CHEM 203. Midterm Exam 2 November 12, 2013 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
|
|
|
- Jasper Hood
- 5 years ago
- Views:
Transcription
1 CEM 203 Midterm Exam 2 November 12, 2013 ANSWERS Your name: This a closed-notes, closed-book exam You may use your set of molecular models This document contains 7 pages Time: 1h 30 min 1. / / / / / / 20 TOTAL / 100 This exam counts for 18.75% of your CEM 203 final grade
2 Chem 203 midterm exam 2 p. 2 of 7 1. (10 pts.) Indicate the approximate pka for the dissociation of the protons in boldface in the following molecules (write your answers in the appropriate boxes): C 3 OSO 2 CF 3 O-C 2 C 3 C=C 2 -O C 3 O -N (15 pts.) The radical chlorination of compound A afforded a mixture of products, from which five distinct mono-chlorinated derivatives were isolated. Draw the structures of the five monochloro products in the boxes below. monochloro product 1 monochloro product 2 A 2 hν monochloro product 3 monochloro product 4 monochloro product 5 3. (15 pts.) Inside the appropriate boxes, draw: a. The structure of an alkyl bromide that furnishes alkene A when treated with potassium tert-butoxide, but alkene B as one of the products obtained upon heating in C 3 O: OK C 3 O heat B A (one of the products)
3 Chem 203 midterm exam 2 p. 3 of 7 b. The structure of an alkyne that yields one product when treated with 2 in the presence of catalyst, and then with OsO 4 followed by aqueous NaSO 3 solution, but a different product when treated with Na in liquid N 3, and then with OsO 4 followed by aqueous NaSO 3 solution. structure of the alkyne in question c. The structure of an alkene that yields a chiral product when treated with 2, but an achiral product when treated with OsO 4 followed by aqueous NaSO 3 solution: structure of the alkene in question d. The structure of an alkene that yields only product C when treated with O 3 and then with Zn / +, followed by C 3 C 2 Mg and then mild 3 O + : 1. O 3 2. Zn, + (or cis isomer) 3. C 3 -C 2 Mg 3. mild 3 O + O C e. The structure of an alkyl halide that cannot undergo E2 reaction other answers are possible
4 Chem 203 midterm exam 2 p. 4 of 7 4. (20 pts.) Provide the structure of the major product expected from the following reaction sequences. If no overall change is expected, answer "NO REACTION." Important: compounds incorporating multiple stereogenic centers must be drawn with the correct relative configuration. Note: it is understood that chiral compounds will be obtained as racemic mixtures. a. 2 hν b. C 3 1., radical 2. C 3 Li Cu no reaction 1. NBS, radical c. 2. Na 3. 2, catalyst C 3 d AIBN, heat OK Ph Ph Ph polystyrene n Ph = phenyl e. 1., radical 2. NaN 3 3. Zn, + N 2
5 Chem 203 midterm exam 2 p. 5 of 7 5. (20 pts.) Propose a method to achieve the transformations shown below. Indicate all the reagents, in the correct order, that are required to induce each transformation. Present your answer as a numbered list displayed above / below each reaction arrow. If a product appears to be unavailable from the indicated starting material by any method known to you, write "INACCESSIBLE" on the reaction arrow Note: it is understood that chiral compounds will be obtained as racemic mixtures. a SO 4, heat O 2. N 2 3. NaN 3 4. Zn, , hv b. S 2. Na 2 S c. O NaN 3 3. Zn, + O N 2 d SO 4, 2 O 2. 2 SO 4, heat 3. 2, 2 O 4. NaSC 3 O SC 3 1. NBS, rad. init. 2. tert-buok e. O 3. O 3, then Zn/ + 4. C 3 Mg, then mild 3 O + other answers are possible
6 Chem 203 midterm exam 2 p. 6 of 7 6. (20 pts.) Propose a method for the preparation of compounds a. e. below starting ONLY with methane (C 4 ) and acetylene ( C C ) as the source of carbon atoms. You may use any additional reagent that might be needed (e.g., borane,, Mg, 2 O 2, potassium tertbutoxide, etc.). Present your answer as a clear flowchart that shows all intermediate steps and products. Substances obtained in a prior sequence may be used in later sequences. It is not necessary to draw mechanisms a. C 4 2, hv C 3 2, cat. C 3 C 3 NaN 2 C 3 C 3 NaN 2 C 3 b. COO cat., 2 1. O O 2, + NaN 2 cat., 2 (twice) c. O C 3 (from a.) cat., 2 1. B O 2, aq. NaO cat., 2 NaN 2 Na/N 3 (liq) instead of 2 / would be OK in the above cases
7 Chem 203 midterm exam 2 p. 7 of 7 d. N 2 1. NaN 3 2. Zn, + product a. NaN 2, rad. cat., 2 e. O O 1. OsO 4 2. aq. NaSO 3 Na liq. N 3 NaN 2 C 3 (from a.) from c. alternative answers may be OK
CHEM 203. Midterm Exam 2 November 13, 2014 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 2 ovember 13, 2014 Your name: ASWERS This a closed-notes, closed-book exam You may use your set of molecular models This document contains 7 pages Time: 1h 30 min 1. / 10 2. / 15 3.
