Year 8 KS3 Science Year Booklet
|
|
|
- Meredith Paul
- 6 years ago
- Views:
Transcription
1 Year 8 KS3 Science Year Booklet Includes: Home-learning Tasks Revision Questions Content Checklists Name: Tutor Group: Science Group: 1
2 Contents: Pg: 4-10 Pg: Pg: Pg: Pg: Pg: Pg: Pg: Pg: Pg: B1: Health and Lifestyle 8C1: The Periodic Table 8P1: Electricity and Magnetism 8B2: Ecosystem Processes 8C2: Separation Techniques 8P2: Energy 8B3: Adaptation and Inheritance 8C3: Metals and Acids 8P3: Motion and Pressure 8C4: The Earth 2
3 FAQs. How many home-learning tasks will I need to do? One a week, which should cover 3 tasks per unit. How will I know which task to complete? Your teacher will tell you which unit and task number to complete. But you are expected to complete the challenge task for each home-learning set. What if I find the challenge task too hard? We want to challenge you to increase your progress during the year, but if you do find it too hard, get your tutor or a parent to make and sign a note in your science book by your home-learning and complete the regular task. You will need to do this each time. What do I do if I don t get it? Go and talk to your teacher (or any science teacher) before the due date, we are here to help. There are revision resources in this book to help, otherwise try or buy a revision guide from the science lab technicians. Can I do my home-learning on the computer? Yes. But make sure you print it out and stick it in your book. If you arrive to your lesson with it on a memory stick, it will not be accepted. Can I complete the tasks ahead of them being set? Yes! Make sure it is easy for your science teacher to find it in your book What are the revision questions and checklists for? It is important to revise before each assessment. Use the checklists to find out if there are any areas you may need to work on before a test and the revision questions to get someone else or to test yourself on the topic. 3
4 8B1: Health and Lifestyle Textbook pages
5 8B1: Home-Learning Tasks: Task Number Regular Challenge Completed? In a table list the 7 groups of nutrients. Give an example of a food from each oneand describe why we need each nutrient. Write a story about a day in the life of a chip as it passes through the digestive system. Draw a table to show examples of medicinal and recreational drugs (including alcohol and tobacco) and their effects on the body Summary Questions: Regular Research what is meant by Kwashikhor. Find out why people get it and the effect it has on people Explain at least two ways that the small intestine is adapted to digestion Explain how tar, nicotine and carbon monoxide damage the breathing system in humans Summary Questions: Challenge Summary Questions: Regular: 1) List the 7 groups of food and their use in the body 2) Describe the food tests for starch, simple sugars, fats/lipids and protein 3) Explain the difference between a balanced diet, starvation, malnutrition and obesity 4) Describe the path of food through the digestive system from mouth to anus 5) Name the 3 types of enzyme and their breakdown products 6) Describe the problems caused by alcohol and tobacco on the body Summary Questions: Challenge: 1) Explain why a fat free diet is not good for the body 2) Is scrambled egg on toast a balanced diet? Explain your answer 3) Explain why explorers in the arctic need a massive amount of food to maintain their body weight. 4) Research the importance of bile in digestion 5) Research the importance of enzymes in digestion 6) Explain why drinking alcohol during pregnancy can cause damage to the foetus 5
6 8B1: Revision Questions: Question 6 Answer 1. Name the 7 components of a healthy diet. Carbohydrates, Lipids, Proteins, Vitamins, Minerals, Water, Fibre. 2. What are lipids? Fats and oils in food. 3. What does the body use carbohydrates for? Energy. 4. What does the body use lipids for? Energy, insulation, protection of organs. 5. What does the body use protein for? Growth and repair of body tissues. 6. What does the body use vitamins and minerals Keeping you healthy in small amounts for? 7. What does the body use water for? Prevention of dehydration. All cells in the body contain water. 8. What does the body use fibre for? It prevents constipation by keeping the food moving through the digestive system. 9. What would you use to test food for starch? Add iodine. 10. What would show starch to be present? Iodine going a blue-black colour. 11. How would you test solid food for lipids? Rub the solid food on a piece of filter paper and hold it up to the light. If it goes transparent, lipids are there. 12. How could you test for lipids in a solution of food? Add a few drops of ethanol to the solution, shake it and leave it for one minute. Tip the ethanol into water. If it goes cloudy, lipids are there. 13. How do you make a solution of food? Crush the food and mix it really well with water. 14. How do you test food for sugar? Add a few drops of Benedict s solution and then heat it in a water bath. If it goes orange-red, sugar is there. 15. How do you test food for protein? Add Buiret s solution to the food. If it turns lilac (purple) then protein is there. 16. What is the energy in food measured in? Joules. 17. What is a kilojoule? 1000 Joules. 18. What do we suffer if we have no food? Starvation. 19. What do we become if we do not eat enough? Underweight. 20. Give 3 problems caused by being underweight. Health problems like a poor immune system, lack of energy or tiredness, lack of vitamins or minerals. 21. What do we become if we eat too much food or eat food too high in fat? 22. What do we call someone who is extremely overweight? Overweight. Obese. 23. Give 4 problems caused by being overweight. Hear t disease, stroke, diabetes, certain cancers. 24. What is the term for being short of a certain We call this a deficiency. vitamin or mineral? 25. What does a deficiency of vitamin A lead to? Night blindness (unable to see in dim light). 26. What does a vitamin D deficiency cause? Rickets (weak bones). 27. How does more exercise affect the amount of It increases it. energy you need? 28. What happens during digestion? Large molecules are broken down into smaller ones. 29. Why does digestion make large molecules into smaller ones? They can then pass into the blood.
7 30. Give the 7 main parts of the digestive system, in order. Mouth, Gullet, Stomach, Small intestine, Large intestine, Rectum, Anus. 31. What happens in the mouth? Food is chewed and mixed with saliva. 32. What does the gullet do? Passes food from the mouth to the anus. 33. What happens in the stomach? Food is mixed with digestive juices and acid. 34. Give 2 things which happen in the small intestine. Digestive juices added from the liver and pancreas. Small molecules are absorbed into the blood. 35. What happens in the large intestine? Water is absorbed back into the body, making faeces. 36. What does the rectum do? Stores faeces. 37. What does the anus do? Allows faeces to pass out of the body. 38. What are Villi? Small finger-like projections on the inside of the small intestine which give it a bigger surface area. 39. What is an enzyme? A biological catalyst, something which speeds up chemical reactions without being used up. 40. What do enzymes do in digestion? They break large molecules down into smaller ones. 41. Name 3 enzymes. Carbohydrase/ amylase, Protease, Lipase. 42. What does carbohydrase do? Breaks down carbohydrates such as starch down into sugar molecules. 43. What does protease do? Breaks down protein into amino acids. 44. What does lipase do? Breaks down lipids into fatty acids and glycerol. 45. What does bile do? Helps lipase to work by breaking up lipids into small droplets which are easier to work on. 46. What is a drug? A chemical substance which affects the way the body works. 47. What are medicinal drugs? Drugs which benefit health in some way. 48. What are recreational drugs? Drugs which people take for enjoyment which may harm the body. 49. Name 2 legal recreational drugs. Alcohol and tobacco. 50. What is addiction? Where someone is dependent on a drug, they have to keep taking it in order to feel normal. 51. What is a depressant? A drug which slows down your body s reactions. 52. What type of alcohol is in alcoholic drinks? Ethanol. 53. What damage can alcohol cause? Stomach ulcers, brain damage, liver damage, heart disease. 54. What is a stimulant? A drug which speeds up the body s reactions. 55. Give 3 health conditions caused by smoking. Lung cancer, heart attacks, strokes. 56. What is the name given to breathing in other Passive smoking. people s smoke? 57. What problems can be caused if a pregnant woman smokes? 58. What health problems are increased in babies whose parents smoke? 59. What sticky substance in smoke causes lung cancer? 60. Name the addictive stimulant drug in tobacco smoke. 61. How does carbon monoxide in tobacco smoke affect people? Higher risk of miscarriage, low birth-weight, development of foetus is affected. Bronchitis, pneumonia, cot death. Tar. Nicotine. It stops the blood from carrying as much oxygen as it could, by binding to red blood cells instead of oxygen. 62. Give 3 diseases caused by smoking. Heart disease, emphysema and respiratory infections. 7
8 8B1: Checklist: Topic Page Spec points Understanding Revised RP I can list the contents of a healthy human diet I can describe why parts of a healthy human diet are needed I can give examples of foods that contain each nutrient I can describe what is meant by a balanced diet I can describe the food tests for starch, lipids, sugars and proteins I can describe the positive results for each nutrient test I can explain the consequences of imbalances in the diet (obesity, starvation and deficiency-related diseases) I can make calculations of energy requirements in a healthy daily diet I can name some tissues and organs in the human digestive system I can describe the functions of some tissues and organs in the human digestive system I can outline the process of digesting food I can link adaptations of different parts of the digestive system to their functions I can describe how food moves through the digestive system I can explain how villi are adapted to their function 8
9 I can explain how digestion happens, with reference to enzymes I can discuss the benefits of bacteria in the human digestive system I can recall the enzymes for different nutrients I can list some effects of recreational drugs on behaviour, health and life processes I can describe some effects of recreational drugs on behaviour, health and life processes I can explain some effects of recreational drugs and substance misuse on behaviour, health and life processes I can evaluate some effects of recreational drugs on behaviour, health and life processes I can describe what a drug is I can list some effects of recreational drugs on behaviour, health and life processes I can describe some effects of recreational drugs on behaviour, health and life processes I can explain some effects of recreational drugs and substance misuse on behaviour, health and life processes I can evaluate some effects of recreational drugs on behaviour, health and life processes I can describe how we measure what a safe amount of alcohol is to drink I can describe what alcohol is I can describe the impact of exercise, asthma and smoking on the human gas exchange system I can list, describe and explain some effects of smoking on behaviour, health and life 9
10 processes I can evaluate some effects of recreational drugs on behaviour, health and life processes I can describe what passive smoking is 10
11 8C1: The Periodic Table Textbook pages
12 8C1: Home-Learning Tasks: Task Number Regular Challenge Completed? Research the history of the periodic table. Explain how it is arranged using the terms groups, periods, atomic number and atomic mass. Make a poster to summarise the reactions and properties of Group 1 elements lithium, sodium, potassium and rubidium Evaluate the uses for Helium. Why is using it for balloons potentially a problem for scientists? Summary Questions: Regular Find out about some other suggestions for laying out the periodic table. Evaluate how they were better and worse than the version we use. Extend your poster by including caesium and francium. Explain why they properties of the elements change in the group Pick an element from group 0. Explain what it is used for, where we get it from and any problems with it. Summary Questions: Challenge Summary Questions: Regular: 1. Where are the non-metals on the periodic table? draw a diagram 2. What are the groups and periods in the periodic table? 3. Describe the reactions of group 1 with water? 4. What are the properties of group 7 elements? 5. What is unusual about group 0 elements? Summary Questions: Challenge: 1. What differences are there between metals and non-metals 2. Why is the pattern of the periodic table useful? 3. What are the trends in group 1? 4. What trends are there in group 7? 5. What are group 0 elements used for and why? 12
