Niels Marquardt Ruhr University Bochum & COMET NanoTec GmbH, Germany
|
|
|
- Austin Gibson
- 5 years ago
- Views:
Transcription
1 A mobile H 2 -solid-state tank based on highly porous nanomaterials for explosion proof storage of large amounts of hydrogen at high density, moderate pressure and room temperature Niels Marquardt Ruhr University Bochum & COMET NanoTec GmbH, Germany Abstract: Hydrogen has the potential to become one of the most important energy sources of the future and will also be a key pillar of the energy transition from fossile to alternative energy sources, due to its high energy content of up to 142 MJ/kg, exceeding that of gazoline by about a factor of 3. Since its combustion product is only pure water, it will make a significant contribution to global CO 2 reduction, and its application will create millions of new jobs and a huge business volume. The production of so-called "green hydrogen" by electrolysis of water using renewable energies is the most sustainable production process. A joint research project, is proposed here, which aims at the development of a light-weight, mobile and operational save tank for the storage of large quantities of hydrogen at very high density in a solid, nano-crystalline material. This new technique will replace the nowadays applied very energy-inefficient and hazardous technology of storage of H 2 gas at the extreme pressure of 700 bar. As novel H 2 storage solid nano-material a 3D-multilayer graphene-on-diamond heterostructure, doped with light elements, has been chosen with especially high thermal conductivity. Due to its very large nano-porosity, corresponding to an extreme specific surface area, this superlight material behaves like a H 2 absorbing sponge, very suitable for its reversible and high-density storage March th European Networking Event, Airporthotel Düsseldorf - 1 -
2 Hydrogen is the fuel of the future Battery-powered Electric Vehicles (BEV) long refueling time: min. 15 min, max. 8 hours limited range: ~200 km (during winter: ~90km) emission-free only if Alternative Energy (so-called "Green Energy") is used for the production of electrical current. H 2 -Compressed-Gas powered Fuel-Cell Electric Vehicles (FCEV) fast refueling time: ~ 5 min (6 kg H 2 ) limited range: ~ 300 km (with very large and expensive tanks) operational unsafe (risk of explosion! ) H2-CG storage is very energy inefficient technology emission-free only if H 2 is produced by Alternative Energies. Advantages of FCEV with H 2 -Storage Solid Material Tanks large driving range (at least 600 km with volume of gazoline tank) operational safe ( no risk of explosion! ) Toyota Mirai L = 4.89 m, M = 1850 kg 2x CG-Tanks: p = 700bar 5 kg H 2 Range ~500km V tank =2. 60 l, V trunk =36 l 114 kw-battery recharged by FC during driving ( 7-8 l H 2 O /100 km). HY4 Emission-Free, Light Aircraft (DLR) L=7.4m,wingspan=21.4m,M=1500kg; 1 st flight: 11/29/ high-pressure tanks: p = 350 bar, 8 kg H 2 Range: ~750 km Low-T. PEM-FC: 45kW (Co."Hydrogenics") Li-Ion battery: 21 kwh 4 passengers E-motor: 80 kw speed: <200 km/h Not suitable for future air traffic! The very high E requirement of large aircrafts can only be achieved by explosion-proof H 2 tanks March th European Networking Event, Airporthotel Düsseldorf - 2 -
3 The Hydrogen Storage Solid Material Tank Disadvantages of 700bar-H 2 -CG-Tank potential hazard due to the high pressure of 700bar-H 2 -CG-tanks when used by laymen danger of oxyhydrogen & pressure explosion, low energy density of H 2 -Compressed Gas, About 30 % of the stored energy are spent for compression of H 2 to high pressures, very voluminous and expensive C-fiber tanks. Advantages of the novel "HySSMat"- Tank moderate-pressure tank with H 2 explosionproof bound in a crystalline lattice structure. For escape of H 2 gas in case of a burst of tank vessel desorption energy is needed. HySSMat - Tank has about 6- to 8-times higher energy density than a H 2 -CG Tank. volume of the HySSMat-Tank is much smaller, about the same size as a normal gasoline tank March th European Networking Event, Airporthotel Düsseldorf - 3 -
4 Necessary Properties of a Novel Nanomaterial Required properties of solid-state storage material: high mech., chem. and thermal stability solid, inorganic 3-dimensional morphology (no organic materials, like MOFs ) no bulk powder (with its rather low thermal conductivity) super-light, sponge-like nano-crystalline molecular 3D-structure consisting of the light carbon atoms cage-like molecular structure nm-porousity and extremely large SSA ( >1000 m 2 g 1 ) superlight very high thermal conductivity of multi-layer graphene storage material and diamond waver as substrate ( graphene: ~5000 W/(mK) & diamond: ~2300 W/(mK) ) As H 2 Storage Solid Material with these optimum properties we have selected a multi-layer Graphene-on-Diamond Heterostructure March th European Networking Event, Airporthotel Düsseldorf - 4 -
5 H 2 Storage Materials HYDROGEN DENSITY H 2 -Speicherwerkstoffe Ref: A. Züttel, Materials for hydrogen storage, materialstoday, Septemper (2003), pp H MH x Metal-Nanocarbon Composites (catalytic destab.effects of CNT s) Complex Hydrides (reversible, physisorpt.) H coval -CH 2 - Non-reversible H 2 binding in highdensity compounds ( liquid hydro-carbons) 2015 DOE system target H 2 H 2 Phys. H 2 Gas Liqu. H DOE system target March th European Networking Event, Airporthotel Düsseldorf - 5 -
6 Diagram of Carbon Allotropes Fullerite Molecular states (covalent C) Graphite Graphene sp 2 Fullerene & CNT Molecules Crystalline states (Van-der-Waals C) Graphene Nanotubes C 60 Fullerene Nano-Crystalline Carbon Diamond sp 3 The spatial 5-component state diagram of carbon allotropes on two levels of a matter morphology, molecular (top) and crystalline (below). ( ref. : D.V. Schur et al., in "Carbon nanomaterials in clean energy hydrogen systems, Dordrecht, NL, Springer (2008) p ) March th European Networking Event, Airporthotel Düsseldorf - 6 -
7 Morphology of van-der-waals Heterostructures H-terminated Nano-Diamond Graphene on Hydrogen-terminated-Nanodiamond Heterostructure (schematic) (Fang Zhao, Sci. Reports 5:13771, DOI: /srep13771) Raman spectra graphene-h-nd graphene-nd H-ND ND Nano-Crystals of layers of graphene held together by van- der- Waals forces are deposited by CVD on both sides of nanodiamond wavers, forming a 3-dimensional Graphene-on-Diamond Fig. (left top): Doping of heterostructure. nano-crystalls with light atoms: carbon atoms are shown by blue spheres, boron atoms in yellow and nitrogen in purple. Instead of light elements also alloys or compounds can be intercalated between the C-layers : (a) hbn layers (hexagonal rings of alternating B and N, with strong covalent sp 2 bonds. (b) Layered oxides used for alloying, combining layers and intercalating of ions and molecules March th European Networking Event, Airporthotel Düsseldorf - 7 -
