Most solids do expand when heated, and its length changes by an amount proportional to the original length and the change in temperature.
|
|
|
- Carmella Daniels
- 5 years ago
- Views:
Transcription
1 A0-E-Y Experiment: Thermal Expansion Apparatus Purpose To use steam heating pot to generate steam. Make the steam flow through the metal rod to make it expand, and then use the dial indicator to measure the length of the metal rod after expansion in order to calculate the coefficient of linear expansion. Theory Most solids do expand when heated, and its length changes by an amount proportional to the original length and the change in temperature. L L0 L0αT () L L0 αt () In the equation, L0: the length at 0 L: the length at T α: the constant of proportionality, called the coefficient of linear expansion In the equation, L: the length at T L: the length at T So we obtain the equations below,
2 L =L 0 ( αt ) (3) L =L 0 ( αt ) (4) L T = L T L = L T - L - L The difference of L and L is minimal, so L ~ L, we obtain, L = L T T (5) (6) In the equation, L: L -L T: T -T Linear expansion coefficient is defined as the increment of length of a solid in a unit of length for a rise in temperature of at constant pressure. Instead of the increase of the length, but also consider the expansion in volume. By equation (), we obtain, V=V 0 ( βt) (7) In the equation, V 0 : the volume at 0 V: the volume at T β: the volume expansion coefficient Assume that the side length of a cube is L, the volume at T V= L 3 = 3 T = 3 L 3 o o should be, 3 3 L 3 T 3 T T Since α is a small number, and 3 can be gotten rid of in the equation, that is V= L 3 o (+3 T) (8) Compare equation (7) to equation (8), we know that the volume coefficient is three times the coefficient of linear expansion, soβ=3α. Although the conclusion above
3 But the volume of an object can be divided into many cubes, so for any solid, this conclusion is correct. Instrument Fig. NO Accessory Quantity Steam Generator Steam Heating Pot 3 Steam Heating Pot Lit 4 Fixer 5 Aluminum Rod 6 Metal Rod 7 Brass Rod 8 Base 3
4 9 Zeroing Knob 0 Metal Rod Supporting Base Dial Indicator Digital Electronic Thermometer 3 Thermometer Socket 4 Water Container 5 Steam Conduit Steam Generator HOW TO USE. Switch: this switch will illuminate when the heater starts heating.. Temperature control knob: temperature control protection switch. The generator starts to heat up when the knob is turned till the switch lights up but No Water Indicator doesn t. 3. No water indicator: the indicator lights up when in a non-heated or no water state. 4
5 A0-E-Y 4. Fuse reversion button: it trips when there is no water or overheating, push this button to turn it on again when the generator cools off. 5. Regularly clean the device using heated citric acid with water to maintain the efficiency. 6. Avoid getting water on sockets and switches. Procedure. Wear gloves when using the device since the temperature is very high.. Measure the original length of the metal rod and record. 3. Fill the pot 80 % full with water. The experiment set is shown as Fig.. 4. Make the metal Fig. of the metal bar and the other end to fit the end of the zeroing knob against the groove dial indicator as shown in Fig. 3. 5
6 Fig.3 4. Record the room temperature and the initial reading of the dial indictor. (start from 4 mm). 5. Turn the switch on to generate the steam. 6. When the steam is generated after 5 minutes, observe the thermometer and dial indicator. 7. When the temperature is stable, record the readings down ( at about 98 to 00 ) 8. Turn the zeroing knot to zero, and then change the metal rod when the metal rod and conduit cool off. Calculate the elongation of metal rod and the coefficient of linear expansion. 9. Replace the metal rod, and repeat steps 3 to 8. Experimental Record Coefficient of linear expansion of metal: α( 0-6 )/ C Substance gold silver Brass steel aluminum lead Coefficient of linear expansion
7 Brass: original length L = Temperature T T T initial reading Length (dial indicator) Elongation L mm Average value = Aluminum:original length L = Temperature T T T initial reading Length (dial indicator) Elongation L mm Average value = Unknown metal: original length L = Temperature T T T initial reading Length (dial indicator) Elongation L mm Average value = 7
8 Questions and Discussions. What causes the errors? 8
9 Atis Scientific Instruments Co.,Ltd Tel: Address:F., No.8, Nanming St., South Dist., Tainan City 70, Taiwan (R.O.C.) Website: 9
1. Make the following conversions: a. 0 ºC to kelvins ( K) c. 273 ºC to kelvins ( K)
 Chapter 4 Heat Practice Problems (answers are in brackets) Name: Temperature Conversions: C = ( F 32) 5 9 F = ( 9 ) C + 32 5 K = C + 273.15 1. Make the following conversions: a. 0 ºC to kelvins (273.15
Chapter 4 Heat Practice Problems (answers are in brackets) Name: Temperature Conversions: C = ( F 32) 5 9 F = ( 9 ) C + 32 5 K = C + 273.15 1. Make the following conversions: a. 0 ºC to kelvins (273.15
CHAPTER 17: Temperature, Thermal Expansion, and the Ideal Gas Law
 CHAPTER 17: Temperature, Thermal Expansion, and the Ideal Gas Law Responses to Questions. Properties of materials that could be exploited in making a thermometer include: a. thermal expansion, both linear
CHAPTER 17: Temperature, Thermal Expansion, and the Ideal Gas Law Responses to Questions. Properties of materials that could be exploited in making a thermometer include: a. thermal expansion, both linear
PAPER 2 THEORY QUESTIONS
 PAPER 2 THEORY QUESTIONS 1 Fig. 1.1 shows the arrangement of atoms in a solid block. Fig. 1.1 (a) End X of the block is heated. Energy is conducted to end Y, which becomes warm. (i) Explain how heat is
PAPER 2 THEORY QUESTIONS 1 Fig. 1.1 shows the arrangement of atoms in a solid block. Fig. 1.1 (a) End X of the block is heated. Energy is conducted to end Y, which becomes warm. (i) Explain how heat is
EXPERIMENT 11 LINEAR EXPANSION OF A SOLID MATERIAL
 EXPERIMENT 11 LINEAR EXPANSION OF A SOLID MATERIAL INTRODUCTION: Earlier this semester, we saw that the length of the pendulum effects the period. Many practical devices, such as the mercury thermometer,
EXPERIMENT 11 LINEAR EXPANSION OF A SOLID MATERIAL INTRODUCTION: Earlier this semester, we saw that the length of the pendulum effects the period. Many practical devices, such as the mercury thermometer,
39th International Physics Olympiad - Hanoi - Vietnam Experimental Problem
 DIFFERENTIAL THERMOMETRIC METHOD In this problem, we use the differential thermometric method to fulfill the two following tasks: 1. Finding the temperature of solidification of a crystalline solid substance.
