Appendix A. Common Abbreviations, Arrows, and Symbols. Abbreviations
|
|
|
- Simon Crawford
- 6 years ago
- Views:
Transcription
1 smi97462_apps.qxd 12/6/04 1:09 PM Page A-1 Appendix A ommon Abbreviations, Arrows, and Symbols Abbreviations Ac acetyl, 3 BBN 9-borabicyclo[3.3.1]nonane BINAP 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl B tert-butoxycarbonyl, ( 3 ) 3 bp boiling point Bu butyl, BS reagent orey Bakshi Shibata reagent DBN 1,5-diazabicyclo[4.3.0]non-5-ene DBU 1,8-diazabicyclo[5.4.0]undec-7-ene D dicyclohexylcarbodiimide DET diethyl tartrate DIBAL- diisobutylaluminum hydride, [( 3 ) 2 2 ] 2 Al DMF dimethylformamide, N( 3 ) 2 DMS dimethyl sulfoxide, ( 3 ) 2 S ee enantiomeric excess Et ethyl, 3 2 FGI functional group interconversion MPA hexamethylphosphoramide, [( 3 ) 2 N] 3 P M highest occupied molecular orbital I infrared LA lithium aluminum hydride, LiAl 4 LDA lithium diisopropylamide, LiN[( 3 ) 2 ] 2 LUM lowest unoccupied molecular orbital m- meta mpba m-chloroperoxybenzoic acid Me methyl, 3 M molecular orbital mp melting point MS mass spectrometry MW molecular weight NBS N-bromosuccinimide NM N-methylmorpholine N-oxide NM nuclear magnetic resonance o- ortho p- para P pyridinium chlorochromate Ph phenyl, 6 5 ppm parts per million Pr propyl, TBDMS tert-butyldimethylsilyl TF tetrahydrofuran TMS tetramethylsilane, ( 3 ) 4 Si UV ultraviolet A-1
2 smi97462_apps.qxd 12/6/04 1:09 PM Page A-2 A-2 APPENDIX A ommon Abbreviations, Arrows, and Symbols Arrows Symbols reaction arrow equilibrium arrows double-headed arrow, used between resonance structures full-headed curved arrow, showing the movement of an electron pair half-headed curved arrow (fishhook), showing the movement of an electron retrosynthetic arrow no reaction dipole hn light heat δ + partial positive charge δ partial negative charge l wavelength n frequency n ~ wavenumber A Brønsted Lowry acid B: Brønsted Lowry base :Nu nucleophile E + electrophile X halogen bond oriented forward bond oriented behind partial bond [ ] transition state [] oxidation [] reduction
3 smi97462_apps.qxd 12/6/04 1:09 PM Page A-3 Appendix B pk a Values for Selected ompounds ompound pk a ompound pk a I 10 Br S S l 7 [( 3 ) 2 ] [ 3 2 ] S N2 0.0 F l ( 3 ) N 3 + N + N N + N N l P F l Br I N F N S S Br N l N Br 4.0 N A-3
4 smi97462_apps.qxd 12/6/04 1:09 PM Page A-4 A-4 APPENDIX B pk a Values for Selected ompounds ompound pk a ompound pk a 3 N 2 Et 3 N N S 10.5 [( 3 ) 3 N] Et 10.7 [ 3 N 3 ] [( 3 ) 2 N 2 ] F N 3 Et N ( 3 ) 3 18 ( 3 ) N 25 l N( 3 ) N N
5 smi97462_apps.qxd 12/6/04 1:09 PM Page A-5 Appendix Bond Dissociation Energies for Some ommon Bonds [A B A + B] Bond o kcal/mol (kj/mol) Z bonds F 136 (569) l 103 (431) Br 88 (368) I 71 (297) 119 (498) Z Z bonds 104 (435) F F 38 (159) l l 58 (242) Br Br 46 (192) I I 36 (151) 51 (213) bonds (435) (410) (410) ( 3 ) 2 95 (397) ( 3 ) 3 91 (381) (435) 125 (523) (364) (460) (356) bonds (368) (356) (385) (489) A-5
6 smi97462_apps.qxd 12/6/04 1:09 PM Page A-6 A-6 APPENDIX Bond Dissociation Energies for Some ommon Bonds [A B A + B] Bond o kcal/mol (kj/mol) X bonds 3 F 109 (456) 3 l 84 (351) 3 Br 70 (293) 3 I 56 (234) 3 2 F 107 (448) 3 2 l 81 (339) 3 2 Br 68 (285) 3 2 I 53 (222) ( 3 ) 2 F 106 (444) ( 3 ) 2 l 80 (335) ( 3 ) 2 Br 68 (285) ( 3 ) 2 I 53 (222) ( 3 ) 3 F 106 (444) ( 3 ) 3 l 79 (331) ( 3 ) 3 Br 65 (272) ( 3 ) 3 I 50 (209) bonds 3 91 (381) (381) (381) ( 3 ) 2 91 (381) ( 3 ) 3 91 (381)
7 smi97462_apps.qxd 12/6/04 1:09 PM Page A-7 Appendix D haracteristic I Absorption Frequencies Bond Functional group Wavenumber (cm 1 ) omment N broad, strong very broad, strong N two peaks 2 N one peak N 2, N one or two peaks; N bending also observed at 1640 cm 1 sp 3300 sharp, often strong sp medium sp strong sp 2 of one or two peaks 2250 medium N 2250 medium strong l 1800 () , 1760 two peaks increasing n ~ with decreasing ring size increasing n ~ with decreasing ring size 2, conjugated N 2, N, increasing n ~ with decreasing N 2 ring size Alkene 1650 medium Arene 1600, 1500 medium N 1650 medium A-7
