Mid-Term #1 (125 points total)
|
|
|
- Gerard Percival Boyd
- 6 years ago
- Views:
Transcription
1 Ocean 520 Name: Chemical Oceanography 20 October 2009 Fall 2009 Points are in parentheses (show all your work) (use back if necessary) MidTerm #1 (125 points total) 1. Doney et al (2008) Ocean Acidification The surface ocean is becoming more acidic due to addition of anthropogenic CO 2. Preindustrial surface seawater ph was Currently the surface seawater ph is a) Calculate how much more [H + ] there is now relative to preindustrial times. Give the numerical concentration. (5) ph 8.21 : H + = = x 10 9 = 6.16 x 10 9 ph 8.10; H + = 7.94 x 10 9 The difference is ( ) x 10 9 = 1.78 x 10 9 ( mol H + / l) ( mol H + / l) = 1.78 x 10 9 mol H + / l = mol H + / l b) By what percentage has the [H + ] concentration changed since preindustrial times (relative to preindustrial times)? (5) [( mol H + / l) / ( mol H + / l)] * 100% = 1.78/6.16 = 28.8% 2. Ocean carbonate reactions Ocean acidification will make calcification by organisms more difficult. a) The concentration of bicarbonate ion (HCO 3 ) is important to know for solubility reactions. Calculate the concentration of HCO 3 in surface seawater if you know the DIC and ph. (10) Assume the apparent equilibrium constants for seawater of K 1 = and K 2 = Assume DIC = x 10 3 and ph = 8.0 DIC = [H 2 CO 3 ] + [HCO3 ] + [CO3 2 ] [H + ][HCO 3 ] = K 1 [H + ][CO 3 2 ] = K 2 [H 2 CO 3 ] [HCO 3 ] [H 2 CO 3 ] = [H + ][HCO 3 ] [CO 3 2 ] = K 2 *[HCO 3 ] K 1 [H + ] DIC = [H + ][ HCO 3 ] + [HCO 3 ] + K 2 *[ HCO 3 ] K 1 [H + ] x 10 3 = (10 8 )*[ HCO 3 ] + [HCO 3 ] + (10 9 )*[ HCO 3 ] x 10 3 = 0.01*[ HCO 3 ] + [HCO 3 ] + 0.1*[ HCO 3 ] = 1.11*[ HCO 3 ] [HCO 3 ] = (2.000 x 10 3 ) / 1.11 = 1.8 x 10 3 mol / l = x 10 3 = or use HCO 3 = C T α 1
2 b) We have learned the following equilibrium reactions and constants for seawater. 1. CO 2 (g) + H 2 O H 2 CO 3 K H = H 2 CO 3 H + + HCO 3 K 1 = HCO 3 H CO 3 K 2 = H 2 O OH + H + K w = CaCO 3 (aragonite) Ca CO 3 K sp = We can write the solubility of CaCO 3 in terms of HCO 3 and CO 2 (g) as follows: CaCO 3 + CO 2 (g) + H 2 O = Ca HCO 3 Derive the equilibrium constant (K ) for this reaction using the values for K H, K 1, K 2 and K s. (10) CO 2 (g) + H 2 O H 2 CO 3 K H = H 2 CO 3 H + + HCO 3 K 1 = H + + CO 2 3 HCO 3 1/K 2 = CaCO 3 (aragonite) Ca +2 + CO 2 3 K sp = CaCO 3(aragonite) + CO 2 (g) + H 2 O = Ca HCO 3 K = K H * K 1 * K sp *(1/K 2 ) K = ( )*( )*( )*( ) = = x 10 5 = 2.04 x 10 5 c) Calculate the equilibrium concentration of HCO 3 in equilibrium with aragonite in seawater assuming we know the concentration of Ca 2+ in seawater and P CO2 in the atmosphere. (10) Assume that the concentration of Ca 2+ = 10.0 x 10 3 M and P CO2 = 380 ppm CaCO 3(aragonite) + CO 2 (g) + H 2 O = Ca HCO 3 K = K = [Ca 2+ ][ HCO 3 ] 2 [HCO 3 ] 2 = K* P CO2 380ppm = 380 x 10 6 P CO2 [Ca 2+ ] = x 10 4 [HCO 3 ] 2 = ( M 2 atm 1 )*( 380 x 10 6 atm) = 7.76 x 10 7 M (10.0 x 10 3 M ) [HCO 3 ] = 8.80 x 10 4 M = x 10 4 = =
3 d) Is surface seawater supersaturated, at equilibrium or undersaturated with respect to aragonite. Explain using the Ω ratio (degree of saturation). (10) From our calculation in part a we know that the concentration of HCO 3 is 1.8 x 10 3 mol / l. In part c we calculated the concentration of HCO 3 necessary for equilibrium with aragonite in seawater which gave us an equilibrium concentration of HCO 3 equal to 8.80 x 10 4 M. HCO 3 in sw HCO 3 in equil (1.8 x 10 3 mol / l) > (8.80 x 10 4 mol / l) Therefore the surface water is super saturated with respect to aragonite. In other words our ion activity product is larger than our K value and Ω is greater than 1. IAP = [Ca 2+ ][ HCO 3 ] 2 Ω = IAP = = = 4.07 P CO2 K = 10 2 (1.8 x 10 3 ) 2 / Ω > 1 so seawater is supersaturated = 10 2 ( ) 2 / = / =
4 3. Gas Exchange Is beer carbonated? a) The ph of your favorite, frosty, carbonated beverage is 5.8 and the HCO 3 content is 2.0 x 10 3 mole liter 1. What is the partial pressure of CO 2 in the can before you open it? Let K 1 = and K H = (10) Write a reaction including both CO 2, HCO 3 and H +. CO 2 (g) + H 2 O H 2 CO 3 K H = H 2 CO 3 H + + HCO 3 K 1 = CO 2 (g) + H 2 O H + + HCO 3 K = K H * K 1 = K = [H + ][ HCO 3 ] rearrange to: P CO2 = [H + ][ HCO 3 ] P CO2 K P CO2 = ( M)*( 2.0 x 10 3 M) = 100 x 10 3 atm = atm M 2 atm 1 b) Calculate the flux of CO 2 (in mol s 1 m 2 ) for your favorite, frosty, carbonated beverage. Assume the surface of the beverage is in equilbrium with the P CO2 of the atmosphere (385 x 10 6 atm.). Let the molecular diffusion coefficient be D CO2 = 2 x 10 9 m 2 s 1 and let the stagnant boundary layer thickness be Z film = 5 x 10 5 m. Which way does the CO 2 flux go? (10) [H 2 CO 3 ] surface = K H P CO2 = M atm 1 * 385 x 10 6 atm = x 10 5 M [H 2 CO 3 ] beer = K H P CO2 = M atm 1 * 100 x 10 3 atm = 3.16 x 10 3 M d[h 2 CO 3 ] = [H 2 CO 3 ] surface [H 2 CO 3 ] beer = 3.15 x 10 3 M F = D(d[H 2 CO 3 ]) / dz F = (2 x 10 9 m 2 s 1 )*( 3.15 x 10 3 M) / (5 x 10 5 m) = 1.26 x 10 7 m mol s 1 l 1 F = (1.26 x 10 7 m mol s 1 l 1 )*(1000 l m 3 ) = 1.26 x 10 4 mol s 1 m 2 The flux is positive so it is out of the beer and into the atmosphere.
