Thermal-Electrochemical Modeling of Battery Systems
|
|
|
- Constance McBride
- 6 years ago
- Views:
Transcription
1 90 Journal of The Electrochemical Society, 47 (8) 90-9 (000) S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. Thermal-Electrochemical Modeling of Battery Sytem W. B. Gu* and C. Y. Wang**,z GATE Center for Advanced Energy Storage, Department of Mechanical and Nuclear Engineering, The Pennylvania State Univerity, Univerity Par, Pennylvania 680, USA A general form of the thermal energy equation for a battery ytem i derived baed on firt principle uing the volume-averaging technique. A thermal-electrochemical coupled modeling approach i preented to imultaneouly predict battery electrochemical and thermal behavior. Thi approach couple the thermal energy equation with the previou multiphae micro-macrocopic electrochemical model via the heat generation and temperature-dependent phyicochemical propertie. The thermal-electrochemical model i multidimenional and capable of predicting the average cell temperature a well a the temperature ditribution inide a cell. Numerical imulation are performed on a Ni-MH battery to demontrate the ignificance of thermal-electrochemical coupling and to invetigate the effect of thermal environment on battery electrochemical and thermal behavior under variou charging condition. 000 The Electrochemical Society. S (00) All right reerved. Manucript ubmitted February 3, 000; revied manucript received May 8, 000. A a follow-up of previou wor,, the preent wor i intended to develop a thermal and electrochemical coupled model capable of predicting the patial ditribution and temporal evolution of temperature inide a battery. It i nown that temperature variation inide a battery may greatly affect it performance, life, and reliability. Battery phyicochemical propertie are generally trong function of temperature. For example, the equilibrium preure of hydrogen aborption-deorption, which ignificantly affect the open-circuit potential of the metal hydride electrode and hence the performance of nicel metal hydride batterie, i trongly dependent on temperature. 3 Capacity loe occur at low temperature due to high internal reitance and at high temperature due to rapid elf-dicharge. 4 Therefore, a proper operating temperature range i eential for a battery to achieve optimal performance. In order to prolong the battery cycle life, balanced utilization of active material i deired, which require a highly uniform temperature profile inide the battery to avoid localized degradation. More important, the battery temperature may increae ignificantly due to the elf-accelerating characteritic of exothermic ide reaction uch a oxygen reaction in aqueou batterie, eventually cauing thermal runaway. 5-8 An optimal operating range and a high uniformity in the internal temperature ditribution contitute two thermal requirement for a battery to operate afely. Thee two are particularly important for advanced electric-vehicle batterie becaue of their high energy and power denitie, large ize, and high charge and dicharge rate. Although experimental teting and microcalorimetric meaurement 9- are neceary to obtain battery thermal data for deign and optimization, a mathematical model baed on firt principle i capable of providing valuable internal information to help optimize the battery ytem in a cot-effective manner. In general, a battery thermal model i formulated baed on the thermal energy balance over a repreentative elementary volume (REV) in a battery. The differential equation that decribe the temperature ditribution in the battery tae the following conervation form,3 cp vt T q accumulation convection conduction heat generation where T i the temperature, v i velocity vector of the electrolyte, q i the volumetric heat generation rate, and, c p, and are the volume-averaged denity, pecific heat, and heat conductivity of the REV, repectively. The thermophyical propertie can be aniotropic due to the inhomogeneity of battery component. ** Electrochemical Society Student Member. ** Electrochemical Society Active Member. * z cxw3@pu.edu [] In batterie with flowing electrolyte, the convection term play an important role. However, it can be neglected in mot tationary batterie, and then Eq. i reduced to the tranient heat conduction equation. Subect to proper boundary condition, Eq. and it variou implified form have been olved to obtain the temperature ditribution in lead-acid, 4-6 nicel-hydrogen, 7 lithium-polymer, 8- and lithium-ion 8, battery module, with a ingle cell a the minimum REV. When the lumped-parameter approach i applicable (cf. Eq. 40) or only the average cell temperature i deired, the conduction term can be further diminihed by integrating boundary condition into the equation. The reulting time-dependent ordinary differential equation (i.e., Eq. 4) ha been widely ued in lead-acid, 3-5 nicel-hydrogen, 6,7 lithium-polymer, 8,9 and lithium-ion 30 battery model. For a thermal model a battery can be thermally and electrochemically coupled or decoupled, depending on how the heat generation term i treated. During battery operation, the heat generation rate depend not only on the cell temperature but alo on charge or dicharge regimen. A fully coupled model ue newly produced current and potential information from the model to calculate the heat generation rate and hence temperature ditribution, which in turn determine the current and potential,,5,7 wherea a decoupled model may employ empirical equation (e.g., the Shepherd equation) decribing experimental battery charge/dicharge curve of different rate at contant temperature. 8-0, The decoupled model i much impler, but accurate only when battery performance i inenitive to temperature. The complexity of the coupled model can be ignificantly reduced by the partially coupled approach propoed by Pal and Newman; 7 that i, etimating the heat generation rate during noniothermal dicharge from that obtained at contant temperature from an iothermal cell model. In other word, heat-generation rate are approximated to be independent of dicharge hitory. The heat generation rate depend on the thermodynamic propertie of the reaction proceeding in a cell, the potential-current characteritic of the cell, and the rate of charge and dicharge. By utilizing the firt law of thermodynamic for an iobaric battery ytem, Bernardi et al. 3 gave a general energy balance equation for a cell in which the rate of heat generation wa given by q I U T U IV enthalpy-of-mixing term T av av phae change term [] where I i the volumetric partial reaction current reulting from electrode reaction, U av i the correponding open-circuit potential (OCP) with upercript av referring to the value evaluated at the average compoition, I the total current in the unit of A/cm 3, and V
2 Journal of The Electrochemical Society, 47 (8) 90-9 (000) 9 S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. the cell potential. The firt term on the right ide (RS) of Eq. repreent the enthalpy of charge-tranfer reaction. The econd term tand for the electrical wor done by the battery. The third term or the enthalpy-of-mixing term repreent the heat effect aociated with concentration gradient developed in the cell. The lat term or the phae-change term tand for the heat effect due to phae tranformation. In arriving at Eq., the energy balance wa performed over the entire cell with the aumption of uniform cell temperature. When a cell i thin and the end effect are negligible, the uniformity in temperature i a good approximation. Apparently, when multiple electrode reaction occur imultaneouly, the partial current of each reaction mut be nown in order to calculate the heat generation rate uing Eq.. For a battery ytem that involve an inertion reaction, uch a lithium-baed and nicel-baed batterie, the OCP i a trong function of the local tate of charge (SOC), which i often controlled by olid-tate pecie diffuion. During operation at high rate, the pecie concentration ditribution in a cell could be highly nonuniform and reult in nonuniform electrochemical reaction rate. Neglecting enthalpy-of-mixing and phae-change term, Rao and Newman 3 mot recently preented a general energy balance equation for inertion battery ytem, in which the rate of heat generation i written a q a i U T U dv IV n c where a i the pecific urface area active for electrode reaction, in the tranfer current denity due to reaction, and U the local OCP of reaction. A imilar expreion of the heat generation rate wa recently preented by De Vidt et al. 7 for a nicel-hydrogen cell, with the preure wor additionally taen into account. Unlie Eq., Eq. 3 relate the heat generation to the local electrochemical reaction rate and the local OCP and thu i capable of calculating heat generation rate when cell undergo relaxation under dynamic condition. 3 It i expected that thermal runaway i firt triggered by the hottet pot in a cell. There i thu a need to predict the temperature ditribution within a cell in order to capture the thermal runaway proce. Baed on overall heat balance of a cell, both Eq. and 3 become inadequate when the temperature profile inide a cell i deired. In the next ection, a thermal energy equation capable of decribing the internal temperature ditribution of the cell i developed baed on firt principle uing the volume-averaging approach. A fully coupled thermal and electrochemical model i then developed by coupling the thermal equation with the previou multiphae tranport and electrochemical model. Simplification to variou calculation of the heat generation rate are dicued. The full capability of the model to predict temperature ditribution inide cell ha been demontrated elewhere. 49 In thi paper, numerical imulation baed on a lumped thermal model are carried out to examine the ignificance of thermal and electrochemical coupling and to invetigate the effect of thermal environment on the electrochemical and thermal behavior of a Ni- MH (metal hydride) cell under variou charging mode. Model Development Conider an electrochemical ytem coniting of porou electrode, an electrolyte, and a ga phae. The electrolyte can be either liquid or olid. The ga phae i preent in batterie due to ga generation accompanying the primary reaction. General thermal energy equation. For a multicomponent ytem uch a electrolytic olution, a general differential equation of thermal energy balance ha been deduced baed on firt principle. 33,34 With the aumption of negligible heat effect due to vicou diipation and preure wor, no body force, and no homogeneou chemical reaction, thi general energy equation i reduced to cp vt q H J ˆ pecie [3] [4] where and c p are the denity and pecific heat, repectively, T i the temperature, v i the velocity vector, q i the heat flux, J i the molar flux of a pecie due to diffuion and migration, and Ĥ i the pecie partial molar enthalpy, with ubcript denoting in phae. The econd term on the RS of Eq. 4 thu repreent thermal tranport due to pecie diffuion and migration, with the ummation carried out over all pecie in phae. In general, the heat flux q include conductive flux (or Fourier flux), flux caued by interdiffuion of variou pecie, and the Dufour energy flux (or diffuion-thermo effect). Becaue Dufour energy flux i uually negligible, 33,34 the heat flux q can be expreed a q T H ˆ J [5] pecie where i the thermal conductivity of phae. Applying Eq. 5 and the continuity equation for phae v 0 [6] Equation 4 become cp ( v T ) ( T ) J Hˆ [7] pecie Ue the thermodynamic relationhip 34 Ĥ T [8] T along with the electrochemical potential defined by 34 o a RT ln zf [9] a,ref where o and a are the tandard chemical potential and the activity of a pecie in phae, repectively, and a,ref i the pecie activity at a reference tate. One ha a ln H ˆ a,ref R T zf T T [0] Equation 0 hold true with no additional aumption. The firt term on the RS of Eq. 0 i cloely related to the enthalpy-of-mixing and ha been generally neglected in practice. 7,30,3 Further ignoring the temperature dependence of phae potential for implicity, Eq. 0 i then implified to Ĥ zf Subtituting Eq. into Eq. 7 yield cp ( vt ) T zfj pecie [] [] Noting that the current through phae reult from diffuion and migration of ionic pecie in the phae under the aumption of electroneutrality, i.e. Equation can be rewritten a i pecie zfj cp ( vt ) T i [3] [4]
3 9 Journal of The Electrochemical Society, 47 (8) 90-9 (000) S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. The econd term on the RS of Eq. 4 repreent the converion of electrical energy to thermal energy (i.e., Joule heating) and i an important difference between electrical and nonelectrical ytem. Electrochemical reaction occur at the interface between the electrode and the electrolyte. Heat balance over the interface reult in 35,36 e Ten e T n in i n [5] where n repreent the normal unit vector pointing outward from a phae, with ubcript e and denoting the phae of electrolyte and the phae of olid active material, repectively, and i n i the local tranfer current denity due to the electrode reaction. The RS of Eq. 5 tand for the heat generated at the electrode/electrolyte interface and i divided into two part. The firt term i the irreverible reaction heat due to the electrochemical reaction reitance at the interface, imilar to Joule heating. It i proportional to the urface overpotential of the electrode reaction and i alway poitive. The econd term i the reverible part of the reaction heat mainly due to the entropy change of the electrode reaction. It i called Peltier heat and change ign with changing current direction. The Peltier coefficient can be determined experimentally. 37 It i worth mentioning that in arriving at Eq. 5, the heat effect aociated with nonelectrochemical procee, uch a water condenation and evaporation occurring in a nicel-hydrogen cell, 6 hydride formation in the MH electrode, and active material decompoition in lithium-ion cell, 30 ha not been conidered. More generally, Eq. 5 can be rewritten a Tn m Tmn m ain( ) ( h hm ) m [6] am for the interface at which phae tranformation a well a multiple electrochemical reaction tae place. Here m repreent the phae tranformation rate at the -m interface from phae m to phae, a i the pecific urface area active for electrode reaction, a m A m /V o i the pecific urface area of the -m interface within the averaging volume V o, and h i the enthalpy with ubcript and m referring to phae and m, repectively. On the RS of Eq. 6, the econd term account for the heat effect due to the phae tranformation taing place at the interface, wherea the firt term include the heat effect due to the electrochemical reaction. For interface at which no electrochemical reaction occur, uch a ga-involved interface, the firt term on the RS imply vanihe. Let V o be the volume of an REV coniting of V ( e,, and g for electrolyte, olid active material, and ga phae, repectively) and follow the procedure decribed in Ref.. Volume-averaging of Eq. 4 over the REV yield < T > cp T < > < v > with and [ < > ] eff a, T < > < > Qm d Qm i Q Joule m Qm d TndA Vo A m Qm cpt ( w v ) nda o A m [7] [8] [9] J Q oule < i > < > nda Vo A m dv [0] o V ( i < i > ) ( < > ) where eff i the effective thermal conductivity of phae and a, i the diperion coefficient in phae. While eff include the effect of tortuoity and may follow the Bruggeman correction (i.e., eff.5 ), a, repreent the effect of hydrodynamic diperion that reult from variation of the microcopic velocity and temperature and vanihe in the abence of fluid motion. The econd term on the RS of Eq. 7 i the um of interfacial heattranfer effect. Qm d repreent the interfacial heat-tranfer rate due to conduction, wherea Q m tand for the thermal effect due to the interface movement at a velocity of w. In view of the mean value for integral, Q m can be modeled a the product of the average interfacial temperature by the phae tranformation rate at the interface, i.e. [] where T m i the area-averaged temperature at the -m interface. The lat term on the RS of Eq. 7, Q Joule, arie from volume-averaging the Joule heating term in Eq. 4. Apparently, it would vanih when electrical equilibrium hold true in a phae, i.e., < >. However, electrical nonequilibrium i expected if the phae conductivity i low and/or the applied current denity i high. The conductivity of emiconductor-lie active material (e.g., NiOOH) can be a low a 0 5 S/cm. Such a low electronic conductivity may caue a ignificant microcopic ohmic drop acro the active material layer coated on a ubtrate. In thi cae, it can be hown that the firt term on the RS of Eq. 0 i till negligible (ee the Appendix). However, the magnitude of the econd term cannot be eaily etimated and, thu, it i left unmodeled in the preent wor. Phyically, thi term arie from fluctuation in the profile of microcopic current and potential. Quantification of thi term will be attempted in future wor. Summation of Eq. 7 over all phae involved in the REV (i.e., the electrolyte phae, the olid active material phae, and the ga phae) and ue of Eq. 8 yield Applying Eq. 6, Eq. become < T > c p < T > < v > T da c T n p mm m m o A m < T > c p < T > < v > Qm cptmm { [ ]} < > eff a, T < > < > i < T > a i eff n Π [ ] ( h hm ) m ( cp cpm) Tmm m m < > < > i [] [3]
