Summary Sheets 8 B. Going for gold!
|
|
|
- Erica Dorsey
- 6 years ago
- Views:
Transcription
1 B Summary Sheets Going for gold! All living cells need to respire to release energy. Energy is needed by organisms to stay alive, to make new substances and to help them move. Respiration normally requires oxygen and so it is called aerobic (with air) respiration. It is a series of chemical reactions that can be shown by a word equation: B glucose + oxygen carbon dioxide + water Glucose and oxygen are the reactants. Carbon dioxide and water are the products. Energy is released but this is not a chemical substance. Glucose is supplied by the digestion of carbohydrates. It is absorbed into the blood by the small intestine and carried around the body dissolved in the plasma of the blood. The blood travels through blood vessels and is pumped by the heart. The heart and the blood vessels form the circulatory system. capillaries in lungs blood to the lungs blood from the lungs blood to the rest of the body blood from rest of body Valves stop the blood flowing in the wrong direction. heart chambers left side of heart right side of heart capillaries in small intestine capillaries in other parts of the body capillaries in leg The oxygen is absorbed from the air by the lungs. The lungs are part of the breathing system (or respiratory system). trachea (windpipe) flowing blood flowing blood Carbon dioxide goes into the alveolus to be breathed out. Oxygen from inhaled air enters the capillary. bronchus (plural = bronchi) air sac Each air sac contains a number of tiny pockets called alveoli (singular = alveolus). capillaries plasma red blood cell to the heart to be pumped around the body carbon dioxide oxygen Exploring Science edition 57 Pearson Education Limited 200
2 B Summary Sheets (continued) The alveoli give the lungs a large surface area so that oxygen can quickly diffuse from the air inside the lungs into the blood contained in capillaries. The walls of the alveoli and the walls of the capillaries are only one cell thick, which also makes it easy for oxygen to diffuse into the blood. The oxygen is then carried by the red blood cells to capillaries around the body. B Tissue fluid comes out of other capillaries around the body and bathes the tissues in the body. Tissue fluid contains oxygen and glucose. The cells take the oxygen and glucose that they need from the tissue fluid and put the carbon dioxide that is produced back into the tissue fluid. The tissue fluid soaks back into other capillaries and the carbon dioxide dissolves in the blood plasma. Waste products, like carbon dioxide dissolve in the tissue fluid and go back into the blood in another capillary. oxygen and glucose Tissue fluid, carrying oxygen and glucose,leaks out of the capillary. In the lungs the dissolved carbon dioxide diffuses out of respiration the blood and into the air in the lungs. That is why we breathe out (exhale) more carbon dioxide than we breathe in (inhale). The carbon dioxide is excreted by the lungs. Carbon dioxide can be tested for by using limewater, which turns from clear to cloudy. Oxygen diffusing into the blood and carbon dioxide diffusing out of the blood is called gas exchange. Cells around the body take the oxygen and food that they need from the tissue fluid. Composition of inhaled and exhaled air. Inhaled air Exhaled air nitrogen gas 7% 7% oxygen gas 21% 16% carbon dioxide gas 0.03% 4% water vapour variable more When you exercise, your breathing rate (number of breaths in one minute) and your pulse rate (number of heart beats in one minute) increase. This is because your cells need more oxygen and glucose for respiration. In some diseases (e.g. emphysema) or when there is little air (e.g. at the top of a mountain) the body cannot get enough oxygen. People in these situations often feel short of breath and tired. If too little oxygen gets to cells, the cells cannot release energy from food and so they die. Most organisms respire using oxygen. Fish and many water organisms have gills to take oxygen out of the water. Oxygen from the water diffuses into the leaves of underwater plants. Exploring Science edition 5 Pearson Education Limited 200
3 C Summary Sheets Microbes Microbes, or micro-organisms, can only be seen using a microscope. There are three main types of microbes: viruses, bacteria and fungi. The most common fungus microbes are yeasts. C Viruses are smaller than bacteria which are smaller than yeast Viruses are usually not considered to be living because they do not carry out any of the seven life processes for themselves. Bacteria and yeasts are important in making some foods and drinks. Yeasts are used to make bread dough rise. The cells use oxygen, from the air found in pockets in the dough, for aerobic respiration. This process produces carbon dioxide, which makes the bread rise. glucose + oxygen carbon dioxide + water Yeast cells are also used to make beer and wine. In this case there is no air and so the yeast cells use anaerobic respiration. When yeasts cells use anaerobic respiration it is called fermentation. The ethanol is a waste product of this reaction. glucose carbon dioxide + ethanol (a sugar) The number of an organism in an area is called a population. In good conditions (warm, moist, plenty of sugar) a population of yeast cells will grow rapidly. The population stops growing if something runs out (e.g. sugar). The thing that stops the population growing is called a limiting factor. the effect of a limiting factor Number of yeast cells Time Page 1 of 2 Exploring Science edition Pearson Education Limited 200
4 C Summary Sheets (continued) Diseases Some microbes cause infectious diseases (diseases that can be spread from person to person). The microbes are said to infect you. The effects the microbes have on your body are known as symptoms. A doctor observes symptoms to come up with a diagnosis. Microbes can be spread by the air, water, touch, food, animals and sex. Disease Microbe that causes it Symptoms How it is spread colds and flu virus sore throat, running nose, fever air food poisoning bacteria vomiting, diarrhoea food cholera bacteria vomiting, diarrhoea water athlete s foot fungus sore cracked skin between the toes touch C Some ways that diseases can be stopped from spreading are: making sure sewage is treated and disposed of properly adding chlorine to water to kill bacteria drying, freezing or refrigerating foods pasteurising milk using disinfectants, antiseptics and soaps immunising people with vaccines. Your body has natural defences to stop microbes getting in (e.g. skin, mucus in the trachea and nose, ciliated epithelial cells to sweep mucus away). Your body also has ways of destroying microbes. These include: a chemical in tears that kills some bacteria acid in the stomach that kills some bacteria white blood cells that engulf microbes other white blood cells that make antibodies to help destroy microbes. Babies do not have fully developed immune systems. Antibodies can pass through the placenta and are found in breast milk. These help the baby to fight infections. For many diseases, once you have had the disease (or been immunised) you will not get it again (e.g. chickenpox). This is because the antibodies against these microbes stay in the blood. Some diseases can be cured using antibiotics. These are medicines that kill off bacteria. Some bacteria, however, are unaffected by antibiotics they are resistant to them. Using too many antibiotics only leaves behind the resistant bacteria, which then cause diseases that are difficult to treat. Page 2 of 2 Exploring Science edition 9 Pearson Education Limited 200