13 8C1: Revision Questions: Question Where are metals on the periodic table? Where are metals on the periodic table? List three properties of a metal? List three properties of a non-metal? What is the name of an element on the border between metals and non-metals? What is made when a metal reacts with oxygen? What might be made when some non-metals dissolve in rain? What are the columns of the periodic table called? What are the rows of the periodic table called? Why is it useful to understand the way the periodic table laid out? What is the pattern in melting point of group 1? What is the pattern in reactivity point of group 1? Describe the general properties of group 1 What is the equation for lithium reacting with water? Are metal oxides acids or bases? What is a physical property? What are chemical properties? 13 Answer On the left On the right Good conductor of electricity, good conductor of heat, shiny, high density, malleable, ductile, sonorous Poor conductor of electricity, poor conductor of heat, dull, low density, brittle, not sonorous Metalloids A metal oxide Acid rain Group number Periods To understand the trends Decreases down the group Increases down the group Low melting and boiling points, low densities, reactive Lithium + water lithium hydroxide + hydrogen Bases Things you can observe and measure They describe substances react What the halogens? Group 7 What types of compounds do fluorine, bromine and iodine make? What happens to the melting point and boiling point down group 7? What happens to the colours of group 7 down the group? What is a displacement reaction? What elements are in group 0? What is another name for group 0? What is the key chemical property of group 0 elements? Where do noble gases come from? What happens when high voltages are passed through noble gases? Fluoride, bromide and iodide Increases They get darker A reaction where a more reactive element replaces a less reactive element He, Ne, Ar, Kr, Xe, Rn Noble gases Unreactive Underground or under the sea They glow
14 8C1: Checklist: Topic Page Spec points Understanding Revised RP I can state that all elements currently known may be found listed in the Periodic Table I can state that the modern Periodic Table was developed by Mendeleev I can identify where metals, nonmetals, periods and groups can be found on the Periodic Table I can list the properties of metals and non-metals I can describe the changes that Mendeleev made when he developed the modern Periodic Table I can describe how the properties of metals and non-metals make them suitable for different uses I can explain some of the properties of metals and nonmetals with reference to their structure I can explain why Mendeleev made the changes he did when developing the modern Periodic Table I can state that the Periodic Table can be used to predict patterns in reactions I can describe what groups and periods are in the periodic table I can state that elements in the same group of the Periodic Table will have similar patterns in reactions I can identify where metals, nonmetals, periods and groups can be 14
15 found on the Periodic Table I can describe how elements with similar physical and chemical properties are grouped together I can predict properties of elements from their position in the periodic table I can identify where Group 1 is in the periodic table I can describe the patterns of reactivity for Group 1 in the Periodic Table I can link group number and electron structure to explain the patterns of reactivity for Group 1 and Group 7 in the Periodic Table I can describe the properties of the Group 1 elements I can describe what happens when Group 1 metals react with water I can create word equations for Group 1 metals reacting with water I can describe the patterns of reactivity for Group 1 and Group 7 in the Periodic Table I can link group number and electron structure to explain the patterns of reactivity for Group 7 in the Periodic Table I can describe the properties of Group 7 elements I can describe what a displacement reaction is I can write word equations for displacement reactions I can identify and describe the properties of the elements in Group 0 I can describe where we can obtain Group 0 elements from I can predict properties of Group 0 elements 15
16 16
17 8P1: Electricity and Magnetism Textbook pages
18 8P1: Home-Learning Tasks: Task Number Regular Challenge Completed? Describe 3 different problems caused by static and describe 2 different ways to prevent problems caused by static electricity Using circuit diagrams, explain why when one bulb breaks in Xmas tree lights, the other lights do not turn off. Describe what a thermistor and a LDR do. Draw their circuit symbols Summary Questions: Regular Describe what Earthing is Describe how static electricity is built up by a moving car and explain why you can get a static shock when you touch it Explain how a light dimmer switch works. Use a diagram to help you Describe what a solenoid is. Explain how it works and describe a use for it Summary Questions: Challenge Summary Questions: Regular: 1. What are the three different types of charge? 2. Draw a series circuit with two bulbs, one cell and a switch in it 3. Describe what you use to measure the current in a circuit. Draw the circuit in question 2 with an ammeter in it 4. Describe what happens to the current in a parallel circuit 5. State which 4 materials are magnetic 6. Describe 3 ways to make an electromagnet stronger Summary Questions: Challenge: 1. Explain how a balloon can be statically charged 2. Draw a circuit with a cell and two bulbs in parallel. Add switches to the circuit so that you can turn each light on and off independently 3. Describe what happens to the potential difference in a series and parallel circuit 4. Describe what a resistor is and explain how it works 5. Describe how to make an electromagnet 18
19 19
20 8P1: Revision Questions: Question Answer 1. What are the 2 types of electrical charge? Positive and negative 2. What 3 particles are atoms made from? Protons, neutrons and electrons 3. What are the charges of protons, neutrons and electrons? 4. What happens when you get 2 positive charges next to each other? 5. What happens when you get a positive charge next to a negative charge? Protons = positive. Neutrons = 0/ neutral. Electrons = negative They repel each other They attract each other 6. What is the overall charge of an atom? Neutral 7. Which particle moves when two insulating surfaces Electrons rub against each other? 8. What force causes particles to move when two Friction insulating surfaces rub against each other? 9. What is an electric field? An area around a charge where another charge will experience a force 10. What causes lightning? Particles move causing a build-up of charge. The charges then move causing a current 11. What is a current? The amount of charge flowing per second 12. What units is current measured in? Amps 13. What equipment can you use to measure current? Ammeter 14. What is the symbol for a bulb, a wire, a switch and a cell? 15. How can a loop of string be used to model a current? 16.What is the job of a cell/ battery in a circuit? 20 Rope represents the charges. The amount of rope moving per second is the current They push charges around the circuit 17. State some examples of circuit components Bulb, buzzer, motor, resistor 18.What is meant by potential difference? The push that makes a charge move around a circuit 19. What unit is potential difference measured in? Volts 20. What piece of equipment can be used to measure Voltmeter potential difference? 21. How do you measure the PD of a power supply? Connect the voltmeter around the battery 22. How do you measure the PD of a component? Connect the voltmeter around the component 23. What happens to the PD in a series circuit? Total PD of components = PD of power supply 24.What happens to the PD in a parallel circuit? The potential difference is the same for each component as the battery 25.What happens to the current in a series circuit? It is the same all the way round the circuit 26. What happens to the current in a parallel circuit? It splits up at the junction
21 27. What is the difference between a series and a parallel circuit? 28. How could you represent a parallel circuit using a model? In series there is one loop of wire. In parallel there is more than one loop of wire. Hold more than one loop of rope that the battery moves around at the same time 29. What does a resistor do? What is its symbol It slows down the current 30. What is resistance? What is it measured in? How easy/ difficult to let the current through. Measured in Ohms 31. What equation can be used to calculate Resistance = Potential difference Current resistance? 32. What do the electrons do as they move through a wire? What does this cause? They collide with the atoms. It causes the wires to heat up. The electrons lose energy 33. What is meant by an electrical conductor? Something that has low resistance and lets a lot of electricity through easily 34. What 4 materials are magnetic? Iron, steel, cobalt, nickel 35. What are the 2 poles for a magnet? North and South 36. What happens when you get 2 different poles The magnets attract each other next to each other? 37. What happens when you get 2 similar poles next to The magnets repel each other each other? 38. What do the magnetic field lines look like around a bar magnet? 39. What happens when a compass is near a magnet? They align up with the magnetic field lines 40. Where is the magnetic field stronger for a bar At the poles. The field lines are closer together magnet? How can you tell this? 41. What else has a magnetic field? The Earth 42. How is a magnetic field made? By charges moving 43. What shape is the magnetic field around a current carrying wire? 44. What is an electromagnet? A strong, electrical magnet 45. What do you need to make an electromagnet? A magnetic core with wire wrapped around it 46. How can you make an electromagnet stronger? Increase the current, PD, loops of wire wrapped around the core 47. Name some uses for electromagnets Motors, sorting out magnetic and non-magnetic metal 48. Name 2 differences between bar magnets and electromagnets 21 Bar magnets are permanent magnets. Electromagnets are stronger 49. What is a relay? An electronic switch containing an electromagnet 50. What is a motor? An electrical component that spins when a current passes through it 31. What is an electrical insulator? Something that has high resistance and does notlet a lot of electricity through easily
22 8P1: Checklist Topic Page Spec points Understanding Revised RP I can link electrical conduction and insulation with the structure of atoms I can state that when two materials are rubbed together electrons will be transferred I can describe the force between two charged objects as electrostatic force I can describe the properties of an electrostatic force I can explain when objects will attract or repel each other due to electrostatic force I can describe how the movement of electrons can result in objects becoming charged with static electricity I can discuss some examples and applications of static electricity I can explain how electrostatic force may attract a non-charged object through the induction of charge I can describe what an electric field is I can state that an electrical circuit must be complete and include a power source, wires and a component for electricity to flow I can state that electrical current is measured in amperes (amps) I can identify some common components of electrical circuits from the circuit symbol I can give the symbol for the unit of current (A) 22
23 I can name some common components of electrical circuits I can name the component used to measure electrical current I can draw the circuit symbols of some common components of electrical circuits I can describe electrical current as the flow of charge in a circuit I can identify electrical current as flowing from positive to negative in a solution or circuit I can describe how to correctly connect an ammeter to a circuit I can model an electric current I can define the term 'direct current' I can state that the potential difference of a battery or cell is what causes the current to flow I can state that potential difference is measured in volts I can state that a battery or cell of a higher potential difference will cause more current to flow I can give the symbol for the unit of potential difference (V) I can name the component used to measure potential difference I can describe how a bulb of an increased potential difference will be brighter I can describe how to correctly connect a voltmeter to a circuit I can model potential difference I can state what happens to the electric current in a series circuit I can state what happens to the potential difference in a series circuit I can identify series and parallel circuits I can describe what happens to an electric current at a junction I can describe what happens to the potential difference in a 23
