Thione-Based Auxiliaries in Organic Synthesis
|
|
|
- Maximilian Owen
- 6 years ago
- Views:
Transcription
1 Thione-Based Auxiliaries in rganic ynthesis C 2 An Evans Group Evening eminar Wade Downey Friday, March 1, title 2/28/02 6:46 PM
2 Thione-Based Auxiliaries in rganic ynthesis Thiazolidinethione xazolidinethione eminar cope 1. Auxiliary synthesis 2. alf-reduction 3. Amine acylation 4. Aldol-type reactions 5. Iminium additions 6. ther reactions a. Claisen condensation b. Diels-Alder c. earrangements d. elenylation e. eductive coupling f. otochemistry elevant eviews: Fujita, E.; agao, Y. Adv. eterocycl. Chem. 1989, 45,1-36. Mukaiyama, T.; Kobayashi,. rg. eact. 1994, 46, scope 3/2/02 10:49 AM
3 Auxiliary ynthesis K, 5 equiv C C, 16 h % 1 a 2 C 3, 1.5 equiv C C, 15 min % Le Corre, M. J. rg. Chem. 1995, 60, Cl 2 C=, Et 3 C 2 Cl 2 95% Thiones were acylated easily with Et 3 and the appropriate acid chloride. Crimmins, M. J. rg. Chem. 2001, 66, Auxiliary ynthesis 3/2/02 10:54 AM
4 alf-eduction: Mukaiyama 1.2 equiv DIBAL, C Yield (%) C 2 C 2 C 2 C 3 C 2 C() C 2 C 2 C 2 Br(C 2 ) 9 C 2 C=C i-bu i-bu Al Proposed intermediate Comparable yields with tri-t-butoxyaluminum hydride Mukaiyama, T. Chem. Lett. 1977, Mukaiyama, T. Bull. Chem. oc. Jpn. 1979, 52, Mukaiyama reduction 3/2/02 10:54 AM
5 alf-eduction: agao and Fujita 20% DIBAL in hexane C 2 Cl 2, hexane, C Yield (%) (C 2 ) 3 C 2 (C 2 ) 7 C 2 (C 2 ) 13 C 2 C=C Yields are comparable to Mukaiyama's. DIBAL was "injected dropwise onto a yellow solution... until the original yellow colour of the reaction medium vanished." Full reduction with ab 4 proceeded with >90% yields. agao, Y.; Fujita, E. J. Chem. oc., Chem. Commun. 1978, agao, Y.; Fujita, E. J. Chem. oc., Perkin Trans , agao reduction 2/28/02 2:42 PM
6 Aminolysis: agao and Fujita C C 2 Cl C t Yield (%) 2 Butyl Cy 4-C 6 4 Bn C 3 (C 2 ) 4 1 min 5 min 6 d 8 min 1 min imilar aminolyses were successful for other -acylated thiones (aryl, alkyl, unsaturated). "tirring was continued until the original yellow color of the solution disappeared." min 7 d 3 min a 95 b + Proposed Transition tate a) solvent = TF b) solvent = Et-TF agao, Y.; Fujita, E. Tetrahedron Lett. 1980, 21, agao aminolysis 1 2/28/02 6:35 PM
7 Macrolactamization: agao and Fujita n 2 2 C 2 Cl 2 n + dimer n Monomer Yield (%) Dimer Yield (%) (C 2 ) 2 (C 2 ) 4 (C 2 ) 6 (C 2 ) 6 (C 2 ) 3 (C 2 ) 4 (C 2 ) 3 (C 2 ) This process has been to applied to the total synthesis of codonocarpine, lunarine, and lunaridine. 06-agao aminolysis 2 2/28/02 2:49 PM agao, Y.; Fujita, E. Tetrahedron Lett. 1980, 21, agao, Y.; Fujita, E. Chem. Lett. 1980, Fujita, E. Pure Appl. Chem. 1981, 53, agao, Y.; Fujita, E. J. Chem. oc., Chem. Commun. 1981, agao, Y.; Fujita, E. eterocycles 1981, 15,
8 Desymmetrization: agao and Fujita 2 C C u, solvent, C 2 Cl 2 u u olvent Yield (%) 2 C C 2 2 C 2 Cl 2 69 Br Et t-bu Et TF TF TF Proposed Model: First nucleophile attacks least favored hindered of four carbonyl faces. Dipole-minimized structure supported by crystal structure Initial amide product isolable (74% yield, 10:1 dr) 07-agao desymmetrization 1 2/28/02 7:35 PM agao, Y.; Fujita, E. J. Am. Chem. oc. 1982, 104, agao, Y.; Fujita, E. J. rg. Chem. 1983, 48, agao, Y.; Fujita, E. Tetrahedron 1984, 40,
9 Aminolysis: agao and Fujita Kinetic esolution of acemic Amines (C 2 ) 14 C equiv C 2 Cl (C 2 ) 14 + ee's typically 25 60% yield of amides >90% agao, Y.; Fujita, E. Tetrahedron Lett. 1982, 23, Absolute tructure Determination of Amines 2 equiv (C 2 ) 14 C C 2 Cl 2 * C 2 (C 2 ) (C 2 ) 14 otation of recovered thione starting material indicates absolute structure of starting amine. 08-agao resolution 2/27/02 12:44 PM agao, Y.; Fujita, E. Tetrahedron Lett. 1982, 23,
10 Thiazolidinethione Adduct Derivatization ab 4 83% DIBAL 69% Bn 2 79% Bn i-bu TE TETf 100% i-bu Imidazole 79% () Cl Imidazole 77% Crimmins, M. rg. Lett. 2000, 2, Thione elaboration 2/28/02 7:36 PM
11 Aldol: Mukaiyama 1. n(tf) 2, -Ethylpiperidine (EP) 1 C 2 Cl 2, 78 C, 20 min 2. 2 C, 78 C, 15 min Yield (%) syn:anti BnC 2 BnC 2 BnC 2 BnC :1 32:1 32:1 32:1 32:1 Prior to this report, Mukaiyama had used these enolization conditions extensively for crossed aldols of ketones. dr's were determined by 13 C M. 10-Mukaiyama ach aldol 2/28/02 7:13 PM Mukaiyama, T. Chem. Lett. 1982,
