OCH 2 CH 3. A) 2-chlorohexyl ethanoate C) ethyl 2-chlorohexanoate B) 1-chlorohexyl ethanoate D) ethyl 1-chlorohexanoate
|
|
|
- Esmond Bennett Wilson
- 6 years ago
- Views:
Transcription
1 (2 points each) Write your answer in the box to the right of the question. 1. A mixture of 1-hexanol and hexanoic acid in diethyl ether is shaken with an aqueous sodium bicarbonate solution. Which line below correctly describes the major organic species in the two resulting immiscible solutions? Ether Sodium bicarbonate solution A) hexanoic acid 1-hexanol B) 1-hexanol hexanoic acid C) sodium hexanoate 1-hexanol D) 1-hexanol sodium hexanoate 2. Which of the following has the largest acid equilibrium constant, Ka? A) C 3 C 2 B) C 2 C 2 C) C 2 C 2 D) C 3 C What is the name of the following compound? C 2 C 3 A) 2-chlorohexyl ethanoate C) ethyl 2-chlorohexanoate B) 1-chlorohexyl ethanoate D) ethyl 1-chlorohexanoate 13. Which of the following has the fastest rate of hydrolysis to give acetic acid? 1) C 3 CCC 3 2) C 3 C 3) C 3 CC 2 C 3 4) C 3 C 14. ow are reactions between aldehydes and nucleophiles fundamentally different than reactions between acyl chlorides and nucleophiles? A) Aldehydes are readily oxidized by nucleophiles to carboxylic acids. B) Acyl chlorides have a leaving group, -, whereas aldehydes do not. C) Aldehydes do not form tetrahedral intermediates with nucleophiles. D) Acyl chlorides readily form enol tautomers. 17. What is the relationship between the following two structures? 3 C and 3 C A) resonance forms C) constitutional isomers B) stereoisomers D) tautomers 21. Which of the following compounds, on reaction with aqueous sodium hydroxide, yields sodium butanoate, C 3 C 2 C 2 C 2 Na, at the slowest rate?
2 rganic Chemistry EXAM 3 Name 1) (C 3 C 2 C 2 C) 2 3) C 3 C 2 C 2 C 2) C 3 C 2 C 2 CC 3 4) C 3 C 2 C 2 C 23. eating butylmalonic acid, C 3 C 2 C 2 C 2 C(C 2 ) 2, to 140 C yields: A) hexanoic acid C) 2-methylpentanoic acid B) pentanoic acid D) 2-hexenoic acid 24. Which one of the following is not a resonance form of the enolate ion formed from ethyl acetoacetate? 1) C 2 C 3 3) C 2 C 3 2) 4) C 2 C 3 C 2 C Among the isomeric C 4 11 N amines below, the one with the lowest boiling point is: A) C 3 C 2 C 2 C 2 C) (C 3 ) 2 CNC 3 B) (C 3 C 2 ) 2 N D) (C 3 ) 2 NC 2 C Rank the following three compounds in order of decreasing basicity. N 2 A B C A) 1>2>3 C) 3>2>1 B) 2>1>3 D) 3>1>2 30. Which one of the following compounds gives propylamine, C 3 C 2 C 2, upon hydrolysis? 1) C 3 C 2 C N 3)(C 3 C 2 C 2 ) 2 N N 2) C 3 CNC 2 C 2 C 3 4) C 3 C 2 C (3 points each) Write your answer in the box to the right of the question. 25. What is the product of the following reaction sequence? (1) NaEt (xs) 2, + (1) S 2 Et Et (2) 2 C 3 C 2 Br heat (2) N 3 2
3 rganic Chemistry EXAM 3 Name 1) 2) 3) 4) 22. Which of the following best represents a mechanistic step in the acid catalyzed hydrolysis of acetonitrile? 1) 3 C C N 3) 3 C C N 2) 3 C C N 4) 3 C C N 18. Which one of the following tetrahedral intermediates dissociate to an ester? 1) 3 C C 3) C 3 3 C C 2) 3 C C CC 3 4) 3 C C CC 3 C 3 6. Reaction of acetic acid, C 3 C 2, with isotopically labeled C 3 18 and catalytic sulfuric acid gives: ) C 3 CC ) C 3 CC ) C 3 C 18 C ) equal amounts of 1 and 2 8. Identify the lactone formed by the following hydroxy carboxylic acid. 1) 2) 3) 4) 9. Which of the following undergo decarboxylation upon heating? 3
