Microscopic Properties of Gases
|
|
|
- Reynard Pearson
- 6 years ago
- Views:
Transcription
1 icroscopic Properties of Gases So far we he seen the gas laws. These came from observations. In this section we want to look at a theory that explains the gas laws: The kinetic theory of gases or The kinetic moleclar theory CHE Gases icroscopic Kinetic Theory of Gases: ssmptions. gas is made p of a large nmber of extremely small particles (molecles or atoms) in constant, random, straight line motion. olecles occpy very little volme (most of the container is free space). olecles collide with one another and with the s of the container 4. There are no forces between the molecles 5. olecles can gain or lose energy on collision bt the total energy remains constant CHE Gases icroscopic Collision between molecles Collision with CHE Gases icroscopic
2 The theory will give information on the speeds of molecles, the freqency with which they collide, and the distribtion of energy It is only sefl if it can predict the gas laws CHE Gases icroscopic 4 Pressre comes from the gas molecles hitting the s of the container. Hence if we can determine the force with which the molecles hit the we can determine the pressre. Sppose yo he a gas with identical molecles of mass m in a container of volme. lso assme that each molecle has a speed, bt that it can be different for different molecles. CHE Gases icroscopic 5 y calclating the force that a molecle exerts on the, the nmber of collisions and eraging the reslt over the different moleclar speeds, one gets: P m where the erage of the sqares of the speeds. CHE Gases icroscopic 6
3 P m This is looking P m a lot like P constant If is a constant at constant temperatre. This is oyle's Law We will not prove this bt assme it is tre and se the P eqations from the macroscopic and microscopic sections to learn abot speed and temperatre CHE Gases icroscopic 7 Temperatre/kinetic energy/speed The kinetic energy of a molecle is: ½m The erage kinetic energy of a molecle is: m The kinetic energy of a mole of molecles is m ( is vogadro' s nmber) CHE Gases icroscopic 8 The kinetic energy of a mole of molecles (E) is E m m E t P nrt given that m P nrt n.e.e E RT n.e CHE Gases icroscopic 9
4 E RT This shows that the temperatre of the gas is a measre of the kinetic energy of the molecles CHE Gases icroscopic 0 Eqating E Gives oleclar Speeds m E m RT RT RT m and E RT The root mean sqare speed rms RT CHE Gases icroscopic What is the speed of a hydrogen molecle at 5 o C? RT rms.9 0 ms.06 0 (7,000km/hr) The factor of 0 - in the denominator is to pt the molar mass in SI nits CHE Gases icroscopic 4
5 Distribtion of moleclar speeds So far we he calclated the root mean sqare speed only What other speeds are possible? What is the real erage speed? CHE Gases icroscopic Distribtion of moleclar speeds m is the most probable speed (mode) is the erage speed (mean) rms is the root mean sqare speed ll speeds are possible bt are not eqally likely CHE Gases icroscopic 4 verage speeds There are three erage speeds. Since the speed distribtion is not symmetric, they are different m rms RT 8RT π RT kt m 8kT πm kt m m : : rms 0.8 : 0.9 : CHE Gases icroscopic 5 5
6 ariation in speed with temperatre We already know that the erage speeds vary with pt The red crves are for O at two temperatres. CHE Gases icroscopic 6 How broad is the speed distribtion We know the erage speeds. Do most molecles he speeds similar to the erage? Do a lot go faster? CHE Gases icroscopic 7 m m m Clearly the distribtion is narrow CHE Gases icroscopic 8 6
7 It is possible to calclate the fraction of molecles that he a speed greater than Speed 0 m 5 m 0 m Fraction of molecles with speeds greater than (all molecles go faster than 0) 4.5x0-4 4x0-0 4x0-4 ost molecles he speeds close to m CHE Gases icroscopic 9 Diffsion and Effsion Diffsion Effsion CHE Gases icroscopic 0 Collisions with the Wall The nmber of collisions that molecles make with the depend on how many molecles there are (per nit volme) and how fast they are moving. Z will he nits of m - s - Z. actally Z 4. CHE Gases icroscopic 7
8 Effsion We can obtain a qantitative nderstanding of effsion by recognizing that effsion is the loss of a molecle that wold normally hit the. Z. 4 The rate at which molecles lee the container is the collision rate times the area of the hole Rate of effsion Z. 4. CHE Gases icroscopic. for rate of effsion of Z rate of effsion of Z gases () () ( and ) in the 8RT 8RT.() (). 4 ()..() 4 π π same container ote that the ratio of the nmber of molecles is the ratio of the partial pressres rate of effsion of P rate of effsion of P and for EQUL pressres rate of effsion of rate of effsion of CHE Gases icroscopic Graham's Law.. side Stdents often he problems with problems involving rates and or time, becase they are inverses lways remember that Rate molecles time So the faster the rate the smaller the time CHE Gases icroscopic 4 8
9 Collisions between molecles When stdying interactions between gas molecles yo need to know how often the molecles actally collide The collision freqency is the nmber of collisions a particlar molecle makes in one second. This will depend on how many molecles there are (per nit volme), how fast they are moving, and how big they are. CHE Gases icroscopic 5 Collisions between molecles For a gas where all the molecles are the same, and he a diameter d, the collision freqency is Z P Z πd πd kt Z will he nits of s - CHE Gases icroscopic 6 Collisions between molecles To calclate the total nmber of collisions for all molecles we mst mltiply Z by / to cont all the molecles, and by ½ so we don t cont them twice. Z πd Z will he nits of s - m - πd CHE Gases icroscopic 7 9
10 ean free path related parameter is the erage distance a molecle trels between collisions. This is the mean free path The collision freqency is Z s - The time between collisions is /Z s The erage speed of the molecles is Since distance is speed times time λ Z πd CHE Gases icroscopic 8 So how big are these nmbers?? itrogen at 98K 8RT π m s - π P m kt d m Z m s Z πd π ( ) λ Z nm s - CHE Gases icroscopic 9 0
PROBLEMS
 PROBLEMS------------------------------------------------ - 7- Thermodynamic Variables and the Eqation of State 1. Compter (a) the nmber of moles and (b) the nmber of molecles in 1.00 cm of an ideal gas
PROBLEMS------------------------------------------------ - 7- Thermodynamic Variables and the Eqation of State 1. Compter (a) the nmber of moles and (b) the nmber of molecles in 1.00 cm of an ideal gas
L = 2 λ 2 = λ (1) In other words, the wavelength of the wave in question equals to the string length,
 PHY 309 L. Soltions for Problem set # 6. Textbook problem Q.20 at the end of chapter 5: For any standing wave on a string, the distance between neighboring nodes is λ/2, one half of the wavelength. The
PHY 309 L. Soltions for Problem set # 6. Textbook problem Q.20 at the end of chapter 5: For any standing wave on a string, the distance between neighboring nodes is λ/2, one half of the wavelength. The