24 parallel circuit I can state what resistance is I can state what resistance is measured in and identify its symbol I can describe what happens to the current if the resistance is increased I can calculate resistance when given potential difference and current I can discuss resistance in terms of electrical conductors and insulators I can suggest some applications for materials of higher or lower resistance I can state that magnets may be temporary or permanent I can state that like poles repel and unlike poles attract I can state that magnetism is a non-contact force that will affect magnetic materials within the magnetic field I can name the three magnetic materials I can draw the field lines surrounding a bar magnet with arrows I can label the north-seeking and south-seeking poles of a magnet, when given the field lines I can describe the Earth and compasses as examples of magnets I can describe the relationship between: strength and distance between field lines I can describe how to find the shape of a magnetic field using a compass I can describe and explain attraction and repulsion in terms of the direction of field lines I can state that a flow of electric current will produce a magnetic 24
25 field I can describe temporary and permanent magnets in terms of hard and soft magnetic materials I can describe how a bar magnet inside an electromagnet field can be made to move I can describe how to make an electromagnet I can describe how to increase the strength of an electromagnet I can describe how magnetism may be induced I can list some uses of electromagnets I can describe how to make a simple motor using electromagnetism and a bar magnet I can describe how a relay works and what it is used for I can list some uses of motors 25
26 8B2: Ecosystem Processes Textbook pages
27 8B2: Home-Learning Tasks: Task Number Regular Challenge Completed? Using the equation for photosynthesis, describe where the reactants come from and what the products are used for by the plant. Describe what you would expect a plant to look like if it was deficient in a) nitrate, b) magnesium, c) phosphorus, d) potassium Compare the equations for aerobic and anaerobic respiration and list the similarities and differences between the processes Summary Questions: Regular Explain the importance of chlorophyll, stomata and xylem vessels in photosynthesis. Draw a table to compare photosynthesis and chemosynthesis Explain why anaerobic respiration in yeast is economically important. Summary Questions: Challenge Summary Questions: Regular: 1) Write the word equation for photosynthesis and explain the importance of light and chlorophyll in this process 2) Describe the role of the stomata in photosynthesis 3) Explain why farmers add minerals to their crops 4) Write the equation for aerobic respiration and describe the importance of haemoglobin in this process 5) Write down the meaning of the term interdependence. In a food web describe what could happen to the other organisms if one of the herbivores population decreased Summary Questions: Challenge: 1) Describe and explain the optimum (best) conditions for photosynthesis 2) Explain how the palisade layer, the spongy layer and stomata in the leaf are adapted for photosynthesis 3) Explain why cropping leads to mineral deficient soils in farming but natural ecosystems rarely show mineral deficiency 4) Compare the processes of photosynthesis, chemosynthesis and aerobic respiration 5) Describe the terms predator and prey and explain how the changes in the numbers of predator or prey can affect the balance in an ecosystem 27
28 8B2: Revision Questions Question Answer 1. What do producers do? Make their own food. 2. Give 2 producers. Plants and algae. 3. Where do algae live? In water. 4. What do algae not have? Leaves, roots or stems. 5. Why are animals called consumers? They eat other organisms to survive. 6. What do we call the process where Photosynthesis. producers make food? 7. What are the reactants in Carbon dioxide and water. photosynthesis? 8. What are the products of Glucose and oxygen. photosynthesis? 9. Give the word equation for Carbon dioxide + Water Glucose + Oxygen photosynthesis. 10. Where does the energy for Light. photosynthesis come from? 11. Which part of a leaf cell carries out The chloroplasts. photosynthesis? 12. What chemical makes chloroplasts Chlorophyll. green? 13. Where does water enter the plant? Through the root hair cells. 14. What transports water through a Xylem tubes. plant? 15. How does carbon dioxide enter a leaf? Through tiny holes in the leaves. 16. How does a leaf being thin help This allows gases to diffuse in and out easily. photosynthesis? 17. Why do leaves have a large surface To absorb as much light as possible. area? 18. What do phloem tubes do? Transport glucose. 19. Where does most of the sunlight hit a The top. leaf? 20. What is the function of the waxy This reduces water loss by evaporation. layer on a leaf? 21. What do we call the tiny holes in Stomata. leaves? 22. What do guard cells do? Open and close stomata. 23. What is the palisade layer? The part of a leaf which contains the most chloroplasts and carries out the most photosynthesis. 24. Where in a leaf is the palisade layer? Near the top. 25. What is the spongy layer? The part of a leaf with air spaces. 26. What do plants need nitrates for? Healthy growth, making amino acids. 28
29 27. What are amino acids used for? Making proteins. 28. What do plants need phosphates for? Healthy roots. 29. What do plants need potassium for? Healthy leaves and flowers. 30. What do plants need magnesium for? Making chlorophyll. 31. Which part of a plant absorbs The root hair cells. minerals? 32. Which part of the plant carries the The xylem. minerals? 33. What are the signs of a plant having a Poor growth and yellow older leaves. nitrate deficiency? 34. What are the signs of a plant having a Leaves turn yellow. magnesium deficiency? 35. What are the signs of a plant having a Poor root growth, purple younger leaves. phosphorous deficiency? 36. What are the signs of a plant having a Yellow leaves with dead patches. potassium deficiency? 37. What is a mineral deficiency? Not having enough of a particular mineral (in plants). 38. What do farmers use to replace Fertilisers. missing minerals in soil? 39. What do NPK fertilisers contain? Nitrogen, phosphorous and potassium. 40. What is chemosynthesis? Making glucose using chemicals as a source of energy. 41. Where do sulfur bacteria live? At the bottom of the sea near volcanic vents. 42. What do sulfur bacteria do? Turn hydrogen sulfide into sulfur by chemosynthesis. 43. Where do nitrogen bacteria live? In the soil and in the roots of plants. 44. What do nitrogen bacteria do? They use nitrogen compounds for chemosynthesis. 45. Sulphur bacteria sometimes live in A symbiotic relationship, or a mutualistic one. tubeworms, benefiting both. What is this type of relationship called? 46. What process uses oxygen to get the Aerobic respiration. energy out of glucose? 47. Give the word equation for aerobic Glucose + Oxygen Carbon dioxide and water. respiration. 48. What part of the cell carries out the Mitochondria. aerobic respiration? 49. What does the small intestine do? Absorbs glucose from food into the blood. 50. Which part of the blood transports The plasma. the glucose? 51. What is the plasma? The liquid part of the blood. 52. Which part of the blood carries The red blood cells. oxygen? 53. What do red blood cells contain which Haemoglobin. carries oxygen? 54. Which part of the blood carries The plasma. carbon dioxide? 55. Which part of the body gets rid of The lungs. the carbon dioxide? 29
30 56. What process releases energy from Anaerobic respiration. food without oxygen? 57. What do humans make during Lactic acid. anaerobic respiration? 58. What does lactic acid cause? Muscle cramps. 59. What is oxygen debt? Breathing heavily after exercise to get oxygen to break down lactic acid. 60. What are the products of anaerobic Ethanol and carbon dioxide. respiration in yeast? 61. What do we call anaerobic respiration Fermentation. in yeast? 62. What is the first organism in a food The producer. chain? 63. What is the 2 nd organism in a food The herbivore (primary consumer). chain? 64. What is the 3 rd organism in the food The carnivore (secondary consumer). chain? 65. What do the arrows show in a food Energy flow. chain? 66. What is a prey animal? One which is eaten by a predator. 67. What is a predator? An animal which eats other animals (prey). 68. Why do most food chains only have 4 or 5 links? 69. What animal is the last link in a food chain? 70. What do we call a number of interconnected food chains? There is not usually enough energy left at the end to support another organism. The top predator. A food web. 71. What is a population? A group of animals of the same species in the same area. 72. What would happen to the population of predators if the population of prey dropped? It would drop (unless they found something else to eat). 73. What is bioaccumulation? Toxins such as insecticides being passed along a food chain and building up in the top predator. 74. What do we call the animals and plants An ecosystem. in the same area, along with the area itself? 75. What do we call all the organisms in an A community. area? 76. What do we call the area where an Its habitat. organism lives? 77. What is an organism s niche? Its role in an ecosystem, in other words where it lives and what it eats. 30
31 8B2: Checklist 31
32 Topic Page Spec points Understanding Revised RP I can explain how plants make carbohydrates in their leaves and gain mineral nutrients and water from the soil I can state that almost all life on Earth depends on photosynthetic organisms I can state how and where plants make carbohydrates I can list the reactants and products of photosynthesis I can summarise the reactants and products of photosynthesis using a word equation I can describe what producers and consumers are I can explain how gases get in and out of a plant I can state that plants exchange gases via the stomata in their leaves I can describe some adaptations of plants leaves I can explain how some structural adaptations of plants' leaves aid photosynthesis I can link the importance of photosynthetic organisms to maintaining levels of oxygen and carbon dioxide in the atmosphere I can state that plants gain mineral nutrients and water from the soil via their roots I can describe the minerals required for plants and what they are used for I can identify the different mineral deficiencies of plants I can explain why farmers use fertilisers I can describe what chemosynthesis is 32
33 29 I can describe the different habitats of bacteria I can compare chemosynthesis with photosynthesis I can describe what aerobic respiration is 31 I can list the reactants and products of aerobic I can describe some applications of aerobic respiration I can summarise the reactants and products of aerobic respiration using word equations I can evaluate the implications of aerobic respiration for organisms based on the reactants and products I can describe how carbon dioxide leaves the body and how oxygen enters the body I can list the reactants and products of anaerobic respiration 33 I can describe some applications of anaerobic respiration I can summarise the reactants and products of anaerobic respiration using word equations I can compare and contrast aerobic and anaerobic respiration I can evaluate the implications of aerobic and anaerobic respiration for organisms based on the reactants and products I can describe what fermentation is I can describe what is meant by an oxygen debt I can describe what foods and drinks are made from fermentation I can state that all organisms in an ecosystem may affect each other and are affected by their environment I can construct and interpret simple food chains 33
34 I can construct and interpret food webs I can describe what a prey and predator organism are I can describe how a change in the numbers of one organism may affect another I can explain how a change in the numbers of one organism may affect another, with reference to competition and predation I can describe what bioaccumulation is and explain how it affects other animals I can describe what is meant by the population of an organism I can describe how organisms may be affected by their environment I can describe what is meant by an ecosystem I can describe what is meant by a habitat and a community I can identify the roles of different organisms in an ecosystem I can describe and identify different niches of an ecosystem 34
35 8C2: Separation Techniques Textbook pages
36 8C2: Home-Learning Tasks: Task Number Regular Challenge Completed? Find 3 mixtures at home. Explain what components there are in each mixture Explain how evaporation is used to make table salt. Include pictures or diagrams Describe how you could use chromatography to identify the different inks in a pen Summary Questions: Regular Create a diagram to show a mixture and suggest what materials you could use to make a model. Explain how distillation can be used to desalinate (remove salt from) water. Why might this become more important in future. Suggest how forensic scientists might be able to use chromatography Summary Questions: Challenge Summary Questions: Regular: 1. What are the definitions of mixture and compound? 2. Draw a diagram of a solution 3. What is solubility? 4. How does filtering work? 5. What is evaporation? 6. What does chromatography do? Summary Questions: Challenge: 1. How can you tell whether a substance is pure by melting and boiling point? 2. Explain why fizzy drinks are fizzy 3. How does temperature affect solubility? 4. Describe the process of filtration using the terms: residue, filtrate, insoluble, solid and liquid. 5. What is distillation? 6. How does chromatography work? 36
