Scanning electron microscopy
|
|
|
- Sheena Burns
- 6 years ago
- Views:
Transcription
1 Scanning electron microscopy Fei Quanta Tabletop Hitachi
2 Example: Tin soldier Pb M Sn L Secondary electrons Backscatter electrons EDS analysis Average composition
3 Learning goals: Understanding the principle Of the instrument Of type of signals detectors Understand how the parameters that you can/must change influences the picture and chemical analysis. Lab: Demonstration and hands on experience with our three different SEMs Table top SEM Environmental SEM High resolution SEM
4 Microscopes then and now
5 Limits to resolution Unaided eye ~ Light microscope ~ Scanning EM ~ Transmission EM ~ 0.1 mm 0.2 mm 1.0 nm 0.1 nm The higher the accelerating voltage, the smaller the wavelength of the electrons and the higher the possible achievable resolution.
6 Instrument: SEM is it like a TEM? In SEM, there are several electromagnetic lenses, including condenser lenses and one objective lens. Electromagnetic lenses are for electron probe formation, not for image formation directly, as in TEM. Two condenser lenses reduce the crossover diameter of the electron beam. The objective lens further reduces the cross-section of the electron beam and focuses the electron beam as probe on the specimen surface.
7 Objective lens The final probe-forming lens has to operate with a relative long working distance (WD= 10 mm), that is the distance between the specimen and lower pole-piece. This is necessary so that the emitted radiation can be collected and detected with desired efficiency. The long working distance increases the spherical aberration of the probeforming lens, which increases the size of the smallest attainable electron-beam spot. Objective lens Cross section Final Lens Probe Forming Labeled as Focus
8 Objective lens designs Out lens In lens Semi-in-lens Electron beam Electron beam Virtual lens Electron beam SED SED Virtual lens SED SED SED Specimen Specimen Virtual lens Features Features Features Easy to observe magnetic sample Possible to observe bigger sample Topographical imaging Deep depth of Focus Ultra high resolution High throughput observation at sample exchange position Variety of signal detecting system to optimize the contrast Specimen Ultra high resolution Possible to observe bigger sample at short WD Variety of signal detecting system to optimize the contrast
9 Compare the three different designs of objective lens : 1:Conventional lens Here the magnetic field of the lens is located inside the lens and the specimen sits away from the field. This leads to that the beam travels unprotected between lens and sample and is more vulnerable to EM disturbance. On the other hand the distance between lens and specimen only has limited effect on the signal strength. 2: Snorkel lens, semi-in-lens or immersion lens Here the magnetic field is projected down below the lens to enclose the specimen if it is located at short working distance. At the same time as the beam is protected from EM disturbance the electrons are effectively captured and led up through the lens to be detected by an in-lens detector. For longer working distance or when a side illumination effect is wanted, a classical SE detector is mounted in the chamber. 3: In-lens Here the sample sits on a TEM-type holder and has a maximum size of ca. 4x4x9 mm. Benefits here are excellent mechanical stability, high electron collection efficiency and very high EDS count rates.
10 Relationship between resolution and focal length
11 Different e - sources and gun types W LaB 6 Schottky FE Cold FE Source size 1 2 mm 1 2 mm nm 3-5 nm Temperature 2300 C 1500 C 1500 C Room temp Brightness [A/cm 2 sr] 1x10 6 1x10 7 5x10 8 2x10 9 Energy spread, E 2.0 ev 1.5 ev 0.5 ev 0.2 ev Stability,%/h 0.1 % 0.2 % 0.2 % 2-3 % Probecurrent 50 na -1 ma 50 na -1 ma >100 na na Life time 1 month 6 months 18 months > 5 years Gun vacuum 10-5 Torr 10-7 Torr 10-9 Torr Torr
12 Magnification of SEM is determined by the ratio of the linear size of the display screen to the linear size of the specimen area being scanned. The linear magnification is given by Electronbeam is scanned across the specimen and the procedure is known as Raster scanning. Raster scanning causes the beam to sequentially cover a rectangular area on the specimen. The signal electrons emitted from the specimen are collected by the detector, amplified and used to reconstruct the image according to one-to-one correlation between scanning points on the specimen and picture points on the screen of cathode ray tube (CRT). CRT converts the electronic signals to a visual display.
13 Condenser Lens Current and Resolution. The current in the condenser lens changes the spot size or diameter of the beam of electrons that scans the sample. More detailed information will be collected when the electron beam scans the same area with a smaller spot size. An increased current or a higher number for the condenser lens (CL) setting, will produce a smaller spot size and in general will result in a better resolution (A).
14
15 Diatome Courtesy of Gokhan Taken by Quanta SEM microscope Magnification: 30000x Sample: Diatome Detector: ETD Voltage: 4.0kV Vacuum: 4.01e-4Pa Horizontal Field Width: 9.93μm Working Distance: 7.7 Spot: Same picture - different size Magnification?
16 Beam samples interaction:
17 Scattering Inelastic scattering refers to a variety of physical processes that act to progressively reduce the energy of the beam electron by transferring that energy to the specimen atoms through interactions with tightly bound inner-shell atomic electrons and loosely bound valence electrons. Although the various inelastic scattering energy loss processes are discrete and independent, Bethe (1930) was able to summarize their collective effects into a continuous energy where E is the beam energy (kev), Z is the atomic number, ρ is the density (g/cm3), A is the atomic weight (g/mol), and J is the mean ionization potential (kev) given by
18 Beam interaction volumes Sample = Si 1μm Vacc : 10kV Vacc : 1kV Images are formed because of beam interaction with the sample This happens in a volume, not in a point The size of this volume varies with beam energy...
19 Beam Excitation Volumes
20 Elastic scattering Simultaneously with inelastic scattering, elastic scattering events occur when the beam electron is deflected by the electrical field of an atom (the positive nuclear charge as partially shielded by the negative charge of the atom s orbital electrons), causing the beam electron to deviate from its previous path onto a new trajectory, as illustrated schematically in the figure. The elastic scattering crossection, can be used to estimate how far the beam electron must travel on average to experience an elastic scattering event, a distance called the mean free path, λ The mean free path is of the order of nm. Elastic scattering is thus likely to occur hundreds to thousands of times along a Bethe range of several hundred to several thousand nanometers.
21 Accelerating voltage
22 Signals in the SEM SE Inner information Electron beam 一一 High energy BSE (I) 一 Low energy BSE (II) 一 一 SE II 一 一 一 一 一 一 SE I SE Surface information Z 一 一 sample SE 一 一 一 一 一 SE escape depth BSE escape depth Z
23 Electron amount Emitted electrons (SE) (BSE) Secondary electron Back scattered electron 50eV Energy of signal electron (ev) (Notice that the scale is logarithmic) When a sample is hit by an electron beam a variety of electron emissions are available.
24 Huskelapp The group of secondary electrons (SE) can be divided into 4 groups. There are SEI, SEII, SEIII, and SEIV. These electrons are classed according to how they are generated. A SEI is an electron is an electron that is generated at the point of primary beam impingement in surface of the specimen. Thus, it carries the highest resolution information. A SEII is an electron that is generated when a backscattered electron leaves the surface of the specimen. Due to the energy of backscattered electrons, this SEII could leave the surface of the specimen microns away from the primary beam impingement site. SEIIs hurt the resolution of the image, but add greatly to overall image brightness. A SEIII is an electron released when an energetic backscattered electron strikes the interior of the specimen chamber, causing a SE to be released. SEIVs are formed when the primary beam strikes an aperature within the electron column. SEIIIs and SEIVs contribute noise to the image. By understanding signal formation, the specimen can be properly prepared for analysis.
