transmetallate displace ox. add. M + (insert) (β-elim.)
|
|
|
- Theodora Goodwin
- 5 years ago
- Views:
Transcription
1 Chapter IV. Transition Metal σ-alkyl Complexes I. General For much of the rest of this course it will be necessary to understand how σ-alkyl metal complexes are formed and how they react. This is summarized below. σ-alkyl transition metal complexes in synthesis. A. Preparation (-) + M(-) + X M X displace displace transmetallate ox. add. M' + MX 'X + M(0) M + insert insert ' M uc. attack cyclomet. M + uc L M + L + M B. Transformation C ox. X M '- (coupling) "M " ' (transmetallate) Me Z nuc. uc-' (ox. cleavage) 'CMe (insert) ' Z (insert) ' (β-elim.) + M- All of these form C-C bonds in unusual ways from unusual starting materials and hence are useful in organic synthesis.
2 II. σ-alkyl metal complexes from the reaction of carbanions with metal halides (MgX, Li + MX) A. rganocopper chemistry [eviews: Lipshutz rg. eact. 41, 135 (1992); Comprehensive rganometallic Chemistry II 12, 59 (1995)] 1. Background - ne of oldest transition metal reactions a. MgX + (Gilman/Kharasch 's) CuX cat b. Stoichiometric diorganocuprates (ouse, Corey, Posner 's) Li + CuI "Cu" + Li "Cu" + LiI insoluble yellow oligomer " 2 CuLi" soluble, unstable eactions (1) 2 CuLi + 'X ' ' = sp 3 1 > 2 >> 3 I > Br > Cl Ts work sp 2 also reactive, both halides and triflates Tf 2 CuLi CCl very reactive as are 's C usually not safe acidic 's, 's, C's, stable to cuprates C millions of examples
3 2. 2 CuLi + ketones > esters for esters: α or β alkylation slows, esp. Fail undergoes 1,2 General Problems: (a) XS reagent required (3-5 fold) and only 1 group transfers (b) thermally unstable (c) until recently, group couldn't contain reactive functional groups - more of this later.
4 2. ecent Trends a. "igher rder" Cuprates and Mixed "igher rder Cuprates" (1) Preparation CuC + 2Li [ 2 CuC]Li 2 Lipshutz claims C stays coordinated to Cu to give a 3-coordinate species with different chemistry. But, Penn-ahn [JACS 118, 8808 (1996); JACS 117, (1995); JC 60, 4310 (1995)] and Bertz and Snyder [JC 60, 4312 (1995); Chem. Comm. 815 (1996)] by EXAFS, XAES and ab initio claim Li Li C whatever, these have different 3 C Cu C reactivity and 3 stability from 2 CuLi Mixed commercially available CuC + S Li S CuC Li Li [(Thien)()CuC]Li 2 nly transfers important if is expensive, hard to make. (2) eactions More reactive, more stable than 2 CuLi, best for 2 X, epoxides.
5 (3) Applications [Kishi JACS 113, 9693 (1991)] S Me Me 1) TMS 2) Ac 2 Cu(C)Li 2 TMS Ac Me Me
6 S Cu(C)Li 2 TIPS Bu 3 Sn TIPS BF 3 Et 2, TF, -78 [Evans JACS 114, 2260 (1992); JACS 115, 4497 (1993)] SnBu 3 Unsymmetrical Biaryl Synthesis Lipshutz, J. Am. Chem. Soc. 113, 8161 (1991) 115, 9276 (1993) ArLi + CuC -78 ArCu(C)Li Ar'Li [ArCuAr']CLi 2 o scrambling at low temp. Ar =, ome, mme, pme, 2Cl-4CF 3, 1 aphth % ArAr' >96:4 Ar' = ome, pme, ome, 3F, 4Me elated Cu Cu + I I [Y. Yamamoto J.C.S. Chem. Comm (1999)]
7 Me Me TBDMS Me X (a) X = Br (b) X = Li 1) Me MeTBDMS Me X ' (a) X = Br (b) X = Li (c) X = Cu(C)Li, ) 2, TMEDA, -131 Me Me Me TBDMS Me ' Me Me TBDMS 58% Me Me Me Me calphostin C Me [Coleman Tet. Letters 34, 2225 (1993)] Me Bn Me 1) CuC TMEDA Me * Bn 2) 2, -78 Me Li [Coleman JACS 117, (1995)] Me Bn Me Bn Bn Me Me Bn 70% 8:1 mix.
8 Me Me Me Br 1) tbuli, -78 Me Me Me Br 2) CuC 3) 2 Me Me Me Me Me Me [Lipshutz Tet. Lett. 35, 815 (1994); Tet. Lett. 38, 1087 (1997)] elated C 2 Et SnMe 3 5 eq CuCl C 2 Et SnMe 3 Cl DMF min 94% Cl many related SnMe 3 SnMe 3 CuCl couple [Piers JACS 118, 1215 (1996); Tetrahedron 54, (1998)] SnBu 3 Bu 3 Sn PdCl 2 CuI DMF [eathcock JC 61, 700 (1996)]
9 I Me 3 Sn Me 2 3 Me Cu S 69% C 2 [Paterson, Angew. Chem. Int. Ed. Engl. 39, 1308 (2000)] ' ' Me
10 b. Functionalized Cuprates (1) From "Cu(0)" LI + aphthalene TF rt, 2 h Li + [dark green] CuI P 3 0 (Cu) n ( 3 P) y [reddish black] [ieke JC 58, 2483 (1993)] Br(C 2 ) 3 C CCl -36 to rt C(C 2 ) 3 C (79%) Br(C 2 ) 3 C 2 Et "Cu(0)" functionalized cuprate* -78 to rt (90%) C 2 Et Br(C 2 ) 6 Cl * FG FG C 2 Cu LiX or C 2 Cu(X)Li -78 to -20 (78%) (C 2 ) 7 Cl C(C 2 ) 6 Br (Cu) n (PBu 3 ) y -45 to rt (C 2 ) 6 C (83%)
11 Li + S Cu(C)Li TF, -78 "(Cu) n (L) y " [Lipshutz Tet. Lett. 28, 9451 (1989)] CuC nlix (X = Cl, Br) Li p TF, -100 "(Cu) n (L) y " [ieke Tet. Lett. 34, 3063 (1993)] Ac Cl "(Cu) n (L) y " TF -100 functionalized allylic cuprate Ac X ArI "Cu(0)" ["XArCu"] 'X XAr' (79%) aaphth + CuX XAr = Br [JC 60, 2361 (1995)] F no benzyne formation vs Li 'X = MeI, BnBr, CCl, etc.
