Bound tritium: Preparation, measurement
|
|
|
- Hubert Wiggins
- 5 years ago
- Views:
Transcription
1 Uëoprotection - Colloques, volume 37, Cl (2002) Cl-973 Bound tritium: Preparation, measurement M. Fournier, N. Coreau and A. Maigret IPSN/DPRE/SERNAT/LMRE, bâtiment 501, Bois des Rames, Orsay cedex, France Abstract. The tritium activity in the ecosystem is measured on various matrices. The tritium bound to organic matter represents the tritium fixed by the operation of living objects. The sample should be prepared to preserve the tritium abundance with no isotopic fractionation. Transformation of the test sample should be conducted in order to obtain the highest possible combustion water yield, with no isotopic exchange. The test samples to be used should be sufficient to perform the measurements with the lowest possible detection threshold. I. INTRODUCTION Tritium is basically incorporated to the food chain via water [1]. The incorporation mechanisms involve isotopic fractionation. The various components generated by organisms are included in the structures or are stored or metabolised. Along the food chain, transformations may result in accumulation effects, through isotopic fractionation or storage. Measurement of the tritium activity in the continental and sea ecosystem has been performed at LMRE since 1997 on various matrices (food products, ground mosses, fish, algae,), applying the methods used for environmental surveys [2,3]. Various terms are proposed to specify the compartment in which the tritium activity is measured: "Free tritium", or tritium as water in the sample ("free water"), "Exchangeable tritium", or tritium bound to an atom, which can undergo isotopic exchange with water or water vapour under the stability conditions of the molecule or sample, "Organically bound tritium", or tritium bound to an atom, which cannot undergo isotopic exchange with water or water vapour under the stability conditions of the molecule or sample, "Bound tritium'", the whole of "exchangeable" and "organically bound" tritium, after extraction of the fee tritium. For better reproducibility and to prevent possible atmospheric contamination, it is recommended to perform a single combustion for each test sample. 2. SAMPLE PREPARATION D»e to easy tritium exchange as hydroxyl or proton, the sample should be prepared in order to preserve tritium abundance with no isotopic fractionation. Dehydration may be performed either by heating at vancus temperatures, oven drying, or vacuum freeze-drying. 2, 1 Selecting the drying mode: oven drying or freeze-drying? Oven drying generates a tritium enrichment of the combustion water resulting from the bound fraction 4 tritium activity of this bound fraction expressed in dry weight of the sample. The durations required Article published by EDP Sciences and available at or
2 Cl-974 RADIOPROTECnON - COLLOQUES for this drying mode generate modifications in the organic matter, either via microbial action (oxidizing fermenting) or via temperature effect (baking). The isotopic fractionation is all the more important as the temperature is high (table 1). Table 1: Oven drying Vs freeze-drying Sample Type Free tritium Bq.l-l Bound tritium +/-1 CT Bq.l-l +/-I0- Bound tritium Bq.kg-1 sec ±la Grass Grass Grass; freeze dried Grass Grass; oven dried: 60 C Grass Apples <1.21 DT Freeze dried Apples O.OF Oven dried: 105 C Apples When it is necessary to measure free tritium, freeze-drying should imperatively be selected. It is also recommended when measuring bound tritium. If exchangeable tritium is to be measured, this is the only technique to check the isotopic quantity and quality of the water used for exchange. Full freeze drying is used to separate the "free water" without denaturing isotopic composition before the transformations required for measurement. This processing applies to all types of matrices (soils, sediments, milk, ground plants, water plants, fish, molluscs, food products,). 2.2 Freeze-drying Freeze drying system LMRE uses a locally procured system (FfETO FD8). This programmable system consists of i evaporation chamber and a condenser separated by a shutoff valve (table 2). Table 2: Features of the freeze-drying system. Chamber Independent cold unit. Number of trays: 6. Each tray is cooled/heated: - 32 C to +70 C, within + 0,5 C, with a temperature check for the contents of each tray. Piranni sensor to measure the vacuum within the chamber. Shutoff valve between chamber and condense^ Condenser - 85 C, 8 kg of ice per 24 hrs. Horizontal, stainless steel, smooth. With heater for fast deicing. Two-stage primary vacuum pump. Drain valve for fluid recovery Preparation The system is dried with dry compressed air before installing the trays into the chamber. Trays i by weighing, in a dry room with regulated temperature (20 to 24 C), and quickly installed chamber.
3 ECORAD 2001 Cl Freeze drying programs The &eeze-drying phases include: pre-freezing, primary drying, and secondary drying. Each phase may be divided into steps, depending on the matrix under processing (table 3). The water/alcohol mixtures generated by freeze-drying are distilled to obtain the optimum water/alcohol separation. The alcohol faction is added to the freeze-dried sample before combustion. Table 3: Examples of freeze drying programs Matrix Milk: 2 litres Honey: 1 kg Grass: 0.7 kg Apple: 3.2 kg Alga: 1.2 kg Freezing 1:-25 C 1:-32 C 1:-32 C 1:-32 C 1-32 C 2: - 25 C, 3 brs 2: - 32 C, 20 hrs 2: - 32 C, 4 hrs 2: - 32 C, 5 hrs 2-32 C J _5-hrs Primary 1:-10 C, 15hrs l:-20 C,24hrs 1:-10 C, 20 hrs 1:-10 C, 10 hrs 1-20 C, 5 hrs drying 2: -5 C, 7 hrs 2: -10 C, 20 hrs 2: 0 C; 10 hrs 2: 0 C, 7 hrs 2-10 C, 5 hrs P: 0.5 hpa 3: -5 C, 10 hrs 3-5 C, 5 hrs 4: 0 C, 20 hrs 4 0 C, 5 hrs Secondary 1:30 C,9brs 1:5 C, 5 hrs 1:30 C, 10 hrs 1:30 C, 10 hrs 1 5 C, 1 hrs drying 2: 10 C, 5 hrs 2 10 C, 1 hrs P: final 3: 15 C, 5 hrs 3 15 C, 1 hrs vacuum 4: 20 C, 5 hrs 4 25 C, 5 hrs 5: 20 C, 40 hrs End of freeze-drying criterion It is essential to check the sample dehydration. The selected criterion is the vacuum pressure: the value read should remain constant over 1 hour minimum and close to vacuum as obtained for the freeze drying system operating with the trays in the evaporation chamber at 30 C and the condenser at -85 C without sample. Practically, this state results in an asymptotic graph of the pressure record. 23 Sample preparation for the test sample After drying, the sample consistency is essential. The test sample for combustion ranges from 20 to 50 g (or 800 g) depending on the matrices and available quantity of sample. The bound tritium distribution wiin the sample should be consistent, for the test sample to be representative. A too coarse crushing results in test samples that may be different in composition (table 4). A 100 um grain size is ideal. LMRE uses a grid-type crashing system with 80 um meshing. Combustion water Bq.1-1 +/-1 o- Activity per kg of dry matter Bq.kg-1 sec +/- 1 a ,55 samples for the same sample result in different values for the combustion water. These test samples are not identical with regard to tritium and composition. However, the expression of the dry