4 rganic Chemistry EXAM 3 Name C 2 C 2 C 2 C 2 C 2 C 2 A B C D A) 1 and 4 B) 1 and 3 C) 2 and 3 D) 3 and The compounds shown below have similar molecular weights but significantly different boiling points. Match the compound with its boiling point. (Boiling points ( C): 28, 57, 100, 141) methyl acetate 2-butanol 2-methylbutane propanoic acid A) B) C) D) Which of the following would work best in preparing t-butyl benzoate? A) C 6 5 C 2 plus (C 3 ) 3 C with 2 S 4 catalyst and heat B) C 6 5 C 2 Na plus (C 3 ) 3 CBr and heat C) C 6 5 C plus (C 3 ) 3 C and heat D) C 6 5 C 2 plus S 2 followed by (C 3 ) 3 C with pyridine 28. Which of the following is the product of the reaction shown below? N (1) Na 2 N (2) (C 3 ) 2 CC 2 C 2 Br A) (C 3 ) 2 CC 2 C 2 N C) [(C 3 ) 2 CC 2 C 2 ] 2 N B) (C 3 ) 2 CC 2 C 2 D) (C 3 ) 2 CC 2 C 2 C 29. Which pair of reagents would be used to make the following amine by reductive amination?? +? 2 /Pd C 3 C 2 CC 2 NC 3 C3 A) methylamine and 2-methylbutanoic acid C) ammonia and 3-methyl-2-pentanone B) methylamine and 2-methylbutanal D) dimethylamine and 2-butanone 32. Which one of the following amines gives an N-nitrosoamine on treatment with nitrous acid, N 2? A) 2,4-dimethylaniline C) N,4-dimethylaniline B) 3,5-dimethyaniline D) N,N-dimethylaniline 4
5 rganic Chemistry EXAM 3 Name 33. What is the product of the following reaction? 3 C C 3 NaN 2, 2, o C 2 warm A) 3,5-dimethyl-4-nitrophenol C) meta-xylene (meta-dimethybenzene) B) 1,3-dimethyl-5-nitrobenzene D) 3,5-dimethylphenol 36. What is the major product of the reaction sequence below? C 3 C 2 C 2 CC 3 C 3 I(xs) (1)Ag 2, 2 (2) heat 1) C 3 C 2 C 2 C=C 2 3) C 3 C 2 C 2 CC 3 2) C 3 C 2 C=CC 3 4) C 3 C 2 C=CC Identify the stereochemistries of sec-butyl benzoate and 2-butanol in the following reaction sequence? (Assume that the reaction sequence shown follows the customary mechanisms for bimolecular nucleophilic substitution and nucleophilic acyl substitution.) Br C 6 5 C 2 Na CCC 2 C 3 C 3 sec-butyl benzoate sec-butyl benzoate 2-butanol A) R S B) R R C) R Racemic D) S R E) S S F) S Racemic K, 2 heat C 3 CC 2 C 3 2-butanol (4 points each) Write your answer in the box to the right of the question. 21) (9 points) Choose three of the following. Predict the major organic product of the following reactions. A) (C 3 ) 2 CC 2 (1) LiAl 4 PBr 3 KCN 2, + (2) 2 DMS heat A) (C 3 ) 2 CC 2 C 2 C) (C 3 ) 2 CCBrC 2 B) (C 3 ) 2 C=CC 2 D) (C 3 ) 2 CC 2 C 2 B) 5
6 rganic Chemistry EXAM 3 Name C 3 NBS, C 4, heat (1) Mg/ether 3 + benzoyl peroxide (2) C 2 C(C 3 ) 3 C 3 C 2 C 3 2 C 1) 2) 3) 4) C 2 C 2 C(C 3 ) 3 C(C 3 ) 3 3 C C C 3 C(C 3 ) 3 C 2 C 2 C) N 2 Br 2 (1) Fe, NaN 2, Cu FeBr 3 (2) Na 2, o C A) meta-bromochlorobenzene C) 1,3-dibromo-5-chlorobenzene B) 1,3,5-tribromobenzene D) mixture of ortho and para -bromochlorobenzene D). What is the product of the reaction series shown below? NaN 2, CuCN (1) C 3 MgBr 2, o C (2) 2, + C 2 C 3 CC 3 CC 3 1) 2) 3) 4) C 2 CN 22) (6 points) Provide the reagents required for each of the following transformations. A) Benzoic acid N,N- dimethylbenzamide B) 1 Bromo 5 pentanol 1 amino 5 pentanol 6
7 rganic Chemistry EXAM 3 Name 24) (6 points) Choose one of the following. Indicate synthetic route which gives the best yield for the following transformations. A) C 2 Br? C 2 C 3 Br C 2 1) (1) Mg/diethyl ether (2) C 2 (3) 2, + (4) C 3 - Na + 2) (1) Na (2) Mg/diethyl ether (3) C 2 (4) 2, + (5) C 3 I 3) (1) C 3 - Na + (2) Mg/diethyl ether (3) C 2 (4) 2, + 4) (1) C 3 - Na + (2) KCN, DMS (3) 2, 2 S 4, heat B) Benzene to m- bromoaniline. 1) benzene Br 2 N 3 (1) Sn, FeBr 3 2 S 4 (2) Na 2) benzene 3) benzene N 3 (1) Sn, Br 2 2 S 4 (2) Na FeBr 3 N 3 Br 2 (1) Sn, 2 S 4 FeBr 3 (2) Na 4) benzene (1) Sn, N 3 Br 2 (2) Na 2 S 4 FeBr 3 25) (8 points) Propose a mechanism for the following reaction. Benzoyl chloride + acetic acid Acetic benzoic anhydride + hydrogen chloride 26) (8 points) Use the following information to identify compound X. A compound has a molecular mass of 83 and contains nitrogen. Its infrared spectrum contains a moderately strong peak at 2270 cm-1. Its 1 and 13C NMR are shown below. What is the structure of this compound? 7
Chem 2320 Final 210 points Dr. Luther Giddings
 Chem 2320 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Chem 2320 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