5 4. Calcite (CaCO 3 (s)) is the main carbonate mineral in modern marine sediments. Ancient marine rocks contain mostly dolomite (CaMg(CO 3 ) 2 (s)). The reaction of calcite to dolomite is written as: 2CaCO 3 (s) + Mg 2+ = CaMg(CO 3 ) 2 (s) + Ca 2+ a) Calculate the equilibrium constant for this reaction at 25 C given the following free energies of formation ( G f ) and using G r = 2.3 RT log K = log K at 25 C (10 pts) Species G f (kj mol 1 ) CaCO 3 (s) CaMg(CO 3 ) 2 (s) Mg Ca Gr = ( ) ((2 x ) ) = 281 kj mol 1 Use Gr = log K Log K = 284 / = b) Write the corresponding equilibrium constant expression ( 5 pts) K = (CaMg(CO 3 ) 2 (s) (Ca 2+ ) / CaCO 3 (s) 2 (Mg 2+ ) = c) Assuming that seawater contains total concentrations of [Ca] = 1.0 x 10 2 mol/l and [Mg] = 5.3 x 10 2 mol/l what is the stable mineral phase, calcite or dolomite. (10 pts) Q = [Ca 2+ ] %free γ Ca2+ / [Mg 2+ ] %free γ Mg2+ = (10 2 ) (0.25) / 5.3 x 10 2 ) (0.31) = = so Q < K The reaction will go to the right. Dolomite is favored as the stable mineral phase.
6 5. The Table below gives the world average composition of river water. a) Correct the concentrations of Na, Mg, SO4, K and Ca for seasalt aerosols using Cl as a tracer? What assumptions do you need to make? (5) To correct the concentrations for sea salt aerosols, we need to subtract the amount of each ion contributed by deposition of sea salt aerosols, since these do not derive directly from weathering of rocks on land. If we assume that: (1) all Cl in rivers comes from sea salt aerosols, and (2) that sea salt aerosols have a composition with proportions identical to seawater, then we can use Cl as a tracer of the ions contributed by deposition of sea salt aerosols by multiplying the river concentration of Cl by the [X]/[Cl] ratio in seawater, where [X] is the concentration of Na, Mg, SO4, K or Ca: [X] aerosol = ([X] seawater /[Cl ] seawater )*[Cl ] river To get the corrected concentration [X*], we just need to subtract the aerosol component we calculated above from the total river concentration of each ion: [X*] = [X] river [X] aerosol Corrected concentrations: Element [X] river (mm) [X] seawater (mm) [X]/[Cl] seawate [X] aerosol [X]* (mm) r (mm) Cl Na Mg SO K Ca
7 Assumptions: We have assumed that: (1) all Cl in rivers comes from sea salt aerosols (this is probably not the case, as some Cl is contributed by weathering of evaporite rocks; see McDuff and Morel, p in coursepack). (2) sea salt aerosols have a composition with proportions identical to seawater (probably a good assumption). Propose an approach to calculate how much CO 2 (atm) is consumed by weathering of b) carbonate rocks (CaCO 3 ) (5 points) c) silicate rocks (5 points) hint: Assume all Si comes from silicate rocks. Calculate the HCO 3 that weathering produces. Then calculate the HCO 3 that comes from weathering of carbonate rocks. As a check, if all the Ca comes from weathering CaCO 3 then Ca should equal 0.5 x HCO 3 from carbonate weathering. You will find that it doesn t. The reason is that some carbonate rocks are MgCO 3 or CaMg(CO 3 ) 2. Approach: Start with the two weathering reactions we are considering: Carbonate rock weathering: CaCO 3 (s) + CO 2 (g) + H 2 O = Ca HCO 3 Note: 2 moles of HCO 3 and 1 mole of Ca 2+ are produced by the reaction of 1 mole of atmospheric CO 2 and 1 mole of CaCO 3. Silicate mineral weathering (using Kfeldspar as an example): KAlSi 3 O 8 (s) + CO 2 (g) + 5/2 H 2 O = ½ Al 2 Si 2 O 5 (OH) 4 (s) + K + + HCO3 o + 2H 4 SiO 4 (Kfeldspar) (kaolinite) Note: 1 mole of HCO 3 and 2 moles of H 4 SiO 4 o is produced by the reaction of 1 mole of atmospheric CO 2 (g) and 1 mole of Kfeldspar. (H 4 SiO 4 o is equivalent to SiO 2 reported in the table) Step 1. Assume all silica comes from silicate rocks. Then we can use the given concentration of SiO 2 (0.218 mmol/l) to calculate the riverine flux of silica: River Flux SiO 2 = River flow rate*[sio 2 ] river = (3.7 x L y 1 )*(0.218 mmol/l) River Flux SiO 2 = mmol/y According to the equation above, 1 mole atmospheric CO 2 is consumed for every 2 moles of silica produced, so: (River SiO 2 flux)/2 = Atmospheric CO 2 consumed by silica weathering (0.807 x mmol/y)/2 = x mmol/y Step 2. Determine the bicarbonate flux due to silicate weathering. Note 1 mole equivalent of HCO 3 is produced by the silicate weathering reaction, so we know that the bicarbonate flux due to silicate weathering is also equal to x mmol/y. Step 3. Assume all river bicarbonate is due to silicate and carbonate weathering combined. If this is true, then the bicarbonate flux due to carbonate weathering is equal to the total river bicarbonate flux minus the bicarbonate flux due to silicate weathering.
