Cosmic rays: Discovery and early research. Florence 29 November 2011 Per Carlson
|
|
|
- Kristopher Paul
- 6 years ago
- Views:
Transcription
1 Cosmic rays: Discovery and early research Florence 29 November 2011 Per Carlson
2 Talk partly based on:
3 Layout A mysterious invisible radiation investigated Wulf and Gockel Domenico Pacini, a partly forgotten scientist August 7, 1912: Hess discovery and confirmation by Kohlhörster World War I , Nationalism Consensus 1926: radiation extraterrestrial The nature of the radiation: Bothe, Kolhörster, Rossi, Millikan and others The Nobel Prize to Hess 1936 Two decades of particle discoveries Antimatter and air showers, two lines of today s research Conclusion
4 Memoires de l Académie Royale 1785, p.612 Charles-Augustin de Coulomb
5 18th and 19th centuries 1785 Coulomb: Spontaneous discharge 1835 Faraday: Confirmes discharge 1879 Crookes: Discharge rate is reduced with reduced pressure. Ionized air the cause.
6 End of 19th century: A steady stream of milestone discoveries Thomson: The electron Röntgen: X-rays Becquerel: Spontaneous radioactivity Curie s: New radioactive elements Radiation produces ionization
7 1900: The scene was set for a more general research on electrical conductivity of air, to solve the puzzle of atmospheric ionization.
8 In action on penetrating radiation: Elster and Geitel, Wilson, Rutherford, Cooke, McLennan, Burton, Mache, Strong, Eve Improvements and experiments: Electroscope improvements Metal shields In tunnels, on sea
9 Wilson, Elster and Geitel Experiments on electroscopes gradually losing charge After experimenting with a gold leaf electroscope, Wilson concludes 1901 It is unlikely, therefore, that the ionization is due to radiation which has traversed our atmosphere; it seems, as Geitel concludes, a property of air itself It is assumed that the ionization is caused by the newly discovered X-rays or gamma-rays coming from outside the electroscope vessel
10 In action on penetrating radiation: Elster and Geitel, Wilson, Rutherford, Cooke, McLennan, Burton, Mache, Strong, Eve Improvements and experiments: Electroscope improvements Metal shields In tunnels, on sea General view 1908: The earth and radioactivity is the source of the radiation
11 Layout A mysterious invisible radiation investigated Wulf and Gockel Domenico Pacini, a partly forgotten scientist August 7, 1912: Hess discovery and confirmation by Kohlhörster World War I , Nationalism Consensus 1926: radiation extraterrestrial The nature of the radiation: Bothe, Kolhörster, Rossi, Millikan and others The Nobel Prize to Hess 1936 Two decades of particle discoveries Antimatter and air showers, two lines of today s research Conclusion
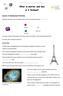
12 Theodore Wulf ( ), German scientist and a Jesuit priest, visits friends in Paris easter He brings his electroscope and climbs the Eiffel tower... Th. Wulf Phys. Zeitschr. 11, 811 (1910) (Phys. Inst. Des Ignat.-Koll., Valkenburg, Holland) Expected with an 80 m absorption length was a few percent of the radiation at ground. Results requires another source for the gamma-radiation or a significantly weaker absortion of gamma..or?
13 OR Is the radiation coming from the tower structure?
14 Albert Gockel First balloon flight 1910 Results inconclusive km Ionization
15 Layout A mysterious invisible radiation investigated Wulf and Gockel Domenico Pacini, a partly forgotten scientist August 7, 1912: Hess discovery and confirmation by Kohlhörster World War I , Nationalism Consensus 1926: radiation extraterrestrial The nature of the radiation: Bothe, Kolhörster, Rossi, Millikan and others The Nobel Prize to Hess 1936 Two decades of particle discoveries Antimatter and air showers, two lines of today s research Conclusion
16 Domenico Pacini Used by Pacini
17 June 1911 With an electroscope 3 m deep in the sea at Livorno and Bracciano Pacini finds a significant 20% decrease in the radiation. He concludes in the Nuovo Cimento article (translated from italian):..a sizable cause of ionization exists in the atmosphere, originating from penetrating radiation, independent of the direct action of radioactive substances in the soil Pacini made important contributions that were not fully appreciated More about Pacini: several articles and talks by Alessandro De Angelis
18 Hess and Eugster published Weltraumstrahlung und ihre biologische Wirkung in The translated edition shown here was published by Fordham University Press in 1949 and incorporated research carried out in the interim.
19 In a very complete 1909 review Kurz concludes that the known amounts of radioactive substances in the soil, in water and in air could fully account for the observed ionizations. Hess and Eugster writes about the contribution of Pacini: "The first who expressed some doubts as to the correctness of this view was D. Pacini, who, in 1910, from measurements over sea and on shores at Livorno concluded that part of the observed ionization might be due to sources other than the known radioactive substances." Pacini, who died in 1934, was never nominated for the Nobel Prize. Hess was first nominated in 1933 and received the prize in Why ignored?
20 Layout A mysterious invisible radiation investigated Wulf and Gockel Domenico Pacini, a partly forgotten scientist August 7, 1912: Hess discovery and confirmation by Kohlhörster World War I , Nationalism Consensus 1926: radiation extraterrestrial The nature of the radiation: Bothe, Kolhörster, Rossi, Millikan and others The Nobel Prize to Hess 1936 Two decades of particle discoveries Antimatter and air showers, two lines of today s research Conclusion
21 Hess 7 August th flight
22 Hess 7th flight 7 August 1912 Following Elbe in the Bohemian (Böhmen) countryside. Diplomarbeit Georg Federmann Institut für Radiumforschung und Kernphysik Wien, 2003
23
24 Data 7th flight Diplomarbeit Georg Federmann Institut für Radiumforschung und Kernphysik Wien, 2003
25 Ionization as function of altitude Hess 1912 Kolhörster
26 V.F. Hess Phys. Zeit. 13(1912)1804 Reported at a meeting in Münster, September 1912 The results of the present observations seem to be most readily explained by the assumption that a radiation of very high penetrating power enters our atmosphere from above, and still produces in the lowest layers a part of the ionization observed in closed vessels. (Transl. A.M Hillas, Cosmic Rays, Pergamon 1972)
27 In action on penetrating radiation: Pacini, Wulf, Gockel, Hess, Kohlhörster Improvements and experiments: Electroscope improvements On sea, in sea, on Eiffel tower, with balloons Common view : There is a penetrating radiation coming from outside the earth But: Not everybody believed an external source for the radiation
28 Layout A mysterious invisible radiation investigated Wulf and Gockel Domenico Pacini, a partly forgotten scientist August 7, 1912: Hess discovery and confirmation by Kohlhörster World War I , Nationalism Consensus 1926: radiation extraterrestrial The nature of the radiation: Bothe, Kolhörster, Rossi, Millikan and others The Nobel Prize to Hess 1936 Two decades of particle discoveries Antimatter and air showers, two lines of today s research Conclusion
29 A difficult time for Europe and for Science: World War I Also on 28th June 1914: Kolhörster measured the ionization at 9300 m!
30 World War I Scientific research almost stopped More nationalism Less communication
31 Percent Central Power Nobel Candidates Source: E Crawford
32 Layout A mysterious invisible radiation investigated Wulf and Gockel Domenico Pacini, a partly forgotten scientist August 7, 1912: Hess discovery and confirmation by Kohlhörster World War I , Nationalism Consensus 1926: radiation extraterrestrial The nature of the radiation: Bothe, Kolhörster, Rossi, Millikan and others The Nobel Prize to Hess 1936 Two decades of particle discoveries Antimatter and air showers, two lines of today s research Conclusion
33 Millikan and Cameron 1926 Experiments in two Californian lakes, Muir Lake (3600 m) and Arrowhead (1500 m) Millikan changed opinion 1926 and accepted the radiation as extraterrestrial... showing that the rays do definitely come from above Figure adapted from Hillas: Cosmic rays (1972)
34 The early 1920s Few measurements in Europe, focus moved to the US. Radiation was uniform, not depending on e.g. thunderstorms or on the time of the day. An extraterrestrial nature of the radiation was still questioned. Millikan (Nobel Prize 1923) at the 1924 APS: The whole of the penetrating radiation is of local origin. Compton was of another opinion. Millikan changed mind in 1926 and coined the name cosmic rays. He suggested that the penetrating gamma-rays were birth cries of atoms in our galaxy. Focus went to properties of the cosmic rays: Radiation or particles? Source?
