What is in Common for the Following Reactions, and How Do They Work?
|
|
|
- Jessie Bond
- 5 years ago
- Views:
Transcription
1 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe Me C 3 3 NaMe 4 2 Na 5 Me C 3 Me NaMe Me C C 3 optically active Na C 3 3 C racemic mixture 4 Things in Common KEY:
2 TYPICAL MECANISM: Via ENLATE Anion 14 = (Aldehyde) = R (Ketone) = R (Ester) R (Base) Br Br C 2 3 -I 2 Me C 3 Enolate Ion 2 Me (original stereo forgotten) Under base conditions, a carbonyl compound with an α-hydrogen can be deprotonated to give a resonance-stablized, delocalized enolate anion, which is nucleophilic at the α-carbon. Normal C- bonds are very non-acidic. But C- bonds α to a carbonyl are much more acidic because the resulting anion is resonance stabilized and is shared by the oxygen. K a = K a = Stabilized Unstabilized The α-carbon has two other attachments in addition to the carbonyl and the shown in this page. The other attachments will remain attached as spectators, and need to be accounted for in drawing products. α-ydrogens are only slightly less acidic than is water or alcohol hydrogens
3 15 Acidity Table Class Structure Ka Acid Strength Anion Base Strength Strong Acids -Cl 10 2 Cl Carboxylic Acid R 10-5 R enol ,3-Dicarbonyl Me Me Water Alcohol R R Ketones and Aldehydes Ester a Me Me Amine (N-) (ipr) 2 N (ipr) 2 N Li LDA Alkane (C-) RC RC 2 -A B A B- Relative stability of anions dictates equilibrium Notes to remember 1. Carbonyls acidify α- s (anion stabilized) 2. 1,3-Dicarbonyls are much more acidic than monocarbonyls (anion is more stabilized) 3. Ketones are more acidic than esters 4. A lower anion on the chart can favorably deprotonate any acid that s higher on chart. Because any acid-base equilibrium will always favor the more stable anion. 5. LDA is strong enough to completely deprotonate ketones, esters, or 1,3-dicarbonyls 6. Na, NaR can completely deprotonate a 1,3-dicarbonyl (but not ketones or esters) 7. Na, NaR do not completely deprotonate ketones or esters, but do provide a usable equilibrium supply of the enolate that can procede to product in some reactions.
4 1. Rank the acidity of the hydrogens at the labeled positions, 1 being most acidic. Draw the three anions that would result from deprotionation at the three spots, and any pertinent resonance structures. a b c For the following compounds, record to what degree they would be deprotonated by NaC 3 or LDA [LiN(iPr) 2 ] respectively. The basic choices are totally (>98%), zero (no enolate whatsoever) or slightly (definitely some equilibrium amount, but <10%). LDA: NaMe: A B C D 3. For the following compounds, rank them according to which would have the greatest amount of enol isomer present at equilibrium, 1 being most enol, 4 being least. A B C D 4. Draw products for the following reactions 2 Br 2, 2Na 2 1 Cl 2, I 2, 3 Na, 2 2.
