Workshop on Isotopes in the Earth System National Center for Atmospheric Research, Boulder, Colorado January 13-15, 2004
|
|
|
- Eugenia Henderson
- 5 years ago
- Views:
Transcription
1 1. Introduction Workshop on Isotopes in the Earth System National Center for Atmospheric Research, Boulder, Colorado January 13-15, 2004 Isotopes are one of the most useful and innovative tools for understanding complex processes in the atmospheric water cycle, paleoclimate, biogeochemistry and chemistry on many timescales. An increasing variety of isotope data is now available, and active research on isotopic physics and chemistry continues to expand the data collected and the species examined. A workshop on isotopes in the climate system was held in Boulder, CO from January13-15 th The workshop, funded by a special grant from the National Science Foundation, had two goals. First, to bring together scientific leaders in observing and modeling isotopes to discuss the most interesting and important state-ofthe-art science using isotopes. Second, the workshop participants discussed modeling isotopes specifically in the context of the Community Climate System Model (CCSM) and developed a plan for the implementation of isotopes within the CCSM. This summary report provides an overview of the workshop. Included are a discussion of science issues and opportunities regarding isotopes and climate (section 2), as well as specific priorities and plans for implementing isotopes in CCSM (section 3). The agenda and participants list are attached as appendices. 2. Science Issues and Opportunities The workshop discussions were broadly organized around different isotopic species, and what can be learned from each one. Various isotopologues of water (H 2 O), carbon dioxide (CO 2 ) and carbon monoxide (CO) were discussed, with some discussion of sulfur and nitrogen atmospheric species (HNO 3 and H2SO 4 ). Discussions were wide ranging, focusing on both observations and modeling, and what can be learned from isotopes. In general, isotopes or isotopologues are valuable tracers in the earth system because their physical properties are slightly different than their parent species. The difference in molecular mass between an isotope and its parent may result in different behaviors during physical and chemical transformations, fluxes between reservoirs or phase changes. This different behavior is known as fractionation : the ratio of the isotope to its parent changes for a given process. For example, the vapor pressure of water is different for its different isotopes, such that the heavier isotopes (HDO, H 2 18 O) preferentially go to the condensed phase at equilibrium. The result is that due to this fractionation the vapor is depleted in the heavy isotope, and the condensate enriched. Fractionation is usually recorded as a ratio of isotope to parent species relative to some standard. In the case of water, this standard is a standard mean ocean water. Depletion or enrichment is often reported in percent terms (or per mil) so that -100 per mil is a 10% depletion (and denoted as δ or delta notation). For water vapor, this fractionation is mainly a function of temperature, which provides a record of a wide range of condensation processes in the climate system. 1
2 In general the isotopic fractionation for similar species (such as H 2 17 O and H 2 18 O) is similar and proportional to their mass. Effects not proportional to mass are known as mass independent fractionation, and because they only occur for certain processes, are useful tracers of those processes. For oxygen, the gas phase reaction creating Ozone (O 2 +O+M O 3 ) has a mass independent fractionation for 17 O and 18 O, creating a signal which is imparted to oxygen containing species (H 2 O, CO, CO 2 ) that react with ozone, mostly in the stratosphere. 2.1 H 2 O isotopes Several isotopologues were discussed. Most prominent were H 2 HO (or HDO) and H 2 18 O. Also discussed were H 2 17 O, and some discussion of H 3 HO (or HTO). The discussions were focused on several themes. Water vapor isotopes are widely known as paleoclimate temperature and ice sheet mass proxies. Their relationship to model and data-based climate reconstructions was also discussed. Water vapor isotopes can also serve as diagnostics of ocean and land surface transport and processes. Much of the discussion focused on the atmospheric hydrologic cycle, and how isotopes can help improve the representation of condensation and evaporation processes in climate system models. There was also discussion of uncertainties in our understanding of isotopic fractionation of water vapor. Figure 1: Illustration of the water cycle, δd and δ 18 O values in the atmosphere (courtesy of Gavin Schmidt.) Values indicate isotopic fractionation observed in various locations or for processes for δ 18 O and (δd) in per mil units. 2
3 Figure 1 presents an overview of the atmospheric hydrologic cycle, with the fractionation observed for the two most important isotopologues of H 2 O: HDO and H 2 18 O. The fractionation is measured relative to standard mean ocean water. Large depletions are found in the stratosphere and upper troposphere, as well as at high latitudes. Several key processes in the upper troposphere are still uncertain Paleoclimate The goal of many paleoclimate researchers is to simulate directly isotopes in climate system models so that transfer functions (e.g. conversion of isotope data to proxy temperature records) are not necessary. Isotopes also enable a better understanding of processes, and provide a new class of validation for paleoclimate models. Paleoclimate isotope effects occur in precipitation, in groundwater, and in ocean carbonates. In precipitation, isotopic fractionation is mainly due to the temperature of evaporation and condensation and to the amount of precipitation (δ 18 O is negatively correlated with precipitation in the tropics). Paleoclimate records thus convolve many processes, including source region, transport, temperature, quantity and seasonal timing of precipitation. Since many of the paleoproxies in oceans involve biological organisms, fractionation within ecosystems also impacts the marine δ 18 O observed. Examples of paleoclimate records include ice cores from tropical glaciers as well as from polar ice caps. Tropical glaciers can record decadal variability, but the signals are often difficult to separate between isotopic variations due to the amount of precipitation or its seasonality and temperature. Carbonate in corals records 18 O in seawater and is sensitive to temperature and rainfall. Interannual correlations with ENSO cycles are found. Cave speleothems (stalactites and stalagmites) also preserve a record of 18 O from precipitation ending up in groundwater calcium carbonates. These records are starting to allow new types of paleoclimate reconstruction of drought patterns in the southwest United States, for example. The deuterium excess (HDO v. H 2 18 O) can also be used as a long term climate proxy. Unfortunately, while the current generation of climate system models is able to correctly simulate the present day deuterium excess, the models still have problems simulating observations of deuterium excess in paleo-climate records such as ice cores. The result is an interesting remaining question for interpreting and modeling the paleo record, and isotope modeling in general Stratospheric Transport Water isotopes can also be used to understand atmospheric transport, and in particular, the exchange of air between the stratosphere and the troposphere. New analyses presented at the workshop help narrow the uncertainties in the isotopic composition of the stratosphere, mostly by new understanding about the isotopic composition of stratospheric hydrogen. The stratosphere is underdepleted relative to a simple Rayleigh fractionation model, in which as an air parcel cools and water condenses, the condensate H 2 O and isotopes are removed from the parcel. Understanding this discrepancy may 3
4 provide useful information on the processes that transport air into the stratosphere. Active laboratory work is also occurring to understand low temperature fractionation processes, kinetic fractionation effects and pure evaporation processes, which may be important for understanding low temperature and low pressure fractionation in the upper troposphere and polar regions. Most participants do not think the uncertainties in these low temperature processes are large enough to explain the observed discrepancies. Most analyses conclude that significant quantities of lofted ice evaporating are necessary to understand the isotopic composition of the stratosphere. Initial modeling results with water vapor isotopes in global models and analytic models do seem to be able to correctly simulate observations of water isotopes in the upper troposphere and stratosphere. This is encouraging for diagnoses of the hydrologic cycle (see below) Ocean Circulation Unstable water vapor isotopes, such as HTO (water with Tritium) have been used effectively as tracers of ocean circulation. Significant quantities of Tritium were injected into the climate system during nuclear bomb tests in the 1950s and 1960s. HTO is now useful because it was absorbed into natural reservoirs (such as the southern ocean) and these reservoirs are now emitting HTO, revealing timescales of the ocean uptake of atmospheric gases (e.g. carbon dioxide). Stable isotopes in ocean waters leave records in carbonates (like corals) that are a useful proxy of temperature and precipitation, as discussed above in the section on paleoclimate Hydrology The focus of the workshop discussions on hydrology were related to isotopic processes in precipitation, chiefly H 2 18 O in precipitation. There are currently active global and North American networks to measure isotopes in precipitation. The US network is not well developed, but is being augmented. The current network is able to pick out basic temperature signals, but the variance of isotopes in precipitation is not strongly related to local temperature at midlatitudes. It is rather related to the amount of precipitation, and trajectories which govern the source water. In studies of North America, for example, there are strong isotopic signals that occur with individual storms depending on their source and trajectory, and the seasonal anomalies are corrected with the phase of the ENSO cycle. The workshop did not focus explicitly on the surface water budget, runoff and the surface hydrologic cycle, but participants noted that this is an area of active research, although outside of the research of most of the participants. There was considerable discussion about how isotopes can be used to improve simulations and understanding of the hydrologic cycle. Water vapor isotopes record an integrated history of condensation and evaporation processes. This makes isotopes useful for several types of diagnosis within the hydrologic cycle that are difficult to observe by other means. In bulk, isotopes are useful for understanding the atmospheric water budget, particularly in the upper troposphere where few observations exist. Isotopes are 4