8 smi97462_apps.qxd 12/6/04 1:09 PM Page A-8
9 smi97462_apps.qxd 12/6/04 1:09 PM Page A-9 Appendix E haracteristic 1 NM Absorptions ompound type hemical shift (ppm) Alcohol Aldehyde 9 10 Alkane ~ ~1.3 3 ~1.7 Alkene sp allylic sp Alkyl halide F l Br I A-9
10 smi97462_apps.qxd 12/6/04 1:09 PM Page A-10 A-10 APPENDIX E haracteristic 1 NM Absorptions ompound type hemical shift (ppm) Alkyne ~2.5 Amide N Amine N N Aromatic compound sp benzylic sp arbonyl compound sp 3 on the α carbon arboxylic acid Ether
11 smi97462_apps.qxd 12/15/04 10:10 AM Page A-11 Appendix F General Types of rganic eactions Substitution eactions [1] Nucleophilic substitution at an sp 3 hybridized carbon atom [a] Alkyl halides (hapter 7) X + Nu Nu + X nucleophile [b] Alcohols (Section 9.11) + X X + 2 [c] Ethers (Section 9.14) ' + X X + ' X + 2 X = Br or I [d] Epoxides (Section 9.15) [1] Nu [2] 2 or Z Nu or Z = nucleophile Nu (Z) [2] Nucleophilic acyl substitution at an sp 2 hybridized carbon atom arboxylic acids and their derivatives (hapter 22) Z + Nu nucleophile Nu + Z Z =, l,, ', N' 2 [3] adical substitution at an sp 3 hybridized bond Alkanes (Section 13.3) + X 2 hν or X + X [4] Electrophilic aromatic substitution Aromatic compounds (hapter 18) + E + E + + electrophile A-11
12 smi97462_apps.qxd 12/6/04 1:09 PM Page A-12 A-12 APPENDIX F General Types of rganic eactions Elimination eactions a Elimination at an sp 3 hybridized carbon atom [a] Alkyl halides (hapter 8) X + B base + B + + X new π bond [b] Alcohols (Section 9.8) A + 2 new π bond Addition eactions [1] Electrophilic addition to carbon carbon multiple bonds [a] Alkenes (hapter 10) [b] Alkynes (Section 11.6) + X Y X Y X Y + X Y X Y [2] Nucleophilic addition to carbon oxygen multiple bonds Aldehydes and ketones (hapter 21) + Nu (') nucleophile 2 (') Nu
13 smi97462_apps.qxd 12/6/04 1:09 PM Page A-13 Appendix G ow to Synthesize Particular Functional Groups Acetals eaction of an aldehyde or ketone with two equivalents of an alcohol (21.14) Acid chlorides eaction of a carboxylic acid with thionyl chloride (22.10) Alcohols Nucleophilic substitution of an alkyl halide with or 2 (9.6) ydration of an alkene (10.12) ydroboration oxidation of an alkene (10.16) eduction of an epoxide with LiAl 4 (12.6) eduction of an aldehyde or ketone (20.4) ydrogenation of an α,β-unsaturated carbonyl compound with 2 + Pd- (20.4) Enantioselective reduction of an aldehyde or ketone with the chiral BS reagent (20.6) eduction of an acid chloride with LiAl 4 (20.7) eduction of an ester with LiAl 4 (20.7) eduction of a carboxylic acid with LiAl 4 (20.7) eaction of an aldehyde or ketone with a Grignard or organolithium reagent (20.10) eaction of an acid chloride with a Grignard or organolithium reagent (20.13) eaction of an ester with a Grignard or organolithium reagent (20.13) eaction of an organometallic reagent with an epoxide (20.14B) Aldehydes ydroboration oxidation of a terminal alkyne (11.10) xidation of a 1 alcohol with P (12.12) xidative cleavage of an alkene with 3 followed by Zn or ( 3 ) 2 S (12.10) eduction of an acid chloride with LiAl[( 3 ) 3 ] 3 (20.7) eduction of an ester with DIBAL- (20.7) ydrolysis of an acetal (21.14B) ydrolysis of an imine or enamine (21.12) eduction of a nitrile (22.18B) Alkanes atalytic hydrogenation of an alkene with 2 + Pd- (12.3) atalytic hydrogenation of an alkyne with two equivalents of 2 + Pd- (12.5A) eduction of an alkyl halide with LiAl 4 (12.6) eduction of a ketone to a methylene group ( 2 ) the Wolff Kishner or lemmensen reaction (18.14B) Protonation of an organometallic reagent with 2,, or acid (20.9) A-13
