Materials education for engineers: a transformation PTAS project
|
|
|
- Denis McDaniel
- 5 years ago
- Views:
Transcription
1 Materials education for engineers: a transformation PTAS project Introduction Materials are at the heart of all branches of engineering. It can be argued and with not much exaggeration that engineering is the creative and rational use of materials for practical purposes Engineers are better engineers if they have a good understanding of the properties of the materials which they use. [Hall 2009]. It is essential that we educate engineers with deep knowledge of materials. To do this the students must understand the basics well. The key aim of this project was to develop new methods, based on Socratic dialogue, to enhance interactive engagement and deepen students fundamental understanding of materials. (Socratic dialogue seeks to get the other person to answer their own questions by making them think and drawing out the answer from them). This report describes our success in developing new methods, parts of the project that proved to be more difficult, and what we can take from the project specifically in terms of Socratic dialogue in Materials teaching and more generally. An RA was employed on the project, full details of the project can be found her MSc thesis (Introducing Socratic Dialogue in Materials Science and Engineering Education, Eirini Theofanidou, MSc thesis, University of Edinburgh, April 2013). The project The key aim of the project was to develop a new Socratic dialogue approach for Materials tutorials to enhance the interactive engagement of the students. In the project new questions were devised and used with students on the course (see Appendix for questions and dialogues). These were highly effective in promoting engagement and curiosity with the undergraduate students (and also the postgraduate tutors). Many of the students were clearly increasingly engaged when they could actually handle the materials, and experience material behaviour with multiple senses. For example: kinaesthetic awareness of materials is powerful in that it is possible to quite literately get a feel for many materials properties: density, modulus, strength, thermal conductivity, surface roughness, deformation and fracture behaviour. Encouraging students to link the qualitative data they can collect through their senses with quantitative data e.g. from data books, promotes deep learning. This approach is very transferable for the students in developing their own learning within much of engineering (however it is worth noting that not all students have the maturity and interest at second year level in engineering to appreciate and apply this approach). The secondary aims of the project were to: make a thorough diagnosis of the problems our engineering students encounter in Materials and evaluate the effectiveness of this Socratic dialogue approach in the course in educationally scientific ways. These turned out to be more difficult to address. Good short/multiple choice style questions are rather hard to devise, especially as we wished to probe initial understanding and how this changed after the students had engaged with the hands- on Socratic dialogue questions. On reflection this methodology may have been somewhat flawed as we had to use the same short- questions before and after the students had engaged with the new Socratic questions, and the short- questions were devised first before new Socratic questions were completed. And, more fundamentally, testing the impact of the deep learning we wished to promote with the hands- on Socratic questions is not well matched to a series of short- questions.
2 The overall nature of this learning may be too fuzzy to assess quantitatively (certainly quantitative assessment is not easy). However, qualitative assessment: from being involved in the tutorials and engaging with the UGs and PG- tutors, from observation the spark and curiosity that was created was clear. More generally, though beyond the scope of this project, it seems there is important learning that can be brought about with this style of deep engagement which is absolutely a part of what we should be doing within a university but which is not easily congruent with quantitative assessment and the culture of metrics. Concluding remarks The approach of Socratic dialogue, particularly hands- on, is extremely good for promoting deep learning. It is a good method to highlight common misconceptions, which can then be addressed in lectures and feed into future iterations of the questions. For those involved in the project, including PG- tutors, it has been an opportunity for developing and refining questioning and listening skills both within the Socratic dialogue approach and more generally. Socratic dialogue works well in the tutorial questions we devised, and with PG- tutors; the approach is highly effective in learning basic concepts in materials science. Equally Socratic dialogue is a good approach for encouraging deep learning in materials science and engineering MSE, however deep subject- specific expertise is required to do this. This project has inspired JB the course lecturer to increasingly use this approach where appropriate in teaching Materials. MSE is a huge subject area, this project has highlighted how extensive my understanding of the whole subject is having spent many years in research, working with strong connections with industry, collaborations within physics, biology, & geosciences, as well as teaching materials to UG students). It is not feasible for PG- tutors to have this level of understanding of the whole subject area, so use of Socratic dialogue methods needs to be carefully thought out. Perhaps the most generic point to take from this project is a subtle shift in ways of thinking and interacting (questioning and listening) to promote learning which may be as applicable to research as it is to teaching. This is reflected in the international outputs of the project: Is ice a good substance for educating engineers about materials? European Geophysical Union, Session organized and sponsored by ESF research networking Programme on the Micro- Dynamics of Ice (Micro- DICE), 2011, Vienna, Austria. This contribution highlighted the use of Socratic questioning approaches, related to ice and snow, and then encouraged students to make links with the structure, manufacturing and behaviour of conventional engineering materials. [A journal paper related to this is in preparation]. Basics of materials: properties and microstructure of ice, and Ice and industry, Summer School on Microstructures of Ice and Snow, 2012, Obergurgl, Tyrol, Austria. This presentation used Socratic questioning in the Summer School to engage students with engineering materials and how everyday awareness of materials can be applied to the material of prime interest in the Summer School: ice. Jane Blackford, Nov 2014.
3 Appendix: Socratic questions & selected dialogues From MSc thesis (chapter 4): Introducing Socratic Dialogue in Materials Science and Engineering Education, Eirini Theofanidou, April Water drops on a lotus leaf The following tutorial was given as the first example of a theme for Socratic dialogue. Water drops on a lotus leaf: Fig 1 The lotus leaf image given during the tutorial. (a) Draw a sketch to indicate the interfacial energies that act on the drops. (b) Give an example of a different system that is hydrophobic (as in the picture), and an example of a systems that is hydrophilic. Consider their use in applications. (c) Use the web to find out about self-cleaning surfaces (potentially amazing!). What structure do they typically have? What are their current disadvantages? Concept Students were asked to develop a deeper understanding on interfacial energies (or tensions), wetting, dewetting, the hydrophobic and hydrophilic behaviour associated with that and also relates these phenomena to examples from real life. Interfacial tension/energy determines the wetting behaviour of a drop of liquid on a flat solid surface. In such a system changing one of the interfacial energies that exist will result in a change of the wetting behaviour of a drop. The example for the lotus leaf was given and the lotus effect was discussed with the students Application A tutor drew a drop on a flat surface and students were asked to determine the different interfacial tensions that exist in this system and the direction of the vector for each of them.
4 Fig 2 Liquid drop on a flat solid surface. Interfacial tension (or energy) is defined by the letter γ; γ lv is the interfacial tension between the drop and the air, γ sv is the interfacial energy between the surface and the air and γ sl is the one between the surface and the liquid. The angle θ is defined as the contact angle and its magnitude determines the wetting behaviour of the drop, i.e. how close the drop will be to a complete sphere (θ =180 o ) or a flat wetting layer (θ =0 o ) on the surface. Of course, these are extreme cases and many a time the angle is between 30 o o. T: Could someone identify the different interfacial energies (or tensions) that exist in the physical system depicted in drawing? S1: We have the interfacial energy between the solid and the water and between the water and the air. S2: And also between the solid and the air. T: Right, which direction do they go? After drawing the tension vectors students were asked to apply force balance and construct the equation that holds (Young s equation). γ sl + γ lv cos θ = γ sv So the angle θ determines the wetting behaviour of the droplet. The bigger the angle the drop becomes closer to full sphere and this means that the contact between the solid and the drop is very small so we have hydrophobic behaviour as the liquid tends to leave the solid surface. So solids with contact angles (with water) of more than 90 degrees exhibit hydrophobicity, while in the case of small contact angles (i.e. θ < 90 ) the water drop is more flat as it attaches to the solid more and the latter is referred to as hydrophilic. T: Could you think of a system which is hydrophobic/hydrophilic? S:.. T: Try to find an example from everyday life. In which cases do we want something to be hydrophobic? S: When it rains? T: Exactly.an example? S: The coats
5 T: Yes the waterproof jacket.in this case we want the garment to be able to repel water, otherwise we will soak. Waterproof clothes are made by fabric which is resistant to water either inherently (natural fabric) or after treatment (synthetic fabrics). In the latter case a waterproofing material like rubber, polyvinyl chloride (PVC), polyurethane (PU), wax etc. are used to coat or laminate to a piece of cloth thus no water can pass through. Later on the discussion, we focused on the lotus leaf. Students were asked whether they searched the web to find any self-cleaning surfaces like the lotus leaf and discover what is actually happening. T: And what about the lotus leaf? Is anyone aware of how it self-cleanses? S: T: Has anyone searched the internet? S:. T: Why do you think this is happening? S: T: The lotus leaf exhibits one property that has to do with what? S: The leaf T: What do you mean? S: The make of the leaf T: You mean the structure of the leaf S: Yes T: What structure do you expect the leaf to have in order to be able to self-cleanse itself? T: Will it help if I describe the surface of the leaf? At this point the picture in figure 4.3 was presented to the students
6 Fig 3 Diagram of Lotus effect. Water forms droplets on the tips of the epidermal protrusions and by rolling off the leaf it collects dirt and small insects. (The picture was taken from iology/ T: The picture is just a schematic drawing of images taken with a scanning electron microscope. As you can see the lotus leaf has a very characteristic structure with a complex micro and nano-roughness; its self-cleaning ability is due to a double structure of the surface. The first layer consists of micro-lumps and bumps of protruding epidermal cells, which are around 10 to 20 µm in height and 10 to 15 µm in width and they are covered by wax crystals of 1 nm size. These superimposed waxes are hydrophobic and they form the second layer. Now if we recall the water droplets we said that they have relatively high surface tension and they tend to minimize their surface by forming spheres. Also we have just discussed the contact angle of a water droplet on a hydrophobic surface. So what contact angle do you expect the lotus leaf to have? S: A quite big one. T: Exactly and in this case due to its double structure the contact angle of the lotus is about 170. So the water doesn t stay flat on the leaf but it stays at the top of the lumps (the roughness enhances the hydrophobic behaviour) as we can see from the picture and it does not slip on the surface, it actually rolls, what about the other dirt and small insects? S: It depends on their contact angles and adherence to the leaf. T: Yes, but these small particles actually make contact only at the tip of the wax crystals so what do you expect? Will they adhere well to the surface of the leaf? S: No. T: In that case the adhesion between the dirt particle and the water is higher than between the lotus surface and the particle (regardless of its chemistry) so the water by rolling down the leaf traps the dirt and it cleans the leaf.