8 Credit: Berkeley Lab. Mg atoms Envelopped by Graphene O 2 H 2 H 2 O impermeable to graphene DOI: /ncomms10804 Graphene layer Mg cluster (acts as spacer) Graphene layer A graphene evelope of Mg prevents nanoparticles (Mg, B, N) to react with molecules from the air (H 2 O, O 2, CO 2 etc.) which would block H 2 from contacting the Mg surface, whereas the very thin oxide layer formed on Mg surface does not hinder MgH 2 formation. Schematically shown : H 2 diffusion pathways (blue) through gaps and pores of over-lapping graphene layers (dark brown) and through the ultra-thin oxide layer (O atoms in red) coating graphene-wrapped Mg particles (light brown). DOI: /acs.nanolett.7b March th European Networking Event, Airporthotel Düsseldorf - 8 -
9 Combined Heat & Hydrogen Storage Tanks Hydrogen-Storage Solid-Material Tank Fuel Cell Electric Vehicle Hydrogen Storage Solid-Material Tank Light-weight aluminum vessel with fiber-reinforced liner, filled with a stack of nano-diamond wavers, double-sided coated with multilayers of Graphene-on-Diamond Vander-Waals heterostructures (doped with light-elements, e.g. B, N, Mg ). PEM-Fuel-Cell Stack Heat Storage Tank H 2 Storage Tank Compact 3-component (FC,Heat, H 2 ) module connected by Heat Pumps backup battery (backup heater) Heat Storage Tank (Heat buffer) Latent heat-storage system of light-weight, highly porous, foam-like Phase Change Nano-Material (N-PCM) with very high thermal conductivity, which absorbs or releases heat very fast, when the PCM changes from solid to liquid and vice versa. Heat conduction is performed by nanocrystalline heatpipes with high thermal conductivity. The purpose of the Heat Tank is two-fold: (a) the H 2 absorption heat is used either for cold start of the FC or desorption of H 2 for generating electricity by FC (b) the FC operation heat is stored in the Heat Tank and used for H 2 desorption and generation of electricity by FC March th European Networking Event, Airporthotel Düsseldorf - 9 -
10 Summary A project has been presented for the development of a mobile, explosion proof, solid-state H 2 -storage tank based on a light, multi-layer nano-crystalline graphene-ondiamond hybrid-material to efficiently store hydrogen with high density. Due to the particular morphology and molecular structure of the chosen multi-layer Van-der-Waals-heterostructure material, both, non-covalent physisorption and a strong, covalent chemisorption forces are simultaneously responsible for a sufficiently large H-bond strength. Whereas the Van-der-Waals or nano-capillary forces of physisorption are enhanced by the nm- and sub-nm-enclosures and predicted by A.K. Geim to lead to enormous nano-capillary pressures (as high as 1GPa = ~10,000 atm) on trapped molecules, the strength of the strong, covalent forces causing light-element hydrogen formation can be adjusted by varying the concentration of the doped light elements. We are looking for partnerships with research institutions and companies, competent in nanomaterial and hydrogen technologies, which are interested in a collaboration March th European Networking Event, Airporthotel Düsseldorf
Hydrogen Storage in Metalfunctionalized
 Hydrogen Storage in Metalfunctionalized Graphene Stefan Heun NEST, Istituto Nanoscienze-CNR and Scuola Normale Superiore Pisa, Italy Outline Introduction to Hydrogen Storage Epitaxial Graphene Hydrogen
Hydrogen Storage in Metalfunctionalized Graphene Stefan Heun NEST, Istituto Nanoscienze-CNR and Scuola Normale Superiore Pisa, Italy Outline Introduction to Hydrogen Storage Epitaxial Graphene Hydrogen
NanoEngineering of Hybrid Carbon Nanotube Metal Composite Materials for Hydrogen Storage Anders Nilsson
 NanoEngineering of Hybrid Carbon Nanotube Metal Composite Materials for Hydrogen Storage Anders Nilsson Stanford Synchrotron Radiation Laboratory (SSRL) and Stockholm University Coworkers and Ackowledgement
NanoEngineering of Hybrid Carbon Nanotube Metal Composite Materials for Hydrogen Storage Anders Nilsson Stanford Synchrotron Radiation Laboratory (SSRL) and Stockholm University Coworkers and Ackowledgement
CVD growth of Graphene. SPE ACCE presentation Carter Kittrell James M. Tour group September 9 to 11, 2014
 CVD growth of Graphene SPE ACCE presentation Carter Kittrell James M. Tour group September 9 to 11, 2014 Graphene zigzag armchair History 1500: Pencil-Is it made of lead? 1789: Graphite 1987: The first
CVD growth of Graphene SPE ACCE presentation Carter Kittrell James M. Tour group September 9 to 11, 2014 Graphene zigzag armchair History 1500: Pencil-Is it made of lead? 1789: Graphite 1987: The first
4.2.1 Chemical bonds, ionic, covalent and metallic
 4.2 Bonding, structure, and the properties of matter Chemists use theories of structure and bonding to explain the physical and chemical properties of materials. Analysis of structures shows that atoms
4.2 Bonding, structure, and the properties of matter Chemists use theories of structure and bonding to explain the physical and chemical properties of materials. Analysis of structures shows that atoms
IEA-HIA Task 32 Hydrogen-based Energy Storage Hydrogen storage in porous materials
 IEA-HIA Task 32 Hydrogen-based Energy Storage Hydrogen storage in porous materials Michael Hirscher Max Planck Institute for Intelligent Systems Stuttgart, Germany MH2018 November 1, 2018 Outline IEA Hydrogen
IEA-HIA Task 32 Hydrogen-based Energy Storage Hydrogen storage in porous materials Michael Hirscher Max Planck Institute for Intelligent Systems Stuttgart, Germany MH2018 November 1, 2018 Outline IEA Hydrogen
Introduction to Nanotechnology Chapter 5 Carbon Nanostructures Lecture 1
 Introduction to Nanotechnology Chapter 5 Carbon Nanostructures Lecture 1 ChiiDong Chen Institute of Physics, Academia Sinica chiidong@phys.sinica.edu.tw 02 27896766 Section 5.2.1 Nature of the Carbon Bond
Introduction to Nanotechnology Chapter 5 Carbon Nanostructures Lecture 1 ChiiDong Chen Institute of Physics, Academia Sinica chiidong@phys.sinica.edu.tw 02 27896766 Section 5.2.1 Nature of the Carbon Bond
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 492 DATE: JANUARY 1, 2018 PROJECT RP0508
 EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 492 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) DEFINITIONS:
EUROPEAN PATENT OFFICE U.S. PATENT AND TRADEMARK OFFICE CPC NOTICE OF CHANGES 492 The following classification changes will be effected by this Notice of Changes: Action Subclass Group(s) DEFINITIONS:
Hydrogen storage in nanotubes & nanostructures
 Hydrogen storage in nanotubes & nanostructures Over the last several years, a significant share of the scientific community has focused its attention on the hydrogen storage problem. Since 1997, when carbon
Hydrogen storage in nanotubes & nanostructures Over the last several years, a significant share of the scientific community has focused its attention on the hydrogen storage problem. Since 1997, when carbon
Materials and Structural Design for Advanced Energy Storage Devices
 Materials and Structural Design for Advanced Energy Storage Devices Imran Shakir Sustainable Energy Technologies Center (SET) King Saud University Saudi Arabia Specific Power (W/kg) Introduction and Motivation
Materials and Structural Design for Advanced Energy Storage Devices Imran Shakir Sustainable Energy Technologies Center (SET) King Saud University Saudi Arabia Specific Power (W/kg) Introduction and Motivation