DIFFERENTIAL THERMOMETRIC METHOD In this problem, we use the differential thermometric method to fulfill the two following tasks: 1. Finding the temperature of solidification of a crystalline solid substance.
Pain-Free Melting Point Determination. James Lee, Ph.D. Stanford Research Systems
 Pain-Free Melting Point Determination James Lee, Ph.D. Stanford Research Systems Who is Stanford Research Systems? In business since 1980 Full catalog is over 200 pages Famous for first digital lock-in
Pain-Free Melting Point Determination James Lee, Ph.D. Stanford Research Systems Who is Stanford Research Systems? In business since 1980 Full catalog is over 200 pages Famous for first digital lock-in
SPECIFIC HEAT CAPACITY
 SPECIFIC HEAT CAPACITY Apparatus: Thermometer, balance, two large double Styrofoam cups, lid, hooked metal cube, lifting tool, hot plate, boiling pot. Any material is capable of storing some heat or thermal
SPECIFIC HEAT CAPACITY Apparatus: Thermometer, balance, two large double Styrofoam cups, lid, hooked metal cube, lifting tool, hot plate, boiling pot. Any material is capable of storing some heat or thermal
Experiment 3. Electrical Energy. Calculate the electrical power dissipated in a resistor.
 Experiment 3 Electrical Energy 3.1 Objectives Calculate the electrical power dissipated in a resistor. Determine the heat added to the water by an immersed heater. Determine if the energy dissipated by
Experiment 3 Electrical Energy 3.1 Objectives Calculate the electrical power dissipated in a resistor. Determine the heat added to the water by an immersed heater. Determine if the energy dissipated by
CHAPTER 30 THERMAL EXPANSION
 CHAPTER 30 THERMAL EXPANSION EXERCISE 140, Page 309 1. A length of lead piping is 50.0 m long at a temperature of 16 C. When hot water flows through it the temperature of the pipe rises to 80 C. Determine
CHAPTER 30 THERMAL EXPANSION EXERCISE 140, Page 309 1. A length of lead piping is 50.0 m long at a temperature of 16 C. When hot water flows through it the temperature of the pipe rises to 80 C. Determine
Energy and Energy Calculations Test Provide the correct answer as a word, phrase or sentence. (3 points each) 1) Define Matter.
 Provide the correct answer as a word, phrase or sentence. (3 points each) 1) Define Matter. 2) What is ENERGY? 3) Give an example of an endothermic process. 4) Give an example of an exothermic process.
Provide the correct answer as a word, phrase or sentence. (3 points each) 1) Define Matter. 2) What is ENERGY? 3) Give an example of an endothermic process. 4) Give an example of an exothermic process.
PHYS102 Previous Exam Problems. Temperature, Heat & The First Law of Thermodynamics
 PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
Chapter 02. Voltage and Current. Atomic Theory Review. Atomic Theory Review. Atomic Theory Review. Electrical Charge.
 Chapter 02 Voltage and Current Atom Atomic Theory Review Contains a nucleus of protons and neutrons Nucleus is surrounded by a group of orbiting electrons Electrons are negative, protons are positive Electrically
Chapter 02 Voltage and Current Atom Atomic Theory Review Contains a nucleus of protons and neutrons Nucleus is surrounded by a group of orbiting electrons Electrons are negative, protons are positive Electrically
Homework: 10, 11, 15, 19, 21 (pages ) 25, 29, 30, 32 (page 501)
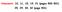 Homework: 1, 11, 15, 19, 1 (pages 5-51) 5, 9, 3, 3 (page 51) 1. An aluminum flagpole is 33m high. By how much does its length increase as the temperature increases by 15 C? For a linear expansion: L LαT
Homework: 1, 11, 15, 19, 1 (pages 5-51) 5, 9, 3, 3 (page 51) 1. An aluminum flagpole is 33m high. By how much does its length increase as the temperature increases by 15 C? For a linear expansion: L LαT
Multi-axis controller V6 / VV6
 The multi-axis controller is available in either single-axis or multi-axis options and is a robust controller used commonly in electro-hydraulic applications. The modular design and many possibilities
The multi-axis controller is available in either single-axis or multi-axis options and is a robust controller used commonly in electro-hydraulic applications. The modular design and many possibilities
Distillation. Boiling
 Distillation The most important technique for separating and purifying organic liquids is distillation 21. A gross oversimplification of the technique is this: the impure liquid in one vessel is vaporized,
Distillation The most important technique for separating and purifying organic liquids is distillation 21. A gross oversimplification of the technique is this: the impure liquid in one vessel is vaporized,
8th Grade. Thermal Energy Study Guide.
 1 8th Grade Thermal Energy Study Guide 2015 10 09 www.njctl.org 2 Thermal Energy Study Guide www.njctl.org 3 Part 1 Define the following terms and/or concepts 4 1 Temperature 5 2 Kinetic Energy 6 3 Thermal
1 8th Grade Thermal Energy Study Guide 2015 10 09 www.njctl.org 2 Thermal Energy Study Guide www.njctl.org 3 Part 1 Define the following terms and/or concepts 4 1 Temperature 5 2 Kinetic Energy 6 3 Thermal
Slide 1 / 67. Slide 2 / 67. 8th Grade. Thermal Energy Study Guide Slide 3 / 67. Thermal Energy. Study Guide.
