Chapter 11 Reaction of Alcohols
|
|
|
- Dwayne Parsons
- 6 years ago
- Views:
Transcription
1 Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C X 3 C 3 C X 3 C Cr 3, 2 S 4 Alcohols can be oxidized to carbon-oxygen double bonds (carbonyl compounds) Primary alcohols will be converted to aldehydes under basic oxidation conditions Primary alcohols will be converted to carboxylic acids under nonbasic oxidation conditions
2 Alcohols as Nucleophiles Neutral alcohols can react as nucleophiles (often observe this with carboxylic acid derivatives or inorganic analogs) To make alcohols more nucleophilic, need to abstract the acidic hydrogen (remember pka s!) With this method, can make nucleophilic oxygen that can react through any S N 2 type reaction already studied
3 NUC Alcohols as Electrophiles In these reactions the alcohol is the leaving group (the C- bond is broken during the reaction) NUC Usually the hydroxide, or alkoxide, is a BAD leaving group, therefore we need to convert the alcohol into a GD leaving group 1) The tosylate, which was seen earlier, is commonly used as a way to make the alcoholic oxygen a good leaving group NUC Ts NUC 2) Another method we have already observed is protonation to form water as the leaving group + 2 X Ts X
4 Conversion of Alcohols into alides The methods to convert alcohols into electrophiles are used to transform alcohols into alkyl halides Can occur with either S N 1 or S N 2 conditions depending upon alcohol used Type of alcohol chloride bromide iodide primary secondary tertiary Best reagents for interconversions: SCl 2 SCl 2 PBr 3 PBr 3 P/I 2 P/I 2 Cl Br I Stereochemistry, and possibility of rearrangements, depends on mechanism for each reaction
5 Chapter 12 Infrared Spectroscopy I is used for Functional Group Identification Position of peaks can be used to differentiate all possible carbonyl compounds in addition to distinguishing common functional groups including nitriles, alkynes and aromatic rings C=C double bond CC triple bond Factors to be considered for peaks: Position of Absorbance (related to energy needed for absorbance)
6 C=C intensity C= intensity Factors to be considered for peaks: Intensity of Absorbance (related to dipole of bond undergoing absorbance) Shape of Absorbance (sharp or broad peaks related to type of bond)
7 Chapter 12 Mass Spectrometry In addition to determining the parent molecular weight for a sample, a MS can also be used to characterize what atoms are present and to differentiate isomers Isotopic Patterns for natural abundance: Fragmentation patterns can also be different for isomers
8 Predicted Mass Spectrometry Differences Isotope Differences Used to Distinguish alogen Substituents I Br m/z = 204 no m+2 for iodine m+1 = 6.4% of m m/z = 156 m+2 ~ equal to m Cl F m/z = 112 m+2 ~ 1/3 of m m/z = 96 no m+2 for fluorine
9 Fragmentation Pattern to Determine Structure e-beam m/z 102!-cleavage C 3 or or loss of water C 3 57 emember: nly charged forms are observed in a MS
10 Chapter 13 NM Spectroscopy Any nucleus with either an odd atomic number or odd mass has a nuclear spin A charge species that is spinning creates a current loop, which in turn creates magnetic field lines In solution, however, there are many hydrogens present and the spinning direction is random In presence of large external magnetic field the spin directions are quantized
11 Shielding Need to remember the structure of a compound (consider only an isolated C- bond) C B 0 B net = B 0 - B electron To reach the nucleus the magnetic field must past through the electron cloud surrounding the nucleus The electrons surrounding the nucleus are charged species that can rotate in the presence of the external magnetic field What this means is that the external magnetic field (B 0 ) is effectively reduced by the time it reaches the nucleus (B 0 minus the field of the electron cloud)
12 Splitting In addition to the electron density surrounding the hydrogen causing shielding, each hydrogen acts like its own magnetic field If one magnet is close to another, it will feel the effect of that magnetic field This occurs in a 1 NM a hydrogen will feel the effect of neighboring hydrogens In the field of one additional hydrogen therefore we would observe two signals N+1 rule: can predict the splitting that will be observed by counting the number of hydrogens on adjacent carbons (N) and adding one
