Hazel Grove High School Year 10 Homework Book Term 2 Spring Focus Biology topic 8, Chemistry topic 7 and Physics topics 12/13.
|
|
|
- Cecily Tate
- 5 years ago
- Views:
Transcription
1 Hazel Grove High School Year 10 Homework Book Term 2 Spring Focus Biology topic 8, Chemistry topic 7 and Physics topics 12/13 Homework Score on Quiz
2 Introduction to Science Homework Science Homework will be set every week by your class teacher. In week 1 you will complete part a and week 2 part b. a. Knowledge notes Homework Part a is the key notes and facts you need to learn for each specific topic. You need to ensure that you learn and remember the information, definitions, key ideas. You are to do this by using the notes to make revision card/mind maps/revision resource etc. that works for you. You will need to bring this resource into school to show your teacher. b. Questions Homework part b. These will be to assess whether you have remembered/learned the knowledge from the topic in section a. This is to be carried out in 2 sections. a. Firstly complete the questions in a blue/black pen. Answering all you can remember. b. Then take a second different coloured pen and use the information and revision guide to answer the questions again. Focusing on making sure you have used key words and definitions. These will also need to be handed in. Happy learning! The Science Department
3 Biology topic 8 Multicellular organisms need exchange surfaces as they have small surface area to volume ratios they can t get enough essentials so instead have an exchange surface so they can make exchange more efficient and effective e.g Gas exchange in the Alveoli The lungs job is to transfer oxygen to blood and remove carbon dioxide. This takes place in alveoli (air sacs.) The concentration gradient of the O 2 and CO 2 is high so diffusion is fast. They are an exchange surface and are adapted to this job: 1. Moist lining to dissolve gases 2. Good blood supply to maintain concentration gradient 3. Very thin for quick diffusion and 4. Large surface area
4 Blood contains Red blood cells (erythocrytes) carry oxygen. Has no nucleus for more room and a biconcave disc shape to fit more haemoglobin (red pigment) in. When oxygen binds to the haemoglobin called oxyhaemoglobin. In tissues opposite happens and splits up into the parts. White blood cells (lymphocytes and phagocytes) to fight disease - immune system Platelets clot. Stop bacteria entering wound and stop bleeding out. They are small fragments of cells with no nucleus. Plasma straw coloured liquid to carry red/white blood cells, platelets, glucose, amino acids, carbon dioxide, urea, hormones, proteins, antibodies and antitoxins Blood vessels Arteries carries blood AWAY from heart. They have thick walls, small lumen to withstand pressure as blood is pumped all over body as carries oxy-haemoglobin. Veins carries blood IN to heart. They have thinner walls and large lumen to carry oxygen back to heart. They contain VALVES which ensures that blood is carried in one direction Capillaries Arteries branch into capillaries. They are few cells thick and have permeable walls to allow diffusion and exchange materials and fit between cells to get blood closer to cells.
5 Cardiac output = heart rate x stroke volume cm 3 /min beats per min cm 3 The Heart The pump that contracts and relaxes 4 chambers, 2 sides, valves to ensure blood flows one direction Cardiac volume = total volume of blood pumped every minute. Stroke volume = volume of blood pumped by one ventricle each time it contracts. Specialist parts of the heart Valves prevent backflow - oxygen is always delivered to cells Try before you buy Left side of heart is thicker than right as higher pressure Aerobic Respiration making energy from the breakdown of organic compounds (usually glucose) at mitochondria with plenty of oxygen supply. (EXOthermic reaction) Route of blood from lungs to - LA - Los Angeles (via pulmonary artery) into LV Las Vegas then all over the Body RA Ryan air (via vena cava) finishing RV and to lungs. Glucose + oxygen carbon dioxide + water C 6 H 12 O O 2 6 CO H 2 O Anaerobic Respiration making energy without oxygen. Glucose lactic acid Anaerobic respiration is much less efficient. The glucose is only partially broken down and lactic acid is produced. When lactic builds up in the muscles it leads to painful cramp and enzymes not working correctly as the ph in your cells changes.
6 Investigating Respiration Using a RESPIROMETER and effect of temperature on rate Organisms use O 2 in respiration. You can measure O 2 used to calculate the organism s rate of respiration. Equipment organism (woodlice/maggots), soda lime (absorb CO 2 ), water bath (change temp), syringe (set level of manometer), manometer (tube containing coloured liquid and a scale to measure), cotton wool 1. Set up equipment as above. Make sure organisms aren t touching soda lime using cotton wool. 2. Glass beads with same mass as organism are placed in control tube 3. Use syringe to set liquid in manometer to a known level 4. Leave in water bath e.g 15⁰C for set time e.g 10mins. During this time total gas will decrease as O2 in by organism and CO2 absorbed by soda lime 5. Decrease in volume, decreased pressure and liquid in manometer moves towards tube containing organisms. Distance moved in a set time = rate of respiration (cm 3 /min) 6. Repeat these steps and change temp of water bath REMEMBER ETHICS
7 Biology topic 8 part b questions 1. Describe gas exchange in the alveoli. 8. Describe the function of each type of blood vessel. 2. Describe 3 ways that the alveoli are adapted for gas exchange. 9. Describe the flow of blood through the heart. 3. Write the definition for diffusion. 10. Explain the difference between the left and right sides of the heart. 4. What are the four components of the blood? 11. Compare aerobic and anaerobic respiration. 5. Describe the function of each component of the blood? 12. Describe a method for an investigation into respiration. 6. How are red blood cells and white blood cells adapted for their functions? 7. What are the four types of blood vessel?