12 ther Enolates: Mukaiyama 1. n(tf) 2, EP C 2 Cl 2, 78 C, 30 min C, temp Yield (%) syn:anti ( 78 C) Cbz(Bn) ( 40 C) Boc(Bn) ( 40 C) BnC 2 C=C 2 Pentyl BnC 2 Pentyl BnC 2 Pentyl :3 5:1 1:1 2:1 6:1 9:1 19:1 19:1 19:1 9:1 19:1 19:1 19:1 19:1 o elimination products were observed. o mention of results with chiral ligand This methodology was employed in the total synthesis of (±)-thienamycin. Mukaiyama, T. Chem. Lett. 1985, Mukaiyama methoxy aldol 2/27/02 1:04 PM
13 Aldol: Mukaiyama 1. n(tf) 2, EP C 2 Cl 2, 78 C, 20 min C, 95 C, 15 min Yield (%) ee (%) BnC 2 Et Pentyl Cy Absolute stereochemistry proven only for benzaldehyde adduct Prior to this report, the "Mukaiyama Ligand" had been used for enantioselective crossed aldols and ketone homoaldols. Mukaiyama, T. Chem. Lett. 1983, Mukaiyama ch ac aldol 2/28/02 2:54 PM
14 Pyruvate Aldol: Mukaiyama 1. n(tf) 2, EP C 2 Cl 2, 78 C, 30 min C 2, 2 4 h i-bu 2 C(C 2 ) 2 Yield (%) ee (%) Previous aldol ligand afforded only 18% ee. A scan of ligands showed the 1-naphthyl moiety to be superior to cyclohexyl, phenyl, 2,6-xylyl, and 2-naphthyl. ee's determined by use of chiral shift reagent Mukaiyama, T. Chem. Lett. 1983, Mukaiyama pyruv aldol 3/1/02 11:20 AM
15 Aldol: Mukaiyama 1. n(tf) 2, EP Bn C 2 Cl 2, 78 C, 20 min 2. C, 78 C, 3 h Bn L* Yield (%) syn:anti ee (%) BnC 2 Pentyl Cy BnC 2 Pentyl Cy none :3 3:1 1.6:1 3:1 1.3:1 1:4 1:7 1:10 1:4.9 1: = TMEDA as ligand resulted in diastereoselectivity similar to that induced by the chiral ligand. ote that even in the absence of ligand, C is anti selective. 14-Mukaiyama Bn aldol 2/27/02 12:59 PM Mukaiyama, T. Chem. Lett. 1984,
16 Aldol: agao and Fujita 1 1. n(tf) 2, EP C 2 Cl 2, 50 C, 3 h equiv 2 C, 78 C, 20 min 1 2 on-evans syn! 1 2 dr Isolated Yield (%) Pr 3.2:1 4.5:1 8:1 10:1 5: Tf n 2 Chelated transition-state structure predicts "on-evans" selectivity. 1 Enolization conditions from Mukaiyama Proposed Transition tate Mukaiyama also postulates a chelated transition state for his racemic tin-mediated aldol. agao, Y.; Fujita, E. J. Chem. oc., Chem. Commun. 1985, agao aldol 1 3/2/02 11:06 AM
17 Acetate Aldol: agao and Fujita 1 1. n(tf) 2, EP C 2 Cl 2, 50 C, 3 h 2. 2 C, 50 C, 4 h Et Et Et 2 dr 13:1 33:1 8:1 36:1 13:1 Total Yield (%) ote the change to thiazolidinethione auxiliary. A footnote suggests that saturated aldehydes are just as selective. 1.2 equiv n(tf) 2 and 1.2 equiv EP were optimal. Excess base led to dehydration products. Excess n(tf) 2 led to lower yields, presumably due to aldehyde polymerization. o mention of propionates agao, Y.; Fujita, E. J. rg. Chem. 1986, 51, agao ac aldol 3/2/02 10:56 AM
18 Aldol: agao equiv n(tf) equiv EP TF, 50 C, 3 h equiv, TF, 50 C, 2 h 3. 5% Cl dr Yield (%) tarting lactone is an aldehyde precursor: Et Pr 99:1 99:1 66:1 99:1 99:1 99: C Aldol 4-acetoxy lactone is unreactive under the reaction conditions. Lactonization occurs during the acid workup. agao, Y. J. rg. Chem. 1989, 54, agao aldol-lactone 2/28/02 7:37 PM
19 δ-lactone: agao equiv n(tf) equiv EP C 2 Cl 2, 40 C, 2 h equiv 2 C, 40 C, 1 h 3. K 2 C 3, DMF, rt, 1 h 1 2 X c 1 2 (Bn)Cbz s-bu exyl Yield (%) Tf n Tf 1 ationale: 18-agao d-lactone 2/28/02 7:37 PM Initial aldol adduct cyclizes under K 2 C 3 /DMF conditions. All product lactones were "optically pure" by PLC and 1 M. tarting material recovered in 5-32% yield In order to avoid steric repulsion between 1 and the isopropyl group, n may coordinate as shown. Dipole-minimized conformation based upon crystal structure agao, Y. Chem. Lett 1992,
20 Aldol: Miller X 1. Bu 2 BTf, 2 Et 1 C 2 Cl 2, 0 C, 30 min equiv 2 C, 78 0 C C 2 X 1 C 2 2 X 1 2 Yield (%) dr Et Et Pr n(tf) 2 afforded "Evans-syn" aldol adducts, unlike the agao/fujita results! Absolute stereochemistry was proven by optical rotation of methanolysis products. dr is calculated from the ee reported by Miller for the methanolysis products. o oxidative workup is required. 19-Miller aldol 1 3/2/02 11:03 AM 66:1 66:1 24:1 99:1 99:1 99:1 99:1 siao, C.; Miller, M. Tetrahedron Lett. 1985, 26, siao, C.; Miller, M. J. rg. Chem. 1987, 52, C 2 L Proposed Transition tate B L
21 Aldol: Miller 1. Bu 2 BTf, 2 Et 1 C 2 Cl 2, 0 C, 30 min 2. 2 C, 78 0 C C 2 1 C C Yield (%) dr Et i() 2 exyl 58 99:1 Boc 73 D The Miller system appears to be compatible with functionalized aldehydes and enolates. C 2 C=C 2 C 28 D Miller, M., Tetrahedron 1990, 46, Boyd,.; siao, C. J. rg. Chem. 1991, 56, Miller, M., Tetrahedron Lett. 1991, 32, Miller aldol apps 2/23/02 1:33 PM