13 1 NM Chemical Shift Scale B molecular - C 3 TMS (standard) C 2 C Aromatic- C=C- C-, C-X, C-N alkane B applied B effective ppm (!) Due to empirically observed effects, chemists can predict the position for functional groups, and the predicted splitting pattern for each signal Applied Magnetic Field: omogeneous for 1 NM therefore spin sample Molecular Magnetic Field: two effects will change the magnetic field experienced by nucleus 1) Density of electron density around nucleus shields nucleus (range ~12 ppm for 1 NM, ~200 ppm for 13 C NM) 2) Nearby magnetic nuclei (spin-spin splitting) The effective magnetic field at the nucleus is thus B applied -B molecular
14 13 C NM Spectroscopy Because a 13 C atom has an odd mass, it also has inherent magnetic field lines and will display NM spectroscopy in the presence of a large external magnetic field The downfield shift is dependent upon the shielding caused by the electrons around the carbon, due to the extra electron density around carbon compared to hydrogen there is a greater amount of shielding in a 13 C NM Instead of ~11 ppm range for 1 NM, 13 C NM is typically in a ~200 ppm range Due to the low probability of having two 13 C isotopes adjacent, there is no splitting observed from the possible spin states of adjacent carbons Typically observe a spin decoupled 13 C NM spectrum which has only singlets for each carbon bserve all carbons though, do not need to have a hydrogen attached as with a 1 NM
15 Amount of Downfield Shift is Comparable carboxylic acid 1 NM aldehyde aromatic alkene ether alkyl halide alkanes ppm (!) carbonyl carbons 13 C NM aromatic alkene alkyne C- C X alkanes ppm (!)
16 Chapter 14 Ethers Ethers are generally synthesized through a nucleophilic method Ethers are often used as solvents for organic reactions because the functional group is relatively unreactive ne of the few reactions that they can undergo is alkyl cleavage with I or Br + Br Br + Br Br Very similar to alcohol reactions observed in chapter 11 I > Br >> Cl
17 Epoxides ne type of ethers that are reactive is a cyclic ether in a 3-membered ring called epoxides Epoxides can be synthesized in one of two ways: 1) eacting alkenes with a peracid 2) eacting halohydrins with a weak base
18 egiochemistry in eaction of Epoxides The base catalyzed opening of epoxides goes through a common S N 2 mechanism, therefore the nucleophile attacks the least hindered carbon of the epoxide C 3 MgBr In the acid catalyzed opening of epoxides, the reaction first protonates the oxygen This protonated oxygen can equilibrate to an open form that places more partial positive charge on more substituted carbon, therefore the more substituted carbon is the preferred reaction site for the nucleophile + C 3 C 3
19 Chapter 15 Conjugated Systems Conjugated systems occur anytime there are p orbitals on adjacent atoms in conjugation Whenever there are p orbitals in conjugations, molecular orbitals result by the mixing of orbitals The difference in energy is due to the number of nodes (different phases overlap), more nodes means higher in energy and electrons are filled in lowest energy orbitals first = E 4 p orbitals = 4 Ms
20 Addition to Conjugated Dienes As we have already seen, alkenes can react with electrophiles to create a carbocation With conjugated dienes this reaction will create an allylic carbocation The nucleophile can then react with either resonance form in the second step
21 Kinetic versus Thermodynamic Control What forms faster (kinetic product) and what is more stable (thermodynamic product) need not be the same Consider the addition to conjugated dienes 2!+ E 2 2!+!+ eaction at 2 cation site has a more stable transition state +!+ Generate allylic cation in first step Allylic cation can have water react at two sites eaction at 2 site eaction at 1 site Thus the kinetic product has water reacting at 2 site eaction a 1 site, though, generates more stable product (more substituted double bond) The thermodynamic product has water reacting at 1 site