5 5. Keto-Enol Mechanisms a. Draw the mechanism for conversion of the keto form to the enol form 17 b. Draw the mechanism for conversion of the enol form to the ketone 6. Draw the mechanism for the following 2 Br 2, Na 7. Try to draw the mechanism for the following. NaEt, Et
6 Draw products for the following alkylation reactions, often involving ester hydrolyses and thermal decarboxylations LDA 2. Me-I 1. LDA Br 1. NaEt 10. Et Et 2. Br 1. NaEt 2. Br 11. Et Et 3. NaEt 4. BrC 2 C=C 2 1. NaEt 2. Br 12. Et Et 3., 2, heat 1. NaEt 2. Br 13. Et 3., 2, heat 1. LDA Et Br
7 15. Which of A, B, C, or D is the correct product for the following reaction? 1. NaC 3 2. C 3 I C 3 C 3 A B C C 3 3 C D 19 Some Synthetic Strategy Tips Alkylation resulting eventually in an acid: from 1,3-diester, via NaR, then subsequent ester hydrolysis/decarboxylation Alkylation resulting eventually in a mono-ester: from ester using LDA Alkylation resulting eventually in a mono-ketone, where unambiguous deprotonation was possible: from ketone using LDA Alkylation resulting in a mono-ketone, where unambiguous LDA deprotonation would not have been possible: from keto-ester using NaR, then subsequent ester hydrolysis/decarboxylation Provide reagents for the following: 16. Et Et Et Et Which of the following would undergo decarboxylation? And which would go fastest? A B C D 19. Draw the mechanism for the following reaction. 1. LDA C 3 2. C 3 I
8 Aldol Examples: Aldehydes/Ketones as Electrophiles 20 Na, 2 Na, 2 1. cold warmup 2. Na, 2 cold Na, 2 warmup With aldehydes, you can usually stop at the β-hydroxy carbonyl stage or proceed on to the α,β-unsaturated carbonyl, depending on time and temperature. NaEt, Et NaEt, Et 3. eat With ketones as electrophiles, the aldol reaction to give the β-hydroxy carbonyl is normally reversible with an unfavorable equilibrium. owever, while it is not possible to isolate high yields of the β-hydroxy ketone, further dehydration to give the enone is irreversible and can give good yields of the enone. 4. NaMe Me 0ºC NaMe Me warmup more time With two different carbonyl compounds, one must function selectively as the enolate precursor, and the other as the electrophile. Since aldehydes are much more electrophilic, when mixed with a ketone the aldehyde will always be the electrophile If there are more than one site where an enolate might form, the most acidic site that would give a stabilized anion will form preferentially 5. Et NaEt Et 0ºC Et NaEt Et warmup more time Et Comments Basic ne carbonyl functions as the enolate nucleophile, a second carbonyl as the neutral electrophile. The enolate precursor and the electrophile carbonyl may be the same (examples 1-3) or different (examples 4 and 5) Loss of an α-, replaced by an α,β C-C bond.
9 21 All of the following molecules can be made by an aldol-type reaction or an aldol-type condensation (aldol followed by loss of 2 ). Draw the carbonyl compound or compounds from which each is derived. example: Strategy: Identify the carbonyl in the product, and mark off which are the α and β carbons. The key bond connection will have been between the α and β carbons. β was originally a carbonyl (the electrophile carbonyl) α originally had s (it was the enolate carbanion) Note: any attachments on the α and β carbons are spectators. If they are there at the end, they must have been attached at the beinning Draw the mechanism for the following reaction. NaMe Me 0ºC
10 Provide products for the following aldol reactions. 22 Na, 2 Na, cold heat NaMe, Me 26. heat 27. NaEt Et 0ºC NaEt Et heat 28. NaEt Et 0ºC NaEt Et heat 29. Na 2 cold Na 2 hot NaC 3 NaC C 3 cold C 3 hot 31. Draw the mechanism for phase one and then phase two of the reaction in problem 28.
11 Provide products for the following Claisen reactions. 23 NaC Me C 3 NaEt 33. Et Et 1. NaMe, Me Me 2. NaMe 3. BrC 2 C=C NaMe, Me Me 2. NaMe 3. BrC 2 C=C 2 4., 2, heat 36. Me 1. NaC 3, C 3 Me 2., 2, heat Me Me NaMe Me 38. Me NaMe Me
12 Draw the product, reagent, or starting material for the following Wittig reactions. 24 Combo 1: 39. Combo 2: 40. Br 1. 3 P 2. BuLi 3. Benzaldehyde 1. 2 Cr PBr 3 2. P 3 3. BuLi 4. acetone 42.
