Mesoscopic Nonequilibrium Thermodynamics Gives the Same Thermodynamic Basis to Butler-Volmer and Nernst Equations
|
|
|
- Erik Black
- 5 years ago
- Views:
Transcription
1 J. Phy. Chem. B 2003, 107, Meocopic Nonequilibrium Thermodynamic Give the Same Thermodynamic Bai to Butler-Volmer and Nernt Equation J. M. Rubi and S. Kjeltrup* Department of Chemitry, Faculty of Natural Science and Technology, Norwegian UniVerity of Science and Technology, Trondheim, 7491-Norway ReceiVed: May 5, 2003; In Final Form: September 16, 2003 Meocopic nonequilibrium thermodynamic i ued to derive the Butler-Volmer equation, or the tationary tate nonlinear relation between the electric current denity and the overpotential of an electrode urface. The equation i derived from a linear flux-force relationhip at the meocopic level for the oxidation of a reactant to it charged component. The urface wa defined with exce variable (a Gibb urface). The Butler- Volmer equation wa derived uing the aumption of local electrochemical equilibrium in the urface on the meocopic level. The reult wa valid for an iothermal electrode with reaction-controlled charge tranfer and with equilibrium for the reactant between the adjacent bulk phae and the urface. The formulation that i ued for the meocopic level i conitent with nonequilibrium thermodynamic for urface and, thu, with the econd law of thermodynamic. The Nernt equation i recovered in the reverible limit. The reverible/ diipative nature of the charge-tranfer proce i dicued on thi bai. 1. Introduction The theory of reaction kinetic 1 ha been ued uccefully to give alo electrode kinetic a broader bai. By introducing the law of ma action, the concept of unidirectional rate, and the tranition tate, the Butler-Volmer equation i derived, and it ha been hown to contain alo the Nernt equation, ee, for example, ref 2-4. Alo, the Marcu theory ue the concept of unidirectional rate and the tranition tate. 5 The Butler-Volmer equation, the central equation in electrode kinetic, give the current denity j a a nonlinear function of the overpotential η: j ) j 0 [e (1-R)ηF/RT - e -RηF/RT ] (1) Here j 0 i the equilibrium exchange current denity, the rate of formation of product or reactant in equilibrium, and R i the tranfer coefficient, that decribe how the electric potential alter the activation energy barrier of the reaction. The ymbol F, R, and T are Faraday contant, the ga contant, and the abolute temperature. The exchange current denity i a function of the activation energy E a and the height of the barrier j 0 ) Be -E a/rt where B i a pre-exponential factor. At equilibrium, one can derive the Nernt equation from thi equation in a imilar way a one derive the equilibrium contant in reaction kinetic theory. The Nernt equation decribe the reverible converion of the reaction Gibb energy r G into electric energy φ, when one mole of charge i * To whom correpondence hould be addreed. On leave from Department of Fundamental Phyic, Univerity of Barcelona, Spain. (2) ( φ) jf0 )- r G/F (3) tranferred in the outer circuit. For large overpotential, one of the exponential term in the Butler-Volmer equation can be neglected, and one obtain the Tafel equation. The ytem i then conidered to be far from equilibrium. More complicated expreion than eq 1 are available; ee, for example, ref 3. The Nernt equation ha it firm bai alo in thermodynamic, but the Butler-Volmer equation ha o far a kinetic bai only. Far from chemical and thermodynamic equilibrium, it i relevant to ak: I there really a bai for uing thermodynamic function to decribe energy converion at the electrode urface? In thi work, we how that a thermodynamic bai can be etablihed for the Butler-Volmer equation and that the Nernt equation i contained in the ame formulation. The aim of thi work i to etablih a well defined thermodynamic bai for the decription of energy converion during electrode reaction. Such a bai can be found from a branch of thermodynamic that ha not yet been applied to electrochemitry, namely meocopic nonequilibrium thermodynamic. 6 The theory ha o far been applied to chemical reaction 7 and particle motion with inertial effect. 8 Through the application of meocopic nonequilibrium thermodynamic to energy converion procee at the electrode, we hall derive eq 1, but with a different et of aumption than uual. The urpriing finding i that we hall need the aumption of local electrochemical equilibrium in the reaction coordinate pace. In reaction kinetic, one normally aume equilibrium between the reactant and the activated pecie, but thi aumption i not needed here. We hall alo ee that the rate equation in eq 1 originate from an expreion that tart with the net rate of reaction, imilar to well-known rate equation uch a Fourier and Fick law. A thermodynamic bai for the equation can thu be given, uing nonequilibrium thermodynamic theory for the meocopic level. We hall accomplih thi by putting together well-known concept in a new way. We hall firt define the electrode urface following Gibb, 9 uing hi exce variable (ection /jp030572g CCC: $ American Chemical Society Publihed on Web 11/08/2003
2 13472 J. Phy. Chem. B, Vol. 107, No. 48, 2003 Rubi and Kjeltrup 2). In ection 3 we give the entropy production rate for the urface. Thi wa done already for everal cae and alo for a polarized electrode. The internal coordinate from meocopic nonequilibrium thermodynamic i in thi context the reaction coordinate. The reaction coordinate i introduced in ection 4, and the particular of the meocopic nonequilibrium thermodynamic are given. By uing thi theory, we can arrive at an expreion for the entropy production rate at the meocopic level under certain condition. The overpotential of an electrode i defined from it meaurement 14 in ection 5. Under iothermal, tationary tate condition, with equilibrium for the reactant at the urface and with local electrochemical equilibrium along the reaction coordinate, we obtain the Butler-Volmer equation in ection 6 without conidering the unidirectional rate in the electrode reaction. The flux-force relationhip on the meocopic level i linear and conform completely to nonequilibrium thermodynamic theory. However, at the integrated level, the level that i related to meaurement, the relationhip become nonlinear. 2. The Sytem The electrode urface i here regarded a an open, autonomou thermodynamic ytem, fully decribed by it exce variable. It decription i tandard in equilibrium 3 and originate from Gibb. 9 A an example we chooe the electrochemical (anodic) oxidation of the metal A at an anode: A f A + + e - (4) The urface ha an extenion in pace that contain A, A +, and e -. The urface contain A in the amount Γ A and the charged particle in the amount Γ A + and Γe -. We chooe the extenion of the urface o that it i electroneutral. For the exce denitie, thi give Γ A + ) Γ e - (5) Since the electron are predominantly preent in the conduction band of the metal and the cation are predominantly facing the electrolyte double layer, the urface i polarized. Mot likely there i therefore alo a pecific adorption of electrolyte from the olution to the polarized urface. The urface exce denitie are determined according to the precription of Gibb 9 and are given for intance in unit of mol/m 2. The charged pecie give the urface a polarization P (in C/m). The additional extenive variable of the urface are the entropy denity and the internal energy denity u. Ma conervation for A give r A ) dγ A+ )- dγ A dt dt where r A i the rate of the anodic reaction. Chemical energy i tranformed into electrical energy in the electrochemical reaction in eq 4. In order for A to eparate into charge, A + and e -, A goe through an activated tate. The beginning and final tate in thi proce are normally, and alo here, thought of a being connected by a path. The ditance along the path i meaured by a reaction coordinate γ. The coordinate i in tate pace and i called an internal variable in (6) meocopic nonequilibrium thermodynamic. There i no poible external control for thi type of variable. The derivation that follow can be carried out for other cae than our example (eq 4), following the procedure that i outlined below. 3. Entropy Production Rate for an Electrode Surface The entropy balance for the urface i where i the urface exce entropy and J i the entropy flux; ee, for example, ref 15 The upercript i mean into the urface, and o mean out of the area element. The area element i now called the thermodynamic urface or imply the urface. The entropy flux contain the meaurable heat flux, J q, and the thermodynamic entropie time their correponding ma flux, here for A only. The tranported entropy of A +, S / A +, i included in J q in thi choice of fluxe. The exce entropy production rate of a polarized electrode urface, σ, wa given by Bedeaux and Kjeltrup Ratkje. 10 It i found in each cae from the Gibb equation and the firt law for the urface. In thi ytem, the Gibb equation i T d ) du - (µ A + where ɛ 0 i the dielectric contant of the vacuum, D eq i the diplacement field of the adjacent phae, and µ i i the chemical potential. The time rate of change in the exce variable in the Gibb equation give T d dt ) du with the reaction Gibb energy for the reaction that take place in the urface The firt law for the urface i d dt ) J i - J o + σ (7) J ) 1 T J q + J A S A (8) + µ e - - µ A )dγ A + - D eq dp (9) ɛ 0 dt - G r A - D eq G ) (µ A + The fluxe are determined by their value in the homogeneou phae adjacent to the urface. There i a heat flux into the urface, J q i, and out of the urface, J q o, and a flux of A into the urface, J A i, that carrie enthalpy H A. The electrical potential jump φ time the electric current denity j give the electric energy delivered by the urface, and D i the diplacement field when the ytem i out of equilibrium. The firt law decribe how upplied electric energy i tranformed into internal energy (enthalpy) and heat, and how the tate of polarization i changed or, vice vera, how fluxe of enthalpy, heat, and polarization can give electric energy. ɛ 0 dp dt (10) + µ e - - µ A ) (11) du dt ) J i q + J i A H A - J o q - j φ + D dp ɛ 0 dt (12)