4 Journal of The Electrochemical Society, 47 (8) 90-9 (000) 93 S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. Auming that local thermal equilibrium exit in the ytem under conideration, i.e. < T > < Tm > m Tm Tm T [4] and dropping the volume-averaged ymbol for convenience, Eq. 3 become where and the heat-ource term, q, i given by cpt vt T q cp cp v cp < v > eff a, [5] [6] [7] [8] q ain h* m m m < > < > ( i ) [9] e& with h* (h h m ) (c p c pm )T [30] The volume-averaged current denity through phae tae the form of eff e eff < i > < e > e i D ln < c > i or [3] for the phae of a concentrated binary electrolyte, and eff < i > < > [3] for the phae of a olid active material, where i the ionic conductivity of the electrolyte, D i the diffuional conductivity, and i the olid electronic conductivity, with upercript eff indicating the effect of poroity and tortuoity included. Note that there i no current flowing through the gaeou phae. Equation 9 how that the thermal effect are due to electrochemical reaction, phae tranformation, and ohmic Joule heating in both the electrolyte and olid active material phae. Becaue the reaction heat i expreed in term of the local tranfer current denity, the heat effect reulting from a highly nonuniform ditribution in the reaction rate can be aeed. Equation 5 enable one to determine temperature ditribution inide a cell rather than to obtain only an average temperature of the cell. In order to calculate the heat generation rate uing Eq. 9, the Peltier coefficient,, mut be nown. An expreion for Peltier coefficient ha been derived by Newman 35 baed on the general multicomponent tranport equation and electrode reaction. With the Dufour energy flux neglected, it i reduced to T S [33] nf where S i the entropy change of electrode reaction. Entropy change of a number of electrode reaction were calculated by Lampinen and Fomino 38 and by Xu et al. 39 Alternatively, uing the following thermodynamic relationhip between the OCP U and the entropy change S 40 S n F U the Peltier coefficient can be rewritten a T U Subtituting Eq. 35 and e e U into Eq. 9 give q a i U T U n ain e e [34] [35] h* m i [36] m m e& Equation 5, along with the expreion for the heat generation rate, Eq. 36, contitute a general thermal model that decribe the temperature field inide a cell. Thermal and electrochemical coupling. Temperature-dependent phyicochemical propertie, uch a the diffuion coefficient and ionic conductivity of the electrolyte, are needed to couple the thermal model with the multiphae ma-tranport and electrochemical inetic model. More pecifically, the dependence of the phyicochemical propertie on the temperature can be decribed by Arrheniu equation,5,36 act, ref exp E [37] R T T ref where i a general variable repreenting the diffuion coefficient of a pecie, conductivity of the electrolyte, exchange current denity of an electrode reaction, etc., with ubcript ref denoting the value at a reference temperature. E act, i the activation energy of the evolution proce of. It magnitude determine the relative enitivity of the cell to temperature. The greater it activation energy, the more enitive i the parameter to temperature. The ionic conductivity and electrolyte diffuion coefficient uually are trong function of the temperature; uch data are available for Ni-MH 4 and Li-baed 7 batterie. In addition, the OCP of electrode reaction, U, i uually approximated a a linear function of temperature U U U,ref ( T Tref ) [38] The heat generation rate due to electrochemical reaction and Joule heating are calculated locally via a detailed electrochemical model, and ubequently are ued in the energy conervation equation to calculate the temperature evolution. Thi temperature information i, in turn, fed bac to update the electrochemical calculation through temperature-dependent phyicochemical propertie. Figure how a chematic diagram of the preent coupled modeling approach. Lumped thermal model. For mot battery ytem, the convection term in Eq. 5 can generally be neglected, and a tranient heat conduction equation i ufficient to decribe thermal phenomena in batterie, i.e. ( cpt) ( T) q [39] Here, the thermal conductivity i highly aniotropic and microtructure-dependent becaue a battery cell i compoed of a variety of complex material. For ingle cell of mall thicne, one may have hl Bi << < > < > [40]
5 94 Journal of The Electrochemical Society, 47 (8) 90-9 (000) S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. Figure. Diagram of the thermal-electrochemical coupled modeling approach. where h i the convective heat-tranfer coefficient, and L and are the thicne and the thermal conductivity of the cell, repectively. Under the condition given by Eq. 40, the aumption of a uniform temperature acro the cell i valid and hence a lump-parameter model of energy balance can be applied. Equation 39 i thu reduced to d( cpt) Q qd [4] dt where the heat removal rate per unit volume from the cell to the urrounding, Q, can be expreed uing an equivalent convective heat tranfer coefficient, h, a follow ha T T Q c ( a ) [4] where V c i the cell volume, A c the urface area through which heat i removed from the cell, and T a the temperature of urrounding. <q> i the volume-averaged heat generation rate in the form of a i U T U n V T dv ain e e dv h m dv c V ( * ) c c m m dv [43] ( < i > < > ) e& We rewrite the econd integral of Eq. 43 to obtain V c e ain e dv e e ( < > ) ( < > ) a i U T U n dv ( < > < > ) ain e V c [ ] e dv ( h* m) dv < i > < > dv V V c m m c e& [44] The third term on the RS of Eq. 44 reult from the electrical nonequilibrium that exit in the olid active material and electrolyte phae. Auming that the heat effect due to the electrical nonequilibrium i negligible, Eq. 44 then become a i U T U n V T dv e a i dv n e ( < > < > ) h dv dv ( * m) c V ( < i > < > ) c m m c e& [45] We aume one-dimenional electrochemical procee acro the cell thicne, which i generally the cae becaue of the high electronic conductivity of the current collector bound to the electrode. Conervation of charge in both olid and electrolyte phae require < ie > < i > ain [46] Applying Eq. 46 to the econd integral in Eq. 45 and integrating it by part yield e nd integral in Eq. 45 < ie >< e > dv < i >< > dv IV dv [47] ( < i > < > ) e& where I ia/v c i/l i the total volumetric current denity (A/cm 3 ) applied to the cell, with A and L denoting the proected electrode area and the cell width, repectively. To obtain Eq. 47, the following boundary condition were ued < i e > 0 at x 0 and L [48] < i > i at x 0 and L [49] with the definition of cell potential V < > xl < > x0 [50] Subtituting Eq. 47 into Eq. 45, one ha a i U T U n dv IV V c h m dv [5] ( * ) m m When the heat effect due to phae tranformation i ignored, Eq. 5 i implified to Eq. 3 preented by Rao and Newman 3 for inertion battery ytem. Two condition can be applied to further implify Eq. 5: (i) the OCP term, a hown in the parenthei in Eq. 5, i contant; and (ii) the electrode reaction rate are patially uniform. In the cae of contant OCP, Eq. 5 can be reduced to the following by taing the OCP term out of the integrand where < q > I U U IV I aindv c [5] [53]
6 Journal of The Electrochemical Society, 47 (8) 90-9 (000) 95 S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. In the cae of uniform reaction rate, the heat generation rate can be rewritten a I U T U dv IV T I U T U IV av [54] Equation 5 or 54 i identical to Eq. preented by Bernardi et al. 3 with neglect of enthalpy-of-mixing and phae-change term. If only one overall cell reaction (i.e., one pair of electrode reaction) mut be conidered, Eq. 5 or 54 can be rewritten a I U T U IV I U V T U T i [55] L U V T U where U i the open-circuit cell potential determined by the difference between poitive and negative electrode OCP. Equation 55 ha been widely ued in lead-acid, 3,4 lithium-polymer, 7,8-0,8,9 and lithium-ion battery thermal model. It i clearly hown by the implification made that Eq. 55 i accurate only for the overall thermal balance of a cell with no ignificant heat effect due to phae change, no concentration gradient preent in the cell, contant OCP or uniform reaction rate, and a ingle overall reaction contributing to reaction heat. Rao and Newman 3 have illutrated that ignificant error may occur when Eq. 55 i ued to calculate the heat generation rate intead of Eq. 3. The extent of error depend greatly on the temporal behavior of the electrode OCP. Equation 55 virtually fail for a dynamic dicharge in which cell relaxation i involved. 3 Application to Ni-MH Cell A thermal and electrochemical coupled model reult by combining the thermal equation derived previouly with the micro-macrocopic multiphae tranport and electrochemical model previouly developed for Ni-MH cell. The model not only account for the microcopic diffuion of proton and hydrogen in olid active material, but alo incorporate oxygen reaction and tranport through both electrolyte and ga phae. Model detail have been preented in Ref. and hence are not repeated here. Equation 4, i.e., the lumpedparameter thermal equation, i employed in the following imulation a a firt tep. Applying Eq. 5 to a Ni-MH cell, one ha a i U T U n dv IV V c [56] where h* hyd i the enthalpy of metal hydride formation. Equation 56 i ued to calculate the volume-averaged heat generation rate of the Ni-MH cell. The model equation are ummarized in Table I, ubect to the following initial and boundary condition. Initial condition. Specie concentration are uniform at time 0, i.e. OH OH O O O O H H hhyd * a3in3 dv c co, ce ce,o, cg cg,o, c co, and T To [57] V c Table I. Summary of model equation for a Ni-MH battery. Specie concentration in liquid phae Specie concentration in ga phae Specie concentration in olid phae Liquid phae potential Solid phae potential Cell temperature OH o ( ec ) OH OH t OH Deff c F O ( ec e ) O O O O D e,eff ce eg t 4F J O cg Vg ( c H ) H F H D c H H H e c le aef eff eff e D OH OH ln c 0 a V eff OH b b 0 Rb OH e Re ae O Jeg dv d( cpt) ha ( T T ) c a dt a i V U T U T dv IV n * ( hyd 3 n3) V c h a i dv
7 96 Journal of The Electrochemical Society, 47 (8) 90-9 (000) S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. Boundary condition. There i no flux of pecie and all current L goe through the olid, therefore < > < > a i U T U P e n e dx L 0 OH O c c e 0, 0, and e 0 x x x and 3 [6] at the electrode/current collector interface [58] The boundary condition for olid-phae potential are dependent on the operation mode. At the poitive electrode/current collector interface (x L) 0 (reference potential) [59] At the negative electrode/current collector interface (x 0) V for floating charge [60] eff i for galvanic charge x where V and i are applied voltage and current denity, repectively. Cell in the previou wor i ued to invetigate the electrochemical and thermal behavior of a Ni-MH cell under variou operation mode and thermal condition. It cell-pecific parameter have been given therein. The operation mode include contant current charging at the C rate (i.e., i 35.7 ma/cm ) and float charging at a contant cell voltage of.5 V. Four thermal condition are conidered in term of an equivalent heat-tranfer coefficient impoed on the cooling urface of the cell. Wherea h 0 and correpond to adiabatic and iothermal condition, repectively, h 5 and 5 W/m K refer to typical value of air-free convection and forced convection (e.g., via a cooling fan). In other word, active thermal management i neceary in order to achieve a convective heat-tranfer coefficient of 5 W/m K. Table II lit the value of parameter ued in the thermal modeling of the Ni-MH battery. Other value of parameter needed in the imulation can be found in Ref.. The partial heat generation rate to be dicued in the next ection are defined a L < > < > a i U T U O e O n e O L dx 0 and 4 [6] H q h* hyda3in3 dx [63] L MH 0 and L J < > < > ( i ) dx [64] L 0 e& where the upercript P, O, H, and J denote the partial heat generation rate due to the primary reaction and 3, oxygen reaction and 4, hydride formation, and Joule heating, repectively. L MH i the thicne of the metal hydride electrode. The partial current preented in the Reult and Dicuion ection are defined a and LMH < > i P LL Ni L L io aindx LL Ni aindx [65] [66] where i p and i O repreent the partial current due to the primary reaction and oxygen generation reaction in the nicel electrode, repectively. L Ni i the thicne of the nicel electrode. Table II. Value of parameter ued in the thermal modeling of a Ni-MH battery. Symbol Value Unit Decription e.5 g/cm 3 Denity of KOH electrolyte 4 Ni 3.55 g/cm 3 Denity of nicel electrode 4 MH 7.49 g/cm 3 Denity of MH electrode 4 ep 0.9 g/cm 3 Denity of polyamide eparator a c p,e 3. J/g K Specific heat of KOH electrolyte 4 c p,ni 0.88 J/g K Specific heat of nicel electrode 4 c p,mh 0.35 J/g K Specific heat of MH electrode 43 c p,ep.9 J/g K Specific heat of polyamide eparator 4 (du/dt) ref.5 mv/k Temperature coefficient of reference electrode OCP 44 (du/dt) Ni.35 mv/k Temperature coefficient of nicel electrode OCP 44 (du/dt) MH mv/k Temperature coefficient of MH electrode OCP 44 b (du/dt) O.68 mv/k Temperature coefficient of oxygen reaction OCP 44 h* hyd 30.4 J/mol H Enthalpy of metal hydride (LaNi 5 H 6 ) formation 4,43 E act,ni 0. J/mol Activation energy of nicel electrode reaction 45 E act,mh 30. J/mol Activation energy of MH electrode reaction 4 E act,o 50. J/mol Activation energy of oxygen reaction 45 E act,d OH 4. J/mol Activation energy of electrolyte diffuion 46 E act,d O 4. J/mol Activation energy of oxygen diffuion in KOH electrolyte 47 E act,d H 9.6 J/mol Activation energy of proton diffuion in nicel active material 48 E act,d H 0. J/mol Activation energy of atomic hydrogen diffuion in MH particle a E act, 3 J/mol Activation energy of electrolyte conductivity 4 c a Etimated value. b Tae the data of H O(l)/H (g), OH electrode. c Evaluated uing the data in Fig. 5 of Ref. 4.
