Functionalization of C O Bonds. Stefan McCarver. MacMillan Lab Group Meeting
|
|
|
- Brendan Douglas
- 5 years ago
- Views:
Transcription
1 Functionalization of C Bonds Stefan McCarver MacMillan Lab Group eting November 23 rd, 2016
2 Functionalization of C Bonds "X" X homolytic cleavage catalyst M oxidative addition
3 Why is C Bond Manipulation Important? Lignin n Second most abundant biopolymer n About 30% of organic carbon on earth n Byproduct of paper production n Potential fine chemical feedstock
4 Why is C Bond Manipulation Important? biomass conversion () n
5 Why is C Bond Manipulation Important? biomass conversion () n startup company (founded 2014) based on one step removal of lignin from biological feedstocks and upgrading to valuable commodity chemicals
6 Why is C Bond Manipulation Important? complex molecule total synthesis S 1) Bu 3 Sn S 2) BBr 3 ( )-colombiasin A most alkyl halides are derived from the corresponding alcohol versatile for cross coupling Br X stable and widely or derivatization available Nicolau, K. C.; Vassilikogiannakis, W.; Mägerlein, W.; Kranich, R. Angew. Chem. Int. Ed. 2001, 40, 2482
7 Activation of C Bonds is Challenging Br > > 112 kcal/mol 101 kcal/mol 84 kcal/mol > > Br 96 kcal/mol 86 kcal/mol 74 kcal/mol carbon oxygen bonds are much stronger than the corresponding carbon halide bonds Blanksby, S. J.; Ellison, G. B. Acc. Chem. Res. 2003, 36, 255
8 Functionalization of C Bonds n Radical alcohol deoxygenation n Thiocarbonyl methods n osphite activation X n Thiol catalysis n Titanium-mediated deoxygenation n Transition metal C bond insertion n Aryl methyl ether electrophiles M n Ru directed C bond insertion n enol cross-coupling
9 Reduction of C Bonds X activating group e.g. Barton-McCombie radical deoxygenation direct deoxygenation (few examples) halide/pseudohalide X Br, Tf, Ms Ac, S 3 R dehalogenation errmann, J. F.; König, B. Eur. J. rg. Chem. 2013, 7017
10 Reduction of C Bonds X activating group e.g. Barton-McCombie radical deoxygenation direct deoxygenation (few examples) halide/pseudohalide X Br, Tf, Ms Ac, S 3 R dehalogenation errmann, J. F.; König, B. Eur. J. rg. Chem. 2013, 7017
11 General Functionalization thods are Not Yet Available direct functionalization? R errmann, J. F.; König, B. Eur. J. rg. Chem. 2013, 7017
12 The Barton-McCombie Alcohol Deoxygenation ipr Bu 3 Sn ipr S, reflux X S S SnBu 3 S SnBu 3 N N Bu 3 Sn N N N N conversion from C=S to C= provides thermodynamic driving force Barton, D.. R.; McCombie, S. W. Perkin Trans. I. 1975, 1574
13 Catalytic Barton-McCombie Deoxygenation S 15 mol% Bu 3 Sn PMS, AIBN, reflux 75% yield (catalytic) 79% (stoichiometric) S Bu 3 Sn R 3 Si TMS Si TMS n R R PMS R R Bu 3 Sn R 3 Si Lopez, R. M.; ays, D. S.; Fu, G. C. J. Am. Chem. Soc. 1997, 119, 6949
14 Tin-Free C Bond Reductions general deoxygenation scheme derivatization X reduction X deoxygenation reactions driven by P 2 n entropy electrochemical reduction n formation of =X bond n typically irreversible TBS polarity-reversal catalysis
15 Electrochemical Reduction of Diphenylphosphinate Esters graphite electrode 100 ma cm 2 P DMF, 60 ºC P 0.15 M NBu 4 BF V (Ag/AgCl) = 2.1 V (SCE) electrolysis at 2.4 V (Ag/AgCl) only provided trace product Lam, K.; Marko, I. E. rg. Lett. 2011, 13, 406
16 Electrochemical Reduction of Diphenylphosphinate Esters fragmentation rates support a radical mechanism R () 2 P + e k R () 2 P R () 2 P R k ethyl 0.19 cyclohexyl adamantyl 0.70 allyl too fast to measure Lam, K.; Marko, I. E. rg. Lett. 2011, 13, 406
17 Electrochemical Reduction of Diphenylphosphinate Esters graphite electrode 33 ma cm 2 P DMF, 110 ºC P 0.15 M NBu 4 BF 4 solvent yield DMS CN NMP DMF 0% 0% 43% 44% Lam, K.; Marko, I. E. rg. Lett. 2011, 13, 406
18 Electrochemical Reduction of Diphenylphosphinate Esters fragmentation rates support a radical mechanism R () 2 P + e k R () 2 P R () 2 P R k ethyl 0.19 cyclohexyl adamantyl 0.70 allyl too fast to measure Lam, K.; Marko, I. E. rg. Lett. 2011, 13, 406
19 Electrochemical Reduction of Diphenylphosphinate Esters graphite electrode 100 ma cm 2 R () 2 P R DMF, 60 ºC 0.15 M NBu 4 BF 4 P Ac P P 55% 81% 74% TMS Lam, K.; Marko, I. E. rg. Lett. 2011, 13, 406
20 In Situ osphine Activation P 3 C 9 19 C 9 19 electrolysis can the phosphine intermediate be formed in situ? Maeda,.; Maki, T.; Eguchi, K.; Koide, T.; hmori,. Tet. Lett. 1994, 35, 4129
21 In Situ osphine Activation phosphine constant current at 25 ma C 9 19 C 9 19 CN,Et 4 NX phosphine triethylammonium salt total electricity yield P 3 BF 4 5 F/mol trace P 3 Cl 5 F/mol 66% P 3 Br 5 F/mol 94% P 3 Br 4 F/mol 70% PBu 3 Br 5 F/mol 68% none Br 5 F/mol 0% phosphine necessary for reduction to occur Maeda,.; Maki, T.; Eguchi, K.; Koide, T.; hmori,. Tet. Lett. 1994, 35, 4129