3 Meocopic Nonequilibrium Thermodynamic J. Phy. Chem. B, Vol. 107, No. 48, By introducing the firt law into eq 10, rearranging the term, and comparing the reult with the entropy balance (eq 7 and 8), one find the exce entropy production rate (ee ref 10 for more detail): σ ) J q i( 1 T - 1 i) T qo( + J 1 T - 1 ) o T - 1 T [µ A,T - µ m A,T ]J o A - The chemical potential, µ A,T r A - 1 T G T j φ + 1 (D - D ) eq dp T ɛ 0 dt and µ m A,T, are taken at the ame temperature, here the temperature of the electrolyte. The upercript m refer to the electrolyte. To compare with experiment, we hall introduce the condition of an iothermal urface, with the ame chemical potential of A a that of it adjacent bulk phae (µ A,T ) µ m A,T ). The deviation of the diplacement field from it equilibrium value i equal and oppoite to the electric potential difference acro the urface (D - D eq ) ɛ 0 (13) )- φ d (14) with d being the urface thickne. The expreion 1/d dp /dt i the diplacement current j dipl, and j tot ) j + j dipl (15) By introducing thee relation, we are left with two term in the exce entropy production rate σ )- 1 T j tot φ - r A T G (16) Thi entropy production rate give two fluxe in the ytem, linearly related to their force. j tot )-L 11 φ - L 12 G (17) r A )-L 21 φ - L 22 G (18) Relation uch a thee were ued to find the urface impedance 13 and Randle equivalent circuit. The urface impedance i found by applying an ocillation potential to the urface and recording the correponding ocillating electric current denity. The chemical force in thi experiment i independent of the electrical force if the urface can accumulate reactant or product. For a urface with contant polarization, like here, the term containing dp /dt diappear, and j ) j tot. Under contant polarization, the two driving force are no longer independent and r A ) j tot /F ) j/f (19) Charge conervation in the urface mean that the electric current denity j i contant through the urface and equal to the flux of electron or the flux of poitive ion. All derivation from now on are made for thi condition. The entropy production rate i reduced to σ )- 1 T j G [ φ + F ] (20) The well-known limiting value of thi expreion for zero electric current can now be found: lim(σ ) jf0 )- j G [ φ + T F ] ) 0 (21) The Nernt equation reult in ( φ) jf0 )- G jf0 F Here refer to the tate of the product and the tate of the reactant in the urface. With repect to the reactant and product, we peak of a two-tate decription on the macrocopic thermodynamic level. Equation 22 expree that the chemical energy i completely tranformed into electrical energy. The potential jump acro the urface i defined by a reverible tranformation of (all) chemical energy in the urface into electrical energy. Under a reverible tranformation, there i a balance between the electrical force and the chemical force. The urface i not in chemical equilibrium a long a G i different from zero, but it may be regarded a being in electrochemical equilibrium, when eq 22 applie. Thi i in agreement with the common perception of electrochemical equilibrium. According to nonequilibrium thermodynamic theory, 15 the exce entropy production rate (eq 20) give the following fluxforce relationhip: The problem to be olved i now how we can reconcile thi eemingly linear relation with the common Butler-Volmer equation. We hall ee that thi can be done by introducing the variable that change continuouly between the two chemical tate, the reaction coordinate or the internal coordinate, mentioned in the preceding ection. 4. A Continuou Reaction Path with Local Electrochemical Equilibrium (22) G j )-L[ φ + F ] (23) The entropy production rate given above refer to the Gibb urface. The electric potential wa the integral acro the urface, and the chemical reaction wa given a the difference between two chemical tate, the tate of the product and the tate of the reactant. It i poible to find another expreion, if we introduce the reaction coordinate γ from reaction kinetic theory 1 and replace the difference by integral. The different value of the γ coordinate correpond to the different configuration that the ytem adopt during it evolution from the inital to the final tate. By doing thi, we change to a meocopic level in the decription. The variable at thi level cannot be controlled from the outide of the ytem. The thermodynamic theory dealing with thi level i therefore called meocopic nonequilibrium thermodynamic. Since j and r A are contant, we are eeking a variation in φ(γ) and G(γ) that obey σ )- j γ T γ1 2[ φ(γ) γ + 1 G (γ) F γ ] dγ (24)
4 13474 J. Phy. Chem. B, Vol. 107, No. 48, 2003 Rubi and Kjeltrup Figure 1. Effective chemical potential a a function of the internal coordinate γ. Thi variation give a poitive driving force and a poitive entropy production at any location in γ pace. The electric potential difference i thu φ ) γ1 γ 2 φ(γ) γ dγ ) φ(γ 2 ) - φ(γ 1 ) (25) and the reaction Gibb energy i likewie recovered by G ) γ1 γ 2 G (γ) γ dγ ) µ A+(γ 2 ) + µ e -(γ 2 ) - µ A (γ 1 ) (26) The difference ign in the above two equation i now referring to difference in tate pace. We can define the effective chemical potential of the reactant in tate 1 µ A(γ 1 ) ) µ A (γ 1 ) + Fφ(γ 1 ) (27) and the effective chemical potential for the product in tate 2 µ A+e-(γ 2 ) ) µ A +(γ 2 ) + µ e -(γ 2 ) + Fφ(γ 2 ) (28) Thi effective chemical potential i equal to the electrochemical potential of A +, when the chemical potential of electron i mall. To make poible a ditinction from the electrochemical potential, we introduce the name effective chemical potential. In the next tep, we aume that thee expreion are valid at any location in the internal coordinate pace, that i, that the normal thermodynamic relation apply in γ-pace: µ A(γ) ) µ A (γ) + Fφ(γ) µ A+e-(γ) ) µ A +(γ) + µ e -(γ) + Fφ(γ) (29) We then move one tep further and introduce the aumption of local electrochemical equilibrium in the internal coordinate pace. With local equilibrium along the coordinate γ, we have µ A +(γ) + µ e -(γ) + Fφ(γ) ) µ A (γ) + Fφ(γ) ) µ (γ) (30) The effective chemical potential of the reactant i alway equal to the effective chemical potential of the product. In other word, there i local equilibrium at any coordinate by thi criterion for equilibrium. The phyical meaning of thi condition i a follow: At each coordinate, along the continuou path between the product and the reactant tate, there i an enemble of particle of a particular energy and tate of polarization. The energy of any tate along the reaction path i now expreed imply by µ (γ). The effective chemical potential µ (γ) i illutrated in Figure 1. The difference between the product and the reactant tate of the effective chemical potential i negative, ince the anode Figure 2. Intrinic barrier C(γ) (lower part of the figure). A linear potential profile i drawn in the upper part of the figure, and the combination of C(γ) and φ(γ) i alo hown. The urface potential drop i indicated to the right in the figure. reaction i pontaneou. In global electrochemical equilibrium, the two tate value are the ame, and µ ) 0 (31) Thi i yet another formulation of the Nernt equation (eq 22). By introducing eq 30 into the entropy production rate (eq 24), we can now write σ )- 1 γ 2 j T γ1 F[ µ (γ) γ ] dγ (32) Thi expreion for the entropy production rate i equivalent to the expreion in eq 20. It mean that all equation above apply when there i local chemical equilibrium along the internal coordinate pace. The effective driving force time it flux, the electric current denity, give the diipation of energy of the electrode urface through eq 32. Only the difference between the chemical force and the electric force, the effective driving force of the reaction, lead to diipation of energy. Chemical reaction that take place in homogeneou phae are purely diipative phenomena. At the electrode urface, however, the reaction can be driven backward or forward, depending on the direction of the electric current. There i thu an element of control or of reveribility in the electrochemical reaction that ditinguihe it from the imple chemical reaction. According to the econd law, σ > 0. We hall make the aumption, a de Groot and Mazur alo did, 15 that the entropy production i poitive, alo on the meocopic level: σ(γ) )- j T F[ µ (γ) γ ] dγ > 0 (33) The effective chemical potential in Figure 1 agree with thi aumption. To comply with the condition in eq 33, we mut have a monotone decreaing function. We now pecify the chemical contribution of eq 30 further: µ (γ) ) C(γ) + RT ln Γ(γ) + Fφ(γ) (34) The econd term on the right in thi equation i the normal configurational contribution to the chemical potential. The exce urface adorption Γ, or the denity of A or A +,ia function of γ. The term C(γ) i the intrinic barrier that the reactant ha to pa on the way to product; ee Figure 2. The
5 Meocopic Nonequilibrium Thermodynamic J. Phy. Chem. B, Vol. 107, No. 48, urface potential drop that appear in the equation of the preceding ection i then equal to φ j in eq 37. The reaction Gibb energy of the electrode i G j.wecan add and ubtract thi value in eq 37. Thi give, with the definition of the effective driving force, η ) µ + ( G j - G jf0 ) 1 F (38) When, furthermore, G j ) G jf0 )- φ jf0, we have from thi equation that the overpotential can be identified with the effective driving force defined by eq 32. η ) µ 1 F (39) Figure 3. Illutration of the configurational part of the chemical potential and the electric potential plu the barrier at equilibrium. There i a ymmetry around the horizontal line of contant effective chemical potential. variation in the electric potential i here illutrated by a traight line, labeled φ(γ). The effective chemical potential can be further rewritten a µ ) RT ln fγ (35) where f(γ) i the activity coefficient, defined a f(γ) ) e (C+Fφ)/RT (36) The activity coefficient expree the variation in the barrier plu the electrical potential in γ pace. The ituation at global electrochemical equilibrium i illutrated in Figure 3. We ee the variation in the um of the barrier and the electric potential, C + φ, a a function of γ. With F φ )-RT ln Γ, the configurational part of the chemical potential i everywhere oppoite and equal to thi function. The equilibrium value of the effective chemical potential i contant and appear a a line of ymmetry in the figure. 5. The Overpotential Meaurement The overpotential i meaured under tationary tate condition with the help of three electrode; ee, for example, ref 14. A net current i paed between the working electrode (the electrode of interet) and an auxiliary electrode. The current denity at the working electrode i j. The potential between the working electrode and a reference electrode of the ame kind that i kept at zero current denity i φ j. When the electric current denity of the working electrode i zero, the meaured potential i φ jf0. The overpotential i then the difference between the potential drop at a given current denity and that at zero current denity: η ) φ j - φ jf0 (37) The overpotential i defined a poitive. In the preent cae of an anodic overpotential, φ j > φ jf0. By definition, when j ) 0, η ) 0. Suppoe now that the overpotential i due to a rate-limiting reaction at the electrode urface and that we have been able to correct the meaurement of φ j for concentration gradient contribution and ohmic reitance drop in the electrolyte. The The aumption G j ) G jf0 i true when the chemical potential are little affected by the current denity. The overpotential time the electric current i then a meaure of the diipation of energy at the electrode urface. We have alo aumed that T ) 0 and µ i ) 0 applie to the urface. 6. The Butler-Volmer Equation We are now in a poition to find the condition for which the Butler-Volmer equation appear. From the entropy production (eq 33) we can define the thermodynamic force, conjugate to the flux j: The force appear a the derivative of the denity. Thi i normal for force on the macrocopic level (for intance in Fick law). The force refer to the urface temperature, T,byeq24. The central aumption in nonequilibrium thermodynamic i that of linear flux-force relationhip. The aumption i normally ued on the macrocopic level. We hall now ue it for the meocopic level. The linear law that follow from the local entropy production rate in γ pace i then We ued a a condition for the derivation in the preceding ection that the electric current i contant. Since the force varie with γ, o will L(γ). The linear law i now rewritten with the coefficient of tranport Thi coefficient can be interpreted a the electric mobility for motion of the charge carrier A + in the complex A + e - in the polarized electrode (the motion of the electron i not ratelimiting). Since the current ha been taken contant along the internal coordinate, we obtain by integration where l ) R/I and X(γ) )- 1 µ FT γ )- R fγ FfΓ γ j )-L(γ) R 1 F fγ fγ γ (40) (41) j )-u + e (-(C+Fφ)/RT) R dγ eµ /RT (42) u + ) L(γ) FΓ (43) j )-l[e µ 2/RT - e µ 1/RT ] (44) I ) 1 2 u+ -1 e (C+Fφ)/RT dγ (45)
