CHAPTER 21 HW: ALDEHYDES + KETONES
|
|
|
- Nathaniel McDonald
- 5 years ago
- Views:
Transcription
1 CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). C Structure 2 ame Structure ame 2. Draw each compound. Structure ame dicyclohexyl ketone 1,1,1-trifluoro-3-pentanone (Z)-3,6-dimethyl-3-heptenal SPECTRSCPY 3. In the 1 MR spectrum of butanal, the signal at 2.40 ppm is a triplet of doublets (approximate J s are 2 z and 7 z). Explain the splitting of this signal, including a sketch of a tree diagram. Page 1
2 4. f compounds A-D, C 3 C 3 Br Br Br A B C D Br a. Which compound would have the following 13 C MR spectra (briefly explain)? δ = 165.9, 132.1, 131.5, 129.1, 127.4, 51.5 ppm Which compound would have the following 13 C MR spectra (briefly explain)? δ = 199.8, 140.3, 135.4, 131.5, 131.0, 127.7, 121.2, 28.6 ppm 5. Below are 1 and 13 C MR spectra of a compound with a formula of C 5 8. Determine the structure, then assign the peaks in the 1 MR spectrum PPM PPM Page 2
3 6. Cyclohexanone has a strong signal in its IR spectrum at 1718 cm -1, while 2-cyclohexenone has a strong signal at 1691 cm -1. Both signals represent vibration of the same kind of bond. Explain why the absorption in 2-cyclohexenone is at a lower wavenumber, including resonance structures. 7. An IR is taken of a mixture of the two compounds below. Two strong signals are noted in the mixed IR spectrum at 1666 and 1692 cm -1, representing the carbonyl stretching modes. Which signal corresponds to which compound? Briefly explain. C= Stretch WITTIG REACTI 8. Give the curved arrow mechanism for each reaction. a. C 3 C 2 Br a. PPh 3 nbuli 3 C C PPh 3 3 C C PPh 3 3 C C PPh 3 Page 3
4 8 continued c. PPh 3 9. Draw both stereoisomers that can be formed in this Wittig reaction. Ph 3 P 10. Give the major product of each reaction (one stereoisomer is sufficient). C 3 C 2 C=PPh a. 3 Ph 3 P c. C C 3 PPh 3 d. 2 C PPh Use a Wittig reaction to produce each alkene, starting from an ylide. C 3 C 2 C 2 C 3 a. C C C 3 C 2 Page 4
5 12. Use a Wittig reaction to produce this alkene, starting from an alkyl halide. YDRATES 13. Give the curved arrow mechanism that shows the formation of hydrate under each set of conditions. a. trace - 2 trace Compound E has a higher percentage of hydrate relative to carbonyl in aqueous solution than compound F. Explain this trend, including energy diagrams with your explanation. E F Page 5
6 15. Compound G has a smaller equilibrium constant (K eq ) for hydrate formation than compound. Explain this trend, including energy diagrams with your explanation. 2 C 3 CF 3 G K eq = 1.06 K eq = 2.9 x 10 4 K eq R R 16. In each pair, predict which would have a greater percentage of hydrate relative to carbonyl when the two forms are at equilibrium in water. Briefly explain each answer. Pair A Brief Explanation: Pair B Brief Explanation: Brief Explanation: Pair C C 3 C 3 Brief Explanation: Pair D 3 Page 6
7 ACETALS 17. Give the curved arrow mechanism for this acetal formation reaction. C 2 C 3 + C 2 C 3 C 3 C Explain why acid is catalytic in the formation of an acetal. (Use the mechanism in the previous problem.) 19. Give the curved arrow mechanism for this reaction. + Page 7
8 20. Give the major organic product for each reaction. a. + C 3 C 2 c. + + d. C + C In order to achieve good yields for most acetal formations, they need to be driven by Le Châtelier s Principle. Explain why good yields are easier to achieve when reacting aldehydes than when reacting ketones. 22. Show all organic products of these reactions. a. C 3 + d. C C + 2 e. 3 C C c. + 2 f. 3 C C 3 C Page 8
9 23. Give the curved arrow mechanism for each reaction. a. C 3 C C c. C 2 C C 3 C 2 d. + Page 9