22 Diels-Alder eaction The reaction between butadiene and ethylene is called a Diels-Alder reaction btain cyclohexene functional units after a Diels-Alder reaction Always try to find the cyclohexene unit in the product, this will indicate what was the initial butadiene and ethylene parts
23 Stereochemistry of Addition Products on previous page did not indicate stereochemistry, but Diels-Alder reaction typically only yields one diastereomer preferentially (does not differentiate enantiomers) Whenever there is a p orbital on the atom attached to the dienophile, the endo product is favored due to orbital interaction between p orbital on dienophile and p orbitals on diene Endo position Interaction of p orbitals NC Exo position N C In endo position, orbitals on alkene substituents can interact with p orbitals of butadiene In exo orientation this interaction is not present
24 egiochemistry of Unsymmetrically Substituted Diels-Alder Products When a monosubstituted butadiene and a monosubstituted alkene react, different regioproducts can be obtained C 3 N 2! C 3 N 2 C 3 or N 2 Can predict favored product by understanding location of charge in molecules 2 3 C 3 C 3 C Consider resonance forms Negative charge is located on C2 and C4 1 2 N N Consider resonance forms Positive charge located on C2 The negative charge will react preferentially with the positive charge to obtain one regioproduct
25 Using this analysis we can predict regioproducts A Diels-Alder reaction can therefore control both regio- and stereochemistry
26 Ultraviolet-Visible (UV-Vis) Spectroscopy Instead of causing molecular vibrations, UV-VIS light causes electronic excitations An electron is excited from the M to the LUM Ethylene LUM Ethylene M E h! If the correct amount of energy is applied (i.e. the correct wavelength of light), the excitation of one electron from the M to the LUM will occur As the amount of conjugation increases, the energy gap between the M and LUM decreases With a lower energy gap, the λmax shifts to a longer wavelength of light to cause excitations
27 Chapter 16 Aromatic Systems When p orbitals are in conjugation in a ring, stability is sometimes much greater than acyclic = = Cyclic systems are thus different than acyclic as seen by how electrons can resonate, -is this difference always better? Depends also on the placement of molecular orbitals (not only due to cyclic) ückels rule: 4n+2 electrons in conjugation = aromatic (more stable) 4n electrons in conjugation = antiaromatic (less stable)
28 Aromatic Ions If p orbitals are in full conjugation in a cyclic system and the number of electrons in conjugation is equal to 4n+2, the compound is more stable regardless of whether the compound is neutral, negatively charged or positively charged = After rehybridization, system has 6 electrons in conjugation in a ring, therefore aromatic pka is ~16 Cyclopentadiene anion is very stable Cyclopentadiene cation is very unstable (will not form) With all cyclic ions, see if p orbitals are conjugated in a ring and then count the number of electrons in conjugation, if 4n+2 then stable, if 4n then unstable
29 Chapter 17 Aromatic eactions Electrophilic Aromatic Substitution Aromatic compounds react through a unique substitution type reaction Initially an electrophile reacts with the aromatic compound to generate an arenium ion (also called sigma complex) The arenium ion has lost aromatic stabilization (one of the carbons of the ring no longer has a conjugated p orbital) In a second step, the arenium ion loses a proton to regenerate the aromatic stabilization The product is thus a substitution (the electrophile has substituted for a hydrogen) and is called an Electrophilic Aromatic Substitution
30 A Variety of Electrophiles Can Be Used The key is generating an electrophilic reagent that can react with the aromatic ring ortho/para director meta director C 3 N 2 C 3 Br 2 FeBr 3 Cl 2 AlCl 3 N 3 2 S 4 Cl AlCl 3 Br C 3 2 N N 2 Electrophile:!+!- Br Br FeBr 3 Cl!+!- Cl Cl AlCl 3 N 2