Carbon Nanomaterials: Nanotubes and Nanobuds and Graphene towards new products 2030
 Carbon Nanomaterials: Nanotubes and Nanobuds and Graphene towards new products 2030 Prof. Dr. Esko I. Kauppinen Helsinki University of Technology (TKK) Espoo, Finland Forecast Seminar February 13, 2009
Carbon Nanomaterials: Nanotubes and Nanobuds and Graphene towards new products 2030 Prof. Dr. Esko I. Kauppinen Helsinki University of Technology (TKK) Espoo, Finland Forecast Seminar February 13, 2009
1.1. Storage of hydrogen
 1.1. Storage of hydrogen 1.1.1. Why to store hydrogen? Three isotopes of hydrogen are known, hydrogen or protium (H), deuterium (D), and the unstable tritium (T). All the isotopes of hydrogen form covalent
1.1. Storage of hydrogen 1.1.1. Why to store hydrogen? Three isotopes of hydrogen are known, hydrogen or protium (H), deuterium (D), and the unstable tritium (T). All the isotopes of hydrogen form covalent
4.2 Bonding, structure, and the properties of matter
 4.2 Bonding, structure, and the properties of matter Chemists use theories of structure and bonding to explain the physical and chemical properties of materials. Analysis of structures shows that atoms
4.2 Bonding, structure, and the properties of matter Chemists use theories of structure and bonding to explain the physical and chemical properties of materials. Analysis of structures shows that atoms
Sustainable Hydrogen and Electrical Energy Storage 6. F.M. Mulder & M. Wagemaker
 Sustainable Hydrogen and Electrical Energy Storage 6 F.M. Mulder & M. Wagemaker 1 Comparison liquid and gaseous H 2 with other liquid fuels Natural gas gasoline Volumetric energy density H 2 is lower than
Sustainable Hydrogen and Electrical Energy Storage 6 F.M. Mulder & M. Wagemaker 1 Comparison liquid and gaseous H 2 with other liquid fuels Natural gas gasoline Volumetric energy density H 2 is lower than
THEORETICAL INVESTIGATIONS ON NANOPOROUS MATERIALS AND IONIC LIQUIDS FOR ENERGY STORAGE
 1 THEORETICAL INVESTIGATIONS ON NANOPOROUS MATERIALS AND IONIC LIQUIDS FOR ENERGY STORAGE A Dissertation by MOUSUMI MANI BISWAS Submitted to the Office of Graduate Studies of Texas A&M University in partial
1 THEORETICAL INVESTIGATIONS ON NANOPOROUS MATERIALS AND IONIC LIQUIDS FOR ENERGY STORAGE A Dissertation by MOUSUMI MANI BISWAS Submitted to the Office of Graduate Studies of Texas A&M University in partial
Designing Graphene for Hydrogen Storage
 Designing Graphene for Hydrogen Storage Stefan Heun NEST, Istituto Nanoscienze-CNR and Scuola Normale Superiore Pisa, Italy Outline Introduction to Hydrogen Storage Epitaxial Graphene Hydrogen Storage
Designing Graphene for Hydrogen Storage Stefan Heun NEST, Istituto Nanoscienze-CNR and Scuola Normale Superiore Pisa, Italy Outline Introduction to Hydrogen Storage Epitaxial Graphene Hydrogen Storage
Synthesis and Characterization of Exfoliated Graphite (EG) and to Use it as a Reinforcement in Zn-based Metal Matrix Composites
 Synthesis and Characterization of Exfoliated Graphite (EG) and to Use it as a Reinforcement in Zn-based Metal Matrix Composites Here H 2 SO 4 was used as an intercalant and H 2 O 2 as an oxidant. Expandable
Synthesis and Characterization of Exfoliated Graphite (EG) and to Use it as a Reinforcement in Zn-based Metal Matrix Composites Here H 2 SO 4 was used as an intercalant and H 2 O 2 as an oxidant. Expandable
High H2 Adsorption by Coordination Framework Materials
 Arianna Marchioro Florian Degueldre High H2 Adsorption by Coordination Framework Materials Xiang Lin, Junhua Jia, Xuebo Zhao, K. Mark Thomas, Alexender J. Black, Gavin S. Walker, Neil R. Champness, Peter
Arianna Marchioro Florian Degueldre High H2 Adsorption by Coordination Framework Materials Xiang Lin, Junhua Jia, Xuebo Zhao, K. Mark Thomas, Alexender J. Black, Gavin S. Walker, Neil R. Champness, Peter
Metal-functionalized Graphene for Hydrogen Storage
 Metal-functionalized Graphene for Hydrogen Storage Stefan Heun NEST, Istituto Nanoscienze-CNR and Scuola Normale Superiore Pisa, Italy NEST Pisa Research themes @ NEST Pisa National Enterprise for nanoscience
Metal-functionalized Graphene for Hydrogen Storage Stefan Heun NEST, Istituto Nanoscienze-CNR and Scuola Normale Superiore Pisa, Italy NEST Pisa Research themes @ NEST Pisa National Enterprise for nanoscience
Application Challenges for Nanostructured Porous Materials
 Application Challenges for Nanostructured Porous Materials Dr Liz Rowsell Director, Johnson Matthey Technology Centre 1 Paradigm Shift in Automotive NOx Control Catalysts A paradigm shift describes a fundamental
Application Challenges for Nanostructured Porous Materials Dr Liz Rowsell Director, Johnson Matthey Technology Centre 1 Paradigm Shift in Automotive NOx Control Catalysts A paradigm shift describes a fundamental
Carbon Nanofibers as a Hydrogen Storage Medium for Fuel Cell Applications in the Transportation Sector
 Carbon Nanofibers as a Hydrogen Storage Medium for Fuel Cell Applications in the Transportation Sector Robert Zeches December 4, 2002 Submitted in partial fulfillment of course requirements for MatE 115,
Carbon Nanofibers as a Hydrogen Storage Medium for Fuel Cell Applications in the Transportation Sector Robert Zeches December 4, 2002 Submitted in partial fulfillment of course requirements for MatE 115,
Carbon 1 of 19 Boardworks Ltd 2016
 Carbon 1 of 19 Boardworks Ltd 2016 Carbon 2 of 19 Boardworks Ltd 2016 The carbon atom 3 of 19 Boardworks Ltd 2016 Carbon is a non-metallic element found in group 4 of the periodic table. It has 6 electrons,
Carbon 1 of 19 Boardworks Ltd 2016 Carbon 2 of 19 Boardworks Ltd 2016 The carbon atom 3 of 19 Boardworks Ltd 2016 Carbon is a non-metallic element found in group 4 of the periodic table. It has 6 electrons,
AQA Chemistry GCSE. Topic 2 - Bonding, Structure and the Properties of Matter. Flashcards.
 AQA Chemistry GCSE Topic 2 - Bonding, Structure and the Properties of Matter Flashcards What is ionic bonding? What is ionic bonding? Ionic bonding is the electrostatic attraction between positive and
AQA Chemistry GCSE Topic 2 - Bonding, Structure and the Properties of Matter Flashcards What is ionic bonding? What is ionic bonding? Ionic bonding is the electrostatic attraction between positive and
IV.D.2 Hydrogen Storage Materials for Fuel Cell-Powered Vehicles
 IV.D.2 Hydrogen Storage Materials for Fuel Cell-Powered Vehicles Andrew Goudy Delaware State University 2 N. Dupont Highway Dover, DE 99 Phone: (32) 857-6534 Email: agoudy@desu.edu DOE Managers Ned Stetson
IV.D.2 Hydrogen Storage Materials for Fuel Cell-Powered Vehicles Andrew Goudy Delaware State University 2 N. Dupont Highway Dover, DE 99 Phone: (32) 857-6534 Email: agoudy@desu.edu DOE Managers Ned Stetson
WJEC England GCSE Chemistry. Topic 5: Bonding, structure and properties. Notes. (Content in bold is for Higher Tier only)
 WJEC England GCSE Chemistry Topic 5: Bonding, structure and properties Notes (Content in bold is for Higher Tier only) Chemical bonds Compounds - substances in which 2 or more elements are chemically combined.