CEM 203 Midterm Exam 2 ovember 13, 2014 Your name: ASWERS This a closed-notes, closed-book exam You may use your set of molecular models This document contains 7 pages Time: 1h 30 min 1. / 10 2. / 15 3.
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CHEM 203. Final Exam December 12, This a closed-notes, closed-book exam. You may use your set of molecular models. This test contains 11 pages
 CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
CHEM 203. Final Exam December 16, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CHEM 203. Final Exam December 18, 2013 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
CHEM 203. Final Exam December 18, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CHEM 203. Midterm Exam 1 October 31, 2008 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CHEM 203 HOMEWORK 7 Alkyl halides. 1. Write the structure of an alkyne that contains at least 4 carbon atoms and that:
 CEM 20 MEWRK 7 lkyl halides 1. Write the structure of an alkyne that contains at least 4 carbon atoms and that: Produces a chiral diol when treated with 2 and ndlar catalyst followed by s 4 and aq. as
CEM 20 MEWRK 7 lkyl halides 1. Write the structure of an alkyne that contains at least 4 carbon atoms and that: Produces a chiral diol when treated with 2 and ndlar catalyst followed by s 4 and aq. as
350 Organic Chemistry I Winona State University
 350 Organic Chemistry I Winona State University Exam #4B, December 9, 2013 Professor T. Nalli Name General Instructions: Write your name in the space provided above and on the provided Scan-tron form.
350 Organic Chemistry I Winona State University Exam #4B, December 9, 2013 Professor T. Nalli Name General Instructions: Write your name in the space provided above and on the provided Scan-tron form.
CHEM 330. Final Exam December 8, 2010 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 8, 2010 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 20 4.
CEM 330 Final Exam December 8, 2010 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 20 4.
CHEM 330. Final Exam December 11, This a closed-notes, closed-book exam. The use of molecular models is allowed. This exam contains 12 pages
 CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
CHEM 330. Final Exam December 11, 2007 A N S W E R S. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 11, 2007 Your name: A S W E R S This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 / 20 3. / 30
CEM 330 Final Exam December 11, 2007 Your name: A S W E R S This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 / 20 3. / 30
Organic Chemistry 1 CHM 2210 Exam 3 (November 9, 2001)
 Organic Chemistry 1 CM 2210 Exam 3 (November 9, 2001) Name (print): Signature: Student ID Number: There are 14 multiple choice problems (5 points each) on this exam, however, you will only be graded out
Organic Chemistry 1 CM 2210 Exam 3 (November 9, 2001) Name (print): Signature: Student ID Number: There are 14 multiple choice problems (5 points each) on this exam, however, you will only be graded out
CH Organic Chemistry I (Katz) Practice Exam #3- Fall 2013
 C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #3 - November 11, 2002 ANSWERS
 Name INSTRUTINS Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 11, 2002 ANSWERS This examination has two parts. The first part is in multiple choice format; the
Name INSTRUTINS Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 11, 2002 ANSWERS This examination has two parts. The first part is in multiple choice format; the
Chem Final Examination August 7, 2004
 Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
Chemistry 232 PRACTICE Midterm 3 October 2010 Your name: 1
 Chemistry 232 PRACTICE Midterm 3 October 2010 Your name: 1 You will need to be able to show picture ID to take the test. DO NOT OPEN TIS TEST UNTIL EVERYONE AS ONE I encourage following instructions: ten
Chemistry 232 PRACTICE Midterm 3 October 2010 Your name: 1 You will need to be able to show picture ID to take the test. DO NOT OPEN TIS TEST UNTIL EVERYONE AS ONE I encourage following instructions: ten