7 1.2 The paper clip Tutorial Experiment Question (can be done before tutorial or in the tutorial if you bring paper clips with you) Consider a metallic paper clip. (a) Bend it. What happens? Explain why by considering the processes that occur at an atomic level. (b) Bend it back. What happens? Again explain why. (c) Bend it repeatedly. What happens? [We ve not covered this in the course yet, but you may be able to work out why this happens. Think about why this behaviour is important in engineering)] Concept This tutorial question focuses on the plastic deformation of steel and how this relates to dislocations. Also it covers the fatigue process of the steel sample Application in the tutorial Most of the students had not brought a paper clip with them so the tutors either brought a few or they discussed what we would expect from common experience. T: Imagine that you have a paper clip made from a typical steel (not a plastic one), and you bend it slightly. What happens? S: It will bend. T: If you bend it slightly? S: No, it comes back to its initial position. T: Right. So what kind of phenomenon do we have? S: A deformation that lasts only during the application of a force. T: How do we call this deformation that ceases to exist upon the removal of the small force? T: Correct. S: Elastic T: Imagine now that you bend it more. What happens? S: The clip bends and it stays in another position T: So in this case what kind of deformation do we have? S: Plastic.
8 T: Right, so if you think in atomic level terms, what happens that leads to this kind of permanent deformation? S: The microstructure changes permanently T: Yes, but what exactly happens to this microstructure, what is the structure of a metal? S: Crystalline. T: Yes, so what happens during elastic deformation? S: The atoms in the crystal move away from each other but upon the removal of the force they return in their initial positions T: And at plastic deformation? S: Again they move away from each other, but they move so much that they cannot return back. T: It is not a bad answer but I will add that when the atoms move away from each other, we have the appearance of linear defects in the crystal lattice called dislocations S: Oh, yes, we have talked about them in the lectures. It is clearer now to me that they are brought about by the deformation. T: Yes, now if we bend it back what do you observe? S: It is actually more difficult to put it back to its initial position. It requires more effort! T: You have to apply higher force, right? S: Yes. T: How do you explain this? S: T: Seems that the plastic deformation has made the material harder. S: Yes. T: What about the dislocations? When we did not have many of them were easy to make and plastically deform the sample, but now that we have probably many of them, they seem to inhibit the movement of the atoms. S: Yes, it seems that having too many of them inhibits the deformation of the sample. T: As we say dislocation motion and plastic deformation are hindered by dislocation accumulation. This is the basis of work hardening. The next step is to think of what is going to happen when we start bending it repeatedly back and forth. S: At some point it will break.
9 T: Correct even if the force is not that high, why do you think this is happening? S: The repeated deformation even if it is small will induce a large density of dislocations, possibly building up slowly but steadily extensive defects. fails. T: In the end, no more elastic or plastic deformation can be sustained and the material T: Could you guess why this process is important for engineers? S: I guess that in many engineering applications we have repeated application of stress which can induce failure in this way. T: Yes, and this can happen because of this repeating motion even if the stresses involved are not that high, they can be much lower than the yield stress. We say that this failure is due to fatigue. 1.3 The silly putty Tutorial question Watch the videos of silly putty (hit with a hammer, dropped and allowed to rest on a surface) on Doitpoms: [use google & underlined words to find the videos]. How does the behaviour of the silly putty change with different strain rates? Think about why Concept The main concept of this and the following tutorial question focuses on how the mechanical behaviour of thermoplastics and rubbers is affected by temperature (T), time (t) and load and also how these parameters interact with each other. It is very important for engineers to develop an awareness of the different possibilities. Before these two tutorial questions students were asked to identify the different types of polymers, i.e. thermoplastics, thermosets and elastomers, to describe the stress-strain curves for each of them and describe the differences in their behaviour. Another tutorial question was related to the glass transition temperature (Tg) of thermoplastics and why it s so important for elastomers Application Students were given real silly putty samples to explore during tutorials; for each tutorial class we had at least one sample which was laid to rest during the previous night. While playing with the samples student were asked what they would expect to happen when different strain rates applied to the silly putty. For safety reasons the strain rate applied with hammer was only asked to be discussed and not performed in the class. During discussion, movies from the web-site were shown to students to see what actually happens while the strain rates applied to silly putty. As one can see from the following snapshots when the silly putty is hit with a hammer it fractures, while it bounces when it is dropped. In the case where is left to rest on the surface it creeps/flows. The latter happens at an extremely slow time-rate and we get an indication by observing the silly putty relative to the graph paper that exists behind.
10 Fig 4 Snapshots of the silly putty hit with a hammer. (Images are snapshots from movies found at Fig 5 Snapshots of the silly putty bouncing and rebouncing on the floor. (Images are snapshots from movies found at
11 Fig 6 Snapshots of the silly putty left to rest on a flat surface. (Images are snapshots from movies found at A typical discussion between tutor and students was the following: T: You are given the silly putty in the form of a ball and we hit it with a hammer; what do you expect to happen? S: It is going to get deformed. T: right but how? S: it is going to change its shape T: what shape do you expect the ball to have now? S: something more flat and elliptical T: imagine that you have extreme power and you hit it VERY hard. What do you expect its shape to be? S: completely flat, like a pastry.. T: well let s see what happens when we do that.through a video demonstration. After the video T: well, as you can see we have not just a simple deformation but a? S: crack. T: it s not a crack S: it breaks T: so we have a fracture.why do you thing this happens? Why a polymer or an elastomer something that is considered soft breaks? How can we explain that? S:
12 T: in order for a material to be able to break it has to exhibit what type of property? Which materials can be broken? S: those we can break. T: how do we call these materials? What kind of behaviour? S: brittle? T: yep and if you can recall form the lecture when a polymer can be brittle? Remember what we did here we applied an extremely high force at a very short time interval. This force stresses the ball so we have applied what kind of strain rate S: a big one. T: exactly by hitting the ball with a hammer we have applied a high strain rate. What does this mean for the material at the molecular scale? S: What do you mean? T: What does this mean for the molecules of the material, the polymer chains? S: They will bend. T: Does really the fracture that we have witnessed indicate bending? S: It doesn t, it indicates break, sudden rupture. T: So, what do you think it happens at the molecular scale when we apply a force at high rate? S: Molecules don t have time to deform to get out of the way and break. This is strange but in the end it makes sense. T: Indeed, if the strain rate is high enough the polymer behaves in a brittle manner and that s why it fractures. However, it is interesting to go a bit deeper. At the molecular scale again, what do you think determines if the behaviour will be brittle or ductile? S: It is the response of the molecules, if they will deform/bend or break. I guess is the magnitude of the force. If it is too high they will break, if low they will bend. T: Do you believe that the result would have been the same if we did not hit the sample with a hammer, to apply the force at high rate, but we just pressed the hammer on the sample slowly, at low rate, from 0 N force to the same maximum force accomplished with the hitting? S: (After some thought) probably not, it wouldn t fracture in a brittle manner, it is not the force, it is the rate of application of the force. T: Correct. But what determines if the rate is high enough? S: It is the molecular properties, if the molecule itself is brittle or ductile?
