Enolates: Z(O,R) (O,R)- and E(O,R) (O,R)-enolates
|
|
|
- Gwendolyn Mosley
- 5 years ago
- Views:
Transcription
1 Enolates: Z(,) (,)- and E(,) (,)-enolates egardless of other groups the encircled ' and - determine whether one is Z(,)- or E(,)- enolate. - - ' ' (E)-enolate (Z)-enolate Enolates: deprotonation 90 ' Most stable conformation 30 ' σ C- ' ' π* C= π* C= σ C- ' ax eq -12 ' - ' (E)-enolate (Z)-enolate Corey, E.J.; neen,.a. J. Am. Chem. oc. 1956, 78, 6269.
2 Effect of base on enolisation! Base must be large and hard.! Thus functions only as a base and not as a nucleophile. i i M Ph Ph i i M - K DA TMP MMD (( 2 Ph) 2 i) t-buk M =, a, K pk a elective formation of E/Z- enolates base TMCl TM TM Z(,) E(,) base Z E Et DA ( 3 i) (Et 3 i) ( 2 Phi) 2 >100 <1 cc 6 11 DA ( 3 i) 2 (Et 3 i) 2 ( 2 Phi) Masamune,. Aldrichimica Acta 1982, 15, 47.
3 Enolisation: Ireland-mechanism! According to the Ireland-mechanism an (E)-enolate is formed via a chair form transition state ( = large alkyl group)! If also is large, a (Z)-enolate is formed!! ote the actual proton abstractor and the role of the metal! " ' - 78 C, Br = Et = i-pr = t-bu : : : >20 Ireland,.E. J. Am. Chem. oc. 1976, 98, Collum, D.B. J. Am. Chem. oc. 1991, 113, Collum, D.B. J. Am. Chem. oc. 1997, 119, Dimeric -enolate - DA complex! First X-ray structure for a dimeric complex. i 200 mol-% DA 3 i i 3 TEE TUCTUE: CCD-code FGIC Willard, P.G. J. Am. Chem. oc. 1987, 109, 5539.
4 Application: Taxol K D t-buk D 2 Ketoni Enolaatti tork, G Approach of the electrophile ouk: 106 o (compare: Burgi-Dunitz angle) B! This angle is similar to the Flippin-odge angle! E - E Agami, C. Tetrahedron ett. 1977, Tetrahedron ett. 1979, Tetrahedron 1979, 35, ouk, K.. J. Am. Chem. oc. 1986,108, side view end view equatorial attack i axial attack E E - e
5 Asymmetric Induction in Enolate and Azaenolate Alkylations Intraligand asymmetric induction intraannular extraannular chelate-mediated intraannular M M M 1 M M * * 1 * M 2 * 2 * * 1,3-1,4-1,2-1,3-1,2-1,3- Interligand asymmetric induction M* M n * Evans, D.A. Asymmetric ynthesis 1984, 3, Controlling Face electivity C 2 tbu I = MPA C 2 tbu 1) DA 2) I tbu tbu I = TF hydrolysis C 2 C 2 Tomioka, K.; Koga, K. J. Am. Chem. oc. 1984, 106, Tomioka, K.; Koga, K. Tetrahedron ett. 1984, 5677.
6 AMP-ydrazones in Ketone Alkylation 2 AMP DA AMP Enders, or 3 -X X = I X = 2 67 %ee 99 %ee Enders, D. Asymmetric ynthesis, vol. 3. Chiral Bicyclic actam Enolates KMn 4 2. Cl xidation of pinene: Carlson,.G.; Pierce, J.K. J. rg. Chem. 1971, 36, eduction of oxime: Masui, M.; hioiri, T. Tetrahedron 1995, 51, acetone 1 1 p-ts, toluene Al 4 2 oth, G.P.; eonard,.f.; Tong,. J. rg. Chem. 1996, 61,
7 Chiral Bicyclic actam Enolates 1 s-bu 1 2 X repeat: s-bu; 3 X =,, Ph 2 =, Bn, allyl 3 =,, Bn exo:endo selectivities typically > 98:2 (except:,, 2:1 oth, G.P.; eonard,.f.; Tong,. J. rg. Chem. 1996, 61, Aldol eaction syn anti Chirality can reside in: nuclephile electrophile catalyst ne of the most extensively studied reactions eview: eathcock, C.. cience 1981, 214, 395.
8 Aldol eaction Ph Ph Ph syn anti Enolate syn anti Z E ipr Z E tbu Z 98 2 Bulky - high selectivity Z-enolate -> syn E-enolate -> anti E 8 92 eathcock, C.. J. rg. Chem. 1980, 45, Generation of E/Z-enolates base TMCl TM TM Z(,) E(,) base Z E Et DA ( 3 i) (Et 3 i) ( 2 Phi) 2 >100 <1 cc 6 11 DA ( 3 i) 2 (Et 3 i) 2 ( 2 Phi) Masamune,. Aldrichimica Acta 1982, 15, 47.