 Slide 1 / 67 Slide 2 / 67 8th Grade Thermal Energy Study Guide 2015-10-09 www.njctl.org Slide 3 / 67 Thermal Energy Study Guide www.njctl.org Slide 4 / 67 Part 1 Define the following terms and/or concepts
Slide 1 / 67 Slide 2 / 67 8th Grade Thermal Energy Study Guide 2015-10-09 www.njctl.org Slide 3 / 67 Thermal Energy Study Guide www.njctl.org Slide 4 / 67 Part 1 Define the following terms and/or concepts
GENERAL PHYSICS (3) LABORATORY PHYS 203 LAB STUDENT MANUAL
 Haifaa altoumah& Rabab Alfaraj By Haifaa altoumah& Rabab Alfaraj GENERAL PHYSICS (3) LABORATORY PHYS 203 LAB STUDENT MANUAL Name:-. ID# KING ABDULAZIZ UNIVERSITY PHYSICS DEPARMENT 1st semester 1430H Contents
Haifaa altoumah& Rabab Alfaraj By Haifaa altoumah& Rabab Alfaraj GENERAL PHYSICS (3) LABORATORY PHYS 203 LAB STUDENT MANUAL Name:-. ID# KING ABDULAZIZ UNIVERSITY PHYSICS DEPARMENT 1st semester 1430H Contents
k T m 8 B P m k T M T
 I. INTRODUCTION AND OBJECTIVE OF THE EXPERIENT The techniques for evaporation of chemicals in a vacuum are widely used for thin film deposition on rigid substrates, leading to multiple applications: production
I. INTRODUCTION AND OBJECTIVE OF THE EXPERIENT The techniques for evaporation of chemicals in a vacuum are widely used for thin film deposition on rigid substrates, leading to multiple applications: production
Memorandum. September 21. To: Terry Cool, Project Manager From: Brian Lim, Lead Scientist Re: Progress Report on Temperature Controllers
 Memorandum September 21 To: Terry Cool, Project Manager From: Brian Lim, Lead Scientist Re: Progress Report on Temperature Controllers Summary I propose using an inexpensive NTC thermistor to maintain
Memorandum September 21 To: Terry Cool, Project Manager From: Brian Lim, Lead Scientist Re: Progress Report on Temperature Controllers Summary I propose using an inexpensive NTC thermistor to maintain
7. Between 0 and 4 C, the volume coefficient of expansion for water: a.is positive. b.is zero. c.is becoming less dense. d.is negative.
 SERWAY QUESTIONS 1. What is the temperature of a system in thermal equilibrium with another system made up of water and steam at one atmosphere of pressure? a.0 F b.273 K c.0 K d.100 C 2. The observation
SERWAY QUESTIONS 1. What is the temperature of a system in thermal equilibrium with another system made up of water and steam at one atmosphere of pressure? a.0 F b.273 K c.0 K d.100 C 2. The observation
Chapter 2 Heat, Temperature and the First Law of Thermodynamics
 Chapter 2 Heat, Temperature and the First Law of Thermodynamics 2.1. Temperature and the Zeroth Law of Thermodynamics 2.2. Thermal Expansion 2.3. Heat and the Absorption of Heat by Solids and Liquids 2.4.
Chapter 2 Heat, Temperature and the First Law of Thermodynamics 2.1. Temperature and the Zeroth Law of Thermodynamics 2.2. Thermal Expansion 2.3. Heat and the Absorption of Heat by Solids and Liquids 2.4.
ICSE Board Class IX Physics Paper 1
 ICSE Board Class IX Physics Paper 1 Time: 2 hrs Total Marks: 80 General Instructions: 1. Answers to this paper must be written on the paper provided separately. 2. You will not be allowed to write during
ICSE Board Class IX Physics Paper 1 Time: 2 hrs Total Marks: 80 General Instructions: 1. Answers to this paper must be written on the paper provided separately. 2. You will not be allowed to write during
Freezing point depression (Item No.: P )
 Freezing point depression (Item No.: P3021101) Curricular Relevance Area of Expertise: Chemistry Education Level: University Topic: General Chemistry Subtopic: Solutions and Mixtures Experiment: Freezing
Freezing point depression (Item No.: P3021101) Curricular Relevance Area of Expertise: Chemistry Education Level: University Topic: General Chemistry Subtopic: Solutions and Mixtures Experiment: Freezing
40P (2 x 60 x 60) = 2.5 x 10 6 (4200)(5) P = 1.82 x 10 5 W
 NAME : F.3C ( ) Marks: /50 Form 3 Physics Assessment on Heat Time allowed: 45 minutes Section A (34 marks) 1. An indoor swimming pool containing 2.5 x 10 6 kg of water uses 40 identical heaters to maintain
NAME : F.3C ( ) Marks: /50 Form 3 Physics Assessment on Heat Time allowed: 45 minutes Section A (34 marks) 1. An indoor swimming pool containing 2.5 x 10 6 kg of water uses 40 identical heaters to maintain
Zeroth Law of Thermodynamics
 Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
Thermal Equilibrium. Zeroth Law of Thermodynamics 2/4/2019. Temperature
 Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
THERMOCOUPLE CHARACTERISTICS TRAINER
 THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
Temperature and Heat. Two systems of temperature. Temperature conversions. PHY heat - J. Hedberg
 Temperature and Heat 1. Two systems of temperature 1. Temperature conversions 2. Real science (one scale to rule them all) 3. Temperature scales 2. Effects of temperature on materials 1. Linear Thermal
Temperature and Heat 1. Two systems of temperature 1. Temperature conversions 2. Real science (one scale to rule them all) 3. Temperature scales 2. Effects of temperature on materials 1. Linear Thermal
CSUS Department of Chemistry Experiment 7 Chem.1A
 EXPERIMENT #7 Gas Laws PRE-LAB ASSIGNMENT Name: Lab Section: 1. An expandable container of gas maintained at constant temperature has an initial volume of 0.532 L at a pressure of 762 torr. On a stormy
EXPERIMENT #7 Gas Laws PRE-LAB ASSIGNMENT Name: Lab Section: 1. An expandable container of gas maintained at constant temperature has an initial volume of 0.532 L at a pressure of 762 torr. On a stormy
High temperature He is hot
 Lecture 9 What is Temperature and Heat? High temperature He is hot Some important definitions * Two objects are in Thermal contact with each other if energy can be exchanged between them. Thermal equilibrium
Lecture 9 What is Temperature and Heat? High temperature He is hot Some important definitions * Two objects are in Thermal contact with each other if energy can be exchanged between them. Thermal equilibrium
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE
 Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
IR 40. Non-Contact Infrared Temperature Sensor/Transmitter. Temperature Range: 0~1000
