Important Concepts. Problems. Chapter Problems. Cuprate additions followed by enolate alkylations. 15. Michael Addition (Section 18-11)
|
|
|
- Marlene Montgomery
- 6 years ago
- Views:
Transcription
1 Problems hapter uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important oncepts A K 2 1. ydrogens next to the carbonyl group (A-hydrogens) are acidic because of the electron-withdrawing nature of the functional group and because the resulting enolate ion is resonance stabilized. 2. Electrophilic attack on enolates can occur at both the a-carbon and the oxygen. aloalkanes usually prefer the a-carbon. Protonation of the oxygen leads to enols. 3. Enamines are neutral analogs of enolates. Resonance donation of the nitrogen lone pair imparts nucleophilic character on the remote double bond carbon, which can be alkylated to give iminium cations that hydrolyze to aldehydes and ketones on aqueous work-up. 4. Aldehydes and ketones are in equilibrium with their tautomeric enol forms; the enol keto conversion is catalyzed by acid or base. This equilibrium allows for facile a-deuteration and stereochemical equilibration. 5. A-alogenation of carbonyl compounds may be acid or base catalyzed. With acid, the enol is halogenated by attack at the double bond; subsequent renewed enolization is slowed down by the halogen substituent. With base, the enolate is attacked at carbon, and subsequent enolate formation is accelerated by the halogens introduced. 6. Enolates are nucleophilic and reversibly attack the carbonyl carbon of an aldehyde or a ketone in the aldol condensation. They also attack the b-carbon of an a,b-unsaturated carbonyl compound in the Michael addition. 7. a,b-unsaturated aldehydes and ketones show the normal chemistry of each individual double bond, but the conjugated system may react as a whole, as revealed by the ability of these compounds to undergo acid- and base-mediated 1,4-additions. uprates add in 1,4-manner, whereas alkyllithiums normally attack the carbonyl function. Problems 32. Underline the a-carbons and circle the a-hydrogens in each of the following structures ( 3 ) 2 3 [ 3 [ 3 3 [ (e) 3 3 (f )
2 862 hapter 18 Enols, Enolates, and the Aldol ondensation (g) ( 3 ) 3 (h) ( 3 ) Write the structures of every enol and enolate ion that can arise from each of the carbonyl compounds illustrated in Problem What product(s) would form if each carbonyl compound in Problem 32 were treated with alkaline D 2 ; 1 equivalent of r 2 in acetic acid; excess l 2 in aqueous base? 35. Describe the experimental conditions that would be best suited for the efficient synthesis of each of the following compounds from the corresponding nonhalogenated ketone. r A l l l l l 36. Propose a mechanism for the following reaction. (int: Take note of all of the products that are formed and base your answer on the mechanism for acid-catalyzed bromination of acetone shown in Section 18-3.) atalytic l, l 4 S 2 l 2 S 2 l l 37. Give the product(s) that would be expected on reaction of 3-pentanone with 1 equivalent of LDA, followed by addition of 1 equivalent of 3 2 r ( 3 ) 2 l ( 3 ) 2 2 S 3 ( 3 ) 3 l 38. Give the product(s) of the following reaction sequences. 3 N 1., 2. ( 3 ) 2 P 2 l 3., 2 N 1., , 2 2 r N 2 N 39. The problem of double compared with single alkylation of ketones by iodomethane and base is mentioned in Section Write a detailed mechanism showing how some double alkylation occurs even when only one equivalent each of the iodide and base is used. Suggest a reason why the enamine alkylation procedure solves this problem. 40. Would the use of an enamine instead of an enolate improve the likelihood of successful alkylation of a ketone by a secondary haloalkane? 41. Formulate a mechanism for the acid-catalyzed hydrolysis of the pyrrolidine enamine of cyclohexanone (shown in the margin). 42. Write the structures of the aldol condensation products of pentanal; 3-methylbutanal; cyclopentanone. 43. Write the structures of the expected major products of crossed aldol condensation at elevated temperature between excess benzaldehyde and 1-phenylethanone (acetophenone see Section 17-1 for structure); acetone; 2,2-dimethylcyclopentanone. 44. Formulate a detailed mechanism for the reaction that you wrote in of Problem 43.