5 J Summary Sheets Forces Balanced forces are forces that are the same size but work in opposite directions. If forces are balanced: J A car or motorbike uses the energy stored in fuel to move at a steady speed because it needs a force from the engine to balance the forces of air resistance and friction. a stationary object stays stationary; a moving object continues to move at the same speed. The amount of air resistance on something can be reduced by giving it a smooth, streamlined shape. The air resistance increases as the speed increases, so cars use up more fuel per mile when they are travelling fast. Air resistance is caused by air particles hitting the moving object. The particles transfer energy to the object, which is why objects moving through air can get hot. Pressure on solids Pressure is the amount of force pushing on a certain area. For a certain area, the bigger the force, the bigger the pressure. For a certain force, the bigger the area, the smaller the pressure. In this picture, the thumb is putting a force onto the head of the pin. The force is transferred to the point of the pin. The point has a very small area, so there is a very large pressure on the board, and the pin goes in. In this picture, the thumb is putting a force directly on the board. The area of the thumb is much larger than the area of the pin point, so there is only a small pressure on the board. The thumb does not go into the board. Sharp knife a small area giving a large pressure. Snow shoes a large area giving a small pressure. Magnets and electromagnets Magnetism is a non-contact force. A magnet does not have to be touching something to attract it. Magnets attract magnetic materials. Iron, nickel and cobalt are magnetic materials. Mixtures, like steel, that include a magnetic material will also be attracted to a magnet. Other metals, such as aluminium or copper, are not magnetic and will not be attracted to a magnet. The two ends of a bar magnet are called the north-seeking pole and the south-seeking pole, or north pole and south pole for short. A north pole and a south pole attract each other. Page 1 of 2 Exploring Science edition 24 Pearson Education Limited 200
6 J Summary Sheets (continued) Two north poles or two south poles repel each other. A bar magnet is a permanent magnet, because it is always magnetic. A wire with electricity flowing through it has a magnetic field around it. An electromagnet is a coil of wire with an electric current flowing through it. It is only magnetic while the current is flowing. You can make an electromagnet stronger by: J increasing the number of coils of wire increasing the size of the current (by increasing the voltage) using an iron core. Magnetic fields The space around a magnet where it has an effect is called its magnetic field. You can find the shape of the magnetic field using iron filings or using a plotting compass. S N The Earth has a magnetic field. A compass is a small magnet that will point towards the Earth s North pole. But magnetic materials placed near a compass can change the direction that the compass points towards. This is the shape of the magnetic fi eld of a bar magnet. The magnetic fi eld of an electro magnet is a similar shape. Levers Forces can be used to turn objects around pivots. A pivot is also known as a fulcrum. Levers work by magnifying the force that is put in (the effort) or they can make the load move further than the effort. The amount the force or distance is multiplied depends on the distances between the load and the pivot and the effort and the pivot. The hammer is acting as a force multiplier. force from han biceps muscle effort triceps muscle radius bone pivot The arm is acting as a distance multiplier. Page 2 of 2 Exploring Science edition 25 Pearson Education Limited 200
7 J Summary Sheets Magnets and electromagnets J Magnetism is a non-contact force. Magnets attract magnetic materials. Iron, nickel and cobalt are magnetic materials. Mixtures, like steel, that include a magnetic material will also be attracted to a magnet. Other metals, like aluminium, are not magnetic and will not be attracted to a magnet. Iron oxide is a compound that is a magnetic material. It is used to make video and music cassettes and computer discs. Magnetic materials can also block magnetism. You can make a magnet from a piece of iron or steel. Always stroke in the same direction. magnet magnetic material The two ends of a bar magnet are called the north seeking pole and the south seeking pole or north pole and south pole for short. A north pole and a south pole attract each other. Two north poles or two south poles will repel each other. The space around a magnet where it has an effect is called its magnetic field. S N This is the shape of the magnetic field of a bar magnet. You can find the shape of the magnetic field using iron filings or using a plotting compass. The Earth has a magnetic field. A compass is a small magnet that always points north. But magnetic materials placed near a compass can change the direction that it points. Magnets can be used to sort iron and aluminium cans for recycling. Only the iron cans are attracted to the magnet. Magnets can also be used for holding fridge doors shut, and in compasses that sailors or walkers use. A wire with electricity flowing through it has a magnetic field around it. An electromagnet is a coil of wire with an electric current flowing through it. Exploring Science for QCA Copymaster File Page 1 of Pearson Education Limited 2002
8 J Summary Sheets (continued) You can make an electromagnet stronger by: increasing the number of coils of wire increasing the size of the current (by increasing the voltage) using an iron core. J Electromagnets can be used for lifting things. They are also used in electric bells, relays and in video and music recording. Electromagnets are used to make bells work. cell pivot electromagnet gong springy metal contact armature A reed switch has two thin pieces of iron inside it. If a magnet is held near the switch, the pieces of iron are magnetised and touch each other. A reed switch can also be switched on using an electromagnet. Any switch that is worked by electricity is called a relay. Relays are used to make things safer. For example, the starter motor in a car uses a high current and needs thick wires for the current to flow through. A relay is used in a car so that the driver does not have to touch any part of the circuit that has a high current. contacts circuit 1 When current flows in circuit 1 the coil becomes an electromagnet. circuit 2 iron armature When the armature moves the iron connects the two contacts and electricity can flow in circuit 2. coil of wire The iron armature is attracted by the electromagnet. Exploring Science for QCA Copymaster File Page 2 of Pearson Education Limited 2002
9 F Summary Sheets Elements An element is a simple substance that cannot be split into anything simpler by chemical reactions. Atoms are the smallest particles of an element. Atoms of one element are all the same, and are different from atoms of all the other elements. All the elements are shown in the periodic table. There are 117 known elements. Each element has a chemical symbol, which is usually one or two letters. A symbol is written with the first letter as a capital, and the second letter lower case, for example F oxygen O carbon C iron Fe aluminium Al Compounds Elements can join together to make compounds. The name of the compound tells you the elements that are in it. Compounds made from two elements always have a name that ends in -ide. These elements join together carbon, oxygen sodium, chlorine magnesium, oxygen to make these compounds carbon dioxide sodium chloride magnesium oxide A chemical formula tells you the name and number of atoms in a compound. The smallest particle of many compounds is called a molecule. Molecules are made up of groups of atoms. Some elements are also made of molecules. For example, a molecule of oxygen contains two oxygen atoms joined together. The formula is O 2. Water contains two atoms of hydrogen and one of oxygen. The formula is H 2 O. Elements Compounds Mixtures atoms of helium (He) molecules of carbon dioxide a mixture of helium and oxygen (CO 2 ) molecules of oxygen (O 2 ) molecules of water (H 2 O) a mixture of carbon dioxide and oxygen a lump of iron (Fe) a lump of sodium chloride (NaCl) a lump of bronze (a mixture of copper and tin) Page 1 of 2 Exploring Science edition 175 Pearson Education Limited 200
10 F Summary Sheets (continued) Metals and non-metals The properties of a substance describe the way the substance behaves, or measurements that we can make on it. Metals and non-metals have different properties. F Metals good conductors of heat and electricity shiny solids with a high melting point (except for mercury) Mainly found on the left-hand side and in the centre of the periodic table three metals are magnetic flexible Non-Metals poor conductors of heat and electricity dull most are solids or gases found on the right-hand side of the periodic table no non-metals are magnetic brittle (break easily instead of bending) Re-use and recycling Materials can be classified according to their properties. All the different materials in the world are made up of about 90 different elements. Some useful materials occur naturally, but others have to be manufactured using chemical reactions. In many cases, raw materials are non-renewable. If we can recycle these materials, then we reduce the demand for raw materials. There may also be energy savings as well. A process that uses recycled or renewable materials is sustainable. Waste can also be reduced by re-using objects such as glass bottles or plastic bags. Page 2 of 2 Exploring Science edition 176 Pearson Education Limited 200