8 Journal of The Electrochemical Society, 47 (8) 90-9 (000) 97 S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. Reult and Dicuion Significance of thermal-electrochemical coupling. The need for a thermal-electrochemical coupled model can be demontrated clearly by comparion of predicted cell potential, preure, and temperature uing the coupled and decoupled model, repectively. The decoupled model aume that the electrochemical ubmodel i eentially temperature-independent. However, becaue temperature i included in the Butler-Volmer equation, the decoupled model reult are till dependent on the thermal hitory and thu on the heat-tranfer coefficient. Figure diplay comparion of predicted cell potential curve during C charging. An apparent dicrepancy between the coupled and decoupled model prediction begin from 0% charge input and become ignificant when approaching the overcharge region. The dicrepancy decreae with increaing heat-tranfer coefficient, becaue a higher heat-tranfer coefficient yield a larger heat diipation rate and hence a maller temperature rie during battery charging. A expected, the decoupled model i applicable only when the variation in cell temperature i ufficiently mall. Unlie the cell potential, the cell temperature and preure are inenitive to the thermal-electrochemical coupling during contantcurrent charging, a hown in Fig. 3. It alo how that the cell temperature decreae ignificantly with increaing heat-tranfer coefficient, while the reduction in cell preure i very mall. Figure 4 plot the current denity applied to the cell during float charging at a voltage of.5 V. A ignificant dicrepancy i oberved between the coupled and decoupled model prediction when the heat diipation i poor (correponding to a mall heat-tranfer coefficient). The dicrepancy become maller when a higher heat-tranfer coefficient i applied. Thi, again, indicate that the decoupled model i valid only when the variation in the cell temperature i inignificant. Figure 5 how the comparion of predicted cell preure and temperature during the float charging. The decoupled model ignificantly underpredict the cell preure and temperature under poorer heat diipation condition. A thermal-electrochemical coupled model i neceary in order to accurately predict preure buildup in a Ni-MH battery and enure it afe operation. In view of the inaccuracy of the decoupled approach, the coupled model i ued to perform all following numerical tudie. Thermal effect during contant-current charging. Figure 6 how electrochemical and thermal behavior of the Ni-MH cell during C charging. A the heat-tranfer coefficient decreae, the cell potential increae more lowly and the potential pea become more pronounced. The cell temperature increae with time in all Figure 3. Comparion of predicted cell temperature and preure evolution between the coupled and decoupled thermal-electrochemical model during C charging. cae, indicating that the cell i exothermic when charged at C. A larger heat-tranfer coefficient correpond to a larger rate of heat diipation, reulting in a maller temperature rie. When the heattranfer coefficient i larger than 5 W/m K, the cell temperature rie i le than 5C when the charge input i le than 90% of the nominal cell capacity. After that tage, the cell temperature increae dra- Figure. Comparion of predicted potential curve between the coupled and decoupled thermal-electrochemical model during C charging. Figure 4. Comparion of predicted current denity variation between the coupled and decoupled thermal-electrochemical model during.5 V charging.
9 98 Journal of The Electrochemical Society, 47 (8) 90-9 (000) S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. Figure 5. Comparion of predicted cell temperature and preure evolution between the coupled and decoupled thermal-electrochemical model during.5 V float charging. Figure 6. Cell potential and temperature evolution during C charging under variou thermal condition. matically up to 64C at 0% charge input when the heat-tranfer coefficient equal 5 W/m K. A the heat-tranfer coefficient increae, the final cell temperature decreae. A le than 0C increae from the original temperature i oberved when the heat-tranfer coefficient i a large a 5 W/m K. The cell temperature exceed the afety limit for an aqueou cell (80C) when the cell i charged in an adiabatic condition, howing the need for thermal management for Ni-MH batterie. The overall trend in the cell temperature during C charging can be explained uing Fig. 7, which plot the total and partial heat generation rate of the cell v. charge input. The total heat generation rate increae lowly before 90% charge input, ump quicly thereafter, and finally reache a teady tate, cloely matching the trend in temperature variation. Surpriingly, the thermal environmental condition ha little effect on the heat generation rate. Becaue the overall reaction current i fixed when a battery i charged at a contant current, light variation in the total heat generation rate reult only from the difference in the ratio of primary to econdary reaction rate under different cooling condition. The primary reaction, oxygen reaction, MH formation, and Joule heating contribute to the total heat generation rate, a decribed by Eq. 9. Figure 7 alo diplay thee contribution of heat generation in the Ni-MH cell for h 5 W/m K. Initially, the heat effect due to primary reaction offet that due to MH formation, while the oxygen reaction are inignificant and the Joule heating negligible. With time, the heat aborbed by the primary reaction decreae becaue of the reduction in their enthalpy potential (i.e., U T U / ) 3,40 and the heat generated from the metal hydride formation remain contant. A a reult, the total heat generation rate gradually increae. When the cell i being charged upon a condition at which the oxygen reaction become ignificant, the total heat generation rate increae dramatically becaue the enthalpy potential of oxygen reaction are comparatively large and the heat aborbed by the primary reaction i negligibly mall. When the cell i being overcharged at a rate a large a C, almot all the current applied to the cell i ued to generate oxygen at the poitive electrode and only a portion of oxygen can be reduced at the negative electrode. In other word, the primary reaction at the MH electrode till account for a large portion of applied current due to the preence of deignated overcharge reerve, a indicated in Fig. 7 by the heat effect due to MH formation. The net heat generation rate i large, but teady tate i reached becaue of a contant reaction rate. Figure 8 how the variation in reaction current during C charging. Initially, the oxygen reaction i negligible, and all the current applied to the cell i ued to convert the active material from the dicharged to charged tate. The oxygen reaction become ignificant when the charge input exceed 90% of nominal cell capacity. It i obviou that the oxygen reaction current increae and the primary reaction current decreae, with the total current remaining the ame. The charge acceptance, defined a the ratio of primary reaction current to the total current, then exactly follow the curve of primary reaction current, with a maximum value of 00%. Apparently, the heat diipation rate affect the charge acceptance only after 00% of cell nominal capacity i applied. The wore the heat diipation, the maller the charge acceptance. Thermal effect during float charging. In addition to the contant-current charging mode, float charging at a contant voltage i alo frequently applied to Ni-MH batterie. Figure 9 how the reaction current when a contant voltage of.5 V i applied to the Ni-
10 Journal of The Electrochemical Society, 47 (8) 90-9 (000) 99 S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. Figure 7. Total and partial heat generation rate during C charging under variou thermal condition. The partial heat generation rate are defined by Eq MH cell. The current due to oxygen reaction i negligible when the charge input i le than 60% of nominal cell capacity in all cae. A poorer cooling condition caue an earlier occurrence of oxygen reaction reulting from the higher cell temperature. The current due to primary reaction decreae very quicly during the firt 0% of Figure 8. Primary and oxygen reaction current at nicel poitive electrode during C charging under variou thermal condition. The partial current are defined by Eq. 65 and 66. Figure 9. Reaction current during.5 V float charging under variou thermal condition. nominal cell capacity becaue the urface overpotential, the driving force for the electrochemical reaction, drop quicly due to the continual increae of the electrode OCP during charging. The primary reaction current i trongly affected by cooling condition. The higher the heat diipation rate, the lower the cell temperature, and hence the lower reaction current. The reaction current increae even after the initial quic drop, indicating that the reaction are facile at high temperature. The total reaction current from both primary and oxygen reaction i expected to have a minimum value when both the primary and oxygen reaction current are mall. A lower teady total current can be obtained at a higher heat diipation rate. Figure 0 how the cell temperature and preure variation during.5 V float charging. The cell temperature i trongly affected by the cooling condition. While the cell temperature continue to increae under poor cooling condition, it decreae continuouly after an initial quic rie. The larger the heat-tranfer coefficient, the earlier the decreae occur. Thi decreae in cell temperature i arreted when the cell i near it fully charged tate and oxygen reaction become dominant. The cell temperature then increae dratically, the magnitude of which depend on the cooling condition. While the cell temperature tend to approach a contant value at high cooling rate, the cell virtually underegoe thermal runaway at low cooling rate. The oxygen reaction contribute to the cell preure buildup. The earlier the oxygen reaction tae place, the more the cell preure build up. While the cell preure can be maintained at a low level when the heat diipation rate i high and hence the cell temperature i low, it build up o quicly under poor cooling condition that afety become a concern.
11 90 Journal of The Electrochemical Society, 47 (8) 90-9 (000) S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. Figure 0. Cell temperature and preure evolution during.5 V float charging under variou thermal condition. A found earlier, the heat generation rate of the cell i nearly independent of thermal condition during contant-current charging. Thi i not the cae during float charging. Figure diplay the heat generation rate of the Ni-MH cell during.5 V float charging. The total heat generation rate continue to decreae before the oxygen reaction, which reult in a dramatic increae in the total heat generation rate, become ignificant. It decreae more quicly at a larger heat diipation rate. The mallet heat generation rate occur when the heat diipation rate i infinitely large and the cell i accordingly iothermal. When the cell i adiabatic, the heat generation rate i nearly contant after an initial quic drop. Figure alo how the partial heat generation rate that contribute to the total rate for h 5 W/m K. The heat effect due to the hydride formation i dominant when the charge input i below 80% of nominal cell capacity, wherea the heat effect due to the oxygen reaction i dominant when the charge input i larger than 95% of nominal cell capacity. The heat effect due to Joule heating experience a minimum cloe to 00%, indicating that total current due to both primary and oxygen reaction ha a minimum value there. The heat effect due to MH formation decreae gradually with time and reache a minimum at a charge input of 0% of nominal cell capacity, meaning that the primary reaction till proceed at the MH electrode and i facile due to the increae in the cell temperature. A a reult, the heat effect due to the primary reaction at both nicel and MH electrode increae with increaing oxygen reaction. Figure how the effect of thermal condition on the charge acceptance of the Ni-MH cell during.5 V float charging. The charge acceptance reflect the efficiency of the charging proce. Figure. Total and partial heat generation rate during.5 V float charging under variou thermal condition. The difference in the charge acceptance i indicernible for all cae when the charge input i le than 60% of nominal cell capacity. However, it drop quicly a oon a the oxygen reaction become ignificant. When the charge input reache 0% of nominal cell Figure. Charge acceptance during.5 V float charging under variou thermal condition.
