Exercise 15 - Preparation of a Key for Reaction-Map of Organic Chemistry (see Appendix C in student manual)
|
|
|
- Mervyn Clinton Arnold
- 5 years ago
- Views:
Transcription
1 125 Exercise 15 - Preparation of a Key for Reaction-Map of Organic Chemistry (see Appendix C in student manual) Abstract: The Reaction -Map of Organic Chemistry has been designed to give organic chemistry students an overview of most of the reactions needed for the organic chemistry course. The chart has been partially organized according to the periodic table on the horizontal axis and according to carbon oxidation level on the vertical axis. In addition the carboxyls are grouped vertically according to decreasing reactivity and carbon - carbon bond forming reactions are emphasized with bold arrows. The chart provides a study aide for students and should help students develop synthetic routes from one functional group to another. The chart should be especially useful for students studying for the final examination for the two semester organic chemistry course. In addition to the chart, three keys are available that organize the reactions according to mechanism, functional group preparations and functional group reactions. Chemistry can be thought of a search for order in matter and this chart attempts to provide some insight into the order that exists in organic chemistry. Please note, Supporting information reprinted with permission from J. Chem. Ed. 2007, 84(7), Copyright 2007 American Chemical Society. Reaction-Map as a Study Aid At the end of a two semester course in organic chemistry, a student should be able to perform the exercises below. (Note: In addition to the exercises below, a student of organic chemistry should be able to demonstrate competency with spectroscopic, stereochemical and multistep synthetic challenges.) By performing the exercises below should result in the preparation of three keys for the Reaction-Map of Organic Chemistry. 1. For each numbered reaction, classify the reaction by mechanism (e.g., substitution, nucleophilic) and list the reagents, conditions, regioselectivity, stereoselectivity and restrictions associated with the reaction. 2. List all methods of preparing each functional group. 3. List all reactions of each functional group. 4. Write mechanisms for reactions: 3 (D2), 10 (B13), 19 (C13), 32 (C11), 33 (E14), 39 (J3) +28 (K2), 42 (G7), 46 (B13), 47 (C11), 62 (K13), 72 (J17), 106 (K19). The problems above should be attempted without reference to the keys but the keys can be used to help check for the correctness of answers for 1-3. The keys are available in the Instructor s Manual and to subscribers of the Journal of Chemical Education at: For the answers to question #4, reference to an organic chemistry textbook may be required.
2 126 KEYS TO REACTION-MAP OF ORGANIC CHEMISTRY General Organization Left to right, compounds in the colored or shaded regions are arranged according to the periodic table. Organolithium and Grignard reagents are under lithium and magnesium but these reagents are used elsewhere on several bold arrows for the synthesis of C-C bonds. Carbon compounds that do not contain other elements are under carbon, carbon - nitrogen (and C, N, O) compounds are under nitrogen, carbon - oxygen compounds are under oxygen and carbon - halogen (and C, O, X) compounds are under fluorine and the halogens. From top to bottom, within groups, the compounds are arranged according to the oxidation level of the compound. The oxidation level of organic compounds is somewhat of a complex concept. Even for propane, the carbons technically have different oxidation states. For the purposes of grouping compounds by oxidation level for this chart, the general guideline used has been that oxidation involves a decrease in the number of bonds to carbon from an atom less electronegative than carbon (most frequently hydrogen) and/or an increase in the number of bonds from carbon to atoms more electronegative than carbon (most frequently N, O, X). Reduction is the reverse. If two carbons change, then the sum of the changes must be considered. When water is added to a double bond, one carbon gains a hydrogen and the other an oxygen and the net oxidation level of the molecule does not change. The increase in oxidation level is indicated by the degree of darkness in the chart in Appendix C and in color in the online version.
3 This organization results in five groups including: 1. alkanes (and organometallics), 2. alkenes (and alkene addition products such as alcohols, ethers and halides), aromatics and amines, 3. alkynes (and alkyne addition products such as carbonyls), 4. carboxyls and 5. carbon dioxide and tetrahalomethane. For the purposes of organizing the numbering of the reactions for this key, the reactions have been grouped according to mechanism of the first step of the reaction. Many reactions fall into more than one group. The addition of hydrogen to π bonds is usually discussed in texts along with electrophilic additions to π bonds but here the hydrogen additions have been placed in the reduction category. Reductions with hydrides such as LiAlH 4 are often grouped with nucleophilic additions but here have also been included in the reduction category. The reactions are listed in the order substitution, addition, elimination, addition-elimination, oxidation, reduction, concerted and miscellaneous. To facilitate the finding of reactions from any of the keys that follow, a roadmap grid has been included. For example, the addition of HX to an alkene is represented by reaction 30 which is in grid position