8 Chemistry topic 7 Rate of reaction is how fast/slow a reaction happens Measured by 1. How quickly reactants are used up or 2. How quickly products are made Activation Energy Minimum energy required for a collision to be successful and break bonds. Catalyst Chemical that speeds up a chemical reaction without altering the products; unchanged chemically and in mass. They decrease activation energy and providing an alternative pathway See the reaction profile diagram Method 1 Precipitation 2 see through solutions which when mixed makes a precipitate (solid) which clouds solution. Can time how quickly cross disappears. E.g sodium thiosulphate & hydrochloric acid Method 2 Change in mass (balance) reaction that produces a gas. Mass will decrease as gas is given off. The quicker it decreases quicker the reaction. (note no bung) e.g HCl and marble chips Method 3 - Volume of gas made Gas syringe collects gas made over a set time interval. E.g HCl and marble chips
9 Graphs to show rate of reaction - Steeper gradient = faster reaction Gradient = Change in y / change in x How to speed up a Rate of reaction 1. Increase temp particles have more kinetic energy so have more successful collisions as collide more and more have above activation energy 2. Increase concentration (liquid)/ pressure (gas) more particles in same volume so more successful collisions 3. Increase surface area (solid) more particles exposed so more successful collisions 4. Add catalyst decreases activation energy To find gradient of a curve a tangent must be drawn Exothermic and endothermic reactions Exothermic reaction = energy given out to surroundings and thermometer goes up. Endothermic reaction = energy taken in from surroundings and thermometer goes down Bond breaking requires energy so is EXO Bond making releases energy so is ENDO In Endo reactions energy used to break bonds is greater than the energy released by forming them In Exo reactions energy released by forming bonds is greater than the energy used to break them
10 Measuring Temp changes Reaction Profiles Activation Energy = difference between reactants and highest point on curve Exothermic profile Endothermic profile 1. Set up equipment and Fill the beaker with cotton wool to help insulate so that less heat loss (with lid) to surroundings. 2. Add known volume of 1st reagent 3. Measure initial temp 4.Add measured mass/volume of 2 nd reagent 5. Measure final temperature and calc diff Bond Energy Calculations = every chem reaction has a bond energy. Energy change = energy to break bonds energy released by forming bonds + result = ENDO - Result = EXO UNIT = kj mol -1 Exo the products are at lower energy than the reactants Height of difference = energy given out Endo products are at higher energy than the reactants Height of difference = energy taken in
11 Chemistry topic 7 part b questions 1. Define a catalyst and give an example of where one could be used. 6. Explain (n terms of bond-making and bond-breaking) the difference between endothermic and exothermic reactions. 2. What is activation energy? 3. Describe 2 methods of how to investigate rates of reaction. 7. Give 2 examples of endothermic and exothermic reactions. 4. Describe how we use a graph to calculate the rate of a reaction. 8. Draw and label profiles for endothermic and exothermic reactions. 5. Describe 4 ways how to increase the rate of a reaction. 9. Describe how to calculate bond energy.
12 Physics topic 12/13 Magnets Bar Magnet = permanent magnet Magnet can attract magnetic materials INC because the space around it is a magnetic field. Shape of a magnetic field Compass can plot the shape (butterfly) Arrows are out of south and into north. Lines that are closer together = stronger magnetic field. Right hand Thumb Rule Point your thumb from + to the magnetic field goes in the direction your fingers are pointing. Induction When a piece of magnetic material is in a magnetic field it becomes a magnet itself = induced magnet Earth has a magnetic field due to the molten iron and nickel outer core evidence is compass Electromagnetism basics Current flowing through a wire causes a magnetic field. Strength of magnetic field depends upon current and distance from the wire. Turn the wire into a solenoid (coils) and you end up with a mag field: To increase the strength of an electromagnet 1. Increase number of coils, 2. Increase current 3. Add a temp magnet (iron core.) FORCE Any wire carrying a current can experience a force near a magnetic field around the wire.
13 Flemming s left hand rule When a wire is moved in the magnetic field of a generator, the movement, magnetic field and current are all at right angles to each other. If the wire is moved in the opposite direction, the induced current also moves in the opposite direction. The size of the induced voltage can be increased by: * rotating the coil or magnet faster * using a magnet with a stronger magnetic field * having more turns of wire in the coil * having an iron core inside the coil Tesla The size of the force on the wire depends upon the magnetic field strength, the current and the length of wire. The strength of the magnetic field is measured in Tesla (T) and is given the symbol B. Force on conductor carrying = magnetic flux density x current x length current at right angles F = B x I x L (N) (N/A m or T) (A) (m)
14 Power The energy transferred per second by an electrical current. National Grid = system of wires and cables called transmission lines. Power station generate electricity at 25kV. A step up transformer will increase to 400kV and a step down will decrease voltage to 230V to enter house. Power Power Equation - Same as Topic 10 = current potential difference Watts = Amps x Volts a.c Electricity Transformers only work with alternating current ( changes direction.) This is because the changing current causes the magnetic field to continuously change so the iron core carries this magnetic field to the secondary coil.
15 Physics topic 12/13 part b questions 1. How can we turn an iron nail into an induced magnet? 6. What is the function of aa transformer? 2. Compare the Earth s magnetic field to that of a bar magnet. 7. Explain how a step-up transformer increases the voltage. 3. Describe what an electromagnet is. 8. Describe the difference between a step up and a step down transformer. 4. Describe how to increase the strength of an electromagnet. 9. Describe how electricity is supplied around the country. 5. Explain Fleming s left hand role.
Science Year 10 Unit 1 Biology
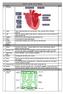 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Paget High School. Preparing for A level Biology
 Paget High School Preparing for A level Biology You will need a copy of the Head Start guide above and use it to make notes on the learning outcomes below. Topic areas Learning outcome Describe with the
Paget High School Preparing for A level Biology You will need a copy of the Head Start guide above and use it to make notes on the learning outcomes below. Topic areas Learning outcome Describe with the
B4 Organising animals and plants. Student Book answers. B4.1 The blood. Question Answer Marks Guidance
 B4. The blood Any three from: 3 transport of blood cells, transport of dissolved gases, transport of food, transport of hormones, removal of waste products, defence against infection, preventing blood
B4. The blood Any three from: 3 transport of blood cells, transport of dissolved gases, transport of food, transport of hormones, removal of waste products, defence against infection, preventing blood
4.7 Magnetism and electromagnetism
 4.7 Magnetism and electromagnetism Electromagnetic effects are used in a wide variety of devices. Engineers make use of the fact that a magnet moving in a coil can produce electric current and also that
4.7 Magnetism and electromagnetism Electromagnetic effects are used in a wide variety of devices. Engineers make use of the fact that a magnet moving in a coil can produce electric current and also that
4-4 Bioenergetics Biology
 4-4 Bioenergetics Biology.0 Figure shows a plant cell. Figure. Draw one line from each part of the cell to its function. [3 marks] Part of the cell Nucleus Chloroplast Mitochondria Function Where most
4-4 Bioenergetics Biology.0 Figure shows a plant cell. Figure. Draw one line from each part of the cell to its function. [3 marks] Part of the cell Nucleus Chloroplast Mitochondria Function Where most
N Goalby chemrevise.org
 4.6 Rate and Extent of Chemical Change Rates of Reaction The rate of a chemical reaction can be found by measuring the amount of a reactant used or the amount of product formed over time: Rate of reaction
4.6 Rate and Extent of Chemical Change Rates of Reaction The rate of a chemical reaction can be found by measuring the amount of a reactant used or the amount of product formed over time: Rate of reaction
3 Movement of substances in living organisms
 Mark schemes Movement of substances in living organisms Using and interpreting data a) (0 to 0 minutes) () decrease (in CO ) () carbon dioxide used in photosynthesis (0 to 0 minutes) () (steep) rise then
Mark schemes Movement of substances in living organisms Using and interpreting data a) (0 to 0 minutes) () decrease (in CO ) () carbon dioxide used in photosynthesis (0 to 0 minutes) () (steep) rise then
Rates. Specification points. Year 10 - Rates of Reaction
 Rates Specification points Year 10 - Rates of Reaction Calculating rates of reactions The rate of a chemical reaction can be found by measuring the quantity of a reactant used or the quantity of product
Rates Specification points Year 10 - Rates of Reaction Calculating rates of reactions The rate of a chemical reaction can be found by measuring the quantity of a reactant used or the quantity of product
Signs of Chemical Reactions
 Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Chemical reactions. C2- Topic 5
 Chemical reactions C2- Topic 5 What is a chemical reaction? A chemical reaction is a change that takes place when one or more substances (called reactants) form one or more new substances (called products)
Chemical reactions C2- Topic 5 What is a chemical reaction? A chemical reaction is a change that takes place when one or more substances (called reactants) form one or more new substances (called products)
Signs of Chemical Reactions
 Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
What does rate of reaction mean?