37 8C2: Revision Questions Question What is a mixture? What happens to melting point if a substance is impure? What is a solvent? What is a solute? What is a solution? Can gases dissolve? What is a saturated solution? What does solubility measure? How does increasing temperature affect solubility? What does it mean if something is insoluble? What does filtering do? What is a filtrate? What is the residue? What do we use evaporation used for? What is distillation? What is distillation used for? What does chromatography do? What is a chromatogram? Answer It is made up of two or more substances mixed together but not chemically bonded It happens over a range of temperatures A substance into which a solute dissolves A substance which dissolves into a solvent A mixture formed when a solute dissolves in a solvent Yes A solution you cannot dissolve any more solute into How much solute you can dissolve into a solvent The higher the temperature the greater the solubility The substance does not dissolve in a particular solvent Separates an insoluble substance from a solution or liquid The solution that passes through the filter The insoluble substance that is left in the filter To separate a solute from a solvent Evaporation followed by condensation To separate mixtures of liquids Separates mixtures, usually dyes The pattern of spots from a chromatography experiment 37
38 8C2: Checklist Topic Page Spec points Understanding Revised RP I can list some mixtures I can describe what a pure substance is I can describe how impurities may affect boiling and melting points of impure substances I can explain how mixtures are different from elements and compounds I can identify pure and impure substances from data I can suggest some applications for making substances impure I can describe a mixture, including dissolved substances I can describe dissolving, with reference to particles I can define and identify solvents and solutes I can explain how substances dissolve in a solvent I can describe what a saturated solution is I can describe what the solubility of a solute is I can describe how solubility is affected by temperature I can identify simple techniques for separating mixtures I can select appropriate simple techniques for separating given mixtures (filtration, distillation and evaporation) I can describe how to carry out filtration of mixtures I can explain how filtering works I can describe what is meant by filtrate and residue 38
39 I can describe how filtration is useful I can describe what evaporation and distillation are I can describe how to carry out distillation and evaporation I can explain how distillation and evaporation work I can describe what chromatography is I can select when to use chromatography I can describe how to carry out chromatography I can explain how chromatography works 39
40 8P2: Energy Textbook pages
41 8P2: Home-Learning Tasks: Task Number Regular Challenge Completed? Give 4 examples of energy transformation. E.g A light bulb Electricity Light + Heat Find out 4 different ways to insulate a house. Explain how one of these methods actually works Describe how energy is generated using geothermal power stations Summary Questions: Regular Explain using energy transformations why when a ball rolls down a hill and up another, the ball will not reach its original height Describe what a thermogram is. Explain what it shows and describe who might use one Describe how energy is generated at Dinorwig (electric mountain. Explain how this method is useful Summary Questions: Challenge Summary Questions: Regular: 1. State 5 different types of energy 2. Give 3 different examples of how energy can be stored 3. Describe what heat is and what it is measured in 4. Describe what temperature is and what it is measured in 5. Describe how heat is transferred through conduction, convection and radiation 6. Describe how energy is generated using a thermal power station Summary Questions: Challenge: 1. Describe what is meant by the conservation of energy 2. Describe what happens to particles when the gain heat energy 3. Explain what happens to the temperature of a pan of water when it is left on the stove of a cooker 4. Explain why solids are good conductors and gases are good insulators 5. Describe which colours are good at reflecting radiation and which colours are good at absorbing and emitting it 6. Explain how a thermos flask keeps the temperature of a substance constant 41
42 8P2: Revision Questions Question Answer 1.What do you measure energy in? Joules/ kilojoules 2. What is a kilo-joule? A thousand Joules 3. State some examples of energy stores Coal, oil, gas, food 4. What do humans need energy for? Keep them warm, let the move around, breathe, talk 5. Describe which people need more energy Athletes 6. Why should do people need to be careful with how much energy they take in? If they take in more than they use, the body stores it as fat to be used in the future 7. What is the law of conservation of energy? Energy cannot be created or destroyed. Only transferred from one form to another 8. What is an energy store? Something that can store energy (food or fuel) 9. How does burning conserve energy? Energy stored in fuel = Energy released from burning 10. How is energy transferred in a light bulb? Electricity Light and Heat 11. How is energy transferred in a motor? Electricity Kinetic and Heat 12. What is most energy wasted as? Heat 13. What does it mean when energy is Energy that has been lost as heat to the surroundings dissipated? 14. How can kinetic energy be stored? Using a flywheel 15. What is temperature? What is it How hot an object is. Measured in measured in? 16. What is heat? What is it measured in? A type of energy. Measured in Joules 17. What can you use to measure A thermometer temperature? 18. What happens when particles heat up? They vibrate/ move around faster (they gain kinetic energy) 19.What does the energy you need to increase the temperature of a material depend on? The mass of material, type of material, temperature rise that you want 20. Which way does heat flow from and to? From hot objects to cold objects. 21. What happens when objects are the same They are in thermal equilibrium temperature? 22.What 3 ways can energy get from one Conduction, convection, infrared radiation place to another 23. Explain how conduction works Particles vibrate when heated. These collide with other particles and transfer the heat energy 24. What materials are good conductors? Solids/ metals 25. Explain how convection works Particles get heated and spread out. They become less dense and rise. When they rise they lose heat to the surroundings and cool. They then become dense and sink. This process is a convection current 26. What materials are good at convection? Fluids (gases and liquids) 42
43 27. Explain how radiation works Objects that have a temperature (that are hot) emit infrared radiation) 28. What objects are good at reflecting Shiny, silver, white objects radiation? 29. What objects are good at emitting and Rough, matt, black objects absorbing radiation? 30. What objects give of infrared radiation? Hotter objects (objects at a higher temperature) 31.Why can solids not carry out convection? The particles cannot move around 32. Which method of heat transfer does not Infrared radiation need particles to work? 33. What happens when something cools It emits more infrared radiation than it absorbs down? 34. What happens when sometime heats up It absorbs more infrared radiation than it emits 35. What can you use to see infrared? An thermal imaging camera 36.Explain why gases are good insulators They are spaced out, so the particles cannot collide with each other 37. What is a fossil fuel? A fuel made from the remains of dead plants/ animals 38. What are the 3 different fossil fuels? Coal, oil and natural gas 39. What does non-renewable mean? It is used up faster than it can be made 40. What does finite mean? That there is a limited amount of a resource 41. How does a thermal power station work? Burn fuel, heat made boils water, steam turns turbine which turns a generator generating electricity 42. Give some disadvantages of fossil fuels Create carbon dioxide when burnt, non-renewable 43. Name some non-renewable energy Fossil fuels, nuclear fuels resources. 44. Give some examples of renewable energy resources Hydro-electricity, wind, solar, geothermal, tidal, biomass 45. What is power? The rate of energy transfer 46. What are the units of power? Watts/ kilowatts 47. What is the equation for power? Power = Energy transferred Time taken 48. How does a house maintain its temperature? 49. What does the cost of electricity depend on? Energy is transferred to heat it at the same rate that it loses heat Cost of electricity, power of appliance, time an appliance is used for (energy used) 50. What is energy measured in? Kilowatt hours or Joules 51. How can we reduce our energy bills? Use low power appliances, use the appliances for less time 52. How does insulating reduce heating bills? It reduces the amount of heat transferred to the surroundings 53. What is meant by work done? Transferring energy by using a force over a distance 54. What is a lever? A simple machine that multiplies the force 55. Give an example of a lever Screwdriver opening a tin of paint, pedal on a bike 56. How can you increase the work done by a lever? Increase the force or distance the force acts from the pivot 7. 43
44 8P2: Checklists 44
45 Topic Page Spec points Understanding Revised RP I can state that energy is stored in food and fuel I can name the energy type that is stored in food and fuel I can calculate energy values of different foods in kj (using food labels) I can state that energy gives the ability to do things I can recall what energy is measured in I can recall the factors that affect the amount of energy we require each day I can describe how the energy in different energy resources can be used I can name different energy stores I can describe several situations where energy is transferred I can state that energy is always conserved I can describe how energy may be wasted and/or dissipated I can fully explain situations that may change the amounts of energy that is wasted I can recall what is meant by the conservation of energy I can state that thermal energy is transferred between objects until thermal equilibrium is reached I can state that thermal energy is transferred from hotter objects to colder objects I can state that the effect of a thermal transfer is that one object increases in temperature whilst the other decreases in temperature I can recall what temperature is 45
Year 8 Tracking Document. Year 8 Science National Curriculum. Health and Lifestyle
 Health and Lifestyle Describe the components of a healthy diet Explain the role of each food group in the body Describe the test for starch, lipids, sugar and proteins Describe the positive test for each
Health and Lifestyle Describe the components of a healthy diet Explain the role of each food group in the body Describe the test for starch, lipids, sugar and proteins Describe the positive test for each
Year 8 Biology Knowledge Organiser Topic 1: Health and Lifestyle
 Year 8 Biology Knowledge Organiser Topic 1: Health and Lifestyle KPI 1: Describe the requirements for a healthy human diet. Kilojoules (kj) Deficiency Disease A unit used to measure energy in foods A disease
Year 8 Biology Knowledge Organiser Topic 1: Health and Lifestyle KPI 1: Describe the requirements for a healthy human diet. Kilojoules (kj) Deficiency Disease A unit used to measure energy in foods A disease
Y8 Science Controlled Assessment Topics & Keywords
 Y8 Science Controlled Assessment Topics & Biology Respiration. Know that respiration in living organisms can be aerobic or anaerobic The word equation for aerobic respiration The process of anaerobic respiration
Y8 Science Controlled Assessment Topics & Biology Respiration. Know that respiration in living organisms can be aerobic or anaerobic The word equation for aerobic respiration The process of anaerobic respiration
- know that in multi-cellular organisms cells are massed together to form tissues, and tissues can be massed together to form organs
 Science Age 12-13 (A) BIOLOGY: ORGANISMS, THEIR BEHAVIOUR AND THE ENVIRONMENT 1. Cells and their functions - know that in multi-cellular organisms cells are massed together to form tissues, and tissues
Science Age 12-13 (A) BIOLOGY: ORGANISMS, THEIR BEHAVIOUR AND THE ENVIRONMENT 1. Cells and their functions - know that in multi-cellular organisms cells are massed together to form tissues, and tissues
KS3 Science PERSONAL LEARNING CHECKLIST. YEAR 88 CONTENT Use this document to monitor your revision and target specific areas.