22 In Situ osphine Activation P 3 constant current at 25 ma C 9 19 C 9 19 CN,Et 4 N + Br 94% + C 9 19 P oxidation reduction P 3 P 3 C 9 19 C 9 19 anode cathode Maeda,.; Maki, T.; Eguchi, K.; Koide, T.; hmori,. Tet. Lett. 1994, 35, 4129
23 In Situ osphine Activation PR 3 constant current at 25 ma R R CN,Et 4 N + Br Br 96% (P 3 ) 89% (P 3 ) 86% (PBu 3 ) C % (PBu 3 ) 48% (PBu 3 ) 79% (P() 3, 50 ma) Maeda,.; Maki, T.; Eguchi, K.; Koide, T.; hmori,. Tet. Lett. 1994, 35, 4129
24 thods for Tertiary Alcohol Deoxygenation are Rare many radical deoxygenation methods exist activated esters often thermally unstable
25 Polarity Reversal Activation of MM Ethers (tbu) 3 SiS (cat.) initiator (cat.) octane, reflux reaction conditions 88% 87% 90%, 10:1 dr Dang,.-S.; Franchi, P.; Roberts, B. P. Chem. Commun. 2000, 499
26 Polarity Reversal Activation of MM Ethers Et tbu tbu Δ R tbu tbu Si S tbu alkyl radical cannot abstract to continue radical chain thiol polarity reversal catalyst enables efficient turnover tbu tbu Si S tbu AT AT tbu tbu Si S tbu Dang,.-S.; Franchi, P.; Roberts, B. P. Chem. Commun. 2000, 499
27 Polarity Reversal Activation of MM Ethers (tbu) 3 SiS (cat.) initiator (cat.) octane, reflux reaction conditions 88% 87% 90%, 10:1 dr Dang,.-S.; Franchi, P.; Roberts, B. P. Chem. Commun. 2000, 499
28 Polarity Reversal Activation of MM Ethers (tbu) 3 SiS (cat.) initiator (cat.) octane, reflux reaction conditions 1) Swern oxidation 2) methyl Grignard 3) MM-Cl protection 4) deoxygenation formal to methyl Dang,.-S.; Franchi, P.; Roberts, B. P. Chem. Commun. 2000, 499
29 Titanium diated Alcohol Reduction 2.0 equiv Cp 2 Ti III Cl 1.5 equiv Mn R R TF or toluene aliphatic alcohol reflux alkane Ti III Cl Ti III Cl Cl Ti III reductant lewis acidic significant bond weakening Barrero, A. F. et al J. Am. Chem. Soc. 2010, 132, 254
30 Titanium diated Alcohol Reduction 2.0 equiv Cp 2 Ti III Cl 1.5 equiv Mn R R TF or toluene aliphatic alcohol reflux alkane R Cp 2 TiCl Cl Cp 2 Ti R R Cl Cp 2 Ti R Cp 2 TiCl fast R TiCp 2 Cl + R alcohol coordination to Ti(III) n inner sphere reduction n trapping of radical by Ti(III) Barrero, A. F. et al J. Am. Chem. Soc. 2010, 132, 254
31 Titanium diated Alcohol Reduction 2.0 equiv Cp 2 Ti III Cl 1.5 equiv Mn R R TF or toluene aliphatic alcohol reflux alkane C 14 90% 83% 45% (45% elimination) Bz Bz 92% 70% Barrero, A. F. et al J. Am. Chem. Soc. 2010, 132, 254
32 Titanium diated Alcohol Reduction 2.0 equiv Cp 2 Ti III Cl 1.5 equiv Mn R R TF or toluene aliphatic alcohol reflux alkane TiCp 2 Cl TiCp 2 Cl TiCp 2 Cl() TiCp 2 Cl TiCp 2 Cl() Barrero, A. F. et al J. Am. Chem. Soc. 2010, 132, 254
33 Titanium diated Alcohol Reduction 2.0 equiv Cp 2 Ti III Cl 1.5 equiv Mn R R TF or toluene aliphatic alcohol reflux alkane C 14 90% 83% 45% (45% elimination) Bz Bz 92% 70% Barrero, A. F. et al J. Am. Chem. Soc. 2010, 132, 254
34 Titanium diated Alcohol Reduction 2.0 equiv Cp 2 Ti III Cl 1.5 equiv Mn R R TF or toluene aliphatic alcohol reflux alkane Bz Bz Bz Bz Bz Ti Cp 2 Cl Ti Cp 2 Cl R Barrero, A. F. et al rg. Biomol. Chem. 2015, 13, 3462
35 Titanium Catalyzed Alcohol Reduction Ti III Cl Ac Ti III Cl Catalytic Titanium Ti IV Cp 2 TiCl 0.3 eq Reduction Mn Cl TiIV Cl TMS TMS 2 TMSCl Ac 85% (38% stoichiometric) Barrero, A. F. et al J. Am. Chem. Soc. 2010, 132, 254
36 Functionalization of C Bonds n Radical alcohol deoxygenation n Thiocarbonyl methods n osphite activation X n Thiol catalysis n Titanium-mediated deoxygenation n Transition metal C bond insertion n Aryl methyl ether electrophiles M n Ru directed C bond insertion n enol cross-coupling
37 First Report of Aryl thyl Ether Cross-Coupling MgBr NiCl 2 (P 3 ) 2 tbu benzene, reflux tbu 75% as above 77% much more efficient for napthyl ethers, although some reactivity observed for anisoles Wenkert, E.; Michelotti, E. L.; Swindell, C. S. J. Am. Chem. Soc. 1979, 101, 2246
38 Extension to Anisole Electrophiles p-tolmgbr 3 eq NiCl 2 (ligand) 2 5% ipr 2, 60 ºC ligand Ar yield PEt 3 PiBu 3 PiPr 3 PCy 3 P 2 Cy 75% 32% < 1% 0% 7% 7% 42% 82% 93% 81% P 3 74% 15% Dankwardt, J. W. Angew. Chem. Int. Ed. 2004, 43, 2428
39 Extension to Anisole Electrophiles ArMgBr 3 eq NiCl 2 (ligand) 2 5% Ar ipr 2, 60 ºC N 2 N 75% 82% 73% N 58% 51% Dankwardt, J. W. Angew. Chem. Int. Ed. 2004, 43, 2428
40 thyl Ether Kumada Cross-Couplings p-tol NiCl 2 (PCy 3 ) 2 NiCl 2 (PCy 2 ) 2 p-tolmgbr N MgBr N non-extended aromatics heterocycles Angew. Chem. Int. Ed. 2004, 43, 2428 Angew. Chem. Int. Ed. 2004, 43, 2428 NiCl 2 (PCy 3 ) 2 NiCl 2 (dppf) MgBr MgBr alkyl Grignards benzylic ethers Chem. Commun. 2008, 1437 J. Am. Chem. Soc. 2008, 130, 3268