25 SE yield variation The rapid change in the incident electron beam range causes a large, characteristic variation in the SE yield Typically the yield rises from ~0.1 at 30keV to in excess of 1 at around 1keV, and as high as 100 for some materials Experimental SE yield data for Ag
26 Why the SE yield changes SE escape depth is ~ 3-5nm At high energies most SE are produced too deep to escape so the SE yield d is low But at lower energies the incident range is so small that most of the SE generated can escape so the SE yield rises rapidly At very low energies fewer SE are produced because less energy is available so the SE yield falls again high voltage low voltage interaction volumes
27 Do high and low kv SE images look the same? compared to the high energy The image looks less 3-D Highlighting is absent Surface junk is more visible
28 Emitted electrons SE:s Change in SE yield at different incident e-beam angles. Occurrence of edge effect in fine structured surfaces. 28
29 Resolution At high energy the SE1 signal typically comes from a volume 3-5nm in diameter, but the SE2 signal from a volume of 1-3µm in diameter. But at low energy the SE1 and SE2 electrons emerge from the same volume because of the reduction in the size of the interaction volume
30 Resolution at ultra-low energies 30eV 500eV 100nm Because C s and C c decrease with the landing energy the imaging resolution is only limited by diffraction
31 BSE Backscatter electrons SE Inner information Electron beam 一一 High energy BSE (I) 一 Low energy BSE (II) 一 一 SE II 一 一 一 一 一 一 SE I SE Surface information Z 一 一 sample SE 一 一 一 一 一 SE escape depth BSE escape depth Z
32 BSE vs. SE detection BSE detector SE Sample : Polyvinyl Alcohol Accelerating Voltage : 3kV, Vacuum: 60Pa, Mag. : 1,000x Sample : Polyvinyl Alcohol Accelerating Voltage : 3kV, Vacuum: 60Pa, Mag. : 1,000x
33 Emitted electrons BSE:s The count ratio of BSE:s depends on the mean atomic number of the specimen. 33
34
35 Detectors on the SEM Electron Detectors: Everhart-Thornley (E-T) detector Located on one side and has a small solid angle of detection.
36 Detectors on the SEM Electron Detectors: Solid-State Diode detector Located on pole piece and has a large solid angle of detection Electron-hole pairs are produced by the action of high energy backscattered electrons. Annular detector split into two semi-circles, A and B. A+B: compositional mode A-B: topographic mode
37 SU8000Series standard optics Variety of signal detection system in SU8200-series Top Upper Signal type Signal name Detector information BSE HA- BSE Top Composition, crystal BSE LA- BSE Upper Composition + Topo (Charge suppression) Electrode SE SE Upper Surface information (Including voltage contrast) Lower SE Lower Lower Topo STEM STEM BF- 1 STEM DF- 1 STEM STEM Lower Sample internal information + Crystal Sample internal information + Composition SE STEM Detector BSE 37 STEM
38
39 Lower detector Electrode LOWER(SE-L) Top EXB Upper Lower Sample SE BSE Sample Vacc : 3.0V Mag. : Photocatalyst : x 50k Nagaoka University of Technology, Faculty of Engineering, Dr. Kazunori Sato courtesy of : Model :SU8020 Topographical image with shadow
40 Upper detector: Pure SE Electrode UPPER(SE) Top EXB Upper Lower Sample Control electrode SE BSE Nagaoka University of Technology, Faculty of Engineering, Sample : Photocatalyst Dr. Kazunori Sato courtesy of : Vacc : 3.0V Mag. : x Surface 50k Model information :SU8020 (incl. Voltage contrast)
41 Upper detector: SE Filtering, LA-BSE signal Electrode UPPER(LA-BSE) Top EXB Upper Lower Sample Control electrode SE BSE Sample Vacc : 3.0V Mag. : Photocatalyst : x 50k Nagaoka University of Technology, Faculty of Engineering, Dr. Kazunori Sato courtesy of : Model :SU8020 Topographical + Compositional information
42 Top detector: High-angle BSE Electrode TOP(HA-BSE) Top EXB Upper Lower SE BSE Sample Control electrode Nagaoka University of Technology, Faculty of Engineering, Sample : Photocatalyst Dr. Kazunori Sato courtesy of : Vacc : 3.0V Mag. : x Compositional 50k Model + Crystal :SU8020 information (Less topographical information)
43 LOWER (SE-L) UPPER (SE) TOP (HA-BSE) UPPER (LA-BSE)
44 Deceleration (cathode lens) In deceleration, a negative voltage is applied to the specimen to decelerate primary electrons before arriving at specimen surface. Primary beam Objective lens Normal mode V R = 0 V Vi = 0.5kV Vi = V o - V R Landing voltage: Vi -V R Specimen -V R : Deceleration voltage Deceleration V R = 1.5kV Vi = 0.5kV Optimum αi Expands Improved resolution
45 But if we accelerate the SE:s? Top Upper Top Upper : Low energy signal : High energy signal V d SE Accelerated SE Accelerated BSE 1 Both of the SE and BSE are accelerated by the deceleration voltage(negative bias) 2 Makes it more difficult to only select the low energy electrons 3 Sample bias and the accelerated BSE:s increase the signal and contrast
46 Lecture 2
47 Microscopy Lecture II EDS, EDX Electron Beam Characteristic X-rays Secondary Electrons (SE) Backscattered Electrons (BSE) Cathodoluminescence Heat Auger Electrons Absorbed Electrons Transmitted Electrons
48 Parameters, imaging, conclusion? We can change 1. Accelerating voltage 2. Magnification 3. Spot size 4. Working distance 5. Beam current 6. Detectors Ask questions in lab: We want: High resolution We have choosen low Accelerating voltage We will repeat inlens detectors Next week
49 Plan: X-rays Detectors Qualitative analyses Quantitative analyses Data from lab(?) Rapport
50 SE and BSE
51 Average PbM Sn L
52 Atomic energy levels and line transition Transitions have different probabilities Lines have different intensities
53 EDS spectra: Origin of Bremsstrahlung and characteristic peaks 1 kev = J
54 EDS spectra: Origin of Bremsstrahlung and characteristic peaks continuum or Bremsstrahlung (breaking radiation) results from deceleration of beam electrons in the electromagnetic field of the atom core combined with energy loss and creation of an X-ray with the same energy
55 Hmmm. Observerte data fra SEM + EDS
56 EDS spectra: Origin of Bremsstrahlung and characteristic peaks cps/ev - Characteristic X-rays are formed by excitation of inner shell electrons - Inner shell electron is ejected and an outer shell electron replaces it - Energy difference is released as an X-ray Fe Mn Cr Mn C Ni Si Cr Fe Ni kev
57 EDS spectra: Origin of Bremsstrahlung and characteristic peaks If beam energy E > E K then a K-electron may be excited Energy of emitted photon can be calculated: E Phot = E 1 E 2 e.g.: Fe L K E 1 = E K = 7.11 kev E 2 = E L = X-ray energy is the difference between two energy levels! 0.71 kev E Ka = 6.40 kev 0.71 kev 7.11 kev 6.40 kev
58 X-ray and AUGER generation process Emission of X-ray Emission of Auger electron Auger and X-ray yield are competing processes
59 Fluorescence yield (ω) ω= fraction of ionisation events producing characteristic X-rays (rest produce Auger electrons) Ge C - ω increases with Z - ω for each shell: ωk ωl ωm - Auger process is favoured for low Z, - fluorescence dominates for high Z + A = for C K 0.5 for Ge K
60 Characteristic peaks: K, L, M series Kα L-familie Fe K-familie Kβ Energy of characteristic peaks is defined by element The higher the atomic number Z the higher the peak energy
61 The K-family of lines S (16) Ca (20) Mn (26) K 1, ev 3692 ev 5900 ev K 2464 ev 4013 ev 6492 ev (K - K 1,2 ) 156 ev 319 ev 592 ev
62 The L-family of lines Mo (42) Ce (58) L ev 4839 ev L 2394 ev 5262 ev L l 2014 ev 4287 ev L l
63 Line intensity relations (2) Lα 1 Lβ 1 Lβ 2 Lγ 1 Ll Lα 2 Lγ 2/3 Spectrum Barium L series, 15 kv α1 : β1 : γ1 = 100 : 52 : 10
64 Line intensity relations (3) Lα 1 (100) Ti-Kα1 BaTiO 3 Barium Ll Lα 2 Lβ 1 (31) Ti-Kβ1 Lβ 2 Lγ 1 (5) Lγ Lγ 2/3 2/3 Spectrum Barium L-series, 15 kv α1 : β1 : γ1 = 100 : 52 : 10 Spectrum BaTiO 3, 15 kv Overlapped Ba L-series and Ti K-series
65
66 Intensity and energy of characteristic lines - Energy of line is defined by - Element - Type of transition - Intensity of line is defined by - probability of producing a hole (vacancy) - probability of electron transition - probability of x-ray emission - concentration
67 Probability of producing a hole: Ionization cross-section for electrons Ionisation cross section for electrons Ionization cross section: probability of excitation maximum ionization cross section: 2,5 x E bind Important for the selection of accelerating voltage.