 KS3 Science PERSONAL LEARNING CHECKLIST YEAR 88 CONTENT Use this document to monitor your revision and target specific areas. Topic Name BIOLOGY the human skeleton Analysing the skeleton the role of skeletal
KS3 Science PERSONAL LEARNING CHECKLIST YEAR 88 CONTENT Use this document to monitor your revision and target specific areas. Topic Name BIOLOGY the human skeleton Analysing the skeleton the role of skeletal
KS3 Science Levelness Posters
 KS Science Levelness Posters Contents Year Year 8 Year 9 A. Cells 8A. Food and digestion 9A. Inheritance and selection B. Reproduction 8B. Respiration 9B. Fit and healthy C. Environment and feeding 8C.
KS Science Levelness Posters Contents Year Year 8 Year 9 A. Cells 8A. Food and digestion 9A. Inheritance and selection B. Reproduction 8B. Respiration 9B. Fit and healthy C. Environment and feeding 8C.
KS3 Science PERSONAL LEARNING CHECKLIST. YEAR 7 CONTENT Use this document to monitor your revision and target specific areas.
 KS3 Science PERSONAL LEARNING CHECKLIST YEAR 7 CONTENT Use this document to monitor your revision and target specific areas. Topic Name BIOLOGY Historical ideas about living things Content Cells as the
KS3 Science PERSONAL LEARNING CHECKLIST YEAR 7 CONTENT Use this document to monitor your revision and target specific areas. Topic Name BIOLOGY Historical ideas about living things Content Cells as the
In the event of snow:
 BIOLOGY Units Learning Objectives: Pupils should be taught (underlined = level 2 only) YEAR 7 Cells and Cell Functions 1a-e Nutrition and movement 2a-e Reproduction 2f-h Breathing and Respiration 2i-l
BIOLOGY Units Learning Objectives: Pupils should be taught (underlined = level 2 only) YEAR 7 Cells and Cell Functions 1a-e Nutrition and movement 2a-e Reproduction 2f-h Breathing and Respiration 2i-l
Year 8 Key Performance Indicators Science (Biology)
 Year 8 Key Performance Indicators Science (Biology) S8.1 B1: To understand key concepts in Biology. I can compare the rate of digestion of different enzymes by linking to their surroundings. S8.2 B3: To
Year 8 Key Performance Indicators Science (Biology) S8.1 B1: To understand key concepts in Biology. I can compare the rate of digestion of different enzymes by linking to their surroundings. S8.2 B3: To
Name Period Date Physical Science 2nd Semester Final Exam Study Guide ( )
 Name Period Date Physical Science 2nd Semester Final Exam Study Guide (2012-2013) 1. Physical Science Basics a. What tool(s) would you want to use to find the: i. Mass of an object? Basic SI Unit? ii.
Name Period Date Physical Science 2nd Semester Final Exam Study Guide (2012-2013) 1. Physical Science Basics a. What tool(s) would you want to use to find the: i. Mass of an object? Basic SI Unit? ii.
Heat: the transfer of thermal energy from one substance to another. Electrons: the small particles inside an atom that have negative charge.
 GRADE 5 0 Energy: the ability to cause change OR to do work Kinetic energy: energy of motion Potential energy: energy of position (stored energy) Thermal energy: the energy that moves particles of matter
GRADE 5 0 Energy: the ability to cause change OR to do work Kinetic energy: energy of motion Potential energy: energy of position (stored energy) Thermal energy: the energy that moves particles of matter
SCIENCE HIGHER LEVEL
 J.37 PRE-JUNIOR CERTIFICATE EXAMINATION, 2014 SCIENCE HIGHER LEVEL TIME: 2 HOURS INSTRUCTIONS 1. Write your name, school s name and teacher s name in the boxes provided on this page. 2. Answer all questions.
J.37 PRE-JUNIOR CERTIFICATE EXAMINATION, 2014 SCIENCE HIGHER LEVEL TIME: 2 HOURS INSTRUCTIONS 1. Write your name, school s name and teacher s name in the boxes provided on this page. 2. Answer all questions.
IGCSE Double Award Extended Coordinated Science
 IGCSE Double Award Extended Coordinated Science Biology 4.2 - Plant Nutrition Photosynthesis You need to know the definition of photosynthesis as: the fundamental process by which plants manufacture carbohydrates
IGCSE Double Award Extended Coordinated Science Biology 4.2 - Plant Nutrition Photosynthesis You need to know the definition of photosynthesis as: the fundamental process by which plants manufacture carbohydrates
Science Year 10 Unit 1 Biology
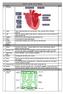 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Chemistry Final Study Guide KEY. 3. Define physical changes. A change in any physical property of a substance, not in the substance itself.
 Chemistry Final Study Guide KEY Unit 2: Matter & Its Properties, Lesson 1: Physical and Chemical Properties & Changes 1. Define physical properties. The characteristics of a substance that can be observed
Chemistry Final Study Guide KEY Unit 2: Matter & Its Properties, Lesson 1: Physical and Chemical Properties & Changes 1. Define physical properties. The characteristics of a substance that can be observed
0654 CO-ORDINATED SCIENCES
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education www.xtremepapers.com MARK SCHEME for the May/June 2010 question paper for the guidance of teachers
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education www.xtremepapers.com MARK SCHEME for the May/June 2010 question paper for the guidance of teachers
Fill in the gaps: You would write this as: we can use for living processes.
 AQA Trilogy Biology Unit 4.4: Bioenergetics Complete the word equation for + + Write the name of each chemical next to its formula. Which elements make up each chemical? How does the rate of photosynthesis
AQA Trilogy Biology Unit 4.4: Bioenergetics Complete the word equation for + + Write the name of each chemical next to its formula. Which elements make up each chemical? How does the rate of photosynthesis
Year 7 Recall Booklet. Name: Class:
 Year 7 Recall Booklet Name: Class: Energy Kinetic energy Moving things have kinetic energy. The heavier a thing is and the faster it moves the more kinetic energy it has. All moving things have kinetic
Year 7 Recall Booklet Name: Class: Energy Kinetic energy Moving things have kinetic energy. The heavier a thing is and the faster it moves the more kinetic energy it has. All moving things have kinetic
Chapter19-Magnetism and Electricity
 Chapter19-Magnetism and Electricity Magnetism: attraction of a magnet for another object. Magnetic poles: north & south ends of a magnet, they exert the strongest forces Like poles repel each other, unlike
Chapter19-Magnetism and Electricity Magnetism: attraction of a magnet for another object. Magnetic poles: north & south ends of a magnet, they exert the strongest forces Like poles repel each other, unlike
Year 7 Science Booklet Name:
 Year 7 Science Booklet Name: Acids and Alkalis Use a dictionary or internet to look up the key words and write a definition. Acid Alkali Litmus Fizz Neutral Reactant Product Irritant Harmful indicator
Year 7 Science Booklet Name: Acids and Alkalis Use a dictionary or internet to look up the key words and write a definition. Acid Alkali Litmus Fizz Neutral Reactant Product Irritant Harmful indicator
CLASS COPY Structure and Properties of Matter Parts of the atom
 CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
CLASS COPY Structure and Properties of Matter Parts of the atom An atom is made up of protons, neutrons, and electrons. Look at the model of a carbon atom from the graphite in the point of a pencil. Protons
YEAR 7- Science Term 1 plan
 Week Topic YEAR 7- Science Term 1 plan 2016-2017 Learning outcomes 1 Cells the building blocks of life Develop models to explain the differences between animal cells and plant cells. Record evidence using
Week Topic YEAR 7- Science Term 1 plan 2016-2017 Learning outcomes 1 Cells the building blocks of life Develop models to explain the differences between animal cells and plant cells. Record evidence using
YEAR 7. St Edmund Arrowsmith CCFL Science Department Curriculum Map New AQA Course Started November 2016
 YEAR 7 St Edmund Arrowsmith CCFL Science Department Curriculum Map 201-2017 New AQA Course Started November 2016 05/09/2016 12/09/2016 19/09/2016 26/09/2016 03/10/2016 10/10/2016 17/10/2016 24/10/2016
YEAR 7 St Edmund Arrowsmith CCFL Science Department Curriculum Map 201-2017 New AQA Course Started November 2016 05/09/2016 12/09/2016 19/09/2016 26/09/2016 03/10/2016 10/10/2016 17/10/2016 24/10/2016
Section 1: Elements Pages 56-59
 Study Guide Chapter 3 Elements, Compounds, and Mixtures Section 1: Elements Pages 56-59 1. Which of the following processes is NOT a physical or chemical change? a. crushing b. weighing c. melting d. passing
Study Guide Chapter 3 Elements, Compounds, and Mixtures Section 1: Elements Pages 56-59 1. Which of the following processes is NOT a physical or chemical change? a. crushing b. weighing c. melting d. passing
Magnets attract some metals but not others
 Electricity and Magnetism Junior Science Magnets attract some metals but not others Some objects attract iron and steel. They are called magnets. Magnetic materials have the ability to attract some materials
Electricity and Magnetism Junior Science Magnets attract some metals but not others Some objects attract iron and steel. They are called magnets. Magnetic materials have the ability to attract some materials
0653 COMBINED SCIENCE
 CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International General Certificate of Secondary Education MARK SCHEME for the October/November 2014 series 0653 COMBINED SCIENCE 0653/31 Paper 3 (Extended
CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International General Certificate of Secondary Education MARK SCHEME for the October/November 2014 series 0653 COMBINED SCIENCE 0653/31 Paper 3 (Extended
STANDARD GRADE CHEMISTRY : GENERAL LEVEL
 STANDARD GRADE CHEMISTRY : GENERAL LEVEL NEED TO KNOW SHEETS (BASED ON 1998 2006 EXAMS) TOPIC NO 1 -ide means two elements only ate/-ite means two elements + oxygen a solution contains a solid (solute)
STANDARD GRADE CHEMISTRY : GENERAL LEVEL NEED TO KNOW SHEETS (BASED ON 1998 2006 EXAMS) TOPIC NO 1 -ide means two elements only ate/-ite means two elements + oxygen a solution contains a solid (solute)
Science Scheme of Work
 Cherry Tree Hill Primary School Science Scheme of Work Essential characteristics: High-quality science education provides the foundations for understanding the world through the specific disciplines of
Cherry Tree Hill Primary School Science Scheme of Work Essential characteristics: High-quality science education provides the foundations for understanding the world through the specific disciplines of
Chapter: Cell Processes
 Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
Year 7. Knowledge/Content Understanding Skills
 Age Related Expectations in KS3 Science 2017-2018 These are the age related expectations for KS3 science for year 7 and 8; they are based on the KS3 core science curriculum. This knowledge, understanding
Age Related Expectations in KS3 Science 2017-2018 These are the age related expectations for KS3 science for year 7 and 8; they are based on the KS3 core science curriculum. This knowledge, understanding
UNIT 9A Inheritance and Selection
 UNIT 9A Inheritance and Selection Know what inheritance means Know what characteristics are Know that sexual reproduction needs two parents Know that animals and plants can be bred for their characteristics
UNIT 9A Inheritance and Selection Know what inheritance means Know what characteristics are Know that sexual reproduction needs two parents Know that animals and plants can be bred for their characteristics
Name Period Date Physical Science 2nd Semester Final Exam Study Guide ( )
 Name Period Date Physical Science 2nd Semester Final Exam Study Guide (2012-2013) 1. Physical Science Basics a. What tool(s) would you want to use to find the: i. Mass of an object? triple beam balance
Name Period Date Physical Science 2nd Semester Final Exam Study Guide (2012-2013) 1. Physical Science Basics a. What tool(s) would you want to use to find the: i. Mass of an object? triple beam balance
Electricity. Prepared by Juan Blázquez, Alissa Gildemann. Electric charge is a property of all objects. It is responsible for electrical phenomena.
