
Chemistry 2202 Unit 3 Test Section 1 &
 Chemistry 2202 Unit 2 Test 2 Section 1 & 2 Page 1 of 6 Chemistry 2202 Unit 3 Test Section 1 & 2-2006 Part 1 Multiple Choice: Complete using the answer form on page 4. (20 points) 1. What is the main idea
Chemistry 2202 Unit 2 Test 2 Section 1 & 2 Page 1 of 6 Chemistry 2202 Unit 3 Test Section 1 & 2-2006 Part 1 Multiple Choice: Complete using the answer form on page 4. (20 points) 1. What is the main idea
I. Multiple Choice Questions (Type-I)
 Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline
 Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
UNIT (7) ORGANIC COMPOUNDS: HYDROCARBONS
 UNIT (7) RGANI MPUNDS: YDRARBNS rganic chemistry is the study carbon containing compounds. 7.1 Bonding in rganic ompounds rganic compounds are made up of only a few elements and the bonding is almost entirely
UNIT (7) RGANI MPUNDS: YDRARBNS rganic chemistry is the study carbon containing compounds. 7.1 Bonding in rganic ompounds rganic compounds are made up of only a few elements and the bonding is almost entirely
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry
 Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
Organic Chemistry. Alkynes
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkynes by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkynes by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) Can act as weak nucleophiles
 Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Alkenes, Alkynes, and Aromatic Compounds
 Alkenes, Alkynes, and Aromatic Compounds Alkenes and Alkynes Unsaturated Contain carbon-carbon DOUBLE and TRIPLE bond to which more hydrogen atoms can be added Alkenes: carbon-carbon double bonds Alkynes:
Alkenes, Alkynes, and Aromatic Compounds Alkenes and Alkynes Unsaturated Contain carbon-carbon DOUBLE and TRIPLE bond to which more hydrogen atoms can be added Alkenes: carbon-carbon double bonds Alkynes:
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
Downloaded from
 1 Class XI Chemistry Ch 13: Hydrocarbons TOP Concepts: 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
1 Class XI Chemistry Ch 13: Hydrocarbons TOP Concepts: 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
Q.1 Draw out suitable structures which fit the molecular formula C 6 H 6
 Aromatic compounds 2814 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out suitable structures which fit the molecular formula
Aromatic compounds 2814 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out suitable structures which fit the molecular formula
Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
 Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
HISTORY OF ORGANIC CHEMISTRY
 hemistry 52 hapter 12 ISTORY OF ORGANI EMISTRY In the early days of chemistry, scientists classified chemical substances into 2 groups: 1. Inorganic: those that were composed of minerals, such as rocks
hemistry 52 hapter 12 ISTORY OF ORGANI EMISTRY In the early days of chemistry, scientists classified chemical substances into 2 groups: 1. Inorganic: those that were composed of minerals, such as rocks
1.3 Reactions of Hydrocarbons
 1.3 Reactions of ydrocarbons All hydrocarbons readily burn in air to give carbon dioxide and water, with the release of large amounts of energy (Figure 1); this chemical reaction accounts for the extensive
1.3 Reactions of ydrocarbons All hydrocarbons readily burn in air to give carbon dioxide and water, with the release of large amounts of energy (Figure 1); this chemical reaction accounts for the extensive
 1 TOP Concepts: Class XI Chemistry Ch 13: Hydrocarbons 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
1 TOP Concepts: Class XI Chemistry Ch 13: Hydrocarbons 1. Alkanes: General formula: C n H 2n+2 2. Preparation of alkanes: 3. Kolbe s electrolytic method: Alkali metal salts of carboxylic acids undergo
Isomerism in Alkanes, Haloalkanes, and Alkenes using Molecular Models
 EXPERIMENT 1 Isomerism in Alkanes, aloalkanes, and Alkenes using Molecular Models Materials Needed - Molecular model kit Relevant Textbook Reading Denniston, chap 11.2-11.4, 12.1-12.3 Background In uncharged,
EXPERIMENT 1 Isomerism in Alkanes, aloalkanes, and Alkenes using Molecular Models Materials Needed - Molecular model kit Relevant Textbook Reading Denniston, chap 11.2-11.4, 12.1-12.3 Background In uncharged,
