Another Equilibrium: Reaction At The α-position
|
|
|
- Tiffany Grant
- 5 years ago
- Views:
Transcription
1 Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3
2 alogenation At The α-position Br 2, 2 C 2 Br I 2, 2 C 2 I Cl 2, 2 C 2 Cl Arthur Lapworth, in 1904, discovered that the above reactions have exactly the same rates and are 1st order in acetone but zero order in halide!
3 alogenation At The α-position Br 2, 2 C 2 Br Arthur Lapworth, in 1904, discovered that the above reactions have exactly the same rates and are 1st order in acetone but zero order in halide! slow C 2 fast C 2 Br + Br Br Br Rate dietermining step Acid, which is produced in the reaction, is also a catalyst in the reaction. This process is therefore autocatalytic. This step is after the rate determining step and does not enter into the rate equation.
4 Carbonyls: Weak Acids At The α-position C 2 C 3 pk a = pk a = 44 pk a = 60+ C 2 C 3 localized anion Reminder alcohols have a pk a range!
5 Carbonyls: Weak Acids At The α-position β γ α D, D 2 D 3 C CD 3 chanism: C 2 C 2 C 2 C 2 an enolate D D D D repeat 6 times D 3 C CD 3
6 The aloform Reaction Excess Br 2, chanism: enolate repeat 2 times C 2 formation C 2 Br Br C 2 Br CBr 3 α-bromo ketone is now more acidic CBr 3 + CBr 3
7 The Aldol Reaction C 2 enol a β-hydroxy carbonyl
8 Mixed Aldol Reactions: Non-Enolizable Aldehydes Acidic: 3 + C 2 a β-hydroxy carbonyl Basic: 2 C 2 2 a β-hydroxy carbonyl
9 Mixed Aldol Reactions: Enolizable Aldehydes C 2 C 2 Mixture of Aldol Addition Products
10 Aldol Reactions With Unsymmetrical Ketones + C 2 + Less stable, less hindered More stable, more hindered Mixture of Aldol Addition Products
11 The Aldol Condensation 3 + E2 Acidic: R R a β-hydroxy carbonyl an α,β-unsaturated carbonyl 2 E2 Basic: R R an α,β-unsaturated carbonyl
12 ow can we make this compound from a linear precursor?
13 ow can we make this compound from a linear precursor? comes from an aldol condensation comes from an aldol addition Linear Precursor (what is this compound called?) + "irreversible" step 2 2
14 Irreversible Enolate Formation: A "Better" Base LDA pk a = only enolate formed N BuLi Li N diisopropyl amine pk a = 35 Lithium Diisopropyl Amide (LDA) LDA is very hindered it will not add to carbonyl groups as a nucleophile Enolate formation is kinetically controlled and irreversible.
15 Reactions of Enolates LDA Li I C 2 C 2 D 2 Br 2 R C 2 D C 2 Br R
16 Enol and Enolate Formation: Stereoelectronic Restrictions An anion can only be resonance stabilized if the orbitals are aligned: base σ C π * C C LP π * C base All 4 α-hydrogens are equivalent (pk a = 19) C LP π * C pk a > 60, no overlap between C LP and π * C
17 Enol Equilibria of Carbonyl Compounds 2 C 2 K eq = 6 X 10-9 the keto form the enol form 2 K eq = 4.0 the keto form the enol form Does this compound exist? Why or why not?
18 Enol and Enolate Formation: Stereoelectronic Restrictions Et base What enolate is formed?
19 Enol and Enolate Formation: Stereoelectronic Restrictions Et base Et no overlap with π * C ring ring b t-bu Et good overlap with π * C a a ring t-bu group "anchors" this chair conformation ring b Et
20 Aldol-Type Reactions with Conjugated Acceptors: The Michael Reaction MgCl 2 CuLi?
21 Aldol-Type Reactions with Conjugated Acceptors: The Michael Reaction The 1,2-addition product Thermodynamically Favored bserved Product The 1,4-addition product
22 Tandem Aldol and Michael Reactions: The Robinson Annulation 3 +
23 Basic Disproportionation of Non-Enolizable Aldehydes: The Cannizaro Reaction K 2 Aldol reaction products K % 50%
24 The Aldol Reaction X base X M R X * * R Forms a Carbon-Carbon bond, generates a β-hydroxy carbonyl compound Creates 2 stereocenters
25 Retro-biosynthesis Et Erythromycin A The Aldol Reaction in Biology N 2 Et Et Erythromycin Seco Acid Polypropionate Biosynthesis: The Elementary Steps R Acylation Reduction R SR SR C 2 R SR Acylation C 2 R Reduction SR R SR Erythromycin Seco Acid 7 Propionate Subunits
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
What is in Common for the Following Reactions, and How Do They Work?