12 (2) From Transmetallation (a) From Zinc L n Zn- or Zn-X [X = halide] + L Cu L n [L = ligand on Cu(I)] Cu Zn X() L Cu L n + L Zn X() functionalized Cu(I) reagent ZnCl 2 + Liaphth FG I Zn(0) TF, FG ZnI (>85%) [Knochel Chem. ev. 93, 2117 (1993)] Zn/Cu in situ C 2 Me I C 2 Me(x's) Tf Zn, CuI ))), rt, 40 min Et/ 2 (7:3) Tf (65%) [Castedo JC 58, 118 (1993)]
13 FG C 2 ZnI (FG 1) neat, 25-50, 2-20 h C 2 ) 2 Zn + + EtI 1.5 Et 2 Zn CuI (0.3 eq) 2) 50, 0.1 mmg, 1 h + Et 2 Zn Preformed Cu(C)ZnX nlix Cu(C)(Znr) nlix ZnX + CuC nlix 2 Zn + CuC nlix 2 C 2 Me 1) (Et) 2 P Cu(C)ZnI TF, 4 h, -78 to rt 2) ef reaction (Et) 2 P Me 2 C (78%) [Angew. Chem. Int. Ed. 28, 351 (1989)] 1) IZn(C)Cu (C 2 ) 5 C 2 Me Si Si 2) TMS-Cl, TF hydrolysis C 2 Me Si JC 56, 3205 (1991) (78%) Si
14 (Et) 2 P "IZn(X)Cu C 2 Me" TBDMS TBDMS C 2 Me Tetrahedron 47, 1861 (1991) TBDMS TBDMS (97%) IZn Boc C 2 Bn CuC 2LiCl TF, -10 to 0 IZn(C)Cu Boc C 2 Bn Zn/Cu (1.7 eq.) /DMA (15:1) sonication 30 min I Boc C 2 Bn 1 2 X X = Cl, Br, Ts 1 =,, C 2 Br, C 2 Me 2 =, Me, C 2 Me 3 =, C 2 Cl Boc [Jackson, J. Chem.Soc., Chem. Comm. 319 (1992)] Can be made catalytic in Cu FGZnI 1) 1.4 MeLi cat. Me 2 CuCLi 2 1) 2 Eq. TMSCl FG = Cl(C 2 ) 4, C(C 2 ) 5, (C 2 ) 3, Et 2 C(C 2 ) 3 2) 1 (32-65%) FG C 2 Bn good yield large scale [Lipshutz JACS 117, 6126 (1995)]
15 ZnCl 2 TMS Et Et C 2 Zn + C 2 Et CuBr SMe 2 (10-25 mol %) MPA, TMS Cl TF, rt, 4-6 h C 2 Et from zinc homoenolate C 2 Et cat. Cu(I) 2 Zn (89%) Me 2 cat. Cu(I) C 2 Et Me 2 TBDMS TBDMS Me cat. Cu(I) (71%) C 2 Me (72%) [Crimmins JC 58, 1038 (1993)]
16 C 2 Et Zn(C 2 C 2 C 2 Et) 2 cat. CuBr Me 2 S (10 mol %) MPA, TF, Et 2 TMS-Cl C 2 Et Me 3 Si (52%) hν [Crimmins JACS 115, 3146 (1993)] C2 Et Carbocupration of Alkynes Me 3 Si FGZnI + Me 2 CuCLi 2 "FGCu(C)LiZnMe 2 Li" ' " FG E E + FG CuC ' " ' " FG = Et, Et 2 C, C, Cl, E + = +, Br, Me 3 SnCl ' = Bu,, " = SMe, [ao, Knochel J. Am. Chem.Soc. 1991, 113, 5735]
17 " I Zn ' ''' " 1) Me 2 CuCLi 2 2) E + ' E ''' " " 55-76% ' "X ' S 2 " S 2 CuMgX 2 + 'C C ' CuMgX 2 D + ' D C 2 1 ' C 2 I 2 ' I (a) Alexakis, A.; Commercon, A.; Coulentianos, C.; ormant, J.F. Pure Appl. Chem. 1983, 55, (b) ormant, J.F.; Alexakis, A. Synthesis 1981, 841. (c) Asymmetric induction in Michael additions 1 MgX 1 CuBF 3 + S 1 ppolzer elv. Chim Acta 72, 1337 (1989) eview Tetrahedron 43, 1969 and 4057 (1987)
18 BuCuI SiMe 3 Bu Me 93% 98% de J. rganomet. Chem. 391, C19 (1990). CuI, MeLi L*, C 3 /TF 6 6 (-) Muscone 89% 100% opt. purity L* = J. Chem. Soc. Perkins I, 1445 (1991).