4 Cl-976 RADIOPROTECTION - COLLOQUES matter activity of both test samples is identical. The tritium distribution is not consistent within the sample. 2.4 Combustion Combustion system The combustion oven access is fitted with a stove (t = 60 C) operating as an airlock, separated from the crystal combustion tube by a valve. The combustion tube goes through a moving oven with adjustable temperature (t= ambient to 1000 C) and a fixed oven (t=1000 C). In the fixed oven, the combustion tube is filled with a catalyst mixture (Deoxo M) and crystal grains. The water vapour generated by comhustioii is condensed in a refrigerating system (t=5 C to 75 C) fitted with a drum and a U-shaped tube, which are in turn cooled down (dry ice or liquid nitrogen). The combustion gases are exhausted through an S-shaped tube containing silica gel. The oxygen/argon mixture is adjustable. The test sample is loaded into one or more crystal boats. The average duration of a combustion operation is equal to 10 hours, over 2 days: automatic warming up, weighing and boat loading, combustion, drying of the refrigerating system and recovery of the drum and U-shaped tube End of combustion criterion The operator assesses the end of combustion by observing the absence of water vapour condensation in the coolants and by observing the contents of the combustion tube. Drying is performed under argon sweeping, with ovens at 1000 C. The ph of the water generated by combustion should be acid. Otherwise, this water undergoes a combustion cycle. 2.5 Distillation Distillation system The distillation glassware was selected. It is kept in stove at 105 C before use. Parts are marked per item. The drum heaters are set to the minimum temperature to obtain distillation. The system includes: a 25 ml drum with glass beads; a column; a three-way coupling; a coolant (t=5 C); an acceptance extension; a 15 ml drum; a refrigerated "Dewar" vessel (dry ice or liquid nitrogen).the water samples are neutralized with calcium carbonate. The duration of a distillation is equal to 5 hours: assembly, weighing, distillation, drying, weighing End of distillation criterion The operator assesses the end of distillation by observing the drum: it is essential to be close to dry distillation. Drying is performed with a hot air gun. The distillation yield should be more than 80%.
5 ECORAD 2001 Cl-977 3, MEASUREMENT 3.1 Measuring conditions ^ the water to be measured is distilled, the bottles forming a counting group are expected to have the same value for the parameter expressing quenching. Weighing is performed on two scales: one 1/10 mg for the background ("blank") and samples, and the other 1/100 mg for the reference. The counting bottles are low-diffusion, anti-static, polyethylene bottles (Packard P/N ). These bottles are stowed at 40 C and stored before use in a tight box containing silica gel. The scintillator quantity (Ultima Gold LLT), distributed by a pipette secured to the scintillator bottle (1L), and the pipetted test samples are weighed for checking. The optimum detection threshold is obtained for a mixture of 10 g of water in 10 ml of scintillator. Counts are performed on Packard 2770 TR-SL and LKB Quantulus, counters. The instruments are cooled down to approximately 14 C, in rooms regulated at 22 C and supplied in overpressure with filtered air at ambient temperature or heated to 20 C. A counting group includes 8 bottles: a "blank", two samples, a reference (detection yield), and four samples. The counting duration is equal to 1000 minutes for each bottle, in 10 cycles of 100 minutes each. Under such conditions, the detection threshold varies between 1 and 1.5 Bq.L" 1, depending on the movement upon measurement. The uncertainty expressed within 1 o" incorporates weighing uncertainties for the reference and sample, the uncertainty on the reference activity and the counting uncertainties on the movement, the sample and the reference [4]. 3.2 Test sample In order to ensure an optimum 10 g quantity of water for the measurement, it is recommended to use test samples (tab.5) the combustion of which generates approximately 15 g of water. Table 5: Examples of test samples for combustion Type Quantity (g) Oxygen (%) Argon (%) Algae Sedimentation sludge 50 to 200 (50) 25 to to0 Cereals 30 to 40 (35) Crustaceans 30 to 50 (50) Water 12,5 (15) Tree leaf 24 to 40 (30) Fruits 30 to 40 (38) Dry milk 25 to 30 (27) Vegetable 30 to 60 (40) Ivy leafs 24 to 30 (30) Mollusc 30 to 45 (35) Ground moss 20 to 110(20) Water moss 30 to 80 (35) Fish 30 to 50 (30) Fat fish 25 to 30 (25) Meadow, pasture 25 to 40 (25) Salad " 30 to 40 (35) B?j1cseamient 50 to 500 (150) 30 to to0 tigtesediment 300 to 800 (800) soil. "c^h. _. 50 to 500 (300) 30 to to0 sugar TvTrr : - values in parentheses are those most frequently used.
6 Cl-978 RADIOPROTECTION - COLLOQUES 4. CONCLUSION The freeze dried product contains the "bound tritium", plus a fraction corresponding to the tritium contained in the exchangeable radicals, as -OH, =N-H, -S-H. The systematic observation of "free tritium" and "bound tritium" values for the same type of vegetal sample does not evidence a systematic relationship between both forms, even in a specific context (La Hague)[5]. It should be noted that the value of the "free tritium" fraction, a priori balanced with the "exchangeable tritium" fraction, indicates the qualitative importance of the error made when considering the "bound tritium" value as being representative of the "organically bound tritium" while not exchanging the "exchangeable tritium". A low value of tritium abundance in the "free tritium" fraction, with regard to the "bound tritium" fraction results in underestimating the value of tritium abundance in the "organically bound tritium" fraction. A high value of tritium abundance in the "free tritium" fraction results in overestimating the valueirftritium abundance in the "organically bound tritium" fraction. These estimate errors (some %) are all the more important, as the freeze-dried samples are rich in hydroxyl or proton functions. The fraction contained in the exchangeable radicals may be balanced with water containing as few tritium as possible, representing the "exchangeable tritium". The methods applied in this specific case are still experimental. References [1] Belot Y., Roy M., Métivier H., Le Tritium de l'environnement à l'homme, (IPSN, Les éditions de physique, 1996), pl-191. [2] IAEA Technical Report Series, Tritium and other environmental isotopes in the hydrological cycle, n 73, (IAEA, 1989). [3] IAEA Technical Report Series, Measurement of radionuclides in food and the environment, n 295, (IAEA, 1989). [4] NF M , 2000, Mesurage de l'activité des émetteurs bêta dans les eaux par scintillation liquide. Partie 1: cas particulier du tritium. [5] Fournier M., Coreau N., Maigret A., Calmet D., Le mesurage du tritium des échantillons de l'environnement à 1TPSN, Journées techniques CETAMA, Cadarache, (CETAMA.1999).