3 Problems hapter Give the likely products for each of the following aldol addition reactions. 2 2 Na, 2 ( 3 ) 2 Na, 2 Na, 2 3 Na, Rotundone (margin) is the natural product responsible for the peppery aroma in peppers, many herbs, and red wines (p. 846). What cyclic diketone will give rotundone upon intramolecular aldol condensation? 47. Write all possible products of the base-catalyzed crossed aldol reactions between each pair of reaction partners given below. (int: Multiple products are possible in every case; be sure to include thermodynamically unfavorable as well as favorable ones.) utanal and acetaldehyde 2,2-Dimethylpropanal and acetophenone enzaldehyde and 2-butanone 48. For each of the three crossed aldol reactions described in Problem 47, indicate which, if any, of the multiple possible products should predominate in the reaction mixture and explain why. 49. Aldol condensations may be catalyzed by acids. Suggest a role for 1 in the acid-catalyzed version. (int: onsider what kind of nucleophile might exist in acidic solution, where enolate ions are unlikely to be present.) 50. Reaction review. Without consulting the Reaction Road Maps on pp , suggest reagents to convert butanal into each of the following compounds. Rotundone l r r (e) (f) (g) 51. Reaction review II. Without consulting the Reaction Road Maps on pp , suggest reagents to convert acetophenone into each of the following compounds l (e) 6 5 (f) r 52. Reaction review III. Without consulting the Reaction Road Maps on pp , suggest reagents to convert 3-buten-2-one into each of the following compounds. 6 5
4 864 hapter 18 Enols, Enolates, and the Aldol ondensation 4-M Polysantol (e) (f) 53. A number of highly conjugated organic compounds have found use as sunscreens. ne of the more widely used is 4-methylbenzylidene camphor (4-M), whose structure is shown in the margin. This compound is effective in absorbing so-called UV- radiation (with wavelengths between 280 and 320 nm, responsible for most sunburns). Suggest a simple synthesis of this compound using a crossed aldol condensation. 54. The distillate from sandalwood is one of the oldest and most highly valued fragrances in perfumery. The natural oil is in short supply and, until recently, synthetic substitutes have been difficult to prepare. Polysantol (margin) is the most successful of these substitutes. Its synthesis in 1984 involved the aldol condensation shown below. K, 2, 3 This synthetic step, although usable, had a significant drawback that was responsible for its modest yield (60). Identify this problem in detail. A solution that avoids conventional aldol condensation was published in A bromoketone is prepared and, upon reaction with Mg metal, gives a magnesium enolate. This enolate then reacts with the aldehyde selectively to give a hydroxyketone that may be dehydrated to the desired product. Discuss how this approach solves the problem outlined in part. (g) (h) Mgr r 2, 3 2, l 4 Mg, ether r 55. Write the expected major product of reaction of each of the carbonyl compounds (i) (iii) with each of the reagents (h). (i) (ii) 3 P 2, Pd, 3 2 l 2, l 4 (e) 3 Li, ( 3 2 ) 2 (g) N 2 NN 2, D G (iii) 56. Give the expected product(s) of each of the following reactions. LiAl 4, ( 3 2 ) 2 KN, 1, 2 (f) ( ) 2 uli, TF (h) ( ) 2 uli, followed by treatment with 2 P 2 l in TF LDA, TF r, MPA 1. LDA, TF 2. r 2 3