BLOOM S TAXONOMY. Following Bloom s Taxonomy to Assess Students
 BLOOM S TAXONOMY Topic Following Bloom s Taonomy to Assess Stdents Smmary A handot for stdents to eplain Bloom s taonomy that is sed for item writing and test constrction to test stdents to see if they
BLOOM S TAXONOMY Topic Following Bloom s Taonomy to Assess Stdents Smmary A handot for stdents to eplain Bloom s taonomy that is sed for item writing and test constrction to test stdents to see if they
Lesson 81: The Cross Product of Vectors
 Lesson 8: The Cross Prodct of Vectors IBHL - SANTOWSKI In this lesson yo will learn how to find the cross prodct of two ectors how to find an orthogonal ector to a plane defined by two ectors how to find
Lesson 8: The Cross Prodct of Vectors IBHL - SANTOWSKI In this lesson yo will learn how to find the cross prodct of two ectors how to find an orthogonal ector to a plane defined by two ectors how to find
sin u 5 opp } cos u 5 adj } hyp opposite csc u 5 hyp } sec u 5 hyp } opp Using Inverse Trigonometric Functions
 13 Big Idea 1 CHAPTER SUMMARY BIG IDEAS Using Trigonometric Fnctions Algebra classzone.com Electronic Fnction Library For Yor Notebook hypotense acent osite sine cosine tangent sin 5 hyp cos 5 hyp tan
13 Big Idea 1 CHAPTER SUMMARY BIG IDEAS Using Trigonometric Fnctions Algebra classzone.com Electronic Fnction Library For Yor Notebook hypotense acent osite sine cosine tangent sin 5 hyp cos 5 hyp tan
Section 7.4: Integration of Rational Functions by Partial Fractions
 Section 7.4: Integration of Rational Fnctions by Partial Fractions This is abot as complicated as it gets. The Method of Partial Fractions Ecept for a few very special cases, crrently we have no way to
Section 7.4: Integration of Rational Fnctions by Partial Fractions This is abot as complicated as it gets. The Method of Partial Fractions Ecept for a few very special cases, crrently we have no way to
5. The Bernoulli Equation
 5. The Bernolli Eqation [This material relates predominantly to modles ELP034, ELP035] 5. Work and Energy 5. Bernolli s Eqation 5.3 An example of the se of Bernolli s eqation 5.4 Pressre head, velocity
5. The Bernolli Eqation [This material relates predominantly to modles ELP034, ELP035] 5. Work and Energy 5. Bernolli s Eqation 5.3 An example of the se of Bernolli s eqation 5.4 Pressre head, velocity
Lecture 3. (2) Last time: 3D space. The dot product. Dan Nichols January 30, 2018
 Lectre 3 The dot prodct Dan Nichols nichols@math.mass.ed MATH 33, Spring 018 Uniersity of Massachsetts Janary 30, 018 () Last time: 3D space Right-hand rle, the three coordinate planes 3D coordinate system:
Lectre 3 The dot prodct Dan Nichols nichols@math.mass.ed MATH 33, Spring 018 Uniersity of Massachsetts Janary 30, 018 () Last time: 3D space Right-hand rle, the three coordinate planes 3D coordinate system:
ρ u = u. (1) w z will become certain time, and at a certain point in space, the value of
 THE CONDITIONS NECESSARY FOR DISCONTINUOUS MOTION IN GASES G I Taylor Proceedings of the Royal Society A vol LXXXIV (90) pp 37-377 The possibility of the propagation of a srface of discontinity in a gas
THE CONDITIONS NECESSARY FOR DISCONTINUOUS MOTION IN GASES G I Taylor Proceedings of the Royal Society A vol LXXXIV (90) pp 37-377 The possibility of the propagation of a srface of discontinity in a gas
Cosmic rays. l Some come from the sun (relatively low energy) and some from catastrophic events elsewhere in the galaxy/universe
 Special relativity The laws of physics are the same in all coordinate systems either at rest or moving at constant speed with respect to one another The speed of light in a vacm has the same vale regardless
Special relativity The laws of physics are the same in all coordinate systems either at rest or moving at constant speed with respect to one another The speed of light in a vacm has the same vale regardless
EXPT. 5 DETERMINATION OF pk a OF AN INDICATOR USING SPECTROPHOTOMETRY
 EXPT. 5 DETERMITIO OF pk a OF IDICTOR USIG SPECTROPHOTOMETRY Strctre 5.1 Introdction Objectives 5.2 Principle 5.3 Spectrophotometric Determination of pka Vale of Indicator 5.4 Reqirements 5.5 Soltions
EXPT. 5 DETERMITIO OF pk a OF IDICTOR USIG SPECTROPHOTOMETRY Strctre 5.1 Introdction Objectives 5.2 Principle 5.3 Spectrophotometric Determination of pka Vale of Indicator 5.4 Reqirements 5.5 Soltions
EDEXCEL NATIONAL CERTIFICATE/DIPLOMA. PRINCIPLES AND APPLICATIONS of FLUID MECHANICS UNIT 13 NQF LEVEL 3 OUTCOME 3 - HYDRODYNAMICS
 EDEXCEL NATIONAL CERTIFICATE/DIPLOMA PRINCIPLES AND APPLICATIONS of FLUID MECHANICS UNIT 3 NQF LEVEL 3 OUTCOME 3 - HYDRODYNAMICS TUTORIAL - PIPE FLOW CONTENT Be able to determine the parameters of pipeline
EDEXCEL NATIONAL CERTIFICATE/DIPLOMA PRINCIPLES AND APPLICATIONS of FLUID MECHANICS UNIT 3 NQF LEVEL 3 OUTCOME 3 - HYDRODYNAMICS TUTORIAL - PIPE FLOW CONTENT Be able to determine the parameters of pipeline
CHEM 116 The Ideal Gas Model: Its Usefulness and Conditions Where it Breaks Down
 Uass oston, Chem 116 CHE 116 The Ideal Gas odel: Its Usefulness and Conditions Where it reaks Down Lecture 3 Prof. Sevian Last Thursday Today Today s agenda Some uses of the ideal gas equation Typical
Uass oston, Chem 116 CHE 116 The Ideal Gas odel: Its Usefulness and Conditions Where it reaks Down Lecture 3 Prof. Sevian Last Thursday Today Today s agenda Some uses of the ideal gas equation Typical
Vectors in Rn un. This definition of norm is an extension of the Pythagorean Theorem. Consider the vector u = (5, 8) in R 2
 MATH 307 Vectors in Rn Dr. Neal, WKU Matrices of dimension 1 n can be thoght of as coordinates, or ectors, in n- dimensional space R n. We can perform special calclations on these ectors. In particlar,
MATH 307 Vectors in Rn Dr. Neal, WKU Matrices of dimension 1 n can be thoght of as coordinates, or ectors, in n- dimensional space R n. We can perform special calclations on these ectors. In particlar,
Momentum Equation. Necessary because body is not made up of a fixed assembly of particles Its volume is the same however Imaginary
 Momentm Eqation Interest in the momentm eqation: Qantification of proplsion rates esign strctres for power generation esign of pipeline systems to withstand forces at bends and other places where the flow
Momentm Eqation Interest in the momentm eqation: Qantification of proplsion rates esign strctres for power generation esign of pipeline systems to withstand forces at bends and other places where the flow
Workshop on Understanding and Evaluating Radioanalytical Measurement Uncertainty November 2007
 1833-3 Workshop on Understanding and Evalating Radioanalytical Measrement Uncertainty 5-16 November 007 Applied Statistics: Basic statistical terms and concepts Sabrina BARBIZZI APAT - Agenzia per la Protezione
1833-3 Workshop on Understanding and Evalating Radioanalytical Measrement Uncertainty 5-16 November 007 Applied Statistics: Basic statistical terms and concepts Sabrina BARBIZZI APAT - Agenzia per la Protezione
Bayes and Naïve Bayes Classifiers CS434