Third Midterm Exam CHEM 255 Organic Chemistry I Prof. Bastin November 19, 2009
 Third Midterm Exam CEM 255 Organic Chemistry I Prof. Bastin November 19, 2009 Name Section Provide CLEAR, CONCISE answers using unambiguous, carefully drawn structures and arrows for all of the appropriate
Third Midterm Exam CEM 255 Organic Chemistry I Prof. Bastin November 19, 2009 Name Section Provide CLEAR, CONCISE answers using unambiguous, carefully drawn structures and arrows for all of the appropriate
Topics in the June 2009 Exam Paper for CHEM1102
 June 009 Topics in the June 009 Exam Paper for CEM110 ick on the links for resources on each topic. 009-J-: 009-J-3: 009-J-4: 009-J-5: 009-J-6: 009-J-7: 009-J-8: 009-J-9: 009-J-10: 009-J-1: 009-J-13: Periodic
June 009 Topics in the June 009 Exam Paper for CEM110 ick on the links for resources on each topic. 009-J-: 009-J-3: 009-J-4: 009-J-5: 009-J-6: 009-J-7: 009-J-8: 009-J-9: 009-J-10: 009-J-1: 009-J-13: Periodic
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
NAME (Print): SIGNATURE: Chemistry 310M/318M Dr. ent Iverson 3rd Midterm November 18, 2010 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit
Chemistry 233 Exam 3 (Green) The Periodic Table
 Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
HOMEWORK PROBLEMS: ALKYNES. 1. Provide the complete IUPAC name for the following compounds:
 CEM 31 MEWRK PRBLEMS: ALKYNES 1. Provide the complete IUPAC name for the following compounds: 2. When the compound below is treated with one equivalent of B 3, where does it react Explain. Where would
CEM 31 MEWRK PRBLEMS: ALKYNES 1. Provide the complete IUPAC name for the following compounds: 2. When the compound below is treated with one equivalent of B 3, where does it react Explain. Where would
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353
 TE UNIVERSITY F ALGARY FAULTY F SIENE MIDTERM EXAMINATIN EMISTRY 353 MAR 8th, 2006 Time: 2 ours PLEASE WRITE YUR NAME AND FULL STUDENT I.D. NUMBER N BT YUR MPUTER ANSWER SEET and on the ANSWER BKLET provided.
TE UNIVERSITY F ALGARY FAULTY F SIENE MIDTERM EXAMINATIN EMISTRY 353 MAR 8th, 2006 Time: 2 ours PLEASE WRITE YUR NAME AND FULL STUDENT I.D. NUMBER N BT YUR MPUTER ANSWER SEET and on the ANSWER BKLET provided.
Departmental Final Examination. Organic Chemistry I Caffein
 Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
 EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
1. (3 pts) Circle the highest priority substituent of the following list:
 Ch 334 Midterm #3 November 17, 2006 Code 1. (3 pts) Circle the highest priority substituent of the following list: 2. (4 pts) Rank the following groups in order of increasing priority. Place the letter
Ch 334 Midterm #3 November 17, 2006 Code 1. (3 pts) Circle the highest priority substituent of the following list: 2. (4 pts) Rank the following groups in order of increasing priority. Place the letter
+ HBr!H = OH CH CH 2. HgOAc
 radical substitution Cl hv + Cl 2 + Cl! = Energy radical addition RR + electrophilic addition + C3 C3! = Energy Energy + 2 acetone water + 2 2 +! =! = Energy Energy acid catalyzed hydration + 2 +! = Energy
radical substitution Cl hv + Cl 2 + Cl! = Energy radical addition RR + electrophilic addition + C3 C3! = Energy Energy + 2 acetone water + 2 2 +! =! = Energy Energy acid catalyzed hydration + 2 +! = Energy
FINAL EXAM Organic Chemistry Chemistry 225b; 9 A.M., Friday, May 9, NAME (print): Section Day: Section Time:
 FINAL EXAM Organic Chemistry Chemistry 225b; 9 A.M., Friday, May 9, 2008 NAME (print): TA: Section Day: Section Time: Take a few moments to look over the exam. Do problems first with which you are most
FINAL EXAM Organic Chemistry Chemistry 225b; 9 A.M., Friday, May 9, 2008 NAME (print): TA: Section Day: Section Time: Take a few moments to look over the exam. Do problems first with which you are most
Practice Homework Iverson CH320M/328M Do not turn in. NAME (Print): Chemistry 320M/328M Dr. Brent Iverson Practice Homework December 3, 2018