41 Aryl thyl Ether Cross-Couplings MgBr R B R poor functional group compatibility limited availability challenging to prepare air and moisture stability widespread availability potentially lower reactivity development of an aryl methyl ether Suzuki-Miyaura coupling would be highly advantageous
42 Aryl thyl Ether Suzuki-Miyaura [Ni(cod) 2 ] / PCy 3 B, CsF, 120 ºC 93% 55% 14% 0% Tobisu, M.; Shimasaki, T.; Chatani, N. Angew. Chem. Int. Ed. 2008, 47, 4866
43 Aryl thyl Ether Suzuki-Miyaura [Ni(cod) 2 ] / PCy 3 Ar R B, CsF, 120 ºC F C 2 N 2 65% 83% 79% 74% Tobisu, M.; Shimasaki, T.; Chatani, N. Angew. Chem. Int. Ed. 2008, 47, 4866
44 Aryl thyl Ether Suzuki-Miyaura [Ni(cod) 2 ] / PCy 3 B, CsF, 120 ºC requirement for extended conjugation suggests an alternative oxidative addition mechanism X X Ni 0 Ni I XNi II Ni 0 Ni II NiII X Ni I Kochi electron transfer A nucleophilic type A Tobisu, M.; Shimasaki, T.; Chatani, N. Angew. Chem. Int. Ed. 2008, 47, 4866
45 Aryl thyl Ether Suzuki-Miyaura Ar effective electrophile in Kumada but not Suzuki-Miyaura coupling B [Ni(cod) 2 ] / PCy 3, CsF, 120 ºC 0% yield Et MgBr NiCl 2 (PCy 3 ) 2 73% yield (Et) 2 C 2, 90 ºC Dankwardt, J. W. Angew. Chem. Int. Ed. 2004, 43, 2428 Wenkert, E.; Michelotti, E. L.; Swindell, C. S. J. Am. Chem. Soc. 1979, 101, 2246
46 Aryl thyl Ether Suzuki-Miyaura Cy 3 P Cy 3 P Ni 0 Cy 3 P Cy 3 P Ni II oxidative addition unfavorable Cy 3 P Cy 3 P Ni 0 Cy 3 P MgBr 2 Ni Cy 3 P MgBr + Cy 3 P Cy 3 P Ni II "ate" complex enhanced nucleophilicity Wenkert, E.; Michelotti, E. L.; Swindell, C. S. J. Am. Chem. Soc. 1979, 101, 2246
47 Directed Ru C Bond Cross Coupling tbu B R Ru 2 (C)(P 3 ) 3 4% toluene, reflux Ar tbu tbu 3 P tbu 3 P tbu tbu [Ru] C activation ArB(R) 2 Ru C P 3 C Ru Ar P 3 Ar Kakiuchi, F.; Tsui, M.; Ueno, S.; Chatani, N.; Murai, S. J. Am. Chem. Soc. 2004, 126, 2706
48 Directed Ru C Bond Cross Coupling tbu B R Ru 2 (C)(P 3 ) 3 4% toluene, reflux Ar tbu tbu tbu tbu tbu 76% 96% 81% 50% Kakiuchi, F.; Tsui, M.; Ueno, S.; Chatani, N.; Murai, S. J. Am. Chem. Soc. 2004, 126, 2706
49 Directed Ru C Bond Cross Coupling tbu Ru 2 (C)(P 3 ) 3 toluene RT, 14 h Ar tbu Ru C P 3 toluene 80 ºC, 3 h tbu 3 P Ru C Ar P 3 P 3 kinetic product thermodynamic product Uno, S.; Mizushima, E.; Chatani, N.; Kakiuchi, F. J. Am. Chem. Soc. 2006, 128, 16516
50 Directed Ru C Bond Cross Coupling tbu Ru 2 (C)(P 3 ) 3 toluene RT, 14 h Ar tbu Ru C P 3 toluene 80 ºC, 3 h tbu 3 P Ru C Ar P 3 P 3 first example of an isolated aryl C bond oxidative addition complex with transition metal Uno, S.; Mizushima, E.; Chatani, N.; Kakiuchi, F. J. Am. Chem. Soc. 2006, 128, 16516
51 Directed Ru C Bond Cross Coupling TMS B Ru 2 (C)(P 3 ) 3 toluene, reflux TMS 2 equiv 2 equiv 93% complete selectivity Uno, S.; Mizushima, E.; Chatani, N.; Kakiuchi, F. J. Am. Chem. Soc. 2006, 128, 16516
52 Activation of C Bonds is Challenging Br > > 112 kcal/mol 101 kcal/mol 84 kcal/mol > > Br 96 kcal/mol 86 kcal/mol 74 kcal/mol carbon oxygen bonds are much stronger than the corresponding carbon halide bonds Blanksby, S. J.; Ellison, G. B. Acc. Chem. Res. 2003, 36, 255
53 enolic C Bond Cross-Coupling reaction conditions M MgX NiF 2, PCy ºC reaction conditions [{2-NapMgBr(TF) 2 } 2 ] Yu, D.-G.; Li, B.-J.; Zheng, S.-F.; Guan, B.-T.; Wang, B.-Q.; Shi, Z.-J. Angew. Chem. Int. Ed. 2010, 49, 4566
54 enolic C Bond Cross-Coupling M MgX NiF 2, PCy ºC metal salt halide GC yield Li + K + Na + Mg +2 Mg +2 Mg +2 Br Br Br Br Cl I 8% 14% 81% 99% 64% 87% Yu, D.-G.; Li, B.-J.; Zheng, S.-F.; Guan, B.-T.; Wang, B.-Q.; Shi, Z.-J. Angew. Chem. Int. Ed. 2010, 49, 4566
55 enolic C Bond Cross-Coupling R Ni(PCy 3 ) n Ar TF Mg Br 2 MgBr L Ni Ar R Nickel Catalytic Cycle L Ar Ni Mg S Br 2 Mg +2 activation of phenol C bond towards oxidative addition R MgX L Ni R Mg Mg X Br 2 S Yu, D.-G.; Li, B.-J.; Zheng, S.-F.; Guan, B.-T.; Wang, B.-Q.; Shi, Z.-J. Angew. Chem. Int. Ed. 2010, 49, 4566
56 enolic C Bond Cross-Coupling M R MgX NiF 2, PCy 3 R R 120 ºC R N TMS 67% 73% 86% TBS N 89% 77% 67% Yu, D.-G.; Li, B.-J.; Zheng, S.-F.; Guan, B.-T.; Wang, B.-Q.; Shi, Z.-J. Angew. Chem. Int. Ed. 2010, 49, 4566
57 Benzylic Alcohol C Bond Cross-Coupling 1.2 equiv MgBr 10 mol% NiCl 2 (PCy 3 ) 2, 20 mol% PCy 3 Ar MgCl Ar nbu 2 : (1:3), 60 ºC, 24 h methyl Grignard reagent to form magnesium alkoxide benzylic alcohol C bond weaker than phenol - reflected in lower T required Yu, D.-G.; Wang, X.; Zhu, R.-Y.; Luo, S.; Zhang, X.-B.; Wang, B.-Q.; Wang, L.; Shi, Z.-J. J. Am. Chem. Soc. 2012, 134, 14638
58 Benzylic Alcohol C Bond Cross-Coupling 1.2 equiv MgBr 10 mol% NiCl 2 (PCy 3 ) 2, 20 mol% PCy 3 Ar MgCl Ar nbu 2 : (1:3), 60 ºC, 24 h N N 48% 61% 50% F 62% 87% 67% alkyl Grignard reagents were not effective Yu, D.-G.; Wang, X.; Zhu, R.-Y.; Luo, S.; Zhang, X.-B.; Wang, B.-Q.; Wang, L.; Shi, Z.-J. J. Am. Chem. Soc. 2012, 134, 14638
