/12 /20 /16 /16 /12 /12 /16 /16 /10 /5 /5 /10 /150
|
|
|
- Bertram Page
- 5 years ago
- Views:
Transcription
1 ``hem Final Exam May 3, 2002 Name: (first) (last) (Please Print) Page Total score Score /12 /20 /16 /16 /12 /12 /16 /16 /10 /5 /5 /10 /150 1
2 I. Multiple choice questions (30 questions, 4 points each). Write down your answer in the provided space in front of every question. There is no partial point if your marked (circled) answer is different from what you write down. Answer Q.1 and Q.2 according to the following reaction I II III IV V VI ( ) 1. Which product(s) can be obtained from S N 2 reaction? I, III II, III I II ( ) 2. Which product(s) can be obtained from E1 reaction? IV, V, VI IV, V V, VI I, II, III ( ) 3. What is the order of decreasing rate in an E2 elimination for the following compounds? I: II: III: I>II>III II>I>III I>III>II III>I>II 2
3 ( ) 4. What is the name for the following compound? 3 p-methylphenol m-dimethylphenol o-methylanisole 3-methylanisole ( ) 5. What is the name for the following compound? N 2 2 p-aminotoluene m-nitrotoluene m-nitrostyrene 3-aminostyrene ( ) 6. What is the name for the following compound? acetic benzoic anhydride acetic phenoic anhydride acetic benzoic ester acetic phenoic ester ( ) 7. What is the name for the following compound? 2 5 N trans-n-ethyl-3-pentenamide trans-1-ethyl-3-pentenamide trans-n-ethyl-4-pentenamide trans-1-ethyl-4-pentenamide ( ) 8. What is the name for the following compound? 3 2 N 2 2 (R)-3-methylhexaneamine (S)-3-methylhexaneamine (R)-3-methylhexanenitrile (S)-3-methylhexanenitrile 3
4 Select the correct product for the following reactions from Q.9 to Q.30 ( ) 9. 1) P 2) 2, ( ) 10. 1) Na 2 r 2 7, +, heat 2) 2, ( ) ) l, All 3 2) NaB ( ) 12. 1), Fel 3 2) Na 2 r 2 7, heat 2 2 4
5 ( ) 13. 1) Mg, Et 2 2) D 2 2 D D D ( ) 14. 1) Pl 3 2) l ( ) ) heat, removal of 2 2) excess N 3, 25 o N 2 N 2 N 2 N 2 N ( ) 16. 1) Mn 2 2) ( ) 2 uli 5
6 ( ) 17. 1) NaN 2) +, 2, hea t 2 N N 2 N 2 ( ) 18. 1) (l) 2, Me 2 S; E t 3 N 2) Ph 3 P 2 l ( ) 19. 1) 2, Lindlar's catalyst 2) 3, then
7 ( ) ) Et Et P 2) (i-bu) 2 Al (DIBAL), -78 o, then ( ) ) 3, then 2 2 2) P 3 3) 2 N, pyridine 4) LiAl 4 N N ( ) 22. 1) 2, Fel 3 2) ( 2 =) 2 uli 3) s 4 7
8 ( ) 23. 1) 2, Fel 3 2) ( 2 =) 2 uli 3) R 3 3 ( ) 24. 1) l 2, All 3 2) Mg, Et 2 3) the n 2 4) Pl 3, pyridine 2 l ( ) 25. 1) l 2, All 3 2) Mg, Et 2 3) then 2 4) Sl 2, pyridine l ( ) ) NBS, heat 2) tert-bu 3) 3, then Me 2 S 4) Et 2 N, NaB 3 N NEt 2 NEt 2 8
9 ( ) 27. 1) 2, Lindlar's catalyst 2) s 4, I 4 3) Zn (g), l, heat 2 ( ) 28. 1) 3, then NaB 4 2) S 2 l (Msl), pyridine 2 3) Na, heat ( ) ) 3, then NaB 4 2) Pl 3 3) NaN, heat 4) +, 2, heat N N 2 ( ) 30. 1) 2, Pt/ 2) Na 2 r 2 7, heat 3) Sl 2 4) 2 N 2 N N 9
10 II. Use the provided table and fill in compound structures and reagents for the following synthesis. Make sure your answer is correctly put in the designated box of the given table. No partial point will be given for the misplaced answer. (10 points) N S 4 Pd/ S 3 2 S 4 N A I II III IV Pl 3 (2 equivalences) K 2 3 S 3 1) +, 2, heat 2) - N 3 VII N 2 B VI V VIII S 3 N 2 1) +, 2, heat 2) - (azo compound) S N 2 Fill in your answers for part II in the following table. A: B: I: II: III: IV: V: VI: VII: VIII: 10
11 III. Propose an electron pushing mechanism for each of the following reactions. (5 points each) NN 2 K, 2 heat 3 2 ontinue to the next page 11
12 2. 3 cat. 2 S 4 ontinue to the next page 12
13 IV. Propose a synthesis for novocain using the provided starting material and any reagents you know: (10 points) starting material 2 2 N from 2 2 N 2 Novocain N 2 13
1. What is the name for the following compound? (a) (b) (c) p-methylphenol m-methylphenol 3-methylanisole. None of the above
 hem 220 Final Exam May 2, 2005 Name: (first) (last) (Please print) Last 4 digits of I.D. I. Multiple hoice ( /0) Score /90 II /20 III /10 IV /10 V /10 Bonus /10 Total score /140 1 I. Multiple choice questions.
hem 220 Final Exam May 2, 2005 Name: (first) (last) (Please print) Last 4 digits of I.D. I. Multiple hoice ( /0) Score /90 II /20 III /10 IV /10 V /10 Bonus /10 Total score /140 1 I. Multiple choice questions.
