On Stress-Strain Responses and Young s Moduli of Single Alkane Molecules, A Molecular Mechanics Study Using the Modified Embedded-Atom Method
|
|
|
- Raymond Lambert
- 5 years ago
- Views:
Transcription
1 On Stress-Strain Responses and Young s Moduli of Single Alkane Molecules, A Molecular Mechanics Study Using the Modified Embedded-Atom Method Sasan Nouranian, *a Steven R. Gwaltney, b Michael I. Baskes, c Mark A. Tschopp, d Mark F. Horstemeyer ae a Center for Advanced Vehicular Systems (CAVS), Mississippi State University, Mississippi State, MS 39762, USA b Department of Chemistry, Mississippi State University, Mississippi State, MS 39762, USA c Department of Aerospace Engineering, Mississippi State University, Mississippi State, MS 39762, USA d U.S. Army Research Laboratory, Aberdeen Proving Ground, MD 21005, USA e Department of Mechanical Engineering, Mississippi State University, Mississippi State, MS 39762, USA ABSTRACT The stress-strain response of a single polymer chain has hitherto remained elusive due to the fact that the cross-sectional area and volume of a single molecule are not well-defined. However, a knowledge of this mechanical response is both essential and a pre-requisite to understanding the nanomechanics of fracture toward developing a multi-scale model for damage prediction in polymers. In this work, molecular mechanics simulations were performed using a modified embedded-atom method (MEAM) potential to generate the stress-strain responses of a series of n- alkane molecules from ethane (C2H6) to undecane (n-c11h24) in tensile deformation up to the point of bond rupture. The results are further generalized to a single polyethylene (PE) chain. Force, true Cauchy stress, true virial stress, and Young s moduli were calculated as a function of true strain for all of the molecules. In calculating the stress of a single molecule, three methods (designated in this work as M1, M2, and M3) are suggested based on three different metrics to quantify both the instantaneous molecular cross-sectional area and volume during deformation. The predictions of these methods are compared to theoretical, first-principles, and experimental data. M1 gives true Cauchy and true virial stress results that are essentially equivalent, suggesting that it is a better method for calculating stress in alkane molecules and, hence, PE single chain. The MEAM predictions of the average elastic modulus for a single PE chain using M1, M2, and M3 are 401 GPa, 172 GPa, and 147 GPa, respectively. Though these results are disparate, M1 gives a modulus value that is strikingly close to the ab initio-calculated value of 405 GPa for the -CH2CH2- repeat unit of PE at 0 K. To further test the MEAM potential, the Young s modulus (C33 elastic constant) of an orthorhombic PE crystal cell was calculated, but its value (184 GPa) underestimates * Present address: Department of Chemical Engineering, The University of Mississippi, University, MS 38677, USA. sasan@olemiss.edu (S. N.) 1
2 the DFT-calculated value of 316 GPa by 42%. Overall, the MEAM potential gives reasonable predictions of the nanomechanics of bond rupture and very good predictions of the Young s modulus of a single PE chain. However, it underestimates the modulus of a PE crystal. 1. Introduction Molecular bond rupture under tensile deformation is of great fundamental interest for damage and failure characterization and modeling of polymers from both mechanochemistry and molecular nanomechanics points of view [1-3]. An understanding and quantification of tensile bond rupture energetics and mechanisms, molecular fragment formation, and stress-strain response of a single molecule provides a basis for developing predictive multi-scale damage models for polymers that ultimately incorporate void formation, growth, and coalescence [4]. While the significance of determining the single-chain stress-strain response is evident from the above discussion, it has remained a point of controversy since the effective cross-sectional area and/or volume of a single molecule for stress calculation is not well-defined. There are numerous theoretical [5, 6], firstprinciples (FP) [7-12], and experimental data [13-15] available for the Young s modulus of a single PE chain, in which various effective chain cross-sectional area are used for the modulus calculation. Furthermore, the reported data are either for an isolated all-trans PE chain or a chain in the crystalline environment. However, the effect of crystalline environment on the modulus of a single PE chain is minimal [15]. The disparity in reported Young s modulus values for a single PE chain compels one to exercise caution when comparing the different methods. One key technique far less used in studying and quantifying the nanomechanics of single polymer chains is molecular mechanics simulation using reactive interatomic potentials. This technique has emerged as an effective tool for studying the mechanochemistry of molecular fracture under tensile deformation [16]. In our previous study [16], we presented the energetics and fragment formation investigation of a series of n-alkane molecules using molecular statics calculations with our recently parameterized reactive modified embedded-atom method (MEAM) potential for saturated hydrocarbons [17]. We further generalized the results to a single PE chain. In this study, we investigate the nanomechanics of the above saturated hydrocarbon series and quantify their stressstrain response. We further determine the Young s modulus of a single PE chain. Since stress definitions have yet not been analyzed at these small length scales in polymers, we employ different definitions in this work and analyze the predictions relative to higher length scales. A similar notion of analyzing stress definitions was successfully accomplished by Horstemeyer et al. [18, 19]. We also calculate the bulk Young s modulus of an orthorhombic PE crystal cell using the MEAM potential and compare the results to the experimental data. One objective of the current work is to introduce proper methods for determining the instantaneous cross-sectional area of a single hydrocarbon molecule during molecular deformation toward investigating molecular nanomechanics of fracture. By correlating the calculated cross-sectional areas and the predicted moduli of a single PE chain to the ab initio calculated and experimentally measured values, we provide a comparison basis for future molecular mechanics investigations of fracture in polymers. 2. Computational method All computations were performed in the open-source large-scale atomic/molecular massively parallel simulator (LAMMPS) software package [20] utilizing its built-in MEAM potential file. The carbon, hydrogen, and CH parameters for saturated hydrocarbons were published previously [17] and can be accessed through the NIST Interatomic Potentials Repository Project website at Alkane molecules from ethane (C2H6) to 2
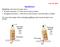
3 undecane (n-c11h24) were created in all-trans configurations and then subjected to a stepwise elongation process that is described in detail in our previous work [16]. We reiterate here that the coordinates of the terminal carbon atom for the molecules with even numbers of carbon atoms in their backbone were zeroed in the first step of the deformation. Force versus true strain and true stress versus true strain behaviors were quantified for the different molecules. Calculating the stress for single molecules is not trivial but very important in the context of multiscale materials modeling. In this work, different methods were used to calculate the true Cauchy and true virial stress. The main difference between the three methods is how to define the length, cross-sectional area, and volume of the single alkane molecules. Calculating the stress-strain response of single alkane molecules is a necessary step for calculating the stiffness (Young s modulus) of longer chain molecules, i.e., PE, and incorporating bond rupture into higher scale formulations for fracture of polymers. In particular, macroscale continuum models use the Cauchy stress, since it the stress metric used in the conservation laws of mass, momentum (linear and angular), and energy; hence, it is important to reconcile potential differences between the Cauchy and virial stress formulations at the molecular level, as well as any differences due to length/area/volume calculations within a molecule. The aforementioned three different methods are described in the next subsections. 2.1.Method 1 for true stress calculation (quadrilateral area) In the first method (M1), the two upper and lower hydrogen atoms situated on the second and the next-to-terminal carbon atoms are taken as the four corners of a quadrilateral seen from the front or back of the molecule (Fig. 1-a). The force at each increment step is divided by the instantaneous area of this quadrilateral to give the true Cauchy stress. To calculate the true virial stress, the xxcomponent of the stress volume tensor at each increment step is divided by the instantaneous volume calculated by multiplying the above-mentioned area by the instantaneous length of the molecule determined from the coordinates of the first and terminal carbon atoms. The true strain calculation in this method utilizes the above-mentioned procedure to determine the instantaneous length of the molecule. Fig. 1 Front and right-side views of an alkane molecule (C11H24), where the cross-sectional area calculated using a) M1, b) M2, and c) M3 are shown schematically. The arrows point to the possible coordinates of the hydrogen atoms comprising the corners of the quadrilaterals in a) and b), and the carbon atoms comprising the diameter of the cricle in c). In c), only carbon atoms are shown. 3