WJEC England GCSE Chemistry Topic 5: Bonding, structure and properties Notes (Content in bold is for Higher Tier only) Chemical bonds Compounds - substances in which 2 or more elements are chemically combined.
Raman studies on potential hydrogen storage materials
 Raman studies on potential hydrogen storage materials The Hydrogen & Fuel Cell Researcher Conference University of Birmingham 17 th December 2013 Daniel Reed, David Book School of Metallurgy and Materials
Raman studies on potential hydrogen storage materials The Hydrogen & Fuel Cell Researcher Conference University of Birmingham 17 th December 2013 Daniel Reed, David Book School of Metallurgy and Materials
Chemistry (separate) for November PPE
 1.1 Elements and 1.2 Atoms, formulae and Chapter 1 Atomic Structure and Periodic Table Identify symbols of elements from the periodic table Recognise the properties of elements and. Identify the elements
1.1 Elements and 1.2 Atoms, formulae and Chapter 1 Atomic Structure and Periodic Table Identify symbols of elements from the periodic table Recognise the properties of elements and. Identify the elements
OCR A GCSE Chemistry. Topic 2: Elements, compounds and mixtures. Properties of materials. Notes.
 OCR A GCSE Chemistry Topic 2: Elements, compounds and mixtures Properties of materials Notes C2.3a recall that carbon can form four covalent bonds C2.3b explain that the vast array of natural and synthetic
OCR A GCSE Chemistry Topic 2: Elements, compounds and mixtures Properties of materials Notes C2.3a recall that carbon can form four covalent bonds C2.3b explain that the vast array of natural and synthetic
Yiping Zhao and Yuping He
 Yiping Zhao and Yuping He Department of Physics and Astronomy Nanoscale Science and Engineering Center The University of Georgia, Athens, GA 30602 April 29, 2010 Outline: Introduction Hydrogenation behaviors
Yiping Zhao and Yuping He Department of Physics and Astronomy Nanoscale Science and Engineering Center The University of Georgia, Athens, GA 30602 April 29, 2010 Outline: Introduction Hydrogenation behaviors
Ab Initio Study of Hydrogen Storage on CNT
 Ab Initio Study of Hydrogen Storage on CNT Zhiyong Zhang, Henry Liu, and KJ Cho Stanford University Presented at the ICNT 2005, San Francisco Financial Support: GCEP (Global Climate and Energy Project)
Ab Initio Study of Hydrogen Storage on CNT Zhiyong Zhang, Henry Liu, and KJ Cho Stanford University Presented at the ICNT 2005, San Francisco Financial Support: GCEP (Global Climate and Energy Project)
Hydrogen storage in boron nitride and carbon nanomaterials studied by TG/DTA and molecular orbital calculations
 ydrogen storage in boron nitride and carbon nanomaterials studied by TG/DTA and molecular orbital calculations Takeo Oku Department of Materials Science, The University of Shiga Prefecture, assaka 25,
ydrogen storage in boron nitride and carbon nanomaterials studied by TG/DTA and molecular orbital calculations Takeo Oku Department of Materials Science, The University of Shiga Prefecture, assaka 25,
The Raman Spectroscopy of Graphene and the Determination of Layer Thickness
 Application Note: 52252 The Raman Spectroscopy of Graphene and the Determination of Layer Thickness Mark Wall, Ph.D., Thermo Fisher Scientific, Madison, WI, USA Key Words DXR Raman Microscope 2D Band D
Application Note: 52252 The Raman Spectroscopy of Graphene and the Determination of Layer Thickness Mark Wall, Ph.D., Thermo Fisher Scientific, Madison, WI, USA Key Words DXR Raman Microscope 2D Band D
National 5 Chemistry
 St Ninian s High School Chemistry Department National 5 Chemistry Unit 1: Chemical Changes & Structure Section 3: Bonding & Properties of Substances Summary Notes Name Learning Outcomes After completing
St Ninian s High School Chemistry Department National 5 Chemistry Unit 1: Chemical Changes & Structure Section 3: Bonding & Properties of Substances Summary Notes Name Learning Outcomes After completing
GCSE CHEMISTRY REVISION LIST
 GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
Chapter 2. Atomic Structure
 Chapter 2 Atomic Structure 2 6 (a) Aluminum foil used for storing food weighs about 0. g per square cm. How many atoms of aluminum are contained in one 6.25 cm 2 size of foil? (b) Using the densities and
Chapter 2 Atomic Structure 2 6 (a) Aluminum foil used for storing food weighs about 0. g per square cm. How many atoms of aluminum are contained in one 6.25 cm 2 size of foil? (b) Using the densities and
1. Reactions can be followed by measuring changes in concentration, mass and volume of reactants and products.
 Higher Chemistry - Traffic Lights Unit 1 CHEMICAL CHANGES AND STRUCTURE I know: Controlling the rate Collision theory and relative rates 1. Reactions can be followed by measuring changes in concentration,
Higher Chemistry - Traffic Lights Unit 1 CHEMICAL CHANGES AND STRUCTURE I know: Controlling the rate Collision theory and relative rates 1. Reactions can be followed by measuring changes in concentration,
Energy Storage. Light-emitting. Nano-Carbons. H 2 Energy. CNT synthesis. Graphene synthesis Top-down. Solar H 2 generation
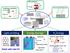 Nano-Carbon battery Graphene synthesis Top-down CNT synthesis CVD reactor hydrocarbon gas Catalyst CNTs Chemical Modification COO O NO 2 COO COO COO Bottom-up O O NO NO 2 2 COO COO Nano-Carbons 20 nm Light-emitting
Nano-Carbon battery Graphene synthesis Top-down CNT synthesis CVD reactor hydrocarbon gas Catalyst CNTs Chemical Modification COO O NO 2 COO COO COO Bottom-up O O NO NO 2 2 COO COO Nano-Carbons 20 nm Light-emitting
Chapter 14. Liquids and Solids
 Chapter 14 Liquids and Solids Section 14.1 Water and Its Phase Changes Reviewing What We Know Gases Low density Highly compressible Fill container Solids High density Slightly compressible Rigid (keeps
Chapter 14 Liquids and Solids Section 14.1 Water and Its Phase Changes Reviewing What We Know Gases Low density Highly compressible Fill container Solids High density Slightly compressible Rigid (keeps
Hydrogen as fuel carrier in PEM fuelcell for automobile applications
 IOP Conference Series: Materials Science and Engineering OPEN ACCESS Hydrogen as fuel carrier in PEM fuelcell for automobile applications To cite this article: Mudassir Ali Sk et al 2015 IOP Conf. Ser.:
IOP Conference Series: Materials Science and Engineering OPEN ACCESS Hydrogen as fuel carrier in PEM fuelcell for automobile applications To cite this article: Mudassir Ali Sk et al 2015 IOP Conf. Ser.:
Chapter 1 Introduction
 Chapter 1 Introduction In our planet carbon forms the basis of all organic molecules which makes it the most important element of life. It is present in over 95% of the known chemical compounds overall
Chapter 1 Introduction In our planet carbon forms the basis of all organic molecules which makes it the most important element of life. It is present in over 95% of the known chemical compounds overall
Introduction Fuel Cells Repetition
 Introduction Fuel Cells Repetition Fuel cell applications PEMFC PowerCell AB, (S1-S3) PEMFC,1-100 kw Toyota Mirai a Fuel Cell Car A look inside The hydrogen tank 1. Inside Layer of polymer closest to the
Introduction Fuel Cells Repetition Fuel cell applications PEMFC PowerCell AB, (S1-S3) PEMFC,1-100 kw Toyota Mirai a Fuel Cell Car A look inside The hydrogen tank 1. Inside Layer of polymer closest to the
Structure and Types of Solids
