Carbonyl Chemistry IV: Enolate Alkylations and Aldols. Aldol Madness O O O N M + substrate. aldehyde. (Z)-enolate H
|
|
|
- Jeffrey Bryan
- 5 years ago
- Views:
Transcription
1 Carbonyl Chemistry IV: nolate Alkylations and Aldols Paul Bracher Chem 30 Section 9 Section Agenda 1) o office hours Thursday 2) The Great Joe Young is covering section next onday 3) andout: Carbonyl Chemistry IV Aldol adness dipoles oppose (minimize net moment) asplode! (S) chiral auxiliary 3 base 2 Your head S 1
2 odel for Asymmetric Alkylation This is relatively easy. Simply draw a 3D structure of the enolate and add the to the least hindered face of the double bond. the one carbon bridge is smaller and allows the easier access to top face of the enolate DA Use of oxazolidinone chiral auxiliaries: top bottom major minor oxazolidinone chiral auxiliary let's analyze this intermediate DA i e preferred bottom top e e major minor i These atoms are roughly in the same plane, held fixed by the chelated. ven a is chelated in these systems. C 3 The group is "up/out", so the has easier access to the bottom face of the enolate plane Addition occurs to the enolate carbon To reverse the product ratios, use the chiral auxiliary with the opposite stereochemistry 2
3 Asymmetric Aldol eactions (S) chiral auxiliary () chiral auxiliary base base Bulky substituent points AWAY from ring Bulky substituent points AWAY from ring S dipoles oppose (minimize net moment) dipoles oppose (minimize net moment) To draw the correct final product, assign and S designations to the two stereocenters that are generated in the transition state, then add solid or dashed wedges to your ewis structure to match these designations 3
4 Conjugate Additions 1,2 addition uc 1,4 addition uc uc via uc a nucleophilic intermediate! ther s add here C add here add here etc. These two additions pathways compete when a nucleophile reacts with an α,β-unsaturated. As a general rule, most 1,2 additions are faster, but they are usually more reversible. ichael reactions are those in which an enolate adds in 1,4 fashion Beware of tandem reactions in which the enolate intermediate acts as a nucleophile 1,2 Additions 1,4 Additions ard nucleophiles not polarizable/high charge density ithium Aluminum ydride (A) Alkyllithium reagents (i) ote that these are the nucleophiles we normally classify as irreversible Soft ucleophiles polarizable/low charge density S, C, enolates rganocuprates ( Cui) (via an organolic mechanism) 4
5 Synthetic Transformations lectrophiles s Synthetic Preparations Swern oxidation of alcohols; DIBA- reduction of nitriles ketones xidation of alcohols; Friedel-Crafts reaction; xidation of Grignard products esters ' Fischer esterification of acids and alcohols; acid chlorides and anhydrides with alcohols; Bayear-Villeger oxidation alkyl halides From alcohols with S 2 or PBr 3 ; alkenes with Br (with or w/o peroxides); alkanes by photohalogenation α,β-unsaturated carbonyls Aldol reactions; α-keto halogenation (ell-volhard-zelinsky) then elimination of Br nitriles C Cyanide substitution of alkyl halides; dehydration of amides by P 2 5 ; ucleophiles kinetic enolates Irreversible bases (DA), bulky bases thermodynamic enolates eversible bases (alkoxides, hydroxides) malonic ester enolates ' ' Bases with pk a >> 8 (a, - ) silyl enol ethers TBS TBS and an amine base in anhydrous solvent enamines ' ' p = 5 catalyzed condensation 5
6 Aldol Additions reversible acid or base irreversible base (e.g. DA) ' ' ' Does not work well with ketone s annich '' ' ' 2 '' Syn Aldol Products ' ' s ' Anti Aldol Products ' ' ()-enolates ' vans' Aldol eaction ' ' ' ' Use A to get terminal alcohols via alonic ster Alkylation hydrolysis and decarboxylation ' '' ' '' ' '' '' '' '' Conjugate Addition ichael eaction rganocuprate Addtion X eview which nucleophiles add 1,4 vs. 1,2 6
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
acetaldehyde (ethanal)
 hem 263 Nov 2, 2010 Preparation of Ketones and Aldehydes from Alkenes zonolysis 1. 3 2. Zn acetone 1. 3 2. Zn acetone acetaldehyde (ethanal) Mechanism: 3 3 3 + - oncerted reaction 3 3 3 + ozonide (explosive)
hem 263 Nov 2, 2010 Preparation of Ketones and Aldehydes from Alkenes zonolysis 1. 3 2. Zn acetone 1. 3 2. Zn acetone acetaldehyde (ethanal) Mechanism: 3 3 3 + - oncerted reaction 3 3 3 + ozonide (explosive)
Chem 263 Nov 3, 2016
 hem 263 Nov 3, 2016 Preparation of Aldehydes from Acid alides? + l l acid chloride aka acyl chloride aldehyde Needed: 2 Actual eagents: 2 /Pd Al This is lithium tri-t-butoxy aluminum hydride, a very sterically
hem 263 Nov 3, 2016 Preparation of Aldehydes from Acid alides? + l l acid chloride aka acyl chloride aldehyde Needed: 2 Actual eagents: 2 /Pd Al This is lithium tri-t-butoxy aluminum hydride, a very sterically
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 23. Alpha Substitution of Carbonyl Compounds.