4 2.2.Method 2 for true stress calculation (van der Waals corrected quadrilateral area) In the second method (M2), the same procedure is followed as described for M1, except the corners of the quadrilateral are determined from the coordinates of the hydrogen atoms corrected by the van der Waals (vdw) radius of atomic hydrogen, which is taken to be 1.2 Å [21]. The correction is made along the diagonals of the quadrilateral (Fig. 1-b). Similarly, the volume is determined by multiplying the instantaneous vdw-corrected area by the instantaneous length of the molecule, corrected for the vdw radius of atomic carbon, which is taken to be 1.7 Å [22]. The correction is made on both sides along the line connecting the initial and terminal carbon atoms. As an example, M2 yields a cross-sectional area for the minimum molecular configuration of n-decane that is roughly three times larger and a volume that is roughly four times larger than those calculated using M1. Both the Cauchy and virial stresses can be calculated using M2 as well. The true strain calculation in this method utilizes the same vdw-corrected instantaneous length of the molecule. 2.3.Method 3 for true stress calculation (circular area) In the third method (M3), the circular instantaneous cross-sectional area of the molecule is calculated by defining a vdw-corrected diameter comprised of the y-distance between two outwardly located carbon atoms (Fig. 1-c) on either side of the molecular backbone. The instantaneous molecular volume is calculated, similar to M2, by multiplying the circular area at each increment step by the vdw-corrected length of the molecule. M3 yields a cross-sectional area and volume for the minimum configuration of the n-decane molecule that are both roughly 30% larger than the values calculated using M2 for the Cauchy and virial stresses. The true strain calculation in this method is the same as that for M2. A comparison between the calculated cross-sectional areas of n-decane and n-undecane molecules using M1, M2, and M3 and the experimentally determined value of the area for a PE chain is given in Table 1. We note here that zeroing of the terminal carbon atom coordinates in even carbon-numbered alkanes (for example n-decane in Table 1) before deformation causes a slight titling of the molecule and, hence, the calculated areas using the three methods (M1, M2, and M3) become larger than those calculated for the odd carbon-numbered alkanes (for example n-undecane in Table 1). Table 1 Cross-sectional areas (Å 2 ) of n-decane and n-undecane molecules calculated using M1, M2, and M3 versus the experimental value for a polyethylene (PE) chain. Molecule M1 M2 M3 Expt. for PE [23] C10H C11H Results and discussion The true stress versus true strain for the n-alkane molecules from C3H8 (propane) to C11H24 (nundecane) are given in Figs. 2 and 3 for the Cauchy and virial stress, respectively. The results are generalized to a single PE chain since higher alkanes, such as undecane, can represent the PE chain adequately [24]. Both Cauchy and virial stress data were calculated based on the three area methods described in detail in the previous section. In general, the molecule s constitutive behavior overlaps separately for higher alkanes with odd and even numbers of carbon atoms in the molecular backbone. As mentioned before, this observation can be attributed to the slight difference in the 4
5 cross-sectional area calculated for the molecules with odd and even numbers of carbon atoms in their backbone. An average between the two can be calculated, and the resulting true stress versus true strain can be generalized for a longer single PE chain. M1 (Method 1) yields a much higher ultimate tensile strength for C10H22 than that calculated using M2 and M3 (Fig. 4). Furthermore, the elongation at fracture is shorter when calculated using M2 and M3. This observation has to do with the vdw correction applied when calculating the true strain (see the previous section). Another important observation is that the true Cauchy and true virial stress versus true strain coincide when M1 is used (Fig. 4). However, there is an 18% difference between the true Cauchy and true virial stress calculated using M2 and M3 at longer true strains. Despite some claims that the atomistic virial stress calculation is not equivalent to the continuum Cauchy stress, Subramaniyan and Sun [25] show that the virial stress is indeed equivalent to the Cauchy stress. Therefore, the near-equivalence of the true Cauchy and true virial stress curves with M1 suggests that M1 is a better method for calculating the stress in alkane molecules and, hence, PE single chains. As shown in Fig. 4, the Cauchy and virial stress-strain curves for M2 and M3 are essentially equivalent. This observation can be attributed to the fact that carbon has a larger van der Waals radius than hydrogen (1.7 Å versus 1.2 Å, respectively) and the diameter for the cross-sectional area calculated in M3 (for example, 4.94 Å for n-decane in its minimum configuration) is very close to the size of the quadrilaterial side calculated in M2 (roughly 5.07 Å for n-decane in its minimum configuration). The MEAM potential predicts an estimated ultimate tensile strength of GPa for a single PE chain when an average is calculated (based on either Cauchy or virial stress and M1) for the n- alkanes with odd and even number of carbon atoms in their molecular backbone (Figs. 2a and 3a). It is again assumed that these values extrapolate to longer hydrocarbon chains. Brower et al. [26] predict an ultimate tensile strength of 66 GPa for the CH2CH2- repeat unit of PE at 0 K. If this value is taken as an estimate of the ultimate tensile strength of a single PE chain, the MEAM prediction using M1 is within 26% of the FP prediction. Hageman et al. [27] used ab initio calculations of the bond scission rate in a PE chain and reported an ultimate tensile strength of 18 GPa at an engineering strain of 8% (true strain of 7.7%). They used a room-temperature crosssectional area of Å 2 for a PE chain [23] to calculate the tensile strength. In this case, the MEAM predictions of the ultimate tensile strength for the n-decane molecule calculated using methods M2 and M3 in the true virial stress versus true strain (Figs. 3b-3c) are 19.4 GPa and 17.8 GPa, respectively. In these two methods, the average initial undeformed cross-sectional area of the n-decane molecule is ~15-19 Å 2 (Table 1), which is close to the cross-sectional area used by Hageman et al. (18.24 Å 2 ) [23]. The MEAM-predicted ultimate strength values could be used as estimated values for a single PE chain by the same arguments as those discussed above. However, the elongation at fracture predicted by the MEAM potential (28% for M1) is significantly larger than that reported by Hageman et al. (7.7%). Another important mechanics parameter is the Young s modulus, which was calculated for the alkane molecules from C5H12 to C11H24 based on the initial slope of the curves presented in Figs. 2 and 3. The smaller alkane molecules were left out from the analysis, since overlapping of the true stress versus true strain curves (Figs. 2 and 3) occur in higher alkanes starting from C5H12. The results for the Young s moduli obtained using all three methods (M1, M2, and M3) are shown in Table 2. Again, the alkane molecules with odd and even numbers of carbon atoms in their molecular backbone tend to give similar moduli, respectively. The Young s modulus is found to be fairly constant for the different n-alkane molecules. This observation is attributed to the fact that the modulus is dominated by the C-C-C angle bending during the initial stages of the molecular 5
6 deformation [16]. However, the stress gets distributed in the chain at each strain increment causing more stretched C-C bonds towards the ends of the molecule. The contribution of C-C-C angle bending gets smaller and the C-C bond stretching gets larger as the deformation moves towards the rupture event. Next, an average Young s modulus was calculated for each method, representing an estimated Young s modulus for a single PE chain. These averages together with the standard deviations are given in Table 2. In general, M1 yields an average modulus that is about twice the values calculated using M2 and M3. In the literature, a range of experimental and FP values are reported for the Young s modulus of a single PE chain. From FP calculations, Crist et al. [12] report a modulus of 405 GPa for the CH2CH2- repeat unit of PE at 0 K, which is strikingly close to the MEAMpredicted average value of 401 GPa using M1 (Table 2). Suhai [7] reports values of GPa for the longitudinal elastic modulus of PE calculated using various quantum mechanical methods. Meier [15] employed a semiempirical method to calculate a Young s modulus of 353 GPa for a single PE chain. On the experimental side, Du et al. [14] used nanoindentation and scanning force microscopy to measure the PE modulus along the chain axis utilizing two different methods and reported values of 168 GPa and 204 GPa for thinner specimens and 267 GPa and 278 GPa for thicker specimens. In short, the range of Young s moduli calculated or measured for a single PE chain is anywhere from 168 GPa to GPa. Interestingly, M1 results are comparable to the FPcomputed values, and M2 and M3 results are comparable to the experimental values. The latter observation can be attributed to the fact that the molecular cross-sectional areas calculated in M2 (~15 Å 2 for relaxed n-decane) and M3 (~19 Å 2 ) are close to the experimental value of Å 2 [23]. Table 2 Young s moduli (GPa) for n-alkane molecules calculated using M1, M2, and M3. The averages and standard deviations represent the estimated values for a single polyethylene chain. Molecule M1 M2 M3 C5H C6H C7H C8H C9H C10H C11H Average St. Dev In addition to the calculation of Young s modulus for a single PE chain, Young s modulus of an orthorhombic PE crystal cell was also calculated in this work to further validate the MEAM potential predictions. An orthorhombic PE cell with unit cell lattice constants of a = 7.39 Å, b = 4.93 Å, and c = 2.54 Å was constructed, and the C33 elastic constant was calculated by an axial deformation of the cell. The MEAM-calculated value of the C33 elastic constant (Young s modulus) at 0 K is 184 GPa, which is an underestimation by 42% of the DFT-calculated value of 316 GPa by Lacks and Rutledge [28]. A value of 334 GPa was reported by Hageman et al. [9], while Barrera et al. [10] reported a value of GPa for the Young s modulus of a PE crystal cell at 0 K. Nevertheless, the MEAM potential minimized the cell to the correct lattice parameters. 6