 Bayes and Naïve Bayes Classifiers CS434 In this lectre 1. Review some basic probability concepts 2. Introdce a sefl probabilistic rle - Bayes rle 3. Introdce the learning algorithm based on Bayes rle (ths
Bayes and Naïve Bayes Classifiers CS434 In this lectre 1. Review some basic probability concepts 2. Introdce a sefl probabilistic rle - Bayes rle 3. Introdce the learning algorithm based on Bayes rle (ths
Lecture 3. The Kinetic Molecular Theory of Gases
 Lecture 3. The Kinetic Molecular Theory of Gases THE IDEAL GAS LAW: A purely empirical law solely the consequence of experimental observations Explains the behavior of gases over a limited range of conditions
Lecture 3. The Kinetic Molecular Theory of Gases THE IDEAL GAS LAW: A purely empirical law solely the consequence of experimental observations Explains the behavior of gases over a limited range of conditions
EXCITATION RATE COEFFICIENTS OF MOLYBDENUM ATOM AND IONS IN ASTROPHYSICAL PLASMA AS A FUNCTION OF ELECTRON TEMPERATURE
 EXCITATION RATE COEFFICIENTS OF MOLYBDENUM ATOM AND IONS IN ASTROPHYSICAL PLASMA AS A FUNCTION OF ELECTRON TEMPERATURE A.N. Jadhav Department of Electronics, Yeshwant Mahavidyalaya, Ned. Affiliated to
EXCITATION RATE COEFFICIENTS OF MOLYBDENUM ATOM AND IONS IN ASTROPHYSICAL PLASMA AS A FUNCTION OF ELECTRON TEMPERATURE A.N. Jadhav Department of Electronics, Yeshwant Mahavidyalaya, Ned. Affiliated to
CONTENTS. INTRODUCTION MEQ curriculum objectives for vectors (8% of year). page 2 What is a vector? What is a scalar? page 3, 4
 CONTENTS INTRODUCTION MEQ crriclm objectives for vectors (8% of year). page 2 What is a vector? What is a scalar? page 3, 4 VECTOR CONCEPTS FROM GEOMETRIC AND ALGEBRAIC PERSPECTIVES page 1 Representation
CONTENTS INTRODUCTION MEQ crriclm objectives for vectors (8% of year). page 2 What is a vector? What is a scalar? page 3, 4 VECTOR CONCEPTS FROM GEOMETRIC AND ALGEBRAIC PERSPECTIVES page 1 Representation
Math 116 First Midterm October 14, 2009
 Math 116 First Midterm October 14, 9 Name: EXAM SOLUTIONS Instrctor: Section: 1. Do not open this exam ntil yo are told to do so.. This exam has 1 pages inclding this cover. There are 9 problems. Note
Math 116 First Midterm October 14, 9 Name: EXAM SOLUTIONS Instrctor: Section: 1. Do not open this exam ntil yo are told to do so.. This exam has 1 pages inclding this cover. There are 9 problems. Note
1 The space of linear transformations from R n to R m :
 Math 540 Spring 20 Notes #4 Higher deriaties, Taylor s theorem The space of linear transformations from R n to R m We hae discssed linear transformations mapping R n to R m We can add sch linear transformations
Math 540 Spring 20 Notes #4 Higher deriaties, Taylor s theorem The space of linear transformations from R n to R m We hae discssed linear transformations mapping R n to R m We can add sch linear transformations
Lecture 26 Chapter 16 Ideal-dilute solutions and Colligative Properties
 Lectre 26 Chapter 16 Ideal-dilte soltions and Colligative Properties nnonce: HW de next Monday Remember Seminars Friday Hledin at 3:00, Bchwalter at 4:00 We ll go over exams on this afternoon Otline: Mixing
Lectre 26 Chapter 16 Ideal-dilte soltions and Colligative Properties nnonce: HW de next Monday Remember Seminars Friday Hledin at 3:00, Bchwalter at 4:00 We ll go over exams on this afternoon Otline: Mixing
Graphs and Networks Lecture 5. PageRank. Lecturer: Daniel A. Spielman September 20, 2007
 Graphs and Networks Lectre 5 PageRank Lectrer: Daniel A. Spielman September 20, 2007 5.1 Intro to PageRank PageRank, the algorithm reportedly sed by Google, assigns a nmerical rank to eery web page. More
Graphs and Networks Lectre 5 PageRank Lectrer: Daniel A. Spielman September 20, 2007 5.1 Intro to PageRank PageRank, the algorithm reportedly sed by Google, assigns a nmerical rank to eery web page. More
FEA Solution Procedure
 EA Soltion Procedre (demonstrated with a -D bar element problem) EA Procedre for Static Analysis. Prepare the E model a. discretize (mesh) the strctre b. prescribe loads c. prescribe spports. Perform calclations
EA Soltion Procedre (demonstrated with a -D bar element problem) EA Procedre for Static Analysis. Prepare the E model a. discretize (mesh) the strctre b. prescribe loads c. prescribe spports. Perform calclations
Chem 4501 Introduction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics. Fall Semester Homework Problem Set Number 10 Solutions
 Chem 4501 Introdction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics Fall Semester 2017 Homework Problem Set Nmber 10 Soltions 1. McQarrie and Simon, 10-4. Paraphrase: Apply Eler s theorem
Chem 4501 Introdction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics Fall Semester 2017 Homework Problem Set Nmber 10 Soltions 1. McQarrie and Simon, 10-4. Paraphrase: Apply Eler s theorem
Gas Laws. Gas Properties. Gas Properties. Gas Properties Gases and the Kinetic Molecular Theory Pressure Gas Laws
 Gas Laws Gas Properties Gases and the Kinetic Molecular Theory Pressure Gas Laws Gas Properties 1) Gases have mass - the density of the gas is very low in comparison to solids and liquids, which make it
Gas Laws Gas Properties Gases and the Kinetic Molecular Theory Pressure Gas Laws Gas Properties 1) Gases have mass - the density of the gas is very low in comparison to solids and liquids, which make it
The Cross Product of Two Vectors in Space DEFINITION. Cross Product. u * v = s ƒ u ƒƒv ƒ sin ud n
 12.4 The Cross Prodct 873 12.4 The Cross Prodct In stdying lines in the plane, when we needed to describe how a line was tilting, we sed the notions of slope and angle of inclination. In space, we want
12.4 The Cross Prodct 873 12.4 The Cross Prodct In stdying lines in the plane, when we needed to describe how a line was tilting, we sed the notions of slope and angle of inclination. In space, we want
Formules relatives aux probabilités qui dépendent de très grands nombers
 Formles relatives ax probabilités qi dépendent de très grands nombers M. Poisson Comptes rends II (836) pp. 603-63 In the most important applications of the theory of probabilities, the chances of events
Formles relatives ax probabilités qi dépendent de très grands nombers M. Poisson Comptes rends II (836) pp. 603-63 In the most important applications of the theory of probabilities, the chances of events
Integration of Basic Functions. Session 7 : 9/23 1
 Integration o Basic Fnctions Session 7 : 9/3 Antiderivation Integration Deinition: Taking the antiderivative, or integral, o some nction F(), reslts in the nction () i ()F() Pt simply: i yo take the integral
Integration o Basic Fnctions Session 7 : 9/3 Antiderivation Integration Deinition: Taking the antiderivative, or integral, o some nction F(), reslts in the nction () i ()F() Pt simply: i yo take the integral
Chapter 10. Gases. The Gas Laws
 Page 1 of 12 10.1 Characteristics of Gases. Chapter 10. Gases. All substances have three phases; solid, liquid and gas. Substances that are liquids or solids under ordinary conditions may also exist as