14 smi97462_apps.qxd 12/6/04 1:09 PM Page A-14 A-14 APPENDIX G ow to Synthesize Particular Functional Groups Alkenes Dehydrohalogenation of an alkyl halide with base (8.3) Dehydration of an alcohol with acid (9.8) Dehydration of an alcohol using Pl 3 and pyridine (9.10) β Elimination of an alkyl tosylate with base (9.13) atalytic hydrogenation of an alkyne with 2 + Lindlar catalyst to form a cis alkene (12.5B) Dissolving metal reduction of an alkyne with Na, N 3 to form a trans alkene (12.5) Wittig reaction (21.10) ofmann elimination of an amine (25.12) Alkyl halides eaction of an alcohol with X (9.11) eaction of an alcohol with Sl 2 or PBr 3 (9.12) leavage of an ether with Br or I (9.14) ydrohalogenation of an alkene with X (10.9) alogenation of an alkene with X 2 (10.13) ydrohalogenation of an alkyne with two equivalents of X (11.7) alogenation of an alkyne with two equivalents of X 2 (11.8) adical halogenation of an alkane (13.3) adical halogenation at an allylic carbon (13.10) adical addition of Br to an alkene (13.13) Electrophilic addition of X to a 1,3-diene (16.10) adical halogenation of an alkyl benzene (18.13) alogenation α to a carbonyl group (23.7) Alkynes Dehydrohalogenation of an alkyl dihalide with base (11.5) S N 2 reaction of an alkyl halide with an acetylide anion, (11.11) Amides eaction of an acid chloride with N 3 or an amine (22.8) eaction of an anhydride with N 3 or an amine (22.9) eaction of a carboxylic acid with N 3 or an amine and D (22.10) eaction of an ester with N 3 or an amine (22.11) Amines eduction of a nitro group (18.14) eduction of an amide with LiAl 4 (20.7B) eduction of a nitrile (22.18B) S N 2 reaction using N 3 or an amine (25.7A) Gabriel synthesis (25.7A) eductive amination of an aldehyde or ketone (25.7) Amino acids S N 2 reaction of an α-halo carboxylic acid with excess N 3 (28.2A) Alkylation of diethyl acetamidomalonate (28.2B) Strecker synthesis (28.2) Enantioselective hydrogenation using a chiral catalyst (28.4)
15 smi97462_apps.qxd 12/6/04 1:09 PM Page A-15 APPENDIX G ow to Synthesize Particular Functional Groups A-15 Anhydrides eaction of an acid chloride with a carboxylate anion (22.8) Dehydration of a dicarboxylic acid (22.10) Aryl halides alogenation of benzene with X 2 + FeX 3 (18.3) eaction of a diazonium salt with ul, ubr, BF 4, NaI, or KI (25.14A) arboxylic acids xidative cleavage of an alkyne with ozone (12.11) xidation of a 1 alcohol with r 3 (or a similar r 6+ reagent), 2, 2 S 4 (12.12B) xidation of an alkyl benzene with KMn 4 (18.14A) xidation of an aldehyde (20.8) eaction of a Grignard reagent with 2 (20.14A) ydrolysis of a cyanohydrin (21.9) ydrolysis of an acid chloride (22.8) ydrolysis of an anhydride (22.9) ydrolysis of an ester (22.11) ydrolysis of an amide (22.13) ydrolysis of a nitrile (22.18A) Malonic ester synthesis (23.9) yanohydrins Addition of N to an aldehyde or ketone (21.9) 1,2-Diols Anti dihydroxylation of an alkene with a peroxyacid, followed by ring opening with or 2 (12.9A) Syn dihydroxylation of an alkene with KMn 4 or s 4 (12.9B) Enamines eaction of an aldehyde or ketone with a 2 amine (21.12) Epoxides Intramolecular S N 2 reaction of a halohydrin using base (9.6) Epoxidation of an alkene with mpba (12.8) Enantioselective epoxidation of an allylic alcohol with the Sharpless reagent (12.14) Esters S N 2 reaction of an alkyl halide with a carboxylate anion, (7.19) eaction of an acid chloride with an alcohol (22.8) eaction of an anhydride with an alcohol (22.9) Fischer esterification of a carboxylic acid with an alcohol (22.10) Ethers Williamson ether synthesis S N 2 reaction of an alkyl halide with an alkoxide, (9.6) eaction of an alkyl tosylate with an alkoxide, (9.13) Addition of an alcohol to an alkene in the presence of acid (10.12) alohydrins eaction of an epoxide with X (9.15) Addition of X and to an alkene (10.15) Imine eaction of an aldehyde or ketone with a 1 o amine (21.11)
16 smi97462_apps.qxd 12/6/04 1:09 PM Page A-16 A-16 APPENDIX G ow to Synthesize Particular Functional Groups Ketones ydration of an alkyne with 2, 2 S 4, and gs 4 (11.9) xidative cleavage of an alkene with 3 followed by Zn or ( 3 ) 2 S (12.10) xidation of a 2 o alcohol with any r 6+ reagent (12.12) Friedel rafts acylation (18.5) eaction of an acid chloride with an organocuprate reagent (20.13) ydrolysis of an imine or enamine (21.12) ydrolysis of an acetal (21.14B) eaction of a nitrile with a Grignard or organolithium reagent (22.18) Acetoacetic ester synthesis (23.10) Nitriles S N 2 reaction of an alkyl halide with NaN (7.19, 22.18) eaction of an aryl diazonium salt with un (25.14A) Phenols eaction of an aryl diazonium salt with 2 (25.14A)