5 particularly valuable as a diagnostic for models. Simulated isotopic distributions of HDO and H 2 18 O can be used to validate whether moisture arrives in the upper troposphere by the right set of processes in a model. This process oriented approach can assist in validating model simulations of water vapor and cloud radiative feedbacks on climate. There was mention that the vertical profile of water itself is not known well in the middle and upper troposphere. Better isotopic observations of water vapor are also needed, particularly vertical profiles. Observations of isotopes in the upper troposphere are difficult. There was some discussion of remote sensing and in-situ techniques. HDO is a tractable measurement problem, H 2 18 O is a much more difficult observation. Few vertical profiles of water isotopes in the atmosphere exist, and a reasonable climatology is not complete. In addition to the atmospheric water budget, isotopes are useful for understanding the sources of water in precipitation. Models can explicitly calculate where water comes from, but validating model results with observations is difficult. Isotopes provide a good diagnostic for validation of models. The source regions or fetch of precipitation are an important and uncertain part of the atmospheric hydrologic cycle. Different source regions (high latitude oceans, low latitude continents, lakes) have different isotopic signals, and these can be traced in precipitation. In addition, isotopes of water can also be used as a detailed diagnostic of cloud condensation and evaporation in models. This can occur both on a global basis, to examine the integrative effect of condensation, or on a cloud scale. Both global models and cloud resolving models can be tested with isotopic observations. Few sets of detailed vertical profiles near clouds exist, and it was urged that isotopic observations become standard in campaigns to observe convective clouds. 2.2 CO 2 isotopes The chief isotopologues of CO 2 discussed were 13 CO 2, 14 CO 2 and C 18 OO. These isotopes provide useful information about various aspects of the global carbon cycle, and are particularly useful for understanding various sources and sinks of carbon, as well as carbon fluxes and exchanges between the atmosphere, oceans and biosphere. 13 CO 2 is a useful diagnostic of the atmospheric CO 2 budget, and can assist in partitioning the terrestrial and oceanic sinks of atmospheric CO 2. C 18 OO can help elucidate the gross exchanges between the atmosphere and the biosphere and is linked to the water cycle. 14 CO 2 provides a useful diagnostic of the age of carbon respired from the biosphere Land/Ocean Carbon Sink 13 CO 2 is a critical tool for trying to understand the sources and sinks of carbon in the earth system. Various sources of CO 2 have different 13 CO 2 signatures (fossil fuel carbon is depleted in 13 C for example), and there are different 13 C burdens in reservoirs. The total flux of carbon to/from each reservoir, and the total flux of 13 C provide two ways to distinguish various reservoirs and the fluxes between them. This closure of the carbon budget using 13 C isotopes is illustrated in Figure 2. Given sources and sinks of carbon, as 5
6 well as the 13 C composition, the magnitude of the net land and ocean sink can be determined. Observations of CO 2 and isotopes over time allow us also to discern interannual variability in carbon sources and sinks.. Figure 2: Illustration of how isotopes can be used as an additional piece of information to distinguish between carbon reservoirs to help determine sources and sinks. If the isotopic composition of a flux is known, then this constrains the magnitude of the flux. (figure from C. Still) In addition, C 18 OO provides a linkage between the global water and carbon cycles as indicated in Figure O in CO 2 is a function of photosynthesis (which enhances C 18 OO) and soil respiration (which depletes C 18 OO). It is also modulated by the H 2 18 O in the water cycle (precipitation mostly). CO 2, in the presence of leaf enzymes, isotopically equilibrates with H 2 O (H 2 18 O). Thus constraints on the gross primary production (GPP) are possible by examining the isotopes. These results indicate that fluxes in and out of leaves are huge: roughly 1/3 of atmospheric CO 2 molecules interact with leaves each year. This also highlights the necessity of understanding the role of the water cycle in regulating the carbon cycle. 6
7 Figure 3: Interactions between the global water and carbon cycles for Oxygen 18 (courtesy of Chris Still). As noted in the text, C18OO isotopic ratios equilibrate with the 18 O ratios in leaves, resetting the isotopic ratio of C18OO. Exchange may also occur in soils, and in root uptake of groundwater. There exist observations of C18OO for the 1980 s and 1990 s which indicate lags with respect to CO2 cycles, and interannual variability. In the tropics, such variability is correlated with ENSO cycles (likely due to precipitation). In high latitudes, there may be interannual anomalies in C18OO which are linked to high latitude circulation patterns (the Arctic Oscillation), due to effects on H218O in the water cycle Carbon cycle Disequilibria & paleo C cycle Currently there exists a disequilibrium between the 13C going into plants and the 13C coming out, because of fossil fuel emissions of carbon dioxide emitted into the atmosphere are more depleted in 13C than other parts of the system. This disequilibrium is illustrated graphically in Figure 2. Note that in Figure 2, changes in the total mass of 13C can occur even with no changes in total mass of carbon as a result of this disequilibrium. The extra constraints on the 13C budget from the 12C budget thus provide a useful extra constraint on understanding the global carbon cycle, and perturbations to the cycle over time. We may also think of constructing a diagram like Figure 2 for different time scales. 7
8 Because of its equilibration in plants, isotopes of oxygen in carbon dioxide can also potentially be an indicator of the paleo-carbon cycle. If we can use it now to discern GPP, then perhaps we can use records of C 18 OO and C 17 OO to understand GPP in the past. The stratospheric production of ozone produces a mass independent anomaly in 17 O in O 2. Because the biosphere removes the isotopically anomalous stratosphere-derived O 2 by respiration, and replaces it with isotopically 'normal' oxygen by photosynthesis, the magnitude of the tropospheric 17 O anomaly can be used as a tracer of global biosphere production on paleo timescales Ocean Biogeochemistry Radiocarbon CO 2 ( 14 CO 2 ) is useful for understanding oceanic carbon reservoirs. Radiocarbon from bomb tests in the 1960 s is now being outgassed from ocean reservoirs into the atmosphere similarly to HTO, providing useful information on the age of carbon in the upper ocean. Some ocean regions (high latitude southern ocean) have been isolated from 14 CO 2 as well, and are depleted in natural and bomb radiocarbon Land Use/Carbon sources Radiocarbon CO 2 ( 14 CO 2 ) has natural cosmogenic sources as well as nuclear bomb sources and is also useful for understanding terrestrial carbon reservoirs. This source provides useful information on carbon reservoirs in tropical forests for example, which differ from fossil fuel CO 2 sources (depleted in radiocarbon). Given knowledge of the natural background of cosmogenic 14 CO 2, it may provide a useful tracer of stratospheretroposphere exchange. Given assumptions about the stratospheric source, 14 CO 2 is then a useful discriminant between various terrestrial and oceanic reservoirs of 14 CO 2 (some of which fixed it at higher levels during the 1960s bomb tests, and are now outgassing it). In addition there was discussion that the mass independent fractionation of C 17 OO can also be used as an indicator of stratosphere-troposphere exchange. A mass independent fractionation signature is imparted to stratospheric CO 2 during its reaction with stratospheric ozone, which has more 17 O than mass dependent theory would presume. This large isotope effect, with known rates of reaction, is a good test of stratospheric models. There are some observations from aircraft and rockets of the 17 O anomaly in stratospheric CO 2. The signal is reset in the terrestrial biosphere by equilibration of CO 2 with the much larger H 2 O reservoir as indicated in Figure 3. In this sense the reset itself may be used as a tracer of the biosphere CO 2 source. Finally, there was discussion of how changes in CO 2 isotopologues over time can be used to understand changes in land use. C 18 OO can help us understand the balance of different types of plants (C3 respiration pathway plants vs. C4 plants), this also may change with changes in the local hydrologic cycle (H 2 O and H 2 18 O variations). Also, 13 C can help us separate biomass burning from fossil fuel emissions over time. 8
9 2.3 CO Several isotopes of carbon monoxide were discussed during the workshop. 13 CO (or C 18 O and C 17 O) is a useful tracer for discriminating the sources of CO since the fossil fuel, C3 and C4 plant biomass burning, and organic compound oxidation sources of CO have different isotopic signatures. Seasonal variations in the isotopic composition of CO at several sites (e.g. Barbados) indicate changes in the sources of CO over the course of a year. In addition, 14 CO provides a useful tracer of stratosphere-troposphere exchange and chemistry in models. 14 CO is produced in the stratosphere, and is basically a stable isotopomer over the lifetime of CO (months). Because the 14CO production rate is well known, then the concentration of 14 CO can also be used to infer stratosphere-troposphere exchange and OH oxidation of CO. 2.4 Summary of scientific priorities Based on the wide ranging discussions above the participants condensed the discussions into a series of summary tables for each of the isotopes. For each isotope, key science questions were described. A summary is provided here. H 2 O 1. Paleo-Climate. Models can be used to directly produce isotopic signatures from past temperature and precipitation patterns to aid in the interpretation of paleoclimate records from ice cores, ground water, corals, etc. This requires an appropriate hydrologic cycle, and H 2 18 O and HDO. 2. Stratospheric Water Vapor and Transport. Understanding isotopic observations of H 2 18 O and HDO in the upper troposphere and lower stratosphere can provide additional information to understand how air and humidity enter the stratosphere. These isotopes provide another constraint on model condensation processes, cloud microphysics and transport. 3. The Atmospheric Water Budget. The large scale humidity budget of the atmosphere is critically important both for understanding the source water for precipitation on weather scales, as well as global scale feedbacks and coupling to radiation on climate scales. Isotopes of water (HDO and H 2 18 O) can help discriminate sources of precipitation, and validate global model simulations of upper level humidity for climate purposes. 4. Simulating and understanding Cloud Processes. There are several related areas of research that are actively concerned with understanding cloud microphysics and condensation processes. Cloud processes are coupled to radiation, latent heat 9