 Properties, type and strength of bonding: Properties could be physical or chemical Structure and Types of Solids Physical Properties M.p., b.p., latent heat, solubility in water and other solvents, conductivity
Properties, type and strength of bonding: Properties could be physical or chemical Structure and Types of Solids Physical Properties M.p., b.p., latent heat, solubility in water and other solvents, conductivity
Basic Semiconductor Physics
 6 Basic Semiconductor Physics 6.1 Introduction With this chapter we start with the discussion of some important concepts from semiconductor physics, which are required to understand the operation of solar
6 Basic Semiconductor Physics 6.1 Introduction With this chapter we start with the discussion of some important concepts from semiconductor physics, which are required to understand the operation of solar
The Solid State. Phase diagrams Crystals and symmetry Unit cells and packing Types of solid
 The Solid State Phase diagrams Crystals and symmetry Unit cells and packing Types of solid Learning objectives Apply phase diagrams to prediction of phase behaviour Describe distinguishing features of
The Solid State Phase diagrams Crystals and symmetry Unit cells and packing Types of solid Learning objectives Apply phase diagrams to prediction of phase behaviour Describe distinguishing features of
On the Possible new High Temperature Superconductors. Zhi Cheng (9 Bairong st. Baiyun District, Guangzhou, China
 On the Possible new High Temperature Superconductors Zhi Cheng (9 Bairong st. Baiyun District, Guangzhou, China. 510400. gzchengzhi@hotmail.com) Abstract: It shows that the hybrid graphene may be the high
On the Possible new High Temperature Superconductors Zhi Cheng (9 Bairong st. Baiyun District, Guangzhou, China. 510400. gzchengzhi@hotmail.com) Abstract: It shows that the hybrid graphene may be the high
Conference Return Seminar- NANO2014,Moscow State University,Moscow,Russia Date: th July 2014
 Conference Return Seminar- NANO2014,Moscow State University,Moscow,Russia Date:13-1818 th July 2014 An electrochemical method for the synthesis of single and few layers graphene sheets for high temperature
Conference Return Seminar- NANO2014,Moscow State University,Moscow,Russia Date:13-1818 th July 2014 An electrochemical method for the synthesis of single and few layers graphene sheets for high temperature
Imaging Carbon materials with correlative Raman-SEM microscopy. Introduction. Raman, SEM and FIB within one chamber. Diamond.
 Imaging Carbon materials with correlative Raman-SEM microscopy Application Example Carbon materials are widely used in many industries for their exceptional properties. Electric conductance, light weight,
Imaging Carbon materials with correlative Raman-SEM microscopy Application Example Carbon materials are widely used in many industries for their exceptional properties. Electric conductance, light weight,
In today s lecture, we will cover:
 In today s lecture, we will cover: Metal and Metal oxide Nanoparticles Semiconductor Nanocrystals Carbon Nanotubes 1 Week 2: Nanoparticles Goals for this section Develop an understanding of the physical
In today s lecture, we will cover: Metal and Metal oxide Nanoparticles Semiconductor Nanocrystals Carbon Nanotubes 1 Week 2: Nanoparticles Goals for this section Develop an understanding of the physical
PROPERTIES OF SOLIDS SCH4U1
 PROPERTIES OF SOLIDS SCH4U1 Intra vs. Intermolecular Bonds The properties of a substance are influenced by the force of attraction within and between the molecules. Intra vs. Intermolecular Bonds Intramolecular
PROPERTIES OF SOLIDS SCH4U1 Intra vs. Intermolecular Bonds The properties of a substance are influenced by the force of attraction within and between the molecules. Intra vs. Intermolecular Bonds Intramolecular
Optimization of MnO2 Electrodeposits using Graphenated Carbon Nanotube Electrodes for Supercapacitors
 Optimization of MnO2 Electrodeposits using Graphenated Carbon Nanotube Electrodes for Supercapacitors Waleed Nusrat, 100425398 PHY 3090U Material Science Thursday April 9 th 2015 Researchers optimize the
Optimization of MnO2 Electrodeposits using Graphenated Carbon Nanotube Electrodes for Supercapacitors Waleed Nusrat, 100425398 PHY 3090U Material Science Thursday April 9 th 2015 Researchers optimize the
Supplementary Figure 1 A schematic representation of the different reaction mechanisms
 Supplementary Figure 1 A schematic representation of the different reaction mechanisms observed in electrode materials for lithium batteries. Black circles: voids in the crystal structure, blue circles:
Supplementary Figure 1 A schematic representation of the different reaction mechanisms observed in electrode materials for lithium batteries. Black circles: voids in the crystal structure, blue circles:
Chemistry: The Central Science. Chapter 20: Electrochemistry
 Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Supplementary Information
 Supplementary Information Supplementary Figure 1. fabrication. A schematic of the experimental setup used for graphene Supplementary Figure 2. Emission spectrum of the plasma: Negative peaks indicate an
Supplementary Information Supplementary Figure 1. fabrication. A schematic of the experimental setup used for graphene Supplementary Figure 2. Emission spectrum of the plasma: Negative peaks indicate an
Adsorption Processes. Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad
 Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
RESEARCH ON BENZENE VAPOR DETECTION USING POROUS SILICON
 Section Micro and Nano Technologies RESEARCH ON BENZENE VAPOR DETECTION USING POROUS SILICON Assoc. Prof. Ersin Kayahan 1,2,3 1 Kocaeli University, Electro-optic and Sys. Eng. Umuttepe, 41380, Kocaeli-Turkey
Section Micro and Nano Technologies RESEARCH ON BENZENE VAPOR DETECTION USING POROUS SILICON Assoc. Prof. Ersin Kayahan 1,2,3 1 Kocaeli University, Electro-optic and Sys. Eng. Umuttepe, 41380, Kocaeli-Turkey
- intermolecular forces forces that exist between molecules
 Chapter 11: Intermolecular Forces, Liquids, and Solids - intermolecular forces forces that exist between molecules 11.1 A Molecular Comparison of Liquids and Solids - gases - average kinetic energy of
Chapter 11: Intermolecular Forces, Liquids, and Solids - intermolecular forces forces that exist between molecules 11.1 A Molecular Comparison of Liquids and Solids - gases - average kinetic energy of
Graphene The Search For Two Dimensions. Christopher Scott Friedline Arizona State University
 Graphene The Search For Two Dimensions Christopher Scott Friedline Arizona State University What Is Graphene? Single atomic layer of graphite arranged in a honeycomb crystal lattice Consists of sp 2 -bonded
Graphene The Search For Two Dimensions Christopher Scott Friedline Arizona State University What Is Graphene? Single atomic layer of graphite arranged in a honeycomb crystal lattice Consists of sp 2 -bonded
Personalised Learning Checklists AQA Chemistry Paper 1
 AQA Chemistry (8462) from 2016 Topics C4.1 Atomic structure and the periodic table State that everything is made of atoms and recall what they are Describe what elements and compounds are State that elements
AQA Chemistry (8462) from 2016 Topics C4.1 Atomic structure and the periodic table State that everything is made of atoms and recall what they are Describe what elements and compounds are State that elements
Understanding Irreducible and Reducible Oxides as Catalysts for Carbon Nanotubes and Graphene Formation