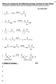 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
Chapter 23. Alpha Substitution of Carbonyl Compounds.
 1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Summary of π Bond Chemistry
 Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
ORGANIC - CLUTCH CH CONDENSATION CHEMISTRY.
 !! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
!! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Chemistry of Carbonyl Compounds
 Chemistry of Carbonyl Compounds ucleophilic addition (1,2-add) / substitution Conjugate addition (1,4 add) obinson annulation (McM 23.12) Alkylation of enolate anions namines as enolate equivs. (McM 23.11)
Chemistry of Carbonyl Compounds ucleophilic addition (1,2-add) / substitution Conjugate addition (1,4 add) obinson annulation (McM 23.12) Alkylation of enolate anions namines as enolate equivs. (McM 23.11)
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
ENOLATES IN ORGANIC SYNTHESIS
 ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
Conjugate Addition Reactions 2:02 PM
 1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
Tautomerism and Keto Enol Equilibrium
 Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
Lecture 24 Two Germans and an Englishman
 Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Chapter 22 The Chemistry of Enolate Ions, Enols, and
 Organic Chemistry, 5th ed. Marc Loudon Chapter 22 The Chemistry of Enolate Ions, Enols, and α,β Unsaturated Carbonyl Compounds Eric J. Kantorowski California Polytechnic State University San Luis Obispo,
Organic Chemistry, 5th ed. Marc Loudon Chapter 22 The Chemistry of Enolate Ions, Enols, and α,β Unsaturated Carbonyl Compounds Eric J. Kantorowski California Polytechnic State University San Luis Obispo,
ANSWER GUIDE APRIL/MAY 2006 EXAMINATIONS CHEMISTRY 249H
 AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Chapter 21 Ester Enolates
 hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
CHAPTER 24 HW: CARBONYL CONDENSATIONS
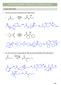 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
Chapter 11 Reaction of Alcohols
 Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
Carbonyl Chemistry IV: Enolate Alkylations and Aldols. Aldol Madness O O O N M + substrate. aldehyde. (Z)-enolate H
 Carbonyl Chemistry IV: nolate Alkylations and Aldols Paul Bracher Chem 30 Section 9 Section Agenda 1) o office hours Thursday 2) The Great Joe Young is covering section next onday 3) andout: Carbonyl Chemistry
Carbonyl Chemistry IV: nolate Alkylations and Aldols Paul Bracher Chem 30 Section 9 Section Agenda 1) o office hours Thursday 2) The Great Joe Young is covering section next onday 3) andout: Carbonyl Chemistry
O Enolate. = Electrophile/ Lewis acid. = Electrophile/ Lewis acid O B. Enolate. = Electrophile/ Lewis acid. Enolate. O O Enolate
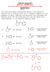 CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
18: Reactions of Enolate Ions and Enols
 18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction.
 Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction. References: Brown & Foote, Chapters 16, 19, 23 INTRODUCTION: This experiment continues the saga of carbon-carbon
Experiment 3. Condensation Reactions of Ketones and Aldehydes: The Aldol Condensation Reaction. References: Brown & Foote, Chapters 16, 19, 23 INTRODUCTION: This experiment continues the saga of carbon-carbon
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry
 Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
State University of New York at Stony Brook Department of Chemistry. CHE 322, Organic Chemistry II Exam II March 17, 2004 Form 1
 State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
OH OH OH CH 2 CH 2 C(CH 3 ) 2 (a) CH 3 CHCH 2 CHCH(CH 3 ) 2. (b)
 hem 226 Problem Set #8 Fundamentals of rganic hemistry, 4 th edition, John McMurry. hapter 8 1. Give IUPA names for the following alcohols. 2 2 ( 3 ) 2 3 2 ( 3 ) 2 Longest chain = 6 carbons:...hexanediol.
hem 226 Problem Set #8 Fundamentals of rganic hemistry, 4 th edition, John McMurry. hapter 8 1. Give IUPA names for the following alcohols. 2 2 ( 3 ) 2 3 2 ( 3 ) 2 Longest chain = 6 carbons:...hexanediol.