/120 /200. Last 4 digits of Banner No. Score. I. Multiple Choice ( /40) II /40 III /10 IV /10 V /20. Total score. Chem 2320 Final Exam.
 hem 2320 Final Exam April 30, 2007 Name: (First) (Last) (Please print) Last 4 digits of Banner No. Score I. Multiple hoice ( /40) /120 II /40 III /10 IV /10 V /20 Total score /200 1 I. Multiple choice
hem 2320 Final Exam April 30, 2007 Name: (First) (Last) (Please print) Last 4 digits of Banner No. Score I. Multiple hoice ( /40) /120 II /40 III /10 IV /10 V /20 Total score /200 1 I. Multiple choice
/60 /100. Last 4 digits of Banner No. Score. I. Multiple Choice ( /20) II /20 III /10 IV /10. Total score. Chem 2320 Exam 3. March 26, 2007.
 hem 2320 Exam 3 March 26, 2007 ame: (first) (last) (Please print) Last 4 digits of Banner o. Score I. Multiple hoice ( /20) /60 II /20 III /10 IV /10 Total score /100 1 I. Multiple choice questions. (3
hem 2320 Exam 3 March 26, 2007 ame: (first) (last) (Please print) Last 4 digits of Banner o. Score I. Multiple hoice ( /20) /60 II /20 III /10 IV /10 Total score /100 1 I. Multiple choice questions. (3
Practice Homework Iverson CH320M/328M Do not turn in. NAME (Print): Chemistry 320M/328M Dr. Brent Iverson Practice Homework December 3, 2018
 Practice omework Iverson C320M/328M Do not turn in NAME (Print): SIGNATURE: Chemistry 320M/328M Dr. ent Iverson Practice omework December 3, 2018 Please print the first three letters of your last name
Practice omework Iverson C320M/328M Do not turn in NAME (Print): SIGNATURE: Chemistry 320M/328M Dr. ent Iverson Practice omework December 3, 2018 Please print the first three letters of your last name
CHEM 203. Final Exam December 12, This a closed-notes, closed-book exam. You may use your set of molecular models. This test contains 11 pages
 CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
CHEM 203. Final Exam December 18, 2013 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
EXAM #3 EXTRA PROBLEMS
 Massachusetts Institute of Technology 5.13, Fall 2006 Dr. Kimberly L. Berkowski rganic chemistry II EXAM #3 EXTRA PRBLEMS What to expect on Exam #3: 1. ~1 Labeling experiment 2. ~2 chanisms 3. ~2 Syntheses
Massachusetts Institute of Technology 5.13, Fall 2006 Dr. Kimberly L. Berkowski rganic chemistry II EXAM #3 EXTRA PRBLEMS What to expect on Exam #3: 1. ~1 Labeling experiment 2. ~2 chanisms 3. ~2 Syntheses
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Organic Chemistry II (CHE ) Final Examination April 30, Name (Print legibly):
 rganic hemistry II (E 232-002) Final Examination April 30, 2007 Name (Print legibly): (last) (first) PLEASE observe the following: You are allowed to have scratch paper (provided by me), and a simple model
rganic hemistry II (E 232-002) Final Examination April 30, 2007 Name (Print legibly): (last) (first) PLEASE observe the following: You are allowed to have scratch paper (provided by me), and a simple model
Chem 342 Organic Chemistry II Final Exam 13 May 2009
 hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
Columbia University C99ORG22ak.DOC Chem S3444Q Summer 99 Professor Irving J. Borowitz Exam No. 2 Answer Key July 28, 1999
 olumbia University 99RG22ak. hem SQ Summer 99 Professor Irving J. Borowitz Exam No. 2 Answer Key July 28, 1999 Name: Grade: Please use a non-red pen. Answer questions in the provided space. If you write
olumbia University 99RG22ak. hem SQ Summer 99 Professor Irving J. Borowitz Exam No. 2 Answer Key July 28, 1999 Name: Grade: Please use a non-red pen. Answer questions in the provided space. If you write
CHEMISTRY 332 FINAL EXAM. December 14, I. (18 points) II. (48 points) III. (50 points) IV. (40 points) V. (26 points) VI.