10 24. Milder conditions can be used to hydrolyze acetal J than to hydrolyze acetal K. Explain their difference in reactivity. C 3 C 3 J C 3 K C3 PRTECTIG GRUPS 25. Design a synthesis that uses cyclopentanone and 4-bromobutanal to efficiently produce the aldehyde shown. + Br 26. The following multi-step synthesis converts benzene into a dicarbonyl species. a. Give the reagents needed to complete each step in the sequence. Br Br Br MgBr Briefly explain why the synthesis below does not work well. Cl Cl AlCl 3 AlCl 3 Page 10
11 IMIES + EAMIES 27. Give the curved arrow mechanism for each reaction. a. C 3 2 trace + C3 + 2 C 3 C trace + 3 C C 3 c. 2 2 trace Page 11
12 28. Draw both stereoisomers that can be formed in this reaction. 2 mild acid 29. Give the major organic product for each reaction (one stereoisomer is sufficient). a. C C 3 2 trace + d. trace + 2 (C3 ) 2 p 5 e. trace + c. f. trace + 3 trace Give the curved arrow mechanism for each reaction. a. + C 2 2 C 3 + C 3 C 2 2 Page 12
13 30 continued C 3 C 2 2 c. 3 C C C 3 C Give the major organic product for each reaction. a. C d. Ph C e. + 2 c. C 3 C f. 3 C Ph + 2 Page 13
14 REACTI SUMMARY 32. Give the major organic product for each reaction. a. K a. a 2 cyclopentanone c. + workup c. + C 3 C 2 d. Br a. Li (s) pentanal c. + workup e. Ph 3 P=CC 3 f. abd 4 C 3 C 2 D g. cat. + C 3 C 2 2 h. + 2 i. a. (C 3 C 2 ) 2 CuLi + workup Page 14
CHAPTER 21 HW: ALDEHYDES + KETONES
 CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). Structure 6 5 4 1 C 2 ame 3,3-dimethyl-2-pentanone (or 1,1-dimethylpropyl methyl ketone) 5-hydroxy-4-methylhexanal
CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). Structure 6 5 4 1 C 2 ame 3,3-dimethyl-2-pentanone (or 1,1-dimethylpropyl methyl ketone) 5-hydroxy-4-methylhexanal
CHE 325 ALDEHYDES AND KETONES I CHAP 18 ASSIGN OCH 3 HCN C 4 H 7 NO. would be: CH 3 B. CH 3CH 2COOCH 3
 CE 325 ALDEYDES AND KETNES I CAP 18 ASSIGN 1. What is the correct IUPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-Isopropyl-4-octanone D. Isobutyl propyl ketone
CE 325 ALDEYDES AND KETNES I CAP 18 ASSIGN 1. What is the correct IUPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-Isopropyl-4-octanone D. Isobutyl propyl ketone
CHAPTER 22 HW: CO 2 H DERIVATIVES
 CHAPTER 22 HW: C 2 H DERIVATIVES MECLATURE 1. Give the name for each compound (IUPAC or common name). Use R/S naming where needed. Structure Br ame Structure ame 2. Give the name for each ester. Structure
CHAPTER 22 HW: C 2 H DERIVATIVES MECLATURE 1. Give the name for each compound (IUPAC or common name). Use R/S naming where needed. Structure Br ame Structure ame 2. Give the name for each ester. Structure
ALDEHYDES AND KETONES
 CE 325 ALDEYDES AND KETNES CAP 17 ASSGN NUCLEPLC ADDTN T TE CARBNYL GRUP 1. What is the correct UPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-sopropyl-4-octanone
CE 325 ALDEYDES AND KETNES CAP 17 ASSGN NUCLEPLC ADDTN T TE CARBNYL GRUP 1. What is the correct UPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-sopropyl-4-octanone
ALDEHYDES AND KETONES
 ALDEHYDES AND KETONES IN WEEK 1, A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name given the structure, and draw the structure given the name, of aldehydes and ketones. Also, draw the structure given
ALDEHYDES AND KETONES IN WEEK 1, A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name given the structure, and draw the structure given the name, of aldehydes and ketones. Also, draw the structure given
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Chemistry 234 Practice Exam 3. The Periodic Table
 ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
CHAPTER 24 HW: CARBONYL CONDENSATIONS