 Practice omework Iverson C320M/328M Do not turn in NAME (Print): SIGNATURE: Chemistry 320M/328M Dr. ent Iverson Practice omework December 3, 2018 Please print the first three letters of your last name
Practice omework Iverson C320M/328M Do not turn in NAME (Print): SIGNATURE: Chemistry 320M/328M Dr. ent Iverson Practice omework December 3, 2018 Please print the first three letters of your last name
Partial Periodic Table
 CEM 3311, Fall 2011 Professor Walba Third our Exam November 17, 2011 scores: 1) 20 2) 20 3) 20 4) 20 5) 20 100 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither
CEM 3311, Fall 2011 Professor Walba Third our Exam November 17, 2011 scores: 1) 20 2) 20 3) 20 4) 20 5) 20 100 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder tudent, I have neither
17.3 REACTIONS INVOLVING ALLYLIC AND BENZYLIC ANIONS
 798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #3 - November 8, 2004 ANSWERS
 Name Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 8, 2004 ANSWERS INSTRUTINS --- This examination has two parts. The first part is in multiple choice format;
Name Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 8, 2004 ANSWERS INSTRUTINS --- This examination has two parts. The first part is in multiple choice format;
Partial Periodic Table
 CEM 33, Fall 00 Professor Walba Third our Exam November 8, 00 Scores: ) 0 ) 0 3) 0 4) 0 5) 0 00 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, have neither given nor received
CEM 33, Fall 00 Professor Walba Third our Exam November 8, 00 Scores: ) 0 ) 0 3) 0 4) 0 5) 0 00 CU onor Code Pledge: n my honor, as a University of Colorado at Boulder Student, have neither given nor received
Lecture 21 Organic Chemistry 1
 CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
CHAPTER 12 HW: REDOX REACTIONS
 CAPTER 12 W: REDX REACTINS XIDATIN STATES AND GENERAL CNCEPTS 1. Are the following statements true or false? When a carbon atom changes its oxidation state from -2 to 0 it is reduce The oxidation state
CAPTER 12 W: REDX REACTINS XIDATIN STATES AND GENERAL CNCEPTS 1. Are the following statements true or false? When a carbon atom changes its oxidation state from -2 to 0 it is reduce The oxidation state
+ HBr ΔH = OH CH CH 2. HgOAc
 radical substitution l hv + l 2 + l Energy radical addition RR + electrophilic addition + 3 3 Energy Energy + 2 acetone water + 2 2 + Energy Energy acid catalyzed hydration + 2 + Energy + + 2 + + 2 = 2
radical substitution l hv + l 2 + l Energy radical addition RR + electrophilic addition + 3 3 Energy Energy + 2 acetone water + 2 2 + Energy Energy acid catalyzed hydration + 2 + Energy + + 2 + + 2 = 2
HONORS ORGANIC CHEM. HAHS MRS. RICHARDS
 NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
there general method to synthesize alkenes a. acidic conditions acid catalyzed dehydration of alcohols to alkene H H H H H OH 2 H H + H 2 O H H
 there general method to synthesize alkenes a. acidic conditions acid catalyzed dehydration of alcohols to alkene O 2 SO 4 heat O 2 SO 4 heat O 2 O 2 2 SO 4 heat + SO 4 + 2 O + SO 4 + 2 O + 2 SO 4 limitation;
there general method to synthesize alkenes a. acidic conditions acid catalyzed dehydration of alcohols to alkene O 2 SO 4 heat O 2 SO 4 heat O 2 O 2 2 SO 4 heat + SO 4 + 2 O + SO 4 + 2 O + 2 SO 4 limitation;
CHEM 8A Summer Student ID # Organic Chemistry FINAL EXAM (400 points)
 CEM 8A Summer 2017 UCSC, Binder Name Student ID # rganic Chemistry FINAL EXAM (400 points) In each of the following problems, you will use your knowledge of organic chemistry conventions to answer the
CEM 8A Summer 2017 UCSC, Binder Name Student ID # rganic Chemistry FINAL EXAM (400 points) In each of the following problems, you will use your knowledge of organic chemistry conventions to answer the
= ( 3 ) + 2 ( 3 ) = ( ) = = ( ) = 20 H H
 Columbia University 92CORG12.DOC CEM S3443D Summer 1992 Professor Grace B. Borowitz Exam No. 2 June 17, 1992 Name: Grade: Please use a non-red pen. Answer questions in the provided space. If you write
Columbia University 92CORG12.DOC CEM S3443D Summer 1992 Professor Grace B. Borowitz Exam No. 2 June 17, 1992 Name: Grade: Please use a non-red pen. Answer questions in the provided space. If you write