10 release and large scale dynamics that are important for organizing clouds in the atmosphere. Isotopes in cloud resolving models and global models (HDO and H 2 18 O) provide strong tests of model performance for various processes. More observations are needed. 5. Ocean Circulation. HTO (tritium), emitted by nuclear tests in the 20 th century, is an important tracer of oceanic transport and ventilation of the ocean on decadal scales. Ocean model transport can be tested with HTO isotope observations, exploring how ocean water comes back into contact with the atmosphere. CO 2 1. Changing Land/Ocean Sinks. Isotopes of carbon dioxide ( 14 CO 2 and 13 CO 2 ) coupled with C 18 OO and water isotopes provide a powerful suite of tracers to understand the current sources and sinks of carbon dioxide. This includes gross photosynthesis and respiration, as well as fire and anthropogenic sources with different isotopic signatures. By properly modeling the different fluxes and reservoirs of both carbon and isotopes in the system, land surface and biogeochemical models can be better constrained. 2. Age Distribution of Carbon Sinks. The current disequilibrium in both radiocarbon ( 14 CO 2 ) and 13 CO 2 from fossil fuels provides a wealth of information about the age of surface (terrestrial and oceanic) carbon sources and sinks, and allows us to close the carbon cycle. Simulations of this cycle are useful for testing the source and sink distributions in models. 3. Ocean Biogeochemistry. As noted, radiocarbon is a useful tracer of ocean carbon reservoirs, as well as transport. Simulations with 14 CO 2 and HTO can be used to understand ocean transport, and uptake and emission from marine biological reservoirs. 4. Paleo Productivity. Interannual variations in carbon isotope sources and sinks ( 13 CO 2, C 18 OO and C1 7 OO) provide an integrated view of the variations in the earth system and interactions between carbon and water cycles when coupled with HDO, H 2 17 O and H 2 18 O. Being able to simulate this system is useful for interpreting paleo isotopic records (as it is for water isotopes) and for testing the coupled variability of the simulated carbon cycle. 5. Land use changes. CO 2 isotopes ( 13 CO 2 ) change with fossil fuel emissions and the distribution of C3 and C4 pathways of respiration in plants. So changing land use and land cover, by changing forests to grassland or crops, can change CO 2 isotopes. Biomass burning sources also have unique isotopic signatures. Correctly simulating these pathways will give us more confidence in projecting future carbon cycle changes, and also assist with paleo record interpretation. 10
11 CO 1. Biomass Burning. 13 CO isotopes provide a strong and unique signature of biomass burning that is important for models to be able to simulate, this helps constrain the overall source of carbon from fires. 2. Stratosphere-Troposphere Exchange. 14 CO has a known and calculable stratospheric source, and can be used as an indicator of the transport of CO from the stratosphere into the upper troposphere. This provides a unique way to quantify a process that models have trouble with: exchange across the tropopause 3. Combustion v. Oxidation. The combination of 14 CO and 13 CO provides insight into the balance of combustion sources of CO and oxidation of CO by OH in the atmosphere. Variations of the distribution of isotopes in time and space help us understand and simulate sources and sinks of CO. 2.5 Summary of Previous Modeling Work There was a brief discussion among several of the participants of previous global modeling work on isotopes. To date, there have been several successful efforts to model isotopes in global models. To date, these efforts have focused on water isotopologues. The first efforts were with the French Laboratoire de Météorologie Dynamique (LMD) general circulation model (Joussaume et al., 1984). Other efforts have included the Goddard Institute for Space Studies (GISS) model (Jouzel et al 1987, Jouzel et al 1991). A second generation of the GISS model with isotopes is currently being developed by G. Schmidt (personal communication). Water vapor isotopes have also been added to the Max Plank Institute Hamburg GCM, ECHAM (Hoffmann, 1998). Isotopes of water have also been added to the Melbourne University GCM (MUGCM) by Noone and Simmonds (2002). The GENESIS model also now has an isotope module (Mathieu et al., 2002). Finally, efforts are underway to add isotopes to the Hadley Center model HadCM2 by P. J. Valdes. References: G. Hoffmann, M. Werner, and M. Heimann. Water isotope module of the ECHAM atmospheric general circulation model: A study on timescales from days to several years. J. Geophys. Res., 103(D14):16,871-16,896, 1998 Joussaume, S., R. Sadourny and J. Jouzel, General circulation model of water isotope cycles in the atmosphere, Nature, 311, 24-29, 1984 J. Jouzel, G. L. Russell, R. J. Suozzo, R. D. Koster, J. W. C. White, and W. S. Broecker. Simulations of the HDO and H 2 18 O atmospheric cycles using the NASA GISS general circulation model: The seasonal cycle for present day conditions. J. Geophys. Res., 92(D12): ,
12 J. Jouzel, R. D. Koster, R. J. Suozzo, G. L. Russell, J. W. C. White, and W. S. Broecker. Simulations of the HDO and H 2 18 O atmospheric cycles using the NASA GISS general circulation model: Sensitivity experiments for present day conditions. J. Geophys. Res., 96(D4): , 1991 R. Mathieu, D. Pollard, J. E. Cole, J. W. C. White, R. S. Webb and S. L. Thompson, Simulation of stable water isotope variations by the GENESIS GCM for modern conditions, J. Geophys. Res., 107, 4037,doi: /2001JD900255, 2002 D. Noone and I. Simmonds. Associations between δ 18 O of water and climate parameters in a simulation of atmospheric circulation for J. Clim., 15(22): , CCSM 3.1 Past and Present isotope work with CCSM There has been some preliminary work using isotopes in the CCSM framework. A version of the land surface model (LSM) from the CSM has been developed with isotopes, the ISOLSM. This effort has been lead by W. Riley (LBL). In addition, some preliminary work on implementing isotopes in the Community Climate Model, version 3 (CCM3) has been completed by Noone (CU-Boulder). Incorporating isotopes into the ocean model is a task for future work. 3.2 Statement of Priorities for CCSM There was a clear statement of priorities from the workshop. Water isotopes are clearly the first set of isotopes to implement in the model. Given the dependence of other processes and species (such as CO 2 isotopes) on water isotopes, as well as other modeling efforts, this is a logical place to start. Because of the many applications, the atmosphere is the highest priority, followed by the land surface and terrestrial biosphere. The latter effort has not be extensively modeled, though some efforts are currently underway with other coupled models. While some of the work has been partially completed in CCM3 and ISOLSM, there is still much to do to upgrade these code to the CAM3 and CLM3 versions. In particular, it was pointed out that there are several aspects of the atmospheric physics parameterizations which are not explicit enough in their description of condensation processes for proper treatment of isotopic fractionation. This is an important area where isotopes can serve as a diagnostic for the atmospheric hydrologic cycle, but a difficulty for modeling isotopes in the near term. 12
13 Carbon dioxide isotopes are an area of importance, and a new frontier for isotope modeling. Properly treating these isotopes requires interactions with the isotopologues of water (for C 18 OO), and thus this is a second task. Fewer modeling efforts have successfully been able to model CO 2 isotopes. Given the details of the CCSM carbon cycle, there is a great potential for cutting edge science here. 3.3 CCSM work plan and tasks The work plan discussed during the workshop focuses on the next 6 months, at which time progress will be re-assessed. The plan is dictated by three milestones. First, the March 2004 CCSM atmosphere and biogeochemistry working group meetings are a good opportunity to raise issues related to isotopes. These issues include (among others); potential future directions for transport schemes in the atmosphere, requirements of conservation for future atmospheric physics packages, and conservation of water substance (through runoff) in the land model. Second, the CCSM annual meeting in July 2004, where it would be nice to be able to illustrate progress. Third, software engineering resources within the CCSM project at NCAR are occupied until the May 2004 release date of CCSM3. After July, it may be possible to get some assistance in developing an isotope implementation in CCSM. Interaction with the IPCC process was also discussed, but it was decided that given the IPCC timeline, it is unlikely that working isotope code will be ready for the planned paleoclimate runs starting soon. It may be possible, but ambitious, to have some paleoclimate simulations performed for science in the IPCC report (submitted by summer 2005 or so). The outline of tasks is detailed below, in approximate order of priority. Tasks: Water isotopes Atmosphere: Goal end of 2004 have working code (not public) 1. Move from ccm3 to cam3 (software) 2. Precip flux negative in convective mass flux scheme (kludge now, fix later) 3. Prognostic water in moist processes different/large scale precip 4. Evaporation under convective clouds new 5. New generic water tracers in atm (software) 6. Debugging + tuning +understanding? Atmosphere Resources Near Term: Group from CU, UCB, UMD and NCAR. After May 18: Possible CCSM assistance for software engineering 13