35 Phys. Zeit. 29(1928) : Millikan vs. Austria/Germany
36 Layout A mysterious invisible radiation investigated Wulf and Gockel Domenico Pacini, a partly forgotten scientist August 7, 1912: Hess discovery and confirmation by Kohlhörster World War I , Nationalism Consensus 1926: radiation extraterrestrial The nature of the radiation: Bothe, Kolhörster, Rossi, Millikan and others The Nobel Prize to Hess 1936 Two decades of particle discoveries Antimatter and air showers, two lines of today s research Conclusion
37 Uncovering the nature of the radiation Clay 1927, 1928: Ionization increased with latitude Clay s work disputed by Millikan Bothe, Kolhörster 1929: Particles, confirmed by Rossi Compton 1932: Detailed latitude survey showed that cosmic rays were charged particles. Millikan attacked Compton. Millikan 1933: Admitted that there was a latitude effect 1933: Three independent experiments, Alvarez and Compton, Johnson and Rossi, showed a significant east-west effect, cosmic ray particles are positive Schein 1941: Cosmic rays mostly protons
38 1932 What are the cosmic rays?
39 The Geiger-Müller tube H. Geiger and W. Müller 1928 A break-through i cosmic ray research!
40 The Bothe Kolhörster experiment 1929 Starting point for Rossi Compton collisions: the only known process of γ-radiation interactions. If Compton e - caused the coincidences, a small absorber should stop them. However 4.1 cm gold only reduced the rate by 24%. The radiation is corpuscular! Conclusion not accepted by Millikan Coincidence resolution by photographic recording about 0.01 s. Lead very old, thus not very radioactive! W. Bothe and W. Kolhörster Z. Physik 56(1929)751, Die Naturwiss. 17(1929)271
41 Rossi: For me, the paper by Bothe and Kolhörster opened a window upon a new, unknown territory, with unlimited opportunities for exploration. B. Rossi: ARCETRI, , in Early History of Cosmic Ray Studies, Ed. Y. Sekido and H. Elliot, (Dreidel Publ. Comp., Dordrecht 1985) Bruno Rossi
42 Bruno Rossi : Florence : Padua 1938: Expelled from the university because of racial laws. Copenhagen, Manchester : With Compton in Chicago : Cornell : Los Alamos, Manhattan project : MIT, institute professor : Offered a chair in any Italian university, chose Palermo 1993: Died at his home in Cambridge, MA
43 On the steps of the laboratory at Arcetri: Left to right: Rossi, Occhialini, Bernadini and Bocciarelli B. Rossi: ARCETRI, , in Early History of Cosmic Ray Studies, Ed. Y. Sekido and H. Elliot, (Dreidel Publ. Comp., Dordrecht 1985)
44 Rossi Physics Today October 1981
45 45 B. Rossi: ARCETRI, , in Early History of Cosmic Ray Studies, Ed. Y. Sekido and H. Elliot, (Dreidel Publ. Comp., Dordrecht 1985)
46 On Rossis right several counters. Batteries were used to provide the high voltage for the counters. B. Rossi: ARCETRI, , in Early History of Cosmic Ray Studies, Ed. Y. Sekido and H. Elliot, (Dreidel Publ. Comp., Dordrecht 1985)
47 Rossi invented the coincidence circuit: Method of Registering Multiple Simultaneous Impulses of Several Geiger Counters, Nature 125,636(1930) 47
48 Rossi proposes the East-West experiments during a visit to Bothe s Berlin laboratory, summer 1930 B. Rossi: Phys. Rev. 36, 606(1930)
49 A cosmic ray telescope consisting of two coincidence counters mounted on pivots so measurements could be made in any direction desired.
50 EAST-WEST EXPERIMENT SETUP, Eritrea 1933
51 The East-West effect was established 1933 Alvarez and Compton Johnson Rossi Particles are positive but Positrons? Protons? Nuclei?
52 Rossi discovered air showers when estimating random coincidences! The frequency of the coincidences recorded with the counters at a distance from one another, shown in the tables as chance coincidences appears to be greater than would have been predicted on the basis of the resolving power of the coincidence circuit. Those observations made us question whether all of these coincidences were actually chance coincidences. This hypothesis appears to be supported by the following observation... Since the interference of possible disturbances was ruled out by suitable tests, it seems that once in a while the recording equipment is struck by very extensive showers [degli sciami molto estesi di corpuscoli] of particles, which cause coincidences between counters, even placed at large distances from one another. Unfortunately, I did not have time to study this phenomenon more closely. B. Rossi: ARCETRI, , in Early History of Cosmic Ray Studies, Ed. Y. Sekido and H. Elliot, (Dreidel Publ. Comp., Dordrecht 1985)
53 The Rome conference October At the invitation of Fermi, I gave an introductory speech on the problem of cosmic rays. The main thrust of this talk was to present what, to my mind, were irrefutable arguments against Millikan s theory of the birth cry of atoms. Such a brash behavior on the part of a mere youngster (I was then 26 years old) clearly did not please Millikan, who for a number of years thereafter, chose to ignore my work altogether. Rossi Physics Today October 1981
54 Rome conference 1931
55 Bruno Rossi, Robert A. Millikan, and Arthur H. Compton at the Rome conference, October 11 18, The Rome conference 1931 marked the beginning of the historical debate about the nature of cosmic rays. B. Rossi: ARCETRI, , in Early History of Cosmic Ray Studies, Ed. Y. Sekido and H. Elliot, (Dreidel Publ. Comp., Dordrecht 1985)
56 Two components in the cosmic rays: a soft and a hard Using a triple coincidence, Rossi showed that about 50% of particles that traversed 10 cm lead also traversed 1 meter! 50 cm Pb B. Rossi Zeit. f. Physik 82(1933)151 L. Bonolis Phys. Perspect. 13(2011)58 From Rossi s notebook
57 Bruno Rossi remebering 1932: In the late fall of 1932, having won a national competition for a chair in experimental physics in the Italian Universities, I was called to fill a vacancy at the University of Padua. I left Arcetri with a heavy heart. I was still young, and I knew that there would be, in my life, other periods of interesting and rewarding work. I also knew, however, that none would have the very special flavor of my years in the Florentine hills. B. Rossi: ARCETRI, , in Early History of Cosmic Ray Studies, Ed. Y. Sekido and H. Elliot, (Dreidel Publ. Comp., Dordrecht 1985)
58 Layout A mysterious invisible radiation investigated Wulf and Gockel Domenico Pacini, a partly forgotten scientist August 7, 1912: Hess discovery and confirmation by Kohlhörster World War I, Nationalism The nature of the radiation: Bothe, Kolhörster, Rossi, Millikan and others The Nobel Prize to Hess Two decades of particle discoveries Antimatter and air showers, two lines of today s research Conclusion
59 1936: The Nobel Prize to Hess
60 Nominations for the 1936 Nobel Prize in Physics The Royal Swedish Academy of Sciences had received a total of 22 Prize proposals from 31 nominators for 18 different Prize Candidates in Physics. Hess was nominated by J. Clay, Amsterdam, for a non-shared prize and by A.H. Compton, Chicago, for a prize shared with J. Clay, Amsterdam. Compton also nominated C.D. Anderson for the discovery of the positron. Hess had been nominated the first time in 1931 by Pohl, from Göttingen, and then in 1933 by Plotnikov, from Zagreb and in 1934 by Willstätter, from Munich. We note that Pacini was never nominated.
61 Arthur H. Compton Nobel Prize in Physics 1927 "for his discovery of the effect named after him"
62 The latitude effect at different altitudes 4360 m The latitude effect is greater for softer radiation. Latitude 2000 m Sea level Phys. Rev. 43(1933)387
63 Compton s (Nobel Prize 1927) nomination 1936 The time has now arrived, it seems to me, when we can say that the so-called cosmic rays definitely have their origin at such remote distances from the Earth that they may properly be called cosmic, and that the use of the rays has by now led to results of such importance that they may be considered a discovery of the first magnitude.... It is, I believe, correct to say that Hess was the first to establish the increase of the ionisation observed in electroscopes with increasing altitude; and he was certainly the first to ascribe with confidence this increased ionisation to radiation coming from outside the Earth.
64 Compton s nomination cont d Before it was appropriate to award the Nobel Prize for the discovery of these rays, it was necessary to await more positive evidence regarding their unique characteristics and importance in various fields of physics.this has now been accomplished. Studies of the magnetic latitude effect on cosmic rays have shown that they include electrical particles of much higher energy than are available from artificial sources, further that these rays come from a source which may be properly called cosmic. The usefulness of the rays has been demonstrated by the experiment which have revealed the existence of the positron.