Page 1 of 12 10.1 Characteristics of Gases. Chapter 10. Gases. All substances have three phases; solid, liquid and gas. Substances that are liquids or solids under ordinary conditions may also exist as
Kinetic Theory of Gases
 Kinetic Theory of Gases Chapter 3 P. J. Grandinetti Chem. 4300 Aug. 28, 2017 P. J. Grandinetti (Chem. 4300) Kinetic Theory of Gases Aug. 28, 2017 1 / 45 History of ideal gas law 1662: Robert Boyle discovered
Kinetic Theory of Gases Chapter 3 P. J. Grandinetti Chem. 4300 Aug. 28, 2017 P. J. Grandinetti (Chem. 4300) Kinetic Theory of Gases Aug. 28, 2017 1 / 45 History of ideal gas law 1662: Robert Boyle discovered
m = Average Rate of Change (Secant Slope) Example:
 Average Rate o Change Secant Slope Deinition: The average change secant slope o a nction over a particlar interval [a, b] or [a, ]. Eample: What is the average rate o change o the nction over the interval
Average Rate o Change Secant Slope Deinition: The average change secant slope o a nction over a particlar interval [a, b] or [a, ]. Eample: What is the average rate o change o the nction over the interval
4 Exact laminar boundary layer solutions
 4 Eact laminar bondary layer soltions 4.1 Bondary layer on a flat plate (Blasis 1908 In Sec. 3, we derived the bondary layer eqations for 2D incompressible flow of constant viscosity past a weakly crved
4 Eact laminar bondary layer soltions 4.1 Bondary layer on a flat plate (Blasis 1908 In Sec. 3, we derived the bondary layer eqations for 2D incompressible flow of constant viscosity past a weakly crved
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 5 GASES INSTR : FİLİZ ALSHANABLEH
 C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 5 GASES 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 5 GASES Properties of Gases Pressure History and Application of the Gas Laws Partial Pressure Stoichiometry of
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 5 GASES 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 5 GASES Properties of Gases Pressure History and Application of the Gas Laws Partial Pressure Stoichiometry of
Uncertainties of measurement
 Uncertainties of measrement Laboratory tas A temperatre sensor is connected as a voltage divider according to the schematic diagram on Fig.. The temperatre sensor is a thermistor type B5764K [] with nominal
Uncertainties of measrement Laboratory tas A temperatre sensor is connected as a voltage divider according to the schematic diagram on Fig.. The temperatre sensor is a thermistor type B5764K [] with nominal
Kinetic theory. Collective behaviour of large systems Statistical basis for the ideal gas equation Deviations from ideality
 Kinetic theory Collective behaviour of large systems Statistical basis for the ideal gas equation Deviations from ideality Learning objectives Describe physical basis for the kinetic theory of gases Describe
Kinetic theory Collective behaviour of large systems Statistical basis for the ideal gas equation Deviations from ideality Learning objectives Describe physical basis for the kinetic theory of gases Describe
ε tran ε tran = nrt = 2 3 N ε tran = 2 3 nn A ε tran nn A nr ε tran = 2 N A i.e. T = R ε tran = 2
 F1 (a) Since the ideal gas equation of state is PV = nrt, we can equate the right-hand sides of both these equations (i.e. with PV = 2 3 N ε tran )and write: nrt = 2 3 N ε tran = 2 3 nn A ε tran i.e. T
F1 (a) Since the ideal gas equation of state is PV = nrt, we can equate the right-hand sides of both these equations (i.e. with PV = 2 3 N ε tran )and write: nrt = 2 3 N ε tran = 2 3 nn A ε tran i.e. T
Technical Note. ODiSI-B Sensor Strain Gage Factor Uncertainty
 Technical Note EN-FY160 Revision November 30, 016 ODiSI-B Sensor Strain Gage Factor Uncertainty Abstract Lna has pdated or strain sensor calibration tool to spport NIST-traceable measrements, to compte
Technical Note EN-FY160 Revision November 30, 016 ODiSI-B Sensor Strain Gage Factor Uncertainty Abstract Lna has pdated or strain sensor calibration tool to spport NIST-traceable measrements, to compte
Chapter 1: Differential Form of Basic Equations
 MEG 74 Energ and Variational Methods in Mechanics I Brendan J. O Toole, Ph.D. Associate Professor of Mechanical Engineering Howard R. Hghes College of Engineering Universit of Nevada Las Vegas TBE B- (7)
MEG 74 Energ and Variational Methods in Mechanics I Brendan J. O Toole, Ph.D. Associate Professor of Mechanical Engineering Howard R. Hghes College of Engineering Universit of Nevada Las Vegas TBE B- (7)
Electric and Magnetic Fields
 Electric and Magnetic Fields Jst as we want to define an electric field for electrostatic forces, we also want to define a magnetic field for magnetic forces. E (nits of N/C, or V/m) B (nits of Tesla)
Electric and Magnetic Fields Jst as we want to define an electric field for electrostatic forces, we also want to define a magnetic field for magnetic forces. E (nits of N/C, or V/m) B (nits of Tesla)
Gases and Kinetic Theory
 Gases and Kinetic Theory Chemistry 35 Fall 2000 Gases One of the four states of matter Simplest to understand both physically and chemically Gas Properties Low density Fluid Can be defined by their: 1.
Gases and Kinetic Theory Chemistry 35 Fall 2000 Gases One of the four states of matter Simplest to understand both physically and chemically Gas Properties Low density Fluid Can be defined by their: 1.
KINETIC THEORY OF GASES
 LECTURE 8 KINETIC THEORY OF GASES Text Sections 0.4, 0.5, 0.6, 0.7 Sample Problems 0.4 Suggested Questions Suggested Problems Summary None 45P, 55P Molecular model for pressure Root mean square (RMS) speed
LECTURE 8 KINETIC THEORY OF GASES Text Sections 0.4, 0.5, 0.6, 0.7 Sample Problems 0.4 Suggested Questions Suggested Problems Summary None 45P, 55P Molecular model for pressure Root mean square (RMS) speed
Why study gases? A Gas 10/17/2017. An understanding of real world phenomena. An understanding of how science works.
 Kinetic Theory and the Behavior of Ideal & Real Gases Why study gases? n understanding of real world phenomena. n understanding of how science works. Gas Uniformly fills any container. Mixes completely
Kinetic Theory and the Behavior of Ideal & Real Gases Why study gases? n understanding of real world phenomena. n understanding of how science works. Gas Uniformly fills any container. Mixes completely
Department of Industrial Engineering Statistical Quality Control presented by Dr. Eng. Abed Schokry
 Department of Indstrial Engineering Statistical Qality Control presented by Dr. Eng. Abed Schokry Department of Indstrial Engineering Statistical Qality Control C and U Chart presented by Dr. Eng. Abed
Department of Indstrial Engineering Statistical Qality Control presented by Dr. Eng. Abed Schokry Department of Indstrial Engineering Statistical Qality Control C and U Chart presented by Dr. Eng. Abed
Relativity II. The laws of physics are identical in all inertial frames of reference. equivalently
 Relatiity II I. Henri Poincare's Relatiity Principle In the late 1800's, Henri Poincare proposed that the principle of Galilean relatiity be expanded to inclde all physical phenomena and not jst mechanics.