5 Problems hapter ( 3 ) 2 uli, TF l 3 ( 2 ) 4 r LDA, TF 57. Write the products of each of the following reactions after aqueous work-up. LDA, TF P 6 5 ( 3 ) 2 P Na, 2 1. ( 2 P) 2 uli, TF 3 1. ( 3 ) 2 uli, TF 2. 2 P 3 2. ( 3 ) 2 P 3 (e) Write the results that you expect from base treatment of the products of reactions and. 58. Write the final products of the following reaction sequences. 2 P 3 Na 3, 3, P 3 K, 3, 1. LDA, TF 2. q 3 Write a detailed mechanism for reaction sequence. (int: Treat the 3-butyn-2-one reagent as a Michael acceptor in the first step.) 59. Propose syntheses of the following compounds by using Michael additions followed by aldol condensations (i.e., Robinson annulation). Each of the compounds shown has been instrumental in one or more total syntheses of steroidal hormones E Would you expect addition of l to the double bond of 3-buten-2-one (shown in the margin) to follow Markovnikov s rule? Explain your answer by a mechanistic argument. 3 P 2 3-uten-2-one
6 866 hapter 18 Enols, Enolates, and the Aldol ondensation 61. Using the following information, propose structures for each of these compounds. 5 10, NMR spectrum A, UV l max (e) 5 280(18) nm; 5 8, NMR spectrum, UV l max (e) 5 220(13,200), 310(40) nm; 6 12, NMR spectrum, UV l max (e) 5 189(8,000) nm; 6 12, NMR spectrum D, UV l max (e) 5 282(25) nm. (See the next page for spectrum D.) ( 3 ) 4 Si 1 1 ( 3 ) 4 Si A Mz 1 NMR spectrum ppm ( δ ) 300-Mz 1 NMR spectrum ppm ( δ ) Next, for each of the following reactions, name an appropriate reagent for the indicated interconversion. (The letters refer to the compounds giving rise to NMR spectra A through D.) (e) A y ; (f) y D; (g) y A Mz 1 NMR spectrum ppm ( δ)
7 Problems hapter ( 3 ) 4 Si D Mz 1 NMR spectrum ppm ( δ ) 62. Treatment of cyclopentane-1,3-dione with iodomethane in the presence of base leads mainly to a mixture of three products. Na, 3 I, A Give a mechanistic description of how these three products are formed. Reaction of product with a cuprate reagent results in loss of the methoxy group. For example, 1. ( ) 2 uli, TF 2., D Suggest a mechanism for this reaction, which is another synthetic route to enones substituted at the b-carbon. (int: See Exercise ) 63. A somewhat unusual synthesis of cortisone-related steroids includes the following two reactions.
8 868 hapter 18 Enols, Enolates, and the Aldol ondensation 3 ( 3 ) 3 ( 3 ) 3 A r ( 3 ) 3 K, benzene 3 ( 3 ) 3 ( 3 ) 3 3 I, ( 3 ) 3 K, benzene 3 ( 3 ) 3 3 ( 3 ) 3 Propose mechanisms for these two transformations. e careful in choosing the initial site of deprotonation in the starting enone. The alkenyl hydrogen, in particular, is not acidic enough to be the one initially removed by base in this reaction. Propose a sequence of reactions that will connect the carbons marked by arrows in the third structure shown to form another six-membered ring. 64. The following steroid synthesis contains modified versions of two key types of reactions presented in this chapter. Identify these reaction types and give detailed mechanisms for each of the transformations shown. 3 K ( 3 ) 3, TF K 2 3, 3 ) 2 3 ) Devise reasonable plans for carrying out the following syntheses. Ignore stereochemistry in your strategies. 3, starting from cyclohexanone N 3 3 3, starting from 2-cyclohexenone (int: Prepare in your first step.) 3 2