65 Who can nominate? FAQ s
66 FAQ s Who can nominate? Physics Members of the Academy Nobel Laureates Nordic professors Chair holders in foreign universities Specially invited
67 FAQ s Who can nominate? Who selects the Nobel Prize Laureates?
68 Who selects the Nobel Prize Laureates? The Royal Swedish Academy of Sciences for the Nobel Prize in Physics and Chemistry. The Nobel Assembly at Karolinska Institutet for the Nobel Prize in Physiology or Medicine. The Swedish Academy for the Nobel Prize in Literature. A committee of five persons to be elected by the Norwegian Parliament (Storting) for the Nobel Peace Prize.
69 FAQ s Who can nominate? Who selects the Nobel Prize Laureates? How many candidates are nominated?
70 FAQ s Who can nominate? Who selects the Nobel Prize Laureates? How many candidates are nominated? Nominate for a posthumous Nobel Prize?
71 10 December 1936, Stockholm
72 Layout A mysterious invisible radiation investigated Wulf and Gockel Domenico Pacini, a partly forgotten scientist August 7, 1912: Hess discovery and confirmation by Kohlhörster World War I , Nationalism Consensus 1926: radiation extraterrestrial The nature of the radiation: Bothe, Kolhörster, Rossi, Millikan and others The Nobel Prize to Hess 1936 Two decades of particle discoveries Antimatter and air showers, two lines of today s research Conclusion
73 1932: The positron discovered in a cloud chamber photo by C. Anderson Mass determined from ionization and range
74 A V 0 particle 1 cm Pb plate Decay 0.5 cm below plate. Incident about 2 GeV/c. Armenteros et al. Nature 167, 501(1951)
75 Nuclear emulsions 1940s π + µ + + ν µ µ+ 0.6 mm e +
76 K + K + π + + π + + π - The first observation of the decay of a kaon into 3 pions, recorded in special photographic emulsion by Cecil Powell's team at Bristol University in 1948.
77
78 Particle discoveries with emulsion and cloud chamber Particle π + and π - π 0 Instrument Nuclear Emulsion Counters and Emulsion Λ K + and K - K 0 Cloud Chamber Nuclear Emulsion Cloud Chamber Σ + Σ - Σ 0 Ξ - Ξ 0 Nuclear Emulsion Cloud Chamber Bubble chamber Cloud Chamber Bubble Chamber Anti Λ 0 Nuclear Emulsion
79 The International Cosmic Ray Conference 1953 marks the birth of subatomic physics and a change of focus for cosmic ray physics. Cronin: EPJ-H 2011
80 Layout A mysterious invisible radiation investigated Wulf and Gockel Domenico Pacini, a partly forgotten scientist August 7, 1912: Hess discovery and confirmation by Kohlhörster World War I , Nationalism Consensus 1926: radiation extraterrestrial The nature of the radiation: Bothe, Kolhörster, Rossi, Millikan and others The Nobel Prize to Hess 1936 Two decades of particle discoveries Antimatter and air showers, two lines of today s research Conclusion
81 Pamela coll. O. Adriani et al. Nature 2 April 2009
82
83 The GZK cut-off confirmed Pierre Auger Observatory Coll. ICRC2011, F. Salmida et al. arxiv
84 Conclusions The discovery of cosmic rays came after detailed studies using electroscopes on land at sea level, on sea, in sea and at high altitudes Scientists in Europe and North America participated in the work characterized by lack of communication and by nationalism caused primarily by World War I. The nature of the radiation was established in steps: first particles, then positive particles, then primarily protons The physics of elementary particles started with discoveries of many new particles using cosmic rays Today cosmic ray physics is a well estabished lively field of research.
85 Thanks for your attention! Thanks to Alessandro de Angelis Alan Watson The Center for History of Science, Royal Swedish Academy of Sciences Florence 29 November 2011 Per Carlson
The Discovery of Cosmic Rays
 The Discovery of Cosmic Rays Centenary Symposium 2012 Denver 27 June 2012 P. Carlson, Stockholm Pre 1900 Discharge, ionization, fundamental discoveries in physics 1900-1910 A mysterious penetrating radiation
The Discovery of Cosmic Rays Centenary Symposium 2012 Denver 27 June 2012 P. Carlson, Stockholm Pre 1900 Discharge, ionization, fundamental discoveries in physics 1900-1910 A mysterious penetrating radiation
Welcome the Centenary!
 Welcome the Centenary! P. Király, Wigner Research Centre for Physics, Budapest In fact I shall cover two centenaries: Discovery of cosmic rays by Victor Hess, 7th August 1912. During this ECRS, we are
Welcome the Centenary! P. Király, Wigner Research Centre for Physics, Budapest In fact I shall cover two centenaries: Discovery of cosmic rays by Victor Hess, 7th August 1912. During this ECRS, we are
ISAPP Gran Sasso June 28-July 9, Observations of Cosmic Rays
 ISAPP 2004 Gran Sasso June 28-July 9, 2003 Observations of Cosmic Rays Tiina Suomijärvi Institut de Physique Nucléaire Université Paris XI-Orsay, IN2P3/CNRS France Why to Study Cosmic Rays? Cosmic rays
ISAPP 2004 Gran Sasso June 28-July 9, 2003 Observations of Cosmic Rays Tiina Suomijärvi Institut de Physique Nucléaire Université Paris XI-Orsay, IN2P3/CNRS France Why to Study Cosmic Rays? Cosmic rays
The Physics of Cosmic Rays
 The Physics of Cosmic Rays QuarkNet summer workshop July 23-27, 2012 1 Recent History Most natural phenomena can be explained by a small number of simple rules. You can determine what these rules are by
The Physics of Cosmic Rays QuarkNet summer workshop July 23-27, 2012 1 Recent History Most natural phenomena can be explained by a small number of simple rules. You can determine what these rules are by
Cosmic Rays. This showed that the energy of cosmic rays was many times that of any other natural or artificial radiation known at that time.
 Cosmic Rays 1. Discovery As long ago as 1900, C. T. R. Wilson and others found that the charge on an electroscope always 'leaked' away in time, and this could never be prevented, no matter how good the
Cosmic Rays 1. Discovery As long ago as 1900, C. T. R. Wilson and others found that the charge on an electroscope always 'leaked' away in time, and this could never be prevented, no matter how good the
Cosmic Rays I. Cosmic rays continually bombard the Earth. In fact, about cosmic rays pass through a person every hour! Astroparticle Course 1
 Cosmic Rays I Cosmic rays continually bombard the Earth. In fact, about 100 000 cosmic rays pass through a person every hour! Astroparticle Course 1 Cosmic Rays I Cosmic rays continually bombard the Earth.
Cosmic Rays I Cosmic rays continually bombard the Earth. In fact, about 100 000 cosmic rays pass through a person every hour! Astroparticle Course 1 Cosmic Rays I Cosmic rays continually bombard the Earth.
The production and properties of positrons
 C ARL D. AN D E R S O N The production and properties of positrons Nobel Lecture, December 12, 1936 Information of fundamental importance to the general problem of atomic structure has resulted from systematic
C ARL D. AN D E R S O N The production and properties of positrons Nobel Lecture, December 12, 1936 Information of fundamental importance to the general problem of atomic structure has resulted from systematic
Lecture 3. lecture slides are at:
 Lecture 3 lecture slides are at: http://www.physics.smu.edu/ryszard/5380fa16/ Proton mass m p = 938.28 MeV/c 2 Electron mass m e = 0.511 MeV/c 2 Neutron mass m n = 939.56 MeV/c 2 Helium nucleus α: 2 protons+2
Lecture 3 lecture slides are at: http://www.physics.smu.edu/ryszard/5380fa16/ Proton mass m p = 938.28 MeV/c 2 Electron mass m e = 0.511 MeV/c 2 Neutron mass m n = 939.56 MeV/c 2 Helium nucleus α: 2 protons+2
arxiv: v1 [physics.hist-ph] 31 Aug 2012
![arxiv: v1 [physics.hist-ph] 31 Aug 2012 arxiv: v1 [physics.hist-ph] 31 Aug 2012](/thumbs/85/93020488.jpg) Atmospheric ionization and cosmic rays: studies and measurements before 1912 Alessandro De Angelis a,b a INFN and Università di Udine, Via delle Scienze 206, I-33100 Udine, Italy b LIP/IST Lisboa, Portugal
Atmospheric ionization and cosmic rays: studies and measurements before 1912 Alessandro De Angelis a,b a INFN and Università di Udine, Via delle Scienze 206, I-33100 Udine, Italy b LIP/IST Lisboa, Portugal
Cosmic rays and the development of the physics of elementary particles and fundamental interactions
 Chapter 3 Cosmic rays and the development of the physics of elementary particles and fundamental interactions By 1785 Coulomb found that electroscopes can discharge spontaneously, and not because of defective
Chapter 3 Cosmic rays and the development of the physics of elementary particles and fundamental interactions By 1785 Coulomb found that electroscopes can discharge spontaneously, and not because of defective
Lecture 14 Cosmic Rays
 Lecture 14 Cosmic Rays 1. Introduction and history 2. Locally observed properties 3. Interactions 4. Demodulation and ionization rate 5. Midplane interstellar pressure General Reference MS Longair, High
Lecture 14 Cosmic Rays 1. Introduction and history 2. Locally observed properties 3. Interactions 4. Demodulation and ionization rate 5. Midplane interstellar pressure General Reference MS Longair, High
6. Atomic and Nuclear Physics
 6. Atomic and Nuclear Physics Chapter 6.2 Radioactivity From IB OCC, prepared by J. Domingues based on Tsokos Physics book Warm Up Define: nucleon atomic number mass number isotope. Radioactivity In 1896,
6. Atomic and Nuclear Physics Chapter 6.2 Radioactivity From IB OCC, prepared by J. Domingues based on Tsokos Physics book Warm Up Define: nucleon atomic number mass number isotope. Radioactivity In 1896,
Early Cosmic Ray Research In France
 Author manuscript, published in "CENTENARY SYMPOSIUM 2012: DISCOVERY OF COSMIC RAYS, Denver : United States (2012)" DOI : 10.1000/ISBN 978-0-7354-1137-1 Early Cosmic Ray Research In France Olivier Ravel
Author manuscript, published in "CENTENARY SYMPOSIUM 2012: DISCOVERY OF COSMIC RAYS, Denver : United States (2012)" DOI : 10.1000/ISBN 978-0-7354-1137-1 Early Cosmic Ray Research In France Olivier Ravel
Populating nucleon states. From the Last Time. Other(less stable) helium isotopes. Radioactivity. Radioactive nuclei. Stability of nuclei.