13 T: Then, why the rate plays a role? If it is the molecule itself that is brittle or ductile the result wouldn t depend on the rate. For the same material, i.e. for the same molecules, the behaviour would be only brittle or ductile at any rate. S: I see, it has indeed to do with how the molecule behaves but I cannot think of what property of the molecule. It has to do with time as there is a rate effect. But I cannot connect time with molecular properties. T: Do the molecules stay still or move? S: The jiggle around in random fashion because of thermal motion. T: Will a polymer chain move more quickly or more slowly compared to a small molecule of a simple liquid at the same temperature? S: I know this, the chain will move more slowly, it is big, there is internal friction, the motion will be sluggish; that s why polymers are tough in the first place. T: What will happen if we try to move the molecules at low rate? S: They will move out of the way, bend, deform. T: What will happen if we try to move the molecules at high rate? S: They won t have time to move out of the way. They could break. So, I see now, as we hit the polymer sample with the hammer at very high rate the chains don t have time to move, they are effectively frozen, the break. T: So, the rate or as we say the characteristic time of testing has to be compared with what? S: With the characteristic time that the molecules take to move. T: Do you know the name of the technical term that gives us the time that a molecule moves a distance of its own size? S: The relaxation time. I knew that but I had no idea that of its practical significance 1.4 The bouncy balls Tutorial Question Watch movies of the behavior of an elastomer during different temperatures. Think about the similarities between the behaviour of elastomers (or silly putty) at different strain rates [the above question] and different temperatures [this question] Concept Continuing from the previous question this one focuses on the glass transition of an elastomer and how the stress strain behavior changes with temperature.
14 1.4.3 Application Four movies were shown to students at different temperatures on the wall screen. Below are snapshots from the movies. In all movies the bouncy ball was left to drop from an initial height of ten units. The first movie was recorded at room temperature of 25 C. Below are five indicative snapshots; the first one is the initial release of the ball, the second shows the first reach on the ground, the third the highest height after the rebound, the fourth the second hit on the ground and the fifth is the last frame of the movie and it shows the highest height after the second rebound. Fig 7 Snapshots of the movie where a bouncy ball is left to drop from an initial height of 10 units at room temperature 25 C. (Images are snapshots from movies found at Students watched the first movie and then we had the following typical dialogue: T: Now let s move to another temperature, lower temperature, what do you anticipate it s going to happen? S: The ball will bounce again up and down until it stops. T: is it going to be the same with the previous one? S: no T: what is going to change? S: the time? T: what do you mean by the time? S: How long it takes to rebounce T: can you explain that
15 S: well the time it will take to hit the ground and rebounce and hit again and rebounce again and the stop will be different. T: So you mean that the time-length of the entire movement until it comes to a rest is going to change S: yes T: Ok but why? I mean if the time changes it means that something else has changed S:.. T: ok let s see what happens Here the second movie at temperature of -50 C was displayed on the screen Fig 8 Snapshots of the movie where a bouncy ball is left to drop from an initial height of 10 units at room temperature -50 C. (Images are snapshots from movies found at T: so what did you see? S: The ball is bouncing at a lower height both times. T: Correct so this explains why it takes less time to come to a rest. Imagine now what is going to happen when we go at an even lower temperature, for example -70 C. What do you expect is going to happen? S: it is going to take even less time T: so? S: it will bounce back at a lower height than at -50 C. And then it will stop quicker.
16 T: ok let s see if this is going to happen The third movie was shown. Fig 9 Snapshots of the movie where a bouncy ball is left to drop from an initial height of 10 units at room temperature -70 C. (Images are snapshots from movies found at T: and now imagine that we are going further down in temperature at -190 C. In this case what will happen? S1: It will take even less time S2: it will drop and stop completely without rebouncing S3: it will stick on the ground. T: ok let s see The movie at -190 C S: is this the correct one? T: yep would you like to have another look? S: yes please. T: so what do you think is happening? How is it possible the ball to behave similarly to -50 C?
17 Fig 10 Snapshots of the movie where a bouncy ball is left to drop from an initial height of 10 units at room temperature -190 C. (Images are snapshots from movies found at S: I really don t know. It is strange. T: It seems that the elastic ball is acting symmetrically around a temperature. S: Yes, I see that. It seems that something changes at that temperature. We discussed in the lecture the so called glass transition temperature, but I was expecting a different behavior bellow and above that temperature, not symmetry around this temperature. T: Could someone remind us what happens at the glass transition temperature? S: It is the temperature at which the material changes behavior. T: What behavior? S/T (in some groups the students did know about that quite well but still had trouble comprehending the symmetric behavior while in some other groups this piece of information had to be provided as the students did not remember the lecture):the viscoelastic it means that at this temperature we have a transition for the material from the rubbery state to glassy and vice versa. T: What actually happens at the molecular scale at the transition? S: The chains become to some extent frozen, they cannot move that much T: This is the glassy state, the chains as you say are frozen (but of course monomers vibrate around their equilibrium positions, the cages ), it is not the transition though. S: Yes, I see, you are right; actually at the transition I guess there will be some jiggling around but quite sluggish, so not so freely. T: What prohibits the movements? S: The temperature is going down, it is more difficult for the chains to move.
18 T: If they are difficult to move, what does this imply for the friction at the molecular scale, the so called internal friction? S: It will be high. T: High friction is associated with what? S: heat losses, energy will be lost. T: So high dissipation of energy. S: I see now, at the glass transition, as the ball hits the ground, a large amount of its kinetic energy is dissipated, lost as thermal energy. T: Correct. Do we now understand why there is this behavior at -70 o C? S: Yes, it indicates that we are at or close to glass transition temperature. T: Now, why we have elastic behavior at higher temperatures? S: Elastomeric behavior. T: What does this mean? S: Elastomers are elastic? T: Yes, but why? Polymers flow at higher temperatures, they are not so elastic. S: You see, elastomers have crosslinks. This inhibits the flow. T: It explains the absence of creep but not necessarily the elasticity. S: Yes, I see we have been taught about the entropic elasticity of the chains between the crosslinks. T: What does it mean? S/T (in some groups the students did know about entropic elasticity while in some other groups this piece of information had to be provided as the students had not comprehended at all the lecture material; a tutorial on entropic elasticity alone could be useful): The chains jiggle around because of thermal motion and have a characteristic size at a certain temperature (above T g ). If they are compressed or stretched there will be a restoring force. Macroscopically this manifests as the high elastic behaviour of rubbers. T: Why the energy loss is low at temperatures well above T g? S: The chains definitely move around, there will be some dissipation. T: You are right but is it big? S: No, not as big as when we are close to T g. T: Yes, there is some internal friction but no so high. What about at very low temperature?
19 ball. S: This is easier. The material is a glass, if it does not break it will bounce like a glass T: Correct, it is more conventional behaviour. But why we do not have high dissipation at the molecular scale at these conditions? S: energy loss is low because the molecules do not move. T: Yes, chains are quite frozen, there is no significant sliding between the chains to generate high internal friction. S: I realize now that the behaviour is symmetric but for quite different reasons. T: Yes, the underlying physics is different above and below the glass transition temperature. Fig 11 Glass transition temperature diagram with energy lost. T: Finally, if we combine the last two tutorial questions what do we conclude about the mechanical behavior of polymers? S: It changes with temperature. S: And strain rates T: Yes so when are they highly elastic and potentially brittle in which regions? S: Low temperature and high strain rates S: And at low strain rates and high temperature are ductile/viscous.