 Unit 11 Electricity 1. Electric charge Electric charge is a property of all objects. It is responsible for electrical phenomena. Electrical phenomena are caused by the forces of attraction and repulsion.
Unit 11 Electricity 1. Electric charge Electric charge is a property of all objects. It is responsible for electrical phenomena. Electrical phenomena are caused by the forces of attraction and repulsion.
10/4/2016. Matter, Energy, and Life
 DISCLAIMER: Principles and concepts on atomic structure, the Periodic Table, atoms, ions, ionic and covalent compounds, metals, and nonmetals will not be covered in this course. You are expected to know
DISCLAIMER: Principles and concepts on atomic structure, the Periodic Table, atoms, ions, ionic and covalent compounds, metals, and nonmetals will not be covered in this course. You are expected to know
0653 COMBINED SCIENCE
 9*CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0653 COMBINED SCIENCE 0653/22 Paper 2 (Core Theory), maximum raw
9*CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0653 COMBINED SCIENCE 0653/22 Paper 2 (Core Theory), maximum raw
B2 Quick Revision Questions. B2 for AQA GCSE examination 2018 onwards
 B2 Quick Revision Questions Question 1 Which raw materials are used in photosynthesis and what are the products of the reaction? Answer 1 Carbon dioxide Water Glucose Oxygen Question 2 What type of reaction
B2 Quick Revision Questions Question 1 Which raw materials are used in photosynthesis and what are the products of the reaction? Answer 1 Carbon dioxide Water Glucose Oxygen Question 2 What type of reaction
What is the role of the nucleus? What is the role of the cytoplasm? What is the role of the mitochondria? What is the role of the cell wall. membrane?
 Page 1 What is the role of the nucleus? What is the role of the cytoplasm? What is the role of the cell membrane? What is the role of the mitochondria? What is the role of ribosomes? What is the role of
Page 1 What is the role of the nucleus? What is the role of the cytoplasm? What is the role of the cell membrane? What is the role of the mitochondria? What is the role of ribosomes? What is the role of
Unit 1 Plants - Extra Worksheets
 Unit 1 Plants - Extra Worksheets 1 Complete the sentences using the words in the box. Plants can make their own using light from the. They also need carbon gas and. 2 Label the parts of the diagram to
Unit 1 Plants - Extra Worksheets 1 Complete the sentences using the words in the box. Plants can make their own using light from the. They also need carbon gas and. 2 Label the parts of the diagram to
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International General Certificate of Secondary Education MARK SCHEME for the May/June 2015 series 0654 CO-ORDINATED SCIENCES 0654/31 Paper 3 (Extended Theory),
CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International General Certificate of Secondary Education MARK SCHEME for the May/June 2015 series 0654 CO-ORDINATED SCIENCES 0654/31 Paper 3 (Extended Theory),
What is Science? Science is both a collection of knowledge and the process for building that knowledge.
 Introduction to Science Junior Science What is Science? Science is both a collection of knowledge and the process for building that knowledge. Science asks questions about the natural world and looks for
Introduction to Science Junior Science What is Science? Science is both a collection of knowledge and the process for building that knowledge. Science asks questions about the natural world and looks for
Stewards Pooi Kei College Integrated Science Secondary One Teaching Schedule in Textbook Chapter 1 Introducing Science Book 1A, 1B
 Stewards Pooi Kei College Integrated Science Secondary One Teaching Schedule 2014-15 Chapter 1 Introducing Science Book 1A, 1B 1 1.1 Science and Us Book 1A Unit 1 (3/9-12/9) Know the scope of science 1.1
Stewards Pooi Kei College Integrated Science Secondary One Teaching Schedule 2014-15 Chapter 1 Introducing Science Book 1A, 1B 1 1.1 Science and Us Book 1A Unit 1 (3/9-12/9) Know the scope of science 1.1
Part 4: Electricity & Magnetism
 Part 4: Electricity & Magnetism Notes: Magnetism Magnetism Magnets: 1.Have a north and south pole 2.Like poles repel; opposite poles attract - The larger the distance between the magnets, the weaker the
Part 4: Electricity & Magnetism Notes: Magnetism Magnetism Magnets: 1.Have a north and south pole 2.Like poles repel; opposite poles attract - The larger the distance between the magnets, the weaker the
HfL Science Topic Maps for Years 1-6. Herts for Learning Ltd.
 HfL Science Topic Maps for Years 1-6 Year 1 Science Map Working scientifically Everyday materials Different animals asking simple questions and recognising that they can be answered in different ways distinguish
HfL Science Topic Maps for Years 1-6 Year 1 Science Map Working scientifically Everyday materials Different animals asking simple questions and recognising that they can be answered in different ways distinguish
Chapter 3-1. proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small
 Chapter 3-1 Sub-atomic Charge Location Mass Particle proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small The most mass of the atom
Chapter 3-1 Sub-atomic Charge Location Mass Particle proton positive nucleus 1 amu neutron zero nucleus 1 amu electron negative on energy levels around the nucleus very small The most mass of the atom
Science subject progression and skills
 Science subject progression and skills Year 1 : Identify and name a variety of common animals that are birds, fish, amphibians, reptiles and mammals Identify and name a variety of common animals that are
Science subject progression and skills Year 1 : Identify and name a variety of common animals that are birds, fish, amphibians, reptiles and mammals Identify and name a variety of common animals that are
Science curriculum overview Yr7 (Draft arrangement as the school may need flexibility as the need arises)
 Science curriculum overview Yr7 (Draft arrangement as the school may need flexibility as the need arises) Term Topic and key questions Assessment structure Autumn 1 CHEMISTRY Test 1: The particle 1.The
Science curriculum overview Yr7 (Draft arrangement as the school may need flexibility as the need arises) Term Topic and key questions Assessment structure Autumn 1 CHEMISTRY Test 1: The particle 1.The
Page 1 / 12. Chemistry Exam. Name: Matter Properties, Structure. Question 1 (1 point) The atomic number of an atom is. A. The mass of the atom.
 Chemistry Exam Matter Properties, Structure Name: Question 1 (1 point) The atomic number of an atom is A. The mass of the atom. B. The number of protons added to the number of neutrons in the nucleus.
Chemistry Exam Matter Properties, Structure Name: Question 1 (1 point) The atomic number of an atom is A. The mass of the atom. B. The number of protons added to the number of neutrons in the nucleus.
