UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, CHEM 241 : APPLIED ORGANIC CHEMISTRY FOR CHEMICAL ENGINEERS JUNE 2006 EXAMINATION MODEL ANSWERS
|
|
|
- Prudence Stephens
- 6 years ago
- Views:
Transcription
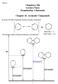
1 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN NE 1.1 Give four reasons why benzene is aromatic. It is cyclic It is has uninterrupted cyclic cloud of π e- above and below the ring. It is planar and each ring atom has unhybridised p orbital. It has an odd number of π e- pairs it satisfies the (4n + 2) π e- rule. ½ mark each. (2) 1.2 Draw resonance forms of cyclopentadienyl anion. (1) cyclopentadienyl anion 1/2 mk 1/2 mk 2
2 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS 1.3 Which ion in each of the following pairs is more stable? Give your reasons. (2) or or is more stable because it is aromatic - it has one π electron pair (it obeys the [4n + 2]π e - rule) { for correct choice and 1.5 for correct reason} is more stable because it is aromatic - it has three π electron pairs (it obeys the [4n + 2]π e - rule) { for correct choice and 1.5 for correct reason} 1.4 Draw structures of the following compounds. m chlorotoluene 4 Bromo -2-chlorophenol c) o Iodobenzene sulphonic acid d) 2,5 Dinitrobenzaldelyde (4) 3
3 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS C 3 Cl c) S 3 I d) Cl C N 2 Br 2 N 4
4 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN NE (Continued) 1.5 Complete the following reactions. Provide answers for A F. (9) I 2 / N 3 A B AlCl 3 c) C + Cl d) C 3 C3C2Cl/ AlCl3 D Cl e) N 2 Br 2 /Fe E f) F S 3 5
5 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS A = I B = Cl 2 mks C 3 C = Cl 2 /FeCl 3 D = 2 mks N 2 C 2 C 3 E = Br 2 mks F = S 3 / 2 S 4 6
6 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN NE (Continued) 1.6 Give a detailed mechanism for the following reactions (6) C 3 C 3 N 3 / 2 S 4 N 2 + C 3 CC 3 2 S 4 Δ 3 C C 3 N 2 S 3 2 N S 3 2 N N 2 + C 3 C 3 C 3 + N N - S 3 N [25] 7
7 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN TW 2.1 Write a structure for each of the following compounds. (5) Phenyl acetate N-Benzylethanamide c) Propanoic anhydride d) Benzoyl chloride e) Cyclohexanone 3 C 3 C N 2 mks c) d) Cl e) 8
8 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN TW (Continued) 2.2 Write structures for the products of the following reactions. + 3 C Cl 3 C (6) 3 C C 3 + C 3 C 2 c) C3 C 2 C C 2 C 3 + C 3 N 2 d) C 3 C + C 3 (excess) + 3 C Cl + 3 C 3 C C 3 + C 3 C 2 + c) C3 C 2 C C 2 C 3 + C 3 N 2 d) C 3 C + C 3 (excess) + N C 3 C 2 9
9 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN TW (Continued) 2.3 Give a detailed mechanism for each of the following reactions. Reaction of acetyl chloride with water to form acetic acid. (4) Reaction of methyl ethanoate with water in the presence acid. 3 C Cl 3 C Cl 3 C Cl - (4) 3 C + 2 1mk C 3 10
10 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN TW (Continued) 2.4 Give the product of the following reactions. (A catalytic amount of acid is present in each reaction.) Cyclopentanone + ethylamine Propanal + ethylamine c) cyclopentanone + diethylamine d) cyclohexanone + ethanol (6) NEt N Et Et C 2 C 3 c) N Et d) [25] 11
11 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN TREE 3.1 Give the products of the following reactions. Indicate whether each reaction is an oxidation or reduction. (9) C 3 C 2 C 2 C 2 C 2 Na 2 Cr S 4 C C 2 KMn 4 -, heat c) C 3 C 2 C 2 CCl d) C 3 C 2 C 2 CNC 3 2 /Pd-C 1. LiAl 4 e) Peracid 2. 2 oxidation C 2 NC 3 oxidation reduction reduction oxidation f) C 3 C 2 C 2 C CC , -78 o C C 3 C 2 oxidation 3.2 Give an example of each of the following named reactions using a chemical equation. Baeyer-Villiger oxidation Swern oxidation c) zonolysis 12 (6)
12 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS [15] Baeyer-Villiger oxidation: Aldehyde RC 2 Acid 2 mks Ketone RC 2 Ester Swern oxidation: Primary alcohol 1. DMS, Cl 2 C 2 2. Et 3 N Aldehyde 2 mks c) Wolff-Kshner reduction: Ketone 1. N /eat Alkane 2 mks QUESTIN FUR 4.1 Which peak would be more intense in the mass spectrum of the following compounds? 3-methylpentane 2-methyl pentane c) 2-hexanol (3) C 4 9 = m/z 57 C 3 7 = m/z 43 c) C 5 11 = m/z 71 13
13 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS QUESTIN FUR (Continued) 4.2 Using curved arrows, show the principal fragment that would be observed in the mass spectrum of each of the following compounds. C 3 (C 2 ) 3 C 2 C 3 C 2 C 2 C 2 C 3 (6) -e 1.5 mk C + i - e iii ii i + C 3 iii ii + C 3 C 2 3 C + C 3 C 2 C 2 14