B11. Three keys are have been designed to accompany the map. The keys are arranged according to mechanism, functional group preparations and functional group reactions. Reactions in the three keys contain the reaction numbers and grid locators. Outside of the main region, bold arrows indicate reactions that form C-C bonds. In the online version, products that result from carbon - carbon bond formation are in the grey areas. In Appendix C, these products are in the area with a graph grid. Dotted arrows represent reactions that result in the breaking of C-C bonds. Also included outside the main region are the reactions of aromatics and miscellaneous reactions. Reaction-Map as a Study Aid At the end of a two semester course in organic chemistry, a student should be able to perform the exercises below. In addition to the exercises below, a student of organic chemistry should be able to demonstrate competency with spectroscopic, stereochemical and multistep synthetic challenges. 1. For each numbered reaction, classify the reaction by mechanism (e.g., substitution, nucleophilic) and list the reagents, conditions, regioselectivity, stereoselectivity and restrictions associated with the reaction. 2. List all methods of preparing each functional group. 3. List all reactions of each functional group. 4. Write mechanisms for reactions: 3 (D2), 10 (B13), 19 (C13), 32 (C11), 33 (E14), 39 (J3) +28 (K2), 42 (G7), 46 (B13), 47 (C11), 62 (K13), 72 (J17), 106 (K19). The problems above should be attempted without reference to the keys but the keys can be used to help check for the correctness of answers. For the answers to question #4, reference to an organic chemistry textbook may be required. 127
4 128 Reaction Mechanism (Listed in the order substitution, addition, elimination, additionelimination, oxidation, reduction, concerted, miscellaneous) SUBSTITUTION Electrophilic (for electrophilic aromatic substitution, ortho-para directors with rate increase compared to benzene in decreasing order of reactivity are (2, p. 632): O O -NH 2 -OH -OR -NHCR -OCR -R -Ar -vinyl The halides are deactivating ortho-para directors. Meta directors arranged approximately in order of decreasing reactivity are: O O O O O -CH -CR -COR -COH -CX -CN -SO 3 H -NH 3 + -NO 2 1 (D1) H 2 SO 4 (can be reversed, see # 2 (E1)), aromatic aromatic derivatives. 2 (E1) H 3 O + /D, aromatic aromatic derivatives. 3 (D2) HNO 3 /H 2 SO 4 produces NO + 2, aromatic aromatic derivatives. 4 (D3) X 2 /FeX 3 (or AlX 3 ), aromatic aromatic derivatives. 5 (D4) RX/AlCl 3, aromatic aromatic derivatives, Friedel-Crafts alkylation, can rearrange and undergo multiple substitution, does not work when ring is deactivated or contains an amino group. 6 (D5) O O O RC-X/AlCl 3 or RCOCR/AlCl 3, aromatic aromatic derivatives, Friedel-Crafts acylation, no rearrangement or multiple substitution, does not work when ring is deactivated or contains an amino group, carbonyl can be reduced to CH 2 (see #87 (C9)). 7 (L2) PhY where Y is a strong activator, aromatic aromatic derivatives (e.g., OH or NH 2 ) for electrophilic aromatic substitution. 8 (A5) H 2 O or any good proton donor, organometallic alkane. Free Radical 9 (E3) NBS (N-bromosuccinimide), aromatics aromatic derivatives, good for benzylic and allylic bromination. 10 (B13) X 2 /hν or Δ, alkane alkyl halide, free radical chain reaction, multiple substitution, because of limited selectivity, useful primarily when there is only one type of hydrogen or for more reactive hydrogens (e.g., benzylic and allylic). 11 (I1) CuCl or CuBr, aromatics aromatic halides, Sandmeyer reaction. 12 (L4) CuCN, aromatics aromatic nitriles, Sandmeyer reaction. Nucleophilic 13 (J1) H 3 O + /Δ, aromatics aromatic phenols. 14 (J1) KI, aromatics aromatic iodides. 15 (K1) HBF 4 /Δ, aromatics aromatic fluorides. 16 (L1) H 3 PO 2, aromatics aromatic derivatives. 17 (B18) CN -, alkyl halide nitrile. 18a (C14) NH 3, alkyl halide amine. The direct synthesis of amines from halides is subject to many problems including multiple substitution. Generally, alternatives (e.g., 18b, 18c) should be used.
5 b (C14) 1. Phthalimide/OH - 2. RX 3. NH 2 NH 2, alkyl halide amine, Gabriel synthesis of primary amines. c (C14) 1. NaN 3 2. Na/ROH or LiAlH 4, alkyl halide amine. 19a (C13) OH -, alkyl halide alcohol, S N 2 for 1 o although steric hindrance in the nucleophile or alkyl halide promotes elimination, 3 o gives elimination. b (C13) H 2 O, alkyl halide alcohol, S N 1 for 3 o, can rearrange, competes with E1. 20a (C15) HX, alcohol alkyl halide, 1 o usually go S N 2 (except that HCl does not work as Cl - is a weak nucleophile) and 3 o usually go S N 1 with rearrangement and competing elimination possible. b (C15) PX 3 or SOCl 2, alcohol alkyl halide. c (C15) 1. TsCl/pyridine 2. X -, alcohol alkyl halide. 21a (D13) 1. Na 2. R X, alcohol ether, Williamson ether synthesis, elimination possible and exclusive for 3 o halide, prevalent with a 2 o halide. b (D13) 1. TsCl/pyridine 2. OR -, alcohol ether. c (D13) H 2 SO 4, alcohol ether, useful for symmetrical ethers and 3 o ROH with 1 o ROH, competes with elimination. 22 (C16) OR -, alkyl halide ether, Williamson ether synthesis, elimination possible and prevalent for 3 o halide. 23 (C17) 1. TsCl/pyridine 2. CN -, alcohol nitrile. 24a (F15) H 3 O +, oxirane diol, overall from the alkene yields anti-addition, with an alcohol instead of water, alcohol adds to most substituted carbon (Markonikov orientation) with anti-addition. b (F15) OH -, oxirane diol, overall from the alkene yields anti-addition, with an alcohol instead of water, alcohol adds to least substituted carbon (anti-markonikov orientation) with anti-addition. 