Measurement of the carbon 14 activity at natural level in air samples
 Radioprotection, Suppl. 1, vol. 40 (2005) S791-S796 EDP Sciences, 2005 DOI: 10.1051/radiopro:2005s1-116 Measurement of the carbon 14 activity at natural level in air samples A. Olivier 1, L. Tenailleau
Radioprotection, Suppl. 1, vol. 40 (2005) S791-S796 EDP Sciences, 2005 DOI: 10.1051/radiopro:2005s1-116 Measurement of the carbon 14 activity at natural level in air samples A. Olivier 1, L. Tenailleau
Tritium along the French coast of the English Channel
 Radioprotection, Suppl. 1, vol. 40 (2005) S621-S627 EDP Sciences, 2005 DOI: 10.1051/radiopro:2005s1-091 Tritium along the French coast of the English Channel M. Masson 1, F. Siclet 2, M. Fournier 3, A.
Radioprotection, Suppl. 1, vol. 40 (2005) S621-S627 EDP Sciences, 2005 DOI: 10.1051/radiopro:2005s1-091 Tritium along the French coast of the English Channel M. Masson 1, F. Siclet 2, M. Fournier 3, A.
Downloaded from
 Matter in Our Surroundings 1. Which state of matter is characterized by the following properties : (0 A substance with a fixed arrangement of particles. (I'O A substance that has large distances between
Matter in Our Surroundings 1. Which state of matter is characterized by the following properties : (0 A substance with a fixed arrangement of particles. (I'O A substance that has large distances between
Topic 19b. Thermal Properties of Matter
 Topic 19b The infra-red image of a head shows the distribution of heat. Different colours indicate different temperatures. Which do you think are the warmest regions? Thermal Properties of Matter contents
Topic 19b The infra-red image of a head shows the distribution of heat. Different colours indicate different temperatures. Which do you think are the warmest regions? Thermal Properties of Matter contents
Principles of Food and Bioprocess Engineering (FS 231) Problems on Heat Transfer
 Principles of Food and Bioprocess Engineering (FS 1) Problems on Heat Transfer 1. What is the thermal conductivity of a material 8 cm thick if the temperature at one end of the product is 0 C and the temperature
Principles of Food and Bioprocess Engineering (FS 1) Problems on Heat Transfer 1. What is the thermal conductivity of a material 8 cm thick if the temperature at one end of the product is 0 C and the temperature
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Science 14 Unit A: Investigating Properties of Matter Chapter 3 Mixtures and Their Uses pp WORKBOOK Name:
 Science 14 Unit A: Investigating Properties of Matter Chapter 3 Mixtures and Their Uses pp. 40-57 WORKBOOK Name: 3.1 Two Kinds of Mixtures pp. 42-44 Read pp. 42-43 Mixtures are represented on the right
Science 14 Unit A: Investigating Properties of Matter Chapter 3 Mixtures and Their Uses pp. 40-57 WORKBOOK Name: 3.1 Two Kinds of Mixtures pp. 42-44 Read pp. 42-43 Mixtures are represented on the right
High-Pressure Volumetric Analyzer
 High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible
 Matter Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible 3 subatomic particles Proton - positively charged particle in the nucleus of an
Matter Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible 3 subatomic particles Proton - positively charged particle in the nucleus of an
Put sufficient ice cubes into water (1 M) and wait for equilibrium (both exist) (1 M)
 NAME : F.5 ( ) Marks: /70 FORM FOUR PHYSICS REVISION TEST on HEAT Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section B (20
NAME : F.5 ( ) Marks: /70 FORM FOUR PHYSICS REVISION TEST on HEAT Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section B (20
NAME: ACTIVITY SHEETS PHYSICS AND CHEMISTRY (SECONDARY 3 rd YEAR)
 NAME: ACTIVITY SHEETS PHYSICS AND CHEMISTRY (SECONDARY 3 rd YEAR) ACTIVITY 1: Matter Lesson 2 THE PARTICULATE NATURE OF MATTER 1-What is matter? 2-What is a particle (corpuscle)? Set some examples 3-What
NAME: ACTIVITY SHEETS PHYSICS AND CHEMISTRY (SECONDARY 3 rd YEAR) ACTIVITY 1: Matter Lesson 2 THE PARTICULATE NATURE OF MATTER 1-What is matter? 2-What is a particle (corpuscle)? Set some examples 3-What
LSUF Update on Organically Bound Tritium Standard project. Arvic Harms. 5 May 2004
 LSUF 2004 Update on Organically Bound Tritium Standard project Arvic Harms 5 May 2004 Contents What is tritium and what is organically bound tritium (OBT) and why is it important? Why do we need an OBT
LSUF 2004 Update on Organically Bound Tritium Standard project Arvic Harms 5 May 2004 Contents What is tritium and what is organically bound tritium (OBT) and why is it important? Why do we need an OBT
(ii) the total kinetic energy of the gas molecules (1 mark) (iii) the total potential energy of the gas molecules (1 mark)
 NAME : F.5 ( ) Marks: /70 FORM FOUR PHYSICS REVISION TEST on HEAT Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section B (20
NAME : F.5 ( ) Marks: /70 FORM FOUR PHYSICS REVISION TEST on HEAT Allowed: 70 minutes This paper consists of two sections. Section A (50 marks) consists of the structure-type questions, and Section B (20
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
Phase Changes and Latent Heat
 Review Questions Why can a person remove a piece of dry aluminum foil from a hot oven with bare fingers without getting burned, yet will be burned doing so if the foil is wet. Equal quantities of alcohol
Review Questions Why can a person remove a piece of dry aluminum foil from a hot oven with bare fingers without getting burned, yet will be burned doing so if the foil is wet. Equal quantities of alcohol
States of Matter: Solid, Liquid, and Gas
 Movie Special Effects Activity 2 States of Matter: Solid, Liquid, and Gas GOALS In this activity you will: Create an animation to illustrate the behavior of particles in different phases of matter, and
Movie Special Effects Activity 2 States of Matter: Solid, Liquid, and Gas GOALS In this activity you will: Create an animation to illustrate the behavior of particles in different phases of matter, and
6 th Grade Introduction to Chemistry
 Lesson 1 (Describing Matter) 6 th Grade Introduction to Chemistry Matter anything that has mass and takes up space All the stuff in the natural world is matter. Chapter 1: Introduction to Matter Chemistry
Lesson 1 (Describing Matter) 6 th Grade Introduction to Chemistry Matter anything that has mass and takes up space All the stuff in the natural world is matter. Chapter 1: Introduction to Matter Chemistry
Science Year 10 Unit 1 Biology
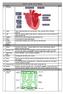 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Uranium from water sample
 Uranium from water sample Analysis of uranium from water sample Determination of uranium is based on radiochemical separation and alpha spectrometric measurements. Detailed description is presented below.