(Refer Slide Time: 00:58)
 Nature and Properties of Materials Professor Bishak Bhattacharya Department of Mechanical Engineering Indian Institute of Technology Kanpur Lecture 18 Effect and Glass Transition Temperature In the last
Nature and Properties of Materials Professor Bishak Bhattacharya Department of Mechanical Engineering Indian Institute of Technology Kanpur Lecture 18 Effect and Glass Transition Temperature In the last
Deformation: Modification of Rocks by Folding and Fracturing
 CHAPTER 7 Deformation: Modification of Rocks by Folding and Fracturing Chapter Summary A geologic map is a scientific model of rock formations that are exposed on the Earth s surface showing outcrops,
CHAPTER 7 Deformation: Modification of Rocks by Folding and Fracturing Chapter Summary A geologic map is a scientific model of rock formations that are exposed on the Earth s surface showing outcrops,
Mechanical properties of polymers: an overview. Suryasarathi Bose Dept. of Materials Engineering, IISc, Bangalore
 Mechanical properties of polymers: an overview Suryasarathi Bose Dept. of Materials Engineering, IISc, Bangalore UGC-NRCM Summer School on Mechanical Property Characterization- June 2012 Overview of polymer
Mechanical properties of polymers: an overview Suryasarathi Bose Dept. of Materials Engineering, IISc, Bangalore UGC-NRCM Summer School on Mechanical Property Characterization- June 2012 Overview of polymer
Chapter 7. Highlights:
 Chapter 7 Highlights: 1. Understand the basic concepts of engineering stress and strain, yield strength, tensile strength, Young's(elastic) modulus, ductility, toughness, resilience, true stress and true
Chapter 7 Highlights: 1. Understand the basic concepts of engineering stress and strain, yield strength, tensile strength, Young's(elastic) modulus, ductility, toughness, resilience, true stress and true
The DiSiDo Project Innovative and motivating
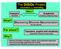 The DiSiDo Project Innovative and motivating Industry WACKER Who? Academia Chemistry Didactics Dept. What? Discovering Silicones Documentation CD and internet For whom? Why? Teachers, pupils and students
The DiSiDo Project Innovative and motivating Industry WACKER Who? Academia Chemistry Didactics Dept. What? Discovering Silicones Documentation CD and internet For whom? Why? Teachers, pupils and students
Abvanced Lab Course. Dynamical-Mechanical Analysis (DMA) of Polymers
 Abvanced Lab Course Dynamical-Mechanical Analysis (DMA) of Polymers M211 As od: 9.4.213 Aim: Determination of the mechanical properties of a typical polymer under alternating load in the elastic range
Abvanced Lab Course Dynamical-Mechanical Analysis (DMA) of Polymers M211 As od: 9.4.213 Aim: Determination of the mechanical properties of a typical polymer under alternating load in the elastic range
Volume vs. Diameter. Teacher Lab Discussion. Overview. Picture, Data Table, and Graph
 5 6 7 Middle olume Length/olume vs. Diameter, Investigation page 1 of olume vs. Diameter Teacher Lab Discussion Overview Figure 1 In this experiment we investigate the relationship between the diameter
5 6 7 Middle olume Length/olume vs. Diameter, Investigation page 1 of olume vs. Diameter Teacher Lab Discussion Overview Figure 1 In this experiment we investigate the relationship between the diameter
Forces and motion. 1 Explaining motion. 2 Identifying forces. 1 of 9
 1 of 9 Forces and motion 1 Explaining motion The reason why force is an important idea in physics is because the motion of any object can be explained by the forces acting on the object. The procedure
1 of 9 Forces and motion 1 Explaining motion The reason why force is an important idea in physics is because the motion of any object can be explained by the forces acting on the object. The procedure
Figure 1: Doing work on a block by pushing it across the floor.
 Work Let s imagine I have a block which I m pushing across the floor, shown in Figure 1. If I m moving the block at constant velocity, then I know that I have to apply a force to compensate the effects
Work Let s imagine I have a block which I m pushing across the floor, shown in Figure 1. If I m moving the block at constant velocity, then I know that I have to apply a force to compensate the effects
Name Date Block LESSON CLUSTER 6: Expansion and Contraction
 LESSON CLUSTER 6: Expansion and Contraction Did you know that when you say that something is hot or cold, you are actually saying something about the molecules of that substance? Words like hot and cold
LESSON CLUSTER 6: Expansion and Contraction Did you know that when you say that something is hot or cold, you are actually saying something about the molecules of that substance? Words like hot and cold
Physics E-1ax, Fall 2014 Experiment 3. Experiment 3: Force. 2. Find your center of mass by balancing yourself on two force plates.
 Learning Goals Experiment 3: Force After you finish this lab, you will be able to: 1. Use Logger Pro to analyze video and calculate position, velocity, and acceleration. 2. Find your center of mass by
Learning Goals Experiment 3: Force After you finish this lab, you will be able to: 1. Use Logger Pro to analyze video and calculate position, velocity, and acceleration. 2. Find your center of mass by
MATERIALS. Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle?
 MATERIALS Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: A. Composition
MATERIALS Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: A. Composition
Mechanics of Earthquakes and Faulting
 Mechanics of Earthquakes and Faulting Lectures & 3, 9/31 Aug 017 www.geosc.psu.edu/courses/geosc508 Discussion of Handin, JGR, 1969 and Chapter 1 Scholz, 00. Stress analysis and Mohr Circles Coulomb Failure
Mechanics of Earthquakes and Faulting Lectures & 3, 9/31 Aug 017 www.geosc.psu.edu/courses/geosc508 Discussion of Handin, JGR, 1969 and Chapter 1 Scholz, 00. Stress analysis and Mohr Circles Coulomb Failure
Fluids: How thick are liquids?
 Fluids: How thick are liquids? Student Advanced Version Introduction: Fluids are substances that can flow under an applied force. What are some examples of fluids? We often think of fluids as substances
Fluids: How thick are liquids? Student Advanced Version Introduction: Fluids are substances that can flow under an applied force. What are some examples of fluids? We often think of fluids as substances
Changes of State. Thermal Energy: Changes of State 1
 Changes of State Last lesson we learned about thermal energy and temperature. Objects whose molecules are moving very quickly are said to have high thermal energy or high temperature. The higher the temperature,
Changes of State Last lesson we learned about thermal energy and temperature. Objects whose molecules are moving very quickly are said to have high thermal energy or high temperature. The higher the temperature,
TA guide Physics 208 Spring 2008 Lab 3 (E-1): Electrostatics
 Name TA guide Physics 208 Spring 2008 Lab 3 (E-1): Electrostatics Section OBJECTIVE: To understand the electroscope as an example of forces between charges, and to use it as a measuring device to explore
Name TA guide Physics 208 Spring 2008 Lab 3 (E-1): Electrostatics Section OBJECTIVE: To understand the electroscope as an example of forces between charges, and to use it as a measuring device to explore
INTRODUCTION TO LESSON CLUSTER 7
 INTRODUCTION TO LESSON CLUSTER 7 EXPLAINING MELTING AND SOLIDIFYING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain melting and solidifying, by reference to the molecular
INTRODUCTION TO LESSON CLUSTER 7 EXPLAINING MELTING AND SOLIDIFYING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain melting and solidifying, by reference to the molecular
The complete lesson plan for this topic is included below.
 Home Connection Parent Information: Magnets provide a simple way to explore force with children. The power of a magnet is somewhat like magic to them and requires exploration to understand. When forces
Home Connection Parent Information: Magnets provide a simple way to explore force with children. The power of a magnet is somewhat like magic to them and requires exploration to understand. When forces
How Does the Sun s Energy Cause Rain?
 1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
GRADE 7: Physical processes 3. UNIT 7P.3 8 hours. Magnetism. Resources. About this unit. Previous learning. Expectations
 GRADE 7: Physical processes 3 Magnetism UNIT 7P.3 8 hours About this unit This unit is the third of five units on physical processes for Grade 7. It builds on Unit 6P.1 and leads on to work on electromagnets
GRADE 7: Physical processes 3 Magnetism UNIT 7P.3 8 hours About this unit This unit is the third of five units on physical processes for Grade 7. It builds on Unit 6P.1 and leads on to work on electromagnets
Rheology. What is rheology? From the root work rheo- Current: flow. Greek: rhein, to flow (river) Like rheostat flow of current
 Rheology What is rheology? From the root work rheo- Current: flow Greek: rhein, to flow (river) Like rheostat flow of current Rheology What physical properties control deformation? - Rock type - Temperature
Rheology What is rheology? From the root work rheo- Current: flow Greek: rhein, to flow (river) Like rheostat flow of current Rheology What physical properties control deformation? - Rock type - Temperature
Lecture 09 Combined Effect of Strain, Strain Rate and Temperature
 Fundamentals of Materials Processing (Part- II) Prof. Shashank Shekhar and Prof. Anshu Gaur Department of Materials Science and Engineering Indian Institute of Technology, Kanpur Lecture 09 Combined Effect
Fundamentals of Materials Processing (Part- II) Prof. Shashank Shekhar and Prof. Anshu Gaur Department of Materials Science and Engineering Indian Institute of Technology, Kanpur Lecture 09 Combined Effect
MECHANICAL PROPERTIES OF MATERIALS
 1 MECHANICAL PROPERTIES OF MATERIALS Pressure in Solids: Pressure in Liquids: Pressure = force area (P = F A ) 1 Pressure = height density gravity (P = hρg) 2 Deriving Pressure in a Liquid Recall that:
1 MECHANICAL PROPERTIES OF MATERIALS Pressure in Solids: Pressure in Liquids: Pressure = force area (P = F A ) 1 Pressure = height density gravity (P = hρg) 2 Deriving Pressure in a Liquid Recall that:
Physic 602 Conservation of Momentum. (Read objectives on screen.)