68 Ionization cross section for electrons A sample with equal amount of Cr,Fe and Ni. Cr: 33% Cr Fe Ni Fe: 33% Ni: 33% U = 10 kev E Cr Fe Ni E exc bind : 1,847 1,561 1,337
69 30 kv and 10 kv Fe/Cr=0,8 Ni/Cr=0,7 Fe/Cr=0,5 Ni/Cr=0,15
70 Isolated Atoms When isolated atoms are considered, the probability of an energetic electron with energy E (kev) ionizing an atom by ejecting an atomic electron bound with ionization energy Ec (kev) can be expressed as a cross section, QI: where ns is the number of electrons in the shell or subshell (e.g., nk = 2), and bs and cs are constants for a given shell (e.g.,bk = 0.35 and ck = 1) E= beam energy Ec= ionization energy
71 5.) X-ray range electron beam (E 0 ) sample surface secondary electrons ca µm ca. 10 µm 3 back-scattered electrons X-rays bremsstrahlung Different excitation ranges for: Bremsstrahlung, - characteristic x-ray radiation and - secondary electrons (SE) - back-scattered electrons (BSE)
72 Detectors on the SEM X-ray Detectors EDS Spectrometer WDS Spectrometer MEF4010 Scanning electron microscopy
73 X-RAY-detectors WDX wavelength dispersive EDX Energy dispersive Energy resolution is 100 times better with the WDX ev EDX 10 ev WDX
74 Kvalitativ analyse
75 Hva har vi tenkt på Valg av akselerasjonsspenning Utsnitt på prøven Vinkel tilting av prøven Arbeidsavstand (WD) Telletid Oppladning (?) Mål: Gode data med god tellestatistikk og god oppløsning
76 Vi kan finne ut mer
77 Quantitative
78 5.) X-ray range Monte Carlo electron-trajectory simulations of interaction volume in iron as function of primary beam energy EHT = 10 kv EHT = 20 kv EHT = 30 kv R d 0,4 µm R d 1,3 µm R d 2,5 µm With higher primary electron energy penetration depth is increasing
79 5.) X-ray range Monte Carlo electron-trajectory simulations of interaction volume as function of atomic number (EHT = 15 kv) Carbon R d 2 µm Iron R d 0,6 µm Gold R d 0,2 µm With higher density penetration depth is decreasing
80 The kv compromise I char increases with increasing E 0 /E c X-ray signal improves R x increases with increasing E 0 /E c X-ray spatial resolution degrades E 0 U ,5 EC
81 Optimum overvoltage The ionization cross-section describes the probability that a particular event will take place: For X-rays: Q = 6.5x10-20 n s b s UE c ln(c s U) n s = number of electrons in a shell E c = critical excitation voltage b s & c s = constants related to the electron shell U = overvoltage (E o /E c ) QE (cm 2 kev 2 ) U = E o /E c Optimum overvoltage U is around Without sufficient overvoltage, x-ray production is dramatically lowered. U
82 ZAF
83 Can't calculate ZAFs unless concentration is known. But we don t know the concentration?? Use k values (I/I = k) to estimate compositions of each element. Then calculate ZAFs, and refine by iteration Z at. no. correction Function of electron backscattering factor & electron stopping power - depend upon the average atomic number of unknown and standard Dependent on ionization cross section Varies with composition and accelerating voltage A absorption correction Varies with m, takeoff angle, accelerating voltage F fluorescence correction primary fluorescent x-rays > secondary fluorescent x-rays Varies with composition and accelerating voltage
84 CalcZAF
85 kv=15 vinkel = 40
86 Mass absorption coefficients Mass absorption coefficients are stored as a matrix of numbers of absorption of a particular X-ray line (the emitter) by an absorber: For example, a portion of the MAC matrix for Kα X-rays for Z = 23 to 29 is shown below. Mass absorption coefficients Emitter V 4952 ev Cr 5415 ev Mn 5899 ev Fe 6403 ev Co 6930 ev Ni 7478 ev Cu 8048 ev V Cr Mn Fe Co Ni Cu Note that the MAC for absorption of Fe Kα by Co (79.0) is different from the MAC for absorption of Co Kα by Fe (57.1). ds/quantitative/
87
88 Sample surface and absorption electron beam SDD rough surface X-rays tilted polished surface take-off angle polished surface negative tilt angle -TA d2 d1 d1: distance to (imaginary) polished surface d2: actual distance to specimen surface
EDS User School. Principles of Electron Beam Microanalysis
 EDS User School Principles of Electron Beam Microanalysis Outline 1.) Beam-specimen interactions 2.) EDS spectra: Origin of Bremsstrahlung and characteristic peaks 3.) Moseley s law 4.) Characteristic
EDS User School Principles of Electron Beam Microanalysis Outline 1.) Beam-specimen interactions 2.) EDS spectra: Origin of Bremsstrahlung and characteristic peaks 3.) Moseley s law 4.) Characteristic
MT Electron microscopy Scanning electron microscopy and electron probe microanalysis
 MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn?
 Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn? EPMA - what is it? Precise and accurate quantitative chemical analyses of micron-size
Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn? EPMA - what is it? Precise and accurate quantitative chemical analyses of micron-size
MT Electron microscopy Scanning electron microscopy and electron probe microanalysis
 MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
MT-0.6026 Electron microscopy Scanning electron microscopy and electron probe microanalysis Eero Haimi Research Manager Outline 1. Introduction Basics of scanning electron microscopy (SEM) and electron
Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist
 12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist
 12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
12.141 Electron Microprobe Analysis 1 Nilanjan Chatterjee, Ph.D. Principal Research Scientist Massachusetts Institute of Technology Electron Microprobe Facility Department of Earth, Atmospheric and Planetary
Basic structure of SEM
 Table of contents Basis structure of SEM SEM imaging modes Comparison of ordinary SEM and FESEM Electron behavior Electron matter interaction o Elastic interaction o Inelastic interaction o Interaction
Table of contents Basis structure of SEM SEM imaging modes Comparison of ordinary SEM and FESEM Electron behavior Electron matter interaction o Elastic interaction o Inelastic interaction o Interaction
Generation of X-Rays in the SEM specimen
 Generation of X-Rays in the SEM specimen The electron beam generates X-ray photons in the beam-specimen interaction volume beneath the specimen surface. Some X-ray photons emerging from the specimen have
Generation of X-Rays in the SEM specimen The electron beam generates X-ray photons in the beam-specimen interaction volume beneath the specimen surface. Some X-ray photons emerging from the specimen have
AP5301/ Name the major parts of an optical microscope and state their functions.