 Seat No. Name (please print and circle your last name) CEMISTRY 332 FINAL EXAM December 14, 2005 I. (18 points) II. (48 points) III. (50 points) IV. (40 points) V. (26 points) VI. (18 points) TTAL (200
Seat No. Name (please print and circle your last name) CEMISTRY 332 FINAL EXAM December 14, 2005 I. (18 points) II. (48 points) III. (50 points) IV. (40 points) V. (26 points) VI. (18 points) TTAL (200
CH310N Spring Anslyn. March 23, Exam 2
 C310N Spring 2010 Anslyn March 23, 2010 Exam 2 Please PRINT the first three letters of your last name in the boxes below. PRINT Full Name UT-EID 1) ( 8 pts ) 2) ( 5 pts ) 3) ( 5 pts ) 4) ( 22 pts ) 5)
C310N Spring 2010 Anslyn March 23, 2010 Exam 2 Please PRINT the first three letters of your last name in the boxes below. PRINT Full Name UT-EID 1) ( 8 pts ) 2) ( 5 pts ) 3) ( 5 pts ) 4) ( 22 pts ) 5)
Chemistry 233 Exam 3 (Green) The Periodic Table
 Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #3 - November 8, 2004 ANSWERS
 Name Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 8, 2004 ANSWERS INSTRUTINS --- This examination has two parts. The first part is in multiple choice format;
Name Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 8, 2004 ANSWERS INSTRUTINS --- This examination has two parts. The first part is in multiple choice format;
(please print and circle your last name) CHEMISTRY 332 FINAL EXAM. December 11, 2006
 Seat No. Name (please print and circle your last name) CEMISTRY 332 FINAL EXAM December 11, 2006 I. (18 points) II. III. IV. (48 points) (50 points) (40 points) V. (26 points) VI. (18 points) TTAL (200
Seat No. Name (please print and circle your last name) CEMISTRY 332 FINAL EXAM December 11, 2006 I. (18 points) II. III. IV. (48 points) (50 points) (40 points) V. (26 points) VI. (18 points) TTAL (200
Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems
 Some Topics to Study (very broad): Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems Reactivity stuff Classify atoms as electrophilic or nucleophilic Definitions of nucleophiles/electrophiles
Some Topics to Study (very broad): Chem 14D Spring 2010 Garg Midterm 1 Review / Practice Problems Reactivity stuff Classify atoms as electrophilic or nucleophilic Definitions of nucleophiles/electrophiles
CHEMISTRY MIDTERM # 4 ANSWER KEY April 16, 2002
 CEMISTY 314-01 MIDTEM # 4 ASWE KEY April 16, 00 1. (6 pts) Mark as true (T) or false (F) the following statements. Do not explain! (F) Amides are less reactive than acid chlorides but more reactive than
CEMISTY 314-01 MIDTEM # 4 ASWE KEY April 16, 00 1. (6 pts) Mark as true (T) or false (F) the following statements. Do not explain! (F) Amides are less reactive than acid chlorides but more reactive than
CHAPTER 22 HW: CO 2 H DERIVATIVES
 APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
Solutions b) OH
 CAPTER 13 279 R R R R Solutions 13.1. 5,5-dibromo-2-methylhexan-2-ol (2S,3R)-2,3,4-trimethylpentan-1-ol 2,2,5,5-tetramethylcyclopentanol d) 2,6-diethylphenol e) (S)-2,2,4,4-tetramethylcyclohexanol 13.2.
CAPTER 13 279 R R R R Solutions 13.1. 5,5-dibromo-2-methylhexan-2-ol (2S,3R)-2,3,4-trimethylpentan-1-ol 2,2,5,5-tetramethylcyclopentanol d) 2,6-diethylphenol e) (S)-2,2,4,4-tetramethylcyclohexanol 13.2.
First 2-Hour Exam. By printing your name below, you pledge that
 Chem 3331 Fall 2004 Exam # 1 1 Name First 2-our Exam By printing your name below, you pledge that "n my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized
Chem 3331 Fall 2004 Exam # 1 1 Name First 2-our Exam By printing your name below, you pledge that "n my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized
Chemistry 234 Practice Exam 3. The Periodic Table
 ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
CH2710-Exam #5 (Katz)
 C2710-Exam #5 (Katz) Name: Score: Section - Multiple Choice-Choose the BEST answer from the choices given and write the letter for that answer in the space provided. (50 pts) Consider the reaction below
C2710-Exam #5 (Katz) Name: Score: Section - Multiple Choice-Choose the BEST answer from the choices given and write the letter for that answer in the space provided. (50 pts) Consider the reaction below
CHEM 203. Final Exam December 18, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
HOMEWORK PROBLEMS: ALKYNES. 1. Provide the complete IUPAC name for the following compounds:
 CEM 31 MEWRK PRBLEMS: ALKYNES 1. Provide the complete IUPAC name for the following compounds: 2. When the compound below is treated with one equivalent of B 3, where does it react Explain. Where would
CEM 31 MEWRK PRBLEMS: ALKYNES 1. Provide the complete IUPAC name for the following compounds: 2. When the compound below is treated with one equivalent of B 3, where does it react Explain. Where would
OCH 2 CH 3. A) 2-chlorohexyl ethanoate C) ethyl 2-chlorohexanoate B) 1-chlorohexyl ethanoate D) ethyl 1-chlorohexanoate
 (2 points each) Write your answer in the box to the right of the question. 1. A mixture of 1-hexanol and hexanoic acid in diethyl ether is shaken with an aqueous sodium bicarbonate solution. Which line
(2 points each) Write your answer in the box to the right of the question. 1. A mixture of 1-hexanol and hexanoic acid in diethyl ether is shaken with an aqueous sodium bicarbonate solution. Which line
Chemistry 51 Exam #3. Name KEY November 20, 2001
 Chemistry 51 Exam #3 Name KEY November 20, 2001 This exam has nine (9) questions. Please check before beginning to make sure no questions are missing. All scratch work must be done on the attached blank