 Physic 602 Conservation of Momentum (Read objectives on screen.) Good. You re back. We re just about ready to start this lab on conservation of momentum during collisions and explosions. In the lab, we
Physic 602 Conservation of Momentum (Read objectives on screen.) Good. You re back. We re just about ready to start this lab on conservation of momentum during collisions and explosions. In the lab, we
Lecture 6 Force and Motion. Identifying Forces Free-body Diagram Newton s Second Law
 Lecture 6 Force and Motion Identifying Forces Free-body Diagram Newton s Second Law We are now moving on from the study of motion to studying what causes motion. Forces are what cause motion. Forces are
Lecture 6 Force and Motion Identifying Forces Free-body Diagram Newton s Second Law We are now moving on from the study of motion to studying what causes motion. Forces are what cause motion. Forces are
Title of Lesson: Can All Things Stretch? RET Project Connection: Failure Modes of Lightweight Sandwich Structures
 Title of Lesson: Can All Things Stretch? RET Project Connection: Failure Modes of Lightweight Sandwich Structures RET Teacher: Michael Wall School: Andover High School Town/District: Andover Public Schools
Title of Lesson: Can All Things Stretch? RET Project Connection: Failure Modes of Lightweight Sandwich Structures RET Teacher: Michael Wall School: Andover High School Town/District: Andover Public Schools
States of Matter: Solid, Liquid, and Gas
 Movie Special Effects Activity 2 States of Matter: Solid, Liquid, and Gas GOALS In this activity you will: Create an animation to illustrate the behavior of particles in different phases of matter, and
Movie Special Effects Activity 2 States of Matter: Solid, Liquid, and Gas GOALS In this activity you will: Create an animation to illustrate the behavior of particles in different phases of matter, and
Thermoplastic. Condensation. Homopolymer. Polymer POLYMERS. Synthetic. Natural. Addition. Copolymer. Polymer. Thermosetting
 Thermoplastic Homopolymer Condensation Polymer Natural POLYMERS Synthetic Addition Polymer Copolymer Thermosetting Polymers are very large covalent molecular substances containing tens of thousands of
Thermoplastic Homopolymer Condensation Polymer Natural POLYMERS Synthetic Addition Polymer Copolymer Thermosetting Polymers are very large covalent molecular substances containing tens of thousands of
Brittle Deformation. Earth Structure (2 nd Edition), 2004 W.W. Norton & Co, New York Slide show by Ben van der Pluijm
 Lecture 6 Brittle Deformation Earth Structure (2 nd Edition), 2004 W.W. Norton & Co, New York Slide show by Ben van der Pluijm WW Norton, unless noted otherwise Brittle deformation EarthStructure (2 nd
Lecture 6 Brittle Deformation Earth Structure (2 nd Edition), 2004 W.W. Norton & Co, New York Slide show by Ben van der Pluijm WW Norton, unless noted otherwise Brittle deformation EarthStructure (2 nd
Johns Hopkins University What is Engineering? M. Karweit MATERIALS
 Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: MATERIALS A. Composition
Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: MATERIALS A. Composition
Year 6 Science Unit 6E Forces in action
 Year 6 Science Unit 6E Forces in action ABOUT THE UNIT In this unit children apply their knowledge of a variety of forces, including magnetic attraction, gravitational attraction and friction. Children
Year 6 Science Unit 6E Forces in action ABOUT THE UNIT In this unit children apply their knowledge of a variety of forces, including magnetic attraction, gravitational attraction and friction. Children
Physics 208 Spring 2008 Lab 3 (E-1): Electrostatics
 Name Section Physics 208 Spring 2008 Lab 3 (E-1): Electrostatics OBJECTIVE: To understand the electroscope as an example of forces between charges, and to use it as a measuring device to explore charge
Name Section Physics 208 Spring 2008 Lab 3 (E-1): Electrostatics OBJECTIVE: To understand the electroscope as an example of forces between charges, and to use it as a measuring device to explore charge
Lesson 1 Solids, Liquids, and Gases
 Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
Please bring the task to your first physics lesson and hand it to the teacher.
 Pre-enrolment task for 2014 entry Physics Why do I need to complete a pre-enrolment task? This bridging pack serves a number of purposes. It gives you practice in some of the important skills you will
Pre-enrolment task for 2014 entry Physics Why do I need to complete a pre-enrolment task? This bridging pack serves a number of purposes. It gives you practice in some of the important skills you will
How materials work. Compression Tension Bending Torsion
 Materials How materials work Compression Tension Bending Torsion Elemental material atoms: A. Composition a) Nucleus: protons (+), neutrons (0) b) Electrons (-) B. Neutral charge, i.e., # electrons = #
Materials How materials work Compression Tension Bending Torsion Elemental material atoms: A. Composition a) Nucleus: protons (+), neutrons (0) b) Electrons (-) B. Neutral charge, i.e., # electrons = #
Introduction to materials modeling and simulation
 1 Introduction to materials modeling and simulation With the development of inexpensive, yet very fast, computers and the availability of software for many applications, computational modeling and simulation
1 Introduction to materials modeling and simulation With the development of inexpensive, yet very fast, computers and the availability of software for many applications, computational modeling and simulation
Forces and motion 3: Friction
 Forces and motion 3: Friction University of York 2003 133 Friction The questions in this set probe pupils understanding of friction. Friction is the name of the interaction between two surfaces when they
Forces and motion 3: Friction University of York 2003 133 Friction The questions in this set probe pupils understanding of friction. Friction is the name of the interaction between two surfaces when they
PHYSICS. Chapter 5 Lecture FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E RANDALL D. KNIGHT Pearson Education, Inc.
 PHYSICS FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E Chapter 5 Lecture RANDALL D. KNIGHT Chapter 5 Force and Motion IN THIS CHAPTER, you will learn about the connection between force and motion.
PHYSICS FOR SCIENTISTS AND ENGINEERS A STRATEGIC APPROACH 4/E Chapter 5 Lecture RANDALL D. KNIGHT Chapter 5 Force and Motion IN THIS CHAPTER, you will learn about the connection between force and motion.
SAM Teachers Guide Phase Change Overview Learning Objectives Possible Student Pre/Misconceptions
 SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion and observing and manipulating
SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion and observing and manipulating
Friction: A Force That Opposes Motion
 3 What You Will Learn The magnitude of the force of can vary. Kinetic is a force that, when unbalanced, can change the velocity of a moving object. Static balances an applied force and can prevent motion.
3 What You Will Learn The magnitude of the force of can vary. Kinetic is a force that, when unbalanced, can change the velocity of a moving object. Static balances an applied force and can prevent motion.