14 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS 4.3 Indicate which of the following compounds has a vibration that is infrared Inactive or active? Acetone active 2 inactive c) 2 active d) ethane active (2) 4.4 A UV spectrum of acetone shows two λ max at 195nm (ε = 9000) and 274nm (ε = 13.6). What electronic transitions are responsible for the two λ max s? (i) λ max at 195 nm: (ii) λ max at 274 nm: π to π n to π (2) [13] QUESTIN FIVE 15
15 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS 5.1 ow many signals would you expect to see in the 1 NMR spectrum of each of the following compounds? C 3 C 2 C 2 C 3 Cl c) 2-pentanone 2 signals 2 mks 3 signals 2 mks c) 4 signals 2 mks (6) 5.2 A signal has been reported to occur at 600z downfield from TMS in an NMR spectrometer? What is the chemical shift of the signal? What would its chemical shift be in an instrument operating at 100Mz? 2.0 ppm 2.0 ppm (3) 5.3 Indicate the number of signals and the multiplicity of each signal in the 16
16 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS 1 NMR spectrum of 1-bromo-3-iodopropane C 4 (3) 1 signal, triplet Br 1 signal; multiplet I 1 signal; triplet 2 mks C 4 1 signal, singlet [10] QUESTIN SIX 6.1 What is a polymer? (1) A polymer is a large molecule made by linking together repeating units of small molecules called monomer. [ ] 6.2 What are the two major classes of synthetic polymers? (2) (i) Addition polymer ( chain-growth polymer) [] (ii) Codensation polymer ( step-growth polymer) [ ] 6.3 The repeating unit of a polymer is called? (0.5) Monomer [ 0.5] 17
17 School of Chemistry, UNIVERSITY F KWAZULU-NATAL, WARD CLLEGE, CEM 241 : APPLIED RGANIC CEMISTRY FR CEMICAL ENGINEERS JUNE 2006 EXAMINATIN MDEL ANSWERS 6.4 What are the three stages of radical polymerization? (i) Chain initiation [ ] (ii) Chain propagation [ ] (iii) Chain termination [ ] (1.5) 6.5 What is a synthetic rubber? A synthetic rubber is a polymer of dienes other than isoprene ( 2-methyl-1,3- butadiene) (2) 6.6 Give the structure of neoprene. (2) Cl Cl n > 5000 [10] END 18
UNIVERSITY OF NATAL DURBAN EXAMINATIONS : NOVEMBER 2001 ORGANIC CHEMISTRY FOR CHEMICAL ENGINEERS DSC 2OE2. Time : 2 Hours Total Marks : 100
 UNIVERSITY F NATAL DURBAN EXAMINATINS : NVEMBER 2001 RGANIC CHEMISTRY FR CHEMICAL ENGINEERS DSC 2E2 Time : 2 Hours Total Marks : 100 INTERNAL EXAMINERS : Professor H C Brookes Dr N Koorbanally EXTERNAL
UNIVERSITY F NATAL DURBAN EXAMINATINS : NVEMBER 2001 RGANIC CHEMISTRY FR CHEMICAL ENGINEERS DSC 2E2 Time : 2 Hours Total Marks : 100 INTERNAL EXAMINERS : Professor H C Brookes Dr N Koorbanally EXTERNAL
1.1 Is the following molecule aromatic or not aromatic? Give reasons for your answer.
 Page 1 QUESTION ONE 1.1 Is the following molecule aromatic or not aromatic? Give reasons for your answer. 1.2 List four criteria which compounds must meet in order to be considered aromatic. Page 2 QUESTION
Page 1 QUESTION ONE 1.1 Is the following molecule aromatic or not aromatic? Give reasons for your answer. 1.2 List four criteria which compounds must meet in order to be considered aromatic. Page 2 QUESTION
CHE 325 ALDEHYDES AND KETONES I CHAP 18 ASSIGN OCH 3 HCN C 4 H 7 NO. would be: CH 3 B. CH 3CH 2COOCH 3
 CE 325 ALDEYDES AND KETNES I CAP 18 ASSIGN 1. What is the correct IUPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-Isopropyl-4-octanone D. Isobutyl propyl ketone
CE 325 ALDEYDES AND KETNES I CAP 18 ASSIGN 1. What is the correct IUPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-Isopropyl-4-octanone D. Isobutyl propyl ketone
ALDEHYDES AND KETONES
 CE 325 ALDEYDES AND KETNES CAP 17 ASSGN NUCLEPLC ADDTN T TE CARBNYL GRUP 1. What is the correct UPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-sopropyl-4-octanone
CE 325 ALDEYDES AND KETNES CAP 17 ASSGN NUCLEPLC ADDTN T TE CARBNYL GRUP 1. What is the correct UPAC name for the following compound A. 2-Methyl-5-heptanone B. 7-Methyl-4-octanone C. 6-sopropyl-4-octanone
Introduction & Definitions Catalytic Hydrogenations Dissolving Metal Reduction Reduction by Addition of H- and H+ Oxidation of Alcohols Oxidation of
 CEM 241- UNIT 4 xidation/reduction Reactions Redox chemistry 1 utline Introduction & Definitions Catalytic ydrogenations Dissolving Metal Reduction Reduction by Addition of - and + xidation of Alcohols
CEM 241- UNIT 4 xidation/reduction Reactions Redox chemistry 1 utline Introduction & Definitions Catalytic ydrogenations Dissolving Metal Reduction Reduction by Addition of - and + xidation of Alcohols