6 13476 J. Phy. Chem. B, Vol. 107, No. 48, 2003 Rubi and Kjeltrup Figure 4. Effective chemical potential and the overpotential. i the integral of the invere mobility weighted with the proper Boltzmann factor. The effective chemical potential at equilibrium µ eq mut have a value between µ 1 and µ 2. We ubtract and add the factor µ eq in the exponential of eq 44 and obtain j )-le µ eq/rt [e (µ 2-µ eq )/RT - e (µ 1-µ eq )/RT ] (46) So far we have only aumed a tationary tate, and eq 46 i the mot general outcome of the derivation. We have een that a proce that i linear in the internal variable lead to a nonlinear proce in the external variable pace. To find the Butler-Volmer equation, we mut relate the effective chemical potential to the overpotential. We make the following identification with the ditance a and b in Figure 4: µ 1 - µ eq ) a ) (1 -R)Fη (47) µ 2 - µ eq )-b )-RFη (48) The difference between the equation make the difference in effective chemical potential equal to -Fη. The fraction R i defined by thee identitie. When thee expreion are introduced into eq 44, we obtain the Butler-Volmer equation j ) j 0 [e (1-R)ηF/RT - e -RηF/RT ] (49) Thi complete the derivation of the Butler-Volmer equation from meocopic nonequilibrium thermodynamic. The equation ha thu been given a thermodynamic bai. Slight difference appear in the interpretation of the variable. The exchange current denity depend on the equilibrium tate through j 0 ) le µ eq (50) In the Butler-Volmer equation there i a imilar dependence on the activation energy for the barrier. The value of j 0 depend here on the barrier C through the variable l. Our variable R i imilar to the ymmetry factor R in the Butler-Volmer equation, eq 1. The value of R in our derivation can be related to the ymmetry of the problem: It meaure the difference of the firt tate and the final tate from the equilibrium tate. The ucce of the Butler-Volmer equation for decription of meaurement i beyond doubt. We have derived thi equation for tationary tate condition, making the aumption that the effective driving force can be replaced by η. When thi aumption i not true, we can ue the more general reult, eq Dicuion The Butler-Volmer equation i uually derived by analyi of unidirectional procee acro an activation barrier, that i, by kinetic theory; ee, for example, ref 4. While there are imilaritie between our thermodynamic approach to the problem and the uual kinetic theory, there are alo difference. The imilaritie and difference hall now be dicued. Both derivation make ue of the reaction coordinate in a decription of the activated proce. Common i that the reaction coordinate meaure the progre of the chemical reaction. In meocopic nonequilibrium thermodynamic the reaction coordinate ha alo the particular tatu of being the internal variable that i ued to decribe the tate of the ytem. The chemical reaction i not in global equilibrium, nor in reaction kinetic, nor in meocopic nonequilibrium thermodynamic. In reaction kinetic, 1 one aume equilibrium between the reactant tate and the activated tate. Our baic aumption i different from thi. We ue the aumption of local chemical equilibrium along the whole reaction path, and we find the Butler-Volmer equation on thi bai. Therefore, we can tate that procee that can be decribed by the Butler-Volmer equation can be regarded a happening in local equilibrium. And, there i no need to quetion the ue of thermodynamic variable in the decription of the urface reaction, in the nonlinear current-potential regime. It i of coure jutified to ak whether the aumption of local equilibrium i good. Some evidence i emerging that it i a good aumption in electroneutral ytem and alo with urface. 16,17 The ue of linear flux-force relation and local equilibrium i very different from the uual premie that are ued to derive the Butler-Volmer equation. In kinetic theory, the difference between the exponential in the Butler-Volmer equation tem from a picture of unidirectional fluxe acro the barrier. Clearly, thi picture decribe a ituation far from equilibrium. One may then rightly wonder whether, for example, the Gibb equation hold. Thi doubt i eliminated by our procedure. We derive the nonlinear flux equation, by conidering only the net rate, and condition are compatible with nonequilibrium thermodynamic. We tart with a linear relation in a proper variable pace. By integration to the macrocopic level, the nonlinear relation i found. Our derivation reolve the problem of the temperature of the reaction, poed by Eyring and Eyring. 1 It i clear from our derivation that the temperature of the reaction i the urface temperature. The urface temperature i defined by Gibb exce variable for the urface. Reaction kinetic theory ha no precription for the temperature. The Butler-Volmer equation and the Nernt equation have for the firt time been given the ame thermodynamic bai by the preent work. The bai i the entropy production rate of the urface. We aw firt that the reverible limit of the entropy production rate gave the well-known Nernt equation. We next ued the condition of local electrochemical equilibrium to find the Butler-Volmer equation from the ame entropy production rate. The entropy production rate of the electrode urface at the tationary tate wa determined by the effective driving force and the electric current denity. The effective driving force wa the um of the chemical and electrical force. The overpotential, a it wa defined by experiment, i not necearily equal to thi effective driving force. Since the overpotential i not necearily equal to the effective driving force, it may not only reflect diipative term only. Thi topic deerve further attention and hould be teted experimentally.
7 Meocopic Nonequilibrium Thermodynamic J. Phy. Chem. B, Vol. 107, No. 48, Meocopic nonequilibrium thermodynamic i a part of claical nonequilibrium thermodynamic. 15 The aumption of a linear flux-force relation and of local chemical equilibrium are central in nonequilibrium thermodynamic. The tarting point in nonequilibrium thermodynamic i alway fluxe that are linear function of their conjugate force. Nonequilibrium thermodynamic become a nonlinear theory, in particular by it incluion of variable on the meocopic level. Meocopic nonequilibrium thermodynamic ue the ame ytematic procedure a claical nonequilibrium thermodynamic. The crucial point, that i olved by the meocopic verion of thi theory, i that a proper definition can be found for the driving force. In our derivation, we have ued a omewhat different procedure than the one originally given by de Groot and Mazur. 15 Thee author tarted with the entropy change of an adiabatic ytem. Since the preent ytem i not adiabatic, it i neceary to find the entropy production on the meocopic level from it counterpart on the macrocopic level. The proper driving force i then more eaily defined. The importance of the preent work lie in the ytematic procedure it can give for further derivation and in the undertanding it can offer. We found that certain premie mut be fulfilled for the Butler-Volmer equation to apply. The urface mut be iothermal, there mut be equilibrium at the urface for adorbed pecie, and one mut be able to identify the overpotential with the effective driving force. Experiment, done for other condition than thee, may give new inight and help develop the theory further. A derivation, tarting with the econd law of thermodynamic, offer inight into the diipative nature of the proce. Chemical energy i alway converted into electrical energy during electrode reaction. The converion i mot clearly decribed in the macrocopic form in eq 23 and 22. The central quetion i alway: Which power i lot in the converion; or how much energy i diipated? In the analyi above, the effective driving force time the electric current gave the complete power lo. The effective driving force wa only equal to the overpotential of the electrode for certain condition, a defined by meaurement. The reaction Gibb energy may be a function of the tate of polarization, G (P ). It i then not correct to replace µ by η. It will be intereting to purue thi point in the future, to find all diipative part of the overpotential. Nonequilibrium thermodynamic give a ytematic bai for including contribution alo from nonzero heat fluxe and thermal force. Bedeaux and Kjeltrup tudied the effect of temperature gradient 10 and lack of equilibrium between the electrolyte and the urface. 11 While it i not practical to tudy all effect combined, it i ueful to have information of the limiting cae and how they all derive from a common bai. Nonequilibrium thermodynamic for the meocopic level expand the poibilitie further to exploit the ue of internal variable and their relation with experiment. Meocopic nonequilibrium themodynamic provide a ytematic way to find kinetic law alo for other condition than the one ued here. 8. Concluion We have hown that the equation ued for reaction controlled charge tranfer, the Butler-Volmer equation, ha a general bai in nonequilibrium thermodynamic. In the derivation we took advantage of the ytematic procedure offered by meocopic nonequilibrium thermodynamic. We arrived at the Butler-Volmer equation uing aumption of local electrochemical equilibrium along the reaction coordinate pace, and a linear flux-force relation for thi coordinate pace. The derivation require a certain identification of the overpotential with the effective driving force to be true. By etting a more general bai for thi important electrochemical equation, we open poibilitie for other tudie when the aumption do not hold, that i, when there are gradient between the urface and it cloe urrounding, and when the tationary tate condition doe not apply. Acknowledgment. Thi work wa completed while J.M.R. wa Onager profeor at NTNU. Nork Hydro ASA i thanked for ponoring the profeorhip. Reference and Note (1) Eyring, H.; Eyring, E. Modern Chemical Kinetic; Chapman and Hall: London, (2) Crow, D. R. Principle and application of electrochemitry, 4th ed.; Blackie Academic & Profeional: London, (3) Bockri, J. O. M.; Khan, S. U. M. Surface Electrochemitry. A molecular level approach; Plenum: (4) Dwayne Miller, R. J.; McLendon, G. L.; Nozik, A. J.; Scmickler, W.; Willig, F. Surface Electron-Tranfer Procee; VCH Publiher: New York, (5) Marcu, R. Can. J. Chem. 1959, 37, 155. (6) Vilar, J. M.; Rubi, J. M. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, (7) Perez-Madrid, A.; Pagonabarraga, I.; Rubi, J. M. Phyica A 1997, 237, 205. (8) Rubi, J. M.; Mazur, P. Phyica A 1999, 276, 477. (9) Gibb, J. W. The Scientific Paper of J. W. Gibb; Dover: New York, (10) Bedeaux, D.; Kjeltrup Ratkje, S. J. Electrochem. Soc. 1996, 136, 767. (11) Kjeltrup Ratkje, S.; Bedeaux, D. J. Electrochem. Soc. 1996, 143, 779. (12) Kjeltrup, S.; Vie, P. J. S.; Bedeaux, D. In Surface Chemitry and Electrochemitry of Membrane; Sørenen, T. S., Ed.; Marcel Dekker: New York, 1999; pp (13) Kjeltrup, S.; Pugazhendi, P.; Bedeaux, D. Z. Phy. Chem. 2000, 214, 895. (14) Atkin, P. W. Phyical Chemitry, 6th ed.; Oxford: (15) de Groot, S. R.; Mazur, P. Non-Equilibrium Thermodynamic; Dover: London, (16) Røjorde, A.; Fomo, D. W.; Kjeltrup, S.; Bedeaux, D.; Hafkjold, B. J. Colloid Interface Sci. 2000, 232, 178. (17) Hafkjold, B.; Kjeltrup, S. J. Stat. Phy. 1995, 78, 463.