13 25 General Routes to Make Alkenes Wittig Reactions. o Very general o Useful for making more elaborate organics, because two subcomponents can be coupled to make a larger product. o Technically longer and more difficult than an aldol condensation, so should not be used to make enones when an aldol condensation could be used instead. Aldol Condensations. o Great for making enones (α,β-unsaturated carbonyls). But limited to making enones. o If you see an enone target, make via aldol condensation. o Useful for making more elaborate organics, because two subcomponents can be coupled to make a larger product. Elimination reactions (from either halides or alcohols). o Not useful for building up carbon chain lengths. Simply involves transforming one functional group into another. 43. For the following alkenes, which method should you use, and what would be the immediate precursors that would be suitable? 44. Synthesis design. Design syntheses of the following products, starting from alcohols of 4 carbons or less. Some key reminder reactions: PCC for oxidizing 1º alcohols to aldehydes 2 Cr 4 for oxidizing 2º alcohols to ketones PBr 3 for converting 1º or 2º alcohols to bromides needed for making Wittig reagents
14 General: Enones as Electrophiles. Nucleophiles that attack enones must chooe between: Carbonyl addition β-addition 26 Carbonyl Addition Alkoxide Formation versus "-Addition Enolate Formation Carbonyl addition normally dominates with: RMgBr RLi NaB 4 LiAl 4 LiCCR β- addition normally dominates with: enolates of dicarbonyls sometimes enolates of monocarbonyls (but not always) Cuprates (R 2 CuLi) Prep: 2RBr 1. 4 Li 2. 1 CuI R 2 CuLi Draw the Products for the following Michael reactions 45. Et NaEt Et 46. Et Et NaEt Et 47. NaEt Et heat, long time -draw in the initial product, second product, and third product
15 Draw the mechanism for the following reaction. (Claisen reaction). NaC 3 Me C 3 Me 49. Draw the mechanism for the following reaction (Michael reaction). Et Et NaEt Et Et Et 50. Draw the mechanism for the following reaction. (Robinson cyclization, involving sequential Michael reaction, aldol reaction, β hydroxyketone dehydration.) NaEt Et heat, long time C via and B A
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
CHAPTER 24 HW: CARBONYL CONDENSATIONS
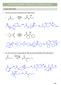 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 23 HW: ENOLS + ENOLATES
 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
What is in Common for the Following Reactions, and How Do They Work?
 Wht is in Common for the Following Rections, nd ow Do They Work? You should eventully be ble to drw the mechnism for these (nd other) rections 13 Key Intermedite 1 Br-Br N Br 2 C 3 -I Me NMe Me Me C 3
Wht is in Common for the Following Rections, nd ow Do They Work? You should eventully be ble to drw the mechnism for these (nd other) rections 13 Key Intermedite 1 Br-Br N Br 2 C 3 -I Me NMe Me Me C 3
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
ORGANIC - CLUTCH CH CONDENSATION CHEMISTRY.
 !! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
!! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Another Equilibrium: Reaction At The α-position
 Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Chapter 23. Alpha Substitution of Carbonyl Compounds.
 1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Conjugate Addition Reactions 2:02 PM
 1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Summary of π Bond Chemistry
 Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Answers to HT2, Chemistry A
 Answers to T2, hemistry 302-302A - 2004 1. There are many ways to do these problems - here is just one. For all three parts we can use diphenyl ketone (benzophenone) so let s make that first: 1. 2 Eq.
Answers to T2, hemistry 302-302A - 2004 1. There are many ways to do these problems - here is just one. For all three parts we can use diphenyl ketone (benzophenone) so let s make that first: 1. 2 Eq.
Chapter 21 Ester Enolates
 hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Answers To Chapter 7 Problems.
 Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
CuI CuI eage lic R tal ome rgan gbr ommon
 Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Expt 9: The Aldol Condensation
 Expt 9: The Aldol Condensation INTRDUCTIN Reactions that form carbon-carbon bonds are particularly important in organic chemistry as they allow the synthesis of more complex structures from simpler molecules.
Expt 9: The Aldol Condensation INTRDUCTIN Reactions that form carbon-carbon bonds are particularly important in organic chemistry as they allow the synthesis of more complex structures from simpler molecules.
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
Carbonyl Chemistry. aldehydes ketones. carboxylic acid and derivatives. Wednesday, April 29, 2009
 Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Synthesis of Ketones and Aldehydes
 Chem 342 Jasperse Ch. 17 Aldehydes and Ketones 1 Synthesis of Ketones and Aldehydes 1 PCC 15.9 2 2 Cr 4 15.9 3 1. B 3 TF 2. Na, 2 2 PCC 6.11 4 2, + 2 Cr 4 6.9 5 1. 3 2. Me 2 S + 6.20 1. MgBr 14.6 6 2.