 Review Problems on Optical Microscopy AP5301/8301-2015 1. Name the major parts of an optical microscope and state their functions. 2. Compare the focal lengths of two glass converging lenses, one with
Review Problems on Optical Microscopy AP5301/8301-2015 1. Name the major parts of an optical microscope and state their functions. 2. Compare the focal lengths of two glass converging lenses, one with
HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS. SCSAM Short Course Amir Avishai
 HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS SCSAM Short Course Amir Avishai RESEARCH QUESTIONS Sea Shell Cast Iron EDS+SE Fe Cr C Objective Ability to ask the
HOW TO APPROACH SCANNING ELECTRON MICROSCOPY AND ENERGY DISPERSIVE SPECTROSCOPY ANALYSIS SCSAM Short Course Amir Avishai RESEARCH QUESTIONS Sea Shell Cast Iron EDS+SE Fe Cr C Objective Ability to ask the
object objective lens eyepiece lens
 Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
h p λ = mν Back to de Broglie and the electron as a wave you will learn more about this Equation in CHEM* 2060
 Back to de Broglie and the electron as a wave λ = mν h = h p you will learn more about this Equation in CHEM* 2060 We will soon see that the energies (speed for now if you like) of the electrons in the
Back to de Broglie and the electron as a wave λ = mν h = h p you will learn more about this Equation in CHEM* 2060 We will soon see that the energies (speed for now if you like) of the electrons in the
= 6 (1/ nm) So what is probability of finding electron tunneled into a barrier 3 ev high?
 STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
Scanning Electron Microscopy
 Scanning Electron Microscopy Field emitting tip Grid 2kV 100kV Anode ZEISS SUPRA Variable Pressure FESEM Dr Heath Bagshaw CMA bagshawh@tcd.ie Why use an SEM? Fig 1. Examples of features resolvable using
Scanning Electron Microscopy Field emitting tip Grid 2kV 100kV Anode ZEISS SUPRA Variable Pressure FESEM Dr Heath Bagshaw CMA bagshawh@tcd.ie Why use an SEM? Fig 1. Examples of features resolvable using
Part II: Thin Film Characterization
 Part II: Thin Film Characterization General details of thin film characterization instruments 1. Introduction to Thin Film Characterization Techniques 2. Structural characterization: SEM, TEM, AFM, STM
Part II: Thin Film Characterization General details of thin film characterization instruments 1. Introduction to Thin Film Characterization Techniques 2. Structural characterization: SEM, TEM, AFM, STM
Modern Optical Spectroscopy
 Modern Optical Spectroscopy X-Ray Microanalysis Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Backscattered
Modern Optical Spectroscopy X-Ray Microanalysis Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Backscattered
Chemical Analysis in TEM: XEDS, EELS and EFTEM. HRTEM PhD course Lecture 5
 Chemical Analysis in TEM: XEDS, EELS and EFTEM HRTEM PhD course Lecture 5 1 Part IV Subject Chapter Prio x-ray spectrometry 32 1 Spectra and mapping 33 2 Qualitative XEDS 34 1 Quantitative XEDS 35.1-35.4
Chemical Analysis in TEM: XEDS, EELS and EFTEM HRTEM PhD course Lecture 5 1 Part IV Subject Chapter Prio x-ray spectrometry 32 1 Spectra and mapping 33 2 Qualitative XEDS 34 1 Quantitative XEDS 35.1-35.4
Massachusetts Institute of Technology. Dr. Nilanjan Chatterjee
 Massachusetts Institute of Technology Dr. Nilanjan Chatterjee Electron Probe Micro-Analysis (EPMA) Imaging and micrometer-scale chemical compositional analysis of solids Signals produced in The Electron
Massachusetts Institute of Technology Dr. Nilanjan Chatterjee Electron Probe Micro-Analysis (EPMA) Imaging and micrometer-scale chemical compositional analysis of solids Signals produced in The Electron
Practical course in scanning electron microscopy
 Practical course in scanning electron microscopy Fortgeschrittenen Praktikum an der Technischen Universität München Wintersemester 2017/2018 Table of contents 1. Introduction 3 2. Formation of an electron
Practical course in scanning electron microscopy Fortgeschrittenen Praktikum an der Technischen Universität München Wintersemester 2017/2018 Table of contents 1. Introduction 3 2. Formation of an electron
SEM. Chemical Analysis in the. Elastic and Inelastic scattering. Chemical analysis in the SEM. Chemical analysis in the SEM
 THE UNIVERSITY Chemical Analysis in the SEM Ian Jones Centre for Electron Microscopy OF BIRMINGHAM Elastic and Inelastic scattering Electron interacts with one of the orbital electrons Secondary electrons,
THE UNIVERSITY Chemical Analysis in the SEM Ian Jones Centre for Electron Microscopy OF BIRMINGHAM Elastic and Inelastic scattering Electron interacts with one of the orbital electrons Secondary electrons,
Analytical Methods for Materials
 Analytical Methods for Materials Lesson 21 Electron Microscopy and X-ray Spectroscopy Suggested Reading Leng, Chapter 3, pp. 83-126; Chapter 4, pp. 127-160; Chapter 6, pp. 191-219 P.J. Goodhew, J. Humphreys
Analytical Methods for Materials Lesson 21 Electron Microscopy and X-ray Spectroscopy Suggested Reading Leng, Chapter 3, pp. 83-126; Chapter 4, pp. 127-160; Chapter 6, pp. 191-219 P.J. Goodhew, J. Humphreys
Gaetano L Episcopo. Scanning Electron Microscopy Focus Ion Beam and. Pulsed Plasma Deposition
 Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
Chapter 9. Electron mean free path Microscopy principles of SEM, TEM, LEEM
 Chapter 9 Electron mean free path Microscopy principles of SEM, TEM, LEEM 9.1 Electron Mean Free Path 9. Scanning Electron Microscopy (SEM) -SEM design; Secondary electron imaging; Backscattered electron
Chapter 9 Electron mean free path Microscopy principles of SEM, TEM, LEEM 9.1 Electron Mean Free Path 9. Scanning Electron Microscopy (SEM) -SEM design; Secondary electron imaging; Backscattered electron
CBE Science of Engineering Materials. Scanning Electron Microscopy (SEM)
 CBE 30361 Science of Engineering Materials Scanning Electron Microscopy (SEM) Scale of Structure Organization Units: micrometer = 10-6 m = 1µm nanometer= 10-9 m = 1nm Angstrom = 10-10 m = 1Å A hair is
CBE 30361 Science of Engineering Materials Scanning Electron Microscopy (SEM) Scale of Structure Organization Units: micrometer = 10-6 m = 1µm nanometer= 10-9 m = 1nm Angstrom = 10-10 m = 1Å A hair is
6. Analytical Electron Microscopy
 Physical Principles of Electron Microscopy 6. Analytical Electron Microscopy Ray Egerton University of Alberta and National Institute of Nanotechnology Edmonton, Canada www.tem-eels.ca regerton@ualberta.ca
Physical Principles of Electron Microscopy 6. Analytical Electron Microscopy Ray Egerton University of Alberta and National Institute of Nanotechnology Edmonton, Canada www.tem-eels.ca regerton@ualberta.ca
Praktikum zur. Materialanalytik
 Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
Praktikum zur Materialanalytik Energy Dispersive X-ray Spectroscopy B513 Stand: 19.10.2016 Contents 1 Introduction... 2 2. Fundamental Physics and Notation... 3 2.1. Alignments of the microscope... 3 2.2.
MSE 321 Structural Characterization
 Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Everhart-Thornley detector
 SEI Detector Everhart-Thornley detector Microscope chamber wall Faraday cage Scintillator Electrons in Light pipe Photomultiplier Electrical signal out Screen Quartz window +200 V +10 kv Always contains
SEI Detector Everhart-Thornley detector Microscope chamber wall Faraday cage Scintillator Electrons in Light pipe Photomultiplier Electrical signal out Screen Quartz window +200 V +10 kv Always contains
X-RAY SPECTRA. Theory:
 12 Oct 18 X-ray.1 X-RAY SPECTRA In this experiment, a number of measurements involving x-rays will be made. The spectrum of x-rays emitted from a molybdenum target will be measured, and the experimental
12 Oct 18 X-ray.1 X-RAY SPECTRA In this experiment, a number of measurements involving x-rays will be made. The spectrum of x-rays emitted from a molybdenum target will be measured, and the experimental
X-ray Energy Spectroscopy (XES).
