Tautomerism and Keto Enol Equilibrium
|
|
|
- Julie Jennings
- 6 years ago
- Views:
Transcription
1
2 Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability of keto and enol forms Enamines & metalloenamines, their nitrogen counterparts, are (almost) as important For simple ketones and aldehydes, keto form is favored by 10 5 to Enol form can be stabilized by increased substitution and conjugation of the alkene. Tautomers: Structural isomers generated as a consequence of the a proton shift. For example:
3 Formation of Metal Enolates General methods for enolate formation The Ireland Model Deprotonation properties of metal enolates Soft Enolization Reduction of a-halocarbonyls Reformatsky reaction Reduction of enones Decarboxylation Acidity of Carbonyls Bulky secondary amines favor formation of E enolates. Large R substituent on carbonyls and weaker amide bases that lead to latter transition states, favor formation of Z enolates. Unless stated otherwise, kinetic distribution of products obtained. Kresge, J. Am. Chem. Soc. 1984, 106, 460. Diastereoselectivity in Enolate Formation Z and E descriptors are used without regard for the nature of the R substituent. It is the matter of definition (convention). Lithium amides are usually used in etherial solvents, THF being the most common. Z enolates are thermodynamically more stable, but the preference is usually small. Proper choice of reaction conditions allows selective formation of either dieastereoisomer.
4 Regioselectivuty: Kinetic vs. Thermodynamic Control Influence of the carbonyl structure. Collum Results Collum, D. B. J. Am. Chem. Soc. 2003, 125, The observed rate acceleration in the presence of Et 3 N has been attributed to the relief of strain in going from the initial complex with a carbonyl to the TS. Only one of the two possible regioisomers is observed. Why? Regioselectivity: Kinetic vs. Thermodynamic control Collum, D. B. J. Am. Chem. Soc. 2008, 130, Aggregation of the lithium amide, its complex with the carbonyl and the TS are all factors in determining the diastereoselectivity of enolization. Every combination of the solvent, Li ligand, and the amide base can in principle lead to a different set of intermediates and TSs. Number of experimental techniques including 6 Li NMR indicate that Ireland model is relevant only for LDA, THF combination. A Sterically hindered and strong bases, and low temperature favor the formation of the less substituted enolate. B More substituted enolates are thermodynamically more stable. Weaker base, small excess of the carbonyl, higher temperature and longer reaction times, all promote equilibration of the enolates and lead to the formation of a thermodynamic mixture of enolates.
5 Dramatic difference in kinetic and thermodynamic selectivity. Enolate Structure For alkali metal enolates (M = Li, Na, K etc.) the O-metal tautomer is strongly favored. This generalization holds for most alkaline earth enolates (Mg+2) as well. These are the generally useful enolate nucleophiles. For some late transition metals C-Metal tautomer is prefered. High selectivity can usually be achieved in deprotonation of enones. General method for the formation of thermodynamic enolates.
6 For NMR solution structures see: Collum, D. B. J. Am. Chem. Soc. 2008, 130, 4859.
7
8 Soft Enolization Lewis acid complexation dramatically enhances acidity of carbonyls. Examples Lithium enolates possible only with relatively acidic carbonyls Horner-Wadsworth-Emmons Reaction Kurti & Czako, Named Reactions in Organic Synthesis p 212. Magnesium enolates Horner-Wadsworth-Emmons Reaction Combination of Lewis acid and week base can also effect enolization. Approach complementary to the use of strong base. Enolate Carboxylation Acid-base complexation has to be reversible Rathke, M. W. J. Org. Chem. 1985, 50, Titanium enolates Kurti & Czako, p Knoevenagel condensation Lehnert, W. Tetrahedron Lett. 1970, 4723.
9 Evans Study Boron enolates More acidic boron reagents favor formation of Z enolate. Larger dialkylboron acids favor E enolates. Structure of the carbonyl compounds has a strong influence on the diastereoselectivity. i-pr 2 NEt, Et 3 N, N-Ethylpiperidine are suitable bases. DBU and tetramethylguanidine do not provide enolate. CH 2 Cl 2 is the only suitable solvent for these enolizations. Order of addition of reagents is important for TiCl 4. Enolization model: Order of addition of reagents is not important for i-proticl 3 or (i-pro) 2 TiCl 2. Enolizable substrates: Problematic substrates: Favored for triflate Favored for chloride
10 Reduction of a-halocarbonyls Reformatsky Reaction Kurti & Czako, Named Reactions in Organic Synthesis p Enolization can be achieved chemoselectively in the presence of other carbonyl compounds. Ideally suited for intramolecular reactions Reduction of Enones Solution of alkali metals (Li, Na, K) in NH 3 used as a reducing reagent at low temperature. Allows regioselective formation of both kinetic and thermodynamic enolates. The oldest method used to control regioselectivity. Other reducing reagents can be used including: L- and K-slectride, triethysilane in the presence of a metal catalyst, hydrogen with a catalyst (and many others). Even relatively sterically hindered ketones can be used. Decarboxylation Decarboxylative enolate formation is one of the key steps in biosynthesis of polyketides and fatty acids. Metals other than zinc can be used, and allow the reaction to be performed at a lower temperature. Sm(II) most commonly used. Molander, "Reductions with Samarium (II) Iodide." Org. Reactions 1994, 46, 211.