Uranium from water sample Analysis of uranium from water sample Determination of uranium is based on radiochemical separation and alpha spectrometric measurements. Detailed description is presented below.
Mr. Carpenter s Biology Biochemistry. Name Pd
 Mr. Carpenter s Biology Biochemistry Name Pd Chapter 2 Vocabulary Atom Element Compound Molecule Ion Cohesion Adhesion Solution Acid Base Carbohydrate Monosaccharide Lipid Protein Amino acid Nucleic acid
Mr. Carpenter s Biology Biochemistry Name Pd Chapter 2 Vocabulary Atom Element Compound Molecule Ion Cohesion Adhesion Solution Acid Base Carbohydrate Monosaccharide Lipid Protein Amino acid Nucleic acid
12. Heat of melting and evaporation of water
 VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
47 Which process best demonstrates a chemical change in distilled water?
 47 Which process best demonstrates a chemical change in distilled water? A B C D Freezing the water Separating the water into its elements Calculating the water s density Dissolving sugar in the water
47 Which process best demonstrates a chemical change in distilled water? A B C D Freezing the water Separating the water into its elements Calculating the water s density Dissolving sugar in the water
Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible
 Matter Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible 3 subatomic particles Proton - positively charged particle in the nucleus of an
Matter Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible 3 subatomic particles Proton - positively charged particle in the nucleus of an
W X gas liquid solid Y Z. C X and Y D Y and Z X Y Z. C Z to X D Z to Y
 1 In which changes do the particles move further apart? W X gas liquid solid Y Z W and X W and Z X and Y Y and Z 2 iagrams X, Y and Z represent the three states of matter. X Y Z Which change occurs during
1 In which changes do the particles move further apart? W X gas liquid solid Y Z W and X W and Z X and Y Y and Z 2 iagrams X, Y and Z represent the three states of matter. X Y Z Which change occurs during
KS3 Science PERSONAL LEARNING CHECKLIST. YEAR 88 CONTENT Use this document to monitor your revision and target specific areas.
 KS3 Science PERSONAL LEARNING CHECKLIST YEAR 88 CONTENT Use this document to monitor your revision and target specific areas. Topic Name BIOLOGY the human skeleton Analysing the skeleton the role of skeletal
KS3 Science PERSONAL LEARNING CHECKLIST YEAR 88 CONTENT Use this document to monitor your revision and target specific areas. Topic Name BIOLOGY the human skeleton Analysing the skeleton the role of skeletal
EXPERIMENT #4 Separation of a Three-Component Mixture
 OBJECTIVES: EXPERIMENT #4 Separation of a Three-Component Mixture Define chemical and physical properties, mixture, solubility, filtration, sublimation, and percent Separate a mixture of sodium chloride
OBJECTIVES: EXPERIMENT #4 Separation of a Three-Component Mixture Define chemical and physical properties, mixture, solubility, filtration, sublimation, and percent Separate a mixture of sodium chloride
for free revision past papers visit:
 NAME ADM NO:. STUNDENT S SIGNATURE DATE.. SCHOOL 233/2 FORM THREE CHEMISTRY THEORY Paper 2 END YEAR 2017 EXAMS. Time: 2 Hrs FORM THREE CHEMISTRY 233/2 INSTRUCTIONS TO CANDIDATES Write your Name and Index
NAME ADM NO:. STUNDENT S SIGNATURE DATE.. SCHOOL 233/2 FORM THREE CHEMISTRY THEORY Paper 2 END YEAR 2017 EXAMS. Time: 2 Hrs FORM THREE CHEMISTRY 233/2 INSTRUCTIONS TO CANDIDATES Write your Name and Index
GRIGNARD REACTION Synthesis of Benzoic Acid
 1 GRIGNARD REACTION Synthesis of Benzoic Acid In the 1920 s, the first survey of the acceleration of chemical transformations by ultrasound was published. Since then, many more applications of ultrasound
1 GRIGNARD REACTION Synthesis of Benzoic Acid In the 1920 s, the first survey of the acceleration of chemical transformations by ultrasound was published. Since then, many more applications of ultrasound
CHEMISTRY WORKSHEET. 1. Anything that occupies space and has weight. 2. The state of matter having maximum compressibility.
 CH.1 Q.1:- Name the Following:- 1. Anything that occupies space and has weight. 2. The state of matter having maximum compressibility. 3. The state which has maximum intermolecular space. 4. The process
CH.1 Q.1:- Name the Following:- 1. Anything that occupies space and has weight. 2. The state of matter having maximum compressibility. 3. The state which has maximum intermolecular space. 4. The process
TRITIUM DETERMINATION IN WATER
 TRITIUM DETERMINATION IN WATER Ricardo M. Gavini 1 Autoridad Regulatoria Nuclear, Av. del Libertador 8250, 1429, Buenos Aires, Argentina. Abstract. An analytical procedure for the determination of tritium
TRITIUM DETERMINATION IN WATER Ricardo M. Gavini 1 Autoridad Regulatoria Nuclear, Av. del Libertador 8250, 1429, Buenos Aires, Argentina. Abstract. An analytical procedure for the determination of tritium
6.3. Theories of Reaction Rates. Collision Theory. The Effect of Concentration on Reactant Rates
 Theories of Reaction Rates 6.3 In section 6.2, you explored the rate law, which defines the relationship between the concentrations of reactants and reaction rate. Why, however, does the rate of a reaction
Theories of Reaction Rates 6.3 In section 6.2, you explored the rate law, which defines the relationship between the concentrations of reactants and reaction rate. Why, however, does the rate of a reaction
Unit 08 Review: The KMT and Gas Laws
 Unit 08 Review: The KMT and Gas Laws It may be helpful to view the animation showing heating curve and changes of state: http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/031_changesstate.mov
Unit 08 Review: The KMT and Gas Laws It may be helpful to view the animation showing heating curve and changes of state: http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/031_changesstate.mov
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. GB Translated English of Chinese Standard: GB5009.
 Translated English of Chinese Standard: GB5009.17-2014 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA National Food Safety Standard-Determination
Translated English of Chinese Standard: GB5009.17-2014 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA National Food Safety Standard-Determination
Page 1 / 12. Chemistry Exam. Name: Matter Properties, Structure. Question 1 (1 point) The atomic number of an atom is. A. The mass of the atom.
 Chemistry Exam Matter Properties, Structure Name: Question 1 (1 point) The atomic number of an atom is A. The mass of the atom. B. The number of protons added to the number of neutrons in the nucleus.
Chemistry Exam Matter Properties, Structure Name: Question 1 (1 point) The atomic number of an atom is A. The mass of the atom. B. The number of protons added to the number of neutrons in the nucleus.