A. B. C. D. 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal?
 Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
OCH 2 CH 3. A) 2-chlorohexyl ethanoate C) ethyl 2-chlorohexanoate B) 1-chlorohexyl ethanoate D) ethyl 1-chlorohexanoate
 (2 points each) Write your answer in the box to the right of the question. 1. A mixture of 1-hexanol and hexanoic acid in diethyl ether is shaken with an aqueous sodium bicarbonate solution. Which line
(2 points each) Write your answer in the box to the right of the question. 1. A mixture of 1-hexanol and hexanoic acid in diethyl ether is shaken with an aqueous sodium bicarbonate solution. Which line
CHEM 3.2 (AS91388) 3 credits. Demonstrate understanding of spectroscopic data in chemistry
 CHEM 3.2 (AS91388) 3 credits Demonstrate understanding of spectroscopic data in chemistry Spectroscopic data is limited to mass, infrared (IR) and 13 C nuclear magnetic resonance (NMR) spectroscopy. Organic
CHEM 3.2 (AS91388) 3 credits Demonstrate understanding of spectroscopic data in chemistry Spectroscopic data is limited to mass, infrared (IR) and 13 C nuclear magnetic resonance (NMR) spectroscopy. Organic
CHEMISTRY 341. Final Exam Tuesday, December 16, Problem 1 15 pts Problem 9 8 pts. Problem 2 5 pts Problem pts
 CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry
 OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
CH 3. mirror plane. CH c d
 CAPTER 20 Practice Exercises 20.1 The index of hydrogen deficiency is two. The structural possibilities include two double bonds, a double do 20.3 (a) As this is an alkane, it contains only C and and has
CAPTER 20 Practice Exercises 20.1 The index of hydrogen deficiency is two. The structural possibilities include two double bonds, a double do 20.3 (a) As this is an alkane, it contains only C and and has
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY
 4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
CHEMISTRY Topic #8: Oxidation and Reduction Reactions Fall 2018 Dr. Susan Findlay
 CEMISTRY 2600 Topic #8: xidation and Reduction Reactions Fall 2018 Dr. Susan Findlay xidation States of Carbon Carbon can have any oxidation state from -4 (C 4 ) to +4 (C 2 ). As a general rule, increasing
CEMISTRY 2600 Topic #8: xidation and Reduction Reactions Fall 2018 Dr. Susan Findlay xidation States of Carbon Carbon can have any oxidation state from -4 (C 4 ) to +4 (C 2 ). As a general rule, increasing
Chapter 7. dehydration
 hapter 7 7.1 ne problem with elimination reactions is that mixtures of products are often formed. For example, treatment of 2-bromo-2-methylbutane with K in ethanol yields a mixture of two alkene products.
hapter 7 7.1 ne problem with elimination reactions is that mixtures of products are often formed. For example, treatment of 2-bromo-2-methylbutane with K in ethanol yields a mixture of two alkene products.
E35 SPECTROSCOPIC TECHNIQUES IN ORGANIC CHEMISTRY
 E35 SPECTRSCPIC TECNIQUES IN RGANIC CEMISTRY Introductory Comments. These notes are designed to introduce you to the basic spectroscopic techniques which are used for the determination of the structure
E35 SPECTRSCPIC TECNIQUES IN RGANIC CEMISTRY Introductory Comments. These notes are designed to introduce you to the basic spectroscopic techniques which are used for the determination of the structure
Q.8. Isomers are the compounds that must have same
 Choose the single correct answer for each of the following questions: (1 Mark each). Q. 1. ow many lone pairs are present in C 3 --C 3 i) 3 ii) 2 iii) 1 iv) 4 Q.2. When any hydrocarbon is added to water,
Choose the single correct answer for each of the following questions: (1 Mark each). Q. 1. ow many lone pairs are present in C 3 --C 3 i) 3 ii) 2 iii) 1 iv) 4 Q.2. When any hydrocarbon is added to water,
ALDEHYDES AND KETONES
 ALDEHYDES AND KETONES IN WEEK 1, A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name given the structure, and draw the structure given the name, of aldehydes and ketones. Also, draw the structure given
ALDEHYDES AND KETONES IN WEEK 1, A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name given the structure, and draw the structure given the name, of aldehydes and ketones. Also, draw the structure given
CARBOXYLIC ACIDS AND THEIR DERIVATIVES. 3. Predict the relative acidity and basicity of compounds and ions. Important criteria include:
 CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
CHEM 2312 practice final. Version I
 CEM 2312 practice final Version The following standardized final examination covers the entire introductory year of organic chemistry (CEM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
CEM 2312 practice final Version The following standardized final examination covers the entire introductory year of organic chemistry (CEM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
Objective 4. Determine (characterize) the structure of a compound using IR, NMR, MS.