22 Aldol: Yan 1. TiCl 4, 2 Et C 2 Cl 2, 0 C, 20 min 2. 1:1 TiCl 4 C (1.5 2 equiv) 78 C, 2 h Yield (%) dr Pr C=C :1 99:1 99:1 50:1 Corresponding oxazolidinones, under the same conditions, afforded ~1:1 mixture of epimers at the α-carbon. Boron enolates yield Evans syn products in high dr. Lithium enolates yield on-evans syn products (dr = 2-9:1). A subsequent paper showed that aldehyde-ticl 4 complex was unnecessary for on-evans syn selectivity. 21-Yan aldol 1 2/27/02 1:21 PM Proposed Transition tate Yan, T. Tetrahedron Lett. 1991, 32, Yan, T. Tetrahedron Lett. 1993, 34, Yan, T. J. Am. Chem. oc. 1993, 115, Ti Cl Cl Cl
23 Acetate Aldol: Yan 1. Lewis Acid, 2 Et C 2 Cl 2, 0 C, 25 min 2. C, 78 C, 2 h + A B Lewis Acid Yield (%) A:B Bu 2 BTf Pr C=C :3 1:3 1:12 1:3 The authors propose that the 9-BB-mediated aldol proceeds through a boat-like transition state, whereas the Bu 2 BTf reaction occurs via the usual chair. 9-BB(Tf) TiCl 4 Pr C=C Pr C=C :1 7:1 99:1 6:1 10:1 19:1 13:1 16:1 The titanium-mediated reaction is believed to proceed via a chelated transition state. Boron enolates of the corresponding oxazolidinone auxiliary show the same trend: Bu 2 BTf affords Evans syn, and 9-BB(Tf) affords non-evans syn, although diastereoselectivity is modest (2-3:1). Yan, T. J. rg. Chem. 1994, 59, Yan, T. J. rg. Chem. 1995, 60, Yan acetate aldol 2/28/02 7:26 PM
24 Bromoacetate Aldol: Yan 1. TiCl 4, 2 Et C 2 Cl 2, 0 C, 10 min 2. Br 2, Et, 78 C 3. C, 78 C, 1.5 h Br Yield (%) dr C=C Pr :1 99:1 99:1 99:1 dr determined by 400 Mz 1 M Epoxide Formation: X c Br 1. Et 3, 2, C 3 C 2. K 2 C 3, C =, 79% = C=C, 86% = Pr, 83% =, 82% Yan, T. Tetrahedron Lett. 1999, 40, Yan, T. Tetrahedron: Asymmetry 1999, 10, Yan bromoacetate aldol 3/1/02 11:22 AM
25 Aldol: Crimmins 1. 1 equiv TiCl 4, sparteine, 0 C equiv C, 0 C, 1 h Bn 1 Bn TiCl 4 Isolated Yield (%) 1. 2 equiv TiCl 4, Et, 0 C C, 78 C, 1 h 1:2 Bn 2 Et C=C C 2 =C Et C=Ca C 2 =C 1 equiv 2 equiv :1 32:1 36:1 37:1 90:1 1:22 1:19 1:41 1:18 1:99 M experiments suggest that a different enolate species forms upon addition of second equivalent of TiCl 4. unig's base or TMEDA in the place of sparteine resulted in lower yields (50-60%) but similar selectivity. The chirality of sparteine was shown to have no impact on diastereoselectivity. a) eaction at 78 C 24-Crimmins aldol 1 3/2/02 10:59 AM Crimmins, M.; King, B.; Tabet, E. J. Am. Chem. oc. 1997, 119,
26 Aldol: Crimmins 1. TiCl 4, 1 equiv sparteine, 0 C equiv C, 0 C, 1 h Bn 1 Bn 1. TiCl 4, 2 equiv sparteine, 0 C equiv C, 0 C, 1 h Bn 2 base Yield (%) 1:2 1 equiv 2 equiv i-bu C=C C 2 =C i-bu C=C C 2 =C :1 19:1 41:1 18:1 142:1 1:44 1:32 1:36 1:37 1:90 Use of 2 Et as base resulted in lower selectivity (typically 10:1). Use of TMEDA in the place of sparteine caused no significant change in selectivity, but lowered yields ~10%. Changes in Lewis acid stoichiometry had no effect on diastereoselectivity. 25-Crimmins Aldol 2 3/2/02 11:00 AM Crimmins, M. rg. Lett. 2000, 2,
27 Aldol: Crimmins Bn L 4 Ti X c Evans syn Bn L 4 Ti TiCl 4 or AgbF 6 Chloride emoval -thylpyrrolidinone or sparteine Ligand Addition Bn Ti Cl Cl Cl X c on-evans syn Bn Ti Cl Cl Cl 26-Crimmins T 3/1/02 11:29 AM Crimmins, M. J. Am. Chem. oc. 1997, 119, Crimmins, M. rg. Lett. 2000, 2, Crimmins, M. J. rg. Chem. 2001, 66,
28 Acetal Addition: Urpi and omea 1. TiCl 4, 2 Et C 2 Cl 2, 0 C 2., Lewis Acid, 40 C Lewis Acid T ( C) Isolated Yield (%) dr Cl n BF 3 Et 2 ncl C ClC C 6 4 Pr i-bu :1 4.3:1 10:1 6:1 13:1 12:1 7:1 Ti econd Lewis acid necessary to activate acetal Less reactive aldehydes require higher temperatures and the stronger ncl 4. Proposed Transition tate Urpi, F.; omea, P. rg. Lett. 2001, 3, Urpi acetal 1 3/2/02 11:07 AM
29 Acetal Addition: Urpi and omea 1. TiCl 4, 2 Et C 2 Cl 2, 0 C 2., Lewis Acid, temperature L.A. T ( C) Isolated Yield (%) dr Cl n BF 3 Et 2 ncl C ClC 6 4 Pr i-bu :1 13:1 7:1 3:1 3:1 4:1 2.4:1 Ti Yields are very similar to propionate case. Compared to the propionate cases, selectivity is similar for BF 3, worse for ncl 4. Proposed Transition tate Urpi, F.; omea, P. Tetrahedron Lett. 2001, 42, Urpi acetal 2 2/28/02 7:41 PM
30 Aldol: Evans Bn 1 1. cat. MgBr 2 Et 2, Et 3 2 C, TMCl, EtAc Cl, TF Bn Mol % Catalyst Isolated Yield (%) dr 4-C C :1 19:1 19:1 L L Et Allyl Bn i-bu C=C C=C() 2-naphthyl C 2 =C() :1 19:1 7:1 7:1 10:1 13:1 8:1 19:1 Mg L L Bn Proposed Transition tate TMCl is necessary to turn over the catalytic cycle. Aliphatic aldehydes are unreactive or self-condense. 29-Evans aldol 2/28/02 6:37 PM Evans, D.; Downey, C.; haw, J.; Tedrow, J. rg. Lett., accepted.