59 Benzylic Alcohol C Suzuki-Miyaura Pd(P 3 ) 4 10% Ar Ar B(R) 2 Ar TF B B B B B 42% 20% 67% Cao, Z.-C.; Yu, D.-G.; Zhu, R.-Y.; Wei, J.-B.; Shi, Z.-J. Chem. Commun. 2015, 51, 2683
60 Benzylic Alcohol C Suzuki-Miyaura Pd(P 3 ) 4 10% Ar Ar B(R) 2 Ar TF coordination of alcohol to phenylboroxine occurs Cao, Z.-C.; Yu, D.-G.; Zhu, R.-Y.; Wei, J.-B.; Shi, Z.-J. Chem. Commun. 2015, 51, 2683
61 Benzylic Alcohol C Suzuki-Miyaura Pd(P 3 ) 4 10% Ar Ar B(R) 2 Ar TF BocN Ac 78% 71% 80% 2 N 85% 41% Cao, Z.-C.; Yu, D.-G.; Zhu, R.-Y.; Wei, J.-B.; Shi, Z.-J. Chem. Commun. 2015, 51, 2683
Use of Cp 2 TiCl in Synthesis
 Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Nickel-Catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides
 ickel-catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides Eunjae Shim Zakarian Group Literature Talk / Dec 13 th, 2018 University of California, Santa Barbara Table of Contents
ickel-catalyzed Reductive Cross-Electrophile-Coupling Between Aryl and Alkyl Halides Eunjae Shim Zakarian Group Literature Talk / Dec 13 th, 2018 University of California, Santa Barbara Table of Contents
CH 3 TMG, DMF N H 3 CO 2 S. (PPh 3 ) 2 Pd 0
 1. (a) rovide a reasonable mechanism for the following transformation. I S 2 C 3 C 3 ( 3 ) 2 2, CuI C 3 TMG, DMF 3 C 2 S TMG = Me 2 Me 2 ICu ( 3 ) 2 0 I S 2 C 3 S 2 C 3 Cu I 3 3 3 C 2 S I 3 3 3 C 2 S 3
1. (a) rovide a reasonable mechanism for the following transformation. I S 2 C 3 C 3 ( 3 ) 2 2, CuI C 3 TMG, DMF 3 C 2 S TMG = Me 2 Me 2 ICu ( 3 ) 2 0 I S 2 C 3 S 2 C 3 Cu I 3 3 3 C 2 S I 3 3 3 C 2 S 3
Additions to Metal-Alkene and -Alkyne Complexes
 Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides
 Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
Organocopper Reagents
 rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
Copper-Catalyzed Reaction of Alkyl Halides with Cyclopentadienylmagnesium Reagent
 Copper-Catalyzed eaction of Alkyl Halides with Cyclopentadienylmagnesium eagent Mg 1) cat. Cu(Tf) 2 i Pr 2, 25 o C, 3 h 2) H 2, Pt 2 Masahiro Sai, Hidenori Someya, Hideki Yorimitsu, and Koichiro shima
Copper-Catalyzed eaction of Alkyl Halides with Cyclopentadienylmagnesium eagent Mg 1) cat. Cu(Tf) 2 i Pr 2, 25 o C, 3 h 2) H 2, Pt 2 Masahiro Sai, Hidenori Someya, Hideki Yorimitsu, and Koichiro shima
O H Hydrogen bonding promotes H-atom transfer from C H bonds for C-alkylation of alcohols
 ydrogen bonding promotes -atom transfer from C bonds for C-alkylation of alcohols Jenna L. Jeffrey, Jack A. Terrett, David W. C. MacMillan Science 2015, 349, 1532-1536 Raffaele Colombo 9/26/2015 Raffaele
ydrogen bonding promotes -atom transfer from C bonds for C-alkylation of alcohols Jenna L. Jeffrey, Jack A. Terrett, David W. C. MacMillan Science 2015, 349, 1532-1536 Raffaele Colombo 9/26/2015 Raffaele
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Mechanistic Insides into Nickamine-Catalyzed Alkyl-Alkyl Cross-Coupling Reactions
 Mechanistic Insides into Nickamine-Catalyzed Alkyl-Alkyl Cross-Coupling Reactions Abstract Within the last decades the transition metal-catalyzed cross-coupling of non-activated alkyl halides has significantly
Mechanistic Insides into Nickamine-Catalyzed Alkyl-Alkyl Cross-Coupling Reactions Abstract Within the last decades the transition metal-catalyzed cross-coupling of non-activated alkyl halides has significantly
Iron Catalysed Coupling Reactions
 LONG LITERATURE REPORT Iron Catalysed Coupling Reactions Mingyu Liu 2017. 8. 31 1 Fe [Ar]3d 6 4s 2 The fourth most common element in the Earth s crust Relatively less understanding and manipulation of
LONG LITERATURE REPORT Iron Catalysed Coupling Reactions Mingyu Liu 2017. 8. 31 1 Fe [Ar]3d 6 4s 2 The fourth most common element in the Earth s crust Relatively less understanding and manipulation of
Sonogashira: in situ, metal assisted deprotonation
 M.C. White, Chem 253 Cross-Coupling -120- Week of ctober 11, 2004 Sonogashira: in situ, metal assisted deprotonation catalytic cycle: ' (h 3 ) n d II The first report: h Sonogashira T 1975 (50) 4467. h
M.C. White, Chem 253 Cross-Coupling -120- Week of ctober 11, 2004 Sonogashira: in situ, metal assisted deprotonation catalytic cycle: ' (h 3 ) n d II The first report: h Sonogashira T 1975 (50) 4467. h
Organic Tutorials 3 rd Year Michaelmas Transition Metals in Organic Synthesis: (General paper level) ! 1! Reading
 rganic Tutorials 3 rd Year Michaelmas 2010 Transition Metals in rganic Synthesis: (General paper level) Reading 1. Lecture Course, and suggested references from this. 2. Clayden, Greaves, Warren and Wothers.
rganic Tutorials 3 rd Year Michaelmas 2010 Transition Metals in rganic Synthesis: (General paper level) Reading 1. Lecture Course, and suggested references from this. 2. Clayden, Greaves, Warren and Wothers.