9 Aldol eaction * 1 * 3 2 Possible Transition tates: E 1 3 Z M E 3 1 M Z n Cyclic (chelated) T pen T eviews: eathcock, C.. cience 1981, 214, 395. eathcock, C.. Aldrichimica Acta 1990, 23, 99. offmann,.w. Angew. Chem. Int. Ed., Engl. 1987, 26, 488. Mukaiyama, T. rg. eact. 1982, 28, 203.! Type I Classification of aldols follow Zimmermann-Traxler T! Type II open T; syn-selective enol silanes, stannanes, borates, zirconates! Type III open T; anti-selective ketene acetals and thioacetals
10 Aldol - Zimmermann-Traxler T 1 M n 2 1 M anti 2 E(,)-enolate 2 13 M syn Aldol - Zimmermann-Traxler T M n 1 M syn 2 1 Z(,)-enolate 2 13 M anti Diastereoselectivity maximized when 1 and 3 large Diastereoselectivity: B > > a > K - Mg- Zn- Al- B- Ti- Zr Å Å Å 1.92 Å Å Å 2.15 Å
11 Aldol - pen T M M 1 E(,)-enolate anti Z(,)-enolate M E(,)-enolate syn M Z(,)-enolate Boron Enolates X 1 X B 2 Tf 1 X B 2 1 e B X 2 i 1 X B 2 1 Z(,)-enolate typical outcome i X 1 B 2 e X B 2 1 E(,)-enolate can be favored: = cc 6 11 X = tbu Evans, D.A. J. Am. Chem. oc. 1981, 103,
12 Boron Enolate diated Aldol CEt 3 B Tf DIPEA B CEt 3 C - 78 C CEt 3 anti:syn 33: %ee For a similar example, see also: eetz, M.T. Tetrahedron ett. 1986, 27, Masamune,. J. Am. Chem. oc. 1986, 108, Evans Aldol: : on-coordinating tal 2 B α-attack B Bu B Bu 2 B β-attack Explanation: opposing dipoles! Evans, D.A. J. Am. Chem. oc. 1981, 103, 2127.
13 Evans Aldol - Coordinating tal M E i attack E Chelated Z(,)-enolate M M E e attack E on-chelated Z(,)-enolate Evans, D.A. J. Am. Chem. oc. 1982, 104, Evans Aldol DA amd M E, 0 o C E, -78 o C E Ph Ph E E = I, EtI, BnBr, allylbr kinetic ratio > 94 : 6 Evans, D.A. J. Am. Chem. oc. 1982, 104, 1737.
14 ynthesis of BMT,, Amino Acid in Cyclosporine Bn 1) amd 2) I 3) A 4) wern C Bn n(tf) 2 1) 3 BF 4-2) 2 3) K 4) 3 X* C X* n Evans, D.A. J. Am. Chem. oc. 1986, 108, on-evans syn-aldol: Ti Enolates 4 Ti 3 Ti GMUP Thornton, E.. J. rg. Chem. 1991, 56, X-ray: interman, T.; Deebach, D. elv. Chim. Acta 1998, 81, 2093.
15 pen Transition tate: Effect of ewis Acid B A small ewis acids syn B A large ewis acids anti eathcock, C.. J. rg. Chem. 1990, 55, 173. eathcock, C.. J. rg. Chem. 1991, 56, hioiri, T. Tetrahedron ett. 1991, 32, All Four Aldols from a ingle Precursor: eathcock t - Bu TB t - Bu B t - Bu TB TB Mg t - Bu TB Ti t - Bu TB eathcock, C.. J. rg. Chem. 1991, 56, eathcock, C.. Aldrichimica Acta 1990, 23, 99.
16 ummary of Best Aldols B-enolate Ti enolate B-enolate A syn (1) syn (2) anti Evans Thornton eatcock Ph B-enolate i-enol ether syn anti ppolzer ppolzer Chiral Catalysis in Aldol: : Corey 2 *BBr B 2 * tbu -C 2*BBr tbu 2 *B Br Et3 CF 3 CF 3 Ph Ph CF 3 2 B 2 CF 3 Br 2 *BBr Ph Ar Ar 2 B Ph 2 Ph Ester enolates: anti products; thioesters: syn products Corey, E.J. J. Am. Chem. oc. 1990, 112, 4976.
17 Chiral Acyloxyborolidines "Anomeric" i 3 ' "C CAB ' " ' B C C 2 anti-coordination C 2 C 2 B3 ' CAB Yamamoto,. J. Am. Chem. oc. 1991, 113, X-ray AVM: Yamamoto,. J. Am. Chem. oc. 1993, 115, syn-elective Boron Aldol approach from least hindered enolate diastereoface BBu 2 dipoles? Bu B Bu i Pr Bu B Bu
18 Acetate aldol i mol-% cat., -10 o C then TBAF t Bu Ti t Bu Bu t t Bu Aldehyde C C C Ph C Ph C C 6 11 Ph C %ee: Carreira, E. J. Am. Chem. oc. 1994, 116, Acetate aldol mith, A.B. rg. ett. 1999, 1,
19 Acetate aldol 1 2 i 3 t Bu 10 mol-% cat., -10 o C then 1 M Cl 2 1 t Bu Bu t Cu t Bu 2 Tf - 1 Bn t Bu Et 2 Et i Bu i Bu %ee: Evans, D.A. J. Am. Chem. oc. 1997, 119, Cyclic enolates: anti-elective Aldols M n M n yield anti:syn B 57 19:1 nph 3 n :1 24:1 n M Ti( i Pr) :1 ayashi, T. Tetrahedron ett. 1991, 32, 5369.
20 Amphotericin 2 C () 2 P 2 P P Calyculin C 2 C Amphotericin B Alkylation Towards Amphotericin B DA, TMCl -78 o C, TF 64 % TiCl 4, PhC C 2 Cl 2, -78 o C i 3 Major product Karisalmi, K. Tetrahedron 2003, 59,
21 Final steps 6 aney ickel (W-2) Et, o C 50 % 7 Karisalmi, K. Tetrahedron 2003, 59, yn-aldol from Z-enolateZ weakly 17-directing BBu 2 Bn TBDM C 2 Cl 2, -78 to -26 o C, 16 h 69 %, 82 %ds Bn TBDM weakly 17-directing Bafilomycin A Paterson, I. Tetrahedron ett. 1995, 36, 175.
22 Anti-aldols from E-enolates Bu t t Bu aldol 55 %; 19:1 ds Bu t t Bu 1. g(ccf 3 ) 2 2. PdCl 2, C,, CuCl % Ac C 2 C1-C8 sequence of pamamycins Pamamycin Walkup,.D.; Kim, Y.. Tetrahedron ett. 1995, 36, Anti-aldol from E-enolateE Bu t t Bu aldol 64 %; 2:1 ds Bu t t Bu eathcock T 1981, 37, Felkin-Anh Ar Bu t t Bu Pilli,.A.; Murta, M.M. J. rg. Chem. 1993, 58, 338.