 1 of 39 2 of 39 What does rate of reaction mean? 3 of 39 The speed of different chemical reactions varies hugely. Some reactions are very fast and others are very slow. The speed of a reaction is called
1 of 39 2 of 39 What does rate of reaction mean? 3 of 39 The speed of different chemical reactions varies hugely. Some reactions are very fast and others are very slow. The speed of a reaction is called
Topic 2 notes Organisms and energy
 Topic 2 notes Organisms and energy AEROBIC RESPIRATION All cells in the body need energy - this energy is released in a process known as respiration Cells that are more active need more energy - e.g during
Topic 2 notes Organisms and energy AEROBIC RESPIRATION All cells in the body need energy - this energy is released in a process known as respiration Cells that are more active need more energy - e.g during
Y8 Science Controlled Assessment Topics & Keywords
 Y8 Science Controlled Assessment Topics & Biology Respiration. Know that respiration in living organisms can be aerobic or anaerobic The word equation for aerobic respiration The process of anaerobic respiration
Y8 Science Controlled Assessment Topics & Biology Respiration. Know that respiration in living organisms can be aerobic or anaerobic The word equation for aerobic respiration The process of anaerobic respiration
N Goalby chemrevise.org
 4.6 Rate and Extent of Chemical Change Rates of Reaction The rate of a chemical reaction can be found by measuring the amount of a reactant used or the amount of product formed over time: Rate of reaction
4.6 Rate and Extent of Chemical Change Rates of Reaction The rate of a chemical reaction can be found by measuring the amount of a reactant used or the amount of product formed over time: Rate of reaction
What does rate of reaction mean?
 Junior Science What does rate of reaction mean? It is not how much of a product is made, but instead how quickly a reaction takes place. The speed of a reaction is called the rate of the reaction. What
Junior Science What does rate of reaction mean? It is not how much of a product is made, but instead how quickly a reaction takes place. The speed of a reaction is called the rate of the reaction. What
Name: Rate of reaction. Class: Higher revision questions. Date: 57 minutes. Time: 56 marks. Marks: Comments: Page 1 of 24
 Rate of reaction Higher revision questions Name: Class: Date: Time: 57 minutes Marks: 56 marks Comments: Page of 24 A student investigated the rate of the reaction between magnesium and dilute hydrochloric
Rate of reaction Higher revision questions Name: Class: Date: Time: 57 minutes Marks: 56 marks Comments: Page of 24 A student investigated the rate of the reaction between magnesium and dilute hydrochloric
Metabolism and energy
 Metabolism and energy Metabolism: chemical reactions in cells Countless chemical reactions take place in cells and are responsible for all the actions of organisms. Together, these reactions make up an
Metabolism and energy Metabolism: chemical reactions in cells Countless chemical reactions take place in cells and are responsible for all the actions of organisms. Together, these reactions make up an
Figure 1. Oxygen. (g) +... (g)... SO 3. The pressure of the reacting gases was increased.
 Q1. Figure 1 represents a reaction in the production of sulfuric acid. Figure 1 Oxygen Sulfur dioxide Sulfur trioxide (a) Complete and balance the equation for the reaction.... SO 2 (g) +... (g)... SO
Q1. Figure 1 represents a reaction in the production of sulfuric acid. Figure 1 Oxygen Sulfur dioxide Sulfur trioxide (a) Complete and balance the equation for the reaction.... SO 2 (g) +... (g)... SO
Lesmahagow High School CfE Higher Chemistry. Chemical Changes & Structure Controlling the Rate
 Lesmahagow High School CfE Higher Chemistry Chemical Changes & Structure Controlling the Rate E a Page 1 of 18 Learning Outcomes Controlling the Rate Circle a face to show how much understanding you have
Lesmahagow High School CfE Higher Chemistry Chemical Changes & Structure Controlling the Rate E a Page 1 of 18 Learning Outcomes Controlling the Rate Circle a face to show how much understanding you have
A student investigated three glow sticks. One was placed in water at 5 C, one in water at 40 C and one in water at 70 C.
 1 The picture shows three glowsticks. Photograph supplied by istockphoto/thinktsock Glow sticks contain several chemicals. When a glow stick is bent the chemicals mix. A chemical reaction takes place which
1 The picture shows three glowsticks. Photograph supplied by istockphoto/thinktsock Glow sticks contain several chemicals. When a glow stick is bent the chemicals mix. A chemical reaction takes place which
C6 Quick Revision Questions
 C6 Quick Revision Questions H = Higher tier only All questions apply for combined and separate science Question 1... of 50 List 3 ways the time of a reaction can be measured. Answer 1... of 50 Loss of
C6 Quick Revision Questions H = Higher tier only All questions apply for combined and separate science Question 1... of 50 List 3 ways the time of a reaction can be measured. Answer 1... of 50 Loss of
4.7.1 Permanent and induced magnetism, magnetic forces and fields. Content Key opportunities for skills development
 4.7 Magnetism and electromagnetism Electromagnetic effects are used in a wide variety of devices. Engineers make use of the fact that a magnet moving in a coil can produce electric current and also that
4.7 Magnetism and electromagnetism Electromagnetic effects are used in a wide variety of devices. Engineers make use of the fact that a magnet moving in a coil can produce electric current and also that
Assessment Schedule 2017 Biology: Demonstrate understanding of life processes at the cellular level (91156)
 NCEA Level 2 Biology (91156) 2017 page 1 of 6 Assessment Schedule 2017 Biology: Demonstrate understanding of life processes at the cellular level (91156) Assessment Criteria Achievement Achievement with
NCEA Level 2 Biology (91156) 2017 page 1 of 6 Assessment Schedule 2017 Biology: Demonstrate understanding of life processes at the cellular level (91156) Assessment Criteria Achievement Achievement with
Enthalpy changes
 2.3.1. Enthalpy changes In an exothermic change energy is transferred from the system (chemicals) to the surroundings. The have less energy than the If an enthalpy change occurs then energy is transferred
2.3.1. Enthalpy changes In an exothermic change energy is transferred from the system (chemicals) to the surroundings. The have less energy than the If an enthalpy change occurs then energy is transferred
Name: Rate of reaction. Class: Foundation revision questions. Date: 47 minutes. Time: 46 marks. Marks: Comments: Page 1 of 21
 Rate of reaction Foundation revision questions Name: Class: Date: Time: 47 minutes Marks: 46 marks Comments: Page of 2 (a) The figure below represents the reaction of sulfur dioxide with oxygen. Oxygen
Rate of reaction Foundation revision questions Name: Class: Date: Time: 47 minutes Marks: 46 marks Comments: Page of 2 (a) The figure below represents the reaction of sulfur dioxide with oxygen. Oxygen
4 Energy and Rates of Chemical Reactions
 CHAPTER 14 4 and Rates of Chemical Reactions SECTION Chemical Reactions BEFORE YOU READ After you read this section, you should be able to answer these questions: How is energy involved in a chemical reaction?