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Direct Catalytic Cross-Coupling of Organolithium
 Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo
 Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Catellani Reaction (Pd-Catalyzed Sequential Reaction) Todd Luo 2014.1.6 1 Content Introduction Progress of Catellani Reaction o-alkylation and Applications o-arylation and Applications Conclusion and Outlook
Functionalization of C(sp 3 ) H Bonds Using a Transient Directing Group
 Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02
![Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02](/thumbs/93/114018431.jpg) Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
CuI CuI eage lic R tal ome rgan gbr ommon
 Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Regioselective Reductive Cross-Coupling Reaction
 Lit. Seminar. 2010. 6.16 Shinsuke Mouri (D3) Regioselective Reductive Cross-Coupling Reaction Glenn C. Micalizio obtained a Ph.D. at the University of Michigan in 2001 under the supervision of Professor
Lit. Seminar. 2010. 6.16 Shinsuke Mouri (D3) Regioselective Reductive Cross-Coupling Reaction Glenn C. Micalizio obtained a Ph.D. at the University of Michigan in 2001 under the supervision of Professor
Radical Reactions. Radical Stability!!! bond dissociation energies X Y X + Y. bond BDE (kcal/mol) bond BDE (kcal/mol) CH 3 CH 3 CH 2 95 O H R 2 C H
 adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
Hybridization of Nickel Catalysis and Photoredox Catalysis. Literature seminar#1 B4 Hiromu Fuse 2017/02/04(Sat)
 Hybridization of Nickel Catalysis and Photoredox Catalysis Literature seminar#1 B4 Hiromu Fuse 2017/02/04(Sat) Introduction Novel cross coupling was reported! Highly selective sp 3 C-H functionalization!
Hybridization of Nickel Catalysis and Photoredox Catalysis Literature seminar#1 B4 Hiromu Fuse 2017/02/04(Sat) Introduction Novel cross coupling was reported! Highly selective sp 3 C-H functionalization!
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols
 A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
Chapter 12. Alcohols from Carbonyl Compounds Oxidation-Reduction & Organometallic Compounds. Structure
 Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Organic Chemistry Laboratory Summer Lecture 6 Transition metal organometallic chemistry and catalysis July
 344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Asymmetric Nucleophilic Catalysis
 Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
Asymmetric Radical Reactions. Zhen Liu 08/30/2018
 Asymmetric adical eactions Zhen Liu 08/30/2018 Contents Introduction eactions Using Chiral Auxiliary Chiral Lewis Acid-diated eactions Transition tal-catalyzed eactions eactions Using Chiral rganocatalysts
Asymmetric adical eactions Zhen Liu 08/30/2018 Contents Introduction eactions Using Chiral Auxiliary Chiral Lewis Acid-diated eactions Transition tal-catalyzed eactions eactions Using Chiral rganocatalysts
Stable gold(iii) catalysts by oxidative addition of a carboncarbon
 Stable gold(iii) catalysts by oxidative addition of a carboncarbon bond Chung-Yeh Wu, Takahiro oribe, Christian Borch Jacobsen & F. Dean Toste ature, 517, 449-454 (2015) presented by Ian Crouch Literature
Stable gold(iii) catalysts by oxidative addition of a carboncarbon bond Chung-Yeh Wu, Takahiro oribe, Christian Borch Jacobsen & F. Dean Toste ature, 517, 449-454 (2015) presented by Ian Crouch Literature
Learning Guide for Chapter 14 - Alcohols (I)
 Learning Guide for Chapter 14 - Alcohols (I) I. Introduction to Alcohols and Thiols II. Acid/base Behavior of Alcohols, Phenols, and Thiols III. Nomenclature of Alcohols IV. Synthesis of Alcohols Previous
Learning Guide for Chapter 14 - Alcohols (I) I. Introduction to Alcohols and Thiols II. Acid/base Behavior of Alcohols, Phenols, and Thiols III. Nomenclature of Alcohols IV. Synthesis of Alcohols Previous
Modern Synthetic Methods
 Modern Synthetic Methods Dr. Dorian Didier dodich@cup.uni-muenchen.de Functionnalized Organometallic Reagents C-N, C-O and C-S Bond Formation Introduction to Organoboron Chemistry Introduction to Organosilicon
Modern Synthetic Methods Dr. Dorian Didier dodich@cup.uni-muenchen.de Functionnalized Organometallic Reagents C-N, C-O and C-S Bond Formation Introduction to Organoboron Chemistry Introduction to Organosilicon
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Palladium-Catalyzed Alkylation of sp2 and sp3 C-H Bonds with Methylboroxine and Alkylboronic Acids: Two Distinct C-H Activation Pathways
 Palladium-Catalyzed Alkylation of sp2 and sp3 C-H Bonds with Methylboroxine and Alkylboronic Acids: Two Distinct C-H Activation Pathways Xiao Cheng, Charles Goodhue, and Jin-Quan Yu Brandeis University
Palladium-Catalyzed Alkylation of sp2 and sp3 C-H Bonds with Methylboroxine and Alkylboronic Acids: Two Distinct C-H Activation Pathways Xiao Cheng, Charles Goodhue, and Jin-Quan Yu Brandeis University
Nickel-Catalyzed Three-Component [3+2+2] Cocyclization of Ethyl Cyclopropylideneacetate and Alkynes
![Nickel-Catalyzed Three-Component [3+2+2] Cocyclization of Ethyl Cyclopropylideneacetate and Alkynes Nickel-Catalyzed Three-Component [3+2+2] Cocyclization of Ethyl Cyclopropylideneacetate and Alkynes](/thumbs/77/74720708.jpg) Nickel-Catalyzed Three-Component [3+2+2] Cocyclization of Ethyl Cyclopropylideneacetate and Alkynes Selective Synthesis of Multisubstituted Cycloheptadienes 1 2 Cat. Ni 0 1 2 Komagawa, S.; Saito, S. Angew.
Nickel-Catalyzed Three-Component [3+2+2] Cocyclization of Ethyl Cyclopropylideneacetate and Alkynes Selective Synthesis of Multisubstituted Cycloheptadienes 1 2 Cat. Ni 0 1 2 Komagawa, S.; Saito, S. Angew.
Metal Hydrides, Alkyls, Aryls, and their Reactions
 Metal Hydrides, Alkyls, Aryls, and their Reactions A Primer on MO Theory σ-bonding in Organotransition Metal Complexes M-C Bond Energies in Organotransition Metal Complexes Thermodynamic Predictions
Metal Hydrides, Alkyls, Aryls, and their Reactions A Primer on MO Theory σ-bonding in Organotransition Metal Complexes M-C Bond Energies in Organotransition Metal Complexes Thermodynamic Predictions
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group.
 Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Chapter 2 The Elementary Steps in TM Catalysis
 hapter 2 The Elementary Steps in TM atalysis + + ligand exchange A oxidative addition > n + A B n+2 reductive elimination < B n n+2 oxidative coupling + M' + M' transmetallation migratory insertion > (carbo-,
hapter 2 The Elementary Steps in TM atalysis + + ligand exchange A oxidative addition > n + A B n+2 reductive elimination < B n n+2 oxidative coupling + M' + M' transmetallation migratory insertion > (carbo-,
Organic Electron Donors
 rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
Short Literature Presentation 10/4/2010 Erika A. Crane
 Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Huang, C.; Gevorgyan, V. J. Am. Chem. Soc. 2009, 131, Daniel Tzvi Cohen Short Literature Feb. 23, MeO HO OH. COOH ( )-Plicatic Acid OH OH
 Asymmetric Total Synthesis of ( )-Plicatic Acid via a Highly Enantioselective and Diastereoselective Nucleophilic Epoxidation of Acyclic Trisubstituted lefins H H H H CH ( )-Plicatic Acid H H Sun, B.F.;
Asymmetric Total Synthesis of ( )-Plicatic Acid via a Highly Enantioselective and Diastereoselective Nucleophilic Epoxidation of Acyclic Trisubstituted lefins H H H H CH ( )-Plicatic Acid H H Sun, B.F.;
Oxidative Addition and Reductive Elimination
 xidative Addition and Reductive Elimination red elim coord 2 ox add ins Peter.. Budzelaar xidative Addition Basic reaction: n + X Y n X Y The new -X and -Y bonds are formed using: the electron pair of
xidative Addition and Reductive Elimination red elim coord 2 ox add ins Peter.. Budzelaar xidative Addition Basic reaction: n + X Y n X Y The new -X and -Y bonds are formed using: the electron pair of
VI. Metal alkyls from oxidative addition / insertion
 V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
Zr-Catalyzed Carbometallation
 -Catalyzed Carbometallation C C C C ML n C C ML n ML n C C C C ML n ML n C C ML n Wipf Group esearch Topic Seminar Juan Arredondo November 13, 2004 Juan Arredondo @ Wipf Group 1 11/14/2004 Carbometallation
-Catalyzed Carbometallation C C C C ML n C C ML n ML n C C C C ML n ML n C C ML n Wipf Group esearch Topic Seminar Juan Arredondo November 13, 2004 Juan Arredondo @ Wipf Group 1 11/14/2004 Carbometallation
Direct Organocatalytic Enantioselective Mannich Reactions of Ketimines: An Approach to Optically Active Quaternary α-amino Acid Derivatives
 Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Lecture 6: Transition-Metal Catalysed C-C Bond Formation
 Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Chemistry 35 Exam 2 Answers - April 9, 2007
 Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
Chemistry 35 Exam 2 Answers - April 9, 2007 Problem 1. (14 points) Provide the products for the reactions shown below. If stereochemistry is important, be sure to indicate stereochemistry clearly. If no
Ethers can be symmetrical or not:
 Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
Carbonyl Ylide Cycloadditions
 Carbonyl Ylide Cycloadditions cond. icholas Anderson Denmark Group eting 07/13/10 Carbonyl Ylides Uncharged 1,3-Dipole Conjugated π-system ighly reactive on-isolable Generate in-situ Carbonyl Ylide Stability
Carbonyl Ylide Cycloadditions cond. icholas Anderson Denmark Group eting 07/13/10 Carbonyl Ylides Uncharged 1,3-Dipole Conjugated π-system ighly reactive on-isolable Generate in-situ Carbonyl Ylide Stability
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
Requirements for an Effective Chiral Auxiliary Enolate Alkylation
 Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Lecture 15. More Carbonyl Chemistry. Alcohols React with Aldehydes and Ketones in two steps first O R'OH, H + OR" 2R"OH R + H 2 O OR" 3/8/16
 Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
Morita Baylis Hillman Reaction. Aaron C. Smith 11/10/04
 Morita Baylis Hillman Reaction Aaron C. Smith 11/10/04 Outline 1. Background 2. Development of Asymmetric Variants 3. Aza-Baylis Hillman Reaction 4. Applications of Baylis Hillman Adducts Outline 1. Background
Morita Baylis Hillman Reaction Aaron C. Smith 11/10/04 Outline 1. Background 2. Development of Asymmetric Variants 3. Aza-Baylis Hillman Reaction 4. Applications of Baylis Hillman Adducts Outline 1. Background
Spiro Monophosphite and Monophosphoramidite Ligand Kit
 Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
An Overview of Organic Reactions. Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants:
 An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
Joseph Salamoun Current Literature 11/21/15 Wipf Group
 Joseph Salamoun Current Literature 11/21/15 Wipf Group Joe Salamoun @ Wipf Group Page 1 of 16 12/29/2015 The mechanism of the oxidative addition-transmetallation-reductive elimination process is very complex
Joseph Salamoun Current Literature 11/21/15 Wipf Group Joe Salamoun @ Wipf Group Page 1 of 16 12/29/2015 The mechanism of the oxidative addition-transmetallation-reductive elimination process is very complex
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Synthesis of 1,3-Diols via Controlled, Radical-Mediated C-H Functionalization
 Synthesis of 1,3-Diols via Controlled, Radical-Mediated C- Functionalization Chen, K.; Richter, J. M.; Baran, P. S. J. Amer. Chem. Soc. 2008, 130, 7247-7249. Literature Group Presentation Wynter Gilson
Synthesis of 1,3-Diols via Controlled, Radical-Mediated C- Functionalization Chen, K.; Richter, J. M.; Baran, P. S. J. Amer. Chem. Soc. 2008, 130, 7247-7249. Literature Group Presentation Wynter Gilson
Direct Oxidative Heck Cyclizations: Intramolecular Fujiwara-Moritani Arylations for the Synthesis of Functionalized Benzofurans and Dihydrobenzofurans
 Direct xidative eck Cyclizations: Intramolecular Fujiwara-Moritani Arylations for the Synthesis of Functionalized Benzofurans and Dihydrobenzofurans by Zhang,.; Ferreira, E. M.; Stoltz, B. M. Angewandte
Direct xidative eck Cyclizations: Intramolecular Fujiwara-Moritani Arylations for the Synthesis of Functionalized Benzofurans and Dihydrobenzofurans by Zhang,.; Ferreira, E. M.; Stoltz, B. M. Angewandte
Valorization of Lignin. M2 Watanabe 11/30/2017