 Non-Contact Infrared Temperature Sensor/Transmitter Temperature Range: 0~1000 IR 40 C-910C, Bupyeong Woolim Lion s Valley, #425, Cheongcheon-Dong, Bupyeong-Gu, Incheon, Korea TEL: +82-32-623-7507 FAX:
Non-Contact Infrared Temperature Sensor/Transmitter Temperature Range: 0~1000 IR 40 C-910C, Bupyeong Woolim Lion s Valley, #425, Cheongcheon-Dong, Bupyeong-Gu, Incheon, Korea TEL: +82-32-623-7507 FAX:
Vapour pressure of water at high temperature
 Vapour pressure of water at high temperature (Item No.: P2340100) Curricular Relevance Area of Expertise: Physics Education Level: University Topic: Thermodynamics Subtopic: Thermal Properties and Processes
Vapour pressure of water at high temperature (Item No.: P2340100) Curricular Relevance Area of Expertise: Physics Education Level: University Topic: Thermodynamics Subtopic: Thermal Properties and Processes
Specific Heat. Power Supply Beaker Beam Balance Conecting wires ice. Assembly
 Specific Heat Objectives a. To measure the specific heat capacity of water b. To measure the specific heat capacity of aluminium c. To measure the heat of fusion of ice (Optional) Apparatus Required Power
Specific Heat Objectives a. To measure the specific heat capacity of water b. To measure the specific heat capacity of aluminium c. To measure the heat of fusion of ice (Optional) Apparatus Required Power
Specific Heat. Power Supply Beaker Beam Balance Conecting wires ice. Assembly
 Specific Heat Objectives a. To measure the specific heat capacity of water b. To measure the specific heat capacity of aluminium c. To measure the heat of fusion of ice (Optional) Apparatus Required Power
Specific Heat Objectives a. To measure the specific heat capacity of water b. To measure the specific heat capacity of aluminium c. To measure the heat of fusion of ice (Optional) Apparatus Required Power
Calorimetry. What is the relationship between heat energy and temperature? 4 qt. saucepan 8 qt. stockpot
 Why? Calorimetry What is the relationship between heat energy and temperature? When a substance is heated, the temperature of that substance increases. Will the same amount of energy cause different substances
Why? Calorimetry What is the relationship between heat energy and temperature? When a substance is heated, the temperature of that substance increases. Will the same amount of energy cause different substances
ME 320L/354L Laboratory 3
 ME 320L/354L Laboratory 3 Verification of numerical solution for transient conduction Introduction As we did last time around, we put on Fourier s hat, and his shoes for good measure, and pretend that
ME 320L/354L Laboratory 3 Verification of numerical solution for transient conduction Introduction As we did last time around, we put on Fourier s hat, and his shoes for good measure, and pretend that
Current and Resistance
 PHYS102 Previous Exam Problems CHAPTER 26 Current and Resistance Charge, current, and current density Ohm s law Resistance Power Resistance & temperature 1. A current of 0.300 A is passed through a lamp
PHYS102 Previous Exam Problems CHAPTER 26 Current and Resistance Charge, current, and current density Ohm s law Resistance Power Resistance & temperature 1. A current of 0.300 A is passed through a lamp
Renewable Energy. Theory: The Ideal Gas Law The equation of state for an ideal gas is written: PV = nrt
 Lab 3 Gas Laws and Heat Engines Fall 2010 Introduction/Purpose: In this exercise you will test some of the aspects of the ideal gas law under conditions of constant pressure, constant temperature, and
Lab 3 Gas Laws and Heat Engines Fall 2010 Introduction/Purpose: In this exercise you will test some of the aspects of the ideal gas law under conditions of constant pressure, constant temperature, and
LD-LP-LL-LC Rope Safety Switches with reset for emergency stop
 TECHNICA DATASHEET D-P--C Rope Safety Switches with reset for emergency stop Metal or polymer housing, from one or three conduit entries Protection degree IP6 In conformity with EN ISO 13850 contact blocks
TECHNICA DATASHEET D-P--C Rope Safety Switches with reset for emergency stop Metal or polymer housing, from one or three conduit entries Protection degree IP6 In conformity with EN ISO 13850 contact blocks
5. TEMPERATURE AND HEAT
 5. TEMPERATURE AND HEAT You will study the concepts of temperature and heat as they apply to a sample of water and you will measure the specific heat capacity of the sample. The measurement of one property
5. TEMPERATURE AND HEAT You will study the concepts of temperature and heat as they apply to a sample of water and you will measure the specific heat capacity of the sample. The measurement of one property
1. Thermal energy is transferred through the glass windows of a house mainly by. D. radiation and convection. (1)
 1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation. C. conduction and convection. D. radiation and convection. 2. The specific latent heat of vaporization
1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation. C. conduction and convection. D. radiation and convection. 2. The specific latent heat of vaporization
Experiment B6 Thermal Properties of Materials Procedure
 Experiment B6 Thermal Properties of Materials Procedure Deliverables: Checked lab notebook, Brief technical memo Overview In this lab, you will examine the thermal properties of various materials commonly
Experiment B6 Thermal Properties of Materials Procedure Deliverables: Checked lab notebook, Brief technical memo Overview In this lab, you will examine the thermal properties of various materials commonly
Physics 4C Chapter 18: Temperature, Heat, and the First Law of Thermodynamics
 Physics 4C Chapter 18: Temperature, Heat, and the First Law of Thermodynamics Anyone who has never made a mistake has never tried anything new. Albert Einstein Experience is the name that everyone gives
Physics 4C Chapter 18: Temperature, Heat, and the First Law of Thermodynamics Anyone who has never made a mistake has never tried anything new. Albert Einstein Experience is the name that everyone gives
Serway_ISM_V1 1 Chapter 10. Thermal Physics. it would if filled with the material making up the rest of the object.
