CHAPTER 21 HW: ALDEHYDES + KETONES
|
|
|
- Julius Dwayne Butler
- 6 years ago
- Views:
Transcription
1 CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). Structure C 2 ame 3,3-dimethyl-2-pentanone (or 1,1-dimethylpropyl methyl ketone) 5-hydroxy-4-methylhexanal m-nitrobenzaldehyde Structure ame 1-penten-3-one or ethyl vinyl ketone 2,4-dioxohexanal 2-methyl-5-heptyn-3-one 2. Draw each compound. Structure F F F C 3 zame zide ame dicyclohexyl ketone 1,1,1-trifluoro-3-pentanone (Z)-3,6-dimethyl-3-heptenal SPECTRSCPY 3. In the 1 MR spectrum of butanal, the signal at 2.40 ppm is a triplet of doublets (approximate J s are 2 z and 7 z). Explain the splitting of this signal, including a sketch of a tree diagram. C C C C Signal at 2.40 ppm (next to carbonyl) The C 2 next to the carbonyl is the signal at 2.40 ppm. It is split in a large way by its two C 2 neighbors (7 z, splitting first level into a triplet), and in a smaller way by the aldehyde (2 z, splitting second level into a doublet). This results in a triplet of doublets. Page 1
2 4. f compounds A-D, C 3 C 3 A B C D a. Which compound would have the following 13 C MR spectra (briefly explain)? δ = 165.9, 132.1, 131.5, 129.1, 127.4, 51.5 ppm Compound A ppm is the C=, and the low range means it could be part of an ester, carboxylic acid or amide. The 51.5 ppm signal is the carbon next to oxygen (could be A or B). There are only 6 total 13 C signals, so it is A (B would have 8 13 C signals). Which compound would have the following 13 C MR spectra (briefly explain)? δ = 199.8, 140.3, 135.4, 131.5, 131.0, 127.7, 121.2, 28.6 ppm Compound D ppm is the C=, and a high range means it could part of an aldehyde or ketone. The 28.6 ppm signal is low, and so is not next to oxygen (could be C or D). There are 8 total 13 C signals, so it is D (C would have 6 13 C signals). 5. Below are 1 and 13 C MR spectra of a compound with a formula of C 5 8. Determine the structure, then assign the peaks in the 1 MR spectrum. 1 a b c d e 2 f PPM PPM a e f b c d e/f assignment could be switched c is a wider ~doublet so must involve a trans coupling (to b). Page 2
3 6. Cyclohexanone has a strong signal in its IR spectrum at 1718 cm -1, while 2-cyclohexenone has a strong signal at 1691 cm -1. Both signals represent vibration of the same kind of bond. Explain why the absorption in 2-cyclohexenone is at a lower wavenumber, including resonance structures. Both signals represent the IR stretching of the C= bonds. 2-cyclohexenone has a lower wavenumber absorbance, as it has a different C= bond strength than cyclohexanone. All carbonyls have a +/- resonance structure (shown with cyclohexanone below), but 2-cyclohexenone has an additional resonance structure, which causes its resonance hybrid to have more single bond character. With greater single bondedness, the C= is weaker, and thus absorbs at a lower wavenumber (since v α f). vs. 7. An IR is taken of a mixture of the two compounds below. Two strong signals are noted in the mixed IR spectrum at 1666 and 1692 cm -1, representing the carbonyl stretching modes. Which signal corresponds to which compound? iefly explain. Both compounds have very conjugated C= bonds, and conjugation lowers the IR wavenumber (more single bond character). The first compound has twice as much conjugation / resonance, C= Stretch 1666 cm -1 C=: 1692 cm -1 so it s C= signal is the lowest. WITTIG REACTI 8. Give the curved arrow mechanism for each reaction. a. C 3 C 2 a. P 3 nbuli 3 C C P 3 3 C C P 3 S 2 P 3 C 3 C 2 3 C C P (nbuli) 3 C C P 3 C C P 3 3 C C P 3 3 C C P 3 C P 3 3 C C C 3 P Page 3