17 smi97462_apps.qxd 12/6/04 1:09 PM Page A-17 Appendix eactions that Form arbon arbon Bonds Section eaction 11.11A S N 2 reaction of an alkyl halide with an acetylide anion, 11.11B pening of an epoxide ring with an acetylide anion, adical polymerization of an alkene Diels Alder reaction 18.5 Friedel rafts alkylation 18.5 Friedel rafts acylation eaction of an aldehyde or ketone with a Grignard or organolithium reagent 20.13A eaction of an acid chloride with a Grignard or organolithium reagent 20.13A eaction of an ester with a Grignard or organolithium reagent 20.13B eaction of an acid chloride with an organocuprate reagent 20.14A eaction of a Grignard reagent with B eaction of an epoxide with an organometallic reagent eaction of an α,β-unsaturated carbonyl compound with an organocuprate reagent 21.9 yanohydrin formation Wittig reaction to form an alkene S N 2 reaction of an alkyl halide with NaN eaction of a nitrile with a Grignard or organolithium reagent 23.8 Direct enolate alkylation using LDA and an alkyl halide 23.9 Malonic ester synthesis to form an α-substituted carboxylic acid Acetoacetic ester synthesis to form an α-substituted ketone 24.1 Aldol reaction to form a β-hydroxy carbonyl compound or an α,β-unsaturated carbonyl compound 24.2 rossed aldol reaction 24.3 Directed aldol reaction 24.5 laisen reaction to form a β-keto ester 24.6 rossed laisen reaction to form a β-dicarbonyl compound 24.7 Dieckmann reaction to form a five- or six-membered ring 24.8 Michael reaction to form a 1,5-dicarbonyl compound 24.9 obinson annulation to form a 2-cyclohexenone 27.10B Kiliani Fischer synthesis of an aldose 28.2B Alkylation of diethyl acetamidomalonate to form an amino acid 28.2 Strecker synthesis of an amino acid A-17
18 smi97462_apps.qxd 12/6/04 1:09 PM Page A-18
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
Chapter 17: Alcohols and Phenols
 hapter 17: Alcohols and Phenols sp 3 alcohol phenol (aromatic alcohol) pka~ 16-18 pka~ 10 Alcohols contain an group connected to a saturated carbon (sp 3 ) Phenols contain an group connected to a carbon
hapter 17: Alcohols and Phenols sp 3 alcohol phenol (aromatic alcohol) pka~ 16-18 pka~ 10 Alcohols contain an group connected to a saturated carbon (sp 3 ) Phenols contain an group connected to a carbon
Chapter 7: Alkenes: Reactions and Synthesis
 hapter 7: Alkenes: Reactions and Synthesis alcohol alkane halohydrin 1,2-diol 1,2-dihalide carbonyl halide halide Addition Y Y Elimination Electrophilic Addition Dehydrohalogenation: loss of from an alkyl
hapter 7: Alkenes: Reactions and Synthesis alcohol alkane halohydrin 1,2-diol 1,2-dihalide carbonyl halide halide Addition Y Y Elimination Electrophilic Addition Dehydrohalogenation: loss of from an alkyl
Synthetic possibilities Chem 315 Beauchamp 1
 Synthetic possibilities hem Beauchamp Propose reasonable syntheses f the following target molecules (TM-#). You can use the given starting materials and any typical ganic reagents studied in our course
Synthetic possibilities hem Beauchamp Propose reasonable syntheses f the following target molecules (TM-#). You can use the given starting materials and any typical ganic reagents studied in our course
Chapter 22: Amines. Organic derivatives of ammonia, NH 3. Nitrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic
 hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
ROADMAP FOR REACTIONS Chapter 6
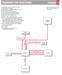 RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
sp 2 geometry tetrahedral trigonal planar linear ΔH C-C ΔH C-H % s character pk a 464 KJ/mol 33% 44