Bogoliubov Transformation in Classical Mechanics
 Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
Bogoliubov Tranformation in Claical Mechanic Canonical Tranformation Suppoe we have a et of complex canonical variable, {a j }, and would like to conider another et of variable, {b }, b b ({a j }). How
A Single Particle Thermal Model for Lithium Ion Batteries
 A Single Particle Thermal Model for Lithium Ion Batterie R. Painter* 1, B. Berryhill 1, L. Sharpe 2 and S. Keith Hargrove 2 1 Civil Engineering, Tenneee State Univerity, Nahville, TN, USA 2 Mechanical
A Single Particle Thermal Model for Lithium Ion Batterie R. Painter* 1, B. Berryhill 1, L. Sharpe 2 and S. Keith Hargrove 2 1 Civil Engineering, Tenneee State Univerity, Nahville, TN, USA 2 Mechanical
Lecture 13. Thermodynamic Potentials (Ch. 5)
 Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
Lecture 13. hermodynamic Potential (Ch. 5) So far we have been uing the total internal energy U and ometime the enthalpy H to characterize variou macrocopic ytem. hee function are called the thermodynamic
A novel protocol for linearization of the Poisson-Boltzmann equation
 Ann. Univ. Sofia, Fac. Chem. Pharm. 16 (14) 59-64 [arxiv 141.118] A novel protocol for linearization of the Poion-Boltzmann equation Roumen Tekov Department of Phyical Chemitry, Univerity of Sofia, 1164
Ann. Univ. Sofia, Fac. Chem. Pharm. 16 (14) 59-64 [arxiv 141.118] A novel protocol for linearization of the Poion-Boltzmann equation Roumen Tekov Department of Phyical Chemitry, Univerity of Sofia, 1164
Molecular Dynamics Simulations of Nonequilibrium Effects Associated with Thermally Activated Exothermic Reactions
 Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
Original Paper orma, 5, 9 7, Molecular Dynamic Simulation of Nonequilibrium Effect ociated with Thermally ctivated Exothermic Reaction Jerzy GORECKI and Joanna Natalia GORECK Intitute of Phyical Chemitry,
Green-Kubo formulas with symmetrized correlation functions for quantum systems in steady states: the shear viscosity of a fluid in a steady shear flow
 Green-Kubo formula with ymmetrized correlation function for quantum ytem in teady tate: the hear vicoity of a fluid in a teady hear flow Hirohi Matuoa Department of Phyic, Illinoi State Univerity, Normal,
Green-Kubo formula with ymmetrized correlation function for quantum ytem in teady tate: the hear vicoity of a fluid in a teady hear flow Hirohi Matuoa Department of Phyic, Illinoi State Univerity, Normal,
Social Studies 201 Notes for November 14, 2003
 1 Social Studie 201 Note for November 14, 2003 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
1 Social Studie 201 Note for November 14, 2003 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor
 NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
NONISOTHERMAL OPERATION OF IDEAL REACTORS Plug Flow Reactor T o T T o T F o, Q o F T m,q m T m T m T mo Aumption: 1. Homogeneou Sytem 2. Single Reaction 3. Steady State Two type of problem: 1. Given deired
The continuous time random walk (CTRW) was introduced by Montroll and Weiss 1.
 1 I. CONTINUOUS TIME RANDOM WALK The continuou time random walk (CTRW) wa introduced by Montroll and Wei 1. Unlike dicrete time random walk treated o far, in the CTRW the number of jump n made by the walker
1 I. CONTINUOUS TIME RANDOM WALK The continuou time random walk (CTRW) wa introduced by Montroll and Wei 1. Unlike dicrete time random walk treated o far, in the CTRW the number of jump n made by the walker
THE THERMOELASTIC SQUARE
 HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
HE HERMOELASIC SQUARE A mnemonic for remembering thermodynamic identitie he tate of a material i the collection of variable uch a tre, train, temperature, entropy. A variable i a tate variable if it integral
AP Physics Charge Wrap up
 AP Phyic Charge Wrap up Quite a few complicated euation for you to play with in thi unit. Here them babie i: F 1 4 0 1 r Thi i good old Coulomb law. You ue it to calculate the force exerted 1 by two charge
AP Phyic Charge Wrap up Quite a few complicated euation for you to play with in thi unit. Here them babie i: F 1 4 0 1 r Thi i good old Coulomb law. You ue it to calculate the force exerted 1 by two charge
Nonlinear Single-Particle Dynamics in High Energy Accelerators
 Nonlinear Single-Particle Dynamic in High Energy Accelerator Part 6: Canonical Perturbation Theory Nonlinear Single-Particle Dynamic in High Energy Accelerator Thi coure conit of eight lecture: 1. Introduction
Nonlinear Single-Particle Dynamic in High Energy Accelerator Part 6: Canonical Perturbation Theory Nonlinear Single-Particle Dynamic in High Energy Accelerator Thi coure conit of eight lecture: 1. Introduction
FUNDAMENTALS OF POWER SYSTEMS
 1 FUNDAMENTALS OF POWER SYSTEMS 1 Chapter FUNDAMENTALS OF POWER SYSTEMS INTRODUCTION The three baic element of electrical engineering are reitor, inductor and capacitor. The reitor conume ohmic or diipative
1 FUNDAMENTALS OF POWER SYSTEMS 1 Chapter FUNDAMENTALS OF POWER SYSTEMS INTRODUCTION The three baic element of electrical engineering are reitor, inductor and capacitor. The reitor conume ohmic or diipative
Social Studies 201 Notes for March 18, 2005
 1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
1 Social Studie 201 Note for March 18, 2005 Etimation of a mean, mall ample ize Section 8.4, p. 501. When a reearcher ha only a mall ample ize available, the central limit theorem doe not apply to the
Convective Heat Transfer
 Convective Heat Tranfer Example 1. Melt Spinning of Polymer fiber 2. Heat tranfer in a Condener 3. Temperature control of a Re-entry vehicle Fiber pinning The fiber pinning proce preent a unique engineering
Convective Heat Tranfer Example 1. Melt Spinning of Polymer fiber 2. Heat tranfer in a Condener 3. Temperature control of a Re-entry vehicle Fiber pinning The fiber pinning proce preent a unique engineering
7.2 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 281
 72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
72 INVERSE TRANSFORMS AND TRANSFORMS OF DERIVATIVES 28 and i 2 Show how Euler formula (page 33) can then be ued to deduce the reult a ( a) 2 b 2 {e at co bt} {e at in bt} b ( a) 2 b 2 5 Under what condition
Vector-Space Methods and Kirchhoff Graphs for Reaction Networks
 Vector-Space Method and Kirchhoff Graph for Reaction Network Joeph D. Fehribach Fuel Cell Center WPI Mathematical Science and Chemical Engineering 00 Intitute Rd. Worceter, MA 0609-2247 Thi article preent
Vector-Space Method and Kirchhoff Graph for Reaction Network Joeph D. Fehribach Fuel Cell Center WPI Mathematical Science and Chemical Engineering 00 Intitute Rd. Worceter, MA 0609-2247 Thi article preent
Chapter 1 Basic Description of Laser Diode Dynamics by Spatially Averaged Rate Equations: Conditions of Validity
 Chapter 1 Baic Decription of Laer Diode Dynamic by Spatially Averaged Rate Equation: Condition of Validity A laer diode i a device in which an electric current input i converted to an output of photon.
Chapter 1 Baic Decription of Laer Diode Dynamic by Spatially Averaged Rate Equation: Condition of Validity A laer diode i a device in which an electric current input i converted to an output of photon.
Control Systems Analysis and Design by the Root-Locus Method
 6 Control Sytem Analyi and Deign by the Root-Locu Method 6 1 INTRODUCTION The baic characteritic of the tranient repone of a cloed-loop ytem i cloely related to the location of the cloed-loop pole. If
6 Control Sytem Analyi and Deign by the Root-Locu Method 6 1 INTRODUCTION The baic characteritic of the tranient repone of a cloed-loop ytem i cloely related to the location of the cloed-loop pole. If
Lecture 17: Analytic Functions and Integrals (See Chapter 14 in Boas)
 Lecture 7: Analytic Function and Integral (See Chapter 4 in Boa) Thi i a good point to take a brief detour and expand on our previou dicuion of complex variable and complex function of complex variable.
Lecture 7: Analytic Function and Integral (See Chapter 4 in Boa) Thi i a good point to take a brief detour and expand on our previou dicuion of complex variable and complex function of complex variable.