 X-ray Energy Spectroscopy (XES). X-ray fluorescence as an analytical tool for element analysis is based on 3 fundamental parameters: A. Specificity: In determining an x-ray emission energy E certainty
X-ray Energy Spectroscopy (XES). X-ray fluorescence as an analytical tool for element analysis is based on 3 fundamental parameters: A. Specificity: In determining an x-ray emission energy E certainty
Electron Microprobe Analysis and Scanning Electron Microscopy
 Electron Microprobe Analysis and Scanning Electron Microscopy Electron microprobe analysis (EMPA) Analytical technique in which a beam of electrons is focused on a sample surface, producing X-rays from
Electron Microprobe Analysis and Scanning Electron Microscopy Electron microprobe analysis (EMPA) Analytical technique in which a beam of electrons is focused on a sample surface, producing X-rays from
MSE 321 Structural Characterization
 Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Auger Spectroscopy Auger Electron Spectroscopy (AES) Scanning Auger Microscopy (SAM) Incident Electron Ejected Electron Auger Electron Initial State Intermediate State Final State Physical Electronics
Chemistry 311: Instrumentation Analysis Topic 2: Atomic Spectroscopy. Chemistry 311: Instrumentation Analysis Topic 2: Atomic Spectroscopy
 Topic 2b: X-ray Fluorescence Spectrometry Text: Chapter 12 Rouessac (1 week) 4.0 X-ray Fluorescence Download, read and understand EPA method 6010C ICP-OES Winter 2009 Page 1 Atomic X-ray Spectrometry Fundamental
Topic 2b: X-ray Fluorescence Spectrometry Text: Chapter 12 Rouessac (1 week) 4.0 X-ray Fluorescence Download, read and understand EPA method 6010C ICP-OES Winter 2009 Page 1 Atomic X-ray Spectrometry Fundamental
Transmission Electron Microscopy
 L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
L. Reimer H. Kohl Transmission Electron Microscopy Physics of Image Formation Fifth Edition el Springer Contents 1 Introduction... 1 1.1 Transmission Electron Microscopy... 1 1.1.1 Conventional Transmission
MSE 321 Structural Characterization
 Optical Microscope Plan Lenses In an "ideal" single-element lens system all planar wave fronts are focused to a point at distance f from the lens; therefore: Image near the optical axis will be in perfect
Optical Microscope Plan Lenses In an "ideal" single-element lens system all planar wave fronts are focused to a point at distance f from the lens; therefore: Image near the optical axis will be in perfect
Interactions with Matter
 Manetic Lenses Manetic fields can displace electrons Manetic field can be produced by passin an electrical current throuh coils of wire Manetic field strenth can be increased by usin a soft ferromanetic
Manetic Lenses Manetic fields can displace electrons Manetic field can be produced by passin an electrical current throuh coils of wire Manetic field strenth can be increased by usin a soft ferromanetic
Interaction X-rays - Matter
 Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
Electron Microscopy I
 Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Characterization of Catalysts and Surfaces Characterization Techniques in Heterogeneous Catalysis Electron Microscopy I Introduction Properties of electrons Electron-matter interactions and their applications
Chapter Six: X-Rays. 6.1 Discovery of X-rays
 Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
X-RAY PRODUCTION. Prepared by:- EN KAMARUL AMIN BIN ABDULLAH
 X-RAY PRODUCTION Prepared by:- EN KAMARUL AMIN BIN ABDULLAH OBJECTIVES Discuss the process of x-ray being produced (conditions) Explain the principles of energy conversion in x-ray production (how energy
X-RAY PRODUCTION Prepared by:- EN KAMARUL AMIN BIN ABDULLAH OBJECTIVES Discuss the process of x-ray being produced (conditions) Explain the principles of energy conversion in x-ray production (how energy
Chemistry Instrumental Analysis Lecture 19 Chapter 12. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
Chemistry 4631 Instrumental Analysis Lecture 19 Chapter 12 There are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry X-ray Techniques include:
PHYS-E0541:Special Course in Physics Gas phase synthesis of carbon nanotubes for thin film application. Electron Microscopy. for
 PHYS-E0541:Special Course in Physics Gas phase synthesis of carbon nanotubes for thin film application Electron Microscopy for Introduction to Electron Microscopy Carbon Nanomaterials (nanotubes) Dr. Hua
PHYS-E0541:Special Course in Physics Gas phase synthesis of carbon nanotubes for thin film application Electron Microscopy for Introduction to Electron Microscopy Carbon Nanomaterials (nanotubes) Dr. Hua
Understanding X-rays: The electromagnetic spectrum
 Understanding X-rays: The electromagnetic spectrum 1 ULa 13.61 kev 0.09 nm BeKa 0.11 kev 11.27 nm E = hn = h c l where, E : energy, h : Planck's constant, n : frequency c : speed of light in vacuum, l
Understanding X-rays: The electromagnetic spectrum 1 ULa 13.61 kev 0.09 nm BeKa 0.11 kev 11.27 nm E = hn = h c l where, E : energy, h : Planck's constant, n : frequency c : speed of light in vacuum, l
Understanding X-rays: The electromagnetic spectrum
 Understanding X-rays: The electromagnetic spectrum 1 ULa 13.61 kev 0.09 nm BeKa 0.11 kev 11.27 nm E = hn = h c l where, E : energy, h : Planck's constant, n : frequency c : speed of light in vacuum, l
Understanding X-rays: The electromagnetic spectrum 1 ULa 13.61 kev 0.09 nm BeKa 0.11 kev 11.27 nm E = hn = h c l where, E : energy, h : Planck's constant, n : frequency c : speed of light in vacuum, l
Part I Basics and Methods
 j1 Part I Basics and Methods In-situ Electron Microscopy: Applications in Physics, Chemistry and Materials Science, First Edition. Edited by Gerhard Dehm, James M. Howe, and Josef Zweck. Ó 2012 Wiley-VCH
j1 Part I Basics and Methods In-situ Electron Microscopy: Applications in Physics, Chemistry and Materials Science, First Edition. Edited by Gerhard Dehm, James M. Howe, and Josef Zweck. Ó 2012 Wiley-VCH
ECE Semiconductor Device and Material Characterization
 ECE 4813 Semiconductor Device and Material Characterization Dr. Alan Doolittle School of Electrical and Computer Engineering Georgia Institute of Technology As with all of these lecture slides, I am indebted
ECE 4813 Semiconductor Device and Material Characterization Dr. Alan Doolittle School of Electrical and Computer Engineering Georgia Institute of Technology As with all of these lecture slides, I am indebted
4. Inelastic Scattering
 1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
Weak-Beam Dark-Field Technique
 Basic Idea recall bright-field contrast of dislocations: specimen close to Bragg condition, s î 0 Weak-Beam Dark-Field Technique near the dislocation core, some planes curved to s = 0 ) strong Bragg reflection
Basic Idea recall bright-field contrast of dislocations: specimen close to Bragg condition, s î 0 Weak-Beam Dark-Field Technique near the dislocation core, some planes curved to s = 0 ) strong Bragg reflection
Introduction to EDX. Energy Dispersive X-ray Microanalysis (EDS, Energy dispersive Spectroscopy) Basics of EDX
 Introduction to EDX Energy Dispersive X-ray Microanalysis (EDS, Energy dispersive Spectroscopy) EDX Marco Cantoni 1 Basics of EDX a) Generation of X-rays b) Detection Si(Li) Detector, SDD Detector, EDS
Introduction to EDX Energy Dispersive X-ray Microanalysis (EDS, Energy dispersive Spectroscopy) EDX Marco Cantoni 1 Basics of EDX a) Generation of X-rays b) Detection Si(Li) Detector, SDD Detector, EDS
Basic physics Questions
 Chapter1 Basic physics Questions S. Ilyas 1. Which of the following statements regarding protons are correct? a. They have a negative charge b. They are equal to the number of electrons in a non-ionized
Chapter1 Basic physics Questions S. Ilyas 1. Which of the following statements regarding protons are correct? a. They have a negative charge b. They are equal to the number of electrons in a non-ionized
X-Ray Photoelectron Spectroscopy (XPS) Auger Electron Spectroscopy (AES)
 X-Ray Photoelectron Spectroscopy (XPS) Auger Electron Spectroscopy (AES) XPS X-ray photoelectron spectroscopy (XPS) is one of the most used techniques to chemically characterize the surface. Also known
X-Ray Photoelectron Spectroscopy (XPS) Auger Electron Spectroscopy (AES) XPS X-ray photoelectron spectroscopy (XPS) is one of the most used techniques to chemically characterize the surface. Also known
The illumination source: the electron beam
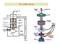 The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
Emphasis on what happens to emitted particle (if no nuclear reaction and MEDIUM (i.e., atomic effects)
 LECTURE 5: INTERACTION OF RADIATION WITH MATTER All radiation is detected through its interaction with matter! INTRODUCTION: What happens when radiation passes through matter? Emphasis on what happens
LECTURE 5: INTERACTION OF RADIATION WITH MATTER All radiation is detected through its interaction with matter! INTRODUCTION: What happens when radiation passes through matter? Emphasis on what happens
Why microscopy?