4 8 continued c. P 3 P 3 P 3 P 3 9. Draw both stereoisomers that can be formed in this Wittig reaction. 3 P trans cis 10. Give the major product of each reaction (one stereoisomer is sufficient). C C 3 C 2 C=P a. 3 C 2 C 3 3 P c. C C 3 C 3 P 3 d. 2 C P 3 2 C 11. Use a Wittig reaction to produce each alkene, starting from an ylide. C 3 C 2 C 2 C 3 a. C C C 3 C 2 Route 1 C 3 C 2 C 2 C 3 C + 3 P C C 3 C 2 product Route 2 C C 3 C 2 C 2 C 3 C P 3 + C 3 C 2 product Route 1 P 3 or P 3 product Route 2 P 3 product Page 4
5 12. Use a Wittig reaction to produce this alkene, starting from an alkyl halide. Route 1 P 3 P 3 nbuli P 3 Route 2 P 3 3 P nbuli 3 P YDRATES 13. Give the curved arrow mechanism that shows the formation of hydrate under each set of conditions. a. trace - 2 trace + 2 trace Compound E has a higher percentage of hydrate relative to carbonyl in aqueous solution than compound F. Explain this trend, including energy diagrams with your explanation. E F-hydrate E-hydrate E F F The difference in reactivity often arises from the relative energies of the carbonyl species (starting reactant energies). The carbonyl carbon is δ+, and EDG lower the energy. The aldehyde has one alkyl group (EDG) attached to the C=, but the ketone has 2 EDG. Therefore, the ketone stabilizes the δ+ more and starts at a lower energy than the aldehyde. This causes the hydrate reaction to be uphill for the ketone (so higher hydrate % for aldehyde). Although it s only a minor factor, we can also be complete by noting that the ketone hydrate energies should also be somewhat higher E because 2 alkyl groups are more crowded than just one. This would make the ketone reaction even more uphill. ne EDG Page 5
6 15. Compound G has a smaller equilibrium constant (K eq ) for hydrate formation than compound. Explain this trend, including energy diagrams with your explanation. 2 C 3 CF 3 G K eq = 1.06 K eq = 2.9 x 10 4 CF 3 EWG CF 3 K eq R R K = hydrate / carbonyl; so a large K eq means a higher % of hydrate. Carbonyl with CF 3 has more hydrate. The reactivity differences arise from different starting carbonyl energies (the hydrate energies are also nearly CF 3 equivalent). CF 3 is a strong EWG, so destabilizes the δ+ of the carbonyl, C 3 CF 3 C making the CF 3 carbonyl higher in 3 G G+-hydrate energy than the aldehyde. This makes the hydrate reaction of the CF 3 carbonyl downhill, resulting in a higher amount of hydrate. 16. In each pair, predict which would have a greater percentage of hydrate relative to carbonyl when the two forms are at equilibrium in water. iefly explain each answer. Pair A ief Explanation: The aromatic group can participate in resonance with the C= which greatly stabilizes the δ+ of the carbonyl. Therefore, the aromatic carbonyl starts at a lower energy, and reacts less (reaction is more uphill). Pair B ief Explanation: C= energies are probably similar. But hydrate energies are more sensitive to steric issues. The dicyclohexyl compound s hydrate will be higher energy, so reaction is more uphill. Pair C C 3 C 3 ief Explanation: thoxy is a stronger EDG than methyl (resonance, not hyperconjugation), so best stabilizes the δ+ of the carbonyl. The methoxy carbonyl starts at a lower energy, so is more uphill. Pair D 3 ief Explanation: The aldehyde is more reactive (makes more hydrate) because it has a higher energy carbonyl form (reaction is downhill). The aromatic has an EWG (- + 3 ), which destabilizes the δ+ of the carbonyl. Page 6