 Valorization of Lignin M2 Watanabe 11/30/2017 1.Introduction 2.Chemical degradation 2-1. aryl C-O bond cleaverage of lignin Hartwig work 2-2. alkyl C-O bond cleaverage of lignin 3. Problem of lignin separation
Valorization of Lignin M2 Watanabe 11/30/2017 1.Introduction 2.Chemical degradation 2-1. aryl C-O bond cleaverage of lignin Hartwig work 2-2. alkyl C-O bond cleaverage of lignin 3. Problem of lignin separation
Recent Developments in Alkynylation
 --New approaches to introduce an alkynyl group Reporter: Zhao-feng Wang Supervisor: Yong Huang 2013-03-27 Contents 1. Introduction of Acetylene Chemistry 2. Nucleophilic alkynylation : Classic text book
--New approaches to introduce an alkynyl group Reporter: Zhao-feng Wang Supervisor: Yong Huang 2013-03-27 Contents 1. Introduction of Acetylene Chemistry 2. Nucleophilic alkynylation : Classic text book
Transition Metal-Catalyzed Carbon-Carbon Bond Cleavage (C-C Activation) Group Meeting Timothy Chang
 Transition Metal-Catalyzed Carbon-Carbon Bond Cleavage (C-C Activation) Group Meeting 01-15-2008 Timothy Chang Outlines - Fundamental considerations, C-H versus C-C activation - Orbital interactions -
Transition Metal-Catalyzed Carbon-Carbon Bond Cleavage (C-C Activation) Group Meeting 01-15-2008 Timothy Chang Outlines - Fundamental considerations, C-H versus C-C activation - Orbital interactions -
Intramolecular Ene Reactions Utilizing Oxazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129,
 Intramolecular Ene Reactions Utilizing xazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129, 3058-3059 - versus -Arylation of Aminoalcohols: rthogonal Selectivity in Copper-Based
Intramolecular Ene Reactions Utilizing xazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129, 3058-3059 - versus -Arylation of Aminoalcohols: rthogonal Selectivity in Copper-Based
Total Syntheses of Minfiensine
 Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
11-Step Enantioselective Synthesis of ( )-Lomaiviticin Aglycon
 11-Step Enantioselective Synthesis of ( )-Lomaiviticin Aglycon Seth B. erzon, Liang Lu, Christina M. Woo, and Shivajirao L. Gholap J. Am. Chem. Soc. ASAP DI 10.1021/ja200034b Melissa Sprachman Current
11-Step Enantioselective Synthesis of ( )-Lomaiviticin Aglycon Seth B. erzon, Liang Lu, Christina M. Woo, and Shivajirao L. Gholap J. Am. Chem. Soc. ASAP DI 10.1021/ja200034b Melissa Sprachman Current
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds*
 Pure Appl. Chem., Vol. 72, No. 9, pp. 1715 1719, 2000. 2000 IUPAC Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds* O. G. Kulinkovich
Pure Appl. Chem., Vol. 72, No. 9, pp. 1715 1719, 2000. 2000 IUPAC Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds* O. G. Kulinkovich
Repeated insertion. Multiple insertion leads to dimerization, oligomerization or polymerization. κ 1: mainly dimerization κ
 Repeated insertion ultiple insertion leads to dimerization, oligomerization or polymerization. k prop Et Key factor: k CT / k prop = κ κ 1: mainly dimerization κ 0.1-1.0: oligomerization (always mixtures)
Repeated insertion ultiple insertion leads to dimerization, oligomerization or polymerization. k prop Et Key factor: k CT / k prop = κ κ 1: mainly dimerization κ 0.1-1.0: oligomerization (always mixtures)
Physical Properties. Alcohols can be: CH CH 2 OH CH 2 CH 3 C OH CH 3. Secondary alcohol. Primary alcohol. Tertiary alcohol
 Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Couplings: Recent Developments in Cross-Couplings: Acids with Carbonyls. and Boronic. Wen Yuan
 Recent Developments in Cross-Couplings: Couplings: Coupling Boronates with Inert C- C Bonds, and Boronic Acids with Carbonyls Wen Yuan 12-02-09 Cross-Coupling Reaction The reaction of organometallic reagents
Recent Developments in Cross-Couplings: Couplings: Coupling Boronates with Inert C- C Bonds, and Boronic Acids with Carbonyls Wen Yuan 12-02-09 Cross-Coupling Reaction The reaction of organometallic reagents
Lecture 18. Oxidation and Reduction. Oxidation. Reduction O CH 4 CH 3 OH H C H. Chemistry 328N
 Lecture 18 xidation and Reduction C 4 C 3 C C C xidation Reduction March 27, 2018 Suppose you want to make this compound????? C + BrC 2 C 2 C?? CC 2 C 2 C 4-ydroxy-4-phenylbutanal It s an alcohol. Use
Lecture 18 xidation and Reduction C 4 C 3 C C C xidation Reduction March 27, 2018 Suppose you want to make this compound????? C + BrC 2 C 2 C?? CC 2 C 2 C 4-ydroxy-4-phenylbutanal It s an alcohol. Use
o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)]
![o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)] o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)]](/thumbs/85/92754396.jpg) 3. Boron -- eview [Suzuki Chem. ev. 95, 2457 (1995)] U77b ydroboration also attractive but B Pd transmetallation difficult - must produce stable B product - solved (by Suzuki) by adding base to make Borates
3. Boron -- eview [Suzuki Chem. ev. 95, 2457 (1995)] U77b ydroboration also attractive but B Pd transmetallation difficult - must produce stable B product - solved (by Suzuki) by adding base to make Borates
Supporting Information for
 Supporting Information for Factors Controlling the Reactivity and Chemoselectivity of Resonance Destabilized Amides in Ni-catalyzed Decarbonylative and Non-decarbonylative Suzuki-Miyaura Coupling Chong-Lei
Supporting Information for Factors Controlling the Reactivity and Chemoselectivity of Resonance Destabilized Amides in Ni-catalyzed Decarbonylative and Non-decarbonylative Suzuki-Miyaura Coupling Chong-Lei
Mechanistic Implications in the Morita Baylis Hillman Alkylation: Isolation and Characterization of an Intermediate
 Mechanistic Implications in the Morita Baylis Hillman Alkylation: Isolation and Characterization of an Intermediate M. E. Krafft,* T. F. N. Haxell, K. A. Seibert, and K. A. Abboud Department of Chemistry
Mechanistic Implications in the Morita Baylis Hillman Alkylation: Isolation and Characterization of an Intermediate M. E. Krafft,* T. F. N. Haxell, K. A. Seibert, and K. A. Abboud Department of Chemistry
Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines
 Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Catalysis by Group IV Elements CHEM 966 (Tunge) Good reference: Titanium and Zirconium in Organic Synthesis Ilan Marek Ed., 2002.