Relatiity II I. Henri Poincare's Relatiity Principle In the late 1800's, Henri Poincare proposed that the principle of Galilean relatiity be expanded to inclde all physical phenomena and not jst mechanics.
Modeling Effort on Chamber Clearing for IFE Liquid Chambers at UCLA
 Modeling Effort on Chamber Clearing for IFE Liqid Chambers at UCLA Presented by: P. Calderoni own Meeting on IFE Liqid Wall Chamber Dynamics Livermore CA May 5-6 3 Otline his presentation will address
Modeling Effort on Chamber Clearing for IFE Liqid Chambers at UCLA Presented by: P. Calderoni own Meeting on IFE Liqid Wall Chamber Dynamics Livermore CA May 5-6 3 Otline his presentation will address
III. Demonstration of a seismometer response with amplitude and phase responses at:
 GG5330, Spring semester 006 Assignment #1, Seismometry and Grond Motions De 30 Janary 006. 1. Calibration Of A Seismometer Using Java: A really nifty se of Java is now available for demonstrating the seismic
GG5330, Spring semester 006 Assignment #1, Seismometry and Grond Motions De 30 Janary 006. 1. Calibration Of A Seismometer Using Java: A really nifty se of Java is now available for demonstrating the seismic
A New Approach to Direct Sequential Simulation that Accounts for the Proportional Effect: Direct Lognormal Simulation
 A ew Approach to Direct eqential imlation that Acconts for the Proportional ffect: Direct ognormal imlation John Manchk, Oy eangthong and Clayton Detsch Department of Civil & nvironmental ngineering University
A ew Approach to Direct eqential imlation that Acconts for the Proportional ffect: Direct ognormal imlation John Manchk, Oy eangthong and Clayton Detsch Department of Civil & nvironmental ngineering University
3.3 Operations With Vectors, Linear Combinations
 Operations With Vectors, Linear Combinations Performance Criteria: (d) Mltiply ectors by scalars and add ectors, algebraically Find linear combinations of ectors algebraically (e) Illstrate the parallelogram
Operations With Vectors, Linear Combinations Performance Criteria: (d) Mltiply ectors by scalars and add ectors, algebraically Find linear combinations of ectors algebraically (e) Illstrate the parallelogram
Physics of the Interstellar and Intergalactic Medium
 Y4A04 Senior Sophister hysics of the Interstellar and Intergalactic edim Lectre 9: Shocks - revised Dr Graham. Harper School of hysics, TCD What a good physicist does best - Simplify eil Nebla ~8000 yr
Y4A04 Senior Sophister hysics of the Interstellar and Intergalactic edim Lectre 9: Shocks - revised Dr Graham. Harper School of hysics, TCD What a good physicist does best - Simplify eil Nebla ~8000 yr
CHEM 116 Characterizing Gas Behavior Mathematically
 CHE 116 Characterizing Gas ehavior athematically Lecture Prof. Sevian Today s agenda Some uses of the ideal gas equation Typical gas law problems Density ( mass density ) of gases olar mass determination
CHE 116 Characterizing Gas ehavior athematically Lecture Prof. Sevian Today s agenda Some uses of the ideal gas equation Typical gas law problems Density ( mass density ) of gases olar mass determination
Strategic Timing of Content in Online Social Networks
 Strategic Timing of Content in Online Social Networks Sina Modaresi Department of Indstrial Engineering, University of Pittsbrgh, Pittsbrgh PA 56, sim3@pitt.ed Jan Pablo Vielma Sloan School of Management,
Strategic Timing of Content in Online Social Networks Sina Modaresi Department of Indstrial Engineering, University of Pittsbrgh, Pittsbrgh PA 56, sim3@pitt.ed Jan Pablo Vielma Sloan School of Management,
Chapter 15 Thermal Properties of Matter
 Chapter 15 Thermal Properties of Matter To understand the mole and Avogadro's number. To understand equations of state. To study the kinetic theory of ideal gas. To understand heat capacity. To learn and
Chapter 15 Thermal Properties of Matter To understand the mole and Avogadro's number. To understand equations of state. To study the kinetic theory of ideal gas. To understand heat capacity. To learn and
Reactions Involving Gases
 Chapter 5 Gases Reactions Involving Gases in reactions of gases, the amount of a gas is often given as a volume the ideal gas law allows us to convert from the volume of the gas to moles; then we can use
Chapter 5 Gases Reactions Involving Gases in reactions of gases, the amount of a gas is often given as a volume the ideal gas law allows us to convert from the volume of the gas to moles; then we can use
19-9 Adiabatic Expansion of an Ideal Gas
 19-9 Adiabatic Expansion of an Ideal Gas Learning Objectives 19.44 On a p-v diagram, sketch an adiabatic expansion (or contraction) and identify that there is no heat exchange Q with the environment. 19.45
19-9 Adiabatic Expansion of an Ideal Gas Learning Objectives 19.44 On a p-v diagram, sketch an adiabatic expansion (or contraction) and identify that there is no heat exchange Q with the environment. 19.45
The Kinetic-Molecular Theory of Gases
 The Kinetic-Molecular Theory of Gases kinetic-molecular theory of gases Originated with Ludwig Boltzman and James Clerk Maxwell in the 19th century Explains gas behavior on the basis of the motion of individual
The Kinetic-Molecular Theory of Gases kinetic-molecular theory of gases Originated with Ludwig Boltzman and James Clerk Maxwell in the 19th century Explains gas behavior on the basis of the motion of individual
WEAR PREDICTION OF A TOTAL KNEE PROSTHESIS TIBIAL TRAY
 APPLIED PHYSICS MEDICAL WEAR PREDICTION OF A TOTAL KNEE PROSTHESIS TIBIAL TRAY L. CÃPITANU, A. IAROVICI, J. ONIªORU Institte of Solid Mechanics, Romanian Academy, Constantin Mille 5, Bcharest Received
APPLIED PHYSICS MEDICAL WEAR PREDICTION OF A TOTAL KNEE PROSTHESIS TIBIAL TRAY L. CÃPITANU, A. IAROVICI, J. ONIªORU Institte of Solid Mechanics, Romanian Academy, Constantin Mille 5, Bcharest Received
1 Differential Equations for Solid Mechanics
 1 Differential Eqations for Solid Mechanics Simple problems involving homogeneos stress states have been considered so far, wherein the stress is the same throghot the component nder std. An eception to
1 Differential Eqations for Solid Mechanics Simple problems involving homogeneos stress states have been considered so far, wherein the stress is the same throghot the component nder std. An eception to
4. 1 mole = 22.4 L at STP mole/volume interconversions at STP
 Ch. 10 Gases and the Ideal Gas Law(s) Chem 210 Jasperse Ch. 10 Handouts 1 10.1 The Atmosphere 1. Earth surrounded by gas 2. Major components: Nitrogen 78% Oxygen 21% Miscellaneous: All
Ch. 10 Gases and the Ideal Gas Law(s) Chem 210 Jasperse Ch. 10 Handouts 1 10.1 The Atmosphere 1. Earth surrounded by gas 2. Major components: Nitrogen 78% Oxygen 21% Miscellaneous: All
Computational Fluid Dynamics Simulation and Wind Tunnel Testing on Microlight Model
 Comptational Flid Dynamics Simlation and Wind Tnnel Testing on Microlight Model Iskandar Shah Bin Ishak Department of Aeronatics and Atomotive, Universiti Teknologi Malaysia T.M. Kit Universiti Teknologi