7 ACETALS 17. Give the curved arrow mechanism for this acetal formation reaction. C 2 C 3 + C 2 C 3 C 3 C 2 C 2 C 3 + C 3 C 2 C 3 C 2 Protonate Attack Deprotonate C 2 C 3 + Protonate C 2 C 3 2 C2 C 3 C2 C 3 C 2 C 3 C 2 C 3 Deprotonate Leave C 3 C 2 C 3 C 2 Attack C 3 C 2 product ote: attack of either resonance structure is acceptable; you don t need to show both. 18. Explain why acid is catalytic in the formation of an acetal. (Use the mechanism in the previous problem.) Acid is a catalyst because it satisfies both criteria: Acid is unconsumed. For every protonate step where it is used, there is a deprotonate step where it is regenerated. Acid lowers the activation barrier of the reaction. It makes the carbonyl more reactive to attack, as it puts charge on the carbonyl, making the carbon of the carbonyl more δ+ (starts at a higher energy, thus lowering E a ). 19. Give the curved arrow mechanism for this reaction Protonate Attack Deprotonate Protonate 2 Leave Attack Deprotonate Page 7
8 20. Give the major organic product for each reaction. a. + C 3 C 2 C 3 C 2 C 2 C 3 c. + + d. C + C 3 C 3 C In order to achieve good yields for most acetal formations, they need to be driven by Le Châtelier s Principle. Explain why good yields are easier to achieve when reacting aldehydes than when reacting ketones. Acetal formation reactions have nearly the same energetics as hydrate formation. For aldehydes, K=1 for acetal formation, while ketones K<1. Ketones have a more stabilized carbonyl (2 EDG stabilizing the δ+ of the carbonyl), so their acetal reaction is uphill. This means it is easier to get good yields with aldehydes (K=1) compared to ketones (uphill). 22. Show all organic products of these reactions. a. C 3 C C 3 d C 3 C 2 3 C + 2 C 3 e. 3 C C C3 c C 3 C f. 3 C C 3 C C + 2 C 3 Page 8
9 23. Give the curved arrow mechanism for each reaction. a. C 3 C C 3 C 3 + C 3 C 3 C3 2 C 3 Protonate C 3 Leave Attack C 3 C 3 + C Deprotonate 3 2 product 2 Protonate Leave Deprotonate acetone c. C 2 C C 3 C 2 + C 2 C 3 (ch could be begun by protonating either oxygen) C 2 C d product R Page 9
10 24. Milder conditions can be used to hydrolyze acetal J than to hydrolyze acetal K. Explain their difference in reactivity. C 3 C 3 J C 3 K C3 Acetal hydrolysis reactions have similar energetics to hydrate reactions. Since J produces a ketone instead of an aldehyde (where the carbonyl is more stabilized by 2 EDGs), it s a more favorable hydrolysis reaction (reaction is easier and can use milder conditions). PRTECTIG GRUPS 25. Design a synthesis that uses cyclopentanone and 4-bromobutanal to efficiently produce the aldehyde shown. + +, heat (protect aldehyde) a) Mg b) c) + workup +, 2, heat (hydrolysis) 26. The following multi-step synthesis converts benzene into a dicarbonyl species. a. Give the reagents needed to complete each step in the sequence. 2 Cl Fe 3 AlCl 3 +, heat Mg +, 2 Cr 3, + or PCC a) Mg heat b) + workup iefly explain why the synthesis below does not work well. Cl AlCl 3 Cl AlCl 3 Step 2 should have problems. The carbonyl is a meta director, and also Friedel Crafts reactions don t work well on deactivated rings. Page 10
11 IMIES + EAMIES 27. Give the curved arrow mechanism for each reaction. a. C 3 2 trace + C C C 3 C 3 2 C 3 2 C 3 C 3 C3 C 3 2 C3 + 2 C 3 C 3 trace + 3 C C C 3 C 3 C 3 C C C 3 3 C C3 C 3 C 3 3 C C 3 3 C C3 3 C C 3 + c. 2 2 trace R 2 2 R R 2 (alf-way point) + 2 R 2 Page 11