8 Total river bicarbonate flux = River flow rate*[hco 3 ] river = = (3.7 x L y 1 )*(0.958 mmol/l) = x mmol/yr HCO 3 flux due to carbonate weathering = (Total river HCO 3 flux) (HCO 3 flux due to silicate weathering) = x mmol/yr x mmol/y = x mmol/y 4. From the bicarbonate flux due to carbonate weathering, calculate the atmospheric CO 2 flux due to carbonate weathering. From the equation above, we can see that for every 2 moles of bicarbonate produced by carbonate weathering, 1 mole of atmospheric CO 2 is consumed. So the flux of CO 2 due to bicarbonate weathering is half that of the HCO 3 flux: Atm. CO 2 consumed by carbonate weathering = (HCO 3 flux due to carbonate weathering)/2 = (3.141 x mmol/y)/2 = x mmol/y 5. Check: Does the corrected Ca 2+ flux equal 0.5 x HCO 3 from carbonate weathering? Ca flux = River flow rate*[ca 2+ ] river = 3.7 x L y 1 * mmol/l = x mmol/y The Ca 2+ flux, at x mmol/y, is smaller than 0.5 x HCO 3 flux due to CaCO 3 weathering, at x mmol/y. The 0.2 mmol/y difference is due to some carbonate rocks being MgCO 3 or CaMg(CO 3 ) 2. d) From your model, what is the total amount of CO 2 consumed by weathering (in PgC y 1 )? Use a total river flow of 3.7 x L y 1. (5 points) To find the total atmospheric CO 2 consumed by weathering, add the carbonate and silicate weathering fluxes together: Atm. CO 2 consumed by carbonate + silicate weathering = x mmol/y x mmol/y = x mmol/y = x mol/y Convert this value to Pg/y: 1 Pg = g x mol CO 2 /y * 1 mol C * g C = x g x 1 Pg = 0.24 Pg/y 1 mol CO2 1 mol C g e) How does your rate compare with that of Sarmiento and Gruber (2002) as shown in their Figure 1. (5 points) Sarmiento and Gruber give 0.2 Pg/y as their estimate of atmospheric CO2 consumed by weathering. The rate calculated by this model is larger by 20% at 0.24 Pg/y, but on the same order of magnitude. f) How does the weathering ratio of CaCO 3 /silicate rocks compare with the outcrop abundance of carbonate sedimentary rocks to silicate rocks. See attached table from Meybeck (1987, American Journal of Science, 287, ). If the weathering ratio differs from the outcrop ratio, suggest why. (5 points) The weathering ratio of CaCO 3 /silicate rocks is: 1.571/0.404 = ~4:1 In the attached table, CaCO 3 composes ~16% of outcrop abundance. Rocks with silicate minerals compose nearly all the rest of rocks (except for evaporites) with an abundance of ~83%. Therefore the outcrop ratio of CaCO 3 /silicate rocks = ~1:5, approximately the
9 inverse of the weathering ratio. Silicate rocks are much more abundant than carbonate rocks. This is because carbonate rocks weather more quickly than silicate rocks. From Meybeck (1987) American Journal of Science, 287,
(4) Give an example of important reactions that are responsible for the composition of river water.
 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Chemical Oceanography 14 March 2012 Points are in parentheses (show all your work) Final Exam
 Ocean 400 Name: Chemical Oceanography 14 March 2012 Winter 2012 Points are in parentheses (show all your work) (give as much detail as you can) (use back if necessary) Final Exam 1. Sarmiento and Gruber
Ocean 400 Name: Chemical Oceanography 14 March 2012 Winter 2012 Points are in parentheses (show all your work) (give as much detail as you can) (use back if necessary) Final Exam 1. Sarmiento and Gruber
Lecture 4 What Controls the Composition of Seawater
 Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Carbon Dioxide, Alkalinity and ph
 Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
Carbon Dioxide, Alkalinity and ph OCN 623 Chemical Oceanography 15 March 2018 Reading: Libes, Chapter 15, pp. 383 389 (Remainder of chapter will be used with the classes Global Carbon Dioxide and Biogenic
Sedimentary Rocks and Processes
 Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero.
![2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero. 2 examples: Mg 2+ Rivers [Mg!! ]! (v r ) [Mg 2+ ] SW. **Answers with OR without vent effluent arrow are acceptable, since [Mg] in vent fluid is zero.](/thumbs/91/107062387.jpg) OCN400 Winter 2015 PS2 - Key 1. You might think it is well known, but there is some debate about what controls the magnesium concentration in seawater. The main input is rivers (see Power Point Lecture
OCN400 Winter 2015 PS2 - Key 1. You might think it is well known, but there is some debate about what controls the magnesium concentration in seawater. The main input is rivers (see Power Point Lecture
Continent-Ocean Interaction: Role of Weathering
 Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.)
 Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
Ocean 421 Your Name Chemical Oceanography Spring 2000 Final Exam (Use the back of the pages if necessary)(more than one answer may be correct.) 1. Due to the water molecule's (H 2 O) great abundance in
organisms CaCO 3 + H 2 O + CO 2 shallow water
 Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Quartz or opaline silica solubility
 Quartz or opaline silica solubility The simplest process that might regulate the concentration of an element in solution is equilibrium with respect to a solid phase containing the element as a major component.
Quartz or opaline silica solubility The simplest process that might regulate the concentration of an element in solution is equilibrium with respect to a solid phase containing the element as a major component.
Global Carbon Cycle - I
 Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Global Carbon Cycle - I Reservoirs and Fluxes OCN 401 - Biogeochemical Systems 13 November 2012 Reading: Schlesinger, Chapter 11 Outline 1. Overview of global C cycle 2. Global C reservoirs 3. The contemporary
Chapter 3: Acid Base Equilibria. HCl + KOH KCl + H 2 O acid + base salt + water
 Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
Global Carbon Cycle - I
 Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Global Carbon Cycle - I OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 11 1. Overview of global C cycle 2. Global C reservoirs Outline 3. The contemporary global C cycle 4. Fluxes and residence
Processes affecting continental shelves
 Marine Sediments Continental Shelves Processes affecting continental shelves 1. Glaciation 2. Sea-level change (±130 m during continental glaciation) 3. Waves and currents 4. Sedimentation 5. Carbonate
Marine Sediments Continental Shelves Processes affecting continental shelves 1. Glaciation 2. Sea-level change (±130 m during continental glaciation) 3. Waves and currents 4. Sedimentation 5. Carbonate
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions
 Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Problem Set #4 ANSWER KEY Fall 2009 Due: 9:30, Monday, Nov 30
 OCN 520 Problem Set #4 ANSWER KEY Fall 2009 Due: 9:30, Monday, Nov 30 1. Two-Box Ocean Model The B Flux Using a 2 box model like the one you have worked on in problem set #4 (question 1) assume the following
OCN 520 Problem Set #4 ANSWER KEY Fall 2009 Due: 9:30, Monday, Nov 30 1. Two-Box Ocean Model The B Flux Using a 2 box model like the one you have worked on in problem set #4 (question 1) assume the following
Hydrological Cycle Rain and rivers OUTLINE
 Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
Hydrological Cycle Rain and rivers The Hydrosphere Rain and rivers OUTLINE 1 Generalizations (non-political conservatism) Conservative (not affected) and Non-Conservative (affected) Ions Distinction: whether
ph and Acid-Base reactions
 ph and Acid-Base reactions http://eps.mcgill.ca/~courses/c220/ Interaction of water with H + (H 3 O + ) Although it is a minor component of most aqueous solutions, the proton, hydrogen ion or H + concentration
ph and Acid-Base reactions http://eps.mcgill.ca/~courses/c220/ Interaction of water with H + (H 3 O + ) Although it is a minor component of most aqueous solutions, the proton, hydrogen ion or H + concentration
Global Carbon Cycle - I Systematics: Reservoirs and Fluxes
 OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
OCN 401-10 Nov. 16, 2010 KCR Global Carbon Cycle - I Systematics: Reservoirs and Fluxes The Global carbon cycle Reservoirs: biomass on land in the oceans, atmosphere, soil and rocks, waters Processes:
Chapter 2: Equilibrium Thermodynamics and Kinetics
 Chapter 2: Equilibrium Thermodynamics and Kinetics Equilibrium Thermodynamics: predicts the concentrations (or more precisely, activities) of various species and phases if a reaction reaches equilibrium.