Lecture 19. Carboxylic Acids O C OH O R C O - + H + O - Chemistry 328N
 Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
Learning Guide for Chapter 7 - Organic Reactions I
 Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
Aldehydes and Ketones
 9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
CHAPTER 23 HW: ENOLS + ENOLATES
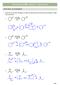 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Chapter 17 Aldehydes and Ketones
 hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
CHEM 330. Final Exam December 5, 2014 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
Reversible Additions to carbonyls: Weak Nucleophiles Relative Reactivity of carbonyls: Hydration of Ketones and Aldehydes
 Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Carbonyl Chemistry. aldehydes ketones. carboxylic acid and derivatives. Wednesday, April 29, 2009
 Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
MITOCW watch?v=gboyppj9ok4
 MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
Conjugated Systems & Pericyclic Reactions
 onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
Answers to HT2, Chemistry A
 Answers to T2, hemistry 302-302A - 2004 1. There are many ways to do these problems - here is just one. For all three parts we can use diphenyl ketone (benzophenone) so let s make that first: 1. 2 Eq.
Answers to T2, hemistry 302-302A - 2004 1. There are many ways to do these problems - here is just one. For all three parts we can use diphenyl ketone (benzophenone) so let s make that first: 1. 2 Eq.
Total synthesis of Spongistatin
 Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of H X 2. Addition of H OH and addition of Y X 3. Addition to allene and
 Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Enolates, Enols, and Enamines Part 3
 Enolates, Enols, and Enamines Part 3 Guanine Tautomerize 2 2 Thymine C 3 Tautomerize C 3 Thursday March 21, 8:00-11:00 AM Final Exam Topics Part A: Carbonyl Fundamentals Carbonyl Survey Enolates, Enols,
Enolates, Enols, and Enamines Part 3 Guanine Tautomerize 2 2 Thymine C 3 Tautomerize C 3 Thursday March 21, 8:00-11:00 AM Final Exam Topics Part A: Carbonyl Fundamentals Carbonyl Survey Enolates, Enols,
Carbonyl Chemistry IV + C O C. Lecture 10. Chemistry /30/02
 arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Nucleophilic Addition Reactions of Carboxylic Acid Derivatives
 Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
(c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
 KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
به نام خدا روشهای سنتز مواد آلی
 به نام خدا روشهای سنتز مواد آلی 1 References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Reactions and Synthesis (Part B), 5th ed., Springer, 2007. 2. Carey, F. A.; Sundberg, R. J. Advanced
به نام خدا روشهای سنتز مواد آلی 1 References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Reactions and Synthesis (Part B), 5th ed., Springer, 2007. 2. Carey, F. A.; Sundberg, R. J. Advanced
CHAPTER 16 CONJUGATE ADDITION
 HAPTER 16 NJUGATE ADDITIN onjugated Pi Bonds onjugated double bonds are separated by only one single bond. Isolated double bonds are separated by two or more single bonds. arbonyl ompounds with onjugated
HAPTER 16 NJUGATE ADDITIN onjugated Pi Bonds onjugated double bonds are separated by only one single bond. Isolated double bonds are separated by two or more single bonds. arbonyl ompounds with onjugated
The problem is that your product still has a-protons, and can keep on forming enolates to get more methyl groups added:
 Lecture 14 ovember 3, 2011 OK I want to continue briefly with the topic of proline catalysis that we discussed last time. In particular, the idea of using secondary amines to catalyze carbonyl chemistry
Lecture 14 ovember 3, 2011 OK I want to continue briefly with the topic of proline catalysis that we discussed last time. In particular, the idea of using secondary amines to catalyze carbonyl chemistry
O or R E + R. - Keto-Enol Tautomerization (enol form usually very minor for simple ketones)
 General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
Modern Organic Synthesis an Introduction
 Modern Organic Synthesis an Introduction G. S. Zweifel M. H. Nantz W.H. Freeman and Company Chapter 1 Synthetic Design 1 What is an ideal or viable synthesis, and how does one approach a synthetic project?