 hapter 10: Alkynes 10.1 Introduction to Alkynes ~ 111 ~ 122 1.06 Å 180 1.1 Å ~ 116 1.08 Å 1.54 Å 1.34 Å 1.20 Å hybridization of sp 3 sp 2 sp geometry tetrahedral trigonal planar linear 368 KJ/mol 632 KJ/mol
hapter 10: Alkynes 10.1 Introduction to Alkynes ~ 111 ~ 122 1.06 Å 180 1.1 Å ~ 116 1.08 Å 1.54 Å 1.34 Å 1.20 Å hybridization of sp 3 sp 2 sp geometry tetrahedral trigonal planar linear 368 KJ/mol 632 KJ/mol
Basic Organic Chemistry
 Basic rganic hemistry ourse code: EM 12162 (Pre-requisites : EM 11122) hapter 06 hemistry of Aldehydes & Ketones Dr. Dinesh R. Pandithavidana ffice: B1 222/3 Phone: (+94)777-745-720 (Mobile) Email: dinesh@kln.ac.lk
Basic rganic hemistry ourse code: EM 12162 (Pre-requisites : EM 11122) hapter 06 hemistry of Aldehydes & Ketones Dr. Dinesh R. Pandithavidana ffice: B1 222/3 Phone: (+94)777-745-720 (Mobile) Email: dinesh@kln.ac.lk
Alkynes. Alkynes-hydrocarbons with a carbon-carbon triple bond.
 Alkynes Alkynes-hydrocarbons with a carbon-carbon triple bond. The carbon-carbon triple bond results from the interaction of two sp hybridized carbon atoms. 180 degree angle. Linear. The carbon-carbon
Alkynes Alkynes-hydrocarbons with a carbon-carbon triple bond. The carbon-carbon triple bond results from the interaction of two sp hybridized carbon atoms. 180 degree angle. Linear. The carbon-carbon
Chapter 11 Reaction of Alcohols
 Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
Aldehydes and Ketones
 Aldehydes and Ketones Preparation of Aldehydes xidation of Primary Alcohols --- 2 P 1o alcohol ydroboration of a Terminal Alkyne, followed by Tautomerization --- 1. B 3, TF 2. 2 2, K 2 terminal alkyne
Aldehydes and Ketones Preparation of Aldehydes xidation of Primary Alcohols --- 2 P 1o alcohol ydroboration of a Terminal Alkyne, followed by Tautomerization --- 1. B 3, TF 2. 2 2, K 2 terminal alkyne
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
When H and OH add to the alkyne, an enol is formed, which rearranges to form a carbonyl (C=O) group:
 Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Chapter 18 Ketones and Aldehydes. Carbonyl Compounds. Chapter 18: Aldehydes and Ketones Slide 18-2
 hapter 18 Ketones and Aldehydes arbonyl ompounds hapter 18: Aldehydes and Ketones Slide 18-2 1 arbonyl Structure arbon is sp 2 hybridized. = bond is shorter, stronger, and more polar than = bond in alkenes.
hapter 18 Ketones and Aldehydes arbonyl ompounds hapter 18: Aldehydes and Ketones Slide 18-2 1 arbonyl Structure arbon is sp 2 hybridized. = bond is shorter, stronger, and more polar than = bond in alkenes.
Chapter 20: Aldehydes and Ketones
 hapter 20: Aldehydes and Ketones [hapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ketone ' aldehyde 2. eview of the Synthesis of Aldehydes and Ketones Br Br f
hapter 20: Aldehydes and Ketones [hapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ketone ' aldehyde 2. eview of the Synthesis of Aldehydes and Ketones Br Br f
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
CH 3 CHCH 3 CH 3 CHCH 3 Isopropyl cation. Oxomium ion intermediate. intermediate (an electrophile)
 Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
235 Organic II. Final Exam Review REACTIONS OF CONJUGATED DIENES 1,2 VS 1,4 ADDITION REACTIONS OF CONJUGATED DIENES
 b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Products from reactions of carbon nucleophiles and carbon electrophiles used in the 14 C Game and our course:
 synthesis strategies, hem / / Beauchamp roducts from reactions of carbon nucleophiles and carbon electrophiles used in the Game and our course: arbon electrophiles methyl primary organolithium reagents
synthesis strategies, hem / / Beauchamp roducts from reactions of carbon nucleophiles and carbon electrophiles used in the Game and our course: arbon electrophiles methyl primary organolithium reagents
ALCOHOLS: Properties & Preparation
 ALLS: Properties & Preparation General formula: -, where is alkyl or substitued alkyl. Ar-: phenol - different properties. Nomenclature 1. ommon names: Name of alkyl group, followed by word alcohol. 2.