7 a) b) Fig. 2 True Cauchy stress versus true strain for a series of alkane molecules from C3H8 to C11H24 calculated using (a) Method 1 (M1), (b) Method 2 (M2), and (c) Method 3 (M3). These methods are described in the Computational Method section. c) 7
8 a) b) Fig. 3 True virial stress versus true strain for a series of alkane molecules from C3H8 to C11H24 calculated using (a) Method 1 (M1), (b) Method 2 (M2), and (c) Method 3 (M3). These methods are described in the Computational Method section. c) 8
9 Fig. 4 Comparisons between the true Cauchy and true virial stress calculated using three methods as a function of true strain for the n-decane (C10H22) molecule. M1, M2, and M3 denote Method 1, Method 2, and Method 3, respectively. Note that M1 gives the greatest hardening rate. 4. Summary and conclusions With respect to a single PE chain, the calculated ultimate tensile strength using one of the methods for determining the stress response (Method 1) is within 26% of the FP prediction. Using the same method, an average Young s modulus of 401 GPa is calculated for a single PE chain, which is remarkably within 1% of the FP results for the CH2CH2- repeat unit of PE at 0 K. However, the calculated Young s modulus for an orthorhombic PE crystal cell using the MEAM potential (184 GPa) underestimates the DFT-calculated value (316 GPa) by 42%. In this work, different methods of quantifying the cross-sectional area to define the true Cauchy stress were introduced, and it was shown that the correlated virial stress gives very close results in these inelastic deformations. The stress and moduli revealed in these molecular statics simulations give fairly close results to the experimental data and FP results. Acknowledgments This work was sponsored by the U.S. Army Engineer Research and Development Center (ERDC) under contract no. W15QKN Special thanks go to Robert Moser for his support. Distribution Statement Approved for public release; distribution unlimited. References [1] S. N. Zhurkov and V. E. Korsukov, "Atomic mechanism of fracture of solid polymers," Journal of Polymer Science: Polymer Physics Edition, vol. 12, pp , [2] M. K. Beyer and H. Clausen-Schaumann, "Mechanochemistry: The Mechanical Activation of Covalent Bonds," Chemical Reviews, vol. 105, pp , 2005/08/ [3] W. Zhang and X. Zhang, "Single molecule mechanochemistry of macromolecules," Progress in Polymer Science, vol. 28, pp , 8//
10 [4] D. K. Francis, J. L. Bouvard, Y. Hammi, and M. F. Horstemeyer, "Formulation of a damage internal state variable model for amorphous glassy polymers," International Journal of Solids and Structures, vol. 51, pp , 8/1/ [5] L. R. G. Treloar, "Calculations of elastic moduli of polymer crystals: I. Polyethylene and nylon 66," Polymer, vol. 1, pp , // [6] I. M. Ward and J. Sweeney, Mechanical Properties of Solid Polymers: Wiley, [7] S. Suhai, "Quantum mechanical calculation of the longitudinal elastic modulus and of deviations from Hooke s law in polyethylene," The Journal of chemical physics, vol. 84, pp , [8] K. Palmö and S. Krimm, "Chain elastic modulus of polyethylene: A spectroscopically determined force field (SDFF) study," Journal of Polymer Science Part B: Polymer Physics, vol. 34, pp , [9] J. Hageman, R. J. Meier, M. Heinemann, and R. De Groot, "Young modulus of crystalline polyethylene from ab initio molecular dynamics," Macromolecules, vol. 30, pp , [10] G. D. Barrera, S. F. Parker, A. J. Ramirez-Cuesta, and P. C. Mitchell, "The vibrational spectrum and ultimate modulus of polyethylene," Macromolecules, vol. 39, pp , [11] A. Karpfen, "Abinitio studies on polymers. V. All trans polyethylene," The Journal of Chemical Physics, vol. 75, pp , [12] B. Crist, M. Ratner, A. Brower, and J. Sabin, "Abinitio calculations of the deformation of polyethylene," Journal of Applied Physics, vol. 50, pp , [13] T. N. Wassermann, J. Thelemann, P. Zielke, and M. A. Suhm, "The stiffness of a fully stretched polyethylene chain: a Raman jet spectroscopy extrapolation," [14] B. Du, J. Liu, Q. Zhang, and T. He, "Experimental measurement of polyethylene chain modulus by scanning force microscopy," Polymer, vol. 42, pp , [15] R. J. Meier, "Regarding the ultimate Young's modulus of a single polyethylene chain," Macromolecules, vol. 26, pp , [16] S. Nouranian, S. R. Gwaltney, M. I. Baskes, M. A. Tschopp, and M. F. Horstemeyer, "Simulations of Tensile Bond Rupture in Single Alkane Molecules Using Reactive Interatomic Potentials," Chemical Physics Letters, vol. 635, pp , [17] S. Nouranian, M. A. Tschopp, S. R. Gwaltney, M. I. Baskes, and M. F. Horstemeyer, "An Interatomic Potential for Saturated Hydrocarbons Based on the Modified Embedded-Atom Method," Physical Chemistry Chemical Physics, vol. 16, pp ,
11 [18] M. F. Horstemeyer and M. Baskes, "Atomistic finite deformation simulations: a discussion on length scale effects in relation to mechanical stresses," Journal of engineering materials and technology, vol. 121, pp , [19] M. F. Horstemeyer, M. I. Baskes, and S. J. Plimpton, "Computational nanoscale plasticity simulations using embedded atom potentials," Theoretical and Applied Fracture Mechanics, vol. 37, pp , [20] S. Plimpton, "Fast parallel algorithms for short-range molecular dynamics," Journal of Computational Physics, vol. 117, pp. 1-19, [21] G. Raj, Advanced Inorganic Chemistry Vol-1: Krishna Prakashan. [22] M. M. Deza and E. Deza, Encyclopedia of Distances: Springer, [23] C. W. Bunn, Transactions of the Faraday Society, vol. 35, p. 482, [24] A. M. Saitta and M. L. Klein, "Polyethylene under tensile load: Strain energy storage and breaking of linear and knotted alkanes probed by first-principles molecular dynamics calculations," The Journal of chemical physics, vol. 111, p. 9434, [25] A. K. Subramaniyan and C. T. Sun, "Continuum interpretation of virial stress in molecular simulations," International Journal of Solids and Structures, vol. 45, pp , 7// [26] A. Brower, J. Sabin, B. Crist, and M. Ratner, "Ab initio molecular orbital studies of polyethylene deformation," International Journal of Quantum Chemistry, vol. 18, pp , [27] J. Hageman, G. de Wijs, R. de Groot, and R. J. Meier, "Bond scission in a perfect polyethylene chain and the consequences for the ultimate strength," Macromolecules, vol. 33, pp , [28] D. J. Lacks and G. C. Rutledge, "Simulation of the temperature dependence of mechanical properties of polyethylene," The Journal of Physical Chemistry, vol. 98, pp , 1994/01/
MECHANICS OF CARBON NANOTUBE BASED COMPOSITES WITH MOLECULAR DYNAMICS AND MORI TANAKA METHODS. Vinu Unnithan and J. N. Reddy
 MECHANICS OF CARBON NANOTUBE BASED COMPOSITES WITH MOLECULAR DYNAMICS AND MORI TANAKA METHODS Vinu Unnithan and J. N. Reddy US-South American Workshop: Mechanics and Advanced Materials Research and Education
MECHANICS OF CARBON NANOTUBE BASED COMPOSITES WITH MOLECULAR DYNAMICS AND MORI TANAKA METHODS Vinu Unnithan and J. N. Reddy US-South American Workshop: Mechanics and Advanced Materials Research and Education
, to obtain a way to calculate stress from the energy function U(r).
 BIOEN 36 014 LECTURE : MOLECULAR BASIS OF ELASTICITY Estimating Young s Modulus from Bond Energies and Structures First we consider solids, which include mostly nonbiological materials, such as metals,
BIOEN 36 014 LECTURE : MOLECULAR BASIS OF ELASTICITY Estimating Young s Modulus from Bond Energies and Structures First we consider solids, which include mostly nonbiological materials, such as metals,
MATERIALS. Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle?
 MATERIALS Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: A. Composition
MATERIALS Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: A. Composition
Johns Hopkins University What is Engineering? M. Karweit MATERIALS
 Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: MATERIALS A. Composition
Why do things break? Why are some materials stronger than others? Why is steel tough? Why is glass brittle? What is toughness? strength? brittleness? Elemental material atoms: MATERIALS A. Composition
How materials work. Compression Tension Bending Torsion
 Materials How materials work Compression Tension Bending Torsion Elemental material atoms: A. Composition a) Nucleus: protons (+), neutrons (0) b) Electrons (-) B. Neutral charge, i.e., # electrons = #
Materials How materials work Compression Tension Bending Torsion Elemental material atoms: A. Composition a) Nucleus: protons (+), neutrons (0) b) Electrons (-) B. Neutral charge, i.e., # electrons = #
IAP 2006: From nano to macro: Introduction to atomistic modeling techniques and application in a case study of modeling fracture of copper (1.
 IAP 2006: From nano to macro: Introduction to atomistic modeling techniques and application in a case study of modeling fracture of copper (1.978 PDF) http://web.mit.edu/mbuehler/www/teaching/iap2006/intro.htm
IAP 2006: From nano to macro: Introduction to atomistic modeling techniques and application in a case study of modeling fracture of copper (1.978 PDF) http://web.mit.edu/mbuehler/www/teaching/iap2006/intro.htm
Intensity (a.u.) Intensity (a.u.) Raman Shift (cm -1 ) Oxygen plasma. 6 cm. 9 cm. 1mm. Single-layer graphene sheet. 10mm. 14 cm
 Intensity (a.u.) Intensity (a.u.) a Oxygen plasma b 6 cm 1mm 10mm Single-layer graphene sheet 14 cm 9 cm Flipped Si/SiO 2 Patterned chip Plasma-cleaned glass slides c d After 1 sec normal Oxygen plasma
Intensity (a.u.) Intensity (a.u.) a Oxygen plasma b 6 cm 1mm 10mm Single-layer graphene sheet 14 cm 9 cm Flipped Si/SiO 2 Patterned chip Plasma-cleaned glass slides c d After 1 sec normal Oxygen plasma
MECE 3321 MECHANICS OF SOLIDS CHAPTER 3
 MECE 3321 MECHANICS OF SOLIDS CHAPTER 3 Samantha Ramirez TENSION AND COMPRESSION TESTS Tension and compression tests are used primarily to determine the relationship between σ avg and ε avg in any material.
MECE 3321 MECHANICS OF SOLIDS CHAPTER 3 Samantha Ramirez TENSION AND COMPRESSION TESTS Tension and compression tests are used primarily to determine the relationship between σ avg and ε avg in any material.