 Electron Microscopy Why microscopy? http://www.cellsalive.com/howbig.htm 2 Microscopes are used as magnifying tools (although not exclusively as will see later on). The resolution of the human eye is limited
Electron Microscopy Why microscopy? http://www.cellsalive.com/howbig.htm 2 Microscopes are used as magnifying tools (although not exclusively as will see later on). The resolution of the human eye is limited
Lecture 5. X-ray Photoemission Spectroscopy (XPS)
 Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
Lecture 5 X-ray Photoemission Spectroscopy (XPS) 5. Photoemission Spectroscopy (XPS) 5. Principles 5.2 Interpretation 5.3 Instrumentation 5.4 XPS vs UV Photoelectron Spectroscopy (UPS) 5.5 Auger Electron
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu
 Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
Auger Electron Spectroscopy (AES) Prof. Paul K. Chu Auger Electron Spectroscopy Introduction Principles Instrumentation Qualitative analysis Quantitative analysis Depth profiling Mapping Examples The Auger
Imaging Methods: Scanning Force Microscopy (SFM / AFM)
 Imaging Methods: Scanning Force Microscopy (SFM / AFM) The atomic force microscope (AFM) probes the surface of a sample with a sharp tip, a couple of microns long and often less than 100 Å in diameter.
Imaging Methods: Scanning Force Microscopy (SFM / AFM) The atomic force microscope (AFM) probes the surface of a sample with a sharp tip, a couple of microns long and often less than 100 Å in diameter.
Characterisation of Catalysts Using Secondary and Backscattered Electron In-lens Detectors
 Platinum Metals Rev., 2014, 58, (2), 106 110 FINAL ANALYSIS Characterisation of Catalysts Using Secondary and Backscattered Electron In-lens Detectors Heterogeneous catalysis often involves the use of
Platinum Metals Rev., 2014, 58, (2), 106 110 FINAL ANALYSIS Characterisation of Catalysts Using Secondary and Backscattered Electron In-lens Detectors Heterogeneous catalysis often involves the use of
1 of 5 14/10/ :21
 X-ray absorption s, characteristic X-ray lines... 4.2.1 Home About Table of Contents Advanced Search Copyright Feedback Privacy You are here: Chapter: 4 Atomic and nuclear physics Section: 4.2 Absorption
X-ray absorption s, characteristic X-ray lines... 4.2.1 Home About Table of Contents Advanced Search Copyright Feedback Privacy You are here: Chapter: 4 Atomic and nuclear physics Section: 4.2 Absorption
Overview of X-Ray Fluorescence Analysis
 Overview of X-Ray Fluorescence Analysis AMPTEK, INC., Bedford, MA 01730 Ph: +1 781 275 2242 Fax: +1 781 275 3470 sales@amptek.com 1 What is X-Ray Fluorescence (XRF)? A physical process: Emission of characteristic
Overview of X-Ray Fluorescence Analysis AMPTEK, INC., Bedford, MA 01730 Ph: +1 781 275 2242 Fax: +1 781 275 3470 sales@amptek.com 1 What is X-Ray Fluorescence (XRF)? A physical process: Emission of characteristic
Electron and electromagnetic radiation
 Electron and electromagnetic radiation Generation and interactions with matter Stimuli Interaction with sample Response Stimuli Waves and energy The energy is propotional to 1/λ and 1/λ 2 λ λ 1 Electromagnetic
Electron and electromagnetic radiation Generation and interactions with matter Stimuli Interaction with sample Response Stimuli Waves and energy The energy is propotional to 1/λ and 1/λ 2 λ λ 1 Electromagnetic
Invited Lecture. "Different Aspects of Electron Microscopy. Sardar Vallabhbhai National Institute of Technology, Surat. Deepak Rajput & S.K.
 Invited Lecture on "Different Aspects of Electron Microscopy at Sardar Vallabhbhai National Institute of Technology, Surat Deepak Rajput & S.K. Tiwary R&D and Product Development Essar Steel Limited Abstract
Invited Lecture on "Different Aspects of Electron Microscopy at Sardar Vallabhbhai National Institute of Technology, Surat Deepak Rajput & S.K. Tiwary R&D and Product Development Essar Steel Limited Abstract
XRF books: Analytical Chemistry, Kellner/Mermet/Otto/etc. 3 rd year XRF Spectroscopy Dr. Alan Ryder (R222, Physical Chemistry) 2 lectures:
 1 3 rd year XRF Spectroscopy Dr. Alan Ryder (R222, Physical Chemistry) 2 lectures: XRF spectroscopy 1 exam question. Notes on: www.nuigalway.ie/nanoscale/3rdspectroscopy.html XRF books: Analytical Chemistry,
1 3 rd year XRF Spectroscopy Dr. Alan Ryder (R222, Physical Chemistry) 2 lectures: XRF spectroscopy 1 exam question. Notes on: www.nuigalway.ie/nanoscale/3rdspectroscopy.html XRF books: Analytical Chemistry,
SEM stands for Scanning Electron Microscopy. The earliest known work describing
 1. HISTORY ABOUT SEM SEM stands for Scanning Electron Microscopy. The earliest known work describing the concept of a Scanning Electron Microscope was by M. Knoll (1935) who, along with other pioneers
1. HISTORY ABOUT SEM SEM stands for Scanning Electron Microscopy. The earliest known work describing the concept of a Scanning Electron Microscope was by M. Knoll (1935) who, along with other pioneers
Analytical Methods for Materials
 Analytical Methods for Materials Lesson 6 Production & Properties of X-rays Suggested Reading Chapter 1 in Waseda et al. Section 2.1 in Leng Other Reference B.D. Cullity and S.R. Stock, Elements of X-ray
Analytical Methods for Materials Lesson 6 Production & Properties of X-rays Suggested Reading Chapter 1 in Waseda et al. Section 2.1 in Leng Other Reference B.D. Cullity and S.R. Stock, Elements of X-ray
Nano-Microscopy. Lecture 2. Scanning and Transmission Electron Microscopies: Principles. Pavel Zinin HIGP, University of Hawaii, Honolulu, USA
 GG 711: Advanced Techniques in Geophysics and Materials Science Nano-Microscopy. Lecture 2 Scanning and Transmission Electron Microscopies: Principles Pavel Zinin HIGP, University of Hawaii, Honolulu,
GG 711: Advanced Techniques in Geophysics and Materials Science Nano-Microscopy. Lecture 2 Scanning and Transmission Electron Microscopies: Principles Pavel Zinin HIGP, University of Hawaii, Honolulu,
LAB REPORT ON XRF OF POTTERY SAMPLES By BIJOY KRISHNA HALDER Mohammad Arif Ishtiaque Shuvo Jie Hong