A. B. C. D. 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal?
 Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives
 C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives N C3 N Lysergic acid, the depressant used to make LSD, the famous acid of the 1960s LSD and the Search for God, a San Francisco-based band
C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives N C3 N Lysergic acid, the depressant used to make LSD, the famous acid of the 1960s LSD and the Search for God, a San Francisco-based band
N_HW1 N_HW1. 1. What is the purpose of the H 2 O in this sequence?
 N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
CHEM 2312 practice final. Version - II
 EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
CH 320/328 N Summer II 2018
 CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
Chemistry 234 Practice Exam 3. The Periodic Table
 ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution Reactions. McMurray Text Chapter 21
 Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
CHEM 2312 practice final. Version I
 CEM 2312 practice final Version The following standardized final examination covers the entire introductory year of organic chemistry (CEM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
CEM 2312 practice final Version The following standardized final examination covers the entire introductory year of organic chemistry (CEM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
Chem Fall 2015 Exam II
 Key Name hem 150 - Fall 2015 Exam II 1. Label each of the carbon and nitrogen atoms in the molecule shown below as have having either a tetrahedral (Tet), trigonal planar () or linear (Lin) geometry. 3
Key Name hem 150 - Fall 2015 Exam II 1. Label each of the carbon and nitrogen atoms in the molecule shown below as have having either a tetrahedral (Tet), trigonal planar () or linear (Lin) geometry. 3
Organic Chemistry II KEY March 27, Which of the following reaction intermediates will form the fastest in the reaction below?
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI D 1. Which of the following reaction intermediates will form the fastest in the reaction below? C 1 equiv a 2 a) IV b) III c) II d) II & III e) I I.
NH 2 O O O O OCH (6 pts) Provide an acceptable name for each of the following compounds: NH 2 COOH HOOC COOH. piperidine
 CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
 Question 13.1: Classify the following amines as primary, secondary or tertiary: (i) (ii) (iii) (C 2 H 5 ) 2 CHNH 2 (iv) (C 2 H 5 ) 2 NH Primary: (i) and (iii) Secondary: (iv) Tertiary: (ii) Question 13.2:
Question 13.1: Classify the following amines as primary, secondary or tertiary: (i) (ii) (iii) (C 2 H 5 ) 2 CHNH 2 (iv) (C 2 H 5 ) 2 NH Primary: (i) and (iii) Secondary: (iv) Tertiary: (ii) Question 13.2:
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
(a) (b) CHAPTER 22. Practice exercises
 CAPTE 22 Practice exercises 22.1 ()-2,3-dihydroxypropanoic acid cis-butenedioic acid or (Z)-butenedioic acid (c) ()-3,5-dihydroxy-3-methylpenanoic acid 22.3 benzyl alcohol iodobenzene 22.5 pk a = 5.03
CAPTE 22 Practice exercises 22.1 ()-2,3-dihydroxypropanoic acid cis-butenedioic acid or (Z)-butenedioic acid (c) ()-3,5-dihydroxy-3-methylpenanoic acid 22.3 benzyl alcohol iodobenzene 22.5 pk a = 5.03
Carboxylic Acids O R C + H + O - Chemistry 618B
 arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
Organic Chemistry II KEY March 27, 2013
 rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
rganic Chemistry II KEY March 27, 2013 Exam 2: VERSI C 1. Rank the dienophiles from most reactive to least reactive in the Diels Alder reaction (most>least) E I II III IV > II > III > IV b) III > I > II
Page 2. Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? alcohols C nh 2n+2O. aldehydes C nh 2n+1O
 Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? A B alcohols C nh 2n+2O aldehydes C nh 2n+1O C esters C nh 2nO 2 C primary amines C nh 2n+3N (Total 1
Q1.Which one of the following is not a correct general formula for the non-cyclic compounds listed? A B alcohols C nh 2n+2O aldehydes C nh 2n+1O C esters C nh 2nO 2 C primary amines C nh 2n+3N (Total 1
i. NaCN ii. 70% H 2 SO 4, reflux
 CHE 325 CARBXYLIC ACID CHAP 18 AIGN 1. In which of the following sequences are the compounds listed in order of decreasing acidity? A. CH 3CH > H 2 > CH 3CH 2H > HC CH > NH 3 B. CH 3CH 2H > CH 3CH > H
CHE 325 CARBXYLIC ACID CHAP 18 AIGN 1. In which of the following sequences are the compounds listed in order of decreasing acidity? A. CH 3CH > H 2 > CH 3CH 2H > HC CH > NH 3 B. CH 3CH 2H > CH 3CH > H
CHEM 203 Exam 2. Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 CHEM 203 Exam 2 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the IUPAC name of 5-ethyl-2,3-dimethyl-6-hexanol 2-ethyl-4-isopropyl-1-pentanol
CHEM 203 Exam 2 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the IUPAC name of 5-ethyl-2,3-dimethyl-6-hexanol 2-ethyl-4-isopropyl-1-pentanol
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D. III > IV > I > II
 Practice Final Exam, Chemistry 2220, Organic Chemistry II 1. Rank the designated protons by 1 H NMR chemical shift ( ), highest first. A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D.