31 The Aldol Comparison Lewis Acid Base / Ac? Yield (%) dr Major Diastereomer Mukaiyama n(tf) 2 EP nly ~20:1 agao/fujita n(tf) 2 EP o Yes ~8:1 ~25:1 on-evans syn on-evans syn Miller Bu 2 BTf 2 Et both o ~20:1 Evans syn Yan Crimmins Urpi Evans TiCl 4 Bu 2 BTf 9-BB(Tf) TiCl 4 TiCl 4 MgBr 2 Et 2 2 Et sparteine 2 Et Et 3 Yes o nly o o Yes o ~50:1 ~20:1 ~20:1 ~25:1 ~25:1 ~8:1 ~15:1 on-evans syn Evans syn Either syn Either syn on-evans anti Evans anti Mukaiyama: stoichiometric chiral ligand agao/fujita: applied to many syntheses Miller: ester substituent on auxiliary Yan: camphor-based auxiliary Crimmins: either syn diastereomer Urpi: anti addition to dimethyl acetals Evans: anti selective, mild Lewis acid 30-aldol comparison 3/1/02 11:30 AM
32 Iminium Addition: agao and Fujita 1.4 equiv equiv n(tf) equiv. EP TF, 78 C, 3 h 2. Ac, TF, 0 C 1 1 dr Total Yield (%) Bn Bn ()Cbz 19:1 "10 50:1" 32:1 32:1 32:1 24:1 99:1 82 "75-85" eactions complete within 30 min Acetate and propionate nucleophiles were enolized at 50 C. Yield of propionate adducts not specified 31-agao imine addn 1 2/28/02 7:29 PM agao, Y.; Fujita, E. J. Am. Chem. oc. 1986,108, agao, Y.; Fujita, E. J. Chem. oc., Chem. Commun. 1986,
33 Iminium Addition: agao and Fujita m Cl 1. 2 equiv n(tf) 2, 2.2 equiv EP TF, 78 C, 3 h equiv Ac n, TF, 0 C m Cl n m n dr 66:1 28:1 39:1 28:1 Total Yield (%) X c Further Elaboration: Alkaloids n LA 0 C reflux n m eactions complete within 2 h m Cl 44 69% agao, Y.; Fujita, E. J. Am. Chem. oc. 1988,110, agao imine addn 2 2/28/02 7:41 PM
34 Iminium Addition: agao 1.4 equiv equiv n(tf) equiv EP TF, 78 C, 3 h TM Ac, TF, 0 C 2 1 TM 1 dr Yield (%) Et 2 9:1 10:1 4:1 6: Matched case required for synthetically useful selectivity electivity much lower than for unsubstituted azetidinones. agao, Y. J. rg. Chem. 1992, 57, agao chiral imine 2/28/02 7:29 PM
35 Iminium Addition: agao equiv n(tf) equiv EP TF, 78 C, 30 min equiv., TF, 0 C, 2 h Ac dr a Isolated Yield (%) purified dr b C 6 11 Bn 1:1 3.6:1 1.6:1 1.6: :1 4.9:1 6:1 Why this turnover to anti selectivity? a) dr = major : Σ minor b) dr of chromatographically purified product agao, Y. Tetrahedron Lett. 1988,47, agao me iminium 2/28/02 7:30 PM
36 Iminium Addition: agao Cyclic Acyclic n Tf n Tf yn Anti agao, Y. Tetrahedron Lett. 1988,47, agao imine model 3/2/02 11:19 AM
37 Crossed Claisen: agao Li, MPA, TF, 78 C 2., 15 min C 6 4 Bu Bu t-bu t-bu t-bu Yield (%) ee (%) Ar Isobutyrylthione behaved analogously in both yield and selectivity. Proposed Transition tate MPA, aromatic 1 appear to be essential for high selectivity. 36-agao Claisen 2/28/02 2:57 PM Large 2 led to decreased yields. agao, Y. J. rg. Chem., 1988, 53,
38 Dieckmann Annulation: agao and Fujita Initial bservation: C 2 2 equiv K TF, rt, 10 h 69%, 96% ee K ationale: C 2 C 2 eaction was later repeated with a methyl ester in the place of the thioester. 37-agao Dieckmann 1 2/28/02 7:44 PM agao, Y., Fujita, E. Chem. Lett. 1987, agao, Y. J. rg. Chem., 1988, 53,
39 Pyrone ynthesis: agao TB n(tf) 2, EP C 2 Cl 2, 40 C, 2 h 63% Tf Tf n n Tf 38-agao pyrone 2/28/02 7:42 PM agao, Y. Chem. Lett 1992,
40 Diels-Alder: Evans 10 mol% Cu(Tf) 2, 11 mol% L* C 2 Cl 2 CEt L* Isolated Yield (%) endo:exo 96:4 92:8 84:16 endo ee (%) CEt :7 92:8 90: L* = 3 C C 1 3 Thiones react at lower temperatures than oxazolidinones (typically 40 C instead of 20 C). Thiones and oxazolidinones react comparably with Cu(II)box. With the bisimine ligand, however, thiones exhibit much better endo:exo selectivity (done typically 2 4:1). Ar Ar 2 (Ar = 2,6-Cl 2 ) 39-Evans DA 2/28/02 7:32 PM Evans, D.; Miller,.; Lectka, T. J. Am. Chem. oc. 1993, 115, Evans, D.; Lectka, T.; Miller,. Tetrahedron Lett. 1993, 34,
41 Thione-Based earrangements ncl 4, C 2 Cl 2 2 ncl 4 2 ncl 3 ncl 3 Et 4-Cl Total Yield (%) dr 50:1 50:1 50:1 7:1 3:1 eactions were conducted at 78 C except for aromatic substrates, which were warmed to room temperature. The camphor-based auxiliary was superior in both yield (typically 85%) and selectivity (by ~5%) but is more difficult to draw. eactions were 0.01 to 0.05 M in substrate. Palomo, C. J. Am. Chem. oc. 2001, 123, Palomo earr 2/28/02 7:44 PM
42 Thione-Based earrangements Aldol Product earrangement C Et C 2 Cl 2 2 C C 1 2 Propionates are unreactive. Competitive with methanolysis for hindered substrates Thermal Carbamate earrangement Adamczyk, A. Tetrahedron Lett. 1995, 36, C', C 2 Kt-Bu C' C 2 oppe, D. Chem. Ber. 1976, 109, earrangements 11/5/63 7:20 AM