Lecture 15 - Current. A Puzzle... Advanced Section: Image Charge for Spheres. Image Charge for a Grounded Spherical Shell
 Lecture 15 - Current Puzzle... Suppoe an infinite grounded conducting plane lie at z = 0. charge q i located at a height h above the conducting plane. Show in three different way that the potential below
Lecture 15 - Current Puzzle... Suppoe an infinite grounded conducting plane lie at z = 0. charge q i located at a height h above the conducting plane. Show in three different way that the potential below
CHAPTER 4 DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL
 98 CHAPTER DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL INTRODUCTION The deign of ytem uing tate pace model for the deign i called a modern control deign and it i
98 CHAPTER DESIGN OF STATE FEEDBACK CONTROLLERS AND STATE OBSERVERS USING REDUCED ORDER MODEL INTRODUCTION The deign of ytem uing tate pace model for the deign i called a modern control deign and it i
Source slideplayer.com/fundamentals of Analytical Chemistry, F.J. Holler, S.R.Crouch. Chapter 6: Random Errors in Chemical Analysis
 Source lideplayer.com/fundamental of Analytical Chemitry, F.J. Holler, S.R.Crouch Chapter 6: Random Error in Chemical Analyi Random error are preent in every meaurement no matter how careful the experimenter.
Source lideplayer.com/fundamental of Analytical Chemitry, F.J. Holler, S.R.Crouch Chapter 6: Random Error in Chemical Analyi Random error are preent in every meaurement no matter how careful the experimenter.
1. Basic introduction to electromagnetic field. wave properties and particulate properties.
 Lecture Baic Radiometric Quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation. Objective:. Baic introduction to electromagnetic field:
Lecture Baic Radiometric Quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation. Objective:. Baic introduction to electromagnetic field:
Correction for Simple System Example and Notes on Laplace Transforms / Deviation Variables ECHE 550 Fall 2002
 Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
Correction for Simple Sytem Example and Note on Laplace Tranform / Deviation Variable ECHE 55 Fall 22 Conider a tank draining from an initial height of h o at time t =. With no flow into the tank (F in
Chapter 2 Sampling and Quantization. In order to investigate sampling and quantization, the difference between analog
 Chapter Sampling and Quantization.1 Analog and Digital Signal In order to invetigate ampling and quantization, the difference between analog and digital ignal mut be undertood. Analog ignal conit of continuou
Chapter Sampling and Quantization.1 Analog and Digital Signal In order to invetigate ampling and quantization, the difference between analog and digital ignal mut be undertood. Analog ignal conit of continuou
Chapter 4. The Laplace Transform Method
 Chapter 4. The Laplace Tranform Method The Laplace Tranform i a tranformation, meaning that it change a function into a new function. Actually, it i a linear tranformation, becaue it convert a linear combination
Chapter 4. The Laplace Tranform Method The Laplace Tranform i a tranformation, meaning that it change a function into a new function. Actually, it i a linear tranformation, becaue it convert a linear combination
Linear Motion, Speed & Velocity
 Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
Add Important Linear Motion, Speed & Velocity Page: 136 Linear Motion, Speed & Velocity NGSS Standard: N/A MA Curriculum Framework (006): 1.1, 1. AP Phyic 1 Learning Objective: 3.A.1.1, 3.A.1.3 Knowledge/Undertanding
Mathematical modeling of control systems. Laith Batarseh. Mathematical modeling of control systems
 Chapter two Laith Batareh Mathematical modeling The dynamic of many ytem, whether they are mechanical, electrical, thermal, economic, biological, and o on, may be decribed in term of differential equation
Chapter two Laith Batareh Mathematical modeling The dynamic of many ytem, whether they are mechanical, electrical, thermal, economic, biological, and o on, may be decribed in term of differential equation
Question 1 Equivalent Circuits
 MAE 40 inear ircuit Fall 2007 Final Intruction ) Thi exam i open book You may ue whatever written material you chooe, including your cla note and textbook You may ue a hand calculator with no communication
MAE 40 inear ircuit Fall 2007 Final Intruction ) Thi exam i open book You may ue whatever written material you chooe, including your cla note and textbook You may ue a hand calculator with no communication
Introduction to Laplace Transform Techniques in Circuit Analysis
 Unit 6 Introduction to Laplace Tranform Technique in Circuit Analyi In thi unit we conider the application of Laplace Tranform to circuit analyi. A relevant dicuion of the one-ided Laplace tranform i found
Unit 6 Introduction to Laplace Tranform Technique in Circuit Analyi In thi unit we conider the application of Laplace Tranform to circuit analyi. A relevant dicuion of the one-ided Laplace tranform i found
V = 4 3 πr3. d dt V = d ( 4 dv dt. = 4 3 π d dt r3 dv π 3r2 dv. dt = 4πr 2 dr
 0.1 Related Rate In many phyical ituation we have a relationhip between multiple quantitie, and we know the rate at which one of the quantitie i changing. Oftentime we can ue thi relationhip a a convenient
0.1 Related Rate In many phyical ituation we have a relationhip between multiple quantitie, and we know the rate at which one of the quantitie i changing. Oftentime we can ue thi relationhip a a convenient
NCAAPMT Calculus Challenge Challenge #3 Due: October 26, 2011
 NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
NCAAPMT Calculu Challenge 011 01 Challenge #3 Due: October 6, 011 A Model of Traffic Flow Everyone ha at ome time been on a multi-lane highway and encountered road contruction that required the traffic
2 States of a System. 2.1 States / Configurations 2.2 Probabilities of States. 2.3 Counting States 2.4 Entropy of an ideal gas
 2 State of a Sytem Motly chap 1 and 2 of Kittel &Kroemer 2.1 State / Configuration 2.2 Probabilitie of State Fundamental aumption Entropy 2.3 Counting State 2.4 Entropy of an ideal ga Phyic 112 (S2012)
2 State of a Sytem Motly chap 1 and 2 of Kittel &Kroemer 2.1 State / Configuration 2.2 Probabilitie of State Fundamental aumption Entropy 2.3 Counting State 2.4 Entropy of an ideal ga Phyic 112 (S2012)
Online supplementary information
 Electronic Supplementary Material (ESI) for Soft Matter. Thi journal i The Royal Society of Chemitry 15 Online upplementary information Governing Equation For the vicou flow, we aume that the liquid thickne
Electronic Supplementary Material (ESI) for Soft Matter. Thi journal i The Royal Society of Chemitry 15 Online upplementary information Governing Equation For the vicou flow, we aume that the liquid thickne
into a discrete time function. Recall that the table of Laplace/z-transforms is constructed by (i) selecting to get
 Lecture 25 Introduction to Some Matlab c2d Code in Relation to Sampled Sytem here are many way to convert a continuou time function, { h( t) ; t [0, )} into a dicrete time function { h ( k) ; k {0,,, }}
Lecture 25 Introduction to Some Matlab c2d Code in Relation to Sampled Sytem here are many way to convert a continuou time function, { h( t) ; t [0, )} into a dicrete time function { h ( k) ; k {0,,, }}
Basics of a Quartz Crystal Microbalance
 Baic of a Quartz Crytal Microbalance Introduction Thi document provide an introduction to the quartz crytal microbalance (QCM) which i an intrument that allow a uer to monitor mall ma change on an electrode.
Baic of a Quartz Crytal Microbalance Introduction Thi document provide an introduction to the quartz crytal microbalance (QCM) which i an intrument that allow a uer to monitor mall ma change on an electrode.
Advanced D-Partitioning Analysis and its Comparison with the Kharitonov s Theorem Assessment
 Journal of Multidiciplinary Engineering Science and Technology (JMEST) ISSN: 59- Vol. Iue, January - 5 Advanced D-Partitioning Analyi and it Comparion with the haritonov Theorem Aement amen M. Yanev Profeor,
Journal of Multidiciplinary Engineering Science and Technology (JMEST) ISSN: 59- Vol. Iue, January - 5 Advanced D-Partitioning Analyi and it Comparion with the haritonov Theorem Aement amen M. Yanev Profeor,
Math Skills. Scientific Notation. Uncertainty in Measurements. Appendix A5 SKILLS HANDBOOK
 ppendix 5 Scientific Notation It i difficult to work with very large or very mall number when they are written in common decimal notation. Uually it i poible to accommodate uch number by changing the SI
ppendix 5 Scientific Notation It i difficult to work with very large or very mall number when they are written in common decimal notation. Uually it i poible to accommodate uch number by changing the SI
CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS
 CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.1 INTRODUCTION 8.2 REDUCED ORDER MODEL DESIGN FOR LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.3
CHAPTER 8 OBSERVER BASED REDUCED ORDER CONTROLLER DESIGN FOR LARGE SCALE LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.1 INTRODUCTION 8.2 REDUCED ORDER MODEL DESIGN FOR LINEAR DISCRETE-TIME CONTROL SYSTEMS 8.3
Geometry and Probablity: Statistical Geometrodynamics with Holography
 EJTP 12, No. IYL15-34 (2015) 83 88 Electronic Journal of Theoretical Phyic Geometry and Probablity: Statitical Geometrodynamic with Holography Koutubh Kabe Department of Mathematic, Gogte Intitute of Technology,
EJTP 12, No. IYL15-34 (2015) 83 88 Electronic Journal of Theoretical Phyic Geometry and Probablity: Statitical Geometrodynamic with Holography Koutubh Kabe Department of Mathematic, Gogte Intitute of Technology,
USPAS Course on Recirculated and Energy Recovered Linear Accelerators
 USPAS Coure on Recirculated and Energy Recovered Linear Accelerator G. A. Krafft and L. Merminga Jefferon Lab I. Bazarov Cornell Lecture 6 7 March 005 Lecture Outline. Invariant Ellipe Generated by a Unimodular
USPAS Coure on Recirculated and Energy Recovered Linear Accelerator G. A. Krafft and L. Merminga Jefferon Lab I. Bazarov Cornell Lecture 6 7 March 005 Lecture Outline. Invariant Ellipe Generated by a Unimodular
A FUNCTIONAL BAYESIAN METHOD FOR THE SOLUTION OF INVERSE PROBLEMS WITH SPATIO-TEMPORAL PARAMETERS AUTHORS: CORRESPONDENCE: ABSTRACT
 A FUNCTIONAL BAYESIAN METHOD FOR THE SOLUTION OF INVERSE PROBLEMS WITH SPATIO-TEMPORAL PARAMETERS AUTHORS: Zenon Medina-Cetina International Centre for Geohazard / Norwegian Geotechnical Intitute Roger
A FUNCTIONAL BAYESIAN METHOD FOR THE SOLUTION OF INVERSE PROBLEMS WITH SPATIO-TEMPORAL PARAMETERS AUTHORS: Zenon Medina-Cetina International Centre for Geohazard / Norwegian Geotechnical Intitute Roger
Research Article A Derivation of the Main Relations of Nonequilibrium Thermodynamics