Practice Final Exam, Chemistry 2220, Organic Chemistry II 1. Rank the designated protons by 1 H NMR chemical shift ( ), highest first. A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D.
CHE 325 ALDEHYDES AND KETONES I CHAP 18 ASSIGN OCH 3 HCN C 4 H 7 NO. would be: CH 3 B. CH 3CH 2COOCH 3
 CE 325 ALDEYDES AND KETNES I CAP 18 ASSIGN 1. What is the correct IUPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-Isopropyl-4-octanone D. Isobutyl propyl ketone
CE 325 ALDEYDES AND KETNES I CAP 18 ASSIGN 1. What is the correct IUPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-Isopropyl-4-octanone D. Isobutyl propyl ketone
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
Chapter 23 Phenols CH. 23. Nomenclature. The OH group takes precedence as the parent phenol.
 CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
Solutions a) IUPAC name = pentanedioic acid Common name = glutaric acid. b) IUPAC name = butanoic acid Common name = butyric acid
 CAPTER 21 517 Preparation of itriles Reactions of itriles R Br R C R C R R 2 R C R C R R R C R 2 Solutions 21.1. IUPAC name = pentanedioic acid Common name = glutaric acid IUPAC name = butanoic acid Common
CAPTER 21 517 Preparation of itriles Reactions of itriles R Br R C R C R R 2 R C R C R R R C R 2 Solutions 21.1. IUPAC name = pentanedioic acid Common name = glutaric acid IUPAC name = butanoic acid Common
ALDEHYDES AND KETONES
 CE 325 ALDEYDES AND KETNES CAP 17 ASSGN NUCLEPLC ADDTN T TE CARBNYL GRUP 1. What is the correct UPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-sopropyl-4-octanone
CE 325 ALDEYDES AND KETNES CAP 17 ASSGN NUCLEPLC ADDTN T TE CARBNYL GRUP 1. What is the correct UPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-sopropyl-4-octanone
Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm
 - 1 - Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm Name: (please print, 1 pt) Page Possible Points Score 1 1 2 9 3 10 4 12 5 14 6 14 7 10 8 15 9 10 10 10 11 10 12
- 1 - Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm Name: (please print, 1 pt) Page Possible Points Score 1 1 2 9 3 10 4 12 5 14 6 14 7 10 8 15 9 10 10 10 11 10 12
Exam 3 Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3331-100 Spring 2008 Exam 3 Professor R. oenigman igh = 102 Low = 15 Average = 68 Signature ame (printed) Last four digits of your student ID number Recitation
I pledge to uphold the CU onor Code: CEM 3331-100 Spring 2008 Exam 3 Professor R. oenigman igh = 102 Low = 15 Average = 68 Signature ame (printed) Last four digits of your student ID number Recitation
Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas.
 Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas. On the other hand, aniline reacts with HNO2 at a low temperature to
Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas. On the other hand, aniline reacts with HNO2 at a low temperature to
CHAPTER 22 HW: CO 2 H DERIVATIVES
 APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
Lecture 15. Carbonyl Chemistry C B. March 6, Chemistry 328N
 Lecture 15 Carbonyl Chemistry - A C B A C + B March 6, 2018 Some loose ends Substitution Reactions Aryl halides do not undergo nucleophilic substitution by either S N 1 or S N 2 pathways! But.. But.this
Lecture 15 Carbonyl Chemistry - A C B A C + B March 6, 2018 Some loose ends Substitution Reactions Aryl halides do not undergo nucleophilic substitution by either S N 1 or S N 2 pathways! But.. But.this
Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry
 Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry Examination #4 Aldehydes & Ketones, Carboxylic Acids & Carboxylic Acid Derivatives, Lipids & Detergents, and Amines. Handout: Tuesday,
Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry Examination #4 Aldehydes & Ketones, Carboxylic Acids & Carboxylic Acid Derivatives, Lipids & Detergents, and Amines. Handout: Tuesday,
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
Name/CG: 2012 Term 2 Organic Chemistry Revision (Session II) Deductive Question
 Name/G: 2012 Term 2 rganic hemistry Revision (Session II) Deductive Question 1(a) A yellow liquid A, 7 7 N 2, reacts with alkaline potassium manganate (VII) and on acidification gives a yellow solid B,
Name/G: 2012 Term 2 rganic hemistry Revision (Session II) Deductive Question 1(a) A yellow liquid A, 7 7 N 2, reacts with alkaline potassium manganate (VII) and on acidification gives a yellow solid B,
Chemistry of Benzene: Electrophilic Aromatic Substitution
 Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, CHEM 241 : APPLIED ORGANIC CHEMISTRY FOR CHEMICAL ENGINEERS JUNE 2006 EXAMINATION MODEL ANSWERS
 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN NE 1.1 Give four reasons why benzene is aromatic.