 Nucleus: From the Last Time System of and neutrons bound by the strong force Proton number determines the element. Different isotopes have different # neutrons. Stable isotopes generally have similar number
Nucleus: From the Last Time System of and neutrons bound by the strong force Proton number determines the element. Different isotopes have different # neutrons. Stable isotopes generally have similar number
Cosmic Rays: A Way to Introduce Modern Physics Concepts. Steve Schnetzer
 Cosmic Rays: A Way to Introduce Modern Physics Concepts Steve Schnetzer Rutgers CR Workshop May 19, 2007 Concepts Astrophysics Particle Physics Radiation Relativity (time dilation) Solar Physics Particle
Cosmic Rays: A Way to Introduce Modern Physics Concepts Steve Schnetzer Rutgers CR Workshop May 19, 2007 Concepts Astrophysics Particle Physics Radiation Relativity (time dilation) Solar Physics Particle
Galactic cosmic rays
 29 FRANK B. MCDONALD* AND VLADIMIR S. PTUSKIN** Galactic cosmic rays Our Galaxy is filled with a relativistic gas of high-energy protons, electrons, and heavy nuclei. The interstellar energy density of
29 FRANK B. MCDONALD* AND VLADIMIR S. PTUSKIN** Galactic cosmic rays Our Galaxy is filled with a relativistic gas of high-energy protons, electrons, and heavy nuclei. The interstellar energy density of
Cosmic Rays. Discovered in 1912 by Viktor Hess using electroscopes to measure ionization at altitudes via balloon
 Cosmic Rays Discovered in 1912 by Viktor Hess using electroscopes to measure ionization at altitudes via balloon Nobel Prize in 1936 Origin of high energy cosmic rays is still not completely understood
Cosmic Rays Discovered in 1912 by Viktor Hess using electroscopes to measure ionization at altitudes via balloon Nobel Prize in 1936 Origin of high energy cosmic rays is still not completely understood
What detectors measure
 What detectors measure As a particle goes through matter, it releases energy Detectors collect the released energy and convert it to electric signals recorded by DAQ Raw event record is a collection of
What detectors measure As a particle goes through matter, it releases energy Detectors collect the released energy and convert it to electric signals recorded by DAQ Raw event record is a collection of
The discovery of the antiproton and other antimatter,
 4 Antibaryons The discovery of the antiproton and other antimatter, 1955 1959 While the existence of antiparticles was established with Anderson s discovery of the positron in 1932, it was not clear in
4 Antibaryons The discovery of the antiproton and other antimatter, 1955 1959 While the existence of antiparticles was established with Anderson s discovery of the positron in 1932, it was not clear in
Physics for Poets Lecture 2
 Physics for Poets Lecture 2 Gaurang Yodh Development of quantum theory (and relativity) was in response to Experimental Observations that defied explanation in terms of Classical Physics (Newton's Laws
Physics for Poets Lecture 2 Gaurang Yodh Development of quantum theory (and relativity) was in response to Experimental Observations that defied explanation in terms of Classical Physics (Newton's Laws
Introduction to cosmic rays
 Introduction to cosmic rays 1 COSMIC RAYS: Messages from exploding stars and even more powerful objects What are cosmic rays? How were they discovered? How do we detect them? What can we learn from them?
Introduction to cosmic rays 1 COSMIC RAYS: Messages from exploding stars and even more powerful objects What are cosmic rays? How were they discovered? How do we detect them? What can we learn from them?
Upper Limit of the Spectrum of Cosmic Rays
 Upper Limit of the Spectrum of Cosmic Rays David Fraebel, Cristian Gaidau, Allycia Gariepy, Rita Garrido Menacho Friday 22.11.2013 G.T. Zatsepin and V.A. Kuzmin 1966, JETP Let. 4, p.78 Outline Motivation
Upper Limit of the Spectrum of Cosmic Rays David Fraebel, Cristian Gaidau, Allycia Gariepy, Rita Garrido Menacho Friday 22.11.2013 G.T. Zatsepin and V.A. Kuzmin 1966, JETP Let. 4, p.78 Outline Motivation
Lecture 3. lecture slides are at:
 Lecture 3 lecture slides are at: http://www.physics.smu.edu/ryszard/5380fa17/ Proton mass m p = 938.28 MeV/c 2 Electron mass m e = 0.511 MeV/c 2 Neutron mass m n = 939.56 MeV/c 2 Helium nucleus α: 2 protons+2
Lecture 3 lecture slides are at: http://www.physics.smu.edu/ryszard/5380fa17/ Proton mass m p = 938.28 MeV/c 2 Electron mass m e = 0.511 MeV/c 2 Neutron mass m n = 939.56 MeV/c 2 Helium nucleus α: 2 protons+2
Isotopes of an element have the same symbol and same atomic number - Mass number refers to the protons plus neutrons in an isotope
 7.1 Atomic Theory and Radioactive Decay Natural background radiation exists all around us. This radiation consists of high energy particles or waves being emitted from a variety of materials Radioactivity
7.1 Atomic Theory and Radioactive Decay Natural background radiation exists all around us. This radiation consists of high energy particles or waves being emitted from a variety of materials Radioactivity
energy loss Ionization + excitation of atomic energy levels Mean energy loss rate de /dx proportional to (electric charge) 2 of incident particle
 Lecture 4 Particle physics processes - particles are small, light, energetic à processes described by quantum mechanics and relativity à processes are probabilistic, i.e., we cannot know the outcome of
Lecture 4 Particle physics processes - particles are small, light, energetic à processes described by quantum mechanics and relativity à processes are probabilistic, i.e., we cannot know the outcome of
The Atomic Nature of Matter
 Chapter 11 The Atomic Nature of Matter I. The Atomic Hypothesis All matter is composed of atoms. Atoms are composed of a positively charged nucleus (containing protons and neutrons) and negatively charged
Chapter 11 The Atomic Nature of Matter I. The Atomic Hypothesis All matter is composed of atoms. Atoms are composed of a positively charged nucleus (containing protons and neutrons) and negatively charged
What happens during nuclear decay? During nuclear decay, atoms of one element can change into atoms of a different element altogether.
 When Henri Becquerel placed uranium salts on a photographic plate and then developed the plate, he found a foggy image. The image was caused by rays that had not been observed before. For his discovery
When Henri Becquerel placed uranium salts on a photographic plate and then developed the plate, he found a foggy image. The image was caused by rays that had not been observed before. For his discovery
Introduction to Experimental Particle Physics : Lecture 1
 Introduction to Experimental Particle Physics : Lecture 1 Graz, September 2012 John Swain, Northeastern University, Boston Very, very, very important... We re just starting!...whole field is about 100
Introduction to Experimental Particle Physics : Lecture 1 Graz, September 2012 John Swain, Northeastern University, Boston Very, very, very important... We re just starting!...whole field is about 100
10.1 RADIOACTIVE DECAY
 10.1 RADIOACTIVE DECAY When Henri Becquerel placed uranium salts on a photographic plate and then developed the plate, he found a foggy image. The image was caused by rays that had not been observed before.