DOSTHILL PRIMARY SCHOOL I am working towards Building Block A1 in Science
 I am working towards Building Block A1 in Science 1 I can observe things closely using some simple equipment 2 I can carry out investigations 3 With some help, I can test out some ideas suggested to me
I am working towards Building Block A1 in Science 1 I can observe things closely using some simple equipment 2 I can carry out investigations 3 With some help, I can test out some ideas suggested to me
Grade 9 Academic Science Review
 Grade 9 Academic Science Review Chapters 4-6: Matter (Chemistry) 1. Review the meanings of the WHMIS safety symbols. 2. a) Define physical property, list and explain examples of physical properties. b)
Grade 9 Academic Science Review Chapters 4-6: Matter (Chemistry) 1. Review the meanings of the WHMIS safety symbols. 2. a) Define physical property, list and explain examples of physical properties. b)
7.1.2 Cell Functions. 104 minutes. 137 marks. Page 1 of 30
 7.1.2 Cell Functions 104 minutes 137 marks Page 1 of 30 ## Most pollen grains are transferred from one flower to another either by wind or by insects. Look at the drawings below which show pollen grains
7.1.2 Cell Functions 104 minutes 137 marks Page 1 of 30 ## Most pollen grains are transferred from one flower to another either by wind or by insects. Look at the drawings below which show pollen grains
Year 8 Chemistry Knowledge Organiser Topic 1: Periodic Table
 KPI 1.1: Identify, with reasons, differences between atoms, elements and compounds Key Terms Element Mixture Compound Elements Definitions A substance that contains only one type of atom A substance that
KPI 1.1: Identify, with reasons, differences between atoms, elements and compounds Key Terms Element Mixture Compound Elements Definitions A substance that contains only one type of atom A substance that
1 Science Stage 9 V2
 Science Stage 9 V This table gives general guidelines on marking answers involving units of length. For questions involving other quantities, correct units are given in the answers. The table shows acceptable
Science Stage 9 V This table gives general guidelines on marking answers involving units of length. For questions involving other quantities, correct units are given in the answers. The table shows acceptable
2018 Version. Photosynthesis Junior Science
 2018 Version Photosynthesis Junior Science 1 Plants fill the role of Producers in a community Plants are special because they have leaves and are able to produce their own food by the process of photosynthesis
2018 Version Photosynthesis Junior Science 1 Plants fill the role of Producers in a community Plants are special because they have leaves and are able to produce their own food by the process of photosynthesis
Band 1 - Science All. Working Scientifically Animals Including Humans Materials. Plants. Seasonal Changes
 Band 1 - Science All Working Scientifically Materials Ask simple questions and recognise that they can be answered in different ways (Year 1 focus). I can ask questions and know they can be answered in
Band 1 - Science All Working Scientifically Materials Ask simple questions and recognise that they can be answered in different ways (Year 1 focus). I can ask questions and know they can be answered in
MATTER & ENERGY STUDY GUIDE. 9 Weeks Test Date: Parent Signature (BONUS!):
 Name: Pd: MATTER & ENERGY STUDY GUIDE 9 Weeks Test Date: Parent Signature (BONUS!): 6.5A MATTER Matter is anything that has mass and takes up space. Give EXAMPLES and NON-EXAMPLES of matter. (42) EXAMPLES
Name: Pd: MATTER & ENERGY STUDY GUIDE 9 Weeks Test Date: Parent Signature (BONUS!): 6.5A MATTER Matter is anything that has mass and takes up space. Give EXAMPLES and NON-EXAMPLES of matter. (42) EXAMPLES
IN THE PAST WEEKS WE SPOKE ABOUT THE 4 ORGANIC MOLECULES THAT ARE INSIDE THE CELL, SUCH AS PROTEINS, CARBOHYDRATES, LIPIDS AND NUCLEIC ACIDS
 IN THE PAST WEEKS WE SPOKE ABOUT THE 4 ORGANIC MOLECULES THAT ARE INSIDE THE CELL, SUCH AS PROTEINS, CARBOHYDRATES, LIPIDS AND NUCLEIC ACIDS CARBOHYDRATES ARE THE IMMEDIATE SOURCE OF ENERGY FOR THE CELL
IN THE PAST WEEKS WE SPOKE ABOUT THE 4 ORGANIC MOLECULES THAT ARE INSIDE THE CELL, SUCH AS PROTEINS, CARBOHYDRATES, LIPIDS AND NUCLEIC ACIDS CARBOHYDRATES ARE THE IMMEDIATE SOURCE OF ENERGY FOR THE CELL
Key Stage 3 Science Revision Guide
 Key Stage 3 Science Revision Guide Use this guide to check that you cover all the topics you have studied at key stage 3. You can use your classwork books, revision guides and the internet to help you
Key Stage 3 Science Revision Guide Use this guide to check that you cover all the topics you have studied at key stage 3. You can use your classwork books, revision guides and the internet to help you
Electric Charge. Conductors A material that transfers charge easily Metals
 Electric Charge An electrical property of matter that creates a force between objects. Like charges repel Opposite charges attract Equal amount of positive and negative = no net charge Electrons: Negative
Electric Charge An electrical property of matter that creates a force between objects. Like charges repel Opposite charges attract Equal amount of positive and negative = no net charge Electrons: Negative
Dushan [δβ] BOODHENA 1
![Dushan [δβ] BOODHENA 1 Dushan [δβ] BOODHENA 1](/thumbs/80/82205169.jpg) CHEMISTRY SUMMARY FOR THE FORM 3 NATIONAL ASSESSMENT 1. Define the term element. An element is a pure substance that cannot be broken down into simpler substances by any ordinary chemical process. 2. Define
CHEMISTRY SUMMARY FOR THE FORM 3 NATIONAL ASSESSMENT 1. Define the term element. An element is a pure substance that cannot be broken down into simpler substances by any ordinary chemical process. 2. Define
Key Skills (to be covered during the year) (from CQ Milestones)
 Science Whole School Unit Overview and Key Skills Checklist Essential Learning Objectives (Chris Quigley): Year 3 National Curriculum Unit To work scientifically Ask relevant questions. Key Skills (to
Science Whole School Unit Overview and Key Skills Checklist Essential Learning Objectives (Chris Quigley): Year 3 National Curriculum Unit To work scientifically Ask relevant questions. Key Skills (to
Skills and Knowledge Progression. Subject: Science KS2 Name: (Child s name) Class: (Child s class) Year 3 Year 4 Year 5 Year 6
 Skills and Progression Subject: Science KS2 Name: (Child s name) Class: (Child s class) Year 3 Year 4 Year 5 Year 6 Plants Can identify and describe the functions of different parts of flowering plants:
Skills and Progression Subject: Science KS2 Name: (Child s name) Class: (Child s class) Year 3 Year 4 Year 5 Year 6 Plants Can identify and describe the functions of different parts of flowering plants:
Main Topic Sub-topics Students should be able to R O G
 Second Year Science Syllabus Biology Revision Check-list Where is your learning at? Green: I know it all. Orange: I have some idea check the answers. Red: I need to start studying this section. Main Topic
Second Year Science Syllabus Biology Revision Check-list Where is your learning at? Green: I know it all. Orange: I have some idea check the answers. Red: I need to start studying this section. Main Topic
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
I. Energy for Life. Energy in Ecosystems Did you know you were solar powered? IN: 11/4/2018. Fill in the blanks to complete the reaction: C H O + 6 2
 11/4/2018 Energy in Ecosystems Did you know you were solar powered? IN: Fill in the blanks to complete the reaction: Light 6 2 + 6 2 Chlorophyll C H O + 6 2 Write the equation for photosynthesis in words.
11/4/2018 Energy in Ecosystems Did you know you were solar powered? IN: Fill in the blanks to complete the reaction: Light 6 2 + 6 2 Chlorophyll C H O + 6 2 Write the equation for photosynthesis in words.
1.1 Characteristics common to organisms
 Biology Form 3 Page 5 Ms. R. Buttigieg 1.1 Characteristics common to organisms see GCSE Biology pg. 292 Biology is the study of living things. We call living things organisms. Plants and animals are ALL
Biology Form 3 Page 5 Ms. R. Buttigieg 1.1 Characteristics common to organisms see GCSE Biology pg. 292 Biology is the study of living things. We call living things organisms. Plants and animals are ALL
Key Stage 3 Science Revision Guide
 Key Stage 3 Science Revision Guide Use this guide to check that you cover all the topics you have studied at key stage 3. You can use your classwork books, revision guides and the internet to help you
Key Stage 3 Science Revision Guide Use this guide to check that you cover all the topics you have studied at key stage 3. You can use your classwork books, revision guides and the internet to help you
Lesson 3.1 Matter and the Environment. Water s abundance is a primary reason there is life on Earth.
 Lesson 3.1 Matter and the Environment Water s abundance is a primary reason there is life on Earth. Lesson 3.1 Matter and the Environment Atoms and Elements Atoms are the basic unit of matter. Nucleus:
Lesson 3.1 Matter and the Environment Water s abundance is a primary reason there is life on Earth. Lesson 3.1 Matter and the Environment Atoms and Elements Atoms are the basic unit of matter. Nucleus:
Section 1: The Science of Energy¹
 SECTION1: THE SCIENCE OF ENERGY Section 1: The Science of Energy¹ What Is Energy? Energy is the ability to do work or the ability to make a change. Everything that happens in the world involves the exchange
SECTION1: THE SCIENCE OF ENERGY Section 1: The Science of Energy¹ What Is Energy? Energy is the ability to do work or the ability to make a change. Everything that happens in the world involves the exchange
Year 11 Science Learning Cycle 3 Overview
 Year 11 Science Learning Cycle 3 Overview Learning Cycle Overview: Biology Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Hypothesis 6 Hypothesis 7 Hypothesis 8 Hypothesis 9 How does
Year 11 Science Learning Cycle 3 Overview Learning Cycle Overview: Biology Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Hypothesis 6 Hypothesis 7 Hypothesis 8 Hypothesis 9 How does
UNIT 3: Cell Energy What is energy? energy is a property of objects which can be transferred to other objects or converted into different forms.
 UNIT 3: Cell Energy What is energy? energy is a property of objects which can be transferred to other objects or converted into different forms. Energy can be found in a number of different forms. 1 Law
UNIT 3: Cell Energy What is energy? energy is a property of objects which can be transferred to other objects or converted into different forms. Energy can be found in a number of different forms. 1 Law
3. Natural gas burns in a bunsen burner. (a) What happens to the temperature of the flame when the air hole is opened. (b) Explain your answer.
 Rate of reactions 1. Different chemical reactions happen at different speeds. (a) Give an example of a reaction in the lab that (i) is almost instantaneous, (ii) takes a few minutes to finish. (b) List
Rate of reactions 1. Different chemical reactions happen at different speeds. (a) Give an example of a reaction in the lab that (i) is almost instantaneous, (ii) takes a few minutes to finish. (b) List
 S1 Science Elements, compounds and mixtures Revision Sheet --------------------------------------------------------------------------------------------------------------------------------- Elements Everything
S1 Science Elements, compounds and mixtures Revision Sheet --------------------------------------------------------------------------------------------------------------------------------- Elements Everything
Primary Science Curriculum Pupil Assessment Record
 Year 1 Plants Can identify and name a variety of common plants, including garden plants, wild plants and trees, and those classified as deciduous and evergreen. Can describe the basic structure of a variety
Year 1 Plants Can identify and name a variety of common plants, including garden plants, wild plants and trees, and those classified as deciduous and evergreen. Can describe the basic structure of a variety
Science. synthesis 2 Analysis and synthesis Using physics to make things work 3 Magnetic fields to keep things moving Energy calculations 4 Energy
 Year 11 (Triple science) 2016-17 Half-term Topic Week 1 Week 2 Week 3 Week 4 Week 5 Week 6 Week 7 1 Exchange of materials Keeping internal conditions constant Analysis and synthesis 2 Analysis and synthesis
Year 11 (Triple science) 2016-17 Half-term Topic Week 1 Week 2 Week 3 Week 4 Week 5 Week 6 Week 7 1 Exchange of materials Keeping internal conditions constant Analysis and synthesis 2 Analysis and synthesis
The Chemistry of Respiration and Photosynthesis
 The Chemistry of Respiration and Photosynthesis Objective- You should be able to write balanced equations for respiration and photosynthesis and explain how the two equations are related. Directions :
The Chemistry of Respiration and Photosynthesis Objective- You should be able to write balanced equations for respiration and photosynthesis and explain how the two equations are related. Directions :
Lymm High School- KS3 Life after levels - Science Y9
 Biology BRONZE SILVER GOLD PLATINUM D and below= GCSE 1,2,3 C= GCSE 4 C/B= GCSE 5,6 A/A*= GCSE 7,8,9 Explain that cells are very Demonstrate an small and a microscope is understanding of the scale needed
Biology BRONZE SILVER GOLD PLATINUM D and below= GCSE 1,2,3 C= GCSE 4 C/B= GCSE 5,6 A/A*= GCSE 7,8,9 Explain that cells are very Demonstrate an small and a microscope is understanding of the scale needed
Year 1 Science overview
 Year 1 Science overview asking simple questions and recognising that they can be answered in different ways observing closely, using simple equipment performing simple tests identifying and classifying
Year 1 Science overview asking simple questions and recognising that they can be answered in different ways observing closely, using simple equipment performing simple tests identifying and classifying
Stamford Green Primary School Science Progression Document. September 2014
 Stamford Green Primary School Science Progression Document September 2014 Early Years Early Learning Goals Area of EYFS curriculum Early Learning Goals The World Children know about the similarities and
Stamford Green Primary School Science Progression Document September 2014 Early Years Early Learning Goals Area of EYFS curriculum Early Learning Goals The World Children know about the similarities and
Science Curriculum Overview
 YEAR 1 Seasonal changes observe changes across the 4 seasons observe and describe weather associated with the seasons and how day length varies Seasonal changes observe changes across the 4 seasons observe
YEAR 1 Seasonal changes observe changes across the 4 seasons observe and describe weather associated with the seasons and how day length varies Seasonal changes observe changes across the 4 seasons observe
In the early version of the periodic table (by Newlands and then Mendeleev), how were the elements arranged?