Calculation of single chain cellulose elasticity using fully atomistic modeling
 PEER-REVIEWED MOLECULAR MODELING Calculation of single chain cellulose elasticity using fully atomistic modeling XIAWA WU, ROBERT J. MOON, AND ASHLIE MARTINI ABSTRACT: Cellulose nanocrystals, a potential
PEER-REVIEWED MOLECULAR MODELING Calculation of single chain cellulose elasticity using fully atomistic modeling XIAWA WU, ROBERT J. MOON, AND ASHLIE MARTINI ABSTRACT: Cellulose nanocrystals, a potential
Tensile stress strain curves for different materials. Shows in figure below
 Tensile stress strain curves for different materials. Shows in figure below Furthermore, the modulus of elasticity of several materials effected by increasing temperature, as is shown in Figure Asst. Lecturer
Tensile stress strain curves for different materials. Shows in figure below Furthermore, the modulus of elasticity of several materials effected by increasing temperature, as is shown in Figure Asst. Lecturer
MOLECULAR SIMULATION FOR PREDICTING MECHANICAL STRENGTH OF 3-D JUNCTIONED CARBON NANOSTRUCTURES
 ECCM16-16 TH EUROPEAN CONFERENCE ON COMPOSITE MATERIALS, Seville, Spain, 22-26 June 214 MOLECULAR SIMULATION FOR PREDICTING MECHANICAL STRENGTH OF 3-D JUNCTIONED CARBON NANOSTRUCTURES S. Sihn a,b*, V.
ECCM16-16 TH EUROPEAN CONFERENCE ON COMPOSITE MATERIALS, Seville, Spain, 22-26 June 214 MOLECULAR SIMULATION FOR PREDICTING MECHANICAL STRENGTH OF 3-D JUNCTIONED CARBON NANOSTRUCTURES S. Sihn a,b*, V.
3.091 Introduction to Solid State Chemistry. Lecture Notes No. 5a ELASTIC BEHAVIOR OF SOLIDS
 3.091 Introduction to Solid State Chemistry Lecture Notes No. 5a ELASTIC BEHAVIOR OF SOLIDS 1. INTRODUCTION Crystals are held together by interatomic or intermolecular bonds. The bonds can be covalent,
3.091 Introduction to Solid State Chemistry Lecture Notes No. 5a ELASTIC BEHAVIOR OF SOLIDS 1. INTRODUCTION Crystals are held together by interatomic or intermolecular bonds. The bonds can be covalent,
Chapter 7. Highlights:
 Chapter 7 Highlights: 1. Understand the basic concepts of engineering stress and strain, yield strength, tensile strength, Young's(elastic) modulus, ductility, toughness, resilience, true stress and true
Chapter 7 Highlights: 1. Understand the basic concepts of engineering stress and strain, yield strength, tensile strength, Young's(elastic) modulus, ductility, toughness, resilience, true stress and true
Supplementary Figures
 Fracture Strength (GPa) Supplementary Figures a b 10 R=0.88 mm 1 0.1 Gordon et al Zhu et al Tang et al im et al 5 7 6 4 This work 5 50 500 Si Nanowire Diameter (nm) Supplementary Figure 1: (a) TEM image
Fracture Strength (GPa) Supplementary Figures a b 10 R=0.88 mm 1 0.1 Gordon et al Zhu et al Tang et al im et al 5 7 6 4 This work 5 50 500 Si Nanowire Diameter (nm) Supplementary Figure 1: (a) TEM image
NORMAL STRESS. The simplest form of stress is normal stress/direct stress, which is the stress perpendicular to the surface on which it acts.
 NORMAL STRESS The simplest form of stress is normal stress/direct stress, which is the stress perpendicular to the surface on which it acts. σ = force/area = P/A where σ = the normal stress P = the centric
NORMAL STRESS The simplest form of stress is normal stress/direct stress, which is the stress perpendicular to the surface on which it acts. σ = force/area = P/A where σ = the normal stress P = the centric
4.MECHANICAL PROPERTIES OF MATERIALS
 4.MECHANICAL PROPERTIES OF MATERIALS The diagram representing the relation between stress and strain in a given material is an important characteristic of the material. To obtain the stress-strain diagram
4.MECHANICAL PROPERTIES OF MATERIALS The diagram representing the relation between stress and strain in a given material is an important characteristic of the material. To obtain the stress-strain diagram
Shear Properties and Wrinkling Behaviors of Finite Sized Graphene
 Shear Properties and Wrinkling Behaviors of Finite Sized Graphene Kyoungmin Min, Namjung Kim and Ravi Bhadauria May 10, 2010 Abstract In this project, we investigate the shear properties of finite sized
Shear Properties and Wrinkling Behaviors of Finite Sized Graphene Kyoungmin Min, Namjung Kim and Ravi Bhadauria May 10, 2010 Abstract In this project, we investigate the shear properties of finite sized
Molecular Dynamics Simulation of Fracture of Graphene
 Molecular Dynamics Simulation of Fracture of Graphene Dewapriya M. A. N. 1, Rajapakse R. K. N. D. 1,*, Srikantha Phani A. 2 1 School of Engineering Science, Simon Fraser University, Burnaby, BC, Canada
Molecular Dynamics Simulation of Fracture of Graphene Dewapriya M. A. N. 1, Rajapakse R. K. N. D. 1,*, Srikantha Phani A. 2 1 School of Engineering Science, Simon Fraser University, Burnaby, BC, Canada
University of Groningen
 University of Groningen Bond Scission in a Perfect Polyethylene Chain and the Consequences for the Ultimate Strength Hageman, J.C.L.; Wijs, G.A. de; Groot, R.A. de; Meier, Robert J. Published in: Macromolecules
University of Groningen Bond Scission in a Perfect Polyethylene Chain and the Consequences for the Ultimate Strength Hageman, J.C.L.; Wijs, G.A. de; Groot, R.A. de; Meier, Robert J. Published in: Macromolecules
Chapter 6: Mechanical Properties of Metals. Dr. Feras Fraige
 Chapter 6: Mechanical Properties of Metals Dr. Feras Fraige Stress and Strain Tension Compression Shear Torsion Elastic deformation Plastic Deformation Yield Strength Tensile Strength Ductility Toughness
Chapter 6: Mechanical Properties of Metals Dr. Feras Fraige Stress and Strain Tension Compression Shear Torsion Elastic deformation Plastic Deformation Yield Strength Tensile Strength Ductility Toughness
Periodic table with the elements associated with commercial polymers in color.
 Polymers 1. What are polymers 2. Polymerization 3. Structure features of polymers 4. Thermoplastic polymers and thermosetting polymers 5. Additives 6. Polymer crystals 7. Mechanical properties of polymers
Polymers 1. What are polymers 2. Polymerization 3. Structure features of polymers 4. Thermoplastic polymers and thermosetting polymers 5. Additives 6. Polymer crystals 7. Mechanical properties of polymers
INTRODUCTION (Cont..)