19 Then 1) MgBr CuBr Me 2 S 2) BnBr Bn 50-89% [ruby Tet. Letters 34, 2561 (1993) JC 60, 5509 (1995)] >99% ee Bn 1) MgX / CuI 2) 2 Pd/C + 2 C 3 [egedus, Lander JACS 116, 8126 (1994)] = t-bu, adamantyl, pme, TMSC 2, cyclohexyl 89-98% yield 88-97% ee ( ) n Bu 2 CuLi L* Et 2-78 ( ) n Bu L* = [ossiter JC 60, 8422 (1995)] n = 2, % yield 92-97% ee + 2 CuLi [Smith JACS 117, (1995)] 1) 2) AcCl MPA 89%
Organocopper Chemistry
 rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
VI. Metal alkyls from oxidative addition / insertion
 V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
Ready; Catalysis Conjugate Addition
 eady; Catalysis Conjugate Addition Topics covered 1. 1,4 addition involving copper a. stoichiometric reactions b. catalytic reactions c. allylic substitution. Conjugate addition without copper a. Ni-based
eady; Catalysis Conjugate Addition Topics covered 1. 1,4 addition involving copper a. stoichiometric reactions b. catalytic reactions c. allylic substitution. Conjugate addition without copper a. Ni-based
Organocopper Reagents
 rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)]
![o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)] o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)]](/thumbs/85/92754396.jpg) 3. Boron -- eview [Suzuki Chem. ev. 95, 2457 (1995)] U77b ydroboration also attractive but B Pd transmetallation difficult - must produce stable B product - solved (by Suzuki) by adding base to make Borates
3. Boron -- eview [Suzuki Chem. ev. 95, 2457 (1995)] U77b ydroboration also attractive but B Pd transmetallation difficult - must produce stable B product - solved (by Suzuki) by adding base to make Borates
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Synthesis of Amphidinolide X and an Exploration of Key Reactions
 PJM 1/12/05 Synthesis of Amphidinolide X and an Exploration of Key eactions Lepage,.; Kattnig, E.; Furstner, A. JACS, 2004, 126, 15970-15971. 7 13 1 6 19 - Produced by marine dinoflagellates, Amphidinium
PJM 1/12/05 Synthesis of Amphidinolide X and an Exploration of Key eactions Lepage,.; Kattnig, E.; Furstner, A. JACS, 2004, 126, 15970-15971. 7 13 1 6 19 - Produced by marine dinoflagellates, Amphidinium
Chapter 5 Three and Four-Membered Ring Systems
 Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Total synthesis of Spongistatin
 Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
CHEM 330. Final Exam December 5, 2014 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
Zr-Catalyzed Carbometallation
 -Catalyzed Carbometallation C C C C ML n C C ML n ML n C C C C ML n ML n C C ML n Wipf Group esearch Topic Seminar Juan Arredondo November 13, 2004 Juan Arredondo @ Wipf Group 1 11/14/2004 Carbometallation
-Catalyzed Carbometallation C C C C ML n C C ML n ML n C C C C ML n ML n C C ML n Wipf Group esearch Topic Seminar Juan Arredondo November 13, 2004 Juan Arredondo @ Wipf Group 1 11/14/2004 Carbometallation
Nickel-Catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides
 ickel-catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides Eunjae Shim Zakarian Group Literature Talk / Dec 13 th, 2018 University of California, Santa Barbara Table of Contents
ickel-catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides Eunjae Shim Zakarian Group Literature Talk / Dec 13 th, 2018 University of California, Santa Barbara Table of Contents
Requirements for an Effective Chiral Auxiliary Enolate Alkylation
 Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Additions to Metal-Alkene and -Alkyne Complexes
 Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Enolates: Z(O,R) (O,R)- and E(O,R) (O,R)-enolates
 Enolates: Z(,) (,)- and E(,) (,)-enolates egardless of other groups the encircled ' and - determine whether one is Z(,)- or E(,)- enolate. - - ' ' (E)-enolate (Z)-enolate Enolates: deprotonation 90 ' Most
Enolates: Z(,) (,)- and E(,) (,)-enolates egardless of other groups the encircled ' and - determine whether one is Z(,)- or E(,)- enolate. - - ' ' (E)-enolate (Z)-enolate Enolates: deprotonation 90 ' Most
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
CuI CuI eage lic R tal ome rgan gbr ommon
 Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Keisuke Suzuki. Baran lab Group Meeting 6/11/16. Shigenobu Umemiya. Akira Suzuki. Takanori Suzuki (Hokkaido University)
 197.D., Teruaki Mukaiyama, University of Tokyo 193 Assistant Professor, Keio University 197 Lecturer, Keio University 199 Assocate Professor, Keio University 1990 Visiting Professor, ET 1994 ull Professor,
197.D., Teruaki Mukaiyama, University of Tokyo 193 Assistant Professor, Keio University 197 Lecturer, Keio University 199 Assocate Professor, Keio University 1990 Visiting Professor, ET 1994 ull Professor,
Radical Reactions. Radical Stability!!! bond dissociation energies X Y X + Y. bond BDE (kcal/mol) bond BDE (kcal/mol) CH 3 CH 3 CH 2 95 O H R 2 C H
 adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
12.5 Organometallic Compounds
 12.5 rganometallic Compounds Compounds that contain carbon-metal bond are called organometallic compounds. C M C δ δ + M C M Primarily ionic Primarily covalent (M = Na + or K + )(M = Mg or Li) (M = Pb,
12.5 rganometallic Compounds Compounds that contain carbon-metal bond are called organometallic compounds. C M C δ δ + M C M Primarily ionic Primarily covalent (M = Na + or K + )(M = Mg or Li) (M = Pb,
Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides
 Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
Lecture 6: Transition-Metal Catalysed C-C Bond Formation
 Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
Huang, C.; Gevorgyan, V. J. Am. Chem. Soc. 2009, 131, Daniel Tzvi Cohen Short Literature Feb. 23, MeO HO OH. COOH ( )-Plicatic Acid OH OH
 Asymmetric Total Synthesis of ( )-Plicatic Acid via a Highly Enantioselective and Diastereoselective Nucleophilic Epoxidation of Acyclic Trisubstituted lefins H H H H CH ( )-Plicatic Acid H H Sun, B.F.;
Asymmetric Total Synthesis of ( )-Plicatic Acid via a Highly Enantioselective and Diastereoselective Nucleophilic Epoxidation of Acyclic Trisubstituted lefins H H H H CH ( )-Plicatic Acid H H Sun, B.F.;
Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines
 Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Organic Tutorials 3 rd Year Michaelmas Transition Metals in Organic Synthesis: (General paper level) ! 1! Reading
 rganic Tutorials 3 rd Year Michaelmas 2010 Transition Metals in rganic Synthesis: (General paper level) Reading 1. Lecture Course, and suggested references from this. 2. Clayden, Greaves, Warren and Wothers.