23 Anti-aldol for macrolide synthesis aldol 50 % Felkin-Anh Ar Tamm, C. ynthesis 1991, 435.
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
Stereoselective reactions of enolates
 1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
Stereoselective reactions of the carbonyl group
 1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
Lewis Base Catalysis: the Aldol Reaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk
 Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
Stereoselective reactions of enolates: auxiliaries
 1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
Requirements for an Effective Chiral Auxiliary Enolate Alkylation
 Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Stereoselective Organic Synthesis
 Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Total synthesis of Spongistatin
 Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Stereoselective Organic Synthesis
 Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Chiral Auxiliaries. attach auxiliary Substrate Substrate Auxiliary
 Chiral Auxiliaries Previously on Advanced ynthesis... Discussed the need for stereoselective synthesis Looked at the use of resolution, the chiral pool and substrate control t there are some potential
Chiral Auxiliaries Previously on Advanced ynthesis... Discussed the need for stereoselective synthesis Looked at the use of resolution, the chiral pool and substrate control t there are some potential
o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)]
![o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)] o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)]](/thumbs/85/92754396.jpg) 3. Boron -- eview [Suzuki Chem. ev. 95, 2457 (1995)] U77b ydroboration also attractive but B Pd transmetallation difficult - must produce stable B product - solved (by Suzuki) by adding base to make Borates
3. Boron -- eview [Suzuki Chem. ev. 95, 2457 (1995)] U77b ydroboration also attractive but B Pd transmetallation difficult - must produce stable B product - solved (by Suzuki) by adding base to make Borates
Chiral Catalyst II. Palladium Catalysed Allylic Displacement ( -allyl complexes) 1. L n Pd(0) 2. Nuc
 Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Synthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives
 ynthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives Russell C. mith Denmark Group Meeting 8-9-2005 most extensive project in organic synthesis this phenomenon is
ynthetic Developments Towards the Preparation of Erythromycin and Erythronolide Derivatives Russell C. mith Denmark Group Meeting 8-9-2005 most extensive project in organic synthesis this phenomenon is
Scope: 1. S N 2' Displacements. 2. Michael additions. Not Covered:
 Is There a "Crams's ule" for lefins? Part A C=C cleophile Additions cleophilic lefin Additions?! Any reaction which proceeds through electron donation (pair or radical) into the π* orbital of the olefin
Is There a "Crams's ule" for lefins? Part A C=C cleophile Additions cleophilic lefin Additions?! Any reaction which proceeds through electron donation (pair or radical) into the π* orbital of the olefin
[3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S
![[3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S [3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S](/thumbs/94/119475672.jpg) Generalized igmatropic Processes [3,3]-sigmatropic Processes 1 3,=C,,, 1 3 3,=C,,, 3 [2,3]-sigmatropic Processes 1 3,=C,,, 1 3 Ene eactions 1 3 1 3 Cope earrangement [3,3]- igmatropic earrangements Transition
Generalized igmatropic Processes [3,3]-sigmatropic Processes 1 3,=C,,, 1 3 3,=C,,, 3 [2,3]-sigmatropic Processes 1 3,=C,,, 1 3 Ene eactions 1 3 1 3 Cope earrangement [3,3]- igmatropic earrangements Transition
CHEM 330. Final Exam December 5, 2014 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
Denmark s Base Catalyzed Aldol/Allylation
 Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
When something goes wrong. Goya: Mother showing her derformed child to two women Louvre, Paris
 1 ew Catalytic Asymmestric eactions Karl Anker Jørgensen Danish ational eserach Foundation: Center for Catalysis Department of Chemistry, Aarhus University Denmark kaj@chem.au.dk When something goes wrong
1 ew Catalytic Asymmestric eactions Karl Anker Jørgensen Danish ational eserach Foundation: Center for Catalysis Department of Chemistry, Aarhus University Denmark kaj@chem.au.dk When something goes wrong
Chiral Catalysis. Chiral Catalyst. Substrate. Chiral Catalyst
 Chiral Catalysis Chiral (stoichiometric) reagents are a very important class of compound but... eed a stoichiometric quantity of the chiral component Unless it is cheap or recoverable this is not very
Chiral Catalysis Chiral (stoichiometric) reagents are a very important class of compound but... eed a stoichiometric quantity of the chiral component Unless it is cheap or recoverable this is not very
transmetallate displace ox. add. M + (insert) (β-elim.)