PAPER 2 THEORY QUESTIONS
 PAPER 2 THEORY QUESTIONS 1 Fig. 1.1 shows the arrangement of atoms in a solid block. Fig. 1.1 (a) End X of the block is heated. Energy is conducted to end Y, which becomes warm. (i) Explain how heat is
PAPER 2 THEORY QUESTIONS 1 Fig. 1.1 shows the arrangement of atoms in a solid block. Fig. 1.1 (a) End X of the block is heated. Energy is conducted to end Y, which becomes warm. (i) Explain how heat is
Exam questions: HEAT. 2. [2003 OL][2004 OL][2005 OL][2006 OL][2007 OL][2008 OL][2009] Name two methods by which heat can be transferred.
![Exam questions: HEAT. 2. [2003 OL][2004 OL][2005 OL][2006 OL][2007 OL][2008 OL][2009] Name two methods by which heat can be transferred. Exam questions: HEAT. 2. [2003 OL][2004 OL][2005 OL][2006 OL][2007 OL][2008 OL][2009] Name two methods by which heat can be transferred.](/thumbs/87/96755378.jpg) Exam questions: HEAT Specific heat capacity of copper = 390 J kg 1 K 1 ; Specific heat capacity of water = 4200 J kg 1 K 1 s.h.c. of aluminium = 910 J kg -1 K -1 ; Specific latent heat of fusion of ice
Exam questions: HEAT Specific heat capacity of copper = 390 J kg 1 K 1 ; Specific heat capacity of water = 4200 J kg 1 K 1 s.h.c. of aluminium = 910 J kg -1 K -1 ; Specific latent heat of fusion of ice
Six decades of environmental radioactivity measurements. Sven P. Nielsen
 Six decades of environmental radioactivity measurements Sven P. Nielsen Research Establishment Risø Established by Government in 1950 s to keep Denmark in line with development of nuclear technology including
Six decades of environmental radioactivity measurements Sven P. Nielsen Research Establishment Risø Established by Government in 1950 s to keep Denmark in line with development of nuclear technology including
Carbon (Organic, and Inorganic)
 DATE: January 2011 Carbon (Organic, and Inorganic) 1. Application This method covers the determination of total organic carbon (TOC), and inorganic carbon (IC) concentrations in soil, plant tissues and
DATE: January 2011 Carbon (Organic, and Inorganic) 1. Application This method covers the determination of total organic carbon (TOC), and inorganic carbon (IC) concentrations in soil, plant tissues and
Chemistry A: States of Matter Packet Name: Hour: Page 1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
Measurement Matter and Density. Name: Period:
 Measurement Matter and Density Name: Period: Studying Physics and Chemistry Physics Tells us how fast objects move or how much it takes to get objects to, turn or stop. Chemistry Explains how different
Measurement Matter and Density Name: Period: Studying Physics and Chemistry Physics Tells us how fast objects move or how much it takes to get objects to, turn or stop. Chemistry Explains how different
STATES OF MATTER INTRODUCTION
 STATES OF MATTER INTRODUCTION In studying chemical reactions, we talk in terms of interactions between molecules, atoms, and electrons. However, in order to understand chemical changes, we must first have
STATES OF MATTER INTRODUCTION In studying chemical reactions, we talk in terms of interactions between molecules, atoms, and electrons. However, in order to understand chemical changes, we must first have
Sodium Chloride - Analytical Standard
 Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
Name Date. Physical and Chemical Changes
 Name Date Physical and Chemical Changes Physical Changes Physical Change: A change in which no new substances form Do not change the type of matter an object is made of You change the shape, size or more
Name Date Physical and Chemical Changes Physical Changes Physical Change: A change in which no new substances form Do not change the type of matter an object is made of You change the shape, size or more
Organic Chemistry. Alkanes are hydrocarbons in which the carbon atoms are joined by single covalent bonds.
 Organic Chemistry Organic compounds: The branch of chemistry which deals with the study of carbon compounds is called organic chemistry. Catenation: The carbon atom has a property to undergo self linking
Organic Chemistry Organic compounds: The branch of chemistry which deals with the study of carbon compounds is called organic chemistry. Catenation: The carbon atom has a property to undergo self linking
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY
 5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY SUB-TOPIC 1.2 METHODS OF PURIFICATION AND ANALYSIS LEARNING
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY SUB-TOPIC 1.2 METHODS OF PURIFICATION AND ANALYSIS LEARNING
Name Partner. Thermal Physics. Part I: Heat of Vaporization of Nitrogen. Introduction:
 Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
WATER IN THE ATMOSPHERE
 WATER IN THE ATMOSPHERE During a rainstorm, the air feels moist On a clear, cloudless day, the air may feel dry As the sun heats the land and oceans, the amount of water in the atmosphere changes Water
WATER IN THE ATMOSPHERE During a rainstorm, the air feels moist On a clear, cloudless day, the air may feel dry As the sun heats the land and oceans, the amount of water in the atmosphere changes Water
Silent Card Shuffle. Dump out the word strips onto your desk.
 Silent Card Shuffle Dump out the word strips onto your desk. With a partner, silently work to arrange the strips into 8 groups. Each group should have a term (purple paper), its definition (white paper),
Silent Card Shuffle Dump out the word strips onto your desk. With a partner, silently work to arrange the strips into 8 groups. Each group should have a term (purple paper), its definition (white paper),
Carbonate content. SCAN-N 32:98 Revised White, green and black liquors and burnt lime sludge
 Revised 1998 White, green and black liquors and burnt lime sludge Carbonate content 0 Introduction This SCAN-test Method replaces SCAN-N 32:88 from which it differs in that it, in addition to white and
Revised 1998 White, green and black liquors and burnt lime sludge Carbonate content 0 Introduction This SCAN-test Method replaces SCAN-N 32:88 from which it differs in that it, in addition to white and
The Flowering Plant and Photosynthesis
 The Flowering Plant and Photosynthesis AIM To name and identify some common Irish trees To identify the parts of a flowering plant To list the function of the flowers, stem, leaves and roots To explain
The Flowering Plant and Photosynthesis AIM To name and identify some common Irish trees To identify the parts of a flowering plant To list the function of the flowers, stem, leaves and roots To explain
Procedure for determining the specific activity of radium-226 in foodstuffs of plant origin
 Procedure for determining the specific activity of radium-6 in foodstuffs of plant origin K-Ra-6-LEBM-01 Authors: M. Beyermann B. Höfs U.-K. Schkade K. Schmidt Federal coordinating office for questions
Procedure for determining the specific activity of radium-6 in foodstuffs of plant origin K-Ra-6-LEBM-01 Authors: M. Beyermann B. Höfs U.-K. Schkade K. Schmidt Federal coordinating office for questions
A simple calorimetric method to avoid artifacts in a controversial field: the ice calorimeter
 Dufour, J., et al. A simple calorimetric method to avoid artifacts in a controversial field: The ice calorimeter. in ICCF-14 International Conference on Condensed Matter Nuclear Science. 2008. Washington,
Dufour, J., et al. A simple calorimetric method to avoid artifacts in a controversial field: The ice calorimeter. in ICCF-14 International Conference on Condensed Matter Nuclear Science. 2008. Washington,
Final Examination ( ) Date: 19/ 06/ 2014
 Class: F.3 ( ) Baptist Lui Ming Choi Secondary School Final Examination (2013-2014) Date: 19/ 06/ 2014 ame: Form 3 Chemistry Time: 8:40-9:50a.m.(70min) Total number of pages: 10 Answer ALL the questions.