Chapter 2: Equilibrium Thermodynamics and Kinetics Equilibrium Thermodynamics: predicts the concentrations (or more precisely, activities) of various species and phases if a reaction reaches equilibrium.
Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments
 Peter Köhler 1 October 2015 Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments enhanced weathering, ocean alkalinization, ocean fertilization Peter Köhler, Judith Hauck
Peter Köhler 1 October 2015 Dissolution of olivine (potential, side effects) in simulated CO 2 removal experiments enhanced weathering, ocean alkalinization, ocean fertilization Peter Köhler, Judith Hauck
Redox, ph, pe OUTLINE 9/12/17. Equilibrium? Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox
 Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
: 1.9 ppm y -1
 Atmospheric CO 2 Concentration Year 2006 Atmospheric CO 2 concentration: 381 ppm 35% above pre-industrial Atmoapheric [CO2] (ppmv) 4001850 1870 1890 1910 1930 1950 1970 1990 2010 380 360 340 320 300 280
Atmospheric CO 2 Concentration Year 2006 Atmospheric CO 2 concentration: 381 ppm 35% above pre-industrial Atmoapheric [CO2] (ppmv) 4001850 1870 1890 1910 1930 1950 1970 1990 2010 380 360 340 320 300 280
Weathering and Erosion
 Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Ocean Acidifica+on: past analogies, present concerns, future predic+ons. Sco8 Wieman
 Ocean Acidifica+on: past analogies, present concerns, future predic+ons Sco8 Wieman Effects Planktonic calcifica+on Carbon and nutrient assimila+on Primary produc+on Acid-base balance Larval stages of
Ocean Acidifica+on: past analogies, present concerns, future predic+ons Sco8 Wieman Effects Planktonic calcifica+on Carbon and nutrient assimila+on Primary produc+on Acid-base balance Larval stages of
EPS 50 Lab 4: Sedimentary Rocks
 Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
CO2 in atmosphere is influenced by pco2 of surface water (partial pressure of water is the CO2 (gas) that would be in equilibrium with water).
 EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
EART 254, Lecture on April 6 & 11, 2011 Introduction (skipped most of this) Will look at C and N (maybe) cycles with respect to how they influence CO2 levels in the atmosphere. Ocean chemistry controls
Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions
 Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions Goals: Refresh your memory on the basics of chemical reactions Ponder the chaos that underlies chemical reactions;
Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions Goals: Refresh your memory on the basics of chemical reactions Ponder the chaos that underlies chemical reactions;
Lecture 5. Introduction to Stable Isotopes
 Lecture 5 Introduction to Stable Isotopes Stable Isotope Geochemistry Primarily concerned with the isotope ratios of H, C, N, O, and S Si and B often included and new instrumentation has opened up others
Lecture 5 Introduction to Stable Isotopes Stable Isotope Geochemistry Primarily concerned with the isotope ratios of H, C, N, O, and S Si and B often included and new instrumentation has opened up others
S= 95.02% S= 4.21% 35. S=radioactive 36 S=0.02% S= 0.75% 34 VI V IV III II I 0 -I -II SO 4 S 2 O 6 H 2 SO 3 HS 2 O 4- S 2 O 3
 SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
SULFUR ISOTOPES 32 S= 95.02% 33 S= 0.75% 34 S= 4.21% 35 S=radioactive 36 S=0.02% S-H S-C S=C S-O S=O S-F S-Cl S-S VI V IV III II I 0 -I -II SO 4 2- S 2 O 6 2- H 2 SO 3 HS 2 O 4- S 2 O 3 2- S 2 F 2 S H
[ ] Sparkling Water and the Carbon Cycle
![[ ] Sparkling Water and the Carbon Cycle [ ] Sparkling Water and the Carbon Cycle](/thumbs/87/96185022.jpg) Materials: Seltzer maker (SodaStream or similar) Some tasty cheese Tap water Glasses or cups for drinking ph paper Many people enjoy carbonated beverages, especially with food. The tiny bubbles and natural
Materials: Seltzer maker (SodaStream or similar) Some tasty cheese Tap water Glasses or cups for drinking ph paper Many people enjoy carbonated beverages, especially with food. The tiny bubbles and natural
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and
 1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
1. Introduction 2. Ocean circulation a) Temperature, salinity, density b) Thermohaline circulation c) Wind-driven surface currents d) Circulation and climate change e) Oceanic water residence times 3.
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza
 Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza General Concepts for Natural Controls on Fresh Water Composition 1. Generalizations about freshwater compositions 2. Conservative/nonconservative
Lecture 16 Guest Lecturer this week. Prof. Greg Ravizza General Concepts for Natural Controls on Fresh Water Composition 1. Generalizations about freshwater compositions 2. Conservative/nonconservative
Activity and Concentration
 Activity and Concentration Activity effective concentration Ion-ion and ion-h 2 O interactions (hydration shell) cause number of ions available to react chemically ("free" ions) to be less than the number
Activity and Concentration Activity effective concentration Ion-ion and ion-h 2 O interactions (hydration shell) cause number of ions available to react chemically ("free" ions) to be less than the number
Predicting Mineral Transformations in Wet Supercritical CO 2 : The Critical Role of Water
 Predicting Mineral Transformations in Wet Supercritical CO 2 : The Critical Role of Water Andrew R. Felmy Eugene S. Ilton Andre Anderko Kevin M. Rosso Ja Hun Kwak Jian Zhi Hu 1 Outline Background Importance
Predicting Mineral Transformations in Wet Supercritical CO 2 : The Critical Role of Water Andrew R. Felmy Eugene S. Ilton Andre Anderko Kevin M. Rosso Ja Hun Kwak Jian Zhi Hu 1 Outline Background Importance
Figure 65: Reservoir in a steady state condition where the input flux is equal to the output flux and the reservoir size remains constant.