 LAB REPORT ON XRF OF POTTERY SAMPLES By BIJOY KRISHNA HALDER Mohammad Arif Ishtiaque Shuvo Jie Hong Introduction: X-ray fluorescence (XRF) spectrometer is an x-ray instrument used for routine, relatively
LAB REPORT ON XRF OF POTTERY SAMPLES By BIJOY KRISHNA HALDER Mohammad Arif Ishtiaque Shuvo Jie Hong Introduction: X-ray fluorescence (XRF) spectrometer is an x-ray instrument used for routine, relatively
X-Ray Emission and Absorption
 X-Ray Emission and Absorption Author: Mike Nill Alex Bryant February 6, 20 Abstract X-rays were produced by two bench-top diffractometers using a copper target. Various nickel filters were placed in front
X-Ray Emission and Absorption Author: Mike Nill Alex Bryant February 6, 20 Abstract X-rays were produced by two bench-top diffractometers using a copper target. Various nickel filters were placed in front
CHEM-E5225 :Electron Microscopy X-Ray Spectrometry
 CHEM-E5225 :Electron Microscopy X-Ray Spectrometry 2016.11 Yanling Ge Outline X-ray Spectrometry X-ray Spectra and Images Qualitative and Quantitative X-ray Analysis and Imaging Discussion of homework
CHEM-E5225 :Electron Microscopy X-Ray Spectrometry 2016.11 Yanling Ge Outline X-ray Spectrometry X-ray Spectra and Images Qualitative and Quantitative X-ray Analysis and Imaging Discussion of homework
X-ray Absorption Spectroscopy
 X-ray Absorption Spectroscopy Nikki Truss November 26, 2012 Abstract In these experiments, some aspects of x-ray absorption spectroscopy were investigated. The x-ray spectrum of molybdenum was recorded
X-ray Absorption Spectroscopy Nikki Truss November 26, 2012 Abstract In these experiments, some aspects of x-ray absorption spectroscopy were investigated. The x-ray spectrum of molybdenum was recorded
FUNDAMENTAL PARAMETER METHOD FOR THE LOW ENERGY REGION INCLUDING CASCADE EFFECT AND PHOTOELECTRON EXCITATION
 Copyright (c)jcpds-international Centre for Diffraction Data 2002, Advances in X-ray Analysis, Volume 45. 511 FUNDAMENTAL PARAMETER METHOD FOR THE LOW ENERGY REGION INCLUDING CASCADE EFFECT AND PHOTOELECTRON
Copyright (c)jcpds-international Centre for Diffraction Data 2002, Advances in X-ray Analysis, Volume 45. 511 FUNDAMENTAL PARAMETER METHOD FOR THE LOW ENERGY REGION INCLUDING CASCADE EFFECT AND PHOTOELECTRON
Information from Every Angle
 pplication Note Information from Every ngle Directional SE Detector for Next-Level Imaging Zinc oxide nanorods with surficial palladium particles imaged at 500 V in high vacuum. dding palladium increases
pplication Note Information from Every ngle Directional SE Detector for Next-Level Imaging Zinc oxide nanorods with surficial palladium particles imaged at 500 V in high vacuum. dding palladium increases
Scanning Electron Microscopy & Ancillary Techniques
 Scanning Electron Microscopy & Ancillary Techniques By Pablo G. Caceres-Valencia The prototype of the first Stereoscan supplied by the Cambridge Instrument Company to the dupont Company, U.S.A. (1965)
Scanning Electron Microscopy & Ancillary Techniques By Pablo G. Caceres-Valencia The prototype of the first Stereoscan supplied by the Cambridge Instrument Company to the dupont Company, U.S.A. (1965)
CHARACTERIZATION of NANOMATERIALS KHP
 CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
Decay Mechanisms. The laws of conservation of charge and of nucleons require that for alpha decay, He + Q 3.1
 Decay Mechanisms 1. Alpha Decay An alpha particle is a helium-4 nucleus. This is a very stable entity and alpha emission was, historically, the first decay process to be studied in detail. Almost all naturally
Decay Mechanisms 1. Alpha Decay An alpha particle is a helium-4 nucleus. This is a very stable entity and alpha emission was, historically, the first decay process to be studied in detail. Almost all naturally
EEE4106Z Radiation Interactions & Detection
 EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
EPMA IMAGES. Figure 9. Energy-dispersive spectra of spot mineral analyses in sample 89GGR-33A for locations 1-5 in Figure 8.
 EPMA IMAGES The attached images and mineral data can be used to supplement an instrument-based lab, or serve as the basis for lab that can be completed without an instrument. Please provide credit for
EPMA IMAGES The attached images and mineral data can be used to supplement an instrument-based lab, or serve as the basis for lab that can be completed without an instrument. Please provide credit for
Introduction to X-ray Photoelectron Spectroscopy (XPS) XPS which makes use of the photoelectric effect, was developed in the mid-1960
 Introduction to X-ray Photoelectron Spectroscopy (XPS) X-ray Photoelectron Spectroscopy (XPS), also known as Electron Spectroscopy for Chemical Analysis (ESCA) is a widely used technique to investigate
Introduction to X-ray Photoelectron Spectroscopy (XPS) X-ray Photoelectron Spectroscopy (XPS), also known as Electron Spectroscopy for Chemical Analysis (ESCA) is a widely used technique to investigate
Atomic Physics. Chapter 6 X ray. Jinniu Hu 24/12/ /20/13
 Atomic Physics Chapter 6 X ray 11/20/13 24/12/2018 Jinniu Hu 1!1 6.1 The discovery of X ray X-rays were discovered in 1895 by the German physicist Wilhelm Roentgen. He found that a beam of high-speed electrons
Atomic Physics Chapter 6 X ray 11/20/13 24/12/2018 Jinniu Hu 1!1 6.1 The discovery of X ray X-rays were discovered in 1895 by the German physicist Wilhelm Roentgen. He found that a beam of high-speed electrons
Nuclear Physics and Astrophysics
 Nuclear Physics and Astrophysics PHY-30 Dr. E. Rizvi Lecture 4 - Detectors Binding Energy Nuclear mass MN less than sum of nucleon masses Shows nucleus is a bound (lower energy) state for this configuration
Nuclear Physics and Astrophysics PHY-30 Dr. E. Rizvi Lecture 4 - Detectors Binding Energy Nuclear mass MN less than sum of nucleon masses Shows nucleus is a bound (lower energy) state for this configuration
X-RAY SCATTERING AND MOSELEY S LAW. OBJECTIVE: To investigate Moseley s law using X-ray absorption and to observe X- ray scattering.