9 Problems hapter Write reagents (a, b, c, d, e) where they have been omitted from the following synthetic sequence. Each letter may correspond to one or more reaction steps. This sequence is the beginning of a synthesis of germanicol, a naturally occurring triterpene. The diol used in the step between and provides selective protection of the more reactive carbonyl group. [int: See Problem 63 when formulating.] 3 3, Li, liquid N 3 * (e) Team Problem Germanicol When 2-methylcyclopentanone is treated with the bulky base triphenylmethyllithium under the two sets of conditions shown, the two possible enolates are generated in differing ratios. Why is this so? Li Li 3 ( 6 5 ) 3 Li 3 3 onditions A: Ketone added to excess base onditions : Excess ketone added to base 6 94 To tackle this problem, you have to invoke the principles of kinetic versus thermodynamic control (review Sections 11-6, 14-6, and 18-2); that is, which enolate is formed faster and which one is more stable? Divide your team so that one group considers conditions A and the other conditions. Use curved arrows to show the flow of electrons leading to each enolate. Then assess whether your set of conditions is subject to enolate equilibration (thermodynamic control) or not (kinetic control). Reconvene to discuss these issues and draw a qualitative potential-energy diagram depicting the progress of deprotonation at the two a sites. *An electron-transfer process (compare alkyne reduction, Section 13-6) that is the equivalent of adding hydride, : 2, to the b-carbon. The product is the enolate of the saturated ketone.
10 870 hapter 18 Enols, Enolates, and the Aldol ondensation Preprofessional Problems 68. When 3-methyl-1,3-diphenyl-2-butanone is treated with excess D 2 in the presence of catalytic acid, some of its hydrogens are replaced by deuterium. ow many? ne; two; three; six; (e) eight. 69. ow would you best classify the following reaction? Wittig reaction; cyanohydrin formation; conjugate addition; aldol addition. E N 2 PPN N 2 PN The aqueous hydroxide-promoted reaction of the compound shown in the margin with ( 3 ) 3 yields exclusively one compound. Which one is it? 3 ( 3 ) 3 f P i 3 A 3 3 A 3 ( 3 ) 3 P 3 ( 3 ) 3 P The 1 NMR spectrum of 2,4-pentanedione indicates the presence of an enol tautomer of the dione. What is its most likely structure? 3 E E PP 3
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones
 9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
C h a p t e r T w e n t y - o n e : Enols, Enolates, and Aldol-like Condensations
 h a p t e r T w e n t y o n e : Enols, Enolates, and Aldollike ondensations Li LDA 1. R TF R 2. 3 R A directed aldol reaction, used in the synthesis of periplanone B, a cockroach attractant M 323: Summary
h a p t e r T w e n t y o n e : Enols, Enolates, and Aldollike ondensations Li LDA 1. R TF R 2. 3 R A directed aldol reaction, used in the synthesis of periplanone B, a cockroach attractant M 323: Summary
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Carbonyl Chemistry IV + C O C. Lecture 10. Chemistry /30/02
 arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
CHAPTER 23 HW: ENOLS + ENOLATES
 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
The problem is that your product still has a-protons, and can keep on forming enolates to get more methyl groups added:
 Lecture 13 Notes November 7, 2012 We are going to start with some examples of proline-catalyzed reactions. In each case, you can draw a mechanism that would involve proline as a chiral catalyst, and one
Lecture 13 Notes November 7, 2012 We are going to start with some examples of proline-catalyzed reactions. In each case, you can draw a mechanism that would involve proline as a chiral catalyst, and one
What is in Common for the Following Reactions, and How Do They Work?