25 (F17, A2) 1. RMgX/Et 2 O 2. H + /H 2 O, oxirane alcohol, Grignard reaction, works best on unsubstituted carbon and can give mixtures. Organocuprates (Gilman reagents) are better. Nucleophilic or Electrophilic (depending on perspective) 26 (E6) - 1. NH 2 2. RX, alkyne and alkyl halide alkyne, 3 o halides eliminate rather than substitute and elimination is prevalent for 2 o halides. 27a (G7) 1. LDA (lithium diisopropylamide) 2. R X, aldehyde or ketone alkylated aldehyde or ketone, works best with 1 o halides, multiple substitution possible, with 2 types of α hydrogens, orientation depends on conditions for kinetic vs thermodynamic control but normally gives the kinetic enolate. b (G7) 1. pyrrolidine (or other 2 o amine)/h + 2. R X 3. H 3 O +, aldehyde or ketone alkylated aldehyde or ketone, alkylation usually takes place on least substituted side of the original carbonyl position, Stork enamine reaction. 28 (K2) H + /ROH, hemiacetal or hemiketal acetal or ketal, reversible, see # 39, 40 (J3), hemiacetals and hemiketals are generally unstable and, except for sugars, not isolated. 29 (K3) H + /H 2 O, acetal or ketal hemiacetal or hemiketal, reversible, see # 28, 39, 40 (J3). 129
6 130 ADDITION Electrophilic (except 30b) 30a (B11) HX, alkene alkyl halide, electrophilic Markovnikov addition. b (B11) HBr/peroxides, alkene alkyl halide, free radical mechanism yields anti- Markovnikov orientation. 31a (C12) H + (H 2 SO 4 or H 3 PO 4 )/ROH, alkene ether, electrophilic Markovnikov addition with possible rearrangement and competing reactions. b (C12) 1. Hg(O 2 CCF 3 ) 2, ROH 2. NaBH 4, alkene ether, alkoxymercuration reaction, Markovnikov addition without rearrangement. 32a (C11) H + (H 2 SO 4 or H 3 PO 4 )/H 2 O, alkene alcohol, electrophilic Markovnikov addition with possible rearrangement and competing reactions. b (C11) 1. Hg(OAc) 2, THF, H 2 O 2. NaBH 4, alkene alcohol, Markovnikov without rearrangement. c (C11) 1. BH 3 2. OH -, H 2 O 2, H 2 O, alkene alcohol, anti-markovnikov 1 orientation without rearrangement. 33 (E14) X 2, alkene dihalide, anti-addition, only practical for X = Cl or Br. 34 (F10) 1. H 2 /Lindlar s catalyst 2. X 2, alkyne dihalide (vicinal). 35 (F10) HX, 2 moles, alkyne dihalide (geminal). 36a (E6) H 2 O/H 2 SO 4 for disubstituted alkynes, H 2 O/H 2 SO 4 /HgSO 4 for terminal alkynes, alkynes ketones (except ethylene acetaldehyde), Markovnikov orientation. b (E6) 1. BH 3 2. H 2 O 2 /OH - for disubstituted alkynes, substitute disiamylborane for borane for terminal alkynes, alkynes aldehydes and ketones, anti-markovnikov orientation. 37,38 (I4) H + /H 2 O or OH - /H 2 O reversible formation of hydrate, aldehyde and ketones hydrates. Equilibrium strongly favors carbonyl for ketones but is very structure dependent for aldehydes (formaldehyde % hydrate and acetaldehyde - 58% hydrate). 39,40 (J3) H + /ROH reversible formation of hemiacetal and hemiketal, aldehyde and ketones hemiacetals and hemiketals, hemiacetals and hemiketals are generally unstable and, except for sugars, not isolated. Sugars usually exist in cyclic hemiacetal or hemiketal form. Addition of second molecule of alcohol (28 (K2), 29 (K3)) to hemiacetals and hemiketals in reversible substitution reaction yields acetal or ketal. Especially important as blocking or protecting group when ethylene glycol is used to form cyclic acetal or ketal. Nucleophilic 41 (F7, A2) 1. RMgX 2. H + /H 2 O, aldehyde or ketone alcohol, Grignard reaction. 42 (G7) H + or OH -, aldehyde or ketone β-hydroxycarbonyl compound (aldol), aldol addition of conjugate base of carbonyl to carbonyl. If R s present are aromatic, addition product eliminates to give enone. If R s are not aromatic, elimination will occur with heating. If elimination occurs, the reaction is called an aldol condensation. Useful primarily when only one kind of α hydrogen is present. 43 (H7) NaCN/HCl, aldehyde or ketone cyanohydrin, forms cyanohydrin that can be hydrolyzed to α-hydroxycarboxylic acid. 44 (M7, A2) 1. RMgX + CO 2 2. H + /H 2 O, carbon dioxide carboxylic acid, Grignard Rxn. 1 Although the orientation of the water in the product is anti-markovnikov, since boron is more electropositive than hydrogen, the intermediate orientation is consistent with Markovnikov s rule (1, p 163).
7 ELIMINATION 45 (G4) see #42 (G7), alcohol alkene. 46a (B13) strong base, strong nucleophile such as OH - /ROH, alkyl halide alkene, Zaitsev product unless R is bulky such as t-butyl or X is fluorine, E2 for 1 o halide competes with S N 2, E2 for 3 o halide, anti elimination, thermodynamic alkene favored but decreases as base strength increases due to earlier TS. b (B13) poor base, poor nucleophile such as H 2 O, alkyl halide alkene, Zaitsev product with thermodynamically favored stereochemistry dominant, doesn t work for 1 o halides, competition between E1 and S N 1 for 2 o and 3 o halides. 47a (C11) H 2 SO 4 or H 3 PO 4, alcohol alkene, Zaitsev product with thermodynamically favored stereochemistry dominant, E2 for 1 o halides, E1 for 2 o and 3 o halides, rearrangement possible. b (C11) POCl 3 /pyridine/o o C, alcohol alkene, milder conditions than 47a, use of this catalyst avoids rearrangements, Zaitsev product with thermodynamically favored stereochemistry dominant, E2 reaction. 48 (D8) 1. CH 3 I (excess) 2.Ag 2 O/H 2 O 3. Δ, amine alkene, Hofmann (least substituted) product. 