Comptational Flid Dynamics Simlation and Wind Tnnel Testing on Microlight Model Iskandar Shah Bin Ishak Department of Aeronatics and Atomotive, Universiti Teknologi Malaysia T.M. Kit Universiti Teknologi
Chem 105 Friday 3 Dec 2010
 Chem 105 Friday 3 Dec 010 Today: Kinetic-molecular theory Diffusion and effusion Course Questionnaires Real gases & Van der Waals equation Hour Exam 4 (Chap 9, 10, 11) Friday. Dec 10 A practice exam will
Chem 105 Friday 3 Dec 010 Today: Kinetic-molecular theory Diffusion and effusion Course Questionnaires Real gases & Van der Waals equation Hour Exam 4 (Chap 9, 10, 11) Friday. Dec 10 A practice exam will
Study of the diffusion operator by the SPH method
 IOSR Jornal of Mechanical and Civil Engineering (IOSR-JMCE) e-issn: 2278-684,p-ISSN: 2320-334X, Volme, Isse 5 Ver. I (Sep- Oct. 204), PP 96-0 Stdy of the diffsion operator by the SPH method Abdelabbar.Nait
IOSR Jornal of Mechanical and Civil Engineering (IOSR-JMCE) e-issn: 2278-684,p-ISSN: 2320-334X, Volme, Isse 5 Ver. I (Sep- Oct. 204), PP 96-0 Stdy of the diffsion operator by the SPH method Abdelabbar.Nait
3.4-Miscellaneous Equations
 .-Miscellaneos Eqations Factoring Higher Degree Polynomials: Many higher degree polynomials can be solved by factoring. Of particlar vale is the method of factoring by groping, however all types of factoring
.-Miscellaneos Eqations Factoring Higher Degree Polynomials: Many higher degree polynomials can be solved by factoring. Of particlar vale is the method of factoring by groping, however all types of factoring
Cosmic Microwave Background Radiation. Carl W. Akerlof April 7, 2013
 Cosmic Microwave Backgrond Radiation Carl W. Akerlof April 7, 013 Notes: Dry ice sblimation temperatre: Isopropyl alcohol freezing point: LNA operating voltage: 194.65 K 184.65 K 18.0 v he terrestrial
Cosmic Microwave Backgrond Radiation Carl W. Akerlof April 7, 013 Notes: Dry ice sblimation temperatre: Isopropyl alcohol freezing point: LNA operating voltage: 194.65 K 184.65 K 18.0 v he terrestrial
MECHANICS OF SOLIDS COMPRESSION MEMBERS TUTORIAL 2 INTERMEDIATE AND SHORT COMPRESSION MEMBERS
 MECHANICS O SOIDS COMPRESSION MEMBERS TUTORIA INTERMEDIATE AND SHORT COMPRESSION MEMBERS Yo shold jdge yor progress by completing the self assessment exercises. On completion of this ttorial yo shold be
MECHANICS O SOIDS COMPRESSION MEMBERS TUTORIA INTERMEDIATE AND SHORT COMPRESSION MEMBERS Yo shold jdge yor progress by completing the self assessment exercises. On completion of this ttorial yo shold be
Calculations involving a single random variable (SRV)
 Calclations involving a single random variable (SRV) Example of Bearing Capacity q φ = 0 µ σ c c = 100kN/m = 50kN/m ndrained shear strength parameters What is the relationship between the Factor of Safety
Calclations involving a single random variable (SRV) Example of Bearing Capacity q φ = 0 µ σ c c = 100kN/m = 50kN/m ndrained shear strength parameters What is the relationship between the Factor of Safety
Numerical Simulation of Three Dimensional Flow in Water Tank of Marine Fish Larvae
 Copyright c 27 ICCES ICCES, vol.4, no.1, pp.19-24, 27 Nmerical Simlation of Three Dimensional Flo in Water Tank of Marine Fish Larvae Shigeaki Shiotani 1, Atsshi Hagiara 2 and Yoshitaka Sakakra 3 Smmary
Copyright c 27 ICCES ICCES, vol.4, no.1, pp.19-24, 27 Nmerical Simlation of Three Dimensional Flo in Water Tank of Marine Fish Larvae Shigeaki Shiotani 1, Atsshi Hagiara 2 and Yoshitaka Sakakra 3 Smmary
VIBRATION MEASUREMENT UNCERTAINTY AND RELIABILITY DIAGNOSTICS RESULTS IN ROTATING SYSTEMS
 VIBRATIO MEASUREMET UCERTAITY AD RELIABILITY DIAGOSTICS RESULTS I ROTATIG SYSTEMS. Introdction M. Eidkevicite, V. Volkovas anas University of Technology, Lithania The rotating machinery technical state
VIBRATIO MEASUREMET UCERTAITY AD RELIABILITY DIAGOSTICS RESULTS I ROTATIG SYSTEMS. Introdction M. Eidkevicite, V. Volkovas anas University of Technology, Lithania The rotating machinery technical state
States of Matter Lesson 3.6 CHEMISTRY 2 HONORS. Jeff Venables Northwestern High School
 States of Matter Lesson 3.6 CHEMISTRY HONORS Molecular Effusion and Diffusion As kinetic energy increases, the velocity of the gas molecules increases. Average kinetic energy of a gas is related to its
States of Matter Lesson 3.6 CHEMISTRY HONORS Molecular Effusion and Diffusion As kinetic energy increases, the velocity of the gas molecules increases. Average kinetic energy of a gas is related to its
The Kinetic Molecular Theory of Gases
 The Kinetic Molecular Theory of Gases Background: It is straightforward to observe that there is an inverse relationship between pressure and volume for a gas at constant temperature. Curious scientists
The Kinetic Molecular Theory of Gases Background: It is straightforward to observe that there is an inverse relationship between pressure and volume for a gas at constant temperature. Curious scientists
ANOVA INTERPRETING. It might be tempting to just look at the data and wing it
 Introdction to Statistics in Psychology PSY 2 Professor Greg Francis Lectre 33 ANalysis Of VAriance Something erss which thing? ANOVA Test statistic: F = MS B MS W Estimated ariability from noise and mean
Introdction to Statistics in Psychology PSY 2 Professor Greg Francis Lectre 33 ANalysis Of VAriance Something erss which thing? ANOVA Test statistic: F = MS B MS W Estimated ariability from noise and mean
FLUCTUATING WIND VELOCITY CHARACTERISTICS OF THE WAKE OF A CONICAL HILL THAT CAUSE LARGE HORIZONTAL RESPONSE OF A CANTILEVER MODEL
 BBAA VI International Colloqim on: Blff Bodies Aerodynamics & Applications Milano, Italy, Jly, 2-24 28 FLUCTUATING WIND VELOCITY CHARACTERISTICS OF THE WAKE OF A CONICAL HILL THAT CAUSE LARGE HORIZONTAL
BBAA VI International Colloqim on: Blff Bodies Aerodynamics & Applications Milano, Italy, Jly, 2-24 28 FLUCTUATING WIND VELOCITY CHARACTERISTICS OF THE WAKE OF A CONICAL HILL THAT CAUSE LARGE HORIZONTAL
Applying Laminar and Turbulent Flow and measuring Velocity Profile Using MATLAB
 IOS Jornal of Mathematics (IOS-JM) e-issn: 78-578, p-issn: 319-765X. Volme 13, Isse 6 Ver. II (Nov. - Dec. 17), PP 5-59 www.iosrjornals.org Applying Laminar and Trblent Flow and measring Velocity Profile
IOS Jornal of Mathematics (IOS-JM) e-issn: 78-578, p-issn: 319-765X. Volme 13, Isse 6 Ver. II (Nov. - Dec. 17), PP 5-59 www.iosrjornals.org Applying Laminar and Trblent Flow and measring Velocity Profile
Engr. Yvonne Ligaya F. Musico Chemical Engineering Department
 GASEOUS STATE Engr. Yvonne Ligaya F. Musico Chemical Engineering Department TOPICS Objective Properties of Gases Kinetic Molecular Theory of Gases Gas Laws OBJECTIVES Determine how volume, pressure and
GASEOUS STATE Engr. Yvonne Ligaya F. Musico Chemical Engineering Department TOPICS Objective Properties of Gases Kinetic Molecular Theory of Gases Gas Laws OBJECTIVES Determine how volume, pressure and
PhysicsAndMathsTutor.com
 . Two smooth niform spheres S and T have eqal radii. The mass of S is 0. kg and the mass of T is 0.6 kg. The spheres are moving on a smooth horizontal plane and collide obliqely. Immediately before the