43 ther eactions elenylation Bn 1. LDA, DMPU, TBCl 2. ecl, TF, C 2 Cl 2 Bn e 37% dr = 6:1 olmes, A. Tetrahedron: Asymmetry 1992, 3, Electroreductive Coupling 2 equiv X Bn e (0.1 A) Et 4 Ts, C 3 C X =, 68%, ee = 74% X =, 69%, ee = 66% Decarboxylation occurs under the reaction conditions. Kise,. J. rg. Chem. 1998, 63, olmes elenylation 2/28/02 7:45 PM
44 otochemical earrangement: akamoto 1 2 hν, C C % dr = 3: Isolable for 1 = 2 = "Impossible to determine stereochemistry" Major byproduct is deacylated thione. Endocyclic-oxygen analog gives similar results. Thiazolidinethiones and oxazolidinethiones yield solely deacylation products. 43-sakamoto 1 2/28/02 7:45 PM akamoto, M. J. Chem. oc., Perkin Trans ,
45 otochemical earrangement: akamoto hν, C 6 6 X X n rt n [2 + 2] X X n n X =, n = 2 X n Yield (%) Product structure confirmed by X-ray crystallography For X =, n = 2, product A was formed in 64% yield: A 44-sakamoto 2 2/28/02 7:45 PM akamoto, M., ishio, T. J. rg. Chem. 1992, 57,
46 otochemical earrangement: akamoto X n hν, C 6 6 rt X + n 1 2 X n X n Yield (%) tereochemistry supported by E studies Proposed mechanism is analogous to previous case sakamoto 3 2/28/02 7:33 PM akamoto, M. J. Chem oc., Perkin Trans ,
47 otochemical [2 + 2]: ishio hν, C 6 6 =, 59% =, 7% Ac imilar results for other -acyl groups in the starting material: isobutyryl, phenylacetyl, isovaleryl, hydrocinnamyl. M calculations suggest that substitution α to sulfur stabilizes the iminothietane. E/Z selectivity of the iminothietane products was not mentioned; in fact, the product in the paper is drawn as E, despite the Z-configured crystal structure featured in the text. ishio, T. J. Chem. oc., Perkin Trans , =, 0% =, 52% 46-ishio /28/02 7:46 PM
48 ummary Thioimides have been used extensively as acylating agents, especially for amide synthesis. alf-reduction of thioimides directly to aldehydes is possible. By far, the aldol is the most common reaction with thioimide auxiliaries. Yield and selectivity vary, but the best systems are viable options to the venerable oxazolidinones. Facile cleavage may be the thioimide's greatest asset. ther enolate reactions include Claisen, Dieckmann, iminium addition, and selenylation. With few exceptions, soft enolization is essential. Thiocarbonyl photochemistry may lead to multiple products. o asymmetric reactions have been reported. earrangement of aldol products and starting acylthiones is a potential danger. More complex enolates and aldehydes have been used in the context of natural product synthesis by the following groups: Crimmins, orikawa, uber, Kibayashi, Kocienski, agao/fujita, Paquette, izzacasa, omo, hiori, ulikowski, Thomas, and Urpi/Vilarrasa. 47-summary 2/28/02 7:47 PM
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
Stereoselective reactions of the carbonyl group
 1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
Cornforth: Nature is an organic chemist with a preference for the aldol reaction.
 I. Basic Principles IC. The Aldol Reaction Cornforth: Nature is an organic chemist with a preference for the aldol reaction. Many natural products contain polyhydroxylated carbon arrays, usually a mix
I. Basic Principles IC. The Aldol Reaction Cornforth: Nature is an organic chemist with a preference for the aldol reaction. Many natural products contain polyhydroxylated carbon arrays, usually a mix
P. Wipf - Chem /29/2006. To achieve high diastereo- and enantioselectivity, it is necessary to:
 P. Wipf - Chem 2320 1 3/29/2006 The Ivanov Reaction rationalization: Zimmerman-Traxler, JACS 1957, 79, 1920. To achieve high diastereo- and enantioselectivity, it is necessary to: - control the enolization
P. Wipf - Chem 2320 1 3/29/2006 The Ivanov Reaction rationalization: Zimmerman-Traxler, JACS 1957, 79, 1920. To achieve high diastereo- and enantioselectivity, it is necessary to: - control the enolization
CHEM 330. Final Exam December 5, 2014 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
Stereoselective reactions of enolates
 1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
Highlights of Schmidt Reaction in the Last Ten Years
 ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
Asymmetric Nucleophilic Catalysis
 Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
Chapter 5 Three and Four-Membered Ring Systems
 Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Stereoselective Organic Synthesis
 Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
CHEM 330. Final Exam December 8, 2010 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 8, 2010 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 20 4.
CEM 330 Final Exam December 8, 2010 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 20 4.