 Objective 4. Determine (characterize) the structure of a compound using IR, NMR, MS. Skills: Draw structure IR: match bond type to IR peak NMR: ID number of non-equivalent H s, relate peak splitting to
Objective 4. Determine (characterize) the structure of a compound using IR, NMR, MS. Skills: Draw structure IR: match bond type to IR peak NMR: ID number of non-equivalent H s, relate peak splitting to
(07) 3 (e) Calculate the ph of this buffer solution at 298 K. Give your answer to 2 decimal places
 7 3 (e) An acidic buffer solution is formed when 10.0 cm3 of 0.125 mol dm 3 aqueous sodium hydroxide are added to 15.0 cm3 of 0.174 mol dm 3 aqueous HX. The value of Ka for the weak acid HX is 3.01 10
7 3 (e) An acidic buffer solution is formed when 10.0 cm3 of 0.125 mol dm 3 aqueous sodium hydroxide are added to 15.0 cm3 of 0.174 mol dm 3 aqueous HX. The value of Ka for the weak acid HX is 3.01 10
Learning Guide for Chapter 3 - Infrared Spectroscopy
 Learning Guide for hapter 3 - Infrared Spectroscopy I. Introduction to spectroscopy - p 1 II. Molecular vibrations - p 3 III. Identifying functional groups - p 6 IV. Interpreting an IR spectrum - p 12
Learning Guide for hapter 3 - Infrared Spectroscopy I. Introduction to spectroscopy - p 1 II. Molecular vibrations - p 3 III. Identifying functional groups - p 6 IV. Interpreting an IR spectrum - p 12
1. Which compound would you expect to have the lowest boiling point? A) NH 2 B) NH 2
 MULTIPLE CICE QUESTINS Topic: Intermolecular forces 1. Which compound would you expect to have the lowest boiling point? A) N 2 B) N 2 C) N D) E) N Ans: : N 2 D Topic: Molecular geometry, dipole moment
MULTIPLE CICE QUESTINS Topic: Intermolecular forces 1. Which compound would you expect to have the lowest boiling point? A) N 2 B) N 2 C) N D) E) N Ans: : N 2 D Topic: Molecular geometry, dipole moment
B. (3 pts) The following values are independent of the operating frequency of the NMR: a. Coupling constant; c. Chemical shift; b. Gyromagnetic ratio;
 CEMISTRY 314-01 MIDTERM # 1 answer key September 29, 2009 Statistics: Average: 70 pts (70%); ighest: 96 pts (96%); Lowest: 37 pts (37%) Number of students performing at or above average: 16 (57%) Number
CEMISTRY 314-01 MIDTERM # 1 answer key September 29, 2009 Statistics: Average: 70 pts (70%); ighest: 96 pts (96%); Lowest: 37 pts (37%) Number of students performing at or above average: 16 (57%) Number
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE FINAL EXAMINATION CHEMISTRY 353
 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 353 April 29 th, 2002 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for TR lectures)
TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 353 April 29 th, 2002 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for TR lectures)
4. NMR spectra. Interpreting NMR spectra. Low-resolution NMR spectra. There are two kinds: Low-resolution NMR spectra. High-resolution NMR spectra
 1 Interpreting NMR spectra There are two kinds: Low-resolution NMR spectra High-resolution NMR spectra In both cases the horizontal scale is labelled in terms of chemical shift, δ, and increases from right
1 Interpreting NMR spectra There are two kinds: Low-resolution NMR spectra High-resolution NMR spectra In both cases the horizontal scale is labelled in terms of chemical shift, δ, and increases from right
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE FINAL EXAMINATION CHEMISTRY 350
 Page 1 of 16 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 350 April, 1997 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for
Page 1 of 16 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 350 April, 1997 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for
CH 320/328 N Summer II 2018
 CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
Nuclear Magnetic Resonance Spectroscopy (NMR)
 OCR Chemistry A 432 Spectroscopy (NMR) What is it? An instrumental method that gives very detailed structural information about molecules. It can tell us - how many of certain types of atom a molecule
OCR Chemistry A 432 Spectroscopy (NMR) What is it? An instrumental method that gives very detailed structural information about molecules. It can tell us - how many of certain types of atom a molecule
IR, MS, UV, NMR SPECTROSCOPY
 CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET All Sections CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET General Instructions for the 318 Spectroscopy Problem Set Consult the Lab Manual,
CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET All Sections CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET General Instructions for the 318 Spectroscopy Problem Set Consult the Lab Manual,
Reactions of Alkenes and Alkynes
 5 2 2 2 2 2 2 2 Reactions of Alkenes and Alkynes APTER SUMMARY Addition is the characteristic reaction of alkenes and alkynes. Since the carbons of a double or triple bond do not have the maximum number
5 2 2 2 2 2 2 2 Reactions of Alkenes and Alkynes APTER SUMMARY Addition is the characteristic reaction of alkenes and alkynes. Since the carbons of a double or triple bond do not have the maximum number
1. How do you account for the formation of ethane during chlorination of methane?
 1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
ST. JOSEPH S COLLEGE OF ARTS & SCIENCE (AUTONOMOUS) ST. JOSEPH S COLLEGE ROAD, CUDDALORE CH101T ORGANIC CHEMISTRY I (SEMESTER-I)
 UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
GCE A level 1094/01 CHEMISTRY CH4
 Surname ther Names Centre 2 Candidate GCE A level 1094/01 CHEMISTRY CH4 P.M. MNDAY, 14 January 2013 1¾ hours ADDITINAL MATERIALS In addition to this examination paper, you will need: Data Sheet Periodic
Surname ther Names Centre 2 Candidate GCE A level 1094/01 CHEMISTRY CH4 P.M. MNDAY, 14 January 2013 1¾ hours ADDITINAL MATERIALS In addition to this examination paper, you will need: Data Sheet Periodic
CHEM Chapter 13. Nuclear Magnetic Spectroscopy (Homework) W
 CHEM 2423. Chapter 13. Nuclear Magnetic Spectroscopy (Homework) W Short Answer 1. For a nucleus to exhibit the nuclear magnetic resonance phenomenon, it must be magnetic. Magnetic nuclei include: a. all
CHEM 2423. Chapter 13. Nuclear Magnetic Spectroscopy (Homework) W Short Answer 1. For a nucleus to exhibit the nuclear magnetic resonance phenomenon, it must be magnetic. Magnetic nuclei include: a. all
Organic Chemistry. Pre-lab Assignment. Purpose. Background. Experiment 14. Before coming to lab: Hydrocarbons. Read the lab thoroughly.