 ISRN Thermodynamic Volume 2013, Article ID 906136, 9 page http://dx.doi.org/10.1155/2013/906136 Reearch Article A Derivation of the Main Relation of Nonequilibrium Thermodynamic Vladimir N. Pokrovkii Mocow
ISRN Thermodynamic Volume 2013, Article ID 906136, 9 page http://dx.doi.org/10.1155/2013/906136 Reearch Article A Derivation of the Main Relation of Nonequilibrium Thermodynamic Vladimir N. Pokrovkii Mocow
DIFFERENTIAL EQUATIONS
 DIFFERENTIAL EQUATIONS Laplace Tranform Paul Dawkin Table of Content Preface... Laplace Tranform... Introduction... The Definition... 5 Laplace Tranform... 9 Invere Laplace Tranform... Step Function...4
DIFFERENTIAL EQUATIONS Laplace Tranform Paul Dawkin Table of Content Preface... Laplace Tranform... Introduction... The Definition... 5 Laplace Tranform... 9 Invere Laplace Tranform... Step Function...4
Jump condition at the boundary between a porous catalyst and a homogeneous fluid
 From the SelectedWork of Francico J. Valde-Parada 2005 Jump condition at the boundary between a porou catalyt and a homogeneou fluid Francico J. Valde-Parada J. Alberto Ochoa-Tapia Available at: http://work.bepre.com/francico_j_valde_parada/12/
From the SelectedWork of Francico J. Valde-Parada 2005 Jump condition at the boundary between a porou catalyt and a homogeneou fluid Francico J. Valde-Parada J. Alberto Ochoa-Tapia Available at: http://work.bepre.com/francico_j_valde_parada/12/
Calculation of the temperature of boundary layer beside wall with time-dependent heat transfer coefficient
 Ŕ periodica polytechnica Mechanical Engineering 54/1 21 15 2 doi: 1.3311/pp.me.21-1.3 web: http:// www.pp.bme.hu/ me c Periodica Polytechnica 21 RESERCH RTICLE Calculation of the temperature of boundary
Ŕ periodica polytechnica Mechanical Engineering 54/1 21 15 2 doi: 1.3311/pp.me.21-1.3 web: http:// www.pp.bme.hu/ me c Periodica Polytechnica 21 RESERCH RTICLE Calculation of the temperature of boundary
CALCULATION OF CHEMICAL POTENTIAL AND ACTIVITY COEFFICIENT OF TWO LAYERS OF CO2 ADSORBED ON A GRAPHITE SURFACE
 hyical Chemitry Chemical hyic CALCULATION OF CHEMICAL OTENTIAL AND ACTIVITY COEFFICIENT OF TWO LAYERS OF CO ADSORBED ON A GRAHITE SURFACE Journal: hyical Chemitry Chemical hyic Manucript ID: C-ART-8-4-378.R
hyical Chemitry Chemical hyic CALCULATION OF CHEMICAL OTENTIAL AND ACTIVITY COEFFICIENT OF TWO LAYERS OF CO ADSORBED ON A GRAHITE SURFACE Journal: hyical Chemitry Chemical hyic Manucript ID: C-ART-8-4-378.R
696 Fu Jing-Li et al Vol. 12 form in generalized coordinate Q ffiq dt = 0 ( = 1; ;n): (3) For nonholonomic ytem, ffiq are not independent of
 Vol 12 No 7, July 2003 cfl 2003 Chin. Phy. Soc. 1009-1963/2003/12(07)/0695-05 Chinee Phyic and IOP Publihing Ltd Lie ymmetrie and conerved quantitie of controllable nonholonomic dynamical ytem Fu Jing-Li(ΛΠ±)
Vol 12 No 7, July 2003 cfl 2003 Chin. Phy. Soc. 1009-1963/2003/12(07)/0695-05 Chinee Phyic and IOP Publihing Ltd Lie ymmetrie and conerved quantitie of controllable nonholonomic dynamical ytem Fu Jing-Li(ΛΠ±)
The Laplace Transform , Haynes Miller and Jeremy Orloff
 The Laplace Tranform 8.3, Hayne Miller and Jeremy Orloff Laplace tranform baic: introduction An operator take a function a input and output another function. A tranform doe the ame thing with the added
The Laplace Tranform 8.3, Hayne Miller and Jeremy Orloff Laplace tranform baic: introduction An operator take a function a input and output another function. A tranform doe the ame thing with the added
III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SUBSTANCES
 III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SBSTANCES. Work purpoe The analyi of the behaviour of a ferroelectric ubtance placed in an eternal electric field; the dependence of the electrical polariation
III.9. THE HYSTERESIS CYCLE OF FERROELECTRIC SBSTANCES. Work purpoe The analyi of the behaviour of a ferroelectric ubtance placed in an eternal electric field; the dependence of the electrical polariation
arxiv: v2 [nucl-th] 3 May 2018
![arxiv: v2 [nucl-th] 3 May 2018 arxiv: v2 [nucl-th] 3 May 2018](/thumbs/89/101162462.jpg) DAMTP-207-44 An Alpha Particle Model for Carbon-2 J. I. Rawlinon arxiv:72.05658v2 [nucl-th] 3 May 208 Department of Applied Mathematic and Theoretical Phyic, Univerity of Cambridge, Wilberforce Road, Cambridge
DAMTP-207-44 An Alpha Particle Model for Carbon-2 J. I. Rawlinon arxiv:72.05658v2 [nucl-th] 3 May 208 Department of Applied Mathematic and Theoretical Phyic, Univerity of Cambridge, Wilberforce Road, Cambridge
online learning Unit Workbook 4 RLC Transients
 online learning Pearon BTC Higher National in lectrical and lectronic ngineering (QCF) Unit 5: lectrical & lectronic Principle Unit Workbook 4 in a erie of 4 for thi unit Learning Outcome: RLC Tranient
online learning Pearon BTC Higher National in lectrical and lectronic ngineering (QCF) Unit 5: lectrical & lectronic Principle Unit Workbook 4 in a erie of 4 for thi unit Learning Outcome: RLC Tranient
THE EXPERIMENTAL PERFORMANCE OF A NONLINEAR DYNAMIC VIBRATION ABSORBER
 Proceeding of IMAC XXXI Conference & Expoition on Structural Dynamic February -4 Garden Grove CA USA THE EXPERIMENTAL PERFORMANCE OF A NONLINEAR DYNAMIC VIBRATION ABSORBER Yung-Sheng Hu Neil S Ferguon
Proceeding of IMAC XXXI Conference & Expoition on Structural Dynamic February -4 Garden Grove CA USA THE EXPERIMENTAL PERFORMANCE OF A NONLINEAR DYNAMIC VIBRATION ABSORBER Yung-Sheng Hu Neil S Ferguon
Radiation Heat Transfer
 CM30 ranport I Part II: Heat ranfer Radiation Heat ranfer Profeor Faith Morrion Department of Chemical Engineering Michigan echnological Univerity CM30 ranport Procee and Unit Operation I Part : Heat ranfer
CM30 ranport I Part II: Heat ranfer Radiation Heat ranfer Profeor Faith Morrion Department of Chemical Engineering Michigan echnological Univerity CM30 ranport Procee and Unit Operation I Part : Heat ranfer
EE Control Systems LECTURE 14
 Updated: Tueday, March 3, 999 EE 434 - Control Sytem LECTURE 4 Copyright FL Lewi 999 All right reerved ROOT LOCUS DESIGN TECHNIQUE Suppoe the cloed-loop tranfer function depend on a deign parameter k We
Updated: Tueday, March 3, 999 EE 434 - Control Sytem LECTURE 4 Copyright FL Lewi 999 All right reerved ROOT LOCUS DESIGN TECHNIQUE Suppoe the cloed-loop tranfer function depend on a deign parameter k We
Lecture 2 Phys 798S Spring 2016 Steven Anlage. The heart and soul of superconductivity is the Meissner Effect. This feature uniquely distinguishes
 ecture Phy 798S Spring 6 Steven Anlage The heart and oul of uperconductivity i the Meiner Effect. Thi feature uniquely ditinguihe uperconductivity fro any other tate of atter. Here we dicu oe iple phenoenological
ecture Phy 798S Spring 6 Steven Anlage The heart and oul of uperconductivity i the Meiner Effect. Thi feature uniquely ditinguihe uperconductivity fro any other tate of atter. Here we dicu oe iple phenoenological
To appear in International Journal of Numerical Methods in Fluids in Stability analysis of numerical interface conditions in uid-structure therm
 To appear in International Journal of Numerical Method in Fluid in 997. Stability analyi of numerical interface condition in uid-tructure thermal analyi M. B. Gile Oxford Univerity Computing Laboratory
To appear in International Journal of Numerical Method in Fluid in 997. Stability analyi of numerical interface condition in uid-tructure thermal analyi M. B. Gile Oxford Univerity Computing Laboratory
The Hassenpflug Matrix Tensor Notation
 The Haenpflug Matrix Tenor Notation D.N.J. El Dept of Mech Mechatron Eng Univ of Stellenboch, South Africa e-mail: dnjel@un.ac.za 2009/09/01 Abtract Thi i a ample document to illutrate the typeetting of
The Haenpflug Matrix Tenor Notation D.N.J. El Dept of Mech Mechatron Eng Univ of Stellenboch, South Africa e-mail: dnjel@un.ac.za 2009/09/01 Abtract Thi i a ample document to illutrate the typeetting of
IEOR 3106: Fall 2013, Professor Whitt Topics for Discussion: Tuesday, November 19 Alternating Renewal Processes and The Renewal Equation
 IEOR 316: Fall 213, Profeor Whitt Topic for Dicuion: Tueday, November 19 Alternating Renewal Procee and The Renewal Equation 1 Alternating Renewal Procee An alternating renewal proce alternate between
IEOR 316: Fall 213, Profeor Whitt Topic for Dicuion: Tueday, November 19 Alternating Renewal Procee and The Renewal Equation 1 Alternating Renewal Procee An alternating renewal proce alternate between
Singular perturbation theory
 Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
Singular perturbation theory Marc R. Rouel June 21, 2004 1 Introduction When we apply the teady-tate approximation (SSA) in chemical kinetic, we typically argue that ome of the intermediate are highly
Digital Control System
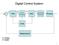 Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
Digital Control Sytem - A D D A Micro ADC DAC Proceor Correction Element Proce Clock Meaurement A: Analog D: Digital Continuou Controller and Digital Control Rt - c Plant yt Continuou Controller Digital
EP225 Note No. 5 Mechanical Waves
 EP5 Note No. 5 Mechanical Wave 5. Introduction Cacade connection of many ma-pring unit conitute a medium for mechanical wave which require that medium tore both kinetic energy aociated with inertia (ma)
EP5 Note No. 5 Mechanical Wave 5. Introduction Cacade connection of many ma-pring unit conitute a medium for mechanical wave which require that medium tore both kinetic energy aociated with inertia (ma)
Automatic Control Systems. Part III: Root Locus Technique
 www.pdhcenter.com PDH Coure E40 www.pdhonline.org Automatic Control Sytem Part III: Root Locu Technique By Shih-Min Hu, Ph.D., P.E. Page of 30 www.pdhcenter.com PDH Coure E40 www.pdhonline.org VI. Root
www.pdhcenter.com PDH Coure E40 www.pdhonline.org Automatic Control Sytem Part III: Root Locu Technique By Shih-Min Hu, Ph.D., P.E. Page of 30 www.pdhcenter.com PDH Coure E40 www.pdhonline.org VI. Root
Lecture 8: Period Finding: Simon s Problem over Z N
 Quantum Computation (CMU 8-859BB, Fall 205) Lecture 8: Period Finding: Simon Problem over Z October 5, 205 Lecturer: John Wright Scribe: icola Rech Problem A mentioned previouly, period finding i a rephraing
Quantum Computation (CMU 8-859BB, Fall 205) Lecture 8: Period Finding: Simon Problem over Z October 5, 205 Lecturer: John Wright Scribe: icola Rech Problem A mentioned previouly, period finding i a rephraing
At the end of this lesson, the students should be able to understand:
 Intructional Objective: At the end of thi leon, the tudent hould be able to undertand: Baic failure mechanim of riveted joint. Concept of deign of a riveted joint. 1. Strength of riveted joint: Strength
Intructional Objective: At the end of thi leon, the tudent hould be able to undertand: Baic failure mechanim of riveted joint. Concept of deign of a riveted joint. 1. Strength of riveted joint: Strength
Bernoulli s equation may be developed as a special form of the momentum or energy equation.