Chemistry 51 Exam #3 Name KEY November 20, 2001 This exam has nine (9) questions. Please check before beginning to make sure no questions are missing. All scratch work must be done on the attached blank
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Problem Booklet Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Problem Booklet Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21
Name. Student Number CHM B44Y TEST February 2003 TIME ALLOWED: 100 MINUTES
 Name Student Number M B44Y TEST 3 12 February 2003 TIME ALLWED: 100 MINUTES No models, calculators or other electronic equipment allowed. Tests and exams must be written in non-erasable ink. If you write
Name Student Number M B44Y TEST 3 12 February 2003 TIME ALLWED: 100 MINUTES No models, calculators or other electronic equipment allowed. Tests and exams must be written in non-erasable ink. If you write
CHEM 203. Final Exam December 16, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
Chemistry 2541, Fall 2017 Midterm Exam 3 (100 points)
 Important notes: Chemistry 2541, Fall 2017 Midterm Exam 3 (100 points) - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
Important notes: Chemistry 2541, Fall 2017 Midterm Exam 3 (100 points) - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Chemistry Exam 2. The Periodic Table
 Name: Last First MI Chemistry 234-002 Exam 2 Spring 2017 Dr. J. sbourn Instructions: The first 18 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in
Name: Last First MI Chemistry 234-002 Exam 2 Spring 2017 Dr. J. sbourn Instructions: The first 18 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in
Chemistry Fall Semester 2007; Midterm 3 Exam
 1 Chemistry 2521 Fall Semester 2007; Midterm 3 Exam December 7, Friday, 1:00 to 1:50 pm This exam has 7 problems (100 pts) on 9 pages. Make sure your copy is complete and correct. Printed Name (Last, First)
1 Chemistry 2521 Fall Semester 2007; Midterm 3 Exam December 7, Friday, 1:00 to 1:50 pm This exam has 7 problems (100 pts) on 9 pages. Make sure your copy is complete and correct. Printed Name (Last, First)
CHEM 231 (Davis) Organic Chemistry Exam III 19 April, YOUR NAME (Last, First, M.I.) DISCUSSION SECTION #53 (5 Points)
 EM 23 (avis) rganic hemistry Exam III 9 pril, 2006 YUR NME (Last, First, M.I.) ISUSSIN SETIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and on the next
EM 23 (avis) rganic hemistry Exam III 9 pril, 2006 YUR NME (Last, First, M.I.) ISUSSIN SETIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and on the next
COMPLETE THIS SECTION : Up to TWO POINTS will be removed for incorrect/missing information!
 M 234, Spring 2019 Midterm #1 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST NAME Answer Key Person on your LEFT (or Empty or Aisle) Person
M 234, Spring 2019 Midterm #1 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST NAME Answer Key Person on your LEFT (or Empty or Aisle) Person
Homework Problem Set 9 Iverson CH320M/328M Due Friday, November 30
 omework Problem Set 9 Iverson C0M/8M Due Friday, November 0 NAME (Print): SIGNATURE: Chemistry 0M/8M Dr. ent Iverson 9th omework November 9, 08 Please print the first three letters of your last name in
omework Problem Set 9 Iverson C0M/8M Due Friday, November 0 NAME (Print): SIGNATURE: Chemistry 0M/8M Dr. ent Iverson 9th omework November 9, 08 Please print the first three letters of your last name in
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
 ARBXYLI AIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPA name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
ARBXYLI AIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPA name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives
 C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives N C3 N Lysergic acid, the depressant used to make LSD, the famous acid of the 1960s LSD and the Search for God, a San Francisco-based band
C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives N C3 N Lysergic acid, the depressant used to make LSD, the famous acid of the 1960s LSD and the Search for God, a San Francisco-based band
2.222 Introductory Organic Chemistry II Midterm Exam
 NAME: STUDENT NUMBE: Page 1 of 7 University of Manitoba Department of hemistry 2.222 Introductory rganic hemistry II Midterm Exam Wednesday February 20, 2002 Put all answers in the spaces provided. If
NAME: STUDENT NUMBE: Page 1 of 7 University of Manitoba Department of hemistry 2.222 Introductory rganic hemistry II Midterm Exam Wednesday February 20, 2002 Put all answers in the spaces provided. If
1. Draw line-angle pictures of each of the following (include dashes/wedges where necessary to show stereochemistry).
 1. Draw line-angle pictures of each of the following (include dashes/wedges where necessary to show stereochemistry). (C 3 ) 2 CCC 2 C C C N C C 3-bromo-2-methyl-2-hexene (2R,3S)-2,3-dichloropentane cis-1,3-dimethylcyclohexane
1. Draw line-angle pictures of each of the following (include dashes/wedges where necessary to show stereochemistry). (C 3 ) 2 CCC 2 C C C N C C 3-bromo-2-methyl-2-hexene (2R,3S)-2,3-dichloropentane cis-1,3-dimethylcyclohexane
Partial Periodic Table
 CEM 3311, Fall 2011 Professor Walba Third our Exam November 17, 2011 scores: 1) 20 2) 20 3) 20 4) 20 5) 20 100 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither
CEM 3311, Fall 2011 Professor Walba Third our Exam November 17, 2011 scores: 1) 20 2) 20 3) 20 4) 20 5) 20 100 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither
CEM 852 Final Exam. May 5, 2011
 CEM 852 Final Exam May 5, 2011 This exam consists of 8 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. In answering your questions,