11 G Summary Sheets Compounds and mixtures Elements are simple substances that cannot be split up in chemical reactions. Atoms are the smallest particles of an element that can exist. Atoms in an element are all the same. Each element has its own chemical symbol. For example, the chemical symbol for oxygen is O. The symbols and names for all the known elements are shown in the periodic table. G Some elements have their atoms joined to each other in small groups called molecules. Oxygen is an example. Elements can join together to make compounds. A compound contains two or more elements joined together by bonds. The name of the compound tells you the elements that are in it. Compounds made from two elements always have a name that ends in -ide. an atom of oxygen O A molecule of oxygen consists of two oxygen atoms joined together. O another atom of oxygen Many compounds exist as atoms attached to each other in small groups molecules. H H O A molecule of water (hydrogen) A compound always contains the same elements in the same ratio. The chemical formula of a compound tells you the ratio of atoms of each element that are bonded together. For compounds that are molecules it tells you the numbers of atoms of each element in a compound. Each element in the chemical formula is shown by its chemical symbol. For example: The properties of a compound are different from the elements that make it up. For example, hydrogen is an explosive gas and oxygen will relight a glowing splint, but water is a liquid that will put fires out. the symbol for hydrogen 2 atoms of hydrogen H 2 O the symbol for oxygen No number after a symbol means that there is only one atom of that element. Chemical reactions Compounds can be made to react by mixing them with other chemicals, or by using heat or electricity. You can tell that a chemical reaction has occurred if there is a colour change or when a gas is given off. Page 1 of 2 Exploring Science edition 202 Pearson Education Limited 200
12 G Summary Sheets (continued) Most chemical reactions also involve an energy change. This is usually in the form of heat, for example in burning (combustion). In a chemical reaction a new substance is always formed. Most chemical reactions are not easily reversed (they are irreversible). Some chemical reactions take place just by mixing substances together. (e.g. when a solid (a precipitate) forms by mixing two liquids in a precipitation reaction). Other chemical reactions need energy to start them off. (e.g. when some compounds are broken up, decomposed, by heat). G Word equations show what happens in a chemical reaction. The chemicals that you start with are called the reactants. The chemicals at the end are called the products. For example: magnesium + oxygen magnesium oxide { reactants { product Physical changes melting evaporating In a physical change no new substance is formed and the changes are usually easily reversed. Melting, evaporating, condensing and freezing are all examples of physical changes. ice water steam Mixtures freezing condensing Elements and compounds can also be mixed together. A mixture is easier to separate than the elements in a compound. Soil, river water and sea water are examples of mixtures that occur naturally. A pure substance contains a single substance, element or compound, nothing else. Elements and compounds melt and boil at a fixed temperature. Mixtures do not have definite melting points and boiling points. Alloys Alloys are mixtures of metals with one or more other elements. Alloys have different properties from the pure metal. Pure gold is too soft for making jewellery. An alloy of gold mixed with other metals, like copper or silver, is used because it is stronger. The original method used to measure the purity of gold alloys was the carat system. One carat is 1 part in 24. So pure gold is 24 carats and 12 carat gold contains 50% gold. Page 2 of 2 Exploring Science edition 203 Pearson Education Limited 200
KS3 revision booklet chemistry
 NAME KS3 revision booklet chemistry Use this booklet to help you revise the chemistry you have studied in Key Stage 3. There are quizzes you can use to test yourself, and diagrams to remind you of key
NAME KS3 revision booklet chemistry Use this booklet to help you revise the chemistry you have studied in Key Stage 3. There are quizzes you can use to test yourself, and diagrams to remind you of key
ELEMENTS, MIXTURES AND COMPOUNDS
 Science Revision for Year 7 7C2 ELEMENTS, MIXTURES AND COMPOUNDS In this document you will find: a) a big ideas for the topic summary sheet b) a list of key words c) a ladder setting out the level standards
Science Revision for Year 7 7C2 ELEMENTS, MIXTURES AND COMPOUNDS In this document you will find: a) a big ideas for the topic summary sheet b) a list of key words c) a ladder setting out the level standards
KS3 Science Levelness Posters
 KS Science Levelness Posters Contents Year Year 8 Year 9 A. Cells 8A. Food and digestion 9A. Inheritance and selection B. Reproduction 8B. Respiration 9B. Fit and healthy C. Environment and feeding 8C.
KS Science Levelness Posters Contents Year Year 8 Year 9 A. Cells 8A. Food and digestion 9A. Inheritance and selection B. Reproduction 8B. Respiration 9B. Fit and healthy C. Environment and feeding 8C.
- know that in multi-cellular organisms cells are massed together to form tissues, and tissues can be massed together to form organs
 Science Age 12-13 (A) BIOLOGY: ORGANISMS, THEIR BEHAVIOUR AND THE ENVIRONMENT 1. Cells and their functions - know that in multi-cellular organisms cells are massed together to form tissues, and tissues
Science Age 12-13 (A) BIOLOGY: ORGANISMS, THEIR BEHAVIOUR AND THE ENVIRONMENT 1. Cells and their functions - know that in multi-cellular organisms cells are massed together to form tissues, and tissues
Modern Chemistry Chapter 1 Matter and Changes. Sections 2 & 3 Matter and Its Properties Elements
 Modern Chemistry Chapter 1 Matter and Changes Sections 2 & 3 Matter and Its Properties Elements 1 Chapter Vocabulary Mass Matter Atom Element Extensive property Intensive property Physical property Physical
Modern Chemistry Chapter 1 Matter and Changes Sections 2 & 3 Matter and Its Properties Elements 1 Chapter Vocabulary Mass Matter Atom Element Extensive property Intensive property Physical property Physical
Y8 Science Controlled Assessment Topics & Keywords
 Y8 Science Controlled Assessment Topics & Biology Respiration. Know that respiration in living organisms can be aerobic or anaerobic The word equation for aerobic respiration The process of anaerobic respiration
Y8 Science Controlled Assessment Topics & Biology Respiration. Know that respiration in living organisms can be aerobic or anaerobic The word equation for aerobic respiration The process of anaerobic respiration
MEG: I can display data in tables With help, stop some variables changing during investigations
 A Food, Glorious Food I can display data in tables With help, stop some variables changing during investigations I can explain why food manufacturers use health slogans I can use data from secondary sources
A Food, Glorious Food I can display data in tables With help, stop some variables changing during investigations I can explain why food manufacturers use health slogans I can use data from secondary sources
Main Topic Sub-topics Students should be able to R O G
 Second Year Science Syllabus Biology Revision Check-list Where is your learning at? Green: I know it all. Orange: I have some idea check the answers. Red: I need to start studying this section. Main Topic
Second Year Science Syllabus Biology Revision Check-list Where is your learning at? Green: I know it all. Orange: I have some idea check the answers. Red: I need to start studying this section. Main Topic
How to use this book. How the book is organised. Answering questions. Learning and using the terminology. Developing skills
 How to use this book Welcome to the beginning of your Human and Social Biology course! We hope that you really enjoy your course, and that this book will help you to understand your work, and to do well
How to use this book Welcome to the beginning of your Human and Social Biology course! We hope that you really enjoy your course, and that this book will help you to understand your work, and to do well
Magnets attract some metals but not others