12 28. Draw both stereoisomers that can be formed in this reaction. 2 mild acid E Z 29. Give the major organic product for each reaction (one stereoisomer is sufficient). a. C C 3 2 trace + C 3 d. trace + 2 (C3 ) 2 p 5 C 3 C 3 e. trace + c. trace + f. 3 trace Give the curved arrow mechanism for each reaction. a. + C 2 2 C 3 + C 3 C C 2 C 3 C 2 C 3 C 2 C 3 2 C 2 C 3 C 2 C 3 2 Page 12
13 30 continued C 3 C C 3 C 2 2 c. 3 C C C 3 C Give the major organic product for each reaction. + C 3 a. 2 + C 3 2 C3 d C e c. C 3 C C 3 C 3 3 C + 3 C f. 2 Page 13
14 REACTI SUMMARY 32. Give the major organic product for each reaction. a. K a. a 2 cyclopentanone c. + workup c. + C 3 C 2 C 2 C 3 C 2 C 3 d. a. Li (s) pentanal c. + workup Li 3 C e. 3 P=CC 3 (The Z isomer is also K) f. abd 4 C 3 C 2 D D D g. C 2 C 3 cat. + C 3 C 2 2 h C 3 C 2 i. a. (C 3 C 2 ) 2 CuLi + workup Page 14
CHAPTER 21 HW: ALDEHYDES + KETONES
 CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). C Structure 2 ame Structure ame 2. Draw each compound. Structure ame dicyclohexyl ketone 1,1,1-trifluoro-3-pentanone
CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). C Structure 2 ame Structure ame 2. Draw each compound. Structure ame dicyclohexyl ketone 1,1,1-trifluoro-3-pentanone
CHAPTER 25 HW: AMINES
 CAPTER 25 W: AMIES MECLATURE 1. Draw each compound. Structure 3 C F ame triphenyl amine 3-fluoro--methyl-1-propanamine,-dipropylaniline 2. Give the IUPAC name for each compound, including R/S, cis/trans
CAPTER 25 W: AMIES MECLATURE 1. Draw each compound. Structure 3 C F ame triphenyl amine 3-fluoro--methyl-1-propanamine,-dipropylaniline 2. Give the IUPAC name for each compound, including R/S, cis/trans
CHAPTER 23 HW: ENOLS + ENOLATES
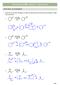 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Chemistry Final Examinations Summer 2006 answers
 Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chemistry 234 Practice Exam 3. The Periodic Table
 ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
CHAPTER 22 HW: CO 2 H DERIVATIVES
 APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
CHAPTER 22 HW: CO 2 H DERIVATIVES
 CHAPTER 22 HW: C 2 H DERIVATIVES MECLATURE 1. Give the name for each compound (IUPAC or common name). Use R/S naming where needed. Structure Br ame Structure ame 2. Give the name for each ester. Structure
CHAPTER 22 HW: C 2 H DERIVATIVES MECLATURE 1. Give the name for each compound (IUPAC or common name). Use R/S naming where needed. Structure Br ame Structure ame 2. Give the name for each ester. Structure
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
CHE 325 ALDEHYDES AND KETONES I CHAP 18 ASSIGN OCH 3 HCN C 4 H 7 NO. would be: CH 3 B. CH 3CH 2COOCH 3
 CE 325 ALDEYDES AND KETNES I CAP 18 ASSIGN 1. What is the correct IUPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-Isopropyl-4-octanone D. Isobutyl propyl ketone
CE 325 ALDEYDES AND KETNES I CAP 18 ASSIGN 1. What is the correct IUPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-Isopropyl-4-octanone D. Isobutyl propyl ketone
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Ketones and Aldehydes Reading Study Problems Key Concepts and Skills Lecture Topics: Structure of Ketones and Aldehydes Structure:
 Ketones and Aldehydes Reading: Wade chapter 18, sections 18-1- 18-21 Study Problems: 18-43, 18-44,18-50, 18-51, 18-52, 18-59, 18-60, 18-62, 18-64, 18-72. Key Concepts and Skills: Interpret the IR, NMR,