 Wright State University CORE Scholar Special Session 5: Carbon and Oxide Based Nanostructured Materials (2011) Special Session 5 6-2011 Understanding Irreducible and Reducible Oxides as Catalysts for Carbon
Wright State University CORE Scholar Special Session 5: Carbon and Oxide Based Nanostructured Materials (2011) Special Session 5 6-2011 Understanding Irreducible and Reducible Oxides as Catalysts for Carbon
ORGANIC SEMICONDUCTOR 3,4,9,10-Perylenetetracarboxylic dianhydride (PTCDA)
 ORGANIC SEMICONDUCTOR 3,4,9,10-Perylenetetracarboxylic dianhydride (PTCDA) Suvranta Tripathy Department of Physics University of Cincinnati Cincinnati, Ohio 45221 March 8, 2002 Abstract In the last decade
ORGANIC SEMICONDUCTOR 3,4,9,10-Perylenetetracarboxylic dianhydride (PTCDA) Suvranta Tripathy Department of Physics University of Cincinnati Cincinnati, Ohio 45221 March 8, 2002 Abstract In the last decade
Introduction to Nanotechnology Chapter 5 Carbon Nanostructures Lecture 1
 Introduction to Nanotechnology Chapter 5 Carbon Nanostructures Lecture 1 ChiiDong Chen Institute of Physics, Academia Sinica chiidong@phys.sinica.edu.tw 02 27896766 Carbon contains 6 electrons: (1s) 2,
Introduction to Nanotechnology Chapter 5 Carbon Nanostructures Lecture 1 ChiiDong Chen Institute of Physics, Academia Sinica chiidong@phys.sinica.edu.tw 02 27896766 Carbon contains 6 electrons: (1s) 2,
4.2 Bonding, structure and the properties of matter. GCSE Chemistry
 4.2 Bonding, structure and the properties of matter GCSE Chemistry 4.2.1 Chemical bonds, ionic, covalent and metallic There are three types of strong chemical bond ionic, covalent and metallic. There are
4.2 Bonding, structure and the properties of matter GCSE Chemistry 4.2.1 Chemical bonds, ionic, covalent and metallic There are three types of strong chemical bond ionic, covalent and metallic. There are
Solutions for Assignment-8
 Solutions for Assignment-8 Q1. The process of adding impurities to a pure semiconductor is called: [1] (a) Mixing (b) Doping (c) Diffusing (d) None of the above In semiconductor production, doping intentionally
Solutions for Assignment-8 Q1. The process of adding impurities to a pure semiconductor is called: [1] (a) Mixing (b) Doping (c) Diffusing (d) None of the above In semiconductor production, doping intentionally
Nanotechnology 5 th lecture
 Nanotechnology 5 th lecture (c) http://www.nccr-nano.org/nccr_data/ gallery/gallery_01/gallery_01_03/pics_06/ internet/nanotube_spiral.jpg Plan for today: http://www.nccr-nano.org/nccr_data/gallery/ gallery_01/gallery_01_03/pics_04/internet/
Nanotechnology 5 th lecture (c) http://www.nccr-nano.org/nccr_data/ gallery/gallery_01/gallery_01_03/pics_06/ internet/nanotube_spiral.jpg Plan for today: http://www.nccr-nano.org/nccr_data/gallery/ gallery_01/gallery_01_03/pics_04/internet/
Shedding New Light on Materials Science with Raman Imaging
 Shedding New Light on Materials Science with Raman Imaging Robert Heintz, Ph.D. Senior Applications Specialist 1 The world leader in serving science Raman Imaging Provides More Information Microscope problems
Shedding New Light on Materials Science with Raman Imaging Robert Heintz, Ph.D. Senior Applications Specialist 1 The world leader in serving science Raman Imaging Provides More Information Microscope problems
Nanostructured complex hydride systems. for solid state hydrogen storage
 Nanostructured complex hydride systems for solid state hydrogen storage by Minchul Jang A thesis presented to the University of Waterloo in fulfilment of the thesis requirement for the degree of Doctor
Nanostructured complex hydride systems for solid state hydrogen storage by Minchul Jang A thesis presented to the University of Waterloo in fulfilment of the thesis requirement for the degree of Doctor
Carbon Nanotubes as Future Energy Storage System
 Carbon Nanotubes as Future Energy Storage System V Vasu, D Silambarasan To cite this version: V Vasu, D Silambarasan. Carbon Nanotubes as Future Energy Storage System. Mechanics, Materials Science Engineering
Carbon Nanotubes as Future Energy Storage System V Vasu, D Silambarasan To cite this version: V Vasu, D Silambarasan. Carbon Nanotubes as Future Energy Storage System. Mechanics, Materials Science Engineering
UNIT 1 CHEMISTRY. How Can the Diversity of Materials Be Explained?
 UNIT 1 CHEMISTRY How Can the Diversity of Materials Be Explained? AoS 1: How Can the Knowledge of Elements Explain the Properties of Matter? AoS 2: How Can the Versatility of Non-Metals be Explained? AoS
UNIT 1 CHEMISTRY How Can the Diversity of Materials Be Explained? AoS 1: How Can the Knowledge of Elements Explain the Properties of Matter? AoS 2: How Can the Versatility of Non-Metals be Explained? AoS
Nanotech for CO2-free energy generation
 Nanotech for CO2-free energy generation - Nano for efficiency - Werner Hoheisel Bayer Technology Services GmbH Bayer Fona, Berlin 2008-09-24 Bayer s contribution to reach the challenging climate targets
Nanotech for CO2-free energy generation - Nano for efficiency - Werner Hoheisel Bayer Technology Services GmbH Bayer Fona, Berlin 2008-09-24 Bayer s contribution to reach the challenging climate targets
Shapes of Molecules & Carbon Allotropes. By: Mahmoud Taha Special thanks to Ms Williams and Ms Matrella for their constant support and inspiration
 Shapes of Molecules & Carbon Allotropes By: Mahmoud Taha Special thanks to Ms Williams and Ms Matrella for their constant support and inspiration Please note that these guides are a collation of my personal
Shapes of Molecules & Carbon Allotropes By: Mahmoud Taha Special thanks to Ms Williams and Ms Matrella for their constant support and inspiration Please note that these guides are a collation of my personal
Computational Investigations of the Adsorption of Molecular Hydrogen on Graphene-Based Nanopore Model
 Wright State University CORE Scholar Browse all Theses and Dissertations Theses and Dissertations 2012 Computational Investigations of the Adsorption of Molecular Hydrogen on Graphene-Based Nanopore Model
Wright State University CORE Scholar Browse all Theses and Dissertations Theses and Dissertations 2012 Computational Investigations of the Adsorption of Molecular Hydrogen on Graphene-Based Nanopore Model
NEM Relays Using 2-Dimensional Nanomaterials for Low Energy Contacts
 NEM Relays Using 2-Dimensional Nanomaterials for Low Energy Contacts Seunghyun Lee, Ji Cao 10/29/2013 A Science & Technology Professor H. -S. Philip Wong Electrical Engineering, Stanford University Center
NEM Relays Using 2-Dimensional Nanomaterials for Low Energy Contacts Seunghyun Lee, Ji Cao 10/29/2013 A Science & Technology Professor H. -S. Philip Wong Electrical Engineering, Stanford University Center
4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes. Unit 1 Unit 2 Unit 3. C2.1.1a Structure and bonding
 Summary of changes This resource outlines the main changes that have been made to the assessment and subject content from our previous GCSE Chemistry (4402) to the new specification (8462). Our new specifications
Summary of changes This resource outlines the main changes that have been made to the assessment and subject content from our previous GCSE Chemistry (4402) to the new specification (8462). Our new specifications
2. As gas P increases and/or T is lowered, intermolecular forces become significant, and deviations from ideal gas laws occur (van der Waal equation).