. Two smooth niform spheres S and T have eqal radii. The mass of S is 0. kg and the mass of T is 0.6 kg. The spheres are moving on a smooth horizontal plane and collide obliqely. Immediately before the
Properties of Gases. 5 important gas properties:
 Gases Chapter 12 Properties of Gases 5 important gas properties: 1) Gases have an indefinite shape 2) Gases have low densities 3) Gases can compress 4) Gases can expand 5) Gases mix completely with other
Gases Chapter 12 Properties of Gases 5 important gas properties: 1) Gases have an indefinite shape 2) Gases have low densities 3) Gases can compress 4) Gases can expand 5) Gases mix completely with other
Diffraction of light due to ultrasonic wave propagation in liquids
 Diffraction of light de to ltrasonic wave propagation in liqids Introdction: Acostic waves in liqids case density changes with spacing determined by the freqency and the speed of the sond wave. For ltrasonic
Diffraction of light de to ltrasonic wave propagation in liqids Introdction: Acostic waves in liqids case density changes with spacing determined by the freqency and the speed of the sond wave. For ltrasonic
Mean Value Formulae for Laplace and Heat Equation
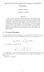 Mean Vale Formlae for Laplace and Heat Eqation Abhinav Parihar December 7, 03 Abstract Here I discss a method to constrct the mean vale theorem for the heat eqation. To constrct sch a formla ab initio,
Mean Vale Formlae for Laplace and Heat Eqation Abhinav Parihar December 7, 03 Abstract Here I discss a method to constrct the mean vale theorem for the heat eqation. To constrct sch a formla ab initio,
The women s heptathlon in the Olympics consists of seven track and field
 CHAPTER 6 The Standard Deviation as a Rler and the Normal Model The women s heptathlon in the Olympics consists of seven track and field events: the 200-m and 800-m rns, 100-m high hrdles, shot pt, javelin,
CHAPTER 6 The Standard Deviation as a Rler and the Normal Model The women s heptathlon in the Olympics consists of seven track and field events: the 200-m and 800-m rns, 100-m high hrdles, shot pt, javelin,
The Kinetic-Molecular Theory of Gases
 The Kinetic-Molecular Theory of Gases kinetic-molecular theory of gases Originated with Ludwig Boltzman and James Clerk Maxwell in the 19th century Explains gas behavior on the basis of the motion of individual
The Kinetic-Molecular Theory of Gases kinetic-molecular theory of gases Originated with Ludwig Boltzman and James Clerk Maxwell in the 19th century Explains gas behavior on the basis of the motion of individual
Videos 1. Crash course Partial pressures: YuWy6fYEaX9mQQ8oGr 2. Crash couse Effusion/Diffusion:
 Videos 1. Crash course Partial pressures: https://youtu.be/jbqtqcunyza?list=pl8dpuualjxtphzz YuWy6fYEaX9mQQ8oGr 2. Crash couse Effusion/Diffusion: https://youtu.be/tlrzafu_9kg?list=pl8dpuualjxtph zzyuwy6fyeax9mqq8ogr
Videos 1. Crash course Partial pressures: https://youtu.be/jbqtqcunyza?list=pl8dpuualjxtphzz YuWy6fYEaX9mQQ8oGr 2. Crash couse Effusion/Diffusion: https://youtu.be/tlrzafu_9kg?list=pl8dpuualjxtph zzyuwy6fyeax9mqq8ogr
Chapter 17 Temperature & Kinetic Theory of Gases 1. Thermal Equilibrium and Temperature
 Chapter 17 Temperature & Kinetic Theory of Gases 1. Thermal Equilibrium and Temperature Any physical property that changes with temperature is called a thermometric property and can be used to measure
Chapter 17 Temperature & Kinetic Theory of Gases 1. Thermal Equilibrium and Temperature Any physical property that changes with temperature is called a thermometric property and can be used to measure
Key Chemistry 102 Discussion #4, Chapter 11 and 12 Student name TA name Section. ; u= M. and T(red)=2*T(yellow) ; t(yellow)=4*t(red) or
 Key Chemisry 0 Discssion #4, Chaper and Sden name TA name Secion. Two idenical conainers, one red and one yellow, are inflaed wih differen gases a he same volme and pressre. Boh conainers have an idenically
Key Chemisry 0 Discssion #4, Chaper and Sden name TA name Secion. Two idenical conainers, one red and one yellow, are inflaed wih differen gases a he same volme and pressre. Boh conainers have an idenically
PhysicsAndMathsTutor.com 1 2 (*) (1)
 PhysicsAndMathsTutor.com 1 1. (a) pressure (*) Pa or N m volume m (*) (*) (not allow kpa) number of moles mol (or none) molar gas constant J K 1 mol 1 (mol 1 implies molar) temperature K 4 (b) (i) W(=
PhysicsAndMathsTutor.com 1 1. (a) pressure (*) Pa or N m volume m (*) (*) (not allow kpa) number of moles mol (or none) molar gas constant J K 1 mol 1 (mol 1 implies molar) temperature K 4 (b) (i) W(=
Transient Approach to Radiative Heat Transfer Free Convection Flow with Ramped Wall Temperature
 Jornal of Applied Flid Mechanics, Vol. 5, No., pp. 9-1, 1. Available online at www.jafmonline.net, ISSN 175-57, EISSN 175-645. Transient Approach to Radiative Heat Transfer Free Convection Flow with Ramped
Jornal of Applied Flid Mechanics, Vol. 5, No., pp. 9-1, 1. Available online at www.jafmonline.net, ISSN 175-57, EISSN 175-645. Transient Approach to Radiative Heat Transfer Free Convection Flow with Ramped
Step-Size Bounds Analysis of the Generalized Multidelay Adaptive Filter
 WCE 007 Jly - 4 007 London UK Step-Size onds Analysis of the Generalized Mltidelay Adaptive Filter Jnghsi Lee and Hs Chang Hang Abstract In this paper we analyze the bonds of the fixed common step-size
WCE 007 Jly - 4 007 London UK Step-Size onds Analysis of the Generalized Mltidelay Adaptive Filter Jnghsi Lee and Hs Chang Hang Abstract In this paper we analyze the bonds of the fixed common step-size
TESTING MEANS. we want to test. but we need to know if 2 1 = 2 2 if it is, we use the methods described last time pooled estimate of variance
 Introdction to Statistics in Psychology PSY Profess Greg Francis Lectre 6 Hypothesis testing f two sample case Planning a replication stdy TESTING MENS we want to test H : µ µ H a : µ µ 6 bt we need to
Introdction to Statistics in Psychology PSY Profess Greg Francis Lectre 6 Hypothesis testing f two sample case Planning a replication stdy TESTING MENS we want to test H : µ µ H a : µ µ 6 bt we need to
Sources of Non Stationarity in the Semivariogram
 Sorces of Non Stationarity in the Semivariogram Migel A. Cba and Oy Leangthong Traditional ncertainty characterization techniqes sch as Simple Kriging or Seqential Gassian Simlation rely on stationary
Sorces of Non Stationarity in the Semivariogram Migel A. Cba and Oy Leangthong Traditional ncertainty characterization techniqes sch as Simple Kriging or Seqential Gassian Simlation rely on stationary
Mixture of gases. Mix 5 moles of CO 2 V = 40L 2 moles of N 2 T = 0 C 1 mole of Cl 2 What is P? Mary J. Bojan Chem 110
 Mixture of gases Mix 5 moles of CO 2 V = 40L 2 moles of N 2 T = 0 C 1 mole of Cl 2 What is P? 1 Partial Pressure Partial pressure: the pressure a gas would have if it was the only gas in the container.