31 eactions on Aromatic Substituents A variety of reactions can be performed on the aromatic substituents N 2 Sn, Fe, or Zn N 2 Cl deactivating activating Carbon side chains: Friedel-Crafts alkylation Friedel-Crafts acylation Cl Cl AlCl 3 AlCl 3 KMn 4 K NBS h! Zn Cl N 2 N 2 K C 2 Br Clemmensen Wolf-Kishner
32 Nucleophilic Aromatic Substitution Mechanism N 2 Cl NaCN N 2 Cl CN N 2 Cl CN N 2 Cl CN 2 N 2 N 2 N 2 N The anion is stabilized by electron withdrawing groups ortho/para to leaving group N 2 Cl CN N 2 CN 2 N 2 N To regain aromatic stabilization, the chloride leaves to give the substituted product 1) Must have EWG s ortho/para to leaving group -the more EWG s present the faster the reaction rate (intermediate is stabilized) 2) The leaving group ability does not parallel S N 2 reactions -follows electronegativity trend (F > Cl > Br > I)
33 Benzyne Mechanism A second nucleophilic aromatic substitution reaction is a benzyne mechanism Benzyne is an extremely unstable intermediate which will react with any nucleophile present N 2 Br NaN 2, N 3 N2 benzyne Need strong base at moderate temperatures, but do not need EWG s on ring
34 Chapter 18 Ketones and Aldehydes New routes to synthesize ketones and aldehydes From carboxylic acids: Li SCl 2 LiAl(tBu) 3 (2 equiv.) Cl 2 CuLi From nitriles: 1) MgBr 2) +, 2 C N 1) DIBAL 2) +, 2 1) DIBAL 2) +, 2 From dithianes: BuLi S S S S X S S +, gcl 2 2
35 eactions of Ketones and Aldehydes Base mechanism Acid mechanism NUC NUC NUC NUC Types of NUC : MgBr LA ylide cyanide Types of neutral NUC: 2 N 2 eactivity As electrophilicity of carbonyl carbon increases, the reactivity increases < < < Cl 3 C
36 Wittig eaction The carbanion of the ylide is nucleophilic and will react with the carbonyl (Ph) 3 P 3 C C 3 (Ph) 3 P 3 C C C 3 3 betaine The betaine structure will form 4-membered ring between phosporous and oxygen (Ph) 3 P (Ph) 3 P 3 C C C C C 3 C 3 oxyphosphetane The oxyphosphetane will collapse to form a second phosphorous-oxygen bond (Ph) 3 P 3 C C 3 C 3 C 3 3 C C 3 (Ph)3P verall an alkene is formed from the initial carbonyl compound
37 Acetals Acetals are related to hydrates, Instead of geminal dialcohols have geminal ethers 3 C C C C 3 This process is once again an equilibrium process Aldehydes (which are more reactive than ketones) typically favor acetals When both alcohols to form an acetal are intramolecular (on same molecule) then a cyclic acetal is formed 3 C + 3 C Cyclic acetals and ketals are often used because they have a higher equilibrium for the acetal form
38 Baeyer-Villiger Allows conversion of ketone to ester C 3 Mechanism of oxygen insertion? Weak oxygen-oxygen single bond Mechanism is not an insertion, but rather a reaction at carbonyl followed by a migration
39 Chapter 19 Amines The lone pair of electrons on nitrogen can act as an acceptor (hence Brønsted-Lowry base) N 2 K b N The basicity, and hence pk b, is determined by the stability of the amine after protonation 3 C N 3 C 3 C sp 3 hybridization pk b = 4.26 N sp 2 hybridization pk b = C C N sp hybridization pk b = 24 As the percent s character increases for the orbital holding the lone pair of electrons, the electrons are held more closely to the positively charged nucleus ther effects, such as whether lone pair is in resonance or involved in aromatic system, also will affect the pk b for the amine
40 Synthesis of Amines Nucleophilic: N 2 N C 3 I N C 3 I N problem is overalkylation 1) K 2) C 3 I N C 3 N 2 N 2 3 C N 2 eduction: oxime N LA N 2 Gabriel allows only 1 addition amide azide N N 3 LA LA N N 2 nitrile CN LA N 2
41 offman Elimination Elimination of Amines F N F (C 3 ) 3 N anti-elimination With quaternary ammonium salts, E2 reaction occurs to eliminate trimethylamine Cope Elimination F N F ( 3 C) 2 N syn-elimination With N-oxide, syn elimination occurs to eliminate dimethylhydroxy amine Both eliminations favor the less substituted alkene to be formed
42 Arenediazonium Salts Arenediazonium salts can be generated from aniline derivatives N 2 NaN 2 Cl N N The diazonium salt can then be converted into a number of different functional groups N N 2 S 4 BF 4 KI CuCN 3 P 2 F I CN Unique phenol derivatives Unique F substitution Easier I substitution Versatile CN (Sandmeyer) educe to