 INTRODUCTION Name : Mohamad Redhwan Abd Aziz Post : Lecturer @ DEAN CENTER OF HND STUDIES Subject : Solid Mechanics Code : BME 2033 Room : CENTER OF HND STUDIES OFFICE H/P No. : 019-2579663 W/SITE : Http://tatiuc.edu.my/redhwan
INTRODUCTION Name : Mohamad Redhwan Abd Aziz Post : Lecturer @ DEAN CENTER OF HND STUDIES Subject : Solid Mechanics Code : BME 2033 Room : CENTER OF HND STUDIES OFFICE H/P No. : 019-2579663 W/SITE : Http://tatiuc.edu.my/redhwan
Module-4. Mechanical Properties of Metals
 Module-4 Mechanical Properties of Metals Contents ) Elastic deformation and Plastic deformation ) Interpretation of tensile stress-strain curves 3) Yielding under multi-axial stress, Yield criteria, Macroscopic
Module-4 Mechanical Properties of Metals Contents ) Elastic deformation and Plastic deformation ) Interpretation of tensile stress-strain curves 3) Yielding under multi-axial stress, Yield criteria, Macroscopic
NE 125 L. Title Page
 NE 125 L Title Page Name: Rajesh Swaminathan ID Number: 20194189 Partners Names: Clayton Szata 20193839 Sarvesh Varma 20203153 Experiment Number: 1 Experiment: Date Experiment was Started: Date Experiment
NE 125 L Title Page Name: Rajesh Swaminathan ID Number: 20194189 Partners Names: Clayton Szata 20193839 Sarvesh Varma 20203153 Experiment Number: 1 Experiment: Date Experiment was Started: Date Experiment
Mechanical properties 1 Elastic behaviour of materials
 MME131: Lecture 13 Mechanical properties 1 Elastic behaviour of materials A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics Deformation of material under the action of a mechanical
MME131: Lecture 13 Mechanical properties 1 Elastic behaviour of materials A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics Deformation of material under the action of a mechanical
ME 2570 MECHANICS OF MATERIALS
 ME 2570 MECHANICS OF MATERIALS Chapter III. Mechanical Properties of Materials 1 Tension and Compression Test The strength of a material depends on its ability to sustain a load without undue deformation
ME 2570 MECHANICS OF MATERIALS Chapter III. Mechanical Properties of Materials 1 Tension and Compression Test The strength of a material depends on its ability to sustain a load without undue deformation
MATERIALS SCIENCE POLYMERS
 POLYMERS 1) Types of Polymer (a) Plastic Possibly the largest number of different polymeric materials come under the plastic classification. Polyethylene, polypropylene, polyvinyl chloride, polystyrene,
POLYMERS 1) Types of Polymer (a) Plastic Possibly the largest number of different polymeric materials come under the plastic classification. Polyethylene, polypropylene, polyvinyl chloride, polystyrene,
A Molecular Modeling Approach to Predicting Thermo-Mechanical Properties of Thermosetting Polymers
 A Molecular Modeling Approach to Predicting Thermo-Mechanical Properties of Thermosetting Polymers Natalia Shenogina, Wright State University Mesfin Tsige, University of Akron Soumya Patnaik, AFRL Sharmila
A Molecular Modeling Approach to Predicting Thermo-Mechanical Properties of Thermosetting Polymers Natalia Shenogina, Wright State University Mesfin Tsige, University of Akron Soumya Patnaik, AFRL Sharmila
Quiz 1 Introduction to Polymers
 090109 Quiz 1 Introduction to Polymers In class we discussed the definition of a polymer first by comparing polymers with metals and ceramics and then by noting certain properties of polymers that distinguish
090109 Quiz 1 Introduction to Polymers In class we discussed the definition of a polymer first by comparing polymers with metals and ceramics and then by noting certain properties of polymers that distinguish
Mechanical Properties of Materials
 Mechanical Properties of Materials Strains Material Model Stresses Learning objectives Understand the qualitative and quantitative description of mechanical properties of materials. Learn the logic of
Mechanical Properties of Materials Strains Material Model Stresses Learning objectives Understand the qualitative and quantitative description of mechanical properties of materials. Learn the logic of
Towards a Unified Framework for Interatomic Potential Development: Application to the Fe-He System
 Towards a Unified Framework for Interatomic Potential Development: Application to the Fe-He System M.A. Tschopp*,1, K.N. Solanki 1, M.I. Baskes 2, F. Gao 3, X. Sun 3, M.F. Horstemeyer 1 1 Center for Advanced
Towards a Unified Framework for Interatomic Potential Development: Application to the Fe-He System M.A. Tschopp*,1, K.N. Solanki 1, M.I. Baskes 2, F. Gao 3, X. Sun 3, M.F. Horstemeyer 1 1 Center for Advanced
Samantha Ramirez, MSE. Stress. The intensity of the internal force acting on a specific plane (area) passing through a point. F 2
 Samantha Ramirez, MSE Stress The intensity of the internal force acting on a specific plane (area) passing through a point. Δ ΔA Δ z Δ 1 2 ΔA Δ x Δ y ΔA is an infinitesimal size area with a uniform force
Samantha Ramirez, MSE Stress The intensity of the internal force acting on a specific plane (area) passing through a point. Δ ΔA Δ z Δ 1 2 ΔA Δ x Δ y ΔA is an infinitesimal size area with a uniform force
1. Demonstrate that the minimum cation-to-anion radius ratio for a coordination number of 8 is
 1. Demonstrate that the minimum cation-to-anion radius ratio for a coordination number of 8 is 0.732. This problem asks us to show that the minimum cation-to-anion radius ratio for a coordination number
1. Demonstrate that the minimum cation-to-anion radius ratio for a coordination number of 8 is 0.732. This problem asks us to show that the minimum cation-to-anion radius ratio for a coordination number
ME 243. Mechanics of Solids
 ME 243 Mechanics of Solids Lecture 2: Stress and Strain Ahmad Shahedi Shakil Lecturer, Dept. of Mechanical Engg, BUET E-mail: sshakil@me.buet.ac.bd, shakil6791@gmail.com Website: teacher.buet.ac.bd/sshakil
ME 243 Mechanics of Solids Lecture 2: Stress and Strain Ahmad Shahedi Shakil Lecturer, Dept. of Mechanical Engg, BUET E-mail: sshakil@me.buet.ac.bd, shakil6791@gmail.com Website: teacher.buet.ac.bd/sshakil
STANDARD SAMPLE. Reduced section " Diameter. Diameter. 2" Gauge length. Radius
 MATERIAL PROPERTIES TENSILE MEASUREMENT F l l 0 A 0 F STANDARD SAMPLE Reduced section 2 " 1 4 0.505" Diameter 3 4 " Diameter 2" Gauge length 3 8 " Radius TYPICAL APPARATUS Load cell Extensometer Specimen
MATERIAL PROPERTIES TENSILE MEASUREMENT F l l 0 A 0 F STANDARD SAMPLE Reduced section 2 " 1 4 0.505" Diameter 3 4 " Diameter 2" Gauge length 3 8 " Radius TYPICAL APPARATUS Load cell Extensometer Specimen
Supplementary Materials
 Supplementary Materials Atomistic Origin of Brittle Failure of Boron Carbide from Large Scale Reactive Dynamics Simulations; Suggestions toward Improved Ductility Qi An and William A. Goddard III * Materials
Supplementary Materials Atomistic Origin of Brittle Failure of Boron Carbide from Large Scale Reactive Dynamics Simulations; Suggestions toward Improved Ductility Qi An and William A. Goddard III * Materials
Modeling aluminum using molecular dynamics simulation
 Journal of Materials and Environmental Sciences ISSN : 2028-2508 CODEN : JMESCN Copyright 2017, University of Mohammed Premier Oujda Morocco J. Mater. Environ. Sci., 2018, Volume 9, Issue 1, Page 93-99
Journal of Materials and Environmental Sciences ISSN : 2028-2508 CODEN : JMESCN Copyright 2017, University of Mohammed Premier Oujda Morocco J. Mater. Environ. Sci., 2018, Volume 9, Issue 1, Page 93-99
Nanoscale Mechanics: A Quantum-Continuum Approach
 Nanoscale Mechanics: A Quantum-Continuum Approach V.Venkatasubramanian a and S.Bharath b a Department of Mechanical Engineering, IIT Madras b Department of Chemical Engineering, IIT Madras 1. Introduction
Nanoscale Mechanics: A Quantum-Continuum Approach V.Venkatasubramanian a and S.Bharath b a Department of Mechanical Engineering, IIT Madras b Department of Chemical Engineering, IIT Madras 1. Introduction
INTRODUCTION TO STRAIN
 SIMPLE STRAIN INTRODUCTION TO STRAIN In general terms, Strain is a geometric quantity that measures the deformation of a body. There are two types of strain: normal strain: characterizes dimensional changes,
SIMPLE STRAIN INTRODUCTION TO STRAIN In general terms, Strain is a geometric quantity that measures the deformation of a body. There are two types of strain: normal strain: characterizes dimensional changes,
For an imposed stress history consisting of a rapidly applied step-function jump in
 Problem 2 (20 points) MASSACHUSETTS INSTITUTE OF TECHNOLOGY DEPARTMENT OF MECHANICAL ENGINEERING CAMBRIDGE, MASSACHUSETTS 0239 2.002 MECHANICS AND MATERIALS II SOLUTION for QUIZ NO. October 5, 2003 For
Problem 2 (20 points) MASSACHUSETTS INSTITUTE OF TECHNOLOGY DEPARTMENT OF MECHANICAL ENGINEERING CAMBRIDGE, MASSACHUSETTS 0239 2.002 MECHANICS AND MATERIALS II SOLUTION for QUIZ NO. October 5, 2003 For
Chapter 2: Elasticity
 OHP 1 Mechanical Properties of Materials Chapter 2: lasticity Prof. Wenjea J. Tseng ( 曾文甲 ) Department of Materials ngineering National Chung Hsing University wenjea@dragon.nchu.edu.tw Reference: W.F.
OHP 1 Mechanical Properties of Materials Chapter 2: lasticity Prof. Wenjea J. Tseng ( 曾文甲 ) Department of Materials ngineering National Chung Hsing University wenjea@dragon.nchu.edu.tw Reference: W.F.
Lecture contents. Stress and strain Deformation potential. NNSE 618 Lecture #23
 1 Lecture contents Stress and strain Deformation potential Few concepts from linear elasticity theory : Stress and Strain 6 independent components 2 Stress = force/area ( 3x3 symmetric tensor! ) ij ji
1 Lecture contents Stress and strain Deformation potential Few concepts from linear elasticity theory : Stress and Strain 6 independent components 2 Stress = force/area ( 3x3 symmetric tensor! ) ij ji
Theory at a Glance (for IES, GATE, PSU)
 1. Stress and Strain Theory at a Glance (for IES, GATE, PSU) 1.1 Stress () When a material is subjected to an external force, a resisting force is set up within the component. The internal resistance force
1. Stress and Strain Theory at a Glance (for IES, GATE, PSU) 1.1 Stress () When a material is subjected to an external force, a resisting force is set up within the component. The internal resistance force
Size Effects In the Crushing of Honeycomb Structures
 45th AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics & Materials Conference 19-22 April 2004, Palm Springs, California AIAA 2004-1640 Size Effects In the Crushing of Honeycomb Structures Erik C.
45th AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics & Materials Conference 19-22 April 2004, Palm Springs, California AIAA 2004-1640 Size Effects In the Crushing of Honeycomb Structures Erik C.
MATERIALS FOR CIVIL AND CONSTRUCTION ENGINEERS
 MATERIALS FOR CIVIL AND CONSTRUCTION ENGINEERS 3 rd Edition Michael S. Mamlouk Arizona State University John P. Zaniewski West Virginia University Solution Manual FOREWORD This solution manual includes
MATERIALS FOR CIVIL AND CONSTRUCTION ENGINEERS 3 rd Edition Michael S. Mamlouk Arizona State University John P. Zaniewski West Virginia University Solution Manual FOREWORD This solution manual includes
High Tech High Top Hat Technicians. An Introduction to Solid Mechanics. Is that supposed to bend there?