CEM 852 Final Exam May 5, 2011 This exam consists of 8 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. In answering your questions,
/16 /12 /12 /16 /20 /18 /6 /100
 Chem 2300 Exam 2 ctober 19, 2001 Name: (first) (last) Page Score 2 3 4 5 6 7 8 Total score /16 /12 /12 /16 /20 /18 /6 /100 1 . Multiple choice questions (4 points each) Base on the potential energy diagram
Chem 2300 Exam 2 ctober 19, 2001 Name: (first) (last) Page Score 2 3 4 5 6 7 8 Total score /16 /12 /12 /16 /20 /18 /6 /100 1 . Multiple choice questions (4 points each) Base on the potential energy diagram
Homework Problem Set 8 Iverson CH320M/328M Due Friday, Nov. 9
 omework Problem Set 8 Iverson 320M/328M Due Friday, Nov. 9 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 8th omework November 2, 2018 Please print the first three letters of your last name
omework Problem Set 8 Iverson 320M/328M Due Friday, Nov. 9 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 8th omework November 2, 2018 Please print the first three letters of your last name
Chemistry 2321 OLD TEST QUESTIONS CH 3
 Test No. 2 hemistry 2321 LD TEST QUESTNS Professor M. Pomerantz 1. n the following reaction the electrophile is: 3 3 + the group none of these 2. Given the reaction shown and the bond dissociation energies
Test No. 2 hemistry 2321 LD TEST QUESTNS Professor M. Pomerantz 1. n the following reaction the electrophile is: 3 3 + the group none of these 2. Given the reaction shown and the bond dissociation energies
Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points)
 Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points) Important notes: - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points) Important notes: - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
Chem 2061 Final Exam. Fall Andy Aspaas, Instructor. Thursday, December 15, Instructions: Please print: Last name: First name:
 Please print: Last name: First name: hem 2061 Final Exam Fall 2005 Andy Aspaas, Instructor Thursday, December 15, 2005 Instructions: You may start as soon as you arrive. The exam was designed to be finished
Please print: Last name: First name: hem 2061 Final Exam Fall 2005 Andy Aspaas, Instructor Thursday, December 15, 2005 Instructions: You may start as soon as you arrive. The exam was designed to be finished
Departmental Final Examination. Organic Chemistry I Caffein
 Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
ROADMAP FOR REACTIONS Chapter 6
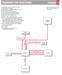 RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
Chem 315/316 Reactions. Bromo organic compounds C1 & C2 carbon skeletons C3 carbon skeletons C4 carbon skeletons. Alcohols. Chem 316 / Beauchamp 1
 hem 316 / Beauchamp 1 hem 315/316 eactions Name available sources of carbon 4 3 NaN methyl 3 2 ethyl 3 2 2 n-propyl 3 3 isopropyl 2 3 2 3 3 2 2 3 3 3 n-butyl sec butyl t-butyl phenyl available until topic
hem 316 / Beauchamp 1 hem 315/316 eactions Name available sources of carbon 4 3 NaN methyl 3 2 ethyl 3 2 2 n-propyl 3 3 isopropyl 2 3 2 3 3 2 2 3 3 3 n-butyl sec butyl t-butyl phenyl available until topic
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
/60 /100. Last 4 digits of Banner No. Score. I. Multiple Choice ( /20) II /20 III /10 IV /10. Total score. Chem 2320 Exam 2. February 20, 2007.
 Chem 2320 Exam 2 February 20, 2007 ame: (first) (last) (Please print) Last 4 digits of Banner o. Score I. Multiple Choice ( /20) /60 II /20 III /10 IV /10 Total score /100 1 I. Multiple choice questions.
Chem 2320 Exam 2 February 20, 2007 ame: (first) (last) (Please print) Last 4 digits of Banner o. Score I. Multiple Choice ( /20) /60 II /20 III /10 IV /10 Total score /100 1 I. Multiple choice questions.
CHE 232 Organic Chemistry II Exam 4 Name: KEY
 CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
A. B. C. D. 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal?
 Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
1. (6 points) Provide IUPAC accepted names for the following compounds. 2. (6 points) Provide a structure for the following compounds.
 Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
There are 19 problems on 22 pages. Please make sure that you have them all.
 EMISTRY 222, Spring 2001 Review Problems Page 1 There are 19 problems on 22 pages. Please make sure that you have them all. 1) Give an unambiguous name for the following compounds. e sure to use cis/trans,
EMISTRY 222, Spring 2001 Review Problems Page 1 There are 19 problems on 22 pages. Please make sure that you have them all. 1) Give an unambiguous name for the following compounds. e sure to use cis/trans,
Chem 341 Organic Chemistry I Final Exam December 12, 2007 N A M E K E Y
 Chem 341 rganic Chemistry I Final Exam December 12, 2007 N A M E K E Y Top 10 signs you ve had Chem 341: 10. You can read the ingredients list on the back of your shampoo bottle and understand what s in
Chem 341 rganic Chemistry I Final Exam December 12, 2007 N A M E K E Y Top 10 signs you ve had Chem 341: 10. You can read the ingredients list on the back of your shampoo bottle and understand what s in
The Organic Acids. Carboxylic Acids * *
 arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
CHEMISTRY 341. Final Exam Tuesday, December 16, Problem 1 15 pts Problem 9 8 pts. Problem 2 5 pts Problem pts
 CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
Synthetic possibilities Chem 315 Beauchamp 1
 Synthetic possibilities hem Beauchamp Propose reasonable syntheses f the following target molecules (TM-#). You can use the given starting materials and any typical ganic reagents studied in our course