 Electricity and Magnetism Junior Science Magnets attract some metals but not others Some objects attract iron and steel. They are called magnets. Magnetic materials have the ability to attract some materials
Electricity and Magnetism Junior Science Magnets attract some metals but not others Some objects attract iron and steel. They are called magnets. Magnetic materials have the ability to attract some materials
Elements,Compounds and Mixtures
 BASIC CONCEPTS: Elements,s and s 1. The smallest fundamental particle of an element that retains the chemical properties of the element is called an atom. 2. A pure substance that cannot be split up into
BASIC CONCEPTS: Elements,s and s 1. The smallest fundamental particle of an element that retains the chemical properties of the element is called an atom. 2. A pure substance that cannot be split up into
Chapter: Cell Processes
 Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
CLASS COPY Structure and Properties of Matter Parts of the atom
 CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
MATTER & ENERGY STUDY GUIDE. 9 Weeks Test Date: Parent Signature (BONUS!):
 Name: Pd: MATTER & ENERGY STUDY GUIDE 9 Weeks Test Date: Parent Signature (BONUS!): 6.5A MATTER Matter is anything that has mass and takes up space. Give EXAMPLES and NON-EXAMPLES of matter. (42) EXAMPLES
Name: Pd: MATTER & ENERGY STUDY GUIDE 9 Weeks Test Date: Parent Signature (BONUS!): 6.5A MATTER Matter is anything that has mass and takes up space. Give EXAMPLES and NON-EXAMPLES of matter. (42) EXAMPLES
1º ESO UNIT 4: Chemical and physical changes. Susana Morales Bernal
 1º ESO UNIT 4: Chemical and physical changes Objectives 1. To know the basic characteristics of chemical reactions. 2. To know the differences between physical changes and chemical changes. 3. To know
1º ESO UNIT 4: Chemical and physical changes Objectives 1. To know the basic characteristics of chemical reactions. 2. To know the differences between physical changes and chemical changes. 3. To know
CALIFORNIA STANDARDS TEST GRADE 5 SCIENCE (Blueprint adopted by the State Board of Education 10/02)
 CALIFORNIA STANDARDS TEST (Blueprint adopted by the State Board of Education 0/02) Physical Sciences 8 30 Physical Sciences Grade 5. Elements and their combinations account for all the varied types of
CALIFORNIA STANDARDS TEST (Blueprint adopted by the State Board of Education 0/02) Physical Sciences 8 30 Physical Sciences Grade 5. Elements and their combinations account for all the varied types of
Name: Chemistry Unit Review Science 9
 Name: Chemistry Unit Review Science 9 Do not forget to study for notes, assignments and quizzes! 1. Classify each of the following as a physical or a chemical change. a) Garbage rotting d) Digesting food
Name: Chemistry Unit Review Science 9 Do not forget to study for notes, assignments and quizzes! 1. Classify each of the following as a physical or a chemical change. a) Garbage rotting d) Digesting food
Section 1: Elements Pages 56-59
 Study Guide Chapter 3 Elements, Compounds, and Mixtures Section 1: Elements Pages 56-59 1. Which of the following processes is NOT a physical or chemical change? a. crushing b. weighing c. melting d. passing
Study Guide Chapter 3 Elements, Compounds, and Mixtures Section 1: Elements Pages 56-59 1. Which of the following processes is NOT a physical or chemical change? a. crushing b. weighing c. melting d. passing
Which farm is likely to have been using too much fertiliser on its land? farm C. farm B
 1 What is produced by anaerobic respiration in yeast? lactic acid carbon dioxide 2 What is the word equation for aerobic respiration in plants? carbon dioxide + water glucose + oxygen glucose + carbon
1 What is produced by anaerobic respiration in yeast? lactic acid carbon dioxide 2 What is the word equation for aerobic respiration in plants? carbon dioxide + water glucose + oxygen glucose + carbon
Vocabulary: Matter: has mass and takes up space (pure substances and mixtures) Pure Substances: composition definite, elements and compounds.
 Unit 2: Composition and Properties of Matter Review Elements, Compounds, Mixtures and Physical/Chemical Properties and Changes, Water Properties and Biogeochemical Cycles Vocabulary: Matter: has mass and
Unit 2: Composition and Properties of Matter Review Elements, Compounds, Mixtures and Physical/Chemical Properties and Changes, Water Properties and Biogeochemical Cycles Vocabulary: Matter: has mass and
3. Which of the following would create a chemical change when it is added to a glass of warm milk?
 Test Name: Science 8 Assessment 6 ReviewScan Test Code: 9668632 Date: Your Name: Teacher: Class Name: 1. Which step in these instructions for making scrambled eggs results in a chemical change? First,
Test Name: Science 8 Assessment 6 ReviewScan Test Code: 9668632 Date: Your Name: Teacher: Class Name: 1. Which step in these instructions for making scrambled eggs results in a chemical change? First,
Chapter 9 Practice Test
 Chapter 9 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which of the following describes a chemical reaction? a) A gas is given off when
Chapter 9 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which of the following describes a chemical reaction? a) A gas is given off when
Hazel Grove High School Year 10 Homework Book Term 2 Spring Focus Biology topic 8, Chemistry topic 7 and Physics topics 12/13.
 Hazel Grove High School Year 10 Homework Book Term 2 Spring Focus Biology topic 8, Chemistry topic 7 and Physics topics 12/13 Homework Score on Quiz Introduction to Science Homework Science Homework will
Hazel Grove High School Year 10 Homework Book Term 2 Spring Focus Biology topic 8, Chemistry topic 7 and Physics topics 12/13 Homework Score on Quiz Introduction to Science Homework Science Homework will
Year 8 Biology Knowledge Organiser Topic 1: Health and Lifestyle
 Year 8 Biology Knowledge Organiser Topic 1: Health and Lifestyle KPI 1: Describe the requirements for a healthy human diet. Kilojoules (kj) Deficiency Disease A unit used to measure energy in foods A disease
Year 8 Biology Knowledge Organiser Topic 1: Health and Lifestyle KPI 1: Describe the requirements for a healthy human diet. Kilojoules (kj) Deficiency Disease A unit used to measure energy in foods A disease
Chemistry Unit 1: Section1 - Elements, Compounds, & Mixtures
 Chemistry Unit 1: Section1 - Elements, Compounds, & Mixtures PURE SUBSTANCES A pure substance is called an element. An element is a pure substance because it cannot be separated into any other substances.
Chemistry Unit 1: Section1 - Elements, Compounds, & Mixtures PURE SUBSTANCES A pure substance is called an element. An element is a pure substance because it cannot be separated into any other substances.
3/1/2010. created by Ms Janelle Tay\2010. Learning Objectives
 1 Learning Objectives Define what elements, compounds and mixtures are. Give the names and symbols of common elements. State how elements are classified. State what the building block of an element is.
1 Learning Objectives Define what elements, compounds and mixtures are. Give the names and symbols of common elements. State how elements are classified. State what the building block of an element is.
In the event of snow:
 BIOLOGY Units Learning Objectives: Pupils should be taught (underlined = level 2 only) YEAR 7 Cells and Cell Functions 1a-e Nutrition and movement 2a-e Reproduction 2f-h Breathing and Respiration 2i-l
BIOLOGY Units Learning Objectives: Pupils should be taught (underlined = level 2 only) YEAR 7 Cells and Cell Functions 1a-e Nutrition and movement 2a-e Reproduction 2f-h Breathing and Respiration 2i-l
K-5 Physical Science Overview with Activities
 K-5 Physical Science Overview with Activities The physical science strand encourages the basic observations of what our physical reality is made of and how it interacts matter, energy, forces, atoms and
K-5 Physical Science Overview with Activities The physical science strand encourages the basic observations of what our physical reality is made of and how it interacts matter, energy, forces, atoms and
Elements and Reactivity Revision Notes
 Elements and Reactivity Revision Notes Elements There are just over 100 elements in the Periodic Table. Elements are made up of one type of atom. Every element has a name, atomic number and symbol. Element
Elements and Reactivity Revision Notes Elements There are just over 100 elements in the Periodic Table. Elements are made up of one type of atom. Every element has a name, atomic number and symbol. Element
Physical Sciences: Matter & Energy. What is physical science? A. Physical science is a field of science that studies matter and energy.