 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
ALDEHYDES AND KETONES
 11 R R R ALDEYDES AND KETNES APTER SUMMARY 111 Structure of Aldehydes and Ketones Aldehydes and ketones both have a carbonyl group (carbonoxygen double bond); aldehydes have at least one carbon bonded
11 R R R ALDEYDES AND KETNES APTER SUMMARY 111 Structure of Aldehydes and Ketones Aldehydes and ketones both have a carbonyl group (carbonoxygen double bond); aldehydes have at least one carbon bonded
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Lecture 19. Carboxylic Acids O C OH O R C O - + H + O - Chemistry 328N
 Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
Conjugate Addition Reactions 2:02 PM
 1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Lecture 24 Two Germans and an Englishman
 Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
(b) (CH 3 ) 2 CCH 2 CH 3 D 2 O. (e) A. CH 3 CCl OSO 2 CH 3 C 6 H 5 H 3 C
 278 h a p t e r 7 Further Reactions of aloalkanes 3. arbocations are stabilized by hyperconjugation: Tertiary are the most stable, followed by secondary. Primary and methyl cations are too unstable to
278 h a p t e r 7 Further Reactions of aloalkanes 3. arbocations are stabilized by hyperconjugation: Tertiary are the most stable, followed by secondary. Primary and methyl cations are too unstable to
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
235 Organic II. Final Exam Review REACTIONS OF CONJUGATED DIENES 1,2 VS 1,4 ADDITION REACTIONS OF CONJUGATED DIENES
 b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
CHAPTER 24 HW: CARBONYL CONDENSATIONS
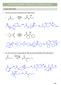 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
Tautomerism and Keto Enol Equilibrium
 Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
18.10 Conjugate Additions O O. O X BrCH 2 CH 2 CH 2 CCH 3 ± ± BrCH 2 CH 2 CH 2 CH 3. TsOH 1 O C O O W 3 CH 3 CCH 3 CH 3 CCH 2 CH 2 CH 2 CH 3
 80 NJUGATE ADDITINS 779 An attempt to form a Grignard reagent from 5-bromo- 2-pentanone is doomed to failure because the Grignard will react with the carbonyl group Therefore, the carbonyl group is first
80 NJUGATE ADDITINS 779 An attempt to form a Grignard reagent from 5-bromo- 2-pentanone is doomed to failure because the Grignard will react with the carbonyl group Therefore, the carbonyl group is first
Reversible Additions to carbonyls: Weak Nucleophiles Relative Reactivity of carbonyls: Hydration of Ketones and Aldehydes
 Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
CHAPTER 21 HW: ALDEHYDES + KETONES
 CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). Structure 6 5 4 1 C 2 ame 3,3-dimethyl-2-pentanone (or 1,1-dimethylpropyl methyl ketone) 5-hydroxy-4-methylhexanal
CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). Structure 6 5 4 1 C 2 ame 3,3-dimethyl-2-pentanone (or 1,1-dimethylpropyl methyl ketone) 5-hydroxy-4-methylhexanal
Basic Organic Chemistry
 Basic rganic hemistry ourse code: EM 12162 (Pre-requisites : EM 11122) hapter 06 hemistry of Aldehydes & Ketones Dr. Dinesh R. Pandithavidana ffice: B1 222/3 Phone: (+94)777-745-720 (Mobile) Email: dinesh@kln.ac.lk
Basic rganic hemistry ourse code: EM 12162 (Pre-requisites : EM 11122) hapter 06 hemistry of Aldehydes & Ketones Dr. Dinesh R. Pandithavidana ffice: B1 222/3 Phone: (+94)777-745-720 (Mobile) Email: dinesh@kln.ac.lk
Chapter 18 Ketones and Aldehydes. Carbonyl Compounds. Chapter 18: Aldehydes and Ketones Slide 18-2
 hapter 18 Ketones and Aldehydes arbonyl ompounds hapter 18: Aldehydes and Ketones Slide 18-2 1 arbonyl Structure arbon is sp 2 hybridized. = bond is shorter, stronger, and more polar than = bond in alkenes.
hapter 18 Ketones and Aldehydes arbonyl ompounds hapter 18: Aldehydes and Ketones Slide 18-2 1 arbonyl Structure arbon is sp 2 hybridized. = bond is shorter, stronger, and more polar than = bond in alkenes.