Chem 342 Jasperse Ch. 17 Aldehydes and Ketones 1 Synthesis of Ketones and Aldehydes 1 PCC 15.9 2 2 Cr 4 15.9 3 1. B 3 TF 2. Na, 2 2 PCC 6.11 4 2, + 2 Cr 4 6.9 5 1. 3 2. Me 2 S + 6.20 1. MgBr 14.6 6 2.
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: hemistry 320N Dr. Brent Iverson 3rd Midterm April 20, 2017 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 320N Dr. Brent Iverson 3rd Midterm April 20, 2017 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
(1) Recall the different types of intermolecular interactions. (2) Look at the structure and determine the correct answer.
 MCAT rganic Chemistry - Problem Drill 11: Carboxylic Acids Question No. 1 of 10 Question 1. What kinds of interactions are holding together the carboxylic acid dimer shown? Question #01 3 C C 3 (A) Van
MCAT rganic Chemistry - Problem Drill 11: Carboxylic Acids Question No. 1 of 10 Question 1. What kinds of interactions are holding together the carboxylic acid dimer shown? Question #01 3 C C 3 (A) Van
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
c. Oxidizing agent shown here oxidizes 2º alcohols to ketones and 1º alcohols to carboxylic acids. 3º alcohols DO NOT REACT.
 Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Practice Synthetic Problems: CHEM 235 Page 2
 Practice Synthetic Problems: CM 235 Page 2 Syntheses based on diethyl malonate, ethyl acetoacetate, etc. Using diethyl malonate and any other necessary organic reagents, show a synthesis of: a) 2,2-dimethyl-1,3-propanediamine
Practice Synthetic Problems: CM 235 Page 2 Syntheses based on diethyl malonate, ethyl acetoacetate, etc. Using diethyl malonate and any other necessary organic reagents, show a synthesis of: a) 2,2-dimethyl-1,3-propanediamine
When H and OH add to the alkyne, an enol is formed, which rearranges to form a carbonyl (C=O) group:
 Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: hemistry 310N Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Chem Final Examination August 7, 2004
 Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
Chem 281 2004-2 Final Examination August 7, 2004 Name: Student Number: Note: You are allowed to use models for this exam. Notes, textbooks and calculators are strictly prohibited. Write your final answers
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
20.10 Conjugate Additions
 894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
Chapter 13: Alcohols and Phenols
 Chapter 13: Alcohols and Phenols [ Chapter 9 Sections: 9.10; Chapter 13 Sections: 13.1-13.3, 13.9-13.10] 1. Nomenclature of Alcohols simple alcohols C3 C3C2 Eddie Sachs 1927-1964 larger alcohols find the
Chapter 13: Alcohols and Phenols [ Chapter 9 Sections: 9.10; Chapter 13 Sections: 13.1-13.3, 13.9-13.10] 1. Nomenclature of Alcohols simple alcohols C3 C3C2 Eddie Sachs 1927-1964 larger alcohols find the
CHEMISTRY MIDTERM # 2 November 02, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
20.3 Alkylation of Enolate Anions
 864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
ALDEHYDES AND KETONES
 11 R R R ALDEYDES AND KETNES APTER SUMMARY 111 Structure of Aldehydes and Ketones Aldehydes and ketones both have a carbonyl group (carbonoxygen double bond); aldehydes have at least one carbon bonded
11 R R R ALDEYDES AND KETNES APTER SUMMARY 111 Structure of Aldehydes and Ketones Aldehydes and ketones both have a carbonyl group (carbonoxygen double bond); aldehydes have at least one carbon bonded
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
ANSWER GUIDE APRIL/MAY 2006 EXAMINATIONS CHEMISTRY 249H
 AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 2nd Midterm March 27, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long,
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
MITOCW watch?v=gboyppj9ok4
 MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
ENOLATES IN ORGANIC SYNTHESIS
 ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
Aldehydes & Ketones LEVEL I. Phenol (enol form) Phenol is aromatic, so equiulibrium is shifted to the right hand side. b) O
 Subjective Problems Aldehydes & Ketones LEVEL I 1. a) 2,4cyclohexadiene-1-one (keto form) Phenol (enol form) Phenol is aromatic, so equiulibrium is shifted to the right hand side. b) Base This ketone is