Ketones and Aldehydes Reading: Wade chapter 18, sections 18-1- 18-21 Study Problems: 18-43, 18-44,18-50, 18-51, 18-52, 18-59, 18-60, 18-62, 18-64, 18-72. Key Concepts and Skills: Interpret the IR, NMR,
1. Predict the structure of the molecules given by the following spectral data: a Mass spectrum:m + = 116
 Additional Problems for practice.. Predict the structure of the molecules given by the following spectral data: a Mass spectrum:m + = IR: weak absorption at 9 cm - medium absorption at cm - NMR 7 3 3 C
Additional Problems for practice.. Predict the structure of the molecules given by the following spectral data: a Mass spectrum:m + = IR: weak absorption at 9 cm - medium absorption at cm - NMR 7 3 3 C
Structure Determination. How to determine what compound that you have? One way to determine compound is to get an elemental analysis
 Structure Determination How to determine what compound that you have? ne way to determine compound is to get an elemental analysis -basically burn the compound to determine %C, %H, %, etc. from these percentages
Structure Determination How to determine what compound that you have? ne way to determine compound is to get an elemental analysis -basically burn the compound to determine %C, %H, %, etc. from these percentages
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
ALDEHYDES AND KETONES
 CE 325 ALDEYDES AND KETNES CAP 17 ASSGN NUCLEPLC ADDTN T TE CARBNYL GRUP 1. What is the correct UPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-sopropyl-4-octanone
CE 325 ALDEYDES AND KETNES CAP 17 ASSGN NUCLEPLC ADDTN T TE CARBNYL GRUP 1. What is the correct UPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-sopropyl-4-octanone
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Chapter 9 Aldehydes and Ketones
 Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Organic Chemistry, Third Edition. Janice Gorzynski Smith University of Hawai i. Chapter 21. Aldehydes and Ketones Nucleophilic Addition
 Organic Chemistry, Third Edition Janice Gorzynski Smith University of Hawai i Chapter 21 Aldehydes and Ketones Nucleophilic Addition Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Organic Chemistry, Third Edition Janice Gorzynski Smith University of Hawai i Chapter 21 Aldehydes and Ketones Nucleophilic Addition Prepared by Rabi Ann Musah State University of New York at Albany Copyright
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Important Note: We will NOT accept papers written in pencil back for re-marking after they have been returned to you. Please do not ask!
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 2 Thursday, March 15, 2012; 7-9 PM This is a 2-hour test, marked out of
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 2 Thursday, March 15, 2012; 7-9 PM This is a 2-hour test, marked out of
Chemistry 3720 Old Exams. Practice Exams & Keys
 Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Chemistry M11 Spring 2010 Examination #3 ANSWER KEY pp. 1
 Chemistry M11 Spring 2010 Examination #3 ASWER KEY pp. 1 or Questions 1 2, consider the hydrogenation of trans-3-methyl-2-pentene in the presence of a transition metal catalyst. 1. C Determine the major
Chemistry M11 Spring 2010 Examination #3 ASWER KEY pp. 1 or Questions 1 2, consider the hydrogenation of trans-3-methyl-2-pentene in the presence of a transition metal catalyst. 1. C Determine the major
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Key ideas: In EAS, pi bond is Nu and undergoes addition.