 7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
7. The carbon cycle 7.1. Box model of the carbon cycle Without the greenhouse effect, our planet would experience a permanent ice age and life as we know it would not be possible. The main contributors
Biogeochemistry of the Earth System QMS Lecture 5 Dr Zanna Chase 16 June 2015
 Biogeochemistry of the Earth System QMS512 2015 Lecture 5 Dr Zanna Chase 16 June 2015 Lecture 5: Inorganic carbon chemistry Outline Inorganic carbon speciation in seawater- conceptual overview Inorganic
Biogeochemistry of the Earth System QMS512 2015 Lecture 5 Dr Zanna Chase 16 June 2015 Lecture 5: Inorganic carbon chemistry Outline Inorganic carbon speciation in seawater- conceptual overview Inorganic
Chemical Sedimentary Rocks: CARBONATES a quick summary
 Chemical Sedimentary Rocks: CARBONATES a quick summary Alessandro Grippo, Ph.D. What are Carbonates? Carbonate rocks are chemical sedimentary rocks composed mainly or only by carbonate minerals Carbonate
Chemical Sedimentary Rocks: CARBONATES a quick summary Alessandro Grippo, Ph.D. What are Carbonates? Carbonate rocks are chemical sedimentary rocks composed mainly or only by carbonate minerals Carbonate
Part 1. Ocean Composition & Circulation
 OCN 401 Biogeochemical Systems (10.19.17) (Schlesinger: Chapter 9) Part 1. Ocean Composition & Circulation 1. Introduction Lecture Outline 2. Ocean Circulation a) Global Patterns in T, S, ρ b) Thermohaline
OCN 401 Biogeochemical Systems (10.19.17) (Schlesinger: Chapter 9) Part 1. Ocean Composition & Circulation 1. Introduction Lecture Outline 2. Ocean Circulation a) Global Patterns in T, S, ρ b) Thermohaline
Ocean Sediments. Key Concepts
 Ocean Sediments Key Concepts 1. What are the processes that control what types of sediments are deposited in which places? 2. Conversely, how can we use the sedimentary record to figure out tectonic and
Ocean Sediments Key Concepts 1. What are the processes that control what types of sediments are deposited in which places? 2. Conversely, how can we use the sedimentary record to figure out tectonic and
Lecture 6 - Determinants of Seawater Composition. Sets up electric dipole because O is more electronegative A o. Figure 3.
 12.742 - Marine Chemistry Fall 2004 Lecture 6 - Determinants of Seawater Composition Prof. Scott Doney What is seawater? Water Dissolved inorganic salts (major ions) Trace species, organics, colloids,
12.742 - Marine Chemistry Fall 2004 Lecture 6 - Determinants of Seawater Composition Prof. Scott Doney What is seawater? Water Dissolved inorganic salts (major ions) Trace species, organics, colloids,
Ocean Acidification the other CO2 problem..
 Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
Ocean Acidification the other CO2 problem.. Recall: Atm CO 2 already above recent planetary history CO 2 Today: What does this do to ocean water? Main Outline: 1. Chemistry. How does ocean absorb CO 2,
Ocean Sediments OCN Nov 2016
 Ocean Sediments OCN 401 10 Nov 2016 Outline Significance & terms Origin & distribution of major types of marine sediments Delivery - dissolution destruction mid-ocean ridges Significance of ocean sediments
Ocean Sediments OCN 401 10 Nov 2016 Outline Significance & terms Origin & distribution of major types of marine sediments Delivery - dissolution destruction mid-ocean ridges Significance of ocean sediments
Weathering Cycle Teacher Notes
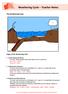 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
WEATHERING. Turning Rock to Sediment and Solutions 10/22/2012
 WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
Chemistry 2000 Lecture 11: Chemical equilibrium
 Chemistry 2000 Lecture 11: Chemical equilibrium Marc R. Roussel February 4, 2019 Marc R. Roussel Chemical equilibrium February 4, 2019 1 / 27 Equilibrium and free energy Thermodynamic criterion for equilibrium
Chemistry 2000 Lecture 11: Chemical equilibrium Marc R. Roussel February 4, 2019 Marc R. Roussel Chemical equilibrium February 4, 2019 1 / 27 Equilibrium and free energy Thermodynamic criterion for equilibrium
1/31/2013. Weathering Includes Physical, Chemical, Biological processes. Weathering Mechanisms. Wind abrasion forming Ventifacts
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
SW Density = kg/l at 20 o C (Pilson 1998)
 Composition of SW To Date We Have Covered: Descriptive Oceanography (Millero chapter 1) Special Properties of H 2 O (Millero chapter 4) Ion-Water & Ion-Ion Interactions (Millero chap 4) Continuing Coverage
Composition of SW To Date We Have Covered: Descriptive Oceanography (Millero chapter 1) Special Properties of H 2 O (Millero chapter 4) Ion-Water & Ion-Ion Interactions (Millero chap 4) Continuing Coverage
Marine Sediments EPSS15 Spring 2017 Lab 4
 Marine Sediments EPSS15 Spring 2017 Lab 4 Why Sediments? Record of Earth s history - Tectonic plate movement - Past changes in climate - Ancient ocean circulation currents - Cataclysmic events 1 Classification
Marine Sediments EPSS15 Spring 2017 Lab 4 Why Sediments? Record of Earth s history - Tectonic plate movement - Past changes in climate - Ancient ocean circulation currents - Cataclysmic events 1 Classification
FRIENDS OF FREZCHEM (Version 5.2)
 FRIENDS OF FREZCHEM (Version 5.2) Attached is a "beta" version of the FREZCHEM model that includes chloride, sulfate, and bicarbonate-carbonate salts (FREZCHEM5.2). This folder includes a FORTRAN program
FRIENDS OF FREZCHEM (Version 5.2) Attached is a "beta" version of the FREZCHEM model that includes chloride, sulfate, and bicarbonate-carbonate salts (FREZCHEM5.2). This folder includes a FORTRAN program
ph of natural waters
 ph of natural waters Na 2 CO 3 10H 2 O (natron) 2 Na + + CO 3 + 10H 2 O 4FeS 2 + 15O 2 + 14H 2 O 4 Fe(OH) 3 + 16H + + 8SO 4 4NaAlSi 3 O 8 + 11H 2 O 4Na + + 4OH - + Al 4 Si 4 O 10 (OH) 8 + 8Si(OH) 4 In
ph of natural waters Na 2 CO 3 10H 2 O (natron) 2 Na + + CO 3 + 10H 2 O 4FeS 2 + 15O 2 + 14H 2 O 4 Fe(OH) 3 + 16H + + 8SO 4 4NaAlSi 3 O 8 + 11H 2 O 4Na + + 4OH - + Al 4 Si 4 O 10 (OH) 8 + 8Si(OH) 4 In
Effect of chemical composition to large scale CO 2 Injection in Morrow Sandstone, Farnsworth Hydrocarbon Field, Texas, USA
 Effect of chemical composition to large scale CO 2 Injection in Morrow Sandstone, Farnsworth Hydrocarbon Field, Texas, USA Bulbul Ahmmed Martin Appold Department of Geological Sciences University of Missouri-Columbia