 Page 1 In the early version of the periodic table (by Newlands and then Mendeleev), how were the elements arranged? What can we say about the elements in the same group of the periodic table? Why is the
Page 1 In the early version of the periodic table (by Newlands and then Mendeleev), how were the elements arranged? What can we say about the elements in the same group of the periodic table? Why is the
National Curriculum 2014: Progression in Science
 Evaluating Concluding Working Scientifically Measuring and Recording Asking Questions Year 1/2 Year 3/4 Year 5/6 ask simple questions and recognise that they can be answered in different ways ask relevant
Evaluating Concluding Working Scientifically Measuring and Recording Asking Questions Year 1/2 Year 3/4 Year 5/6 ask simple questions and recognise that they can be answered in different ways ask relevant
Angel International School - Manipay 1 st Term Examination November, 2016 Chemistry
 c Grade 9 Angel International School - Manipay 1 st Term Examination November, 2016 Chemistry Duration: 01:30 Hours Index No:- PART I Underline the correct answers 1) Identify the correct statement about
c Grade 9 Angel International School - Manipay 1 st Term Examination November, 2016 Chemistry Duration: 01:30 Hours Index No:- PART I Underline the correct answers 1) Identify the correct statement about
Which row in the chart below identifies the lettered substances in this process?
 1. A biological process that occurs in both plants and animals is shown below. Which row in the chart below identifies the lettered substances in this process? A) 1 B) 2 C) 3 D) 4 2. All life depends on
1. A biological process that occurs in both plants and animals is shown below. Which row in the chart below identifies the lettered substances in this process? A) 1 B) 2 C) 3 D) 4 2. All life depends on
8/25/ Opening Questions: What is ENERGY? Chapter 4 Energy and Life: How do you make it through your day? How does a plant eat?
 Chapter 4 Energy and Life: 4.1 What is energy? 4.2 Energy flow 4.3 Sunlight is used to produce sugars 4.4 Photosynthesis has 2 linked stages 4.5 Light reactions: a closer look 4.6 Calvin cycle: a closer
Chapter 4 Energy and Life: 4.1 What is energy? 4.2 Energy flow 4.3 Sunlight is used to produce sugars 4.4 Photosynthesis has 2 linked stages 4.5 Light reactions: a closer look 4.6 Calvin cycle: a closer
Mapping progression across KS1 to KS2. Science. Adapted from Mapping Progression in the Primary Science Curriculum written by Sue Atkinson CYC
 Mapping progression across KS1 to KS2 Science Adapted from Mapping Progression in the Primary Science Curriculum written by Sue Atkinson CYC Sc1 Working Scientifically Asking simple questions and recognising
Mapping progression across KS1 to KS2 Science Adapted from Mapping Progression in the Primary Science Curriculum written by Sue Atkinson CYC Sc1 Working Scientifically Asking simple questions and recognising
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education www.xtremepapers.com MARK SCHEME for the October/November 2013 series 0654 CO-ORDINATED SCIENCES 0654/23 Paper
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education www.xtremepapers.com MARK SCHEME for the October/November 2013 series 0654 CO-ORDINATED SCIENCES 0654/23 Paper
8.L.5.1 Practice Questions
 Name: ate: 1. The diagram below represents a series of events that occur in living cells. Which molecule is indicated by X?. glucose. TP. carbon dioxide. protein 2. The diagram represents one metabolic
Name: ate: 1. The diagram below represents a series of events that occur in living cells. Which molecule is indicated by X?. glucose. TP. carbon dioxide. protein 2. The diagram represents one metabolic
Additional Science Chemistry
 Additional Science Chemistry C2 Core Questions and Keywords and Definitions Question How did Mendeleev arrange the elements known at the time into a periodic table? How did Mendeleev use his table? Where
Additional Science Chemistry C2 Core Questions and Keywords and Definitions Question How did Mendeleev arrange the elements known at the time into a periodic table? How did Mendeleev use his table? Where
Time (s) Velocity (m/s)
 29.01.18 22.01.18 15.01.18 w/c Weekly Combined Science revision countdown plan (revision guide / workbook page numbers) *content across both paper 1 and paper 2 15 14 13 Revise *Key concepts in biology
29.01.18 22.01.18 15.01.18 w/c Weekly Combined Science revision countdown plan (revision guide / workbook page numbers) *content across both paper 1 and paper 2 15 14 13 Revise *Key concepts in biology
Y7 Science Controlled Assessment Topics & Keywords
 Y7 Science Controlled Assessment Topics & Biology Cells. Cells as the fundamental unit of living organisms, including how to observe cell structure using a light microscope. The functions of the cell wall,
Y7 Science Controlled Assessment Topics & Biology Cells. Cells as the fundamental unit of living organisms, including how to observe cell structure using a light microscope. The functions of the cell wall,
Fairlawn Primary School Science Curriculum
 Materials Plants Animals and Humans At Fairlawn, we follow the curriculum set out in the Science Bug resource Year 1 SCIENTIFIC KNOWLEDGE Identify and name a variety of common animals including fish, amphibians,
Materials Plants Animals and Humans At Fairlawn, we follow the curriculum set out in the Science Bug resource Year 1 SCIENTIFIC KNOWLEDGE Identify and name a variety of common animals including fish, amphibians,
Electricity. What is electricity?
 Words attract = pull towards an object back and forth = to go in one direction and then in the other balanced = the same as stable carbon = a chemical material that is in coal or petrol. It is in its purest
Words attract = pull towards an object back and forth = to go in one direction and then in the other balanced = the same as stable carbon = a chemical material that is in coal or petrol. It is in its purest
National 4 Unit Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes. Homework
 National 4 Unit 1 1. Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes Homework 1 2 Homework 1 - Rates of Reaction Decide which of the following are True or False: 1. Increasing
National 4 Unit 1 1. Rates of Reaction 2. Atomic Structure 3. Acids & Bases 4. Energy Changes Homework 1 2 Homework 1 - Rates of Reaction Decide which of the following are True or False: 1. Increasing
Q1. Methane and oxygen react together to produce carbon dioxide and water.
 Chemistry C3 Higher Questions Part 2 Q1. Methane and oxygen react together to produce carbon dioxide and water. The methane gas will not burn in oxygen until a flame is applied, but once lit it continues
Chemistry C3 Higher Questions Part 2 Q1. Methane and oxygen react together to produce carbon dioxide and water. The methane gas will not burn in oxygen until a flame is applied, but once lit it continues
Mowbray School Long Term Science Plan KS1. Two Year rolling programme KS1
 Mowbray School Long Term Science Plan KS1 Two Year rolling programme KS1 Pupils should be taught to; Ø develop scientific knowledge and conceptual understanding through the specific disciplines of biology,
Mowbray School Long Term Science Plan KS1 Two Year rolling programme KS1 Pupils should be taught to; Ø develop scientific knowledge and conceptual understanding through the specific disciplines of biology,
Science Curriculum Map: KS1 & KS
 Subject In KS1 pupils should be taught to use the following practical scientific methods, processes and skills through the teaching of the programme content of study content: Key stage Asking simple questions
Subject In KS1 pupils should be taught to use the following practical scientific methods, processes and skills through the teaching of the programme content of study content: Key stage Asking simple questions
Big idea (age 11-14) PEM: Electricity and magnetism
 Physics Big idea (age 11-14) PEM: Electricity and magnetism What s the big idea? The familiar everyday world we live in is largely a consequence of the properties and behaviour of electric charge. Matter
Physics Big idea (age 11-14) PEM: Electricity and magnetism What s the big idea? The familiar everyday world we live in is largely a consequence of the properties and behaviour of electric charge. Matter
Science Curriculum Overview
 YEAR 1 Seasonal changes Seasonal changes Seasonal changes observe changes across the 4 seasons observe and describe weather associated with the seasons and how day length varies observe changes across
YEAR 1 Seasonal changes Seasonal changes Seasonal changes observe changes across the 4 seasons observe and describe weather associated with the seasons and how day length varies observe changes across
Cells the Building Blocks of Life. Cells the Building Blocks of Life. Cells the Building Blocks of Life. Cells the Building Blocks of Life
 1 What is gestation? 2 Give three ways in which alveoli are adapted for gas exchange. 3 Describe what happens to someone when they suffer from an asthma attack. 4 What is meant by the term antagonistic
1 What is gestation? 2 Give three ways in which alveoli are adapted for gas exchange. 3 Describe what happens to someone when they suffer from an asthma attack. 4 What is meant by the term antagonistic
Chemistry CH3FP Unit Chemistry C3 Written Paper Monday 20 May pm to 2.30 pm For this paper you must have: Time allowed Instructions all
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark General Certificate of Secondary Education Foundation Tier June 2013 1 2 Chemistry
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark General Certificate of Secondary Education Foundation Tier June 2013 1 2 Chemistry