Class: F.3 ( ) Baptist Lui Ming Choi Secondary School Final Examination (2013-2014) Date: 19/ 06/ 2014 ame: Form 3 Chemistry Time: 8:40-9:50a.m.(70min) Total number of pages: 10 Answer ALL the questions.
Grade 8 Science Unit 2 Test» Form A (Master Copy) Directions: Please choose the best answer choice for each of the following questions.
 Directions: Please choose the best answer choice for each of the following questions. 1. Which of the following could be a molecule BUT not a compound? a pair of atoms of the same element a collection
Directions: Please choose the best answer choice for each of the following questions. 1. Which of the following could be a molecule BUT not a compound? a pair of atoms of the same element a collection
Unit 11: Temperature and heat
 Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Corn Sugar (crude & refined) Analysis F MOISTURE (Karl Fischer)
 Corn Sugar (crude & refined) Analysis F-32-1 MOISTURE (Karl Fischer) PRINCIPLE SCOPE The sugar sample is dissolved in a mixture of methanol and formamide (50:50 v/v) and then titrated with standardized
Corn Sugar (crude & refined) Analysis F-32-1 MOISTURE (Karl Fischer) PRINCIPLE SCOPE The sugar sample is dissolved in a mixture of methanol and formamide (50:50 v/v) and then titrated with standardized
SPECIFIC HEAT OF WATER LAB 11-2
 CONCEPT Heat of Fusion Changes of state (phase changes) involve the conversion or transition of matter from one of the common states (solid, liquid or gas) to another. Examples include fusion or melting
CONCEPT Heat of Fusion Changes of state (phase changes) involve the conversion or transition of matter from one of the common states (solid, liquid or gas) to another. Examples include fusion or melting
ISO Water quality Determination of carbon 14 activity Liquid scintillation counting method
 INTERNATIONAL STANDARD ISO 13162 First edition 2011-11-01 Water quality Determination of carbon 14 activity Liquid scintillation counting method Qualité de l eau Détermination de l activité volumique du
INTERNATIONAL STANDARD ISO 13162 First edition 2011-11-01 Water quality Determination of carbon 14 activity Liquid scintillation counting method Qualité de l eau Détermination de l activité volumique du
Paper Reference. London Examinations IGCSE. Foundation Tier. Tuesday 10 November 2009 Afternoon Time: 1 hour 30 minutes
 Centre No. Candidate No. Paper Reference(s) 4335/1F London Examinations IGCSE Chemistry Paper 1F Foundation Tier Tuesday 10 November 2009 Afternoon Time: 1 hour 30 minutes Materials required for examination
Centre No. Candidate No. Paper Reference(s) 4335/1F London Examinations IGCSE Chemistry Paper 1F Foundation Tier Tuesday 10 November 2009 Afternoon Time: 1 hour 30 minutes Materials required for examination
METHOD 3520C CONTINUOUS LIQUID-LIQUID EXTRACTION
 METHOD 3520C CONTINUOUS LIQUID-LIQUID EXTRACTION 1.0 SCOPE AND APPLICATION 1.1 This method describes a procedure for isolating organic compounds from aqueous samples. The method also describes concentration
METHOD 3520C CONTINUOUS LIQUID-LIQUID EXTRACTION 1.0 SCOPE AND APPLICATION 1.1 This method describes a procedure for isolating organic compounds from aqueous samples. The method also describes concentration
Name Class Date. What is a change of state? What happens during a change of state? What can happen when a substance loses or gains energy?
 CHAPTER 2 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
CHAPTER 2 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
Freezing point depression (Item No.: P )
 Freezing point depression (Item No.: P3021101) Curricular Relevance Area of Expertise: Chemistry Education Level: University Topic: General Chemistry Subtopic: Solutions and Mixtures Experiment: Freezing
Freezing point depression (Item No.: P3021101) Curricular Relevance Area of Expertise: Chemistry Education Level: University Topic: General Chemistry Subtopic: Solutions and Mixtures Experiment: Freezing
CHAPTER 2 & 3 WARM-UP
 Name Period Date 1. What is the definition of energy? 2. What is work? CHAPTER 2 & 3 WARM-UP 3. What are the three forms of energy? Give an example of each. 4. Explain chemical potential energy and give
Name Period Date 1. What is the definition of energy? 2. What is work? CHAPTER 2 & 3 WARM-UP 3. What are the three forms of energy? Give an example of each. 4. Explain chemical potential energy and give
CHAPTER 13. States of Matter. Kinetic = motion. Polar vs. Nonpolar. Gases. Hon Chem 13.notebook
 CHAPTER 13 States of Matter States that the tiny particles in all forms of matter are in constant motion. Kinetic = motion A gas is composed of particles, usually molecules or atoms, with negligible volume
CHAPTER 13 States of Matter States that the tiny particles in all forms of matter are in constant motion. Kinetic = motion A gas is composed of particles, usually molecules or atoms, with negligible volume
CHAPTER 8 ENTROPY. Blank
 CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
Citric Acid Analysis L-6-1. MOISTURE (Karl Fischer)
 Citric Acid Analysis L-6-1 MOISTURE (Karl Fischer) PRINCIPLE SCOPE The sample is dissolved in a mixture of methanol and formamide (50:50 v/v) and then titrated with standardized Karl Fischer reagent. The
Citric Acid Analysis L-6-1 MOISTURE (Karl Fischer) PRINCIPLE SCOPE The sample is dissolved in a mixture of methanol and formamide (50:50 v/v) and then titrated with standardized Karl Fischer reagent. The
What is a change of state? What happens during a change of state? What can happen when a substance loses or gains energy?