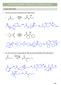 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
TOPIC 3 - ALDEHYDES AND KETONES (Chapters 12 & 16)
 TPIC 3 - ALDEYDES AND KETNES (Chapters 12 & 16) Lecture 15 Web12 12.1 Introduction 16.1 Introduction 16.2 Nomenclature of Aldehydes and Ketones 16.3 ysical Properties 12.2 xidation Reduction Reactions
TPIC 3 - ALDEYDES AND KETNES (Chapters 12 & 16) Lecture 15 Web12 12.1 Introduction 16.1 Introduction 16.2 Nomenclature of Aldehydes and Ketones 16.3 ysical Properties 12.2 xidation Reduction Reactions
ORGANIC - EGE 5E CH UV AND INFRARED MASS SPECTROMETRY
 !! www.clutchprep.com CONCEPT: IR SPECTROSCOPY- FREQUENCIES There are specific absorption frequencies in the functional group region that we should be familiar with EXAMPLE: What are the major IR absorptions
!! www.clutchprep.com CONCEPT: IR SPECTROSCOPY- FREQUENCIES There are specific absorption frequencies in the functional group region that we should be familiar with EXAMPLE: What are the major IR absorptions
1. Following are structural formulas for two steroid hormones.
 CHEM 122: Introduction to rganic Chemistry Chapter 9: Aldehydes and Ketones. 1. Following are structural formulas for two steroid hormones. Cortisone Aldosterone Name the functional groups in each. b)
CHEM 122: Introduction to rganic Chemistry Chapter 9: Aldehydes and Ketones. 1. Following are structural formulas for two steroid hormones. Cortisone Aldosterone Name the functional groups in each. b)
Topic 4 Aldehydes and Ketones
 4-1 Topic 4 Aldehydes and Ketones 16.1 4-2 Aldehydes and Ketones ' aldehyde ketone The polarized oxygen-carbon -bond renders aldehydes and ketones electrophilic: ' The electrophilicity of the oxygen-carbon
4-1 Topic 4 Aldehydes and Ketones 16.1 4-2 Aldehydes and Ketones ' aldehyde ketone The polarized oxygen-carbon -bond renders aldehydes and ketones electrophilic: ' The electrophilicity of the oxygen-carbon
FIRST EXAMINATION. Name: CHM 332
 ame: CM 332 FIRST EXAMIATI All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is,
ame: CM 332 FIRST EXAMIATI All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is,
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
C HAPTER 21: A LDEHYDES + K ETONES
 C APTER 21: A LDEYDES + K ETNES GENERAL INF EXAMPLES F ALDEYDES + KETNES Formaldehyde Acetaldehyde Cinnamaldehyde Citronellal Preservant Main cause of hangovers Taste + odor of cinnamon Mosquito repellant
C APTER 21: A LDEYDES + K ETNES GENERAL INF EXAMPLES F ALDEYDES + KETNES Formaldehyde Acetaldehyde Cinnamaldehyde Citronellal Preservant Main cause of hangovers Taste + odor of cinnamon Mosquito repellant
CHEM 203. Final Exam December 18, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CHEM 203. Final Exam December 12, This a closed-notes, closed-book exam. You may use your set of molecular models. This test contains 11 pages
 CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
CHAPTER 23 HW: ENOLS + ENOLATES
 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
ORGANIC - BRUICE 8E CH MASS SPECT AND INFRARED SPECTROSCOPY
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
CHEM 3.2 (AS91388) 3 credits. Demonstrate understanding of spectroscopic data in chemistry
 CHEM 3.2 (AS91388) 3 credits Demonstrate understanding of spectroscopic data in chemistry Spectroscopic data is limited to mass, infrared (IR) and 13 C nuclear magnetic resonance (NMR) spectroscopy. Organic
CHEM 3.2 (AS91388) 3 credits Demonstrate understanding of spectroscopic data in chemistry Spectroscopic data is limited to mass, infrared (IR) and 13 C nuclear magnetic resonance (NMR) spectroscopy. Organic
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
ORGANIC - BROWN 8E CH INFRARED SPECTROSCOPY.