Forces and Newton s Laws
 chapter 3 section 1 Forces Forces and Newton s Laws What You ll Learn how force and motion are related what friction is between objects the difference between mass and weight Before You Read When you hit
chapter 3 section 1 Forces Forces and Newton s Laws What You ll Learn how force and motion are related what friction is between objects the difference between mass and weight Before You Read When you hit
MITOCW MITRES18_005S10_DiffEqnsMotion_300k_512kb-mp4
 MITOCW MITRES18_005S10_DiffEqnsMotion_300k_512kb-mp4 PROFESSOR: OK, this lecture, this day, is differential equations day. I just feel even though these are not on the BC exams, that we've got everything
MITOCW MITRES18_005S10_DiffEqnsMotion_300k_512kb-mp4 PROFESSOR: OK, this lecture, this day, is differential equations day. I just feel even though these are not on the BC exams, that we've got everything
(Refer Slide Time: 00:10)
 Chemical Reaction Engineering 1 (Homogeneous Reactors) Professor R. Krishnaiah Department of Chemical Engineering Indian Institute of Technology Madras Lecture No 10 Design of Batch Reactors Part 1 (Refer
Chemical Reaction Engineering 1 (Homogeneous Reactors) Professor R. Krishnaiah Department of Chemical Engineering Indian Institute of Technology Madras Lecture No 10 Design of Batch Reactors Part 1 (Refer
Section 2: Friction, Gravity, and Elastic Forces
 Chapter 10, Section 2 Friction, Gravity, & Elastic Forces Section 2: Friction, Gravity, and Elastic Forces What factors determine the strength of the friction force between two surfaces? What factors affect
Chapter 10, Section 2 Friction, Gravity, & Elastic Forces Section 2: Friction, Gravity, and Elastic Forces What factors determine the strength of the friction force between two surfaces? What factors affect
SCED 204 Sample Activity SCED 204: Matter and Energy in Chemical Systems
 SCED 204 Sample Activity SCED 204: Matter and Energy in Chemical Systems Activity #2: DO THE SMALL PARTICLES OF MATTER MOVE? IF SO, HOW? Purpose: We have been developing a model of matter that involves
SCED 204 Sample Activity SCED 204: Matter and Energy in Chemical Systems Activity #2: DO THE SMALL PARTICLES OF MATTER MOVE? IF SO, HOW? Purpose: We have been developing a model of matter that involves
SAM Teachers Guide Phase Change Overview Learning Objectives Possible Student Pre/Misconceptions
 SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion. Students observe and manipulate
SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion. Students observe and manipulate
ACTIVITY 2: Motion with a Continuous Force
 CHAPTER 2 Developing Ideas ACTIVITY 2: Motion with a Continuous Force Purpose In Activity 1 you saw the effect that quick pushes had on the motion of a cart. This is like the situation in many sports,
CHAPTER 2 Developing Ideas ACTIVITY 2: Motion with a Continuous Force Purpose In Activity 1 you saw the effect that quick pushes had on the motion of a cart. This is like the situation in many sports,
AP Physics Ch 6 Day 4
 Textbook Reference: Goal/Objectives: Sections 6.3 (Collisions) - Understand how momentum and kinetic energy relate to the 3 types of collisions: elastic, inelastic, and perfectly inelastic - Use projectile
Textbook Reference: Goal/Objectives: Sections 6.3 (Collisions) - Understand how momentum and kinetic energy relate to the 3 types of collisions: elastic, inelastic, and perfectly inelastic - Use projectile
Friction Can Be Rough
 10.1 Observe and Find a Pattern Friction Can Be Rough Observe the following experiment: Rest a brick on a rough surface. Tie a string around the brick and attach a large spring scale to it. Pull the scale
10.1 Observe and Find a Pattern Friction Can Be Rough Observe the following experiment: Rest a brick on a rough surface. Tie a string around the brick and attach a large spring scale to it. Pull the scale
Force. Chapter Definition: Force W ~ F ~ N r ~
 Chapter 3 Force Before we do any real dynamics, we need to define what a force is. I know that you ve already taken at least one physics course and a statics course. So, I know that you have worked with
Chapter 3 Force Before we do any real dynamics, we need to define what a force is. I know that you ve already taken at least one physics course and a statics course. So, I know that you have worked with
Chapter 5, Lesson 2 Surface Tension
 Chapter 5, Lesson 2 Surface Tension Key Concepts The attraction of molecules at the surface of a liquid is called surface tension. The polarity of water molecules can help explain why water has a strong
Chapter 5, Lesson 2 Surface Tension Key Concepts The attraction of molecules at the surface of a liquid is called surface tension. The polarity of water molecules can help explain why water has a strong
WHAT EXACTLY IS SLIME???
 SLIME Pre-Lab - Read the following passages below out loud with your partner. After you are done reading answer the questions that follow in complete sentences. WHAT EXACTLY IS SLIME??? The mixture of
SLIME Pre-Lab - Read the following passages below out loud with your partner. After you are done reading answer the questions that follow in complete sentences. WHAT EXACTLY IS SLIME??? The mixture of
Section 19.1: Forces Within Earth Section 19.2: Seismic Waves and Earth s Interior Section 19.3: Measuring and Locating.
 CH Earthquakes Section 19.1: Forces Within Earth Section 19.2: Seismic Waves and Earth s Interior Section 19.3: Measuring and Locating Earthquakes Section 19.4: Earthquakes and Society Section 19.1 Forces
CH Earthquakes Section 19.1: Forces Within Earth Section 19.2: Seismic Waves and Earth s Interior Section 19.3: Measuring and Locating Earthquakes Section 19.4: Earthquakes and Society Section 19.1 Forces
Caution! Stick-slip motion should not be confused with strike-slip motions along lateral faults.
 Lesson 5: Earthquake Machine As concluded in Lesson 4, earthquakes are associated with displacements on faults. Faults lock and a displacement occurs when the stress across the fault builds up to a sufficient
Lesson 5: Earthquake Machine As concluded in Lesson 4, earthquakes are associated with displacements on faults. Faults lock and a displacement occurs when the stress across the fault builds up to a sufficient
Engage I 1. What do you think about this design? If the car were to suddenly stop, what would happen to the child? Why?
 AP Physics 1 Lesson 4.a Nature of Forces Outcomes Define force. State and explain Newton s first Law of Motion. Describe inertia and describe its relationship to mass. Draw free-body diagrams to represent
AP Physics 1 Lesson 4.a Nature of Forces Outcomes Define force. State and explain Newton s first Law of Motion. Describe inertia and describe its relationship to mass. Draw free-body diagrams to represent
Bell Ringer: What is Newton s 3 rd Law? Which force acts downward? Which force acts upward when two bodies are in contact?
 Bell Ringer: What is Newton s 3 rd Law? Which force acts downward? Which force acts upward when two bodies are in contact? Does the moon attract the Earth with the same force that the Earth attracts the
Bell Ringer: What is Newton s 3 rd Law? Which force acts downward? Which force acts upward when two bodies are in contact? Does the moon attract the Earth with the same force that the Earth attracts the
, to obtain a way to calculate stress from the energy function U(r).
 BIOEN 36 014 LECTURE : MOLECULAR BASIS OF ELASTICITY Estimating Young s Modulus from Bond Energies and Structures First we consider solids, which include mostly nonbiological materials, such as metals,
BIOEN 36 014 LECTURE : MOLECULAR BASIS OF ELASTICITY Estimating Young s Modulus from Bond Energies and Structures First we consider solids, which include mostly nonbiological materials, such as metals,
Donald P. Shiley School of Engineering ME 328 Machine Design, Spring 2019 Assignment 1 Review Questions
 Donald P. Shiley School of Engineering ME 328 Machine Design, Spring 2019 Assignment 1 Review Questions Name: This is assignment is in workbook format, meaning you may fill in the blanks (you do not need
Donald P. Shiley School of Engineering ME 328 Machine Design, Spring 2019 Assignment 1 Review Questions Name: This is assignment is in workbook format, meaning you may fill in the blanks (you do not need
Lecture No. (1) Introduction of Polymers
 Lecture No. (1) Introduction of Polymers Polymer Structure Polymers are found in nature as proteins, cellulose, silk or synthesized like polyethylene, polystyrene and nylon. Some natural polymers can also
Lecture No. (1) Introduction of Polymers Polymer Structure Polymers are found in nature as proteins, cellulose, silk or synthesized like polyethylene, polystyrene and nylon. Some natural polymers can also
Special Theory of Relativity Prof. Shiva Prasad Department of Physics Indian Institute of Technology, Bombay. Lecture - 15 Momentum Energy Four Vector
 Special Theory of Relativity Prof. Shiva Prasad Department of Physics Indian Institute of Technology, Bombay Lecture - 15 Momentum Energy Four Vector We had started discussing the concept of four vectors.
Special Theory of Relativity Prof. Shiva Prasad Department of Physics Indian Institute of Technology, Bombay Lecture - 15 Momentum Energy Four Vector We had started discussing the concept of four vectors.