 CHAPTER 3 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
CHAPTER 3 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
baking soda a solid material in the form of a white powder; also called sodium bicarbonate (IG)
 FOSS Mixtures and Solutions Module Glossary NGSS Edition 2019 analyze to examine carefully (IG) atmosphere the layer of gases surrounding Earth (air) baking soda a solid material in the form of a white
FOSS Mixtures and Solutions Module Glossary NGSS Edition 2019 analyze to examine carefully (IG) atmosphere the layer of gases surrounding Earth (air) baking soda a solid material in the form of a white
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION. Instructions
 THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION 032/1 CHEMISTRY 1 (For Both School and Private Candidates) TIME: 3 Hours Thursday, 05 th October
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION 032/1 CHEMISTRY 1 (For Both School and Private Candidates) TIME: 3 Hours Thursday, 05 th October
CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level. PHYSICS 5054/02 Paper 2 Theory October/November 2003
 Centre Number Candidate Number Name CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level PHYSICS 5054/02 Paper 2 Theory October/November 2003 Candidates answer on the Question
Centre Number Candidate Number Name CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level PHYSICS 5054/02 Paper 2 Theory October/November 2003 Candidates answer on the Question
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *1137125136* PHYSICS 0625/33 Paper 3 Theory (Core) May/June 2016 1 hour 15 minutes Candidates answer
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *1137125136* PHYSICS 0625/33 Paper 3 Theory (Core) May/June 2016 1 hour 15 minutes Candidates answer
2B Air, Oxygen, Carbon Dioxide and Water
 Air, Oxygen, Carbon Dioxide and Water Air, oxygen and carbon dioxide are important chemicals in our everyday lives. Knowledge of their properties helps us to develop an understanding of the role they play.
Air, Oxygen, Carbon Dioxide and Water Air, oxygen and carbon dioxide are important chemicals in our everyday lives. Knowledge of their properties helps us to develop an understanding of the role they play.
3. EFFECTS OF HEAT. Thus, heat can be defined as a form of energy that gives the sensation of hotness or coldness
 3. EFFECTS OF HEAT In the previous class you have learnt that heat is a form of energy. Heat can be obtained from various sources like the sun, fire, etc. When we read the weather forecast we observe that
3. EFFECTS OF HEAT In the previous class you have learnt that heat is a form of energy. Heat can be obtained from various sources like the sun, fire, etc. When we read the weather forecast we observe that
Outline Chapter 5 Matter and Energy Temperature. Measuring Temperature Temperature Temperature. Measuring Temperature
 Outline Chapter 5 Matter and Energy 5-1. Temperature 5-2. Heat 5-3. Metabolic Energy 5-4. Density 5-5. Pressure 5-6. Buoyancy 5-7. Gas Laws 5-8. Kinetic Theory of Gases 5-9. Molecular Motion and Temperature
Outline Chapter 5 Matter and Energy 5-1. Temperature 5-2. Heat 5-3. Metabolic Energy 5-4. Density 5-5. Pressure 5-6. Buoyancy 5-7. Gas Laws 5-8. Kinetic Theory of Gases 5-9. Molecular Motion and Temperature
IGCSE (9-1) Edexcel - Chemistry
 IGCSE (9-1) Edexcel - Chemistry Principles of Chemistry Element, Compounds and Mixtures NOTES 1.8: Understand how to classify a substance as an element, compound or mixture Classifications: S Class Element
IGCSE (9-1) Edexcel - Chemistry Principles of Chemistry Element, Compounds and Mixtures NOTES 1.8: Understand how to classify a substance as an element, compound or mixture Classifications: S Class Element
Matter & Energy. Objectives: properties and structures of the different states of matter.
 Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
The Water Molecule. Like all molecules, a water molecule is neutral. Water is polar. Why are water molecules polar?
 Properties of Water The Water Molecule Like all molecules, a water molecule is neutral. Water is polar Why are water molecules polar? Polarity oxygen atom 8 protons in its nucleus has a much stronger attraction
Properties of Water The Water Molecule Like all molecules, a water molecule is neutral. Water is polar Why are water molecules polar? Polarity oxygen atom 8 protons in its nucleus has a much stronger attraction
Chapter #6 Properties of Matter
 Chapter #6 Properties of Matter Matter anything that occupies space and has mass. Pure Substance is matter with fixed composition, can be an element or a compound. Element a type of atom. About 90 are
Chapter #6 Properties of Matter Matter anything that occupies space and has mass. Pure Substance is matter with fixed composition, can be an element or a compound. Element a type of atom. About 90 are
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level *0955802702* CHEMISTRY 9701/23 Paper 2 Structured Questions AS Core October/November
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Advanced Subsidiary Level and Advanced Level *0955802702* CHEMISTRY 9701/23 Paper 2 Structured Questions AS Core October/November
3. In what state do the following exist at room temperature and standard pressure? a) Diamond c) Mercury e) Oxygen b) Clay d) Cooking oil f) Neon
 CH30S Physical Properties & Change Review 1. Explain why the melting of ice is a physical change, while the rusting of iron is a chemical change. 2. List 4 physical properties of an iron nail. 3. In what
CH30S Physical Properties & Change Review 1. Explain why the melting of ice is a physical change, while the rusting of iron is a chemical change. 2. List 4 physical properties of an iron nail. 3. In what
Ch. 36 Transport in Vascular Plants
 Ch. 36 Transport in Vascular Plants Feb 4 1:32 PM 1 Essential Question: How does a tall tree get the water from its roots to the top of the tree? Feb 4 1:38 PM 2 Shoot architecture and Light Capture: Phyllotaxy
Ch. 36 Transport in Vascular Plants Feb 4 1:32 PM 1 Essential Question: How does a tall tree get the water from its roots to the top of the tree? Feb 4 1:38 PM 2 Shoot architecture and Light Capture: Phyllotaxy
Question 4. Calculate q when 0.10 g of ice is cooled from 10.ºC to -75ºC. (c ice = J/g ºC) A) -18 J B) -14 J C) -8.5 J D) + 14 J E) +18 J 5-4
 Question 1 A system conducts 1.07 kj of heat to the surroundings while delivering 1.79 kj of work. What is the change in internal energy of the system? A) +0.72 kj B) -0.72 kj C) +2.86 kj D) -2.86 kj 5-1
Question 1 A system conducts 1.07 kj of heat to the surroundings while delivering 1.79 kj of work. What is the change in internal energy of the system? A) +0.72 kj B) -0.72 kj C) +2.86 kj D) -2.86 kj 5-1
10 WATER SAMPLING AND LABORATORY TREATMENT
 10 WATER SAMPLING AND LABORATORY TREATMENT This chapter on field and specifically on laboratory practice serves to give the hydrologist as well as the beginning laboratory worker an impression of the experimental
10 WATER SAMPLING AND LABORATORY TREATMENT This chapter on field and specifically on laboratory practice serves to give the hydrologist as well as the beginning laboratory worker an impression of the experimental
LESSON 1: DESCRIBING MATTER pg.5. Chemistry = Is the study of matter & how matter changes. Liquid/Solid/Gas
 Chemistry..CHAPTER 1: INTRO TO MATTER LESSON 1: DESCRIBING MATTER pg.5 Chemistry = Is the study of matter & how matter changes A. Matter = anything that has mass & takes up space à You, air, plastic, metal,