49 (E8) NaNH 2 /NH 3 or K t-butoxide/dmso, (di)halide alkyne. ADDITION-ELIMINATION 50 (E10) 1. NH 3 (or RNH 2 )/H + 2. H 2 /Ni or hydride such as NaBH 3 CN, aldehyde or ketone amine, reductive amination, technically this should be classed as two reactions with the addition-elimination (51 (G11)) first and the hydrogenation second. 51 (G11) NH 2 R, aldehyde or ketone imine, R s commonly used to make classic derivatives of carbonyls yield oximes (R = OH, NH 2 OH = hydroxylamine), hydrazones (R = NH 2 or NHR, if 2,4-dintrophenylhydrazine is used, a 2,4- dinitrophenylhydrazone results), semicarbazones (R = NHCONH 2, NH 2 NHCONH 2 = semicarbazide). 52 (H15) RCOO -, acyl halide anhydride. 53 (K10) NH 3 (or RNH 2 or R 2 NH), acyl halide amide. 54a (J15) H + /H 2 O, acyl halide carboxylic acid. b (J15) 1. OH - /H 2 O 2. H + /H 2 O, acyl halide carboxylic acid. 55 (J16) ROH, acyl halide ester. 56 (K10) NH 3 (or RNH 2 or R 2 NH), anhydride amide. 57a (J12) H + /H 2 O, anhydride carboxylic acid. b (J12) 1. OH - /H 2 O 2. H + /H 2 O, anhydride carboxylic acid. 58 (I12) ROH, anhydride ester. 59a (J11) RCOCl (with conjugate base of acid), carboxylic acid anhydride. b (J11) Δ for formation of intramolecular anhydrides such as phthalic anhydride. 60 (I16) SOCl 2 or PCl 3 or PCl 5 or PBr 3, carboxylic acid acyl halide. 61 (K11) NH 3, carboxylic acid amide, primarily an industrial reaction as it requires extreme conditions. 62 (K13) H + /ROH, carboxylic acid ester, position of equilibrium important in this reaction. 63 (K14) H + /H 2 O, ester carboxylic acid, position of equilibrium important in this reaction. 64 (L11) NH 3 (or RNH 2 or R 2 NH), ester amide. 65a (J10) H + /H 2 O, nitrile carboxylic acid. b (J10) 1. OH - /H 2 O 2. H + /H 2 O, nitrile carboxylic acid. 131
8 132 66a (K9) H + /H 2 O or OH - /H 2 O, nitrile amide, mild conditions as strong conditions yield carboxylic acids b (K9) H + /ROH, nitrile N-substituted amide, Ritter reaction, does not work with 1 o alcohols 67a (K11) H + /H 2 O, amide carboxylic acid. b (K11) 1. OH - /H 2 O 2. H + /H 2 O, amide carboxylic acid. 68 (H18) 1. R 2 CuLi/Et 2 O/-78 o C 2. H 2 O, acyl halide and organometallic ketone, with Gilman reagent, addition stops at ketone level. 69 (K11, A3) 1.RMgX/Et 2 O 2. H + /H 2 O, acyl halide and organometallic 3 o alcohol. 70 (I17, A3) 1.RMgX/Et 2 O 2. H + /H 2 O, ester and organometallic 3 o alcohol. 71 (J7, A3) 1.R MgX/Et 2 O 2. H + /H 2 O, nitrile and organometallic ketone. 72 (J17) RO -, ester β-keto ester, Claisen condensation, ester must have 2 α hydrogens (the 2 nd one prevents a reverse reaction). OXIDATION 73 (B3) 1. O 3 2. Zn/HCl, alkene aldehydes and/or ketones depending on alkene substitution. 74 (H2) NaNO 2 /HCl, amine diazonium salt, for 1 o aromatic amines. 75a (F3) KMnO 4 /H 3 O + /Δ, alkyl benzene with α alkyl hydrogens a benzoic acid. b (F3) K 2 Cr 2 O 7 /H 3 O + /Δ, alkyl benzene with α alkyl hydrogens a benzoic acid. 76 (D11) RCO 3 H, alkene oxirane. 77a (E11) KMnO 4 /OH -, syn-addition, alkene diol. b (E11) 1. OsO 4 2. H 2 O 2, syn-addition, alkene diol. 78a (F12) for 2 o alcohols - CrO 3 /H 2 SO 4 or Na 2 Cr 2 O 7 /H 2 SO 4 or KMnO 4 /H 2 SO 4, alcohol ketone. b (F12) for 1 o alcohols - PCC (pyridinium chlorochromate), alcohol aldehyde. 79 (I13) CrO 3 /H 2 SO 4 or Na 2 Cr 2 O 7 /H 2 SO 4 or KMnO 4 /H 2 SO 4 for 1 o alcohols, alcohol carboxylic acid. 80a (H12) CrO 3 /H 2 SO 4 or Na 2 Cr 2 O 7 /H 2 SO 4 or KMnO 4 /H 2 SO 4, aldehyde carboxylic acid. b (H12) 1. Ag 2 O/NH 3 2. H 3 O + Tollens test for aldehydes yields silver mirror, aldehyde carboxylic acid. c (H12) Cu 2+ /sodium tartrate, aldehyde carboxylic acid, Fehling s test for aldehydes yields red Cu 2 O. d (H12) Cu 2+ /sodium citrate, aldehyde carboxylic acid, Benedict s test for aldehydes yields red Cu 2 O. REDUCTION 81a (A9) Mg/Et 2 O, alkyl halide organometallic, formation of Grignard, water must be excluded. b (A9) Li/Et 2 O, alkyl halide organometallic formation of organolithium compound, more reactive than Grignard, water must be excluded. 82 (B7) H 2 /Pt or Pd or Ni, alkene alkane, syn-addition. 83 (B6) H 2 /Pt or Pd or Ni, alkyne alkane. 84a (D7) H 2 /Lindlar s catalyst (palladium precipitated on CaCO 3 and treated with Pb(C 2 H 3 O 2 ) 2 and quinoline), alkyne alkene, poisoned catalyst necessary to prevent addition of 2 nd mole of H 2, syn-addition. b (D7) Na or Li/NH 3, non-terminal alkyne alkene, gives anti-addition resulting in trans alkene. 85 (B9) 1. TsCl/pyridine 2. LiAlH 4, alcohol alkane. 86 (G2) Sn/HCl, aromatic nitro compound aromatic amine.
9 87a (C9) Zn(Hg)/HCl/Δ, aldehyde or ketone alkane, Clemmensen reduction for acid insensitive compounds. b (C9) NH 2 NH 2 //OH - /Δ, aldehyde or ketone alkane, Wolff-Kishner reduction for base insensitive compounds. c (C9) 1. HS(CH 2 ) 2 SH/HCl 2. H 2 /Raney Ni, aldehyde or ketone alkane, for compounds insensitive to acid or base 88a (E12) 1. LiAlH 4 2. H 3 O +, aldehyde or ketone alcohol. b (E12) 1. NaBH 4 2. H 3 O +, aldehyde or ketone alcohol. c (E12) H 2 /Raney Ni, aldehyde or ketone alcohol. 89 (E14) 1. LiAlH 4 2. H 2 O, oxirane alcohol, hydride attacks least substituted carbon. 90a (G16) 1. LiAlH 4 2. H 3 O +, acyl halide alcohol. b (G16) H 2 /Pd, acyl halide alcohol. 91 (H15) 1. LiAlH[OC(CH 3 ) 3 ] 3 H, -78 o C 2. H + or H 2 /partially deactivated Pd similar to Lindlar s catalyst (Rosenmund reduction), acyl halide aldehyde 92a (I9) H 2 /Pd/C, nitrile amine. b (I9) 1. LiAlH 4 2. H 2 O, nitrile amine 93 (I10) 1. DIBAH (diisobutylaluminium hydride)/-80 o C 2. H +, nitrile aldehyde. 94 (H12) 1. LiAlH 4 2. H +, carboxylic acid alcohol. 95 (I13) 1. DIBAH (diisobutylaluminium hydride)/-80 o C 2. H +, ester aldehyde. 96 (H14) 1. LiAlH 4 2. H +, ester alcohol. 