43 Chapters 20&21 Carboxylic Acids & Derivatives eactions of Acyl Compounds There is a commonality amongst carbonyl reactions, they have a nucleophile react at the carbonyl carbon X X NUC NUC X NUC Generate a tetrahedral intermediate that can expel a leaving group
44 Interconversion of Carboxylic Acid Derivatives Cl Acid chloride Anhydride Ester N 2 Amide Carboxylate The carboxylic acid can thus be converted into any other carboxylic acid derivative, In addition each carboxylic acid derivative can be converted to a carboxylic acid
45 eactivity of Carboxylic Acid Derivatives Cl Acid chloride Anhydride reactivity Ester N 2 Amide Carboxylate All carboxylic acid derivatives can also be converted back to the carboxylic acid (by either acidic or basic hydrolysis) or the derivatives can be directly interconverted to a less reactive form But cannot interconvert a less reactive acyl derivative into a more reactive
46 Predicting eactivity Patterns for Carboxylic Acid Derivatives All of the carboxylic acid derivatives can react with a nucleophile to generate the same carbonyl product the difference is the leaving group Cl NUC NUC Cl pka of conjugate for leaving group -7 NUC NUC ~4-5 NUC NUC 16 N NUC NUC N 35 The stability of the leaving group affects the reactivity pattern for the acid derivatives
47 Chapter 22 eactions at α-carbon A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles reacting at the electrophilic carbonyl carbon NUC NUC
48 eactions of Enols The enol form can react with electrophiles A common reaction is halogenation Na Br Br Br Under basic conditions it is hard to stop at one addition due to hydrogen abstraction of product is more favored than starting material Na Br 2 Br Br Br Br
49 Enolates Enolates are similar to enols but they are far more nucleophilic In order to generate an enolate, need a base to abstract an α-hydrogen LDA will quantitatively form enolate 3 C C 3 LDA 3 C C 2 Using LDA the enolate will be formed quantitatively, with weaker bases will only form the enolate in a small fraction
50 Enolate as a Nucleophile ave already observed many reactions with a negatively charged nucleophile (most S N 2 reactions) An enolate is simply another type of nucleophile, it can react in similar manner as other nucleophiles 3 C C 2 E+ 3 C C 2 E ne common reaction is to alkylate the enolate C 3 Br 3 C C 2 3 C C 2 C 3 This reaction will place an alkyl substituent at the α-position of a carbonyl Any electrophile that will react in a S N 2 reaction can be used
51 Thermodynamic vs. Kinetic Control With unsymmetrical ketones, different enolates can be generated The enolate can be preferentially generated at either site depending upon conditions LDA Kinetic enolate easier hydrogen to abstract Thermodynamic enolate more stable double bond Lower temperature favors kinetic product igher temperatures (in this case usually room temperature and above) favors thermodynamic product
52 Aldol Condensation Instead of reacting the enolate with an alkyl halide, we can also react the enolate with a carbonyl compound The carbonyl can react as an electrophile - 2 Greater the conjugation, the easier for loss of water Upon work-up obtain a β-hydroxy ketone The β-hydroxy ketone that is formed can also lose water to form an α,β-unsaturated ketone
53 Claisen Condensation There are many Name reactions that are modifications of the aldol condensation, A Claisen condensation is an aldol where one carbonyl compound is an ester By using an ester, the chemistry is changed due to the presence of a leaving group C 3 Na Can run reaction with both carbonyls present with weak base due to differences in pka (ketones ~20, esters ~24) With ester leaving group, obtain diketone product
54 Dieckmann A Dieckmann condensation is an intramolecular Claisen condensation C 3 C 3 Convenient method to form 5- or 6-membered rings
55 Michael Addition If we add stabilized enolates to α,β-unsaturated system, the reaction can occur with 1,4-addition NUC NUC Michael product (1,4 addition) Whether a reaction occurs with 1,2- or 1,4-addition, selectively often depends on the stability of the nucleophilic anion. A more stable anion occurs with 1,4 selectivity while a less stable anion occurs with 1,2 selectivity. N C 3 MgBr (C 3 ) 2 CuLi 1,2 1,4 1,4 1,2 1,4 Enolate anions prefer 1,2 but β-diketone or enamines favor 1,4 Grignard reagents prefer 1,2 but Gilman reagents prefer 1,4