 A. Introduction. (Section 11.1) CHAPTER 11: STATES OF MATTER, LIQUIDS AND SOLIDS 1. Gases are easily treated mathematically because molecules behave independently. 2. As gas P increases and/or T is lowered,
A. Introduction. (Section 11.1) CHAPTER 11: STATES OF MATTER, LIQUIDS AND SOLIDS 1. Gases are easily treated mathematically because molecules behave independently. 2. As gas P increases and/or T is lowered,
Supplementary Figures
 Supplementary Figures Supplementary Figure 1: Microstructure, morphology and chemical composition of the carbon microspheres: (a) A SEM image of the CM-NFs; and EDS spectra of CM-NFs (b), CM-Ns (d) and
Supplementary Figures Supplementary Figure 1: Microstructure, morphology and chemical composition of the carbon microspheres: (a) A SEM image of the CM-NFs; and EDS spectra of CM-NFs (b), CM-Ns (d) and
Chapter 10. Liquids and Solids
 Chapter 10 Liquids and Solids Chapter 10 Table of Contents 10.1 Intermolecular Forces 10.2 The Liquid State 10.3 An Introduction to Structures and Types of Solids 10.4 Structure and Bonding in Metals 10.5
Chapter 10 Liquids and Solids Chapter 10 Table of Contents 10.1 Intermolecular Forces 10.2 The Liquid State 10.3 An Introduction to Structures and Types of Solids 10.4 Structure and Bonding in Metals 10.5
Direct Line-of-site Gas Desorption Study of LiBH 4 in Nanoporous Carbons: The Size Effect
 Direct Line-of-site Gas Desorption Study of LiBH 4 in Nanoporous Carbons: The Size Effect David Peaslee Center for Nanoscience and Department of Physics and Astronomy University of Missouri St. Louis Advised
Direct Line-of-site Gas Desorption Study of LiBH 4 in Nanoporous Carbons: The Size Effect David Peaslee Center for Nanoscience and Department of Physics and Astronomy University of Missouri St. Louis Advised
SWCNTs Single Wall Carbon Nanotubes
 Carbon Nanotubes - CNTs 1 SWCNTs Single Wall Carbon Nanotubes 2 Carbon Nanotubes - Growth 3 Carbon Nanotubes Building Principles 4 Carbon Nanotubes Building Principle 5 Carbon Nanotubes Building Principle
Carbon Nanotubes - CNTs 1 SWCNTs Single Wall Carbon Nanotubes 2 Carbon Nanotubes - Growth 3 Carbon Nanotubes Building Principles 4 Carbon Nanotubes Building Principle 5 Carbon Nanotubes Building Principle
GECP Hydrogen Project: "Nanomaterials Engineering for Hydrogen Storage"
 GECP Hydrogen Project: "Nanomaterials Engineering for Hydrogen Storage" PI: KJ Cho Students and Staff Members: Zhiyong Zhang, Wei Xiao, Byeongchan Lee, Experimental Collaboration: H. Dai, B. Clemens, A.
GECP Hydrogen Project: "Nanomaterials Engineering for Hydrogen Storage" PI: KJ Cho Students and Staff Members: Zhiyong Zhang, Wei Xiao, Byeongchan Lee, Experimental Collaboration: H. Dai, B. Clemens, A.
Chemistry in Biology. Section 1. Atoms, Elements, and Compounds
 Section 1 Atoms, Elements, and Compounds Atoms! Chemistry is the study of matter.! Atoms are the building blocks of matter.! Neutrons and protons are located at the center of the atom.! Protons are positively
Section 1 Atoms, Elements, and Compounds Atoms! Chemistry is the study of matter.! Atoms are the building blocks of matter.! Neutrons and protons are located at the center of the atom.! Protons are positively
Crystalline Solids have atoms arranged in an orderly repeating pattern. Amorphous Solids lack the order found in crystalline solids
 Ch 12: Solids and Modern Materials Learning goals and key skills: Classify solids base on bonding/intermolecular forces and understand how difference in bonding relates to physical properties Know the
Ch 12: Solids and Modern Materials Learning goals and key skills: Classify solids base on bonding/intermolecular forces and understand how difference in bonding relates to physical properties Know the
For more information, please contact: or +1 (302)
 Introduction Graphene Raman Analyzer: Carbon Nanomaterials Characterization Dawn Yang and Kristen Frano B&W Tek Carbon nanomaterials constitute a variety of carbon allotropes including graphene, graphene
Introduction Graphene Raman Analyzer: Carbon Nanomaterials Characterization Dawn Yang and Kristen Frano B&W Tek Carbon nanomaterials constitute a variety of carbon allotropes including graphene, graphene
AS LEVEL CHEMISTRY BONDING AND STRUCTURE PERIODICITY
 AS LEVEL CHEMISTRY BONDING AND STRUCTURE PERIODICITY Answer all questions Max 90 marks Name.. Mark../90...% Grade Paddington Academy 1 1. Draw a dot-and-cross diagram for CaCl 2. [Total 2 marks] 2. Magnesium
AS LEVEL CHEMISTRY BONDING AND STRUCTURE PERIODICITY Answer all questions Max 90 marks Name.. Mark../90...% Grade Paddington Academy 1 1. Draw a dot-and-cross diagram for CaCl 2. [Total 2 marks] 2. Magnesium
In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium salt to produce potassium
 Q1. This question is about potassium. (a) Humphrey Davy was a professor of chemistry. In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium
Q1. This question is about potassium. (a) Humphrey Davy was a professor of chemistry. In 1807 Davy did an electrolysis experiment to produce potassium. Davy first tried to electrolyse a solid potassium
Q1. The chart shows the processes involved in the manufacture of nitric acid from ammonia.