Mixture of gases Mix 5 moles of CO 2 V = 40L 2 moles of N 2 T = 0 C 1 mole of Cl 2 What is P? 1 Partial Pressure Partial pressure: the pressure a gas would have if it was the only gas in the container.
CS 450: COMPUTER GRAPHICS VECTORS SPRING 2016 DR. MICHAEL J. REALE
 CS 45: COMPUTER GRPHICS VECTORS SPRING 216 DR. MICHEL J. RELE INTRODUCTION In graphics, we are going to represent objects and shapes in some form or other. First, thogh, we need to figre ot how to represent
CS 45: COMPUTER GRPHICS VECTORS SPRING 216 DR. MICHEL J. RELE INTRODUCTION In graphics, we are going to represent objects and shapes in some form or other. First, thogh, we need to figre ot how to represent
Chapter 6. Inverse Circular Functions and Trigonometric Equations. Section 6.1 Inverse Circular Functions y = 0
 Chapter Inverse Circlar Fnctions and Trigonometric Eqations Section. Inverse Circlar Fnctions. onetoone. range. cos... = tan.. Sketch the reflection of the graph of f across the line =. 7. (a) [, ] é ù
Chapter Inverse Circlar Fnctions and Trigonometric Eqations Section. Inverse Circlar Fnctions. onetoone. range. cos... = tan.. Sketch the reflection of the graph of f across the line =. 7. (a) [, ] é ù
Microscale physics of fluid flows
 Microscale physics of flid flows By Nishanth Dongari Senior Undergradate Department of Mechanical Engineering Indian Institte of Technology, Bombay Spervised by Dr. Sman Chakraborty Ot line What is microflidics
Microscale physics of flid flows By Nishanth Dongari Senior Undergradate Department of Mechanical Engineering Indian Institte of Technology, Bombay Spervised by Dr. Sman Chakraborty Ot line What is microflidics
1 JAXA Special Pblication JAXA-SP-1-E Small-scale trblence affects flow fields arond a blff body and therefore it governs characteristics of cross-sec
 First International Symposim on Fltter and its Application, 1 11 IEXPERIMENTAL STUDY ON TURBULENCE PARTIAL SIMULATION FOR BLUFF BODY Hiroshi Katschi +1 and Hitoshi Yamada + +1 Yokohama National University,
First International Symposim on Fltter and its Application, 1 11 IEXPERIMENTAL STUDY ON TURBULENCE PARTIAL SIMULATION FOR BLUFF BODY Hiroshi Katschi +1 and Hitoshi Yamada + +1 Yokohama National University,
This should serve a s a study guide as you go on to do the problems in Sapling and take the quizzes and exams.
 CHM 111 Chapter 9 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the corresponding
CHM 111 Chapter 9 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the corresponding
Chapter 3 MATHEMATICAL MODELING OF DYNAMIC SYSTEMS
 Chapter 3 MATHEMATICAL MODELING OF DYNAMIC SYSTEMS 3. System Modeling Mathematical Modeling In designing control systems we mst be able to model engineered system dynamics. The model of a dynamic system
Chapter 3 MATHEMATICAL MODELING OF DYNAMIC SYSTEMS 3. System Modeling Mathematical Modeling In designing control systems we mst be able to model engineered system dynamics. The model of a dynamic system
Model Explaining the Gravitational Force
 Model Explaining the Gravitational Force Clade Mercier eng., Agst nd, 015 Rev. October 17 th, 015 clade.mercier@gctda.com The niversal gravitation eqation of Newton is widely sed in the calclations in
Model Explaining the Gravitational Force Clade Mercier eng., Agst nd, 015 Rev. October 17 th, 015 clade.mercier@gctda.com The niversal gravitation eqation of Newton is widely sed in the calclations in
Discussion of The Forward Search: Theory and Data Analysis by Anthony C. Atkinson, Marco Riani, and Andrea Ceroli
 1 Introdction Discssion of The Forward Search: Theory and Data Analysis by Anthony C. Atkinson, Marco Riani, and Andrea Ceroli Søren Johansen Department of Economics, University of Copenhagen and CREATES,
1 Introdction Discssion of The Forward Search: Theory and Data Analysis by Anthony C. Atkinson, Marco Riani, and Andrea Ceroli Søren Johansen Department of Economics, University of Copenhagen and CREATES,
Chapter 5. The Gas Laws
 Chapter 5 The Gas Laws 1 Pressure Force per unit area. Gas molecules fill container. Molecules move around and hit sides. Collisions are the force. Container has the area. Measured with a barometer. 2
Chapter 5 The Gas Laws 1 Pressure Force per unit area. Gas molecules fill container. Molecules move around and hit sides. Collisions are the force. Container has the area. Measured with a barometer. 2
An Investigation into Estimating Type B Degrees of Freedom
 An Investigation into Estimating Type B Degrees of H. Castrp President, Integrated Sciences Grop Jne, 00 Backgrond The degrees of freedom associated with an ncertainty estimate qantifies the amont of information
An Investigation into Estimating Type B Degrees of H. Castrp President, Integrated Sciences Grop Jne, 00 Backgrond The degrees of freedom associated with an ncertainty estimate qantifies the amont of information