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
Reactions of Aromatic Compounds. Aromatic compounds do not react like other alkenes. With an appropriate catalyst, however, benzene will react
 Reactions of Aromatic Compounds Aromatic compounds do not react like other alkenes 2 Fe 3 2 Does not form A major part of the problem for this reaction is the product has lost all aromatic stabilization,
Reactions of Aromatic Compounds Aromatic compounds do not react like other alkenes 2 Fe 3 2 Does not form A major part of the problem for this reaction is the product has lost all aromatic stabilization,
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Amines. Amines are organic compounds containing a nitrogen functionality. primary secondary tertiary quaternary
 Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
and Ultraviolet Spectroscopy
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Loudon Chapter 23 Review: Amines Jacquie Richardson, CU Boulder Last updated 4/22/2018
 This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions that are specific to nitrogen. Amines can be subdivided based on how many
This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions that are specific to nitrogen. Amines can be subdivided based on how many
Conjugated Systems. With conjugated double bonds resonance structures can be drawn
 Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Amines Reading Study Problems Key Concepts and Skills Lecture Topics: Amines: structure and nomenclature
 Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Alcohols. Have seen many reactions to synthesize alcohols: In this chapter we will study reactions of the alcohols
 Alcohols ave seen many reactions to synthesize alcohols: In this chapter we will study reactions of the alcohols Oxidation Need to understand the nomenclature of organic reduction/oxidation In general
Alcohols ave seen many reactions to synthesize alcohols: In this chapter we will study reactions of the alcohols Oxidation Need to understand the nomenclature of organic reduction/oxidation In general
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Chemistry of Benzene: Electrophilic Aromatic Substitution
 Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
When H and OH add to the alkyne, an enol is formed, which rearranges to form a carbonyl (C=O) group:
 Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Chapter 22: Amines. Organic derivatives of ammonia, NH 3. Nitrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic
 hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Chapter 20: Aldehydes and Ketones
 Chapter 20: Aldehydes and Ketones [Chapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ' ketone aldehyde f both aldehydes and ketones, the parent chain is the longest
Chapter 20: Aldehydes and Ketones [Chapter 20 Sections: 20.1-20.7, 20.9-10.10, 20.13] 1. Nomenclature of Aldehydes and Ketones ' ketone aldehyde f both aldehydes and ketones, the parent chain is the longest
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Cape Cod Community College
 Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
235 Organic II. Final Exam Review REACTIONS OF CONJUGATED DIENES 1,2 VS 1,4 ADDITION REACTIONS OF CONJUGATED DIENES
 b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
Chapter 17 Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
2016 Pearson Education, Inc. Isolated and Conjugated Dienes
 2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
Chapter 17 Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution o General reaction - an electrophile replaces a hydrogen Electrons of pi system attack strong electrophile, generating resonancestabilized
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution o General reaction - an electrophile replaces a hydrogen Electrons of pi system attack strong electrophile, generating resonancestabilized
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Carbonyl Chemistry. aldehydes ketones. carboxylic acid and derivatives. Wednesday, April 29, 2009
 Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry
 OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II. 5 Credit Hours. Prepared by: Richard A. Pierce
 JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Aldehydes and Ketones: Nucleophilic Addition Reactions
 Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
CHAPTER 16 - CHEMISTRY OF BENZENE: ELECTROPHILIC AROMATIC SUBSTITUTION
 CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
Loudon Chapter 23 Review: Amines CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 23 eview: Amines CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions
Loudon Chapter 23 eview: Amines CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 This chapter is about the chemistry of nitrogen. We ve seen it before in several places, but now we can look at several reactions
Loudon Chapter 19 Review: Aldehydes and Ketones CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
R N R N R N. primary secondary tertiary
 Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY
 4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
PAPER No. 5: REACTION MECHANISM MODULE No. 2: Types of Organic Reaction Mechanisms
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Organic Chemistry, 7 L. G. Wade, Jr. Chapter , Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Solution problem 22: Non-Benzoid Aromatic Sytems
 Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
11/5/ Conjugated Dienes. Conjugated Dienes. Conjugated Dienes. Heats of Hydrogenation
 8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
Structure and Reactivity: Prerequired Knowledge
 Structure and eactivity: Prerequired Knowledge!!! The concepts presented in this summary are required for lecture and examination!!! 1. Important Principles in rganic Chemistry In general, structures which
Structure and eactivity: Prerequired Knowledge!!! The concepts presented in this summary are required for lecture and examination!!! 1. Important Principles in rganic Chemistry In general, structures which
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
Ketones and Aldehydes Reading Study Problems Key Concepts and Skills Lecture Topics: Structure of Ketones and Aldehydes Structure:
 Ketones and Aldehydes Reading: Wade chapter 18, sections 18-1- 18-21 Study Problems: 18-43, 18-44,18-50, 18-51, 18-52, 18-59, 18-60, 18-62, 18-64, 18-72. Key Concepts and Skills: Interpret the IR, NMR,
Ketones and Aldehydes Reading: Wade chapter 18, sections 18-1- 18-21 Study Problems: 18-43, 18-44,18-50, 18-51, 18-52, 18-59, 18-60, 18-62, 18-64, 18-72. Key Concepts and Skills: Interpret the IR, NMR,
Answers To Chapter 7 Problems.
 Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Chapter 13: Alcohols and Phenols
 Chapter 13: Alcohols and Phenols [ Chapter 9 Sections: 9.10; Chapter 13 Sections: 13.1-13.3, 13.9-13.10] 1. Nomenclature of Alcohols simple alcohols C3 C3C2 Eddie Sachs 1927-1964 larger alcohols find the
Chapter 13: Alcohols and Phenols [ Chapter 9 Sections: 9.10; Chapter 13 Sections: 13.1-13.3, 13.9-13.10] 1. Nomenclature of Alcohols simple alcohols C3 C3C2 Eddie Sachs 1927-1964 larger alcohols find the
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Nucleophilic Addition Reactions of Carboxylic Acid Derivatives
 Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Chapter 15. Reactions of Aromatic Compounds. 1. Electrophilic Aromatic Substitution Reactions
 hapter 15 eactions of Aromatic ompounds 1. Electrophilic Aromatic Substitution eactions v verall reaction reated by Professor William Tam & Dr. Phillis hang opyright S 3 2 S 4 S 3 2. A General Mechanism
hapter 15 eactions of Aromatic ompounds 1. Electrophilic Aromatic Substitution eactions v verall reaction reated by Professor William Tam & Dr. Phillis hang opyright S 3 2 S 4 S 3 2. A General Mechanism
Chapter 13. Conjugated Unsaturated Systems. +,., - Allyl. What is a conjugated system? AllylicChlorination (High Temperature)
 What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
Topic 9. Aldehydes & Ketones
 Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Ch.16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 Ch.16 Chemistry of Benzene: Electrophilic Aromatic Substitution Electrophilic aromatic substitution: E + E + + Some electrophilic aromatic substitution: X N 2 S 3 R C R alogenation Nitration Sulfonation