12 Journal of The Electrochemical Society, 47 (8) 90-9 (000) 9 S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. capacity, the charge acceptance virtually diappear. The oxygen reaction i much more facile at a higher cell temperature that reult from a poorer cooling condition. Accordingly, the charge acceptance drop earlier when the heat-tranfer coefficient i maller. Thi behavior i different from that diplayed in Fig. 8 during C charging. Concluion A general thermal energy equation ha been derived uing the volume-averaging technique, along with a local heat generation rate reulting from electrochemical reaction, phae tranformation, and Joule heating. The thermal model i fully coupled to the micro-macrocopic electrochemical model previouly developed via temperaturedependent phyicochemical propertie. The thermal-electrochemical coupled model i multidimenional and capable of predicting the temperature ditribution inide a cell in addition to the average cell temperature, thu providing a cot-effective tool to accurately predict the cell electrochemical and thermal behavior, and mot important, to identify the mechanim reponible for thermal runaway. Numerical imulation were performed to illutrate the ignificance of thermal and electrochemical coupling. The electrochemical and thermal behavior of a Ni-MH battery were then explored numerically uing the fully coupled thermal-electrochemical model. Variou operation mode and thermal condition were examined. The cell temperature rie i ignificant when the cell i charged at high rate and under poor cooling condition, primarily due to the oxygen reaction occurring near the end of full charge. The cell thermal behavior during contant-current charging wa found to differ ignificantly from that during float charging. Wor i underway to examine the predictablity of the preent model for hot pot within a cell. The thermal effect on the active material degradation and hence battery cycle life will be incorporated in future wor. Acnowledgment Thi wor wa upported, in part, by the Defene Advanced Reearch Proect Agency (DARPA), Tactical Technology Office, Electric and Hybrid Vehicle Technology Program, under cooperative agreement no. MDA and The Pennylvania State univerity aited in meeting the publication cot of thi article. Appendix An Order-of-Magnitude Etimate of the Firt Term in Eq. 0 The firt term in Eq. 0, Q Joule, repreent the Joule heating rate due to fluctuation at the microcopic level. It can be rewritten a < i t term in Eq. 0 > am m [A-] ( < > ) Note that the microcopic ohmic drop can be etimated from the tranfer current denity normal to the electrode/electrolyte interface a follow m < > r i n [A-] where r i the microcopic length cale (or the particle radiu). Subtituting Eq. A- into A- reult in eff < i t term in Eq. 0 > r a e i < > n e n r a i [A-3] where ue ha been made of the volume-averaged Ohm law, Eq. 3. Furthermore, it follow from the conervation of charge over an entire electrode that i ae i < > n [A-4] Le where L e i the macrocopic thicne of the electrode. Subtituting Eq. A-4 into A-3 and comparing thi term to the macrocopic Joule heating rate yield eff t term in Eq. 0 r r < i > < > Le Le << [A-5] ince the ratio of micro to macro length cale i much maller than unity. Lit of Symbol A proected electrode area, cm A c urface area through which heat i removed from the cell, cm a activity of a pecie a pecific urface area active for electrode reaction, cm /cm 3 c i volume-averaged concentration of pecie i over a phae, mol/cm 3 c p pecific heat, J/g K D i diffuion coefficient of pecie i, cm / E act activation energy, J/mol F Faraday contant, 96,487 C/mol Ĥ partial molar enthalpy of a pecie, J/mol h enthalpy of pecie participating in the phae tranformation, J/mol h equivalent heat-tranfer coefficient, W/cm K I volumetric current, A/cm 3 i current denity vector, A/cm i applied current denity, A/cm in tranfer current denity of reaction, A/cm J molar flux of a pecie, mol/cm J O eg ma-tranfer rate of oxygen acro ga/electrolyte interface, mol/cm 3 i reaction current reulting in production or conumption of pecie i, A/cm 3 L cell width, cm L e electrode thicne, cm l e microcopic diffuion length of pecie in a olid phae, cm n number of electron tranferred in reaction Q volumetric heat removal rate from the cell, J/cm 3 Q Joule microcopic Joule heating term due to electrical nonequilibrium, J/cm 3 Q d m interfacial heat-tranfer rate due to conduction, J/cm 3 Qm thermal effect due to the interface movement, J/cm3 q heat flux, J/cm q volumetric heat generation rate, J/cm 3 R univeral ga contant, J/mol K R o applied load, R b, area-pecific electrical reitance acro the olid/ubtrate interface, R e cm Ŝ partial molar entropy, J/mol K toichiometric coefficient of a pecie involved in reaction T abolute temperature of the cell ytem, K t time, t o tranference number of OH with repect to the olvent velocity U open-circuit potential of electrode reaction, V V applied voltage, V V c cell volume, cm 3 V g volume occupied by the ga phae, cm 3 v velocity vector, cm/ w interface velocity vector, cm/ x coordinate along the cell width, cm z charge number of an ionic pecie Gree volume fraction of a phae urface overpotential of electrode reaction, V the Peltier coefficient, V phae tranformation rate, mol/cm conductivity of an electrolyte, S/cm D diffuional conductivity of a pecie, A/cm denity, g/cm 3 conductivity of olid active material, S/cm thermal conductivity, W/cm K electrochemical potential of a pecie, J/mol o tandard chemical potential of a pecie, J/mol h* enthalpy change due to phae tranformation, J/mol potential in a phae, V urface potential in a phae, V Subcript b ubtrate e electrolyte phae eff effective eg electrolyte/ga interface g ga phae hyd hydride formation
13 9 Journal of The Electrochemical Society, 47 (8) 90-9 (000) S (00)0-05- CCC: $7.00 The Electrochemical Society, Inc. m interface between phae and m MH metal hydride active material Ni nicel active material ref with repect to a reference tate olid phae b olid/ubtrate interface e olid/electrolyte interface ep eparator o initial value Supercript av average eff effective H pecie hydrogen or proton H O olvent water OH pecie OH overbar, average over an interface Reference. C. Y. Wang, W. B. Gu, and B. Y. Liaw, J. Electrochem. Soc., 45, 3407 (998).. W. B. Gu, C. Y. Wang, S. L. Li, B. Y. Liaw, and M. M. Geng, Electrochim. Acta, 44, 455 (999). 3. T. F. Fuller and J. Newman, in Modern Apect of Electrochemitry, Vol. 7, R. E. White, J. Bocri, and B. Conway, Editor, p. 359, Plenum Pre, New Yor (995). 4. G. Zheng, B. N. Popov, and R. E. White, J. Appl. Electrochem., 7, 38 (997). 5. D. Berndt and E. Meiner, INTELEC 90 Proceeding of th International Telecommunication Energy Conference, p. 48 (990). 6. D. Pavlov, J. Power Source, 64, 3 (997). 7. C. R. Pal and J. Newman, J. Electrochem. Soc., 4, 374, 38 (995). 8. Y. Chen and J. W. Evan, J. Electrochem. Soc., 43, 708 (996). 9. F. Joubert, J. Bouet, and J.-F. Fauvarque, in Batterie for Portable Application and Electric Vehicle, C. F. Holme and A. R. Landgrebe, Editor, PV 97-8, p. 837, The Electrochemical Society Proceeding Serie, Pennington, NJ (997). 0. M. S. Wu, Y. Y. Wang, and C. C. Wan, J. Power Source, 74, 0 (998).. Z. L. Zhang, M. H. Zhong, F. M. Liu, F. P. Zhong, and F. Wu, J. Power Source, 70, 76 (998).. J. Lee, in Electrochemical and Thermal Modeling of Battery, Fuel cell, and Photoenergy Converion Sytem, J. R. Selman and H. C. Maru, Editor, PV 86-, p. 06, The Electrochemical Society Proceeding Serie, Pennington, NJ (986). 3. J. Newman and W. Tiedemann, J. Electrochem. Soc., 4, 054 (995). 4. K. W. Choi and N. P. Yao, J. Electrochem. Soc., 6, 3 (979). 5. J. Lee, K. W. Choi, N. P. Yao, and C. C. Chritianon, J. Electrochem. Soc., 33, 86 (986). 6. H. Huang and T. V. Nguyen, J. Electrochem. Soc., 44, 06 (997). 7. J. Kim, T. V. Nguyen, and R. E. White, J. Electrochem. Soc., 39, 78 (99). 8. Y. Chen and J. W. Evan, J. Electrochem. Soc., 40, 833 (993). 9. Y. Chen and J. W. Evan, J. Electrochem. Soc., 4, 947 (994). 0. Y. Chen and J. W. Evan, Electrochim. Acta, 39, 57 (994).. M. K. Verbrugge AIChE J., 4, 550 (995).. Y. Chen and J. W. Evan, INTELEC 96 Proceeding of the 8th International Telecommunication Energy Conference, p. 465 (996). 3. W. Tiedemann and J. Newman, in Battery Deign and Optimization, S. Gro, Editor, PV 79-, p. 3, The Electrochemical Society Proceeding Serie, Pennington, NJ (979). 4. W. Tiedemann and J. Newman, in Advance in Lead-Acid Batterie, K. R. Bulloc and D. Pavlov, Editor, PV 84-4, p. 336, The Electrochemical Society Proceeding Serie, Pennington, NJ (984). 5. H. Huang and T. V. Nguyen, J. Electrochem. Soc., 44, 40 (997). 6. J. Kim, T. V. Nguyen, and R. E. White, J. Electrochem. Soc., 4, 333 (994). 7. P. De Vidt, J. Delgado, B. Wu, D. See, K. Koanovicb, and R. E. White, J. Electrochem. Soc., 45, 3874 (998). 8. C. R. Pal, M. Doyle, T. F. Fuller, and J. Newman, in Dougla N. Bennion Memorial Sympoium, R. E. White and J. Newman, Editor, PV 94-, p. 48, The Electrochemical Society Proceeding Serie, Pennington, NJ (994). 9. M. Doyle and J. Newman, Electrochim. Acta, 40, 9 (995). 30. G. G. Botte, B. A. Johnon, and R. E. White, J. Electrochem. Soc., 46, 94 (999). 3. D. Bernardi, E. Pawliowi, and J. Newman, J. Electrochem. Soc., 3, 5 (985). 3. L. Rao and J. Newman, J. Electrochem. Soc., 44, 697 (997). 33. R. B. Bird, W. E. Stewart, and E. N. Lightfoot, Tranport Phenomena, John Wiley & Son, New Yor (960). 34. J. Newman, Electrochemical Sytem, Prentice-Hall, Englewood Cliff, NJ (99). 35. J. Newman, Ind. Eng. Chem. Re., 34, 308 (995). 36. Y. V. Kuzminii, L. I. Nyova, and A. A. Andriio, J. Power Source, 46, 9 (993). 37. Y. V. Kuzminii and A. V. Gorodyii, J. Electroanal. Chem., 5, (988). 38. M. J. Lampinen and M. Fomino, J. Electrochem. Soc., 40, 3537 (993). 39. Q. Xu, S. Keltrup, and B. Hafold, Electrochim. Acta, 43, 597 (998). 40. H. F. Gibbard, J. Electrochem. Soc., 5, 353 (978). 4. D. Berndt, Maintenance-Free Batterie Lead-acid, Nicel/Cadmium, Nicel/ Hydride, John Wiley & Son, New Yor (993). 4. B. Paxton and J. Newman, J. Electrochem. Soc., 44, 388 (997). 43. D. Ohlendorf and H. E. Flotow, J. Le-Common. Met., 73, 5 (980). 44. S. G. Bratch, J. Phy. Chem. Ref. Data, 8, (989). 45. B. V. Ratnaumar, P. Timmerman, C. Sanchez, S. Di Stefano, and G. Halpert, J. Electrochem. Soc., 43, 803 (996). 46. Y. Oinaa, J. Electrochem. Soc., 7, 89 (970). 47. R. E. Davi, G. L. Horvath, and C. W. Tobia, Electrochim. Acta,, 87 (967). 48. D. M. MacArthur, J. Electrochem. Soc., 7, 79 (970). 49. W. B. Gu and C. Y. Wang, in Lithium Batterie, R. A. Marh, Z. Ogumi, J. Praah, and S. Surampudi, Editor, PV 99-5, p. 748, The Electrochemical Society Proceeding Serie, Pennington, NJ (999).