rganic Tutorials 3 rd Year Michaelmas 2010 Transition Metals in rganic Synthesis: (General paper level) Reading 1. Lecture Course, and suggested references from this. 2. Clayden, Greaves, Warren and Wothers.
Catalytic Reactions in Organic Synthesis
 17 April, 2008 Catalytic eactions in rganic Synthesis hodium Complexes and edox Catalysts Koichi AASAKA, Motoki YAMAE, Shunsuke CIBA Division of Chemistry and Biological Chemistry, School of ysical and
17 April, 2008 Catalytic eactions in rganic Synthesis hodium Complexes and edox Catalysts Koichi AASAKA, Motoki YAMAE, Shunsuke CIBA Division of Chemistry and Biological Chemistry, School of ysical and
Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization
![Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization](/thumbs/82/85235286.jpg) Total Synthesis of (+/-)-Goniomitine via a Formal itrile/donor-acceptor Cyclopropane [3 + 2] Cyclization (-)-Goniomitine Christian L. Morales and Brian Pagenkopf* rganic Letters, ASAP Current Literature
Total Synthesis of (+/-)-Goniomitine via a Formal itrile/donor-acceptor Cyclopropane [3 + 2] Cyclization (-)-Goniomitine Christian L. Morales and Brian Pagenkopf* rganic Letters, ASAP Current Literature
Use of Cp 2 TiCl in Synthesis
 Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Metal Catalyzed Outer Sphere Alkylations of Unactivated Olefins and Alkynes
 Metal Catalyzed uter Sphere Alkylations of Unactivated lefins and Alkynes Stephen Goble rganic Super-Group Meeting Literature Presentation ctober 6, 2004 1 utline I. Background Introduction to Carbometallation
Metal Catalyzed uter Sphere Alkylations of Unactivated lefins and Alkynes Stephen Goble rganic Super-Group Meeting Literature Presentation ctober 6, 2004 1 utline I. Background Introduction to Carbometallation
Nickel-Catalyzed Multicomponent Coupling of Alkynes. -Recent development in methodologies and applications. Zhenjie Lu. Department of Chemistry, MSU
 28 58.69 i ickel ickel-catalyzed Multicomponent Coupling of Alkynes -ecent development in methodologies and applications Zhenjie u Department of Chemistry, MSU January 28, 2004 Background Introduction
28 58.69 i ickel ickel-catalyzed Multicomponent Coupling of Alkynes -ecent development in methodologies and applications Zhenjie u Department of Chemistry, MSU January 28, 2004 Background Introduction
Organomagnesium (Grignard) and organolithium reagents
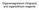 rganomagnesium (Grignard) and organolithium reagents Different polarization of non-metallic and organometallic reagents H CH 3 - I H - CH 3 + I H 3 H 3 C H 3 C H 3 + H 3 C H 3 C H 2 H 3 C H3C H I - CH
rganomagnesium (Grignard) and organolithium reagents Different polarization of non-metallic and organometallic reagents H CH 3 - I H - CH 3 + I H 3 H 3 C H 3 C H 3 + H 3 C H 3 C H 2 H 3 C H3C H I - CH
CH 3 TMG, DMF N H 3 CO 2 S. (PPh 3 ) 2 Pd 0
 1. (a) rovide a reasonable mechanism for the following transformation. I S 2 C 3 C 3 ( 3 ) 2 2, CuI C 3 TMG, DMF 3 C 2 S TMG = Me 2 Me 2 ICu ( 3 ) 2 0 I S 2 C 3 S 2 C 3 Cu I 3 3 3 C 2 S I 3 3 3 C 2 S 3
1. (a) rovide a reasonable mechanism for the following transformation. I S 2 C 3 C 3 ( 3 ) 2 2, CuI C 3 TMG, DMF 3 C 2 S TMG = Me 2 Me 2 ICu ( 3 ) 2 0 I S 2 C 3 S 2 C 3 Cu I 3 3 3 C 2 S I 3 3 3 C 2 S 3
Mechanistic Studies in Copper Catalysis
 chanistic Studies in Copper Catalysis Jen Alleva May 1st 2013 Timeline of Achievements in Copper Chemistry General istorical verview first cross-couplings 1869 Ullmann Goldberg Glaser 1903 Glaser, C. Ann.
chanistic Studies in Copper Catalysis Jen Alleva May 1st 2013 Timeline of Achievements in Copper Chemistry General istorical verview first cross-couplings 1869 Ullmann Goldberg Glaser 1903 Glaser, C. Ann.