 Chapter IV. Transition Metal σ-alkyl Complexes I. General For much of the rest of this course it will be necessary to understand how σ-alkyl metal complexes are formed and how they react. This is summarized
Chapter IV. Transition Metal σ-alkyl Complexes I. General For much of the rest of this course it will be necessary to understand how σ-alkyl metal complexes are formed and how they react. This is summarized
Asymmetric Lewis Base Strategies for Heterocycle Synthesis
 Asymmetric Lewis Base trategies for eterocycle ynthesis Dr Andrew mith EatCEM, chool of Chemistry, University of t Andrews 1st cottish-japanese ymposium of rganic Chemistry, University of Glasgow Friday
Asymmetric Lewis Base trategies for eterocycle ynthesis Dr Andrew mith EatCEM, chool of Chemistry, University of t Andrews 1st cottish-japanese ymposium of rganic Chemistry, University of Glasgow Friday
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chemistry of the Double Bond
 Chemistry of the Double ond. eactions of the Carbonyl Group. At Carbonyl.. eduction (hydride addition)..2 kylation..3 lylation/propargylation.2 At α-center (Enolate Chemistry).2. kylation.2.2 dol eaction.3
Chemistry of the Double ond. eactions of the Carbonyl Group. At Carbonyl.. eduction (hydride addition)..2 kylation..3 lylation/propargylation.2 At α-center (Enolate Chemistry).2. kylation.2.2 dol eaction.3
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of H X 2. Addition of H OH and addition of Y X 3. Addition to allene and
 Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Structure and Reactivity
 Structure and eactivity Fall Semester 2007 Summary Prof. Jérôme Waser C 4306 021 693 93 88 jerome.waser@epfl.ch Assistant: Simone onazzi C 4401 021 693 94 46 simone.bonazzi@epfl.ch Structure and eactivity
Structure and eactivity Fall Semester 2007 Summary Prof. Jérôme Waser C 4306 021 693 93 88 jerome.waser@epfl.ch Assistant: Simone onazzi C 4401 021 693 94 46 simone.bonazzi@epfl.ch Structure and eactivity
Strategies for Catalytic Asymmetric Electrophilic a Halogenation of Carbonyl Compounds
 Strategies for Catalytic Asymmetric Electrophilic a alogenation of Carbonyl Compounds 1 2 Y Catalyst [X + ] 1 X! 2 Y intermann, L. ; Togni, A. Angew. Chem. Int. Ed. 2000, 39, 4359 4362 amashima, Y.; Sodeoka,
Strategies for Catalytic Asymmetric Electrophilic a alogenation of Carbonyl Compounds 1 2 Y Catalyst [X + ] 1 X! 2 Y intermann, L. ; Togni, A. Angew. Chem. Int. Ed. 2000, 39, 4359 4362 amashima, Y.; Sodeoka,
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Chiral Diol Promoted Boronates Addi3on Reac3ons. Lu Yan Morken Group Boston College
 Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Additions to Metal-Alkene and -Alkyne Complexes
 Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Asymmetric Alklylation of Enolates
 Asymmetric Alklylation of Enolates M with material from A G Meyers http://faculty.chemistry.harvard.edu/myers/pages/chem-215-handouts 745 rganic Synthesis Spring 2015 Asymmetric Alkylation - eed to control
Asymmetric Alklylation of Enolates M with material from A G Meyers http://faculty.chemistry.harvard.edu/myers/pages/chem-215-handouts 745 rganic Synthesis Spring 2015 Asymmetric Alkylation - eed to control
CHEM 330. Final Exam December 8, 2010 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 8, 2010 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 20 4.
CEM 330 Final Exam December 8, 2010 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 20 4.
[3,3]-Sigmatropic rearrangements
![[3,3]-Sigmatropic rearrangements [3,3]-Sigmatropic rearrangements](/thumbs/75/71504906.jpg) 1 [3,3]-Sigmatropic rearrangements heat R 1 R 3 R 1 R 3 R 1 R 3 A class of pericyclic reactions whose stereochemical outcome is governed by the geometric requirements of the cyclic transition state Reactions
1 [3,3]-Sigmatropic rearrangements heat R 1 R 3 R 1 R 3 R 1 R 3 A class of pericyclic reactions whose stereochemical outcome is governed by the geometric requirements of the cyclic transition state Reactions
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation Reactions of Substituted Ketenes
 Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
MECHANISMS. Croomine. Key reaction is the vinylogous Mannich reaction. (CH 2 ) 4 Br H N P. CO 2 Me. Iminium ion formation via decarboxylation
 MECAM Croomine Key reaction is the vinylogous Mannich reaction T C 2 Me T C 2 Me (C 2 ) 4 C 2 Me minium ion formation via decarboxylation C 2 Cl 3 Cl ndanomycin The Julia lefination Classical Julia Ar
MECAM Croomine Key reaction is the vinylogous Mannich reaction T C 2 Me T C 2 Me (C 2 ) 4 C 2 Me minium ion formation via decarboxylation C 2 Cl 3 Cl ndanomycin The Julia lefination Classical Julia Ar
TMSCl imidazole DMF. Ph Ph OTMS. Michael reaction. Michael reaction Ph R 3. epoxidation O R
 eaction using diarylprolinol silyl ether derivatives as catalyst 1) C Et K C 3, ) MgBr, TF TMS hexane, 0 o C TBS p- C 6 4, T C Et 85%, 99% ee Angew. Chem., nt. Ed., 44, 41 (005). rg. Synth., 017, 94, 5.
eaction using diarylprolinol silyl ether derivatives as catalyst 1) C Et K C 3, ) MgBr, TF TMS hexane, 0 o C TBS p- C 6 4, T C Et 85%, 99% ee Angew. Chem., nt. Ed., 44, 41 (005). rg. Synth., 017, 94, 5.