CHAPTER 14 4 and Rates of Chemical Reactions SECTION Chemical Reactions BEFORE YOU READ After you read this section, you should be able to answer these questions: How is energy involved in a chemical reaction?
1.5 Kinetics. Reacting molecules have to collide with enough energy to break the initial bonds, the activation energy.
 1.5 Kinetics Collision theory: Reacting molecules have to collide with enough energy to break the initial bonds, the activation energy. Activation energy Activation energy The minimum amount of energy
1.5 Kinetics Collision theory: Reacting molecules have to collide with enough energy to break the initial bonds, the activation energy. Activation energy Activation energy The minimum amount of energy
Chapter Introduction Lesson 1 Understanding Chemical Reactions Lesson 2 Types of Chemical Reactions Lesson 3 Energy Changes and Chemical Reactions
 Chapter Introduction Lesson 1 Understanding Chemical Reactions Lesson 2 Types of Chemical Reactions Lesson 3 Energy Changes and Chemical Reactions Chapter Wrap-Up Changes in Matter A physical change does
Chapter Introduction Lesson 1 Understanding Chemical Reactions Lesson 2 Types of Chemical Reactions Lesson 3 Energy Changes and Chemical Reactions Chapter Wrap-Up Changes in Matter A physical change does
Which farm is likely to have been using too much fertiliser on its land? farm C. farm B
 1 What is produced by anaerobic respiration in yeast? lactic acid carbon dioxide 2 What is the word equation for aerobic respiration in plants? carbon dioxide + water glucose + oxygen glucose + carbon
1 What is produced by anaerobic respiration in yeast? lactic acid carbon dioxide 2 What is the word equation for aerobic respiration in plants? carbon dioxide + water glucose + oxygen glucose + carbon
Unit 13 Kinetics & Equilibrium Page 1 of 14 Chemistry Kinetics, Entropy, Equilibrium, LeChatelier s Principle, K, Unit 13 Quiz: Unit 13 Test:
 Unit 13 Kinetics & Equilibrium Page 1 of 14 Chemistry Kinetics, Entropy, Equilibrium, LeChatelier s Principle, K, Unit 13 Quiz: Unit 13 Test: Final Project: VOCABULARY: 1 Chemical equilibrium 2 equilibrium
Unit 13 Kinetics & Equilibrium Page 1 of 14 Chemistry Kinetics, Entropy, Equilibrium, LeChatelier s Principle, K, Unit 13 Quiz: Unit 13 Test: Final Project: VOCABULARY: 1 Chemical equilibrium 2 equilibrium
P7 MAGNETISM AND ELECTROMAGNETISM
 P7 MAGNETISM AND ELECTROMAGNETISM Question Practice Name: Class: Date: Time: 0 minutes Marks: 98 marks Comments: HIGHER TIER Page of 35 An electric current is a flow of electrical charge through a circuit.
P7 MAGNETISM AND ELECTROMAGNETISM Question Practice Name: Class: Date: Time: 0 minutes Marks: 98 marks Comments: HIGHER TIER Page of 35 An electric current is a flow of electrical charge through a circuit.
UNIT 4 INTRODUCTION TO PHYSICAL CHEMISTRY
 UNIT 4 INTRODUCTION TO PHYSICAL CHEMISTRY Student Version www.swotrevision.com www.chemguide.co.uk www.khanacademy.org Contents a) Energy Changes b) Rates of Reaction c) Equilibrium Key words: enthalpy,
UNIT 4 INTRODUCTION TO PHYSICAL CHEMISTRY Student Version www.swotrevision.com www.chemguide.co.uk www.khanacademy.org Contents a) Energy Changes b) Rates of Reaction c) Equilibrium Key words: enthalpy,
Chapter 6 and 7 Study Guide Reactions and Bonds
 Name_ Per. Block _ Multiple Choice: Chapter 6 and 7 Study Guide Reactions and Bonds 1. Copper is a good conductor of electricity because its electrons A. are positively charged B. are free to move and
Name_ Per. Block _ Multiple Choice: Chapter 6 and 7 Study Guide Reactions and Bonds 1. Copper is a good conductor of electricity because its electrons A. are positively charged B. are free to move and
Unit Packet Table of Contents Notes 1: Magnetism Intro Notes 2: Electromagnets Notes 3: Electromagnetic Induction Guided Practice: Left Hand Rule #3
 Unit Packet Table of Contents Notes 1: Magnetism Intro Notes 2: Electromagnets Notes 3: Electromagnetic Induction Guided Practice: Left Hand Rule #3 Name Date Notes: Magnetism intro. Regents Physics Objectives:
Unit Packet Table of Contents Notes 1: Magnetism Intro Notes 2: Electromagnets Notes 3: Electromagnetic Induction Guided Practice: Left Hand Rule #3 Name Date Notes: Magnetism intro. Regents Physics Objectives:
Reactions Rates
 3.2.2. Reactions Rates Collision theory Reactions can only occur when collisions take place between particles having sufficient energy. The energy is usually needed to break the relevant bonds in one or
3.2.2. Reactions Rates Collision theory Reactions can only occur when collisions take place between particles having sufficient energy. The energy is usually needed to break the relevant bonds in one or
Chapter 17: Chemical Reactions
 Chapter 17: Chemical Reactions Section 1: Chemical Formulas and Equations Chemical Equations Balancing Chemical Equations Law of Conservation of Mass Evidences of Chemical Reactions Symbols to represent
Chapter 17: Chemical Reactions Section 1: Chemical Formulas and Equations Chemical Equations Balancing Chemical Equations Law of Conservation of Mass Evidences of Chemical Reactions Symbols to represent
Unit 6 Kinetics and Equilibrium.docx
 6-1 Unit 6 Kinetics and Equilibrium At the end of this unit, you ll be familiar with the following: Kinetics: Reaction Rate Collision Theory Reaction Mechanism Factors Affecting Rate of Reaction: o Nature
6-1 Unit 6 Kinetics and Equilibrium At the end of this unit, you ll be familiar with the following: Kinetics: Reaction Rate Collision Theory Reaction Mechanism Factors Affecting Rate of Reaction: o Nature
3.2.2 Kinetics. Effect of Concentration. 135 minutes. 134 marks. Page 1 of 13
 3.. Kinetics Effect of Concentration 35 minutes 34 marks Page of 3 M. (a) Activation energy;- The minimum energy needed for a reaction to occur / start () Catalyst effect:- Alternative route (or more molecules
3.. Kinetics Effect of Concentration 35 minutes 34 marks Page of 3 M. (a) Activation energy;- The minimum energy needed for a reaction to occur / start () Catalyst effect:- Alternative route (or more molecules
2-4 Chemical Reactions and Enzymes
 2-4 Chemical Reactions and Enzymes Living things, as you have seen, are made up of chemical compounds-some simple and some complex. But chemistry isn t just what life is made of-chemistry is also what
2-4 Chemical Reactions and Enzymes Living things, as you have seen, are made up of chemical compounds-some simple and some complex. But chemistry isn t just what life is made of-chemistry is also what
2 4 Chemical Reactions
 SECTION 2 4 Chemical Reactions ^^^^^ ^^^ ^^ tff'\f f**^*.tffn*f i***i i i «i f.' KEY CONCEPT Life depends on chemical reactions. Student text pages 50-53 Bonds break and form during chemical reactions.