 High Tech High Top Hat Technicians An Introduction to Solid Mechanics Or Is that supposed to bend there? Why don't we fall through the floor? The power of any Spring is in the same proportion with the
High Tech High Top Hat Technicians An Introduction to Solid Mechanics Or Is that supposed to bend there? Why don't we fall through the floor? The power of any Spring is in the same proportion with the
Multiscale modeling of failure in ABS materials
 Institute of Mechanics Multiscale modeling of failure in ABS materials Martin Helbig, Thomas Seelig 15. International Conference on Deformation, Yield and Fracture of Polymers Kerkrade, April 2012 Institute
Institute of Mechanics Multiscale modeling of failure in ABS materials Martin Helbig, Thomas Seelig 15. International Conference on Deformation, Yield and Fracture of Polymers Kerkrade, April 2012 Institute
Introduction to Engineering Materials ENGR2000. Dr. Coates
 Introduction to Engineering Materials ENGR2 Chapter 6: Mechanical Properties of Metals Dr. Coates 6.2 Concepts of Stress and Strain tension compression shear torsion Tension Tests The specimen is deformed
Introduction to Engineering Materials ENGR2 Chapter 6: Mechanical Properties of Metals Dr. Coates 6.2 Concepts of Stress and Strain tension compression shear torsion Tension Tests The specimen is deformed
Elastic Deformation Mechanics of Cellulose Nanocrystals
 Elastic Deformation Mechanics of Cellulose Nanocrystals Xiawa Wu 1, Ryan Wagner 1,2, Arvind Raman 1,2, Robert Moon 2,3,4, Ashlie Martini 1 1 School of Mechanical Engineering, Purdue University 2 Birck
Elastic Deformation Mechanics of Cellulose Nanocrystals Xiawa Wu 1, Ryan Wagner 1,2, Arvind Raman 1,2, Robert Moon 2,3,4, Ashlie Martini 1 1 School of Mechanical Engineering, Purdue University 2 Birck
Fundamentals of Durability. Unrestricted Siemens AG 2013 All rights reserved. Siemens PLM Software
 Fundamentals of Durability Page 1 Your single provider of solutions System simulation solutions 3D simulation solutions Test-based engineering solutions Engineering services - Deployment services Troubleshooting
Fundamentals of Durability Page 1 Your single provider of solutions System simulation solutions 3D simulation solutions Test-based engineering solutions Engineering services - Deployment services Troubleshooting
[5] Stress and Strain
![[5] Stress and Strain [5] Stress and Strain](/thumbs/95/123344550.jpg) [5] Stress and Strain Page 1 of 34 [5] Stress and Strain [5.1] Internal Stress of Solids [5.2] Design of Simple Connections (will not be covered in class) [5.3] Deformation and Strain [5.4] Hooke s Law
[5] Stress and Strain Page 1 of 34 [5] Stress and Strain [5.1] Internal Stress of Solids [5.2] Design of Simple Connections (will not be covered in class) [5.3] Deformation and Strain [5.4] Hooke s Law
4/14/11. Chapter 12 Static equilibrium and Elasticity Lecture 2. Condition for static equilibrium. Stability An object is in equilibrium:
 About Midterm Exam 3 When and where Thurs April 21 th, 5:45-7:00 pm Rooms: Same as Exam I and II, See course webpage. Your TA will give a brief review during the discussion session. Coverage: Chapts 9
About Midterm Exam 3 When and where Thurs April 21 th, 5:45-7:00 pm Rooms: Same as Exam I and II, See course webpage. Your TA will give a brief review during the discussion session. Coverage: Chapts 9
An Atomistic-based Cohesive Zone Model for Quasi-continua
 An Atomistic-based Cohesive Zone Model for Quasi-continua By Xiaowei Zeng and Shaofan Li Department of Civil and Environmental Engineering, University of California, Berkeley, CA94720, USA Extended Abstract
An Atomistic-based Cohesive Zone Model for Quasi-continua By Xiaowei Zeng and Shaofan Li Department of Civil and Environmental Engineering, University of California, Berkeley, CA94720, USA Extended Abstract
The science of elasticity
 The science of elasticity In 1676 Hooke realized that 1.Every kind of solid changes shape when a mechanical force acts on it. 2.It is this change of shape which enables the solid to supply the reaction
The science of elasticity In 1676 Hooke realized that 1.Every kind of solid changes shape when a mechanical force acts on it. 2.It is this change of shape which enables the solid to supply the reaction
MECHANICAL PROPERTIES OF MATERIALS
 1 MECHANICAL PROPERTIES OF MATERIALS Pressure in Solids: Pressure in Liquids: Pressure = force area (P = F A ) 1 Pressure = height density gravity (P = hρg) 2 Deriving Pressure in a Liquid Recall that:
1 MECHANICAL PROPERTIES OF MATERIALS Pressure in Solids: Pressure in Liquids: Pressure = force area (P = F A ) 1 Pressure = height density gravity (P = hρg) 2 Deriving Pressure in a Liquid Recall that:
Outline. Tensile-Test Specimen and Machine. Stress-Strain Curve. Review of Mechanical Properties. Mechanical Behaviour
 Tensile-Test Specimen and Machine Review of Mechanical Properties Outline Tensile test True stress - true strain (flow curve) mechanical properties: - Resilience - Ductility - Toughness - Hardness A standard
Tensile-Test Specimen and Machine Review of Mechanical Properties Outline Tensile test True stress - true strain (flow curve) mechanical properties: - Resilience - Ductility - Toughness - Hardness A standard
Stress Strain Elasticity Modulus Young s Modulus Shear Modulus Bulk Modulus. Case study
 Stress Strain Elasticity Modulus Young s Modulus Shear Modulus Bulk Modulus Case study 2 In field of Physics, it explains how an object deforms under an applied force Real rigid bodies are elastic we can
Stress Strain Elasticity Modulus Young s Modulus Shear Modulus Bulk Modulus Case study 2 In field of Physics, it explains how an object deforms under an applied force Real rigid bodies are elastic we can
XI. NANOMECHANICS OF GRAPHENE
 XI. NANOMECHANICS OF GRAPHENE Carbon is an element of extraordinary properties. The carbon-carbon bond possesses large magnitude cohesive strength through its covalent bonds. Elemental carbon appears in
XI. NANOMECHANICS OF GRAPHENE Carbon is an element of extraordinary properties. The carbon-carbon bond possesses large magnitude cohesive strength through its covalent bonds. Elemental carbon appears in
Application to modeling brittle materials
 1.01, 3.01, 10.333,.00 Introduction to Modeling and Simulation Spring 011 Part I Continuum and particle methods Application to modeling brittle materials Lecture 7 Markus J. Buehler Laboratory for Atomistic
1.01, 3.01, 10.333,.00 Introduction to Modeling and Simulation Spring 011 Part I Continuum and particle methods Application to modeling brittle materials Lecture 7 Markus J. Buehler Laboratory for Atomistic
The influence of water on the elastic modulus of paper
 PEER REVIEWED In Part 1, the theory of hydrogenband-dominated solids is extended to explain phenomena concerning the elastic behavior of paper, based on the postulate that the number density of effective
PEER REVIEWED In Part 1, the theory of hydrogenband-dominated solids is extended to explain phenomena concerning the elastic behavior of paper, based on the postulate that the number density of effective
Mechanical Properties of Tetra-Polyethylene and Tetra-Polyethylene Oxide Diamond Networks via Molecular Dynamics Simulations
 Supplemental Information Mechanical Properties of Tetra-Polyethylene and Tetra-Polyethylene Oxide Diamond Networks via Molecular Dynamics Simulations Endian Wang and Fernando A. Escobedo Table S1 Lennard-Jones
Supplemental Information Mechanical Properties of Tetra-Polyethylene and Tetra-Polyethylene Oxide Diamond Networks via Molecular Dynamics Simulations Endian Wang and Fernando A. Escobedo Table S1 Lennard-Jones
DEVELOPMENT OF A CONTINUUM PLASTICITY MODEL FOR THE COMMERCIAL FINITE ELEMENT CODE ABAQUS
 DEVELOPMENT OF A CONTINUUM PLASTICITY MODEL FOR THE COMMERCIAL FINITE ELEMENT CODE ABAQUS Mohsen Safaei, Wim De Waele Ghent University, Laboratory Soete, Belgium Abstract The present work relates to the
DEVELOPMENT OF A CONTINUUM PLASTICITY MODEL FOR THE COMMERCIAL FINITE ELEMENT CODE ABAQUS Mohsen Safaei, Wim De Waele Ghent University, Laboratory Soete, Belgium Abstract The present work relates to the
SIMPLE MICROMECHANICAL MODEL OF PROTEIN CRYSTALS FOR THEIR MECHANICAL CHARACTERIZATIONS
 EPJ Web of Conferences 6, 6 51 (21) DOI:1.151/epjconf/21651 Owned by the authors, published by EDP Sciences, 21 SIMPLE MICROMECHANICAL MODEL OF PROTEIN CRYSTALS FOR THEIR MECHANICAL CHARACTERIZATIONS Gwonchan
EPJ Web of Conferences 6, 6 51 (21) DOI:1.151/epjconf/21651 Owned by the authors, published by EDP Sciences, 21 SIMPLE MICROMECHANICAL MODEL OF PROTEIN CRYSTALS FOR THEIR MECHANICAL CHARACTERIZATIONS Gwonchan
CHAPTER 3 THE EFFECTS OF FORCES ON MATERIALS
 CHAPTER THE EFFECTS OF FORCES ON MATERIALS EXERCISE 1, Page 50 1. A rectangular bar having a cross-sectional area of 80 mm has a tensile force of 0 kn applied to it. Determine the stress in the bar. Stress
CHAPTER THE EFFECTS OF FORCES ON MATERIALS EXERCISE 1, Page 50 1. A rectangular bar having a cross-sectional area of 80 mm has a tensile force of 0 kn applied to it. Determine the stress in the bar. Stress
Mechanical Properties of Polymers. Scope. MSE 383, Unit 3-1. Joshua U. Otaigbe Iowa State University Materials Science & Engineering Dept.