A Single Particle Thermal Model for Lithium Ion Batteries
 A Single Particle Thermal Model for Lithium Ion Batterie R. Painter* 1, B. Berryhill 1, L. Sharpe 2 and S. Keith Hargrove 2 1 Civil Engineering, Tenneee State Univerity, Nahville, TN, USA 2 Mechanical
A Single Particle Thermal Model for Lithium Ion Batterie R. Painter* 1, B. Berryhill 1, L. Sharpe 2 and S. Keith Hargrove 2 1 Civil Engineering, Tenneee State Univerity, Nahville, TN, USA 2 Mechanical
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor
 NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
Molecular Dynamics Simulations of Nonequilibrium Effects Associated with Thermally Activated Exothermic Reactions
 Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
Modeling of Transport and Reaction in a Catalytic Bed Using a Catalyst Particle Model.
 Excerpt from the Proceeding of the COMSOL Conference 2010 Boton Modeling of Tranport and Reaction in a Catalytic Bed Uing a Catalyt Particle Model. F. Allain *,1, A.G. Dixon 1 1 Worceter Polytechnic Intitute
Excerpt from the Proceeding of the COMSOL Conference 2010 Boton Modeling of Tranport and Reaction in a Catalytic Bed Uing a Catalyt Particle Model. F. Allain *,1, A.G. Dixon 1 1 Worceter Polytechnic Intitute
Parameter Sensitivity Analysis to Improve Material Design for Novel Zn-MnO 2 Batteries with Ionic Liquid Electrolytes
 Parameter Senitivity Analyi to Improve Material Deign for Novel Zn-MnO Batterie with Ionic Liquid Electrolyte Zachary T. Gima & Bernard J. Kim CE 95: Spring 06 Abtract Battery deign at the material level
Parameter Senitivity Analyi to Improve Material Deign for Novel Zn-MnO Batterie with Ionic Liquid Electrolyte Zachary T. Gima & Bernard J. Kim CE 95: Spring 06 Abtract Battery deign at the material level
FUNDAMENTALS OF POWER SYSTEMS
 1 FUNDAMENTALS OF POWER SYSTEMS 1 Chapter FUNDAMENTALS OF POWER SYSTEMS INTRODUCTION The three baic element of electrical engineering are reitor, inductor and capacitor. The reitor conume ohmic or diipative
1 FUNDAMENTALS OF POWER SYSTEMS 1 Chapter FUNDAMENTALS OF POWER SYSTEMS INTRODUCTION The three baic element of electrical engineering are reitor, inductor and capacitor. The reitor conume ohmic or diipative
Green-Kubo formulas with symmetrized correlation functions for quantum systems in steady states: the shear viscosity of a fluid in a steady shear flow
 Green-Kubo formula with ymmetrized correlation function for quantum ytem in teady tate: the hear vicoity of a fluid in a teady hear flow Hirohi Matuoa Department of Phyic, Illinoi State Univerity, Normal,
Green-Kubo formula with ymmetrized correlation function for quantum ytem in teady tate: the hear vicoity of a fluid in a teady hear flow Hirohi Matuoa Department of Phyic, Illinoi State Univerity, Normal,
Jump condition at the boundary between a porous catalyst and a homogeneous fluid
 From the SelectedWork of Francico J. Valde-Parada 2005 Jump condition at the boundary between a porou catalyt and a homogeneou fluid Francico J. Valde-Parada J. Alberto Ochoa-Tapia Available at: http://work.bepre.com/francico_j_valde_parada/12/
From the SelectedWork of Francico J. Valde-Parada 2005 Jump condition at the boundary between a porou catalyt and a homogeneou fluid Francico J. Valde-Parada J. Alberto Ochoa-Tapia Available at: http://work.bepre.com/francico_j_valde_parada/12/
Entropy Minimization in Design of Extractive Distillation System with Internal Heat Exchangers
 Entropy Minimization in Deign of Extractive Ditillation Sytem with Internal Heat Exchanger Diego F. Mendoza, Carlo.M. Riaco * Group of Proce Sytem Engineering, Department of Chemical and Environmental
Entropy Minimization in Deign of Extractive Ditillation Sytem with Internal Heat Exchanger Diego F. Mendoza, Carlo.M. Riaco * Group of Proce Sytem Engineering, Department of Chemical and Environmental
7.2 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 281
 72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
Lecture Notes II. As the reactor is well-mixed, the outlet stream concentration and temperature are identical with those in the tank.
 Lecture Note II Example 6 Continuou Stirred-Tank Reactor (CSTR) Chemical reactor together with ma tranfer procee contitute an important part of chemical technologie. From a control point of view, reactor
Lecture Note II Example 6 Continuou Stirred-Tank Reactor (CSTR) Chemical reactor together with ma tranfer procee contitute an important part of chemical technologie. From a control point of view, reactor
Social Studies 201 Notes for November 14, 2003
 1 Social Studie 201 Note for November 14, 2003 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
1 Social Studie 201 Note for November 14, 2003 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
Performance Modeling of the Metal Hydride Electrode
 Performance Modeling of the Metal Hydride Electrode by Bala S. S. Haran, Anand Durairajan, Branko N. Popov and Ralph E. E. White Center for Electrochemical Engineering Department of of Chemical Engineering
Performance Modeling of the Metal Hydride Electrode by Bala S. S. Haran, Anand Durairajan, Branko N. Popov and Ralph E. E. White Center for Electrochemical Engineering Department of of Chemical Engineering
Lecture 13. Thermodynamic Potentials (Ch. 5)
 Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
THE EXPERIMENTAL PERFORMANCE OF A NONLINEAR DYNAMIC VIBRATION ABSORBER
 Proceeding of IMAC XXXI Conference & Expoition on Structural Dynamic February -4 Garden Grove CA USA THE EXPERIMENTAL PERFORMANCE OF A NONLINEAR DYNAMIC VIBRATION ABSORBER Yung-Sheng Hu Neil S Ferguon
Proceeding of IMAC XXXI Conference & Expoition on Structural Dynamic February -4 Garden Grove CA USA THE EXPERIMENTAL PERFORMANCE OF A NONLINEAR DYNAMIC VIBRATION ABSORBER Yung-Sheng Hu Neil S Ferguon
Digital Control System
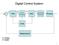 Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Convective Heat Transfer
 Convective Heat Tranfer Example 1. Melt Spinning of Polymer fiber 2. Heat tranfer in a Condener 3. Temperature control of a Re-entry vehicle Fiber pinning The fiber pinning proce preent a unique engineering
Convective Heat Tranfer Example 1. Melt Spinning of Polymer fiber 2. Heat tranfer in a Condener 3. Temperature control of a Re-entry vehicle Fiber pinning The fiber pinning proce preent a unique engineering
Detonation Initiation by Gradient Mechanism in Propane Oxygen and Propane Air Mixtures
 Detonation Initiation by Gradient Mechanim in Propane Oxygen and Propane Air Mixture A.E. Rakitin, I.B. Popov, NEQLab Reearch BV, The Hague, 5AL, The Netherland A.Yu. Starikovkiy Princeton Univerity, Princeton,
Detonation Initiation by Gradient Mechanim in Propane Oxygen and Propane Air Mixture A.E. Rakitin, I.B. Popov, NEQLab Reearch BV, The Hague, 5AL, The Netherland A.Yu. Starikovkiy Princeton Univerity, Princeton,
MAE 101A. Homework 3 Solutions 2/5/2018
 MAE 101A Homework 3 Solution /5/018 Munon 3.6: What preure gradient along the treamline, /d, i required to accelerate water upward in a vertical pipe at a rate of 30 ft/? What i the anwer if the flow i
MAE 101A Homework 3 Solution /5/018 Munon 3.6: What preure gradient along the treamline, /d, i required to accelerate water upward in a vertical pipe at a rate of 30 ft/? What i the anwer if the flow i
A novel protocol for linearization of the Poisson-Boltzmann equation
 Ann. Univ. Sofia, Fac. Chem. Pharm. 16 (14) 59-64 [arxiv 141.118] A novel protocol for linearization of the Poion-Boltzmann equation Roumen Tekov Department of Phyical Chemitry, Univerity of Sofia, 1164
Ann. Univ. Sofia, Fac. Chem. Pharm. 16 (14) 59-64 [arxiv 141.118] A novel protocol for linearization of the Poion-Boltzmann equation Roumen Tekov Department of Phyical Chemitry, Univerity of Sofia, 1164
Gain and Phase Margins Based Delay Dependent Stability Analysis of Two- Area LFC System with Communication Delays
 Gain and Phae Margin Baed Delay Dependent Stability Analyi of Two- Area LFC Sytem with Communication Delay Şahin Sönmez and Saffet Ayaun Department of Electrical Engineering, Niğde Ömer Halidemir Univerity,
Gain and Phae Margin Baed Delay Dependent Stability Analyi of Two- Area LFC Sytem with Communication Delay Şahin Sönmez and Saffet Ayaun Department of Electrical Engineering, Niğde Ömer Halidemir Univerity,
CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS
 CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.1 INTRODUCTION 8.2 REDUCED ORDER MODEL DESIGN FOR LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.3
CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.1 INTRODUCTION 8.2 REDUCED ORDER MODEL DESIGN FOR LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.3
Determination of Flow Resistance Coefficients Due to Shrubs and Woody Vegetation
 ERDC/CL CETN-VIII-3 December 000 Determination of Flow Reitance Coefficient Due to hrub and Woody Vegetation by Ronald R. Copeland PURPOE: The purpoe of thi Technical Note i to tranmit reult of an experimental
ERDC/CL CETN-VIII-3 December 000 Determination of Flow Reitance Coefficient Due to hrub and Woody Vegetation by Ronald R. Copeland PURPOE: The purpoe of thi Technical Note i to tranmit reult of an experimental
Cake ltration analysis the eect of the relationship between the pore liquid pressure and the cake compressive stress
 Chemical Engineering Science 56 (21) 5361 5369 www.elevier.com/locate/ce Cake ltration analyi the eect of the relationhip between the pore liquid preure and the cake compreive tre C. Tien, S. K. Teoh,
Chemical Engineering Science 56 (21) 5361 5369 www.elevier.com/locate/ce Cake ltration analyi the eect of the relationhip between the pore liquid preure and the cake compreive tre C. Tien, S. K. Teoh,
Characterization of the heat transfer in open-cell metal foam
 Characterization of the heat tranfer in open-cell metal foam C. Briano-Calcagno, J. Fontánez-Delgado & N. Dukhan Department of Mechanical Engineering, Univerity of Puerto Rico Mayagüez, Mayagüez, P.R.,
Characterization of the heat tranfer in open-cell metal foam C. Briano-Calcagno, J. Fontánez-Delgado & N. Dukhan Department of Mechanical Engineering, Univerity of Puerto Rico Mayagüez, Mayagüez, P.R.,
Determination of the local contrast of interference fringe patterns using continuous wavelet transform
 Determination of the local contrat of interference fringe pattern uing continuou wavelet tranform Jong Kwang Hyok, Kim Chol Su Intitute of Optic, Department of Phyic, Kim Il Sung Univerity, Pyongyang,
Determination of the local contrat of interference fringe pattern uing continuou wavelet tranform Jong Kwang Hyok, Kim Chol Su Intitute of Optic, Department of Phyic, Kim Il Sung Univerity, Pyongyang,
SOLVING THE KONDO PROBLEM FOR COMPLEX MESOSCOPIC SYSTEMS
 SOLVING THE KONDO POBLEM FO COMPLEX MESOSCOPIC SYSTEMS V. DINU and M. ÞOLEA National Intitute of Material Phyic, Bucharet-Magurele P.O. Box MG-7, omania eceived February 21, 2005 Firt we preent the calculation
SOLVING THE KONDO POBLEM FO COMPLEX MESOSCOPIC SYSTEMS V. DINU and M. ÞOLEA National Intitute of Material Phyic, Bucharet-Magurele P.O. Box MG-7, omania eceived February 21, 2005 Firt we preent the calculation
CHAPTER 4 DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL
 98 CHAPTER DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL INTRODUCTION The deign of ytem uing tate pace model for the deign i called a modern control deign and it i
98 CHAPTER DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL INTRODUCTION The deign of ytem uing tate pace model for the deign i called a modern control deign and it i
Unified Design Method for Flexure and Debonding in FRP Retrofitted RC Beams
 Unified Deign Method for Flexure and Debonding in FRP Retrofitted RC Beam G.X. Guan, Ph.D. 1 ; and C.J. Burgoyne 2 Abtract Flexural retrofitting of reinforced concrete (RC) beam uing fibre reinforced polymer
Unified Deign Method for Flexure and Debonding in FRP Retrofitted RC Beam G.X. Guan, Ph.D. 1 ; and C.J. Burgoyne 2 Abtract Flexural retrofitting of reinforced concrete (RC) beam uing fibre reinforced polymer
Journal of Power Sources
 Journal of Power Source 196 (2011) 196 207 Content lit available at ScienceDirect Journal of Power Source journal homepage: wwweleviercom/locate/jpowour Modeling and control of tubular olid-oxide fuel
Journal of Power Source 196 (2011) 196 207 Content lit available at ScienceDirect Journal of Power Source journal homepage: wwweleviercom/locate/jpowour Modeling and control of tubular olid-oxide fuel
Source slideplayer.com/fundamentals of Analytical Chemistry, F.J. Holler, S.R.Crouch. Chapter 6: Random Errors in Chemical Analysis
 Source lideplayer.com/fundamental of Analytical Chemitry, F.J. Holler, S.R.Crouch Chapter 6: Random Error in Chemical Analyi Random error are preent in every meaurement no matter how careful the experimenter.