 Experiment 14 Pre-lab Assignment Before coming to lab: Read the lab thoroughly. rganic hemistry Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered
Experiment 14 Pre-lab Assignment Before coming to lab: Read the lab thoroughly. rganic hemistry Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered
(please print and circle your last name) CHEMISTRY 332 FINAL EXAM. December 11, 2006
 Seat No. Name (please print and circle your last name) CEMISTRY 332 FINAL EXAM December 11, 2006 I. (18 points) II. III. IV. (48 points) (50 points) (40 points) V. (26 points) VI. (18 points) TTAL (200
Seat No. Name (please print and circle your last name) CEMISTRY 332 FINAL EXAM December 11, 2006 I. (18 points) II. III. IV. (48 points) (50 points) (40 points) V. (26 points) VI. (18 points) TTAL (200
C h a p t e r S i x t e e n: Nuclear Magnetic Resonance Spectroscopy. An 1 H NMR FID of ethanol
 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 3.6 C h a p t e r S i x t e e n: Nuclear Magnetic Resonance Spectroscopy An 1 NMR FID of ethanol Note: Problems with italicized numbers
0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2.2 2.4 2.6 2.8 3.0 3.2 3.4 3.6 C h a p t e r S i x t e e n: Nuclear Magnetic Resonance Spectroscopy An 1 NMR FID of ethanol Note: Problems with italicized numbers
Name: Date: I. Multiple Choice (Circle the letter for the best answer)
 Organic Chemistry II Sample Exam 3 You should also be able to name compounds, draw structures from names, and complete reactions given the reactants and conditions of the reaction. Name: Date: I. Multiple
Organic Chemistry II Sample Exam 3 You should also be able to name compounds, draw structures from names, and complete reactions given the reactants and conditions of the reaction. Name: Date: I. Multiple
Write your name and date on the cover page Do not open exam until instructed to do so
 Write your name and date on the cover page Do not open exam until instructed to do so Name: Date: Exam III hem. 210 Do not open exam until told to do so. Get out your pencil, eraser, and scientific nongraphing
Write your name and date on the cover page Do not open exam until instructed to do so Name: Date: Exam III hem. 210 Do not open exam until told to do so. Get out your pencil, eraser, and scientific nongraphing
Mechanism Summary for A-level AQA Chemistry
 Mechanism Summary for Alevel AQA hemistry Electrophilic Addition of Alkenes with omine Electrophilic Addition of Alkenes with sulphuric acid 3 S 2 3 3 S 2 S 2 Electrophilic Addition of Alkenes with hydrogen
Mechanism Summary for Alevel AQA hemistry Electrophilic Addition of Alkenes with omine Electrophilic Addition of Alkenes with sulphuric acid 3 S 2 3 3 S 2 S 2 Electrophilic Addition of Alkenes with hydrogen
2 Answer all the questions. CH(NH 2. )COOH, R is CH [1] (ii) Draw the structures of the ions formed by alanine at ph 6.0 and at ph 1.5.
![2 Answer all the questions. CH(NH 2. )COOH, R is CH [1] (ii) Draw the structures of the ions formed by alanine at ph 6.0 and at ph 1.5. 2 Answer all the questions. CH(NH 2. )COOH, R is CH [1] (ii) Draw the structures of the ions formed by alanine at ph 6.0 and at ph 1.5.](/thumbs/94/121313851.jpg) 2 Answer all the questions. 1 This question looks at the properties and chemistry of some α-amino acids. The general formula of an α-amino acid is R(N 2 ). (a) In the α-amino acid alanine, 3 (N 2 ), R
2 Answer all the questions. 1 This question looks at the properties and chemistry of some α-amino acids. The general formula of an α-amino acid is R(N 2 ). (a) In the α-amino acid alanine, 3 (N 2 ), R
Name: 1. Ignoring C-H absorptions, what characteristic IR absorption(s) would be expected for the functional group shown below?
 Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
Name: 1. Ignoring C-H absorptions, what characteristic IR absorption(s) would be expected for the functional group shown below?
 Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
Chemistry 262 Winter 2018 Exam 3 Practice The following practice contains 20 questions. Thursday s 90 exam will also contain 20 similar questions, valued at 4 points/question. There will also be 2 unknown
CHAPTER 24 Organic Chemistry
 CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
OH OH OH CH 2 CH 2 C(CH 3 ) 2 (a) CH 3 CHCH 2 CHCH(CH 3 ) 2. (b)
 hem 226 Problem Set #8 Fundamentals of rganic hemistry, 4 th edition, John McMurry. hapter 8 1. Give IUPA names for the following alcohols. 2 2 ( 3 ) 2 3 2 ( 3 ) 2 Longest chain = 6 carbons:...hexanediol.
hem 226 Problem Set #8 Fundamentals of rganic hemistry, 4 th edition, John McMurry. hapter 8 1. Give IUPA names for the following alcohols. 2 2 ( 3 ) 2 3 2 ( 3 ) 2 Longest chain = 6 carbons:...hexanediol.
Name Date Class. aryl halides substitution reaction
 23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
Closed book exam, no books, notebooks, notes, etc. allowed. However, calculators, rulers, and molecular model sets are permitted.