Asymmetric Deprotonation
 ( )-sparteine i-pr, t 2, -98 C 84% ee Asymmetric Deprotonation gands, Bases, and Applications CF 3 TMS t-bu TMSCl, MPA TF, -100 C t-bu 93% ee Fe (i-pr) 2 1. n-bu ( )-sparteine t 2, -78 C 2. 2 PCl P 2 Fe
( )-sparteine i-pr, t 2, -98 C 84% ee Asymmetric Deprotonation gands, Bases, and Applications CF 3 TMS t-bu TMSCl, MPA TF, -100 C t-bu 93% ee Fe (i-pr) 2 1. n-bu ( )-sparteine t 2, -78 C 2. 2 PCl P 2 Fe
Suggested solutions for Chapter 40
 s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
Total Synthesis of Oxazolomycin A
 Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
Functionalization of C O Bonds. Stefan McCarver. MacMillan Lab Group Meeting
 Functionalization of C Bonds Stefan McCarver MacMillan Lab Group eting November 23 rd, 2016 Functionalization of C Bonds "X" X homolytic cleavage catalyst M oxidative addition Why is C Bond Manipulation
Functionalization of C Bonds Stefan McCarver MacMillan Lab Group eting November 23 rd, 2016 Functionalization of C Bonds "X" X homolytic cleavage catalyst M oxidative addition Why is C Bond Manipulation
Chiral Diol Promoted Boronates Addi3on Reac3ons. Lu Yan Morken Group Boston College
 Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Spiro Monophosphite and Monophosphoramidite Ligand Kit
 Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
A Concise Synthesis of ( )- Aplyviolene Facilitated by a Stragetegic Ter<ary Radical Conjugate Addi<on
 A Concise Synthesis of ( )- Aplyviolene Facilitated by a Stragetegic Ter
A Concise Synthesis of ( )- Aplyviolene Facilitated by a Stragetegic Ter
Asymmetric Nucleophilic Catalysis
 Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
Denmark s Base Catalyzed Aldol/Allylation
 Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
Copper-Catalyzed Reaction of Alkyl Halides with Cyclopentadienylmagnesium Reagent
 Copper-Catalyzed eaction of Alkyl Halides with Cyclopentadienylmagnesium eagent Mg 1) cat. Cu(Tf) 2 i Pr 2, 25 o C, 3 h 2) H 2, Pt 2 Masahiro Sai, Hidenori Someya, Hideki Yorimitsu, and Koichiro shima
Copper-Catalyzed eaction of Alkyl Halides with Cyclopentadienylmagnesium eagent Mg 1) cat. Cu(Tf) 2 i Pr 2, 25 o C, 3 h 2) H 2, Pt 2 Masahiro Sai, Hidenori Someya, Hideki Yorimitsu, and Koichiro shima
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Reduction. Boron based reagents. NaBH 4 / NiCl 2. Uses: Zn(BH 4 ) 2. Preparation: Good for base sensitive groups Chelation control model.
 Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
Large-Scale Synthesis of the Anti-Cancer Marine Natural Product (+)-Discodermolide
 61.7 g prepared in 39 steps 43 chemists worked on the project which lasted 20 months Chris Kendall @ Wipf Group 1 3/27/04 8 22 1 5 24 carbon linear polypropionate chain containing stereocenters (6 hydroxyl
61.7 g prepared in 39 steps 43 chemists worked on the project which lasted 20 months Chris Kendall @ Wipf Group 1 3/27/04 8 22 1 5 24 carbon linear polypropionate chain containing stereocenters (6 hydroxyl
TMSCl imidazole DMF. Ph Ph OTMS. Michael reaction. Michael reaction Ph R 3. epoxidation O R
 eaction using diarylprolinol silyl ether derivatives as catalyst 1) C Et K C 3, ) MgBr, TF TMS hexane, 0 o C TBS p- C 6 4, T C Et 85%, 99% ee Angew. Chem., nt. Ed., 44, 41 (005). rg. Synth., 017, 94, 5.
eaction using diarylprolinol silyl ether derivatives as catalyst 1) C Et K C 3, ) MgBr, TF TMS hexane, 0 o C TBS p- C 6 4, T C Et 85%, 99% ee Angew. Chem., nt. Ed., 44, 41 (005). rg. Synth., 017, 94, 5.
Syntheses of Leucascandrolide A. Supergroup Meeting August 4 th, 2004 Yu Yuan
 Syntheses of Leucascandrolide A Supergroup Meeting August 4 th, 2004 Yu Yuan Leucascandrolide A Me Me Me Dambrosio, M.; Guerriero, A.; Debitus, C.; Pietra, F. elvetica Chimica Acta 1996, 79, 51-60 Me 1
Syntheses of Leucascandrolide A Supergroup Meeting August 4 th, 2004 Yu Yuan Leucascandrolide A Me Me Me Dambrosio, M.; Guerriero, A.; Debitus, C.; Pietra, F. elvetica Chimica Acta 1996, 79, 51-60 Me 1
Recent Development in. Tandem Radical Reactions (TRR)
 ecent Development in Tandem adical eactions (T) Feng u Jan. 13, 2006 Contents Brief Introduction of the istory of T Definition of T Intramolecular T Intermolecular T T as Key Steps in Total Synthesis of
ecent Development in Tandem adical eactions (T) Feng u Jan. 13, 2006 Contents Brief Introduction of the istory of T Definition of T Intramolecular T Intermolecular T T as Key Steps in Total Synthesis of
Asymmetric Alklylation of Enolates
 Asymmetric Alklylation of Enolates M with material from A G Meyers http://faculty.chemistry.harvard.edu/myers/pages/chem-215-handouts 745 rganic Synthesis Spring 2015 Asymmetric Alkylation - eed to control
Asymmetric Alklylation of Enolates M with material from A G Meyers http://faculty.chemistry.harvard.edu/myers/pages/chem-215-handouts 745 rganic Synthesis Spring 2015 Asymmetric Alkylation - eed to control
Chapter 12. Alcohols from Carbonyl Compounds Oxidation-Reduction & Organometallic Compounds. Structure
 Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Asymmetric Radical Reactions. Zhen Liu 08/30/2018