 Mechanical Properties of Polymers Scope MSE 383, Unit 3-1 Joshua U. Otaigbe Iowa State University Materials Science & Engineering Dept. Structure - mechanical properties relations Time-dependent mechanical
Mechanical Properties of Polymers Scope MSE 383, Unit 3-1 Joshua U. Otaigbe Iowa State University Materials Science & Engineering Dept. Structure - mechanical properties relations Time-dependent mechanical
A Molecular Dynamics Simulation of a Homogeneous Organic-Inorganic Hybrid Silica Membrane
 A Molecular Dynamics Simulation of a Homogeneous Organic-Inorganic Hybrid Silica Membrane Supplementary Information: Simulation Procedure and Physical Property Analysis Simulation Procedure The molecular
A Molecular Dynamics Simulation of a Homogeneous Organic-Inorganic Hybrid Silica Membrane Supplementary Information: Simulation Procedure and Physical Property Analysis Simulation Procedure The molecular
EFFECT OF VACANCY DEFECTS ON THE MECHANICAL PROPERTIES OF CARBON NANOTUBE REINFORCED POLYPROPYLENE
 International Journal of Mechanical Engineering and Technology (IJMET) Volume 8, Issue 7, July 2017, pp. 1370 1375, Article ID: IJMET_08_07_148 Available online at http://www.iaeme.com/ijmet/issues.asp?jtype=ijmet&vtype=8&itype=7
International Journal of Mechanical Engineering and Technology (IJMET) Volume 8, Issue 7, July 2017, pp. 1370 1375, Article ID: IJMET_08_07_148 Available online at http://www.iaeme.com/ijmet/issues.asp?jtype=ijmet&vtype=8&itype=7
SIZE EFFECTS IN THE COMPRESSIVE CRUSHING OF HONEYCOMBS
 43rd AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics, and Materials Con 22-25 April 2002, Denver, Colorado SIZE EFFECTS IN THE COMPRESSIVE CRUSHING OF HONEYCOMBS Erik C. Mellquistand Anthony M.
43rd AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics, and Materials Con 22-25 April 2002, Denver, Colorado SIZE EFFECTS IN THE COMPRESSIVE CRUSHING OF HONEYCOMBS Erik C. Mellquistand Anthony M.
3.021J / 1.021J / J / J / 22.00J Introduction to Modeling and Simulation Markus Buehler, Spring 2008
 MIT OpenCourseWare http://ocw.mit.edu 3.021J / 1.021J / 10.333J / 18.361J / 22.00J Introduction to Modeling and Simulation Markus Buehler, Spring 2008 For information about citing these materials or our
MIT OpenCourseWare http://ocw.mit.edu 3.021J / 1.021J / 10.333J / 18.361J / 22.00J Introduction to Modeling and Simulation Markus Buehler, Spring 2008 For information about citing these materials or our
Molecular Dynamics Simulations of Mechanical Properties of Boron-Nitride Nanotubes Embedded in Si-B-N Ceramics
 Molecular Dynamics Simulations of Mechanical Properties of Boron-Nitride Nanotubes Embedded in Si-B-N Ceramics Michael Griebel and Jan Hamaekers Department of Numerical Simulation, University of Bonn,
Molecular Dynamics Simulations of Mechanical Properties of Boron-Nitride Nanotubes Embedded in Si-B-N Ceramics Michael Griebel and Jan Hamaekers Department of Numerical Simulation, University of Bonn,
Exercise: concepts from chapter 8
 Reading: Fundamentals of Structural Geology, Ch 8 1) The following exercises explore elementary concepts associated with a linear elastic material that is isotropic and homogeneous with respect to elastic
Reading: Fundamentals of Structural Geology, Ch 8 1) The following exercises explore elementary concepts associated with a linear elastic material that is isotropic and homogeneous with respect to elastic
Kendra Erk is an Assistant Professor in the School of Materials Engineering at Purdue University in West Lafayette, Indiana.
 Paper ID #15426 Using Mechanical Testing of Disposable Plastic Cups to Illustrate Processing- Structure-Property Relationships in an Introductory Materials Laboratory Course Dr. Kendra A. Erk, Purdue University
Paper ID #15426 Using Mechanical Testing of Disposable Plastic Cups to Illustrate Processing- Structure-Property Relationships in an Introductory Materials Laboratory Course Dr. Kendra A. Erk, Purdue University
Prediction of Young s Modulus of Graphene Sheets by the Finite Element Method
 American Journal of Mechanical Engineering, 15, Vol. 3, No. 6, 5-9 Available online at http://pubs.sciepub.com/ajme/3/6/14 Science and Education Publishing DOI:1.1691/ajme-3-6-14 Prediction of Young s
American Journal of Mechanical Engineering, 15, Vol. 3, No. 6, 5-9 Available online at http://pubs.sciepub.com/ajme/3/6/14 Science and Education Publishing DOI:1.1691/ajme-3-6-14 Prediction of Young s
3.22 Mechanical Properties of Materials Spring 2008
 MIT OpenCourseWare http://ocw.mit.edu 3.22 Mechanical Properties of Materials Spring 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Problem Set
MIT OpenCourseWare http://ocw.mit.edu 3.22 Mechanical Properties of Materials Spring 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Problem Set
BioMechanics and BioMaterials Lab (BME 541) Experiment #5 Mechanical Prosperities of Biomaterials Tensile Test
 BioMechanics and BioMaterials Lab (BME 541) Experiment #5 Mechanical Prosperities of Biomaterials Tensile Test Objectives 1. To be familiar with the material testing machine(810le4) and provide a practical
BioMechanics and BioMaterials Lab (BME 541) Experiment #5 Mechanical Prosperities of Biomaterials Tensile Test Objectives 1. To be familiar with the material testing machine(810le4) and provide a practical
Biomaterial Scaffolds
 Biomaterial Scaffolds Biomaterial Properties Surface properties Bulk properties Biological properties Types of Biomaterials Biological materials Synthetic materials Surface Properties The body reads the
Biomaterial Scaffolds Biomaterial Properties Surface properties Bulk properties Biological properties Types of Biomaterials Biological materials Synthetic materials Surface Properties The body reads the
ME 207 Material Science I
 ME 207 Material Science I Chapter 3 Properties in Tension and Compression Dr. İbrahim H. Yılmaz http://web.adanabtu.edu.tr/iyilmaz Automotive Engineering Adana Science and Technology University Introduction
ME 207 Material Science I Chapter 3 Properties in Tension and Compression Dr. İbrahim H. Yılmaz http://web.adanabtu.edu.tr/iyilmaz Automotive Engineering Adana Science and Technology University Introduction
Chapter 3 Entropy elasticity (rubbery materials) Review basic thermal physics Chapter 5.1 to 5.5 (Nelson)
 Chapter 3 Entropy elasticity (rubbery materials) Review basic thermal physics Chapter 5.1 to 5.5 (Nelson) Outline: 3.1 Strain, stress and Young modulus 3. Energy density 3.3 Typical stress-strain curve
Chapter 3 Entropy elasticity (rubbery materials) Review basic thermal physics Chapter 5.1 to 5.5 (Nelson) Outline: 3.1 Strain, stress and Young modulus 3. Energy density 3.3 Typical stress-strain curve
Geology 229 Engineering Geology. Lecture 5. Engineering Properties of Rocks (West, Ch. 6)
 Geology 229 Engineering Geology Lecture 5 Engineering Properties of Rocks (West, Ch. 6) Common mechanic properties: Density; Elastic properties: - elastic modulii Outline of this Lecture 1. Uniaxial rock
Geology 229 Engineering Geology Lecture 5 Engineering Properties of Rocks (West, Ch. 6) Common mechanic properties: Density; Elastic properties: - elastic modulii Outline of this Lecture 1. Uniaxial rock
CHAPTER 6 MECHANICAL PROPERTIES OF METALS PROBLEM SOLUTIONS
 CHAPTER 6 MECHANICAL PROPERTIES OF METALS PROBLEM SOLUTIONS Concepts of Stress and Strain 6.1 Using mechanics of materials principles (i.e., equations of mechanical equilibrium applied to a free-body diagram),
CHAPTER 6 MECHANICAL PROPERTIES OF METALS PROBLEM SOLUTIONS Concepts of Stress and Strain 6.1 Using mechanics of materials principles (i.e., equations of mechanical equilibrium applied to a free-body diagram),
9 MECHANICAL PROPERTIES OF SOLIDS
 9 MECHANICAL PROPERTIES OF SOLIDS Deforming force Deforming force is the force which changes the shape or size of a body. Restoring force Restoring force is the internal force developed inside the body
9 MECHANICAL PROPERTIES OF SOLIDS Deforming force Deforming force is the force which changes the shape or size of a body. Restoring force Restoring force is the internal force developed inside the body
Weight and contact forces: Young's modulus, Hooke's law and material properties
 Weight and contact forces: Young's modulus, Hooke's law and material properties Many objects deform according to Hooke's law; many materials behave elastically and have a Young's modulus. In this section,
Weight and contact forces: Young's modulus, Hooke's law and material properties Many objects deform according to Hooke's law; many materials behave elastically and have a Young's modulus. In this section,