Effect of chemical composition to large scale CO 2 Injection in Morrow Sandstone, Farnsworth Hydrocarbon Field, Texas, USA Bulbul Ahmmed Martin Appold Department of Geological Sciences University of Missouri-Columbia
Stable Isotopes OUTLINE
 Stable Isotopes OUTLINE Reading: White Ch 9.1 to 9.7.1 (or digital p370-400) Exercise answer? What does the salt do? Today 1. 2 leftovers 2. Stable Isotopes for hydrologic and climate applications 1 CaCO
Stable Isotopes OUTLINE Reading: White Ch 9.1 to 9.7.1 (or digital p370-400) Exercise answer? What does the salt do? Today 1. 2 leftovers 2. Stable Isotopes for hydrologic and climate applications 1 CaCO
Chapter 17: Solubility Equilibria
 Previous Chapter Table of Contents Next Chapter Chapter 17: Solubility Equilibria Sections 17.1-17.2: Solubility Equilibria and the K sp Table In this chapter, we consider the equilibrium associated with
Previous Chapter Table of Contents Next Chapter Chapter 17: Solubility Equilibria Sections 17.1-17.2: Solubility Equilibria and the K sp Table In this chapter, we consider the equilibrium associated with
2.2 Acid mine drainage
 PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
Geology 560, Prof. Thomas Johnson, Fall 2009 Class Notes: Unit III Part 2. Weak Acids- Speciation
 Geology 560, Prof. Thomas Johnson, Fall 2009 Class Notes: Unit III Part 2. Weak Acids Speciation Reading: White 6.2; Walther Ch. 6 Sections on Acid/Base equilibria, buffers, and carbonate solubility We
Geology 560, Prof. Thomas Johnson, Fall 2009 Class Notes: Unit III Part 2. Weak Acids Speciation Reading: White 6.2; Walther Ch. 6 Sections on Acid/Base equilibria, buffers, and carbonate solubility We
Lect. 2: Chemical Water Quality
 The Islamic University of Gaza Faculty of Engineering Civil Engineering Department M.Sc. Water Resources Water Quality Management (ENGC 6304) Lect. 2: Chemical Water Quality ١ Chemical water quality parameters
The Islamic University of Gaza Faculty of Engineering Civil Engineering Department M.Sc. Water Resources Water Quality Management (ENGC 6304) Lect. 2: Chemical Water Quality ١ Chemical water quality parameters
The Lithosphere. Definition
 10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
Wednesday week 12. These ions move through the soil to streams and eventually to the ocean. In the ocean; CaCO 3 + H 2 O + CO 2 H 2 O + H 2 O
 Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Wednesday week 12 I. Control of CO 2 content of atmosphere by the ocean H 4 SiO 4 A. Consider a hypothetical planet with a crust made of single mineral (Wallastonite) CaSiO3. We could use the composition
Carbon is widely distributed in our
 C H A P T E R WATER SOFTENING Carbon is widely distributed in our ecosystem through five major spheres, namely the lithosphere, hydrosphere, atmosphere, pedosphere, and biosphere (see Table -). Sediments
C H A P T E R WATER SOFTENING Carbon is widely distributed in our ecosystem through five major spheres, namely the lithosphere, hydrosphere, atmosphere, pedosphere, and biosphere (see Table -). Sediments
Evolution of Earth Environments Bio-Geo-Chemical Cycling
 Evolution of Earth Environments Bio-Geo-Chemical Cycling Evolution of the Earliest Atmospheres of Mars and Earth Volcanic Outgassing Evolving to Equilibrium Atmosphere To Atmosphere Lost to space (Abundant)
Evolution of Earth Environments Bio-Geo-Chemical Cycling Evolution of the Earliest Atmospheres of Mars and Earth Volcanic Outgassing Evolving to Equilibrium Atmosphere To Atmosphere Lost to space (Abundant)
Overview of CO 2 -induced Changes in Seawater Chemistry
 Overview of CO 2 -induced Changes in Seawater Chemistry Joan Kleypas & Chris Langdon I. Background II. Facts vs Hypotheses III. Future directions 10-Oct-00 9th Int Coral Reef Symp. 1 Effects of CO 2 on
Overview of CO 2 -induced Changes in Seawater Chemistry Joan Kleypas & Chris Langdon I. Background II. Facts vs Hypotheses III. Future directions 10-Oct-00 9th Int Coral Reef Symp. 1 Effects of CO 2 on
SCOPE 35 Scales and Global Change (1988)
 1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
1. Types and origins of marine sediments 2. Distribution of sediments: controls and patterns 3. Sedimentary diagenesis: (a) Sedimentary and organic matter burial (b) Aerobic and anaerobic decomposition
Salinity. foot = 0.305m yard = 0.91m. Length. Area m 2 square feet ~0.09m2. Volume m 3 US pint ~ 0.47 L fl. oz. ~0.02 L.
 Length m foot = 0.305m yard = 0.91m Area m 2 square feet ~0.09m2 Volume m 3 US pint ~ 0.47 L, L (liters) fl. oz. ~0.02 L Speed m/s mph Acceleration m/s 2 mph/s Weight kg, gram pound ~0.45kg Temperature
Length m foot = 0.305m yard = 0.91m Area m 2 square feet ~0.09m2 Volume m 3 US pint ~ 0.47 L, L (liters) fl. oz. ~0.02 L Speed m/s mph Acceleration m/s 2 mph/s Weight kg, gram pound ~0.45kg Temperature
CEE 370 Environmental Engineering Principles. Equilibrium Chemistry
 Updated: 9 September 015 Print version CEE 370 Environmental Engineering Principles Lecture #6 Environmental Chemistry IV: Thermodynamics, Equilibria, Acids-bases I Reading: Mihelcic & Zimmerman, Chapter
Updated: 9 September 015 Print version CEE 370 Environmental Engineering Principles Lecture #6 Environmental Chemistry IV: Thermodynamics, Equilibria, Acids-bases I Reading: Mihelcic & Zimmerman, Chapter
Overview. Open system flush with kinetic constraints Closed system with kinetic constraints
 Rationale Minerals associated with lung diseases How do lungs function? Health threats of minerals The paper researched Approach and methodology Saturation indices Activity-Ratio diagrams Reaction-path
Rationale Minerals associated with lung diseases How do lungs function? Health threats of minerals The paper researched Approach and methodology Saturation indices Activity-Ratio diagrams Reaction-path
Fall 2016 Due: 10:30 a.m., Tuesday, September 13
 Geol 330_634 Problem Set #1_Key Fall 2016 Due: 10:30 a.m., Tuesday, September 13 1. Why is the surface circulation in the central North Pacific dominated by a central gyre with clockwise circulation? (5)
Geol 330_634 Problem Set #1_Key Fall 2016 Due: 10:30 a.m., Tuesday, September 13 1. Why is the surface circulation in the central North Pacific dominated by a central gyre with clockwise circulation? (5)
Learning Outcomes: At the end of this assignment, students will be able to:
 Chemical Equilibria & Sample Preparation Purpose: The purpose of this assignment is to predict how solute concentrations are controlled by chemical equilibria, understand the chemistry involved with sample