 Serway_ISM_V1 1 Chapter 10 10 Thermal Physics ANSWERS TO MULTIPLE CHOICE QUESTIONS 1., and the correct response is choice (e). 2. The correct choice is (b). When an object, containing a cavity, is heated,
Serway_ISM_V1 1 Chapter 10 10 Thermal Physics ANSWERS TO MULTIPLE CHOICE QUESTIONS 1., and the correct response is choice (e). 2. The correct choice is (b). When an object, containing a cavity, is heated,
Week 14 The Simple Pendulum
 Week 14 The Simple Pendulum 1. Scope 1.1 Goal Conduct experiment to study the simple harmonic motion of an oscillatory pendulum and analyze and interpret the data 1.2 Units of measurement to use United
Week 14 The Simple Pendulum 1. Scope 1.1 Goal Conduct experiment to study the simple harmonic motion of an oscillatory pendulum and analyze and interpret the data 1.2 Units of measurement to use United
Laboratory 12: Three Thermodynamics Experiments
 Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Determination of freezing points of pure substances with Cobra4 TEC
 Determination of freezing points of pure substances TEC Related concept Crystallization point, Gibbs free energy, enthalpy, entropy, heat of fusion, freezing point depression. Principle When a pure substance
Determination of freezing points of pure substances TEC Related concept Crystallization point, Gibbs free energy, enthalpy, entropy, heat of fusion, freezing point depression. Principle When a pure substance
Handout 10: Heat and heat transfer. Heat capacity
 1 Handout 10: Heat and heat transfer Heat capacity Consider an experiment in Figure 1. Heater is inserted into a solid substance of mass m and the temperature rise T degrees Celsius is measured by a thermometer.
1 Handout 10: Heat and heat transfer Heat capacity Consider an experiment in Figure 1. Heater is inserted into a solid substance of mass m and the temperature rise T degrees Celsius is measured by a thermometer.
Rate in Thermal Systems
 Rate in Thermal Systems Overview Rate in Thermal Systems 1 Fundamental Concepts What is the prime mover in the thermal system? temperature difference ( T) What does rate measure in the thermal system?
Rate in Thermal Systems Overview Rate in Thermal Systems 1 Fundamental Concepts What is the prime mover in the thermal system? temperature difference ( T) What does rate measure in the thermal system?
Electricity. Electrolysis. Current and the transport of charge DETERMINATION OF THE FARADAY CONSTANT BASIC PRINCIPLES
 Electricity Current and the transport of charge Electrolysis DETERMINATION OF THE FARADAY CONSTANT Production of hydrogen by means of electrolysis and determining the volume of the hydrogen V. Determining
Electricity Current and the transport of charge Electrolysis DETERMINATION OF THE FARADAY CONSTANT Production of hydrogen by means of electrolysis and determining the volume of the hydrogen V. Determining
4 th International Junior Science Olympiad
 4 th International Junior Science Olympiad Practical Examination December 08, 2007 Important Remarks 1. While you are in the laboratory, you should wear safety spectacles at all times. 2. Eating of any
4 th International Junior Science Olympiad Practical Examination December 08, 2007 Important Remarks 1. While you are in the laboratory, you should wear safety spectacles at all times. 2. Eating of any
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
Self-Testing Prevectron 2 Millenium Lightning Conductor Set-up and Installation Manual
 Self testing Prevectron 2 Millenium Lightning Conductor ENGLISH Self-Testing Prevectron 2 Millenium Lightning Conductor Set-up and Installation Manual S6.60T S4.50T S3.40T N.B. : Information and Specifications
Self testing Prevectron 2 Millenium Lightning Conductor ENGLISH Self-Testing Prevectron 2 Millenium Lightning Conductor Set-up and Installation Manual S6.60T S4.50T S3.40T N.B. : Information and Specifications
LAB 1 PRE-LAB. residuals (cm)
 LAB 1 PRE-LAB 1. The table below records measurements of the lengths l of five goldfish. Calculate the average length l avg of this population of goldfish, and the residual, or deviation from average length
LAB 1 PRE-LAB 1. The table below records measurements of the lengths l of five goldfish. Calculate the average length l avg of this population of goldfish, and the residual, or deviation from average length
Exam 4--PHYS 101--Fall 2016
 Name: Exam 4--PHYS 101--Fall 2016 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A bus contains a 2000 kg flywheel (a disk that has a 0.500 m radius)
Name: Exam 4--PHYS 101--Fall 2016 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A bus contains a 2000 kg flywheel (a disk that has a 0.500 m radius)
Chapter 17 Temperature and heat
 Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
HEAT HISTORY. D. Whitehall
 1 HEAT HISTORY 18 th Century In the 18 th century it was assumed that there was an invisible substance called caloric. When objects got it was assumed that they gained caloric, therefore hot objects should