 BERNOULLI S EQUATION Bernoulli equation may be developed a a pecial form of the momentum or energy equation. Here, we will develop it a pecial cae of momentum equation. Conider a teady incompreible flow
BERNOULLI S EQUATION Bernoulli equation may be developed a a pecial form of the momentum or energy equation. Here, we will develop it a pecial cae of momentum equation. Conider a teady incompreible flow
Preemptive scheduling on a small number of hierarchical machines
 Available online at www.ciencedirect.com Information and Computation 06 (008) 60 619 www.elevier.com/locate/ic Preemptive cheduling on a mall number of hierarchical machine György Dóa a, Leah Eptein b,
Available online at www.ciencedirect.com Information and Computation 06 (008) 60 619 www.elevier.com/locate/ic Preemptive cheduling on a mall number of hierarchical machine György Dóa a, Leah Eptein b,
Dimensional Analysis A Tool for Guiding Mathematical Calculations
 Dimenional Analyi A Tool for Guiding Mathematical Calculation Dougla A. Kerr Iue 1 February 6, 2010 ABSTRACT AND INTRODUCTION In converting quantitie from one unit to another, we may know the applicable
Dimenional Analyi A Tool for Guiding Mathematical Calculation Dougla A. Kerr Iue 1 February 6, 2010 ABSTRACT AND INTRODUCTION In converting quantitie from one unit to another, we may know the applicable
Cake ltration analysis the eect of the relationship between the pore liquid pressure and the cake compressive stress
 Chemical Engineering Science 56 (21) 5361 5369 www.elevier.com/locate/ce Cake ltration analyi the eect of the relationhip between the pore liquid preure and the cake compreive tre C. Tien, S. K. Teoh,
Chemical Engineering Science 56 (21) 5361 5369 www.elevier.com/locate/ce Cake ltration analyi the eect of the relationhip between the pore liquid preure and the cake compreive tre C. Tien, S. K. Teoh,
11.2 Stability. A gain element is an active device. One potential problem with every active circuit is its stability
 5/7/2007 11_2 tability 1/2 112 tability eading Aignment: pp 542-548 A gain element i an active device One potential problem with every active circuit i it tability HO: TABIITY Jim tile The Univ of Kana
5/7/2007 11_2 tability 1/2 112 tability eading Aignment: pp 542-548 A gain element i an active device One potential problem with every active circuit i it tability HO: TABIITY Jim tile The Univ of Kana
two equations that govern the motion of the fluid through some medium, like a pipe. These two equations are the
 Fluid and Fluid Mechanic Fluid in motion Dynamic Equation of Continuity After having worked on fluid at ret we turn to a moving fluid To decribe a moving fluid we develop two equation that govern the motion
Fluid and Fluid Mechanic Fluid in motion Dynamic Equation of Continuity After having worked on fluid at ret we turn to a moving fluid To decribe a moving fluid we develop two equation that govern the motion
BASIC INDUCTION MOTOR CONCEPTS
 INDUCTION MOTOS An induction motor ha the ame phyical tator a a ynchronou machine, with a different rotor contruction. There are two different type of induction motor rotor which can be placed inide the
INDUCTION MOTOS An induction motor ha the ame phyical tator a a ynchronou machine, with a different rotor contruction. There are two different type of induction motor rotor which can be placed inide the
Lecture 10 Filtering: Applied Concepts
 Lecture Filtering: Applied Concept In the previou two lecture, you have learned about finite-impule-repone (FIR) and infinite-impule-repone (IIR) filter. In thee lecture, we introduced the concept of filtering
Lecture Filtering: Applied Concept In the previou two lecture, you have learned about finite-impule-repone (FIR) and infinite-impule-repone (IIR) filter. In thee lecture, we introduced the concept of filtering
A Constraint Propagation Algorithm for Determining the Stability Margin. The paper addresses the stability margin assessment for linear systems
 A Contraint Propagation Algorithm for Determining the Stability Margin of Linear Parameter Circuit and Sytem Lubomir Kolev and Simona Filipova-Petrakieva Abtract The paper addree the tability margin aement
A Contraint Propagation Algorithm for Determining the Stability Margin of Linear Parameter Circuit and Sytem Lubomir Kolev and Simona Filipova-Petrakieva Abtract The paper addree the tability margin aement
Hybrid Projective Dislocated Synchronization of Liu Chaotic System Based on Parameters Identification
 www.ccenet.org/ma Modern Applied Science Vol. 6, No. ; February Hybrid Projective Dilocated Synchronization of Liu Chaotic Sytem Baed on Parameter Identification Yanfei Chen College of Science, Guilin
www.ccenet.org/ma Modern Applied Science Vol. 6, No. ; February Hybrid Projective Dilocated Synchronization of Liu Chaotic Sytem Baed on Parameter Identification Yanfei Chen College of Science, Guilin
Time [seconds]
![Time [seconds] Time [seconds]](/thumbs/92/109361387.jpg) .003 Fall 1999 Solution of Homework Aignment 4 1. Due to the application of a 1.0 Newton tep-force, the ytem ocillate at it damped natural frequency! d about the new equilibrium poition y k =. From the
.003 Fall 1999 Solution of Homework Aignment 4 1. Due to the application of a 1.0 Newton tep-force, the ytem ocillate at it damped natural frequency! d about the new equilibrium poition y k =. From the
Standard Guide for Conducting Ruggedness Tests 1
 Deignation: E 69 89 (Reapproved 996) Standard Guide for Conducting Ruggedne Tet AMERICA SOCIETY FOR TESTIG AD MATERIALS 00 Barr Harbor Dr., Wet Conhohocken, PA 948 Reprinted from the Annual Book of ASTM
Deignation: E 69 89 (Reapproved 996) Standard Guide for Conducting Ruggedne Tet AMERICA SOCIETY FOR TESTIG AD MATERIALS 00 Barr Harbor Dr., Wet Conhohocken, PA 948 Reprinted from the Annual Book of ASTM
Parameter Sensitivity Analysis to Improve Material Design for Novel Zn-MnO 2 Batteries with Ionic Liquid Electrolytes
 Parameter Senitivity Analyi to Improve Material Deign for Novel Zn-MnO Batterie with Ionic Liquid Electrolyte Zachary T. Gima & Bernard J. Kim CE 95: Spring 06 Abtract Battery deign at the material level
Parameter Senitivity Analyi to Improve Material Deign for Novel Zn-MnO Batterie with Ionic Liquid Electrolyte Zachary T. Gima & Bernard J. Kim CE 95: Spring 06 Abtract Battery deign at the material level
Midterm Review - Part 1
 Honor Phyic Fall, 2016 Midterm Review - Part 1 Name: Mr. Leonard Intruction: Complete the following workheet. SHOW ALL OF YOUR WORK. 1. Determine whether each tatement i True or Fale. If the tatement i
Honor Phyic Fall, 2016 Midterm Review - Part 1 Name: Mr. Leonard Intruction: Complete the following workheet. SHOW ALL OF YOUR WORK. 1. Determine whether each tatement i True or Fale. If the tatement i
Clustering Methods without Given Number of Clusters
 Clutering Method without Given Number of Cluter Peng Xu, Fei Liu Introduction A we now, mean method i a very effective algorithm of clutering. It mot powerful feature i the calability and implicity. However,
Clutering Method without Given Number of Cluter Peng Xu, Fei Liu Introduction A we now, mean method i a very effective algorithm of clutering. It mot powerful feature i the calability and implicity. However,
CONTROL SYSTEMS, ROBOTICS AND AUTOMATION Vol. VIII Decoupling Control - M. Fikar
 DECOUPLING CONTROL M. Fikar Department of Proce Control, Faculty of Chemical and Food Technology, Slovak Univerity of Technology in Bratilava, Radlinkého 9, SK-812 37 Bratilava, Slovakia Keyword: Decoupling:
DECOUPLING CONTROL M. Fikar Department of Proce Control, Faculty of Chemical and Food Technology, Slovak Univerity of Technology in Bratilava, Radlinkého 9, SK-812 37 Bratilava, Slovakia Keyword: Decoupling:
Stability. ME 344/144L Prof. R.G. Longoria Dynamic Systems and Controls/Lab. Department of Mechanical Engineering The University of Texas at Austin
 Stability The tability of a ytem refer to it ability or tendency to eek a condition of tatic equilibrium after it ha been diturbed. If given a mall perturbation from the equilibrium, it i table if it return.