14 Land (2 nd priority) Move from LSM to CLM Plan is in place for this to occur. New generic water tracer scheme in clm Leaf water may need development in next version of CLM Resources there for next 6 months??? Perhaps done by July More development (longer develop with CO 2 ) Need river tracers (longer term) possible to add tracer without too much trouble?? but to do right need lakes (longer time-need new version of runnoff) Other model components ocean model (advection of del18o, HDO, HTO in POP)? (kludge rivers or do right?) ~1 week) Coding easy, spinup problems Advection issues??? Fresh water vs. isotopes consistent funky adjustments Sea ice tracers (multi layer snow and sea ice may not matter) (longer term) (new model has some tracers, vague plan currently CO2 isotopes 1. CLM-CN integrations of CLM work by CSU 1. Scheme similar to the water isotopes 2. Move CSU into CLM-CN 13 C ISO/LSM CLM-CN: entrepreneurial 1. Soil diffusion 2. Leaf water 5. Need plan for 14 C passive tracer (relatively easy but no plan) 6. for all isotope applications, new state, new flux, new diagnostics BGC meeting discuss again. Chose which to include. 14
15 Appendix 1: Workshop agenda AGENDA Workshop on Isotopes in the Earth System at Mesa Lab, Walter Orr Roberts Board Room, Fleischmann Building Boulder, Colorado January 13-15, 2004 PHASE I: ISOTOPES AS A TOOL FOR UNDERSTANDING THE EARTH SYSTEM Each session has two speakers focusing on current status and future directions, with plenty of time for discussion. Tuesday, January 13, 2004, Walter Orr Roberts Board Room Day 1, AM: Water isotope data: Interesting questions, innovative new data approaches 8:00 Continental Breakfast 8:30-9:00 Introductions 9:00-10:30 Betty Otto-Bliesner, NCAR, Chair Speakers: Evaporation and condensation of water (Ronald Cohen, University of California, Berkeley) Stable isotopes in paleoclimate and climate modeling (Julia Cole, University of Arizona) Isotopic patterns and processes in precipitation: Recent findings and future directions (Jeffrey Welker, Colorado State University) 10:30-10:45 Break 10:45-12:30 Other Speakers (1-2 slides), discussion 12:30-1:45 Lunch Day 1, PM: Water isotope modeling: Results and future modeling approaches 1:45-3:15 David Noone, University of Colorado, Boulder, Chair Speakers: Water stable isotopes using GCMs: an overview (Martin Werner, Max Planck Institute for Biogeochemistry) Water isotope modeling: Land surface and plants (William Riley, Lawrence Berkeley National Laboratory) 15
16 Coupled modeling of isotopes in the GISS GCM (Gavin Schmidt, NASA Goddard Institute for Space Studies) 3:15-3:30 Break 3:30-5:30 Other Speakers, discussion 5:30-7:30 Reception Wednesday, January 14, 2004, Walter Orr Roberts Board Room Day 2, AM: Carbon dioxide data and modeling 8:30-10:30 Inez Fung, University of California, Berkeley, Chair Speakers: Oxygen-18 in atmospheric CO2: a linkage between the global cycle of carbon and water (Christopher Still, University of California, Berkeley) Why do we want carbon isotopes in the CCSM? (Neil Suits, Colorado State University) Isotopic fractionation of CO2 in the stratosphere (Kristie Boering, University of California, Berkeley) Estimating carbon sources and reservoir lifetimes with C13 & C14 (Jim Randerson, University of California, Irvine) 10:30-10:45 Break 10:45-12:30 Other Speakers, discussion 12:30-1:45 Lunch Day 2, PM: Other isotopes and approaches? 1:45-2:45 Andrew Gettelman, NCAR, Chair Speakers: Observations of carbon isotopes in CO (John Mak, State University of New York) Oxygen mass independent fractionation in sulfur and nitrogen compounds (Mark Thiemens, University of California, San Diego) 2:45-3:00 Break 3:00-5:00 Other Speakers, discussion PHASE II: ISOTOPES IN THE CCSM Goal: Establish plan for implementing isotopes into CCSM, including identifying resources required and persons responsible for specific tasks. 16
17 Thursday, January 15, 2004, Walter Orr Roberts Board Room Day 3, AM: Isotopes in CCSM 8:00 Continental Breakfast 8:30-9:45 Natalie Mahowald, NCAR, Chair Discussion of Priorities for Isotopes in CCSM 9:45-10:00 Break 10:00-12:00 Current Status of Entrepreneurial Work in CCSM Individual (10 minute) presentations 12:00-1:00 Lunch Day 3, PM: Discussion of future work: Plan for needs and assign tasks 1:00-3:00 Andrew Gettelman, NCAR, Chair Discussion of Work Plan and Wrap Up 17
18 Appendix 2: List of Participants Workshop on Isotopes in the Earth System Participant List Kristie Boering, University of California, Berkeley Rod Chimner, Colorado State University Ronald Cohen, University of California, Berkeley Julia Cole, University of Arizona Scott Doney, Woods Hole Oceanographic Institution Pru Foster, Lawrence Berkeley Laboratory Inez Fung, University of California, Berkeley Jung- Eun Lee, University of California, Berkeley John Mak, The State University of New York Liz Moyer, Harvard University David Noone, University of Colorado, Boulder James Randerson, University of California, Irvine William Riley, Lawrence Berkeley Laboratory Gavin Schmidt, NASA GISS/Columbia University Heidi Steltzer, Colorado State University Christopher Still, University of California, Santa Barbara Neil Suites, Colorado State University Paddy Sullivan, Colorado State University Mark Thiemens, University of California, San Diego Ryan Vachon, INSTAAR Jeffrey Welker, Colorado State University Martin Werner, Max Planck Institute for Biogeochemistry, Jena Sun Wong, University of Maryland Peter Hess, NCAR Jean-Francois Lamarque, NCAR Caspar Ammann, NCAR Louisa Emmons, NCAR Andrew Gettelman, NCAR Natalie Mahowald, NCAR Bette Otto-Bliesner, NCAR Philip Rasch, NCAR Peter Thornton, NCAR Gordon Bonan, NCAR Keith Lindsay, NCAR Esther Brady, NCAR Jeff Kiehl, NCAR Kevin Trenberth, NCAR Bill Collins, NCAR Carrie Morrill, NCAR 18
Coupled modelling of water isotopes in the GISS GCM
 IAEA: Feb 2004 Coupled modelling of water isotopes in the GISS GCM Gavin Schmidt NASA GISS and Center for Climate Systems Research, Columbia University Focus of GISS water isotope research Forward modelling
IAEA: Feb 2004 Coupled modelling of water isotopes in the GISS GCM Gavin Schmidt NASA GISS and Center for Climate Systems Research, Columbia University Focus of GISS water isotope research Forward modelling
Stable Water Isotopes in the Atmosphere
 Stable Water Isotopes in the Atmosphere Jonathon S. Wright jswright@tsinghua.edu.cn Overview 1. Stable water isotopes (SWI) illustrate the tightly coupled nature of the earth system, and are useful tools
Stable Water Isotopes in the Atmosphere Jonathon S. Wright jswright@tsinghua.edu.cn Overview 1. Stable water isotopes (SWI) illustrate the tightly coupled nature of the earth system, and are useful tools
State of the Community Climate System Model
 State of the Community Climate System Model Peter Gent Chairman CCSM Scientific Steering Committee gent@ucar.edu Recent Science Highlights CCSM IJHPCA Special Issue Objectives Describe SE for climate models
State of the Community Climate System Model Peter Gent Chairman CCSM Scientific Steering Committee gent@ucar.edu Recent Science Highlights CCSM IJHPCA Special Issue Objectives Describe SE for climate models
CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION
 i CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION 1 1.1 MIXING RATIO 1 1.2 NUMBER DENSITY 2 1.3 PARTIAL PRESSURE 6 PROBLEMS 10 1.1 Fog formation 10 1.2 Phase partitioning of water in cloud 10 1.3 The ozone
i CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION 1 1.1 MIXING RATIO 1 1.2 NUMBER DENSITY 2 1.3 PARTIAL PRESSURE 6 PROBLEMS 10 1.1 Fog formation 10 1.2 Phase partitioning of water in cloud 10 1.3 The ozone
Isotope modeling with CESM
 Isotope modeling with CESM Alexandra Jahn Depart. of Atmospheric and Oceanic Sciences & Institute for Arctic and Alpine Research University of Colorado at Boulder With contribu,ons from B. O3o- Bliesner
Isotope modeling with CESM Alexandra Jahn Depart. of Atmospheric and Oceanic Sciences & Institute for Arctic and Alpine Research University of Colorado at Boulder With contribu,ons from B. O3o- Bliesner
3. Carbon Dioxide (CO 2 )
 3. Carbon Dioxide (CO 2 ) Basic information on CO 2 with regard to environmental issues Carbon dioxide (CO 2 ) is a significant greenhouse gas that has strong absorption bands in the infrared region and
3. Carbon Dioxide (CO 2 ) Basic information on CO 2 with regard to environmental issues Carbon dioxide (CO 2 ) is a significant greenhouse gas that has strong absorption bands in the infrared region and
An Introduction to Climate Modeling
 An Introduction to Climate Modeling A. Gettelman & J. J. Hack National Center for Atmospheric Research Boulder, Colorado USA Outline What is Climate & why do we care Hierarchy of atmospheric modeling strategies
An Introduction to Climate Modeling A. Gettelman & J. J. Hack National Center for Atmospheric Research Boulder, Colorado USA Outline What is Climate & why do we care Hierarchy of atmospheric modeling strategies
An Introduction to Physical Parameterization Techniques Used in Atmospheric Models
 An Introduction to Physical Parameterization Techniques Used in Atmospheric Models J. J. Hack National Center for Atmospheric Research Boulder, Colorado USA Outline Frame broader scientific problem Hierarchy
An Introduction to Physical Parameterization Techniques Used in Atmospheric Models J. J. Hack National Center for Atmospheric Research Boulder, Colorado USA Outline Frame broader scientific problem Hierarchy
2/18/2013 Estimating Climate Sensitivity From Past Climates Outline
 Estimating Climate Sensitivity From Past Climates Outline Zero-dimensional model of climate system Climate sensitivity Climate feedbacks Forcings vs. feedbacks Paleocalibration vs. paleoclimate modeling
Estimating Climate Sensitivity From Past Climates Outline Zero-dimensional model of climate system Climate sensitivity Climate feedbacks Forcings vs. feedbacks Paleocalibration vs. paleoclimate modeling
Example Biases and Development Needs for CESM
 Example Biases and Development Needs for CESM Marika Holland NCAR (On behalf of the CESM Project)!"#$%"&'())"*+& 1 CESM Status! CESM2 currently being assembled! CAM5.5, POP2+, CLM5, CICE5! CESM has benefitted
Example Biases and Development Needs for CESM Marika Holland NCAR (On behalf of the CESM Project)!"#$%"&'())"*+& 1 CESM Status! CESM2 currently being assembled! CAM5.5, POP2+, CLM5, CICE5! CESM has benefitted
Influence of condensate evaporation on water vapor and its stable isotopes in a GCM
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L12804, doi:10.1029/2009gl038091, 2009 Influence of condensate evaporation on water vapor and its stable isotopes in a GCM Jonathon S.
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L12804, doi:10.1029/2009gl038091, 2009 Influence of condensate evaporation on water vapor and its stable isotopes in a GCM Jonathon S.