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
C h a p t e r T w e n t y: Aldehydes and Ketones
 C h a p t e r T w e n t y: Aldehydes and Ketones (S)-Warfarin (named for the Wisconsin Alumni Research Foundation), a useful clinical anticoagulant which as a racemate is also a rat poison Note: Problems
C h a p t e r T w e n t y: Aldehydes and Ketones (S)-Warfarin (named for the Wisconsin Alumni Research Foundation), a useful clinical anticoagulant which as a racemate is also a rat poison Note: Problems
ORGANIC - CLUTCH CH ANALYTICAL TECHNIQUES: IR, NMR, MASS SPECT
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
Ketones and Aldehydes Reading Study Problems Key Concepts and Skills Lecture Topics: Structure of Ketones and Aldehydes Structure:
 Ketones and Aldehydes Reading: Wade chapter 18, sections 18-1- 18-21 Study Problems: 18-43, 18-44,18-50, 18-51, 18-52, 18-59, 18-60, 18-62, 18-64, 18-72. Key Concepts and Skills: Interpret the IR, NMR,
Ketones and Aldehydes Reading: Wade chapter 18, sections 18-1- 18-21 Study Problems: 18-43, 18-44,18-50, 18-51, 18-52, 18-59, 18-60, 18-62, 18-64, 18-72. Key Concepts and Skills: Interpret the IR, NMR,
Carbonyl Chemistry. aldehydes ketones. carboxylic acid and derivatives. Wednesday, April 29, 2009
 Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Lecture 15. More Carbonyl Chemistry. Alcohols React with Aldehydes and Ketones in two steps first O R'OH, H + OR" 2R"OH R + H 2 O OR" 3/8/16
 Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
ORGANIC - CLUTCH CH ANALYTICAL TECHNIQUES: IR, NMR, MASS SPECT
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
Final Exam April 30, 2014
 Bennett Department of Chemistry Chemistry 234 Final Exam April 30, 2014 ame: This exam is a closed book, closed notes. Calculators and a molecular model set are allowed. You must show your work in order
Bennett Department of Chemistry Chemistry 234 Final Exam April 30, 2014 ame: This exam is a closed book, closed notes. Calculators and a molecular model set are allowed. You must show your work in order
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Hour Examination # 2
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Exam # 2 Solutions Key Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Hour Examination # 2 Tuesday,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Exam # 2 Solutions Key Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Hour Examination # 2 Tuesday,
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
STUDYING VIBRATIONS IN MOLECULES
 Name Partner(s) Section Date STUDYING VIBRATIONS IN MOLECULES To complete this activity go to the following Internet site: http://academic.pgcc.edu/~ssinex/vibmol/vibrations_in_molecules.htm Images will
Name Partner(s) Section Date STUDYING VIBRATIONS IN MOLECULES To complete this activity go to the following Internet site: http://academic.pgcc.edu/~ssinex/vibmol/vibrations_in_molecules.htm Images will
You must have your answers written in PERMANENT ink if you want a regrade!!!! This means no test written in pencil or ERASABLE INK will be regraded.
 NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 13, 2014 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 320N Dr. Brent Iverson 1st Midterm Feb. 13, 2014 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson 1st Midterm Feb. 22, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson 1st Midterm Feb. 22, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
CHEM 203. Final Exam December 18, 2013 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
MIDTERM #3 PRACTICE TEST #1
 EM 234 PRITED FIRST AME the exam cover sheets look kind of like this PRITED LAST AME ASU ID or Posting ID Ian R. Gould Person on your LEFT (or Aisle) Person on your RIGT (or Aisle) PRIT YUR AME EA PAGE!