Hydraulics Prof. Dr. Arup Kumar Sarma Department of Civil Engineering Indian Institute of Technology, Guwahati
 Hydraulics Prof. Dr. Arup Kumar Sarma Department of Civil Engineering Indian Institute of Technology, Guwahati Module No. # 08 Pipe Flow Lecture No. # 05 Water Hammer and Surge Tank Energy cannot be consumed
Hydraulics Prof. Dr. Arup Kumar Sarma Department of Civil Engineering Indian Institute of Technology, Guwahati Module No. # 08 Pipe Flow Lecture No. # 05 Water Hammer and Surge Tank Energy cannot be consumed
Mechanics of Earthquakes and Faulting
 Mechanics of Earthquakes and Faulting www.geosc.psu.edu/courses/geosc508 Surface and body forces Tensors, Mohr circles. Theoretical strength of materials Defects Stress concentrations Griffith failure
Mechanics of Earthquakes and Faulting www.geosc.psu.edu/courses/geosc508 Surface and body forces Tensors, Mohr circles. Theoretical strength of materials Defects Stress concentrations Griffith failure
Thermal properties of Engineering Materials
 Thermal properties of Engineering Materials Engineering materials are important in everyday life because of their versatile structural properties. Other than these properties, they do play an important
Thermal properties of Engineering Materials Engineering materials are important in everyday life because of their versatile structural properties. Other than these properties, they do play an important
Forces. Unit 2. Why are forces important? In this Unit, you will learn: Key words. Previously PHYSICS 219
 Previously Remember From Page 218 Forces are pushes and pulls that can move or squash objects. An object s speed is the distance it travels every second; if its speed increases, it is accelerating. Unit
Previously Remember From Page 218 Forces are pushes and pulls that can move or squash objects. An object s speed is the distance it travels every second; if its speed increases, it is accelerating. Unit
Medical Biomaterials Prof. Mukesh Doble Department of Biotechnology Indian Institute of Technology, Madras. Lecture - 04 Properties (Mechanical)
 Medical Biomaterials Prof. Mukesh Doble Department of Biotechnology Indian Institute of Technology, Madras Lecture - 04 Properties (Mechanical) Welcome to the course on medical biomaterials. Today we are
Medical Biomaterials Prof. Mukesh Doble Department of Biotechnology Indian Institute of Technology, Madras Lecture - 04 Properties (Mechanical) Welcome to the course on medical biomaterials. Today we are
Activity Title: It s Either Very Hot or Very Cold Up There!
 Grades 3-5 Teacher Pages Activity Title: It s Either Very Hot or Very Cold Up There! Activity Objective(s): In this activity, and the follow-up activity next week, teams will design and conduct experiments
Grades 3-5 Teacher Pages Activity Title: It s Either Very Hot or Very Cold Up There! Activity Objective(s): In this activity, and the follow-up activity next week, teams will design and conduct experiments
Performance script for sixth graders By Thomas Kuo and Kimberly Kline LEAPS Fellows, University of California, Santa Barbara
 Performance script for sixth graders By Thomas Kuo and Kimberly Kline LEAPS Fellows, 2007-08 University of California, Santa Barbara [Remember to get answers from a wide variety of students in the audience.
Performance script for sixth graders By Thomas Kuo and Kimberly Kline LEAPS Fellows, 2007-08 University of California, Santa Barbara [Remember to get answers from a wide variety of students in the audience.
Gravitational Potential Energy
 Name: Directions: Read and answer the following questions. You can then go on to my web page and check your answers. At the conclusion, go to schoology.com and complete the PE assignment. Gravitational
Name: Directions: Read and answer the following questions. You can then go on to my web page and check your answers. At the conclusion, go to schoology.com and complete the PE assignment. Gravitational
Instability & Patterning of Thin Polymer Films Prof. R. Mukherjee Department of Chemical Engineering Indian Institute Of Technology, Kharagpur
 Instability & Patterning of Thin Polymer Films Prof. R. Mukherjee Department of Chemical Engineering Indian Institute Of Technology, Kharagpur Lecture No. # 33 Spontaneous Instability & Dewetting of Thin
Instability & Patterning of Thin Polymer Films Prof. R. Mukherjee Department of Chemical Engineering Indian Institute Of Technology, Kharagpur Lecture No. # 33 Spontaneous Instability & Dewetting of Thin
3 Friction: A Force That Opposes Motion
 CHAPTER 1 SECTION Matter in Motion 3 Friction: A Force That Opposes Motion BEFORE YOU READ After you read this section, you should be able to answer these questions: What is friction? How does friction
CHAPTER 1 SECTION Matter in Motion 3 Friction: A Force That Opposes Motion BEFORE YOU READ After you read this section, you should be able to answer these questions: What is friction? How does friction
Introduction. Introductory Remarks
 Introductory Remarks This is probably your first real course in quantum mechanics. To be sure, it is understood that you have encountered an introduction to some of the basic concepts, phenomenology, history,
Introductory Remarks This is probably your first real course in quantum mechanics. To be sure, it is understood that you have encountered an introduction to some of the basic concepts, phenomenology, history,
Design Calculations & Real Behaviour (Hambly s Paradox)
 Design Calculations & Real Behaviour (Hambly s Paradox) Carol Serban 1. Introduction The present work is meant to highlight the idea that engineering design calculations (lately most of the time using
Design Calculations & Real Behaviour (Hambly s Paradox) Carol Serban 1. Introduction The present work is meant to highlight the idea that engineering design calculations (lately most of the time using
Dynamics of Ocean Structures Prof. Dr. Srinivasan Chandrasekaran Department of Ocean Engineering Indian Institute of Technology, Madras
 Dynamics of Ocean Structures Prof. Dr. Srinivasan Chandrasekaran Department of Ocean Engineering Indian Institute of Technology, Madras Module - 01 Lecture - 09 Characteristics of Single Degree - of -
Dynamics of Ocean Structures Prof. Dr. Srinivasan Chandrasekaran Department of Ocean Engineering Indian Institute of Technology, Madras Module - 01 Lecture - 09 Characteristics of Single Degree - of -
GRADE 5: Physical processes 4. UNIT 5P.4 5 hours. Magnetic forces. Resources. About this unit. Previous learning. Expectations
 GRADE 5: Physical processes 4 Magnetic forces UNIT 5P.4 5 hours About this unit This unit is the fourth of five units on physical processes for Grade 5. The unit is designed to guide your planning and
GRADE 5: Physical processes 4 Magnetic forces UNIT 5P.4 5 hours About this unit This unit is the fourth of five units on physical processes for Grade 5. The unit is designed to guide your planning and
Lecture 21. Temperature. Thermal Expansion. Heat and Internal Energy. Cutnell+Johnson: , Temperature
 Lecture 21 Temperature Thermal Expansion Heat and Internal Energy Cutnell+Johnson: 12.1-12.7, 14.3 Temperature So far in this class we ve usually talked about large objects, and we ve treated the object
Lecture 21 Temperature Thermal Expansion Heat and Internal Energy Cutnell+Johnson: 12.1-12.7, 14.3 Temperature So far in this class we ve usually talked about large objects, and we ve treated the object
5.4 The Kinetic Molecular Theory and Changes of State
 5.4 The Kinetic Molecular Theory and Changes of State Chemists know that they will probably never be able to observe exactly what is happening in a chemical reaction. Observation is a powerful tool of
5.4 The Kinetic Molecular Theory and Changes of State Chemists know that they will probably never be able to observe exactly what is happening in a chemical reaction. Observation is a powerful tool of
What Is Air Temperature?
 2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
Atoms and molecules are in motion and have energy
 Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
BREAKING SPAGHETTI. Yulia Peregud. Find the conditions under which dry spaghetti falling on a hard floor does not break.
 BREAKING SPAGHETTI Yulia Peregud Belarusian State University Lyceum, Belarus Formulation of the problem: Find the conditions under which dry spaghetti falling on a hard floor does not break. Introduction:
BREAKING SPAGHETTI Yulia Peregud Belarusian State University Lyceum, Belarus Formulation of the problem: Find the conditions under which dry spaghetti falling on a hard floor does not break. Introduction:
ATOMIC AND MOLECULAR ATTRACTION
 ATOMIC AND MOLECULAR ATTRACTION Name(s) PART 1 DROPS ON A PENNY Assemble the following materials: three pennies, two eye droppers, several paper towels, a small cup of water, a small cup of alcohol, and
ATOMIC AND MOLECULAR ATTRACTION Name(s) PART 1 DROPS ON A PENNY Assemble the following materials: three pennies, two eye droppers, several paper towels, a small cup of water, a small cup of alcohol, and
Physics 3 Summer 1989 Lab 7 - Elasticity
 Physics 3 Summer 1989 Lab 7 - Elasticity Theory All materials deform to some extent when subjected to a stress (a force per unit area). Elastic materials have internal forces which restore the size and
Physics 3 Summer 1989 Lab 7 - Elasticity Theory All materials deform to some extent when subjected to a stress (a force per unit area). Elastic materials have internal forces which restore the size and
PHYSICS 107. Lecture 8 Conservation Laws. For every action there is an equal and opposite reaction.