 Objective 7. Apply addition and elimination concepts to predict electrophilic aromatic substitution reactions (EAS) of benzene and monosubstituted benzenes. Skills: Draw structure ID structural features
Objective 7. Apply addition and elimination concepts to predict electrophilic aromatic substitution reactions (EAS) of benzene and monosubstituted benzenes. Skills: Draw structure ID structural features
CH 3. mirror plane. CH c d
 CAPTER 20 Practice Exercises 20.1 The index of hydrogen deficiency is two. The structural possibilities include two double bonds, a double do 20.3 (a) As this is an alkane, it contains only C and and has
CAPTER 20 Practice Exercises 20.1 The index of hydrogen deficiency is two. The structural possibilities include two double bonds, a double do 20.3 (a) As this is an alkane, it contains only C and and has
Carbonyl Compounds. Introduction
 Carbonyl Compounds Introduction 1 Introduction Two broad classes of compounds contain the carbonyl group: [1] Compounds that have only carbon and hydrogen atoms bonded to the carbonyl [2] Compounds that
Carbonyl Compounds Introduction 1 Introduction Two broad classes of compounds contain the carbonyl group: [1] Compounds that have only carbon and hydrogen atoms bonded to the carbonyl [2] Compounds that
FIRST EXAMINATION. Name: CHM 332
 ame: CM 332 FIRST EXAMIATI All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is,
ame: CM 332 FIRST EXAMIATI All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is,
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Loudon Chapter 19 Review: Aldehydes and Ketones CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
WITTIG REACTION. Solo Experiment 2 Individual Lab Report (due at 12:00 pm one week after the lab is performed)
 WITTIG REACTION Solo Experiment 2 Individual Lab Report (due at 2:00 pm one week after the lab is performed) Last Name: Atique First Name: Anika TA Name: Davin Date Lab Performed: 2/26/204 Date Lab Submitted:
WITTIG REACTION Solo Experiment 2 Individual Lab Report (due at 2:00 pm one week after the lab is performed) Last Name: Atique First Name: Anika TA Name: Davin Date Lab Performed: 2/26/204 Date Lab Submitted:
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
CH 320/328 N Summer II 2018
 CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
ALDEHYDES AND KETONES
 ALDEHYDES AND KETONES IN WEEK 1, A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name given the structure, and draw the structure given the name, of aldehydes and ketones. Also, draw the structure given
ALDEHYDES AND KETONES IN WEEK 1, A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name given the structure, and draw the structure given the name, of aldehydes and ketones. Also, draw the structure given
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies
 Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Unit 5: Organic Chemistry
 Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
Reversible Additions to carbonyls: Weak Nucleophiles Relative Reactivity of carbonyls: Hydration of Ketones and Aldehydes
 Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
N_HW1 N_HW1. 1. What is the purpose of the H 2 O in this sequence?
 N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
TOPIC 3 - ALDEHYDES AND KETONES (Chapters 12 & 16)
 TPIC 3 - ALDEYDES AND KETNES (Chapters 12 & 16) Lecture 15 Web12 12.1 Introduction 16.1 Introduction 16.2 Nomenclature of Aldehydes and Ketones 16.3 ysical Properties 12.2 xidation Reduction Reactions
TPIC 3 - ALDEYDES AND KETNES (Chapters 12 & 16) Lecture 15 Web12 12.1 Introduction 16.1 Introduction 16.2 Nomenclature of Aldehydes and Ketones 16.3 ysical Properties 12.2 xidation Reduction Reactions
11/30/ Substituent Effects in Electrophilic Substitutions. Substituent Effects in Electrophilic Substitutions
 Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Chapter 17 Aldehydes and Ketones
 hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
hapter 17 Aldehydes and Ketones arbonyl Groups polarized (1) Aldehydes and Ketones ' aldehydes ketones : and : are poor leaving groups (2) arboxylic Acid Derivatives l ' ' 2 carboxylic acid substituent
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
1.1 Is the following molecule aromatic or not aromatic? Give reasons for your answer.
 Page 1 QUESTION ONE 1.1 Is the following molecule aromatic or not aromatic? Give reasons for your answer. 1.2 List four criteria which compounds must meet in order to be considered aromatic. Page 2 QUESTION
Page 1 QUESTION ONE 1.1 Is the following molecule aromatic or not aromatic? Give reasons for your answer. 1.2 List four criteria which compounds must meet in order to be considered aromatic. Page 2 QUESTION
Aldehydes and Ketones 2. Based on Organic Chemistry, J. G. Smith 3rde.
 Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
Organic Chemistry 1 CHM 2210 Exam 4 (December 10, 2001)
 Exam 4 (December 10, 2001) Name (print): Signature: Student ID Number: There are 12 multiple choice problems (4 points each) on this exam. Record the answers to the multiple choice questions on THIS PAGE.
Exam 4 (December 10, 2001) Name (print): Signature: Student ID Number: There are 12 multiple choice problems (4 points each) on this exam. Record the answers to the multiple choice questions on THIS PAGE.