 X-RAY SCATTERING AND MOSELEY S LAW OBJECTIVE: To investigate Moseley s law using X-ray absorption and to observe X- ray scattering. READING: Krane, Section 8.5. BACKGROUND: In 1913, Henry Moseley measured
X-RAY SCATTERING AND MOSELEY S LAW OBJECTIVE: To investigate Moseley s law using X-ray absorption and to observe X- ray scattering. READING: Krane, Section 8.5. BACKGROUND: In 1913, Henry Moseley measured
Shell Atomic Model and Energy Levels
 Shell Atomic Model and Energy Levels (higher energy, deeper excitation) - Radio waves: Not absorbed and pass through tissue un-attenuated - Microwaves : Energies of Photos enough to cause molecular rotation
Shell Atomic Model and Energy Levels (higher energy, deeper excitation) - Radio waves: Not absorbed and pass through tissue un-attenuated - Microwaves : Energies of Photos enough to cause molecular rotation
X-Ray Photoelectron Spectroscopy (XPS)-2
 X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
X-Ray Photoelectron Spectroscopy (XPS)-2 Louis Scudiero http://www.wsu.edu/~scudiero; 5-2669 Fulmer 261A Electron Spectroscopy for Chemical Analysis (ESCA) The 3 step model: 1.Optical excitation 2.Transport
CHEM*3440. X-Ray Energies. Bremsstrahlung Radiation. X-ray Line Spectra. Chemical Instrumentation. X-Ray Spectroscopy. Topic 13
 X-Ray Energies very short wavelength radiation 0.1Å to 10 nm (100 Å) CHEM*3440 Chemical Instrumentation Topic 13 X-Ray Spectroscopy Visible - Ultraviolet (UV) - Vacuum UV (VUV) - Extreme UV (XUV) - Soft
X-Ray Energies very short wavelength radiation 0.1Å to 10 nm (100 Å) CHEM*3440 Chemical Instrumentation Topic 13 X-Ray Spectroscopy Visible - Ultraviolet (UV) - Vacuum UV (VUV) - Extreme UV (XUV) - Soft
Nova 600 NanoLab Dual beam Focused Ion Beam IITKanpur
 Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
X-ray spectroscopy: Experimental studies of Moseley s law (K-line x-ray fluorescence) and x-ray material s composition determination
 Uppsala University Department of Physics and Astronomy Laboratory exercise X-ray spectroscopy: Experimental studies of Moseley s law (K-line x-ray fluorescence) and x-ray material s composition determination
Uppsala University Department of Physics and Astronomy Laboratory exercise X-ray spectroscopy: Experimental studies of Moseley s law (K-line x-ray fluorescence) and x-ray material s composition determination
SEM Doctoral Course MS-636. April 11-13, 2016
 Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Electron Sources, Optics and Detectors SEM Doctoral Course MS-636 April 11-13, 2016 Summary Electron propagation is only possible through
Thomas LaGrange, Ph.D. Faculty Lecturer and Senior Staff Scientist Electron Sources, Optics and Detectors SEM Doctoral Course MS-636 April 11-13, 2016 Summary Electron propagation is only possible through
X-ray Microanalysis in Nanomaterials
 3 X-ray Microanalysis in Nanomaterials Robert Anderhalt 1. Introduction Traditionally, energy dispersive x-ray spectroscopy (EDS) in the scanning electron microscope (SEM) has been called microanalysis,
3 X-ray Microanalysis in Nanomaterials Robert Anderhalt 1. Introduction Traditionally, energy dispersive x-ray spectroscopy (EDS) in the scanning electron microscope (SEM) has been called microanalysis,
LAB 01 X-RAY EMISSION & ABSORPTION
 LAB 0 X-RAY EMISSION & ABSORPTION REPORT BY: TEAM MEMBER NAME: Ashley Tsai LAB SECTION No. 05 GROUP 2 EXPERIMENT DATE: Feb., 204 SUBMISSION DATE: Feb. 8, 204 Page of 3 ABSTRACT The goal of this experiment
LAB 0 X-RAY EMISSION & ABSORPTION REPORT BY: TEAM MEMBER NAME: Ashley Tsai LAB SECTION No. 05 GROUP 2 EXPERIMENT DATE: Feb., 204 SUBMISSION DATE: Feb. 8, 204 Page of 3 ABSTRACT The goal of this experiment
Auger Electron Spectroscopy
 Auger Electron Spectroscopy Auger Electron Spectroscopy is an analytical technique that provides compositional information on the top few monolayers of material. Detect all elements above He Detection
Auger Electron Spectroscopy Auger Electron Spectroscopy is an analytical technique that provides compositional information on the top few monolayers of material. Detect all elements above He Detection
MS482 Materials Characterization ( 재료분석 ) Lecture Note 4: XRF
 2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 4: XRF Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
2016 Fall Semester MS482 Materials Characterization ( 재료분석 ) Lecture Note 4: XRF Byungha Shin Dept. of MSE, KAIST 1 Course Information Syllabus 1. Overview of various characterization techniques (1 lecture)
EDS Mapping. Ian Harvey Fall Practical Electron Microscopy
 EDS Mapping Ian Harvey Fall 2008 1 From: Energy Dispersive X-ray Microanalysis, An Introduction Kevex Corp. 1988 Characteristic X-ray generation p.2 1 http://www.small-world.net/efs.htm X-ray generation
EDS Mapping Ian Harvey Fall 2008 1 From: Energy Dispersive X-ray Microanalysis, An Introduction Kevex Corp. 1988 Characteristic X-ray generation p.2 1 http://www.small-world.net/efs.htm X-ray generation
CHARGED PARTICLE INTERACTIONS
 CHARGED PARTICLE INTERACTIONS Background Charged Particles Heavy charged particles Charged particles with Mass > m e α, proton, deuteron, heavy ion (e.g., C +, Fe + ), fission fragment, muon, etc. α is
CHARGED PARTICLE INTERACTIONS Background Charged Particles Heavy charged particles Charged particles with Mass > m e α, proton, deuteron, heavy ion (e.g., C +, Fe + ), fission fragment, muon, etc. α is
MICRO-TOMOGRAPHY AND X-RAY ANALYSIS OF GEOLOGICAL SAMPLES
 THE PUBLISHING HOUSE PROCEEDINGS OF THE ROMANIAN ACADEMY, Series A, OF THE ROMANIAN ACADEMY Volume 18, Number 1/2017, pp. 42 49 MICRO-TOMOGRAPHY AND X-RAY ANALYSIS OF GEOLOGICAL SAMPLES Ion GRUIA University
THE PUBLISHING HOUSE PROCEEDINGS OF THE ROMANIAN ACADEMY, Series A, OF THE ROMANIAN ACADEMY Volume 18, Number 1/2017, pp. 42 49 MICRO-TOMOGRAPHY AND X-RAY ANALYSIS OF GEOLOGICAL SAMPLES Ion GRUIA University
Auger Electron Spectroscopy (AES)
 1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
Chemical Analysis. Energy Dispersive X-Ray Spectroscopy (EDS)
 Chemical Analysis We have so far discussed several of the signals detected on interaction of a high-energy electron beam with a solid sample (secondary, backscattered, transmitted, and diffracted electrons);
Chemical Analysis We have so far discussed several of the signals detected on interaction of a high-energy electron beam with a solid sample (secondary, backscattered, transmitted, and diffracted electrons);
Geology 777 Monte Carlo Exercise I
 Geology 777 Monte Carlo Exercise I Purpose The goal of this exercise is to get you to think like an electron... to start to think about where electrons from the stream of high energy electrons go when
Geology 777 Monte Carlo Exercise I Purpose The goal of this exercise is to get you to think like an electron... to start to think about where electrons from the stream of high energy electrons go when
Techniques EDX, EELS et HAADF en TEM: possibilités d analyse et applications
 Techniques EDX, EELS et HAADF en TEM: possibilités d analyse et applications Thomas Neisius Université Paul Cézanne Plan Imaging modes HAADF Example: supported Pt nanoparticles Electron sample interaction
Techniques EDX, EELS et HAADF en TEM: possibilités d analyse et applications Thomas Neisius Université Paul Cézanne Plan Imaging modes HAADF Example: supported Pt nanoparticles Electron sample interaction
X Rays & Crystals. Characterizing Mineral Chemistry & Structure. J.D. Price
 X Rays & Crystals Characterizing Mineral Chemistry & Structure J.D. Price Light - electromagnetic spectrum Wave behavior vs. particle behavior If atoms are on the 10-10 m scale, we need to use sufficiently
X Rays & Crystals Characterizing Mineral Chemistry & Structure J.D. Price Light - electromagnetic spectrum Wave behavior vs. particle behavior If atoms are on the 10-10 m scale, we need to use sufficiently
Nanoelectronics 09. Atsufumi Hirohata Department of Electronics. Quick Review over the Last Lecture
 Nanoelectronics 09 Atsufumi Hirohata Department of Electronics 13:00 Monday, 12/February/2018 (P/T 006) Quick Review over the Last Lecture ( Field effect transistor (FET) ): ( Drain ) current increases
Nanoelectronics 09 Atsufumi Hirohata Department of Electronics 13:00 Monday, 12/February/2018 (P/T 006) Quick Review over the Last Lecture ( Field effect transistor (FET) ): ( Drain ) current increases