Carbon Isotopes in the icesm
 Carbon Isotopes in the icesm Alexandra Jahn Collaborators: Keith Lindsay, Mike Levy, Esther Brady, Synte Peacock, Bette Otto-Bliesner NCAR is sponsored by the National Science Foundation The icesm project
Carbon Isotopes in the icesm Alexandra Jahn Collaborators: Keith Lindsay, Mike Levy, Esther Brady, Synte Peacock, Bette Otto-Bliesner NCAR is sponsored by the National Science Foundation The icesm project
Stable Water Isotopes in CAM5: Current development and initial results
 Stable Water Isotopes in CAM5: Current development and initial results Jesse Nusbaumer (nusbaume@colorado.edu) David Noone, Mariana Vertenstein, Bette Otto- Bliesner, Esther Brady, Andrew Gettelman, and
Stable Water Isotopes in CAM5: Current development and initial results Jesse Nusbaumer (nusbaume@colorado.edu) David Noone, Mariana Vertenstein, Bette Otto- Bliesner, Esther Brady, Andrew Gettelman, and
5. General Circulation Models
 5. General Circulation Models I. 3-D Climate Models (General Circulation Models) To include the full three-dimensional aspect of climate, including the calculation of the dynamical transports, requires
5. General Circulation Models I. 3-D Climate Models (General Circulation Models) To include the full three-dimensional aspect of climate, including the calculation of the dynamical transports, requires
Some remarks on climate modeling
 Some remarks on climate modeling A. Gettelman & J. J. Hack National Center for Atmospheric Research Boulder, Colorado USA Selected overheads by Doug Nychka Outline Hierarchy of atmospheric modeling strategies
Some remarks on climate modeling A. Gettelman & J. J. Hack National Center for Atmospheric Research Boulder, Colorado USA Selected overheads by Doug Nychka Outline Hierarchy of atmospheric modeling strategies
Climate Modeling Research & Applications in Wales. John Houghton. C 3 W conference, Aberystwyth
 Climate Modeling Research & Applications in Wales John Houghton C 3 W conference, Aberystwyth 26 April 2011 Computer Modeling of the Atmosphere & Climate System has revolutionized Weather Forecasting and
Climate Modeling Research & Applications in Wales John Houghton C 3 W conference, Aberystwyth 26 April 2011 Computer Modeling of the Atmosphere & Climate System has revolutionized Weather Forecasting and
Terrestrial Climate Change Variables
 Terrestrial Climate Change Variables Content Terrestrial Climate Change Variables Surface Air Temperature Land Surface Temperature Sea Level Ice Level Aerosol Particles (acid rain) Terrestrial Climate
Terrestrial Climate Change Variables Content Terrestrial Climate Change Variables Surface Air Temperature Land Surface Temperature Sea Level Ice Level Aerosol Particles (acid rain) Terrestrial Climate
Energy Systems, Structures and Processes Essential Standard: Analyze patterns of global climate change over time Learning Objective: Differentiate
 Energy Systems, Structures and Processes Essential Standard: Analyze patterns of global climate change over time Learning Objective: Differentiate between weather and climate Global Climate Focus Question
Energy Systems, Structures and Processes Essential Standard: Analyze patterns of global climate change over time Learning Objective: Differentiate between weather and climate Global Climate Focus Question
Environmental Isotopes in Hydrology. Woocay substituting for Walton
 Environmental Isotopes in Hydrology Oct 7, 2010 1 What is an Isotope? An element is defined by the number of protons (Z) in the nucleus The number of neutrons (N) defines the isotope(s) of that element
Environmental Isotopes in Hydrology Oct 7, 2010 1 What is an Isotope? An element is defined by the number of protons (Z) in the nucleus The number of neutrons (N) defines the isotope(s) of that element
Why 17 O-excess? (And, what is it?)
 Why 17 O-excess? (And, what is it?) Advances in cavity ring-down spectroscopy (CRDS) have enabled scientists around the world to make quick, easy and highly precise measurements of the stable isotopes
Why 17 O-excess? (And, what is it?) Advances in cavity ring-down spectroscopy (CRDS) have enabled scientists around the world to make quick, easy and highly precise measurements of the stable isotopes
Global Carbon Cycle - I
 Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Recent Climate History - The Instrumental Era.
 2002 Recent Climate History - The Instrumental Era. Figure 1. Reconstructed surface temperature record. Strong warming in the first and late part of the century. El Ninos and major volcanic eruptions are
2002 Recent Climate History - The Instrumental Era. Figure 1. Reconstructed surface temperature record. Strong warming in the first and late part of the century. El Ninos and major volcanic eruptions are
The Climate Sensitivity of the Community Climate System Model Version 3 (CCSM3)
 2584 J O U R N A L O F C L I M A T E VOLUME 19 The Climate Sensitivity of the Community Climate System Model Version 3 (CCSM3) JEFFREY T. KIEHL, CHRISTINE A. SHIELDS, JAMES J. HACK, AND WILLIAM D. COLLINS
2584 J O U R N A L O F C L I M A T E VOLUME 19 The Climate Sensitivity of the Community Climate System Model Version 3 (CCSM3) JEFFREY T. KIEHL, CHRISTINE A. SHIELDS, JAMES J. HACK, AND WILLIAM D. COLLINS
Measurements of Ozone. Why is Ozone Important?
 Anthropogenic Climate Changes CO 2 CFC CH 4 Human production of freons (CFCs) Ozone Hole Depletion Human production of CO2 and CH4 Global Warming Human change of land use Deforestation (from Earth s Climate:
Anthropogenic Climate Changes CO 2 CFC CH 4 Human production of freons (CFCs) Ozone Hole Depletion Human production of CO2 and CH4 Global Warming Human change of land use Deforestation (from Earth s Climate:
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle
 Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Final Exam: Monday March 17 3:00-6:00 pm (here in Center 113) Slides from Review Sessions are posted on course website:
 Final Exam: Monday March 17 3:00-6:00 pm (here in Center 113) 35% of total grade Format will be all multiple choice (~70 questions) Final exam will cover entire course - material since 2 nd midterm weighted
Final Exam: Monday March 17 3:00-6:00 pm (here in Center 113) 35% of total grade Format will be all multiple choice (~70 questions) Final exam will cover entire course - material since 2 nd midterm weighted
Name the surface winds that blow between 0 and 30. GEO 101, February 25, 2014 Monsoon Global circulation aloft El Niño Atmospheric water
 GEO 101, February 25, 2014 Monsoon Global circulation aloft El Niño Atmospheric water Name the surface winds that blow between 0 and 30 What is the atmospheric pressure at 0? What is the atmospheric pressure
GEO 101, February 25, 2014 Monsoon Global circulation aloft El Niño Atmospheric water Name the surface winds that blow between 0 and 30 What is the atmospheric pressure at 0? What is the atmospheric pressure
Stable Isotope Tracers
 Stable Isotope Tracers OCN 623 Chemical Oceanography 5 March 2015 Reading: Emerson and Hedges, Chapter 5, p.134-153 (c) 2015 David Ho and Frank Sansone Outline Stable Isotopes - Introduction & Notation
Stable Isotope Tracers OCN 623 Chemical Oceanography 5 March 2015 Reading: Emerson and Hedges, Chapter 5, p.134-153 (c) 2015 David Ho and Frank Sansone Outline Stable Isotopes - Introduction & Notation
Features of Global Warming Review. GEOG/ENST 2331 Lecture 23 Ahrens: Chapter 16
 Features of Global Warming Review GEOG/ENST 2331 Lecture 23 Ahrens: Chapter 16 The Greenhouse Effect 255 K 288 K Ahrens, Fig. 2.12 What can change the global energy balance? Incoming energy Solar strength
Features of Global Warming Review GEOG/ENST 2331 Lecture 23 Ahrens: Chapter 16 The Greenhouse Effect 255 K 288 K Ahrens, Fig. 2.12 What can change the global energy balance? Incoming energy Solar strength
Climate Variability Natural and Anthropogenic
 Climate Variability Natural and Anthropogenic Jim Renwick NIWA Climate Research j.renwick@niwa.co.nz Climate equilibrium and climate forcings Natural forcings Anthropogenic forcings Feedbacks Natural variability
Climate Variability Natural and Anthropogenic Jim Renwick NIWA Climate Research j.renwick@niwa.co.nz Climate equilibrium and climate forcings Natural forcings Anthropogenic forcings Feedbacks Natural variability
Projections of future climate change
 Projections of future climate change Matthew Collins 1,2 and Catherine A. Senior 2 1 Centre for Global Atmospheric Modelling, Department of Meteorology, University of Reading 2 Met Office Hadley Centre,
Projections of future climate change Matthew Collins 1,2 and Catherine A. Senior 2 1 Centre for Global Atmospheric Modelling, Department of Meteorology, University of Reading 2 Met Office Hadley Centre,
Diagnosis of Relative Humidity Changes in a Warmer Climate Using Tracers of Last Saturation
 Diagnosis of Relative Humidity Changes in a Warmer Climate Using Tracers of Last Saturation 8 March, 2011 Jonathon Wright Department of Applied Mathematics & Theoretical Physics University of Cambridge
Diagnosis of Relative Humidity Changes in a Warmer Climate Using Tracers of Last Saturation 8 March, 2011 Jonathon Wright Department of Applied Mathematics & Theoretical Physics University of Cambridge
Stable Water Isotope Cycle
 Regional Atmospheric Modelling of the Stable Water Isotope Cycle SIMS workshop, Vienna Mars 2004. Kristof Sturm 1,3 Georg Hoffmann 2, Bärbel Langmann 1 1 Max-Planck-Institut für Meteorologie, Hamburg 2