Chemical Equilibria & Sample Preparation Purpose: The purpose of this assignment is to predict how solute concentrations are controlled by chemical equilibria, understand the chemistry involved with sample
Solubility of gases in water: Henry s Law concentration dissolved / partial pressure of the gas K H (units mol L -1 atm -1 ) = c X /p X
 CHEM/TOX 336 Lecture 11/12 Dissolved Gases in Natural Water Dissolved Solids in Natural Water Solubility of gases in water: Henry s Law concentration dissolved / partial pressure of the gas K H (units
CHEM/TOX 336 Lecture 11/12 Dissolved Gases in Natural Water Dissolved Solids in Natural Water Solubility of gases in water: Henry s Law concentration dissolved / partial pressure of the gas K H (units
Groundwater chemistry
 Read: Ch. 3, sections 1, 2, 3, 5, 7, 9; Ch. 7, sections 2, 3 PART 14 Groundwater chemistry Introduction Matter present in water can be divided into three categories: (1) Suspended solids (finest among
Read: Ch. 3, sections 1, 2, 3, 5, 7, 9; Ch. 7, sections 2, 3 PART 14 Groundwater chemistry Introduction Matter present in water can be divided into three categories: (1) Suspended solids (finest among
/ Past and Present Climate
 MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
MIT OpenCourseWare http://ocw.mit.edu 12.842 / 12.301 Past and Present Climate Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. The Faint Young
Dissolved Gases in Natural Water Dissolved Solids in Natural Water
 Dissolved Gases in Natural Water Dissolved Solids in Natural Water Solubility of gases in water: Henry's Law concentration dissolved / partial pressure of the gas K H (units mol L -1 atm -1 ) = c X /p
Dissolved Gases in Natural Water Dissolved Solids in Natural Water Solubility of gases in water: Henry's Law concentration dissolved / partial pressure of the gas K H (units mol L -1 atm -1 ) = c X /p
Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington
 Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington Last lecture was concerned with gas exchange and one example we looked at was the solubility of CO 2. Next we have
Chapter 12: Acids and Bases: Ocean Carbonate System James Murray 4/30/01 Univ. Washington Last lecture was concerned with gas exchange and one example we looked at was the solubility of CO 2. Next we have
XI. the natural carbon cycle. with materials from J. Kasting (Penn State)
 XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
XI. the natural carbon cycle with materials from J. Kasting (Penn State) outline properties of carbon the terrestrial biological cycle of carbon the ocean cycle of carbon carbon in the rock cycle overview
INTRODUCTION TO CO2 CHEMISTRY
 INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego Mauna Loa Observatory, Hawaii Monthly Average Carbon Dioxide Concentration Data from Scripps
INTRODUCTION TO CO2 CHEMISTRY IN SEA WATER Andrew G. Dickson Scripps Institution of Oceanography, UC San Diego Mauna Loa Observatory, Hawaii Monthly Average Carbon Dioxide Concentration Data from Scripps
Geological Sciences 4550: Geochemistry
 1. Consider the following hypothetical gaseous solution: gases 1 and form an ideal binary solution; at 1000 K, the free energies of formation from the elements are - 50kJ/mol for component 1 and - 40kJ/mol
1. Consider the following hypothetical gaseous solution: gases 1 and form an ideal binary solution; at 1000 K, the free energies of formation from the elements are - 50kJ/mol for component 1 and - 40kJ/mol
Lecture 6. Physical Properties. Solid Phase. Particle Composition
 Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
FRIENDS OF FREZCHEM (Version 6.2)
 FRIENDS OF FREZCHEM (Version 6.2) Attached is a "beta" version of the FREZCHEM model that includes chloride, nitrate, sulfate, and bicarbonate-carbonate salts, and strong acid chemistry (FREZCHEM6.2).
FRIENDS OF FREZCHEM (Version 6.2) Attached is a "beta" version of the FREZCHEM model that includes chloride, nitrate, sulfate, and bicarbonate-carbonate salts, and strong acid chemistry (FREZCHEM6.2).
CO 2 and the carbonate system. 1/45
 CO 2 and the carbonate system http://eps.mcgill.ca/~courses/c542/ 1/45 The Atmospheric CO 2 -Climate Connection From: http://www.google.ca/imgres?imgurl=http:/ /www.tallbergfoundation.org/ From: Ruddiman,
CO 2 and the carbonate system http://eps.mcgill.ca/~courses/c542/ 1/45 The Atmospheric CO 2 -Climate Connection From: http://www.google.ca/imgres?imgurl=http:/ /www.tallbergfoundation.org/ From: Ruddiman,
CO 2 sequestration via direct mineral carbonation of Mg-silicates. Natalie Johnson GCEP Symposium 4 October 2011
 CO 2 sequestration via direct mineral carbonation of Mg-silicates Natalie Johnson GCEP Symposium 4 October 2011 CO 2 release/year (Gt) 2 CCS: Part of climate change mitigation Projection based on current
CO 2 sequestration via direct mineral carbonation of Mg-silicates Natalie Johnson GCEP Symposium 4 October 2011 CO 2 release/year (Gt) 2 CCS: Part of climate change mitigation Projection based on current
Nutrients; Aerobic Carbon Production and Consumption
 Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography Reading: Libes, Chapters 8 and 9 Formation and respiration of organic matter DINutrients POM Primary Producers Autotrophs
Chapter 15. Chemical Equilibrium
 Chapter 15. Chemical Equilibrium 15.1 The Concept of Equilibrium Consider colorless frozen N 2 O 4. At room temperature, it decomposes to brown NO 2. N 2 O 4 (g) 2NO 2 (g) At some time, the color stops
Chapter 15. Chemical Equilibrium 15.1 The Concept of Equilibrium Consider colorless frozen N 2 O 4. At room temperature, it decomposes to brown NO 2. N 2 O 4 (g) 2NO 2 (g) At some time, the color stops
OCN400 Problem Set #1 Winter 2015 Due: 9:30 a.m., Monday, Jan 12
 OCN400 Problem Set #1 Winter 2015 Due: 9:30 a.m., Monday, Jan 12 1. What determines if an ion is a major ion in seawater? (5) - Major ions are those that contribute to salinity. - If salinity can be determined
OCN400 Problem Set #1 Winter 2015 Due: 9:30 a.m., Monday, Jan 12 1. What determines if an ion is a major ion in seawater? (5) - Major ions are those that contribute to salinity. - If salinity can be determined
INTRODUCTION TO GEOCHEMICAL AND REACTIVE TRANSPORT MODELING. Ondra Sracek
 INTRODUCTION TO GEOCHEMICAL AND REACTIVE TRANSPORT MODELING Ondra Sracek Principal types of geochemical modeling: 1. Speciation (for exemple, for lead); 2. Inverse geochemical modeling; 3. Direct geochemical