1 HEAT HISTORY 18 th Century In the 18 th century it was assumed that there was an invisible substance called caloric. When objects got it was assumed that they gained caloric, therefore hot objects should
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *8792218070* PHYSICS 0625/23 Paper 2 Core October/November 2010 1 hour 15 minutes Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *8792218070* PHYSICS 0625/23 Paper 2 Core October/November 2010 1 hour 15 minutes Candidates
Casio G SHOCK watches Specifications
 Casio G SHOCK watches Specifications 1. Design AW-590-1A Case dimension: 52mm X 46.4mm X 14.9mm - Case and band: Resin - Bezel: Stainless steel Weight: 57.2g Analogue display: hour and minute hands (moves
Casio G SHOCK watches Specifications 1. Design AW-590-1A Case dimension: 52mm X 46.4mm X 14.9mm - Case and band: Resin - Bezel: Stainless steel Weight: 57.2g Analogue display: hour and minute hands (moves
Basic Electricity Video Exam
 Name: Class: Date: Basic Electricity Video Exam Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Matter is made of. a. plasma, gas, and solid b. solid,
Name: Class: Date: Basic Electricity Video Exam Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Matter is made of. a. plasma, gas, and solid b. solid,
Equation of state of ideal gases Students worksheet
 3.2.1 Tasks For a constant amount of gas (in our case air) investigate the correlation between 1. Volume and pressure at constant temperature (Boyle-Marriotte s law) 2. Temperature and volume at constant
3.2.1 Tasks For a constant amount of gas (in our case air) investigate the correlation between 1. Volume and pressure at constant temperature (Boyle-Marriotte s law) 2. Temperature and volume at constant
Tells us the average translational kinetic energy of the particles
 Temperature and Heat What is temperature? Kinetic Energy What is heat? Thermal Expansion Specific Heat Latent Heat and phase changes Unit 03, Slide 1 Temperature Tells us the average translational kinetic
Temperature and Heat What is temperature? Kinetic Energy What is heat? Thermal Expansion Specific Heat Latent Heat and phase changes Unit 03, Slide 1 Temperature Tells us the average translational kinetic
PART I: MEASURING MASS
 Chemistry I Name Dr. Saulmon 2014-15 School Year Laboratory 1 Measuring Mass, Volume, and Temperature Monday, August 25, 2014 This laboratory is broken into three parts, each with its own introduction,
Chemistry I Name Dr. Saulmon 2014-15 School Year Laboratory 1 Measuring Mass, Volume, and Temperature Monday, August 25, 2014 This laboratory is broken into three parts, each with its own introduction,
Experiment 1. Measurement of Thermal Conductivity of a Metal (Brass) Bar
 Experiment 1 Measurement of Thermal Conductivity of a Metal (Brass) Bar Introduction: Thermal conductivity is a measure of the ability of a substance to conduct heat, determined by the rate of heat flow
Experiment 1 Measurement of Thermal Conductivity of a Metal (Brass) Bar Introduction: Thermal conductivity is a measure of the ability of a substance to conduct heat, determined by the rate of heat flow
Introduction to Blackbody Sources
 Introduction to s This section contains dedicated blackbody sources for low uncertainty calibration of infrared thermometers. A range of portable primary blackbody sources combine high emissivity with
Introduction to s This section contains dedicated blackbody sources for low uncertainty calibration of infrared thermometers. A range of portable primary blackbody sources combine high emissivity with
Table of Contents. Experiment 1: Vapour Pressure of Water at High Temperature 2. Experiment 2: Heat Capacity of Gases 5
 1 Table of Contents EXPERIMENT PAGE Experiment 1: Vapour Pressure of Water at High Temperature 2 Experiment 2: Heat Capacity of Gases 5 Experiment 3: Joule-Thomson Effect 11 Experiment 4: Thermal and Electrical
1 Table of Contents EXPERIMENT PAGE Experiment 1: Vapour Pressure of Water at High Temperature 2 Experiment 2: Heat Capacity of Gases 5 Experiment 3: Joule-Thomson Effect 11 Experiment 4: Thermal and Electrical
Lab 10: Harmonic Motion and the Pendulum
 Lab 10 Harmonic Motion and the Pendulum 119 Name Date Partners Lab 10: Harmonic Motion and the Pendulum OVERVIEW A body is said to be in a position of stable equilibrium if, after displacement in any direction,
Lab 10 Harmonic Motion and the Pendulum 119 Name Date Partners Lab 10: Harmonic Motion and the Pendulum OVERVIEW A body is said to be in a position of stable equilibrium if, after displacement in any direction,
Module 3 - Thermodynamics. Thermodynamics. Measuring Temperatures. Temperature and Thermal Equilibrium
 Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Electricity 1.notebook. May 04, 2016 ELECTRICITY. objects.