Chemistry 210 Organic Chemistry I Winter Semester 2001 Dr. Rainer Glaser
 hemistry 210 rganic hemistry I Winter Semester 2001 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, April 20, 2001, 9:00-9:50 Name: Answer Key Question
hemistry 210 rganic hemistry I Winter Semester 2001 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, April 20, 2001, 9:00-9:50 Name: Answer Key Question
Option II: Chiral + Achiral = Optically Active Diastereomers
 Option II: Chiral + Achiral = Optically Active Diastereomers What about additions to chiral alkenes? The previous examples were reactions done on achiral alkenes. What is the difference when an alkene
Option II: Chiral + Achiral = Optically Active Diastereomers What about additions to chiral alkenes? The previous examples were reactions done on achiral alkenes. What is the difference when an alkene
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chem 232. Problem Set 9. Question 1. D. J. Wardrop
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
Sample Final Examination Organic Chemistry I
 Sample Final Examination rganic Chemistry I CEM 2423 Rofecoxib (Vioxx)- is a nonsteroidal anti-inflammatory drug (NSAID) developed by Merck & Co.to treat osteoarthritis, acute pain conditions, and dysmenorrhoea
Sample Final Examination rganic Chemistry I CEM 2423 Rofecoxib (Vioxx)- is a nonsteroidal anti-inflammatory drug (NSAID) developed by Merck & Co.to treat osteoarthritis, acute pain conditions, and dysmenorrhoea
Homework for Chapter 17 Chem 2320
 Homework for Chapter 17 Chem 2320 I. Cumulated, isolated, and conjugated dienes Name 1. Draw structures which fit the following descriptions. Use correct geometry! a conjugated diene with the formula C
Homework for Chapter 17 Chem 2320 I. Cumulated, isolated, and conjugated dienes Name 1. Draw structures which fit the following descriptions. Use correct geometry! a conjugated diene with the formula C
Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron sheet.
 Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
Problem Set 8: Substitution Reactions-ANSWER KEY. (b) nucleophile NH 3 H C. (d) H 3 N
 Problem Set 8: Substitution Reactions-ANSWER KEY hemistry 260 rganic hemistry 1. The answers are (1), (3) and (5). Nucleophiles generally have lone pair and/or negative charge. 2. (a) neither 4 (c) nucleophile
Problem Set 8: Substitution Reactions-ANSWER KEY hemistry 260 rganic hemistry 1. The answers are (1), (3) and (5). Nucleophiles generally have lone pair and/or negative charge. 2. (a) neither 4 (c) nucleophile
CHEM 3311 (Richardson) Third Exam Nov. 27, 2018
 CHEM 3311 (Richardson) Third Exam Nov. 27, 2018 Your Name: Question Score Out of 1 15 Student ID: 2 20 3 20 Recitation (check one) O 10:00 Mon (Shafer Soars) 4 10 O 11:00 Mon (Matthew Farmer) O 1:00 Mon
CHEM 3311 (Richardson) Third Exam Nov. 27, 2018 Your Name: Question Score Out of 1 15 Student ID: 2 20 3 20 Recitation (check one) O 10:00 Mon (Shafer Soars) 4 10 O 11:00 Mon (Matthew Farmer) O 1:00 Mon
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CHEMISTRY 341. Final Exam Tuesday, December 16, Problem 1 15 pts Problem 9 8 pts. Problem 2 5 pts Problem pts
 CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
CHEM 330. Final Exam December 5, 2014 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 READ ALL THE INSTRUCTIONS CAREFULLY
 WEDNESDAY MARCH 9th, 2016 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
WEDNESDAY MARCH 9th, 2016 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
PLEASE DO NOT OPEN THIS EXAM UNTIL YOU ARE INSTRUCTED TO DO SO.
 CEMISTRY 0 :5 AM Section EXAM 2 Nov 2009 Name: Note: Your exam should consist of 5 pages including the cover page and grade tabulation sheet. Skim the entire exam, and solve the easiest problems first.
CEMISTRY 0 :5 AM Section EXAM 2 Nov 2009 Name: Note: Your exam should consist of 5 pages including the cover page and grade tabulation sheet. Skim the entire exam, and solve the easiest problems first.