STRUCTURE, ISOMERISM AND NOMENCLATURE OF ORGANIC COMPOUNDS
 II STRUCTURE, ISOMERISM AND NOMENCLATURE OF ORGANIC COMPOUNDS I. OBJECTIVES AND BACKGROUND This exercise will give you an opportunity to experience the three-dimensional nature of molecules and to visualize
II STRUCTURE, ISOMERISM AND NOMENCLATURE OF ORGANIC COMPOUNDS I. OBJECTIVES AND BACKGROUND This exercise will give you an opportunity to experience the three-dimensional nature of molecules and to visualize
Straight. C C bonds are sp 3 hybridized. Butane, C 4 H 10 H 3 C
 Hydrocarbons Straight Chain Alkanes aren t Straight C C bonds are sp 3 hybridized Butane, C 4 H 10 Structural Shorthand Explicit hydrogens (those required to complete carbon s valence) are usually left
Hydrocarbons Straight Chain Alkanes aren t Straight C C bonds are sp 3 hybridized Butane, C 4 H 10 Structural Shorthand Explicit hydrogens (those required to complete carbon s valence) are usually left
Chemical Reactions of Unsaturated Compounds
 hemical Reactions of Unsaturated ompounds hemical Reactions of Alkenes hemical Reactions of Alkenes Structure of the Double Bond in Alkenes The two components of the double bond are pictured the same,
hemical Reactions of Unsaturated ompounds hemical Reactions of Alkenes hemical Reactions of Alkenes Structure of the Double Bond in Alkenes The two components of the double bond are pictured the same,
H H C C. Alkenes C n H 2n unsaturated hydrocarbons. C 2 H 4 ethylene. Functional group = carbon-carbon double bond
 Alkenes C n H 2n unsaturated hydrocarbons C 2 H 4 ethylene H H C C H H Functional group = carbon-carbon double bond sp 2 hybridization => flat, 120 o bond angles σ bond & π bond => H 2 C=CH 2 No rotation
Alkenes C n H 2n unsaturated hydrocarbons C 2 H 4 ethylene H H C C H H Functional group = carbon-carbon double bond sp 2 hybridization => flat, 120 o bond angles σ bond & π bond => H 2 C=CH 2 No rotation
ORGANIC CHEMISTRY- 1
 ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
Objectives. Organic molecules. Carbon. Hydrocarbon Properties. Organic Chemistry Introduction. Organic versus Hydrocarbon 1/1/17
 Objectives Organic Chemistry Introduction 8.1 To determine the properties of organic molecules and recognize a hydrocarbon. Use table P and Q to write structural and molecular formulas for hydrocarbons.
Objectives Organic Chemistry Introduction 8.1 To determine the properties of organic molecules and recognize a hydrocarbon. Use table P and Q to write structural and molecular formulas for hydrocarbons.
Chemistry 2.5 AS WORKBOOK. Working to Excellence Working to Excellence
 Chemistry 2.5 AS 91165 Demonstrate understanding of the properties of selected organic compounds WORKBOOK Working to Excellence Working to Excellence CONTENTS 1. Writing Excellence answers to Cis-Trans
Chemistry 2.5 AS 91165 Demonstrate understanding of the properties of selected organic compounds WORKBOOK Working to Excellence Working to Excellence CONTENTS 1. Writing Excellence answers to Cis-Trans
Ch 16 Electrophilic Aromatic Substitution
 Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Chapter 15 Answers to Questions
 Chapter 15 Answers to Questions 1. Structural formulas show all the covalent bonds between atoms. In condensed formulas, the lines depicting carbon-hydrogen bonds are removed. 2. Space-filling models provide
Chapter 15 Answers to Questions 1. Structural formulas show all the covalent bonds between atoms. In condensed formulas, the lines depicting carbon-hydrogen bonds are removed. 2. Space-filling models provide
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Unit 5: Organic Chemistry
 Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
Carbon Bonding Isomers Naming Reference Tables Functional Groups. Reactions
 arbon Bonding Isomers Naming Reference Tables Functional Groups 2 Reactions Not electrolytes; they do not generally conduct electricity. Low melting points; they are nonpolar with weak forces of attraction.
arbon Bonding Isomers Naming Reference Tables Functional Groups 2 Reactions Not electrolytes; they do not generally conduct electricity. Low melting points; they are nonpolar with weak forces of attraction.