Source lideplayer.com/fundamental of Analytical Chemitry, F.J. Holler, S.R.Crouch Chapter 6: Random Error in Chemical Analyi Random error are preent in every meaurement no matter how careful the experimenter.
ESTIMATION OF THE HEAT TRANSFER COEFFICIENT IN THE SPRAY COOLING OF CONTINUOUSLY CAST SLABS
 ESTIMATION OF THE HEAT TRANSFER COEFFICIENT IN THE SPRAY COOLING OF CONTINUOUSLY CAST SLABS Helcio R. B. Orlande and Marcelo J. Colaço Federal Univerity of Rio de Janeiro, UFRJ Department of Mechanical
ESTIMATION OF THE HEAT TRANSFER COEFFICIENT IN THE SPRAY COOLING OF CONTINUOUSLY CAST SLABS Helcio R. B. Orlande and Marcelo J. Colaço Federal Univerity of Rio de Janeiro, UFRJ Department of Mechanical
Introduction to Laplace Transform Techniques in Circuit Analysis
 Unit 6 Introduction to Laplace Tranform Technique in Circuit Analyi In thi unit we conider the application of Laplace Tranform to circuit analyi. A relevant dicuion of the one-ided Laplace tranform i found
Unit 6 Introduction to Laplace Tranform Technique in Circuit Analyi In thi unit we conider the application of Laplace Tranform to circuit analyi. A relevant dicuion of the one-ided Laplace tranform i found
84 ZHANG Jing-Shang Vol. 39 of which would emit 5 He rather than 3 He. 5 He i untable and eparated into n + pontaneouly, which can alo be treated a if
 Commun. Theor. Phy. (Beijing, China) 39 (003) pp. 83{88 c International Academic Publiher Vol. 39, No. 1, January 15, 003 Theoretical Analyi of Neutron Double-Dierential Cro Section of n+ 11 B at 14. MeV
Commun. Theor. Phy. (Beijing, China) 39 (003) pp. 83{88 c International Academic Publiher Vol. 39, No. 1, January 15, 003 Theoretical Analyi of Neutron Double-Dierential Cro Section of n+ 11 B at 14. MeV
Radiation Heat Transfer
 CM30 ranport I Part II: Heat ranfer Radiation Heat ranfer Profeor Faith Morrion Department of Chemical Engineering Michigan echnological Univerity CM30 ranport Procee and Unit Operation I Part : Heat ranfer
CM30 ranport I Part II: Heat ranfer Radiation Heat ranfer Profeor Faith Morrion Department of Chemical Engineering Michigan echnological Univerity CM30 ranport Procee and Unit Operation I Part : Heat ranfer
Non-Equilibrium Phonon Distributions in Sub-100 nm Silicon Transistors
 S. Sinha Thermocience Diviion, Mechanical Engineering Department, Stanford Univerity, California 94305-3030 e-mail: anjiv@tanfordalumni.org E. Pop R. W. Dutton Electrical Engineering Department, Stanford
S. Sinha Thermocience Diviion, Mechanical Engineering Department, Stanford Univerity, California 94305-3030 e-mail: anjiv@tanfordalumni.org E. Pop R. W. Dutton Electrical Engineering Department, Stanford
Calculation of the temperature of boundary layer beside wall with time-dependent heat transfer coefficient
 Ŕ periodica polytechnica Mechanical Engineering 54/1 21 15 2 doi: 1.3311/pp.me.21-1.3 web: http:// www.pp.bme.hu/ me c Periodica Polytechnica 21 RESERCH RTICLE Calculation of the temperature of boundary
Ŕ periodica polytechnica Mechanical Engineering 54/1 21 15 2 doi: 1.3311/pp.me.21-1.3 web: http:// www.pp.bme.hu/ me c Periodica Polytechnica 21 RESERCH RTICLE Calculation of the temperature of boundary
Spot-on: Safe Fuel/Air Compression
 Spot-on: Safe Fuel/Air Compreion Problem preented by Robert Hart and Kevin Hughe Veeder-Root Participant: Jeffrey Bank Joeph Fehribach Alitair Fitt John Ockendon Colin Pleae Don Schwendeman Burt Tilley
Spot-on: Safe Fuel/Air Compreion Problem preented by Robert Hart and Kevin Hughe Veeder-Root Participant: Jeffrey Bank Joeph Fehribach Alitair Fitt John Ockendon Colin Pleae Don Schwendeman Burt Tilley
Lecture 15 - Current. A Puzzle... Advanced Section: Image Charge for Spheres. Image Charge for a Grounded Spherical Shell
 Lecture 15 - Current Puzzle... Suppoe an infinite grounded conducting plane lie at z = 0. charge q i located at a height h above the conducting plane. Show in three different way that the potential below
Lecture 15 - Current Puzzle... Suppoe an infinite grounded conducting plane lie at z = 0. charge q i located at a height h above the conducting plane. Show in three different way that the potential below
Numerical Simulations of Coriolis Flow Meters for Low Reynolds Number Flows
 MAPAN - Journal Numerical of Metrology Simulation Society of of Corioli India, Vol. Flow 26, Meter No. 3, 2011; for Low pp. Reynold 225-235 Number Flow ORIGINAL ARTICLE Numerical Simulation of Corioli
MAPAN - Journal Numerical of Metrology Simulation Society of of Corioli India, Vol. Flow 26, Meter No. 3, 2011; for Low pp. Reynold 225-235 Number Flow ORIGINAL ARTICLE Numerical Simulation of Corioli
Alternate Dispersion Measures in Replicated Factorial Experiments
 Alternate Diperion Meaure in Replicated Factorial Experiment Neal A. Mackertich The Raytheon Company, Sudbury MA 02421 Jame C. Benneyan Northeatern Univerity, Boton MA 02115 Peter D. Krau The Raytheon
Alternate Diperion Meaure in Replicated Factorial Experiment Neal A. Mackertich The Raytheon Company, Sudbury MA 02421 Jame C. Benneyan Northeatern Univerity, Boton MA 02115 Peter D. Krau The Raytheon
Supplementary Figures
 Supplementary Figure Supplementary Figure S1: Extraction of the SOF. The tandard deviation of meaured V xy at aturated tate (between 2.4 ka/m and 12 ka/m), V 2 d Vxy( H, j, hm ) Vxy( H, j, hm ) 2. The
Supplementary Figure Supplementary Figure S1: Extraction of the SOF. The tandard deviation of meaured V xy at aturated tate (between 2.4 ka/m and 12 ka/m), V 2 d Vxy( H, j, hm ) Vxy( H, j, hm ) 2. The
SERIES COMPENSATION: VOLTAGE COMPENSATION USING DVR (Lectures 41-48)
 Chapter 5 SERIES COMPENSATION: VOLTAGE COMPENSATION USING DVR (Lecture 41-48) 5.1 Introduction Power ytem hould enure good quality of electric power upply, which mean voltage and current waveform hould
Chapter 5 SERIES COMPENSATION: VOLTAGE COMPENSATION USING DVR (Lecture 41-48) 5.1 Introduction Power ytem hould enure good quality of electric power upply, which mean voltage and current waveform hould
Lecture 7 Grain boundary grooving
 Lecture 7 Grain oundary grooving The phenomenon. A polihed polycrytal ha a flat urface. At room temperature, the urface remain flat for a long time. At an elevated temperature atom move. The urface grow
Lecture 7 Grain oundary grooving The phenomenon. A polihed polycrytal ha a flat urface. At room temperature, the urface remain flat for a long time. At an elevated temperature atom move. The urface grow
12th International Congress on the Deterioration and Conservation of Stone Columbia University, New York, 2012
 THE INFLUENCE OF OSMOTIC PRESSURE ON POULTICING TREATMENTS Leo Pel, 1 Victoria Voronina 1 and Alion Heritage 2 1 Tranport in Permeable Media, Department of Applied Phyic, Eindhoven Univerity of Technology,
THE INFLUENCE OF OSMOTIC PRESSURE ON POULTICING TREATMENTS Leo Pel, 1 Victoria Voronina 1 and Alion Heritage 2 1 Tranport in Permeable Media, Department of Applied Phyic, Eindhoven Univerity of Technology,
Thermal Resistance Measurements and Thermal Transient Analysis of Power Chip Slug-Up and Slug-Down Mounted on HDI Substrate
 Intl Journal of Microcircuit and Electronic Packaging Thermal Reitance Meaurement and Thermal Tranient Analyi of Power Chip Slug-Up and Slug-Down Mounted on HDI Subtrate Claudio Sartori Magneti Marelli
Intl Journal of Microcircuit and Electronic Packaging Thermal Reitance Meaurement and Thermal Tranient Analyi of Power Chip Slug-Up and Slug-Down Mounted on HDI Subtrate Claudio Sartori Magneti Marelli
What lies between Δx E, which represents the steam valve, and ΔP M, which is the mechanical power into the synchronous machine?
 A 2.0 Introduction In the lat et of note, we developed a model of the peed governing mechanim, which i given below: xˆ K ( Pˆ ˆ) E () In thee note, we want to extend thi model o that it relate the actual
A 2.0 Introduction In the lat et of note, we developed a model of the peed governing mechanim, which i given below: xˆ K ( Pˆ ˆ) E () In thee note, we want to extend thi model o that it relate the actual
A FUNCTIONAL BAYESIAN METHOD FOR THE SOLUTION OF INVERSE PROBLEMS WITH SPATIO-TEMPORAL PARAMETERS AUTHORS: CORRESPONDENCE: ABSTRACT
 A FUNCTIONAL BAYESIAN METHOD FOR THE SOLUTION OF INVERSE PROBLEMS WITH SPATIO-TEMPORAL PARAMETERS AUTHORS: Zenon Medina-Cetina International Centre for Geohazard / Norwegian Geotechnical Intitute Roger
A FUNCTIONAL BAYESIAN METHOD FOR THE SOLUTION OF INVERSE PROBLEMS WITH SPATIO-TEMPORAL PARAMETERS AUTHORS: Zenon Medina-Cetina International Centre for Geohazard / Norwegian Geotechnical Intitute Roger
PRESSURE WORK EFFECTS IN UNSTEADY CONVECTIVELY DRIVEN FLOW ALONG A VERTICAL PLATE
 Proceeding of 3ICCHMT 3 rd International Conference on Computational Heat and Ma Tranfer May 6 3, 3, Banff, CANADA Paper Number 87 PRESSURE WORK EFFECTS IN UNSTEADY CONVECTIVELY DRIVEN FLOW ALONG A VERTICAL
Proceeding of 3ICCHMT 3 rd International Conference on Computational Heat and Ma Tranfer May 6 3, 3, Banff, CANADA Paper Number 87 PRESSURE WORK EFFECTS IN UNSTEADY CONVECTIVELY DRIVEN FLOW ALONG A VERTICAL
Social Studies 201 Notes for March 18, 2005
 1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
Reliability Analysis of Embedded System with Different Modes of Failure Emphasizing Reboot Delay
 International Journal of Applied Science and Engineering 3., 4: 449-47 Reliability Analyi of Embedded Sytem with Different Mode of Failure Emphaizing Reboot Delay Deepak Kumar* and S. B. Singh Department
International Journal of Applied Science and Engineering 3., 4: 449-47 Reliability Analyi of Embedded Sytem with Different Mode of Failure Emphaizing Reboot Delay Deepak Kumar* and S. B. Singh Department
Q.1. x A =0.8, ε A =δ A *y A = 0.8*5=4 (because feed contains 80 mol% A, y A = 0.8, δ A =((6-1)/1)=5) k= 0.3 hr -1. So, θ = hr Q.