SECTION 2 4 Chemical Reactions ^^^^^ ^^^ ^^ tff'\f f**^*.tffn*f i***i i i «i f.' KEY CONCEPT Life depends on chemical reactions. Student text pages 50-53 Bonds break and form during chemical reactions.
GCSE COMBINED SCIENCE: SYNERGY
 GCSE COMBINED SCIENCE: SYNERGY Foundation Tier Paper 4F F Specimen 2018 Time allowed: 1 hour 45 minutes Materials For this paper you must have: a ruler a calculator the periodic table (enclosed) the Physics
GCSE COMBINED SCIENCE: SYNERGY Foundation Tier Paper 4F F Specimen 2018 Time allowed: 1 hour 45 minutes Materials For this paper you must have: a ruler a calculator the periodic table (enclosed) the Physics
Unit 2: Cellular Chemistry, Structure, and Physiology Module 4: Cellular Physiology
 Unit 2: Cellular Chemistry, Structure, and Physiology Module 4: Cellular Physiology NC Essential Standard: 1.2.1 Explain how homeostasis is maintained in a cell and within an organism in various environments
Unit 2: Cellular Chemistry, Structure, and Physiology Module 4: Cellular Physiology NC Essential Standard: 1.2.1 Explain how homeostasis is maintained in a cell and within an organism in various environments
OCR (A) Biology A-level
 OCR (A) Biology A-level Module 1: Development of practical skills in Biology PAG 11: Investigation Into The Measurement of Plant or Animal Responses Please note: You only need to do one from each PAG,
OCR (A) Biology A-level Module 1: Development of practical skills in Biology PAG 11: Investigation Into The Measurement of Plant or Animal Responses Please note: You only need to do one from each PAG,
4.7 Magnetism and electromagnetism
 4.7 Magnetism and electromagnetism Electromagnetic effects are used in a wide variety of devices. Engineers make use of the fact that a magnet moving in a coil can produce electric current and also that
4.7 Magnetism and electromagnetism Electromagnetic effects are used in a wide variety of devices. Engineers make use of the fact that a magnet moving in a coil can produce electric current and also that
Since reactions want to minimize energy you would think that the reaction would be spontaneous like a ball rolling down a hill
 Notes 1.1 Exothermic reactions give off heat 120 100 80 60 40 20 0 0 2 4 6 Heat Content Since reactions want to minimize energy you would think that the reaction would be spontaneous like a ball rolling
Notes 1.1 Exothermic reactions give off heat 120 100 80 60 40 20 0 0 2 4 6 Heat Content Since reactions want to minimize energy you would think that the reaction would be spontaneous like a ball rolling
How fast or slow will a reaction be? How can the reaction rate may be changed?
 Part I. 1.1 Introduction to Chemical Kinetics How fast or slow will a reaction be? How can the reaction rate may be changed? *In order to understand how these factors affect reaction rates, you will also
Part I. 1.1 Introduction to Chemical Kinetics How fast or slow will a reaction be? How can the reaction rate may be changed? *In order to understand how these factors affect reaction rates, you will also
Biology Chapter 2: The Chemistry of Life. title 4 pictures, with color (black and white don t count!)
 33 Biology Chapter 2: The Chemistry of Life title 4 pictures, with color (black and white don t count!) 34 Chapter 2: The Chemistry of Life Goals Highlight all unknown words 35-36 Chapter 2: The Chemistry
33 Biology Chapter 2: The Chemistry of Life title 4 pictures, with color (black and white don t count!) 34 Chapter 2: The Chemistry of Life Goals Highlight all unknown words 35-36 Chapter 2: The Chemistry
DO NOT WRITE ON THIS TEST Topic 3- Cells and Transport
 Topic 3- Cells and Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma membranes D) All cells can
Topic 3- Cells and Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma membranes D) All cells can
1. Gas Reactions Page 4 2. Measuring the Speed Page 6 3. Increasing the Speed Page Making Foam Page Putting Out a Fire Page 18
 P & L Johnson 2012 A foam fire extinguisher puts out fires by producing a blanket of foam that contains carbon dioxide rather than air. This smothers the fire preventing oxygen getting to the fuel. In
P & L Johnson 2012 A foam fire extinguisher puts out fires by producing a blanket of foam that contains carbon dioxide rather than air. This smothers the fire preventing oxygen getting to the fuel. In
Atoms. - Proton - Neutron. - Electron
 Chemistry of Life Atoms - The basic unit of matter is called an Atom - Atoms are incredibly small, but despite its extremely small size, an atom contains subatomic particles that are even smaller - Three
Chemistry of Life Atoms - The basic unit of matter is called an Atom - Atoms are incredibly small, but despite its extremely small size, an atom contains subatomic particles that are even smaller - Three
A-Level Biology Bridging Unit
 A-Level Biology Bridging Unit Welcome to Biology at A-Level! You are about to embark on a learning journey involving many different aspects of Biology! Studying at A-Level is a challenge, but it also gives
A-Level Biology Bridging Unit Welcome to Biology at A-Level! You are about to embark on a learning journey involving many different aspects of Biology! Studying at A-Level is a challenge, but it also gives
CELL PRACTICE TEST
 Name: Date: 1. As a human red blood cell matures, it loses its nucleus. As a result of this loss, a mature red blood cell lacks the ability to (1) take in material from the blood (2) release hormones to
Name: Date: 1. As a human red blood cell matures, it loses its nucleus. As a result of this loss, a mature red blood cell lacks the ability to (1) take in material from the blood (2) release hormones to
CHAPTER 9: Rate of Reaction
 CHAPTER 9: Rate of Reaction 9.1 Rate of Reaction 9.2 Factors Affecting Rate of Reaction 9.3 Catalysis Learning outcomes: (a) explain and use the terms: rate of reaction, activation energy and catalysis.
CHAPTER 9: Rate of Reaction 9.1 Rate of Reaction 9.2 Factors Affecting Rate of Reaction 9.3 Catalysis Learning outcomes: (a) explain and use the terms: rate of reaction, activation energy and catalysis.