Look for absorption bands in decreasing order of importance:
 1. Match the following to their IR spectra (30 points) Look for absorption bands in decreasing order of importance: a e a 2941 1716 d f b 3333 c b 1466 1.the - absorption(s) between 3100 and 2850 cm-1.
1. Match the following to their IR spectra (30 points) Look for absorption bands in decreasing order of importance: a e a 2941 1716 d f b 3333 c b 1466 1.the - absorption(s) between 3100 and 2850 cm-1.
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Answers to Assignment #5
 Answers to Assignment #5 A. 9 8 l 2 5 DBE (benzene + 1 DBE) ( 9 2(9)+2-9 8+1+1 = 10 ˆ 5 DBE) nmr pattern of two doublets of equal integration at δ7.4 and 7.9 ppm means the group (the δ7.9 shift) IR band
Answers to Assignment #5 A. 9 8 l 2 5 DBE (benzene + 1 DBE) ( 9 2(9)+2-9 8+1+1 = 10 ˆ 5 DBE) nmr pattern of two doublets of equal integration at δ7.4 and 7.9 ppm means the group (the δ7.9 shift) IR band
Synthesis Using Aromatic Materials
 Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
Topic 9. Aldehydes & Ketones
 Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
b.(12) Where is pyrrole protonated under strong acidic conditions? Why this site of protonation?
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank the following dienes by rate of Diels-Alder reaction. The diene which reacts the fastest with an alkene is 1, while the
1. Rank the following compounds in the trend requested. (15 points each) a. Rank the following dienes by rate of Diels-Alder reaction. The diene which reacts the fastest with an alkene is 1, while the
Chapter 18: Ketones and Aldehydes. I. Introduction
 1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
CHEMISTRY 341. Final Exam Tuesday, December 16, Problem 1 15 pts Problem 9 8 pts. Problem 2 5 pts Problem pts
 CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
4. NMR spectra. Interpreting NMR spectra. Low-resolution NMR spectra. There are two kinds: Low-resolution NMR spectra. High-resolution NMR spectra
 1 Interpreting NMR spectra There are two kinds: Low-resolution NMR spectra High-resolution NMR spectra In both cases the horizontal scale is labelled in terms of chemical shift, δ, and increases from right
1 Interpreting NMR spectra There are two kinds: Low-resolution NMR spectra High-resolution NMR spectra In both cases the horizontal scale is labelled in terms of chemical shift, δ, and increases from right
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson 3rd Midterm April 26, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. ent Iverson 3rd Midterm April 26, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
Learning Guide for Chapter 14 - Alcohols (I)
 Learning Guide for Chapter 14 - Alcohols (I) I. Introduction to Alcohols and Thiols II. Acid/base Behavior of Alcohols, Phenols, and Thiols III. Nomenclature of Alcohols IV. Synthesis of Alcohols Previous
Learning Guide for Chapter 14 - Alcohols (I) I. Introduction to Alcohols and Thiols II. Acid/base Behavior of Alcohols, Phenols, and Thiols III. Nomenclature of Alcohols IV. Synthesis of Alcohols Previous
Name: Student Number: University of Manitoba - Department of Chemistry CHEM Introductory Organic Chemistry II - Term Test 1
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 11, 2016; 7-9 PM This is a 2-hour test, marked out
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 1 Thursday, February 11, 2016; 7-9 PM This is a 2-hour test, marked out
Chapter 17: Alcohols and Phenols. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Name: Student Number:
 Page 1 of 5 Name: Student Number: l University of Manitoba - Department of Chemistry CEM 2220 - Introductory rganic Chemistry II - Term Test 1 Thursday, February 14, 2008 This is a 2-hour test, marked
Page 1 of 5 Name: Student Number: l University of Manitoba - Department of Chemistry CEM 2220 - Introductory rganic Chemistry II - Term Test 1 Thursday, February 14, 2008 This is a 2-hour test, marked