Regional Atmospheric Modelling of the Stable Water Isotope Cycle SIMS workshop, Vienna Mars 2004. Kristof Sturm 1,3 Georg Hoffmann 2, Bärbel Langmann 1 1 Max-Planck-Institut für Meteorologie, Hamburg 2
Climate Change 2007: The Physical Science Basis
 Climate Change 2007: The Physical Science Basis Working Group I Contribution to the IPCC Fourth Assessment Report Presented by R.K. Pachauri, IPCC Chair and Bubu Jallow, WG 1 Vice Chair Nairobi, 6 February
Climate Change 2007: The Physical Science Basis Working Group I Contribution to the IPCC Fourth Assessment Report Presented by R.K. Pachauri, IPCC Chair and Bubu Jallow, WG 1 Vice Chair Nairobi, 6 February
Remote Sensing Data Assimilation for a Prognostic Phenology Model
 June 2008 Remote Sensing Data Assimilation for a Prognostic Phenology Model How to define global-scale empirical parameters? Reto Stöckli 1,2 (stockli@atmos.colostate.edu) Lixin Lu 1, Scott Denning 1 and
June 2008 Remote Sensing Data Assimilation for a Prognostic Phenology Model How to define global-scale empirical parameters? Reto Stöckli 1,2 (stockli@atmos.colostate.edu) Lixin Lu 1, Scott Denning 1 and
Environmental Science Chapter 13 Atmosphere and Climate Change Review
 Environmental Science Chapter 13 Atmosphere and Climate Change Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Climate in a region is a. the long-term,
Environmental Science Chapter 13 Atmosphere and Climate Change Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Climate in a region is a. the long-term,
Website Lecture 3 The Physical Environment Part 1
 Website http://websites.rcc.edu/halama Lecture 3 The Physical Environment Part 1 1 Lectures 3 & 4 1. Biogeochemical Cycling 2. Solar Radiation 3. The Atmosphere 4. The Global Ocean 5. Weather and Climate
Website http://websites.rcc.edu/halama Lecture 3 The Physical Environment Part 1 1 Lectures 3 & 4 1. Biogeochemical Cycling 2. Solar Radiation 3. The Atmosphere 4. The Global Ocean 5. Weather and Climate
Introduction to Climate ~ Part I ~
 2015/11/16 TCC Seminar JMA Introduction to Climate ~ Part I ~ Shuhei MAEDA (MRI/JMA) Climate Research Department Meteorological Research Institute (MRI/JMA) 1 Outline of the lecture 1. Climate System (
2015/11/16 TCC Seminar JMA Introduction to Climate ~ Part I ~ Shuhei MAEDA (MRI/JMA) Climate Research Department Meteorological Research Institute (MRI/JMA) 1 Outline of the lecture 1. Climate System (
Observed State of the Global Climate
 WMO Observed State of the Global Climate Jerry Lengoasa WMO June 2013 WMO Observations of Changes of the physical state of the climate ESSENTIAL CLIMATE VARIABLES OCEANIC ATMOSPHERIC TERRESTRIAL Surface
WMO Observed State of the Global Climate Jerry Lengoasa WMO June 2013 WMO Observations of Changes of the physical state of the climate ESSENTIAL CLIMATE VARIABLES OCEANIC ATMOSPHERIC TERRESTRIAL Surface
Components of the Climate System. Lecture 2: Earth s Climate System. Pop Quiz. Sub-components Global cycles What comes in What goes out
 Lecture 2: Earth s Climate System Components of the Climate System terrestrial radiation Atmosphere Ocean solar radiation Land Energy, Water, and Biogeochemistry Cycles Sub-components Global cycles What
Lecture 2: Earth s Climate System Components of the Climate System terrestrial radiation Atmosphere Ocean solar radiation Land Energy, Water, and Biogeochemistry Cycles Sub-components Global cycles What
Table of Contents. Chapter: Atmosphere. Section 1: Earth's Atmosphere. Section 2: Energy Transfer in the Atmosphere. Section 3: Air Movement
 Table of Contents Chapter: Atmosphere Section 1: Earth's Atmosphere Section 2: Energy Transfer in the Atmosphere Section 3: Air Movement Table of Contents Chapter 4: Atmosphere Section 1: Earth's Atmosphere
Table of Contents Chapter: Atmosphere Section 1: Earth's Atmosphere Section 2: Energy Transfer in the Atmosphere Section 3: Air Movement Table of Contents Chapter 4: Atmosphere Section 1: Earth's Atmosphere
GEO1010 tirsdag
 GEO1010 tirsdag 31.08.2010 Jørn Kristiansen; jornk@met.no I dag: Først litt repetisjon Stråling (kap. 4) Atmosfærens sirkulasjon (kap. 6) Latitudinal Geographic Zones Figure 1.12 jkl TØRR ATMOSFÆRE Temperature
GEO1010 tirsdag 31.08.2010 Jørn Kristiansen; jornk@met.no I dag: Først litt repetisjon Stråling (kap. 4) Atmosfærens sirkulasjon (kap. 6) Latitudinal Geographic Zones Figure 1.12 jkl TØRR ATMOSFÆRE Temperature
Lecture 2: Earth s Climate System
 Lecture 2: Earth s Climate System terrestrial radiation solar radiation Atmosphere Ocean Solid Earth Land Energy, Water, and Biogeochemistry Cycles Sub-components Global cycles What comes in What goes
Lecture 2: Earth s Climate System terrestrial radiation solar radiation Atmosphere Ocean Solid Earth Land Energy, Water, and Biogeochemistry Cycles Sub-components Global cycles What comes in What goes
Match (one-to-one) the following (1 5) from the list (A E) below.
 GEO 302C EXAM 1 Spring 2009 Name UID You may not refer to any other materials during the exam. For each question (except otherwise explicitly stated), select the best answer for that question. Read all
GEO 302C EXAM 1 Spring 2009 Name UID You may not refer to any other materials during the exam. For each question (except otherwise explicitly stated), select the best answer for that question. Read all
Historical Changes in Climate
 Historical Changes in Climate Medieval Warm Period (MWP) Little Ice Age (LIA) Lamb, 1969 Hunters in the snow by Pieter Bruegel, 1565 Retreat of the Rhone Glacier shown by comparing the drawing from 1750
Historical Changes in Climate Medieval Warm Period (MWP) Little Ice Age (LIA) Lamb, 1969 Hunters in the snow by Pieter Bruegel, 1565 Retreat of the Rhone Glacier shown by comparing the drawing from 1750
Lecture 28: Observed Climate Variability and Change
 Lecture 28: Observed Climate Variability and Change 1. Introduction This chapter focuses on 6 questions - Has the climate warmed? Has the climate become wetter? Are the atmosphere/ocean circulations changing?
Lecture 28: Observed Climate Variability and Change 1. Introduction This chapter focuses on 6 questions - Has the climate warmed? Has the climate become wetter? Are the atmosphere/ocean circulations changing?
Sulfur Biogeochemical Cycle
 Sulfur Biogeochemical Cycle Chris Moore 11/16/2015 http://www.inorganicventures.com/element/sulfur 1 Sulfur Why is it important? 14 th most abundant element in Earth s crust Sulfate is second most abundant
Sulfur Biogeochemical Cycle Chris Moore 11/16/2015 http://www.inorganicventures.com/element/sulfur 1 Sulfur Why is it important? 14 th most abundant element in Earth s crust Sulfate is second most abundant
Introduction to Climate Change
 Ch 19 Climate Change Introduction to Climate Change Throughout time, the earth's climate has always been changing produced ice ages Hence, climate variations have been noted in the past what physical processes
Ch 19 Climate Change Introduction to Climate Change Throughout time, the earth's climate has always been changing produced ice ages Hence, climate variations have been noted in the past what physical processes
Stable Isotope Tracers OCN 623 Chemical Oceanography
 Stable Isotope Tracers OCN 623 Chemical Oceanography 21 March 2017 Reading: Emerson and Hedges, Chapter 5, p.134-153 2017 Frank Sansone and David Ho Student Learning Outcomes At the completion of this
Stable Isotope Tracers OCN 623 Chemical Oceanography 21 March 2017 Reading: Emerson and Hedges, Chapter 5, p.134-153 2017 Frank Sansone and David Ho Student Learning Outcomes At the completion of this
The Atmosphere Made up of mainly two gases: Nitrogen 78% Oxygen 21% Trace Gases 1%
 The Atmosphere 18.1 The Atmosphere Made up of mainly two gases: Nitrogen 78% Oxygen 21% Trace Gases 1% Layers of the Atmosphere made made up of 5 layers: Troposphere Stratosphere Mesosphere Ionosphere
The Atmosphere 18.1 The Atmosphere Made up of mainly two gases: Nitrogen 78% Oxygen 21% Trace Gases 1% Layers of the Atmosphere made made up of 5 layers: Troposphere Stratosphere Mesosphere Ionosphere
Day 1 of Global Warming. Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Day 1 of Global Warming Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Atmosphere Atmosphere = the thin layer (1/100 th of Earth s diameter) of gases that surrounds
Day 1 of Global Warming Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Atmosphere Atmosphere = the thin layer (1/100 th of Earth s diameter) of gases that surrounds
Updated Dust-Iron Dissolution Mechanism: Effects Of Organic Acids, Photolysis, and Dust Mineralogy
 Updated Dust-Iron Dissolution Mechanism: Effects Of Organic Acids, Photolysis, and Dust Mineralogy Nicholas Meskhidze & Matthew Johnson First International Workshop on the Long Range Transport and Impacts
Updated Dust-Iron Dissolution Mechanism: Effects Of Organic Acids, Photolysis, and Dust Mineralogy Nicholas Meskhidze & Matthew Johnson First International Workshop on the Long Range Transport and Impacts
Climate and the Atmosphere
 Climate and Biomes Climate Objectives: Understand how weather is affected by: 1. Variations in the amount of incoming solar radiation 2. The earth s annual path around the sun 3. The earth s daily rotation
Climate and Biomes Climate Objectives: Understand how weather is affected by: 1. Variations in the amount of incoming solar radiation 2. The earth s annual path around the sun 3. The earth s daily rotation
Chapter 14: The Changing Climate
 Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