97 (I9) 1. LiAlH 4 2. H 2 O, amide amine. CONCERTED 98 (B4) Δ for some, alkene + diene cyclohexene, Diels-Alder reaction, a concerted syn addition that also falls under classifications of pericyclic and [4 + 2] cycloaddition reactions, electronic releasing groups on the diene and electron withdrawing groups on the dienophile generally increase the rate of reaction. The diene must be capable of achieving an s-cis conformation and cyclic dienes such as cyclopentadiene react rapidly. There is a stereochemical preference for electron withdrawing groups on the dienophile to end up in the endo position. MISCELLANEOUS 99a (C5) 1. CH 2 N 2 /Δ or hν 2. alkene, carbene + alkene cyclopropane, generally a syn addition, also see 107 (L18). b (C5) 1. CH 2 I 2 /Zn(Cu) 2. alkene, Simmons-Smith reagent, carbenoid + alkene cyclopropane (also see 107 (L18). 100 (B18) 1. Li/Et 2 O 2. CuI 3. R X, alkyl halide uses Gilman reagent to make symmetrical and unsymmetrical hydrocarbons. 101 (F6) (Ph) 3 P=CHR, Wittig reagent synthesized from 1. Ph 3 P + RBr 2. RLi, aldehyde or ketone alkene. 102 (H13) 1. X 2 /OH - 2. H +, methyl aldehydes and ketones haloform + carboxylic acid, haloform reaction used to test for methyl ketones (and acetaldehyde and ethanol and other 2-alcohols). 103 (K8) P 2 O 5 or SOCl 2, amide nitrile. 104 (L6) Br 2 /OH -, Hofmann rearrangement, amide amine with one less carbon. 105 (L12) Δ, carboxylic acid carbon dioxide + residue (usually aromatic or β-keto caboxylic acids, others do not easily decarboxylate). 106 (K19) 1. OR - 2. R Br 3. H + /H 2 O/Δ, β-keto ester ketone, acetoacetic ester synthesis (also consider malonic ester synthesis). 107 (L18) 1. t-butoxide 2. alkene, dichlorocarbene + alkene dichlorocyclopropane, a syn addition, also see 99 (C5). 133
10 134 Preparation of Functional Groups Acetals and Ketals Acid catalyzed addition of 2 moles of alcohol to aldehyde or ketone (39 (J3), 28 (K2)). Acyl halides Reaction of carboxylic acid with SOCl 2 or PCl 3 or PCl 5 or PBr 3 (60 (I16)). Alcohols Reaction of an alkyl halide with OH - (19 (C13)). Reaction of an oxirane with a Grignard reagent, an organolithium reagent or a Gilman reagent (organocuprate) (25 (F17, A2)). Hydration of an alkene (32 (C11)). Reaction of a carbonyl compound with a Grignard reagent or an organolithium reagent (41 (F7, A2)). Aldol reaction (42 (G7)) Reaction of an acyl halide (69 (K11, A3)) or an ester (70 (I17, A3)) with a Grignard reagent or an organolithium reagent. Reduction of an oxirane with LiAlH 4 (89 (E14)). Reduction of carbonyl (88 (E12)) and carboxyl compounds (90 (G16), 94 (H12), 96 (H14)). Aldehydes Hydration of ethylene (36a (E6)) or other terminal alkynes (36b (E6)). Alkylation of conjugate base with an alkyl halide (27 (G7)). Formation from acetals and hemiacetals (38 (I4), 40 (J3)). Aldol reaction (42 (G7)). Oxidation of an alkene with ozone followed by reductive workup (73 (B3)). Oxidation of a primary alcohol with PCC. Reduction of an acyl halide (91 (H15)) or an ester (95 (I13)). Reduction of a nitrile (93 (I10)). Aldehyde and ketone derivatives Reaction of appropriate reagent with carbonyl compound (51 (G11)). Alkanes Reaction of water with a Grignard reagent or an organolithium reagent (8 (A5)) Reduction of an alkene (82 (B7)) or an alkyne (83 (B6)) Reduction of an alcohol (85 (B9)). Reduction of carbonyl compounds (87 (C9)). Coupling of two alkyl halides (100 (B18)). Carbene and carbenoid reactions (99 (C5)). Alkenes Elimination of water from an alcohol (45 (G4), 47 (C11)). Elimination of HX from an alkyl halide (46 (B13)). Hofmann elimination from an amine (48 (D8)). Reduction of an alkyne (84 (D7)). Diels-Alder reaction (98 (B4)). Wittig reaction (101 (F6)). Alkyl halides Reaction of NBS at a benzylic or allylic position (9 (E3)). Radical bromination or chlorination of an alkane (10 (B13)). Nucleophilic substitution of an alcohol (20 (C15)). Addition of HX to an alkene (30 (B11)) or alkyne (35 (F10)).
11 Addition of X 2 to an alkene (33 (E14)). Haloform reaction (102 (H13)). Addition of dichlorocarbene to an alkene (107 (L18)). Alkynes Alkylation of an acetylide with an alkyl halide (26 (E6)). Elimination of 2 moles of HX from geminal or vicinal dihalides (49 (E8)). Amides Reaction of an acid chloride (53 (K10)) or anhydride (56 (K10)) or ester (64 (L11)) with NH 3 or a 1 o or 2 o amine. Reaction of a carboxylic acid with NH 3 or a 1 o or 2 o amine under extreme conditions (61 (K11)). Nitrile hydrolysis for un-substituted amide and Ritter reaction for N-substituted amide (66 (K9)). Amines Reaction of an alkyl halide (18 (C14)) with NH 3, RNH 2, or R 2 NH. Reductive amination of a carbonyl compound (50 (E10)). Reduction of an aromatic nitro compound (86 (G2)). Reduction of an amide (97 (I9)) or nitrile (92 (I9)). Hofmann rearrangement starting with an amide (104 (L6)). Anhydrides Reaction of an acyl halide with a carboxylate ion (52 (H15), 59a (J11)). Preparation of a cyclic anhydride by dehydration of a dicarboxylic acid (59b (J11)). Aromatic derivatives Sulfonation with sulfuric acid and reverse reaction (1 (D1), 2 (E1)) Nitration with nitric acid/sulfuric acid (3 (D2)). Reaction of aromatic with X 2 /Lewis acid (4 (D3)). Friedel-Crafts alkylation and acylation (5 (D4), 6 (D5)). Reaction of alkyl halide with NBS to give α-bromo aromatic (9 (E3)). Reaction of diazonium salts to give phenols (13 (J1)), halides (11 (I1),14 (J1),15 (K1)), hydrocarbons (16 (L1)), nitriles (12 (L4)). Carbon-carbon bonds Friedel-Crafts alkylations (5 (D4)) and acylations (6 (D5)). Reaction of a diazonium salt with CuCN (16 (L1)). Reaction of cyanide with an alkyl halide (17 (B18)). Conversion of an alcohol to a nitrile (23 (C17)) via a tosylate. Reaction of an acetylide with an alkyl halide (26 (E6)). Alkylation of carbons α to a carbonyl (27 (G7)). Reactions of carbonyls (41 (F7, A2)), carbon dioxide (44 (M7, A2)), carboxyls (68 (H18), 69 (K11, A3), 70 (I17, A3)), oxiranes (25 (F17, A2)), nitriles (71 (J7, A3)) with a Grignard reagent or an organolithium reagent. Aldol and related reactions (42 (G7)). Addition of cyanide to a carbonyl (43 (H7)). Claisen condensation and related reactions (72 (J17)). Diels-Alder reaction (98 (B4)). Carbene and carbenoid additions to alkenes (99 (C5), 107 (L18)). Coupling of two alkyl halides (100 (B18)). Wittig reaction (101 (F6)). Acetoacetic ester and malonic ester synthesis (106 (K19)). 135