REVIEW : INTRODUCTION TO THE MOLECULAR ORIGINS OF MECHANICAL PROPERTIES QUANTITATIVE TREATMENT OF INTERATOMIC BONDING : THE LENNARD-JONES POTENTIAL
 LECTURE #19 : 3.11 MECANICS OF MATERIALS F3 INSTRUCTOR : Professor Christine Ortiz OFFICE : 13-422 PONE : 452-384 WWW : http://web.mit.edu/cortiz/www REVIEW : INTRODUCTION TO TE MOLECULAR ORIGINS OF MECANICAL
LECTURE #19 : 3.11 MECANICS OF MATERIALS F3 INSTRUCTOR : Professor Christine Ortiz OFFICE : 13-422 PONE : 452-384 WWW : http://web.mit.edu/cortiz/www REVIEW : INTRODUCTION TO TE MOLECULAR ORIGINS OF MECANICAL
Fatigue Analysis of Wind Turbine Composites using Multi-Continuum Theory and the Kinetic Theory of Fracture
 Fatigue Analysis of Wind Turbine Composites using Multi-Continuum Theory and the Kinetic Theory of Fracture P. Greaves a, P. McKeever a, R. G. Dominy b, T. Koziara b a Narec, Offshore House, Albert Street,
Fatigue Analysis of Wind Turbine Composites using Multi-Continuum Theory and the Kinetic Theory of Fracture P. Greaves a, P. McKeever a, R. G. Dominy b, T. Koziara b a Narec, Offshore House, Albert Street,
12/8/2009. Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka
 Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Introduction and classes of properties Case studies showing selection of the right material for the job Deformation of material under the action of a
Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Introduction and classes of properties Case studies showing selection of the right material for the job Deformation of material under the action of a
MECHANICAL AND RHEOLOGICAL PROPERTIES
 MECHANICAL AND RHEOLOGICAL PROPERTIES MECHANICAL PROPERTIES OF SOLIDS Extension Shear δ τ xy l 0 l l 0 θ σ Hooke's law σ = Eε Hooke's law τ = G γ xy xy MECHANICAL AND RHEOLOGICAL PROPERTIES RHEOLOGICAL
MECHANICAL AND RHEOLOGICAL PROPERTIES MECHANICAL PROPERTIES OF SOLIDS Extension Shear δ τ xy l 0 l l 0 θ σ Hooke's law σ = Eε Hooke's law τ = G γ xy xy MECHANICAL AND RHEOLOGICAL PROPERTIES RHEOLOGICAL
Report on Atomistic Modeling of Bonding in Carbon-Based Nanostructures
 Report on Atomistic Modeling of Bonding in Carbon-Based Nanostructures Timothy Stillings Department of Physics, Astronomy and Materials Science Missouri State University Advisor: Ridwan Sakidja Abstract
Report on Atomistic Modeling of Bonding in Carbon-Based Nanostructures Timothy Stillings Department of Physics, Astronomy and Materials Science Missouri State University Advisor: Ridwan Sakidja Abstract
Geology 2112 Principles and Applications of Geophysical Methods WEEK 1. Lecture Notes Week 1
 Lecture Notes Week 1 A Review of the basic properties and mechanics of materials Suggested Reading: Relevant sections from any basic physics or engineering text. Objectives: Review some basic properties
Lecture Notes Week 1 A Review of the basic properties and mechanics of materials Suggested Reading: Relevant sections from any basic physics or engineering text. Objectives: Review some basic properties
Effect of different crosslink densities on the thermomechanical properties of polymer nanocomposites
 Effect of different crosslink densities on the thermomechanical properties of polymer nanocomposites *Byungjo Kim 1), Joonmyung Choi 2), Suyoung Yu 3), Seunghwa Yang 4) and Maenghyo Cho 5) 1), 2), 3),
Effect of different crosslink densities on the thermomechanical properties of polymer nanocomposites *Byungjo Kim 1), Joonmyung Choi 2), Suyoung Yu 3), Seunghwa Yang 4) and Maenghyo Cho 5) 1), 2), 3),
Stress-Strain Behavior
 Stress-Strain Behavior 6.3 A specimen of aluminum having a rectangular cross section 10 mm 1.7 mm (0.4 in. 0.5 in.) is pulled in tension with 35,500 N (8000 lb f ) force, producing only elastic deformation.
Stress-Strain Behavior 6.3 A specimen of aluminum having a rectangular cross section 10 mm 1.7 mm (0.4 in. 0.5 in.) is pulled in tension with 35,500 N (8000 lb f ) force, producing only elastic deformation.
Equilibrium. the linear momentum,, of the center of mass is constant
 Equilibrium is the state of an object where: Equilibrium the linear momentum,, of the center of mass is constant Feb. 19, 2018 the angular momentum,, about the its center of mass, or any other point, is
Equilibrium is the state of an object where: Equilibrium the linear momentum,, of the center of mass is constant Feb. 19, 2018 the angular momentum,, about the its center of mass, or any other point, is
13 Solid materials Exam practice questions
 Pages 206-209 Exam practice questions 1 a) The toughest material has the largest area beneath the curve the answer is C. b) The strongest material has the greatest breaking stress the answer is B. c) A
Pages 206-209 Exam practice questions 1 a) The toughest material has the largest area beneath the curve the answer is C. b) The strongest material has the greatest breaking stress the answer is B. c) A
Nanomechanics Measurements and Standards at NIST
 Nanomechanics Measurements and Standards at NIST Robert F. Cook Deputy Chief, Ceramics Division Leader, Nanomechanical Properties Group robert.cook@nist.gov NIST Mission Promote U.S. innovation and industrial
Nanomechanics Measurements and Standards at NIST Robert F. Cook Deputy Chief, Ceramics Division Leader, Nanomechanical Properties Group robert.cook@nist.gov NIST Mission Promote U.S. innovation and industrial
22 Which of the following correctly defines the terms stress, strain and Young modulus? stress strain Young modulus
 PhysicsndMathsTutor.com Which of the following correctly defines the terms stress, strain and Young modulus? 97/1/M/J/ stress strain Young modulus () x (area) (extension) x (original length) (stress) /
PhysicsndMathsTutor.com Which of the following correctly defines the terms stress, strain and Young modulus? 97/1/M/J/ stress strain Young modulus () x (area) (extension) x (original length) (stress) /
Mechanics of Materials Primer
 Mechanics of Materials rimer Notation: A = area (net = with holes, bearing = in contact, etc...) b = total width of material at a horizontal section d = diameter of a hole D = symbol for diameter E = modulus
Mechanics of Materials rimer Notation: A = area (net = with holes, bearing = in contact, etc...) b = total width of material at a horizontal section d = diameter of a hole D = symbol for diameter E = modulus
Nonlinear Finite Element Modeling of Nano- Indentation Group Members: Shuaifang Zhang, Kangning Su. ME 563: Nonlinear Finite Element Analysis.
 ME 563: Nonlinear Finite Element Analysis Spring 2016 Nonlinear Finite Element Modeling of Nano- Indentation Group Members: Shuaifang Zhang, Kangning Su Department of Mechanical and Nuclear Engineering,
ME 563: Nonlinear Finite Element Analysis Spring 2016 Nonlinear Finite Element Modeling of Nano- Indentation Group Members: Shuaifang Zhang, Kangning Su Department of Mechanical and Nuclear Engineering,
Materials and Structures. Indian Institute of Technology Kanpur
 Introduction to Composite Materials and Structures Nachiketa Tiwari Indian Institute of Technology Kanpur Lecture 16 Behavior of Unidirectional Composites Lecture Overview Mt Material ilaxes in unidirectional
Introduction to Composite Materials and Structures Nachiketa Tiwari Indian Institute of Technology Kanpur Lecture 16 Behavior of Unidirectional Composites Lecture Overview Mt Material ilaxes in unidirectional
Example questions for Molecular modelling (Level 4) Dr. Adrian Mulholland
 Example questions for Molecular modelling (Level 4) Dr. Adrian Mulholland 1) Question. Two methods which are widely used for the optimization of molecular geometies are the Steepest descents and Newton-Raphson
Example questions for Molecular modelling (Level 4) Dr. Adrian Mulholland 1) Question. Two methods which are widely used for the optimization of molecular geometies are the Steepest descents and Newton-Raphson
Section 2.5 Atomic Bonding
 Section 2.5 Atomic Bonding Metallic bond, Covalent bond, Ionic bond, van der Waals bond are the different types of bonds. Van der Waals interactions: London forces, Debye interaction, Keesom interaction
Section 2.5 Atomic Bonding Metallic bond, Covalent bond, Ionic bond, van der Waals bond are the different types of bonds. Van der Waals interactions: London forces, Debye interaction, Keesom interaction
VISCOELASTIC PROPERTIES OF POLYMERS
 VISCOELASTIC PROPERTIES OF POLYMERS John D. Ferry Professor of Chemistry University of Wisconsin THIRD EDITION JOHN WILEY & SONS New York Chichester Brisbane Toronto Singapore Contents 1. The Nature of
VISCOELASTIC PROPERTIES OF POLYMERS John D. Ferry Professor of Chemistry University of Wisconsin THIRD EDITION JOHN WILEY & SONS New York Chichester Brisbane Toronto Singapore Contents 1. The Nature of