10.1 RADIOACTIVE DECAY When Henri Becquerel placed uranium salts on a photographic plate and then developed the plate, he found a foggy image. The image was caused by rays that had not been observed before.
EXPERIMENT 11: NUCLEAR RADIATION
 Introduction: radioactive nuclei. third is electromagnetic radiation. EXPERIMENT 11: NUCLEAR RADIATION In this lab, you will be investigating three types of emissions from Two types of these emissions
Introduction: radioactive nuclei. third is electromagnetic radiation. EXPERIMENT 11: NUCLEAR RADIATION In this lab, you will be investigating three types of emissions from Two types of these emissions
Alpha decay usually occurs in heavy nuclei such as uranium or plutonium, and therefore is a major part of the radioactive fallout from a nuclear
 Radioactive Decay Radioactivity is the spontaneous disintegration of atomic nuclei. This phenomenon was first reported in 1896 by the French physicist Henri Becquerel. Marie Curie and her husband Pierre
Radioactive Decay Radioactivity is the spontaneous disintegration of atomic nuclei. This phenomenon was first reported in 1896 by the French physicist Henri Becquerel. Marie Curie and her husband Pierre
Radioactivity. The Nobel Prize in Physics 1903 for their work on radioactivity. Henri Becquerel Pierre Curie Marie Curie
 Radioactivity Toward the end of the 19 th century, minerals were found that would darken a photographic plate even in the absence of light. This phenomenon is now called radioactivity. Marie and Pierre
Radioactivity Toward the end of the 19 th century, minerals were found that would darken a photographic plate even in the absence of light. This phenomenon is now called radioactivity. Marie and Pierre
The Development of Particle Physics. Dr. Vitaly Kudryavtsev E45, Tel.:
 The Development of Particle Physics Dr. Vitaly Kudryavtsev E45, Tel.: 0114 2224531 v.kudryavtsev@sheffield.ac.uk Discovery of the muon and the pion Energy losses of charged particles. This is an important
The Development of Particle Physics Dr. Vitaly Kudryavtsev E45, Tel.: 0114 2224531 v.kudryavtsev@sheffield.ac.uk Discovery of the muon and the pion Energy losses of charged particles. This is an important
What is Radiation? Física da Radiação MEBiom 2016/2017 Patrícia Gonçalves. Supporting Slides and images
 What is Radiation? Física da Radiação MEBiom 2016/2017 Patrícia Gonçalves Supporting Slides and images Radiation? Radiation is energy that comes from a source and travels through some material or through
What is Radiation? Física da Radiação MEBiom 2016/2017 Patrícia Gonçalves Supporting Slides and images Radiation? Radiation is energy that comes from a source and travels through some material or through
Two centenaries: The discovery of cosmic rays and the birth of Lajos Jánossy
 Journal of Physics: Conference Series Two centenaries: The discovery of cosmic rays and the birth of Lajos Jánossy To cite this article: Péter Király 2013 J. Phys.: Conf. Ser. 409 012001 View the article
Journal of Physics: Conference Series Two centenaries: The discovery of cosmic rays and the birth of Lajos Jánossy To cite this article: Péter Király 2013 J. Phys.: Conf. Ser. 409 012001 View the article
The neutron and its properties
 JA M E S C H A D W I C K The neutron and its properties Nobel Lecture, December 12, 1935 The idea that there might exist small particles with no electrical charge has been put forward several times. Nernst,
JA M E S C H A D W I C K The neutron and its properties Nobel Lecture, December 12, 1935 The idea that there might exist small particles with no electrical charge has been put forward several times. Nernst,
CLASS 29. THE NUCLEUS IS MADE OF PROTONS AND NEUTRONS
 CLASS 29. THE NUCLEUS IS MADE OF PROTONS AND NEUTRONS 29.1. INTRODUCTION Nuclear physics is the subfield of physics that studies the building blocks of the nucleus and how those building blocks are put
CLASS 29. THE NUCLEUS IS MADE OF PROTONS AND NEUTRONS 29.1. INTRODUCTION Nuclear physics is the subfield of physics that studies the building blocks of the nucleus and how those building blocks are put
CHEMISTRY. Matter and Change. Table Of Contents. Section 4.1 Early Ideas About Matter. Unstable Nuclei and Radioactive Decay
 CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
Discovery of antiproton
 Discovery of antiproton Antonia Mos I chose the article Observation of Antiprotons because, during the course, I was very intersted in the lectures about the first hadron s discoveries, and particularly
Discovery of antiproton Antonia Mos I chose the article Observation of Antiprotons because, during the course, I was very intersted in the lectures about the first hadron s discoveries, and particularly
STUDY OF EXTENSIVE AIR SHOWERS IN THE EARTH S ATMOSPHERE
 STUDY OF EXTENSIVE AIR SHOWERS IN THE EARTH S ATMOSPHERE I. BACIOIU * Institute of Space Science, P.O. Box MG-23, RO-077125 Bucharest-Magurele, Romania, E-mail: iuliana.bacioiu@spacescience.ro Abstract.
STUDY OF EXTENSIVE AIR SHOWERS IN THE EARTH S ATMOSPHERE I. BACIOIU * Institute of Space Science, P.O. Box MG-23, RO-077125 Bucharest-Magurele, Romania, E-mail: iuliana.bacioiu@spacescience.ro Abstract.
Cosmic Rays - R. A. Mewaldt - California Institute of Technology
 Cosmic Rays - R. A. Mewaldt - California Institute of Technology Cosmic rays are high energy charged particles, originating in outer space, that travel at nearly the speed of light and strike the Earth
Cosmic Rays - R. A. Mewaldt - California Institute of Technology Cosmic rays are high energy charged particles, originating in outer space, that travel at nearly the speed of light and strike the Earth
Cosmic Rays in the earth s atmosphere. Ilya Usoskin Sodankylä Geophysical Observatory ReSoLVE Center of Excellence, University of Oulu, Finland
 1 Cosmic Rays in the earth s atmosphere Ilya Usoskin Sodankylä Geophysical Observatory ReSoLVE Center of Excellence, University of Oulu, Finland Outline 2 Atmosphere Cosmic-ray induced atmospheric cascade
1 Cosmic Rays in the earth s atmosphere Ilya Usoskin Sodankylä Geophysical Observatory ReSoLVE Center of Excellence, University of Oulu, Finland Outline 2 Atmosphere Cosmic-ray induced atmospheric cascade
Cosmic Rays Detector. Use of Coincidence Detector for Measures of Cosmic Rays. Lodovico Lappetito. RivelatoreRaggiCosmici_ENG - 6/22/2015 Page 1
 Cosmic Rays Detector Use of Coincidence Detector for Measures of Cosmic Rays Lodovico Lappetito RivelatoreRaggiCosmici_ENG - 6/22/2015 Page 1 Table of Contents Design and Components... 3 Detector Design...
Cosmic Rays Detector Use of Coincidence Detector for Measures of Cosmic Rays Lodovico Lappetito RivelatoreRaggiCosmici_ENG - 6/22/2015 Page 1 Table of Contents Design and Components... 3 Detector Design...
Chapter 44. Nuclear Structure
 Chapter 44 Nuclear Structure Milestones in the Development of Nuclear Physics 1896: the birth of nuclear physics Becquerel discovered radioactivity in uranium compounds Rutherford showed the radiation
Chapter 44 Nuclear Structure Milestones in the Development of Nuclear Physics 1896: the birth of nuclear physics Becquerel discovered radioactivity in uranium compounds Rutherford showed the radiation
4.1 Structure of the Atom
 4.1 Structure of the Atom How do atoms differ from each other? What are atoms composed of? What are the subatomic particles? 2-1 Structure of the Atom Atoms actually are divisible. They are composed of
4.1 Structure of the Atom How do atoms differ from each other? What are atoms composed of? What are the subatomic particles? 2-1 Structure of the Atom Atoms actually are divisible. They are composed of
The Physics of Particle Detectors
 The Physics of Particle Detectors Lecture Notes WS 2010/11 Erika Garutti (DESY) Heinz Graafsma (XFEL) 1 On tools and instrumentation New directions in science are launched by new tools much more often
The Physics of Particle Detectors Lecture Notes WS 2010/11 Erika Garutti (DESY) Heinz Graafsma (XFEL) 1 On tools and instrumentation New directions in science are launched by new tools much more often
DIETRICH MÜLLER University of Chicago SLAC SUMMER INSTITUTE 2011
 SEARCHES FOR ANTIMATTER DIETRICH MÜLLER University of Chicago SLAC SUMMER INSTITUTE 2011 OUTLINE Early History Baryon Asymmetry of the Universe? Current Limits on Antimatter Nuclei from Distant Galaxies
SEARCHES FOR ANTIMATTER DIETRICH MÜLLER University of Chicago SLAC SUMMER INSTITUTE 2011 OUTLINE Early History Baryon Asymmetry of the Universe? Current Limits on Antimatter Nuclei from Distant Galaxies
Chapter 3. Radioactivity. Table of Contents
 Radioactivity Table of Contents Introduction 1. Radioactivity 2. Types of Radioactive Decays 3. Natural Radioactivity 4. Artificial Radioactivity 5. The Rate of Radioactive Decay 6. The Effects of Radiation
Radioactivity Table of Contents Introduction 1. Radioactivity 2. Types of Radioactive Decays 3. Natural Radioactivity 4. Artificial Radioactivity 5. The Rate of Radioactive Decay 6. The Effects of Radiation
Glencoe: Chapter 4. The Structure of the Atom
 Glencoe: Chapter 4 The Structure of the Atom Section One: Early Ideas about Matter Atomists and Democritus : 400 B.C. From Thrace in Greece. Atoms- Uncut-Table Indivisible parts which cannot be broken
Glencoe: Chapter 4 The Structure of the Atom Section One: Early Ideas about Matter Atomists and Democritus : 400 B.C. From Thrace in Greece. Atoms- Uncut-Table Indivisible parts which cannot be broken
What does rate of reaction mean?