11 The timing of the decarboxylation is not firmly established but the order of events depicted below is most commonly encountered. Kinetic Acylation with methyl cyanoformate (The Mender reagent) At low temperature decomposition of the tetrahedral intermediate is much slower than acylation. Enolate Acylation Jaz, J. M. et al. Chem. Biol. 2000, 7, 919. Carbon Acylation with N-Methoxy-N-methylamides Nucleophilic addition to Weinreb amide results in the formation of a particularly stable tetrahedral intermediate. Reaction Thermodynamics: Overall Keq ~ 1 Final enolization Step: Keq ~ final enolization step does not render the process irreversible just thermodynamically favorable
12 In reactions with organometallic reagents ketones are formed. Aldehydes obtained in reactions with strong hydride donors. The Dieckmann Condensation Provide a detailed explanation for the observed selectivity in the two reactions.
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
به نام خدا روشهای سنتز مواد آلی
 به نام خدا روشهای سنتز مواد آلی 1 References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Reactions and Synthesis (Part B), 5th ed., Springer, 2007. 2. Carey, F. A.; Sundberg, R. J. Advanced
به نام خدا روشهای سنتز مواد آلی 1 References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Reactions and Synthesis (Part B), 5th ed., Springer, 2007. 2. Carey, F. A.; Sundberg, R. J. Advanced
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Another Equilibrium: Reaction At The α-position
 Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Chem 263 Nov 3, 2016
 hem 263 Nov 3, 2016 Preparation of Aldehydes from Acid alides? + l l acid chloride aka acyl chloride aldehyde Needed: 2 Actual eagents: 2 /Pd Al This is lithium tri-t-butoxy aluminum hydride, a very sterically
hem 263 Nov 3, 2016 Preparation of Aldehydes from Acid alides? + l l acid chloride aka acyl chloride aldehyde Needed: 2 Actual eagents: 2 /Pd Al This is lithium tri-t-butoxy aluminum hydride, a very sterically
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
acetaldehyde (ethanal)
 hem 263 Nov 2, 2010 Preparation of Ketones and Aldehydes from Alkenes zonolysis 1. 3 2. Zn acetone 1. 3 2. Zn acetone acetaldehyde (ethanal) Mechanism: 3 3 3 + - oncerted reaction 3 3 3 + ozonide (explosive)
hem 263 Nov 2, 2010 Preparation of Ketones and Aldehydes from Alkenes zonolysis 1. 3 2. Zn acetone 1. 3 2. Zn acetone acetaldehyde (ethanal) Mechanism: 3 3 3 + - oncerted reaction 3 3 3 + ozonide (explosive)
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Chapter 23. Alpha Substitution of Carbonyl Compounds.
 1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
Chem 263 Nov 7, elimination reaction. There are many reagents that can be used for this reaction. Only three are given in this course:
 hem 263 Nov 7, 2013 Preparation of Ketones and Aldehydes from Alcohols xidation of Alcohols [] must have at least 1 E elimination reaction [] = oxidation; removal of electrons [] = reduction; addition
hem 263 Nov 7, 2013 Preparation of Ketones and Aldehydes from Alcohols xidation of Alcohols [] must have at least 1 E elimination reaction [] = oxidation; removal of electrons [] = reduction; addition
Chapter 19 Substitutions at the Carbonyl Group
 Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
ENOLATES IN ORGANIC SYNTHESIS
 ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
The problem is that your product still has a-protons, and can keep on forming enolates to get more methyl groups added:
 Lecture 13 Notes November 7, 2012 We are going to start with some examples of proline-catalyzed reactions. In each case, you can draw a mechanism that would involve proline as a chiral catalyst, and one
Lecture 13 Notes November 7, 2012 We are going to start with some examples of proline-catalyzed reactions. In each case, you can draw a mechanism that would involve proline as a chiral catalyst, and one
Important Concepts. Problems. Chapter Problems. Cuprate additions followed by enolate alkylations. 15. Michael Addition (Section 18-11)
 Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
THE CHEMISTRY OF THE CARBONYL GROUP
 TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
First Year Organic Chemistry THE CHEMISTRY OF THE CARBONYL GROUP: CORE CARBONYL CHEMISTRY
 First Year rganic Chemistry TE CEMISTY F TE CABNYL GUP: CE CABNYL CEMISTY Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2015 Wednesdays at 9am; Fridays at 10am (Dyson Perrins) andout A You will be able
First Year rganic Chemistry TE CEMISTY F TE CABNYL GUP: CE CABNYL CEMISTY Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2015 Wednesdays at 9am; Fridays at 10am (Dyson Perrins) andout A You will be able