Organic Chemistry I (Chem340), Spring Final Exam
 rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
ENOLATES IN ORGANIC SYNTHESIS
 ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
The problem is that your product still has a-protons, and can keep on forming enolates to get more methyl groups added:
 Lecture 14 ovember 3, 2011 OK I want to continue briefly with the topic of proline catalysis that we discussed last time. In particular, the idea of using secondary amines to catalyze carbonyl chemistry
Lecture 14 ovember 3, 2011 OK I want to continue briefly with the topic of proline catalysis that we discussed last time. In particular, the idea of using secondary amines to catalyze carbonyl chemistry
Carbonyl Condensation Reactions
 24 arbonyl ondensation eactions 24.1 The aldol reaction 24.2 rossed aldol reactions 24.3 Directed aldol reactions 24.4 Intramolecular aldol reactions 24.5 The laisen reaction 24.6 The crossed laisen and
24 arbonyl ondensation eactions 24.1 The aldol reaction 24.2 rossed aldol reactions 24.3 Directed aldol reactions 24.4 Intramolecular aldol reactions 24.5 The laisen reaction 24.6 The crossed laisen and
ORGANIC - CLUTCH CH CONDENSATION CHEMISTRY.
 !! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
!! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Aldehydes and Ketones 2. Based on Organic Chemistry, J. G. Smith 3rde.
 Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
Aldehydes and Ketones Reactions. Dr. Sapna Gupta
 Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Chapter 7: Alkene reactions conversion to new functional groups
 hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Also note here that the product is always a six membered ring with a double bond in it.
 Diels Alder Class 1 March 3, 2011 I want to talk in some detail over the next few classes about Diels Alder reactions. I am sure that most of you have heard of Diels Alder reactions before, but we will
Diels Alder Class 1 March 3, 2011 I want to talk in some detail over the next few classes about Diels Alder reactions. I am sure that most of you have heard of Diels Alder reactions before, but we will
20.3 Alkylation of Enolate Anions
 864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
Topic 9. Aldehydes & Ketones
 Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Organic Chemistry, Third Edition. Janice Gorzynski Smith University of Hawai i. Chapter 21. Aldehydes and Ketones Nucleophilic Addition
 Organic Chemistry, Third Edition Janice Gorzynski Smith University of Hawai i Chapter 21 Aldehydes and Ketones Nucleophilic Addition Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Organic Chemistry, Third Edition Janice Gorzynski Smith University of Hawai i Chapter 21 Aldehydes and Ketones Nucleophilic Addition Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Chapter 17 Aldehydes and Ketones
 hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CHEMISTRY Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay
 EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
Carbonyl Compounds. Introduction
 Carbonyl Compounds Introduction 1 Introduction Two broad classes of compounds contain the carbonyl group: [1] Compounds that have only carbon and hydrogen atoms bonded to the carbonyl [2] Compounds that
Carbonyl Compounds Introduction 1 Introduction Two broad classes of compounds contain the carbonyl group: [1] Compounds that have only carbon and hydrogen atoms bonded to the carbonyl [2] Compounds that
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Chem 342 Organic Chemistry II Final Exam 13 May 2009
 hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
CuI CuI eage lic R tal ome rgan gbr ommon
 Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
1. Following are structural formulas for two steroid hormones.
 CHEM 122: Introduction to rganic Chemistry Chapter 9: Aldehydes and Ketones. 1. Following are structural formulas for two steroid hormones. Cortisone Aldosterone Name the functional groups in each. b)
CHEM 122: Introduction to rganic Chemistry Chapter 9: Aldehydes and Ketones. 1. Following are structural formulas for two steroid hormones. Cortisone Aldosterone Name the functional groups in each. b)
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
Chemistry 263 Laboratory Experiment 5: Dibenzalacetone 5/10/18. Introduction
 Chemistry 263 Laboratory Experiment 5: Dibenzalacetone 5/10/18 Introduction We have been examining the electrophilic nature of the carbonyl of late. In particular, we have been trying to determine how
Chemistry 263 Laboratory Experiment 5: Dibenzalacetone 5/10/18 Introduction We have been examining the electrophilic nature of the carbonyl of late. In particular, we have been trying to determine how
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
12. Aldehydes & Ketones (text )
 2009, Department of Chemistry, The University of Western ntario 12.1 12. Aldehydes & Ketones (text 13.1 13.11) A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is
2009, Department of Chemistry, The University of Western ntario 12.1 12. Aldehydes & Ketones (text 13.1 13.11) A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is
Chapter 23. Alpha Substitution of Carbonyl Compounds.