Changing Reaction Rates
 Changing Reaction Rates 1 of 30 Boardworks Ltd 2016 Changing Reaction Rates 2 of 30 Boardworks Ltd 2016 Rates of reaction 3 of 30 Boardworks Ltd 2016 Why are some reactions faster than others? Reactions,
Changing Reaction Rates 1 of 30 Boardworks Ltd 2016 Changing Reaction Rates 2 of 30 Boardworks Ltd 2016 Rates of reaction 3 of 30 Boardworks Ltd 2016 Why are some reactions faster than others? Reactions,
8 th Grade Science. Directed Reading Packet. Chemistry. Name: Teacher: Period:
 8 th Grade Science Directed Reading Packet Chemistry Name: Teacher: Period: Chapter 1, Section 1: Inside the Atom Introduction 1. Atoms are the particles of an element that still have the element s. 2.
8 th Grade Science Directed Reading Packet Chemistry Name: Teacher: Period: Chapter 1, Section 1: Inside the Atom Introduction 1. Atoms are the particles of an element that still have the element s. 2.
CHAPTER 8 CHEMICAL REACTIONS AND EQUATIONS
 CHAPTER 8 CHEMICAL REACTIONS AND EQUATIONS CHEMICAL REACTIONS Occurs when matter combines or breaks apart to produce new kinds of matter with different properties with a change in energy. EVIDENCE FOR
CHAPTER 8 CHEMICAL REACTIONS AND EQUATIONS CHEMICAL REACTIONS Occurs when matter combines or breaks apart to produce new kinds of matter with different properties with a change in energy. EVIDENCE FOR
EB Education Revision Guide. How to work with Magnetism and Electromagnetism
 EB Education Revision Guide How to work with Magnetism and Electromagnetism Magnets Magnetic fields Magnets have two poles, north and south. They produce a magnetic field, this is a region where other
EB Education Revision Guide How to work with Magnetism and Electromagnetism Magnets Magnetic fields Magnets have two poles, north and south. They produce a magnetic field, this is a region where other
Main Topic Sub-topics Students should be able to R O G
 Second Year Science Syllabus Biology Revision Check-list Where is your learning at? Green: I know it all. Orange: I have some idea check the answers. Red: I need to start studying this section. Main Topic
Second Year Science Syllabus Biology Revision Check-list Where is your learning at? Green: I know it all. Orange: I have some idea check the answers. Red: I need to start studying this section. Main Topic
Name: Photosynthesis. Class: Date: 76 minutes. Time: 76 marks. Marks: level 1, 2 and 3. Increasing demand. Comments:
 Photosynthesis Name: Class: Date: Time: 76 minutes Marks: 76 marks Comments: level, 2 and 3. Increasing demand Q. Complete the word equation for photosynthesis. carbon dioxide + water energy glucose +
Photosynthesis Name: Class: Date: Time: 76 minutes Marks: 76 marks Comments: level, 2 and 3. Increasing demand Q. Complete the word equation for photosynthesis. carbon dioxide + water energy glucose +
Animal structure and function
 Animal structure and function The nervous system Parts of the nervous system 43C, 44B, 45D Brain structure and function Eyes Retina Neurons: How neurons communicate: Resting potential: The resting
Animal structure and function The nervous system Parts of the nervous system 43C, 44B, 45D Brain structure and function Eyes Retina Neurons: How neurons communicate: Resting potential: The resting
2. Name the part of the brain which controls posture and balance of the body?
 . Give two characteristics of magnetic field lines. SECTION-A 2. Name the part of the brain which controls posture and balance of the body?. Mention two different ways of harnessing energy from ocean.
. Give two characteristics of magnetic field lines. SECTION-A 2. Name the part of the brain which controls posture and balance of the body?. Mention two different ways of harnessing energy from ocean.
Science Home Learning Task. Year 9. GCSE Cell structure and transport
 Science Home Learning Task Year 9 GCSE Cell structure and transport Name Tutor Group Teacher Given out: Monday 23 April Hand in: Monday 30 April Parent/Carer Comment Staff Comment GCSE level Target Investigating
Science Home Learning Task Year 9 GCSE Cell structure and transport Name Tutor Group Teacher Given out: Monday 23 April Hand in: Monday 30 April Parent/Carer Comment Staff Comment GCSE level Target Investigating
2803/01 Transport January 2005 Mark Scheme
 2803/01 Transport January 2005 ADVICE TO EXAMINERS ON THE ANNOTATION OF SCRIPTS 1. Please ensure that you use the final version of the. You are advised to destroy all draft versions. 2. Please mark all
2803/01 Transport January 2005 ADVICE TO EXAMINERS ON THE ANNOTATION OF SCRIPTS 1. Please ensure that you use the final version of the. You are advised to destroy all draft versions. 2. Please mark all
GCSE COMBINED SCIENCE: SYNERGY
 GCSE COMBINED SCIENCE: SYNERGY Foundation Tier Paper 3F F Specimen 2018 Time allowed: 1 hour 45 minutes Materials For this paper you must have: a ruler a calculator the periodic table (enclosed) the Physics
GCSE COMBINED SCIENCE: SYNERGY Foundation Tier Paper 3F F Specimen 2018 Time allowed: 1 hour 45 minutes Materials For this paper you must have: a ruler a calculator the periodic table (enclosed) the Physics
Topic C6 Chemical Synthesis Homework booklet Graph paper required for homework two Name Key terms and spellings on back page
 Topic C6 Chemical Synthesis Homework booklet Graph paper required for homework two Name Key terms and spellings on back page Due Date Teacher Comment Homework 1 Homework 2 Homework 3 Homework 4 Homework
Topic C6 Chemical Synthesis Homework booklet Graph paper required for homework two Name Key terms and spellings on back page Due Date Teacher Comment Homework 1 Homework 2 Homework 3 Homework 4 Homework
Page 2. Q1.Marble chips are mainly calcium carbonate (CaCO 3 ).