 Asymmetric adical eactions Zhen Liu 08/30/2018 Contents Introduction eactions Using Chiral Auxiliary Chiral Lewis Acid-diated eactions Transition tal-catalyzed eactions eactions Using Chiral rganocatalysts
Asymmetric adical eactions Zhen Liu 08/30/2018 Contents Introduction eactions Using Chiral Auxiliary Chiral Lewis Acid-diated eactions Transition tal-catalyzed eactions eactions Using Chiral rganocatalysts
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols
 A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
Chiral Bronsted Acids as Catalysts
 Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
Reactivity Umpolung-1 Ready
 eactivity Umpolung-1 eady eactivity Umpolung: reversal of normal polarity electrophiles become nucleophiles nucleophiles become electrophiles complimentary disconnections ormal reactivity: x = heteroatom
eactivity Umpolung-1 eady eactivity Umpolung: reversal of normal polarity electrophiles become nucleophiles nucleophiles become electrophiles complimentary disconnections ormal reactivity: x = heteroatom
Total Synthesis of (+)-Suaveolindole
 1 Total Synthesis of (+)-Suaveolindole 15 2 C 16 11 Emile J. Velthuisen and Samuel J. Danishefsky J. Am. Chem. Soc. 2007, 9, 10640-10641 Julia Vargas September 15, 2007 2 utline Isolation and Elucidation
1 Total Synthesis of (+)-Suaveolindole 15 2 C 16 11 Emile J. Velthuisen and Samuel J. Danishefsky J. Am. Chem. Soc. 2007, 9, 10640-10641 Julia Vargas September 15, 2007 2 utline Isolation and Elucidation
Enan$oselec$ve Total Synthesis of Amphidinolide F
 Enan$oselec$ve Total Synthesis of Amphidinolide F Subham Mahapatra and ich G. Carter regon State University Angew. Chem. nt. Ed., 2012, 51, 7948 Nicolas Millius Bern, 07.02.2013 ntroduc$on isolated from
Enan$oselec$ve Total Synthesis of Amphidinolide F Subham Mahapatra and ich G. Carter regon State University Angew. Chem. nt. Ed., 2012, 51, 7948 Nicolas Millius Bern, 07.02.2013 ntroduc$on isolated from
CEM 852 Exam LDA, THF, 0 C, 15 min; then
 CEM 85 Exam- April, 005 This exam consists of 5 pages. Please write ALL your answers in the answer books. Please write legibly and draw all structures clearly. Good luck. 1. Provide examples of the following
CEM 85 Exam- April, 005 This exam consists of 5 pages. Please write ALL your answers in the answer books. Please write legibly and draw all structures clearly. Good luck. 1. Provide examples of the following
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group.
 Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Short Literature Presentation 10/4/2010 Erika A. Crane
 Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
BURIED TREASURES: CASCADES WE HAVEN'T SEEN
 BURIED TREASURES: CASCADES WE AVE'T SEE Me Me Me 2 3 C C 3 3 C C 3 C 2 Me R. Robinson, J. Chem. Soc., 1917, 762 Cascades Articulated Main Entry: 1cas cade Pronunciation: (")kas-'kad Function: noun Etymology:
BURIED TREASURES: CASCADES WE AVE'T SEE Me Me Me 2 3 C C 3 3 C C 3 C 2 Me R. Robinson, J. Chem. Soc., 1917, 762 Cascades Articulated Main Entry: 1cas cade Pronunciation: (")kas-'kad Function: noun Etymology:
A 1,3 Strain and the Anomeric Effect. Michael Shaghafi Chem. Topics Feb. 6, 2012
 A 1,3 Strain and the Anomeric Effect Michael Shaghafi Chem. Topics Feb. 6, 2012 Introduction: Definition of A 1,3 Strain m L L m m 3 L 3 1 1 otation about σ-bond between α-stereocenter and olefin is associated
A 1,3 Strain and the Anomeric Effect Michael Shaghafi Chem. Topics Feb. 6, 2012 Introduction: Definition of A 1,3 Strain m L L m m 3 L 3 1 1 otation about σ-bond between α-stereocenter and olefin is associated
Organic Chemistry Laboratory Summer Lecture 6 Transition metal organometallic chemistry and catalysis July
 344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
Total Syntheses of Minfiensine
 Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Strained Molecules in Organic Synthesis
 Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid
 Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid Shiqing Xu, Akimichi Oda, Thomas Bobinski, Haijun Li, Yohei Matsueda, and Ei-ichi Negishi Angew. Chem. Int. Ed. 2015,
Highly Efficient, Convergent, and Enantioselective Synthesis of Phthioceranic Acid Shiqing Xu, Akimichi Oda, Thomas Bobinski, Haijun Li, Yohei Matsueda, and Ei-ichi Negishi Angew. Chem. Int. Ed. 2015,
Asymmetric Lewis Base Strategies for Heterocycle Synthesis
 Asymmetric Lewis Base trategies for eterocycle ynthesis Dr Andrew mith EatCEM, chool of Chemistry, University of t Andrews 1st cottish-japanese ymposium of rganic Chemistry, University of Glasgow Friday
Asymmetric Lewis Base trategies for eterocycle ynthesis Dr Andrew mith EatCEM, chool of Chemistry, University of t Andrews 1st cottish-japanese ymposium of rganic Chemistry, University of Glasgow Friday
11-Step Enantioselective Synthesis of ( )-Lomaiviticin Aglycon
 11-Step Enantioselective Synthesis of ( )-Lomaiviticin Aglycon Seth B. erzon, Liang Lu, Christina M. Woo, and Shivajirao L. Gholap J. Am. Chem. Soc. ASAP DI 10.1021/ja200034b Melissa Sprachman Current
11-Step Enantioselective Synthesis of ( )-Lomaiviticin Aglycon Seth B. erzon, Liang Lu, Christina M. Woo, and Shivajirao L. Gholap J. Am. Chem. Soc. ASAP DI 10.1021/ja200034b Melissa Sprachman Current
Chem 253 Problem Set 7 Due: Friday, December 3, 2004