Organocopper Reagents
 rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
Memory of Chirality: A Strategy for Asymmetric Synthesis
 Memory of Chirality: A trategy for Asymmetric ynthesis David J. Richard eptember 14, 2005 Two Forms of Chirality Absolute (tatic) Chirality 2 - Absolute chirality - orientation of functional groups at
Memory of Chirality: A trategy for Asymmetric ynthesis David J. Richard eptember 14, 2005 Two Forms of Chirality Absolute (tatic) Chirality 2 - Absolute chirality - orientation of functional groups at
VI. Metal alkyls from oxidative addition / insertion
 V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols
 A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Keisuke Suzuki. Baran lab Group Meeting 6/11/16. Shigenobu Umemiya. Akira Suzuki. Takanori Suzuki (Hokkaido University)
 197.D., Teruaki Mukaiyama, University of Tokyo 193 Assistant Professor, Keio University 197 Lecturer, Keio University 199 Assocate Professor, Keio University 1990 Visiting Professor, ET 1994 ull Professor,
197.D., Teruaki Mukaiyama, University of Tokyo 193 Assistant Professor, Keio University 197 Lecturer, Keio University 199 Assocate Professor, Keio University 1990 Visiting Professor, ET 1994 ull Professor,
Enantioselective Protonations
 Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Renaud Group Exercise Set
 Renaud Group Exercise Set Prepared by ick Tappin 08/07/16 Spectroscopy 1. Deduce the structures for compounds A, B, and C C 6 10 3 A IR: 1745 and 1720 cm -1 13 C-MR: δ 208, 172, 51, 37, 32, and 27 ab 4
Renaud Group Exercise Set Prepared by ick Tappin 08/07/16 Spectroscopy 1. Deduce the structures for compounds A, B, and C C 6 10 3 A IR: 1745 and 1720 cm -1 13 C-MR: δ 208, 172, 51, 37, 32, and 27 ab 4
James D. White. A very productive professor 64 students graduated from his lab 94 postdocs have worked in his lab. Education Experience
 A very productive professor 64 students graduated from his lab 94 postdocs have worked in his lab Education Experience Fraser Fleming University of Drexel Pavel agory University of Michigan Cambridge University,
A very productive professor 64 students graduated from his lab 94 postdocs have worked in his lab Education Experience Fraser Fleming University of Drexel Pavel agory University of Michigan Cambridge University,
Lecture 1 ADVANCED SYNTHESIS Stereochemistry Introduction
 ecture 1 ADVACED YTEI tereochemistry Introduction ne of the most important issues in modern organic synthesis ost natural compounds are enantiomerically pure Frequently different enantiomers have different
ecture 1 ADVACED YTEI tereochemistry Introduction ne of the most important issues in modern organic synthesis ost natural compounds are enantiomerically pure Frequently different enantiomers have different
a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of Homoallylic Primary Amines
 a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of omoallylic Primary Amines 1 3 2 3 ML n 1 2 2 3 Masaharu Sugiura, Keiichi irano and Shu Kobayashi JACS ASAP ryan Wakefield @ Wipf
a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of omoallylic Primary Amines 1 3 2 3 ML n 1 2 2 3 Masaharu Sugiura, Keiichi irano and Shu Kobayashi JACS ASAP ryan Wakefield @ Wipf
Highlights of Schmidt Reaction in the Last Ten Years
 ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
Synthesis of Amphidinolide X and an Exploration of Key Reactions
 PJM 1/12/05 Synthesis of Amphidinolide X and an Exploration of Key eactions Lepage,.; Kattnig, E.; Furstner, A. JACS, 2004, 126, 15970-15971. 7 13 1 6 19 - Produced by marine dinoflagellates, Amphidinium
PJM 1/12/05 Synthesis of Amphidinolide X and an Exploration of Key eactions Lepage,.; Kattnig, E.; Furstner, A. JACS, 2004, 126, 15970-15971. 7 13 1 6 19 - Produced by marine dinoflagellates, Amphidinium
Lecture 6: Transition-Metal Catalysed C-C Bond Formation
 Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Carbonyl Chemistry IV: Enolate Alkylations and Aldols. Aldol Madness O O O N M + substrate. aldehyde. (Z)-enolate H
 Carbonyl Chemistry IV: nolate Alkylations and Aldols Paul Bracher Chem 30 Section 9 Section Agenda 1) o office hours Thursday 2) The Great Joe Young is covering section next onday 3) andout: Carbonyl Chemistry
Carbonyl Chemistry IV: nolate Alkylations and Aldols Paul Bracher Chem 30 Section 9 Section Agenda 1) o office hours Thursday 2) The Great Joe Young is covering section next onday 3) andout: Carbonyl Chemistry
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
Asymmetric Radical Reactions. Zhen Liu 08/30/2018
 Asymmetric adical eactions Zhen Liu 08/30/2018 Contents Introduction eactions Using Chiral Auxiliary Chiral Lewis Acid-diated eactions Transition tal-catalyzed eactions eactions Using Chiral rganocatalysts
Asymmetric adical eactions Zhen Liu 08/30/2018 Contents Introduction eactions Using Chiral Auxiliary Chiral Lewis Acid-diated eactions Transition tal-catalyzed eactions eactions Using Chiral rganocatalysts
Asymmetric Nucleophilic Catalysis
 Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
Guideline 5: Tactical Bonds
 Guideline 5: Tactical Bonds f the molecule contains more than one functional group and hence there is a choice of which bonds to disconnect, how do you decide? Practice, but here are a few rough guidelines
Guideline 5: Tactical Bonds f the molecule contains more than one functional group and hence there is a choice of which bonds to disconnect, how do you decide? Practice, but here are a few rough guidelines
Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang!