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
Chapter 10 Radical Reactions
 Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Partial Periodic Table
 Easily Legible Printed Name: CEM 3351 (100), all 2015 Professor Walba Third our Exam November 17, 2015 scores: 1) 20 2) 20 3) 20 4) 20 5) 20 100 CU onor Code Pledge: n my honor, as a University of Colorado
Easily Legible Printed Name: CEM 3351 (100), all 2015 Professor Walba Third our Exam November 17, 2015 scores: 1) 20 2) 20 3) 20 4) 20 5) 20 100 CU onor Code Pledge: n my honor, as a University of Colorado
CHAPTER PRACTICE PROBLEMS CHEMISTRY
 APTER PRACTICE PRBLEMS EMISTRY Electrophilic Aromatic Substitution Name : Batch : Date : rientation influence of groups 1. Predict the characteristics of -NH + as a substituent. activating, o/p directing
APTER PRACTICE PRBLEMS EMISTRY Electrophilic Aromatic Substitution Name : Batch : Date : rientation influence of groups 1. Predict the characteristics of -NH + as a substituent. activating, o/p directing
CHEM 240: Survey of Organic Chemistry at North Dakota State University Midterm Exam 02 - Tue, 23 Sep 2014!! Name:! KEY!
 CEM 240: Survey of rganic Chemistry at orth Dakota State University Midterm Exam 02 - Tue, 23 Sep 2014!! ame:! KEY! Please read through each question carefully and answer in the spaces provided. A good
CEM 240: Survey of rganic Chemistry at orth Dakota State University Midterm Exam 02 - Tue, 23 Sep 2014!! ame:! KEY! Please read through each question carefully and answer in the spaces provided. A good
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTION AND/OR GSI IF YOU ARE IN THE LABORATORY COURSE:
 CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
CEM 351 2nd EXAM/Version A Friday, October 17, :50 2:40 p.m. Room 138, Chemistry
 Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
Name (print) Signature Student # Section Number CEM 351 2nd EXAM/Version A Friday, October 17, 2003 1:50 2:40 p.m. Room 138, Chemistry Ann Swerkey Grade? 1.(20 2.(20. 3.(20 4.(20 5.(20 6.(20 TOTAL 100
CHEM1902/ N-9 November 2014
 CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CHEM 341: Organic Chemistry I at North Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:!
 CHEM 341: rganic Chemistry I at Nth Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:! You may use the blank pages of your test f scratch paper. Please read through each question carefully
CHEM 341: rganic Chemistry I at Nth Dakota State University Midterm Exam 02 - Fri, Mar 9, 2012!! Name:! You may use the blank pages of your test f scratch paper. Please read through each question carefully
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
CHEM Exam 2 ANSWER KEY
 CEM 3311-200 Exam 2 ANSWER KEY ctober 23, 2012 Time: 2 ours Please copy and sign the onor Pledge on the scantron sheet in the space below the double lines. I pledge that n my honor, as a University of
CEM 3311-200 Exam 2 ANSWER KEY ctober 23, 2012 Time: 2 ours Please copy and sign the onor Pledge on the scantron sheet in the space below the double lines. I pledge that n my honor, as a University of
Lecture 20 Organic Chemistry 1
 CEM 232 rganic Chemistry I at Chicago Lecture 20 rganic Chemistry 1 Professor Duncan Wardrop March 18, 2010 1 Self-Test Question Capnellene (4) is a marine natural product that was isolated from coral
CEM 232 rganic Chemistry I at Chicago Lecture 20 rganic Chemistry 1 Professor Duncan Wardrop March 18, 2010 1 Self-Test Question Capnellene (4) is a marine natural product that was isolated from coral
Chem 30A Winter Mon March 21st
 Last Name First Name MI Student ID Number: Total Score / 200 Chem 30A Winter 2005 FINAL (180 Min) Mon March 21st Course Grade ***DO NOT OPEN THIS EXAM UNTIL INSTRUCTED TO DO SO*** ONLY ANSWERS WRITTEN
Last Name First Name MI Student ID Number: Total Score / 200 Chem 30A Winter 2005 FINAL (180 Min) Mon March 21st Course Grade ***DO NOT OPEN THIS EXAM UNTIL INSTRUCTED TO DO SO*** ONLY ANSWERS WRITTEN
Chem 341 Organic Chemistry I Midterm Exam 2 October 19, 2007
 Chem 341 rganic Chemistry I Midterm Exam 2 ctober 19, 2007 NAME What happens to an organic chemistry student during an exam... rganic Student organic chemistry exam You may use the back of the pages for
Chem 341 rganic Chemistry I Midterm Exam 2 ctober 19, 2007 NAME What happens to an organic chemistry student during an exam... rganic Student organic chemistry exam You may use the back of the pages for