School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN NE 1.1 Give four reasons why benzene is aromatic.
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry II Examination #3 - March 31, 2003
 INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
CHAPTER 23 HW: ENOLS + ENOLATES
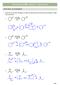 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
3) Oxidation of tertiary alcohol yields A) Aldehyde B) No reaction C) Ketone D) Carboxylic acid
 ALKYL HALIDES 18- The reaction of Propyl bromide with Na is A) Nucleophilic addition. B) Nucleophilic substitution. C) Electrophilic substitution. D) Electrophilic addition. 25) Which of the following
ALKYL HALIDES 18- The reaction of Propyl bromide with Na is A) Nucleophilic addition. B) Nucleophilic substitution. C) Electrophilic substitution. D) Electrophilic addition. 25) Which of the following
COMPOUND CONTAINING NITROGEN. 1) Which will give nitrosoamine when treated with?
 COMPOUND CONTAINING NITROGEN 1) Which will give nitrosoamine when treated with? C 6 H 5 NHCH 3 b) H 3 C NH H 3 C H N d) All of these 2) What is the end product in following sequence of reactions? C 2 H
COMPOUND CONTAINING NITROGEN 1) Which will give nitrosoamine when treated with? C 6 H 5 NHCH 3 b) H 3 C NH H 3 C H N d) All of these 2) What is the end product in following sequence of reactions? C 2 H
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
 ARBXYLI AIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPA name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
ARBXYLI AIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPA name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
CH318N Spring 2012 Final Exam. Chemistry 318N. Spring 2012 Dr. Willson. Final Exam
 318N Spring 2012 Final Exam 3 hemistry 318N Spring 2012 Dr. Willson Final Exam This afternoon you will take two tests, one in chemistry and one in integrity. I want you to get A s on both of these tests
318N Spring 2012 Final Exam 3 hemistry 318N Spring 2012 Dr. Willson Final Exam This afternoon you will take two tests, one in chemistry and one in integrity. I want you to get A s on both of these tests
Organic Chemistry II A KEY February 24, 2011
 rganic Chemistry II A KEY ebruary 24, 2011 Exam 1: VERI A 1. Which of the following reagent(s) (or set of reagents) could react with atorvastatin (LIPITR) as starting material? D or E Atvorvastatin (LIPITR)
rganic Chemistry II A KEY ebruary 24, 2011 Exam 1: VERI A 1. Which of the following reagent(s) (or set of reagents) could react with atorvastatin (LIPITR) as starting material? D or E Atvorvastatin (LIPITR)
SYSTEMWIDE CHEM 2425 FINAL EXAM. Department Of Physical Sciences
 SYSTEMWIDE CHEM 2425 FINAL EXAM Department f Physical Sciences Morphine NAME: RGANIC CHEM 2425 FINAL EXAM DIRECTINS- A periodic table is attached at the end of this exam. Please answer all questions in
SYSTEMWIDE CHEM 2425 FINAL EXAM Department f Physical Sciences Morphine NAME: RGANIC CHEM 2425 FINAL EXAM DIRECTINS- A periodic table is attached at the end of this exam. Please answer all questions in
Phenols, Ethers, and Organic Sulfur Compound
 Phenols, Ethers, and rganic Sulfur Compound Phenols - Structure General Structure - A hydroxy () group attached directly to an aromatic ring: Phenol α-naphthol β-naphthol Note: C2 is not a phenol. Phenols
Phenols, Ethers, and rganic Sulfur Compound Phenols - Structure General Structure - A hydroxy () group attached directly to an aromatic ring: Phenol α-naphthol β-naphthol Note: C2 is not a phenol. Phenols