 Q1.Marble chips are mainly calcium carbonate (CaCO 3 ). A student investigated the rate of reaction between marble chips and hydrochloric acid (HCl). Figure 1 shows the apparatus the student used. Figure
Q1.Marble chips are mainly calcium carbonate (CaCO 3 ). A student investigated the rate of reaction between marble chips and hydrochloric acid (HCl). Figure 1 shows the apparatus the student used. Figure
6.1 Collision Theory & Rates of Reaction IB SL CHEMISTRY MRS. PAGE
 6.1 Collision Theory & Rates of Reaction IB SL CHEMISTRY MRS. PAGE Understandings: Species react as a result of collisions of sufficient energy and proper orientation. The rate of reaction is expressed
6.1 Collision Theory & Rates of Reaction IB SL CHEMISTRY MRS. PAGE Understandings: Species react as a result of collisions of sufficient energy and proper orientation. The rate of reaction is expressed
Energetics and Rates
 Energetics and Rates Specification points Year 0 Energetics Energy transfer during exothermic and endothermic reactions - know that an exothermic reaction transfers energy to the surroundings so the surrounding
Energetics and Rates Specification points Year 0 Energetics Energy transfer during exothermic and endothermic reactions - know that an exothermic reaction transfers energy to the surroundings so the surrounding
Kinetics & Equilibrium
 Kinetics & Equilibrium Name: Essential Questions How can one explain the structure, properties, and interactions of matter? Learning Objectives Explain Collision Theory Molecules must collide in order
Kinetics & Equilibrium Name: Essential Questions How can one explain the structure, properties, and interactions of matter? Learning Objectives Explain Collision Theory Molecules must collide in order
Additional Science AS2HP. (Jun15AS2HP01) General Certificate of Secondary Education Higher Tier June Unit 6. Time allowed 1 hour 30 minutes
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark Additional Science General Certificate of Secondary Education Higher Tier June
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark Additional Science General Certificate of Secondary Education Higher Tier June
Surname. Other Names. Candidate Signature
 A Surname Other Names Centre Number For Examiner s Use Candidate Number Candidate Signature Additional Science Unit 6 AS2HP General Certificate of Secondary Education Higher Tier June 2015 Wednesday 20
A Surname Other Names Centre Number For Examiner s Use Candidate Number Candidate Signature Additional Science Unit 6 AS2HP General Certificate of Secondary Education Higher Tier June 2015 Wednesday 20
SCIENCE (REVISED SYLLABUS) HIGHER LEVEL
 2009. S26 Coimisiún na Scrúduithe Stáit State Examinations Commission JUNIOR CERTIFICATE EXAMINATION, 2009 SCIENCE (REVISED SYLLABUS) HIGHER LEVEL THURSDAY, 11 JUNE MORNING 9.30 to 11.30 INSTRUCTIONS 1.
2009. S26 Coimisiún na Scrúduithe Stáit State Examinations Commission JUNIOR CERTIFICATE EXAMINATION, 2009 SCIENCE (REVISED SYLLABUS) HIGHER LEVEL THURSDAY, 11 JUNE MORNING 9.30 to 11.30 INSTRUCTIONS 1.
Why do cold packs get cold?
 Assignment #5 Temperature in Reactions LO: To determine which solute dissolves most endothermically and exothermically in water. EQ: What makes an endothermic reaction feel cold? (explain using bonds and
Assignment #5 Temperature in Reactions LO: To determine which solute dissolves most endothermically and exothermically in water. EQ: What makes an endothermic reaction feel cold? (explain using bonds and
Chemistry of Life Essential Questions
 Chemistry of Life Essential Questions VMHS Standards 8.6b; 8.6c; 1h; 4e; 4f; 5a;1b; 1. What is an atom? What are elements? An atom is the basic unit of o Consist of,, and An element is a type of atom o
Chemistry of Life Essential Questions VMHS Standards 8.6b; 8.6c; 1h; 4e; 4f; 5a;1b; 1. What is an atom? What are elements? An atom is the basic unit of o Consist of,, and An element is a type of atom o
Unit 5 A3: Energy changes in industry
 1. ENTHALPY CHANGES Unit 5 A3: Energy changes in industry 1.1 Introduction to enthalpy and enthalpy changes 2 1.2 Enthalpy profile diagrams 2 1.3 Activation energy 3 1.4 Standard conditions 5 1.5 Standard
1. ENTHALPY CHANGES Unit 5 A3: Energy changes in industry 1.1 Introduction to enthalpy and enthalpy changes 2 1.2 Enthalpy profile diagrams 2 1.3 Activation energy 3 1.4 Standard conditions 5 1.5 Standard
4 Bioenergetics higher (import)
 4 Bioenergetics higher (import) Name: Class: Date: Time: 56 minutes Marks: 56 marks Comments: Page of 2 Plants are grown in glasshouses to protect them from the weather or extend the growing season. Plants
4 Bioenergetics higher (import) Name: Class: Date: Time: 56 minutes Marks: 56 marks Comments: Page of 2 Plants are grown in glasshouses to protect them from the weather or extend the growing season. Plants
Experiment 5. Heat and Temperature
 Experiment 5 Heat and Temperature This coffee isn t hot enough! E5-1 E5-2 The Task In this experiment you will study the heat flow associated with a range of processes and examine the relationship between
Experiment 5 Heat and Temperature This coffee isn t hot enough! E5-1 E5-2 The Task In this experiment you will study the heat flow associated with a range of processes and examine the relationship between
C1a The particulate nature of matter
 C1a The particulate nature of matter Introduction This topic may go back over ideas that students have already met, so it does not need to take up much teaching time. Nevertheless, it is important for
C1a The particulate nature of matter Introduction This topic may go back over ideas that students have already met, so it does not need to take up much teaching time. Nevertheless, it is important for
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-DINATED SCIENCES 0654/32 Paper 3 (Extended Theory), maximum
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-DINATED SCIENCES 0654/32 Paper 3 (Extended Theory), maximum
Chemical Reactions. Unit 4
 Chemical Reactions Unit 4 Lesson 1: Chemical Bonds Unit 4: Reactions Compounds Most substances around you are NOT elements. There are around 100 elements, but millions of different substances. Most substances
Chemical Reactions Unit 4 Lesson 1: Chemical Bonds Unit 4: Reactions Compounds Most substances around you are NOT elements. There are around 100 elements, but millions of different substances. Most substances
6.1 Rates of Reaction
 6.1 Rates of Reaction 6.1.1 Define the term rate of reaction The change in concentration of reactants or products with time. In other words, how quickly the reactants are converted into products. These
6.1 Rates of Reaction 6.1.1 Define the term rate of reaction The change in concentration of reactants or products with time. In other words, how quickly the reactants are converted into products. These
TOPIC: ELECTRODYNAMICS - MOTORS AND GENERATORS AND ALTERNATING CURRENT. (Taken from the DoE Physical Sciences Preparatory Examination Paper )
 TOPIC: ELECTRODYNAMICS - MOTORS AND GENERATORS AND ALTERNATING CURRENT SECTION A: TYPICAL EXAM QUESTIONS QUESTION 1: 13 minutes (Taken from the DoE Physical Sciences Preparatory Examination Paper 1 2008)
TOPIC: ELECTRODYNAMICS - MOTORS AND GENERATORS AND ALTERNATING CURRENT SECTION A: TYPICAL EXAM QUESTIONS QUESTION 1: 13 minutes (Taken from the DoE Physical Sciences Preparatory Examination Paper 1 2008)
Magnets & Magnetic Fields
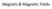 Magnets & Magnetic Fields Magnets Magnets have 2 poles, North and South if broken in half, each half will have both poles at the ends. Like poles repel, unlike poles attract. Hard Magnets- materials that
Magnets & Magnetic Fields Magnets Magnets have 2 poles, North and South if broken in half, each half will have both poles at the ends. Like poles repel, unlike poles attract. Hard Magnets- materials that
Chemical Reac+ons and Enzymes. Lesson Overview. Lesson Overview. 2.4 Chemical Reactions and Enzymes
 Lesson Overview Chemical Reac+ons and Enzymes Lesson Overview 2.4 Chemical Reactions and Enzymes THINK ABOUT IT Living things are made up of chemical compounds, but chemistry isn t just what life is made
Lesson Overview Chemical Reac+ons and Enzymes Lesson Overview 2.4 Chemical Reactions and Enzymes THINK ABOUT IT Living things are made up of chemical compounds, but chemistry isn t just what life is made
Answer all the questions.