Exam 2 Chem 109a Fall 2004
 Exam 2 Chem 109a Fall 2004 Please put your name and perm number on both the exam and the scantron sheet. Next, answer the following 34 multiple-choice questions on the scantron sheet. Then choose one A-type
Exam 2 Chem 109a Fall 2004 Please put your name and perm number on both the exam and the scantron sheet. Next, answer the following 34 multiple-choice questions on the scantron sheet. Then choose one A-type
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22
 Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical
 C h a p t e r E l e v e n: Radical Reactions α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical CM 321: Summary of Important Concepts YConcepts for Chapter 11: Radical
C h a p t e r E l e v e n: Radical Reactions α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical CM 321: Summary of Important Concepts YConcepts for Chapter 11: Radical
Chemistry 201. MW 12:00pm 1:15pm Examination #2 August 15 th Bronco ID. Question Score Possible Points. 1 (12pts) 2 (24pts) 3 (25pts)
 Chemistry 201 MW 12:00pm 1:15pm Examination #2 August 15 th 2016 Name Bronco ID. Question Score Possible Points 1 (12pts) 2 (24pts) 3 (25pts) 4... (12pts) 5 (27pts). Total (100pts) 1. Read each question
Chemistry 201 MW 12:00pm 1:15pm Examination #2 August 15 th 2016 Name Bronco ID. Question Score Possible Points 1 (12pts) 2 (24pts) 3 (25pts) 4... (12pts) 5 (27pts). Total (100pts) 1. Read each question
Chapter 8. Alkenes and Alkynes II: Addition Reactions. Ch. 8-1
 hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
CHEM 2423 Unit 8 Homework Answers
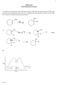 1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat #
 Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm
 - 1 - Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm Name: (please print, 1 pt) Page Possible Points Score 1 1 2 9 3 10 4 12 5 14 6 14 7 10 8 15 9 10 10 10 11 10 12
- 1 - Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm Name: (please print, 1 pt) Page Possible Points Score 1 1 2 9 3 10 4 12 5 14 6 14 7 10 8 15 9 10 10 10 11 10 12
Partial Periodic Table
 Easily Legible Printed Name: CHEM 3351 (100), Fall 2016 Professor Walba Second Hour Exam October 18, 2016 CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student, I have neither
Easily Legible Printed Name: CHEM 3351 (100), Fall 2016 Professor Walba Second Hour Exam October 18, 2016 CU Honor Code Pledge: On my honor, as a University of Colorado at Boulder Student, I have neither
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
1. Which statement is not true of nucleophillic substitution reactions?!
 Mock Exam 3 CH 235-2F Ch. 7, 8, 9, and 11 Multiple Choice: 1. Which statement is not true of nucleophillic substitution reactions? a. SN1 proceeds through a cation intermediate. b. SN2 occurs with inversion
Mock Exam 3 CH 235-2F Ch. 7, 8, 9, and 11 Multiple Choice: 1. Which statement is not true of nucleophillic substitution reactions? a. SN1 proceeds through a cation intermediate. b. SN2 occurs with inversion
Final Exam December 13, 2005 Professor Rebecca Hoenigman
 CEM 3311-200, all 2005 inal Exam December 13, 2005 Professor Rebecca oenigman Average core = 158 igh core = 276 Low core = 20 I pledge to uphold the CU onor Code: ignature Name (printed) Last four digits
CEM 3311-200, all 2005 inal Exam December 13, 2005 Professor Rebecca oenigman Average core = 158 igh core = 276 Low core = 20 I pledge to uphold the CU onor Code: ignature Name (printed) Last four digits
Chemistry 51 Exam #3. Name KEY November 20, 2001
 Chemistry 51 Exam #3 Name KEY November 20, 2001 This exam has nine (9) questions. Please check before beginning to make sure no questions are missing. All scratch work must be done on the attached blank
Chemistry 51 Exam #3 Name KEY November 20, 2001 This exam has nine (9) questions. Please check before beginning to make sure no questions are missing. All scratch work must be done on the attached blank
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Topics in the November 2014 Exam Paper for CHEM1102
 November 2014 Topics in the November 2014 Exam Paper for CEM1102 Click on the links for resources on each topic. 2014-N-2: 2014-N-3: 2014-N-4: 2014-N-5: 2014-N-6: 2014-N-7: 2014-N-8: 2014-N-9: 2014-N-10:
November 2014 Topics in the November 2014 Exam Paper for CEM1102 Click on the links for resources on each topic. 2014-N-2: 2014-N-3: 2014-N-4: 2014-N-5: 2014-N-6: 2014-N-7: 2014-N-8: 2014-N-9: 2014-N-10:
CHEM 263 Oct 25, stronger base stronger acid weaker acid weaker base
 CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
Chem 3719 Example Exams. Chemistry 3719 Practice Exams
 Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
CHEM 3311 Exam 2 ANSWER KEY
 CEM 3311 Exam 2 ANSWER KEY March 11, 2014 Time: 2 ours Please sign the onor Pledge h in this sheet with your scantron. I pledge that n my honor, as a University of Colorado-Boulder student, I have neither
CEM 3311 Exam 2 ANSWER KEY March 11, 2014 Time: 2 ours Please sign the onor Pledge h in this sheet with your scantron. I pledge that n my honor, as a University of Colorado-Boulder student, I have neither
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
CHEM 203. Topics Discussed on Oct. 16
 EM 203 Topics Discussed on Oct. 16 ydrogenation (= saturation) of olefins in the presence of finely divided transition metal catalysts (Ni, Pd, Pt, Rh, Ru...): generic alkene R 1 finely divided Pd (or
EM 203 Topics Discussed on Oct. 16 ydrogenation (= saturation) of olefins in the presence of finely divided transition metal catalysts (Ni, Pd, Pt, Rh, Ru...): generic alkene R 1 finely divided Pd (or
Chem 112A: Final Exam
 Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Partial Periodic Table 1A
 CEMISTRY 3311, all 2001 Professor Walba irst our Exam September 27, 2001 scores: 1) 20 2) 20 3) 15 This is a closed-book "open model" exam. You may use models, but no notes or books. Please put all your
CEMISTRY 3311, all 2001 Professor Walba irst our Exam September 27, 2001 scores: 1) 20 2) 20 3) 15 This is a closed-book "open model" exam. You may use models, but no notes or books. Please put all your
FINAL EXAMINATION Chemistry 3A
 1 FINAL EXAMINATION Chemistry 3A Key Name: Print first name before second! Use capital letters! SID #: Peter Vollhardt May 11, 2016 GSI (if you are taking Chem 3AL): Please provide the following information
1 FINAL EXAMINATION Chemistry 3A Key Name: Print first name before second! Use capital letters! SID #: Peter Vollhardt May 11, 2016 GSI (if you are taking Chem 3AL): Please provide the following information
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
Chemistry 14D Winter 2016 Midterm Exam 1 Page 1
 Chemistry 14D Winter 2016 Midterm Exam 1 Page 1 OK to use "" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer. t
Chemistry 14D Winter 2016 Midterm Exam 1 Page 1 OK to use "" anywhere on this exam where appropriate. Exceeding the specified word limit on an answer will result in a point deduction for that answer. t
OChem1 Old Exams. Chemistry 3719 Practice Exams
 OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
Dr. Steven Pedersen November 9, Chemistry 3A. Midterm 2
 Dr. Steven Pedersen November 9, 2016 Student name: ANSWER KEY Chemistry 3A Midterm 2 Student ID: (Also include your SID in the top left corner of each page) Student signature: Problem 1 Problem 2 Problem
Dr. Steven Pedersen November 9, 2016 Student name: ANSWER KEY Chemistry 3A Midterm 2 Student ID: (Also include your SID in the top left corner of each page) Student signature: Problem 1 Problem 2 Problem
EXAM 2 CHEMISTRY 220a Friday, October 14, Section Day: Section Time:
 EXAM 2 CHEMISTRY 220a Friday, October 14, 2005 NAME (print): Your ID#: TA: Section Day: Section Time: No Calculators! Take a few moments to look over the exam. Answer each question on the exam paper. Important
EXAM 2 CHEMISTRY 220a Friday, October 14, 2005 NAME (print): Your ID#: TA: Section Day: Section Time: No Calculators! Take a few moments to look over the exam. Answer each question on the exam paper. Important
CHEM 2311 HW1. COMPLETELY FILL THE BOXES IN DARK PENCIL, i.e.:, NOT or or STRUCTURE
 EM 2311 W1 Provide answers to the following multiple choice questions on the Scantron form provided in lecture. Make sure that you put your name and bubble in your GTID # on the answer form. MPLETELY FILL
EM 2311 W1 Provide answers to the following multiple choice questions on the Scantron form provided in lecture. Make sure that you put your name and bubble in your GTID # on the answer form. MPLETELY FILL
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections
 Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