 1 of 39 What does rate of reaction mean? 2 of 39 The speed of different chemical reactions varies hugely. Some reactions are very fast and others are very slow. The speed of a reaction is called the rate
1 of 39 What does rate of reaction mean? 2 of 39 The speed of different chemical reactions varies hugely. Some reactions are very fast and others are very slow. The speed of a reaction is called the rate
Ch05. Radiation. Energy and matter that comes from the nucleus of an atom. version 1.6
 Ch05 Radiation Energy and matter that comes from the nucleus of an atom. version 1.6 Nick DeMello, PhD. 2007-2016 Ch05 Radiation The Discovery of Radioactivity Phosphorescence Radioactive history Antoine
Ch05 Radiation Energy and matter that comes from the nucleus of an atom. version 1.6 Nick DeMello, PhD. 2007-2016 Ch05 Radiation The Discovery of Radioactivity Phosphorescence Radioactive history Antoine
The Nucleus Came Next
 The Nucleus Came Next Ernest Rutherford The New Zealand born British chemist and physicist who became known as the father of nuclear physics. He discovered the atomic nucleus, and thereby pioneered the
The Nucleus Came Next Ernest Rutherford The New Zealand born British chemist and physicist who became known as the father of nuclear physics. He discovered the atomic nucleus, and thereby pioneered the
Lecture 32 April
 Lecture 32 April 08. 2016. Hydrogen Discharge Tube and Emission of Discrete Wavelengths Description of the discrete Hydrogen Emission Spectrum by the Balmer (1884) Rydberg Ritz formula (1908) Cathode Ray
Lecture 32 April 08. 2016. Hydrogen Discharge Tube and Emission of Discrete Wavelengths Description of the discrete Hydrogen Emission Spectrum by the Balmer (1884) Rydberg Ritz formula (1908) Cathode Ray
Chapter 2 From the Discovery of Radioactivity to the First Accelerator Experiments
 Chapter 2 From the Discovery of Radioactivity to the First Accelerator Experiments Michael Walter 2.1 Introduction The article reviews the historical phases of cosmic ray research from the very beginning
Chapter 2 From the Discovery of Radioactivity to the First Accelerator Experiments Michael Walter 2.1 Introduction The article reviews the historical phases of cosmic ray research from the very beginning
SiPM & Plastic Scintillator
 SiPM & Plastic Scintillator Silicon photomultiplier coupled to plastic scintillator Lodovico Lappetito SiPM_PlasticScint_ENG - 28/04/2016 Pag. 1 Table of contents Introduction... 3 Plastic Scintillators...
SiPM & Plastic Scintillator Silicon photomultiplier coupled to plastic scintillator Lodovico Lappetito SiPM_PlasticScint_ENG - 28/04/2016 Pag. 1 Table of contents Introduction... 3 Plastic Scintillators...
Phys 328 Nuclear Physics and Particles Introduction Murat
 Phys 328 Nuclear Physics and Particles Introduction 1 Syllabus COURSE OUTLINE PHYS328 NUCLEAR PHYSICS AND PARTICLES Instructor: Prof. Dr. A. Murat Güler Room: 333, e-mail: mguler@newton.physics.metu.edu.tr
Phys 328 Nuclear Physics and Particles Introduction 1 Syllabus COURSE OUTLINE PHYS328 NUCLEAR PHYSICS AND PARTICLES Instructor: Prof. Dr. A. Murat Güler Room: 333, e-mail: mguler@newton.physics.metu.edu.tr
What is matter and how is it formed?
 What is matter and how is it formed? Lesson 6: Subatomic Particles Subatomic particles refers to particles that are more "fundamental" than... Are these fundamental particles or are they made up of smaller,
What is matter and how is it formed? Lesson 6: Subatomic Particles Subatomic particles refers to particles that are more "fundamental" than... Are these fundamental particles or are they made up of smaller,
Democritus of Abdera. John Dalton. Dalton s Atom. Dalton s Atomic Theory Ancient Greece - 4th century BC. Eaglesfield, England
 Democritus of Abdera Ancient Greece - 4th century BC first suggested the existence of tiny fundamental particles that make up matter. atoms = indestructible did not agree with the current sci theory -
Democritus of Abdera Ancient Greece - 4th century BC first suggested the existence of tiny fundamental particles that make up matter. atoms = indestructible did not agree with the current sci theory -
Review of Particle Properties: (http://pdg.lbl.gov/ ). See the review section on Cosmic Rays under Astrophysics and Cosmology.
 XI: Cosmic Rays I. References E. Fermi, Nuclear Physics, Notes compiled by Orear, Rosenfeld, and Schluter, Revised Edition, The University of Chicago Press, 195, chapter on Cosmic Rays, pp. 215-224. Review
XI: Cosmic Rays I. References E. Fermi, Nuclear Physics, Notes compiled by Orear, Rosenfeld, and Schluter, Revised Edition, The University of Chicago Press, 195, chapter on Cosmic Rays, pp. 215-224. Review
Radioactivity and Ionizing Radiation
 Radioactivity and Ionizing Radiation QuarkNet summer workshop June 24-28, 2013 1 Recent History Most natural phenomena can be explained by a small number of simple rules. You can determine what these rules
Radioactivity and Ionizing Radiation QuarkNet summer workshop June 24-28, 2013 1 Recent History Most natural phenomena can be explained by a small number of simple rules. You can determine what these rules
Evolution of Atomic Theory
 Evolution of Atomic Theory Mrs. Baldessari Chemistry Scientists that changed our view of the atom?greatest Chemistry Discoveries?? YouTube Aristotle What: All matter is a combo of fire, air, earth or water
Evolution of Atomic Theory Mrs. Baldessari Chemistry Scientists that changed our view of the atom?greatest Chemistry Discoveries?? YouTube Aristotle What: All matter is a combo of fire, air, earth or water
Chapter 3 Radioactivity
 Chapter 3 Radioactivity Marie Curie 1867 1934 Discovered new radioactive elements Shared Nobel Prize in physics in 1903 Nobel Prize in Chemistry in 1911 Radioactivity Radioactivity is the spontaneous emission
Chapter 3 Radioactivity Marie Curie 1867 1934 Discovered new radioactive elements Shared Nobel Prize in physics in 1903 Nobel Prize in Chemistry in 1911 Radioactivity Radioactivity is the spontaneous emission
Chapter 4 The Atom. Philosophers and scientists have proposed many ideas on the structure of atoms.