 Q1. The chart shows the processes involved in the manufacture of nitric acid from ammonia. (a) Complete the word equation for the reaction that takes place in the first reaction vessel. ammonia +... nitrogen
Q1. The chart shows the processes involved in the manufacture of nitric acid from ammonia. (a) Complete the word equation for the reaction that takes place in the first reaction vessel. ammonia +... nitrogen
Modelling hydrogen adsorption within spherical, cylindrical and slit-shaped cavities
 University of Wollongong Research Online Faculty of Informatics - Papers (Archive) Faculty of Engineering and Information Sciences 2009 Modelling hydrogen adsorption within spherical, cylindrical and slit-shaped
University of Wollongong Research Online Faculty of Informatics - Papers (Archive) Faculty of Engineering and Information Sciences 2009 Modelling hydrogen adsorption within spherical, cylindrical and slit-shaped
Nanomaterials for Solid State Hydrogen Storage
 Nanomaterials for Solid State Hydrogen Storage Fuel Cells and Hydrogen Energy Series Editor: Narottam P. Bansal NASA Glenn Research Center Cleveland, OH 44135 narottam.p.bansal@nasa.gov Aims and Scope
Nanomaterials for Solid State Hydrogen Storage Fuel Cells and Hydrogen Energy Series Editor: Narottam P. Bansal NASA Glenn Research Center Cleveland, OH 44135 narottam.p.bansal@nasa.gov Aims and Scope
Learning Model Answers Year 11 Double Chemistry
 1 Describe ionic bonding Describe covalent bonding Occurs between metals and non-metals. Electrons are transferred. There is an electrostatic force of attraction between oppositely charged ions. Occurs
1 Describe ionic bonding Describe covalent bonding Occurs between metals and non-metals. Electrons are transferred. There is an electrostatic force of attraction between oppositely charged ions. Occurs
Hydrogen Storage in Single- and Multi-walled Carbon Nanotubes and Nanotube Bundles
 Australian Journal of Basic and Applied Sciences, 5(7): 483-490, 2011 ISSN 1991-8178 Hydrogen Storage in Single- and Multi-walled Carbon Nanotubes and Nanotube Bundles 1 S. Hamidi and 2 H. Golnabi 1 Physics
Australian Journal of Basic and Applied Sciences, 5(7): 483-490, 2011 ISSN 1991-8178 Hydrogen Storage in Single- and Multi-walled Carbon Nanotubes and Nanotube Bundles 1 S. Hamidi and 2 H. Golnabi 1 Physics
Hydrogenation of Single Walled Carbon Nanotubes
 Hydrogenation of Single Walled Carbon Nanotubes Anders Nilsson Stanford Synchrotron Radiation Laboratory (SSRL) and Stockholm University Coworkers and Ackowledgement A. Nikitin 1), H. Ogasawara 1), D.
Hydrogenation of Single Walled Carbon Nanotubes Anders Nilsson Stanford Synchrotron Radiation Laboratory (SSRL) and Stockholm University Coworkers and Ackowledgement A. Nikitin 1), H. Ogasawara 1), D.
554 Chapter 10 Liquids and Solids
 554 Chapter 10 Liquids and Solids above 7376 kpa, CO 2 is a supercritical fluid, with properties of both gas and liquid. Like a gas, it penetrates deep into the coffee beans; like a liquid, it effectively
554 Chapter 10 Liquids and Solids above 7376 kpa, CO 2 is a supercritical fluid, with properties of both gas and liquid. Like a gas, it penetrates deep into the coffee beans; like a liquid, it effectively
Part 6- Chemistry Paper 1 Bonding Application Questions Triple Science
 Part 6- Chemistry Paper 1 Bonding Application Questions Triple Science How bonding and structure are related to the properties of substances A simple model of the atom, symbols, relative atomic mass, electronic
Part 6- Chemistry Paper 1 Bonding Application Questions Triple Science How bonding and structure are related to the properties of substances A simple model of the atom, symbols, relative atomic mass, electronic
MOFs: Metal organic frameworks
 MOFs: Metal organic frameworks BY KATRINA KRÄMER 27 APRIL 2017 A holey material that promises to solve fuel storage problems and store chemical information. Ben Valsler This week, looks at a versatile
MOFs: Metal organic frameworks BY KATRINA KRÄMER 27 APRIL 2017 A holey material that promises to solve fuel storage problems and store chemical information. Ben Valsler This week, looks at a versatile
performance electrocatalytic or electrochemical devices. Nanocrystals grown on graphene could have
 Nanocrystal Growth on Graphene with Various Degrees of Oxidation Hailiang Wang, Joshua Tucker Robinson, Georgi Diankov, and Hongjie Dai * Department of Chemistry and Laboratory for Advanced Materials,
Nanocrystal Growth on Graphene with Various Degrees of Oxidation Hailiang Wang, Joshua Tucker Robinson, Georgi Diankov, and Hongjie Dai * Department of Chemistry and Laboratory for Advanced Materials,
Growth of fullerene thin films and oxygen diffusion in fullerites (C 60 and C 70 )
 Growth of fullerene thin films and oxygen diffusion in fullerites (C 60 and C 70 ) Undergraduate project in solid state physics Supervisor: Dr. Eugene Katz Dept. of Solar Energy and Environmental Physics
Growth of fullerene thin films and oxygen diffusion in fullerites (C 60 and C 70 ) Undergraduate project in solid state physics Supervisor: Dr. Eugene Katz Dept. of Solar Energy and Environmental Physics
[1] Answer all the questions. Crude oil can be separated in the laboratory into fractions which have different boiling points.
![[1] Answer all the questions. Crude oil can be separated in the laboratory into fractions which have different boiling points. [1] Answer all the questions. Crude oil can be separated in the laboratory into fractions which have different boiling points.](/thumbs/80/82115448.jpg) Answer all the questions.. Crude oil can be separated in the laboratory into fractions which have different boiling points. Look at the table. It shows possible relationships between: boiling point number
Answer all the questions.. Crude oil can be separated in the laboratory into fractions which have different boiling points. Look at the table. It shows possible relationships between: boiling point number
Supporting Information
 Supporting Information MoSe2 embedded CNT-Reduced Graphene Oxide (rgo) Composite Microsphere with Superior Sodium Ion Storage and Electrocatalytic Hydrogen Evolution Performances Gi Dae Park, Jung Hyun
Supporting Information MoSe2 embedded CNT-Reduced Graphene Oxide (rgo) Composite Microsphere with Superior Sodium Ion Storage and Electrocatalytic Hydrogen Evolution Performances Gi Dae Park, Jung Hyun
Covalent bonding does not involve electrostatic attraction between oppositely charged particles.
 SCH3U7 - Topic 4: Bonding Review SL Which of these bonding types would not be classified as strong? Metallic Covalent Ionic Dipole dipole The bond dissociation energy of NaCl is 411 kj mol -1, while that
SCH3U7 - Topic 4: Bonding Review SL Which of these bonding types would not be classified as strong? Metallic Covalent Ionic Dipole dipole The bond dissociation energy of NaCl is 411 kj mol -1, while that
CARBON. Electrochemical ond Physicochemicol Properties KIM KINOSHITA. Lawrence Berkeley Laboratory Berkeley, California
 CARBON Electrochemical ond Physicochemicol Properties KIM KINOSHITA Lawrence Berkeley Laboratory Berkeley, California A Wiley-Interscience Publicotion JOHN WILEY & SONS New York / Chichester / Brisbane
CARBON Electrochemical ond Physicochemicol Properties KIM KINOSHITA Lawrence Berkeley Laboratory Berkeley, California A Wiley-Interscience Publicotion JOHN WILEY & SONS New York / Chichester / Brisbane
Save My Exams! The Home of Revision For more awesome GCSE and A level resources, visit us at
 Covalent Bonding Mark Scheme Level Subject Exam Board Topic Sub Topic Booklet Paper Type A Level Chemistry Edexcel Bonding & Structure Covalent Bonding Mark Scheme Open-Response Time Allowed: Score: Percentage:
Covalent Bonding Mark Scheme Level Subject Exam Board Topic Sub Topic Booklet Paper Type A Level Chemistry Edexcel Bonding & Structure Covalent Bonding Mark Scheme Open-Response Time Allowed: Score: Percentage:
1.4 Crystal structure
 1.4 Crystal structure (a) crystalline vs. (b) amorphous configurations short and long range order only short range order Abbildungen: S. Hunklinger, Festkörperphysik, Oldenbourg Verlag represenatives of
1.4 Crystal structure (a) crystalline vs. (b) amorphous configurations short and long range order only short range order Abbildungen: S. Hunklinger, Festkörperphysik, Oldenbourg Verlag represenatives of