Chemistry Organic Chemistry II: Reactivity and Synthesis FINAL EXAM Winter 2003
 Name: Student No: Page 1 of 13 Chemistry 2.222 rganic Chemistry II: Reactivity and Synthesis FINAL EXAM Winter 2003 Paper Number 546 Thursday April 24, 2003 6:00 9:00 pm Frank Kennedy own Gym Students
Name: Student No: Page 1 of 13 Chemistry 2.222 rganic Chemistry II: Reactivity and Synthesis FINAL EXAM Winter 2003 Paper Number 546 Thursday April 24, 2003 6:00 9:00 pm Frank Kennedy own Gym Students
Fundamentals of Organic Chemistry
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 3. AROMATIC HYDROCARBONS Aromatic
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 3. AROMATIC HYDROCARBONS Aromatic
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
Chemistry Questions ans Answers BASED ON HIGH ORDER THINKING SKILL (HOTS) UNIT- 12 ALDEHYDES, KETONES AND CARBXYLIC ACID
 Chemistry Questions ans Answers BASED N IG RDER TINKING SKILL (TS) UNIT- 12 ALDEYDES, KETNES AND CARBXYLIC ACID 1 MARK QUESTINS Q. 1. Name the reaction and the reagent used for the conversion of acid chlorides
Chemistry Questions ans Answers BASED N IG RDER TINKING SKILL (TS) UNIT- 12 ALDEYDES, KETNES AND CARBXYLIC ACID 1 MARK QUESTINS Q. 1. Name the reaction and the reagent used for the conversion of acid chlorides
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Hour Examination # 1
 CEM 347 rganic Chemistry II Spring 2015 Exam # 1 Solutions Key Page 1 of 11 CEM 347 rganic Chemistry II Spring 2015 Instructor: Paul Bracher our Examination # 1 Wednesday, February 11 th, 2015 6:00 8:00
CEM 347 rganic Chemistry II Spring 2015 Exam # 1 Solutions Key Page 1 of 11 CEM 347 rganic Chemistry II Spring 2015 Instructor: Paul Bracher our Examination # 1 Wednesday, February 11 th, 2015 6:00 8:00
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Answers to Problem Set #2
 hem 242 Spring 2008 Answers to Problem Set #2 1. For this question we have been given the molecular formula, 3 5 l. Looking at the IR, the strong signal at 1720 cm 1 tells us that we have a carbonyl (we
hem 242 Spring 2008 Answers to Problem Set #2 1. For this question we have been given the molecular formula, 3 5 l. Looking at the IR, the strong signal at 1720 cm 1 tells us that we have a carbonyl (we
Aldehydes and Ketones
 9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
9 Aldehydes and Ketones hapter Summary The carbonyl group, =, is present in both aldehydes (=) and ketones ( 2 =). The IUPA ending for naming aldehydes is -al, and numbering begins with the carbonyl carbon.
Exam (6 pts) Show which starting materials are used to produce the following Diels-Alder products:
 Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
CARBOXYLIC ACIDS AND THEIR DERIVATIVES. 3. Predict the relative acidity and basicity of compounds and ions. Important criteria include:
 CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
Organic Chemistry I Exam 3 Fall 2001 November 30, Which of the following compounds corresponds to the spectral data given below?
 . Which of the following compounds corresponds to the spectral data given below? one of these. The reaction energy diagram given below corresponds to which of the following reactions? TS TS TS Br + R RI
. Which of the following compounds corresponds to the spectral data given below? one of these. The reaction energy diagram given below corresponds to which of the following reactions? TS TS TS Br + R RI
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
CHEM 3.2 (AS91388) 3 credits. Demonstrate understanding of spectroscopic data in chemistry
 CHEM 3.2 (AS91388) 3 credits Demonstrate understanding of spectroscopic data in chemistry Spectroscopic data is limited to mass, infrared (IR) and 13 C nuclear magnetic resonance (NMR) spectroscopy. Organic
CHEM 3.2 (AS91388) 3 credits Demonstrate understanding of spectroscopic data in chemistry Spectroscopic data is limited to mass, infrared (IR) and 13 C nuclear magnetic resonance (NMR) spectroscopy. Organic
Chapter 17. Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