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
[3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S
![[3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S [3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S](/thumbs/94/119475672.jpg) Generalized igmatropic Processes [3,3]-sigmatropic Processes 1 3,=C,,, 1 3 3,=C,,, 3 [2,3]-sigmatropic Processes 1 3,=C,,, 1 3 Ene eactions 1 3 1 3 Cope earrangement [3,3]- igmatropic earrangements Transition
Generalized igmatropic Processes [3,3]-sigmatropic Processes 1 3,=C,,, 1 3 3,=C,,, 3 [2,3]-sigmatropic Processes 1 3,=C,,, 1 3 Ene eactions 1 3 1 3 Cope earrangement [3,3]- igmatropic earrangements Transition
Stereoselective reactions of enolates: auxiliaries
 1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
Lewis Base Catalysis: the Aldol Reaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk
 Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
Asymmetric Alklylation of Enolates
 Asymmetric Alklylation of Enolates M with material from A G Meyers http://faculty.chemistry.harvard.edu/myers/pages/chem-215-handouts 745 rganic Synthesis Spring 2015 Asymmetric Alkylation - eed to control
Asymmetric Alklylation of Enolates M with material from A G Meyers http://faculty.chemistry.harvard.edu/myers/pages/chem-215-handouts 745 rganic Synthesis Spring 2015 Asymmetric Alkylation - eed to control
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation Reactions of Substituted Ketenes
 Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
CEM 852 Final Exam. May 6, 2010
 CEM 852 Final Exam May 6, 2010 This exam consists of 7 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. n answering your questions,
CEM 852 Final Exam May 6, 2010 This exam consists of 7 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. n answering your questions,
Chiral Auxiliaries. attach auxiliary Substrate Substrate Auxiliary
 Chiral Auxiliaries Previously on Advanced ynthesis... Discussed the need for stereoselective synthesis Looked at the use of resolution, the chiral pool and substrate control t there are some potential
Chiral Auxiliaries Previously on Advanced ynthesis... Discussed the need for stereoselective synthesis Looked at the use of resolution, the chiral pool and substrate control t there are some potential
Mechanism Problem. 1. NaH allyl bromide, THF N H
 Mechanism Problem 1. a allyl bromide, TF 2. 9-BB (1.2 equiv), TF, rt; ame (1.2 equiv); t-buli (2.4 equiv), TMEDA (2.4 equiv) 30 to rt; allyl bromide; 30% 2 2, aq. a, 0 C (58% yield) Mechanism Problem 9-BB
Mechanism Problem 1. a allyl bromide, TF 2. 9-BB (1.2 equiv), TF, rt; ame (1.2 equiv); t-buli (2.4 equiv), TMEDA (2.4 equiv) 30 to rt; allyl bromide; 30% 2 2, aq. a, 0 C (58% yield) Mechanism Problem 9-BB
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Chiral Catalyst II. Palladium Catalysed Allylic Displacement ( -allyl complexes) 1. L n Pd(0) 2. Nuc
 Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Organocopper Reagents
 rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
Requirements for an Effective Chiral Auxiliary Enolate Alkylation
 Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Shi Asymmetric Epoxidation
 Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Use of Cp 2 TiCl in Synthesis
 Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Answers To Chapter 7 Problems.
 Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Dual enantioselective control by heterocycles of (S)-indoline derivatives*
 Pure Appl. Chem., Vol. 77, No. 12, pp. 2053 2059, 2005. DOI: 10.1351/pac200577122053 2005 IUPAC Dual enantioselective control by heterocycles of (S)-indoline derivatives* Yong Hae Kim, Doo Young Jung,
Pure Appl. Chem., Vol. 77, No. 12, pp. 2053 2059, 2005. DOI: 10.1351/pac200577122053 2005 IUPAC Dual enantioselective control by heterocycles of (S)-indoline derivatives* Yong Hae Kim, Doo Young Jung,
Denmark s Base Catalyzed Aldol/Allylation
 Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
Enolates: Z(O,R) (O,R)- and E(O,R) (O,R)-enolates
 Enolates: Z(,) (,)- and E(,) (,)-enolates egardless of other groups the encircled ' and - determine whether one is Z(,)- or E(,)- enolate. - - ' ' (E)-enolate (Z)-enolate Enolates: deprotonation 90 ' Most
Enolates: Z(,) (,)- and E(,) (,)-enolates egardless of other groups the encircled ' and - determine whether one is Z(,)- or E(,)- enolate. - - ' ' (E)-enolate (Z)-enolate Enolates: deprotonation 90 ' Most
Tautomerism and Keto Enol Equilibrium
 Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
TMSCl imidazole DMF. Ph Ph OTMS. Michael reaction. Michael reaction Ph R 3. epoxidation O R
 eaction using diarylprolinol silyl ether derivatives as catalyst 1) C Et K C 3, ) MgBr, TF TMS hexane, 0 o C TBS p- C 6 4, T C Et 85%, 99% ee Angew. Chem., nt. Ed., 44, 41 (005). rg. Synth., 017, 94, 5.
eaction using diarylprolinol silyl ether derivatives as catalyst 1) C Et K C 3, ) MgBr, TF TMS hexane, 0 o C TBS p- C 6 4, T C Et 85%, 99% ee Angew. Chem., nt. Ed., 44, 41 (005). rg. Synth., 017, 94, 5.
Asymmetric Lewis Base Strategies for Heterocycle Synthesis
 Asymmetric Lewis Base trategies for eterocycle ynthesis Dr Andrew mith EatCEM, chool of Chemistry, University of t Andrews 1st cottish-japanese ymposium of rganic Chemistry, University of Glasgow Friday
Asymmetric Lewis Base trategies for eterocycle ynthesis Dr Andrew mith EatCEM, chool of Chemistry, University of t Andrews 1st cottish-japanese ymposium of rganic Chemistry, University of Glasgow Friday
Total synthesis of Spongistatin
 Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Suggested solutions for Chapter 40
 s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr.