 Chapter4 TheAtom 4.1 Early Models of the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. Philosophers and scientists have proposed many ideas on the
Chapter4 TheAtom 4.1 Early Models of the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. Philosophers and scientists have proposed many ideas on the
8Subatomic particles. CHaPTer
 CHaPTer 8Subatomic particles remember Before beginning this chapter you should be able to: recall that all matter is made up of atoms explain the arrangement of particles in atoms, in particular that atoms
CHaPTer 8Subatomic particles remember Before beginning this chapter you should be able to: recall that all matter is made up of atoms explain the arrangement of particles in atoms, in particular that atoms
Physics 30 Modern Physics Unit: Atomic Basics
 Physics 30 Modern Physics Unit: Atomic Basics Models of the Atom The Greeks believed that if you kept dividing matter into smaller and smaller pieces, you would eventually come to a bit of matter that
Physics 30 Modern Physics Unit: Atomic Basics Models of the Atom The Greeks believed that if you kept dividing matter into smaller and smaller pieces, you would eventually come to a bit of matter that
Nuclear Physics and Astrophysics
 Nuclear Physics and Astrophysics PHY-30 Dr. E. Rizvi Lecture 4 - Detectors Binding Energy Nuclear mass MN less than sum of nucleon masses Shows nucleus is a bound (lower energy) state for this configuration
Nuclear Physics and Astrophysics PHY-30 Dr. E. Rizvi Lecture 4 - Detectors Binding Energy Nuclear mass MN less than sum of nucleon masses Shows nucleus is a bound (lower energy) state for this configuration
Radioattivita' naturale
 Radioattivita' naturale 1 2 Raggi Cosmici 3 Preamble Early 1900: the discharge of electroscopes due to ionization induced by natural radioactivity (Rutherford) Discovery In 1909 Theodor Wulf used an enhanced
Radioattivita' naturale 1 2 Raggi Cosmici 3 Preamble Early 1900: the discharge of electroscopes due to ionization induced by natural radioactivity (Rutherford) Discovery In 1909 Theodor Wulf used an enhanced
Chapter 30 Nuclear Physics and Radioactivity
 Chapter 30 Nuclear Physics and Radioactivity 30.1 Structure and Properties of the Nucleus Nucleus is made of protons and neutrons Proton has positive charge: Neutron is electrically neutral: 30.1 Structure
Chapter 30 Nuclear Physics and Radioactivity 30.1 Structure and Properties of the Nucleus Nucleus is made of protons and neutrons Proton has positive charge: Neutron is electrically neutral: 30.1 Structure
Leptons and Weak interactions
 PHY771, 8/28/2014 Tomasz Skwarnicki 1 Historical introduction to Elementary Particles: Leptons and Weak interactions Tomasz Skwarnicki Syracuse University Griffiths, 2 nd ed., 1.3-1.5,1.10 PHY771, 8/28/2014
PHY771, 8/28/2014 Tomasz Skwarnicki 1 Historical introduction to Elementary Particles: Leptons and Weak interactions Tomasz Skwarnicki Syracuse University Griffiths, 2 nd ed., 1.3-1.5,1.10 PHY771, 8/28/2014
Chapter One. The Old Quantum Theory. 1-1 Why Quantum Mechanics.
 Chapter One The Old Quantum Theory 1-1 Why Quantum Mechanics. The birth of quantum mechanics can be dated to 1925, when physicists such as Werner Heisenberg and Erwin Schrödinger invented mathematical
Chapter One The Old Quantum Theory 1-1 Why Quantum Mechanics. The birth of quantum mechanics can be dated to 1925, when physicists such as Werner Heisenberg and Erwin Schrödinger invented mathematical
Experiments on the interaction of high-speed nucleons with atomic nuclei
 J O H N D. C O C K C R O F T Experiments on the interaction of high-speed nucleons with atomic nuclei Nobel Lecture, December 11, 1951 The experimental researches in nuclear physics with which I have been
J O H N D. C O C K C R O F T Experiments on the interaction of high-speed nucleons with atomic nuclei Nobel Lecture, December 11, 1951 The experimental researches in nuclear physics with which I have been
Chapter 29. Nuclear Physics
 Chapter 29 Nuclear Physics Ernest Rutherford 1871 1937 Discovery that atoms could be broken apart Studied radioactivity Nobel prize in 1908 Some Properties of Nuclei All nuclei are composed of protons
Chapter 29 Nuclear Physics Ernest Rutherford 1871 1937 Discovery that atoms could be broken apart Studied radioactivity Nobel prize in 1908 Some Properties of Nuclei All nuclei are composed of protons
GLOSSARY OF BASIC RADIATION PROTECTION TERMINOLOGY
 GLOSSARY OF BASIC RADIATION PROTECTION TERMINOLOGY ABSORBED DOSE: The amount of energy absorbed, as a result of radiation passing through a material, per unit mass of material. Measured in rads (1 rad
GLOSSARY OF BASIC RADIATION PROTECTION TERMINOLOGY ABSORBED DOSE: The amount of energy absorbed, as a result of radiation passing through a material, per unit mass of material. Measured in rads (1 rad
Ch 17 Radioactivity & Nuc. Chemistry Study Guide Accelerated Chemistry SCANTRON
 Ch 17 Radioactivity & Nuc. Chemistry Study Guide Accelerated Chemistry SCANTRON Name No-Calculators Allowed /65 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
Ch 17 Radioactivity & Nuc. Chemistry Study Guide Accelerated Chemistry SCANTRON Name No-Calculators Allowed /65 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
RADIOACTIVITY MATERIALS: PURPOSE: LEARNING OBJECTIVES: DISCUSSION:
 RADIOACTIVITY This laboratory experiment was largely adapted from an experiment from the United States Naval Academy Chemistry Department MATERIALS: (total amounts per lab) small bottle of KCl; isogenerator
RADIOACTIVITY This laboratory experiment was largely adapted from an experiment from the United States Naval Academy Chemistry Department MATERIALS: (total amounts per lab) small bottle of KCl; isogenerator
The atomic theory explains
 The atomic theory explains radioactivity. Radioactive the photograph elements shown release here, energy radioactive as a result iron-59 of changes is used to in make their nuclei. an image In of the circulatory
The atomic theory explains radioactivity. Radioactive the photograph elements shown release here, energy radioactive as a result iron-59 of changes is used to in make their nuclei. an image In of the circulatory
Ionization Energy Loss of Charged Projectiles in Matter. Steve Ahlen Boston University
 Ionization Energy Loss of Charged Projectiles in Matter Steve Ahlen Boston University Almost all particle detection and measurement techniques in high energy physics are based on the energy deposited by
Ionization Energy Loss of Charged Projectiles in Matter Steve Ahlen Boston University Almost all particle detection and measurement techniques in high energy physics are based on the energy deposited by
DEVIL PHYSICS THE BADDEST CLASS ON CAMPUS IB PHYSICS
 DEVIL PHYSICS THE BADDEST CLASS ON CAMPUS IB PHYSICS TSOKOS LESSON 7-1B RADIOACTIVITY Essential Idea: In the microscopic world energy is discrete. Nature Of Science: Accidental discovery: Radioactivity
DEVIL PHYSICS THE BADDEST CLASS ON CAMPUS IB PHYSICS TSOKOS LESSON 7-1B RADIOACTIVITY Essential Idea: In the microscopic world energy is discrete. Nature Of Science: Accidental discovery: Radioactivity
Topic III Quest Study Guide
 Topic III Quest Study Guide A. Early Concepts: Democritus: Democritus: Greek Philosopher 400 B.C. Matter is composed of atoms, which move through empty space Atoms are solid, homogeneous indestructible
Topic III Quest Study Guide A. Early Concepts: Democritus: Democritus: Greek Philosopher 400 B.C. Matter is composed of atoms, which move through empty space Atoms are solid, homogeneous indestructible
High Energy Physics. QuarkNet summer workshop June 24-28, 2013
 High Energy Physics QuarkNet summer workshop June 24-28, 2013 1 The Birth of Particle Physics In 1896, Thompson showed that electrons were particles, not a fluid. In 1905, Einstein argued that photons
High Energy Physics QuarkNet summer workshop June 24-28, 2013 1 The Birth of Particle Physics In 1896, Thompson showed that electrons were particles, not a fluid. In 1905, Einstein argued that photons
Y2 Neutrino Physics (spring term 2017)
 Y2 Neutrino Physics (spring term 2017) Lecture 5 Discoveries of the leptons Dr E Goudzovski eg@hep.ph.bham.ac.uk http://epweb2.ph.bham.ac.uk/user/goudzovski/y2neutrino Previous lecture In 1940s, nuclear
Y2 Neutrino Physics (spring term 2017) Lecture 5 Discoveries of the leptons Dr E Goudzovski eg@hep.ph.bham.ac.uk http://epweb2.ph.bham.ac.uk/user/goudzovski/y2neutrino Previous lecture In 1940s, nuclear
Atomic Theory Timeline
 Atomic Theory Timeline Democritus 450 B.C. Democritus was a Greek philosopher who came to the conclusion that everything was made up of tiny particles. He used the term atomos. Unfortunately, since Democritus
Atomic Theory Timeline Democritus 450 B.C. Democritus was a Greek philosopher who came to the conclusion that everything was made up of tiny particles. He used the term atomos. Unfortunately, since Democritus