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a
 Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Organic and Biochemical Molecules. 1. Compounds composed of carbon and hydrogen are called hydrocarbons.
 Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
MOLECULER MODELS/ISOMERS ORGANIC STRUCTURES AND NAMING
 REVISED 10/14 EMISTRY 1101L MOLEULER MODELS/ISOMERS ORGANI STRUTURES AND NAMING NOTE: This lab does not require safety glasses or lab coats. INTRODUTION Electron Dot Structures: Electron dot structures,
REVISED 10/14 EMISTRY 1101L MOLEULER MODELS/ISOMERS ORGANI STRUTURES AND NAMING NOTE: This lab does not require safety glasses or lab coats. INTRODUTION Electron Dot Structures: Electron dot structures,
Chapter 25 Organic and Biological Chemistry
 Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
ExA1. Unsaturated Hydrocarbons. Olefins. Experiment: Next Week. Structure Addition Reactions Mechanisms
 ExA1 Unsaturated Hydrocarbons Olefins Structure Addition Reactions Mechanisms Experiment: part A - Setup part B - Reaction part C - Isolation part D - Analysis Next Week 1 Alkenes & Alkynes Hydrocarbons
ExA1 Unsaturated Hydrocarbons Olefins Structure Addition Reactions Mechanisms Experiment: part A - Setup part B - Reaction part C - Isolation part D - Analysis Next Week 1 Alkenes & Alkynes Hydrocarbons
As time allows, additional practice questions for Exam 2 follow. This is an incomplete collection. Content & emphasis will vary.
 Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
1. Which of the following reactions would have the smallest energy of activation?.
 Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
2 ethane CH 3 CH 3. 3 propane CH 3 CH 2 CH 3
 #100 Notes Unit 12: Introduction to Organic and Biochemistry Ch. Organic/ Biochemistry I. Alkanes, C n H 2n+2 (saturated hydrocarbons: no C=C or C C) *always 4 bonds on carbon # Carbons parent chain name
#100 Notes Unit 12: Introduction to Organic and Biochemistry Ch. Organic/ Biochemistry I. Alkanes, C n H 2n+2 (saturated hydrocarbons: no C=C or C C) *always 4 bonds on carbon # Carbons parent chain name
Chapter 4. An Introduction to Organic Compounds
 Chapter 4 An Introduction to Organic Compounds Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line bond structure. 2. Understand and construct condensed
Chapter 4 An Introduction to Organic Compounds Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line bond structure. 2. Understand and construct condensed
BIOB111 - Tutorial activities for session 8
 BIOB111 - Tutorial activities for session 8 General topics for week 4 Session 8 Physical and chemical properties and examples of these functional groups (methyl, ethyl in the alkyl family, alkenes and
BIOB111 - Tutorial activities for session 8 General topics for week 4 Session 8 Physical and chemical properties and examples of these functional groups (methyl, ethyl in the alkyl family, alkenes and
Chem101 General Chemistry. Lecture 11 Unsaturated Hydrocarbons
 hem101 General hemistry Lecture 11 Unsaturated ydrocarbons Unsaturated ydrocarbons ontain one or more double or triple carbon-carbon bond. University of Wisconsin-Eau laire hem101 - Lecture 11 2 Unsaturated
hem101 General hemistry Lecture 11 Unsaturated ydrocarbons Unsaturated ydrocarbons ontain one or more double or triple carbon-carbon bond. University of Wisconsin-Eau laire hem101 - Lecture 11 2 Unsaturated
Chem 121 Winter 2016: Section 03, Sample Problems. Alkenes and Alkynes
 Chem 121 Winter 2016: Section 03, Sample Problems Alkenes and Alkynes Problems adapted from Chemistry - The Central Science, 3 rd edition. 24.2 Consider the hydrocarbon drawn below. (a) What is the hybridisation
Chem 121 Winter 2016: Section 03, Sample Problems Alkenes and Alkynes Problems adapted from Chemistry - The Central Science, 3 rd edition. 24.2 Consider the hydrocarbon drawn below. (a) What is the hybridisation