 Chem 253 roblem Set 7 ue: Friday, ecember 3, 2004 Name TF. Starting with the provided starting material, provide a concise synthesis of. You may use any other reagents for your synthesis. It can be assumed
Chem 253 roblem Set 7 ue: Friday, ecember 3, 2004 Name TF. Starting with the provided starting material, provide a concise synthesis of. You may use any other reagents for your synthesis. It can be assumed
Carbonyl Ylide Cycloadditions
 Carbonyl Ylide Cycloadditions cond. icholas Anderson Denmark Group eting 07/13/10 Carbonyl Ylides Uncharged 1,3-Dipole Conjugated π-system ighly reactive on-isolable Generate in-situ Carbonyl Ylide Stability
Carbonyl Ylide Cycloadditions cond. icholas Anderson Denmark Group eting 07/13/10 Carbonyl Ylides Uncharged 1,3-Dipole Conjugated π-system ighly reactive on-isolable Generate in-situ Carbonyl Ylide Stability
Stereoselective reactions of enolates: auxiliaries
 1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02
![Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02](/thumbs/93/114018431.jpg) Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
eatles Oasis - 199
 eatles - 1964 asis - 199 Biography 2001-present: University of 1997-2000: Professor, Shef 1988-1997: Various Reader 1986-1988: Post-doc with G 1983-1986: D at Universi 1983: Undergrad at Cambrid Enantioselective
eatles - 1964 asis - 199 Biography 2001-present: University of 1997-2000: Professor, Shef 1988-1997: Various Reader 1986-1988: Post-doc with G 1983-1986: D at Universi 1983: Undergrad at Cambrid Enantioselective
Classics in Tetrahedron Letters
 Classics in Tetrahedron Letters Jeremy Richter Baran Group Meeting: 9/24/03 The Plan Methodology Protecting Groups atural Products Syntheses Methodology xidation of Vicinal Diols R R' R'' 1. Cl 2, DMS,
Classics in Tetrahedron Letters Jeremy Richter Baran Group Meeting: 9/24/03 The Plan Methodology Protecting Groups atural Products Syntheses Methodology xidation of Vicinal Diols R R' R'' 1. Cl 2, DMS,
Topic 18: Nucleophilic Sigma Bonds
 Professor David L. Van Vranken Chemistry 201: rganic eaction Mechanisms I Topic 18: ucleophilic Sigma Bonds E E C E eferences: terature cited ecall the Six Types of Canonical Frontier rbitals We ve already
Professor David L. Van Vranken Chemistry 201: rganic eaction Mechanisms I Topic 18: ucleophilic Sigma Bonds E E C E eferences: terature cited ecall the Six Types of Canonical Frontier rbitals We ve already
Sonogashira: in situ, metal assisted deprotonation
 M.C. White, Chem 253 Cross-Coupling -120- Week of ctober 11, 2004 Sonogashira: in situ, metal assisted deprotonation catalytic cycle: ' (h 3 ) n d II The first report: h Sonogashira T 1975 (50) 4467. h
M.C. White, Chem 253 Cross-Coupling -120- Week of ctober 11, 2004 Sonogashira: in situ, metal assisted deprotonation catalytic cycle: ' (h 3 ) n d II The first report: h Sonogashira T 1975 (50) 4467. h
Ynolate Chemistry. Jeff Kallemeyn October 22, 2002
 Ynolate Chemistry While enolates have numbered among the most important reagents of organic chemistry for more than a century, ynolates have hitherto remained unknown although their chemistry should be
Ynolate Chemistry While enolates have numbered among the most important reagents of organic chemistry for more than a century, ynolates have hitherto remained unknown although their chemistry should be
Shi Asymmetric Epoxidation
 Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
!"#$%&&'!&(!)*+,-./!01"2.3$*4!"!#$!%$!%&'(') *+,!-$!%&'(').!'/ *&%&*,$.&-!"!3$!4$!5)01+!.*!06'2
 !"#$%&&'!&(!)*+,-./!01"2.3$*4!"!#$!%$!%&'(') *+,!-$!%&'(').!'/012 546*&%&*,$.&-!"!3$!4$!5)01+!.*!06'2 C-C Bond Formation: Cross-coupling Reaction of rganometal Compounds with rganic alids M C-m + X-C C-C
!"#$%&&'!&(!)*+,-./!01"2.3$*4!"!#$!%$!%&'(') *+,!-$!%&'(').!'/012 546*&%&*,$.&-!"!3$!4$!5)01+!.*!06'2 C-C Bond Formation: Cross-coupling Reaction of rganometal Compounds with rganic alids M C-m + X-C C-C
Sonogashira Couplings of Aryl Bromides: Room Temperature, Water Only, No Copper
 Sonogashira Couplings of Aryl omides: Room Temperature, Water nly, o Copper uce. Lipshutz, David W. Chung, and ian Rich rg.lett. ASAP Article Presentation By ora Jameson Current Lit 08/30/2008 ora Jameson
Sonogashira Couplings of Aryl omides: Room Temperature, Water nly, o Copper uce. Lipshutz, David W. Chung, and ian Rich rg.lett. ASAP Article Presentation By ora Jameson Current Lit 08/30/2008 ora Jameson
Arylhalide-Tolerated Electrophilic Amination of Arylboronic Acids with N-Chloroamides Catalyzed by CuCl at Room Temperature
 Current Literature July 19, 08 Jitendra Mishra Arylhalide-Tolerated Electrophilic Amination of Arylboronic Acids with -Chloroamides Catalyzed by CuCl at Room Temperature Aiwen Lei et.al. College of the
Current Literature July 19, 08 Jitendra Mishra Arylhalide-Tolerated Electrophilic Amination of Arylboronic Acids with -Chloroamides Catalyzed by CuCl at Room Temperature Aiwen Lei et.al. College of the
Development of Chiral Phosphine Olefin Ligands and Their Use in Asymmetric Catalysis
 Development of Chiral osphine lefin Ligands and Their Use in Asymmetric Catalysis 2 Wei-Liang Duan July 31, 2007 Research Works in Hayashi Group, Kyoto University (ct, 2003 Mar, 2007) Conventional Chiral