 ELECTRICITY is objects. 1 2 3 4 5 6 Insulators and Conductors You should now know that electricity. 1. Electrical Insulator - Any substance in which Examples: 7 If atoms in an become charged with, these
ELECTRICITY is objects. 1 2 3 4 5 6 Insulators and Conductors You should now know that electricity. 1. Electrical Insulator - Any substance in which Examples: 7 If atoms in an become charged with, these
Nucleophilic Substitution
 1 Introduction: Nucleophilic Substitution The purpose of this experiment is to compare the reactivities of a two nucleophiles, iodide and chloride, in a substitution reaction of 1-bromooctane. A quaternary
1 Introduction: Nucleophilic Substitution The purpose of this experiment is to compare the reactivities of a two nucleophiles, iodide and chloride, in a substitution reaction of 1-bromooctane. A quaternary
Archimedes Principle
 Archimedes Principle applies in air the more air an object displaces, the greater the buoyant force on it if an object displaces its weight, it hovers at a constant altitude if an object displaces less
Archimedes Principle applies in air the more air an object displaces, the greater the buoyant force on it if an object displaces its weight, it hovers at a constant altitude if an object displaces less
SPECIFIC HEAT CAPACITY AND HEAT OF FUSION
 SPECIFIC HEAT CAPACITY AND HEAT OF FUSION Apparatus on each table: Thermometer, metal cube, complete calorimeter, outer calorimeter can (aluminum only), balance, 4 styrofoam cups, graduated container,
SPECIFIC HEAT CAPACITY AND HEAT OF FUSION Apparatus on each table: Thermometer, metal cube, complete calorimeter, outer calorimeter can (aluminum only), balance, 4 styrofoam cups, graduated container,
Conceptual Physics Fundamentals
 Conceptual Physics Fundamentals Chapter 8: TEMPERATURE, HEAT, AND THERMODYNAMICS This lecture will help you understand: Temperature Absolute Zero Internal Energy Heat Quantity of Heat The Laws of Thermodynamics
Conceptual Physics Fundamentals Chapter 8: TEMPERATURE, HEAT, AND THERMODYNAMICS This lecture will help you understand: Temperature Absolute Zero Internal Energy Heat Quantity of Heat The Laws of Thermodynamics
Chapter 3 Metric Units and Conversions
 Chapter 3 Metric Units and Conversions 3.1 The Metric System and Prefixes Metric system: a simple decimal system of measurement that uses the following basic units: Quantity Basic Unit Symbol length meter
Chapter 3 Metric Units and Conversions 3.1 The Metric System and Prefixes Metric system: a simple decimal system of measurement that uses the following basic units: Quantity Basic Unit Symbol length meter
f Static Electricity:
 ELECTRICITV VOCflB WORDS Electricity: f Static Electricity: Current Electricity: Electron: Neutron: Proton: Attraction: Repulsion: / ^ Source: Conductor: Insulator: Load: Switch: Series Circuit: Parallel
ELECTRICITV VOCflB WORDS Electricity: f Static Electricity: Current Electricity: Electron: Neutron: Proton: Attraction: Repulsion: / ^ Source: Conductor: Insulator: Load: Switch: Series Circuit: Parallel
Chapter 4: Heat Capacity and Heat Transfer
 Chapter 4: Heat Capacity and Heat Transfer Chapter 4: Heat Capacity and Heat Transfer Chapter 4: Heat Capacity and Heat Transfer 4.1 Material Structure 4.2 Temperature and Material Properties 4.3 Heating
Chapter 4: Heat Capacity and Heat Transfer Chapter 4: Heat Capacity and Heat Transfer Chapter 4: Heat Capacity and Heat Transfer 4.1 Material Structure 4.2 Temperature and Material Properties 4.3 Heating
Unit 11: Temperature and heat
 Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Thermal Conductivity, k
 Homework # 85 Specific Heats at 20 C and 1 atm (Constant Pressure) Substance Specific Heat, c Substance Specific Heat, c kcal/kg C J/kg C kcal/kg C J/kg C Solids Aluminum 0.22 900 Brass 0.090 377 Copper
Homework # 85 Specific Heats at 20 C and 1 atm (Constant Pressure) Substance Specific Heat, c Substance Specific Heat, c kcal/kg C J/kg C kcal/kg C J/kg C Solids Aluminum 0.22 900 Brass 0.090 377 Copper
Temperature control for Varian Cary line of UV/Vis Spectrophotometers and the Eclipse Fluorometer
 Temperature control for Varian Cary line of UV/Vis Spectrophotometers and the Eclipse Fluorometer Temperature Control without Compromise: rapid changes, precise temperatures, powerful complex functionality.
Temperature control for Varian Cary line of UV/Vis Spectrophotometers and the Eclipse Fluorometer Temperature Control without Compromise: rapid changes, precise temperatures, powerful complex functionality.
Measurements in the Laboratory
 Measurements in the Laboratory Objectives The objectives of this laboratory are: a) Use standard laboratory measurement devices to measure length, volume and mass amounts. b) Use these measurements to
Measurements in the Laboratory Objectives The objectives of this laboratory are: a) Use standard laboratory measurement devices to measure length, volume and mass amounts. b) Use these measurements to
Study of Temperature Distribution Along the Fin Length
 Heat Transfer Experiment No. 2 Study of Temperature Distribution Along the Fin Length Name of the Student: Roll No: Department of Mechanical Engineering for Women, Pune. Aim: ˆ Measuring the temperature
Heat Transfer Experiment No. 2 Study of Temperature Distribution Along the Fin Length Name of the Student: Roll No: Department of Mechanical Engineering for Women, Pune. Aim: ˆ Measuring the temperature
34000 Series. Compact High Power DC Electronic Load. Prodigit Electronics Co., Ltd. 60V 600V 1000V. 20 Models Type. 5kW~ 40kW. Features.
 Compact High DC Electronic Load 4000 Series 60V 600V 1000V 5kW~ 40kW 0 Models Type Features 5 digit V/A/W Meter. High-speed measurement and communication transmission. Large LCD Display setting values
Compact High DC Electronic Load 4000 Series 60V 600V 1000V 5kW~ 40kW 0 Models Type Features 5 digit V/A/W Meter. High-speed measurement and communication transmission. Large LCD Display setting values
Lecture 13 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics
 Lecture 13 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Lecture 13 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Temperature and Thermal Equilibrium Linear Expansion
Lecture 13 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Lecture 13 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Temperature and Thermal Equilibrium Linear Expansion
Investigation #9 OBSERVATION OF THE PHOTOELECTRIC EFFECT
 Name: Investigation #9 Partner(s): OBSERVATION OF THE PHOTOELECTRIC EFFECT As mentioned in the previous investigation, one well-known phenomenon that defied explanation based on the well-established theories
Name: Investigation #9 Partner(s): OBSERVATION OF THE PHOTOELECTRIC EFFECT As mentioned in the previous investigation, one well-known phenomenon that defied explanation based on the well-established theories
IR 80-H. Non-Contact Infrared Temperature Sensor/Transmitter. Temperature Range: 500~1700
 Non-Contact Infrared Temperature Sensor/Transmitter Temperature Range: 500~1700 IR 80-H C-910C, Bupyeong Woolim Lion s Valley, #425, Cheongcheon-Dong, Bupyeong-Gu, Incheon, Korea TEL: +82-32-623-7507 FAX:
Non-Contact Infrared Temperature Sensor/Transmitter Temperature Range: 500~1700 IR 80-H C-910C, Bupyeong Woolim Lion s Valley, #425, Cheongcheon-Dong, Bupyeong-Gu, Incheon, Korea TEL: +82-32-623-7507 FAX:
Thermal Energy. Thermal Energy is the TRANSFER of kinetic energy between two objects that are at different temperatures.