 Physical Sciences: Matter & Energy What is physical science? A. Physical science is a field of science that studies matter and energy. B. Physical science has 2 main branches: 1.PHYSICS: the study of how
Physical Sciences: Matter & Energy What is physical science? A. Physical science is a field of science that studies matter and energy. B. Physical science has 2 main branches: 1.PHYSICS: the study of how
Matter and Energy. Section 2.1 Chapter 2. Representations of Matter: Models and Symbols. Goal 1. Goal 2
 Section 2.1 Chapter 2 Matter and Energy Representations of Matter: Models and Symbols Goal 1 Goal 2 Identify and explain the difference among observations of matter at the macroscopic, microscopic, and
Section 2.1 Chapter 2 Matter and Energy Representations of Matter: Models and Symbols Goal 1 Goal 2 Identify and explain the difference among observations of matter at the macroscopic, microscopic, and
Science Grade 5 Chapter 5: Comparing Kinds of Matter Lesson2: Elements
 Element: is a material that cannot be broken down into anything simpler by chemical reactions. o There are 118 elements o Most elements are solids, some are gasses and few are liquid at room temperature
Element: is a material that cannot be broken down into anything simpler by chemical reactions. o There are 118 elements o Most elements are solids, some are gasses and few are liquid at room temperature
Classification of Matter. Elements, Compounds, Mixtures
 Classification of Matter Elements, Compounds, Mixtures Introducing Little Miss Element Hi! I am Little Miss Element I am PURE SUBSTANCE I cannot be broken down into any simpler substance by means of a
Classification of Matter Elements, Compounds, Mixtures Introducing Little Miss Element Hi! I am Little Miss Element I am PURE SUBSTANCE I cannot be broken down into any simpler substance by means of a
Foundation Tier 3 6 Test
 Foundation Tier 3 6 Test ame Class 1 Blood is pumped around the body by the heart. a Write the following in the order blood passes through them after leaving the heart. veins arteries capillaries First
Foundation Tier 3 6 Test ame Class 1 Blood is pumped around the body by the heart. a Write the following in the order blood passes through them after leaving the heart. veins arteries capillaries First
Answers to Review #1: Classification of Matter
 1. Definitions: Answers to Review #1: Classification of Matter a) Chemistry: The study of matter, its properties and its transformations (how it can change). b) Matter: Anything that has mass and volume
1. Definitions: Answers to Review #1: Classification of Matter a) Chemistry: The study of matter, its properties and its transformations (how it can change). b) Matter: Anything that has mass and volume
Science Is A Verb! Elementary. Part 2 LET S DO IT! ISBN
 LET S DO IT! Science Is A Verb! Elementary Part 2 ISBN 978-1-847003-58-4 Contents INTRODUCTION Lab Title What Happens When Steel Rusts? Students know that during chemical reactions the atoms in the reactants
LET S DO IT! Science Is A Verb! Elementary Part 2 ISBN 978-1-847003-58-4 Contents INTRODUCTION Lab Title What Happens When Steel Rusts? Students know that during chemical reactions the atoms in the reactants
Chapter 1. Matter. 1.1 What is Chemistry. 1.2 The Scientific Method:
 Chapter 1. Matter 1.1 What is Chemistry CHEMISTRY The study of the structure, composition, properties and reactions of matter and the energy changes associated with matter. In other words the study of
Chapter 1. Matter 1.1 What is Chemistry CHEMISTRY The study of the structure, composition, properties and reactions of matter and the energy changes associated with matter. In other words the study of
Chemistry Final Study Guide KEY. 3. Define physical changes. A change in any physical property of a substance, not in the substance itself.
 Chemistry Final Study Guide KEY Unit 2: Matter & Its Properties, Lesson 1: Physical and Chemical Properties & Changes 1. Define physical properties. The characteristics of a substance that can be observed
Chemistry Final Study Guide KEY Unit 2: Matter & Its Properties, Lesson 1: Physical and Chemical Properties & Changes 1. Define physical properties. The characteristics of a substance that can be observed
I. Tick ( ) the most appropriate answer. 1. Physical or chemical changes are a result of absorption of :
 4 CHANGES AROUND US I. Tick ( ) the most appropriate answer. 1. Physical or chemical changes are a result of absorption of : (a) heat energy only (b) light energy only (c) sound energy only (d) some kind
4 CHANGES AROUND US I. Tick ( ) the most appropriate answer. 1. Physical or chemical changes are a result of absorption of : (a) heat energy only (b) light energy only (c) sound energy only (d) some kind
Matter and Its Properties
 Section 2 4A, 4B, 4C, 4D Main Ideas Atoms are the building blocks of matter. All substances have characteristic properties. Matter can be a pure substance or a mixture. 4A differentiate between physical
Section 2 4A, 4B, 4C, 4D Main Ideas Atoms are the building blocks of matter. All substances have characteristic properties. Matter can be a pure substance or a mixture. 4A differentiate between physical
Electromagnetism Review Sheet
 Electromagnetism Review Sheet Electricity Atomic basics: Particle name Charge location protons electrons neutrons + in the nucleus - outside of the nucleus neutral in the nucleus What would happen if two
Electromagnetism Review Sheet Electricity Atomic basics: Particle name Charge location protons electrons neutrons + in the nucleus - outside of the nucleus neutral in the nucleus What would happen if two
WHAT IS CHEMISTRY? Chapter Preview Questions
 WHAT IS CHEMISTRY? 1. A piece of iron is made up of a. iron molecules. b. iron compounds. c. iron atoms. d. iron salts. 1 1. A piece of iron is made up of a. iron molecules. b. iron compounds. c. iron
WHAT IS CHEMISTRY? 1. A piece of iron is made up of a. iron molecules. b. iron compounds. c. iron atoms. d. iron salts. 1 1. A piece of iron is made up of a. iron molecules. b. iron compounds. c. iron
Changes in Matter. Introduction to Chemistry
 Changes in Matter Introduction to Chemistry Classifying Matter Matter: is anything that has mass and volume. Volume: the amount of space that something takes up Property: a characteristic of a material
Changes in Matter Introduction to Chemistry Classifying Matter Matter: is anything that has mass and volume. Volume: the amount of space that something takes up Property: a characteristic of a material
4 Energy and Rates of Chemical Reactions
 CHAPTER 14 4 and Rates of Chemical Reactions SECTION Chemical Reactions BEFORE YOU READ After you read this section, you should be able to answer these questions: How is energy involved in a chemical reaction?
CHAPTER 14 4 and Rates of Chemical Reactions SECTION Chemical Reactions BEFORE YOU READ After you read this section, you should be able to answer these questions: How is energy involved in a chemical reaction?