Development of Chiral osphine lefin Ligands and Their Use in Asymmetric Catalysis 2 Wei-Liang Duan July 31, 2007 Research Works in Hayashi Group, Kyoto University (ct, 2003 Mar, 2007) Conventional Chiral
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2
 Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of Homoallylic Primary Amines
 a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of omoallylic Primary Amines 1 3 2 3 ML n 1 2 2 3 Masaharu Sugiura, Keiichi irano and Shu Kobayashi JACS ASAP ryan Wakefield @ Wipf
a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of omoallylic Primary Amines 1 3 2 3 ML n 1 2 2 3 Masaharu Sugiura, Keiichi irano and Shu Kobayashi JACS ASAP ryan Wakefield @ Wipf
A Stille or Suzuki reaction is a good choice for this coupling O O because they are functional group tolerant, no radical chemistry F
 Chemistry 253 roblem et 3 Due: Friday, ctober 15th ame TF 1. For the following products of cross coupling reactions and indicated bond disconnections, please indicate a reasonable cross coupling protocol
Chemistry 253 roblem et 3 Due: Friday, ctober 15th ame TF 1. For the following products of cross coupling reactions and indicated bond disconnections, please indicate a reasonable cross coupling protocol
Total Synthesis of Cyclosporine: Access to N-Methylated Peptides via Isonitrile Coupling Reactions
 Total Synthesis of Cyclosporine: Access to -thylated Peptides via Isonitrile Coupling Reactions Xiangyang Wu, Jennifer L. Stockdill, Ping Wang, Samuel J. Danishefsky* J. Am. Chem. Soc. 2010,132, 4098-4100
Total Synthesis of Cyclosporine: Access to -thylated Peptides via Isonitrile Coupling Reactions Xiangyang Wu, Jennifer L. Stockdill, Ping Wang, Samuel J. Danishefsky* J. Am. Chem. Soc. 2010,132, 4098-4100
Investigations of Organocuprates
 Investigations of rganocuprates Department of Chemistry University of North Carolina at Charlotte Andy Thomas Rapid injection NMR Why is RINMR so useful? Time intervals between spectra can be modified
Investigations of rganocuprates Department of Chemistry University of North Carolina at Charlotte Andy Thomas Rapid injection NMR Why is RINMR so useful? Time intervals between spectra can be modified
Rhenium-Catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage
 henium-catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage Kuninobu, Y.; Takata,.; Kawata, A.; Takai, K. rg. Lett. ASAP Et 5 6 cat. [ebr(c) 3 (thf)] 2 5 6 Current Literature
henium-catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage Kuninobu, Y.; Takata,.; Kawata, A.; Takai, K. rg. Lett. ASAP Et 5 6 cat. [ebr(c) 3 (thf)] 2 5 6 Current Literature
Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction Mechanisms. Group Meeting Aaron Bailey 12 May 2009
 Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction chanisms Group eting Aaron Bailey 12 May 2009 What is a Non-Linear Effect? In asymmetric catalysis, the ee (er) of the
Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction chanisms Group eting Aaron Bailey 12 May 2009 What is a Non-Linear Effect? In asymmetric catalysis, the ee (er) of the
Functionalization of C(sp 3 ) H Bonds Using a Transient Directing Group
 Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Functionalized Organometallic Reagents
 Availability Availability Preparation via Insertion Grignard s Synthesis Generally Considered as a Radical Process Schlenk Equilibrium Parasite Reactions Reversible Reaction in THF Substitution Reactions
Availability Availability Preparation via Insertion Grignard s Synthesis Generally Considered as a Radical Process Schlenk Equilibrium Parasite Reactions Reversible Reaction in THF Substitution Reactions
Suggested solutions for Chapter 32
 s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
Prof. Ang Li. Literature Seminar Kosuke Minagawa (D2)
 Prof. Ang Li Literature Seminar 2017. 10. 28 Kosuke Minagawa (D2) 1 2 3 aspidodasycarpine 1) 2 C sespenine 3) atural Products Synthesized by Ang Li group clostrubin 6) drimentine A 7) i-bu rubriflordilactone
Prof. Ang Li Literature Seminar 2017. 10. 28 Kosuke Minagawa (D2) 1 2 3 aspidodasycarpine 1) 2 C sespenine 3) atural Products Synthesized by Ang Li group clostrubin 6) drimentine A 7) i-bu rubriflordilactone
Total Synthesis of (+)-Sieboldine A J. Am. Chem. Soc. 2010, 132,
 Total Synthesis of (+)-Sieboldine A J. Am. Chem. Soc. 2010, 132, 7876 7877. Current Literature Presentation 10JUL2010 Michael Yang Mike Yang @ Wipf Group Page 1 of 15 7/10/2010 Sieboldine A Background
Total Synthesis of (+)-Sieboldine A J. Am. Chem. Soc. 2010, 132, 7876 7877. Current Literature Presentation 10JUL2010 Michael Yang Mike Yang @ Wipf Group Page 1 of 15 7/10/2010 Sieboldine A Background
Strategies for Stereocontrolled Synthesis
 Chemistry. Synthetic rganic Chemistry II Lecture 3 March, 2007 Rick L. Danheiser Massachusetts Institute of Technology! Thermodynamic Control Strategies! Kinetic Control Strategies! Strategies for the
Chemistry. Synthetic rganic Chemistry II Lecture 3 March, 2007 Rick L. Danheiser Massachusetts Institute of Technology! Thermodynamic Control Strategies! Kinetic Control Strategies! Strategies for the