 Answer all the questions.. A student investigates the reaction between sodium thiosulfate and hydrochloric acid. Look at the diagram below. It shows the apparatus he uses. After a time he cannot see the
Answer all the questions.. A student investigates the reaction between sodium thiosulfate and hydrochloric acid. Look at the diagram below. It shows the apparatus he uses. After a time he cannot see the
Thermodynamics. Standard enthalpy change, H
 Standard enthalpy change, H Thermodynamics Enthalpy change, H, is defined as the heat energy change measured under conditions of constant pressure. The value of the enthalpy change for a particular reaction
Standard enthalpy change, H Thermodynamics Enthalpy change, H, is defined as the heat energy change measured under conditions of constant pressure. The value of the enthalpy change for a particular reaction
Date: SCH 4U Name: ENTHALPY CHANGES
 Date: SCH 4U Name: ENTHALPY CHANGES Enthalpy (H) = heat content of system (heat, latent heat) Enthalpy = total energy of system + pressure volume H = E + PV H = E + (PV) = final conditions initial conditions
Date: SCH 4U Name: ENTHALPY CHANGES Enthalpy (H) = heat content of system (heat, latent heat) Enthalpy = total energy of system + pressure volume H = E + PV H = E + (PV) = final conditions initial conditions
Science 1.5 AS Demonstrate understanding of aspects of acids and bases WORKBOOK. Working to Excellence
 Science 1.5 AS 90944 Demonstrate understanding of aspects of acids and bases WORKBOOK Working to Excellence CONTENTS 1. Writing Excellence answers to Ion Formation questions 2. Writing Excellence answers
Science 1.5 AS 90944 Demonstrate understanding of aspects of acids and bases WORKBOOK Working to Excellence CONTENTS 1. Writing Excellence answers to Ion Formation questions 2. Writing Excellence answers
MODULE 4.2 MAGNETISM ELECTRIC CURRENTS AND MAGNETISIM VISUAL PHYSICS ONLINE
 VISUAL PHYSICS ONLINE MODULE 4.2 MAGNETISM ELECTRIC CURRENTS AND MAGNETISIM When electric charges are in motion they exert forces on each other that can t be explained by Coulomb s law. If two parallel
VISUAL PHYSICS ONLINE MODULE 4.2 MAGNETISM ELECTRIC CURRENTS AND MAGNETISIM When electric charges are in motion they exert forces on each other that can t be explained by Coulomb s law. If two parallel
States of Matter. Reviewing Vocabulary. Match the definition in Column A with the term in Column B.
 Name Date Class States of Matter Reviewing Vocabulary Match the definition in Column A with the term in Column B. Column A 1. A measure of the resistance of a liquid to flow 2. The energy required to increase
Name Date Class States of Matter Reviewing Vocabulary Match the definition in Column A with the term in Column B. Column A 1. A measure of the resistance of a liquid to flow 2. The energy required to increase
Fill in the gaps: You would write this as: we can use for living processes.
 AQA Trilogy Biology Unit 4.4: Bioenergetics Complete the word equation for + + Write the name of each chemical next to its formula. Which elements make up each chemical? How does the rate of photosynthesis
AQA Trilogy Biology Unit 4.4: Bioenergetics Complete the word equation for + + Write the name of each chemical next to its formula. Which elements make up each chemical? How does the rate of photosynthesis
The Chemistry of Life
 The Chemistry of Life Things you should be able to do 1. Describe how the unique properties of water support life on Earth. 2. Explain how carbon is uniquely suited to form biological macromolecules. 3.
The Chemistry of Life Things you should be able to do 1. Describe how the unique properties of water support life on Earth. 2. Explain how carbon is uniquely suited to form biological macromolecules. 3.
Chemical Bonds & Reactions
 Chemical Bonds & Reactions Chemical Bonding Do you understand how it works? What do you think when I pull out a bag of candy? I want that candy cause I don t have any! Does everyone think the same thing?
Chemical Bonds & Reactions Chemical Bonding Do you understand how it works? What do you think when I pull out a bag of candy? I want that candy cause I don t have any! Does everyone think the same thing?
1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time
 Name answer key period IB topic 6 Kinetics 1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time b. the reaction between C
Name answer key period IB topic 6 Kinetics 1. A. Define the term rate of reaction. The measure of the amount of reactants being converted into products per unit amount of time b. the reaction between C
Electricity. Year 10 Science
 Electricity Year 10 Science What is electricity? The collection or flow of electrons in the form of an electric charge What is static electricity? A stationary electrical charge that is built up on the
Electricity Year 10 Science What is electricity? The collection or flow of electrons in the form of an electric charge What is static electricity? A stationary electrical charge that is built up on the
Fairfield Public Schools Science Curriculum. Draft Units
 Fairfield Public Schools Science Curriculum Draft Units Human Anatomy and Physiology - Blood, Guts, Senses, and Defenses 1 Human Anatomy and Physiology - Blood, Guts, Senses, and Defenses: Description
Fairfield Public Schools Science Curriculum Draft Units Human Anatomy and Physiology - Blood, Guts, Senses, and Defenses 1 Human Anatomy and Physiology - Blood, Guts, Senses, and Defenses: Description
Cambridge Assessment International Education Cambridge International General Certificate of Secondary Education. Published
 Cambridge Assessment International Education Cambridge International General Certificate of Secondary Education CO-ORDINATED SCIENCES 0654/4 Paper 4 Theory (Extended) 07 MARK SCHEME Maximum Mark: 0 Published
Cambridge Assessment International Education Cambridge International General Certificate of Secondary Education CO-ORDINATED SCIENCES 0654/4 Paper 4 Theory (Extended) 07 MARK SCHEME Maximum Mark: 0 Published
Photosynthesis and Cellular Respiration
 Name Date Class CHAPTER 5 TEST PREP PRETEST Photosynthesis and Cellular Respiration In the space provided, write the letter of the term or phrase that best completes each statement or best answers each
Name Date Class CHAPTER 5 TEST PREP PRETEST Photosynthesis and Cellular Respiration In the space provided, write the letter of the term or phrase that best completes each statement or best answers each
Cellular Energy: Respiration. Goals: Anaerobic respiration
 Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
Cellular Energy: Respiration Anaerobic respiration Goals: Define and describe the 3 sets of chemical reactions that comprise aerobic cellular respiration Describe the types of anaerobic respiration Compare