 Thermal Energy Thermal Energy is the TRANSFER of kinetic energy between two objects that are at different temperatures. And remember: heat will always transfer from a warm object to a cold object. HEAT
Thermal Energy Thermal Energy is the TRANSFER of kinetic energy between two objects that are at different temperatures. And remember: heat will always transfer from a warm object to a cold object. HEAT
HL-800K Infrared Laser Thermometer. -50 C to +800 C (13:1 ratio) User Manual
 HL-800K Infrared Laser Thermometer -50 C to +800 C (13:1 ratio) User Manual TABLE OF CONTENTS INTRODUCTION... 3 FEATURES... 3 WIDE APPLICATION RANGE... 3 SAFETY... 3 DISTANCE & SPOT SIZE... 4 SPECIFICATIONS...
HL-800K Infrared Laser Thermometer -50 C to +800 C (13:1 ratio) User Manual TABLE OF CONTENTS INTRODUCTION... 3 FEATURES... 3 WIDE APPLICATION RANGE... 3 SAFETY... 3 DISTANCE & SPOT SIZE... 4 SPECIFICATIONS...
DISCONTINUED PRECISION MEASURING FOWLER CALIPERS 1 - VERNIER CALIPERS 4 - ELECTRONIC CALIPERS
 FOWLER CALIPERS 1 - VERNIER CALIPERS 4 - ELECTRONIC CALIPERS 52-058-016 Fine quality vernier calipers are constructed of stainless steel. 52-057-004 offers 3-way measurement to accuracy. 52-058-XXX series
FOWLER CALIPERS 1 - VERNIER CALIPERS 4 - ELECTRONIC CALIPERS 52-058-016 Fine quality vernier calipers are constructed of stainless steel. 52-057-004 offers 3-way measurement to accuracy. 52-058-XXX series
Temperature Scales. Temperature, and Temperature Dependent on Physical Properties. Temperature. Temperature Scale
 Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
1. How much heat was needed to raise the bullet to its final temperature?
 Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
SPECIFIC HEAT OF WATER LAB 11-2
 CONCEPT Heat of Fusion Changes of state (phase changes) involve the conversion or transition of matter from one of the common states (solid, liquid or gas) to another. Examples include fusion or melting
CONCEPT Heat of Fusion Changes of state (phase changes) involve the conversion or transition of matter from one of the common states (solid, liquid or gas) to another. Examples include fusion or melting
Heat and Temperature
 Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
Chapter 4 Heat Heat and Temperature Heat is a form of energy Heat is the energy of random motion of molecules constituting the body. It flows from a hot body to a cold body. Unit of heat is joule (J) and
Static Electricity. Electric Field. the net accumulation of electric charges on an object
 Static Electricity the net accumulation of electric charges on an object Electric Field force exerted by an e - on anything that has an electric charge opposite charges attract like charges repel Static
Static Electricity the net accumulation of electric charges on an object Electric Field force exerted by an e - on anything that has an electric charge opposite charges attract like charges repel Static
'-. MICROMEr II. The MICROMET8 II is shipped assembled except for the Filar Eyepiece, Micrometer Stage, and Standard Vise.
 MICROMEr II '-. UNPACKING: Carefully unpack and check contents. If any components are missing or damaged, save the packing list and material, and advise the carrier and Buehler Ltd. of the discrepancy.
MICROMEr II '-. UNPACKING: Carefully unpack and check contents. If any components are missing or damaged, save the packing list and material, and advise the carrier and Buehler Ltd. of the discrepancy.
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
STATIC ELECTRICITY. I. Tick ( ) the most appropriate answer. 1. When an ebonite rod is rubbed with fur, the charge acquired by the fur is:
 6 STATIC ELECTRICITY I. Tick ( ) the most appropriate answer. 1. When an ebonite rod is rubbed with fur, the charge acquired by the fur is: (a) negative (b) positive (c) both positive and negative (d)
6 STATIC ELECTRICITY I. Tick ( ) the most appropriate answer. 1. When an ebonite rod is rubbed with fur, the charge acquired by the fur is: (a) negative (b) positive (c) both positive and negative (d)
Experiment Two (2) Torsional testing of Circular Shafts
 Experiment Two (2) Torsional testing of Circular Shafts Introduction: Torsion occurs when any shaft is subjected to a torque. This is true whether the shaft is rotating (such as drive shafts on engines,
Experiment Two (2) Torsional testing of Circular Shafts Introduction: Torsion occurs when any shaft is subjected to a torque. This is true whether the shaft is rotating (such as drive shafts on engines,
Phy 100 s Lab - Measurement techniques for mass, size and density. Name Course & Sec. Lab Partner
 Phy 100 s Lab - techniques for mass, size and density. Name Course & Sec Lab Partner Date 1. You should have a metal block and a metal cylinder both made of the same material. If you are unsure if the
Phy 100 s Lab - techniques for mass, size and density. Name Course & Sec Lab Partner Date 1. You should have a metal block and a metal cylinder both made of the same material. If you are unsure if the