 April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr. Rainer Glaser Announced Reading: Prins Cyclization Reactions 1 Question 1. Aldol-Prins
April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr. Rainer Glaser Announced Reading: Prins Cyclization Reactions 1 Question 1. Aldol-Prins
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Conjugate (1,4-) addition
 1 Conjugate (1,4-) addition uc R 1 R 2 uc R 1 R 2 uc R 1 E R 2 E ucleophilic attack on C=C bond normally requires electron deficient alkene Know as 1,4-addition or conjugate addition As enolate formed
1 Conjugate (1,4-) addition uc R 1 R 2 uc R 1 R 2 uc R 1 E R 2 E ucleophilic attack on C=C bond normally requires electron deficient alkene Know as 1,4-addition or conjugate addition As enolate formed
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
Carbonyl Chemistry IV: Enolate Alkylations and Aldols. Aldol Madness O O O N M + substrate. aldehyde. (Z)-enolate H
 Carbonyl Chemistry IV: nolate Alkylations and Aldols Paul Bracher Chem 30 Section 9 Section Agenda 1) o office hours Thursday 2) The Great Joe Young is covering section next onday 3) andout: Carbonyl Chemistry
Carbonyl Chemistry IV: nolate Alkylations and Aldols Paul Bracher Chem 30 Section 9 Section Agenda 1) o office hours Thursday 2) The Great Joe Young is covering section next onday 3) andout: Carbonyl Chemistry
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Loudon Chapter 20 & 21 Review: Carboxylic Acids & Derivatives CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
CHEM 330. Final Exam December 11, This a closed-notes, closed-book exam. The use of molecular models is allowed. This exam contains 12 pages
 CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
Development of Small Organic Molecules as Catalysts for Asymmetric
 Development of Small Organic Molecules as Catalysts for Asymmetric Organic Transformations The development of new and efficient catalysts capable of catalyzing enantioselective transformation in a controlled
Development of Small Organic Molecules as Catalysts for Asymmetric Organic Transformations The development of new and efficient catalysts capable of catalyzing enantioselective transformation in a controlled
Suggested solutions for Chapter 30
 s for Chapter 30 30 PRBLEM 1 uggest a mechanism for this synthesis of a tricyclic aromatic heterocycle. 2 Cl base A simple exercise in the synthesis of a pyridine fused to a pyrrole (or an indole with
s for Chapter 30 30 PRBLEM 1 uggest a mechanism for this synthesis of a tricyclic aromatic heterocycle. 2 Cl base A simple exercise in the synthesis of a pyridine fused to a pyrrole (or an indole with
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization
![Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization](/thumbs/82/85235286.jpg) Total Synthesis of (+/-)-Goniomitine via a Formal itrile/donor-acceptor Cyclopropane [3 + 2] Cyclization (-)-Goniomitine Christian L. Morales and Brian Pagenkopf* rganic Letters, ASAP Current Literature
Total Synthesis of (+/-)-Goniomitine via a Formal itrile/donor-acceptor Cyclopropane [3 + 2] Cyclization (-)-Goniomitine Christian L. Morales and Brian Pagenkopf* rganic Letters, ASAP Current Literature
A Modular Approach to Polyketide Building Blocks: Cycloadditions of Nitrile Oxides and Homoallylic Alcohols
 A Modular Approach to Polyketide Building Blocks: Cycloadditions of itrile xides and Homoallylic Alcohols rganic Letters, 2005, ASAP ina Lohse-Fraefel and Erick M. Carreira * H H H + ' 1. t-bucl, -78 C
A Modular Approach to Polyketide Building Blocks: Cycloadditions of itrile xides and Homoallylic Alcohols rganic Letters, 2005, ASAP ina Lohse-Fraefel and Erick M. Carreira * H H H + ' 1. t-bucl, -78 C
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
MECHANISMS. Croomine. Key reaction is the vinylogous Mannich reaction. (CH 2 ) 4 Br H N P. CO 2 Me. Iminium ion formation via decarboxylation
 MECAM Croomine Key reaction is the vinylogous Mannich reaction T C 2 Me T C 2 Me (C 2 ) 4 C 2 Me minium ion formation via decarboxylation C 2 Cl 3 Cl ndanomycin The Julia lefination Classical Julia Ar
MECAM Croomine Key reaction is the vinylogous Mannich reaction T C 2 Me T C 2 Me (C 2 ) 4 C 2 Me minium ion formation via decarboxylation C 2 Cl 3 Cl ndanomycin The Julia lefination Classical Julia Ar
Wilkinson s other (ruthenium) catalyst
 Wilkinson s other (ruthenium) catalyst Cl 3 ; 2 h 3, reflux 3h h 3 Cl h 3 h Cl 3 Good catalyst especially for 2 1-alkenes 2, base toluene Cl h 3 h 3 h 3 Et 3 Cl h 3 Cl h 3 h 3 R h 3 h 3 Cl h 3 R RC 2 C
Wilkinson s other (ruthenium) catalyst Cl 3 ; 2 h 3, reflux 3h h 3 Cl h 3 h Cl 3 Good catalyst especially for 2 1-alkenes 2, base toluene Cl h 3 h 3 h 3 Et 3 Cl h 3 Cl h 3 h 3 R h 3 h 3 Cl h 3 R RC 2 C
Suggested solutions for Chapter 34
 s for Chapter 34 34 PRBLEM 1 Predict the structure of the product of this Diels- Alder reaction. C 2 +? 3 Si Can you deal with a moderately complicated Diels- Alder? The diene is electron- rich and will
s for Chapter 34 34 PRBLEM 1 Predict the structure of the product of this Diels- Alder reaction. C 2 +? 3 Si Can you deal with a moderately complicated Diels- Alder? The diene is electron- rich and will
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
Chiral Diol Promoted Boronates Addi3on Reac3ons. Lu Yan Morken Group Boston College
 Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Only five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those five.
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Synthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives
 ynthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives Russell C. mith Denmark Group Meeting 8-9-2005 most extensive project in organic synthesis this phenomenon is
ynthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives Russell C. mith Denmark Group Meeting 8-9-2005 most extensive project in organic synthesis this phenomenon is
CH 3 TMG, DMF N H 3 CO 2 S. (PPh 3 ) 2 Pd 0
 1. (a) rovide a reasonable mechanism for the following transformation. I S 2 C 3 C 3 ( 3 ) 2 2, CuI C 3 TMG, DMF 3 C 2 S TMG = Me 2 Me 2 ICu ( 3 ) 2 0 I S 2 C 3 S 2 C 3 Cu I 3 3 3 C 2 S I 3 3 3 C 2 S 3
1. (a) rovide a reasonable mechanism for the following transformation. I S 2 C 3 C 3 ( 3 ) 2 2, CuI C 3 TMG, DMF 3 C 2 S TMG = Me 2 Me 2 ICu ( 3 ) 2 0 I S 2 C 3 S 2 C 3 Cu I 3 3 3 C 2 S I 3 3 3 C 2 S 3
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines
 Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Organocopper Chemistry
 rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
Radical Reactions. Radical Stability!!! bond dissociation energies X Y X + Y. bond BDE (kcal/mol) bond BDE (kcal/mol) CH 3 CH 3 CH 2 95 O H R 2 C H
 adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Enantioselective Protonations
 Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
Asymmetric Deprotonation
 ( )-sparteine i-pr, t 2, -98 C 84% ee Asymmetric Deprotonation gands, Bases, and Applications CF 3 TMS t-bu TMSCl, MPA TF, -100 C t-bu 93% ee Fe (i-pr) 2 1. n-bu ( )-sparteine t 2, -78 C 2. 2 PCl P 2 Fe
( )-sparteine i-pr, t 2, -98 C 84% ee Asymmetric Deprotonation gands, Bases, and Applications CF 3 TMS t-bu TMSCl, MPA TF, -100 C t-bu 93% ee Fe (i-pr) 2 1. n-bu ( )-sparteine t 2, -78 C 2. 2 PCl P 2 Fe
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02
![Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02](/thumbs/93/114018431.jpg) Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Molybdenum-Catalyzed Asymmetric Allylic Alkylation
 Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
[3,3]-Sigmatropic rearrangements
![[3,3]-Sigmatropic rearrangements [3,3]-Sigmatropic rearrangements](/thumbs/75/71504906.jpg) 1 [3,3]-Sigmatropic rearrangements heat R 1 R 3 R 1 R 3 R 1 R 3 A class of pericyclic reactions whose stereochemical outcome is governed by the geometric requirements of the cyclic transition state Reactions
1 [3,3]-Sigmatropic rearrangements heat R 1 R 3 R 1 R 3 R 1 R 3 A class of pericyclic reactions whose stereochemical outcome is governed by the geometric requirements of the cyclic transition state Reactions
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Discussion Addendum for: Trifluoromethylation at the -Position of, -Unsaturated Ketones: 4-Phenyl-3-Trifluoromethyl-2-Butanone
 DI:10.15227/orgsyn.089.0374 Discussion Addendum for: Trifluoromethylation at the -Position of, -Unsaturated Ketones: 4-Phenyl-3-Trifluoromethyl-2-Butanone C 3 Ph hcl(pph 3 ) 3 C 3 I C 3 Ph T C 3 Prepared
DI:10.15227/orgsyn.089.0374 Discussion Addendum for: Trifluoromethylation at the -Position of, -Unsaturated Ketones: 4-Phenyl-3-Trifluoromethyl-2-Butanone C 3 Ph hcl(pph 3 ) 3 C 3 I C 3 Ph T C 3 Prepared
Chem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below.