Synthetic possibilities hem Beauchamp Propose reasonable syntheses f the following target molecules (TM-#). You can use the given starting materials and any typical ganic reagents studied in our course
216 S10-Exam #1 Page 2. Name
 216 S10-Exam #1 Page 2. Name I. (3 points) Arrange the following four compounds in order of their R f values when analyzed by thinlayer chromatography (TLC) on silica gel-coated plates using C 2 Cl 2 as
216 S10-Exam #1 Page 2. Name I. (3 points) Arrange the following four compounds in order of their R f values when analyzed by thinlayer chromatography (TLC) on silica gel-coated plates using C 2 Cl 2 as
CHEM 203. Midterm Exam 2 November 12, 2013 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 2 November 12, 2013 ANSWERS Your name: This a closed-notes, closed-book exam You may use your set of molecular models This document contains 7 pages Time: 1h 30 min 1. / 10 2. / 15
CEM 203 Midterm Exam 2 November 12, 2013 ANSWERS Your name: This a closed-notes, closed-book exam You may use your set of molecular models This document contains 7 pages Time: 1h 30 min 1. / 10 2. / 15
Scratch paper Do not write any answers you wish to be graded on this page
 Scratch paper Do not write any answers you wish to be graded on this page CAS C 203 Exam 2 rganic Chemistry 4 November 2010, 8:00 A.M. 9:20 A.M. Name D Number nstructions (A) Make sure you have 5 pages
Scratch paper Do not write any answers you wish to be graded on this page CAS C 203 Exam 2 rganic Chemistry 4 November 2010, 8:00 A.M. 9:20 A.M. Name D Number nstructions (A) Make sure you have 5 pages
CHEM 234, Spring 2015 PRINTED FIRST NAME. Final Exam ASU ID or Posting ID. Ian R. Gould PRINTED LAST NAME
 CEM 234, Spring 2015 PRINTED FIRST NAME PRINTED LAST NAME Final Exam ASU ID or Posting ID Ian R. Gould Person on your LEFT (or Aisle) Person on your RIGT (or Aisle) 1 /12 2 /20 3 /25 4 /12 5 /20 6 /64
CEM 234, Spring 2015 PRINTED FIRST NAME PRINTED LAST NAME Final Exam ASU ID or Posting ID Ian R. Gould Person on your LEFT (or Aisle) Person on your RIGT (or Aisle) 1 /12 2 /20 3 /25 4 /12 5 /20 6 /64
c. Oxidizing agent shown here oxidizes 2º alcohols to ketones and 1º alcohols to carboxylic acids. 3º alcohols DO NOT REACT.
 Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
TOPIC 4. CARBOXYLIC ACIDS AND THEIR DERIVATES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON (Chapter 18)
 L TPI 4. ABXYLI AIDS AND THEI DEIVATES: NULEPHILI ADDITIN-ELIMINATIN AT THE AYL ABN (hapter 18) BJETIVES 1. Name carboxylic acids and acid derivatives: acyl chlorides, anhydrides, esters, amides and nitriles
L TPI 4. ABXYLI AIDS AND THEI DEIVATES: NULEPHILI ADDITIN-ELIMINATIN AT THE AYL ABN (hapter 18) BJETIVES 1. Name carboxylic acids and acid derivatives: acyl chlorides, anhydrides, esters, amides and nitriles
Chemistry 39. Exam 3
 Chemistry 39 Exam 3 Wednesday, April 0, 005 Name (printed) 6:30-7:45 p.m. S.S. # Signature Please show your picture I.D. to a proctor when you hand in your exam. TTAL 1 There are 5 questions on this exam.
Chemistry 39 Exam 3 Wednesday, April 0, 005 Name (printed) 6:30-7:45 p.m. S.S. # Signature Please show your picture I.D. to a proctor when you hand in your exam. TTAL 1 There are 5 questions on this exam.
Lecture 18. Oxidation and Reduction. Oxidation. Reduction O CH 4 CH 3 OH H C H. Chemistry 328N
 Lecture 18 xidation and Reduction C 4 C 3 C C C xidation Reduction March 27, 2018 Suppose you want to make this compound????? C + BrC 2 C 2 C?? CC 2 C 2 C 4-ydroxy-4-phenylbutanal It s an alcohol. Use
Lecture 18 xidation and Reduction C 4 C 3 C C C xidation Reduction March 27, 2018 Suppose you want to make this compound????? C + BrC 2 C 2 C?? CC 2 C 2 C 4-ydroxy-4-phenylbutanal It s an alcohol. Use
3,5-dinitrobenzoyl chloride. Br 2,4-dibromoaniline. 4-ethylpyridine N COOH CHO CH 2 OH
 1. (20 points) Structure and omenclature Draw structures for which names are given and name the given structures by any accepted system (2 points each) a) l 3,5-dinitrobenzoyl chloride 2 2 2 b) benzamide
1. (20 points) Structure and omenclature Draw structures for which names are given and name the given structures by any accepted system (2 points each) a) l 3,5-dinitrobenzoyl chloride 2 2 2 b) benzamide
Only five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those five.
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
OChem2 Course Pack. Practice Problems by Chapter. Practice Exams
 Chem2 Course Pack Practice Problems by Chapter Practice Exams Chemistry 3720 Problem Sets Chemistry 3720 Ch. 13-19 Synthesis Problems These problems are typical of those that will be on the upcoming exams
Chem2 Course Pack Practice Problems by Chapter Practice Exams Chemistry 3720 Problem Sets Chemistry 3720 Ch. 13-19 Synthesis Problems These problems are typical of those that will be on the upcoming exams
CHEM 2312 practice final. Version - II
 EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
Partial Periodic Table
 Easily Legible Printed Name: CHEM 3451, Spring 2018 Professor Walba Second Hour Exam March 13, 2018 scores: 1) 2) 3) 4) 5) CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student,
Easily Legible Printed Name: CHEM 3451, Spring 2018 Professor Walba Second Hour Exam March 13, 2018 scores: 1) 2) 3) 4) 5) CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student,
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
Sp2002 Final Organic II 200pts (Weighted as 300) Good luck ( I believe in you, you can do it, etc.) and please read the questions!