Subjective Problems Aldehydes & Ketones LEVEL I 1. a) 2,4cyclohexadiene-1-one (keto form) Phenol (enol form) Phenol is aromatic, so equiulibrium is shifted to the right hand side. b) Base This ketone is
Important Concepts. Problems. Chapter Problems. Cuprate additions followed by enolate alkylations. 15. Michael Addition (Section 18-11)
 Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
Advanced Organic Chemistry: Retrosynthesis
 123.312 Advanced rganic Chemistry: Retrosynthesis Tutorial Question 1. Propose a retrosynthetic analysis of the following two compounds. Your answer should include both the synthons, showing your thinking,
123.312 Advanced rganic Chemistry: Retrosynthesis Tutorial Question 1. Propose a retrosynthetic analysis of the following two compounds. Your answer should include both the synthons, showing your thinking,
Organic Synthesis and Carbon-Carbon Bond Forming Reactions. 1. To introduce basic concepts of organic synthesis:
 rganic Synthesis and Carbon-Carbon Bond Forming eactions 1. To introduce basic concepts of organic synthesis: etrosynthesis thinking backwards from relatively complex molecules to simpler ones the disconnection
rganic Synthesis and Carbon-Carbon Bond Forming eactions 1. To introduce basic concepts of organic synthesis: etrosynthesis thinking backwards from relatively complex molecules to simpler ones the disconnection
Allyl radicals are especially stable due to resonance ( and double bond switch places):
 Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Chemistry 3720, Spring 2004 Exam 3 Name:
 Chemistry 3720, Spring 2004 Exam 3 Name: This exam is worth 100 points out of a total of 600 points for Chemistry 3720/3720L. You have 50 minutes to complete the exam and you may use the spectroscopy data
Chemistry 3720, Spring 2004 Exam 3 Name: This exam is worth 100 points out of a total of 600 points for Chemistry 3720/3720L. You have 50 minutes to complete the exam and you may use the spectroscopy data
Chem 312 SS05 Page 1 of 6 Kantorowski. 17 August 2005 Name:
 Chem 32 SS05 Page of 6 Kantorowski Exam III Key 7 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
Chem 32 SS05 Page of 6 Kantorowski Exam III Key 7 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
18: Reactions of Enolate Ions and Enols
 18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
Chapter 17 Aldehydes and Ketones
 hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
O Enolate. = Electrophile/ Lewis acid. = Electrophile/ Lewis acid O B. Enolate. = Electrophile/ Lewis acid. Enolate. O O Enolate
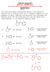 CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
Answers to Hour Examination #3, Chemistry 302X, 2006
 Answers to our Examination #, Chemistry 02X, 2006 1. (a). That SN2 shown just can t happen with the required inversion. The back of that methyl group is not reachable by the putative nucleophile, the pi
Answers to our Examination #, Chemistry 02X, 2006 1. (a). That SN2 shown just can t happen with the required inversion. The back of that methyl group is not reachable by the putative nucleophile, the pi
Loudon Chapter 19 Review: Aldehydes and Ketones CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
OChem2 Course Pack. Practice Problems by Chapter. Practice Exams
 Chem2 Course Pack Practice Problems by Chapter Practice Exams Chemistry 3720 Problem Sets Chemistry 3720 Ch. 13-19 Synthesis Problems These problems are typical of those that will be on the upcoming exams
Chem2 Course Pack Practice Problems by Chapter Practice Exams Chemistry 3720 Problem Sets Chemistry 3720 Ch. 13-19 Synthesis Problems These problems are typical of those that will be on the upcoming exams
Lecture 24 Two Germans and an Englishman
 Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Oxidation of alcohols Chromic Acid KMnO4 PCC Swern 1 ROH RCOOH RCOOH RCHO RCHO 2 ROH Ketone Ketone Ketone Ketone 3 ROH NR NR NR NR
 Chapter 11 xidation in rganic terms xidation Reduction Neither Addition of, X2; loss of 2 Addition of 2; loss of or X2 Anytime you oxidize one carbon but reduce another (ex. Adding and to adjacent carbons)
Chapter 11 xidation in rganic terms xidation Reduction Neither Addition of, X2; loss of 2 Addition of 2; loss of or X2 Anytime you oxidize one carbon but reduce another (ex. Adding and to adjacent carbons)