Stability The tability of a ytem refer to it ability or tendency to eek a condition of tatic equilibrium after it ha been diturbed. If given a mall perturbation from the equilibrium, it i table if it return.
Fermi Distribution Function. n(e) T = 0 T > 0 E F
 LECTURE 3 Maxwell{Boltzmann, Fermi, and Boe Statitic Suppoe we have a ga of N identical point particle in a box ofvolume V. When we ay \ga", we mean that the particle are not interacting with one another.
LECTURE 3 Maxwell{Boltzmann, Fermi, and Boe Statitic Suppoe we have a ga of N identical point particle in a box ofvolume V. When we ay \ga", we mean that the particle are not interacting with one another.
Supplementary Figures
 Supplementary Figure Supplementary Figure S1: Extraction of the SOF. The tandard deviation of meaured V xy at aturated tate (between 2.4 ka/m and 12 ka/m), V 2 d Vxy( H, j, hm ) Vxy( H, j, hm ) 2. The
Supplementary Figure Supplementary Figure S1: Extraction of the SOF. The tandard deviation of meaured V xy at aturated tate (between 2.4 ka/m and 12 ka/m), V 2 d Vxy( H, j, hm ) Vxy( H, j, hm ) 2. The
UNITS FOR THERMOMECHANICS
 UNITS FOR THERMOMECHANICS 1. Conitent Unit. Every calculation require a conitent et of unit. Hitorically, one et of unit wa ued for mechanic and an apparently unrelated et of unit wa ued for heat. For
UNITS FOR THERMOMECHANICS 1. Conitent Unit. Every calculation require a conitent et of unit. Hitorically, one et of unit wa ued for mechanic and an apparently unrelated et of unit wa ued for heat. For
ANALYTICAL BEARING MODEL FOR ANALYSIS OF INNER LOAD DISTRIBUTION AND ESTIMATION OF OPERATIONAL LUBRICATION REGIME
 58 th ICMD 017 6-8 September 017, Prague, Czech Republic ANALYTICAL BEARING MODEL FOR ANALYSIS OF INNER LOAD DISTRIBUTION AND ESTIMATION OF OPERATIONAL LUBRICATION REGIME Jakub CHMELAŘ 1, Votěch DYNYBYL
58 th ICMD 017 6-8 September 017, Prague, Czech Republic ANALYTICAL BEARING MODEL FOR ANALYSIS OF INNER LOAD DISTRIBUTION AND ESTIMATION OF OPERATIONAL LUBRICATION REGIME Jakub CHMELAŘ 1, Votěch DYNYBYL
1 year n0tes chemistry new st CHAPTER 7 THERMOCHEMISTRY MCQs Q.1 Which of the following statements is contrary to the first law of thermodynamics?
 year n0te chemitry new t CHAPTER 7 THERMOCHEMISTRY MCQ Q.1 Which of the following tatement i contrary to the firt law of thermodynamic? (a) energy can neither be created nor detroyed (b) one form of energy
year n0te chemitry new t CHAPTER 7 THERMOCHEMISTRY MCQ Q.1 Which of the following tatement i contrary to the firt law of thermodynamic? (a) energy can neither be created nor detroyed (b) one form of energy
Lecture 6: Resonance II. Announcements
 EES 5 Spring 4, Lecture 6 Lecture 6: Reonance II EES 5 Spring 4, Lecture 6 Announcement The lab tart thi week You mut how up for lab to tay enrolled in the coure. The firt lab i available on the web ite,
EES 5 Spring 4, Lecture 6 Lecture 6: Reonance II EES 5 Spring 4, Lecture 6 Announcement The lab tart thi week You mut how up for lab to tay enrolled in the coure. The firt lab i available on the web ite,
Gain and Phase Margins Based Delay Dependent Stability Analysis of Two- Area LFC System with Communication Delays
 Gain and Phae Margin Baed Delay Dependent Stability Analyi of Two- Area LFC Sytem with Communication Delay Şahin Sönmez and Saffet Ayaun Department of Electrical Engineering, Niğde Ömer Halidemir Univerity,
Gain and Phae Margin Baed Delay Dependent Stability Analyi of Two- Area LFC Sytem with Communication Delay Şahin Sönmez and Saffet Ayaun Department of Electrical Engineering, Niğde Ömer Halidemir Univerity,
Modeling of Transport and Reaction in a Catalytic Bed Using a Catalyst Particle Model.
 Excerpt from the Proceeding of the COMSOL Conference 2010 Boton Modeling of Tranport and Reaction in a Catalytic Bed Uing a Catalyt Particle Model. F. Allain *,1, A.G. Dixon 1 1 Worceter Polytechnic Intitute
Excerpt from the Proceeding of the COMSOL Conference 2010 Boton Modeling of Tranport and Reaction in a Catalytic Bed Uing a Catalyt Particle Model. F. Allain *,1, A.G. Dixon 1 1 Worceter Polytechnic Intitute
in a circular cylindrical cavity K. Kakazu Department of Physics, University of the Ryukyus, Okinawa , Japan Y. S. Kim
 Quantization of electromagnetic eld in a circular cylindrical cavity K. Kakazu Department of Phyic, Univerity of the Ryukyu, Okinawa 903-0, Japan Y. S. Kim Department of Phyic, Univerity of Maryland, College
Quantization of electromagnetic eld in a circular cylindrical cavity K. Kakazu Department of Phyic, Univerity of the Ryukyu, Okinawa 903-0, Japan Y. S. Kim Department of Phyic, Univerity of Maryland, College
Lecture 3 Basic radiometric quantities.
 Lecture 3 Baic radiometric quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation.. Baic introduction to electromagnetic field: Definition,
Lecture 3 Baic radiometric quantitie. The Beer-Bouguer-Lambert law. Concept of extinction cattering plu aborption and emiion. Schwarzchild equation.. Baic introduction to electromagnetic field: Definition,
Computers and Mathematics with Applications. Sharp algebraic periodicity conditions for linear higher order
 Computer and Mathematic with Application 64 (2012) 2262 2274 Content lit available at SciVere ScienceDirect Computer and Mathematic with Application journal homepage: wwweleviercom/locate/camwa Sharp algebraic
Computer and Mathematic with Application 64 (2012) 2262 2274 Content lit available at SciVere ScienceDirect Computer and Mathematic with Application journal homepage: wwweleviercom/locate/camwa Sharp algebraic
The Secret Life of the ax + b Group
 The Secret Life of the ax + b Group Linear function x ax + b are prominent if not ubiquitou in high chool mathematic, beginning in, or now before, Algebra I. In particular, they are prime exhibit in any
The Secret Life of the ax + b Group Linear function x ax + b are prominent if not ubiquitou in high chool mathematic, beginning in, or now before, Algebra I. In particular, they are prime exhibit in any
A BATCH-ARRIVAL QUEUE WITH MULTIPLE SERVERS AND FUZZY PARAMETERS: PARAMETRIC PROGRAMMING APPROACH
 Mathematical and Computational Application Vol. 11 No. pp. 181-191 006. Aociation for Scientific Reearch A BATCH-ARRIVA QEE WITH MTIPE SERVERS AND FZZY PARAMETERS: PARAMETRIC PROGRAMMING APPROACH Jau-Chuan
Mathematical and Computational Application Vol. 11 No. pp. 181-191 006. Aociation for Scientific Reearch A BATCH-ARRIVA QEE WITH MTIPE SERVERS AND FZZY PARAMETERS: PARAMETRIC PROGRAMMING APPROACH Jau-Chuan
Convex Hulls of Curves Sam Burton
 Convex Hull of Curve Sam Burton 1 Introduction Thi paper will primarily be concerned with determining the face of convex hull of curve of the form C = {(t, t a, t b ) t [ 1, 1]}, a < b N in R 3. We hall
Convex Hull of Curve Sam Burton 1 Introduction Thi paper will primarily be concerned with determining the face of convex hull of curve of the form C = {(t, t a, t b ) t [ 1, 1]}, a < b N in R 3. We hall
Laplace Transformation
 Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
Univerity of Technology Electromechanical Department Energy Branch Advance Mathematic Laplace Tranformation nd Cla Lecture 6 Page of 7 Laplace Tranformation Definition Suppoe that f(t) i a piecewie continuou
Diffusion equation and spin drag in spin-polarized transport
 Downloaded from orbit.dtu.dk on: Apr 7, 29 Diffuion equation and pin drag in pin-polarized tranport Flenberg, Karten; Jenen, Thoma Stibiu; Mortenen, Ager Publihed in: Phyical Review B Condened Matter Link
Downloaded from orbit.dtu.dk on: Apr 7, 29 Diffuion equation and pin drag in pin-polarized tranport Flenberg, Karten; Jenen, Thoma Stibiu; Mortenen, Ager Publihed in: Phyical Review B Condened Matter Link
PHYS 110B - HW #2 Spring 2004, Solutions by David Pace Any referenced equations are from Griffiths Problem statements are paraphrased
 PHYS 11B - HW # Spring 4, Solution by David Pace Any referenced equation are from Griffith Problem tatement are paraphraed [1.] Problem 7. from Griffith A capacitor capacitance, C i charged to potential
PHYS 11B - HW # Spring 4, Solution by David Pace Any referenced equation are from Griffith Problem tatement are paraphraed [1.] Problem 7. from Griffith A capacitor capacitance, C i charged to potential