 PHYSICS 107 Lecture 8 Conservation Laws Newton s Third Law This is usually stated as: For every action there is an equal and opposite reaction. However in this form it's a little vague. I prefer the form:
PHYSICS 107 Lecture 8 Conservation Laws Newton s Third Law This is usually stated as: For every action there is an equal and opposite reaction. However in this form it's a little vague. I prefer the form:
INTRODUCING NEWTON TO SECONDARY SCHOOL STUDENTS
 INTRODUCING NEWTON TO SECONDARY SCHOOL STUDENTS K. P. Mohanan and Tara Mohanan This write-up is a draft that could serve as a starting point for a project. The goal of the project is to design learning
INTRODUCING NEWTON TO SECONDARY SCHOOL STUDENTS K. P. Mohanan and Tara Mohanan This write-up is a draft that could serve as a starting point for a project. The goal of the project is to design learning
Using Models to Enhance How Students Learn Science
 Using Models to Enhance How Students Learn Science Brooke Bourdélat-Parks and Betty Stennett NSTA San Antonio, TX April 12, 2013 BSCS Mission The mission of BSCS is to transform science teaching and learning
Using Models to Enhance How Students Learn Science Brooke Bourdélat-Parks and Betty Stennett NSTA San Antonio, TX April 12, 2013 BSCS Mission The mission of BSCS is to transform science teaching and learning
This Rocks! Author: Sara Kobilka Institute for Chemical Education and Nanoscale Science and Engineering Center University of Wisconsin-Madison
 This Rocks! Author: Sara Kobilka Institute for Chemical Education and Nanoscale Science and Engineering Center University of Wisconsin-Madison Purpose: To learn about the rock cycle and the role that weather
This Rocks! Author: Sara Kobilka Institute for Chemical Education and Nanoscale Science and Engineering Center University of Wisconsin-Madison Purpose: To learn about the rock cycle and the role that weather
Friction Contact friction is the force that develops between two surfaces in contact as they attempt to move parallel to their interface.
 Friction Contact friction is the force that develops between two surfaces in contact as they attempt to move parallel to their interface. Typically, friction is separated into two cases: static and kinetic.
Friction Contact friction is the force that develops between two surfaces in contact as they attempt to move parallel to their interface. Typically, friction is separated into two cases: static and kinetic.
Covalent Compounds 1 of 30 Boardworks Ltd 2016
 Covalent Compounds 1 of 30 Boardworks Ltd 2016 Covalent Compounds 2 of 30 Boardworks Ltd 2016 What are covalent bonds? 3 of 30 Boardworks Ltd 2016 When atoms share pairs of electrons, they form covalent
Covalent Compounds 1 of 30 Boardworks Ltd 2016 Covalent Compounds 2 of 30 Boardworks Ltd 2016 What are covalent bonds? 3 of 30 Boardworks Ltd 2016 When atoms share pairs of electrons, they form covalent
2. be aware of the thermal properties of materials and their practical importance in everyday life;
 MODULE 3: THERMAL AND MECHANICAL PROPERTIES OF MATTER GENERAL OBJECTIVES On completion of this Module, students should: 1. understand the principles involved in the design and use of thermometers; 2. be
MODULE 3: THERMAL AND MECHANICAL PROPERTIES OF MATTER GENERAL OBJECTIVES On completion of this Module, students should: 1. understand the principles involved in the design and use of thermometers; 2. be
Introduction to Algebra: The First Week
 Introduction to Algebra: The First Week Background: According to the thermostat on the wall, the temperature in the classroom right now is 72 degrees Fahrenheit. I want to write to my friend in Europe,
Introduction to Algebra: The First Week Background: According to the thermostat on the wall, the temperature in the classroom right now is 72 degrees Fahrenheit. I want to write to my friend in Europe,
ADHESIVE TAPE. Figure 1. Peeling weight is 0.5 kg, velocity is (2.38±0.02) mm/min.
 ADHESIVE TAPE Stanislav Krasulin BSU Lyceum, Belarus Introduction This task asks us to determine force necessary to remove a piece of adhesive tape from a horizontal surface. Relevant parameters are also
ADHESIVE TAPE Stanislav Krasulin BSU Lyceum, Belarus Introduction This task asks us to determine force necessary to remove a piece of adhesive tape from a horizontal surface. Relevant parameters are also
Grade Six: Earthquakes/Volcanoes Lesson 6.2: Fault Formations
 Lesson Concept Link Time Grade Six: Earthquakes/Volcanoes Lesson 6.2: Fault Formations Forces in the Earth (tension, compression, shearing) cause stress at plate boundaries. Lesson 6.2 builds on the earthquake
Lesson Concept Link Time Grade Six: Earthquakes/Volcanoes Lesson 6.2: Fault Formations Forces in the Earth (tension, compression, shearing) cause stress at plate boundaries. Lesson 6.2 builds on the earthquake
for any object. Note that we use letter, m g, meaning gravitational
 Lecture 4. orces, Newton's Second Law Last time we have started our discussion of Newtonian Mechanics and formulated Newton s laws. Today we shall closely look at the statement of the second law and consider
Lecture 4. orces, Newton's Second Law Last time we have started our discussion of Newtonian Mechanics and formulated Newton s laws. Today we shall closely look at the statement of the second law and consider
An Earthquake in Your Community
 Activity 1 An Earthquake in Your Community Goals In this activity you will: Generate and describe two types of waves. Determine the relative speeds of compressional and shear waves. Simulate some of the
Activity 1 An Earthquake in Your Community Goals In this activity you will: Generate and describe two types of waves. Determine the relative speeds of compressional and shear waves. Simulate some of the
Talk Science Professional Development
 Talk Science Professional Development Transcript for Grade 5 Scientist Case: The Water to Ice Investigations 1. The Water to Ice Investigations Through the Eyes of a Scientist We met Dr. Hugh Gallagher
Talk Science Professional Development Transcript for Grade 5 Scientist Case: The Water to Ice Investigations 1. The Water to Ice Investigations Through the Eyes of a Scientist We met Dr. Hugh Gallagher
Solving Quadratic & Higher Degree Equations
 Chapter 7 Solving Quadratic & Higher Degree Equations Sec 1. Zero Product Property Back in the third grade students were taught when they multiplied a number by zero, the product would be zero. In algebra,
Chapter 7 Solving Quadratic & Higher Degree Equations Sec 1. Zero Product Property Back in the third grade students were taught when they multiplied a number by zero, the product would be zero. In algebra,
Gravity Well Demo - 1 of 9. Gravity Well Demo
 Gravity Well Demo - 1 of 9 Gravity Well Demo Brief Summary This demo/activity in Space Odyssey will give visitors a hands-on feel for how gravity works. Specifically, how Newton interpreted the force of
Gravity Well Demo - 1 of 9 Gravity Well Demo Brief Summary This demo/activity in Space Odyssey will give visitors a hands-on feel for how gravity works. Specifically, how Newton interpreted the force of
LABORATORY V ENERGY. Use the conservation of energy to predict the outcome of interactions between objects.
 LABORATORY V ENERGY In this lab, you will begin to use the principle of conservation of energy to determine the motion resulting from interactions that are difficult to analyze using force concepts alone.
LABORATORY V ENERGY In this lab, you will begin to use the principle of conservation of energy to determine the motion resulting from interactions that are difficult to analyze using force concepts alone.
Lesson 14: Friction. a) Fill in the table that follows by constructing a force diagram for the block (the system) for these five situations.
 Lesson 14: Friction 14.1 Observe and Find a Pattern Perform the following experiment: Rest a wooden block (or some other object, like your shoe) on a table. Attach a large spring scale to a string attached
Lesson 14: Friction 14.1 Observe and Find a Pattern Perform the following experiment: Rest a wooden block (or some other object, like your shoe) on a table. Attach a large spring scale to a string attached
PHY131H1F - Class 9. Today, finishing Chapter 5: Kinetic Friction Static Friction Rolling without slipping (intro) Drag
 PHY131H1F - Class 9 Today, finishing Chapter 5: Kinetic Friction Static Friction Rolling without slipping (intro) Drag Microscopic bumps and holes crash into each other, causing a frictional force. Kinetic
PHY131H1F - Class 9 Today, finishing Chapter 5: Kinetic Friction Static Friction Rolling without slipping (intro) Drag Microscopic bumps and holes crash into each other, causing a frictional force. Kinetic
Natural Sciences 1: Laws and Models in Chemistry
 Natural Sciences 1: Laws and Models in Chemistry Natural Sciences 1: Laws and Models in Chemistry, traces the efforts in Western thought to understand what makes up the physical world what remains the
Natural Sciences 1: Laws and Models in Chemistry Natural Sciences 1: Laws and Models in Chemistry, traces the efforts in Western thought to understand what makes up the physical world what remains the