ALLS: Properties & Preparation General formula: -, where is alkyl or substitued alkyl. Ar-: phenol - different properties. Nomenclature 1. ommon names: Name of alkyl group, followed by word alcohol. 2.
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II. 5 Credit Hours. Prepared by: Richard A. Pierce
 JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
Cape Cod Community College
 Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
N.b. A catalyst is a species which speeds up a chemical reaction but which remains chemically unchanged. Reverse process of dehydration of an alcohol
 An Introduction to Organic hemistry N.b. A catalyst is a species which speeds up a chemical reaction but which remains chemically unchanged. ydration (Addition) Reverse process of dehydration of an alcohol
An Introduction to Organic hemistry N.b. A catalyst is a species which speeds up a chemical reaction but which remains chemically unchanged. ydration (Addition) Reverse process of dehydration of an alcohol
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
24.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols. + Electron-withdrawing groups make an O
 Chapter 24: Phenols. Alcohols contain an group bonded to an sp 3 -hybridized carbon. Phenols contain an group bonded to an sp 2 -hybridized carbon of a benzene ring 24.1: Nomenclature (please read) 24.2:
Chapter 24: Phenols. Alcohols contain an group bonded to an sp 3 -hybridized carbon. Phenols contain an group bonded to an sp 2 -hybridized carbon of a benzene ring 24.1: Nomenclature (please read) 24.2:
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
 ARBXYLI AIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPA name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
ARBXYLI AIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPA name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
Chapter 16: Ethers, Epoxides, and Sulfides
 Chapter 16: Ethers, Epoxides, and Sulfides 16.1: omenclature of Ethers, Epoxides, and Sulfides (Please read) 16.2: Structure and Bonding in Ethers and Epoxides The ether oxygen is sp 3 -hybridized and
Chapter 16: Ethers, Epoxides, and Sulfides 16.1: omenclature of Ethers, Epoxides, and Sulfides (Please read) 16.2: Structure and Bonding in Ethers and Epoxides The ether oxygen is sp 3 -hybridized and
Alcohol Synthesis. Dr. Sapna Gupta
 Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Chapter 7: Alkene reactions conversion to new functional groups
 hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
Chapter 17 Aldehydes and Ketones
 hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
acetaldehyde (ethanal)
 hem 263 Nov 2, 2010 Preparation of Ketones and Aldehydes from Alkenes zonolysis 1. 3 2. Zn acetone 1. 3 2. Zn acetone acetaldehyde (ethanal) Mechanism: 3 3 3 + - oncerted reaction 3 3 3 + ozonide (explosive)
hem 263 Nov 2, 2010 Preparation of Ketones and Aldehydes from Alkenes zonolysis 1. 3 2. Zn acetone 1. 3 2. Zn acetone acetaldehyde (ethanal) Mechanism: 3 3 3 + - oncerted reaction 3 3 3 + ozonide (explosive)
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Bond length (pm) Bond strength (KJ/mol)
 hapter 10: Alkyl alides = F, l,, I Alkyl halide Aryl halide Vinyl halide 10.1 Naming alkyl halides- ead 10.2 Structure of alkyl halides Table 10.1 alomethane 3 -F 3 -l 3-3 -I Bond length (pm) 139 178 193
hapter 10: Alkyl alides = F, l,, I Alkyl halide Aryl halide Vinyl halide 10.1 Naming alkyl halides- ead 10.2 Structure of alkyl halides Table 10.1 alomethane 3 -F 3 -l 3-3 -I Bond length (pm) 139 178 193
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Chem 342 Organic Chemistry II Final Exam 13 May 2009
 hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
Lecture 19. Carboxylic Acids O C OH O R C O - + H + O - Chemistry 328N
 Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Chapter 12. Alcohols from Carbonyl Compounds Oxidation-Reduction & Organometallic Compounds. Structure
 Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Chapter 12 Alcohols from Carbonyl Compounds xidation-eduction & rganometallic Compounds Created by Professor William Tam & Dr. Phillis Chang Structure ~ 120 o ~ 120 o C ~ 120 o Carbonyl carbon: sp 2 hybridized
Conjugated Systems & Pericyclic Reactions
 onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
Modern Organic Synthesis an Introduction
 Modern Organic Synthesis an Introduction G. S. Zweifel M. H. Nantz W.H. Freeman and Company Chapter 1 Synthetic Design 1 What is an ideal or viable synthesis, and how does one approach a synthetic project?