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Chapter 9 Aldehydes and Ketones
 Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
روشهای 2 سنتز مواد آلی
 به نام خدا Advanced Organic Chemistry روشهای 2 سنتز مواد آلی Dr Morteza Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir 2 Francis A. Carey University of Virginia
به نام خدا Advanced Organic Chemistry روشهای 2 سنتز مواد آلی Dr Morteza Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir 2 Francis A. Carey University of Virginia
CHAPTER 23 HW: ENOLS + ENOLATES
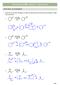 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Alkynes Nomenclature of Alkynes
 Chapter 7 Alkynes Alkynes - hydrocarbons containing a carbon-carbon triple bond (2 bonds) Acyclic alkanes = C n H 2n+2 Alkenes and cyclic alkanes = C n H 2n Alkynes (and cyclic alkenes) = C n H 2n-2 The
Chapter 7 Alkynes Alkynes - hydrocarbons containing a carbon-carbon triple bond (2 bonds) Acyclic alkanes = C n H 2n+2 Alkenes and cyclic alkanes = C n H 2n Alkynes (and cyclic alkenes) = C n H 2n-2 The
Topic 9. Aldehydes & Ketones
 Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
A. Loupy, B.Tchoubar. Salt Effects in Organic and Organometallic Chemistry
 A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
Physical Properties. Alcohols can be: CH CH 2 OH CH 2 CH 3 C OH CH 3. Secondary alcohol. Primary alcohol. Tertiary alcohol
 Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
(c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
 KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
B X A X. In this case the star denotes a chiral center.
 Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Grant MacEwan University Fall 2012 CHEM 362 (AS 40) Advanced Organic Chemistry. Organic Chemistry by Clayden, Greeves, Warren and Wothers
 1 Instructor: Office: Grant MacEwan University Fall 2012 CHEM 362 (AS 40) Advanced Organic Chemistry Dr. Manzar Saberi 5-138 G, City Center Campus Phone: 497-4634 Lectures: Monday, Wednesday, Friday 11:00-12:00
1 Instructor: Office: Grant MacEwan University Fall 2012 CHEM 362 (AS 40) Advanced Organic Chemistry Dr. Manzar Saberi 5-138 G, City Center Campus Phone: 497-4634 Lectures: Monday, Wednesday, Friday 11:00-12:00
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Grant MacEwan University Fall 2010 CHEM 362 (270) Advanced Organic Chemistry. Organic Chemistry by Clayden, Greeves, Warren and Wothers
 1 Instructor: Office: Grant MacEwan University Fall 2010 CHEM 362 (270) Advanced Organic Chemistry Dr. Manzar Saberi 5-172 K, City Center Campus Phone: 497-4634 Lectures: Tuesday, Thursday, 12:30-2:00
1 Instructor: Office: Grant MacEwan University Fall 2010 CHEM 362 (270) Advanced Organic Chemistry Dr. Manzar Saberi 5-172 K, City Center Campus Phone: 497-4634 Lectures: Tuesday, Thursday, 12:30-2:00
Carboxylic Acids O R C + H + O - Chemistry 618B
 arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
Chapter 12: Carbonyl Compounds II
 Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton)
 314 Arrow Pushing practice/eauchamp 1 Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton) ucleophile = nucleus/positive loving = any general electron
314 Arrow Pushing practice/eauchamp 1 Electrophile = electron loving = any general electron pair acceptor = Lewis acid, (often an acidic proton) ucleophile = nucleus/positive loving = any general electron
O or R E + R. - Keto-Enol Tautomerization (enol form usually very minor for simple ketones)
 General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
Synthesis of Amines Amine Alkylation by SN2 reaction II. Reductive Amination
 Synthesis of Amines I. Amine Alkylation by SN2 reaction Amines can be alkylated in SN2 fashion by alkyl halides; primary halides are best for this purpose. This is not a practical reaction for formation
Synthesis of Amines I. Amine Alkylation by SN2 reaction Amines can be alkylated in SN2 fashion by alkyl halides; primary halides are best for this purpose. This is not a practical reaction for formation
Module No and Title. PAPER No: 5 ; TITLE : Organic Chemistry-II MODULE No: 25 ; TITLE: S E 1 reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Reversible Additions to carbonyls: Weak Nucleophiles Relative Reactivity of carbonyls: Hydration of Ketones and Aldehydes
 Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
(5) a. b. (6) N H CH 3 N H O H O (7) CH 3 O (8) OCH 3 2. explanation:
 ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