EM 234 PRITED FIRST AME the exam cover sheets look kind of like this PRITED LAST AME ASU ID or Posting ID Ian R. Gould Person on your LEFT (or Aisle) Person on your RIGT (or Aisle) PRIT YUR AME EA PAGE!
Organic Chemistry, Third Edition. Janice Gorzynski Smith University of Hawai i. Chapter 21. Aldehydes and Ketones Nucleophilic Addition
 Organic Chemistry, Third Edition Janice Gorzynski Smith University of Hawai i Chapter 21 Aldehydes and Ketones Nucleophilic Addition Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Organic Chemistry, Third Edition Janice Gorzynski Smith University of Hawai i Chapter 21 Aldehydes and Ketones Nucleophilic Addition Prepared by Rabi Ann Musah State University of New York at Albany Copyright
CHAPTER 25 HW: AMINES
 CAPTER 25 W: AMIES MECLATURE 1. Draw each compound. Structure ame triphenyl amine 3-fluoro--methyl-1-propanamine,-dipropylaniline 2. Give the IUPAC name for each compound, including R/S, cis/trans or E/Z
CAPTER 25 W: AMIES MECLATURE 1. Draw each compound. Structure ame triphenyl amine 3-fluoro--methyl-1-propanamine,-dipropylaniline 2. Give the IUPAC name for each compound, including R/S, cis/trans or E/Z
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
216 S10-Exam #1 Page 2. Name
 216 S10-Exam #1 Page 2. Name I. (3 points) Arrange the following four compounds in order of their R f values when analyzed by thinlayer chromatography (TLC) on silica gel-coated plates using C 2 Cl 2 as
216 S10-Exam #1 Page 2. Name I. (3 points) Arrange the following four compounds in order of their R f values when analyzed by thinlayer chromatography (TLC) on silica gel-coated plates using C 2 Cl 2 as
Please note: We routinely xerox a number of exams following initial grading to guard against receiving altered answers during the regrading process.
 NAME (Print): SIGNATURE: Chemistry 320N Dr. ent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 320N Dr. ent Iverson 1st Midterm Feb. 18, 2016 Please print the first three letters of your LAST name in the three boxes Please Note: This test may be a bit long, but
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 21, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 21, 2008 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Objective 4. Determine (characterize) the structure of a compound using IR, NMR, MS.
 Objective 4. Determine (characterize) the structure of a compound using IR, NMR, MS. Skills: Draw structure IR: match bond type to IR peak NMR: ID number of non-equivalent H s, relate peak splitting to
Objective 4. Determine (characterize) the structure of a compound using IR, NMR, MS. Skills: Draw structure IR: match bond type to IR peak NMR: ID number of non-equivalent H s, relate peak splitting to
Aldehydes and Ketones 2. Based on Organic Chemistry, J. G. Smith 3rde.
 Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
Aldehydes and Ketones 2 Based on Organic Chemistry, J. G. Smith 3rde. The Wittig Reaction Wittig reaction, named for German chemist Georg Wittig, who was awarded the Nobel Prize in Chemistry in 1979 for
CHEMISTRY 216 WINTER TERM 2007 END OF TERM EXAM. Time Allowed 2 hours
 EMISTRY 216 WITER TERM 2007 ED F TERM EXAM Time Allowed 2 hours ame KEY GSI ame ID umber Lab Section Write legibly. Illegible or messy answers will not be graded. Read these instructions carefully. In
EMISTRY 216 WITER TERM 2007 ED F TERM EXAM Time Allowed 2 hours ame KEY GSI ame ID umber Lab Section Write legibly. Illegible or messy answers will not be graded. Read these instructions carefully. In
Chemistry Organic Chemistry II: Reactivity and Synthesis ANSWERS FOR FINAL EXAM Winter 2004
 ASWER KEY Page 1 of 18 Chemistry 2.222 rganic Chemistry II: Reactivity and Synthesis ASWERS FR FIAL EXAM Winter 2004 Paper umber 631 Thursday April 22, 2004 1:30 4:30 pm University Centre Rm 210-224 Students
ASWER KEY Page 1 of 18 Chemistry 2.222 rganic Chemistry II: Reactivity and Synthesis ASWERS FR FIAL EXAM Winter 2004 Paper umber 631 Thursday April 22, 2004 1:30 4:30 pm University Centre Rm 210-224 Students
Chemistry 234 Exam 3. The Periodic Table
 ame: Last First MI Chemistry 234 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 24 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
ame: Last First MI Chemistry 234 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 24 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Loudon Chapter 19 Review: Aldehydes and Ketones CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES
 ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name when given the structure, and draw the structure given the name of open-chain and monocyclic
ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name when given the structure, and draw the structure given the name of open-chain and monocyclic