 Massachusetts Institute of Technology Organic Chemistry 5.13 Friday, September 26, 2003 Prof. Timothy F. Jamison Hour Exam #1 Name (please both print and sign your name) Official Recitation Instructor
Massachusetts Institute of Technology Organic Chemistry 5.13 Friday, September 26, 2003 Prof. Timothy F. Jamison Hour Exam #1 Name (please both print and sign your name) Official Recitation Instructor
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
 NMR Spectroscopy 1 NULEAR MAGNETI RESONANE SPETROSOPY Involves interaction of materials with the low-energy radiowave region of the electromagnetic spectrum Origin of Spectra Theory All nuclei possess
NMR Spectroscopy 1 NULEAR MAGNETI RESONANE SPETROSOPY Involves interaction of materials with the low-energy radiowave region of the electromagnetic spectrum Origin of Spectra Theory All nuclei possess
Chemistry 51 Summer 2007 Final Exam
 Chemistry 51 Summer 2007 Final Exam 1. This is a two-hour, closed book exam. All answers are to be put on the question sheets. 2. Use the backsides of these pages for scratch paper. None other permitted.
Chemistry 51 Summer 2007 Final Exam 1. This is a two-hour, closed book exam. All answers are to be put on the question sheets. 2. Use the backsides of these pages for scratch paper. None other permitted.
CHEM Review for test 1 (ch12-15). F17. Stafford
 CHEM Multiple Choice Identify the choice that best completes the statement or answers the question. 1. What range of wavelengths corresponds to the infrared region of the electromagnetic spectrum? a. 200-400
CHEM Multiple Choice Identify the choice that best completes the statement or answers the question. 1. What range of wavelengths corresponds to the infrared region of the electromagnetic spectrum? a. 200-400
CHEMISTRY MIDTERM # 1 answer key February 10, 2005
 CEMSTRY 314-0 MDTERM # 1 answer key February 10, 005 Statistics: Average: 77 pts (77%); ighest: 99 pts (99%); Lowest: 40 pts (40%) Number of students performing at or above average: 5 (54%) 1. (8 pts)
CEMSTRY 314-0 MDTERM # 1 answer key February 10, 005 Statistics: Average: 77 pts (77%); ighest: 99 pts (99%); Lowest: 40 pts (40%) Number of students performing at or above average: 5 (54%) 1. (8 pts)
Cyclooctatetraene (2R)-2-phenylbutane benzyl chloride cycloocta-1,3,5,7-tetraene
 CM238-02 S05 Practice Material for xam 1 This is a compilation from relevant problems from last spring s exam 1&2. This is too long! 1. (4 pts) Draw 2 of the following 3: Cross one out or graded in order.
CM238-02 S05 Practice Material for xam 1 This is a compilation from relevant problems from last spring s exam 1&2. This is too long! 1. (4 pts) Draw 2 of the following 3: Cross one out or graded in order.
Chemistry Organic Chemistry II: Reactivity and Synthesis ANSWERS FOR FINAL EXAM Winter 2004
 ASWER KEY Page 1 of 18 Chemistry 2.222 rganic Chemistry II: Reactivity and Synthesis ASWERS FR FIAL EXAM Winter 2004 Paper umber 631 Thursday April 22, 2004 1:30 4:30 pm University Centre Rm 210-224 Students
ASWER KEY Page 1 of 18 Chemistry 2.222 rganic Chemistry II: Reactivity and Synthesis ASWERS FR FIAL EXAM Winter 2004 Paper umber 631 Thursday April 22, 2004 1:30 4:30 pm University Centre Rm 210-224 Students
Chapter 18 Ketones and Aldehydes
 Chapter 18 Ketones and Aldehydes omenclature o Ketones have priority over alcohols Find the longest chain with the carbonyl in it. ame the parent and replace the e with one Say where the carbonyl is. octan-3-one
Chapter 18 Ketones and Aldehydes omenclature o Ketones have priority over alcohols Find the longest chain with the carbonyl in it. ame the parent and replace the e with one Say where the carbonyl is. octan-3-one
Chem 342 Organic Chemistry II Final Exam 13 May 2009
 hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
CHEM 2312 practice final. Version - II
 EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
CHEM J-8 June Complete the following table. Make sure you give the name of the starting material where indicated. REAGENTS/ CONDITIONS
 CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
Unit 5: Organic Chemistry
 Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE FINAL EXAMINATION CHEMISTRY 353
 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EAMINATIN CEMISTRY 353 April 25 th, 2000 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for TR lectures)
TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EAMINATIN CEMISTRY 353 April 25 th, 2000 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for TR lectures)
Chapter 16: Aromatic Compounds
 Chamras Chemistry 106 Lecture otes xamination 2 Materials Chapter 16: Aromatic Compounds Benzene, the Most Commonly Known Aromatic Compound: The aromatic nature of benzene stabilizes it 36 kcal.mol 1.
Chamras Chemistry 106 Lecture otes xamination 2 Materials Chapter 16: Aromatic Compounds Benzene, the Most Commonly Known Aromatic Compound: The aromatic nature of benzene stabilizes it 36 kcal.mol 1.