Science Year 10 Unit 1 Biology
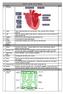 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key)
 The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key) The City School/ PAF Chapter/ Comprehensive Worksheet/ May 2016/ Science/ Class 7 /Ans Key Page 1 of 7 OBJECTIVE
The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key) The City School/ PAF Chapter/ Comprehensive Worksheet/ May 2016/ Science/ Class 7 /Ans Key Page 1 of 7 OBJECTIVE
STANDARD GRADE CHEMISTRY : GENERAL LEVEL
 STANDARD GRADE CHEMISTRY : GENERAL LEVEL NEED TO KNOW SHEETS (BASED ON 1998 2006 EXAMS) TOPIC NO 1 -ide means two elements only ate/-ite means two elements + oxygen a solution contains a solid (solute)
STANDARD GRADE CHEMISTRY : GENERAL LEVEL NEED TO KNOW SHEETS (BASED ON 1998 2006 EXAMS) TOPIC NO 1 -ide means two elements only ate/-ite means two elements + oxygen a solution contains a solid (solute)
Unit 1: Cells, Tissues, Organs, and Systems
 Unit 1: Cells, Tissues, Organs, and Systems Big Ideas The cell is the basic scientific unit of all living things. Cells must interact with the external environment to meet their basic needs. Your health
Unit 1: Cells, Tissues, Organs, and Systems Big Ideas The cell is the basic scientific unit of all living things. Cells must interact with the external environment to meet their basic needs. Your health
The Fundamental Ideas in Chemistry
 The Fundamental Ideas in Chemistry Question Paper Level Subject Exam Board Unit Topic Difficulty Level Booklet GCSE Chemistry AQA C1 The Fundamental Ideas in Chemistry Silver Level Question Paper Time
The Fundamental Ideas in Chemistry Question Paper Level Subject Exam Board Unit Topic Difficulty Level Booklet GCSE Chemistry AQA C1 The Fundamental Ideas in Chemistry Silver Level Question Paper Time
Chemistry Chapter 1 Test Review
 Chemistry Chapter 1 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A chemical can be defined as a. a toxic substance. b. an unnatural additive
Chemistry Chapter 1 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A chemical can be defined as a. a toxic substance. b. an unnatural additive
Cells the Building Blocks of Life. Cells the Building Blocks of Life. Cells the Building Blocks of Life. Cells the Building Blocks of Life
 1 What is gestation? 2 Give three ways in which alveoli are adapted for gas exchange. 3 Describe what happens to someone when they suffer from an asthma attack. 4 What is meant by the term antagonistic
1 What is gestation? 2 Give three ways in which alveoli are adapted for gas exchange. 3 Describe what happens to someone when they suffer from an asthma attack. 4 What is meant by the term antagonistic
Unit 2 Benchmark Review. Disease Review:
 Match the term with the definition: Unit 2 Benchmark Review Disease Review: 1. Caused by tiny organisms called pathogens B 2. This is responsible for distinguishing between the different kinds of pathogens
Match the term with the definition: Unit 2 Benchmark Review Disease Review: 1. Caused by tiny organisms called pathogens B 2. This is responsible for distinguishing between the different kinds of pathogens
LESSON 1: DESCRIBING MATTER pg.5. Chemistry = Is the study of matter & how matter changes. Liquid/Solid/Gas
 Chemistry..CHAPTER 1: INTRO TO MATTER LESSON 1: DESCRIBING MATTER pg.5 Chemistry = Is the study of matter & how matter changes A. Matter = anything that has mass & takes up space à You, air, plastic, metal,
Chemistry..CHAPTER 1: INTRO TO MATTER LESSON 1: DESCRIBING MATTER pg.5 Chemistry = Is the study of matter & how matter changes A. Matter = anything that has mass & takes up space à You, air, plastic, metal,
The Particulate Nature of Matter
 Matter Objectives Learn about the composition of matter. Learn the difference between elements and compounds. Learn to distinguish between physical and chemical properties and changes. Learn to distinguish
Matter Objectives Learn about the composition of matter. Learn the difference between elements and compounds. Learn to distinguish between physical and chemical properties and changes. Learn to distinguish
Chapter 2 Matter and Change. Charles Page High School Pre-AP Chemistry Stephen L. Cotton
 Chapter 2 Matter and Change 1 Charles Page High School Pre-AP Chemistry Stephen L. Cotton Section 2.1 Properties of Matter OBJECTIVES: Identify properties of matter as extensive or intensive. 2 Section
Chapter 2 Matter and Change 1 Charles Page High School Pre-AP Chemistry Stephen L. Cotton Section 2.1 Properties of Matter OBJECTIVES: Identify properties of matter as extensive or intensive. 2 Section
Matter: Properties and Changes. Chapter 3.1: Properties of Matter
 Matter: Properties and Changes Chapter 3.1: Properties of Matter Substances Review: Matter is anything that has mass and takes up space. Matter with uniform and unchanging composition is pure substance.
Matter: Properties and Changes Chapter 3.1: Properties of Matter Substances Review: Matter is anything that has mass and takes up space. Matter with uniform and unchanging composition is pure substance.
Chemical reactions. C2- Topic 5
 Chemical reactions C2- Topic 5 What is a chemical reaction? A chemical reaction is a change that takes place when one or more substances (called reactants) form one or more new substances (called products)
Chemical reactions C2- Topic 5 What is a chemical reaction? A chemical reaction is a change that takes place when one or more substances (called reactants) form one or more new substances (called products)
Class X. Exercises solution
 Exercises solution Question 1: Which of the statements about the reaction below are incorrect? Lead is getting reduced. Carbon dioxide is getting oxidised. Carbon is getting oxidised. Lead oxide is getting
Exercises solution Question 1: Which of the statements about the reaction below are incorrect? Lead is getting reduced. Carbon dioxide is getting oxidised. Carbon is getting oxidised. Lead oxide is getting
Indicate the answer choice that best completes the statement or answers the question.
 Indicate the answer choice that best completes the statement or answers the question. 1. Which of the following bonds is polar? a. F F b. O H c. O O d. H H 2. In the compound, H 2 O, the electrons in the
Indicate the answer choice that best completes the statement or answers the question. 1. Which of the following bonds is polar? a. F F b. O H c. O O d. H H 2. In the compound, H 2 O, the electrons in the
Chapter 1 Section 1- Pages 4-7: Electrons and Chemical Bonding COMBINING ATOMS THROUGH CHEMICAL BONDING
 Study Guide Chapter 1 and 2 Interactions of Matter Chapter 1 Section 1- Pages 4-7: Electrons and Chemical Bonding COMBINING ATOMS THROUGH CHEMICAL BONDING 1. Which of these substances is a combination
Study Guide Chapter 1 and 2 Interactions of Matter Chapter 1 Section 1- Pages 4-7: Electrons and Chemical Bonding COMBINING ATOMS THROUGH CHEMICAL BONDING 1. Which of these substances is a combination
Electricity. What is electricity?
 Words attract = pull towards an object back and forth = to go in one direction and then in the other balanced = the same as stable carbon = a chemical material that is in coal or petrol. It is in its purest
Words attract = pull towards an object back and forth = to go in one direction and then in the other balanced = the same as stable carbon = a chemical material that is in coal or petrol. It is in its purest
An acid made from sulphur, oxygen and hydrogen. A chemical with a ph value. of 8 or more.
 A with a ph value of 8 or more. An acid made from sulphur, oxygen and hydrogen. Alkali Sulfuric Acid Red and blue paper used to tell if a substance is an acid or alkali. Very reactive metal element. Half
A with a ph value of 8 or more. An acid made from sulphur, oxygen and hydrogen. Alkali Sulfuric Acid Red and blue paper used to tell if a substance is an acid or alkali. Very reactive metal element. Half
ì<(sk$m)=cdfged< +^-Ä-U-Ä-U
 Standards Preview Physical Sciences Standard Set 1. Physical Sciences 1. Elements and their combinations account for all the varied types of matter in the world. As a basis for understanding this concept:
Standards Preview Physical Sciences Standard Set 1. Physical Sciences 1. Elements and their combinations account for all the varied types of matter in the world. As a basis for understanding this concept:
Lesson (1) The cell The basic unit of structure and function
 Cairo Governorate Department : Science Nozha Directorate of Education Form : 4 th. Primary Nozha Language Schools Second Term Ismailia Road Branch Lesson (1) The cell The basic unit of structure and function
Cairo Governorate Department : Science Nozha Directorate of Education Form : 4 th. Primary Nozha Language Schools Second Term Ismailia Road Branch Lesson (1) The cell The basic unit of structure and function
Same theme covered in Combined but extra content Extra parts atomic symbols (first 20, Group 1 and Group 7)
 Co-teaching document new ELC Science 5960 and Foundation Level GCSE Combined Science: Trilogy (8464) Chemistry: Component 3 Elements, mixtures and compounds ELC Outcomes Summary of content covered in ELC
Co-teaching document new ELC Science 5960 and Foundation Level GCSE Combined Science: Trilogy (8464) Chemistry: Component 3 Elements, mixtures and compounds ELC Outcomes Summary of content covered in ELC
Cells Key Words. Task. Key words. Write a definition for each of the key words listed below. Microscope. Plant cell. Animal Cell.