Ch.16 Chemistry of Benzene: Electrophilic Aromatic Substitution Electrophilic aromatic substitution: E + E + + Some electrophilic aromatic substitution: X N 2 S 3 R C R alogenation Nitration Sulfonation
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
CH 3 CHCH 3 CH 3 CHCH 3 Isopropyl cation. Oxomium ion intermediate. intermediate (an electrophile)
 Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Lecture 28 Organic Chemistry 1
 CEM 232 rganic Chemistry I University of Illinois at ChicagoUIC Lecture 28 rganic Chemistry 1 Professor Duncan Wardrop April 22, 2010 1 Today s Lecture Topics Covered: 1. Aryl alides - Bonding, Physical
CEM 232 rganic Chemistry I University of Illinois at ChicagoUIC Lecture 28 rganic Chemistry 1 Professor Duncan Wardrop April 22, 2010 1 Today s Lecture Topics Covered: 1. Aryl alides - Bonding, Physical
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA
 CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
ORGANIC CHEMISTRY. Fifth Edition. Stanley H. Pine
 ORGANIC CHEMISTRY Fifth Edition Stanley H. Pine Professor of Chemistry California State University, Los Angeles McGraw-Hill, Inc. New York St. Louis San Francisco Auckland Bogota Caracas Lisbon London
ORGANIC CHEMISTRY Fifth Edition Stanley H. Pine Professor of Chemistry California State University, Los Angeles McGraw-Hill, Inc. New York St. Louis San Francisco Auckland Bogota Caracas Lisbon London
What is in Common for the Following Reactions, and How Do They Work?
 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
b.(12) Where is pyrrole protonated under strong acidic conditions? Why this site of protonation?
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank the following dienes by rate of Diels-Alder reaction. The diene which reacts the fastest with an alkene is 1, while the
1. Rank the following compounds in the trend requested. (15 points each) a. Rank the following dienes by rate of Diels-Alder reaction. The diene which reacts the fastest with an alkene is 1, while the
Chapter 19: Amines. Introduction
 Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
ROADMAP FOR REACTIONS Chapter 6
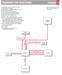 RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry
 Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Allyl radicals are especially stable due to resonance ( and double bond switch places):
 Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Chapter 5. Aromatic Compounds
 Chapter 5. Aromatic Compounds 5.1 Structure of Benzene: The Kekule Proposal Mid-1800s, benzene was known to have the molecular formula C 6 6. Benzene reacts with 2 in the presence of iron to give substitution
Chapter 5. Aromatic Compounds 5.1 Structure of Benzene: The Kekule Proposal Mid-1800s, benzene was known to have the molecular formula C 6 6. Benzene reacts with 2 in the presence of iron to give substitution
Module No and Title. PAPER No: 5 ; TITLE : Organic Chemistry-II MODULE No: 25 ; TITLE: S E 1 reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II. 5 Credit Hours. Prepared by: Richard A. Pierce. Revised by: Sean Birke October, 2013
 JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised by: Sean Birke October, 2013 Ms. Linda Abernathy, Math, Science & Business Division Chair
JEFFERSON COLLEGE COURSE SYLLABUS CHM201 ORGANIC CHEMISTRY II 5 Credit Hours Prepared by: Richard A. Pierce Revised by: Sean Birke October, 2013 Ms. Linda Abernathy, Math, Science & Business Division Chair
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
BENZENE AND AROMATIC COMPOUNDS
 BENZENE AND AROMATIC COMPOUNDS The discovery of benzene: 1825 - Michael Faraday, empirical formula of C 1834 - Eilhard Mitscherlich synthesized benzin from gum benzoin, empirical formula C Aromatic The
BENZENE AND AROMATIC COMPOUNDS The discovery of benzene: 1825 - Michael Faraday, empirical formula of C 1834 - Eilhard Mitscherlich synthesized benzin from gum benzoin, empirical formula C Aromatic The
Organic Chemistry Curriculum Content Outline
 Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