12 136 Carboxylic acids Reaction of carbon dioxide with a Grignard reagent or an organolithium reagent (44 (M7, A2)). Hydrolysis of an acyl halide (54 (J15)), anhydride (57 (J12)), ester (63 (K14)), nitrile (65 (J10)) or amide (67 (K11)). Oxidation of an alkyl benzene (75 (F3)). Oxidation of a primary alcohol (79 (I13)) or an aldehyde (80 (H12)). Haloform reaction of a methyl ketone (102 (H13)). Cyanohydrins Reaction of a carbonyl compound with cyanide (43 (H7)). Diols (1,2) Hydrolysis of an oxirane (24 (F15)). Oxidation of an alkene (77 (E11)). Esters Reaction of an acyl halide (55 (J16)) or anhydride (58 (I12)) with an alcohol, avoids equilibrium problem involved in direct conversion from the carboxylic acid. Acid catalyzed reaction of a carboxylic acid with an alcohol (62 (K13)). Claisen condensation (72 (J17)). Ethers Williamson ether synthesis between an alkyl halide and an alkoxide ion (21 (D13), 22 (C16)). Addition of an alcohol to an alkene including alkoxymercuration (31 (C12)). Hemiacetals and hemiketals Acid catalyzed addition of alcohol to a carbonyl (39 (J3)). Ketones Friedel-Crafts acylation (6 (D5)). Alkylation of conjugate base with an alkyl halide (27 (G7)). Hydration of an alkyne (36 (E6)). Formation from ketals and hemiketals (38 (I4), 40 (J3)). Aldol reaction and condensation (42 (G7)). Reaction of acyl halide with Gilman reagent (68 (H18)). Reaction of a nitrile with a Grignard reagent (71 (J7, A3)). Claisen condensation (72 (J17)). Oxidation of an alkene with ozone followed by reductive workup (73 (B3)). Oxidation of a 2 o alcohol (78a (F12)). Acetoacetic ester synthesis (106 (K19)) Nitriles Reaction of a diazonium salt with CuCN (Sandmeyer reaction) (16 (L1)). Nucleophilic substitution of an alkyl halide (17 (B18)). Conversion of an alcohol (23 (C17)). Reaction of a carbonyl compound with hydrogen cyanide (43 (H7)). Dehydration of an amide (103 (K8)). Organometallics Reaction of magnesium (Grignard reagent) or lithium with an alkyl halide in ether (81 (A9)). Formation of a Gilman reagent (organocuprate) by reacting an organolithium reagent with CuI. This procedure is used in three synthetic pathways on map (25 (F17, A2), 68 (H18), 100 (B18)). Oxiranes Reaction of an alkene with a peroxyacid (76 (D11)).
13 137 Reactions of Functional Groups Acetals and Ketals Acid catalyzed reaction to carbonyl compounds via hemiacetals and hemiketals (29 (K3)). Acyl halides Conversion to anhydrides (52 (H15)), amides (53 (K10)), acids (54 (J15)), esters (55 (J16)). Reaction with Gilman reagent to give ketones (68 (H18)). Reaction with Grignards and organolithium reagents to give 3 o alcohols (69 (K11, A3)). Reduction to 1 o alcohols (90 (G16)). Reduction to aldehydes (91 (H15)). Alcohols Conversion to alkyl halides (20 (C15)), ethers (21 (D13)), nitriles (23 (C17)). Dehydration to alkenes (45 (G4), 47 (C11)). Reaction with acyl halides (55 (J16)) and anhydrides (58 (I12)) to give esters. Oxidation to carbonyls (78 (F12)) and carboxylic acids (79 (I13)). Reduction to alkanes (85 (B9)). Aldehydes Alkylation of α-carbons (27 (G7)). Reaction with alcohols to give hemiacetals (39 (J3)) and subsequently acetals (28 (K2)). Reaction with water to give hydrates (37 (I4)). Reaction with Grignards and organolithium reagents to give 2 o alcohols (41 (F7, A2)). Aldol condensation to give β-hydroxy carbonyl compounds (42 (G7)). Reaction with cyanide to give cyanohydrins (43 (H7)). Reductive amination to amines (50 (E10)). Reaction with nitrogen compounds to give carbonyl derivatives (51 (G11)). Reduction to alkanes (87 (C9)). Reduction to 1 o alcohols (88 (E12)). Wittig reaction to give alkenes (101 (F6)). For acetaldehyde, haloform reaction to haloform and formic acid (102 (H13)). Alkanes Reaction with chlorine or bromine with heat or light to give alkyl halides (10 (B13)). Alkenes Addition of HX to give alkyl halides (30 (B11)). Addition of alcohols to give ethers (31 (C12)). Addition of water to give alcohols (32 (C11)). Addition of X 2 to give vicinal halides (33 (E14)). Ozonolysis to give carbonyl compounds (73 (B3)). Reaction with a peroxyacid to give an oxirane (76 (D11)). Oxidation to a 1,2-diol (77 (E11)). Hydrogenation to give alkanes (82 (B7)). Reaction with a diene to give a cyclohexene, Diels-Alder reaction (98 (B4)). Reaction with a carbene or carbenoid to give a cyclopropane (99 (C5), 107 (L18)). Alkyl halides Substitution to give alcohols (19 (C13)), ethers (22 (C16)), nitriles (17 (B18)). Conversion to amines (18 (C14)). Reaction with acetylide to give larger alkynes (26 (E6)). Elimination to give alkenes (46 (B13)). Elimination of dihalides to give alkynes (49 (E8)). Reaction with magnesium or lithium to give Grignards (81a (A9)) or organolithium reagents (81b (A9)). Coupling to give alkanes (100 (B18)).