Modern Organic Synthesis an Introduction G. S. Zweifel M. H. Nantz W.H. Freeman and Company Chapter 1 Synthetic Design 1 What is an ideal or viable synthesis, and how does one approach a synthetic project?
Chem 263 Nov 7, elimination reaction. There are many reagents that can be used for this reaction. Only three are given in this course:
 hem 263 Nov 7, 2013 Preparation of Ketones and Aldehydes from Alcohols xidation of Alcohols [] must have at least 1 E elimination reaction [] = oxidation; removal of electrons [] = reduction; addition
hem 263 Nov 7, 2013 Preparation of Ketones and Aldehydes from Alcohols xidation of Alcohols [] must have at least 1 E elimination reaction [] = oxidation; removal of electrons [] = reduction; addition
Organic Chemistry Curriculum Content Outline
 Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
Structure and Reactivity: Prerequired Knowledge
 Structure and eactivity: Prerequired Knowledge!!! The concepts presented in this summary are required for lecture and examination!!! 1. Important Principles in rganic Chemistry In general, structures which
Structure and eactivity: Prerequired Knowledge!!! The concepts presented in this summary are required for lecture and examination!!! 1. Important Principles in rganic Chemistry In general, structures which
O C. Aldehyde: O C H 3 CH 2 C H propanal propionaldehyde. O C H 3 CH 2 C CH 2 CH 3 3-pentanone diethylketone
 Aldehydes and Ketones Both contain the functional group Aldehyde: R Ketone R R' Aldehydes omenclature. IUPA: drop the 'e' from the name of the alkane and add 'al'. There are also common names which are
Aldehydes and Ketones Both contain the functional group Aldehyde: R Ketone R R' Aldehydes omenclature. IUPA: drop the 'e' from the name of the alkane and add 'al'. There are also common names which are
I. Write Structures for the compounds named below: (12 points) H 2 N NH 2. Acetone Hydrazine Cyclohexane carbaldehyde H 3 C
 I. Write Structures for the compounds named below: (12 points) 3 2 N N 2 Acetone ydrazine yclohexane carbaldehyde N P( 6 5 ) 3 3 An ethyl ylide of Any imine 3-xo-6-phenylhexanal triphenylphosphine II.
I. Write Structures for the compounds named below: (12 points) 3 2 N N 2 Acetone ydrazine yclohexane carbaldehyde N P( 6 5 ) 3 3 An ethyl ylide of Any imine 3-xo-6-phenylhexanal triphenylphosphine II.
Chapter 13: Alcohols and Phenols
 Chapter 13: Alcohols and Phenols [ Chapter 9 Sections: 9.10; Chapter 13 Sections: 13.1-13.3, 13.9-13.10] 1. Nomenclature of Alcohols simple alcohols C3 C3C2 Eddie Sachs 1927-1964 larger alcohols find the
Chapter 13: Alcohols and Phenols [ Chapter 9 Sections: 9.10; Chapter 13 Sections: 13.1-13.3, 13.9-13.10] 1. Nomenclature of Alcohols simple alcohols C3 C3C2 Eddie Sachs 1927-1964 larger alcohols find the
Aldehydes and Ketones
 9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
Carbonyl Chemistry. aldehydes ketones. carboxylic acid and derivatives. Wednesday, April 29, 2009
 Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
R N R N R N. primary secondary tertiary
 Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Amines Amines (e.g. RNH 2 ) are organic derivatives of ammonia, NH 3 similar to ammonia
 148Exam 2 notes rganic hemistry (e.g. 2 ) are organic derivatives of ammonia, 3 similar to ammonia omenclature: suffix 2 prefix 2 ( 3 ) 2 Types: Primary (1 o ), secondary (2 o ), tertiary (3 o ) or quaternary
148Exam 2 notes rganic hemistry (e.g. 2 ) are organic derivatives of ammonia, 3 similar to ammonia omenclature: suffix 2 prefix 2 ( 3 ) 2 Types: Primary (1 o ), secondary (2 o ), tertiary (3 o ) or quaternary
Reactivity of C=C. Chapter 8 Reactions of Alkenes. Types of Additions. Electrophilic Addition. Addition of HX (1) Addition of HX (2)
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 8 Reactions of Alkenes Jo Blackburn Richland ollege, allas, TX allas ounty ommunity ollege istrict 2003, Prentice all Reactivity of = Electrons in pi
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 8 Reactions of Alkenes Jo Blackburn Richland ollege, allas, TX allas ounty ommunity ollege istrict 2003, Prentice all Reactivity of = Electrons in pi
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES
 ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name when given the structure, and draw the structure given the name of open-chain and monocyclic
ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name when given the structure, and draw the structure given the name of open-chain and monocyclic
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of H X 2. Addition of H OH and addition of Y X 3. Addition to allene and
 Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Aldehydes and Ketones: Nucleophilic Addition Reactions
 Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
Carbonyl Chemistry IV + C O C. Lecture 10. Chemistry /30/02
 arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
S N 2 and E2 Mechanisms (strong base/nucleophile competition reacting at a carbon or reacting at a proton)