 1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Chem 263 Nov 7, elimination reaction. There are many reagents that can be used for this reaction. Only three are given in this course:
 hem 263 Nov 7, 2013 Preparation of Ketones and Aldehydes from Alcohols xidation of Alcohols [] must have at least 1 E elimination reaction [] = oxidation; removal of electrons [] = reduction; addition
hem 263 Nov 7, 2013 Preparation of Ketones and Aldehydes from Alcohols xidation of Alcohols [] must have at least 1 E elimination reaction [] = oxidation; removal of electrons [] = reduction; addition
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
THE CHEMISTRY OF THE CARBONYL GROUP
 TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Cape Cod Community College
 Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
Carbonyl Chemistry. aldehydes ketones. carboxylic acid and derivatives. Wednesday, April 29, 2009
 Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
Chapter 11 Reaction of Alcohols
 Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Chemistry of Carbonyl Compounds
 Chemistry of Carbonyl Compounds ucleophilic addition (1,2-add) / substitution Conjugate addition (1,4 add) obinson annulation (McM 23.12) Alkylation of enolate anions namines as enolate equivs. (McM 23.11)
Chemistry of Carbonyl Compounds ucleophilic addition (1,2-add) / substitution Conjugate addition (1,4 add) obinson annulation (McM 23.12) Alkylation of enolate anions namines as enolate equivs. (McM 23.11)
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16: Ethers, Epoxides, and Sulfides
 Chapter 16: Ethers, Epoxides, and Sulfides 16.1: omenclature of Ethers, Epoxides, and Sulfides (Please read) 16.2: Structure and Bonding in Ethers and Epoxides The ether oxygen is sp 3 -hybridized and
Chapter 16: Ethers, Epoxides, and Sulfides 16.1: omenclature of Ethers, Epoxides, and Sulfides (Please read) 16.2: Structure and Bonding in Ethers and Epoxides The ether oxygen is sp 3 -hybridized and
TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See
 Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Nucleophilic Addition Reactions of Carboxylic Acid Derivatives
 Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry
 Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Chapter 20: Carboxylic Acids
 1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Organic Chemistry Curriculum Content Outline
 Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
Synthetic possibilities Chem 315 Beauchamp 1
 Synthetic possibilities hem Beauchamp Propose reasonable syntheses f the following target molecules (TM-#). You can use the given starting materials and any typical ganic reagents studied in our course
Synthetic possibilities hem Beauchamp Propose reasonable syntheses f the following target molecules (TM-#). You can use the given starting materials and any typical ganic reagents studied in our course
Chem 251 Fall Learning Objectives
 Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Organic Chemistry Review: Topic 10 & Topic 20
 Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
CH 3 CHCH 3 CH 3 CHCH 3 Isopropyl cation. Oxomium ion intermediate. intermediate (an electrophile)
 Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Another Equilibrium: Reaction At The α-position
 Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Chapter 22: Amines. Organic derivatives of ammonia, NH 3. Nitrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic
 hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
O or R E + R. - Keto-Enol Tautomerization (enol form usually very minor for simple ketones)
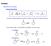 General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
September [KV 804] Sub. Code: 3804
![September [KV 804] Sub. Code: 3804 September [KV 804] Sub. Code: 3804](/thumbs/89/97855699.jpg) September 2009 [KV 804] Sub. Code: 3804 (Regulations 2008-2009) (Candidates admitted from 2008-2009 onwards) Paper IV PHARMACEUTICAL ORGANIC CHEMISTRY Time : Three hours Maximum : 70 marks Answer All questions
September 2009 [KV 804] Sub. Code: 3804 (Regulations 2008-2009) (Candidates admitted from 2008-2009 onwards) Paper IV PHARMACEUTICAL ORGANIC CHEMISTRY Time : Three hours Maximum : 70 marks Answer All questions
First Year Organic Chemistry THE CHEMISTRY OF THE CARBONYL GROUP: CORE CARBONYL CHEMISTRY
 First Year rganic Chemistry TE CEMISTY F TE CABNYL GUP: CE CABNYL CEMISTY Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2015 Wednesdays at 9am; Fridays at 10am (Dyson Perrins) andout A You will be able
First Year rganic Chemistry TE CEMISTY F TE CABNYL GUP: CE CABNYL CEMISTY Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2015 Wednesdays at 9am; Fridays at 10am (Dyson Perrins) andout A You will be able
What is in Common for the Following Reactions, and How Do They Work?