 1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
EWG EWG EWG EDG EDG EDG
 Functional Group Interconversions Lecture 4 2.1 rganic Synthesis A. Armstrong 20032004 3.4 eduction of aromatic systems We can reduce aromatic systems to cyclohexanes under very forcing hydrogenolytic
Functional Group Interconversions Lecture 4 2.1 rganic Synthesis A. Armstrong 20032004 3.4 eduction of aromatic systems We can reduce aromatic systems to cyclohexanes under very forcing hydrogenolytic
California State Polytechnic University, Pomona. Exam Points 1. Nomenclature (1) 25
 hem 316 Final Spring, 2004 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 25 redit Tautomers (acidic or base conditions) 20 3. Reactions Page (10 x 3 = 30) 30 4. Aromatic Mechanism and
hem 316 Final Spring, 2004 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 25 redit Tautomers (acidic or base conditions) 20 3. Reactions Page (10 x 3 = 30) 30 4. Aromatic Mechanism and
Summary of π Bond Chemistry
 Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
(5) a. b. (6) N H CH 3 N H O H O (7) CH 3 O (8) OCH 3 2. explanation:
 ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Ninth Edition
 hemistry 110 Bettelheim, Brown, ampbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry hapter 17 Aldehydes & Ketones hemistry of the arbonyl Group Aldehydes & Ketones The functional
hemistry 110 Bettelheim, Brown, ampbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry hapter 17 Aldehydes & Ketones hemistry of the arbonyl Group Aldehydes & Ketones The functional
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
N.b. A catalyst is a species which speeds up a chemical reaction but which remains chemically unchanged. Reverse process of dehydration of an alcohol
 An Introduction to Organic hemistry N.b. A catalyst is a species which speeds up a chemical reaction but which remains chemically unchanged. ydration (Addition) Reverse process of dehydration of an alcohol
An Introduction to Organic hemistry N.b. A catalyst is a species which speeds up a chemical reaction but which remains chemically unchanged. ydration (Addition) Reverse process of dehydration of an alcohol
CHE 232 Organic Chemistry II Exam 4 Name: KEY
 CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Carbonyl Chemistry V + C O C. Chemistry /30/02
 arbonyl hemistry V Ō - + Keto-enol enol Tautomerism H 3 H 3 Ketone H H R 2 H R' H H 3 H 2 H Enol H Acid atalyzed α Halogenation R 2 R' + X 2 H + R 2 R' + HX H X X 2 can be l 2, Br 2, or I 2. Substitution
arbonyl hemistry V Ō - + Keto-enol enol Tautomerism H 3 H 3 Ketone H H R 2 H R' H H 3 H 2 H Enol H Acid atalyzed α Halogenation R 2 R' + X 2 H + R 2 R' + HX H X X 2 can be l 2, Br 2, or I 2. Substitution
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chapter 7. dehydration
 hapter 7 7.1 ne problem with elimination reactions is that mixtures of products are often formed. For example, treatment of 2-bromo-2-methylbutane with K in ethanol yields a mixture of two alkene products.
hapter 7 7.1 ne problem with elimination reactions is that mixtures of products are often formed. For example, treatment of 2-bromo-2-methylbutane with K in ethanol yields a mixture of two alkene products.
Chapter 9 Aldehydes and Ketones
 Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