 Catalysis by Group IV Elements CEM 966 (Tunge) Good reference: Titanium and Zirconium in rganic Synthesis Ilan Marek Ed., 2002. Electronegativity: Ti(1.54); (1.33); f(1.3) Much of the chemistry is dominated
Catalysis by Group IV Elements CEM 966 (Tunge) Good reference: Titanium and Zirconium in rganic Synthesis Ilan Marek Ed., 2002. Electronegativity: Ti(1.54); (1.33); f(1.3) Much of the chemistry is dominated
Chiral Diol Promoted Boronates Addi3on Reac3ons. Lu Yan Morken Group Boston College
 Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Highlights of Schmidt Reaction in the Last Ten Years
 ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
CHEM 344 Organometallic Chemistry Practice Problems (not for credit)
 CHEM 344 Organometallic Chemistry Practice Problems (not for credit) Name (print): TA name (print): 1) Careful choice of solvent is essential for the successful generation and reaction of a Grignard reagent.
CHEM 344 Organometallic Chemistry Practice Problems (not for credit) Name (print): TA name (print): 1) Careful choice of solvent is essential for the successful generation and reaction of a Grignard reagent.
12.5 Organometallic Compounds
 12.5 rganometallic Compounds Compounds that contain carbon-metal bond are called organometallic compounds. C M C δ δ + M C M Primarily ionic Primarily covalent (M = Na + or K + )(M = Mg or Li) (M = Pb,
12.5 rganometallic Compounds Compounds that contain carbon-metal bond are called organometallic compounds. C M C δ δ + M C M Primarily ionic Primarily covalent (M = Na + or K + )(M = Mg or Li) (M = Pb,
Reduction. Boron based reagents. NaBH 4 / NiCl 2. Uses: Zn(BH 4 ) 2. Preparation: Good for base sensitive groups Chelation control model.
 Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Ready; Catalysis Conjugate Addition
 eady; Catalysis Conjugate Addition Topics covered 1. 1,4 addition involving copper a. stoichiometric reactions b. catalytic reactions c. allylic substitution. Conjugate addition without copper a. Ni-based
eady; Catalysis Conjugate Addition Topics covered 1. 1,4 addition involving copper a. stoichiometric reactions b. catalytic reactions c. allylic substitution. Conjugate addition without copper a. Ni-based
Asymmetric Palladium Catalyzed Cross-Coupling Reactions. Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1
 Asymmetric Palladium Catalyzed Cross-Coupling Reactions Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1 Palladium Catalyzed Cross-Coupling Reactions 2 Kumada/Negishi Cross-Coupling Kumada:
Asymmetric Palladium Catalyzed Cross-Coupling Reactions Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1 Palladium Catalyzed Cross-Coupling Reactions 2 Kumada/Negishi Cross-Coupling Kumada:
Development of Chiral Phosphine Olefin Ligands and Their Use in Asymmetric Catalysis
 Development of Chiral osphine lefin Ligands and Their Use in Asymmetric Catalysis 2 Wei-Liang Duan July 31, 2007 Research Works in Hayashi Group, Kyoto University (ct, 2003 Mar, 2007) Conventional Chiral
Development of Chiral osphine lefin Ligands and Their Use in Asymmetric Catalysis 2 Wei-Liang Duan July 31, 2007 Research Works in Hayashi Group, Kyoto University (ct, 2003 Mar, 2007) Conventional Chiral
R 2 R 4 Ln catalyst. This manuscript describes the methods for the synthesis and application of group 4 metallocene bis(trimethylsilyl)acetylene
 VII Abstracts 2011 p1 2.12.15 rganometallic Complexes of Scandium, Yttrium, and the Lanthanides P. Dissanayake, D. J. Averill, and M. J. Allen This manuscript is an update to the existing Science of Synthesis
VII Abstracts 2011 p1 2.12.15 rganometallic Complexes of Scandium, Yttrium, and the Lanthanides P. Dissanayake, D. J. Averill, and M. J. Allen This manuscript is an update to the existing Science of Synthesis
Insertion Reactions. 1) 1,1 insertion in which the metal and the X ligand end up bound to the same (1,1) atom
 Insertion Reactions xidative addition and substitution allow us to assemble 1e and 2e ligands on the metal, respectively. With insertion, and its reverse reaction, elimination, we can now combine and transform
Insertion Reactions xidative addition and substitution allow us to assemble 1e and 2e ligands on the metal, respectively. With insertion, and its reverse reaction, elimination, we can now combine and transform
Massachusetts Institute of Technology Organic Chemistry Problem Set 1. Functional Group Transformations Study Guide
 Massachusetts Institute of Technology rganic Chemistry 5.511 Problem Set 1 September, 2007 Prof. Rick L. Danheiser Functional Group Transformations Study Guide The purpose of this three-part study guide
Massachusetts Institute of Technology rganic Chemistry 5.511 Problem Set 1 September, 2007 Prof. Rick L. Danheiser Functional Group Transformations Study Guide The purpose of this three-part study guide
Three Type Of Carbene Complexes
 Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Suggested solutions for Chapter 40
 s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
Homework - Chapter 14 Chem 2320
 omework - Chapter 14 Chem 2320 Name 1. Label each alcohol in the the following compounds as primary, secondary, tertiary, enol, or aryl. 2. Fill in the blanks in the following sentences. Enols are in equilibrium
omework - Chapter 14 Chem 2320 Name 1. Label each alcohol in the the following compounds as primary, secondary, tertiary, enol, or aryl. 2. Fill in the blanks in the following sentences. Enols are in equilibrium
1. Radical Substitution on Alkanes. 2. Radical Substitution with Alkenes. 3. Electrophilic Addition
 1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
Enantioselective Protonations
 Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Chapter 17: Alcohols and Phenols
 hapter 17: Alcohols and Phenols sp 3 alcohol phenol (aromatic alcohol) pka~ 16-18 pka~ 10 Alcohols contain an group connected to a saturated carbon (sp 3 ) Phenols contain an group connected to a carbon
hapter 17: Alcohols and Phenols sp 3 alcohol phenol (aromatic alcohol) pka~ 16-18 pka~ 10 Alcohols contain an group connected to a saturated carbon (sp 3 ) Phenols contain an group connected to a carbon
O or R E + R. - Keto-Enol Tautomerization (enol form usually very minor for simple ketones)
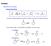 General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
Strategies for Catalytic Asymmetric Electrophilic a Halogenation of Carbonyl Compounds
 Strategies for Catalytic Asymmetric Electrophilic a alogenation of Carbonyl Compounds 1 2 Y Catalyst [X + ] 1 X! 2 Y intermann, L. ; Togni, A. Angew. Chem. Int. Ed. 2000, 39, 4359 4362 amashima, Y.; Sodeoka,
Strategies for Catalytic Asymmetric Electrophilic a alogenation of Carbonyl Compounds 1 2 Y Catalyst [X + ] 1 X! 2 Y intermann, L. ; Togni, A. Angew. Chem. Int. Ed. 2000, 39, 4359 4362 amashima, Y.; Sodeoka,