CHEM 303 Organic Chemistry II Exam II 11-April-2006 Answers
 CEM 303 rganic Chemistry II Exam II 11-April-2006 Answers 1) Compound D, C 8 14, is converted by C 2 =PPh 3 into E, C 9 16. Treatment of D with LiAl 4 yields two isomeric products, F and G, both of formula
CEM 303 rganic Chemistry II Exam II 11-April-2006 Answers 1) Compound D, C 8 14, is converted by C 2 =PPh 3 into E, C 9 16. Treatment of D with LiAl 4 yields two isomeric products, F and G, both of formula
CHAPTER 21 HW: ALDEHYDES + KETONES
 CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). C Structure 2 ame Structure ame 2. Draw each compound. Structure ame dicyclohexyl ketone 1,1,1-trifluoro-3-pentanone
CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). C Structure 2 ame Structure ame 2. Draw each compound. Structure ame dicyclohexyl ketone 1,1,1-trifluoro-3-pentanone
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
CHEM 213 FALL 2018 MIDTERM EXAM 2 - VERSION A
 CEM 213 FALL 2018 MIDTERM EXAM 2 - VERSIN A Answer multiple choice questions on the green computer sheet provided with a PENCIL. Be sure to encode both your NAME and Registration Number (V#). You will
CEM 213 FALL 2018 MIDTERM EXAM 2 - VERSIN A Answer multiple choice questions on the green computer sheet provided with a PENCIL. Be sure to encode both your NAME and Registration Number (V#). You will
CHAPTER 21 HW: ALDEHYDES + KETONES
 CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). Structure 6 5 4 1 C 2 ame 3,3-dimethyl-2-pentanone (or 1,1-dimethylpropyl methyl ketone) 5-hydroxy-4-methylhexanal
CAPTER 21 W: ALDEYDES + KETES MECLATURE 1. Give the name for each compound (IUPAC or common name). Structure 6 5 4 1 C 2 ame 3,3-dimethyl-2-pentanone (or 1,1-dimethylpropyl methyl ketone) 5-hydroxy-4-methylhexanal
Exam I 19 April 2004 Name:
 Chem 317 S04 Page 1 of 7 Kantorowski Exam I 19 April 2004 Name: This exam contains 6 pages of questions confirm this once you begin The last page is a spectral data table that can be removed You will have
Chem 317 S04 Page 1 of 7 Kantorowski Exam I 19 April 2004 Name: This exam contains 6 pages of questions confirm this once you begin The last page is a spectral data table that can be removed You will have
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Departmental Final Examination. Organic Chemistry I Caffein
 Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
CHEMISTRY MIDTERM # 2 November 02, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
Introduction to Organic Chemistry. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Introduction to Organic Chemistry Copyright The McGraw-ill Companies, Inc. Permission required for reproduction or display. 1 Common Elements in Organic Compounds 2 Classification of ydrocarbons ydrocarbons
Introduction to Organic Chemistry Copyright The McGraw-ill Companies, Inc. Permission required for reproduction or display. 1 Common Elements in Organic Compounds 2 Classification of ydrocarbons ydrocarbons
Chem 213 Final 2012 Detailed Solution Key for Structures A H
 Chem 213 Final 2012 Detailed Solution Key for Structures A H COMPOUND A on Exam Version A (B on Exam Version B) C 8 H 6 Cl 2 O 2 DBE = 5 (aromatic + 1) IR: 1808 cm 1 suggests an acid chloride since we
Chem 213 Final 2012 Detailed Solution Key for Structures A H COMPOUND A on Exam Version A (B on Exam Version B) C 8 H 6 Cl 2 O 2 DBE = 5 (aromatic + 1) IR: 1808 cm 1 suggests an acid chloride since we
Chem 2320 Final 210 points Dr. Luther Giddings
 Chem 2320 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Chem 2320 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Faculty of Science Mid-Term Examination I. Chem 222/234 Introductory Organic Chemistry I
 Student Name: Student ID #: Faculty of Science Mid-Term Examination I Chem 222/234 Introductory rganic Chemistry I Examiner: Professor James L. Gleason Tuesday, ctober 6, 2009 Associate Examiner: Professor
Student Name: Student ID #: Faculty of Science Mid-Term Examination I Chem 222/234 Introductory rganic Chemistry I Examiner: Professor James L. Gleason Tuesday, ctober 6, 2009 Associate Examiner: Professor
Organic Chemistry. It s all about the charges!
 Organic Chemistry It s all about the charges! Hydrocarbons So far, we ve mostly looked at hydrocarbons: alkanes, alkenes, alkynes, and benzene. Hydrocarbons are NON-polar molecules: the C-H bond has an
Organic Chemistry It s all about the charges! Hydrocarbons So far, we ve mostly looked at hydrocarbons: alkanes, alkenes, alkynes, and benzene. Hydrocarbons are NON-polar molecules: the C-H bond has an
Infrared Spectroscopy used to analyze the presence of functional groups (bond types) in organic molecules How IR spectroscopy works:
 Infrared Spectroscopy used to analyze the presence of functional groups (bond types) in organic molecules It is the study of the interaction of infrared energy with organic molecules; the process analyzes
Infrared Spectroscopy used to analyze the presence of functional groups (bond types) in organic molecules It is the study of the interaction of infrared energy with organic molecules; the process analyzes
Common Elements in Organic Compounds
 Organic hemistry ommon Elements in Organic ompounds lassification of ydrocarbons Alkanes Alkanes have the general formula n 2n+2 where n = 1,2,3, only single covalent bonds saturated hydrocarbons because
Organic hemistry ommon Elements in Organic ompounds lassification of ydrocarbons Alkanes Alkanes have the general formula n 2n+2 where n = 1,2,3, only single covalent bonds saturated hydrocarbons because
, by reacting CH 3 with ethanoic anhydride, (CH 3
 1 A chemist prepares and analyses some esters. (a) The chemist prepares an ester of propan-2-ol, H 3 H(H)H 3, by reacting H 3 H(H)H 3 with ethanoic anhydride, (H 3 ) 2. Using structural formulae, write
1 A chemist prepares and analyses some esters. (a) The chemist prepares an ester of propan-2-ol, H 3 H(H)H 3, by reacting H 3 H(H)H 3 with ethanoic anhydride, (H 3 ) 2. Using structural formulae, write
235 Organic II. Final Exam Review REACTIONS OF CONJUGATED DIENES 1,2 VS 1,4 ADDITION REACTIONS OF CONJUGATED DIENES
 b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
Organic Chemistry II KEY March 25, a) I only b) II only c) II & III d) III & IV e) I, II, III & IV
 rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
The resonance frequency of the H b protons is dependent upon the orientation of the H a protons with respect to the external magnetic field:
 Spin-Spin Splitting in Alkanes The signal arising from a proton or set of protons is split into (N+1) lines by the presence of N adjacent nuclei Example 1: Bromoethane The resonance frequency of the H
Spin-Spin Splitting in Alkanes The signal arising from a proton or set of protons is split into (N+1) lines by the presence of N adjacent nuclei Example 1: Bromoethane The resonance frequency of the H
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE FINAL EXAMINATION CHEMISTRY 353
 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 353 April 20 th, 1999 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for TR lectures)
TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 353 April 20 th, 1999 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for TR lectures)