Modern Organic Synthesis an Introduction G. S. Zweifel M. H. Nantz W.H. Freeman and Company Chapter 1 Synthetic Design 1 What is an ideal or viable synthesis, and how does one approach a synthetic project?
8-3 This exercise is worked out on page 293 as "Working with Concepts".
 Copyright 2009 James K Whitesell 8-1 This exercise combines your knowledge of basic nomenclature rules and concepts of stereochemistry introduced in Chapters 4 and 5. 8-2 Keep in mind that faculty at UCSD
Copyright 2009 James K Whitesell 8-1 This exercise combines your knowledge of basic nomenclature rules and concepts of stereochemistry introduced in Chapters 4 and 5. 8-2 Keep in mind that faculty at UCSD
Suggested solutions for Chapter 31
 s for Chapter 31 31 PRBLEM 1 Predict the most favourable conformation for these insect pheromones. Practice drawing the conformations of cyclic acetals. There are many good ways to draw these conformations
s for Chapter 31 31 PRBLEM 1 Predict the most favourable conformation for these insect pheromones. Practice drawing the conformations of cyclic acetals. There are many good ways to draw these conformations
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
THE CHEMISTRY OF THE CARBONYL GROUP
 TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
Solution problem 22: Non-Benzoid Aromatic Sytems
 Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
CuI CuI eage lic R tal ome rgan gbr ommon
 Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Introduction to Alkenes and Alkynes
 Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Important Concepts. Problems. Chapter Problems. Cuprate additions followed by enolate alkylations. 15. Michael Addition (Section 18-11)
 Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Aldehydes & Ketones LEVEL I. Phenol (enol form) Phenol is aromatic, so equiulibrium is shifted to the right hand side. b) O
 Subjective Problems Aldehydes & Ketones LEVEL I 1. a) 2,4cyclohexadiene-1-one (keto form) Phenol (enol form) Phenol is aromatic, so equiulibrium is shifted to the right hand side. b) Base This ketone is
Subjective Problems Aldehydes & Ketones LEVEL I 1. a) 2,4cyclohexadiene-1-one (keto form) Phenol (enol form) Phenol is aromatic, so equiulibrium is shifted to the right hand side. b) Base This ketone is
20.10 Conjugate Additions
 894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
September [KV 804] Sub. Code: 3804
![September [KV 804] Sub. Code: 3804 September [KV 804] Sub. Code: 3804](/thumbs/89/97855699.jpg) September 2009 [KV 804] Sub. Code: 3804 (Regulations 2008-2009) (Candidates admitted from 2008-2009 onwards) Paper IV PHARMACEUTICAL ORGANIC CHEMISTRY Time : Three hours Maximum : 70 marks Answer All questions
September 2009 [KV 804] Sub. Code: 3804 (Regulations 2008-2009) (Candidates admitted from 2008-2009 onwards) Paper IV PHARMACEUTICAL ORGANIC CHEMISTRY Time : Three hours Maximum : 70 marks Answer All questions
The problem is that your product still has a-protons, and can keep on forming enolates to get more methyl groups added:
 Lecture 13 Notes November 7, 2012 We are going to start with some examples of proline-catalyzed reactions. In each case, you can draw a mechanism that would involve proline as a chiral catalyst, and one
Lecture 13 Notes November 7, 2012 We are going to start with some examples of proline-catalyzed reactions. In each case, you can draw a mechanism that would involve proline as a chiral catalyst, and one
CH 3 CHCH 3 CH 3 CHCH 3 Isopropyl cation. Oxomium ion intermediate. intermediate (an electrophile)
 Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Aldehydes and Ketones
 Reading Chapter 12: 12.1-12.3, 12.6-12.9 Practice problems: in text problems and 19, 21-24, 28 Carbonyl Compounds II: Reactions of More Reactions of Carboxylic Acid Derivatives The Structure of 1 The Structure
Reading Chapter 12: 12.1-12.3, 12.6-12.9 Practice problems: in text problems and 19, 21-24, 28 Carbonyl Compounds II: Reactions of More Reactions of Carboxylic Acid Derivatives The Structure of 1 The Structure