Chemistry..CHAPTER 1: INTRO TO MATTER LESSON 1: DESCRIBING MATTER pg.5 Chemistry = Is the study of matter & how matter changes A. Matter = anything that has mass & takes up space à You, air, plastic, metal,
Chapter 2: Properties of Matter Student Outline 2.1 Classifying Matter A. Pure Substances
 Name: Date: Physical Science Period: Chapter 2: Properties of Matter Student Outline GA Performance Standards SPS1. Students will investigate our current understanding of the atom. SPS2. Students will
Name: Date: Physical Science Period: Chapter 2: Properties of Matter Student Outline GA Performance Standards SPS1. Students will investigate our current understanding of the atom. SPS2. Students will
CLASSIFIED 2 PRESSURE THERMAL PHYSICS MR. HUSSAM SAMIR
 CLASSIFIED 2 PRESSURE THERMAL PHYSICS MR. HUSSAM SAMIR 1. The diagram shows a simple mercury barometer. If atmospheric pressure increases, what happens to level X and to level Y? 2. Four flower vases have
CLASSIFIED 2 PRESSURE THERMAL PHYSICS MR. HUSSAM SAMIR 1. The diagram shows a simple mercury barometer. If atmospheric pressure increases, what happens to level X and to level Y? 2. Four flower vases have
2/22/2019 NEW UNIT! Chemical Interactions. Atomic Basics #19
 NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
1st Nine Weeks Midterm Study Guide
 Name: ate: 1. Which statement correctly describes a property of a type of matter?. ir is a mixture of gases.. Ice is a mixture of gases.. ir is a liquid.. Ice is a liquid. 4. Why is cobalt (o) placed before
Name: ate: 1. Which statement correctly describes a property of a type of matter?. ir is a mixture of gases.. Ice is a mixture of gases.. ir is a liquid.. Ice is a liquid. 4. Why is cobalt (o) placed before
file:///biology Exploring Life/BiologyExploringLife04/
 Objectives Describe the structure of a water molecule. List and describe water's unique properties. Distinguish between an acid and a base. Explain how Earth's conditions are fit for life. Key Terms polar
Objectives Describe the structure of a water molecule. List and describe water's unique properties. Distinguish between an acid and a base. Explain how Earth's conditions are fit for life. Key Terms polar
Lesson Plan: Diffusion
 Lesson Plan: Diffusion Background Particles in cells show rapid back and forth movement, or Brownian motion, which is also known as diffusion. The back and forth motion consists of random steps from a
Lesson Plan: Diffusion Background Particles in cells show rapid back and forth movement, or Brownian motion, which is also known as diffusion. The back and forth motion consists of random steps from a
Describe plant meristems. Where are they located? perpetually embryonic cells found at tips of roots and shoots (apical vs.
 Which conditions have the higher rate of transpiration? Light or dark: Humid or dry: Breezy or still air: Hot or warm: light (need CO 2 for photosyn.) dry (lower H 2 O potential out) breezy (greater evaporation)
Which conditions have the higher rate of transpiration? Light or dark: Humid or dry: Breezy or still air: Hot or warm: light (need CO 2 for photosyn.) dry (lower H 2 O potential out) breezy (greater evaporation)
3. Which of the following would create a chemical change when it is added to a glass of warm milk?
 Test Name: Science 8 Assessment 6 ReviewScan Test Code: 9668632 Date: Your Name: Teacher: Class Name: 1. Which step in these instructions for making scrambled eggs results in a chemical change? First,
Test Name: Science 8 Assessment 6 ReviewScan Test Code: 9668632 Date: Your Name: Teacher: Class Name: 1. Which step in these instructions for making scrambled eggs results in a chemical change? First,
A. GENERAL NOTICES. Ninth Edition, which may be abbreviated as JSFA-IX.
 A. GENERAL NOTICES A. GENERAL NOTICES 1. The title of this book is Japan s Specifications and Standards for Food Additives, Ninth Edition, which may be abbreviated as JSFA-IX. 2. Unless otherwise specified,
A. GENERAL NOTICES A. GENERAL NOTICES 1. The title of this book is Japan s Specifications and Standards for Food Additives, Ninth Edition, which may be abbreviated as JSFA-IX. 2. Unless otherwise specified,
CALORIEMETRY. Similar to the other forms of the energy, The S.I unit of heat is joule. joule is represented as J.
 CALORIEMETRY CALORIMETRY Heat is the kinetic energy due to random motion of the molecules of a substance is called heat energy. Heat is a an invisible energy, that causes in us the sensation of hotness
CALORIEMETRY CALORIMETRY Heat is the kinetic energy due to random motion of the molecules of a substance is called heat energy. Heat is a an invisible energy, that causes in us the sensation of hotness
The CONSORT REACTOR - Analytical Services Group
 The CONSORT REACTOR - Analytical Services Group Nasser Baghini to provide a national neutron activation analysis service The Delayed Neutron Counting (DNC) System consists of: A suitable neutron source
The CONSORT REACTOR - Analytical Services Group Nasser Baghini to provide a national neutron activation analysis service The Delayed Neutron Counting (DNC) System consists of: A suitable neutron source
The Chemistry of Seawater. Unit 3
 The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
The Chemistry of Seawater Unit 3 Water occurs naturally on earth in 3 phases: solid, liquid, or gas (liquid is most abundant) Water Phases Basic Chemistry Review What is an atom? Smallest particles of
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION
 THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION 032/1 CHEMISTRY 1 (For Both School and Private Candidates) Time: 3 Hours Thursday, 06 th November
THE UNITED REPUBLIC OF TANZANIA NATIONAL EXAMINATIONS COUNCIL CERTIFICATE OF SECONDARY EDUCATION EXAMINATION 032/1 CHEMISTRY 1 (For Both School and Private Candidates) Time: 3 Hours Thursday, 06 th November
"In Terms Of" 1. Explain, in terms of electron configuration, why arsenic and antimony are chemically similar.
 Name: Mrs. Vandergoot "In Terms Of" Regents Chemistry 1. Explain, in terms of electron configuration, why arsenic and antimony are chemically similar. 2. Base your answer to the following question on the
Name: Mrs. Vandergoot "In Terms Of" Regents Chemistry 1. Explain, in terms of electron configuration, why arsenic and antimony are chemically similar. 2. Base your answer to the following question on the
Speed Distribution at CONSTANT Temperature is given by the Maxwell Boltzmann Speed Distribution
 Temperature ~ Average KE of each particle Particles have different speeds Gas Particles are in constant RANDOM motion Average KE of each particle is: 3/2 kt Pressure is due to momentum transfer Speed Distribution
Temperature ~ Average KE of each particle Particles have different speeds Gas Particles are in constant RANDOM motion Average KE of each particle is: 3/2 kt Pressure is due to momentum transfer Speed Distribution
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certifi cate of Secondary Education
 *8106479956* UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certifi cate of Secondary Education CHEMISTRY 0620/61 Paper 6 Alternative to Practical October/November 2013 1 hour
*8106479956* UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certifi cate of Secondary Education CHEMISTRY 0620/61 Paper 6 Alternative to Practical October/November 2013 1 hour