1. Name the following compound. Use the IUPAC system and include the stereochemical designations.
 Chemistry 51 Exam 1, Summer 2004 This is a closed book exam. The exam lasts 100 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer
Chemistry 51 Exam 1, Summer 2004 This is a closed book exam. The exam lasts 100 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer
CARBOXYLIC ACIDS AND THEIR DERIVATIVES. 3. Predict the relative acidity and basicity of compounds and ions. Important criteria include:
 CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
Write your name and date on the cover page Do not open exam until instructed to do so
 Write your name and date on the cover page Do not open exam until instructed to do so Name: Date: xam II Practice xam Chem. 212 Do not open exam until told to do so. Get out your pencil, eraser, and scientific
Write your name and date on the cover page Do not open exam until instructed to do so Name: Date: xam II Practice xam Chem. 212 Do not open exam until told to do so. Get out your pencil, eraser, and scientific
The following 30 point quiz contains 20 multiple choice questions valued at 1.5 points/question, and an unknown analysis question valued at 6 points.
 Chemistry 262 Quiz 2 Spring 2018 Principally Chapters 17, 20, and 21 ame: KEY The following 30 point quiz contains 20 multiple choice questions valued at 1.5 points/question, and an unknown analysis question
Chemistry 262 Quiz 2 Spring 2018 Principally Chapters 17, 20, and 21 ame: KEY The following 30 point quiz contains 20 multiple choice questions valued at 1.5 points/question, and an unknown analysis question
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below?
 1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below? 2. Which of the following does not have an octet of electrons surrounding the central atom? A. B 3 B. C
1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below? 2. Which of the following does not have an octet of electrons surrounding the central atom? A. B 3 B. C
Chemistry Questions ans Answers BASED ON HIGH ORDER THINKING SKILL (HOTS) UNIT- 12 ALDEHYDES, KETONES AND CARBXYLIC ACID
 Chemistry Questions ans Answers BASED N IG RDER TINKING SKILL (TS) UNIT- 12 ALDEYDES, KETNES AND CARBXYLIC ACID 1 MARK QUESTINS Q. 1. Name the reaction and the reagent used for the conversion of acid chlorides
Chemistry Questions ans Answers BASED N IG RDER TINKING SKILL (TS) UNIT- 12 ALDEYDES, KETNES AND CARBXYLIC ACID 1 MARK QUESTINS Q. 1. Name the reaction and the reagent used for the conversion of acid chlorides
Name Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry II Examination #2 - March 12, 2001
 Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #2 - March 12, 2001 INSTRUTINS This examination has two parts. Part I is in multiple choice format and the answers should
Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #2 - March 12, 2001 INSTRUTINS This examination has two parts. Part I is in multiple choice format and the answers should
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Problem Booklet Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Problem Booklet Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21
Alcohol, Phenols & Ethers
 Alcohol, Phenols & Ethers 1) Conc. H 2 S 4 Heated with excess of C 2 H 5 at 140 o c to form CH 2 b) CH 2 CH 2 CH 2 CH 2 d) CH 2 = CH 2 2) In the following series of chemical reactions identify Z C 3 H
Alcohol, Phenols & Ethers 1) Conc. H 2 S 4 Heated with excess of C 2 H 5 at 140 o c to form CH 2 b) CH 2 CH 2 CH 2 CH 2 d) CH 2 = CH 2 2) In the following series of chemical reactions identify Z C 3 H
There are 19 problems on 22 pages. Please make sure that you have them all.
 EMISTRY 222, Spring 2001 Review Problems Page 1 There are 19 problems on 22 pages. Please make sure that you have them all. 1) Give an unambiguous name for the following compounds. e sure to use cis/trans,
EMISTRY 222, Spring 2001 Review Problems Page 1 There are 19 problems on 22 pages. Please make sure that you have them all. 1) Give an unambiguous name for the following compounds. e sure to use cis/trans,
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
NBS, CCl 4 heat A B C D
 1. What is(are) the expected product(s) of the following reaction? 2 C=CC( ) 2 NBS, CCl 4 heat A B C D 1) only B 2) only C 3) A and C 4) B and D 2. Which of the following is the 1,4-addition product in
1. What is(are) the expected product(s) of the following reaction? 2 C=CC( ) 2 NBS, CCl 4 heat A B C D 1) only B 2) only C 3) A and C 4) B and D 2. Which of the following is the 1,4-addition product in
HALOALKANES AND HALOARENES
 Unit - 10 HALOALKANES AND HALOARENES 1. Write the IUPAC names of the following compounds. Br CH = CH C CH (vi) (vii) (ix) (CCl 3 ) 3 CCl 103 XII Chemistry 2. Write the structure of following halogen compounds