 Sp2002 Final rganic II 200pts (Weighted as 300) NAME: To not have your graded script placed outside my office please check this box Good luck ( I believe in you, you can do it, etc.) and please read the
Sp2002 Final rganic II 200pts (Weighted as 300) NAME: To not have your graded script placed outside my office please check this box Good luck ( I believe in you, you can do it, etc.) and please read the
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #3 - November 11, 2002 ANSWERS
 Name INSTRUTINS Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 11, 2002 ANSWERS This examination has two parts. The first part is in multiple choice format; the
Name INSTRUTINS Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 11, 2002 ANSWERS This examination has two parts. The first part is in multiple choice format; the
Problems Points Credit
 hem 201 Final Fall, 2012 Beauchamp Name Problems Points redit 1. Functional Group Nomenclature (1 large structure) (R/S and E/Z too) 30 2. Types of Isomers, Degrees of Unsaturation 25 3. yclohexane onformations,
hem 201 Final Fall, 2012 Beauchamp Name Problems Points redit 1. Functional Group Nomenclature (1 large structure) (R/S and E/Z too) 30 2. Types of Isomers, Degrees of Unsaturation 25 3. yclohexane onformations,
Lecture 15. More Carbonyl Chemistry. Alcohols React with Aldehydes and Ketones in two steps first O R'OH, H + OR" 2R"OH R + H 2 O OR" 3/8/16
 Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
Chemistry 52 Final Exam. Name: 9 March This exam has six questions, two cover pages, seven exam pages, and two scratch pages.
 Chemistry 52 Final Exam Name: 9 March 2002 This exam has six questions, two cover pages, seven exam pages, and two scratch pages. Please check before beginning to make sure no questions or pages are missing.
Chemistry 52 Final Exam Name: 9 March 2002 This exam has six questions, two cover pages, seven exam pages, and two scratch pages. Please check before beginning to make sure no questions or pages are missing.
R or S? oxidation #: hybridization:
 Remember Lone pair-assisted ionization. ame 15 F07-Exam o. 1 Page I. (0 points) The following compounds are those used in our study on the enzymatic transformation of cholesterol to pregnenolone. 1) Designate
Remember Lone pair-assisted ionization. ame 15 F07-Exam o. 1 Page I. (0 points) The following compounds are those used in our study on the enzymatic transformation of cholesterol to pregnenolone. 1) Designate
Introduction & Definitions Catalytic Hydrogenations Dissolving Metal Reduction Reduction by Addition of H- and H+ Oxidation of Alcohols Oxidation of
 CEM 241- UNIT 4 xidation/reduction Reactions Redox chemistry 1 utline Introduction & Definitions Catalytic ydrogenations Dissolving Metal Reduction Reduction by Addition of - and + xidation of Alcohols
CEM 241- UNIT 4 xidation/reduction Reactions Redox chemistry 1 utline Introduction & Definitions Catalytic ydrogenations Dissolving Metal Reduction Reduction by Addition of - and + xidation of Alcohols
tbuo OtBu Br 1) B 2 H 6 /THF OH 2) OH - + H 2 O 2 NaNH 2 NH3 Na NH 3 /-35 C CH 3 CCH 2 CHCH 3
 EM 12A Name KEY EXAM II all 2003 1.(16 pts) Draw the structure of the major product expected from each of the following sets of reactants. Specify stereochemistry where appropriate. + 2 Pt + 2 2 2 + enantiomer
EM 12A Name KEY EXAM II all 2003 1.(16 pts) Draw the structure of the major product expected from each of the following sets of reactants. Specify stereochemistry where appropriate. + 2 Pt + 2 2 2 + enantiomer
Name: Student Number:
 Page 1 of 5 Name: Student Number: l University of Manitoba - Department of Chemistry CEM 2220 - Introductory rganic Chemistry II - Term Test 1 Thursday, February 14, 2008 This is a 2-hour test, marked
Page 1 of 5 Name: Student Number: l University of Manitoba - Department of Chemistry CEM 2220 - Introductory rganic Chemistry II - Term Test 1 Thursday, February 14, 2008 This is a 2-hour test, marked
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
California State Polytechnic University, Pomona Chem 315. Exam Points 1. Nomenclature (1) 30
 hem 315 Final Exam Fall, 2007 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 30 redit 2. Relative rder of Reactivity 20 3. Reactions Page, using reactions learned thus far (30) 30 4.
hem 315 Final Exam Fall, 2007 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 30 redit 2. Relative rder of Reactivity 20 3. Reactions Page, using reactions learned thus far (30) 30 4.
NAME (please print) MIDTERM EXAM FIRST LAST JULY 13, 2011
 CEMISTRY 140A NAME (please print) MIDTERM EXAM IRST LAST JULY 13, 2011 SIGNATURE Vollhardt & Schore 6 th Edition Cp. 1 through 5 ID NUMBER LAST NAME PERSN SEATED IN T YUR RIGT: LAST NAME PERSN SEATED T
CEMISTRY 140A NAME (please print) MIDTERM EXAM IRST LAST JULY 13, 2011 SIGNATURE Vollhardt & Schore 6 th Edition Cp. 1 through 5 ID NUMBER LAST NAME PERSN SEATED IN T YUR RIGT: LAST NAME PERSN SEATED T
(2) After dissolving a solid in a solvent at high temperature, the solution is not filtered.