 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
Chapter 17 Aldehydes and Ketones
 hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
Chapter 11 - Alcohols and Ethers 1
 Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
Chapter 17: Alcohols and Phenols
 hapter 17: Alcohols and Phenols sp 3 alcohol phenol (aromatic alcohol) pka~ 16-18 pka~ 10 Alcohols contain an group connected to a saturated carbon (sp 3 ) Phenols contain an group connected to a carbon
hapter 17: Alcohols and Phenols sp 3 alcohol phenol (aromatic alcohol) pka~ 16-18 pka~ 10 Alcohols contain an group connected to a saturated carbon (sp 3 ) Phenols contain an group connected to a carbon
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
CHEMISTRY. Module No and Title Module-7, Nucleophilic and Free Radical Addition. Reactions of Alkenes
 Subject Chemistry Paper No and Title Paper-9, Organic Chemistry-III (Reaction Mechanism-2) Module No and Title Module-7, Nucleophilic and Free Radical Addition Module Tag CHE_P9_M7 TABLE OF CONTENTS 1.
Subject Chemistry Paper No and Title Paper-9, Organic Chemistry-III (Reaction Mechanism-2) Module No and Title Module-7, Nucleophilic and Free Radical Addition Module Tag CHE_P9_M7 TABLE OF CONTENTS 1.
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
ALCOHOLS: Properties & Preparation
 ALLS: Properties & Preparation General formula: -, where is alkyl or substitued alkyl. Ar-: phenol - different properties. Nomenclature 1. ommon names: Name of alkyl group, followed by word alcohol. 2.
ALLS: Properties & Preparation General formula: -, where is alkyl or substitued alkyl. Ar-: phenol - different properties. Nomenclature 1. ommon names: Name of alkyl group, followed by word alcohol. 2.
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.
 Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
ORGANIC - BROWN 8E CH CARBOXYLIC ACIDS.
 RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS!! www.clutchprep.com RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS CNCEPT: CARBXYLIC ACID NMENCLATURE IUPAC: Replace alkane -e with Substituents are located using
RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS!! www.clutchprep.com RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS CNCEPT: CARBXYLIC ACID NMENCLATURE IUPAC: Replace alkane -e with Substituents are located using
Chiral Catalyst II. Palladium Catalysed Allylic Displacement ( -allyl complexes) 1. L n Pd(0) 2. Nuc
 Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Appendix A. Common Abbreviations, Arrows, and Symbols. Abbreviations
 smi97462_apps.qxd 12/6/04 1:09 PM Page A-1 Appendix A ommon Abbreviations, Arrows, and Symbols Abbreviations Ac acetyl, 3 BBN 9-borabicyclo[3.3.1]nonane BINAP 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl
smi97462_apps.qxd 12/6/04 1:09 PM Page A-1 Appendix A ommon Abbreviations, Arrows, and Symbols Abbreviations Ac acetyl, 3 BBN 9-borabicyclo[3.3.1]nonane BINAP 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl
Alcohol Synthesis. Dr. Sapna Gupta
 Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
ORGANIC - EGE 5E CH. 2 - COVALENT BONDING AND CHEMICAL REACTIVITY
 !! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
!! www.clutchprep.com CONCEPT: HYBRID ORBITAL THEORY The Aufbau Principle states that electrons fill orbitals in order of increasing energy. If carbon has only two unfilled orbitals, why does it like to
Química Orgânica I. Ciências Farmacêuticas Bioquímica Química AFB QO I 2007/08 1
 Química rgânica I Ciências Farmacêuticas Bioquímica Química AFB Q I 2007/08 1 alcohols Adaptado de rganic Chemistry, 6th Edition; Wade rganic Chemistry, 6 th Edition; McMurry AFB Q I 2007/08 2 Typical
Química rgânica I Ciências Farmacêuticas Bioquímica Química AFB Q I 2007/08 1 alcohols Adaptado de rganic Chemistry, 6th Edition; Wade rganic Chemistry, 6 th Edition; McMurry AFB Q I 2007/08 2 Typical
Option G: Further organic chemistry (15/22 hours)
 Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
Advanced Organic Chemistry: Retrosynthesis
 123.312 Advanced rganic Chemistry: Retrosynthesis Tutorial Question 1. Propose a retrosynthetic analysis of the following two compounds. Your answer should include both the synthons, showing your thinking,
123.312 Advanced rganic Chemistry: Retrosynthesis Tutorial Question 1. Propose a retrosynthetic analysis of the following two compounds. Your answer should include both the synthons, showing your thinking,
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Text Sections (N0 1.9, 9-11) Homework: Chapter 1:
 CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
Aldehydes, Ketones and Carboxylic acids
 Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