INTRODUCTION TO GEOCHEMICAL AND REACTIVE TRANSPORT MODELING Ondra Sracek Principal types of geochemical modeling: 1. Speciation (for exemple, for lead); 2. Inverse geochemical modeling; 3. Direct geochemical
Ocean Chemical Dynamics. EAS 2200 Lecture 21
 Ocean Chemical Dynamics EAS 2200 Lecture 21 Summary of Water s H-bonding accounts for high heat capacity, latent heats of fusion and evaporation. important for heat transport, transfer to atmosphere, and
Ocean Chemical Dynamics EAS 2200 Lecture 21 Summary of Water s H-bonding accounts for high heat capacity, latent heats of fusion and evaporation. important for heat transport, transfer to atmosphere, and
Aqueous Solutions O 2-
 6 Aqueous Solutions Because of its ubiquity and importance in sustaining life, the chemistry of water and aqueous solutions has been studied extensively. Water, H 2 O, is prevalent in the atmosphere, exists
6 Aqueous Solutions Because of its ubiquity and importance in sustaining life, the chemistry of water and aqueous solutions has been studied extensively. Water, H 2 O, is prevalent in the atmosphere, exists
The Martian Sedimentary Mass: Constraints on its Composition, Age and Size. Scott McLennan Department of Geosciences, SUNY Stony Brook
 The Martian Sedimentary Mass: Constraints on its Composition, Age and Size Scott McLennan Department of Geosciences, SUNY Stony Brook Exploring Mars Habitability Lisbon 14 June, 2011 Martian Crustal Chemistry
The Martian Sedimentary Mass: Constraints on its Composition, Age and Size Scott McLennan Department of Geosciences, SUNY Stony Brook Exploring Mars Habitability Lisbon 14 June, 2011 Martian Crustal Chemistry
Sedimentary Rocks, Stratigraphy, and Geologic Time
 Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
Section 3 Environmental Chemistry
 Section 3 Environmental Chemistry 1 Environmental Chemistry Definitions Chemical Reactions Stoichiometry Photolytic Reactions Enthalpy and Heat of Reaction Chemical Equilibria ph Solubility Carbonate Systems
Section 3 Environmental Chemistry 1 Environmental Chemistry Definitions Chemical Reactions Stoichiometry Photolytic Reactions Enthalpy and Heat of Reaction Chemical Equilibria ph Solubility Carbonate Systems
Exam3Fall2009thermoelectro
 Exam3Fall2009thermoelectro Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Thermodynamics can be used to determine all of the following EXCEPT
Exam3Fall2009thermoelectro Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Thermodynamics can be used to determine all of the following EXCEPT
Weathering: the disintegration, or breakdown of rock material
 Weathering: the disintegration, or breakdown of rock material Mechanical Weathering: no change in chemical composition--just disintegration into smaller pieces Chemical Weathering: breakdown as a result
Weathering: the disintegration, or breakdown of rock material Mechanical Weathering: no change in chemical composition--just disintegration into smaller pieces Chemical Weathering: breakdown as a result
) and/or dolomite (CaMg(CO 3
 Environmental Chemistry I Week 4 Alkalinity 1) Alkalinity Alkalinity is defined as the capacity of water to accept H + ions (protons). It can also be defined as the capacity of water to neutralize acids
Environmental Chemistry I Week 4 Alkalinity 1) Alkalinity Alkalinity is defined as the capacity of water to accept H + ions (protons). It can also be defined as the capacity of water to neutralize acids
Chemistry 12 August 2007 Form A Provincial Examination Multiple-Choice Key
 Chemistry 12 August 2007 Form A Provincial Examination Multiple-Choice Key Cognitive Processes K = Knowledge U = Understanding H = Higher Mental Processes Weightings 11% 78% 11% Question Types 50 = Multiple
Chemistry 12 August 2007 Form A Provincial Examination Multiple-Choice Key Cognitive Processes K = Knowledge U = Understanding H = Higher Mental Processes Weightings 11% 78% 11% Question Types 50 = Multiple
Announcements. First problem set due next Tuesday. Review for first exam next Thursday. Quiz on Booth (1994) after break today.
 Announcements First problem set due next Tuesday. Review for first exam next Thursday. Quiz on Booth (1994) after break today. Intertidal, Lowes Cove, ME Marine Sediments: Clues to the ocean s past There
Announcements First problem set due next Tuesday. Review for first exam next Thursday. Quiz on Booth (1994) after break today. Intertidal, Lowes Cove, ME Marine Sediments: Clues to the ocean s past There
Equilibrium Practice Problems page 1
 Equilibrium Practice Problems page 1 1988 D NH 4 HS(s) NH 3 (g) + H 2 S(g) ΔHº = +93 kilojoules The equilibrium above is established by placing solid NH 4 HS in an evacuated container at 25ºC. At equilibrium,
Equilibrium Practice Problems page 1 1988 D NH 4 HS(s) NH 3 (g) + H 2 S(g) ΔHº = +93 kilojoules The equilibrium above is established by placing solid NH 4 HS in an evacuated container at 25ºC. At equilibrium,
CERAMIC GLAZING as an IGNEOUS PROCESS
 GEOL 640: Geology through Global Arts and Artifacts CERAMIC GLAZING as an IGNEOUS PROCESS GLAZE COMPONENTS A glaze is a waterproof silica glass on the surface of a ceramic pot, and was first produced by
GEOL 640: Geology through Global Arts and Artifacts CERAMIC GLAZING as an IGNEOUS PROCESS GLAZE COMPONENTS A glaze is a waterproof silica glass on the surface of a ceramic pot, and was first produced by
Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) There are 7 common ions in freshwater: (e.g.
 Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) A. Major ions 1. Common Ions There are 7 common ions in freshwater: (e.g. Table 13-3, p207) cations: Ca +2, Mg +2, Na +1, K +1 anions:
Major Ions, Carbonate System, Alkalinity, ph (Kalff Chapters 13 and 14) A. Major ions 1. Common Ions There are 7 common ions in freshwater: (e.g. Table 13-3, p207) cations: Ca +2, Mg +2, Na +1, K +1 anions:
OCEAN ACIDIFICATION. Effects of an increasing atmospheric CO2 concentration on the ph of the Baltic Sea. Ville Berg Malmborg and Marcus Sjöstedt
 OCEAN ACIDIFICATION Effects of an increasing atmospheric CO2 concentration on the ph of the Baltic Sea Ville Berg Malmborg and Marcus Sjöstedt Department of Physics, Lund University Department of Physical
OCEAN ACIDIFICATION Effects of an increasing atmospheric CO2 concentration on the ph of the Baltic Sea Ville Berg Malmborg and Marcus Sjöstedt Department of Physics, Lund University Department of Physical
Sediment and sedimentary rocks Sediment
 Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