Radioactivity. General Physics II PHYS 111. King Saud University College of Applied Studies and Community Service Department of Natural Sciences
 King Saud University College of Applied Studies and Community Service Department of Natural Sciences Radioactivity General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa Outline Radioactive Decay
King Saud University College of Applied Studies and Community Service Department of Natural Sciences Radioactivity General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa Outline Radioactive Decay
Cosmic Rays, GammaRays and the Universal. Background Radiation
 Cosmic Rays, GammaRays and the Universal Background Radiation Malcolm Longair Cavendish Laboratory, Cambridge Celebrating Occhialini I met Occhialini only a few times, in connection with ESA projects and
Cosmic Rays, GammaRays and the Universal Background Radiation Malcolm Longair Cavendish Laboratory, Cambridge Celebrating Occhialini I met Occhialini only a few times, in connection with ESA projects and
Carl David Anderson ( )
 Carl David Anderson (1905-1991) Carl David Anderson was born on September 3 rd, 1905, in New York City. At the age of 7, his family moved to Los Angeles where he attended public school. Shortly after the
Carl David Anderson (1905-1991) Carl David Anderson was born on September 3 rd, 1905, in New York City. At the age of 7, his family moved to Los Angeles where he attended public school. Shortly after the
Evidence for the Strong Interaction
 Evidence for the Strong Interaction Scott Wilbur Scott Wilbur Evidence for the Strong Interaction 1 Overview Continuing search inside fundamental particles Scott Wilbur Evidence for the Strong Interaction
Evidence for the Strong Interaction Scott Wilbur Scott Wilbur Evidence for the Strong Interaction 1 Overview Continuing search inside fundamental particles Scott Wilbur Evidence for the Strong Interaction
Data Visualization in Particle Physics Past and Presence. Otto Schaile, LMU München Interdisciplinary Cluster Workshop on Visualization, 5 Nov, 2015
 Data Visualization in Particle Physics Past and Presence Otto Schaile, LMU München Interdisciplinary Cluster Workshop on Visualization, 5 Nov, 2015 Our current picture of particles and forces 2 elementary
Data Visualization in Particle Physics Past and Presence Otto Schaile, LMU München Interdisciplinary Cluster Workshop on Visualization, 5 Nov, 2015 Our current picture of particles and forces 2 elementary
Atomic and nuclear physics
 Chapter 4 Atomic and nuclear physics INTRODUCTION: The technologies used in nuclear medicine for diagnostic imaging have evolved over the last century, starting with Röntgen s discovery of X rays and Becquerel
Chapter 4 Atomic and nuclear physics INTRODUCTION: The technologies used in nuclear medicine for diagnostic imaging have evolved over the last century, starting with Röntgen s discovery of X rays and Becquerel
The Physics of Ultrahigh Energy Cosmic Rays. Example Poster Presentation Physics 5110 Spring 2009 Reminder: Posters are due Wed April 29 in class.
 The Physics of Ultrahigh Energy Cosmic Rays Example Poster Presentation Physics 5110 Spring 2009 Reminder: Posters are due Wed April 29 in class. 1 Introduction to Cosmic Rays References: http://www.phy.bris.ac.uk/groups/particle/pus/affo
The Physics of Ultrahigh Energy Cosmic Rays Example Poster Presentation Physics 5110 Spring 2009 Reminder: Posters are due Wed April 29 in class. 1 Introduction to Cosmic Rays References: http://www.phy.bris.ac.uk/groups/particle/pus/affo
Radioactivity. General Physics II PHYS 111. King Saud University College of Applied Studies and Community Service Department of Natural Sciences
 King Saud University College of Applied Studies and Community Service Department of Natural Sciences Radioactivity General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa Outline Radioactive Decay
King Saud University College of Applied Studies and Community Service Department of Natural Sciences Radioactivity General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa Outline Radioactive Decay
PSI AP Physics How was it determined that cathode rays possessed a negative charge?
 PSI AP Physics 2 Name Chapter Questions 1. How was it determined that cathode rays possessed a negative charge? 2. J. J. Thomson found that cathode rays were really particles, which were subsequently named
PSI AP Physics 2 Name Chapter Questions 1. How was it determined that cathode rays possessed a negative charge? 2. J. J. Thomson found that cathode rays were really particles, which were subsequently named
3.1 Early History of Atomic Theories
 Figure 1 In Dalton s atomic model, an atom is a solid sphere, similar to a billiard ball. This simple model is still used today to represent the arrangement of atoms in molecules. DID YOU KNOW? William
Figure 1 In Dalton s atomic model, an atom is a solid sphere, similar to a billiard ball. This simple model is still used today to represent the arrangement of atoms in molecules. DID YOU KNOW? William
Physics in Italy
 Physics in Italy 1950-2000 Nicola Cabibbo Dipartimento di Fisica Università di Roma La Sapienza INFN Sezione di Roma Lares, 3 July 2009 Nicola Cabibbo Physics in Italy Lares, 3 July 2009 1 / 27 The Conversi,
Physics in Italy 1950-2000 Nicola Cabibbo Dipartimento di Fisica Università di Roma La Sapienza INFN Sezione di Roma Lares, 3 July 2009 Nicola Cabibbo Physics in Italy Lares, 3 July 2009 1 / 27 The Conversi,
Chapter 20 Nuclear Chemistry. 1. Nuclear Reactions and Their Characteristics
 Chapter 2 Nuclear Chemistry 1. Nuclear Reactions and Their Characteristics Nuclear reactions involve the particles located in the nucleus of the atom: nucleons:. An atom is characterized by its atomic
Chapter 2 Nuclear Chemistry 1. Nuclear Reactions and Their Characteristics Nuclear reactions involve the particles located in the nucleus of the atom: nucleons:. An atom is characterized by its atomic
Chemistry 6A F2007. Dr. J.A. Mack 12/3/07. What do I need to bring? Exam 3: Friday 12/7/07 (here in lecture)
 Chemistry 6A F2007 Dr. J.A. Mack Exam 3: Friday 12/7/07 (here in lecture) What will be covered on the exam? Chapter 6: 6.9-6.15 Chapter 7: All Chapter 8: All Chapter 9: 9.1-9.9 Any thing from lab as well
Chemistry 6A F2007 Dr. J.A. Mack Exam 3: Friday 12/7/07 (here in lecture) What will be covered on the exam? Chapter 6: 6.9-6.15 Chapter 7: All Chapter 8: All Chapter 9: 9.1-9.9 Any thing from lab as well
Nobel Prize for missing piece in neutrino mass puzzle (Update) 6 October 2015
 Nobel Prize for missing piece in neutrino mass puzzle (Update) 6 October 2015 The work dispelled the long-held notion that neutrinos had no mass. Neutrinos come in three types, or "flavors," and what the
Nobel Prize for missing piece in neutrino mass puzzle (Update) 6 October 2015 The work dispelled the long-held notion that neutrinos had no mass. Neutrinos come in three types, or "flavors," and what the
Sources of Radiation
 Radioactivity Sources of Radiation Natural Sources Cosmic Radiation The Earth is constantly bombarded by radiation from outside our solar system. interacts in the atmosphere to create secondary radiation
Radioactivity Sources of Radiation Natural Sources Cosmic Radiation The Earth is constantly bombarded by radiation from outside our solar system. interacts in the atmosphere to create secondary radiation
charge. Gamma particles turned out to be electromagnetic radiation, the same as light, but with much higher energy.
 Simple, sensitive Geiger counter Radioactivity is a fascinating subject. The more you learn about it, the more questions there are to ask! This project is simple to construct and relatively inexpensive,
Simple, sensitive Geiger counter Radioactivity is a fascinating subject. The more you learn about it, the more questions there are to ask! This project is simple to construct and relatively inexpensive,
SECTION A Quantum Physics and Atom Models
 AP Physics Multiple Choice Practice Modern Physics SECTION A Quantum Physics and Atom Models 1. Light of a single frequency falls on a photoelectric material but no electrons are emitted. Electrons may
AP Physics Multiple Choice Practice Modern Physics SECTION A Quantum Physics and Atom Models 1. Light of a single frequency falls on a photoelectric material but no electrons are emitted. Electrons may
Nuclear Reactions: Chemistry 5.1 AN INTRODUCTION TO NUCLEAR CHEMISTRY
 Nuclear Reactions: Chemistry 5.1 AN INTRODUCTION TO NUCLEAR CHEMISTRY Discovery of Radioactivity Roentgen In 1895 Wilhelm Konrad Roentgen discovered Xrays. Roentgen observed that a vacuum discharge tube
Nuclear Reactions: Chemistry 5.1 AN INTRODUCTION TO NUCLEAR CHEMISTRY Discovery of Radioactivity Roentgen In 1895 Wilhelm Konrad Roentgen discovered Xrays. Roentgen observed that a vacuum discharge tube