14 138 Alkynes Alkylation via conjugate base and alkyl halide (26 (E6)). Addition of H 2, X 2 (34 (F10)), and HX (35 (F10)) to give vicinal and geminal halides Hydration to give carbonyl compounds (36 (E6)). Reduction to alkanes (83 (B6)) and alkenes (84 (D7)). Amides Hydrolysis to carboxylic acids (67 (K11)). Reduction to an amine (97 (I9)). Dehydration to nitriles (103 (K8)). Hofmann rearrangement to amines (104 (L6)). Amines Hofmann elimination to alkene (48 (D8)). Reaction with acyl halides (53 (K10)) and anyhdrides (56 (K10)) to give amides. Reaction with nitrous acid to give diazonium ion (74 (H2)). Anhydrides Conversion to amides (56 (K10)), carboxylic acids (57 (J12)), esters (58 (I12)). Aromatics Electrophilic aromatic substitution to give sulfonates (1 (D1)), nitro compounds (3 (D2)), halides (4 (D3)), alkyl (5 (D4))and acyl (6 (D5)) derivatives. Reaction of diazonium salts to give azo compounds (7 (L2)), phenols (13 (J1)), halides (11 (I1), 14 (J1), 15 (K1)), hydrocarbons (16 (L1)), nitriles (12 (L4)). Replacement of benzylic α-hydrogens with bromine using NBS (9 (E3)). Oxidation of alkyl groups (except t-butyl) to carboxylic acids (75 (F3)). Reduction of aromatic nitro compounds to give aromatic amines (86 (G2)). Carboxylic acids Conversion to anhydrides (59 (J11)), acyl halides (60 (I16)), amides (61 (K11)), esters (62 (K13)). Reduction to alcohols (94 (H12)). Decarboxylation of aromatic carboxylic acids and β-keto acids (105 (L12)). Esters Reaction with Grignards and organolithium reagents to give 3 o alcohols (70 (I17, A3)). Claisen condensation to give β-keto esters (72 (J17)). Conversion to carboxylic acids (63 (K14)), amides (64 (L11)). Reduction to aldehydes (95 (I13)) and alcohols (96 (H14)). Decarboxylation of β-keto esters to give ketones (106 (K19)). Hemiacetals and hemiketals Acid catalyzed reaction with alcohol to give acetals and ketals (28 (K2)). Acid catalyzed reaction to give carbonyl compounds (40 (J3)). Ketones Alkylation of α-carbons (27 (G7)). Reaction with alcohols to give hemiketals (39 (J3)) and subsequently ketals (28 (K2)). Reaction with water to give hydrates (37 (I4)). Reaction with Grignard and organolithium reagents to give 3 o alcohols (41 (F7, A2)). Aldol condensation to give β-hydroxy carbonyl compounds (42 (G7)). Reaction with cyanide to give cyanohydrins (43 (H7)). Reductive amination to amines (50 (E10)). Reaction with nitrogen compounds to give carbonyl derivatives (51 (G11)). Reduction to alkanes (87 (C9)). Reduction to 2 o alcohols (88 (E12)). Wittig reaction to give alkenes (101 (F6)). Haloform reaction to haloform and carboxylic acid (102 (H13)).
15 Nitriles Hydrolysis to give a carboxylic acid (65 (J10)) or an amide (66 (K9)). Reduction to give an amine (92 (I9)) or aldehyde (93 (I10)). Organometallics Grignard and organolithium reagents Reaction with water to give an alkane (8). Reaction with oxiranes (25 (F17, A2)), carbonyls (41 (F7, A2)), acyl halides (69 (K11, A3)), esters (70 (I17, A3)) to give alcohols. Reaction with carbon dioxide to give carboxylic acids (44 (M7, A2)). Reaction with nitriles (71 (J7, A3)) to give ketones. Gilman reagents Reaction with oxiranes to give alcohols (25 (F17, A2)). Reaction with acyl halides to give ketones (68 (H18)). Reaction with alkyl halides to give alkanes (100 (B18)). Oxiranes Hydrolysis to 1,2-diols (24 (F15)). Reaction with a Grignard or a Gilman reagent (organocuprate) to give an alcohol (25 (F17, A2)). Reduction to alcohols (89 (E14)). 139
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
ROADMAP FOR REACTIONS Chapter 6
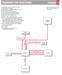 RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
18.8 Oxidation. Oxidation by silver ion requires an alkaline medium
 18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
1. Radical Substitution on Alkanes. 2. Radical Substitution with Alkenes. 3. Electrophilic Addition
 1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Chemistry of Benzene: Electrophilic Aromatic Substitution
 Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Aldehydes and Ketones: Nucleophilic Addition Reactions
 Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Chapter 18: Ketones and Aldehydes. I. Introduction
 1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
Cape Cod Community College
 Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See
 Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Available chemicals from the catalog (the starting sources of carbon compounds will continually decrease as we learn new reactions.
 ucleophilic ubstitution & Elimination Chemistry Beauchamp 1 Available chemicals from the catalog (the starting sources of carbon compounds will continually decrease as we learn new reactions. ources of
ucleophilic ubstitution & Elimination Chemistry Beauchamp 1 Available chemicals from the catalog (the starting sources of carbon compounds will continually decrease as we learn new reactions. ources of
Chem 251 Fall Learning Objectives
 Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 17: Alcohols and Phenols. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Double and Triple Bonds. The addition of an electrophile and a
 Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Aldehydes and Ketones. Dr. Munther A. M. Ali
 Aldehydes and Ketones Dr. Munther A. M. Ali ALDYHYDES AND KETONES Aldehydes are compounds of the general formula RCHO Ketones are compounds of the general formula RR'CO Aldehydes A ketone Both aldehydes
Aldehydes and Ketones Dr. Munther A. M. Ali ALDYHYDES AND KETONES Aldehydes are compounds of the general formula RCHO Ketones are compounds of the general formula RR'CO Aldehydes A ketone Both aldehydes
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water.
 Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Chapter 8 Alkenes and Alkynes II: Addition Reactions "
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Ch 16 Electrophilic Aromatic Substitution
 Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Chapter 7. Alkenes: Reactions and Synthesis
 Chapter 7. Alkenes: Reactions and Synthesis 1 Synthesis of Alkenes: Elimination Reactions 1. Dehydrohalogenation of alkyl halides. loss of requires CH 2 CH 2 Cl Zaitsev s Rule: CH 2 C 2. Dehydration of
Chapter 7. Alkenes: Reactions and Synthesis 1 Synthesis of Alkenes: Elimination Reactions 1. Dehydrohalogenation of alkyl halides. loss of requires CH 2 CH 2 Cl Zaitsev s Rule: CH 2 C 2. Dehydration of
Loudon Chapter 23 Review: Amines Jacquie Richardson, CU Boulder Last updated 4/22/2018
 This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions that are specific to nitrogen. Amines can be subdivided based on how many
This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions that are specific to nitrogen. Amines can be subdivided based on how many
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
Aldehydes, Ketones and Carboxylic acids
 Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
Option G: Further organic chemistry (15/22 hours)
 Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Organic Chemistry I: Reactions and Overview
 Organic Chemistry I: Reactions and Overview Andrew Rosen Editor: Raghav Malik January 13, 2013 Contents I Library of Synthetic Reactions 3 II Organic Trends and Essentials 4 1 The Basics: Bonding and Molecular
Organic Chemistry I: Reactions and Overview Andrew Rosen Editor: Raghav Malik January 13, 2013 Contents I Library of Synthetic Reactions 3 II Organic Trends and Essentials 4 1 The Basics: Bonding and Molecular
Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.
 Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Chapter 20: Carboxylic Acids
 1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions
 Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions
 Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic
Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton)
 314 Arrow Pushing practice/eauchamp 1 Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton) ucleophile = nucleus/positive loving = any general electron
314 Arrow Pushing practice/eauchamp 1 Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton) ucleophile = nucleus/positive loving = any general electron
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry
 Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15. Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution on Arenes. The first step is the slow, rate-determining step
 Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Ch 17 Alcohols and Phenols
 Ch 17 Alcohols and Phenols Alcohols are compounds with a hydroxyl group (OH) attached to an sp 3 C. Phenols are compounds with a hydroxyl group (OH) attached to an aromatic sp 2 C. Classification of Alcohols
Ch 17 Alcohols and Phenols Alcohols are compounds with a hydroxyl group (OH) attached to an sp 3 C. Phenols are compounds with a hydroxyl group (OH) attached to an aromatic sp 2 C. Classification of Alcohols
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Aldehydes and Ketones Reactions. Dr. Sapna Gupta
 Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
Chapter 9 Aldehydes and Ketones
 Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
2016 Pearson Education, Inc. Isolated and Conjugated Dienes
 2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
OCR (A) Chemistry A-level. Module 6: Organic Chemistry and Analysis
 OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
Chapter 19 Substitutions at the Carbonyl Group
 Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Química Orgânica I. Ciências Farmacêuticas Bioquímica Química AFB QO I 2007/08 1
 Química rgânica I Ciências Farmacêuticas Bioquímica Química AFB Q I 2007/08 1 alcohols Adaptado de rganic Chemistry, 6th Edition; Wade rganic Chemistry, 6 th Edition; McMurry AFB Q I 2007/08 2 Typical
Química rgânica I Ciências Farmacêuticas Bioquímica Química AFB Q I 2007/08 1 alcohols Adaptado de rganic Chemistry, 6th Edition; Wade rganic Chemistry, 6 th Edition; McMurry AFB Q I 2007/08 2 Typical
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Alcohols, Ethers and Epoxides. Chapter Organic Chemistry, 8th Edition John McMurry
 Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Reducing Agents. Linda M. Sweeting 1998
 Reducing Agents Linda M. Sweeting 1998 Reduction is defined in chemistry as loss of oxygen, gain of hydrogen or gain of electrons; the gain of electrons enables you to calculate an oxidation state. Hydride
Reducing Agents Linda M. Sweeting 1998 Reduction is defined in chemistry as loss of oxygen, gain of hydrogen or gain of electrons; the gain of electrons enables you to calculate an oxidation state. Hydride
Dr. Mohamed El-Newehy
 By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Aldehydes and Ketones 1 Structure of Aldehydes and Ketones - Aldehydes and ketones
By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Aldehydes and Ketones 1 Structure of Aldehydes and Ketones - Aldehydes and ketones
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Chapter 20: Aldehydes and Ketones
 hem A225 Notes Page 67 I. Introduction hapter 20: Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group (=) with no other heteroatoms attached. An aldehyde has at least one hydrogen attached;
hem A225 Notes Page 67 I. Introduction hapter 20: Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group (=) with no other heteroatoms attached. An aldehyde has at least one hydrogen attached;
10. Alkyl Halides. What Is an Alkyl Halide. An organic compound containing at least one carbonhalogen
 10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
Benzene and Aromatic Compounds. Chapter 15 Organic Chemistry, 8 th Edition John McMurry
 Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Chapter 11 Reaction of Alcohols
 Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
LECTURE #22 Thurs., Nov.15, 2007
 Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Provide a rxn sequence to make these as the major products Answers: 1. i Pr-Cl, AlCl 3 2. conc. fuming? H 2 S 4 3. Cl 2, FeCl 3 or AlCl 3 4. dilute H 2 S 4 note: normally aqueous workup after step 1, but
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Organic Chemistry, 7 L. G. Wade, Jr. Chapter , Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Chapter 8 - Alkenes and Alkynes II Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles
 Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
Products from reactions of carbon nucleophiles and carbon electrophiles used in the 14 C Game and our course:
 synthesis strategies, hem / / Beauchamp roducts from reactions of carbon nucleophiles and carbon electrophiles used in the Game and our course: arbon electrophiles methyl primary organolithium reagents
synthesis strategies, hem / / Beauchamp roducts from reactions of carbon nucleophiles and carbon electrophiles used in the Game and our course: arbon electrophiles methyl primary organolithium reagents
N_HW1 N_HW1. 1. What is the purpose of the H 2 O in this sequence?
 N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
Appendix A. Common Abbreviations, Arrows, and Symbols. Abbreviations
 smi97462_apps.qxd 12/6/04 1:09 PM Page A-1 Appendix A ommon Abbreviations, Arrows, and Symbols Abbreviations Ac acetyl, 3 BBN 9-borabicyclo[3.3.1]nonane BINAP 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl
smi97462_apps.qxd 12/6/04 1:09 PM Page A-1 Appendix A ommon Abbreviations, Arrows, and Symbols Abbreviations Ac acetyl, 3 BBN 9-borabicyclo[3.3.1]nonane BINAP 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II. 5 Credit Hours. Prepared by: Richard A. Pierce
 JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
CHAPTER 16 - CHEMISTRY OF BENZENE: ELECTROPHILIC AROMATIC SUBSTITUTION
 CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
ORGANIC - BROWN 8E CH. 22- REACTIONS OF BENZENE AND ITS DERIVATIVES
 !! www.clutchprep.com CONCEPT: ELECTROPHILIC AROMATIC SUBSTITUTION GENERAL MECHANISM Benzene reacts with very few reagents. It DOES NOT undergo typical addition reactions. Why? If we can get benzene to
!! www.clutchprep.com CONCEPT: ELECTROPHILIC AROMATIC SUBSTITUTION GENERAL MECHANISM Benzene reacts with very few reagents. It DOES NOT undergo typical addition reactions. Why? If we can get benzene to