2 Answer all the questions. 1 Alkenes and benzene both react with bromine but alkenes are much more reactive.
 2 Answer all the questions. 1 Alkenes and benzene both react with bromine but alkenes are much more reactive. (a) Explain the relative resistance to bromination of benzene compared with alkenes. In your
2 Answer all the questions. 1 Alkenes and benzene both react with bromine but alkenes are much more reactive. (a) Explain the relative resistance to bromination of benzene compared with alkenes. In your
Topic 4 Aldehydes and Ketones
 4-1 Topic 4 Aldehydes and Ketones 16.1 4-2 Aldehydes and Ketones ' aldehyde ketone The polarized oxygen-carbon -bond renders aldehydes and ketones electrophilic: ' The electrophilicity of the oxygen-carbon
4-1 Topic 4 Aldehydes and Ketones 16.1 4-2 Aldehydes and Ketones ' aldehyde ketone The polarized oxygen-carbon -bond renders aldehydes and ketones electrophilic: ' The electrophilicity of the oxygen-carbon
Paper 12: Organic Spectroscopy
 Subject hemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 34: ombined problem on UV, IR, 1 H NMR, 13 NMR and Mass- Part 6 HE_P12_M34 TABLE OF ONTENTS 1. Learning
Subject hemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 34: ombined problem on UV, IR, 1 H NMR, 13 NMR and Mass- Part 6 HE_P12_M34 TABLE OF ONTENTS 1. Learning
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
*Assignments could be reversed. *
 Name Key 5 W-Exam No. Page I. (6 points) Identify the indicated pairs of hydrogens in each of the following compounds as (i) homotopic, (ii) enantiotopic, or (iii) diastereotopic s. Write the answers as
Name Key 5 W-Exam No. Page I. (6 points) Identify the indicated pairs of hydrogens in each of the following compounds as (i) homotopic, (ii) enantiotopic, or (iii) diastereotopic s. Write the answers as
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
STRUCTURE ELUCIDATION BY INTEGRATED SPECTROSCOPIC METHODS
 Miscellaneous Methods UNIT 14 STRUCTURE ELUCIDATION BY INTEGRATED SPECTROSCOPIC METHODS Structure 14.1 Introduction Objectives 14.2 Molecular Formula and Index of Hydrogen Deficiency 14.3 Structural Information
Miscellaneous Methods UNIT 14 STRUCTURE ELUCIDATION BY INTEGRATED SPECTROSCOPIC METHODS Structure 14.1 Introduction Objectives 14.2 Molecular Formula and Index of Hydrogen Deficiency 14.3 Structural Information
Organic Chemistry. Radical Reactions
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Radical Reactions by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Radical Reactions by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
2. Examining the infrared spectrum of a compound allows us to:
 CHEM 204 2010 Ass. 1 Problem 1. The amount of energy in infrared light corresponds to: a. the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital b. the amount
CHEM 204 2010 Ass. 1 Problem 1. The amount of energy in infrared light corresponds to: a. the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital b. the amount
NH 2 O O O O OCH (6 pts) Provide an acceptable name for each of the following compounds: NH 2 COOH HOOC COOH. piperidine
 CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
Organic Chemistry. A. Introduction
 Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
N_HW1 N_HW1. 1. What is the purpose of the H 2 O in this sequence?
 N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
PAPER No.12 :Organic Spectroscopy MODULE No.30: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part II
 Subject Chemistry Paper No and Title Module No and Title Module Tag 12 : rganic Spectroscopy 30: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass Part-II CHE_P12_M30 TABLE F CNTENTS 1. Learning utcomes
Subject Chemistry Paper No and Title Module No and Title Module Tag 12 : rganic Spectroscopy 30: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass Part-II CHE_P12_M30 TABLE F CNTENTS 1. Learning utcomes
CHAPTER 16 - CHEMISTRY OF BENZENE: ELECTROPHILIC AROMATIC SUBSTITUTION
 CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
 Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY Purpose: This is an exercise to introduce the use of nuclear magnetic resonance spectroscopy, in conjunction with infrared spectroscopy, to determine
Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY Purpose: This is an exercise to introduce the use of nuclear magnetic resonance spectroscopy, in conjunction with infrared spectroscopy, to determine