Stereoselective Organic Synthesis
 Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
20.3 Alkylation of Enolate Anions
 864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry
 UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
Enolates, Enols, and Enamines Part 3
 Enolates, Enols, and Enamines Part 3 Guanine Tautomerize 2 2 Thymine C 3 Tautomerize C 3 Thursday March 21, 8:00-11:00 AM Final Exam Topics Part A: Carbonyl Fundamentals Carbonyl Survey Enolates, Enols,
Enolates, Enols, and Enamines Part 3 Guanine Tautomerize 2 2 Thymine C 3 Tautomerize C 3 Thursday March 21, 8:00-11:00 AM Final Exam Topics Part A: Carbonyl Fundamentals Carbonyl Survey Enolates, Enols,
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
Carbonyl Chemistry IV + C O C. Lecture 10. Chemistry /30/02
 arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
Stereoselective reactions of enolates
 1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Keywords: amination ate complexes carbomagnesiation carbometalation Grignard reagents homocoupling magnesates magnesium compounds metalation
 VII Abstracts 2010 p1 7.6.5.6 Aryl Grignard Reagents H. Yorimitsu Halogen magnesium exchange between aryl iodides or bromides and an isopropylmagnesium chloride lithium chloride complex or lithium trialkylmagnesates
VII Abstracts 2010 p1 7.6.5.6 Aryl Grignard Reagents H. Yorimitsu Halogen magnesium exchange between aryl iodides or bromides and an isopropylmagnesium chloride lithium chloride complex or lithium trialkylmagnesates
ORGANIC - CLUTCH CH CONDENSATION CHEMISTRY.
 !! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
!! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Mild and Selective Hydrozirconation of Amides to Aldehydes Using Cp 2 Zr(H)Cl: Scope and Mechanistic Insight
 Mild and Selective Hydrozirconation of Amides to Aldehydes Using Cp 2 Zr(H)Cl: Scope and Mechanistic Insight Jared T. Spletstoser, Jonathan M. White, Ashok Rao Tunoori, and Gunda I. George J. Am. Chem.
Mild and Selective Hydrozirconation of Amides to Aldehydes Using Cp 2 Zr(H)Cl: Scope and Mechanistic Insight Jared T. Spletstoser, Jonathan M. White, Ashok Rao Tunoori, and Gunda I. George J. Am. Chem.
Organometallic Compounds of Magnesium *
 OpenStax-CNX module: m32494 1 Organometallic Compounds of Magnesium * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 While beryllium
OpenStax-CNX module: m32494 1 Organometallic Compounds of Magnesium * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 While beryllium
CHEM 345 Problem Set 07 Key
 CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
Page 1 of 9. Sessional Examination (November 2017) Max Marks: 20 Date: Time: One Hour. Model Answers
 Page 1 of 9 Sessional Examination (November 2017) Class: B. Pharm-II yr (III sem) Subject: Pharma Org. Chem-II Max Marks: 20 Date: 14.11.2017 Time: One Hour Model Answers Q. 1. Solve the following (ANY
Page 1 of 9 Sessional Examination (November 2017) Class: B. Pharm-II yr (III sem) Subject: Pharma Org. Chem-II Max Marks: 20 Date: 14.11.2017 Time: One Hour Model Answers Q. 1. Solve the following (ANY
Chapter 22 The Chemistry of Enolate Ions, Enols, and
 Organic Chemistry, 5th ed. Marc Loudon Chapter 22 The Chemistry of Enolate Ions, Enols, and α,β Unsaturated Carbonyl Compounds Eric J. Kantorowski California Polytechnic State University San Luis Obispo,
Organic Chemistry, 5th ed. Marc Loudon Chapter 22 The Chemistry of Enolate Ions, Enols, and α,β Unsaturated Carbonyl Compounds Eric J. Kantorowski California Polytechnic State University San Luis Obispo,
MITOCW watch?v=gboyppj9ok4
 MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 5 N S. Typical Reactivity of Pyridines, Quinolines and Isoquinolines
 Chapter 5 S Typical Reactivity of Pyridines, Quinolines and Isoquinolines 1 2 Typical Reactivity of Pyridines pyridines are much less susceptible to electrophilic substitution than benzene and much more
Chapter 5 S Typical Reactivity of Pyridines, Quinolines and Isoquinolines 1 2 Typical Reactivity of Pyridines pyridines are much less susceptible to electrophilic substitution than benzene and much more
CO 2 and CO activation
 2 and activation Most organic chemicals are currently made commercially from ethylene, a product of oil refining. Itispossiblethatinthenextseveraldecadeswemayhavetoshifttowardothercarbonsources for these
2 and activation Most organic chemicals are currently made commercially from ethylene, a product of oil refining. Itispossiblethatinthenextseveraldecadeswemayhavetoshifttowardothercarbonsources for these
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