The Challenge of. Guy Brasseur
 The Challenge of Monitoring and Predicting Chemical Weather Guy Brasseur Introduction: What is Chemical Weather? What is Chemical Weather? Local, regional, and global distributions of important trace gases
The Challenge of Monitoring and Predicting Chemical Weather Guy Brasseur Introduction: What is Chemical Weather? What is Chemical Weather? Local, regional, and global distributions of important trace gases
Torben Königk Rossby Centre/ SMHI
 Fundamentals of Climate Modelling Torben Königk Rossby Centre/ SMHI Outline Introduction Why do we need models? Basic processes Radiation Atmospheric/Oceanic circulation Model basics Resolution Parameterizations
Fundamentals of Climate Modelling Torben Königk Rossby Centre/ SMHI Outline Introduction Why do we need models? Basic processes Radiation Atmospheric/Oceanic circulation Model basics Resolution Parameterizations
XI. the natural carbon cycle. with materials from J. Kasting (Penn State)
 XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
NOTES AND CORRESPONDENCE. On the Radiative and Dynamical Feedbacks over the Equatorial Pacific Cold Tongue
 15 JULY 2003 NOTES AND CORRESPONDENCE 2425 NOTES AND CORRESPONDENCE On the Radiative and Dynamical Feedbacks over the Equatorial Pacific Cold Tongue DE-ZHENG SUN NOAA CIRES Climate Diagnostics Center,
15 JULY 2003 NOTES AND CORRESPONDENCE 2425 NOTES AND CORRESPONDENCE On the Radiative and Dynamical Feedbacks over the Equatorial Pacific Cold Tongue DE-ZHENG SUN NOAA CIRES Climate Diagnostics Center,
Global Carbon Cycle - I
 Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
1. The frequency of an electromagnetic wave is proportional to its wavelength. a. directly *b. inversely
 CHAPTER 3 SOLAR AND TERRESTRIAL RADIATION MULTIPLE CHOICE QUESTIONS 1. The frequency of an electromagnetic wave is proportional to its wavelength. a. directly *b. inversely 2. is the distance between successive
CHAPTER 3 SOLAR AND TERRESTRIAL RADIATION MULTIPLE CHOICE QUESTIONS 1. The frequency of an electromagnetic wave is proportional to its wavelength. a. directly *b. inversely 2. is the distance between successive
Lecture 16 - Stable isotopes
 Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
Lecture 16 - Stable isotopes 1. The fractionation of different isotopes of oxygen and their measurement in sediment cores has shown scientists that: (a) ice ages are common and lasted for hundreds of millions
Observed Climate Variability and Change: Evidence and Issues Related to Uncertainty
 Observed Climate Variability and Change: Evidence and Issues Related to Uncertainty David R. Easterling National Climatic Data Center Asheville, North Carolina Overview Some examples of observed climate
Observed Climate Variability and Change: Evidence and Issues Related to Uncertainty David R. Easterling National Climatic Data Center Asheville, North Carolina Overview Some examples of observed climate
Today s Lecture: Land, biosphere, cryosphere (All that stuff we don t have equations for... )
 Today s Lecture: Land, biosphere, cryosphere (All that stuff we don t have equations for... ) 4 Land, biosphere, cryosphere 1. Introduction 2. Atmosphere 3. Ocean 4. Land, biosphere, cryosphere 4.1 Land
Today s Lecture: Land, biosphere, cryosphere (All that stuff we don t have equations for... ) 4 Land, biosphere, cryosphere 1. Introduction 2. Atmosphere 3. Ocean 4. Land, biosphere, cryosphere 4.1 Land
Website Lecture 3 The Physical Environment Part 1
 Website http://websites.rcc.edu/halama Lecture 3 The Physical Environment Part 1 1 Lectures 3 & 4 1. Biogeochemical Cycling 2. Solar Radiation 3. The Atmosphere 4. The Global Ocean 5. Weather and Climate
Website http://websites.rcc.edu/halama Lecture 3 The Physical Environment Part 1 1 Lectures 3 & 4 1. Biogeochemical Cycling 2. Solar Radiation 3. The Atmosphere 4. The Global Ocean 5. Weather and Climate
COURSE CLIMATE SCIENCE A SHORT COURSE AT THE ROYAL INSTITUTION
 COURSE CLIMATE SCIENCE A SHORT COURSE AT THE ROYAL INSTITUTION DATE 4 JUNE 2014 LEADER CHRIS BRIERLEY Course Outline 1. Current climate 2. Changing climate 3. Future climate change 4. Consequences 5. Human
COURSE CLIMATE SCIENCE A SHORT COURSE AT THE ROYAL INSTITUTION DATE 4 JUNE 2014 LEADER CHRIS BRIERLEY Course Outline 1. Current climate 2. Changing climate 3. Future climate change 4. Consequences 5. Human
From Isotopes to Temperature: Using Ice Core Data!
 From Isotopes to Temperature: Using Ice Core Data! Spruce W. Schoenemann schoes@uw.edu UWHS Atmospheric Sciences 211 May 2013 Dept. of Earth and Space Sciences University of Washington Seattle http://www.uwpcc.washington.edu
From Isotopes to Temperature: Using Ice Core Data! Spruce W. Schoenemann schoes@uw.edu UWHS Atmospheric Sciences 211 May 2013 Dept. of Earth and Space Sciences University of Washington Seattle http://www.uwpcc.washington.edu
Carbon Cycling Internal
 Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
Carbon Cycling Internal The 4 subcycles Atmosphere The Earth s Atmosphere The Earth has a radius of some 6400 km. Ninety-nine percent of the earth's atmosphere is contained within a layer approximately
Course Outline CLIMATE SCIENCE A SHORT COURSE AT THE ROYAL INSTITUTION. 1. Current climate. 2. Changing climate. 3. Future climate change
 COURSE CLIMATE SCIENCE A SHORT COURSE AT THE ROYAL INSTITUTION DATE 4 JUNE 2014 LEADER CHRIS BRIERLEY Course Outline 1. Current climate 2. Changing climate 3. Future climate change 4. Consequences 5. Human
COURSE CLIMATE SCIENCE A SHORT COURSE AT THE ROYAL INSTITUTION DATE 4 JUNE 2014 LEADER CHRIS BRIERLEY Course Outline 1. Current climate 2. Changing climate 3. Future climate change 4. Consequences 5. Human
The Climate Sensitivity of the Community Climate System Model: CCSM3. Jeffrey T. Kiehl*, Christine A. Shields, James J. Hack and William D.
 For JCLI CCSM Special Issue The Climate Sensitivity of the Community Climate System Model: CCSM3 Jeffrey T. Kiehl*, Christine A. Shields, James J. Hack and William D. Collins National Center for Atmospheric
For JCLI CCSM Special Issue The Climate Sensitivity of the Community Climate System Model: CCSM3 Jeffrey T. Kiehl*, Christine A. Shields, James J. Hack and William D. Collins National Center for Atmospheric
Final Review Meteorology
 Final Review Meteorology Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is an example of climate? a. A sudden snowstorm resulted
Final Review Meteorology Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is an example of climate? a. A sudden snowstorm resulted
2018 Science Olympiad: Badger Invitational Meteorology Exam. Team Name: Team Motto:
 2018 Science Olympiad: Badger Invitational Meteorology Exam Team Name: Team Motto: This exam has 50 questions of various formats, plus 3 tie-breakers. Good luck! 1. On a globally-averaged basis, which
2018 Science Olympiad: Badger Invitational Meteorology Exam Team Name: Team Motto: This exam has 50 questions of various formats, plus 3 tie-breakers. Good luck! 1. On a globally-averaged basis, which
Climate Change Lecture Notes
 Climate Change Lecture Notes (Topic 12A) page 1 Climate Change Lecture Notes Learning Outcomes for the Climate Change Unit 1. Students can list observations which suggest that the world is warming, and
Climate Change Lecture Notes (Topic 12A) page 1 Climate Change Lecture Notes Learning Outcomes for the Climate Change Unit 1. Students can list observations which suggest that the world is warming, and
Lecture 7: The Monash Simple Climate
 Climate of the Ocean Lecture 7: The Monash Simple Climate Model Dr. Claudia Frauen Leibniz Institute for Baltic Sea Research Warnemünde (IOW) claudia.frauen@io-warnemuende.de Outline: Motivation The GREB
Climate of the Ocean Lecture 7: The Monash Simple Climate Model Dr. Claudia Frauen Leibniz Institute for Baltic Sea Research Warnemünde (IOW) claudia.frauen@io-warnemuende.de Outline: Motivation The GREB
The scientific basis for climate change projections: History, Status, Unsolved problems
 The scientific basis for climate change projections: History, Status, Unsolved problems Isaac Held, Princeton, Feb 2008 Katrina-like storm spontaneously generated in atmospheric model Regions projected
The scientific basis for climate change projections: History, Status, Unsolved problems Isaac Held, Princeton, Feb 2008 Katrina-like storm spontaneously generated in atmospheric model Regions projected
Weather and Climate Change
 Weather and Climate Change What if the environmental lapse rate falls between the moist and dry adiabatic lapse rates? The atmosphere is unstable for saturated air parcels but stable for unsaturated air
Weather and Climate Change What if the environmental lapse rate falls between the moist and dry adiabatic lapse rates? The atmosphere is unstable for saturated air parcels but stable for unsaturated air
anemometer a weather instrument that measures wind speed with wind-catching cups (SRB, IG)
 FOSS Weather on Earth Module Glossary 3 rd Edition 2012 absorb to soak in air the mixture of gases surrounding Earth air pressure the force exerted on a surface by the mass of the air above it anemometer
FOSS Weather on Earth Module Glossary 3 rd Edition 2012 absorb to soak in air the mixture of gases surrounding Earth air pressure the force exerted on a surface by the mass of the air above it anemometer
This presentation was assembled as part of the outreach initiative for the Canadian Network for the Detection of Atmospheric Change.