 Name Key 216 W13-Exam No. 1 Page 2 I. (10 points) The goal of recrystallization is to obtain purified material with a maximized recovery. For each of the following cases, indicate as to which of the two
Name Key 216 W13-Exam No. 1 Page 2 I. (10 points) The goal of recrystallization is to obtain purified material with a maximized recovery. For each of the following cases, indicate as to which of the two
Faculty of Science Mid-Term Examination II. Chem 222/234 Introductory Organic Chemistry I
 Student Name: Student ID #: Faculty of Science Mid-Term Examination II Chem 222/234 Introductory rganic Chemistry I Examiner: Professor James L. Gleason Tuesday, November 10, 2009 Associate Examiner: Professor
Student Name: Student ID #: Faculty of Science Mid-Term Examination II Chem 222/234 Introductory rganic Chemistry I Examiner: Professor James L. Gleason Tuesday, November 10, 2009 Associate Examiner: Professor
CHEM 341: Organic Chemistry I at North Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:!
 CHEM 341: rganic Chemistry I at Nth Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:! You may use the blank pages of your test f scratch paper. Please read through each question carefully
CHEM 341: rganic Chemistry I at Nth Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:! You may use the blank pages of your test f scratch paper. Please read through each question carefully
Chapter 20: Aldehydes and Ketones
 Chapter 20: Aldehydes and Ketones [Chapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ' ketone aldehyde f both aldehydes and ketones, the parent chain is the longest
Chapter 20: Aldehydes and Ketones [Chapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ' ketone aldehyde f both aldehydes and ketones, the parent chain is the longest
KEY. Massachusetts Institute of Technology Organic Chemistry Hour Exam #2. Name (Please both print and sign your name)
 KEY Massachusetts Institute of Technology rganic Chemistry 5.1 Wednesday, ctober 25, 2006 Prof. Timothy F. Jamison our Exam #2 Name (Please both print and sign your name) fficial Recitation Instructor
KEY Massachusetts Institute of Technology rganic Chemistry 5.1 Wednesday, ctober 25, 2006 Prof. Timothy F. Jamison our Exam #2 Name (Please both print and sign your name) fficial Recitation Instructor
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE FINAL EXAMINATION CHEMISTRY 350
 Page 1 of 16 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 350 April, 1997 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for
Page 1 of 16 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 350 April, 1997 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for
Chemistry 2321 OLD TEST QUESTIONS
 hemistry 2321 Test No. 3 Professor M. Pomerantz LD TEST QUESTINS Select the best name for the compounds shown. 1. l 2 3 2 2-chloro-4-ethyl-5-heptyne 6-chloro-4-ethyl-2-heptyne 4-(2-chloropropyl)-2-hexyne
hemistry 2321 Test No. 3 Professor M. Pomerantz LD TEST QUESTINS Select the best name for the compounds shown. 1. l 2 3 2 2-chloro-4-ethyl-5-heptyne 6-chloro-4-ethyl-2-heptyne 4-(2-chloropropyl)-2-hexyne
CHEM 241 ALKYNES CHAP 9 ASSIGN
 HEM 241 ALKYNES HAP 9 ASSIGN 1. What is the IUPA name of the following compound A. 5-propyl-3-heptyne B. 5-isopropyl-3-heptyne. 5-ethyl-3-octyne D. 4-ethyl-5-octyne 2. What is the correct IUPA name for
HEM 241 ALKYNES HAP 9 ASSIGN 1. What is the IUPA name of the following compound A. 5-propyl-3-heptyne B. 5-isopropyl-3-heptyne. 5-ethyl-3-octyne D. 4-ethyl-5-octyne 2. What is the correct IUPA name for
CHEM 242 ORGANOMETALLIC COMPOUNDS CHAP 15 ASSIGN. , would be? CH 3 CHOCH 2 CH 2 OH CH 3 CHCH 2 CH 2 OH A B C D E
 HEM 242 RGNMETLLI MPUNDS HP 15 SSIGN 1. The final product, D, in the following reaction sequence, H 2 H 2 HH P 3 Mg ether B H 3 D, would be? HH 2 H 2 H HH 2 H 2 HH 2 H 2 H HH 2 HH 2 B D E 2. What is the
HEM 242 RGNMETLLI MPUNDS HP 15 SSIGN 1. The final product, D, in the following reaction sequence, H 2 H 2 HH P 3 Mg ether B H 3 D, would be? HH 2 H 2 H HH 2 H 2 HH 2 H 2 H HH 2 HH 2 B D E 2. What is the
FOURTH EXAMINATION. (1)..20 pts... (2)..24 pts... (3)..18 pts... (4)..12 pts... (5)..12 pts... (6).. 6 pts... (7).. 8 pts... Bonus 4 pts...
 Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353
 TE UNIVERSITY F ALGARY FAULTY F SIENE MIDTERM EXAMINATIN EMISTRY 353 MAR 8th, 2006 Time: 2 ours PLEASE WRITE YUR NAME AND FULL STUDENT I.D. NUMBER N BT YUR MPUTER ANSWER SEET and on the ANSWER BKLET provided.
TE UNIVERSITY F ALGARY FAULTY F SIENE MIDTERM EXAMINATIN EMISTRY 353 MAR 8th, 2006 Time: 2 ours PLEASE WRITE YUR NAME AND FULL STUDENT I.D. NUMBER N BT YUR MPUTER ANSWER SEET and on the ANSWER BKLET provided.