 hem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below. TP l Et step 8 4 5 eagents used in synthesis A B D E F a DMF (solvent) 1. (i-pr) 2 Li (LDA)/TF
hem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below. TP l Et step 8 4 5 eagents used in synthesis A B D E F a DMF (solvent) 1. (i-pr) 2 Li (LDA)/TF
Chiral Proton Catalysis in Organic Synthesis. Samantha M. Frawley Organic Seminar September 14 th, 2005
 Chiral Proton Catalysis in rganic Synthesis Samantha M. Frawley rganic Seminar September 14 th, 2005 Seminar utline Introduction Lewis Acid-assisted Chiral Brønsted Acids Enantioselective protonation for
Chiral Proton Catalysis in rganic Synthesis Samantha M. Frawley rganic Seminar September 14 th, 2005 Seminar utline Introduction Lewis Acid-assisted Chiral Brønsted Acids Enantioselective protonation for
Scope: 1. S N 2' Displacements. 2. Michael additions. Not Covered:
 Is There a "Crams's ule" for lefins? Part A C=C cleophile Additions cleophilic lefin Additions?! Any reaction which proceeds through electron donation (pair or radical) into the π* orbital of the olefin
Is There a "Crams's ule" for lefins? Part A C=C cleophile Additions cleophilic lefin Additions?! Any reaction which proceeds through electron donation (pair or radical) into the π* orbital of the olefin
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine N-oxide
 Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols
 A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
Strategies for Catalytic Asymmetric Electrophilic a Halogenation of Carbonyl Compounds
 Strategies for Catalytic Asymmetric Electrophilic a alogenation of Carbonyl Compounds 1 2 Y Catalyst [X + ] 1 X! 2 Y intermann, L. ; Togni, A. Angew. Chem. Int. Ed. 2000, 39, 4359 4362 amashima, Y.; Sodeoka,
Strategies for Catalytic Asymmetric Electrophilic a alogenation of Carbonyl Compounds 1 2 Y Catalyst [X + ] 1 X! 2 Y intermann, L. ; Togni, A. Angew. Chem. Int. Ed. 2000, 39, 4359 4362 amashima, Y.; Sodeoka,
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Short Literature Presentation 10/4/2010 Erika A. Crane
 Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Additions to Metal-Alkene and -Alkyne Complexes
 Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
O H HO H. !-D-galactopyranose
 ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
CHAPTER 24 HW: CARBONYL CONDENSATIONS
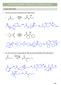 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
B X A X. In this case the star denotes a chiral center.
 Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
CHEM 330. Final Exam December 11, 2007 A N S W E R S. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 11, 2007 Your name: A S W E R S This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 / 20 3. / 30
CEM 330 Final Exam December 11, 2007 Your name: A S W E R S This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 / 20 3. / 30
Answers To Chapter 1 Problems.
 Answers To Chapter Problems.. (a) Both and in amides have lone pairs that can react with electrophiles. When the reacts with an electrophile +, a product is obtained for which two good resonance structures
Answers To Chapter Problems.. (a) Both and in amides have lone pairs that can react with electrophiles. When the reacts with an electrophile +, a product is obtained for which two good resonance structures
Organic Chemistry I (Chem340), Spring Final Exam
 rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
Chem 315/316 Reactions. Bromo organic compounds C1 & C2 carbon skeletons C3 carbon skeletons C4 carbon skeletons. Alcohols. Chem 316 / Beauchamp 1
 hem 316 / Beauchamp 1 hem 315/316 eactions Name available sources of carbon 4 3 NaN methyl 3 2 ethyl 3 2 2 n-propyl 3 3 isopropyl 2 3 2 3 3 2 2 3 3 3 n-butyl sec butyl t-butyl phenyl available until topic
hem 316 / Beauchamp 1 hem 315/316 eactions Name available sources of carbon 4 3 NaN methyl 3 2 ethyl 3 2 2 n-propyl 3 3 isopropyl 2 3 2 3 3 2 2 3 3 3 n-butyl sec butyl t-butyl phenyl available until topic
Corey-Bakshi. Bakshi-Shibata Reduction. Name Reaction Nilanjana Majumdar
 Corey-Bakshi Bakshi-Shibata Reduction Name Reaction Nilanjana Majumdar 02.27.09 utline Introduction Background CBS Reaction Application to Synthesis Introduction Born: 12 th July, 1928 in Methuen, Massachusetts,
Corey-Bakshi Bakshi-Shibata Reduction Name Reaction Nilanjana Majumdar 02.27.09 utline Introduction Background CBS Reaction Application to Synthesis Introduction Born: 12 th July, 1928 in Methuen, Massachusetts,