 1! Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang! 2! utline! 1. Brief Introduction! 2. ucleophilic Dominoes! 3. Electrophilc Dominoes! 4. Radical
1! Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang! 2! utline! 1. Brief Introduction! 2. ucleophilic Dominoes! 3. Electrophilc Dominoes! 4. Radical
Shi Asymmetric Epoxidation
 Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Chiral Proton Catalysis in Organic Synthesis. Samantha M. Frawley Organic Seminar September 14 th, 2005
 Chiral Proton Catalysis in rganic Synthesis Samantha M. Frawley rganic Seminar September 14 th, 2005 Seminar utline Introduction Lewis Acid-assisted Chiral Brønsted Acids Enantioselective protonation for
Chiral Proton Catalysis in rganic Synthesis Samantha M. Frawley rganic Seminar September 14 th, 2005 Seminar utline Introduction Lewis Acid-assisted Chiral Brønsted Acids Enantioselective protonation for
ANSWER GUIDE APRIL/MAY 2006 EXAMINATIONS CHEMISTRY 249H
 AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
Back to Sugars: Enzymatic Synthesis
 Back to Sugars: Enzymatic Synthesis Zhensheng Ding ov. 04 orthrup, A. B.; M acm illan, D. W. C. Science 2004, 305, 1752 orthrup, A. B. and MacMillan, D. W. C. J. Am. Chem. Soc. 2002, 124, 6798-6799 orthrup,
Back to Sugars: Enzymatic Synthesis Zhensheng Ding ov. 04 orthrup, A. B.; M acm illan, D. W. C. Science 2004, 305, 1752 orthrup, A. B. and MacMillan, D. W. C. J. Am. Chem. Soc. 2002, 124, 6798-6799 orthrup,
Chapter 5 Three and Four-Membered Ring Systems
 Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Thione-Based Auxiliaries in Organic Synthesis
 Thione-Based Auxiliaries in rganic ynthesis C 2 An Evans Group Evening eminar Wade Downey Friday, March 1, 2002 00-title 2/28/02 6:46 PM Thione-Based Auxiliaries in rganic ynthesis Thiazolidinethione xazolidinethione
Thione-Based Auxiliaries in rganic ynthesis C 2 An Evans Group Evening eminar Wade Downey Friday, March 1, 2002 00-title 2/28/02 6:46 PM Thione-Based Auxiliaries in rganic ynthesis Thiazolidinethione xazolidinethione
Mild Cobalt-Catalyzed Hydrocyanation of Olefins with Tosyl Cyanide
 Mild Cobalt-Catalyzed ydrocyanation of lefins with Tosyl Cyanide 1 3 2 + Ts Co cat., Si 3 Et, 1-3 h, T 1 2 3 Gaspar, B.; Carreira, E. M. Angew. Chem. Int. Ed. ASAP Current Literature Kalyani Patil 12 May
Mild Cobalt-Catalyzed ydrocyanation of lefins with Tosyl Cyanide 1 3 2 + Ts Co cat., Si 3 Et, 1-3 h, T 1 2 3 Gaspar, B.; Carreira, E. M. Angew. Chem. Int. Ed. ASAP Current Literature Kalyani Patil 12 May
Direct Organocatalytic Enantioselective Mannich Reactions of Ketimines: An Approach to Optically Active Quaternary α-amino Acid Derivatives
 Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Disulfonimide-Catalyzed Asymmetric Vinylogous and Bisvinylogous Mukaiyama Aldol Reactions
 Disulfonimide-Catalyzed Asymmetric Vinylogous and Bisvinylogous Mukaiyama Aldol eactions atjen, L. Garcia-Garcia, P., Lay, F., Beck, M. E., List, B.; Angew. Chem. Int. Ed. 2010, ASAP. Convergent Total
Disulfonimide-Catalyzed Asymmetric Vinylogous and Bisvinylogous Mukaiyama Aldol eactions atjen, L. Garcia-Garcia, P., Lay, F., Beck, M. E., List, B.; Angew. Chem. Int. Ed. 2010, ASAP. Convergent Total
A Concise Synthesis of ( )- Aplyviolene Facilitated by a Stragetegic Ter<ary Radical Conjugate Addi<on
 A Concise Synthesis of ( )- Aplyviolene Facilitated by a Stragetegic Ter
A Concise Synthesis of ( )- Aplyviolene Facilitated by a Stragetegic Ter
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Enantioselective Benzoin Reactions
 Enantioselective Benzoin Reactions GUAQU ZAG DEMARK GRUP PREETATI 2016.02.16 1 Benzoin benzoin benzoic acid Industrially used in powder coating to prevent pinholes In organic chemistry used to prepare
Enantioselective Benzoin Reactions GUAQU ZAG DEMARK GRUP PREETATI 2016.02.16 1 Benzoin benzoin benzoic acid Industrially used in powder coating to prevent pinholes In organic chemistry used to prepare
A Modular Approach to Polyketide Building Blocks: Cycloadditions of Nitrile Oxides and Homoallylic Alcohols
 A Modular Approach to Polyketide Building Blocks: Cycloadditions of itrile xides and Homoallylic Alcohols rganic Letters, 2005, ASAP ina Lohse-Fraefel and Erick M. Carreira * H H H + ' 1. t-bucl, -78 C
A Modular Approach to Polyketide Building Blocks: Cycloadditions of itrile xides and Homoallylic Alcohols rganic Letters, 2005, ASAP ina Lohse-Fraefel and Erick M. Carreira * H H H + ' 1. t-bucl, -78 C
Use of Cp 2 TiCl in Synthesis
 Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
A 1,3 Strain and the Anomeric Effect. Michael Shaghafi Chem. Topics Feb. 6, 2012
 A 1,3 Strain and the Anomeric Effect Michael Shaghafi Chem. Topics Feb. 6, 2012 Introduction: Definition of A 1,3 Strain m L L m m 3 L 3 1 1 otation about σ-bond between α-stereocenter and olefin is associated
A 1,3 Strain and the Anomeric Effect Michael Shaghafi Chem. Topics Feb. 6, 2012 Introduction: Definition of A 1,3 Strain m L L m m 3 L 3 1 1 otation about σ-bond between α-stereocenter and olefin is associated
Organic Electron Donors
 rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
Organocopper Chemistry
 rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
Hennoxazole A. Philip Williams Group Meeting December 12, OMe. OMe 1 6 O H
 ennoxazole A 1 6 11 17 24 Philip Williams Group eting December 12, 2007 Discovered Discovered by Paul cheuer at the University of awaii in 1991. Isolated 480mg ennoxazole A from 4.5kg from the sponge Polyfibrospongia
ennoxazole A 1 6 11 17 24 Philip Williams Group eting December 12, 2007 Discovered Discovered by Paul cheuer at the University of awaii in 1991. Isolated 480mg ennoxazole A from 4.5kg from the sponge Polyfibrospongia
STEREOELECTRONIC EFFECTS (S.E.) IN ORGANIC CHEMISTRY
 STEEELECTNIC EFFECTS (S.E.) IN GANIC CEMISTY Pierre Deslongchamps (version du 5 février 2010) Cf. pour le livre: http://pages.usherbrooke.ca/pdeslongchamps/cv.htm 1 SECTIN 2 Stereoelectronic Effects (S.E.)