 Electrophile (Lewis Acid) = electron loving = any general electron pair acceptor (can also be an acidic proton = onsted acid) ucleophile (Lewis Base) = nucleus/positive loving = any general electron pair
Electrophile (Lewis Acid) = electron loving = any general electron pair acceptor (can also be an acidic proton = onsted acid) ucleophile (Lewis Base) = nucleus/positive loving = any general electron pair
Summary of π Bond Chemistry
 Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chapter 11 Reactions of Alcohols. Types of Alcohol Reactions
 hapter 11 Reactions of Alcohols Types of Alcohol Reactions Dehydration to alkene (Discussed in hap 7) xidation to aldehyde, ketone Substitution to form alkyl halide Reduction to alkane Esterification Tosylation
hapter 11 Reactions of Alcohols Types of Alcohol Reactions Dehydration to alkene (Discussed in hap 7) xidation to aldehyde, ketone Substitution to form alkyl halide Reduction to alkane Esterification Tosylation
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Chapter 17: Alcohols and Phenols. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton)
 314 Arrow Pushing practice/eauchamp 1 Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton) ucleophile = nucleus/positive loving = any general electron
314 Arrow Pushing practice/eauchamp 1 Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton) ucleophile = nucleus/positive loving = any general electron
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Electrophilic Addition
 . Reactivity of = Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. arbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the
. Reactivity of = Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. arbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the
The Organic Acids. Carboxylic Acids * *
 arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
arboxylic Acids The rganic Acids Some Notation: Acids and their conjugate bases pka ~ 15 - weak acid arboxylic Acid pka ~ 4 moderate acid - arboxylate Anion pka ~ -7 very strong acid l - l arboxylic acids
18.8 Oxidation. Oxidation by silver ion requires an alkaline medium
 18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Introductory Organic Chemistry II Midterm Exam
 NAME: STUDENT NUMBE: Page 1 of 7 University of Manitoba Department of hemistry 2.222 Introductory rganic hemistry II Midterm Exam Wednesday February 20, 2002 Put all answers in the spaces provided. If
NAME: STUDENT NUMBE: Page 1 of 7 University of Manitoba Department of hemistry 2.222 Introductory rganic hemistry II Midterm Exam Wednesday February 20, 2002 Put all answers in the spaces provided. If
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson 3rd Midterm April 26, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson 3rd Midterm April 26, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
CH318N Spring 2012 Final Exam. Chemistry 318N. Spring 2012 Dr. Willson. Final Exam
 318N Spring 2012 Final Exam 3 hemistry 318N Spring 2012 Dr. Willson Final Exam This afternoon you will take two tests, one in chemistry and one in integrity. I want you to get A s on both of these tests
318N Spring 2012 Final Exam 3 hemistry 318N Spring 2012 Dr. Willson Final Exam This afternoon you will take two tests, one in chemistry and one in integrity. I want you to get A s on both of these tests
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Available chemicals from the catalog (the starting sources of carbon compounds will continually decrease as we learn new reactions.
 ucleophilic ubstitution & Elimination Chemistry Beauchamp 1 Available chemicals from the catalog (the starting sources of carbon compounds will continually decrease as we learn new reactions. ources of
ucleophilic ubstitution & Elimination Chemistry Beauchamp 1 Available chemicals from the catalog (the starting sources of carbon compounds will continually decrease as we learn new reactions. ources of
Carboxylic Acids O R C + H + O - Chemistry 618B
 arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
Week 6 notes CHEM
 Week 6 notes EM1002 2009 Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, hemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 1 Note
Week 6 notes EM1002 2009 Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, hemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 1 Note
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
MOLECULAR REPRESENTATIONS AND INFRARED SPECTROSCOPY
 MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
Carbonyls (Ch ketones and aldehydes and carboxylic acids derivatives)
 arbonyls (h 16-19 ketones and aldehydes and carboxylic acids derivatives) +δ -δ ' - sp 2 - trigonal planar (120 0 ) - strongly polarized double bond eactivity? addition nucleophilic 1 Nucleophilic Addition
arbonyls (h 16-19 ketones and aldehydes and carboxylic acids derivatives) +δ -δ ' - sp 2 - trigonal planar (120 0 ) - strongly polarized double bond eactivity? addition nucleophilic 1 Nucleophilic Addition