1. Name the following compound. Use the IUPAC system and include stereochemical designations.
 Chemistry 51 Exam 1, Fall 2004, Evening Division This is a closed book exam. The exam lasts 90 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name
Chemistry 51 Exam 1, Fall 2004, Evening Division This is a closed book exam. The exam lasts 90 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name
Topic 9. Aldehydes & Ketones
 Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 11, 2006 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 11, 2006 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
CHEM 203. Final Exam December 16, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CH 320/328 N Summer II 2018
 CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
Physical Properties. Alcohols can be: CH CH 2 OH CH 2 CH 3 C OH CH 3. Secondary alcohol. Primary alcohol. Tertiary alcohol
 Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Name: Student No: Page 1 of 10
 Name: Student No: Page 1 of 10 CEM 2220 rganic Chemistry II: Reactivity and Synthesis FINAL EXAM Winter Session 08R Paper 102 University centre, Room 210 224 Tuesday April 15, 2008 9:00 am 12:00 pm Students
Name: Student No: Page 1 of 10 CEM 2220 rganic Chemistry II: Reactivity and Synthesis FINAL EXAM Winter Session 08R Paper 102 University centre, Room 210 224 Tuesday April 15, 2008 9:00 am 12:00 pm Students
Organic Chemistry 321 Workshop: Spectroscopy NMR-IR Problem Set
 Organic Chemistry 321 Workshop: Spectroscopy NMR-IR Problem Set 1. Draw an NMR spectrum for each of the following compounds. Indicate each peak by a single vertical line (for example, a quartet would be
Organic Chemistry 321 Workshop: Spectroscopy NMR-IR Problem Set 1. Draw an NMR spectrum for each of the following compounds. Indicate each peak by a single vertical line (for example, a quartet would be
TOPIC 3. ALDEHYDES AND KETONES (Chapters 12 and 16)
 L TPIC 3. ALDEYDES AND KETNES (Chapters 12 and 16) BJECTIVES 1. Describe the synthesis aldehydes and ketones. 2. Describe the carbonyl group and oxidation-reductions reactions associated with alcohols
L TPIC 3. ALDEYDES AND KETNES (Chapters 12 and 16) BJECTIVES 1. Describe the synthesis aldehydes and ketones. 2. Describe the carbonyl group and oxidation-reductions reactions associated with alcohols
Lecture 18. Oxidation and Reduction. Oxidation. Reduction O CH 4 CH 3 OH H C H. Chemistry 328N
 Lecture 18 xidation and Reduction C 4 C 3 C C C xidation Reduction March 27, 2018 Suppose you want to make this compound????? C + BrC 2 C 2 C?? CC 2 C 2 C 4-ydroxy-4-phenylbutanal It s an alcohol. Use
Lecture 18 xidation and Reduction C 4 C 3 C C C xidation Reduction March 27, 2018 Suppose you want to make this compound????? C + BrC 2 C 2 C?? CC 2 C 2 C 4-ydroxy-4-phenylbutanal It s an alcohol. Use
N_HW1 N_HW1. 1. What is the purpose of the H 2 O in this sequence?