Unit - 10 HALOALKANES AND HALOARENES 1. Write the IUPAC names of the following compounds. Br CH = CH C CH (vi) (vii) (ix) (CCl 3 ) 3 CCl 103 XII Chemistry 2. Write the structure of following halogen compounds
Organic Chemistry II KEY March 25, a) I only b) II only c) II & III d) III & IV e) I, II, III & IV
 rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
2. Examining the infrared spectrum of a compound allows us to:
 CHEM 204 2010 Ass. 1 Problem 1. The amount of energy in infrared light corresponds to: a. the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital b. the amount
CHEM 204 2010 Ass. 1 Problem 1. The amount of energy in infrared light corresponds to: a. the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital b. the amount
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Aldehydes and Ketones Reactions. Dr. Sapna Gupta
 Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
4.4, 4.5 HW MS 1. (a) nucleophilic addition 1 M2. (b) (i) 2-hydroxybutanenitrile 1 (ii) 2 H 3C O H
 4.4, 4.5 W MS 1. (a) nucleophilic addition 1 M N N M1 M M4 4 (b) (i) -hydroxybutanenitrile 1 (ii) N (allow 1 for amide even if not 4 7 N, i.e. RN ) (if not amide, allow one for any isomer of 4 7 N which
4.4, 4.5 W MS 1. (a) nucleophilic addition 1 M N N M1 M M4 4 (b) (i) -hydroxybutanenitrile 1 (ii) N (allow 1 for amide even if not 4 7 N, i.e. RN ) (if not amide, allow one for any isomer of 4 7 N which
Lecture 15. More Carbonyl Chemistry. Alcohols React with Aldehydes and Ketones in two steps first O R'OH, H + OR" 2R"OH R + H 2 O OR" 3/8/16
 Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
CH Organic Chemistry I (Katz) Practice Exam #3- Fall 2013
 C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
CHEMISTRY 104 Practice Problems #1 Organic Chem and Ch. 11 Kinetics Do the topics appropriate for your lecture
 EMISTRY 104 Practice Problems #1 rganic hem and h. 11 Kinetics Do the topics appropriate for your lecture Prepared by Dr. Tony Jacob http://www.chem.wisc.edu/areas/clc (Resource page) Suggestions on preparing
EMISTRY 104 Practice Problems #1 rganic hem and h. 11 Kinetics Do the topics appropriate for your lecture Prepared by Dr. Tony Jacob http://www.chem.wisc.edu/areas/clc (Resource page) Suggestions on preparing
FUNCTIONAL GROUPS Functional Group Suffix Formula Other Info O. Ester. Amide --- R C N R' or R(CO)NR R
 EMISTRY 10 elp Sheet # rganic (Part III hapters.7 (condensed, structural drawings, 6.3 (line drawings, 6.9a (benzene, 7.e (hybrid orbitals in organic structures, and Appendix E (functional groups Do topics
EMISTRY 10 elp Sheet # rganic (Part III hapters.7 (condensed, structural drawings, 6.3 (line drawings, 6.9a (benzene, 7.e (hybrid orbitals in organic structures, and Appendix E (functional groups Do topics
Chemistry 52 Exam #2. Name: 5 February This exam has six (6) questions, two cover pages, six exam pages, and three scratch pages.
 Chemistry 52 Exam #2 Name: 5 February 2003 This exam has six (6) questions, two cover pages, six exam pages, and three scratch pages. Please check before beginning to make sure no questions are missing.
Chemistry 52 Exam #2 Name: 5 February 2003 This exam has six (6) questions, two cover pages, six exam pages, and three scratch pages. Please check before beginning to make sure no questions are missing.
CHEM Chapter 16. Chemistry of Benzene: Electrophilic Aromatic Substitution (homework) W
 CHEM 2425. Chapter 16. Chemistry of Benzene: Electrophilic Aromatic Substitution (homework) W Short Answer Exhibit 16-1 MATCH a structure or term from the following list with each description below. Place
CHEM 2425. Chapter 16. Chemistry of Benzene: Electrophilic Aromatic Substitution (homework) W Short Answer Exhibit 16-1 MATCH a structure or term from the following list with each description below. Place
UCF - ORGANIC CHEMISTRY 1 - PROF. DAOUDI UCF PROF. DAOUDI EXAM 3 REVIEW.
 UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
What is in Common for the Following Reactions, and How Do They Work?
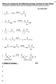 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
Section A. 1 at a given temperature. The rate was found to be first order with respect to the ester and first order with respect to hydroxide ions.
 2 Section A Answer all questions in the spaces provided. Question 1:The N/Arate of hydrolysis of an ester X (HCOOCH2CH2CH3) was studied in alkaline 1 at a given temperature. The rate was found to be first
2 Section A Answer all questions in the spaces provided. Question 1:The N/Arate of hydrolysis of an ester X (HCOOCH2CH2CH3) was studied in alkaline 1 at a given temperature. The rate was found to be first
Chemistry 52 Exam #1. Name: 22 January This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages.