 KS3 Science Cells Cells Key Words Task Write a definition for each of the key words listed below Key words Microscope Plant cell Animal Cell Nucleus Cell Membrane Cytoplasm Cell wall Chloroplasts Mitochondria
KS3 Science Cells Cells Key Words Task Write a definition for each of the key words listed below Key words Microscope Plant cell Animal Cell Nucleus Cell Membrane Cytoplasm Cell wall Chloroplasts Mitochondria
How to Use This Presentation
 How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
Year 7 - Cells Summary Notes
 Year 7 - Cells Summary Notes Life Processes All living things do all seven of the life processes. Things that are not living may do some but do not do all seven of the life processes. These are: Movement
Year 7 - Cells Summary Notes Life Processes All living things do all seven of the life processes. Things that are not living may do some but do not do all seven of the life processes. These are: Movement
Biology Unit 4 Energy and Life. 4:1 Energy All living things require a constant supply of ENERGY.
 Biology Unit 4 Energy and Life 4:1 Energy All living things require a constant supply of ENERGY. GLUCOSE: (C 6 H 12 O 6 ) the form of energy used for fuel by ALL living cells It requires energy to form
Biology Unit 4 Energy and Life 4:1 Energy All living things require a constant supply of ENERGY. GLUCOSE: (C 6 H 12 O 6 ) the form of energy used for fuel by ALL living cells It requires energy to form
CHEMISTRY. Everything is made of matter. Matter is composed of tiny particles called atoms.
 CHEMISTRY Everything is made of matter. Matter is composed of tiny particles called atoms. Everything in the world (every substance) is composed of one or more elements. Elements are pure substances, which
CHEMISTRY Everything is made of matter. Matter is composed of tiny particles called atoms. Everything in the world (every substance) is composed of one or more elements. Elements are pure substances, which
Volume. measures how much space matter takes up. solubility. The amount of mass for an object is called. matter
 measures how much space matter takes up. Volume is the resistance of an object to sink, and it depends on. The ability of a substance to dissolve into another substance is called. The amount of mass for
measures how much space matter takes up. Volume is the resistance of an object to sink, and it depends on. The ability of a substance to dissolve into another substance is called. The amount of mass for
Lesson 1: What are chemical changes?
 Lesson 1 Summary Lesson 1: What are chemical changes? Vocabulary Use with pp. 375 377 physical change a change in which the material keeps its identify chemical change a change in which one substance or
Lesson 1 Summary Lesson 1: What are chemical changes? Vocabulary Use with pp. 375 377 physical change a change in which the material keeps its identify chemical change a change in which one substance or
Website: Page 1. Page 14»Exercise» Page 15» Question 1:
 Page 14»Exercise» Question 1: Which of the statements about the reaction below are incorrect? (a) Lead is getting reduced. (b) Carbon dioxide is getting oxidised. (c) Carbon is getting oxidised. (d) Lead
Page 14»Exercise» Question 1: Which of the statements about the reaction below are incorrect? (a) Lead is getting reduced. (b) Carbon dioxide is getting oxidised. (c) Carbon is getting oxidised. (d) Lead
Q1. Methane and oxygen react together to produce carbon dioxide and water.
 Chemistry C3 Higher Questions Part 2 Q1. Methane and oxygen react together to produce carbon dioxide and water. The methane gas will not burn in oxygen until a flame is applied, but once lit it continues
Chemistry C3 Higher Questions Part 2 Q1. Methane and oxygen react together to produce carbon dioxide and water. The methane gas will not burn in oxygen until a flame is applied, but once lit it continues
Chapter 1. Matter. Table of Contents. 1. Matter 2. States of Matter 3. Classification of Matter 4. Properties of Matter 5. Separation of Mixtures
 Matter Table of Contents 1. Matter 2. States of Matter 3. Classification of Matter 4. Properties of Matter 5. Separation of Mixtures 1. Matter Warm up Look at the list of words below: peanut butter, water,
Matter Table of Contents 1. Matter 2. States of Matter 3. Classification of Matter 4. Properties of Matter 5. Separation of Mixtures 1. Matter Warm up Look at the list of words below: peanut butter, water,
CHAPTER 3: MATTER. Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64
 CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
1. C + O2 CO2 2. H2O2 H2 + O2. 3. acid + base salt + water 4. CH4 + O2 CO2 + H2O
 SNC 2P Final Exam- PRACTICE EXAM Part 2: Multiple Choice Portion 50 minutes NAME All questions must be answered on the Scantron card provided. You must fill in your answer using pencil. Identify the following
SNC 2P Final Exam- PRACTICE EXAM Part 2: Multiple Choice Portion 50 minutes NAME All questions must be answered on the Scantron card provided. You must fill in your answer using pencil. Identify the following
SCIENCE HIGHER LEVEL
 J.37 PRE-JUNIOR CERTIFICATE EXAMINATION, 2014 SCIENCE HIGHER LEVEL TIME: 2 HOURS INSTRUCTIONS 1. Write your name, school s name and teacher s name in the boxes provided on this page. 2. Answer all questions.
J.37 PRE-JUNIOR CERTIFICATE EXAMINATION, 2014 SCIENCE HIGHER LEVEL TIME: 2 HOURS INSTRUCTIONS 1. Write your name, school s name and teacher s name in the boxes provided on this page. 2. Answer all questions.
Objectives. Inertia. Is air matter? Is Light matter? Chapter 2. Chapter 2. Table of Contents. Chapter 2. Chapter 2. Section 1 What Is Matter?
 The Properties of Matter Section 1 What Is Matter? Table of Contents Section 1 What Is Matter? Section 2 Physical Properties Section 3 Chemical Properties Objectives Describe the two properties of all
The Properties of Matter Section 1 What Is Matter? Table of Contents Section 1 What Is Matter? Section 2 Physical Properties Section 3 Chemical Properties Objectives Describe the two properties of all
5. Researching the properties of particular materials and understand why they are used for particular products.
 1. The difference between a physical and chemical change. 2. How to identify whether a physical or chemical change has taken place. 3. Understand what reactants and products are. 4. Writing and understanding
1. The difference between a physical and chemical change. 2. How to identify whether a physical or chemical change has taken place. 3. Understand what reactants and products are. 4. Writing and understanding
ammonia carbon dioxide hydrogen nitrogen electrical heat solar sound (a) In air, the two most common gases are oxygen and...