 N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
Chapter 18: Ketones and Aldehydes. I. Introduction
 1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
Chemistry 3720 Old Exams. Practice Exams & Keys
 Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
Chemistry 3720 ld Exams Practice Exams & Keys 2015-17 Spring 2017 Page File 3 Spring 2017 Exam 1 10 Spring 2017 Exam 1 Key 16 Spring 2017 Exam 2 23 Spring 2017 Exam 2 Key 29 Spring 2017 Exam 3 36 Spring
Chemistry 333. Final Examination
 Chemistry 333 Final Examination December 9, 2010 Professor Charonnat Be certain that your examination has eleven (11) pages including this one. Put your name on each page of this examination booklet. By
Chemistry 333 Final Examination December 9, 2010 Professor Charonnat Be certain that your examination has eleven (11) pages including this one. Put your name on each page of this examination booklet. By
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Still More Carbonyl Chemistry
 Lecture 17 Still More arbonyl hemistry ' ' A B P( 6 5 ) 3 A P( 6 5 ) 3 B March 22, 2018 eaction Theme The most common reaction of a carbonyl group is addition of a nucleophile to form a tetrahedral addition
Lecture 17 Still More arbonyl hemistry ' ' A B P( 6 5 ) 3 A P( 6 5 ) 3 B March 22, 2018 eaction Theme The most common reaction of a carbonyl group is addition of a nucleophile to form a tetrahedral addition
Important Concepts. Problems. Chapter Problems. Cuprate additions followed by enolate alkylations. 15. Michael Addition (Section 18-11)
 Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
12. Aldehydes & Ketones (text )
 2009, Department of Chemistry, The University of Western ntario 12.1 12. Aldehydes & Ketones (text 13.1 13.11) A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is
2009, Department of Chemistry, The University of Western ntario 12.1 12. Aldehydes & Ketones (text 13.1 13.11) A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is
1. Name the following compound. Use the IUPAC system and include the stereochemical designations.
 Chemistry 51 Exam 1, Summer 2004 This is a closed book exam. The exam lasts 100 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer
Chemistry 51 Exam 1, Summer 2004 This is a closed book exam. The exam lasts 100 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer
Chemistry Final Examinations Summer 2006 answers
 Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
18.10 Conjugate Additions O O. O X BrCH 2 CH 2 CH 2 CCH 3 ± ± BrCH 2 CH 2 CH 2 CH 3. TsOH 1 O C O O W 3 CH 3 CCH 3 CH 3 CCH 2 CH 2 CH 2 CH 3
 80 NJUGATE ADDITINS 779 An attempt to form a Grignard reagent from 5-bromo- 2-pentanone is doomed to failure because the Grignard will react with the carbonyl group Therefore, the carbonyl group is first
80 NJUGATE ADDITINS 779 An attempt to form a Grignard reagent from 5-bromo- 2-pentanone is doomed to failure because the Grignard will react with the carbonyl group Therefore, the carbonyl group is first
Chemistry 51 Summer 2006 Final Exam
 Chemistry 51 Summer 2006 Final Exam 1. This is a two-hour, closed book exam. All answers are to be put on the question sheets. 2. Use the backsides of these pages for scratch paper. None other permitted.
Chemistry 51 Summer 2006 Final Exam 1. This is a two-hour, closed book exam. All answers are to be put on the question sheets. 2. Use the backsides of these pages for scratch paper. None other permitted.
EXAM #3 EXTRA PROBLEMS
 Massachusetts Institute of Technology 5.13, Fall 2006 Dr. Kimberly L. Berkowski rganic chemistry II EXAM #3 EXTRA PRBLEMS What to expect on Exam #3: 1. ~1 Labeling experiment 2. ~2 chanisms 3. ~2 Syntheses
Massachusetts Institute of Technology 5.13, Fall 2006 Dr. Kimberly L. Berkowski rganic chemistry II EXAM #3 EXTRA PRBLEMS What to expect on Exam #3: 1. ~1 Labeling experiment 2. ~2 chanisms 3. ~2 Syntheses
Note: You must have your answers written in pen if you want a regrade!!!!
 NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 23, 2006 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
NAME (Print): SIGNATURE: Chemistry 310N Dr. Brent Iverson 1st Midterm Feb. 23, 2006 Please print the first three letters of your last name in the three boxes Please Note: This test may be a bit long, but
Name: Student Number: University of Manitoba - Department of Chemistry CHEM Introductory Organic Chemistry II - Term Test 2
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 2 Thursday, March 12, 2015; 7-9 PM This is a 2-hour test, marked out of
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 2 Thursday, March 12, 2015; 7-9 PM This is a 2-hour test, marked out of
Aldehydes and Ketones Reactions. Dr. Sapna Gupta
 Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Exam 3 Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3331-100 Spring 2008 Exam 3 Professor R. oenigman igh = 102 Low = 15 Average = 68 Signature ame (printed) Last four digits of your student ID number Recitation
I pledge to uphold the CU onor Code: CEM 3331-100 Spring 2008 Exam 3 Professor R. oenigman igh = 102 Low = 15 Average = 68 Signature ame (printed) Last four digits of your student ID number Recitation
CHEM 2220 Organic Chemistry II: Reactivity and Synthesis Prof. P.G. Hultin. FINAL EXAM Winter Session 2017R
 CHEM 2220 Organic Chemistry II: Reactivity and Synthesis Prof. P.G. Hultin FINAL EXAM Winter Session 2017R Tuesday April 25, 2017 8:00 am 11:00 am Frank Kennedy Gold Gym PRINT LEGIBLY PLEASE Name: Student
CHEM 2220 Organic Chemistry II: Reactivity and Synthesis Prof. P.G. Hultin FINAL EXAM Winter Session 2017R Tuesday April 25, 2017 8:00 am 11:00 am Frank Kennedy Gold Gym PRINT LEGIBLY PLEASE Name: Student
Name: 1. Ignoring C-H absorptions, what characteristic IR absorption(s) would be expected for the functional group shown below?
 Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
Organic Chemistry II (CHE ) Examination I February 11, Name (Print legibly): Key. Student ID#:
 rganic hemistry II (HE 232-001) Examination I February 11, 2009 Name (Print legibly): Key (last) (first) Student ID#: PLEASE observe the following: You are allowed to have scratch paper (provided by me),
rganic hemistry II (HE 232-001) Examination I February 11, 2009 Name (Print legibly): Key (last) (first) Student ID#: PLEASE observe the following: You are allowed to have scratch paper (provided by me),
CHEMISTRY 252 Exam pts. Section 703 Grand Rapids 27 July 2006
 ame PID CMISTRY 252 xam 1 100 pts. Section 70 Grand Rapids 27 July 2006 Make sure you have all 12 exam pages You will have 90 minutes to complete the 5 questions Please sign your name at the bottom of
ame PID CMISTRY 252 xam 1 100 pts. Section 70 Grand Rapids 27 July 2006 Make sure you have all 12 exam pages You will have 90 minutes to complete the 5 questions Please sign your name at the bottom of
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry
 Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
CARBOXYLIC ACIDS AND THEIR DERIVATIVES. 3. Predict the relative acidity and basicity of compounds and ions. Important criteria include:
 CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
CHEMISTRY 341. Final Exam Tuesday, December 16, Problem 1 15 pts Problem 9 8 pts. Problem 2 5 pts Problem pts
 CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Name: 1. Ignoring C-H absorptions, what characteristic IR absorption(s) would be expected for the functional group shown below?
 Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, CHEM 241 : APPLIED ORGANIC CHEMISTRY FOR CHEMICAL ENGINEERS JUNE 2006 EXAMINATION MODEL ANSWERS
 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN NE 1.1 Give four reasons why benzene is aromatic.
School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN NE 1.1 Give four reasons why benzene is aromatic.
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE FINAL EXAMINATION CHEMISTRY 350
 Page 1 of 16 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 350 April, 1997 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for
Page 1 of 16 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 350 April, 1997 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for