 Chemistry 52 Exam #1 Name: 22 January 2003 This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages. Please check before beginning to make sure no questions are missing. 65 minutes
Chemistry 52 Exam #1 Name: 22 January 2003 This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages. Please check before beginning to make sure no questions are missing. 65 minutes
Isomerism and Carbonyl Compounds
 Isomerism and Carbonyl Compounds 18 Section B Answer all questions in the spaces provided. 7 Esters have many important commercial uses such as solvents and artificial flavourings in foods. Esters can
Isomerism and Carbonyl Compounds 18 Section B Answer all questions in the spaces provided. 7 Esters have many important commercial uses such as solvents and artificial flavourings in foods. Esters can
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
I pledge to uphold the CU onor Code: CEM 3311-100 Spring 2007 Final Exam Professor R. oenigman Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation number, day,
Chem 263 Oct. 12, 2010
 Chem 263 ct. 12, 2010 Alkyl Side Chain xidation Reaction If the carbon directly attached to the aromatic ring has > 1 hydrogen attached to it, it can be oxidized to the corresponding carboxylic acid with
Chem 263 ct. 12, 2010 Alkyl Side Chain xidation Reaction If the carbon directly attached to the aromatic ring has > 1 hydrogen attached to it, it can be oxidized to the corresponding carboxylic acid with
CHEM Review for test 1 (ch12-15). F17. Stafford
 CHEM Multiple Choice Identify the choice that best completes the statement or answers the question. 1. What range of wavelengths corresponds to the infrared region of the electromagnetic spectrum? a. 200-400
CHEM Multiple Choice Identify the choice that best completes the statement or answers the question. 1. What range of wavelengths corresponds to the infrared region of the electromagnetic spectrum? a. 200-400
CHEM1902/ N-8 November Consider the following reaction sequences beginning with the carboxylic acid, E.
 CEM1902/4 2014--8 ovember 2014 Consider the following reaction sequences beginning with the carboxylic acid, E. 6 ame compounds E and G. E: propionic acid G: methyl propionate Propose structures for compounds
CEM1902/4 2014--8 ovember 2014 Consider the following reaction sequences beginning with the carboxylic acid, E. 6 ame compounds E and G. E: propionic acid G: methyl propionate Propose structures for compounds
Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid R C N + H 2 O + H O R
 arboxylic Acid Derivatives Addition/Elimination Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid X = l, Br addition/elimination X acid
arboxylic Acid Derivatives Addition/Elimination Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid X = l, Br addition/elimination X acid
a) b) c) d) e) none of these Organic Chemistry II KEY April 25, 2016
 rganic Chemistry II KEY April 25, 2016 Exam 3: VERSI B 1. Tamoxifen is a selective estrogen receptor modulator (SERM) used to treat breast cancer. Which of the following reaction energy diagrams describes
rganic Chemistry II KEY April 25, 2016 Exam 3: VERSI B 1. Tamoxifen is a selective estrogen receptor modulator (SERM) used to treat breast cancer. Which of the following reaction energy diagrams describes
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Pearson Edexcel Level 3 GCE Chemistry Advanced Paper 2: Advanced Organic and Physical Chemistry
 Write your name here Surname Other names Pearson Edexcel Level 3 GCE Centre Number Candidate Number Chemistry Advanced Paper 2: Advanced Organic and Physical Chemistry Specimen Paper for first teaching
Write your name here Surname Other names Pearson Edexcel Level 3 GCE Centre Number Candidate Number Chemistry Advanced Paper 2: Advanced Organic and Physical Chemistry Specimen Paper for first teaching
Unit 2 Review: Organic Chemistry. 1. Terms for which you should be able to write or apply the definitions:
 Unit 2 Review: Organic Chemistry 1. Terms for which you should be able to write or apply the definitions: organic compound aliphatic hydrocarbons saturated miscible functional group aromatic hydrocarbons
Unit 2 Review: Organic Chemistry 1. Terms for which you should be able to write or apply the definitions: organic compound aliphatic hydrocarbons saturated miscible functional group aromatic hydrocarbons
Homework 5 Organic Chemistry MCAT Review Summer 2012 Brent Iverson
 omework 5 rganic Chemistry MCAT Review Summer 2012 ent Iverson 1 1. For the following reactions, draw the predominant product or products. When a new chiral center is created, mark it with an asterisk
omework 5 rganic Chemistry MCAT Review Summer 2012 ent Iverson 1 1. For the following reactions, draw the predominant product or products. When a new chiral center is created, mark it with an asterisk
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Nitro compounds are named by writing the word nitro before the name of the parent compound.
 Nitro compounds are an important class of organic compounds which may be regarded as derived from hydrocarbons by the replacement of one or more hydrogen atoms by nitro (NO₂) groups. Nitro arenes(i.e.
Nitro compounds are an important class of organic compounds which may be regarded as derived from hydrocarbons by the replacement of one or more hydrogen atoms by nitro (NO₂) groups. Nitro arenes(i.e.
1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES. Vikasana - CET 2012
 CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
As time allows, additional practice questions for Exam 2 follow. This is an incomplete collection. Content & emphasis will vary.
 Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