 Q.1 k [ 1 ln(1 x)] x x =.8, ε =δ *y =.8*5=4 (becaue feed contain 8 mol%, y =.8, δ =((6-1)/1)=5) k=. hr -1 So, θ = 16.157 hr Q.2 Q.2 Continue (c) V PFR
Q.1 k [ 1 ln(1 x)] x x =.8, ε =δ *y =.8*5=4 (becaue feed contain 8 mol%, y =.8, δ =((6-1)/1)=5) k=. hr -1 So, θ = 16.157 hr Q.2 Q.2 Continue (c) V PFR
Non-linearity parameter B=A of binary liquid mixtures at elevated pressures
 PRAMANA cfl Indian Academy of Science Vol. 55, No. 3 journal of September 2000 phyic pp. 433 439 Non-linearity parameter B=A of binary liquid mixture at elevated preure J D PANDEY, J CHHABRA, R DEY, V
PRAMANA cfl Indian Academy of Science Vol. 55, No. 3 journal of September 2000 phyic pp. 433 439 Non-linearity parameter B=A of binary liquid mixture at elevated preure J D PANDEY, J CHHABRA, R DEY, V
Section Induction motor drives
 Section 5.1 - nduction motor drive Electric Drive Sytem 5.1.1. ntroduction he AC induction motor i by far the mot widely ued motor in the indutry. raditionally, it ha been ued in contant and lowly variable-peed
Section 5.1 - nduction motor drive Electric Drive Sytem 5.1.1. ntroduction he AC induction motor i by far the mot widely ued motor in the indutry. raditionally, it ha been ued in contant and lowly variable-peed
Earth Potential Rise (EPR) Computation for a Fault on Transmission Mains Pole
 ACN: 32586675 ABN: 8632586675 NATIONAL ELECTRICAL ENGINEERING CONULTANCY Earth otential Rie (ER) Computation for a Fault on Tranmiion Main ole repared by: M. Naereddine ACN: 32586675 ABN: 8632586675 Abtract
ACN: 32586675 ABN: 8632586675 NATIONAL ELECTRICAL ENGINEERING CONULTANCY Earth otential Rie (ER) Computation for a Fault on Tranmiion Main ole repared by: M. Naereddine ACN: 32586675 ABN: 8632586675 Abtract
THE PARAMETERIZATION OF ALL TWO-DEGREES-OF-FREEDOM SEMISTRONGLY STABILIZING CONTROLLERS. Tatsuya Hoshikawa, Kou Yamada and Yuko Tatsumi
 International Journal of Innovative Computing, Information Control ICIC International c 206 ISSN 349-498 Volume 2, Number 2, April 206 pp. 357 370 THE PARAMETERIZATION OF ALL TWO-DEGREES-OF-FREEDOM SEMISTRONGLY
International Journal of Innovative Computing, Information Control ICIC International c 206 ISSN 349-498 Volume 2, Number 2, April 206 pp. 357 370 THE PARAMETERIZATION OF ALL TWO-DEGREES-OF-FREEDOM SEMISTRONGLY
STUDY OF THE INFLUENCE OF CONVECTIVE EFFECTS IN INCIDENT RADIATIVE HEAT FLUX DENSITY MEASUREMENT UNCERTAINTY
 XIX IMEKO World Congre Fundamental and Applied Metrology September 6, 009, Libon, Portugal SUDY OF HE INFLUENCE OF CONVECIVE EFFECS IN INCIDEN RADIAIVE HEA FLUX DENSIY MEASUREMEN UNCERAINY L. Lage Martin,
XIX IMEKO World Congre Fundamental and Applied Metrology September 6, 009, Libon, Portugal SUDY OF HE INFLUENCE OF CONVECIVE EFFECS IN INCIDEN RADIAIVE HEA FLUX DENSIY MEASUREMEN UNCERAINY L. Lage Martin,
Chapter 1 Basic Description of Laser Diode Dynamics by Spatially Averaged Rate Equations: Conditions of Validity
 Chapter 1 Baic Decription of Laer Diode Dynamic by Spatially Averaged Rate Equation: Condition of Validity A laer diode i a device in which an electric current input i converted to an output of photon.
Chapter 1 Baic Decription of Laer Diode Dynamic by Spatially Averaged Rate Equation: Condition of Validity A laer diode i a device in which an electric current input i converted to an output of photon.
DEVELOPMENT OF A STRUCTURED THERMOCLINE THERMAL ENERGY STORAGE SYSTEM
 DEVELOPMENT OF A STRUCTURED THERMOCLINE THERMAL ENERGY STORAGE SYSTEM Brad M. Brown Matt N. Straer R. Paneer Selvam Univerity of Arkana Department of Civil Engineering 4190 Bell Engineering Center Fayetteville,
DEVELOPMENT OF A STRUCTURED THERMOCLINE THERMAL ENERGY STORAGE SYSTEM Brad M. Brown Matt N. Straer R. Paneer Selvam Univerity of Arkana Department of Civil Engineering 4190 Bell Engineering Center Fayetteville,
Research Article Reliability of Foundation Pile Based on Settlement and a Parameter Sensitivity Analysis
 Mathematical Problem in Engineering Volume 2016, Article ID 1659549, 7 page http://dxdoiorg/101155/2016/1659549 Reearch Article Reliability of Foundation Pile Baed on Settlement and a Parameter Senitivity
Mathematical Problem in Engineering Volume 2016, Article ID 1659549, 7 page http://dxdoiorg/101155/2016/1659549 Reearch Article Reliability of Foundation Pile Baed on Settlement and a Parameter Senitivity
A Compensated Acoustic Actuator for Systems with Strong Dynamic Pressure Coupling
 A Compenated Acoutic Actuator for Sytem with Strong Dynamic Preure Coupling Submitted to ASME Journal of Vibration and Acoutic July.997 Charle Birdong and Clark J. Radcliffe Department of Mechanical Engineering
A Compenated Acoutic Actuator for Sytem with Strong Dynamic Preure Coupling Submitted to ASME Journal of Vibration and Acoutic July.997 Charle Birdong and Clark J. Radcliffe Department of Mechanical Engineering
Mesoscopic Nonequilibrium Thermodynamics Gives the Same Thermodynamic Basis to Butler-Volmer and Nernst Equations
 J. Phy. Chem. B 2003, 107, 13471-13477 13471 Meocopic Nonequilibrium Thermodynamic Give the Same Thermodynamic Bai to Butler-Volmer and Nernt Equation J. M. Rubi and S. Kjeltrup* Department of Chemitry,
J. Phy. Chem. B 2003, 107, 13471-13477 13471 Meocopic Nonequilibrium Thermodynamic Give the Same Thermodynamic Bai to Butler-Volmer and Nernt Equation J. M. Rubi and S. Kjeltrup* Department of Chemitry,
Module 1: Learning objectives
 Heat and Ma Tranfer Module 1: Learning objective Overview: Although much of the material of thi module will be dicued in greater detail, the objective of thi module i to give you a reaonable overview of
Heat and Ma Tranfer Module 1: Learning objective Overview: Although much of the material of thi module will be dicued in greater detail, the objective of thi module i to give you a reaonable overview of
Study of a Freely Falling Ellipse with a Variety of Aspect Ratios and Initial Angles
 Study of a Freely Falling Ellipe with a Variety of Apect Ratio and Initial Angle Dedy Zulhidayat Noor*, Ming-Jyh Chern*, Tzyy-Leng Horng** *Department of Mechanical Engineering, National Taiwan Univerity
Study of a Freely Falling Ellipe with a Variety of Apect Ratio and Initial Angle Dedy Zulhidayat Noor*, Ming-Jyh Chern*, Tzyy-Leng Horng** *Department of Mechanical Engineering, National Taiwan Univerity
THE THERMOELASTIC SQUARE
 HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
BASIC INDUCTION MOTOR CONCEPTS
 INDUCTION MOTOS An induction motor ha the ame phyical tator a a ynchronou machine, with a different rotor contruction. There are two different type of induction motor rotor which can be placed inide the
INDUCTION MOTOS An induction motor ha the ame phyical tator a a ynchronou machine, with a different rotor contruction. There are two different type of induction motor rotor which can be placed inide the
EP225 Note No. 5 Mechanical Waves
 EP5 Note No. 5 Mechanical Wave 5. Introduction Cacade connection of many ma-pring unit conitute a medium for mechanical wave which require that medium tore both kinetic energy aociated with inertia (ma)
EP5 Note No. 5 Mechanical Wave 5. Introduction Cacade connection of many ma-pring unit conitute a medium for mechanical wave which require that medium tore both kinetic energy aociated with inertia (ma)
Robust Decentralized Design of H -based Frequency Stabilizer of SMES
 International Energy Journal: Vol. 6, No., Part, June 005-59 Robut Decentralized Deign of H -baed Frequency Stabilizer of SMES www.erd.ait.ac.th/reric C. Vorakulpipat *, M. Leelajindakrirerk *, and I.
International Energy Journal: Vol. 6, No., Part, June 005-59 Robut Decentralized Deign of H -baed Frequency Stabilizer of SMES www.erd.ait.ac.th/reric C. Vorakulpipat *, M. Leelajindakrirerk *, and I.
CONTROL SYSTEMS, ROBOTICS AND AUTOMATION Vol. VIII Decoupling Control - M. Fikar
 DECOUPLING CONTROL M. Fikar Department of Proce Control, Faculty of Chemical and Food Technology, Slovak Univerity of Technology in Bratilava, Radlinkého 9, SK-812 37 Bratilava, Slovakia Keyword: Decoupling:
DECOUPLING CONTROL M. Fikar Department of Proce Control, Faculty of Chemical and Food Technology, Slovak Univerity of Technology in Bratilava, Radlinkého 9, SK-812 37 Bratilava, Slovakia Keyword: Decoupling:
Efficient Methods of Doppler Processing for Coexisting Land and Weather Clutter
 Efficient Method of Doppler Proceing for Coexiting Land and Weather Clutter Ça gatay Candan and A Özgür Yılmaz Middle Eat Technical Univerity METU) Ankara, Turkey ccandan@metuedutr, aoyilmaz@metuedutr
Efficient Method of Doppler Proceing for Coexiting Land and Weather Clutter Ça gatay Candan and A Özgür Yılmaz Middle Eat Technical Univerity METU) Ankara, Turkey ccandan@metuedutr, aoyilmaz@metuedutr
LOAD FREQUENCY CONTROL OF MULTI AREA INTERCONNECTED SYSTEM WITH TCPS AND DIVERSE SOURCES OF POWER GENERATION
 G.J. E.D.T.,Vol.(6:93 (NovemberDecember, 03 ISSN: 39 793 LOAD FREQUENCY CONTROL OF MULTI AREA INTERCONNECTED SYSTEM WITH TCPS AND DIVERSE SOURCES OF POWER GENERATION C.Srinivaa Rao Dept. of EEE, G.Pullaiah
G.J. E.D.T.,Vol.(6:93 (NovemberDecember, 03 ISSN: 39 793 LOAD FREQUENCY CONTROL OF MULTI AREA INTERCONNECTED SYSTEM WITH TCPS AND DIVERSE SOURCES OF POWER GENERATION C.Srinivaa Rao Dept. of EEE, G.Pullaiah
Determination of Load Dependent Thermal Conductivity of Porous Adsorbents
 Determination of Load Dependent Thermal Conductivity of Porou Adorbent Kraft 1, Gaier 1, Stripf 1, Hee 2 1 Univerity of Applied Science Karlruhe, Baden-ürttemberg, Germany 2 TU Dreden Abtract: Standard
Determination of Load Dependent Thermal Conductivity of Porou Adorbent Kraft 1, Gaier 1, Stripf 1, Hee 2 1 Univerity of Applied Science Karlruhe, Baden-ürttemberg, Germany 2 TU Dreden Abtract: Standard
Lecture 4 Topic 3: General linear models (GLMs), the fundamentals of the analysis of variance (ANOVA), and completely randomized designs (CRDs)
 Lecture 4 Topic 3: General linear model (GLM), the fundamental of the analyi of variance (ANOVA), and completely randomized deign (CRD) The general linear model One population: An obervation i explained
Lecture 4 Topic 3: General linear model (GLM), the fundamental of the analyi of variance (ANOVA), and completely randomized deign (CRD) The general linear model One population: An obervation i explained
IEOR 3106: Fall 2013, Professor Whitt Topics for Discussion: Tuesday, November 19 Alternating Renewal Processes and The Renewal Equation
 IEOR 316: Fall 213, Profeor Whitt Topic for Dicuion: Tueday, November 19 Alternating Renewal Procee and The Renewal Equation 1 Alternating Renewal Procee An alternating renewal proce alternate between
IEOR 316: Fall 213, Profeor Whitt Topic for Dicuion: Tueday, November 19 Alternating Renewal Procee and The Renewal Equation 1 Alternating Renewal Procee An alternating renewal proce alternate between
R. W. Erickson. Department of Electrical, Computer, and Energy Engineering University of Colorado, Boulder
 R. W. Erickon Department of Electrical, Computer, and Energy Engineering Univerity of Colorado, Boulder Cloed-loop buck converter example: Section 9.5.4 In ECEN 5797, we ued the CCM mall ignal model to
R. W. Erickon Department of Electrical, Computer, and Energy Engineering Univerity of Colorado, Boulder Cloed-loop buck converter example: Section 9.5.4 In ECEN 5797, we ued the CCM mall ignal model to
Optimal Coordination of Samples in Business Surveys