 Chemistry C1 Foundation Questions Q1. Choose words from this list to complete the sentences, ammonia carbon dioxide hydrogen nitrogen electrical heat solar sound (a) In air, the two most common gases are
Chemistry C1 Foundation Questions Q1. Choose words from this list to complete the sentences, ammonia carbon dioxide hydrogen nitrogen electrical heat solar sound (a) In air, the two most common gases are
Year 8 Chemistry Knowledge Organiser Topic 1: Periodic Table
 KPI 1.1: Identify, with reasons, differences between atoms, elements and compounds Key Terms Element Mixture Compound Elements Definitions A substance that contains only one type of atom A substance that
KPI 1.1: Identify, with reasons, differences between atoms, elements and compounds Key Terms Element Mixture Compound Elements Definitions A substance that contains only one type of atom A substance that
MATTER: CLASSIFICATION AND PROPERTIES
 MATTER: CLASSIFICATION AND PROPERTIES Chemistry: Is the science concerned with the properties, composition and behaviour of matter. Matter: Anything that has mass and occupies space. (volume) (Matter is
MATTER: CLASSIFICATION AND PROPERTIES Chemistry: Is the science concerned with the properties, composition and behaviour of matter. Matter: Anything that has mass and occupies space. (volume) (Matter is
Classification of Matter
 Classification of Matter All the objects that we see in the world around us are made of matter. Matter makes up the air we breathe, the ground we walk on, the food we eat and the animals and plants that
Classification of Matter All the objects that we see in the world around us are made of matter. Matter makes up the air we breathe, the ground we walk on, the food we eat and the animals and plants that
Although they are composed of ions, ionic compounds are electrically neutral. Most ionic compounds are crystalline solids at room temperature.
 Key Concepts Although they are composed of ions, ionic compounds are electrically neutral. Most ionic compounds are crystalline solids at room temperature. Ionic compounds generally have high melting points.
Key Concepts Although they are composed of ions, ionic compounds are electrically neutral. Most ionic compounds are crystalline solids at room temperature. Ionic compounds generally have high melting points.
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
Chapter 3 Matter and Energy
 Introductory Chemistry, 3 rd Edition Nivaldo Tro Matter and Energy The chapter opening (page 52) showing a room and highlighting the structure of water and the carbon atoms in a graphite tennis racket
Introductory Chemistry, 3 rd Edition Nivaldo Tro Matter and Energy The chapter opening (page 52) showing a room and highlighting the structure of water and the carbon atoms in a graphite tennis racket
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0654 CO-ORDINATED SCIENCES 0654/23 Paper 2 (Core Theory), maximum
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0654 CO-ORDINATED SCIENCES 0654/23 Paper 2 (Core Theory), maximum
ESSENTIAL PHYSICAL SCIENCE VOCABULARY
 ESSENTIAL PHYSICAL SCIENCE VOCABULARY I. MATTER: ANYTHING THAT HAS MASS AND VOLUME A. mass 1. amount of matter in an object 2. measured in grams B. volume 1. amount of space 2. measured in Liters for liquid
ESSENTIAL PHYSICAL SCIENCE VOCABULARY I. MATTER: ANYTHING THAT HAS MASS AND VOLUME A. mass 1. amount of matter in an object 2. measured in grams B. volume 1. amount of space 2. measured in Liters for liquid
2B Air, Oxygen, Carbon Dioxide and Water
 Air, Oxygen, Carbon Dioxide and Water Air, oxygen and carbon dioxide are important chemicals in our everyday lives. Knowledge of their properties helps us to develop an understanding of the role they play.
Air, Oxygen, Carbon Dioxide and Water Air, oxygen and carbon dioxide are important chemicals in our everyday lives. Knowledge of their properties helps us to develop an understanding of the role they play.
Grade 9 Academic Homework Answers
 Grade 9 Academic Homework Answers From Particles to Solutions Lesson p. 178 # 1-6, 8, 10 1. All matter is composed of tiny particles separated by empty spaces. Different substances are made up of different
Grade 9 Academic Homework Answers From Particles to Solutions Lesson p. 178 # 1-6, 8, 10 1. All matter is composed of tiny particles separated by empty spaces. Different substances are made up of different
5129 COMBINED SCIENCE
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Ordinary Level MARK SCHEME for the October/November 2011 question paper for the guidance of teachers 5129 COMBINED SCIENCE 5129/21 Paper 2 (Theory),
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Ordinary Level MARK SCHEME for the October/November 2011 question paper for the guidance of teachers 5129 COMBINED SCIENCE 5129/21 Paper 2 (Theory),
Name Class Date. How do mixtures differ from elements and compounds? How can mixtures be separated? What are solutions?
 CHAPTER 3 3 Mixtures SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: How do mixtures differ from elements and compounds?
CHAPTER 3 3 Mixtures SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: How do mixtures differ from elements and compounds?
ST EDWARD S OXFORD. 14+ Entrance Assessment 2014 Science 1 hour. Candidate Name:.
 ST EDWARD S OXFORD 14+ Entrance Assessment 2014 Science 1 hour Candidate Name: PHYSICS 1 Electricity may be produced from a number of different energy resources (a) (i) Complete the table below The first
ST EDWARD S OXFORD 14+ Entrance Assessment 2014 Science 1 hour Candidate Name: PHYSICS 1 Electricity may be produced from a number of different energy resources (a) (i) Complete the table below The first
Midterm Study Guide Major Concepts
 Midterm Study Guide Name 7 th Grade PSI Major Concepts 1. What is an atom? 2. What is a molecule? 3. What is an element? 4. What is a compound? 5. What are physical properties? Describe a few examples.
Midterm Study Guide Name 7 th Grade PSI Major Concepts 1. What is an atom? 2. What is a molecule? 3. What is an element? 4. What is a compound? 5. What are physical properties? Describe a few examples.
Section 1: The Science of Energy¹
 SECTION1: THE SCIENCE OF ENERGY Section 1: The Science of Energy¹ What Is Energy? Energy is the ability to do work or the ability to make a change. Everything that happens in the world involves the exchange
SECTION1: THE SCIENCE OF ENERGY Section 1: The Science of Energy¹ What Is Energy? Energy is the ability to do work or the ability to make a change. Everything that happens in the world involves the exchange
BASIC CHEMISTRY Organisms and all other things in the universe consist of matter Matter: Elements and Compounds Matter is
 Chapter 2 Lecture Notes Essential Chemistry for Biology Biol 100 K. Marr 2009 Topics Discussed in these notes Matter, Elements and Compounds Periodic Table of the Elements: Metals vs. Nonmetals Atomic
Chapter 2 Lecture Notes Essential Chemistry for Biology Biol 100 K. Marr 2009 Topics Discussed in these notes Matter, Elements and Compounds Periodic Table of the Elements: Metals vs. Nonmetals Atomic
Downloaded from
 Science For Class IX Is Matter Around Us Pure (Q.1) Name the process which can be used to recover sugar from an aqueous sugar solution. (Q.2) What happens when a saturated solution is heated?
Science For Class IX Is Matter Around Us Pure (Q.1) Name the process which can be used to recover sugar from an aqueous sugar solution. (Q.2) What happens when a saturated solution is heated?
Mixtures 1 of 38 Boardworks Ltd 2016
 Mixtures 1 of 38 Boardworks Ltd 2016 Mixtures 2 of 38 Boardworks Ltd 2016 Pure and impure substances 3 of 38 Boardworks Ltd 2016 All materials can be classified as either a pure substance or an impure
Mixtures 1 of 38 Boardworks Ltd 2016 Mixtures 2 of 38 Boardworks Ltd 2016 Pure and impure substances 3 of 38 Boardworks Ltd 2016 All materials can be classified as either a pure substance or an impure
Properties of Matter
 Properties of Matter OBJECTIVES: Identify properties of matter as extensive or intensive. Define physical property, and list several common physical properties of substances. Differentiate among three
Properties of Matter OBJECTIVES: Identify properties of matter as extensive or intensive. Define physical property, and list several common physical properties of substances. Differentiate among three