 This will be a lesson for students in grades 9-12. The subject matter is climate change - the greenhouse effect, greenhouse gases, how greenhouse gases are measured and studied, and the impacts of climate
This will be a lesson for students in grades 9-12. The subject matter is climate change - the greenhouse effect, greenhouse gases, how greenhouse gases are measured and studied, and the impacts of climate
Global Biogeochemical Cycles and. II. Biological Metabolism
 Global Biogeochemical Cycles and Biological Metabolism I. Biogeochemistry & Biogeochemical Cycles A. Global cycles: nitrogen, water, carbon B. Carbon cycle through time II. Biological Metabolism A. Redox
Global Biogeochemical Cycles and Biological Metabolism I. Biogeochemistry & Biogeochemical Cycles A. Global cycles: nitrogen, water, carbon B. Carbon cycle through time II. Biological Metabolism A. Redox
Overview of Dust in the Earth System
 AAAS Symposium 1 Overview of Dust in the Earth System Dr. Karen E. Kohfeld School of Resource and Environmental Management, Simon Fraser University, CANADA What is dust? Soil mineral fragments Quartz,
AAAS Symposium 1 Overview of Dust in the Earth System Dr. Karen E. Kohfeld School of Resource and Environmental Management, Simon Fraser University, CANADA What is dust? Soil mineral fragments Quartz,
Centennial-scale Climate Change from Decadally-paced Explosive Volcanism
 Centennial-scale Climate Change from Decadally-paced Explosive Volcanism Yafang Zhong and Gifford Miller INSTAAR, University of Colorado at Boulder, USA Bette Otto-Bliesner, Caspar Ammann, Marika Holland,
Centennial-scale Climate Change from Decadally-paced Explosive Volcanism Yafang Zhong and Gifford Miller INSTAAR, University of Colorado at Boulder, USA Bette Otto-Bliesner, Caspar Ammann, Marika Holland,
Weather Forecasts and Climate AOSC 200 Tim Canty. Class Web Site: Lecture 27 Dec
 Weather Forecasts and Climate AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Climate Natural Variations Feedback Mechanisms Lecture 27 Dec 4 2018 1 Climate
Weather Forecasts and Climate AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Climate Natural Variations Feedback Mechanisms Lecture 27 Dec 4 2018 1 Climate
Thermal / Solar. When air is warmed it... Rises. Solar Energy. Evaporation. Condensation Forms Clouds
 Thermal / Solar Light from the Sun is transformed into what type of energy when it hits Earth's surface? Rises When air is warmed it... Solar Energy Water moves through the water cycle using what type
Thermal / Solar Light from the Sun is transformed into what type of energy when it hits Earth's surface? Rises When air is warmed it... Solar Energy Water moves through the water cycle using what type
An Introduction to Coupled Models of the Atmosphere Ocean System
 An Introduction to Coupled Models of the Atmosphere Ocean System Jonathon S. Wright jswright@tsinghua.edu.cn Atmosphere Ocean Coupling 1. Important to climate on a wide range of time scales Diurnal to
An Introduction to Coupled Models of the Atmosphere Ocean System Jonathon S. Wright jswright@tsinghua.edu.cn Atmosphere Ocean Coupling 1. Important to climate on a wide range of time scales Diurnal to
A B C D PROBLEMS Dilution of power plant plumes. z z z z
 69 PROBLEMS 4. Dilution of power plant plumes Match each power plant plume (-4) to the corresponding atmospheric lapse rate (A-D, solid lines; the dashed line is the adiabatic lapse rate Γ). Briefly comment
69 PROBLEMS 4. Dilution of power plant plumes Match each power plant plume (-4) to the corresponding atmospheric lapse rate (A-D, solid lines; the dashed line is the adiabatic lapse rate Γ). Briefly comment
Global Carbon Cycle - I Systematics: Reservoirs and Fluxes
 OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
Paleoclimate: What can the past tell us about the present and future? Global Warming Science February 14, 2012 David McGee
 Paleoclimate: What can the past tell us about the present and future? 12.340 Global Warming Science February 14, 2012 David McGee 1 Recent observed trends: Greenhouse gases Image courtesy of NOAA. 2 Recent
Paleoclimate: What can the past tell us about the present and future? 12.340 Global Warming Science February 14, 2012 David McGee 1 Recent observed trends: Greenhouse gases Image courtesy of NOAA. 2 Recent
Thermodynamics of Atmospheres and Oceans
 Thermodynamics of Atmospheres and Oceans Judith A. Curry and Peter J. Webster PROGRAM IN ATMOSPHERIC AND OCEANIC SCIENCES DEPARTMENT OF AEROSPACE ENGINEERING UNIVERSITY OF COLORADO BOULDER, COLORADO USA
Thermodynamics of Atmospheres and Oceans Judith A. Curry and Peter J. Webster PROGRAM IN ATMOSPHERIC AND OCEANIC SCIENCES DEPARTMENT OF AEROSPACE ENGINEERING UNIVERSITY OF COLORADO BOULDER, COLORADO USA
The Climate Sensitivity of the Community Climate System Model: CCSM3. Jeffrey T. Kiehl*, Christine A. Shields, James J. Hack and William D.
 For JCLI CCSM Special Issue The Climate Sensitivity of the Community Climate System Model: CCSM3 Jeffrey T. Kiehl*, Christine A. Shields, James J. Hack and William D. Collins National Center for Atmospheric
For JCLI CCSM Special Issue The Climate Sensitivity of the Community Climate System Model: CCSM3 Jeffrey T. Kiehl*, Christine A. Shields, James J. Hack and William D. Collins National Center for Atmospheric
2. Fargo, North Dakota receives more snow than Charleston, South Carolina.
 2015 National Tournament Division B Meteorology Section 1: Weather versus Climate Chose the answer that best answers the question 1. The sky is partly cloudy this morning in Lincoln, Nebraska. 2. Fargo,
2015 National Tournament Division B Meteorology Section 1: Weather versus Climate Chose the answer that best answers the question 1. The sky is partly cloudy this morning in Lincoln, Nebraska. 2. Fargo,
Figure 65: Reservoir in a steady state condition where the input flux is equal to the output flux and the reservoir size remains constant.
 7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
Weather and Climate. Weather the condition of the Earth s atmosphere at a particular time and place
 Weather and Climate Weather the condition of the Earth s atmosphere at a particular time and place Climate the average year-after-year conditions of temperature, precipitation, winds and clouds in an area
Weather and Climate Weather the condition of the Earth s atmosphere at a particular time and place Climate the average year-after-year conditions of temperature, precipitation, winds and clouds in an area
7/5/2018. Global Climate Change
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Global Climate Change Earth, Chapter 21 Chapter 21 Global Climate Change Climate and Geology The climate system is a multidimensional system of many interacting parts,
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Global Climate Change Earth, Chapter 21 Chapter 21 Global Climate Change Climate and Geology The climate system is a multidimensional system of many interacting parts,
Lecture 9: Climate Sensitivity and Feedback Mechanisms
 Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
GEOL/ENVS 3520 Spring 2009 Hour Exam #2
 GEOL/ENVS 3520 Spring 2009 Hour Exam #2 Enter your name, the date, your ID number, and a made-up 4-digit code (for later recall and identification of your test results) on the separate test sheet. Carefully
GEOL/ENVS 3520 Spring 2009 Hour Exam #2 Enter your name, the date, your ID number, and a made-up 4-digit code (for later recall and identification of your test results) on the separate test sheet. Carefully
GEOL 437 Global Climate Change 2/1/18: Solar radiation and the annual cycle
 GEOL 437 Global Climate Change 2/1/18: Solar radiation and the annual cycle Why are there seasons? How does the climate respond to the radiative annual cycle? How does the climate respond to changes in
GEOL 437 Global Climate Change 2/1/18: Solar radiation and the annual cycle Why are there seasons? How does the climate respond to the radiative annual cycle? How does the climate respond to changes in
AT350 EXAM #1 September 23, 2003
 AT350 EXAM #1 September 23, 2003 Name and ID: Enter your name and student ID number on the answer sheet and on this exam. Record your answers to the questions by using a No. 2 pencil to completely fill
AT350 EXAM #1 September 23, 2003 Name and ID: Enter your name and student ID number on the answer sheet and on this exam. Record your answers to the questions by using a No. 2 pencil to completely fill
Climate Modeling: From the global to the regional scale
 Climate Modeling: From the global to the regional scale Filippo Giorgi Abdus Salam ICTP, Trieste, Italy ESA summer school on Earth System Monitoring and Modeling Frascati, Italy, 31 July 11 August 2006
Climate Modeling: From the global to the regional scale Filippo Giorgi Abdus Salam ICTP, Trieste, Italy ESA summer school on Earth System Monitoring and Modeling Frascati, Italy, 31 July 11 August 2006
Consequences for Climate Feedback Interpretations
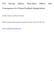 CO 2 Forcing Induces Semi-direct Effects with Consequences for Climate Feedback Interpretations Timothy Andrews and Piers M. Forster School of Earth and Environment, University of Leeds, Leeds, LS2 9JT,
CO 2 Forcing Induces Semi-direct Effects with Consequences for Climate Feedback Interpretations Timothy Andrews and Piers M. Forster School of Earth and Environment, University of Leeds, Leeds, LS2 9JT,
Which Climate Model is Best?
 Which Climate Model is Best? Ben Santer Program for Climate Model Diagnosis and Intercomparison Lawrence Livermore National Laboratory, Livermore, CA 94550 Adapting for an Uncertain Climate: Preparing
Which Climate Model is Best? Ben Santer Program for Climate Model Diagnosis and Intercomparison Lawrence Livermore National Laboratory, Livermore, CA 94550 Adapting for an Uncertain Climate: Preparing
Chapter outline. Reference 12/13/2016
 Chapter 2. observation CC EST 5103 Climate Change Science Rezaul Karim Environmental Science & Technology Jessore University of science & Technology Chapter outline Temperature in the instrumental record
Chapter 2. observation CC EST 5103 Climate Change Science Rezaul Karim Environmental Science & Technology Jessore University of science & Technology Chapter outline Temperature in the instrumental record