STEEELECTNIC EFFECTS (S.E.) IN GANIC CEMISTY Pierre Deslongchamps (version du 5 février 2010) Cf. pour le livre: http://pages.usherbrooke.ca/pdeslongchamps/cv.htm 1 SECTIN 2 Stereoelectronic Effects (S.E.)
Total Synthesis of ( )-Virginiamycin M2
 Total Synthesis of ( )-Virginiamycin M2 Jie Wu and James S. Panek, Angewandte Chemie International Edition, 2010, 49, 6165-6168 btained from the CDC Public ealth Image Library. Image credit: CDC/Dr. David
Total Synthesis of ( )-Virginiamycin M2 Jie Wu and James S. Panek, Angewandte Chemie International Edition, 2010, 49, 6165-6168 btained from the CDC Public ealth Image Library. Image credit: CDC/Dr. David
O or R E + R. - Keto-Enol Tautomerization (enol form usually very minor for simple ketones)
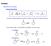 General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
Asymmetric Organocatalysis. Andrew Satterfield
 Asymmetric rganocatalysis Andrew Satterfield 2-24 - 06 utline ase Transfer Catalysis Cinchona alkaloid derived catalysts Catalyst developed by Lygo Catalysts developed by Maruoka Enamine Catalysis Proline
Asymmetric rganocatalysis Andrew Satterfield 2-24 - 06 utline ase Transfer Catalysis Cinchona alkaloid derived catalysts Catalyst developed by Lygo Catalysts developed by Maruoka Enamine Catalysis Proline
R 2 R 4 Ln catalyst. This manuscript describes the methods for the synthesis and application of group 4 metallocene bis(trimethylsilyl)acetylene
 VII Abstracts 2011 p1 2.12.15 rganometallic Complexes of Scandium, Yttrium, and the Lanthanides P. Dissanayake, D. J. Averill, and M. J. Allen This manuscript is an update to the existing Science of Synthesis
VII Abstracts 2011 p1 2.12.15 rganometallic Complexes of Scandium, Yttrium, and the Lanthanides P. Dissanayake, D. J. Averill, and M. J. Allen This manuscript is an update to the existing Science of Synthesis
Huang, C.; Gevorgyan, V. J. Am. Chem. Soc. 2009, 131, Daniel Tzvi Cohen Short Literature Feb. 23, MeO HO OH. COOH ( )-Plicatic Acid OH OH
 Asymmetric Total Synthesis of ( )-Plicatic Acid via a Highly Enantioselective and Diastereoselective Nucleophilic Epoxidation of Acyclic Trisubstituted lefins H H H H CH ( )-Plicatic Acid H H Sun, B.F.;
Asymmetric Total Synthesis of ( )-Plicatic Acid via a Highly Enantioselective and Diastereoselective Nucleophilic Epoxidation of Acyclic Trisubstituted lefins H H H H CH ( )-Plicatic Acid H H Sun, B.F.;
Answers To Chapter 7 Problems.
 Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
CEM 852 Exam What is the ratio of (S) / (R) alcohol formed during this reaction? (2 pts) baker's yeast. H 2 O, sucrose 25 C
 CEM 852 Exam-1 February 19, 2005 This exam consists of 5 pages. Please write ALL your answers in the answer books. Please write legibly and draw all structures clearly. Good luck. 1. What is the ratio
CEM 852 Exam-1 February 19, 2005 This exam consists of 5 pages. Please write ALL your answers in the answer books. Please write legibly and draw all structures clearly. Good luck. 1. What is the ratio
I. Liu Lab. Ka<e Boknevitz 1
 A ighly Convergent Total Synthesis of Leustroducsin B Barry M. Trost,* Berenger Biannic, Cheyenne S. Brindle, B. Michael Keefe, Thomas J. unger, and Ming-Yu gai Department of Chemistry, Stanford University,
A ighly Convergent Total Synthesis of Leustroducsin B Barry M. Trost,* Berenger Biannic, Cheyenne S. Brindle, B. Michael Keefe, Thomas J. unger, and Ming-Yu gai Department of Chemistry, Stanford University,
O H HO H. !-D-galactopyranose
 ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
UNIVERSITY OF MANITOBA DEPARTMENT OF CHEMISTRY
 PAGE 1 of 7 UNIVERSITY F MANITBA DEPARTMENT F CEMISTRY 2.339 STRUCTURAL TRANSFRMATINS IN RGANIC CEMISTRY FINAL EAMINATIN Dr. Phil ultin Thursday December 14, 2000. NAME: ANSWERS STUDENT NUMBER: 1) (15
PAGE 1 of 7 UNIVERSITY F MANITBA DEPARTMENT F CEMISTRY 2.339 STRUCTURAL TRANSFRMATINS IN RGANIC CEMISTRY FINAL EAMINATIN Dr. Phil ultin Thursday December 14, 2000. NAME: ANSWERS STUDENT NUMBER: 1) (15
Zr-Catalyzed Carbometallation
 -Catalyzed Carbometallation C C C C ML n C C ML n ML n C C C C ML n ML n C C ML n Wipf Group esearch Topic Seminar Juan Arredondo November 13, 2004 Juan Arredondo @ Wipf Group 1 11/14/2004 Carbometallation
-Catalyzed Carbometallation C C C C ML n C C ML n ML n C C C C ML n ML n C C ML n Wipf Group esearch Topic Seminar Juan Arredondo November 13, 2004 Juan Arredondo @ Wipf Group 1 11/14/2004 Carbometallation
Reduction. Boron based reagents. NaBH 4 / NiCl 2. Uses: Zn(BH 4 ) 2. Preparation: Good for base sensitive groups Chelation control model.
 Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
Syntheses of Leucascandrolide A. Supergroup Meeting August 4 th, 2004 Yu Yuan
 Syntheses of Leucascandrolide A Supergroup Meeting August 4 th, 2004 Yu Yuan Leucascandrolide A Me Me Me Dambrosio, M.; Guerriero, A.; Debitus, C.; Pietra, F. elvetica Chimica Acta 1996, 79, 51-60 Me 1
Syntheses of Leucascandrolide A Supergroup Meeting August 4 th, 2004 Yu Yuan Leucascandrolide A Me Me Me Dambrosio, M.; Guerriero, A.; Debitus, C.; Pietra, F. elvetica Chimica Acta 1996, 79, 51-60 Me 1
CHEM 330. Final Exam December 11, 2007 A N S W E R S. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 11, 2007 Your name: A S W E R S This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 / 20 3. / 30
CEM 330 Final Exam December 11, 2007 Your name: A S W E R S This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 / 20 3. / 30
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02
![Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02](/thumbs/93/114018431.jpg) Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Conjugate (1,4-) addition
 1 Conjugate (1,4-) addition uc R 1 R 2 uc R 1 R 2 uc R 1 E R 2 E ucleophilic attack on C=C bond normally requires electron deficient alkene Know as 1,4-addition or conjugate addition As enolate formed
1 Conjugate (1,4-) addition uc R 1 R 2 uc R 1 R 2 uc R 1 E R 2 E ucleophilic attack on C=C bond normally requires electron deficient alkene Know as 1,4-addition or conjugate addition As enolate formed
Ready; Catalysis Conjugate Addition
 eady; Catalysis Conjugate Addition Topics covered 1. 1,4 addition involving copper a. stoichiometric reactions b. catalytic reactions c. allylic substitution. Conjugate addition without copper a. Ni-based
eady; Catalysis Conjugate Addition Topics covered 1. 1,4 addition involving copper a. stoichiometric reactions b. catalytic reactions c. allylic substitution. Conjugate addition without copper a. Ni-based
π-alkyne metal complex and vinylidene metal complex in organic synthesis
 Literature Seminar 080220 Kenzo YAMATSUGU (D1) π-alkyne metal complex and vinylidene metal complex in organic synthesis 0. Introduction ' ' = π-alkyne metal complex vinylidene metal complex ecently, electrophilic
Literature Seminar 080220 Kenzo YAMATSUGU (D1) π-alkyne metal complex and vinylidene metal complex in organic synthesis 0. Introduction ' ' = π-alkyne metal complex vinylidene metal complex ecently, electrophilic
Selected topics in metal- free catalysis: Carbenes (and Lewis Base) Catalysis
 elected topics in metal- free catalysis: Carbenes (and Lewis Base) Catalysis Mar$n mith ffice: CL 1 st floor 30.087 Telephone: (2) 85103 Email: mar$n.smith@chem.ox.ac.uk ! elected topics Enamine Iminium
elected topics in metal- free catalysis: Carbenes (and Lewis Base) Catalysis Mar$n mith ffice: CL 1 st floor 30.087 Telephone: (2) 85103 Email: mar$n.smith@chem.ox.ac.uk ! elected topics Enamine Iminium
April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr.
 April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr. Rainer Glaser Announced Reading: Prins Cyclization Reactions 1 Question 1. Aldol-Prins
April 2002 CUME Organic Chemistry Department of Chemistry University of Missouri Columbia Saturday, April 6th, 2002 Dr. Rainer Glaser Announced Reading: Prins Cyclization Reactions 1 Question 1. Aldol-Prins
H H H OH OH H OH H O 1 CH 2 OH
 Name 215 F07-Exam No. 3 Page 2 I. (29 points) Streptomycetes are soil-dwelling, filamentous, Gram-positive saprophytic bacteria. They are responsible for over 50% of the known microbial metabolites, including
Name 215 F07-Exam No. 3 Page 2 I. (29 points) Streptomycetes are soil-dwelling, filamentous, Gram-positive saprophytic bacteria. They are responsible for over 50% of the known microbial metabolites, including
Update to 2011 Bode Research Group TOPIC: ENANTIOSELECTIVE ALDOL REACTIONS
 Update to 2011 ode esearch Group http://www.bode.ethz.ch/ TPIC: EATIELECTIVE ALDL EACTI 1 ITDUCTI Formation of a new C-C bond with the possibility of forming two new stereocentersà 4 diastereomers. First
Update to 2011 ode esearch Group http://www.bode.ethz.ch/ TPIC: EATIELECTIVE ALDL EACTI 1 ITDUCTI Formation of a new C-C bond with the possibility of forming two new stereocentersà 4 diastereomers. First
C-O C-O C-N A A. (KHMDS) C-N (scheme 1) C-O C-N (1)
 C- C- C- A A 2007 2008 -t- (Boc)-- (MM)- () (KMDS) C- (scheme ) C- C- () C- C- C- C- C- C- B C- B (scheme 2) C- B (2) C- manzacidin A Manzacidine A (5) - ATP (fig. ) C- 5 Ph MM Boc ethyl lactate X n=,2
C- C- C- A A 2007 2008 -t- (Boc)-- (MM)- () (KMDS) C- (scheme ) C- C- () C- C- C- C- C- C- B C- B (scheme 2) C- B (2) C- manzacidin A Manzacidine A (5) - ATP (fig. ) C- 5 Ph MM Boc ethyl lactate X n=,2
Chapter 17 Aldehydes and Ketones
 hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
Chapter 14 Aldehydes and Ketones: Addition Reactions at Electrophilic Carbons Overview of Chapter Structures of aldehydes and ketones
 hem 215 F12 otes Dr. Masato Koreeda - Page 1 of 11. Date: September 19, 2012 hapter 14 Aldehydes and Ketones: Addition eactions at Electrophilic arbons verview of hapter 14 1. Structures of aldehydes and
hem 215 F12 otes Dr. Masato Koreeda - Page 1 of 11. Date: September 19, 2012 hapter 14 Aldehydes and Ketones: Addition eactions at Electrophilic arbons verview of hapter 14 1. Structures of aldehydes and