 Paper preented at the ICES-III, June 8-, 007, Montreal, Quebec, Canada Optimal Coordination of Sample in Buine Survey enka Mach, Ioana Şchiopu-Kratina, Philip T Rei, Jean-Marc Fillion Statitic Canada New
Paper preented at the ICES-III, June 8-, 007, Montreal, Quebec, Canada Optimal Coordination of Sample in Buine Survey enka Mach, Ioana Şchiopu-Kratina, Philip T Rei, Jean-Marc Fillion Statitic Canada New
III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SUBSTANCES
 III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SBSTANCES. Work purpoe The analyi of the behaviour of a ferroelectric ubtance placed in an eternal electric field; the dependence of the electrical polariation
III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SBSTANCES. Work purpoe The analyi of the behaviour of a ferroelectric ubtance placed in an eternal electric field; the dependence of the electrical polariation
Designing scroll expanders for use in heat recovery Rankine cycles
 Deigning croll expander for ue in heat recovery Rankine cycle V Lemort, S Quoilin Thermodynamic Laboratory, Univerity of Liège, Belgium ABSTRACT Thi paper firt invetigate experimentally the performance
Deigning croll expander for ue in heat recovery Rankine cycle V Lemort, S Quoilin Thermodynamic Laboratory, Univerity of Liège, Belgium ABSTRACT Thi paper firt invetigate experimentally the performance
Bogoliubov Transformation in Classical Mechanics
 Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
External Flow: Flow over Bluff Objects (Cylinders, Spheres, Packed Beds) and Impinging Jets
 External Flow: Flow over Bluff Object (Cylinder, Sphere, Packed Bed) and Impinging Jet he Cylinder in Cro Flow - Condition depend on pecial feature of boundary layer development, including onet at a tagnation
External Flow: Flow over Bluff Object (Cylinder, Sphere, Packed Bed) and Impinging Jet he Cylinder in Cro Flow - Condition depend on pecial feature of boundary layer development, including onet at a tagnation
Swelling Behavior of Anionic Acrylamide-Based Hydrogels in Aqueous Salt Solutions: Comparison of Experiment with Theory
 Swelling Behavior of Anionic Acrylamide-Baed Hydrogel in Aqueou Salt Solution: Comparion of Experiment with Theory OĞUZ OKAY, 1,2 SAFIYE B. SARIIŞIK, 1 SIBEL D. ZOR 1 1 Department of Chemitry, Kocaeli
Swelling Behavior of Anionic Acrylamide-Baed Hydrogel in Aqueou Salt Solution: Comparion of Experiment with Theory OĞUZ OKAY, 1,2 SAFIYE B. SARIIŞIK, 1 SIBEL D. ZOR 1 1 Department of Chemitry, Kocaeli
CHARGING OF DUST IN A NEGATIVE ION PLASMA (APS DPP-06 Poster LP )
 1 CHARGING OF DUST IN A NEGATIVE ION PLASMA (APS DPP-06 Poter LP1.00128) Robert Merlino, Ro Fiher, Su Hyun Kim And Nathan Quarderer Department of Phyic and Atronomy, The Univerity of Iowa Abtract. We invetigate
1 CHARGING OF DUST IN A NEGATIVE ION PLASMA (APS DPP-06 Poter LP1.00128) Robert Merlino, Ro Fiher, Su Hyun Kim And Nathan Quarderer Department of Phyic and Atronomy, The Univerity of Iowa Abtract. We invetigate
Hybrid Projective Dislocated Synchronization of Liu Chaotic System Based on Parameters Identification
 www.ccenet.org/ma Modern Applied Science Vol. 6, No. ; February Hybrid Projective Dilocated Synchronization of Liu Chaotic Sytem Baed on Parameter Identification Yanfei Chen College of Science, Guilin
www.ccenet.org/ma Modern Applied Science Vol. 6, No. ; February Hybrid Projective Dilocated Synchronization of Liu Chaotic Sytem Baed on Parameter Identification Yanfei Chen College of Science, Guilin
Advanced D-Partitioning Analysis and its Comparison with the Kharitonov s Theorem Assessment
 Journal of Multidiciplinary Engineering Science and Technology (JMEST) ISSN: 59- Vol. Iue, January - 5 Advanced D-Partitioning Analyi and it Comparion with the haritonov Theorem Aement amen M. Yanev Profeor,
Journal of Multidiciplinary Engineering Science and Technology (JMEST) ISSN: 59- Vol. Iue, January - 5 Advanced D-Partitioning Analyi and it Comparion with the haritonov Theorem Aement amen M. Yanev Profeor,
Fundamental Physics of Force and Energy/Work:
 Fundamental Phyic of Force and Energy/Work: Energy and Work: o In general: o The work i given by: dw = F dr (5) (One can argue that Eqn. 4 and 5 are really one in the ame.) o Work or Energy are calar potential
Fundamental Phyic of Force and Energy/Work: Energy and Work: o In general: o The work i given by: dw = F dr (5) (One can argue that Eqn. 4 and 5 are really one in the ame.) o Work or Energy are calar potential
Finding the location of switched capacitor banks in distribution systems based on wavelet transform
 UPEC00 3t Aug - 3rd Sept 00 Finding the location of witched capacitor bank in ditribution ytem baed on wavelet tranform Bahram nohad Shahid Chamran Univerity in Ahvaz bahramnohad@yahoo.com Mehrdad keramatzadeh
UPEC00 3t Aug - 3rd Sept 00 Finding the location of witched capacitor bank in ditribution ytem baed on wavelet tranform Bahram nohad Shahid Chamran Univerity in Ahvaz bahramnohad@yahoo.com Mehrdad keramatzadeh
Clustering Methods without Given Number of Clusters
 Clutering Method without Given Number of Cluter Peng Xu, Fei Liu Introduction A we now, mean method i a very effective algorithm of clutering. It mot powerful feature i the calability and implicity. However,
Clutering Method without Given Number of Cluter Peng Xu, Fei Liu Introduction A we now, mean method i a very effective algorithm of clutering. It mot powerful feature i the calability and implicity. However,
To appear in International Journal of Numerical Methods in Fluids in Stability analysis of numerical interface conditions in uid-structure therm
 To appear in International Journal of Numerical Method in Fluid in 997. Stability analyi of numerical interface condition in uid-tructure thermal analyi M. B. Gile Oxford Univerity Computing Laboratory
To appear in International Journal of Numerical Method in Fluid in 997. Stability analyi of numerical interface condition in uid-tructure thermal analyi M. B. Gile Oxford Univerity Computing Laboratory
Coupling of Three-Phase Sequence Circuits Due to Line and Load Asymmetries
 Coupling of Three-Phae Sequence Circuit Due to Line and Load Aymmetrie DEGO BELLAN Department of Electronic nformation and Bioengineering Politecnico di Milano Piazza Leonardo da inci 01 Milano TALY diego.ellan@polimi.it
Coupling of Three-Phae Sequence Circuit Due to Line and Load Aymmetrie DEGO BELLAN Department of Electronic nformation and Bioengineering Politecnico di Milano Piazza Leonardo da inci 01 Milano TALY diego.ellan@polimi.it
THEORY FOR HOPPER SEDIMENTATION.
 THEORY FOR HOPPER SEDIMENTATION. Dr.ir. S.A. Miedema 1 Prof.ir. W.J. Vlablom ABSTRACT. The edimentation proce in the hopper of a Trailing Suction Hopper Dredge (TSHD) i very complex. However it i debatable
THEORY FOR HOPPER SEDIMENTATION. Dr.ir. S.A. Miedema 1 Prof.ir. W.J. Vlablom ABSTRACT. The edimentation proce in the hopper of a Trailing Suction Hopper Dredge (TSHD) i very complex. However it i debatable
Recent progress in fire-structure analysis
 EJSE Special Iue: Selected Key Note paper from MDCMS 1 1t International Conference on Modern Deign, Contruction and Maintenance of Structure - Hanoi, Vietnam, December 2007 Recent progre in fire-tructure
EJSE Special Iue: Selected Key Note paper from MDCMS 1 1t International Conference on Modern Deign, Contruction and Maintenance of Structure - Hanoi, Vietnam, December 2007 Recent progre in fire-tructure
High-field behavior: the law of approach to saturation (Is there an equation for the magnetization at high fields?)
 High-field behavior: the law of approach to aturation (I there an equation for the magnetization at high field? In the high-field region the magnetization approache aturation. The firt attempt to give
High-field behavior: the law of approach to aturation (I there an equation for the magnetization at high field? In the high-field region the magnetization approache aturation. The firt attempt to give
Improving the Efficiency of a Digital Filtering Scheme for Diabatic Initialization
 1976 MONTHLY WEATHER REVIEW VOLUME 15 Improving the Efficiency of a Digital Filtering Scheme for Diabatic Initialization PETER LYNCH Met Éireann, Dublin, Ireland DOMINIQUE GIARD CNRM/GMAP, Météo-France,
1976 MONTHLY WEATHER REVIEW VOLUME 15 Improving the Efficiency of a Digital Filtering Scheme for Diabatic Initialization PETER LYNCH Met Éireann, Dublin, Ireland DOMINIQUE GIARD CNRM/GMAP, Météo-France,
Fluid-structure coupling analysis and simulation of viscosity effect. on Coriolis mass flowmeter
 APCOM & ISCM 11-14 th December, 2013, Singapore luid-tructure coupling analyi and imulation of vicoity effect on Corioli ma flowmeter *Luo Rongmo, and Wu Jian National Metrology Centre, A*STAR, 1 Science
APCOM & ISCM 11-14 th December, 2013, Singapore luid-tructure coupling analyi and imulation of vicoity effect on Corioli ma flowmeter *Luo Rongmo, and Wu Jian National Metrology Centre, A*STAR, 1 Science
Liquid cooling
 SKiiPPACK no. 3 4 [ 1- exp (-t/ τ )] + [( P + P )/P ] R [ 1- exp (-t/ τ )] Z tha tot3 = R ν ν tot1 tot tot3 thaa-3 aa 3 ν= 1 3.3.6. Liquid cooling The following table contain the characteritic R ν and
SKiiPPACK no. 3 4 [ 1- exp (-t/ τ )] + [( P + P )/P ] R [ 1- exp (-t/ τ )] Z tha tot3 = R ν ν tot1 tot tot3 thaa-3 aa 3 ν= 1 3.3.6. Liquid cooling The following table contain the characteritic R ν and
Singular perturbation theory
 Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
AMS 212B Perturbation Methods Lecture 20 Part 1 Copyright by Hongyun Wang, UCSC. is the kinematic viscosity and ˆp = p ρ 0
 Lecture Part 1 Copyright by Hongyun Wang, UCSC Prandtl boundary layer Navier-Stoke equation: Conervation of ma: ρ t + ( ρ u) = Balance of momentum: u ρ t + u = p+ µδ u + ( λ + µ ) u where µ i the firt
Lecture Part 1 Copyright by Hongyun Wang, UCSC Prandtl boundary layer Navier-Stoke equation: Conervation of ma: ρ t + ( ρ u) = Balance of momentum: u ρ t + u = p+ µδ u + ( λ + µ ) u where µ i the firt
Lecture 23 Date:
 Lecture 3 Date: 4.4.16 Plane Wave in Free Space and Good Conductor Power and Poynting Vector Wave Propagation in Loy Dielectric Wave propagating in z-direction and having only x-component i given by: E
Lecture 3 Date: 4.4.16 Plane Wave in Free Space and Good Conductor Power and Poynting Vector Wave Propagation in Loy Dielectric Wave propagating in z-direction and having only x-component i given by: E
( 7) ( 9) ( 8) Applying Thermo: an Example of Kinetics - Diffusion. Applying Thermo: an Example of Kinetics - Diffusion. dw = F dr = dr (6) r
 Fundamental Phyic of Force and Energy/Work: Energy and Work: o In general: o The work i given by: dw = F dr (5) (One can argue that Eqn. 4 and 5 are really one in the ame.) o Work or Energy are calar potential
Fundamental Phyic of Force and Energy/Work: Energy and Work: o In general: o The work i given by: dw = F dr (5) (One can argue that Eqn. 4 and 5 are really one in the ame.) o Work or Energy are calar potential
Equivalent Strain in Simple Shear Deformations
 Equivalent Strain in Simple Shear Deformation Yan Beygelzimer Donetk Intitute of Phyic and Engineering The National Academy of Science of Ukraine Abtract We how that imple hear and pure hear form two group
Equivalent Strain in Simple Shear Deformation Yan Beygelzimer Donetk Intitute of Phyic and Engineering The National Academy of Science of Ukraine Abtract We how that imple hear and pure hear form two group
